Modifiable Nutritional Biomarkers in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Vitamin D, B12, and Homocysteine Exposure Spanning Prenatal Development Through Late Adolescence
Abstract
1. Introduction
1.1. Main Review Question
- Null Hypothesis (H0): There is no statistically significant association between levels of vitamin D, vitamin B12, or homocysteine (measured before or after birth) and the risk, severity, or treatment outcomes of ASD.
- Alternative Hypothesis (H1): Deviations in vitamin D, vitamin B12, or homocysteine levels are significantly associated with ASD risk, symptom severity, or therapeutic outcomes, and may represent modifiable determinants of neurodevelopmental trajectories.
1.2. Background
1.2.1. The Role of Vitamin D, Vitamin B12, and Homocysteine in Neurodevelopment
1.2.2. Limitations of the Evidence
- Observational Designs Dominate the Field
- Small Sample Sizes and Methodological Heterogeneity in Trials
- Variability in Biomarker Definitions and Timing
- U-Shaped Associations Require Caution
1.2.3. How Up-to-Date Is This Evidence?
1.3. Rationale for This Review
1.3.1. Why Is This Review Scientifically and Clinically Significant?
1.3.2. Scientific Justifications for This Review
- a.
- Evidence Integration & Consensus Building
- b.
- Clinical Relevance & Public Health Implications
- c.
- Identification of Research Gaps & Methodological Limitations
- d.
- Lifespan and Mechanistic Perspective
1.4. Objectives and Methodological Framework
2. Materials and Methods
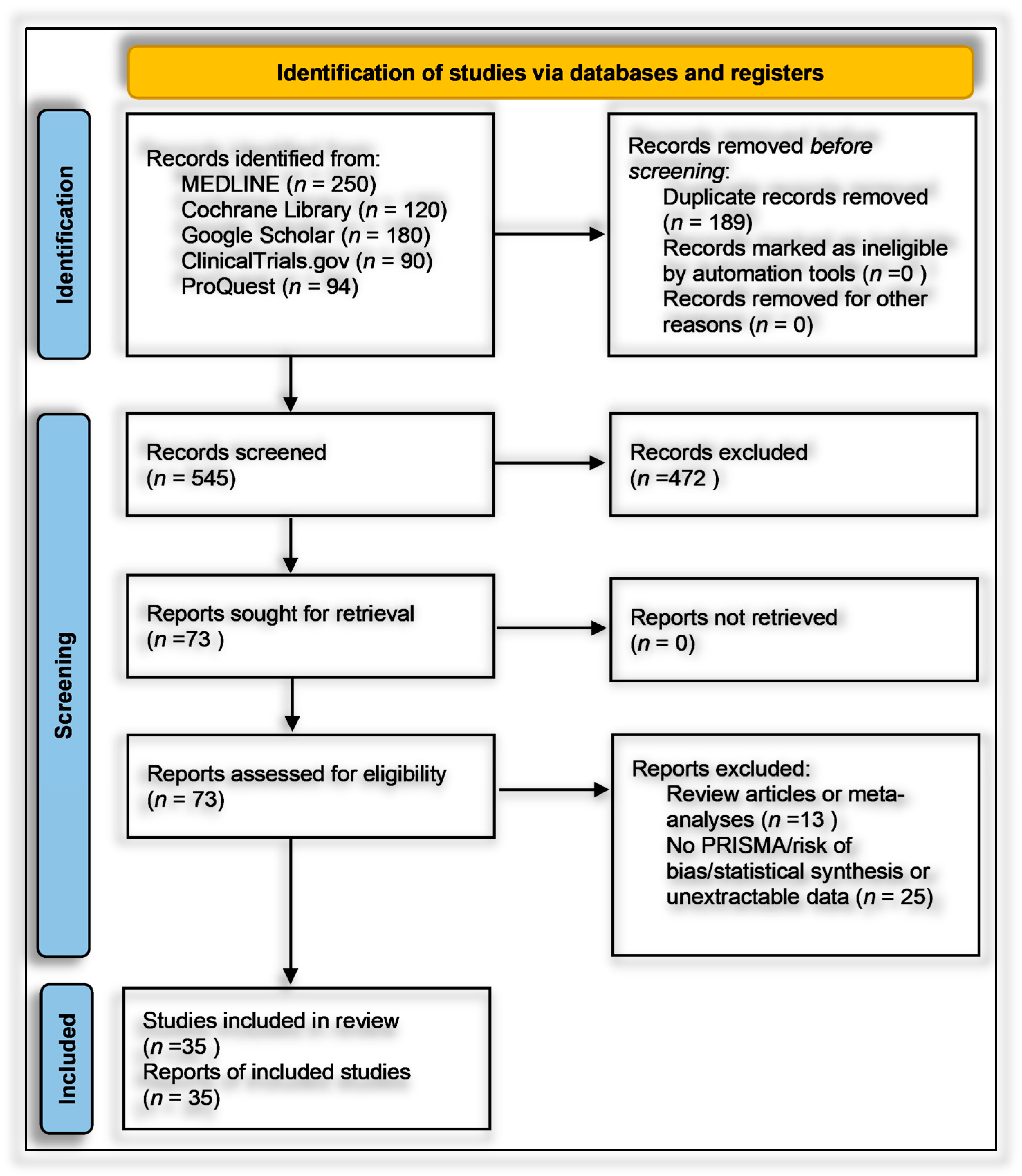
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Types of Interventions
2.4. Scientific Inference or Imputation
2.5. Software and Analytical Tools
2.6. Information Sources and Search Strategy
2.7. Study Selection
2.8. Data Extraction and Management
2.9. Risk of Bias and Quality Assessment
2.10. Data Synthesis and Statistical Analysis
3. Results
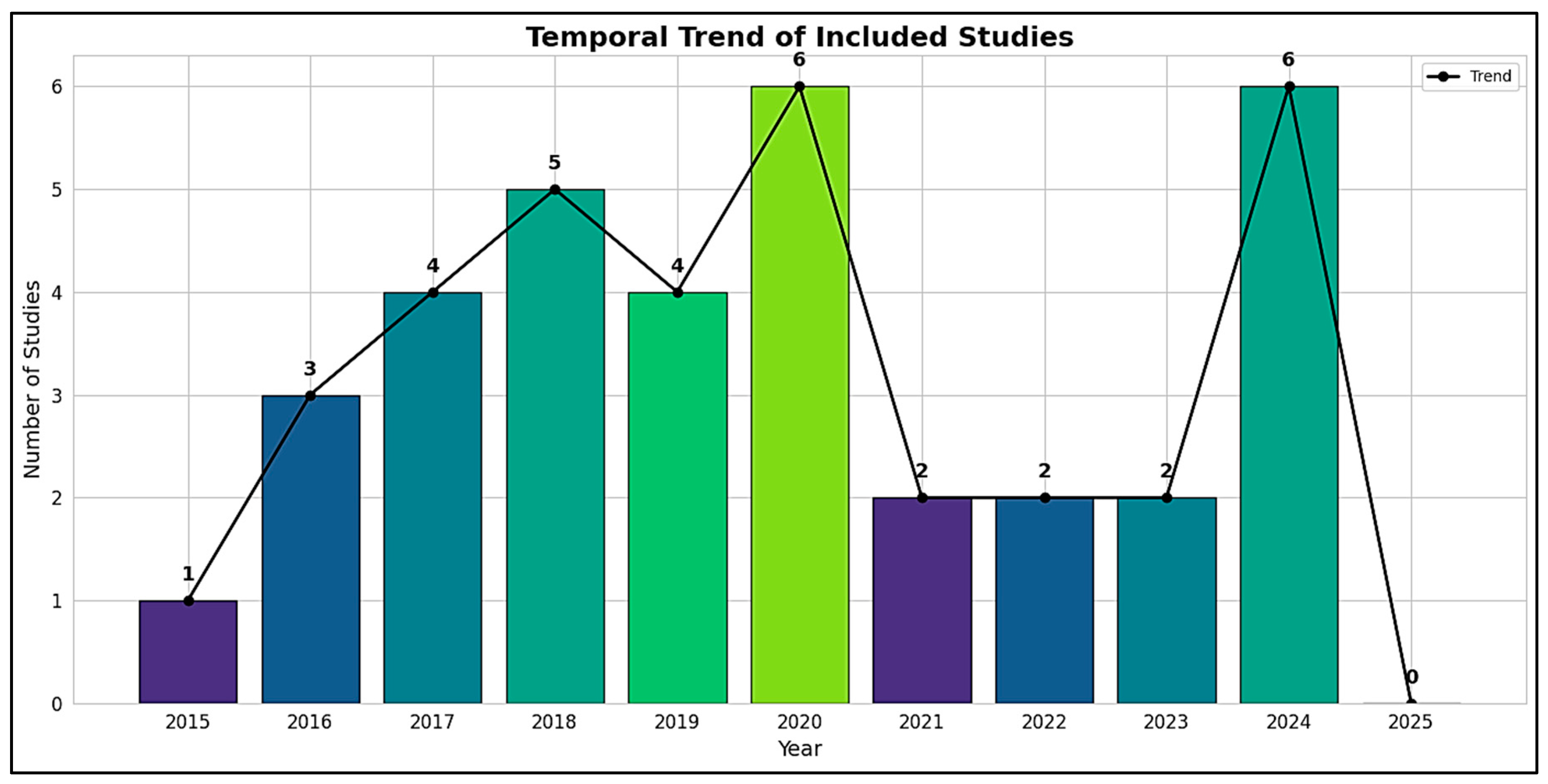
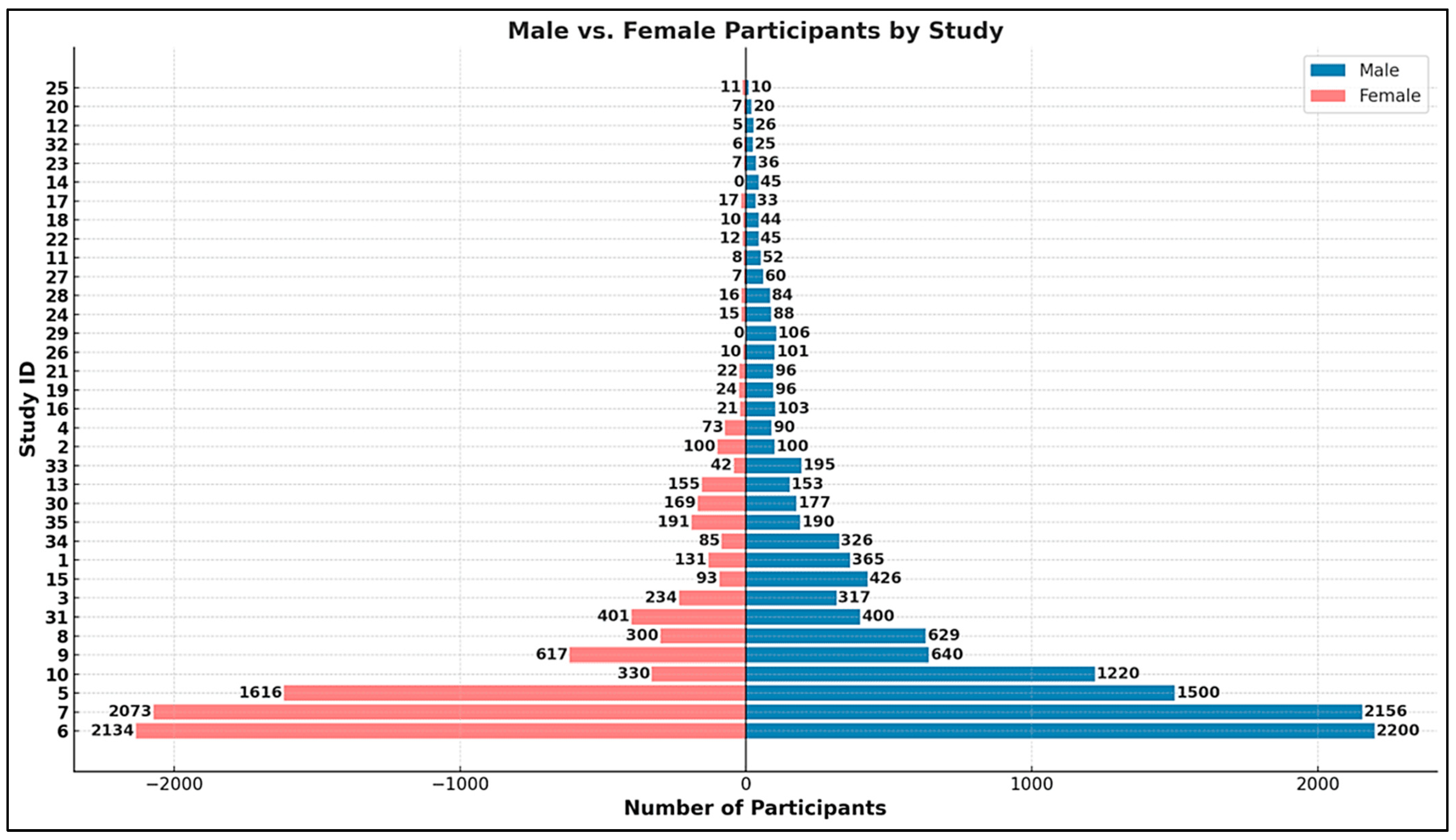
3.1. Statistical Analysis: Characteristics of Included Studies
- Europe (n = 14): Ireland (n = 1), Denmark (n = 2), Turkey (n = 3), Czech Republic (n = 1), Italy (n = 1), Netherlands (n = 2), Sweden (n = 1), Finland (n = 3);
- Middle East (n = 1): Qatar (n = 1);
- Asia (n = 10): Malaysia (n = 1), Iran (n = 3), China (n = 4), India (n = 1), Bangladesh (n = 1);
- Africa (n = 2): Egypt (n = 1), Libya (n = 1);
- North America (n = 6): United States of America (n = 6);
- Oceania (n = 2): New Zealand (n = 2).
