Biphasic Catalytic Conversion of Olefins in Aqueous Media: A Systematic Review
Abstract
1. Introduction
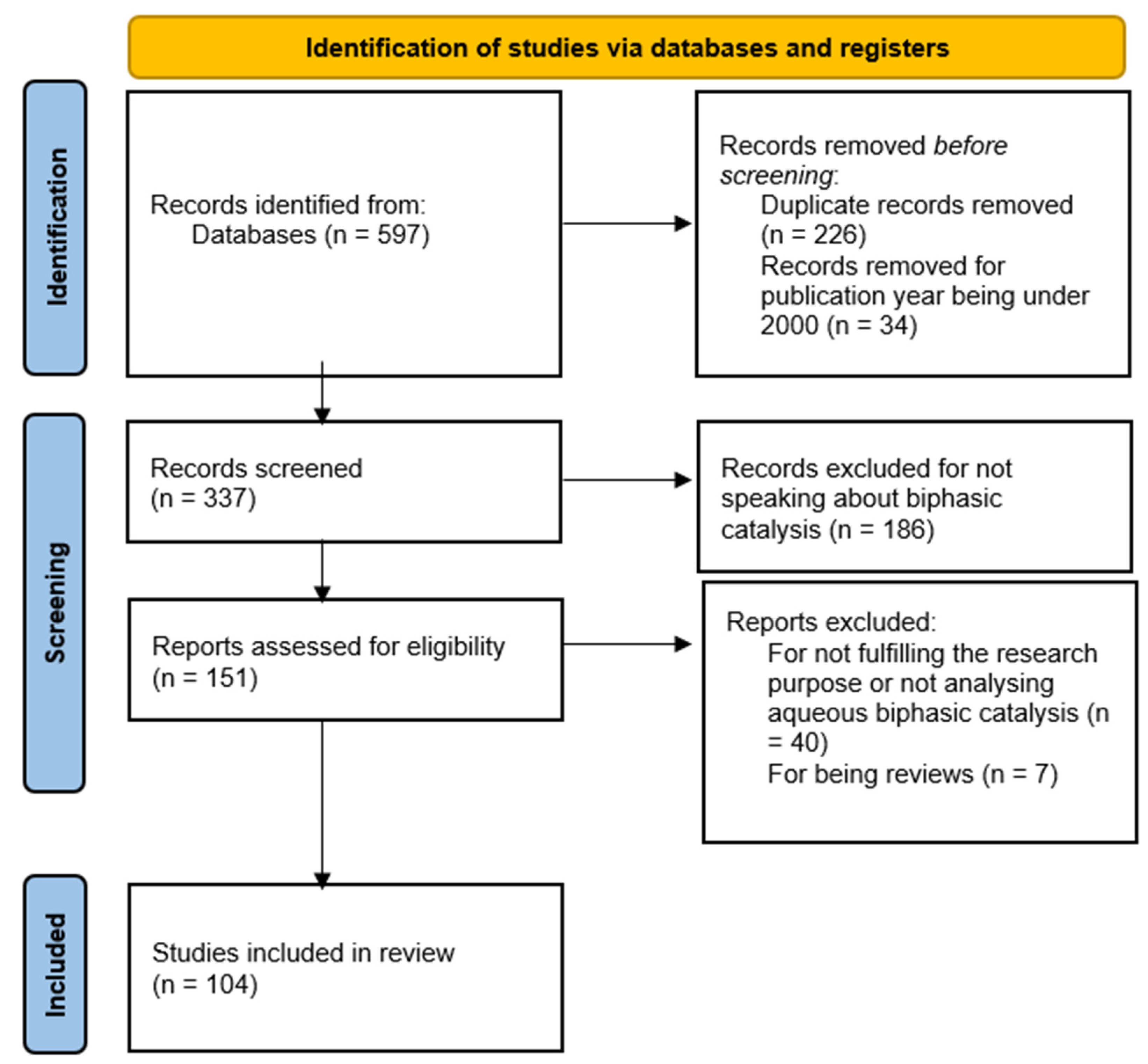
2. Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsalahi, W.; Trzeciak, A.M. Rhodium-catalyzed hydroformylation under green conditions: Aqueous/organic biphasic, “on water”, solventless and Rh nanoparticle based systems. Coord. Chem. Rev. 2021, 430, 213732. [Google Scholar] [CrossRef]
- Kohlpaintner, C.W.; Fischer, R.W.; Cornils, B. Aqueous biphasic catalysis: Ruhrchemie/Rhône-Poulenc oxo process. Appl. Catal. Gen. 2001, 221, 219–225. [Google Scholar] [CrossRef]
- Peng, Q. Aqueous Biphasic Hydroformylation of Higher Olefins Catalyzed by Rhodium Complexes with Amphiphilic Ligands of Sulfonated Triphenylphosphine Analog. Catal. Lett. 2003, 88, 219–225. [Google Scholar] [CrossRef]
- Modroño-Alonso, M.; Castro, W.; Lopez-Linares, F.; Rosales, M.; Baricelli, P.J. Aqueous-biphasic hydroformylation of 1-hexene catalyzed by the complex HCo(CO)[P(o-C6H4SO3Na)]3. J. Mex. Chem. Soc. 2017, 61, 128–137. [Google Scholar] [CrossRef]
- Matsinha, L.C.; Siangwata, S.; Smith, G.S.; Makhubela, B.C.E. Aqueous biphasic hydroformylation of olefins: From classical phosphine-containing systems to emerging strategies based on water-soluble nonphosphine ligands. Catal. Rev. 2019, 61, 111–133. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Pereira, J.C.; Rosales, M. Aqueous-biphasic catalysis: A technological alternative for the use of organometallic complexes in hydrogenation and hydroformylation reactions with possible industrial application. Catal. Today 2025, 443, 114969. [Google Scholar] [CrossRef]
- Kokkinos, N.C. Modeling and simulation of biphasic catalytic hydrogenation of a hydroformylated fuel. Int. J. Hydrogen Energy 2021, 46, 19731–19736. [Google Scholar] [CrossRef]
- Cornils, B. Bulk and fine chemicals via aqueous biphasic catalysis. J. Mol. Catal. Chem. 1999, 143, 1–10. [Google Scholar] [CrossRef]
- Escola, J.M.; Botas, J.A.; Vargas, C.; Bravo, M. Oxidation of heavy 1-olefins (C12=–C20=) with TBHP using a modified Wacker system. J. Catal. 2010, 270, 34–39. [Google Scholar] [CrossRef]
- Brink, G.-J.T.; Arends, I.W.C.E.; Papadogianakis, G.; Sheldon, R.A. Catalytic conversions in water: Part 13. Aerobic oxidation of olefins to methyl ketones catalysed by a water-soluble palladium complex—Mechanistic investigations. Appl. Catal. Gen. 2000, 194, 435–442. [Google Scholar] [CrossRef]
- Mac Leod, T.C.O.; Palaretti, V.; Barros, V.P.; Faria, A.L.; Silva, T.A.; Assis, M.D. Jacobsen catalyst immobilized on chitosan membrane as interface catalyst in organic/aqueous system for alkene oxidation. Appl. Catal. Gen. 2009, 361, 152–159. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Baricelli, D.; Lujano, E.; Melean, L.G.; Borusiak, M.; López-Linares, F.; Bastidas, L.J.; Rosales, M. Catalytic hydrogenation of olefins and their mixtures using HRh(CO)(TPPMS)3 complex in an aqueous biphasic medium. J. Mol. Catal. Chem. 2007, 271, 180–184. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Ascanio, T.; Lujano, E.; Melean, L.G.; Borusiak, M.; López-Linares, F.; Bastidas, L.J.; Rosales, M. Aqueous biphasic catalytic hydrogenation of olefins and olefin mixtures by the [Rh(μ-Pz)(CO)(TPPMS)]2 complex, Pz=pyrazolate, TPPMS=(C6H5)2P(C6H4SO3Na). J. Mol. Catal. Chem. 2007, 278, 107–111. [Google Scholar] [CrossRef]
- Parmar, D.U.; Bhatt, S.D.; Bajaj, H.C.; Jasra, R.V. Hydrogenation of alkenes and aromatic hydrocarbons using water-soluble RuCl2(TPPTS)3 in aqueous medium. J. Mol. Catal. Chem. 2003, 202, 9–15. [Google Scholar] [CrossRef]
- Kokkinos, N.C.; Nikolaou, N.; Psaroudakis, N.; Mertis, K.; Mitkidou, S.; Mitropoulos, A.C. Two-step conversion of LLCN olefins to strong anti-knocking alcohol mixtures catalysed by Rh, Ru/TPPTS complexes in aqueous media. Catal. Today 2015, 247, 132–138. [Google Scholar] [CrossRef]
- Debouttière, P.; Coppel, Y.; Denicourt-Nowicki, A.; Roucoux, A.; Chaudret, B.; Philippot, K. PTA-Stabilized Ruthenium and Platinum Nanoparticles: Characterization and Investigation in Aqueous Biphasic Hydrogenation Catalysis. Eur. J. Inorg. Chem. 2012, 2012, 1229–1236. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Jiang, J.; Jin, Z. Novel Aqueous/Organic Biphasic System for Thermoregulated Phase-Transfer Catalysis with Rhodium Nanoparticles. Chin. J. Catal. 2011, 32, 1133–1137. [Google Scholar] [CrossRef]
