Abstract
Neurological diseases, including neurodegenerative disorders and stroke, represent significant medical challenges due to their complexity and the limitations of current treatment approaches. This review explores the potential of stem cell (SC)-derived exosomes (Exos) as a transformative therapeutic strategy for these diseases. Exos, especially those derived from SCs, exhibit natural targeting ability, biocompatibility, and the capacity to cross the blood–brain barrier (BBB), making them ideal vehicles for drug delivery. This review provides an in-depth discussion of the properties and advantages of SC-Exos. It highlights their potential synergistic benefits in therapeutic approaches to treat neurological diseases. This article discusses the mechanisms of action of SC-Exos, highlighting their ability to target specific cells, modulate disease pathways, and provide controlled release of therapeutic agents. Applications in specific neurological disorders have been investigated, demonstrating the potential to improve outcomes in conditions such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and stroke. Moreover, Exos-coated nanoparticles (NPs) combine the natural properties of Exos with the multifunctionality of NPs. This integration takes advantage of exosome membrane biocompatibility and targeting capabilities while preserving NPs’ beneficial features, such as drug loading and controlled release. As a result, Exos-coated NPs may enhance the precision, efficacy, and safety of therapeutic interventions. In conclusion, SC-Exos represent a promising and innovative approach to treating neurological diseases.
1. Introduction
1.1. Overview of Neurological Diseases
Neurological diseases encompass many disorders that affect the central and peripheral nervous systems [1]. These conditions can significantly impair quality of life and pose substantial challenges in diagnosis and treatment. Neurological disorders can be categorized based on various criteria, including the primary location affected, the type of dysfunction, or the underlying cause [2]. Some key categories include neurodegenerative diseases, which are characterized by the progressive loss of neuronal structure and function. An example of this condition is AD, which leads to memory loss and cognitive decline [3]. PD impacts motor function, leading to symptoms like tremors and stiffness [4]. Cerebrovascular Diseases involve disorders related to the blood vessels in the brain. Stroke occurs when the blood supply to the brain is interrupted, resulting in brain damage [5]. Treatment approaches for AD typically involve cholinesterase inhibitors and NMDA receptor antagonists to manage symptoms. Recent advancements include immunotherapy that targets amyloid beta plaques. However, some therapies have been associated with severe side effects, such as brain bleeds [6]. Dopaminergic medications for PD are the standard treatment. Innovations such as adaptive deep brain stimulation (DBS) have emerged, offering responsive modulation of brain activity and enhancing symptom management [7]. The acute management of a stroke includes thrombolytic therapy and mechanical thrombectomy [8].
Current treatments are typically limited to symptomatic relief and do not effectively halt or reverse disease progression. Treating neurological diseases involves overcoming several key challenges. The BBB is a highly selective barrier that protects the brain from harmful substances in the bloodstream. However, it also restricts the systemic delivery of therapeutic agents, making it difficult for them to cross [9]. The brain’s complexity requires targeted treatment due to its intricate morphology and localized neural damage [10]. Many current drugs have significant side effects because they cannot precisely target diseased brain regions. The brain has a limited ability to regenerate neurons, making recovery from neural damage particularly challenging [11]. Recent advances in biotechnology and nanotechnology offer promising new avenues for overcoming some of these challenges when treating neurological diseases [12,13], including the use of Exos-based therapies and NP-mediated drug delivery, which have shown significant potential [14].
1.2. Potential of Exos
Exos are small, membrane-bound vesicles secreted by cells that play a crucial role in cell-to-cell communication [15,16]. They can transfer proteins, lipids, and genetic material between cells, influencing various physiological and pathological processes. The unique properties of Exos, such as their biocompatibility, ability to cross the BBB, and intrinsic targeting capabilities, make them attractive candidates for drug delivery systems [17,18]. SCs can differentiate into various neural cell types and secrete Exos that carry neuroprotective and regenerative factors [19,20,21]. SC-Exos are particularly promising for treating neurological diseases due to their natural affinity for brain tissues and their content of bioactive molecules that support neural health and repair [19,22,23].
1.3. Integrating Exos and NPs
NPs are tiny particles, typically 1 to 100 nanometers, engineered to deliver therapeutic agents to specific cells or tissues [24]. They can be designed to improve drug solubility, protect therapeutic agents from degradation, and ensure controlled release. NPs have shown potential in improving the delivery of drugs across the BBB and ensuring targeted action within the brain [25]. Exos-coated NPs were created by merging the advantages of Exos with those of NPs. This hybrid approach leverages the natural targeting and communication abilities of Exos with the versatility and efficiency of NPs [26,27,28], creating a potent delivery system for neurological therapies.
1.4. Aim
This review aims to explore the potential of Exos in treating neurological diseases. It will cover Exos’ properties and therapeutic attributes, the advantages of their use in drug delivery systems, and how the combination of Exos and NPs can address current challenges in Neurotherapy. Furthermore, it will discuss their mechanisms of action, applications in the preclinical and clinical research of specific diseases, and future directions in this emerging field.
2. Exos
2.1. Definition of Exos
“Exos” is short for exosomes, an extracellular vesicle (EV) type. Exos are small, membrane-bound vesicles typically measuring 30–150 nm in diameter [29,30,31]. They originate from the endosomal system and are formed through the inward budding of multivesicular bodies (MVBs). Exos are released into the extracellular environment upon fusion of these MVBs with the plasma membrane. Exos play a crucial role in intercellular communication by transferring bioactive molecules, including proteins, lipids, mRNA, microRNA (miRNA), and other non-coding RNAs to recipient cells [32]. They are vital in various physiological processes, such as immune response, tissue regeneration, and neural communication. Additionally, Exos are implicated in several pathological conditions, including cancer, neurodegenerative diseases, and cardiovascular disorders [33]. The term “Exos” is often used interchangeably with “extracellular vesicles” (EVs). Still, it is important to note that Exos represent a specific class of EVs with unique biogenesis, size, and molecular composition characteristics. While EVs is a broad term that includes Exos, microvesicles (MVs), apoptotic bodies, and other types of vesicles, using “Exos” helps to highlight those vesicles that are derived from endosomes, as opposed to microvesicles, which bud directly from the plasma membrane. Exos are especially relevant in therapeutic applications, particularly in treating neurological diseases, regenerative medicine, and drug delivery [4]. This relevance is due to their ability to cross the BBB, their high stability, and their intrinsic targeting capabilities.
2.2. Sources of Exos
Exos differ significantly based on their cellular origin, which affects their composition, biological functions, and therapeutic applications [20,34]. A comparison of stem cell-derived exosomes (SC-Exos) with Exos from other donor cells regarding their characterization and functions can be seen below (Table 1, [32,35,36,37,38,39,40,41,42,43,44]). SC-Exos are particularly well-suited for regenerative and neuroprotective applications because they contain a unique cargo of growth factors, neurotrophic factors, and anti-inflammatory molecules [45]. In contrast, Exos from other donor cells exhibit functions specific to their cell type. These functions can be beneficial, such as neuron-derived Exos that support synaptic function, or detrimental, such as cancer-derived Exos that promote tumor progression. Due to their immunomodulatory, anti-apoptotic, and regenerative properties, SC-Exos hold superior therapeutic potential for treating neurodegenerative diseases and stroke [46,47].

Table 1.
Comparison of stem cell (SC)- derived exosomes (SC-Exos) and Exos from other donor cells. Exos differ significantly based on their cellular origin, which affects their composition, biological functions, and therapeutic applications.
2.3. Exos Extraction and Isolation Methods
Efficient and reliable isolation techniques are crucial to studying and utilizing Exos for therapeutic and diagnostic purposes [22,31,34]. The most common methods include Ultracentrifugation (UC), the gold standard for Exos isolation. It involves differential centrifugation at high speeds (approximately 100,000× g). Size Exclusion Chromatography (SEC) is a technique that separates Exos based on their size. This results in high purity while preserving their functionality. Polymer-based precipitation, such as ExoQuick, utilizes polyethylene glycol (PEG) to aggregate Exos and aid their precipitation. Ultrafiltration is a technique that uses membrane filters to separate Exos based on molecular weight, ensuring high recovery efficiency. Immunoaffinity capture involves targeting specific surface markers on Exos, such as CD9, CD63, and CD81, using antibody-coated magnetic beads to achieve high specificity.
2.4. Biological Properties of Exos
Exos have unique characteristics that make them valuable for therapeutic applications: they are biocompatible and exhibit low immunogenicity [41]. Because Exos are derived from natural cellular processes, they are generally well-tolerated in living organisms. Exos can traverse the BBB, making them suitable for delivering drugs and biomolecules to the central nervous system (CNS) [48]. The surface markers of Exos facilitate selective interactions with particular cells and tissues. Cargo delivery and controlled-release Exos naturally transport therapeutic biomolecules to target cells, promoting precision medicine [49]. Exos represent a powerful tool in regenerative medicine, drug delivery, and biomarker discovery.
2.5. Therapeutic Potential in Neurological Therapies
The therapeutic potential of SC-Exos lies in their unique properties and bioactive contents. SC-Exos may deliver neuroprotective proteins and RNAs that help to shield neurons from apoptosis and other forms of cellular stress [31,34,40]. SC-Exos may also modulate immune responses by carrying anti-inflammatory cytokines and other regulatory molecules, reducing the neuroinflammation associated with many neurological disorders [34]. In addition, the growth factors and signaling molecules within SC-Exos may stimulate the proliferation and differentiation of endogenous neural stem cells, aiding in tissue repair and regeneration [50]. Furthermore, SC-Exos may improve synaptic plasticity and support neuronal network remodeling, crucial for recovery in neurodegenerative conditions and brain injuries [34,51]. SC-Exos offer several advantages for neurological therapies [52,53,54]. Due to their biocompatibility, Exos derived from human cells are less likely to provoke immune responses than synthetic delivery systems. Their natural origin enables better integration and acceptance by neural tissues, promoting the targeted delivery of therapeutic agents. Exos possess the unique ability to cross the BBB, a critical challenge in treating neurological diseases. The bioactive molecules in SC-Exos are naturally optimized for neural health and repair.
