Development of Palladium and Magnetite-Coated Diatomite as a Magnetizable Catalyst for Hydrogenation of Benzophenone
Abstract
1. Introduction
2. Results and Discussion
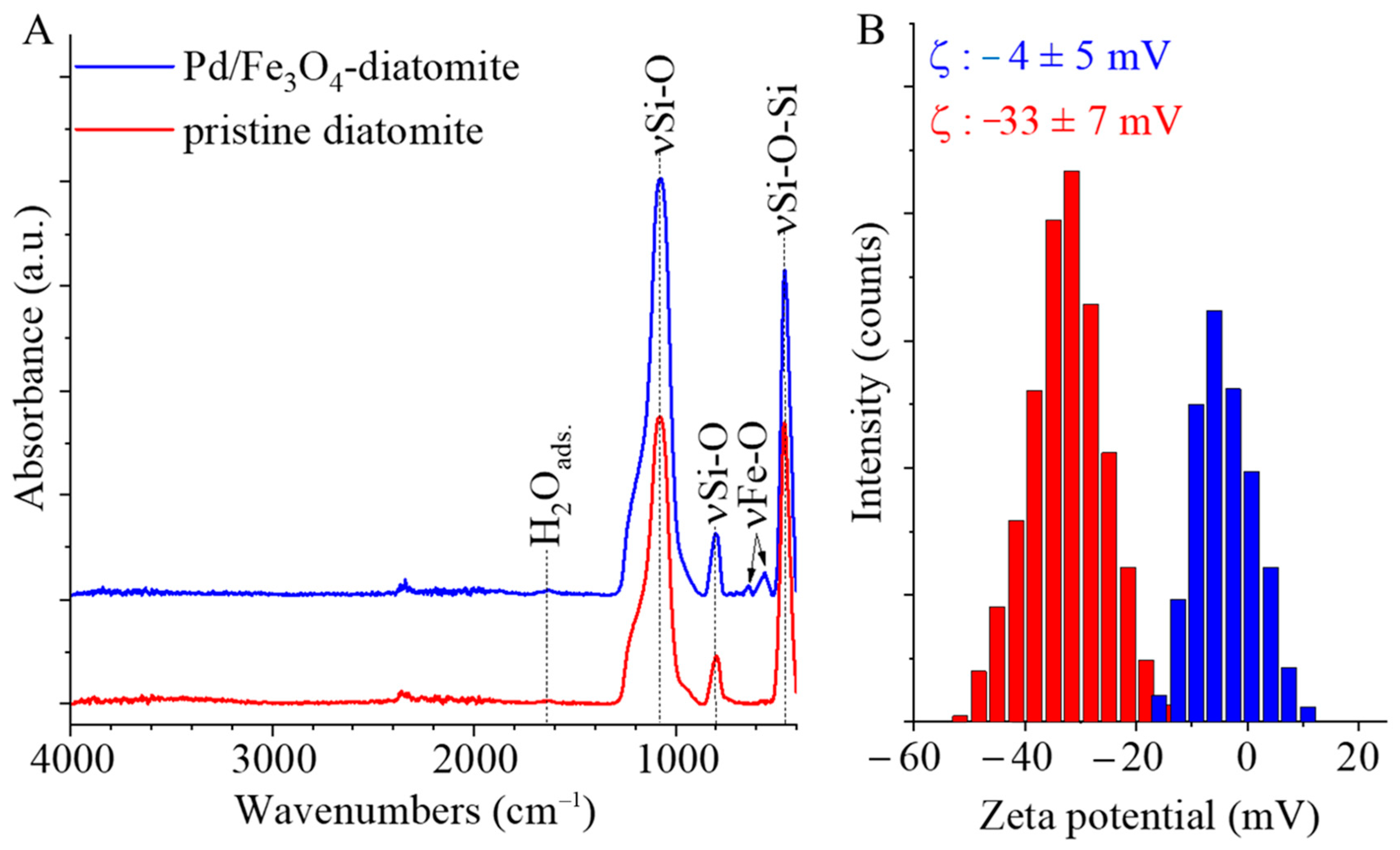
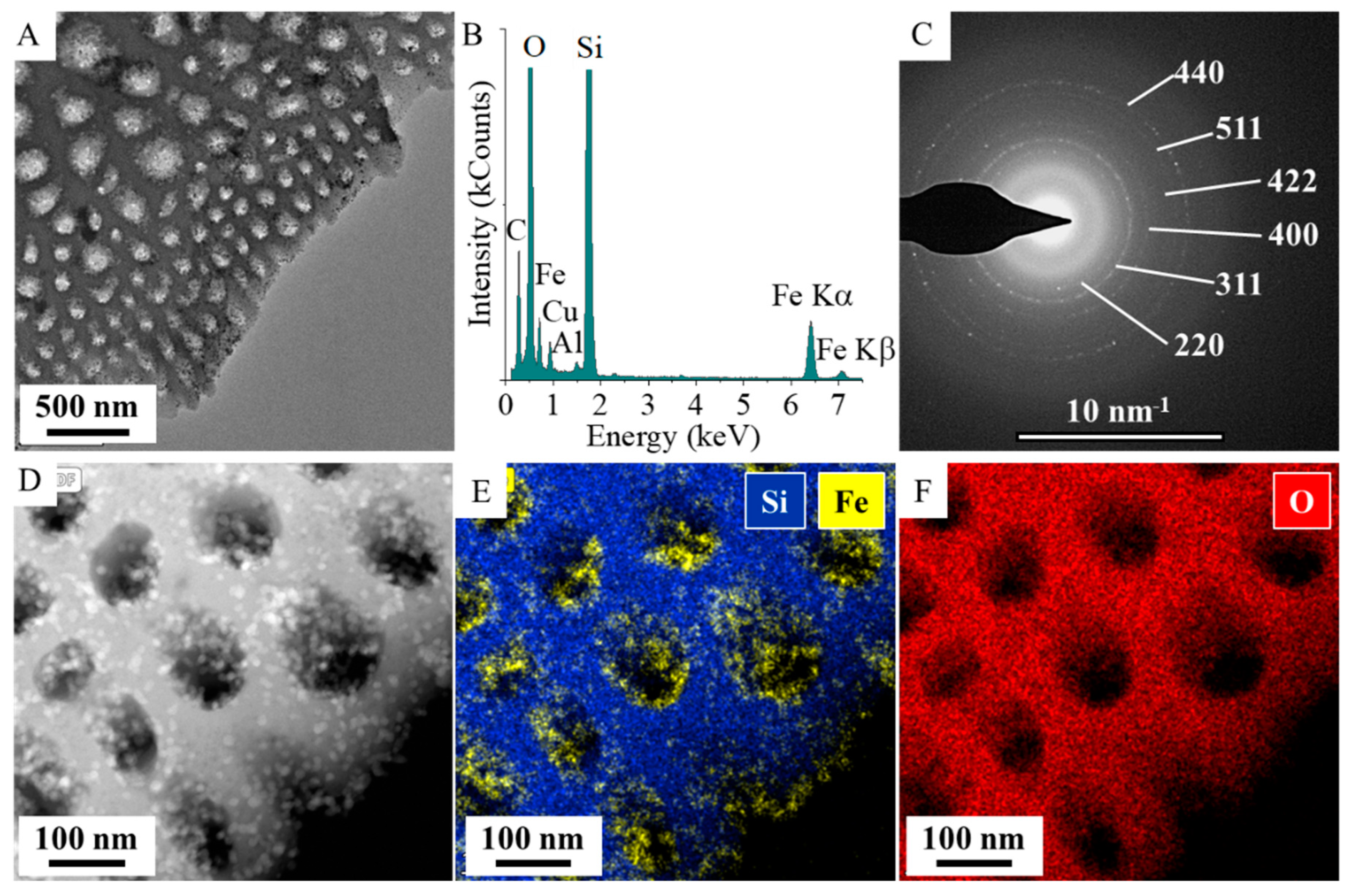
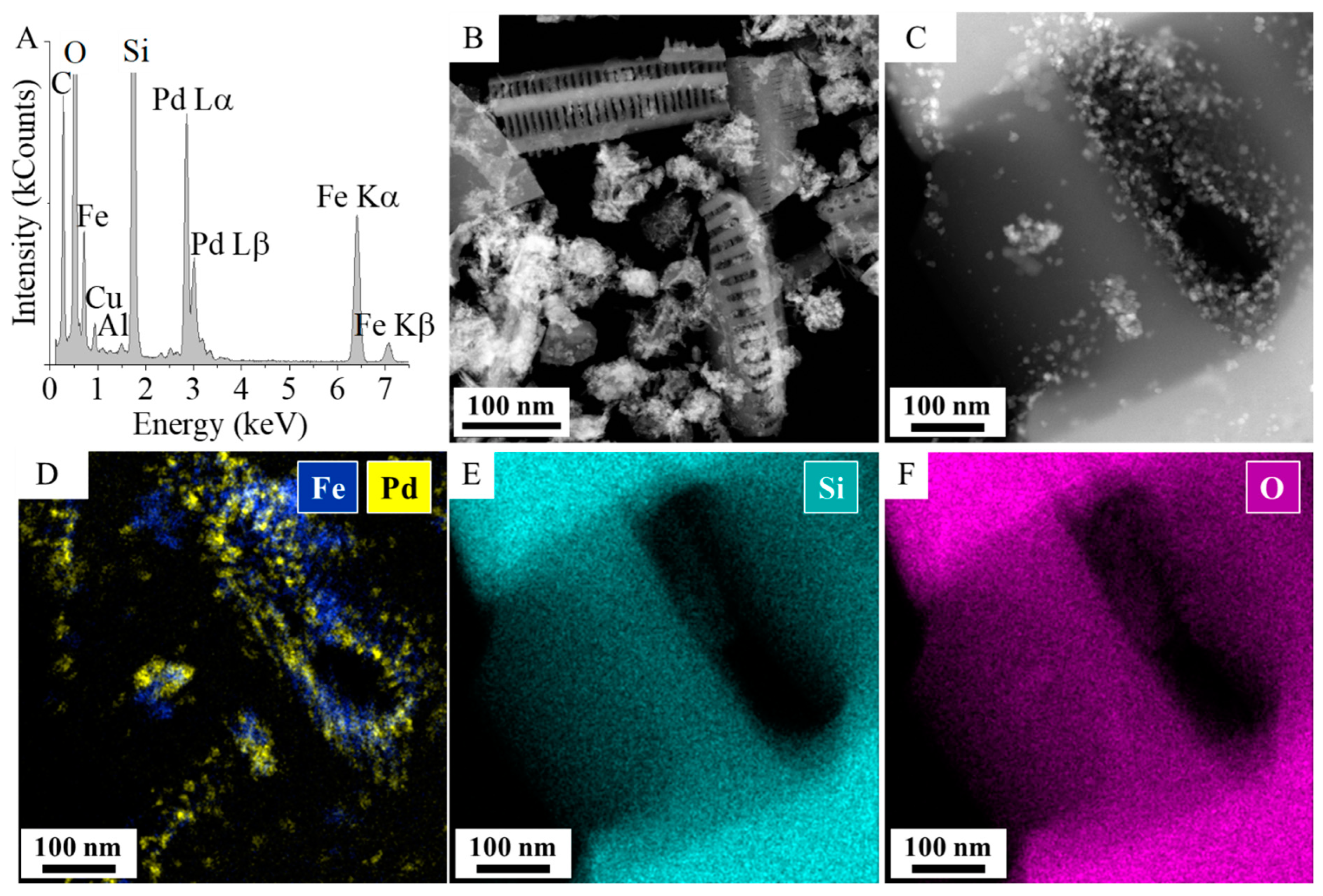
2.1. Results of the Characterization
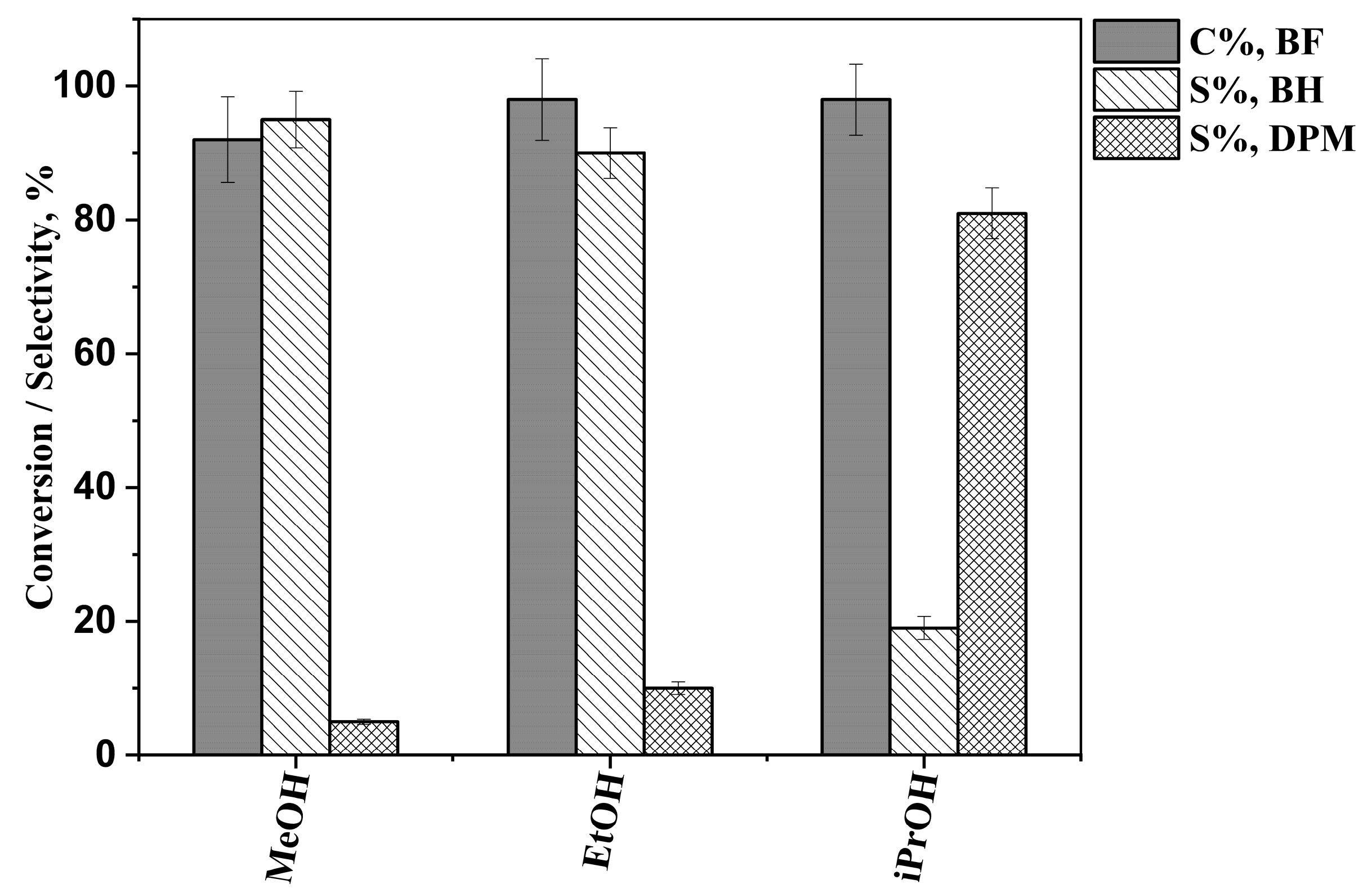
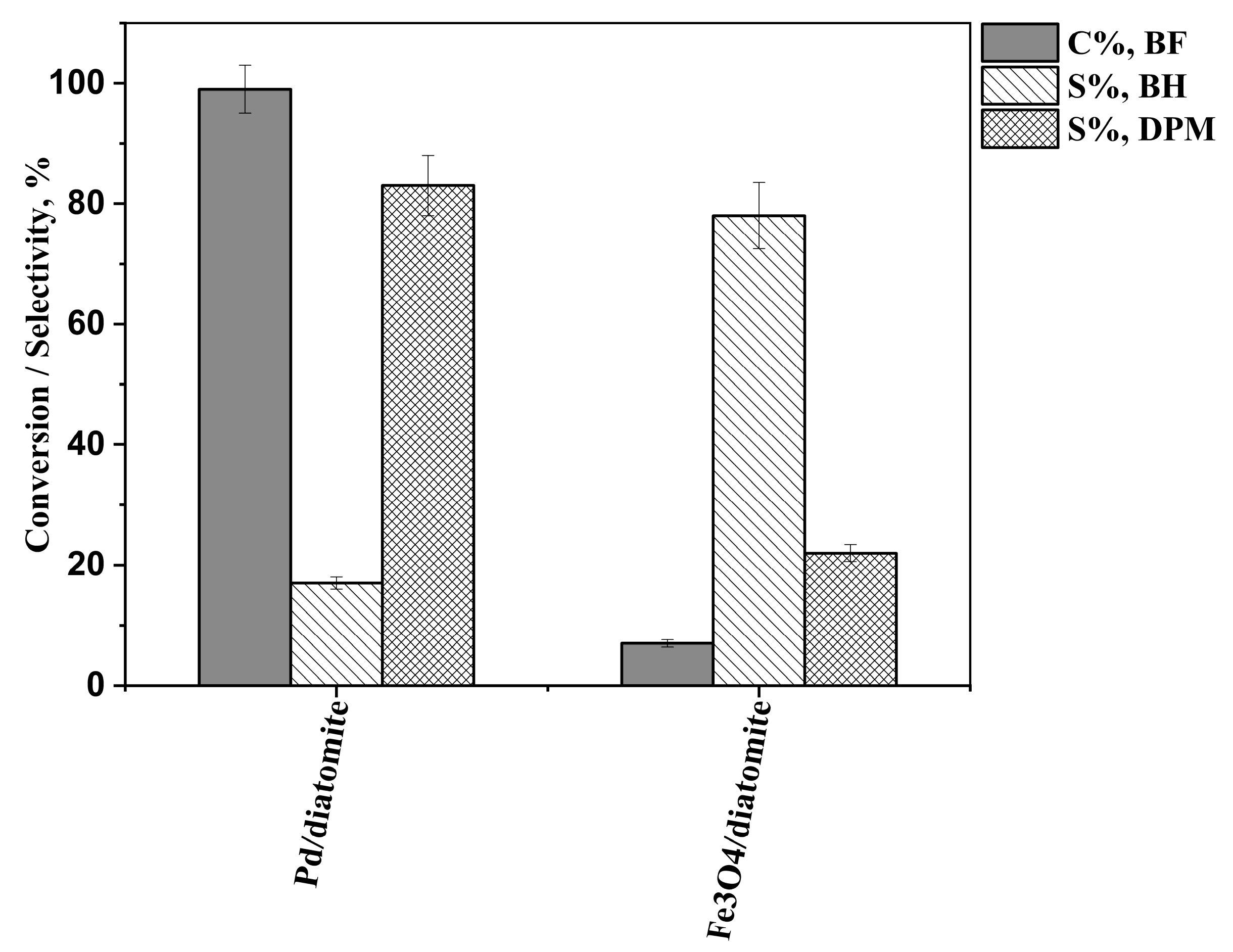
2.2. Results of the Hydrogenation Reactions
3. Experimental
3.1. Materials
3.2. Synthesis of the Magnetite-Coated Diatomite-Supported Palladium Catalyst
3.3. Characterization Technics
3.4. Catalytic Tests of the Catalyst in Benzophenone Hydrogenation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, M.S.; Yadav, G.D.; Ng, F.T. Selective Hydrogenation of 3,4-Dimethoxybenzophenone in Liquid Phase over Pd/C Catalyst in a Slurry Reactor. Can. J. Chem. Eng. 2014, 92, 2157–2165. [Google Scholar] [CrossRef]
- Lee, S.H.; Suh, D.J.; Park, T.J.; Kim, K.L. The Effect of Heat Treatment Conditions on the Textural and Catalytic Properties of Nickel–Titania Composite Aerogel Catalysts. Catal. Commun. 2002, 3, 441–447. [Google Scholar] [CrossRef]
- Carr, C.R.; Vesto, J.I.; Xing, X.; Fettinger, J.C.; Berben, L.A. Aluminum-Ligand Cooperative O−H Bond Activation Initiates Catalytic Transfer Hydrogenation. ChemCatChem 2022, 14, e202101869. [Google Scholar] [CrossRef]
