Prediction and Prioritisation of Novel Anthelmintic Candidates from Public Databases Using Deep Learning and Available Bioactivity Data Sets
Abstract
1. Introduction
2. Results
2.1. Modelling Approach
2.2. Data Curation and Establishment of Publicly Accessible Database
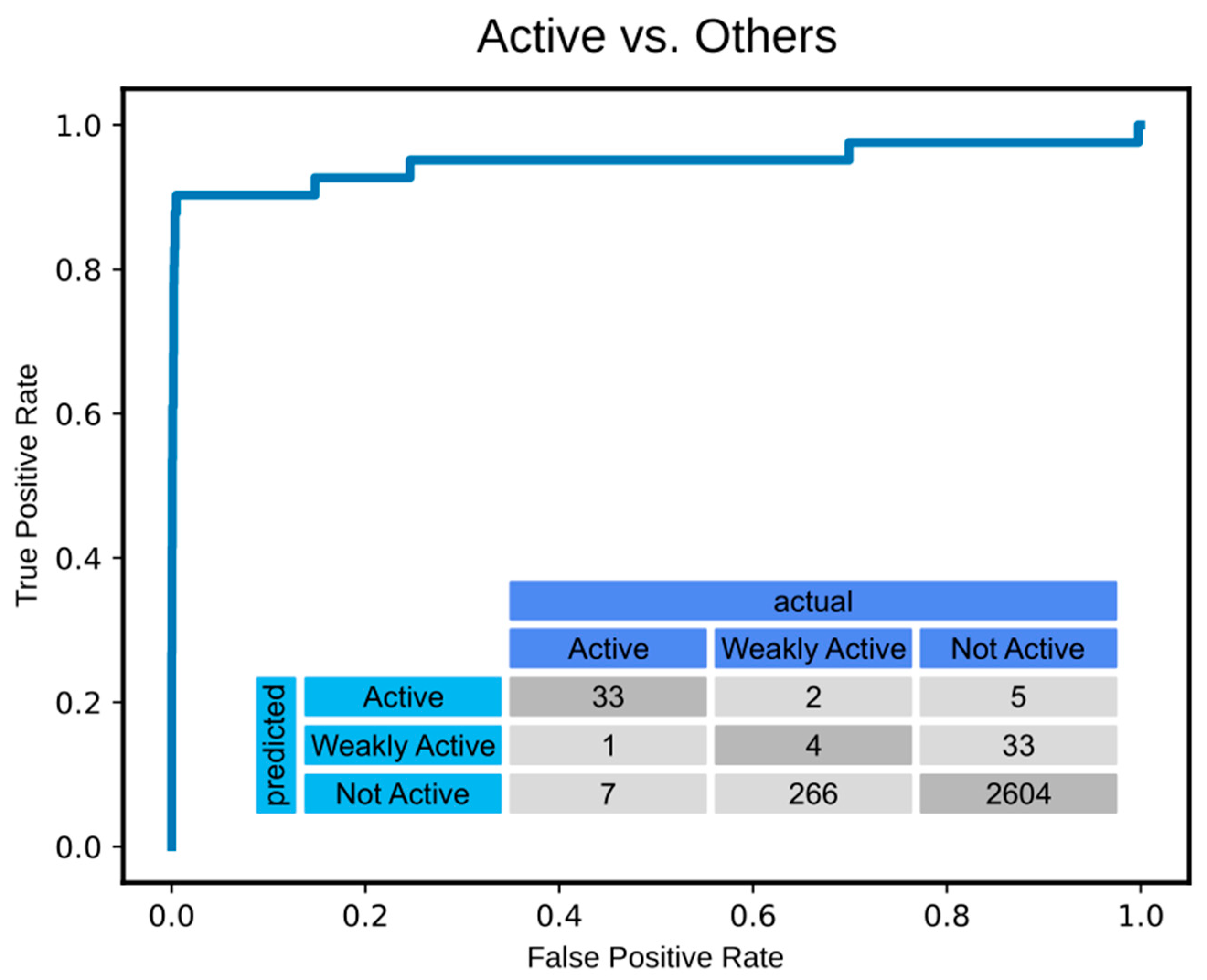
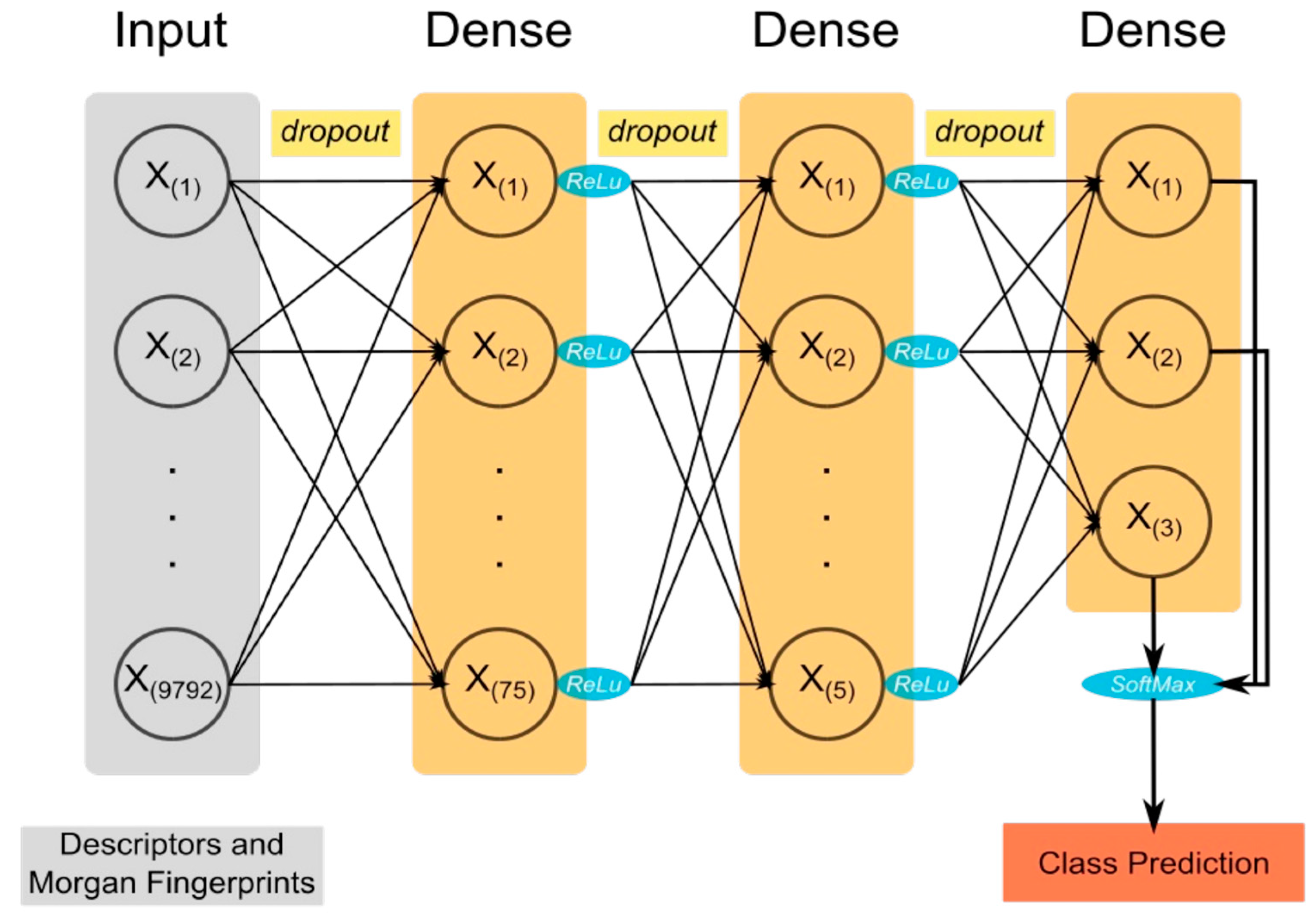
2.3. Feature Generation, Model Architecture, and Training