2.6. Current Research and Developments
Recent research has focused on optimizing the production, isolation, and therapeutic application of SC-Exos [19,50,55]. Engineered Exos loaded with specific drugs, RNA molecules, and gene-editing tools are increasingly being used due to their ability to enhance therapeutic efficacy. An improved mechanistic understanding of how SC-Exos exert their effects has also facilitated their optimized use in various neurological conditions. Numerous animal studies have reported the potential of SC-Exos in models of neurodegenerative diseases and stroke, showing promising results regarding functional recovery and neuroprotection [34,56]. This comprehensive understanding of SC-Exos provides a foundation for their potential use in advanced therapies for neurological disorders, leveraging their natural properties and therapeutic potential.
3. Mechanisms of Action
3.1. Targeting and Uptake
Upon administration, the Exos travel through the bloodstream and cross the BBB using mechanisms such as receptor-mediated transcytosis [32,57]. The exosome surface proteins interact with receptors on the target cells, facilitating specific binding and uptake [58]. The mechanisms by which SC-Exos targets and is taken up by neural cells are intricate and multifaceted. Exos are naturally equipped with various surface proteins and ligands that can recognize and bind to specific receptors on target cells [59]. SC-Exos may leverage these surface molecules to home in on neural cells, expressing corresponding receptors [22,60]. For example, Exos may target neurons or glial cells by binding to cell surface markers like CD63, CD81, and L1CAM. In addition, upon binding to target cell receptors, Exos are internalized through endocytic pathways such as clathrin-mediated endocytosis, caveolin-mediated endocytosis, or micropinocytosis [61,62]. In some cases, phagocytic pathways can also be involved, especially in microglia, the brain’s resident immune cells. In specific scenarios, Exos may fuse directly with the plasma membrane of the recipient cell, releasing their cargo directly into the cytoplasm [58,63]. Specific membrane fusion proteins and lipid compositions of the exosome and target cell membranes facilitate this process.
3.2. Cargo Delivery and Release
The therapeutic agents, including drugs, RNA molecules, or proteins, are delivered to the site of action within the target cells [34]. This can lead to the modulation of cellular pathways, a reduction in pathological processes, and the promotion of cellular repair and regeneration. The delivery and release of therapeutic cargo from SC-Exos involves several critical steps [34], including Controlled Release—once in the cytoplasm, the cargo (which could include drugs, RNA molecules, or proteins) is released in a controlled manner [49]—and Nuclear Targeting—some Exos cargo needs to reach the cell nucleus for gene therapy applications [50,64].
3.3. Cellular and Molecular Effects
The SC-Exos components may exert therapeutic effects by influencing gene expression, reducing inflammation, and promoting cell survival and growth [4,19]. The therapeutic effects of the cargo delivered by SC-Exos occur at multiple cellular and molecular levels. Therapeutic proteins delivered by SC-Exos can replace defective proteins, inhibit harmful enzymes, or stimulate protective pathways [34]. For example, delivering neurotrophic factors like BDNF (Brain-Derived Neurotrophic Factor) can promote neuronal survival and growth [40]. The anti-inflammatory targeting of SC-Exos includes cytokines and molecules that modulate the immune response, reducing neuroinflammation, a hallmark of many neurological diseases [34,40]. For example, visualization studies have shown that SC-Exos delivers anti-inflammatory agents (e.g., cytokines, small molecules) to brain regions affected by inflammation [31]. Reduction in toxicity can be achieved through various approaches [65]. Studies have shown that SC-Exos delivers targeted drugs precisely to diseased cells, reducing off-target effects and systemic toxicity. Similarly, therapeutic agents encapsulated within SC-Exos provide a protective barrier that prevents degradation and reduces toxic side effects. In addition, the natural biocompatibility of SC-Exos and their efficient clearance from the body minimizes long-term toxicity and immune reactions [34]. Exos’ cargo can support neuronal health by reducing oxidative stress, inhibiting apoptosis, and promoting cellular repair mechanisms [33,47,66,67,68,69,70]. Molecules such as growth factors and miRNAs in the Exos enhance neurogenesis and synaptic plasticity, facilitating recovery from neural damage. For example, SC-Exos delivery of neurotrophic factors (e.g., BDNF, GDNF) to support neuron survival, growth, and repair was reported [19,71]. Other studies showed SC-Exos delivering antioxidants that neutralize reactive oxygen species (ROS) and reduce oxidative stress in neuronal cells. SC-Exos can also promote the proliferation and differentiation of neural stem cells, contributing to the regeneration of damaged neural tissue [19,20,31]. Another study shows the role of SC-Exos in enhancing synaptic plasticity and improving both neural network function and cognitive outcomes [72]. Target cells internalizing SC-Exos via receptor-mediated endocytosis enable the intracellular delivery of therapeutic agents [73]. Other studies showed the controlled and sustained release of therapeutic cargo from SC-Exos within target cells, ensuring prolonged therapeutic effects [34,74]. The mechanisms of action by which SC-Exos modulates neuroinflammation, reduce toxicity, and promote neuroprotection highlight their multifaceted therapeutic potential in treating neurological diseases [75]. Mitochondrial dysfunction and endoplasmic reticulum (ER) stress are key characteristics of several health conditions, such as neurological diseases [76]. These conditions are often associated with impaired energy metabolism, increased oxidative stress, and cellular damage from mitochondrial failure and ER stress. As a result [77], using Exos for mitochondrial-targeted therapy to restore or enhance mitochondrial function has emerged as a promising strategy to alleviate the effects of these debilitating diseases. The relationship between Exos and ER stress pathways has garnered significant attention due to their roles in developing various human diseases and potential therapeutic applications [78]. In neurodegenerative diseases, ER stress, which involves misfolded proteins such as tau and α-synuclein, impacts the biogenesis and release of Exos. The interplay between exosome and ER stress pathways is a crucial factor in the progression of many diseases. The mechanisms by which Exos contribute to neuroprotection is summarized in Figure 1. The source and cargo of Exos influence their effects on brain health. Utilizing their therapeutic potential while reducing harmful effects presents a promising approach to treating neurodegenerative diseases.
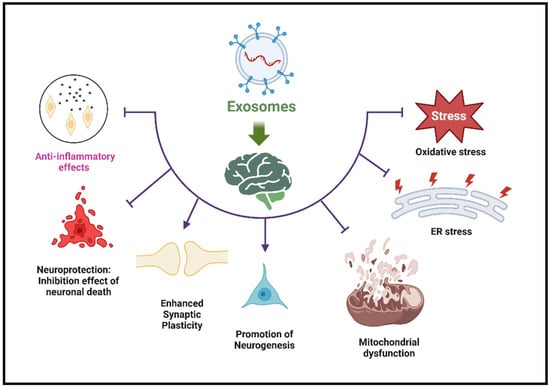
Figure 1.
Mechanisms of Exos’ effects on brain health. This figure illustrates the cellular and molecular effects of Exos on brain health. It highlights beneficial anti-inflammatory effects that reduce neuroinflammation and neuroprotection that prevents neuronal death. Enhances synaptic plasticity—supports learning and memory. Promotes neurogenesis—encourages the formation of new neurons. Antioxidative stress—reduces ROS damage. Fights ER stress—reduces effects on protein folding and cellular homeostasis. Anti-mitochondrial dysfunction—reduces damage to energy metabolism.
3.4. Crosstalk with the Microenvironment
SC-Exos interact dynamically with the brain’s microenvironment [19,34,51,53]. Exos facilitate communication between different cell types in the brain, including neurons, astrocytes, and microglia. This intercellular communication is crucial for coordinating repair processes and maintaining homeostasis [79,80,81]. Components of SC-Exos can influence the ECM, promoting an environment conducive to tissue repair and regeneration. For example, Exos carry matrix metalloproteinases (MMPs) that can remodel the ECM, facilitating cell migration and tissue integration. By delivering anti-inflammatory agents and immunomodulatory molecules, SC-Exos can alter the behavior of immune cells in the brain, such as microglia and infiltrating lymphocytes, promoting a more regenerative and less destructive immune response [31]. SC-Exos are particularly effective at overcoming biological barriers that impede traditional drug delivery [82]. The BBB is a significant obstacle in treating neurological diseases. Exos naturally possess mechanisms to cross the BBB via transcytosis, making them ideal for delivering NPs loaded with therapeutic agents to the brain [32,44]. The dense and complex extracellular matrix in the brain can also hinder the movement of therapeutic agents. SC-Exos can navigate this environment more effectively due to their small size and natural composition [34,74].
4. Applications in Specific Neurological Diseases
The characteristics of SC-Exos make them suitable for various neurological applications [19,21,34,50,74].
4.1. AD
AD is characterized by the accumulation of amyloid beta plaques and tau tangles, leading to neuronal death, synaptic dysfunction, and cognitive decline [83,84,85,86]. The BBB poses a significant challenge in delivering therapeutic agents to the brain [87,88]. Therapeutic strategies with SC-Exos include Anti-Amyloid Therapies. SC-Exos can deliver siRNA or small molecules targeting amyloid precursor protein (APP) or beta-secretase, enzymes involved in amyloid beta production. This reduces plaque formation and promotes plaque clearance. In addition, the delivery of microRNAs or small interfering RNA (siRNA) can downregulate tau protein expression and prevent hyperphosphorylation, thereby reducing neurofibrillary tangles [89]. Exos from neural stem cells also contain neuroprotective factors such as BDNF, which can be delivered to support neuron survival and promote neurogenesis [31,81,90]. Finally, SC-Exos naturally carry anti-inflammatory cytokines that can modulate microglial activation and reduce neuroinflammation, a key component of AD pathology [91].
