Endometrial Stromal Senescence Mediates the Progression of Intrauterine Adhesions
Abstract
1. Introduction
2. Results
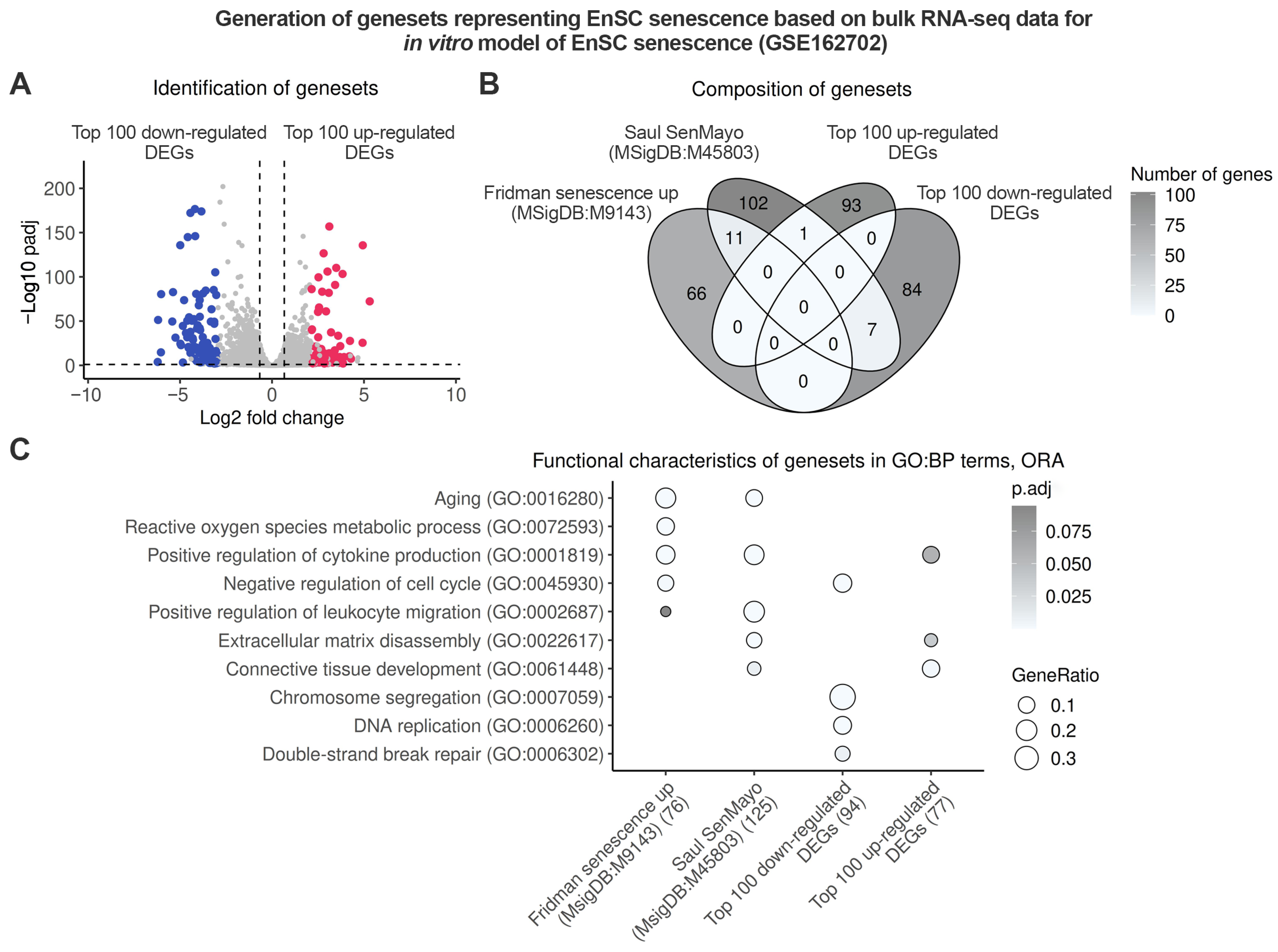
2.1. Endometrium-Specific Gene Signatures for Identifying Stromal Senescence in Transcriptomic Data
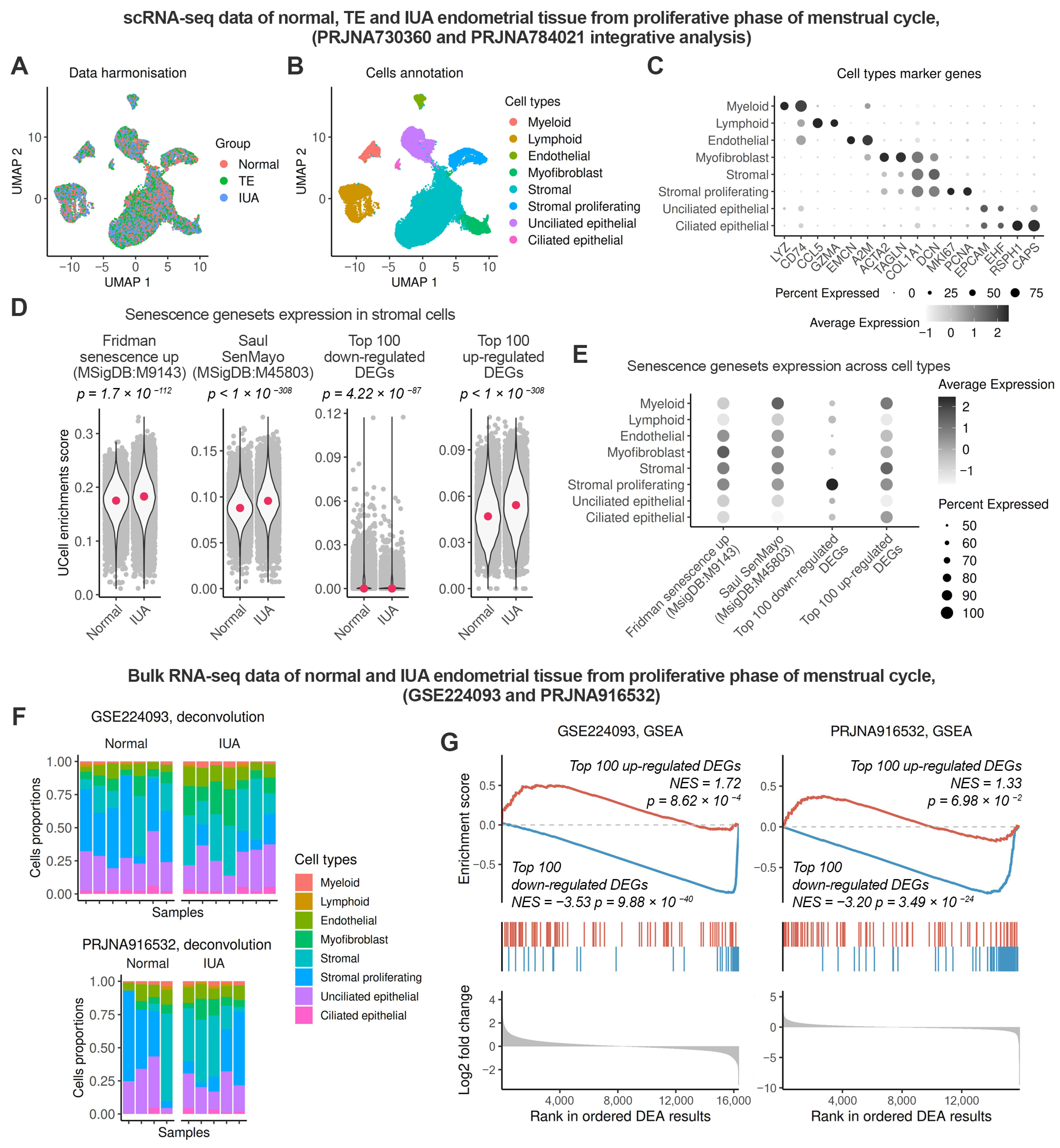
2.2. Enhanced Senescence in the Endometrial Stroma of Patients with IUA During the Proliferative Phase
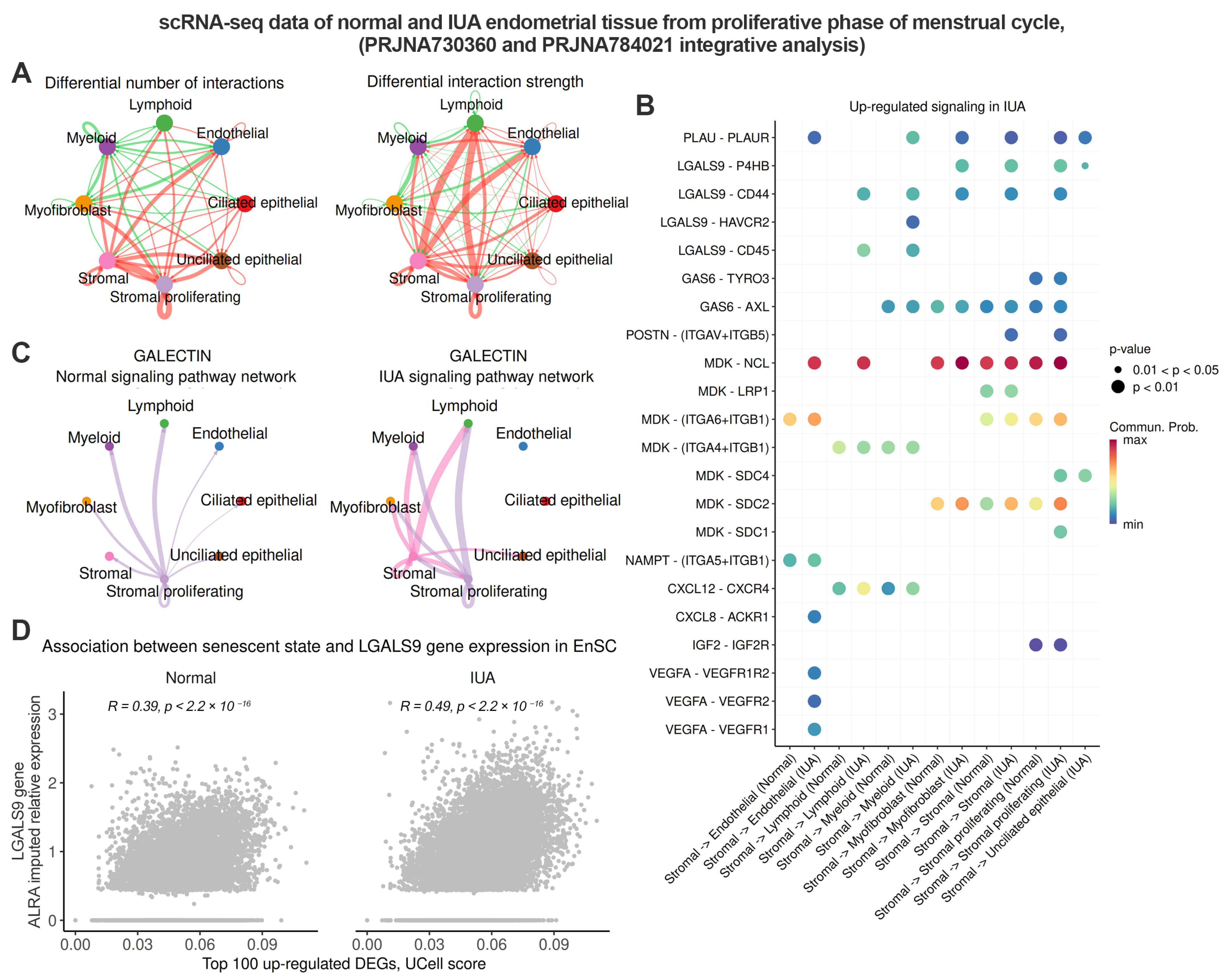
2.3. Senescent Stromal Cells in the IUA Endometrium Create an Immunosuppressive and Profibrotic Microenvironment Through Increased Expression of LGALS9
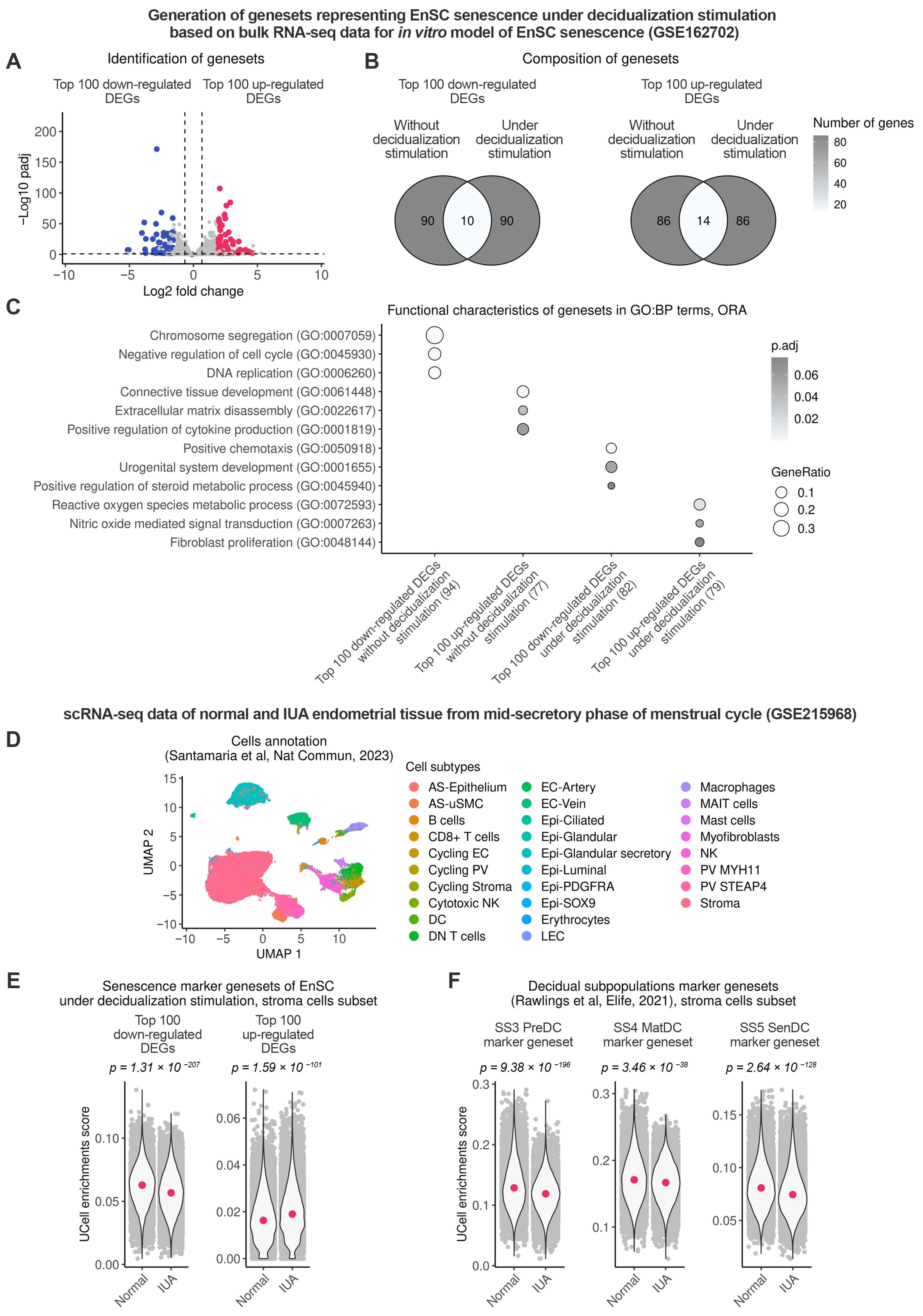
2.4. Endometrial Stromal Senescence Persists During the Window of Implantation (WOI) and Is Associated with Impaired Decidualization of the IUA Endometrium
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Data Processing
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BH | Benjamini–Hochberg |
| DEA | differential expression analysis |
| DEGs | differentially expressed genes |
| EnSC | endometrial stromal cells |
| GEO | Gene Expression Omnibus |
| IUA | intrauterine adhesions |
| RNA-seq | RNA sequencing |
| SASP | senescence-associated secretory phenotype |
| ScRNA-seq | single-cell RNA-sequencing |
| WOI | window of implantation |
References
- Zhao, G.; Hu, Y. Mechanistic insights into intrauterine adhesions. Semin. Immunopathol. 2024, 47, 3. [Google Scholar] [CrossRef]
- Capella-Allouc, S. Hysteroscopic treatment of severe Asherman’s syndrome and subsequent fertility. Hum. Reprod. 1999, 14, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Lasmar, R.B.; Lasmar, B.P.; Haimovich, S.; Pacheco, L.A.; Moawad, N.S. Proposal for a new classification of intrauterine adhesions by sites. Int. J. Gynecol. Obstet. 2024, 169, 9–14. [Google Scholar] [CrossRef]