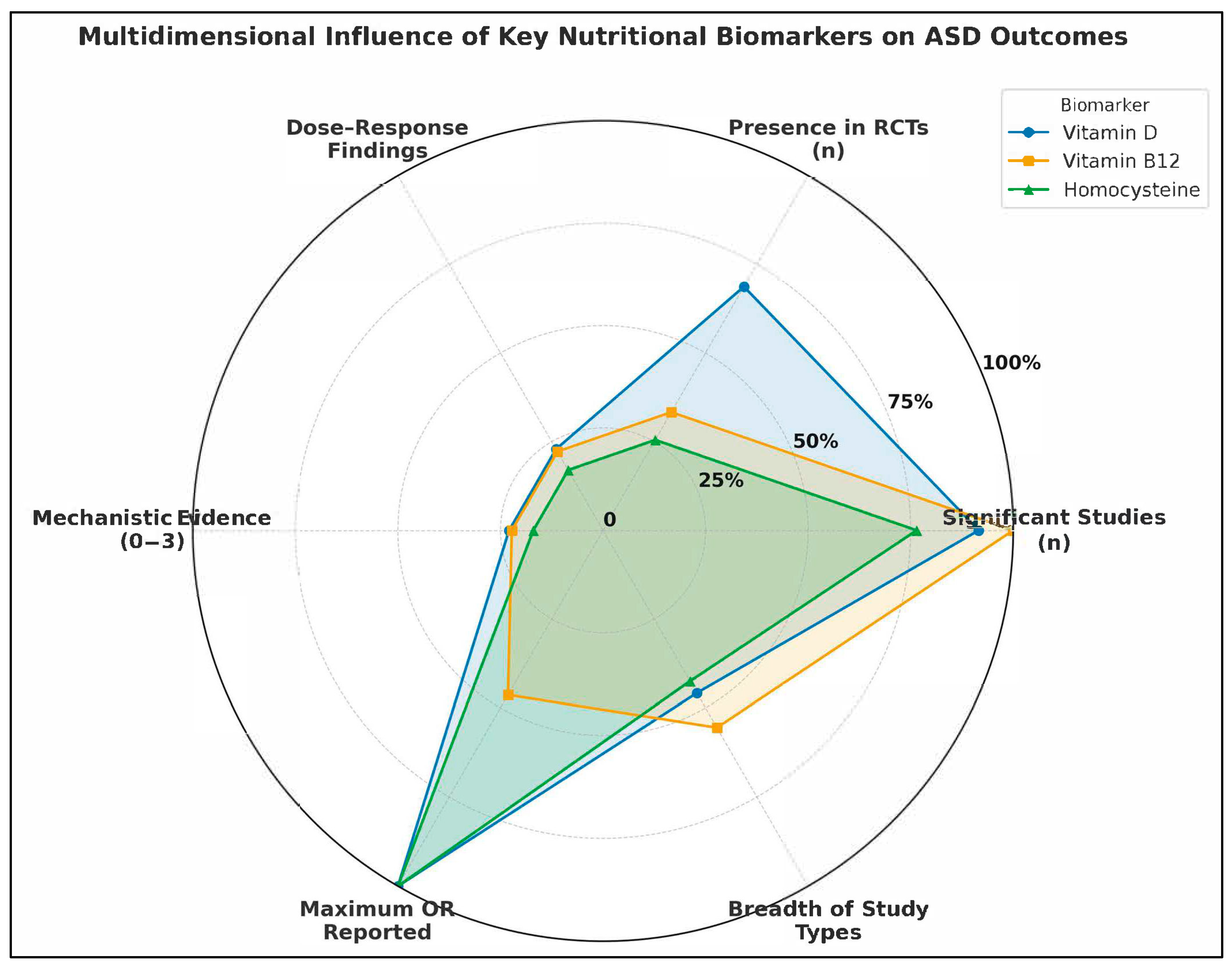
- Number of Studies Reporting Statistically Significant Associations (n): Reflects the empirical consistency across the literature.
- Presence in Randomized Controlled Trials (RCTs): Indicates causal inference strength and translational potential.
- Documented Dose-Response Relationships (binary count): Highlights biologically plausible gradient effects.
- Mechanistic Evidence Strength (scaled 0–3): Encodes pathway-based plausibility, such as links to methylation, inflammation, or neurotransmission.
- Maximum Reported Odds Ratio (OR): Reflects extremity of observed association, signaling possible high-risk subgroups.
- Breadth of Study Type Representation (0–6): Captures the diversity of methodological contexts in which the biomarker was studied (e.g., prenatal, postnatal, intervention, systems biology).
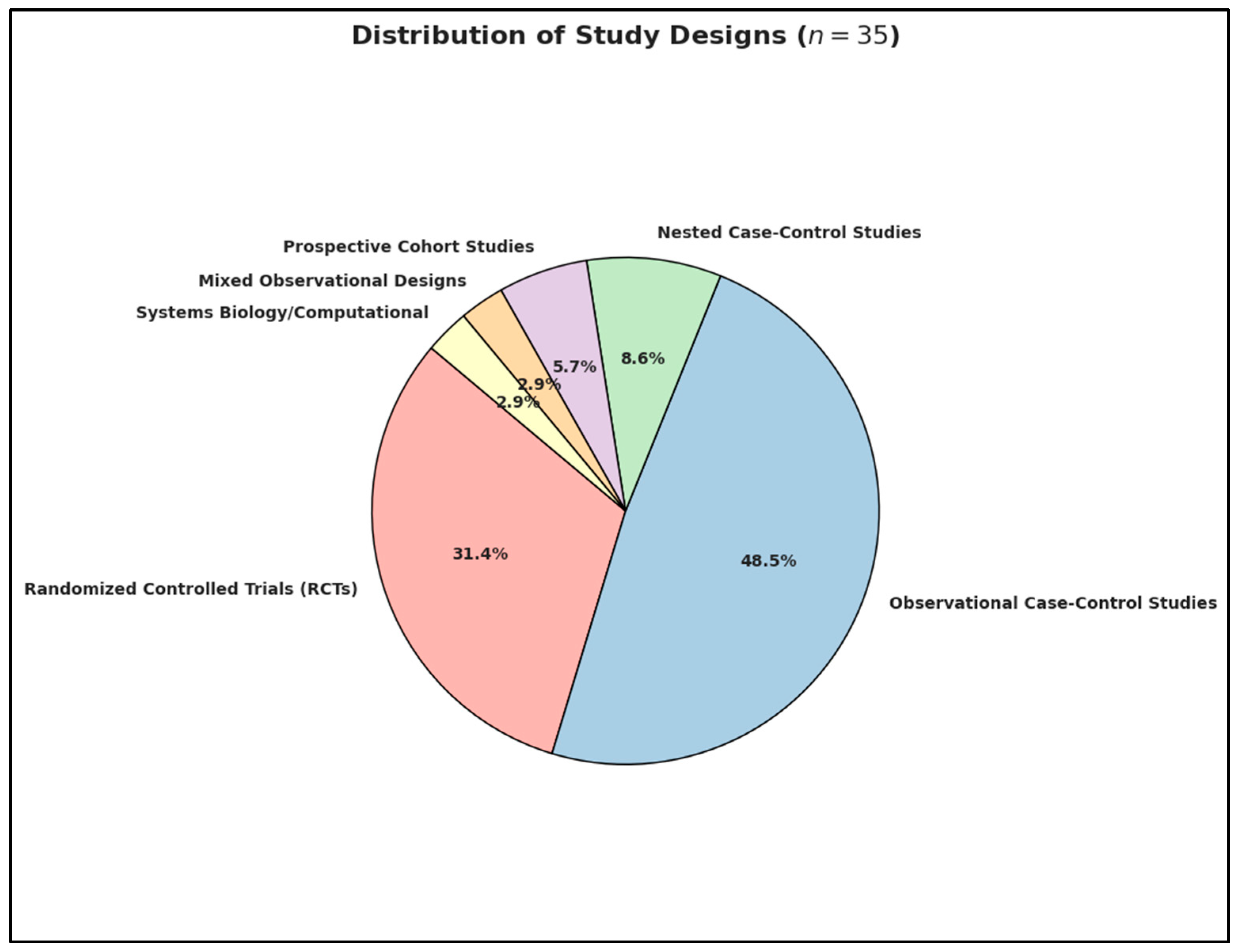
3.2. Overview of Included Studies and Synthesis Strategy
3.2.1. Prenatal Nutrient Exposure
3.2.2. Neonatal Nutrient Biomarkers
3.2.3. Postnatal/Early Childhood Nutrient Status
3.2.4. Postnatal Nutritional Interventions
3.2.5. Genetic–Nutrient Interaction Studies
3.2.6. Combined Behavioral–Nutritional Interventions
- Moradi et al.—Four-arm RCT: vitamin D, structured motor training, both, or placebo. The combined vitamin D + exercise group showed the greatest improvement on the Gilliam Autism Rating Scale (social interaction domain improved most; p < 0.001 for combination vs. control) and also significantly modulated inflammatory cytokines (↓IL-6, ↑IL-10). Motor training alone helped, and vitamin D alone helped, but the combination had an additive effect, highlighting that addressing nutritional deficits can potentiate behavioral interventions and vice versa [12].