- Lopes, J.C.; Sampaio, M.J.; Rosa, B.; Lima, M.J.; Faria, J.L.; Silva, C.G. Role of TiO2-Based Photocatalysts on the Synthesis of the Pharmaceutical Precursor Benzhydrol by UVA-LED Radiation. J. Photochem. Photobiol. A Chem. 2020, 391, 112350. [Google Scholar] [CrossRef]
- Albiter Escobar, E.; Valenzuela Zapata, M.Á.; Alfaro Hernández, S.; Flores Valle, S.O.; Ríos Berny, O.; González Ángeles, V.J.; Córdova Reyes, I. Photocatalytic Reduction of Benzophenone on TiO2: Effect of Preparation Method and Reaction Conditions. J. Mex. Chem. Soc. 2010, 54, 133–138. [Google Scholar]
- Mulero, C.M.; Sáez, A.; Iniesta, J.; Montiel, V. An Alternative to Hydrogenation Processes. Electrocatalytic Hydrogenation of Benzophenone. Beilstein J. Org. Chem. 2018, 14, 537–546. [Google Scholar] [CrossRef]
- Villalba, M.; Del Pozo, M.; Calvo, E.J. Electrocatalytic Hydrogenation of Acetophenone and Benzophenone Using Palladium Electrodes. Electrochim. Acta 2015, 164, 125–131. [Google Scholar] [CrossRef]
- Ling, F.; Hou, H.; Chen, J.; Nian, S.; Yi, X.; Wang, Z.; Song, D.; Zhong, W. Highly Enantioselective Synthesis of Chiral Benzhydrols via Manganese Catalyzed Asymmetric Hydrogenation of Unsymmetrical Benzophenones Using an Imidazole-Based Chiral PNN Tridentate Ligand. Org. Lett. 2019, 21, 3937–3941. [Google Scholar]
- Touge, T.; Nara, H.; Fujiwhara, M.; Kayaki, Y.; Ikariya, T. Efficient Access to Chiral Benzhydrols via Asymmetric Transfer Hydrogenation of Unsymmetrical Benzophenones with Bifunctional Oxo-Tethered Ruthenium Catalysts. J. Am. Chem. Soc. 2016, 138, 10084–10087. [Google Scholar]