A summary of these papers on SC-Exos and their potential in AD are provided (Table 2). Adipose-derived Stem Cells (ASCs) Exos decreased Aβ deposition and apoptosis in neuronal cells derived from AD transgenic mice, highlighting their therapeutic effect in reducing neuronal damage [92]. Mesenchymal Stem Cells (MSCs)-derived Exos promoted neurogenesis and cognitive recovery in AD mouse models, supporting their potential to restore lost neuronal connections and cognitive functions [93]. MSC-derived Exos delivered intranasally exhibited immunomodulatory and neuroprotective effects in a 3xTg AD mouse model, effectively reducing neuroinflammation and preventing neuronal degeneration [94]. MSC-derived exosomal miR-223 regulated neuronal apoptosis by inhibiting pro-apoptotic pathways, highlighting a molecular mechanism for neuroprotection in AD [95]. MSC-derived Exos improved cognitive function and reduced Aβ aggregation in AD mouse models, showcasing their neuroprotective potential [96]. MSC-derived Exos enhanced autophagy and regulated insulin signaling by modulating the PI3K/Akt/mTOR pathway, suggesting a mechanism to alleviate AD pathology [97]. Neural Stem Cells (NSCs)-derived Exos promoted mitochondrial biogenesis and restored abnormal protein distribution in AD mouse models, indicating the potential for addressing mitochondrial dysfunction in AD [98]. NSC-derived Exos repaired the disrupted BBB in an in vitro model of AD, demonstrating their capacity to restore vascular integrity in neurodegenerative conditions [99].

Table 2.
SC-Exos and AD. Exos derived from SCs (including ASCs, MSCs, and NSCs) show promising therapeutic effects in AD by reducing Aβ levels, enhancing autophagy, promoting mitochondrial biogenesis, increasing neurogenesis, and modulating the immune response.
In conclusion, SC-Exos show great potential for treating AD due to their immunomodulatory, anti-apoptotic, and neurodegenerative properties. They function through various mechanisms, including enhancing mitochondrial function, regulating autophagy, and repairing the BBB. These diverse abilities make them versatile tools for addressing the pathology of AD and improving cognitive function.
4.2. PD
PD involves the degeneration of dopaminergic neurons in the substantia nigra, leading to motor dysfunction [100,101]. Effective delivery of therapeutic agents to the brain is crucial for slowing disease progression and alleviating symptoms [102,103]. Therapeutic strategies with hNSC-Exos include encapsulating dopamine or dopamine agonists in SC-Exos, ensuring targeted delivery to dopaminergic neurons, enhancing therapeutic efficacy, and reducing peripheral side effects. The delivery of neuroprotective agents such as GDNF (Glial cell line-derived Neurotrophic Factor) also supports the survival of dopaminergic neurons [104]. Targeting the aggregation of alpha-synuclein with siRNA or small molecules delivered via SC-Exos to reduce toxic oligomer formation was also reported [105]. Finally, the delivery of genes encoding for enzymes involved in dopamine synthesis (e.g., tyrosine hydroxylase) restores dopamine levels in the brain [106].
These studies show that SC-Exos may reduce neuroinflammation, minimize neuronal apoptosis, enhance neurotrophic signaling, and improve metabolism in the diverse applications of Exos-based therapies for PD (Table 3). Exos derived from bone marrow-derived MSCs (BM-MSCs) containing the Gli1 protein were found to inhibit Sp1 signaling, reducing microglial activation and neuronal apoptosis [107]. These findings suggest a possible mechanism for reducing inflammation and cell death in PD. Exos derived from MSCs modulated cholesterol metabolism in neurons via the Wnt5a-LRP1 axis, improving cognitive function in a progressive PD model [108]. This study connects metabolic regulation to cognitive enhancements in PD. Exos derived from ASCs demonstrated therapeutic effects in a transgenic mouse model of PD by modulating neuroinflammatory and neurotrophic pathways [109]. This indicates their potential for enhancing motor function and alleviating PD pathology. Treatment with Ginkgolide A improved the functions of MSC-derived Exos, including their antioxidative and anti-inflammatory effects, in a 6-OHDA-induced PD cell model [110]. This study highlights the importance of pharmacologically enhancing Exos in treating PD. MSC-derived Exos delivered FTO-targeted siRNA, regulating m6A-dependent ATM mRNA to prevent the death of dopaminergic neurons [111]. This innovative approach combines Exos therapy with RNA interference to specifically target molecular mechanisms in PD. Human umbilical cord MSC-derived Exos loaded with BDNF enhance neuroregeneration and functional recovery in a PD model [112]. Neurotrophic factors significantly improve the therapeutic potential of Exos-based treatments.

Table 3.
SC-Exos and PD. These articles explore the role of SC-Exos in providing neuroprotection in PD.
4.3. Stroke
Stroke results in significant neuronal loss, neuroinflammation, and the disruption of the BBB [113,114]. The rapid and effective delivery of therapeutic agents is critical for minimizing damage and promoting recovery [115,116]. Therapeutic strategies with SC-Exos include the rapid delivery of neuroprotective agents such as antioxidants, anti-apoptotic molecules, and calcium channel blockers to protect neurons during the acute phase of injury [117,118]. Similarly, the delivery of growth factors such as VEGF (Vascular Endothelial Growth Factor) and BDNF stimulate the formation of new neurons and blood vessels, supporting tissue repair and functional recovery [119]. Also, the encapsulation of anti-inflammatory agents reduces secondary injury caused by neuroinflammation [120,121], as does the delivery of agents that promote the repair of the BBB, restore its integrity, and prevent further damage [122,123].
These studies highlight the potential of SC-Exos as a stroke treatment, emphasizing mechanisms like neuroprotection, anti-inflammation, and the reduction in oxidative stress (Table 4). IFN-γ stimulation improves the therapeutic potential of NSC-derived Exos, modulating immune responses and leading to better neuroprotection in an ischemic stroke model [22]. The miR-146a-5p found in Exos suppresses neuroinflammation by inhibiting the microglia’s IRAK1/TRAF6 signaling pathway, thereby reducing pro-inflammatory responses [124]. Exos miR-150-5p helps to reduce ischemia–reperfusion injury by targeting TLR5, which modulates inflammatory responses [125]. Exos lncRNA-ZFAS1 alleviates oxidative stress and inflammation by inhibiting miR-15a-5p, restoring homeostasis following a stroke [126]. Exos KLF4 reduces damage caused by ischemic stroke by modulating lncRNA-ZFAS1 to inhibit Drp1 methylation, thus preventing mitochondrial dysfunction [127]. Exos miR-193b-5p inhibits pyroptosis following an ischemic stroke by targeting AIM2, which helps to reduce inflammation-driven cell death [128]. NSC-derived Exos serve as nanocarriers for BDNF delivery, promoting neuronal repair and functional recovery after a stroke [40]. PD-L1- and HGF-decorated Exos enhance neuroplasticity by activating the STAT3-FOXO3 signaling pathway, which supports neuronal repair and functional recovery [129].

Table 4.
SC-Exos and stroke. These studies indicate advancements in the application of SC-Exos for treating ischemic stroke.
5. Exosome-Coated Nanoparticles (Exos-NPs)
5.1. Advantages of NPs in Neurological Disorders
Due to their ability to overcome significant therapeutic challenges, NPs have emerged as promising platforms for drug delivery, particularly in treating neurological diseases [13,76,130]. Some key advantages include improved drug solubility and bioavailability. NPs can encapsulate hydrophobic drugs, enhancing their solubility and extending their circulation time in the body [24]. Engineered NPs allow for sustained drug release, reducing dosing frequency and enhancing therapeutic efficacy [131]. Targeted drug delivery can be achieved by functionalizing NPs with targeting ligands, such as antibodies or peptides. This ensures selective delivery to diseased cells while minimizing off-target effects [132]. NPs can be designed to effectively cross the BBB, a significant challenge in neurological treatments [25]. This method utilizes the enhanced permeability and retention (EPR) effect in areas where the BBB is compromised, such as inflamed or tumorous regions [133]. Altering the surface of NPs with ligands that specifically bind to receptors on brain cells can improve their uptake [134]. Stimuli-responsive release NPs can be engineered to release drugs in response to specific stimuli, such as changes in pH, temperature, or enzymatic activity, ensuring drug activation occurs at the targeted site [135]. Intranasal delivery uses the olfactory and trigeminal nerve pathways to bypass the BBB, allowing direct access to the brain [136]. Magnetic targeting allows the precise delivery of magnetic NPs to specific brain regions using external magnetic fields, ensuring localized therapy [137].
5.2. Merging Exos and NPs: Strategies and Benefits
Exos combined with NPs create a highly effective delivery system that leverages both strengths [26,43]. Various methods, including direct adsorption, have been developed to coat, fuse, or merge Exos with NPs [138]. This method involves mixing Exos with NPs under controlled conditions, allowing for spontaneous adsorption while maintaining the integrity of the Exos. Layer-by-layer assembly involves alternating layers of oppositely charged molecules, including Exos, which result in a tunable coating with adjustable thickness and composition. Chemically modifying NPs can enhance covalent bonding, establishing strong covalent interactions with exosomal membrane proteins or lipids to ensure stable attachment [41]. This method utilizes charge-based attraction between Exos and NPs, creating a stable coating especially effective for charged nanoparticle surfaces. Bioorthogonal click chemistry involves engineered functional groups on Exos and NPs for precise, biocompatible conjugation, ensuring no interference with biological processes. These strategies showcase the complex yet advantageous interactions between Exos and NPs in developing advanced delivery systems. Exosomal coating enhances the properties and therapeutic potential of NPs through various mechanisms. Compared to synthetic coatings, it increases biocompatibility by reducing immunogenicity and toxicity, thereby improving the safety of therapies [139]. The surface proteins of Exos facilitate specific cell recognition and enhance uptake, enabling precise delivery to targeted brain regions [140]. Enhanced stability Exos protect NPs from premature degradation and clearance, prolonging their circulation time and increasing drug bioavailability. Exos naturally carry bioactive molecules such as neurotrophic factors, anti-inflammatory cytokines, and siRNA, which can enhance NPs-mediated therapies [37,138]. Exos provide better targeting, uptake, and biocompatibility, while synthetic NPs offer higher drug-loading capacity and controlled release (Table 5, [13,17,25,34,131,133,138,141,142,143,144]). Combining these natural and engineered systems in Exos-coated NPs creates an advanced platform for precision medicine, which is particularly beneficial for treating neurodegenerative diseases and stroke.