- Salazar, C.A.; Isaacson, K.; Morris, S. A comprehensive review of Asherman’s syndrome: Causes, symptoms and treatment options. Curr. Opin. Obstet. Gynecol. 2017, 29, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Urman, B.; Yakin, K.; Ertas, S.; Alper, E.; Aksakal, E.; Riemma, G.; Angioni, S.; Vitale, S.G. Fertility and anatomical outcomes following hysteroscopic adhesiolysis: An 11-year retrospective cohort study to validate a new classification system for intrauterine adhesions (Urman-Vitale Classification System). Int. J. Gynecol. Obstet. 2023, 165, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhan, H.; Li, W.; Zhang, L.; Yun, F.; Wu, R.; Lin, J.; Li, Y. Recent trends in therapeutic strategies for repairing endometrial tissue in intrauterine adhesion. Biomater. Res. 2021, 25, 40. [Google Scholar] [CrossRef]
- Turano, P.S.; Herbig, U. Cellular senescence, p21, and the path to fibrosis. EMBO J. 2024, 43, 5332–5334. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Ng, B.; Cook, S.A.; Schafer, S. Interleukin-11 signaling underlies fibrosis, parenchymal dysfunction, and chronic inflammation of the airway. Exp. Mol. Med. 2020, 52, 1871–1878. [Google Scholar] [CrossRef]
- Maus, M.; López-Polo, V.; Mateo, L.; Lafarga, M.; Aguilera, M.; De Lama, E.; Meyer, K.; Sola, A.; Lopez-Martinez, C.; López-Alonso, I.; et al. Iron accumulation drives fibrosis, senescence and the senescence-associated secretory phenotype. Nat. Metab. 2023, 5, 2111–2130. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, F.; Prats, N.; Ramponi, V.; López-Domínguez, J.A.; Meyer, K.; Aguilera, M.; Martín, M.I.M.; Martínez, D.; Agusti, A.; Faner, R.; et al. Human senescent fibroblasts trigger progressive lung fibrosis in mice. Aging 2023, 15, 6641–6657. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Senolytics for kidney repair. Nat. Rev. Nephrol. 2021, 17, 512. [Google Scholar] [CrossRef]