- Ohkuma, T.; Koizumi, M.; Ikehira, H.; Yokozawa, T.; Noyori, R. Selective Hydrogenation of Benzophenones to Benzhydrols. Asymmetric Synthesis of Unsymmetrical Diarylmethanols. Org. Lett. 2000, 2, 659–662. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, J.; Yang, X.; Niu, L.; Zhang, H.; Bai, G. Hydrogelator as Growth-Controlling Agent for Enhancing the Catalytic Activity of NiB Amorphous Alloy Catalyst. Res. Chem. Intermed. 2018, 44, 7861–7872. [Google Scholar] [CrossRef]
- Bai, G.; Niu, L.; Qiu, M.; He, F.; Fan, X.; Dou, H.; Zhang, X. Liquid-Phase Selective Hydrogenation of Benzophenone over Ultrasonic-Assisted Ni–La–B Amorphous Alloy Catalyst. Catal. Commun. 2010, 12, 212–216. [Google Scholar] [CrossRef]
- Santori, G.F.; Moglioni, A.G.; Vetere, V.; Iglesias, G.Y.M.; Casella, M.L.; Ferretti, O.A. Hydrogenation of Aromatic Ketones with Pt- and Sn-Modified Pt Catalysts. Appl. Catal. A Gen. 2004, 269, 215–223. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Rajadhyaksha, R.A. Liquid Phase Catalytic Hydrogenation of Benzophenone: Role of Metal Support Interaction, Bimetallic Catalysts, Solvents and Additives. Stud. Surf. Sci. Catal. 1993, 78, 251–258. [Google Scholar] [CrossRef]