Table 5.
Comparison of targeting and internalization mechanisms: Exos vs. NPs. Understanding the differences in targeting and internalization mechanisms between Exos and synthetic NPs is crucial. Exos-coated NPs present a significant advantage in drug delivery and neuroprotection.
5.3. Properties of Exos-NPs
Exos-NPs have shown great potential in diagnosis and therapy (Table 6, [48,140,145,146,147,148,149]). These hybrid nanocarriers leverage the advantages of Exos, including their biocompatibility, ability to cross the BBB, and natural targeting capabilities, combined with the customizable properties of NPs to improve drug delivery. According to recent studies, Exos-NPs have become an emerging platform in nanomedicine due to their unique combination of synthetic NPs versatility and the natural targeting ability of Exos. Integrating Exos with NPs can enhance drug solubility, immune evasion, and targeted delivery, providing a powerful and versatile approach to treating complex diseases. Lopes et al. review bioengineered exosomal membrane-coated nanocarriers designed for neurodegenerative diseases and regenerative medicine [17]. Ribeiro et al. suggest using Exos-like liposomes to deliver natural compounds and RNA therapies to treat AD [150]. Liu et al. developed an Exos-coated gene-chem nanocomplex that acts as a nanoscavenger, effectively clearing alpha-synuclein aggregates and activating immune responses in PD [151]. In the context of ischemic stroke, Kim et al. developed magnetic extracellular nanovesicles derived from mesenchymal stem cells for targeted therapy [152]. Techniques such as surface coating, membrane fusion, and hybridization offer distinct advantages, particularly in drug delivery, gene therapy, and regenerative medicine. Incorporating Exos-NPs can enhance targeted drug delivery and improve the effectiveness of treatments. Exos-NP technologies are currently in preclinical stages, and research is ongoing to optimize their design, safety, and efficacy.

Table 6.
Biomedical applications of exosome-coated nanoparticles (Exos-NPs) for diagnosis and therapy. Exos-NPs have gained significant attention in biomedical research for their multifaceted capabilities in both diagnosis and therapy. By harnessing the intrinsic properties of Exos—such as biocompatibility, low immunogenicity, and natural targeting ability—Exo-NPs provide an advanced platform that enhances the therapeutic efficacy and diagnostic precision of conventional NPs.
Mesenchymal stromal/stem cell-derived exosomes (MSC-Exos) are under clinical investigation in immunology, regenerative medicine, neurology, dermatology, endocrinology, and infectious diseases [153]. In one trial for chronic kidney disease, UCB-MSC-Exos administered intravenously at 100 g/kg produced significant improvements in estimated glomerular filtration rate, serum creatinine, blood urea, and urine albumin-to-creatinine ratio. In skin applications, ASC-Exos delivered by local injection (0.2 g twice daily) reduced melanin levels and enhanced skin texture, while an intravenous regimen (ExoFlo at 1–10 × 106 MSCs/kg) in a COVID-19 trial improved survival and reduced inflammatory markers. Other trials report significant gains in pain reduction and disability scores in osteoarthritis, although some studies did not report clinical outcomes. Dosing strategies span microgram amounts to particle counts, with varying frequencies and routes (intravenous, local injection, inhalation). No clinical trial data on Exos-coated NPs were found.
5.4. The Translational Advances, Challenges, and Future Perspectives of Exos and Exos-NPs
Extracellular vesicles and their engineered derivatives, NPs, have emerged as promising tools in precision medicine, drug delivery, and regenerative therapies. Recent advances in Exos engineering, therapeutic cargo loading, and clinical applications highlight these biological NPs’ potential and challenges. Despite their promise, significant hurdles remain in optimizing their therapeutic efficacy, ensuring large-scale production, and establishing standardized data reporting frameworks for clinical comparability. Engineering and loading therapeutic Exos focuses on improving their natural capacity to carry bioactive molecules, such as proteins, RNAs, and small-molecule drugs. Tian et al. comprehensively review recent developments in Exos engineering for drug delivery [154]. Their study emphasizes various strategies for modifying Exos to improve targeting efficiency, including surface modifications with peptides and antibodies and genetic engineering to enhance endogenous cargo loading. These strategies have demonstrated improved therapeutic outcomes in preclinical neurodegeneration, cancer, and metabolic disease models. Similarly, Piffoux et al. discuss key exosome loading aspects, focusing on passive and active loading strategies [155]. Passive loading, which relies on diffusion-based methods, has limitations in achieving high cargo concentrations. In contrast, active loading techniques such as electroporation and sonication significantly enhance the efficiency of therapeutic payload delivery. However, these methods may also compromise the structural integrity of Exos, leading to functional alterations. A critical challenge remains to balance high-efficiency loading with preserving exosomal stability and bioactivity. Chen et al. further examine the encapsulation of therapeutic molecules within Exos [156]. They provide a systematic review of various techniques used to assess exosome loading and therapeutic effectiveness. Their findings underscore the importance of rigorous characterization methods, including high-resolution imaging and advanced omics-based profiling, to ensure that engineered Exos maintain their functional properties post-modification. The clinical translation of Exos-based therapeutics is a focus of intense research. Lee et al. critically review the clinical applications of Exos across multiple disease areas, including oncology, neurodegenerative disorders, and cardiovascular diseases [157]. They highlight several ongoing clinical trials where Exos formulations have shown promising therapeutic potential, particularly in targeted drug delivery. One example is using MSC-derived Exos to treat inflammatory diseases and regenerate tissue. However, Lee et al. also emphasize the variability in Exos production methods, which complicates regulatory approval and large-scale manufacturing. Further supporting this perspective, Perocheau et al. analyze the readiness of Exos-based therapies for clinical deployment [158]. They argue that while numerous preclinical studies have demonstrated efficacy, very few have progressed beyond early-stage trials due to concerns over batch-to-batch variability, immunogenicity, and stability during storage. These issues necessitate the development of standardized protocols for Exos isolation, purification, and quality control. Maumus et al. extensively explore the therapeutic potential of MSC-derived Exos, examining their regenerative capabilities in musculoskeletal and immune-related disorders [159]. Their study highlights the challenges in transitioning these vesicles from research models to clinical use, particularly regarding reproducibility and ensuring consistent therapeutic effects across different patient populations. Lener et al. proposed a foundational framework for addressing these translational barriers [160]. In their study, the international society for extracellular vesicles (ISEV) outlined key recommendations for using extracellular vesicles in clinical trials. Their position paper stresses the need for standardized manufacturing protocols and robust safety assessments to facilitate regulatory approval.
Data reporting and standardization pose critical challenges in this field, as the lack of a standardized framework limits the comparability of Exos-based studies. Piffoux et al. propose a structured framework for reporting exosome characteristics, cargo composition, and functional assays [155]. This initiative aims to improve reproducibility and ensure that data generated in preclinical models can be reliably translated into clinical applications. The study also highlights the importance of defining Exos purity metrics, as contamination with non-vesicular components can significantly alter therapeutic outcomes. Beyond technical standardization, Syn et al. explore the implications of Exos heterogeneity in cancer nanomedicine and immunotherapy [161]. Their research demonstrates that exosomal cargo composition varies depending on cell source, isolation method, and culture conditions, further complicating clinical standardization efforts. This variability underscores the need for a consensus on best practices for exosome production, characterization, and therapeutic evaluation. While Exos-based therapeutics hold immense potential, their clinical translation requires addressing several key bottlenecks. As Sharma et al. discussed, advances in engineering methods are improving Exos functionality, but large-scale manufacturing and quality control remain significant challenges [162]. Innovations in bioreactor-based production systems and cryopreservation techniques could solve these issues. Furthermore, Butreddy et al. discuss the broader implications of Exos-based biopharmaceuticals, including their use as delivery vehicles for nucleic acid-based therapies such as siRNA and mRNA [163]. The study highlights the need for more in-depth safety evaluations, particularly in assessing human subjects’ potential off-target effects and immune responses.
Engineering Exos-based NPs for therapeutic applications represents one of the most significant advancements in exosome research. This innovation aims to enhance therapeutic outcomes. These hybrid systems combine the biological advantages of Exos—such as biocompatibility, immune evasion, and natural targeting ability—with synthetic NPs tunability and enhanced stability. Several studies have provided insights into how these engineered Exos can be leveraged for clinical applications. For example, Chen et al. demonstrated the therapeutic potential of Exos-coated polydatin NPs in treating radiation-induced intestinal damage [146]. Their study highlighted the protective role of Exos functionalization, which improved NPs stability, enhanced cellular uptake, and increased therapeutic efficacy through anti-inflammatory and antioxidant mechanisms. This research underscores how Exos engineering can enhance drug bioavailability and mitigate side effects in oxidative stress and tissue injury conditions. Similarly, Hill et al. developed Exos-coated Prussian blue NPs for targeted glioblastoma therapy [149]. The study found that when used to coat Prussian blue NPs, tumor-derived Exos enabled the more precise targeting of glioblastoma cells while maintaining the ROS-scavenging properties of Prussian blue. This hybrid approach demonstrated increased therapeutic efficacy and reduced off-target toxicity, illustrating the potential of Exos-based targeting in oncology. In another study, Ye et al. investigated the role of Exos-based NPs in cancer immunotherapy [164]. Their work emphasized how engineered Exos could enhance immune responses by acting as antigen-presenting vesicles or delivering immune checkpoint inhibitors. The ability to modulate immune cell interactions through Exos engineering presents new opportunities for improving the efficacy of cancer immunotherapy. Furthermore, Yong et al. explored the use of tumor-derived Exos as drug carriers for chemotherapy [148]. Their research demonstrated that Exos-based NPs loaded with chemotherapeutic agents exhibited improved drug retention and enhanced tumor-targeting capabilities. The findings suggest that Exos-based carriers can help to overcome common limitations associated with conventional chemotherapy, such as systemic toxicity and poor drug penetration in solid tumors. Exos-based NPs are an innovative drug delivery and regenerative medicine solution, providing a promising alternative to synthetic nanoparticle systems. Recent research has shown significant advancements in Exos engineering, cargo loading, and targeting strategies, with applications in oncology, neurology, and inflammatory diseases. With ongoing advancements, Exos-engineered NPs have the potential to revolutionize precision medicine and targeted therapy in the years ahead.