- Mazahery et al.—Post-hoc inflammatory subgroup: as mentioned, vitamin D (and omega-3) supplementation led to greater SRS improvements in children with high baseline inflammation. Although all participants also received behavioral interventions as part of standard care, this finding suggests a biological subtype (inflammation-associated ASD) that might particularly benefit from nutritional add-ons. It underscores the principle of combined intervention tailoring: e.g., treating co-existing immune dysregulation nutritionally to allow behavioral therapies to be more effective [26].
- Saad et al.—Open-label D trial with behavioral assessment: though no formal non-nutrient therapy was introduced, caregivers reported qualitative improvements in behavior, attention, and eye contact post–vitamin D. The authors hypothesized that better sensory processing and engagement (possibly via vitamin D’s neuroactive properties) made the children more receptive to ongoing behavioral therapies and education. This anecdotal evidence complements the more structured combined interventions [85].
- No study in our set combined B12 with behavioral therapy explicitly in a factorial design, but Hendren et al. allowed stable behavioral therapies during the trial. Some secondary analyses indicated children in skill training programs improved slightly more if on B12 vs. placebo (non-significant trend), again hinting at synergy [25].
3.2.7. Synthesis of Patterns and Interpretation
3.3. Risk of Bias, Newcastle-Ottawa Scale (NOS) and Certainty of Evidence Evaluation According to GRADE
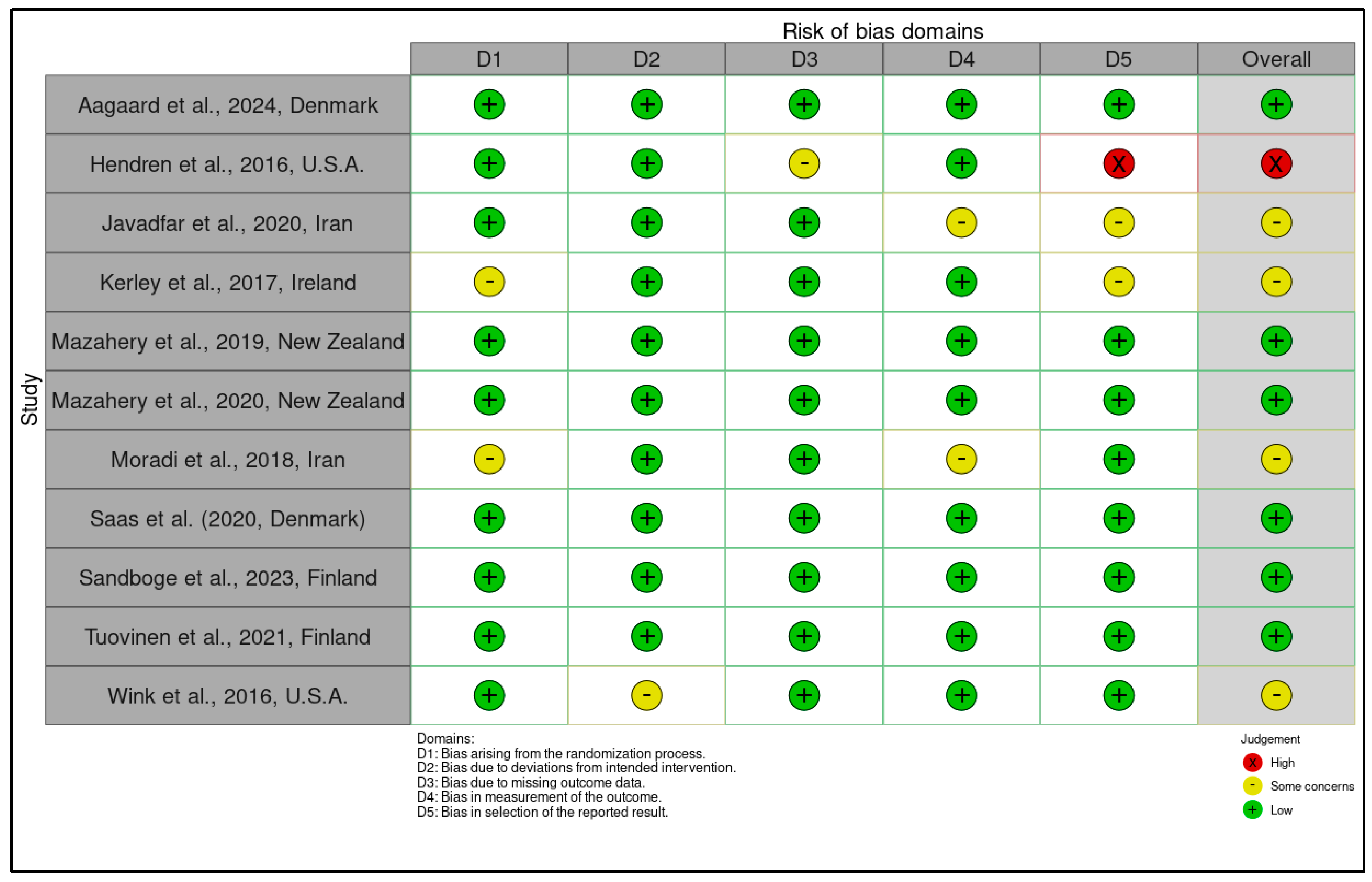
3.3.1. Risk of Bias Assessment Using the RoB 2 Tool for Randomized Controlled Trials and Certainty of Evidence Ratings According to the GRADE Framework
3.3.2. Quality Assessment of Observational Studies Using the Newcastle-Ottawa Scale (NOS) and Certainty of Evidence Evaluation According to GRADE Criteria
3.4. Stratified Meta-Analysis of Nutritional Exposures and Autism Risk
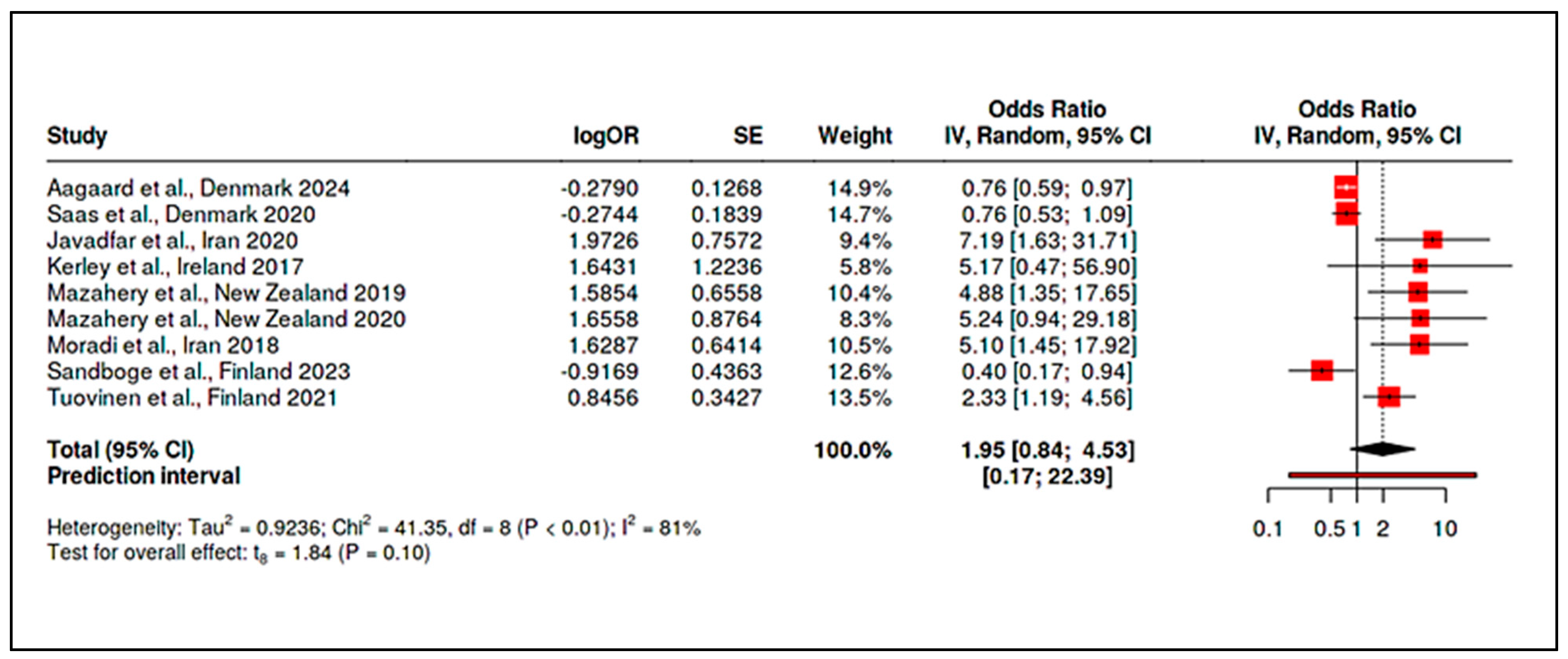
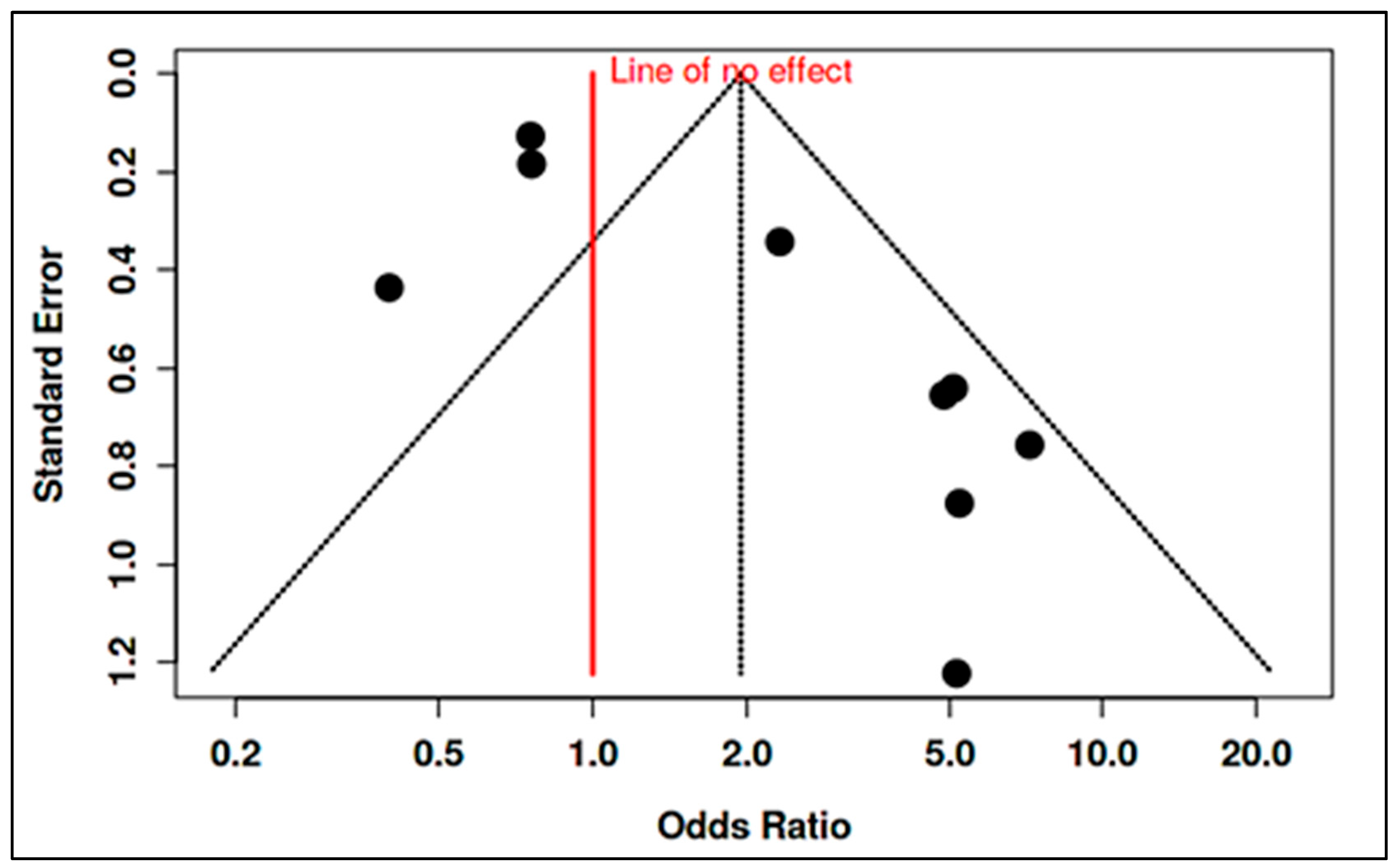
3.4.1. Meta-Analysis of Randomized Controlled Trials on Vitamin D
3.4.2. Meta-Analysis of Observational Studies on Vitamin D
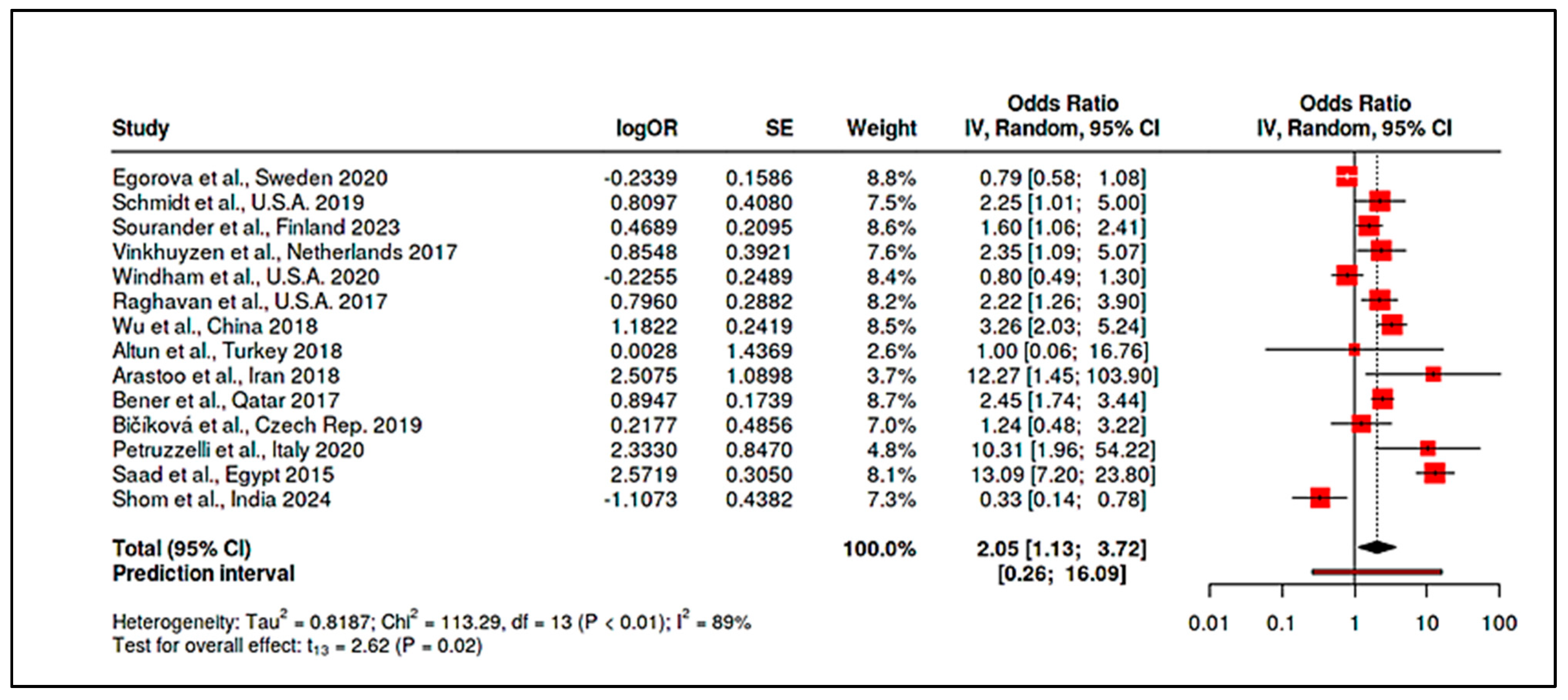
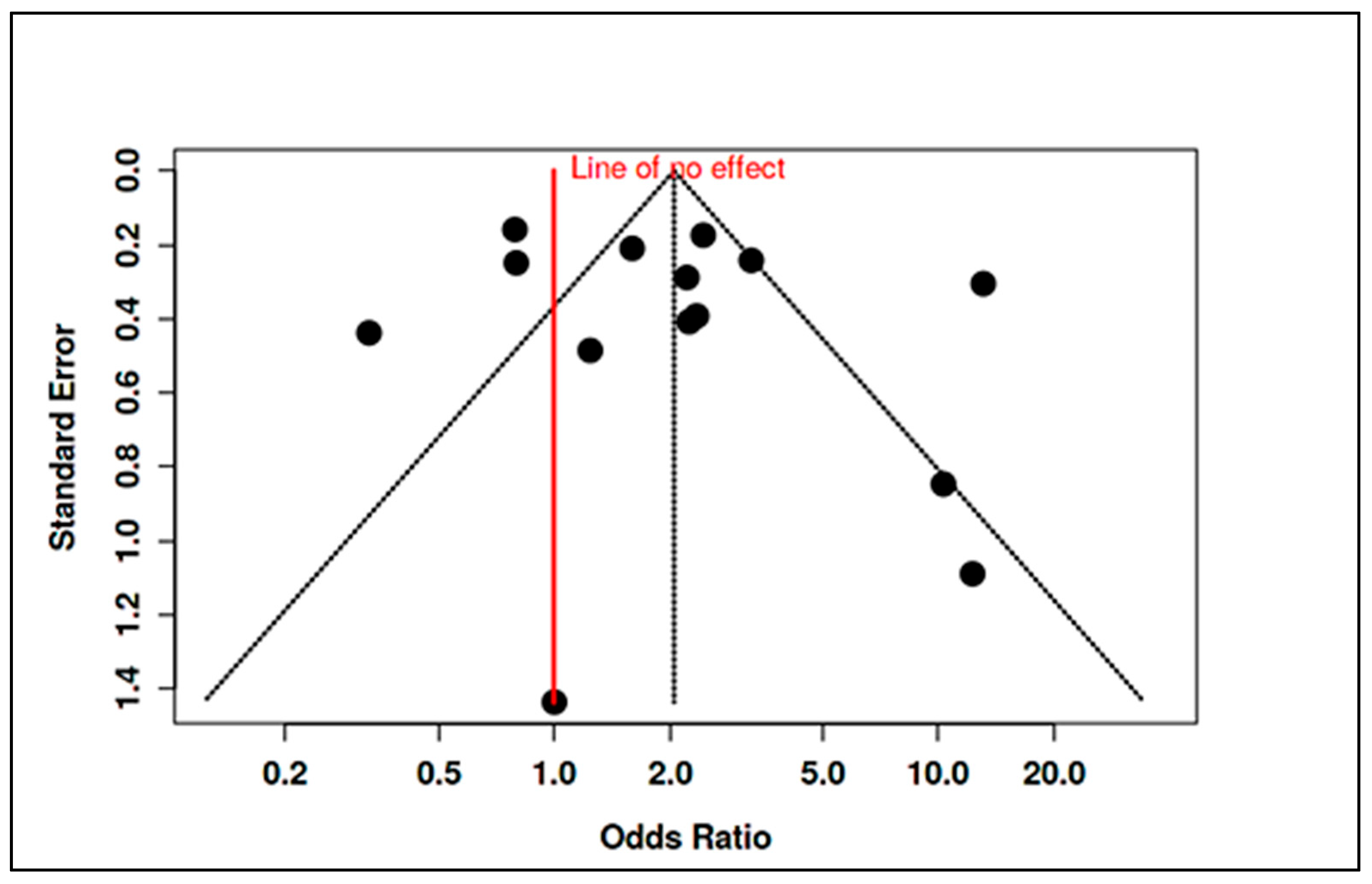
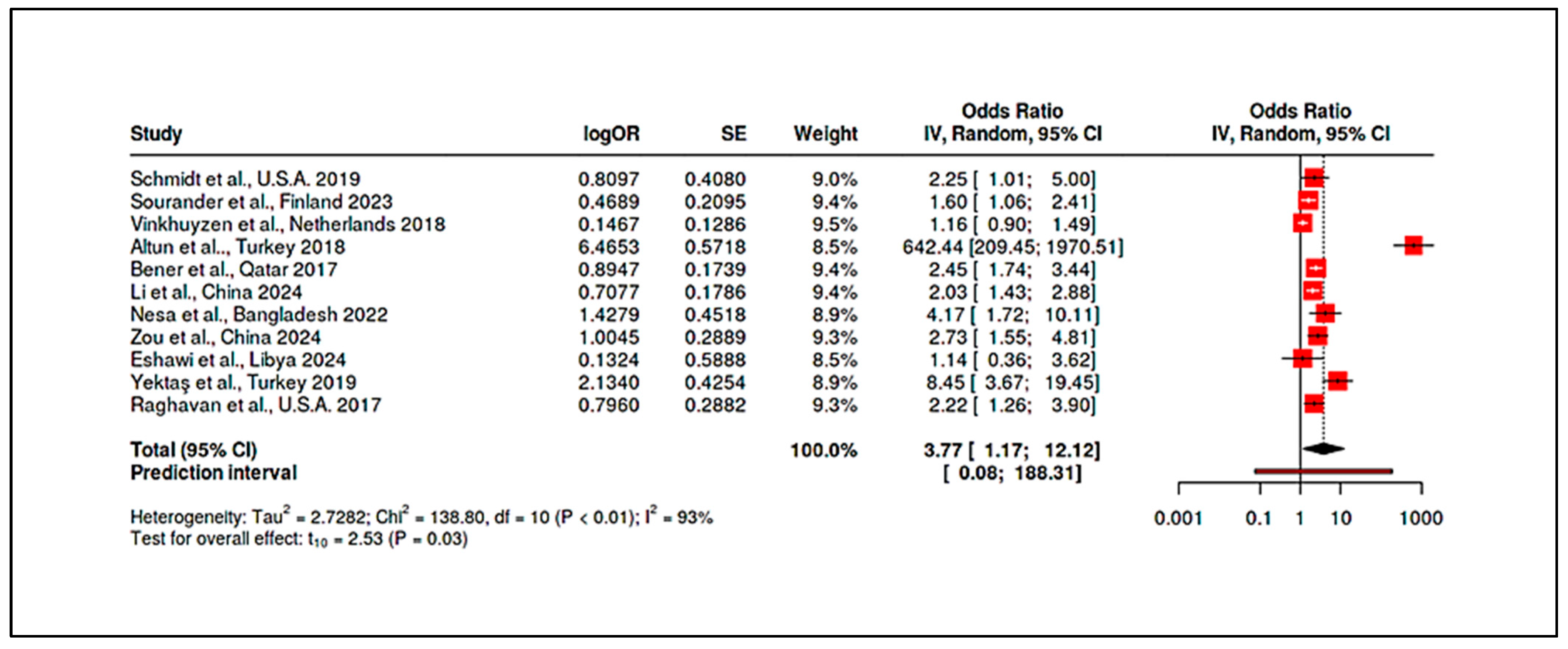
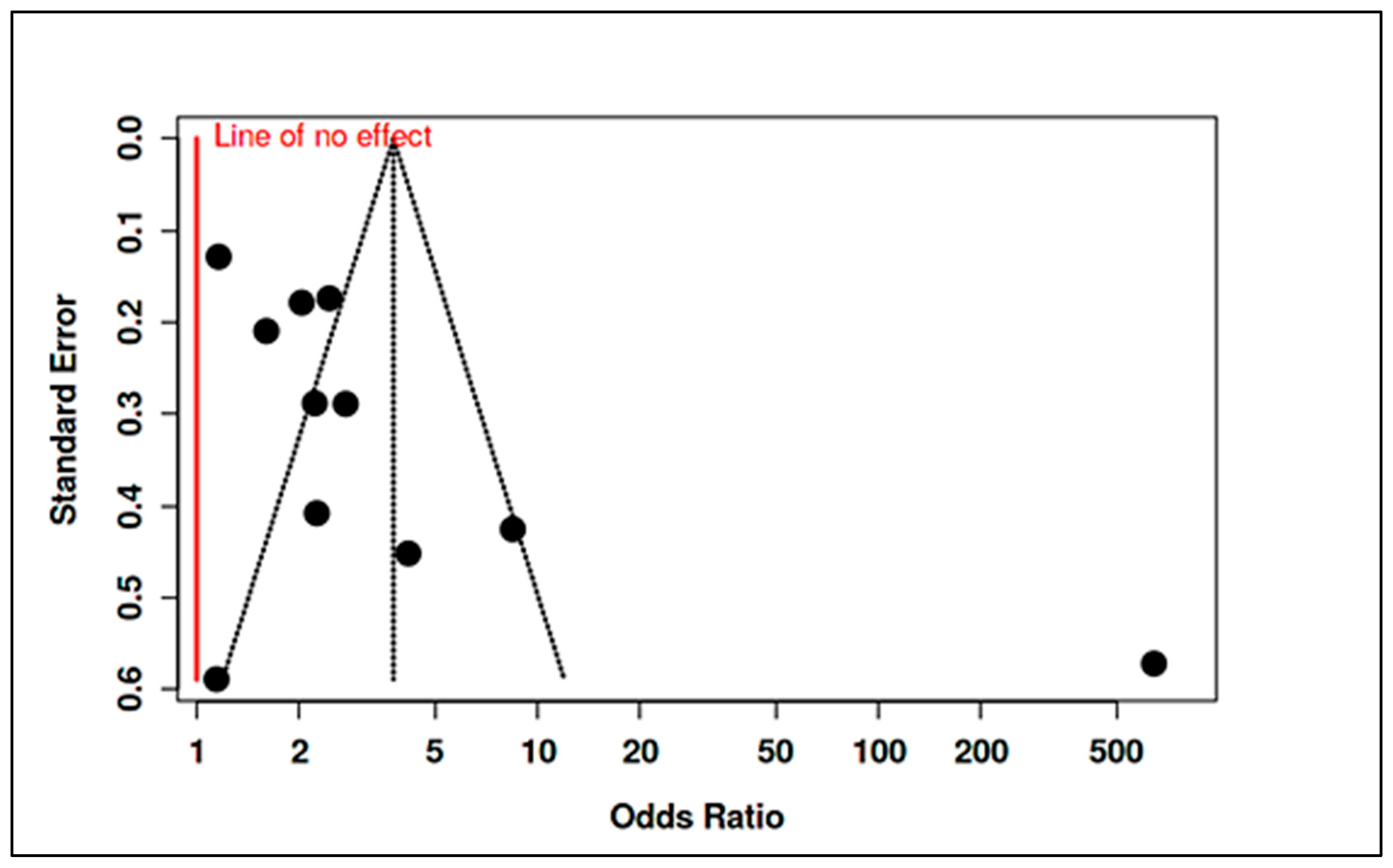
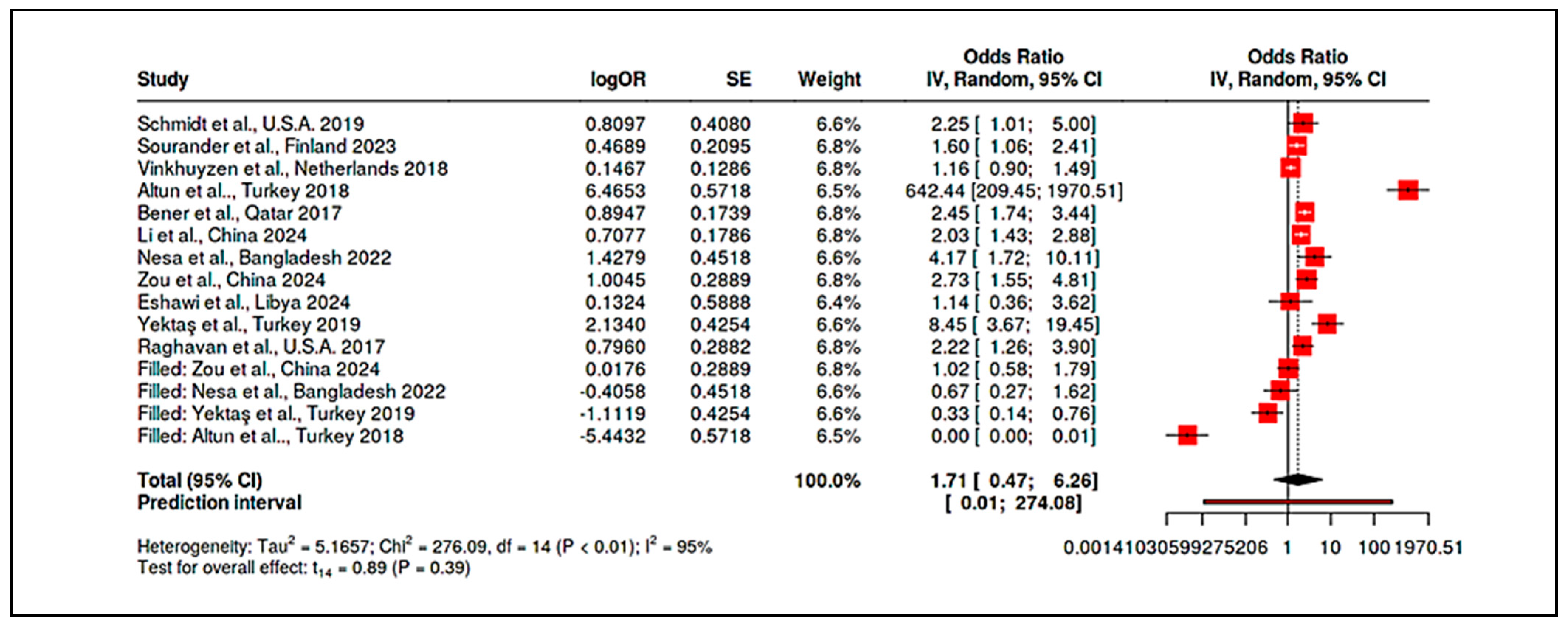
3.4.3. Meta-Analysis of Randomized Controlled Trials on Vitamin B12
3.4.4. Meta-Analysis of Observational Studies on Vitamin B12
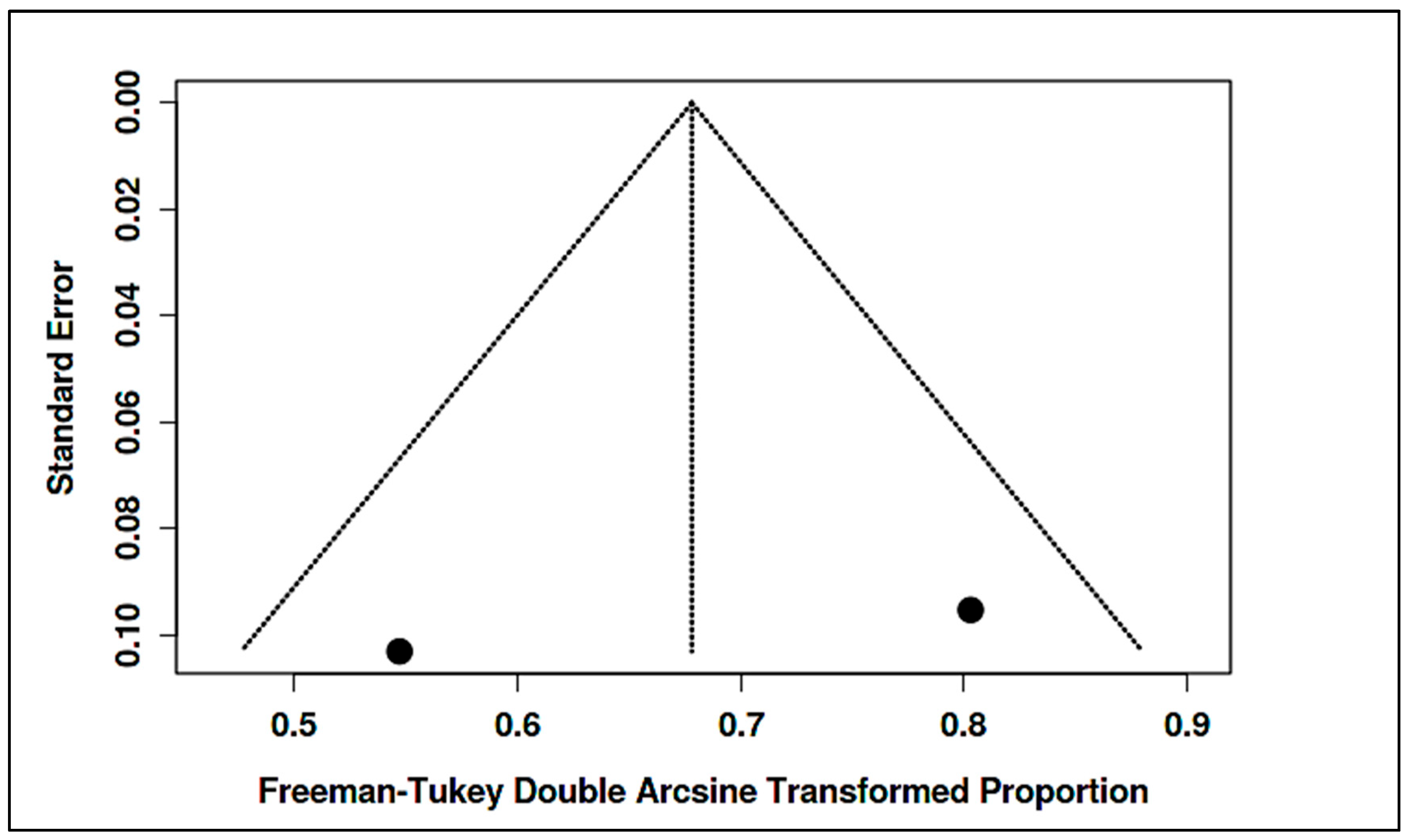
3.4.5. Meta-Analysis of Randomized Controlled Trials & Observational Studies on Homocysteine
4. Discussion
4.1. Answering the Research Hypothesis
4.2. Summary of Findings
4.3. Comparison with Previous Literature
4.4. Plausible Biological Mechanisms
4.5. Limitations
4.6. Implications