- Mourad, O.; Mastikhina, O.; Khan, S.; Sun, X.; Hatkar, R.; Williams, K.; Nunes, S.S. Antisenescence therapy improves function in a human model of cardiac Fibrosis-on-a-Chip. ACS Mater. Au 2023, 3, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal–epithelial transition in endometrial function. Hum. Reprod. Update 2018, 25, 114–133. [Google Scholar] [CrossRef]
- Zhu, Q.; Yao, S.; Ye, Z.; Jiang, P.; Wang, H.; Zhang, X.; Liu, D.; Lv, H.; Cao, C.; Zhou, Z.; et al. Ferroptosis contributes to endometrial fibrosis in intrauterine adhesions. Free Radic. Biol. Med. 2023, 205, 151–162. [Google Scholar] [CrossRef]
- Min, J.; Lu, N.; Huang, S.; Chai, X.; Wang, S.; Peng, L.; Wang, J. Phenotype and biological characteristics of endometrial mesenchymal stem/stromal cells: A comparison between intrauterine adhesion patients and healthy women. Am. J. Reprod. Immunol. 2020, 85, 13379. [Google Scholar] [CrossRef]
- Burova, E.; Borodkina, A.; Shatrova, A.; Nikolsky, N. Sublethal Oxidative Stress Induces the Premature Senescence of Human Mesenchymal Stem Cells Derived from Endometrium. Oxidative Med. Cell. Longev. 2013, 2013, 474931. [Google Scholar] [CrossRef]
- Alekseenko, L.L.; Zemelko, V.I.; Domnina, A.P.; Lyublinskaya, O.G.; Zenin, V.V.; Pugovkina, N.A.; Kozhukharova, I.V.; Borodkina, A.V.; Grinchuk, T.M.; Fridlyanskaya, I.I.; et al. Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell Stress Chaperones 2013, 19, 355–366. [Google Scholar] [CrossRef]