6. Advantages of SC-Exos Therapies
SC-Exos offers superior targeting capabilities compared to traditional therapies [50,165]. Conventional drug delivery systems often suffer from non-specific distribution, leading to off-target effects and reduced therapeutic efficacy. Conventional systemic therapies often affect non-target tissues, leading to undesirable side effects. SC-Exos mitigates this issue through targeted delivery [34,50]. SC-Exos can address challenges related to drug resistance in neurological diseases. By facilitating efficient drug delivery to the brain, SC-Exos can ensure higher local concentrations of the therapeutic agent, potentially overcoming resistance mechanisms that limit drug efficacy [34,106]. Delivering siRNA or CRISPR-Cas9 components can also precisely silence or edit genes associated with drug resistance, providing a novel approach to overcoming therapeutic challenges [12]. Neurological diseases are often characterized by complex pathologies that necessitate a multifaceted approach to treatment. SC-Exos can deliver a combination of therapies that address various aspects of disease pathology, such as inflammation, oxidative stress, and synaptic dysfunction, offering a comprehensive treatment strategy [50,166]. The flexibility of SC-Exos allows for adapting the therapeutic payload to different stages of disease progression, from early intervention to advanced stages, ensuring continuous and effective disease management. By providing these significant advantages over traditional therapies, Exos-coated NPs represent a promising and innovative approach to treating neurological diseases [167], potentially transforming patient outcomes and paving the way for more effective and personalized medical interventions.
7. Challenges and Future Directions
7.1. Challenges
Overcoming biological barriers, such as the BBB and ECM, remains a significant challenge in the clinical translation of SC-Exos. Exos can cross the BBB, but efficiently delivering larger therapeutic cargoes to the brain remains challenging [57]. The complex and dense ECM in the brain can impede the movement of SC-Exos, limiting their distribution within target tissues and impacting their therapeutic efficacy [168]. Ensuring consistency and reproducibility in producing and characterizing SC-Exos is essential for clinical translation. In addition, the variability in exosome isolation methods can lead to differences in size, composition, and functionality, affecting the performance of the SC-Exos [169]. Standardized methods for characterizing SC-Exos are needed to accurately assess their physicochemical properties, stability, and cargo loading efficiency [170]. Modifying the surface properties of Exos and NPs (e.g., by incorporating targeting ligands or stealth coatings) may improve their stability, circulation time, and targeting specificity [145,171].
7.2. Immunogenicity and Safety
One of the key considerations in using SC-Exos is their immunogenicity and safety profile. SC-Exos tend to have lower immunogenicity than synthetic particles because they are derived from human cells. This reduces the risk of immune rejection and adverse inflammatory responses [21]. The use of biodegradable materials in SC-Exos and the natural clearance mechanisms of Exos contribute to a favorable safety profile, minimizing long-term accumulation and toxicity [74]. Addressing concerns related to immunogenicity and long-term safety is critical for the clinical success of SC-Exos. While Exos are generally considered immunologically inert, concerns remain about potential immune reactions to foreign components in the SC-Exos, such as synthetic polymers or therapeutic cargoes [172]. Long-term studies are needed to evaluate the potential accumulation and persistence of SC-Exos in the body and their impact on systemic health over time [50].
8. Conclusions
SC-Exos represent a cutting-edge and promising approach to revolutionizing the treatment of neurological diseases. By leveraging the natural targeting capabilities of Exos and the advanced delivery mechanisms of NPs, therapeutic agents can be delivered to the brain with unprecedented precision and efficacy, and give us the ability to explore the potential of SC-Exos in treating a variety of neurological diseases, including neurodegenerative diseases such as AD and PD, as well as stroke. The advantages of SC-Exos over conventional therapies are clear: enhanced targeting and delivery capabilities, reduced side effects and toxicity, multifunctionality, improved patient compliance, and versatility across various neurological diseases. These advantages stem from the unique properties of Exos, such as their ability to cross biological barriers, their biocompatibility and biodegradability, and their potential for customized therapeutic approaches. However, several challenges must be addressed to fully realize the clinical potential of SC-Exos. Overcoming biological barriers, ensuring standardization and quality control, addressing immunogenicity and long-term safety issues, and advancing delivery strategies are key areas for future research. In conclusion, SC-Exos offers a promising avenue for advancement in neurotherapeutics, potentially improving outcomes in patients affected by and treated for these diseases.
Author Contributions
Y.-P.Y.: writing—review and editing, supervision. C.J.B.N.: writing—review and editing. M.-C.C.: investigation, project administration, writing—original Draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Science and Technology Council (NSTC 113-2314-B-030-007, NSTC 113-2515-S-030-001, and NSTC 114-2918-I-030-001), Taiwan.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank Tairui Chiang for the methodology and visualization of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef]
- Chiang, M.C.; Yang, Y.P.; Nicol, C.J.B.; Chiang, T.; Yen, C. Resveratrol-Enhanced Human Neural Stem Cell-Derived Exosomes Mitigate MPP+-Induced Neurotoxicity Through Activation of AMPK and Nrf2 Pathways and Inhibition of the NLRP3 Inflammasome in SH-SY5Y Cells. Life 2025, 15, 294. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Lo, S.S.; Hung, S.W.; Wang, C.J.; Lin, C.H. Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture. Int. J. Mol. Sci. 2022, 23, 1678. [Google Scholar] [CrossRef]
- Sharma, A.; Rudrawar, S.; Bharate, S.B.; Jadhav, H.R. Recent advancements in the therapeutic approaches for Alzheimer’s disease treatment: Current and future perspective. RSC Med. Chem. 2025, 16, 652–693. [Google Scholar] [CrossRef]
- Franca, C.; Carra, R.B.; Diniz, J.M.; Munhoz, R.P.; Cury, R.G. Deep brain stimulation in Parkinson’s disease: State of the art and future perspectives. Arq Neuropsiquiatr 2022, 80 (Suppl. S1), 105–115. [Google Scholar] [CrossRef]
- Tawil, S.E.; Muir, K.W. Thrombolysis and thrombectomy for acute ischaemic stroke. Clin. Med. 2017, 17, 161–165. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef]
- Pessoa, L. Understanding brain networks and brain organization. Phys. Life Rev. 2014, 11, 400–435. [Google Scholar] [CrossRef]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central nervous system regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef]
- Vashist, A.; Manickam, P.; Raymond, A.D.; Arias, A.Y.; Kolishetti, N.; Vashist, A.; Arias, E.; Nair, M. Recent Advances in Nanotherapeutics for Neurological Disorders. ACS Appl. Bio. Mater. 2023, 6, 2614–2621. [Google Scholar] [CrossRef]
- Chiang, M.C.; Yang, Y.P.; Nicol, C.J.B.; Wang, C.J. Gold Nanoparticles in Neurological Diseases: A Review of Neuroprotection. Int. J. Mol. Sci. 2024, 25, 2360. [Google Scholar] [CrossRef]
- Sen, S.; Xavier, J.; Kumar, N.; Ahmad, M.Z.; Ranjan, O.P. Exosomes as natural nanocarrier-based drug delivery system: Recent insights and future perspectives. 3 Biotech 2023, 13, 101. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Lopes, D.; Lopes, J.; Pereira-Silva, M.; Peixoto, D.; Rabiee, N.; Veiga, F.; Moradi, O.; Guo, Z.H.; Wang, X.D.; Conde, J.; et al. Bioengineered exosomal-membrane-camouflaged abiotic nanocarriers: Neurodegenerative diseases, tissue engineering and regenerative medicine. Mil. Med. Res. 2023, 10, 19. [Google Scholar] [CrossRef]
- Huang, L.; Wu, E.; Liao, J.; Wei, Z.; Wang, J.; Chen, Z. Research Advances of Engineered Exosomes as Drug Delivery Carrier. ACS Omega 2023, 8, 43374–43387. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, J.; Wang, P.; Liu, X.; Liu, P.; Cheng, X.; Cao, L.; Wu, H.; Chen, J.; Zhou, L. Neural stem cell-derived exosomes and regeneration: Cell-free therapeutic strategies for traumatic brain injury. Stem Cell Res. Ther. 2023, 14, 198. [Google Scholar] [CrossRef]