4.7. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J. Vitamin D and Autism, What’s New? Rev. Endocr. Metab. Disord. 2017, 18, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, L.M.S.; Groot, N.A.; Díaz Mosquera, E.N.; Andrade Zúñiga, I.P.; Delfos, M.F. Prevalence of Autism Spectrum Disorders in Ecuador: A Pilot Study in Quito. J. Autism Dev. Disord. 2015, 45, 4165–4173. [Google Scholar] [CrossRef]
- Arrhenius, B.; Upadhyaya, S.; Hinkka-Yli-Salomäki, S.; Brown, A.S.; Cheslack-Postava, K.; Öhman, H.; Sourander, A. Prenatal Vitamin D Levels in Maternal Sera and Offspring Specific Learning Disorders. Nutrients 2021, 13, 3321. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.; Camargo, C.A., Jr.; Grant, C.C. Global Summary of Maternal and Newborn Vitamin D Status—A Systematic Review. Matern. Child. Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- Thakkar, K.; Billa, G. Treatment of Vitamin B12 Deficiency—Methylcobalamin? Cyanocobalamin? Hydroxocobalamin?—Clearing the Confusion. Eur. J. Clin. Nutr. 2015, 69, 1–2. [Google Scholar] [CrossRef]
- Hewitson, L.; Mathews, J.A.; Devlin, M.; Schutte, C.; Lee, J.; German, D.C. Blood Biomarker Discovery for Autism Spectrum Disorder: A Proteomic Analysis. PLoS ONE 2024, 19, e0302951. [Google Scholar] [CrossRef]
- Altun, H.; Kurutaş, E.B.; Şahin, N.; Güngör, O.; Fındıklı, E. The Levels of Vitamin D, Vitamin D Receptor, Homocysteine and Complex B Vitamin in Children with Autism Spectrum Disorders. Clin. Psychopharmacol. Neurosci. 2018, 16, 383–390. [Google Scholar] [CrossRef]
- Li, B.; Xu, Y.; Pang, D.; Zhao, Q.; Zhang, L.; Li, M.; Li, W.; Duan, G.; Zhu, C. Interrelation between Homocysteine Metabolism and the Development of Autism Spectrum Disorder in Children. Front. Mol. Neurosci. 2022, 15, 947513. [Google Scholar] [CrossRef]
- Sass, L.; Vinding, R.K.; Stokholm, J.; Bjarnadóttir, E.; Noergaard, S.; Thorsen, J.; Sunde, R.B.; Mcgrath, J.; Bønnelykke, K.; Chawes, B.; et al. High-Dose Vitamin D Supplementation in Pregnancy and Neurodevelopment in Childhood: A Prespecified Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, E2026018. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Sohrabi, M.; Taheri, H.; Khodashenas, E.; Movahedi, A. Comparison of the Effects of Perceptual-Motor Exercises, Vitamin D Supplementation and the Combination of These Interventions on Decreasing Stereotypical Behavior in Children with Autism Disorder. Int. J. Dev. Disabil. 2018, 66, 122–132. [Google Scholar] [CrossRef]
- Keehn, B.; Monahan, P.; Enneking, B.; Ryan, T.; Swigonski, N.; McNally Keehn, R. Eye-Tracking Biomarkers and Autism Diagnosis in Primary Care. JAMA Netw. Open 2024, 7, e2411190. [Google Scholar] [CrossRef]
- Sandboge, S.; Räikkönen, K.; Lahti-Pulkkinen, M.; Hauta-Alus, H.; Holmlund-Suila, E.; Girchenko, P.; Kajantie, E.; Mäkitie, O.; Andersson, S.; Heinonen, K. Effect of Vitamin D3 Supplementation in the First 2 Years of Life on Psychiatric Symptoms at Ages 6 to 8 Years: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, E2314319. [Google Scholar] [CrossRef]