- Ogweno, A.O.; Ojwach, S.O.; Akerman, M.P. Cationic pyridyl(benzoazole) ruthenium(II) complexes: Efficient and recyclable catalysts in biphasic hydrogenation of alkenes and alkynes. Appl. Catal. Gen. 2014, 486, 250–258. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Rodríguez, G.; Rodríguez, M.; Lujano, E.; López-Linares, F. Synthesis, characterization and aqueous-biphase hydrogenation of olefins by the ruthenium complexes Ru(CO)3(TPPMS)2 and RuH2(CO)(TPPMS)3. Appl. Catal. Gen. 2003, 239, 25–34. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Jiang, J.; Jin, Z. Thermoregulated phase-transfer rhodium nanoparticle catalyst for hydrogenation in an aqueous/organic biphasic system. Catal. Commun. 2010, 11, 542–546. [Google Scholar] [CrossRef]
- Wang, X.; Yu, F.; Xie, C.; Yu, S. Highly selective hydrogenation of α-pinene in aqueous medium using PVA-stabilized Ru nanoparticles. Mol. Catal. 2018, 444, 62–69. [Google Scholar] [CrossRef]
- Duval, M.; Navarre, S.; Sagorin, G.; Denicourt-Nowicki, A.; Roucoux, A. Multigram Scale-up of the Selective Hydrogenation of α-Pinene with Ruthenium Nanoparticles in Water. ACS Sustain. Chem. Eng. 2020, 8, 5985–5993. [Google Scholar] [CrossRef]
- Kotzabasakis, V.; Hadjichristidis, N.; Papadogianakis, G. Catalytic conversions in aqueous media: Part 3. Biphasic hydrogenation of polybutadiene catalyzed by Rh/TPPTS complexes in micellar systems. J. Mol. Catal. Chem. 2009, 304, 95–100. [Google Scholar] [CrossRef]
- Püschel, S.; Störtte, S.; Topphoff, J.; Vorholt, A.J.; Leitner, W. Green Process Design for Reductive Hydroformylation of Renewable Olefin Cuts for Drop-In Diesel Fuels. ChemSusChem 2021, 14, 5226–5234. [Google Scholar] [CrossRef]
- Joumaa, A.; Chen, S.; Vincendeau, S.; Gayet, F.; Poli, R.; Manoury, E. Rhodium-catalyzed aqueous biphasic hydrogenation of alkenes with amphiphilic phosphine-containing core-shell polymers. Mol. Catal. 2017, 438, 267–271. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, M.; Zhang, W.; Yang, L. Enhanced Pd-Catalyzed Hydrogenation of Olefins within Polymeric Microreactors under Organic/Aqueous Biphasic Conditions. Chem. Eur. J. 2009, 15, 3670–3673. [Google Scholar] [CrossRef]
- Suárez, T.; Fontal, B.; Reyes, M.; Bellandi, F.; Contreras, R.R.; Bahsas, A.; León, G.; Cancines, P.; Castillo, B. Catalytic hydrogenation of 1-hexene with RuCl2 (TPPMS)3 (DMSO), Part I: Aqueous biphasic system. React. Kinet. Catal. Lett. 2004, 82, 317–324. [Google Scholar] [CrossRef]
- Guerrero, M.; Than Chau, N.T.; Noel, S.; Denicourt-Nowicki, A.; Hapiot, F.; Roucoux, A.; Monflier, E.; Philippot, K. About the Use of Rhodium Nanoparticles in Hydrogenation and Hydroformylation Reactions. COC 2013, 17, 364–399. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Li, G.; Jiang, J.; Jin, Z. Thermoregulated phase-transfer cobalt catalyst for production of linear higher alcohols from C11–12 internal olefins in aqueous/organic biphasic system. Catal. Commun. 2014, 44, 54–56. [Google Scholar] [CrossRef]
- Herwig, J.; Fischer, R. Aqueous biphasic hydroformylation. In Rhodium Catalyzed Hydroformylation; Van Leeuwen, P.W.N.M., Claver, C., Eds.; Catalysis by Metal Complexes; Springer: Dordrecht, The Netherlands, 2000; Volume 22, pp. 189–202. ISBN 978-0-7923-6551-8. [Google Scholar]
- Chen, H.; Zheng, X.; Li, X. Hydroformylation of Olefins in Aqueous–Organic Biphasic Catalytic Systems. In Bridging Heterogeneous and Homogeneous Catalysis; Can, L., Yan, L., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 489–510. ISBN 978-3-527-33583-1. [Google Scholar]
- Kokkinos, N.C.; Kazou, E.; Lazaridou, A.; Papadopoulos, C.E.; Psaroudakis, N.; Mertis, K.; Nikolaou, N. A potential refinery process of light–light naphtha olefins conversion to valuable oxygenated products in aqueous media—Part 1: Biphasic hydroformylation. Fuel 2013, 104, 275–283. [Google Scholar] [CrossRef]
- Guanipa, Q.V.J.; Melean, L.G.; Alonzo, M.M.; Gonzalez, A.; Rosales, M.; Lopez-Linares, F.; Baricelli, P.J. Evaluation of the catalytic activity of the water-soluble organometallic complex [Rh(μ-Pz)(CO)(TPPTS)]2 in the hydroformylation of short-chain olefins in a refinery’s naphtha cut. Appl. Catal. Gen. 2009, 358, 21–25. [Google Scholar] [CrossRef]
- Suárez, T.; Fontal, B.; León, G.; Reyes, M.; Bellandi, F.; Contreras, R.R.; Cancines, P. Aqueous biphasic olefin hydroformylation catalyzed by water-soluble rhodium complexes. Transit. Met. Chem. 2006, 31, 974–976. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Segovia, K.; Lujano, E.; Modroño-Alonso, M.; López-Linares, F.; Sánchez-Delgado, R.A. Synthesis and characterization of [HRu(CO)(CH3CN)(TPPTS)3]BF4. J. Mol. Catal. Chem. 2006, 252, 70–75. [Google Scholar] [CrossRef]
- Alonso, M.M.; Guanipa, V.; Melean, L.G.; Rosales, M.; Gonzalez, A.; Baricelli, P.J. Catalytic activity of the RhH(CO)(TPPTS)3 precursor in the biphasic hydroformylation reaction of C5–C7 alkenes from a real naphtha cut. Appl. Catal. Gen. 2009, 358, 211–214. [Google Scholar] [CrossRef]