- Bai, G.; Zhao, Z.; Niu, L.; Dong, H.; Qiu, M.; Li, F.; Chen, Q.; Chen, G. Effect of Polymers and Alkaline Earth Metals on the Catalytic Performance of Ni–B Amorphous Alloy in Benzophenone Hydrogenation. Catal. Commun. 2012, 23, 34–38. [Google Scholar] [CrossRef]
- Bejblová, M.; Zámostný, P.; Červený, L.; Čejka, J. Hydrodeoxygenation of Benzophenone on Pd Catalysts. Appl. Catal. A Gen. 2005, 296, 169–175. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lai, C.W.; Hsien, K.J. Characterization and Adsorption Properties of Diatomaceous Earth Modified by Hydrofluoric Acid Etching. J. Colloid Interface Sci. 2006, 297, 749–754. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Nemkayeva, R.R.; Nurgain, A.; Seitkazinova, A.R.; Dinistanova, B.K.; Issanbekova, A.T.; Zhylybayeva, N.; Bergeneva, N.S.; Mamatova, G.U. The Use of Diatomite as a Catalyst Carrier for the Synthesis of Carbon Nanotubes. Nanomaterials 2022, 12, 1817. [Google Scholar] [CrossRef]
- Jabbour, K.; El, N.; Davidson, A.; Massiani, P.; Casale, S. Characterizations and Performances of Ni/Diatomite Catalysts for Dry Reforming of Methane. Chem. Eng. J. 2015, 264, 351–358. [Google Scholar] [CrossRef]
- Cherrak, R.; Hadjel, M.; Benderdouche, N. Heterogenous Photocatalysis Treatement of Azo Dye Methyl Orange by Nano Composite TiO2/Diatomite. Orient. J. Chem. 2015, 31, 1611–1620. [Google Scholar] [CrossRef]