- Borodkina, A.; Shatrova, A.; Abushik, P.; Nikolsky, N.; Burova, E. Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging 2014, 6, 481–495. [Google Scholar] [CrossRef]
- Griukova, A.; Deryabin, P.; Shatrova, A.; Burova, E.; Severino, V.; Farina, A.; Nikolsky, N.; Borodkina, A. Molecular basis of senescence transmitting in the population of human endometrial stromal cells. Aging 2019, 11, 9912–9931. [Google Scholar] [CrossRef]
- Vassilieva, I.; Kosheverova, V.; Vitte, M.; Kamentseva, R.; Shatrova, A.; Tsupkina, N.; Skvortsova, E.; Borodkina, A.; Tolkunova, E.; Nikolsky, N.; et al. Paracrine senescence of human endometrial mesenchymal stem cells: A role for the insulin-like growth factor binding protein 3. Aging 2020, 12, 1987–2004. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Borodkina, A.V. Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells. Hum. Reprod. 2022, 37, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Ivanova, J.S.; Borodkina, A.V. Senescent endometrial stromal cells transmit reactive oxygen species to the trophoblast-like cells and impair spreading of blastocyst-like spheroids. Mol. Hum. Reprod. 2022, 28, gaac039. [Google Scholar] [CrossRef]
- Fridman, A.L.; Tainsky, M.A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene 2008, 27, 5975–5987. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, G.; Jiang, P.; Wang, H.; Wang, Z.; Yao, S.; Zhou, Z.; Wang, L.; Liu, D.; Deng, W.; et al. Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2115912119. [Google Scholar] [CrossRef]
- Yan, P.; Jimenez, E.R.; Li, Z.; Bui, T.; Seehawer, M.; Nishida, J.; Foidart, P.; Stevens, L.E.; Xie, Y.; Gomez, M.M.; et al. Midkine as a driver of age-related changes and increase in mammary tumorigenesis. Cancer Cell 2024, 42, 1936–1954.e9. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, X.; Ma, Y.; Du, Y.; Feng, J. A new emerging target in cancer immunotherapy: Galectin-9 (LGALS9). Genes Dis. 2022, 10, 2366–2382. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Molè, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 2021, 10, 69603. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-Da-Costa, M.M.; Brighton, P.J.; Kong, C.-S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Kong, C.-S.; Alkhoury, M.; Tryfonos, M.; Brighton, P.J.; Rawlings, T.M.; Muter, J.; Gori, M.S.; Leirós, C.P.; Lucas, E.S.; et al. The endoplasmic reticulum protein HSPA5/BiP is essential for decidual transformation of human endometrial stromal cells. Sci. Rep. 2024, 14, 25992. [Google Scholar] [CrossRef]
- O’Reilly, S.; Tsou, P.-S.; Varga, J. Senescence and tissue fibrosis: Opportunities for therapeutic targeting. Trends Mol. Med. 2024, 30, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, L.; Liu, Y. Cellular senescence in kidney fibrosis: Pathologic significance and therapeutic strategies. Front. Pharmacol. 2020, 11, 601325. [Google Scholar] [CrossRef]
- Osorio, J.M.; Espinoza-Pérez, C.; Rimassa-Taré, C.; Machuca, V.; Bustos, J.O.; Vallejos, M.; Vargas, H.; Díaz-Araya, G. Senescent cardiac fibroblasts: A key role in cardiac fibrosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166642. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Pei, Z.; Zhu, Y.; Zhang, X.; Zhou, Z.; Ye, C.; Song, M.; Hu, Y.; Xue, P.; et al. Endothelial senescence induced by PAI-1 promotes endometrial fibrosis. Cell Death Discov. 2025, 11, 89. [Google Scholar] [CrossRef]
- Xu, P.; Wang, M.; Song, W.-M.; Wang, Q.; Yuan, G.-C.; Sudmant, P.H.; Zare, H.; Tu, Z.; Orr, M.E.; Zhang, B. The landscape of human tissue and cell type specific expression and co-regulation of senescence genes. Mol. Neurodegener. 2022, 17, 5. [Google Scholar] [CrossRef]