- Borrmann, T.; Roesky, H.W.; Ritter, U. Biphasic hydroformylation of olefins using a novel water soluble rhodium polyethylene glycolate catalyst. J. Mol. Catal. Chem. 2000, 153, 31–48. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Alonso, M.M.; Rosales, M. Kinetic and Mechanisms of the Aqueous-Biphasic Hydroformylation of Olefins Contained in Naphtha Cuts Catalyzed by RhH(CO)(TPPTS)3 [TPPTS: Tri(sodium m-sulfonated-phenyl)phosphine]. Catal. Lett. 2018, 148, 1150–1161. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Jin, Z. Thermoregulated Liquid/Liquid Biphasic Catalysis and Its Application. Catal. Surv. Asia 2004, 8, 119–126. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Melean, L.G.; Alonso, M.M.; Rodríguez, A.; Rosales, M.; González, Á. Advances in the aqueous-phase hydroformylation of olefins from a refinery naphtha cut: The effect of monoethanolamine in the catalytic activity of the Rh/TPPTS system. Catal. Today 2015, 247, 124–131. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Lujano, E.; Modroño, M.; Marrero, A.C.; García, Y.M.; Fuentes, A.; Sánchez-Delgado, R.A. Rhodium-catalyzed hydroformylation of C6 alkenes and alkene mixtures—A comparative study in homogeneous and aqueous-biphasic media using PPh3, TPPTS and TPPMS ligands. J. Organomet. Chem. 2004, 689, 3782–3792. [Google Scholar] [CrossRef]
- Baricelli, P.J.; Lujano, E.; Rodríguez, M.; Fuentes, A.; Sánchez-Delgado, R.A. Synthesis and characterization of Ru(H)2(CO)(TPPMS)3 and catalytic properties in the aqueous-biphasic hydroformylation of olefins. Appl. Catal. Gen. 2004, 263, 187–191. [Google Scholar] [CrossRef]
- Baricelli, P.J.; López-Linares, F.; Rivera, S.; Melean, L.G.; Guanipa, V.; Rodriguez, P.; Rodriguez, M.; Rosales, M. Influence of the addition of thiophenes on the catalytic activity of the rhodium binuclear complex [Rh(CO)(μ-Pz)(TPPTS)]2 during the biphasic hydroformylation of 1-hexene. J. Mol. Catal. Chem. 2008, 291, 12–16. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Jiang, J.; Jin, Z. Hydroformylation of Higher Olefins by Thermoregulated Phase-Transfer Catalysis with Rhodium Nanoparticles. Chin. J. Catal. 2010, 31, 1191–1194. [Google Scholar] [CrossRef]
- Zhao, J.; Yi, J.; Yang, C.; Wan, K.; Duan, X.; Tang, S.; Fu, H.; Zheng, X.; Yuan, M.; Li, R.; et al. A Novel Strategy of Homogeneous Catalysis and Highly Efficient Recycling of Aqueous Catalyst for the Hydroformylation of Higher Olefins Based on a Simple Methanol/Water Mixed Solvent. Catal. Lett. 2021, 151, 1273–1281. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Chen, J.; Cheng, P.; Li, X. Synergistic effect of TPPTS and TPPDS on the regioselectivity of olefin hydroformylation in two-phase catalytic system. Catal. Today 2002, 74, 131–135. [Google Scholar] [CrossRef]
- Peng, Q.; Liao, X.; Yuan, Y. Micelle effect of disulfonated cetyldiphenyl phosphine in biphasic hydroformylation of higher olefins. Catal. Commun. 2004, 5, 447–451. [Google Scholar] [CrossRef]
- Hejun, Z.; Yunjie, D.; Fu, Y.; Li, Y.; Jianmin, X.; Hongmei, Y.; Liwu, L. Water/Oil Biphasic Hydroformylation of Higher Olefins over TPPTS-Rh/SiO2 Catalyst. Energy Chem. 2004, 13, 87. [Google Scholar]
- Desset, S.L.; Reader, S.W.; Cole-Hamilton, D.J. Aqueous-biphasic hydroformylation of alkenes promoted by “weak” surfactants. Green. Chem. 2009, 11, 630. [Google Scholar] [CrossRef]
- Matsinha, L.C.; Mapolie, S.F.; Smith, G.S. Recoverable and recyclable water-soluble sulphonated salicylaldimine Rh(I) complexes for 1-octene hydroformylation in aqueous biphasic media. Dalton Trans. 2015, 44, 1240–1248. [Google Scholar] [CrossRef]
- Hager, E.B.; Makhubela, B.C.E.; Smith, G.S. Aqueous-phase hydroformylation of 1-octene using hydrophilic sulfonate salicylaldimine dendrimers. Dalton Trans. 2012, 41, 13927. [Google Scholar] [CrossRef]
- Schrimpf, M.; Graefe, P.A.; Holl, A.; Vorholt, A.J.; Leitner, W. Effect of Liquid–Liquid Interfacial Area on Biphasic Catalysis Exemplified by Hydroformylation. ACS Catal. 2022, 12, 7850–7861. [Google Scholar] [CrossRef]
- Naße, K.E.; Heinen, F.S.; Pawlowsky, N.; Schrimpf, M.; Monflier, E.; Tilloy, S.; Leitner, W.; Vorholt, A.J. The role of cyclodextrins in the acceleration of the reaction rate in a biphasic hydroformylations. Chem. Eng. J. 2024, 497, 154114. [Google Scholar] [CrossRef]
- Siangwata, S.; Goosen, N.J.; Smith, G.S. Aqueous olefin hydroformylation using water-soluble mono- and trinuclear N,O-chelate rhodium(I)-aryl ether precatalysts. Appl. Catal. Gen. 2020, 603, 117736. [Google Scholar] [CrossRef]
- Lobry, E.; Cardozo, A.F.; Barthe, L.; Blanco, J.-F.; Delmas, H.; Chen, S.; Gayet, F.; Zhang, X.; Lansalot, M.; D’Agosto, F.; et al. Core phosphine-functionalized amphiphilic nanogels as catalytic nanoreactors for aqueous biphasic hydroformylation. J. Catal. 2016, 342, 164–172. [Google Scholar] [CrossRef]