- Athar, S.D.; Asilian, H. Catalytic Oxidation of Carbon Monoxide Using Copper-Zinc Mixed Oxide Nanoparticles Supported on Diatomite. Health Scope 2012, 1, 52–56. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Jiguang, D.; Jing, L.; Hou, Z.; Gao, R.; Dai, H. Pt/CeMnOx/Diatomite: A Highly Active Catalyst for the Oxidative Removal of Toluene and Ethyl Acetate. Catalysts 2023, 13, 676. [Google Scholar] [CrossRef]
- Zno, T.; Composites, D.; Yang, B.; Ma, Z.; Wang, Q.; Yang, J. Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites. Catalysts 2022, 12, 268. [Google Scholar] [CrossRef]
- Toshtay, K.; Auyezov, A.B.; Bizhanov, Z.A.; Yeraliyeva, A.T. Effect of Catalyst Preparation on the Selective Hydrogenation of Vegetable Oil Over Low Percentage Pd/Diatomite Catalysts. Eurasian Chem. J. 2015, 17, 33–39. [Google Scholar] [CrossRef]
- Zhu, Z.; Liang, S.; Sun, H.; Zhang, W.; Yang, J.; Gao, Z. Magnetic Pd–Fe Nanoparticles for Sustainable Suzuki–Miyaura Cross-Coupling Reactions. Catal. Sci. Technol. 2024, 14, 3176–3183. [Google Scholar] [CrossRef]
- Rossi, L.M.; Garcia, M.A.S.; Vono, L.L.R. Recent Advances in the Development of Magnetically Recoverable Metal Nanoparticle Catalysts. J. Braz. Chem. Soc. 2012, 23, 1959–1971. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Bronstein, L.M. Magnetically Recoverable Catalysts: Beyond Magnetic Separation. Front. Chem. 2018, 6, 386821. [Google Scholar]
- Bawane, S.P.; Sawant, S.B. Kinetics of Liquid-Phase Catalytic Hydrogenation of Benzophenone to Benzhydrol. Org. Process Res. Dev. 2003, 7, 769–773. [Google Scholar]