- Bonetto, V.; Grilli, M. Neural stem cell-derived extracellular vesicles: Mini players with key roles in neurogenesis, immunomodulation, neuroprotection and aging. Front. Mol. Biosci. 2023, 10, 1187263. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.E.I.; Lin, W.; Cai, H.; Zhu, Z.; Ahmed, W.; Zhang, Q.; Liu, J.; Zhang, Y.; Li, R.; et al. Neural stem cell-derived exosomes: A cell-free transplant for potential cure of neurological diseases. Biocell 2024, 48, 1405–1418. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, Z.; Wang, H.; Yu, Y.; Chen, W.; Waqas, A.; Wang, Y.; Chen, L. Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J. Adv. Res. 2020, 24, 435–445. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Z.; Zhang, M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J. Transl. Med. 2022, 20, 291. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood-Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Shao, M.M.; Pei, X.B.; Chen, Q.Y.; Wang, F.; Wang, Z.; Zhai, K. Macrophage-derived exosome promotes regulatory T cell differentiation in malignant pleural effusion. Front. Immunol. 2023, 14, 1161375. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Coffey, R.J. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat. Protoc. 2023, 18, 1462–1487. [Google Scholar] [CrossRef]
- Gao, G.; Li, C.; Ma, Y.; Liang, Z.; Li, Y.; Li, X.; Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Neural stem cell-derived extracellular vesicles mitigate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Signal Transduct. Target. Ther. 2023, 8, 228. [Google Scholar] [CrossRef]
- Gu, C.; Li, Y.; Liu, J.; Liu, S.; Long, J.; Zhang, Q.; Duan, W.; Feng, T.; Huang, J.; Qiu, Y.; et al. Neural stem cell-derived exosomes-loaded adhesive hydrogel controlled-release promotes cerebral angiogenesis and neurological function in ischemic stroke. Exp. Neurol. 2023, 370, 114547. [Google Scholar] [CrossRef]
- Li, Y.; Fang, B. Neural stem cell-derived extracellular vesicles: The light of central nervous system diseases. Biomed. Pharmacother. 2023, 165, 115092. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood-Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef]
- Fallahi, S.; Zangbar, H.S.; Farajdokht, F.; Rahbarghazi, R.; Mohaddes, G.; Ghiasi, F. Exosomes as a therapeutic tool to promote neurorestoration and cognitive function in neurological conditions: Achieve two ends with a single effort. CNS Neurosci. Ther. 2024, 30, e14752. [Google Scholar] [CrossRef]
- Singh, G.; Mehra, A.; Arora, S.; Gugulothu, D.; Vora, L.K.; Prasad, R.; Khatri, D.K. Exosome-mediated delivery and regulation in neurological disease progression. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130728. [Google Scholar] [CrossRef]
- Chen, Y.; Qi, W.; Wang, Z.; Niu, F. Exosome Source Matters: A Comprehensive Review from the Perspective of Diverse Cellular Origins. Pharmaceutics 2025, 17, 147. [Google Scholar] [CrossRef]
- Zou, J.; Xia, H.; Jiang, Q.; Su, Z.; Wen, S.; Liang, Z.; Ouyang, Y.; Liu, J.; Zhang, Z.; Chen, D.; et al. Exosomes derived from odontogenic stem cells: Its role in the dentin-pulp complex. Regen. Ther. 2023, 24, 135–146. [Google Scholar] [CrossRef]
- Abdulmalek, O.; Husain, K.H.; AlKhalifa, H.; Alturani, M.; Butler, A.E.; Moin, A.S.M. Therapeutic Applications of Stem Cell-Derived Exosomes. Int. J. Mol. Sci. 2024, 25, 3562. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Chen, Y.F.; Luh, F.; Ho, Y.S.; Yen, Y. Exosomes: A review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Jia, F.; Ahmed, W.; Zhang, G.L.; Wang, H.; Lin, C.Q.; Chen, W.H.; Chen, L.K. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural. Regen. Res. 2023, 18, 404–409. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Chen, Z. Emerging role of exosomes in cancer therapy: Progress and challenges. Mol. Cancer 2025, 24, 13. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, L.; Cao, W.; Shen, C.; Qiu, Y.; Li, C.; Xiong, Y.; Yang, S.B.; Chen, Z.; Yin, X.; et al. Present and future use of exosomes containing proteins and RNAs in neurodegenerative diseases for synaptic function regulation: A comprehensive review. Int. J. Biol. Macromol. 2024, 280 Pt 3, 135826. [Google Scholar] [CrossRef]
- Bahadorani, M.; Nasiri, M.; Dellinger, K.; Aravamudhan, S.; Zadegan, R. Engineering Exosomes for Therapeutic Applications: Decoding Biogenesis, Content Modification, and Cargo Loading Strategies. Int. J. Nanomed. 2024, 19, 7137–7164. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Gursoy-Ozdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Liu, H.; Zhu, R.; He, H.; Zhou, Y.; Zhang, Y.; Li, C.; Liang, D.; Zeng, Q.; et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 2021, 341, 113700. [Google Scholar] [CrossRef]
- Lee, E.C.; Ha, T.W.; Lee, D.H.; Hong, D.Y.; Park, S.W.; Lee, J.Y.; Lee, M.R.; Oh, J.S. Utility of Exosomes in Ischemic and Hemorrhagic Stroke Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 8367. [Google Scholar] [CrossRef]
- Saikia, B.; Dhanushkodi, A. Engineered exosome therapeutics for neurodegenerative diseases. Life Sci. 2024, 356, 123019. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.S.; Chen, C.A.; Zhou, Q.A. Exosomes horizontal line Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Yu, T.; Yang, L.L.; Zhou, Y.; Wu, M.F.; Jiao, J.H. Exosome-mediated repair of spinal cord injury: A promising therapeutic strategy. Stem Cell Res. Ther. 2024, 15, 6. [Google Scholar] [CrossRef]
- Ahmed, W.; Huang, S.; Chen, L. Engineered exosomes derived from stem cells: A new brain-targeted strategy. Expert Opin. Drug Deliv. 2024, 21, 91–110. [Google Scholar] [CrossRef]
- Nieland, L.; Mahjoum, S.; Grandell, E.; Breyne, K.; Breakefield, X.O. Engineered EVs designed to target diseases of the CNS. J. Control. Release 2023, 356, 493–506. [Google Scholar] [CrossRef]
- Fatima, S.; Qaiser, A.; Andleeb, S.; Hashmi, A.H.; Manzoor, S. Navigating the brain: The role of exosomal shuttles in precision therapeutics. Front. Neurol. 2023, 14, 1324216. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Riza, Y.M.; Eid, T.M.; Almotairi, R.; Scherschinski, L.; Contreras, J.; Nadeem, M.; Perez, S.E.; Raikwar, S.P.; Jha, R.M.; et al. Exosomes in Vascular/Neurological Disorders and the Road Ahead. Cells 2024, 13, 670. [Google Scholar] [CrossRef]
- Liu, W.; Bai, X.; Zhang, A.; Huang, J.; Xu, S.; Zhang, J. Role of Exosomes in Central Nervous System Diseases. Front. Mol. Neurosci. 2019, 12, 240. [Google Scholar] [CrossRef]
- Osaid, Z.; Haider, M.; Hamoudi, R.; Harati, R. Exosomes Interactions with the Blood-Brain Barrier: Implications for Cerebral Disorders and Therapeutics. Int. J. Mol. Sci. 2023, 24, 5635. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef]
- Lyu, C.; Sun, H.; Sun, Z.; Liu, Y.; Wang, Q. Roles of exosomes in immunotherapy for solid cancers. Cell Death Dis. 2024, 15, 106. [Google Scholar] [CrossRef]
- Fruhbeis, C.; Frohlich, D.; Kramer-Albers, E.M. Emerging roles of exosomes in neuron-glia communication. Front. Physiol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Lau, N.C.H.; Yam, J.W.P. From Exosome Biogenesis to Absorption: Key Takeaways for Cancer Research. Cancers 2023, 15, 1992. [Google Scholar] [CrossRef]
- Khan, H.; Pan, J.J.; Li, Y.; Zhang, Z.; Yang, G.Y. Native and Bioengineered Exosomes for Ischemic Stroke Therapy. Front. Cell Dev. Biol. 2021, 9, 619565. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Wallen, M.; Aqil, F.; Spencer, W.; Gupta, R.C. Exosomes as an Emerging Plasmid Delivery Vehicle for Gene Therapy. Pharmaceutics 2023, 15, 1832. [Google Scholar] [CrossRef]
- Liang, Y.; Iqbal, Z.; Lu, J.; Wang, J.; Zhang, H.; Chen, X.; Duan, L.; Xia, J. Cell-derived nanovesicle-mediated drug delivery to the brain: Principles and strategies for vesicle engineering. Mol. Ther. 2023, 31, 1207–1224. [Google Scholar] [CrossRef]
- Fallahi, S.; Zangbar, H.S.; Farajdokht, F.; Rahbarghazi, R.; Ghiasi, F.; Mohaddes, G. Mesenchymal stem cell-derived exosomes improve neurogenesis and cognitive function of mice with methamphetamine addiction: A novel treatment approach. CNS Neurosci. Ther. 2024, 30, e14719. [Google Scholar] [CrossRef]
- Chai, M.; Su, G.; Chen, W.; Gao, J.; Wu, Q.; Song, J.; Zhang, Z. Effects of Bone Marrow Mesenchymal Stem Cell-Derived Exosomes in Central Nervous System Diseases. Mol. Neurobiol. 2024, 61, 7481–7499. [Google Scholar] [CrossRef]
- Gotoh, S.; Kawabori, M.; Fujimura, M. Intranasal administration of stem cell-derived exosomes for central nervous system diseases. Neural. Regen. Res. 2024, 19, 1249–1255. [Google Scholar] [CrossRef]
- Hu, S.; Feng, L.; Yang, Z.; Fan, X.; Gao, H.; Yang, T. A recognition of exosomes as regulators of epigenetic mechanisms in central nervous system diseases. Front. Mol. Neurosci. 2024, 17, 1370449. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H.; Duan, H.; Sheng, G.; Tian, N.; Liu, D.; Sun, Z. Isolation and usage of exosomes in central nervous system diseases. CNS Neurosci. Ther. 2024, 30, e14677. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, P.; Chen, F.; Zhao, Y.; Li, Y.; He, X.; Huselstein, C.; Ye, Q.; Tong, Z.; Chen, Y. Brain Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor-Transfected Bone Mesenchymal Stem Cells for the Repair of Periphery Nerve Injury. Front. Bioeng. Biotechnol. 2020, 8, 874. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Dong, Y.; Gao, P.; Zhao, X.; Wang, M.; Wu, X.; Shen, J.; Zhang, X.; Lu, Z.; et al. Stem cell-derived extracellular vesicles in the therapeutic intervention of Alzheimer’s Disease, Parkinson’s Disease, and stroke. Theranostics 2024, 14, 3358–3384. [Google Scholar] [CrossRef] [PubMed]