- Chen, S.; Cardozo, A.F.; Julcour, C.; Blanco, J.-F.; Barthe, L.; Gayet, F.; Lansalot, M.; D’Agosto, F.; Delmas, H.; Manoury, E.; et al. Amphiphilic core-cross-linked micelles functionalized with bis(4-methoxyphenyl)phenylphosphine as catalytic nanoreactors for biphasic hydroformylation. Polymer 2015, 72, 327–335. [Google Scholar] [CrossRef]
- Ramarou, D.S.; Makhubela, B.C.E.; Smith, G.S. Synthesis of Rh(I) alkylated-PTA complexes as catalyst precursors in the aqueous-biphasic hydroformylation of 1-octene. J. Organomet. Chem. 2018, 870, 23–31. [Google Scholar] [CrossRef]
- Warmeling, H.; Hafki, D.; Von Söhnen, T.; Vorholt, A.J. Kinetic investigation of lean aqueous hydroformylation—An engineer’s view on homogeneous catalysis. Chem. Eng. J. 2017, 326, 298–307. [Google Scholar] [CrossRef]
- Obrecht, L.; Kamer, P.C.J.; Laan, W. Alternative approaches for the aqueous–organic biphasic hydroformylation of higher alkenes. Catal. Sci. Technol. 2013, 3, 541–551. [Google Scholar] [CrossRef]
- Warmeling, H.; Schneider, A.-C.; Vorholt, A.J. Considerations on film reactivity in the aqueous biphasic hydroformylation. AIChE J. 2018, 64, 161–171. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Wang, Y.; Jiang, J.; Jin, Z. Aqueous/organic biphasic hydroformylation of 1-octene catalyzed by Co2(CO)8 /Ph2P(CH2CH2O)nMe. Appl. Organom. Chemis 2012, 26, 718–721. [Google Scholar] [CrossRef]
- Kunna, K.; Müller, C.; Loos, J.; Vogt, D. Aqueous-Phase Hydroformylation of 1-Octene: Styrene Latices as Phase-Transfer Agents. Angew. Chem. Int. Ed. 2006, 45, 7289–7292. [Google Scholar] [CrossRef]
- Joumaa, A.; Gayet, F.; Garcia-Suarez, E.J.; Himmelstrup, J.; Riisager, A.; Poli, R.; Manoury, E. Synthesis of Nixantphos Core-Functionalized Amphiphilic Nanoreactors and Application to Rhodium-Catalyzed Aqueous Biphasic 1-Octene Hydroformylation. Polymers 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Warmeling, H.; Janz, D.; Peters, M.; Vorholt, A.J. Acceleration of lean aqueous hydroformylation in an innovative jet loop reactor concept. Chem. Eng. J. 2017, 330, 585–595. [Google Scholar] [CrossRef]
- Bischoff, S.; Kant, M. Efficient Catalysts for the Two-Phase Hydroformylation of Long-Chain α-Olefins. Ind. Eng. Chem. Res. 2000, 39, 4908–4913. [Google Scholar] [CrossRef]
- Wei, J.-Z.; Lang, J.-W.; Fu, H.-Y.; Li, R.-X.; Zheng, X.-L.; Yuan, M.-L.; Chen, H. Aqueous biphasic hydroformylation of higher alkenes and highly efficient catalyst recycling in the presence of a polar low boiling solvent. Transit. Met. Chem. 2016, 41, 599–603. [Google Scholar] [CrossRef]
- Miyagawa, C.C.; Kupka, J.; Schumpe, A. Rhodium-catalyzed hydroformylation of 1-octene in micro-emulsions and micellar media. J. Mol. Catal. Chem. 2005, 234, 9–17. [Google Scholar] [CrossRef]
- Nowothnick, H.; Rost, A.; Hamerla, T.; Schomäcker, R.; Müller, C.; Vogt, D. Comparison of phase transfer agents in the aqueous biphasic hydroformylation of higher alkenes. Catal. Sci. Technol. 2013, 3, 600–605. [Google Scholar] [CrossRef]
- Hapiot, F.; Bricout, H.; Tilloy, S.; Monflier, E. Hydroformylation in Aqueous Biphasic Media Assisted by Molecular Receptors. In Hydroformylation for Organic Synthesis; Taddei, M., Mann, A., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 342, pp. 49–78. ISBN 978-3-642-45058-7. [Google Scholar]
- Leblond, J.; Potier, J.; Menuel, S.; Bricout, H.; Machut-Binkowski, C.; Landy, D.; Tilloy, S.; Monflier, E.; Hapiot, F. Water-soluble phosphane-substituted cyclodextrin as an effective bifunctional additive in hydroformylation of higher olefins. Catal. Sci. Technol. 2017, 7, 3823–3830. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, J.; Wang, Y.; Cheng, F.; Jin, Z. Thermoregulated phase transfer ligands and catalysis XVIII: Synthesis of N,N-dipolyoxyethylene-substituted-2-(diphenylphosphino)phenylamine (PEO–DPPPA) and the catalytic activity of its rhodium complex in the aqueous–organic biphasic hydroformylation of 1-decene. J. Mol. Catal. Chem. 2003, 198, 23–27. [Google Scholar] [CrossRef]
- Elard, M.; Denis, J.; Ferreira, M.; Bricout, H.; Landy, D.; Tilloy, S.; Monflier, E. Rhodium catalyzed hydroformylation assisted by cyclodextrins in biphasic medium: Can sulfonated naphthylphosphanes lead to active, selective and recyclable catalytic species? Catal. Today 2015, 247, 47–54. [Google Scholar] [CrossRef]
- Pagar, N.S.; Deshpande, R.M. Kinetics of 1-decene hydroformylation in an aqueous biphasic medium using a water-soluble Rh-sulfoxantphos catalyst in the presence of a cosolvent. Int. J. Chem. Kinet. 2021, 53, 333–344. [Google Scholar] [CrossRef]
- Sieffert, N.; Wipff, G. Importance of Interfacial Adsorption in the Biphasic Hydroformylation of Higher Olefins Promoted by Cyclodextrins: A Molecular Dynamics Study at the Decene/Water Interface. Chem. A Eur. J. 2007, 13, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Künnemann, K.U.; Schurm, L.; Lange, D.; Seidensticker, T.; Tilloy, S.; Monflier, E.; Vogt, D.; Dreimann, J.M. Continuous hydroformylation of 1-decene in an aqueous biphasic system enabled by methylated cyclodextrins. Green. Chem. 2020, 22, 3809–3819. [Google Scholar] [CrossRef]