- Zhao, S.; Cai, J.; Chen, H.; Shen, J. Understanding the Effects of Solvents on the Hydrogenation of Toluene over Supported Pd and Ru Catalysts. Catal. Commun. 2021, 157, 106330. [Google Scholar] [CrossRef]
- Li, Z.; Tang, X.; Liu, K.; Huang, J.; Xu, Y.; Peng, Q.; Ao, M. Synthesis of a MnO2/Fe3O4/Diatomite Nanocomposite as an Efficient Heterogeneous Fenton-like Catalyst for Methylene Blue Degradation. Beilstein J. Nanotechnol. 2018, 9, 1940–1950. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Q.; Liu, K.; Li, Z.; Tang, X.; Li, G. Highly Efficient Fluoride Adsorption from Aqueous Solution by Nepheline Prepared from Kaolinite through Alkali-Hydrothermal Process. J. Environ. Manag. 2017, 196, 72–79. [Google Scholar] [CrossRef]
- Liu, K.; Feng, Q.; Yang, Y.; Zhang, G.; Ou, L.; Lu, Y. Preparation and Characterization of Amorphous Silica Nanowires from Natural Chrysotile. J. Non-Cryst. Solids 2007, 353, 1534–1539. [Google Scholar] [CrossRef]
- Capelli, S.; Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Iron as Modifier of Pd and Pt-Based Catalysts for Sustainable and Green Processes. Inorg. Chim. Acta 2022, 535, 120856. [Google Scholar] [CrossRef]
- Kong, Z.; Li, D.; Cai, R.; Li, T.; Diao, L.; Chen, X.; Wang, X.; Zheng, H.; Jia, Y.; Yang, D. Electron-Rich Palladium Regulated by Cationic Vacancies in CoFe Layered Double Hydroxide Boosts Electrocatalytic Hydrodechlorination. J. Hazard. Mater. 2024, 463, 132964. [Google Scholar] [CrossRef] [PubMed]