- Javadfar, Z.; Abdollahzad, H.; Moludi, J.; Rezaeian, S.; Amirian, H.; Foroughi, A.A.; Nachvak, S.M.; Goharmehr, N.; Mostafai, R. Effects of Vitamin D Supplementation on Core Symptoms, Serum Serotonin, and Interleukin-6 in Children with Autism Spectrum Disorders: A Randomized Clinical Trial. Nutrition 2020, 79–80, 110986. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2017, 32, 100. [Google Scholar] [CrossRef]
- Petruzzelli, M.G.; Marzulli, L.; Margari, F.; De Giacomo, A.; Gabellone, A.; Giannico, O.V.; Margari, L. Vitamin D Deficiency in Autism Spectrum Disorder: A Cross-Sectional Study. Dis. Markers 2020, 9292560. [Google Scholar] [CrossRef]
- Jayanath, S.; Fong, C.Y.; Sarvananthan, R. Autism Spectrum Disorder and Vitamin D Status: A Cross-Sectional Study of Children in a Developing Country in Southeast Asia. Res. Autism Spectr. Disord. 2021, 84, 101786. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; White, T.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational Vitamin D Deficiency and Autism Spectrum Disorder. BJPsych Open 2017, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Wen, X.; Han, X.R.; Wang, S.; Wang, Y.J.; Shen, M.; Fan, S.H.; Zhuang, J.; Li, M.Q.; Hu, B.; et al. Relationship Between Neonatal Vitamin D at Birth and Risk of Autism Spectrum Disorders: The NBSIB Study. J. Bone Miner. Res. 2018, 33, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Windham, G.; Pearl, M.; Poon, V.; Berger, K.; Soriano, J.W.; Eyles, D.; Lyall, K.; Kharrazi, M.; Croen, L.A. Maternal Vitamin D Levels During Pregnancy in Association with Autism Spectrum Disorders (ASD) or Intellectual Disability (ID) in Offspring; Exploring Non-Linear. Wiley Online Libr. 2020, 13, 2216–2229. [Google Scholar]
- Nesa, A.; Sultana, G.S. Study of Homocysteine, Vitamin B12 and Folate in Children with Autism Spectrum Disorder: Vitamin B12 and Folate in Children with Autism Spectrum Disorder. Bangladesh Med. Res. Counc. Bull. 2022, 48, 127–132. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational Vitamin D Deficiency and Autism-Related Traits: The Generation R Study. Mol. Psychiatry 2018, 23, 240–246. [Google Scholar] [CrossRef]
- Tuovinen, S.; Räikkönen, K.; Holmlund-Suila, E.; Hauta-Alus, H.; Helve, O.; Rosendahl, J.; Enlund-Cerullo, M.; Kajantie, E.; Valkama, S.; Viljakainen, H.; et al. Effect of High-Dose vs. Standard-Dose Vitamin D Supplementation on Neurodevelopment of Healthy Term Infants: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2124493. [Google Scholar] [CrossRef]
- Hendren, R.L.; James, S.J.; Widjaja, F.; Lawton, B.; Rosenblatt, A.; Bent, S. Randomized, Placebo-Controlled Trial of Methyl B12 for Children with Autism. J. Child Adolesc. Psychopharmacol. 2016, 26, 774–783. [Google Scholar] [CrossRef]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A.; Meyer, B.J.; Tsang, B.; von Hurst, P.R. Inflammation (IL-1β) Modifies the Effect of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids on Core Symptoms of Autism Spectrum Disorder-An Exploratory Pilot Study. Nutrients 2020, 12, 661. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: New York, NY, USA, 1985. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- Deeks, J.J. Issues in the Selection of a Summary Statistic for Meta-Analysis of Clinical Trials with Binary Outcomes. Stat. Med. 2002, 21, 1575–1600. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; The Cochrane Collaboration: London, UK, 2021. [Google Scholar]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-Analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Mendeley Ltd. Mendeley Desktop; Version 1.19.8; Elsevier: London, UK, 2019. [Google Scholar]
- JASP Team. JASP (Version 0.19.3); [Computer Software]; JASP Team: Amsterdam, The Netherlands, 2024. [Google Scholar]
- The Nordic Cochrane Centre; The Cochrane Collaboration. Review Manager (RevMan); Version 5.3; [Computer Software]; The Nordic Cochrane Centre: Copenhagen, Denmark, 2014. [Google Scholar]
- Fekete, J.T.; Gyorffy, B. MetaAnalysisOnline.com: An Online Tool for the Rapid Meta-Analysis of Clinical and Epidemiological Studies. J. Med. Internet Res. 2025, 27, e64016. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 18; StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Brister, J.R.; Chan, J.; Connor, R.; Feldgarden, M.; Fine, A.M.; Funk, K.; Hoffman, J.; et al. Database resources of the National Center for Biotechnology Information in 2025. Nucleic Acids Res. 2025, 53, D20–D29. [Google Scholar] [CrossRef]
- Cochrane Central Register of Controlled Trials (CENTRAL). Wiley: Hoboken, NJ, USA. Available online: https://www.cochranelibrary.com/central (accessed on 18 February 2025).
- Vine, R. Google Scholar. J. Med. Libr. Assoc. 2006, 94, 97–99. [Google Scholar] [PubMed Central]
- Zarin, D.A.; Tse, T.; Williams, R.J.; Califf, R.M.; Ide, N.C. The ClinicalTrials.gov results database—update and key issues. N. Engl. J. Med. 2011, 364, 852–860. [Google Scholar] [CrossRef]
- ProQuest LLC. ProQuest; Ann Arbor, MI, USA. Available online: https://www.proquest.com (accessed on 8 May 2024).
- Eshawi, Z.M.; Al-Zwayi, M.M.; Ibrahim, N.M. Study of Homocysteine Concentration and Its Relationship with Vitamin B12 in Children with Autism Spectrum and Attention-Deficit Hyperactivity Disorders in Southern Libya. Wadi AlShatti Univ. J. Pure Appl. Sci. 2024, 2, 72–78. [Google Scholar]
- Schünemann, H.J.; Brożek, J.; Guyatt, G.; Oxman, A.D. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 1 April 2025).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Aagaard, K.; Møllegaard Jepsen, J.R.; Sevelsted, A.; Horner, D.; Vinding, R.; Rosenberg, J.B.; Brustad, N.; Eliasen, A.; Mohammadzadeh, P.; Følsgaard, N.; et al. High-Dose Vitamin D3 Supplementation in Pregnancy and Risk of Neurodevelopmental Disorders in the Children at Age 10: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2024, 119, 362–370. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 April 2025).