- Legrand, F.-X.; Sauthier, M.; Flahaut, C.; Hachani, J.; Elfakir, C.; Fourmentin, S.; Tilloy, S.; Monflier, E. Aqueous hydroformylation reaction mediated by randomly methylated β-cyclodextrin: How substitution degree influences catalytic activity and selectivity. J. Mol. Catal. Chem. 2009, 303, 72–77. [Google Scholar] [CrossRef]
- Benatmane, M.; Cousin, K.; Laggoune, N.; Menuel, S.; Monflier, E.; Woisel, P.; Hapiot, F.; Potier, J. Pillar5arenes as Supramolecular Hosts in Aqueous Biphasic Rhodium-Catalyzed Hydroformylation of Long Alkyl-chain Alkenes. ChemCatChem 2018, 10, 5306–5313. [Google Scholar] [CrossRef]
- Ji, Z.J.; Jiang, J.Y.; Wang, Y.H. A novel thermoregulated phosphine ligand used for the Rh-catalyzed hydroformylation of mixed C11–12 olefins in aqueous/organic biphasic system. Chin. Chem. Lett. 2010, 21, 515–518. [Google Scholar] [CrossRef]
- Fu, H.; Li, M.; Chen, J.; Zhang, R.; Jiang, W.; Yuan, M.; Chen, H.; Li, X. Application of a new amphiphilic phosphine in the aqueous biphasic catalytic hydroformylation of long chain olefins. J. Mol. Catal. Chem. 2008, 292, 21–27. [Google Scholar] [CrossRef]
- Fu, H.; Li, M.; Chen, H.; Li, X. Higher olefin hydroformylation in organic/aqueous biphasic system accelerated by double long-chain cationic surfactants. J. Mol. Catal. Chem. 2006, 259, 156–160. [Google Scholar] [CrossRef]
- Cocq, A.; Bricout, H.; Djedaïni-Pilard, F.; Tilloy, S.; Monflier, E. Rhodium-Catalyzed Aqueous Biphasic Olefin Hydroformylation Promoted by Amphiphilic Cyclodextrins. Catalysts 2020, 10, 56. [Google Scholar] [CrossRef]
- Fu, H.; Li, M.; Mao, H.; Lin, Q.; Yuan, M.; Li, X.; Chen, H. Aqueous biphasic catalytic hydroformylation of higher olefins: Promotion effect of cationic gemini and trimeric surfactants. Catal. Commun. 2008, 9, 1539–1544. [Google Scholar] [CrossRef]
- Six, N.; Guerriero, A.; Landy, D.; Peruzzini, M.; Gonsalvi, L.; Hapiot, F.; Monflier, E. Supramolecularly controlled surface activity of an amphiphilic ligand. Application to aqueous biphasic hydroformylation of higher olefins. Catal. Sci. Technol. 2011, 1, 1347. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; He, Y.; Li, Y.; Li, M.; Li, X. Long chain olefin hydroformylation in biphasic catalytic system—How the reaction is accelerated. Appl. Catal. Gen. 2003, 242, 85–88. [Google Scholar] [CrossRef]
- Wang, X.; Fu, H.Y.; Li, X.; Chen, H. Hydroformylation of high olefin in biphasic catalytic system: Effect of electronic and steric factor of phosphine ligands. Catal. Commun. 2004, 5, 739–741. [Google Scholar] [CrossRef]
- Li, M.; Fu, H.; Yang, M.; Zheng, H.; He, Y.; Chen, H.; Li, X. Micellar effect of cationic gemini surfactants on organic/aqueous biphasic catalytic hydroformylation of 1-dodecene. J. Mol. Catal. Chem. 2005, 235, 130–136. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Chen, H.; He, Y.; Li, X. Studies on 1-dodecene hydroformylation in biphasic catalytic system containing mixed micelle. J. Mol. Catal. Chem. 2003, 194, 13–17. [Google Scholar] [CrossRef]
- Yang, C.; Bi, X.; Mao, Z.-S. Effect of reaction engineering factors on biphasic hydroformylation of 1-dodecene catalyzed by water-soluble rhodium complex. J. Mol. Catal. Chem. 2002, 187, 35–46. [Google Scholar] [CrossRef]
- Parmar, D. Hydroformylation of 1-hexene catalyzed by water soluble CoCl2(TPPTS)2 in biphasic medium. J. Mol. Catal. Chem. 2004, 211, 83–87. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Bajaj, H.C.; Bricout, H.; Monflier, E. Biphasic hydroformylation of 1-octene catalyzed by cobalt complex of trisulfonated tris(biphenyl)phosphine. Appl. Catal. Gen. 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Parmar, D.U.; Bajaj, H.C.; Jasra, R.V. CoCl2(TPPTS)2 catalyzed hydroformylation of 1-octene and 1-decene in the presence of surfactant and co-solvents in a biphasic medium. J. Mol. Catal. Chem. 2008, 282, 99–106. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Parmar, J.N.; Jasra, R.V.; Bajaj, H.C.; Monflier, E. Cobalt catalyzed hydroformylation of higher olefins in the presence of chemically modified cyclodextrins. Catal. Commun. 2009, 10, 1808–1812. [Google Scholar] [CrossRef]
- Ferreira, M.; Bricout, H.; Roth, T.F.H.; Seidensticker, T.; Tilloy, S.; Monflier, E. Aqueous biphasic hydroformylation and hydroaminomethylation assisted by cyclodextrins: From benchtop to industrial perspective. Catal. Today 2024, 442, 114951. [Google Scholar] [CrossRef]