- Cohn, R.L.; Gasek, N.S.; Kuchel, G.A.; Xu, M. The heterogeneity of cellular senescence: Insights at the single-cell level. Trends Cell Biol. 2022, 33, 9–17. [Google Scholar] [CrossRef]
- Tao, W.; Yu, Z.; Han, J.-D.J. Single-cell senescence identification reveals senescence heterogeneity, trajectory, and modulators. Cell Metab. 2024, 36, 1126–1143.e5. [Google Scholar] [CrossRef]
- Sanborn, M.A.; Wang, X.; Gao, S.; Dai, Y.; Rehman, J. SenePy: Unveiling the Cell-Type Specific Landscape of Cellular Senescence through Single-Cell Analysis in Living Organisms. Biorxiv 2023. [Google Scholar] [CrossRef]
- Lo, S.T.; Ramsay, P.; Pierson, R.; Manconi, F.; Munro, M.G.; Fraser, I.S. Endometrial thickness measured by ultrasound scan in women with uterine outlet obstruction due to intrauterine or upper cervical adhesions. Hum. Reprod. 2007, 23, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.M.; Hurst, B.S. Comprehensive management of severe Asherman syndrome and amenorrhea. Fertil. Steril. 2011, 97, 160–164. [Google Scholar] [CrossRef]
- Hamada, M.; Varkoly, K.S.; Riyadh, O.; Beladi, R.; Munuswamy-Ramanujam, G.; Rawls, A.; Wilson-Rawls, J.; Chen, H.; McFadden, G.; Lucas, A.R. Urokinase-Type Plasminogen Activator Receptor (UPAR) in Inflammation and disease: A unique inflammatory pathway activator. Biomedicines 2024, 12, 1167. [Google Scholar] [CrossRef]
- Fan, G.; Xie, T.; Tan, Q.; Lou, N.; Wang, S.; Han, X.; Shi, Y. An immunosuppressive subtype of senescent tumor cells predicted worse immunotherapy response in lung adenocarcinoma. iScience 2023, 26, 107894. [Google Scholar] [CrossRef]