- Youmbi, B.S.; Pélisson, C.H.; Denicourt-Nowicki, A.; Roucoux, A.; Greneche, J.M. Impact of the Charge Transfer Process on the Fe2+/Fe3+distribution at Fe3O4 Magnetic Surface Induced by Deposited Pd Clusters. Surf. Sci. 2021, 712, 121879. [Google Scholar] [CrossRef]
- Coker, V.S.; Bennett, J.A.; Telling, N.D.; Henkel, T.; Charnock, J.M.; Van Der Laan, G.; Pattrick, R.A.D.; Pearce, C.I.; Cutting, R.S.; Shannon, I.J.; et al. Microbial Engineering of Nanoheterostructures: Biological Synthesis of a Magnetically Recoverable Palladium Nanocatalyst. ACS Nano 2010, 4, 2577–2584. [Google Scholar]
- Li, T.; Ji, N.; Jia, Z.; Diao, X.; Wang, Z.; Liu, Q.; Song, C.; Lu, X. Effects of Metal Promoters in Bimetallic Catalysts in Hydrogenolysis of Lignin Derivatives into Value-Added Chemicals. ChemCatChem 2020, 12, 5288–5302. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, T. Fabrication of Diatomite/Silicalite-1 Composites and Their Property for VOCs Adsorption. Materials 2019, 12, 551. [Google Scholar] [CrossRef]
- Augustine, R.L. Heterogeneous Catalysis for the Synthetic Chemist, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Potts, D.S.; Bregante, D.T.; Adams, J.S.; Torres, C.; Flaherty, D.W. Influence of Solvent Structure and Hydrogen Bonding on Catalysis at Solid–Liquid Interfaces. Chem. Soc. Rev. 2021, 50, 12308–12337. [Google Scholar] [CrossRef]

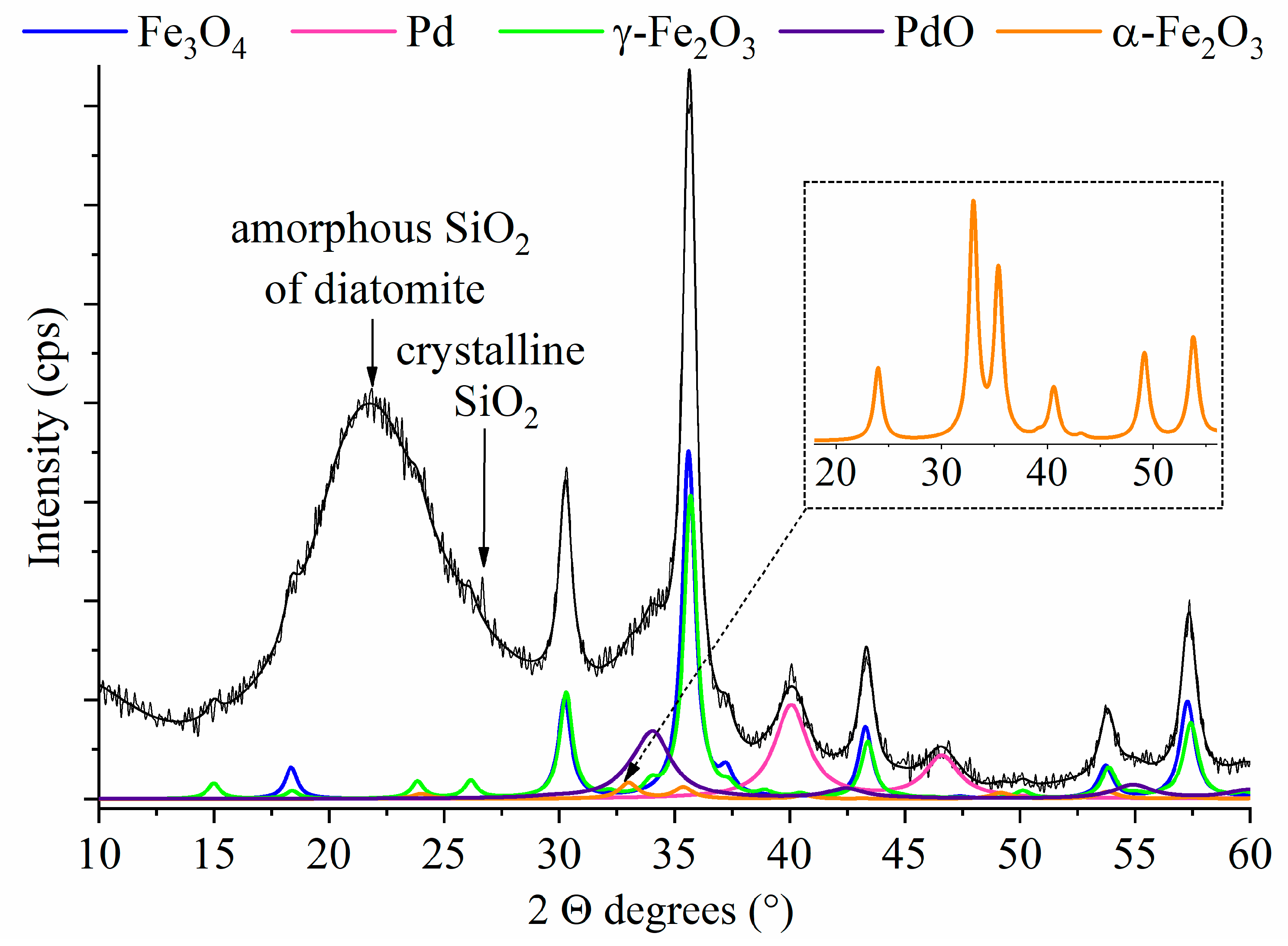