- Künnemann, K.U.; Weber, D.; Becquet, C.; Tilloy, S.; Monflier, E.; Seidensticker, T.; Vogt, D. Aqueous Biphasic Hydroaminomethylation Enabled by Methylated Cyclodextrins: Sensitivity Analysis for Transfer into a Continuous Process. ACS Sustain. Chem. Eng. 2021, 9, 273–283. [Google Scholar] [CrossRef]
- Suárez, T.; Fontal, B.; Zambrano, G.; Reyes, M.; Bellandi, F.; Contreras, R.R.; Diaz, J.C.; Cancines, P.; Fonseca, Y.; Romero, I. Hydroformylation reactions of the trans-Mo(CO)4(p-C5NH4SO3NA)2 complex in biphasic medium. React. Kinet. Catal. Lett. 2008, 94, 27–34. [Google Scholar] [CrossRef][Green Version]
- Gao, S.; Xi, Z. Reaction-Controlled Phase-Transfer Catalysis for Epoxidation of Olefins. In Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 429–447. ISBN 978-0-444-53188-9. [Google Scholar]
- Gao, J.; Chen, Y.; Han, B.; Feng, Z.; Li, C.; Zhou, N.; Gao, S.; Xi, Z. A spectroscopic study on the reaction-controlled phase transfer catalyst in the epoxidation of cyclohexene. J. Mol. Catal. Chem. 2004, 210, 197–204. [Google Scholar] [CrossRef]
- Campestrini, S.; Tonellato, U. Highly efficient cascade-oxygen-transfer from H2O2 to olefins mediated by halogenated carbonyl compounds and metalloporphyrins. J. Mol. Catal. Chem. 2001, 171, 37–42. [Google Scholar] [CrossRef]
- Schmidt, F.; Cokoja, M. Supramolecular concepts for the biphasic epoxidation of olefins using aqueous hydrogen peroxide. Green. Chem. 2021, 23, 708–722. [Google Scholar] [CrossRef]
- Mahamat Ahmat, Y.; Kaliaguine, S. Epoxidation of limonene and pinenes by dimethyldioxirane in microemulsions. Catal. Today 2023, 407, 146–155. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Xu, Y.; Li, W.; Niu, M.; Jiang, J.; Jin, Z. Thermoregulated phase-transfer rhodium nanoparticle catalyst for hydroaminomethylation of olefins. Catal. Commun. 2013, 34, 73–77. [Google Scholar] [CrossRef]
- Behr, A.; Becker, M.; Reyer, S. A highly efficient method for the hydroaminomethylation of long-chain alkenes under aqueous, biphasic conditions. Tetrahedron Lett. 2010, 51, 2438–2441. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Luo, M.M.; Li, Y.Z.; Chen, H.; Li, X.J. The catalytic hydroaminomethylation of long chain alkenes with dimethylamine in aqueous–organic two-phase system. Appl. Catal. Gen. 2004, 272, 151–155. [Google Scholar] [CrossRef]
- Faßbach, T.A.; Sommer, F.O.; Vorholt, A.J. Hydroaminomethylation in Aqueous Solvent Systems—An Efficient Pathway to Highly Functionalized Amines. Adv. Synth. Catal. 2018, 360, 1473–1482. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Luo, M.; Chen, H.; Li, X. Hydroaminomethylation of high alkenes with dual-metal catalysts in aqueous/organic biphasic system. Arkivoc 2008, 2008, 165–174. [Google Scholar] [CrossRef]
- Albarella, L.; Musumeci, D.; Sica, D. Reactions of 1,5-Dienes with Ruthenium Tetraoxide: Stereoselective Synthesis of Tetrahydrofurandiols. Eur. J. Org. Chem. 2001, 2001, 997–1003. [Google Scholar] [CrossRef]
- Akiri, S.O.; Ojwach, S.O. Structural studies and applications of water soluble (phenoxy)imine palladium(II) complexes as catalysts in biphasic methoxycarbonylation of 1-hexene. J. Organomet. Chem. 2021, 942, 121812. [Google Scholar] [CrossRef]
- Bauers, F.M.; Chowdhry, M.M.; Mecking, S. Catalytic Polymerization of Ethylene in Aqueous Emulsion with a Simple in Situ Catalyst. Macromolecules 2003, 36, 6711–6715. [Google Scholar] [CrossRef]
- Kuntz, E.G.; Godard, G.; Basset, J.M.; Vittori, O.M. Nickel(0)-TPPTS-Cyanide Complex in Water. An Efficient and Flexible Catalyst for the Isomerisation of Olefinic Compounds at Room Temperature. Oil Gas. Sci. Technol.—Rev. IFP 2007, 62, 781–785. [Google Scholar] [CrossRef]
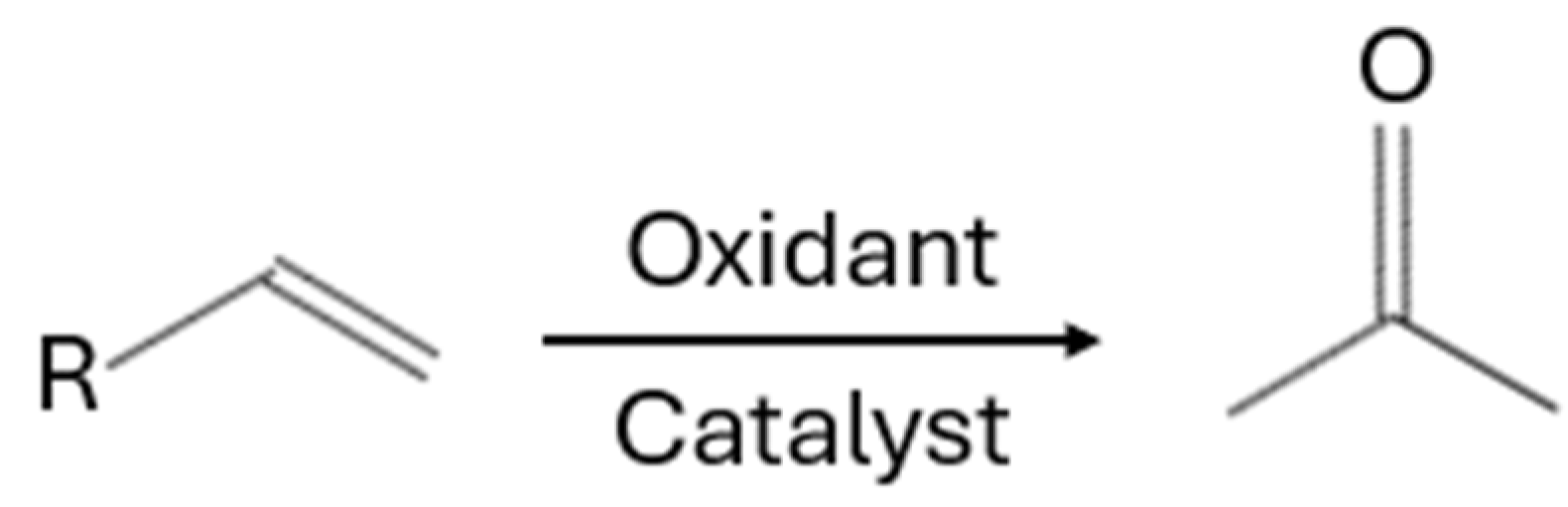
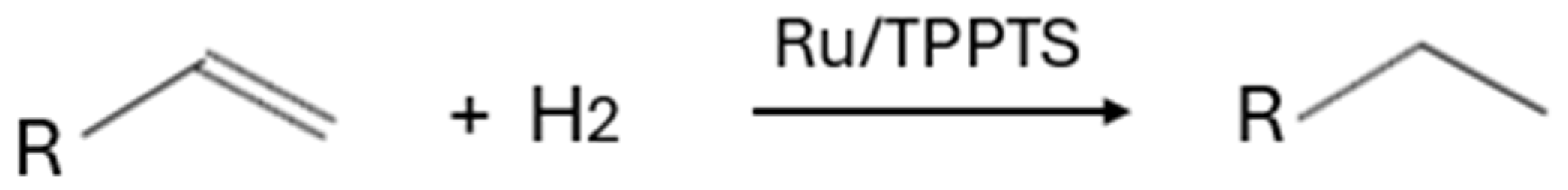
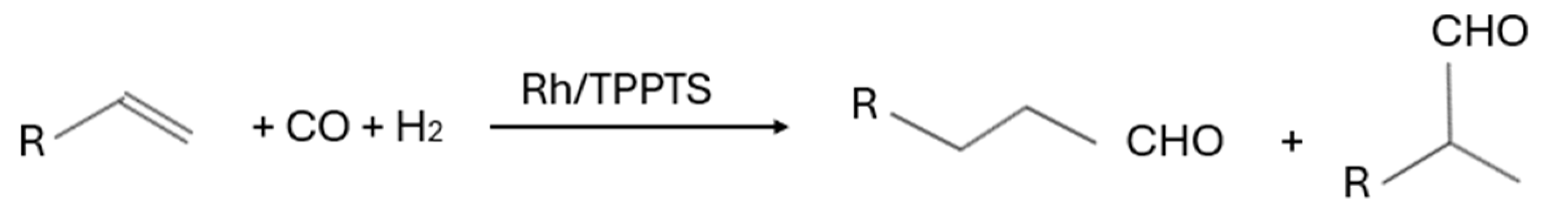

| Substrate | Products | Catalyst | Conditions | Additives | Catalytic Activity | References |
|---|---|---|---|---|---|---|
| C12–C20 olefins | 2-methyl ketones | PdCl2 | Oxidant: tert-butyl-hydroperoxide Temperature: 80 °C Time: 2–7 h Stirring: 300 rpm | n-paraffins | 90–95% (Conversion) | [9] |
| Terminal C6 Olefins | 2-alkanones | PhenS*Pd(OAc)2 | Oxidant: O2, Temperature: 100 °C Pressure: 30 bar | NaOAc, NaOH | >99% (Selectivity), 99% (Yield) | [10] |
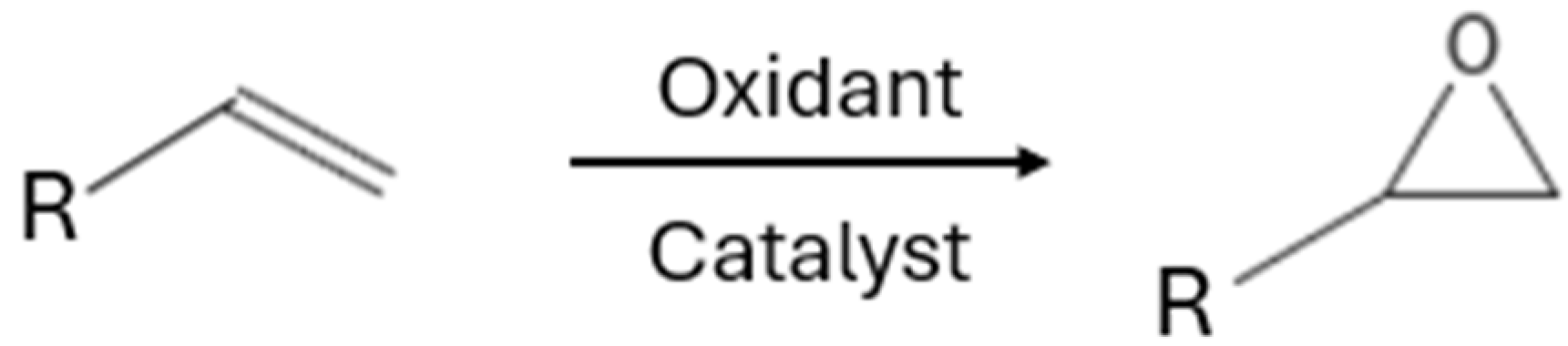
| C8 olefins | cyclooctene oxide and styrene oxidants | Mn(salen)-Chit | Oxidants: m-CPBA, t-BuOOH, H2O2 Temperature: 25 °C Time: 24 h | - | 3–39% (Yield), 12–160 h−1 (TOF) | [11] |
| Substrate | Products | Catalyst | Conditions | Additives | Catalytic Activity | References |
|---|---|---|---|---|---|---|
| C4–C8 olefins | C4–C8 alkanes, and alcohols derived from hydroformylated olefins | HRh(CO)(TPPMS)3, Rh(μ-Pz)(CO)(TPPMS)]2, RuCl2(TPPTS)3, RhH(CO)(TPPTS)3 | Temperature: 70–150 °C Time: 1–24 h Pressure: 9.8–98.7 atm H2 | ZnCl2, NaCl | 18.81–100% (Conversion), 91–100% (Selectivity), 78–95% (Yield). | [12,13,14,15] |