- Quilbe, A.; Mustapha, R.; Duchêne, B.; Kumar, A.; Werkmeister, E.; Leteurtre, E.; Moralès, O.; Jonckheere, N.; Van Seuningen, I.; Delhem, N. A novel anti-galectin-9 immunotherapy limits the early progression of pancreatic neoplastic lesions in transgenic mice. Front. Immunol. 2023, 14, 1267279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Lee, H.-J.; Jeon, M.-S.; Yi, T.; Song, S.U. Galectin-9 is Involved in Immunosuppression Mediated by Human Bone Marrow-derived Clonal Mesenchymal Stem Cells. Immune Netw. 2015, 15, 241. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhou, W.-H.; Tao, Y.; Wang, S.-C.; Jiang, Y.-L.; Zhang, D.; Piao, H.-L.; Fu, Q.; Li, D.-J.; Du, M.-R. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal–fetal interface in early pregnancy. Cell. Mol. Immunol. 2015, 13, 73–81. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Sagiv, A.; Krizhanovsky, V. Immunosurveillance of senescent cells: The bright side of the senescence program. Biogerontology 2013, 14, 617–628. [Google Scholar] [CrossRef]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, 31274. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.B.; De Leeuw, R.A.; Emanuel, M.H.; Mijatovic, V.; Brolmann, H.A.M.; Huirne, J.A.F. The link between intrauterine adhesions and impaired reproductive performance: A systematic review of the literature. BMC Pregnancy Childbirth 2022, 22, 837. [Google Scholar] [CrossRef] [PubMed]
- Antelo-Iglesias, L.; Picallos-Rabina, P.; Estévez-Souto, V.; Da Silva-Álvarez, S.; Collado, M. The role of cellular senescence in tissue repair and regeneration. Mech. Ageing Dev. 2021, 198, 111528. [Google Scholar] [CrossRef]
- Xu, J.; He, X.; Zhang, S.; Li, L.; Li, P. Expression of co-signaling molecules TIM-3/Galectin-9 at the maternal-fetal interface. Placenta 2025, 163, 43–50. [Google Scholar] [CrossRef]