- Bener, A.; Khattab, A.; Bhugra, D.; Hoffmann, G. Iron and Vitamin D Levels among Autism Spectrum Disorders Children. Ann. Afr. Med. 2017, 16, 186. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
- Adibsaber, F.; Ansari, S.; Elmieh, A.; Barkadehi, B. Vitamin D3 Supplementation and Aquatic Exercise Combination as a Safe- Efficient Therapeutic Strategy to Ameliorate Interleukin-6 and 10, and Social Interaction in Children with Autism. Iran. J. Child. Neurol. 2024, 18, 91. [Google Scholar]
- Brîndușe, L.A.; Eclemea, I.; Neculau, A.E.; Cucu, M.A. Vitamin D Status in the Adult Population of Romania-Results of the European Health Examination Survey. Nutrients 2024, 16, 867. [Google Scholar] [CrossRef]
- Bonetti, F.; Brombo, G.; Zuliani, G. The Relationship between Hyperhomocysteinemia and Neurodegeneration. Neurodegener. Dis. Manag. 2016, 6, 133–145. [Google Scholar] [CrossRef]
- Chirita-Emandi, A.; Socolov, D.; Haivas, C.; Calapiş, A.; Gheorghiu, C.; Puiu, M. Vitamin D Status: A Different Story in the Very Young versus the Very Old Romanian Patients. PLoS ONE 2015, 10, e0128010. [Google Scholar] [CrossRef]
- Dias, C.M.; Walsh, C.A. Recent Advances in Understanding the Genetic Architecture of Autism. Annu. Rev. Genom. Human. Genet. 2020, 21, 289–304. [Google Scholar] [CrossRef]
- Doi, H.; Furui, A.; Ueda, R.; Shimatani, K.; Yamamoto, M.; Eguchi, A.; Sagara, N.; Sakurai, K.; Mori, C.; Tsuji, T. Risk of Autism Spectrum Disorder at 18 Months of Age Is Associated with Prenatal Level of Polychlorinated Biphenyls Exposure in a Japanese Birth Cohort. Sci. Rep. 2024, 14, 31872. [Google Scholar] [CrossRef]
- Endres, D.; Dersch, R.; Stich, O.; Buchwald, A.; Perlov, E.; Feige, B.; Maier, S.; Riedel, A.; van Elst, L.T. Vitamin D Deficiency in Adult Patients with Schizophreniform and Autism Spectrum Syndromes: A One-Year Cohort Study at a German Tertiary Care Hospital. Front. Psychiatry 2016, 7, 168. [Google Scholar] [CrossRef]
- Gao, Q.; Bi, D.; Li, B.; Ni, M.; Pang, D.; Li, X.; Zhang, X.; Xu, Y.; Zhao, Q.; Zhu, C. The Association Between Branched-Chain Amino Acid Concentrations and the Risk of Autism Spectrum Disorder in Preschool-Aged Children. Mol. Neurobiol. 2024, 61, 6031. [Google Scholar] [CrossRef]
- Gerges, P.; Bangarusamy, D.K.; Bitar, T.; Alameddine, A.; Nemer, G.; Hleihel, W. Turicibacter and Catenibacterium as Potential Biomarkers in Autism Spectrum Disorders. Sci. Rep. 2024, 14, 23184. [Google Scholar] [CrossRef]
- Ghiga, G.; Țarcă, E.; Țarcă, V.; Spoială, E.L.; Păduraru, G.; Gimiga, N.; Boca, L.O.; Iftinchi, O.; Donos, M.A.; Manole, L.M.; et al. Vitamin D Deficiency: Insights and Perspectives from a Five-Year Retrospective Analysis of Children from Northeastern Romania. Nutrients 2024, 16, 3808. [Google Scholar] [CrossRef]
- Grant, W.B. Vitamin D and Health in the Mediterranean Countries. Hormones 2019, 18, 23–35. [Google Scholar] [CrossRef]
- Guerini, F.R.; Bolognesi, E.; Mensi, M.M.; Zanette, M.; Agliardi, C.; Zanzottera, M.; Chiappedi, M.; Annunziata, S.; García-García, F.; Cavallini, A.; et al. HLA-A, -B, -C and -DRB1 Association with Autism Spectrum Disorder Risk: A Sex-Related Analysis in Italian ASD Children and Their Siblings. Int. J. Mol. Sci. 2024, 25, 9879. [Google Scholar] [CrossRef]
- Herdea, A.; Marie, H.; Ionescu, A.; Sandu, D.-M.; Pribeagu, S.-T.; Ulici, A. Vitamin D Deficiency—A Public Health Issue in Children. Children 2024, 11, 1061. [Google Scholar] [CrossRef]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and Preliminary Evidence on Altered Oral and Gut Microbiota in Individuals with Autism Spectrum Disorder (ASD): Implications for ASD Diagnosis and Subtyping Based on Microbial Biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goines, P.E.; Tancredi, D.J.; Ashwood, P.; Hansen, R.L.; Hertz-Picciotto, I.; Van de Water, J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol. Psychiatry 2015, 81, 442. [Google Scholar] [CrossRef]
- Li, D.; Karnath, H.O.; Xu, X. Candidate Biomarkers in Children with Autism Spectrum Disorder: A Review of MRI Studies. Neurosci. Bull. 2017, 33, 219. [Google Scholar] [CrossRef]
- Manghi, P.; Filosi, M.; Zolfo, M.; Casten, L.G.; Garcia-Valiente, A.; Mattevi, S.; Heidrich, V.; Golzato, D.; Perini, S.; Thomas, A.M.; et al. Large-Scale Metagenomic Analysis of Oral Microbiomes Reveals Markers for Autism Spectrum Disorders. Nat. Commun. 2024, 15, 9743. [Google Scholar] [CrossRef]
- Mogire, R.M.; Morovat, A.; Muriuki, J.M.; Mentzer, A.J.; Webb, E.L.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Cutland, C.L.; Sirima, S.B.; et al. Prevalence and Predictors of Vitamin D Deficiency in Young African Children. BMC Med. 2021, 19, 115. [Google Scholar] [CrossRef]
- Myat, P.; John, J.R.; Montgomery, A.; Eapen, V. Sociocultural and Perinatal Health Factors Associated with Autism Spectrum Disorder (ASD) in Children. Compr. Psychiatry 2025, 138, 152576. [Google Scholar] [CrossRef]
- Niculescu, D.A.; Capatina, C.A.M.; Dusceac, R.; Caragheorgheopol, A.; Ghemigian, A.; Poiana, C. Seasonal Variation of Serum Vitamin D Levels in Romania. Arch. Osteoporos. 2017, 12, 113. [Google Scholar] [CrossRef]
- Noori, A.S.; Rajabi, P.; Sargolzaei, J.; Alaghmand, A. Correlation of Biochemical Markers and Inflammatory Cytokines in Autism Spectrum Disorder (ASD). BMC Pediatr. 2024, 24, 696. [Google Scholar] [CrossRef]
- Peralta-Marzal, L.N.; Prince, N.; Bajic, D.; Roussin, L.; Naudon, L.; Rabot, S.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P. The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int. J. Mol. Sci. 2021, 22, 10052. [Google Scholar] [CrossRef]
- Ramirez-Celis, A.; Croen, L.A.; Yoshida, C.K.; Alexeeff, S.E.; Schauer, J.; Yolken, R.H.; Ashwood, P.; Van de Water, J. Maternal Autoantibody Profiles as Biomarkers for ASD and ASD with Co-Occurring Intellectual Disability. Mol. Psychiatry 2022, 27, 3760. [Google Scholar] [CrossRef]
- Ranjan, S.; Nasser, J.A. Nutritional Status of Individuals with Autism Spectrum Disorders: Do We Know Enough? Adv. Nutr. 2015, 6, 397–407. [Google Scholar] [CrossRef]
- Russell-Jones, G. Functional Vitamin B2 Deficiency in Autism. J. Pediatr. Perinatol. Child Health 2022, 6, 324–328. [Google Scholar] [CrossRef]
- Sharma, M.; Tiwari, M.; Tiwari, R.K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic Clin. Pharmacol. Toxicol. 2015, 117, 287–296. [Google Scholar] [CrossRef]
- Soares, R.D.C.S.; Cândido, F.G.; Filgueiras, M.D.S.; Rosa, C.D.O.B.; Novaes, J.F.D.; Araujo, R.M.A. Problematic Behaviors at Mealtimes and the Nutritional Status of Brazilian Children with Autism Spectrum Disorder. Front. Public Health 2024, 12, 1392478. [Google Scholar] [CrossRef]
- Sultan, S.; Alhejin, N.; Serafi, R.; Alrahi, M.A.; Afifi, G.; Al-Adawi, L.; Serafi, M.; Madhoun, N.E. Does Vitamin D Deficiency Increase the Risk of Autism Spectrum Disorder? Linking Evidence with Theory—A Narrative Review. Psychiatry Int. 2025, 6, 22. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, V.T.; Nguyen, M.P.; Huynh Nguyen, P.Q.; Le, T.T.H.; Nguyen, H.Y.; Nguyen, T.H.; Nguyen, T.L.; Le, C.T.; Nguyen, V.T. Prevalence of Autism Spectrum Disorder Diagnosed According to DSM-5 Criteria and Associated Factors in Preschoolers in Southern Vietnam. La. Clin. Ter. 2024, 175, 405–411. [Google Scholar]
- Ye, M.; Yang, X.; Yan, J.; Yao, Y.; Lv, H.; Yue, Z.; Lin, X.; Qian, C.; Liu, Z. Causal Relationship Between B Vitamins and Neuropsychiatric Disorders: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2025, 170, 106068. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Ren, Y.; Zhang, H.; Qiu, W.; Wang, H. Assessment of Serum Vitamin D Levels in Children Aged 0–17 Years Old in a Chinese Population: A Comprehensive Study. Sci. Rep. 2024, 14, 12562. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Yu, L.; Liu, J. Polymorphisms in Vitamin D Receptor Genes in Association with Childhood Autism Spectrum Disorder. Dis. Markers 2018, 2018. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Gao, X.; Wang, D.; Wang, N.; Xie, R.; Tong, X.; He, Y.; Yang, L. Early Biomarkers of Neurodevelopmental Disorders in Preterm Infants: Protocol for a Longitudinal Cohort Study. BMJ Open 2023, 13, e070230. [Google Scholar] [CrossRef]