| C5–C10 olefins | C5–C10 alkanes, alcohols derived from hydroformylated olefins and ethylbenzene derived from styrene | Raney Ni, [Rh(COD)Cl]2, palladium nanoparticles (immobilized within the walls of hollow polymeric microspheres), RuCl2(TPPMS)3(DMSO), PVP stabilized Rh nanoparticles | Temperature: 25–120 °C Time: 1–22 h Pressure: 0.99–34 atm H2 Stirring: 1600–2000 rpm | Catalytic nanoreactors (TPP@CCM1 or TPP@CCM2), electrolytes | 34–100% (Conversion), 86–95% (Selectivity), 90–99% (Yield) | [24,25,26,27,28] |
| C6–C12 olefins | C6–C12 alcohols derived from hydroformylated olefins | Co/Ph2P(CH2CH2O)nCH3, Co/nBuPhP(CH2CH2O)nCH3, Ru/PTA, Pt/PTA, Rh nanoparticles/Ph2P(CH2CH2O)22CH3, [η6-(2-phenoxyethanol)RuCl(NH)]Cl, [η6-(2-phenoxyethanol)RuCl(S)]Cl, [η6-(2-phenoxyethanol)RuCl(O)]Cl, Ru(CO)3(TPPMS)2 (I), RuH2(CO)(TPPMS)3 (II) | Temperature: 20–100 °C Time: 1–8 h Pressure: 1–27.6 atm H2 Stirring: 500–630 rpm | - | 41–100% (Conversion), 92–100% (Selectivity) | [16,17,18,19,29] |
| C7–C18 olefins | C7–C18 alkenes | Rh nanoparticles/Ph2P(CH2CH2O)16CH3 (thermoregulated) | Temperature: 60 °C Time: 1–2 h Pressure: 9.8 atm H2 | - | 87–100% (Conversion), 500–2000 h−1 (TOF) | [20] |
| Alfa-pinene | Cis and trans pinane | Rh nanoparticles/PVA, Ru nanoparticles stabilized by ammonium surfacants | Temperature: 25–70 °C Time: 1–3 h Pressure: 9.8–19.7 atm H2 | - | 96–99.9% (Conversion), 98.9–99% (Selectivity) | [21,22] |
| Polybutadiene | Hydrogenated polybutadiene | Rh/TPPTS | Temperature: 100 °C Time: 20–30 min Pressure: 19.7 atm H2 pH: 7 | Cationic DTAC and Brij-35 | 255–1245 h−1 (TOF) | [23] |
| Substrate | Catalyst | Conditions | Additives | Catalytic Activity | References |
|---|---|---|---|---|---|
| C2–C4 olefins | Rh/TPPTS, HRh(CO)(TPPTS)3 | Temperature: 65–130 °C Pressure: 14.8–49.3 atm syngas pH: 5–6 | - | 92.5–99% (Selectivity) | [30,31] |
| C3–C7 olefins | RhH(CO)(TPPTS)3, [Rh(m-Pz)(CO)(TPPTS)]2, trans-Mo(CO)4(p-PySO3Na)2, RhCl(CO)(TPPMS)2, RhCl(CO)(TPPDS)2, RhCl(CO)(TPPTS)2, [HRu(CO)(CH3CN)(TPPTS)3]BF4 | Temperature: 50–150 °C Time: 3–72 h Pressure: 13.8–98.7 atm syngas Stirring: 760–1200 rpm | - | 40–100% (Conversion), 8–50% (Selectivity), 3–95% (Yield) | [32,33,34,35,95] |
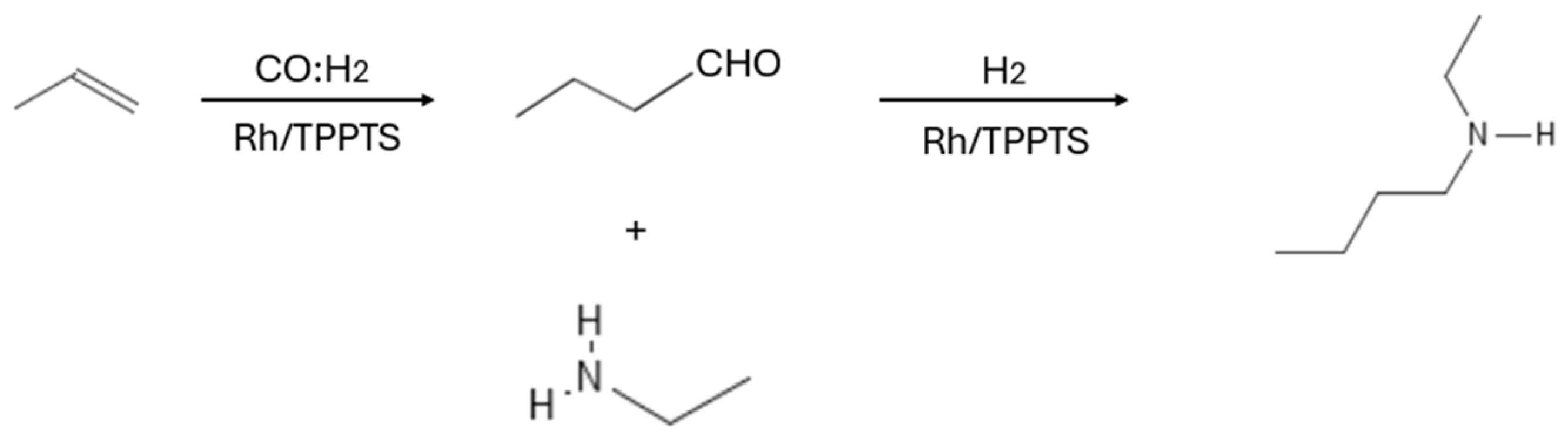
| C5–C12 | RhH(CO)(TPPTS)3, rhodium polyethylene glycolate, (RhH(CO)(TPPTS)3, Rh/PETPP, RhH(CO)(TPPTS)3 | Temperature: 40–130 °C Time: 0.66–200 h Pressure: 39.5–118.4 atm syngas Stirring: 600–760 rpm | Monoethanolamine (MEA) | 17–99% (Conversion), 98% (Selectivity), 72–95.5% (Yield) | [36,37,38,39,40] |
| C6 olefins | CoCl2(TPPTS)2, RhH(CO)(TPPTS)3, RhH(CO)(TPPTS)3, H2Ru(CO)(TPPMS)3, [Rh(CO)(μ-Pz)(TPPTS)]2 | Temperature: 69.8–100 °C Time: 3–26 h Pressure: 17.2–88.8 atm syngas Stirring: 600–760 rpm | - | 87–100% (Conversion), 68% (Selectivity), 90% (Yield) | [41,42,43,89] |
| C6–C12 olefins | Ph2P(CH2CH2O)16CH3, HRh(CO)(TPPTS)3, RhCl(CO)(TPPTS)2 modified with TPPDS, Rh(acac)(CO)2 with TPPTS/TPPDS/CDPPDS, TPPTS-Rh/SiO2, [Rh(acac)(CO)2] and TPPTS, Rh(acac)(CO)2 and water-soluble phosphine ligands, [RhH(CO)(TPPTS)3], [Rh(μ-Pz)(CO)(m-TPPTS)]2 | Temperature: 70–120 °C Time: 0.5–200 h Pressure: 9.9–69.1 atm syngas Stirring: 760–1200 rpm | CTAB, [OctMim]Br | 24.3–99.5% (Conversion), 57.9–95.7% (Selectivity), 93–97% (Yield), 8.95–11.88 (TON), 10–1614 h−1 (TOF) | [3,6,44,45,46,47,48,49] |
| C8 olefins | [Rh(sulphsal-X-R)(COD)], CoCl2(BiphTS)2, [RhCl(COD)]2, Rh(acac)(CO)2/TPPTS, mononuclear Rh(I)-salicylaldimine complex (9), trinuclear Rh(I)-salicylaldimine complex (10), rhodium complex ([Rh(acac)(CO)2]) embedded in phosphine-functionalized amphiphilic nanogels (TPP@NG), [Rh(acac)(CO)2] coordinated to BMOPPP ligands within the hydrophobic core of CCM, BMOPPP-functionalized micelles synthesized via RAFT polymerization, rhodium(I)-based mono-, di-, and trinuclear PTA complexes, CO-modified analogs, [Rh(cod)Cl]2/TPPTS, Rh/TPPTS, [Rh(cod)Cl]2/TPPTS, Ph2P(CH2CH2O)ₙMe, [Rh(acac)(CO)2]/TPPTS, Rh-nixantphos@CCM, [Rh(cod)Cl]2/TPPTS | Temperature: 75–180 °C Time: 3–20 h Pressure: 19.7–88.8 atm syngas Stirring: 300–2750 rpm pH: 5.5 | CTAB, RAME-β-CD, AC-WV, cyclodextrins, nonionic latex, anionic latex (sodium 4-vinylbenzylsulfonate), cationic latex (4-vinylbenzyltrimethylammonium tetrafluoroborate) | 98–99% (Conversion), 49–99% (Selectivity), 8–98.5% (Yield), 365 (TON), 4.6–742 h−1 (TOF) | [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,90] |
| C8–C14 olefins | CoCl2(TPPTS)2, Rh/Ph2P-(CH2)10-PO3Na2, Rh/Ph2P-(CH2)12-PO3Na2), Rh/TPPTS, HRh(CO)(TPPTS)3, [Rh(acac)(CO)2] combined with SulfoXantPhos, CoCl2(TPPTS)2, Rh(acac)(CO)2/TPPTS | Temperature: 80–140 °C Time: 2–10 h Pressure: 19.7–78.9 atm syngas Stirring: 600–100 rpm | CTAB, Lutensol® ON 70 (C10E7, non-ionic amphiphile), nonionic surfacants (Marlophen NP 9), Polymer latices, RAME-β-CD | 75–98% (Conversion), 40–98% (Selectivity), 71.6–88% (Yield), 65–5046 h−1 (TOF) | [65,66,67,68,69,91,92] |