- Zhu, Y.; Bao, M.; Wang, T.; Ai, X.; Qiu, D.; Wang, C. Novel therapeutic targets, including IGFBP3, of umbilical cord mesenchymal stem-cell-conditioned medium in intrauterine adhesion. Biol. Open 2024, 13, 60141. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, X.; Roson, B.; Perez-Moraga, R.; Venkatesan, N.; Pardo-Figuerez, M.; Gonzalez-Fernandez, J.; Llera-Oyola, J.; Fernández, E.; Moreno, I.; Salumets, A.; et al. Decoding the endometrial niche of Asherman’s Syndrome at single-cell resolution. Nat. Commun. 2023, 14, 5890. [Google Scholar] [CrossRef]
- Lv, H.; Sun, H.; Wang, L.; Yao, S.; Liu, D.; Zhang, X.; Pei, Z.; Zhou, J.; Wang, H.; Dai, J.; et al. Targeting CD301+ macrophages inhibits endometrial fibrosis and improves pregnancy outcome. EMBO Mol. Med. 2023, 15, e17601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deryabin, P.I.; Borodkina, A.V. Endometrial Stromal Senescence Mediates the Progression of Intrauterine Adhesions. Int. J. Mol. Sci. 2025, 26, 4183. https://doi.org/10.3390/ijms26094183
Deryabin PI, Borodkina AV. Endometrial Stromal Senescence Mediates the Progression of Intrauterine Adhesions. International Journal of Molecular Sciences. 2025; 26(9):4183. https://doi.org/10.3390/ijms26094183
Chicago/Turabian StyleDeryabin, Pavel I., and Aleksandra V. Borodkina. 2025. "Endometrial Stromal Senescence Mediates the Progression of Intrauterine Adhesions" International Journal of Molecular Sciences 26, no. 9: 4183. https://doi.org/10.3390/ijms26094183
APA StyleDeryabin, P. I., & Borodkina, A. V. (2025). Endometrial Stromal Senescence Mediates the Progression of Intrauterine Adhesions. International Journal of Molecular Sciences, 26(9), 4183. https://doi.org/10.3390/ijms26094183