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol. Cancer Res. 2019, 17, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Si, Q.; Wu, L.; Pang, D.; Jiang, P. Exosomes in brain diseases: Pathogenesis and therapeutic targets. MedComm 2023, 4, e287. [Google Scholar] [CrossRef]
- Cheng, C.H.; Hao, W.R.; Cheng, T.H. Stem cell exosomes: New hope for recovery from diabetic brain hemorrhage. World J. Diabetes 2024, 15, 2264–2271. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B. GSH-AuNP anti-oxidative stress, ER stress and mitochondrial dysfunction in amyloid-beta peptide-treated human neural stem cells. Free Radic. Biol. Med. 2022, 187, 185–201. [Google Scholar] [CrossRef]
- Yan, X.; Chen, X.; Shan, Z.; Bi, L. Engineering Exosomes to Specifically Target the Mitochondria of Brain Cells. ACS Omega 2023, 8, 48984–48993. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, B.; Saei, A.K.; Obi, P.O.; Asghari, N.; Lorzadeh, S.; Hekmatirad, S.; Rahmati, M.; Velayatipour, F.; Asghari, M.H.; Saleem, A.; et al. Exosomes, autophagy and ER stress pathways in human diseases: Cross-regulation and therapeutic approaches. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166484. [Google Scholar] [CrossRef]
- Kumari, M.; Anji, A. Small but Mighty-Exosomes, Novel Intercellular Messengers in Neurodegeneration. Biology 2022, 11, 413. [Google Scholar] [CrossRef]
- Lu, D.; Sun, H.; Fan, H.; Li, N.; Li, Y.; Yin, X.; Fan, Y.; Sun, H.; Wang, S.; Xin, T. Regulation of nerve cells and therapeutic potential in central nervous system injury using microglia-derived exosomes. Neuroscience 2024, 563, 84–92. [Google Scholar] [CrossRef]
- Huo, L.; Du, X.; Li, X.; Liu, S.; Xu, Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 738442. [Google Scholar] [CrossRef]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid beta-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, M.; Davies, C.; Sirisi, S.; Lee, J.E.; Simzer, E.M.; Tzioras, M.; Querol-Vilaseca, M.; Sanchez-Aced, E.; Chang, Y.Y.; Holt, K.; et al. Synaptic oligomeric tau in Alzheimer’s disease—A potential culprit in the spread of tau pathology through the brain. Neuron 2023, 111, 2170–2183.e6. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Yang, Y.P.; Chiang, T.; Yen, C. The alpha-MG exhibits neuroprotective potential by reducing amyloid beta peptide-induced inflammation, oxidative stress, and tau aggregation in human neural stem cells. Brain Res. 2025, 1852, 149506. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Alajangi, H.K.; Kaur, M.; Sharma, A.; Rana, S.; Thakur, S.; Chatterjee, M.; Singla, N.; Jaiswal, P.K.; Singh, G.; Barnwal, R.P. Blood-brain barrier: Emerging trends on transport models and new-age strategies for therapeutics intervention against neurological disorders. Mol. Brain 2022, 15, 49. [Google Scholar] [CrossRef]
- Facal, C.L.; Fernandez Bessone, I.; Muniz, J.A.; Pereyra, A.E.; Pedroncini, O.; Paez-Paz, I.; Clerici-Delville, R.; Arnaiz, C.; Urrutia, L.; Falasco, G.; et al. Tau reduction with artificial microRNAs modulates neuronal physiology and improves tauopathy phenotypes in mice. Mol. Ther. 2024, 32, 1080–1095. [Google Scholar] [CrossRef]
- Spinelli, M.; Fusco, S.; Grassi, C. Therapeutic potential of stem cell-derived extracellular vesicles in neurodegenerative diseases associated with cognitive decline. Stem Cells 2024, 43, sxae074. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, P.; Lei, S.; Guo, S.; Zhou, P.; Zhao, L.; Qi, Y.; Wei, X.; Wu, W.; Wang, N.; et al. An Exosome-Based Therapeutic Strategy Targeting Neuroinflammation in Alzheimer’s Disease with Berberine and Palmatine. Drug Des. Devel. Ther. 2023, 17, 2401–2420. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ban, J.J.; Yang, S.; Im, W.; Kim, M. The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018, 1691, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Reza-Zaldivar, E.E.; Hernandez-Sapiens, M.A.; Gutierrez-Mercado, Y.K.; Sandoval-Avila, S.; Gomez-Pinedo, U.; Marquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E.; Canales-Aguirre, A.A. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural. Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084. [Google Scholar] [CrossRef]
- Wei, H.; Xu, Y.; Chen, Q.; Chen, H.; Zhu, X.; Li, Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020, 11, 290. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Ebrahim, N.; Al Saihati, H.A.; Alali, Z.; Aleniz, F.Q.; Mahmoud, S.Y.M.; Badr, O.A.; Dessouky, A.A.; Mostafa, O.; Hussien, N.I.; Farid, A.S.; et al. Exploring the molecular mechanisms of MSC-derived exosomes in Alzheimer’s disease: Autophagy, insulin and the PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2024, 176, 116836. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhou, Y.; Feng, X.; Gu, G.; Han, S.; Cheng, N.; Sun, Y.; Zhang, Y.; Cheng, J.; et al. Neural stem cell-derived exosomes promote mitochondrial biogenesis and restore abnormal protein distribution in a mouse model of Alzheimer’s disease. Neural. Regen. Res. 2024, 19, 1593–1601. [Google Scholar] [CrossRef]
- Liu, Y.; Huber, C.C.; Wang, H. Disrupted blood-brain barrier in 5×FAD mouse model of Alzheimer’s disease can be mimicked and repaired in vitro with neural stem cell-derived exosomes. Biochem. Biophys. Res. Commun. 2020, 525, 192–196. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef]
- Baggett, D.; Olson, A.; Parmar, M.S. Novel approaches targeting α-Synuclein for Parkinson’s Disease: Current progress and future directions for the disease-modifying therapies. Brain Disord. 2024, 16, 100163. [Google Scholar] [CrossRef]
- Ntetsika, T.; Papathoma, P.E.; Markaki, I. Novel targeted therapies for Parkinson’s disease. Mol. Med. 2021, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Mahato, A.K.; Kopra, J.; Renko, J.M.; Visnapuu, T.; Korhonen, I.; Pulkkinen, N.; Bespalov, M.M.; Domanskyi, A.; Ronken, E.; Piepponen, T.P.; et al. Glial cell line-derived neurotrophic factor receptor Rearranged during transfection agonist supports dopamine neurons in Vitro and enhances dopamine release In Vivo. Mov. Disord 2020, 35, 245–255. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, Y.; Xue, F.; Zheng, Y.; Huang, H.; Wang, W.; Chang, Y.; Yang, H.; Zhang, J. Exosomal DNA Aptamer Targeting alpha-Synuclein Aggregates Reduced Neuropathological Deficits in a Mouse Parkinson’s Disease Model. Mol. Ther. Nucleic Acids 2019, 17, 726–740. [Google Scholar] [CrossRef]
- Nouri, Z.; Barfar, A.; Perseh, S.; Motasadizadeh, H.; Maghsoudian, S.; Fatahi, Y.; Nouri, K.; Yektakasmaei, M.P.; Dinarvand, R.; Atyabi, F. Exosomes as therapeutic and drug delivery vehicle for neurodegenerative diseases. J. Nanobiotechnol. 2024, 22, 463. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, M.M.; Wang, M.; Jiang, Z.H.; Tan, Z.G. Bone Marrow-Derived Mesenchymal Stem Cell-Derived Exosomes Containing Gli1 Alleviate Microglial Activation and Neuronal Apoptosis In Vitro and in a Mouse Parkinson Disease Model by Direct Inhibition of Sp1 Signaling. J. Neuropathol. Exp. Neurol. 2022, 81, 522–534. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Zuo, H.; Chen, H.; Gui, Y. Mesenchymal stem cell-derived exosomes altered neuron cholesterol metabolism via Wnt5a-LRP1 axis and alleviated cognitive impairment in a progressive Parkinson’s disease model. Neurosci. Lett. 2022, 787, 136810. [Google Scholar] [CrossRef]
- Chan, L.; Hsu, W.; Chen, K.Y.; Wang, W.; Hung, Y.C.; Hong, C.T. Therapeutic Effect of Human Adipocyte-derived Stem Cell-derived Exosomes on a Transgenic Mouse Model of Parkinson’s Disease. In Vivo 2023, 37, 2028–2038. [Google Scholar] [CrossRef]
- Chen, W.S.; Lin, T.Y.; Kuo, C.H.; Hsieh, D.J.; Kuo, W.W.; Liao, S.C.; Kao, H.C.; Ju, D.T.; Lin, Y.J.; Huang, C.Y. Ginkgolide A improves the pleiotropic function and reinforces the neuroprotective effects by mesenchymal stem cell-derived exosomes in 6-OHDA-induced cell model of Parkinson’s disease. Aging 2023, 15, 1358–1370. [Google Scholar] [CrossRef]
- Geng, Y.; Long, X.; Zhang, Y.; Wang, Y.; You, G.; Guo, W.; Zhuang, G.; Zhang, Y.; Cheng, X.; Yuan, Z.; et al. FTO-targeted siRNA delivery by MSC-derived exosomes synergistically alleviates dopaminergic neuronal death in Parkinson’s disease via m6A-dependent regulation of ATM mRNA. J. Transl. Med. 2023, 21, 652. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Hu, X.M.; Long, Y.F.; Huang, H.R.; He, Y.; Xu, Z.R.; Qi, Z.Q. Treatment of Parkinson’s disease model with human umbilical cord mesenchymal stem cell-derived exosomes loaded with BDNF. Life Sci. 2024, 356, 123014. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, X.; Yang, Z.H.; Si, X.K.; Sun, X. Stroke-induced damage on the blood-brain barrier. Front. Neurol. 2023, 14, 1248970. [Google Scholar] [CrossRef]