| Pd | PdO | Fe3O4 | γ-Fe3O4 | α-Fe3O4 | SiO2 | |
|---|---|---|---|---|---|---|
| Content | 4.40 wt% | 3.31 wt% | 20.9 wt% | 22.1 wt% | 2.23 wt% | 47.1 wt% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prekob, Á.; Szeleczki, B.; Veréb, Z.; Nagy, C.; Vanyorek, L.; Kristály, F.; Fejes, Z. Development of Palladium and Magnetite-Coated Diatomite as a Magnetizable Catalyst for Hydrogenation of Benzophenone. Int. J. Mol. Sci. 2025, 26, 3157. https://doi.org/10.3390/ijms26073157
Prekob Á, Szeleczki B, Veréb Z, Nagy C, Vanyorek L, Kristály F, Fejes Z. Development of Palladium and Magnetite-Coated Diatomite as a Magnetizable Catalyst for Hydrogenation of Benzophenone. International Journal of Molecular Sciences. 2025; 26(7):3157. https://doi.org/10.3390/ijms26073157
Chicago/Turabian StylePrekob, Ádám, Balázs Szeleczki, Zsolt Veréb, Csenge Nagy, László Vanyorek, Ferenc Kristály, and Zsolt Fejes. 2025. "Development of Palladium and Magnetite-Coated Diatomite as a Magnetizable Catalyst for Hydrogenation of Benzophenone" International Journal of Molecular Sciences 26, no. 7: 3157. https://doi.org/10.3390/ijms26073157
APA StylePrekob, Á., Szeleczki, B., Veréb, Z., Nagy, C., Vanyorek, L., Kristály, F., & Fejes, Z. (2025). Development of Palladium and Magnetite-Coated Diatomite as a Magnetizable Catalyst for Hydrogenation of Benzophenone. International Journal of Molecular Sciences, 26(7), 3157. https://doi.org/10.3390/ijms26073157