- Saad, K.; Abdel-rahman, A.A.; Elserogy, Y.M.; Al-Atram, A.A.; Cannell, J.J.; Bjørklund, G.; Abdel-Reheim, M.K.; Othman, H.A.K.; El-Houfey, A.A.; Abd El-Aziz, N.H.R.; et al. Vitamin D Status in Autism Spectrum Disorders and the Efficacy of Vitamin D Supplementation in Autistic Children. Nutr. Neurosci. 2016, 19, 346–351. [Google Scholar] [CrossRef]
- Wink, L.K.; Adams, R.; Wang, Z.; Klaunig, J.E.; Plawecki, M.H.; Posey, D.J.; McDougle, C.J.; Erickson, C.A. A Randomized Placebo-Controlled Pilot Study of N-Acetylcysteine in Youth with Autism Spectrum Disorder. Mol. Autism 2016, 7, 26. [Google Scholar] [CrossRef]
- Coşkun, S.; Şimşek, Ş.; Camkurt, M.A.; Çim, A.; Çelik, S.B. Association of Polymorphisms in the Vitamin D Receptor Gene and Serum 25-Hydroxyvitamin D Levels in Children with Autism Spectrum Disorder. Gene 2016, 588, 109–114. [Google Scholar] [CrossRef]
- Kerley, C.P.; Power, C.; Gallagher, L.; Coghlan, D. Lack of Effect of Vitamin D3 Supplementation in Autism: A 20-Week, Placebo-Controlled RCT. Arch. Dis. Child. 2017, 102, 1030–1036. [Google Scholar] [CrossRef]
- Arastoo, A.A.; Khojastehkia, H.; Rahimi, Z.; Khafaie, M.A.; Hosseini, S.A.; Mansoori, S.M.; Yosefyshad, S.; Abshirini, M.; Karimimalekabadi, N.; Cheraghi, M. Evaluation of Serum 25-Hydroxy Vitamin D Levels in Children with Autism Spectrum Disorder. Ital. J. Pediatr. 2018, 44, 150. [Google Scholar] [CrossRef]
- Li, H.; Dang, Y.; Yan, Y. Serum Interleukin-17 A and Homocysteine Levels in Children with Autism. BMC Neurosci. 2024, 25, 17. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, Y.; Li, D.; Li, S.; Hu, J.; Gao, Y.; Cheng, Z.; Liu, S.; Wu, L.; Sun, C. Correlation of Co-Morbidities with Symptom Severity of Children with Autism Spectrum Disorder: A Cross-Sectional Survey. Nutrients 2024, 16, 2960. [Google Scholar] [CrossRef]
- Shom, S.; Saha, S.; Chatterjee, M.; Sinha, S.; Mukhopadhyay, K. Indian ASD Probands with 25(OH)D and Vitamin D Binding Protein Deficiency Exhibited Higher Severity. Sci. Rep. 2024, 14, 19242. [Google Scholar] [CrossRef]
- De Marzio, M.; Lasky-Su, J.; Chu, S.H.; Prince, N.; Litonjua, A.A.; Weiss, S.T.; Kelly, R.S.; Glass, K.R. The Metabolic Role of Vitamin D in Children’s Neurodevelopment: A Network Study. Sci. Rep. 2024, 14, 16929. [Google Scholar] [CrossRef]
- Sourander, A.; Silwal, S.; Surcel, H.M.; Hinkka-Yli-Salomäki, S.; Upadhyaya, S.; McKeague, I.W.; Cheslack-Postava, K.; Brown, A.S. Maternal Serum Vitamin B12 during Pregnancy and Offspring Autism Spectrum Disorder. Nutrients 2023, 15, 2009. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Niu, Q.; Eyles, D.W.; Hansen, R.L.; Iosif, A.M. Neonatal Vitamin D Status in Relation to Autism Spectrum Disorder and Developmental Delay in the CHARGE Case-Control Study. Autism Res. 2019, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Yektaş, Ç.; Alpay, M.; Tufan, A.E. Comparison of Serum B12, Folate and Homocysteine Concentrations in Children with Autism Spectrum Disorder or Attention Deficit Hyperactivity Disorder and Healthy Controls. Neuropsychiatr. Dis. Treat. 2019, 15, 2213–2219. [Google Scholar] [CrossRef]
- Bičíková, M.; Máčová, L.; Ostatníková, D.; Hanzlíková, L. Vitamin D in Autistic Children and Healthy Controls. Physiol. Res. 2019, 68, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Egorova, O.; Myte, R.; Schneede, J.; Hägglöf, B.; Bölte, S.; Domellöf, E.; Ivars A’Roch, B.; Elgh, F.; Ueland, P.M.; Silfverdal, S.A. Maternal Blood Folate Status during Early Pregnancy and Occurrence of Autism Spectrum Disorder in Offspring: A Study of 62 Serum Biomarkers. Mol. Autism 2020, 11, 7. [Google Scholar] [CrossRef]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A.; Meyer, B.J.; Jones, B.; von Hurst, P.R. A Randomised Controlled Trial of Vitamin D and Omega-3 Long Chain Polyunsaturated Fatty Acids in the Treatment of Irritability and Hyperactivity among Children with Autism Spectrum Disorder. J. Steroid Biochem. Mol. Biol. 2019, 187, 9–16. [Google Scholar] [CrossRef]
- Curis, C.; Ardeleanu, V.; Moroianu, L.A.; Manole, C.; Stoica, R.A.; Gherghiceanu, F.; Pantea Stoian, A. Obesity in Children: Systematic Review over a 6-Year Period, Including the COVID-19 Pandemic. J. Mind Med. Sci. 2024, 11, 310–320. [Google Scholar] [CrossRef]
- Curis, C.; Moroianu, M.; Moroianu, L.-A.; Reurean Pintilei, D.; Ardeleanu, V.; Bobirca, A.; Mahler, B.; Pantea Stoian, A. Intuitive Nutrition Co-factor of Emotional Equilibrium for Maintaining Nutritional Balance: A Prospective Study. Mediterr. J. Clin. Psychol. 2023, 11. [Google Scholar] [CrossRef]

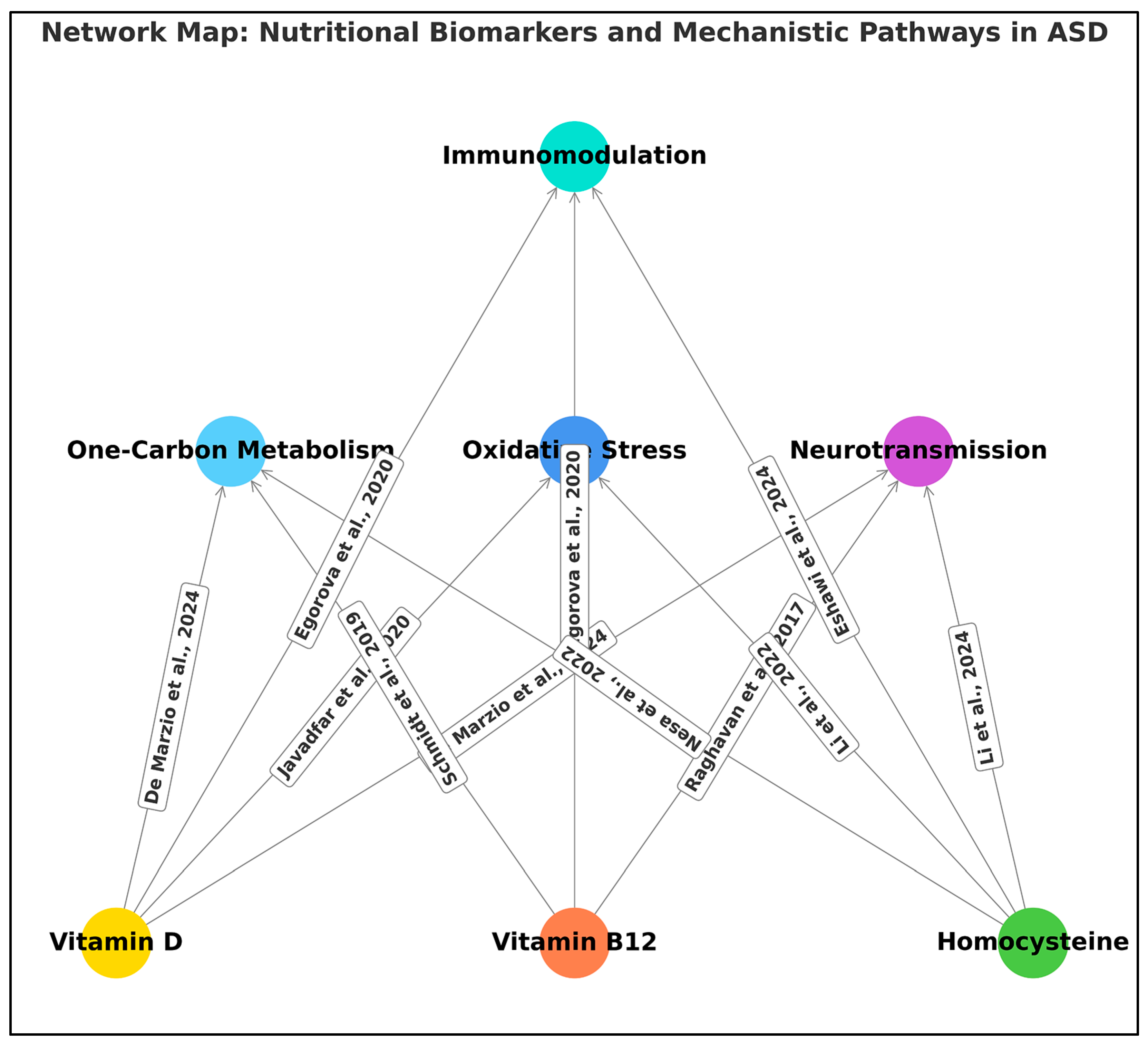








| Type | Definition and Scientific Basis |
|---|---|
| Prenatal Nutrient Exposure Studies | Studies that measure maternal plasma or serum concentrations of vitamin D, B12, or homocysteine during gestation (typically spanning the first to third trimester), intended to capture fetal exposure during critical stages of brain development. |
| Neonatal Nutrient Biomarker Studies | Studies assessing immediate postnatal biomarker levels—often via cord blood or neonatal dried blood spots—as retrospective proxies for intrauterine nutritional status. |
| Postnatal/Early Childhood Nutrient Status | Studies examining vitamin and homocysteine levels in toddlers and young children (generally up to age 6), who are either diagnosed with ASD or considered at elevated risk. |
| Postnatal Nutritional Intervention Studies | Studies that evaluate the efficacy of nutritional supplementation (e.g., vitamin D, B12) administered postnatally, often employing pre-post or placebo-controlled designs. |
| Gene–Nutrient Interaction Studies | Investigations exploring the modulatory effects of genetic polymorphisms (e.g., Methylenetetrahydrofolate Reductase, and Vitamin D Receptor) on biomarker levels or ASD phenotypes, emphasizing gene–environment interplay in neurodevelopment. |
| Mechanistic/Systems Biology Models | In silico models, computational simulations, or pathway-based analyses that investigate nutrient-influenced molecular networks relevant to ASD, typically without involving human subjects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, O.-E.; Bratu, E.-A.; Curis, C.; Moroianu, L.-A.; Drima, E. Modifiable Nutritional Biomarkers in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Vitamin D, B12, and Homocysteine Exposure Spanning Prenatal Development Through Late Adolescence. Int. J. Mol. Sci. 2025, 26, 4410. https://doi.org/10.3390/ijms26094410
Avram O-E, Bratu E-A, Curis C, Moroianu L-A, Drima E. Modifiable Nutritional Biomarkers in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Vitamin D, B12, and Homocysteine Exposure Spanning Prenatal Development Through Late Adolescence. International Journal of Molecular Sciences. 2025; 26(9):4410. https://doi.org/10.3390/ijms26094410
Chicago/Turabian StyleAvram, Oana-Elisabeta, Elena-Alexandra Bratu, Cecilia Curis, Lavinia-Alexandra Moroianu, and Eduard Drima. 2025. "Modifiable Nutritional Biomarkers in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Vitamin D, B12, and Homocysteine Exposure Spanning Prenatal Development Through Late Adolescence" International Journal of Molecular Sciences 26, no. 9: 4410. https://doi.org/10.3390/ijms26094410
APA StyleAvram, O.-E., Bratu, E.-A., Curis, C., Moroianu, L.-A., & Drima, E. (2025). Modifiable Nutritional Biomarkers in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Vitamin D, B12, and Homocysteine Exposure Spanning Prenatal Development Through Late Adolescence. International Journal of Molecular Sciences, 26(9), 4410. https://doi.org/10.3390/ijms26094410