- Nozohouri, S.; Sifat, A.E.; Vaidya, B.; Abbruscato, T.J. Novel approaches for the delivery of therapeutics in ischemic stroke. Drug Discov. Today 2020, 25, 535–551. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Li, H.; Xie, Y.; Bai, J.; Guan, J.; Qi, H.; Sun, J. Targeted pathophysiological treatment of ischemic stroke using nanoparticle-based drug delivery system. J. Nanobiotechnol. 2024, 22, 499. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, J.; Li, K.; Shi, L.; Wen, X.; Fang, F. Progresses and Prospects of Neuroprotective Agents-Loaded Nanoparticles and Biomimetic Material in Ischemic Stroke. Front. Cell Neurosci. 2022, 16, 868323. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Rust, R.; Nih, L.R.; Liberale, L.; Yin, H.; El Amki, M.; Ong, L.K.; Zlokovic, B.V. Brain repair mechanisms after cell therapy for stroke. Brain 2024, 147, 3286–3305. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Lukomska, B.; Janowski, M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J. Neuroinflamm. 2019, 16, 178. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Wang, F.; Sun, X.; Li, H.; Zhang, K.; Li, J. Recent advances in tissue repair of the blood-brain barrier after stroke. J. Tissue Eng. 2024, 15, 20417314241226551. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Chen, H.; Cui, G.Y.; Hu, J.X. Damage mechanism and therapy progress of the blood-brain barrier after ischemic stroke. Cell Biosci. 2023, 13, 196. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 2021, 13, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, T.; Yang, S. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered 2022, 13, 3030–3043. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J. Bone marrow mesenchymal stem cell-derived exosomes carrying long noncoding RNA ZFAS1 alleviate oxidative stress and inflammation in ischemic stroke by inhibiting microRNA-15a-5p. Metab. Brain Dis. 2022, 37, 2545–2557. [Google Scholar] [CrossRef]
- Wang, Q.S.; Xiao, R.J.; Peng, J.; Yu, Z.T.; Fu, J.Q.; Xia, Y. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal KLF4 Alleviated Ischemic Stroke Through Inhibiting N6-Methyladenosine Modification Level of Drp1 by Targeting lncRNA-ZFAS1. Mol. Neurobiol. 2023, 60, 3945–3962. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Fan, X.; Xu, C.; Li, M.; Sun, H.; Song, J.; Jia, F.; Wei, W.; Jiang, F.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-193b-5p reduces pyroptosis after ischemic stroke by targeting AIM2. J. Stroke Cerebrovasc. Dis. 2023, 32, 107235. [Google Scholar] [CrossRef]
- Lin, S.L.; Chang, Y.W.; Lee, W.; Chiang, C.S.; Liu, S.P.; Lee, H.T.; Jeng, L.B.; Shyu, W.C. Role of STAT3-FOXO3 Signaling in the Modulation of Neuroplasticity by PD-L1-HGF-Decorated Mesenchymal Stem Cell-Derived Exosomes in a Murine Stroke Model. Adv. Sci. 2024, 11, e2404882. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Devel. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Prajapati, B.G.; Singh, S. A critical review on the dissemination of PH and stimuli-responsive polymeric nanoparticular systems to improve drug delivery in cancer therapy. Crit. Rev. Oncol. Hematol. 2023, 185, 103961. [Google Scholar] [CrossRef]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Molecules 2018, 23, 9. [Google Scholar] [CrossRef]
- Chavda, V.P.; Pandya, A.; Kumar, L.; Raval, N.; Vora, L.K.; Pulakkat, S.; Patravale, V.; Salwa; Duo, Y.; Tang, B.Z. Exosome nanovesicles: A potential carrier for therapeutic delivery. Nano Today 2023, 49, 101771. [Google Scholar] [CrossRef]
- Li, L.; Wang, F.; Zhu, D.; Hu, S.; Cheng, K.; Li, Z. Engineering Exosomes and Exosome-like Nanovesicles for Improving Tissue Targeting and Retention. Fundam. Res. 2024, 5, 851–867. [Google Scholar] [CrossRef]
- Shao, M.; Lopes, D.; Lopes, J.; Yousefiasl, S.; Macário-Soares, A.; Peixoto, D.; Ferreira-Faria, I.; Veiga, F.; Conde, J.; Huang, Y.; et al. Exosome membrane-coated nanosystems: Exploring biomedical applications in cancer diagnosis and therapy. Matter 2023, 6, 761–799. [Google Scholar] [CrossRef]
- Mondal, J.; Pillarisetti, S.; Junnuthula, V.; Surwase, S.S.; Hwang, S.R.; Park, I.-K.; Lee, Y.-k. Extracellular vesicles and exosome-like nanovesicles as pioneering oral drug delivery systems. Front. Bioeng. Biotechnol. 2024, 11, 1307878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Y.; Zhang, C.; Bi, R.; Qiu, Y.; Li, Y.; Hu, B. Cell-Membrane-Coated Nanoparticles for Targeted Drug Delivery to the Brain for the Treatment of Neurological Diseases. Pharmaceutics 2023, 15, 621. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Sousa de Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Soltanmohammadi, F.; Gharehbaba, A.M.; Zangi, A.R.; Adibkia, K.; Javadzadeh, Y. Current knowledge of hybrid nanoplatforms composed of exosomes and organic/inorganic nanoparticles for disease treatment and cell/tissue imaging. Biomed. Pharmacother. 2024, 178, 117248. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, L.; Liu, Q.; Hou, J.; Qiu, X.; Chen, M.; Wu, Z.; Hu, D.; Cui, F.; Yan, T. Exosome-coated polydatin nanoparticles in the treatment of radiation-induced intestinal damage. Aging 2023, 15, 6905–6920. [Google Scholar] [CrossRef]
- Tanziela, T.; Shaikh, S.; Jiang, H.; Lu, Z.; Wang, X. Efficient encapsulation of biocompatible nanoparticles in exosomes for cancer theranostics. Nano Today 2020, 35, 100964. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef]
- Hill, M.L.; Chung, S.J.; Woo, H.J.; Park, C.R.; Hadrick, K.; Nafiujjaman, M.; Kumar, P.P.P.; Mwangi, L.; Parikh, R.; Kim, T. Exosome-Coated Prussian Blue Nanoparticles for Specific Targeting and Treatment of Glioblastoma. ACS Appl. Mater. Interfaces 2024, 16, 20286–20301. [Google Scholar] [CrossRef]
- Ribeiro, J.; Lopes, I.; Gomes, A.C. A New Perspective for the Treatment of Alzheimer’s Disease: Exosome-like Liposomes to Deliver Natural Compounds and RNA Therapies. Molecules 2023, 28, 6015. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Peng, H.; Liu, R.; Ji, W.; Shi, Z.; Shen, J.; Ma, G.; Zhang, X. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing alpha-synuclein and immune activation of Parkinson’s disease. Sci. Adv. 2020, 6, e3967. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, T.J.; Kang, L.; Kim, Y.J.; Kang, M.K.; Kim, J.; Ryu, J.H.; Hyeon, T.; Yoon, B.W.; Ko, S.B.; et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 2020, 243, 119942. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.A.; Gazeau, F. Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, M.; Tian, J.; Song, D.; Duan, S.; Zhang, L. Encapsulation and assessment of therapeutic cargo in engineered exosomes: A systematic review. J. Nanobiotechnol. 2024, 22, 18. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Phoebe, L.K.W.; Park, Y.; Yi, K.H. Clinical Applications of Exosomes: A Critical Review. Int. J. Mol. Sci. 2024, 25, 7794. [Google Scholar] [CrossRef]
- Perocheau, D.; Touramanidou, L.; Gurung, S.; Gissen, P.; Baruteau, J. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 2021, 178, 2375–2392. [Google Scholar] [CrossRef]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noel, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Syn, N.L.; Wang, L.; Chow, E.K.; Lim, C.T.; Goh, B.C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, A.; Nandy, A.; Ghatak, S. Insight into the Functional Dynamics and Challenges of Exosomes in Pharmaceutical Innovation and Precision Medicine. Pharmaceutics 2024, 16, 709. [Google Scholar] [CrossRef]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, D.; Jie, Y.; Luo, H.; Zhang, W.; Qiu, C. Exosome-based nanoparticles and cancer immunotherapy. Biomed. Pharmacother. 2024, 179, 117296. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2022, 12, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Shahzad, K.A.; Marryum, M.; Singh, S.; Zhou, Q.; Du, S.; Wang, S.; Shao, C.; Shaikh, I.I. Exosome-based therapies for inflammatory disorders: A review of recent advances. Stem Cell Res. Ther. 2024, 15, 477. [Google Scholar] [CrossRef]
- Choi, H.K.; Chen, M.; Goldston, L.L.; Lee, K.B. Extracellular vesicles as nanotheranostic platforms for targeted neurological disorder interventions. Nano Converg. 2024, 11, 19. [Google Scholar] [CrossRef]
- Fayazi, N.; Sheykhhasan, M.; Soleimani Asl, S.; Najafi, R. Stem Cell-Derived Exosomes: A New Strategy of Neurodegenerative Disease Treatment. Mol. Neurobiol. 2021, 58, 3494–3514. [Google Scholar] [CrossRef]
- Sonbhadra, S.; Mehak; Pandey, L.M. Biogenesis, Isolation, and Detection of Exosomes and Their Potential in Therapeutics and Diagnostics. Biosensors 2023, 13, 802. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C.; et al. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef]
- Koh, H.B.; Kim, H.J.; Kang, S.W.; Yoo, T.H. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics 2023, 15, 2042. [Google Scholar] [CrossRef] [PubMed]
- Gangadaran, P.; Madhyastha, H.; Madhyastha, R.; Rajendran, R.L.; Nakajima, Y.; Watanabe, N.; Velikkakath, A.K.G.; Hong, C.M.; Gopi, R.V.; Muthukalianan, G.K.; et al. The emerging role of exosomes in innate immunity, diagnosis and therapy. Front. Immunol. 2022, 13, 1085057. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).