| C10 olefins | Rh/β-cyclodextrin-based phosphane ligand, PEO–DPPPA/Rh, Rh(acac)(CO)2/M1NPS, Rh(acac)(CO)2/D2NPS, Rh(CO)2(acac)/2,7-bis(SO3Na)-xantphos, [RhH(CO)(TPPTS)2]6−, Rh/TPPTS, Rh/sulfoxantphos, [Rh(acac)(CO)2]/TPPTS | Temperature: 76.8–130 °C Time: 3–240 h Pressure: 40.8–50 atm syngas Stirring: 500–1500 rpm | RAME-β-CD, 2,6-dimethyl-β-CD | 99.5–100% (Conversion), 93–97% (Selectivity), 39–99% (Yield) | [70,71,72,73,74,75,76] |
| C10–C18 olefins | Rh(CO)2(acac)/TPPTS, Rh/Ph2P(CH2CH2O)22CH3, RhCl(CO)(TPPTS)2, Rh(acac)(CO)2/TPPTS, RhCl(CO)(TPPTS)2, Rh(acac)(CO)2/1-(4-tert-butylbenzyl)-1-azonia-3,5-diaza-7-phosphaadamantyl bromide | Temperature: 80–120 °C Time: 1–6 h Pressure: 19.7–49.3 atm syngas Stirring: 400–1500 rpm | PEG-substituded pillar[5]arene, DLCS, OS-CDs, β-CD-(OSG–Me)1, cationic gemini and trimeric surfactants, RAME-β-CD, native β-CD | 72–100% (Conversion), 51–95% (Selectivity), 94% (Yield), 157–1111 h−1 (TOF) | [77,78,79,80,81,82,83] |
| C12 olefins | RhCl(CO)(TPPTS)2, RhCl(CO)(2-MOTPPTS)2, RhCl(CO)(4-MOTPPTS)2, [Rh(acac)(CO)2], Rh/TPPTS, RhCl(CO)(TPPTS)2 | Temperature: 80–130 °C Time: 2–6 h Pressure: 9.9–49.3 atm syngas Stirring: 0–1000 rpm | CTAB, cyclodextrins, RAME-β-CD, Gemini surfactants (cationic) with varying spacers, CPB, SDS, DBS, Triton X-100, Brij 35, 1-pentanol, 1-heptanol | 8–94% (Conversion), 81.8–90% (Selectivity), 94% (Yield), 883–1200 h−1 (TOF) | [1,84,85,86,87,88] |
| Substrate | Catalyst | Conditions | Additives | Catalytic Activity | References |
|---|---|---|---|---|---|
| C3–C6 olefins | [p-C5H5NC16H33]3[PW4O16], [C5H5N(CH2)15CH3]3[PW4O16], Mn(TDCPP)Cl and Iron porphyrins | Oxidant: H2O2 Temperature: 25–65 °C Time: 1–4 h | Hexafluoroacetone hydrate (HFAH) | 90–99% (Conversion), 99.5% (Selectivity) | [96,97,98] |
| C6–C8 olefins | Polyoxometalate (POM) derivatives | Oxidant: H2O2 Temperature: 70 °C Time: 4 h | - | 95% (Conversion), 87–95% (Selectivity) | [99] |
| Terpenes (Limonene + alfa-pinene) | Dimethyldioxirane (DMDO) generated in situ from oxone (potassium peroxymonosulfate) and acetone | Oxidant: Oxone Temperature: 25 °C Time: 45–90 min | - | 100% (Conversion), 99–100% (Yield) | [100] |
| Substrate | Catalyst | Conditions | Additives | Catalytic Activity | References |
|---|---|---|---|---|---|
| C6–C12 olefins | Rh nanoparticles/Ph2P(CH2CH2O)16CH3, [Rh(cod)Cl]2 combined with Na-TPPTS, RhCl(CO)(TPPTS)2, [Rh(cod)Cl]2/Sulfoxantphos, RhCl(CO)(TPPTS)2 | Temperature: 100–130 °C Time: 4–6 h Pressure: 29.6–59.2 atm syngas or CO2 or CO:H2 | Morpholine salts, CTAB | 80.1–99% (Conversion), 51.4–98% (Selectivity) | [101,102,103,104,105] |
| C10–C16 olefins | Rh/TPPTS, Rh/Sulfoxantphos, Rh(acac)(CO)2, combined with the ligand SulfoXantphos | Temperature: 80–125 °C Time: 30 h Pressure: 29.6–49.3 atm syngas | RAME-β-CD | 80% (Selectivity) | [93,94] |
| Substrate | Reaction | Products | Catalyst | Conditions | Additives | Catalytic Activity | References |
|---|---|---|---|---|---|---|---|
| 1,5-dienes | Cyclization | cis-Tetrahydrofuran derivatives | RuO2·2H2O | Oxidant: NaIO4 Temperature: 25 °C Time: Few min | - | 37–50% (Selectivity) | [106] |
| 1-hexene | Methoxycarbonylation | Esters | Water-soluble palladium(II) complexes with phenoxyimine ligands | Temperature: 90 °C Time: 20 h Pressure: 59.2 atm CO | - | 92% (Conversion), 92% (Yield) | [107] |
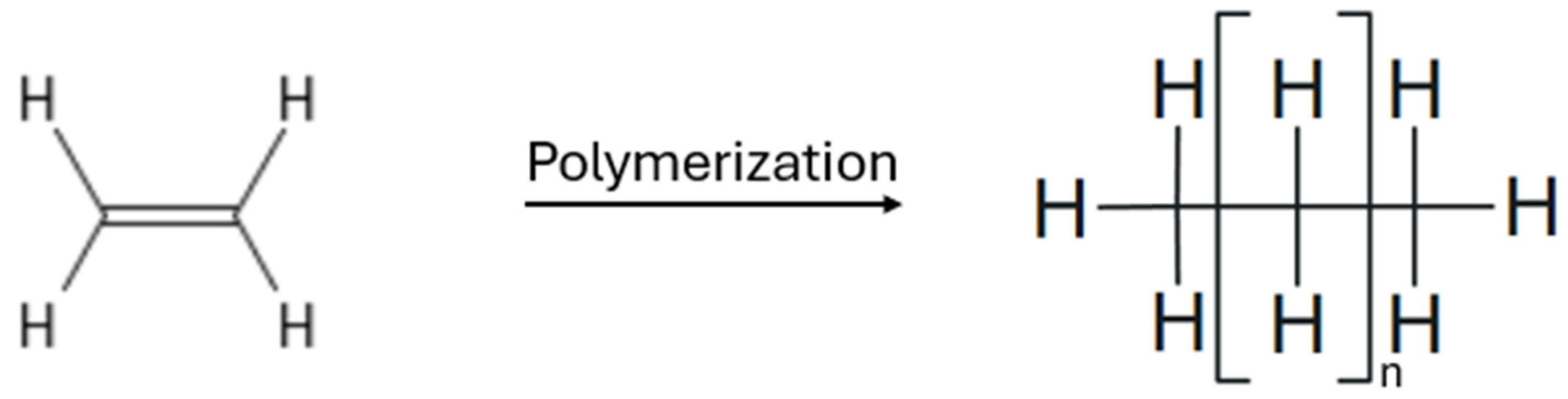
| Ethylene | Polymerization | Linear semicrystalline polyethylene | P∧O-chelated nickel(II) complex | Temperature: 50–70 °C Pressure: 39.5 atm | - | - | [108] |
| C4 olefins | Isomerization | Butenes | Nickel(0)–TPPTS–cyanide complex | Temperature: 0–20 °C Time: 15 min to 1 h pH: 9.5 | NaBH4 | 3600 h−1 (TOF) | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chira, A.; Kokkinos, N.C. Biphasic Catalytic Conversion of Olefins in Aqueous Media: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 4028. https://doi.org/10.3390/ijms26094028
Chira A, Kokkinos NC. Biphasic Catalytic Conversion of Olefins in Aqueous Media: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(9):4028. https://doi.org/10.3390/ijms26094028
Chicago/Turabian StyleChira, Angeliki, and Nikolaos C. Kokkinos. 2025. "Biphasic Catalytic Conversion of Olefins in Aqueous Media: A Systematic Review" International Journal of Molecular Sciences 26, no. 9: 4028. https://doi.org/10.3390/ijms26094028
APA StyleChira, A., & Kokkinos, N. C. (2025). Biphasic Catalytic Conversion of Olefins in Aqueous Media: A Systematic Review. International Journal of Molecular Sciences, 26(9), 4028. https://doi.org/10.3390/ijms26094028