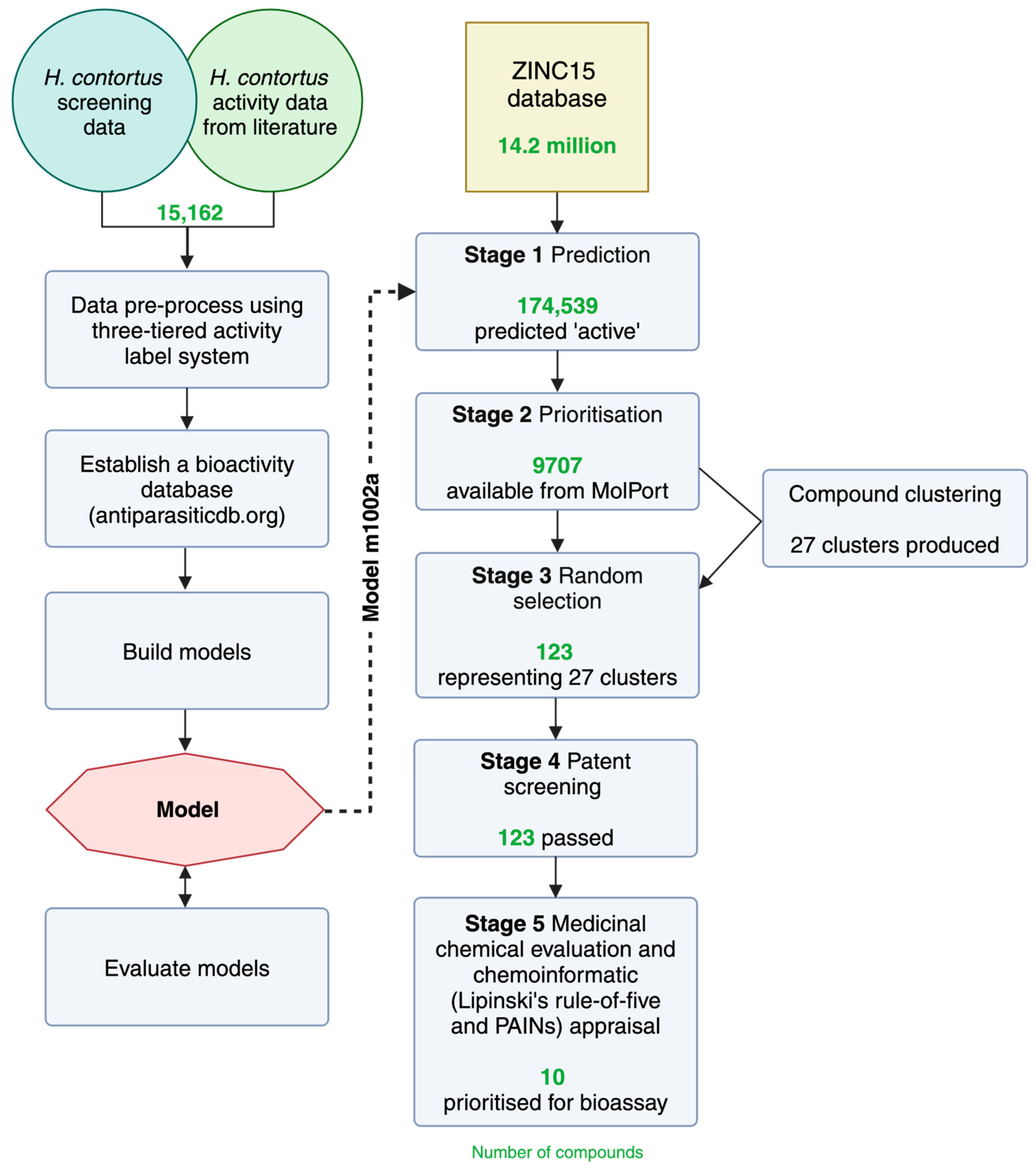
2.4. In Silico Screening and Post-Processing
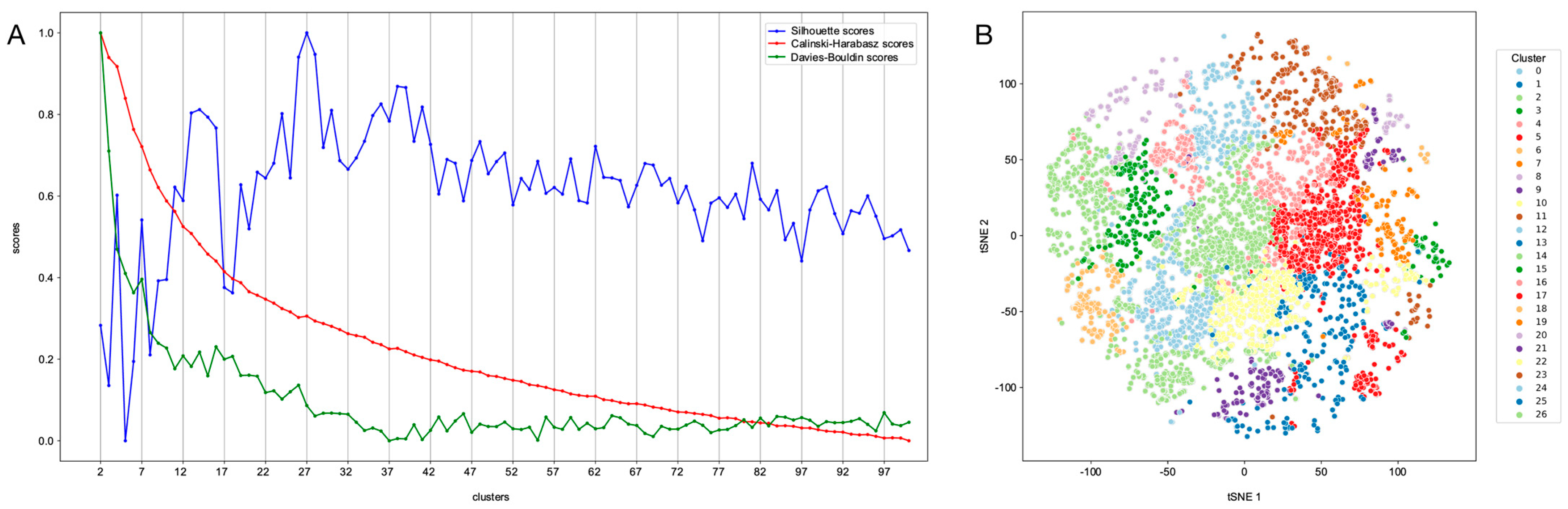
2.5. Clustering of Compounds with Predicted Nematocidal Activity
2.6. Selection of Molecules for Experimental Evaluation
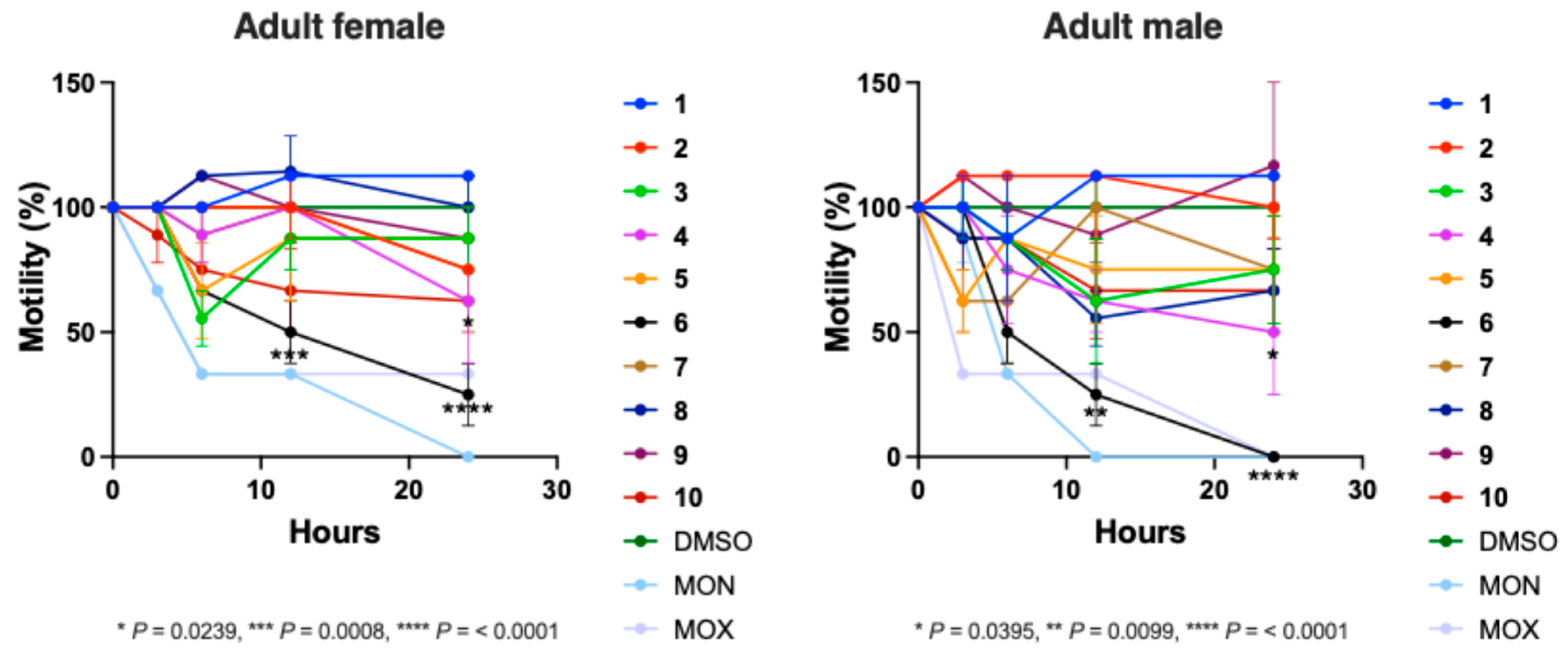
2.7. Experimental Evaluation of Molecules with Predicted Bioactivity Against H. contortus
3. Discussion
3.1. Harnessing Existing In-House and Literature Data to Apply Machine Learning Methodologies
3.2. Prediction and Prioritisation of Novel Compounds with Nematocidal Properties and Assessment of Potential Leads
4. Materials and Methods
4.1. Small-Molecule Bioactivity Data
4.2. Model
4.3. Input Features
4.4. Hyperparameter Tuning
4.5. In Silico Screening of ZINC Database
4.6. Clustering of Compounds with Predicted Nematocidal Activity
4.7. Post-Processing and Prioritisation
4.8. Assay to Evaluate Bioactivity of Prioritised Compounds
4.9. Method to Assess Cytotoxicity of Prioritised Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casulli, A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl. Trop. Dis. 2021, 15, e0009373. [Google Scholar] [CrossRef] [PubMed]
- Loukas, A.; Maizels, R.M.; Hotez, P.J. The yin and yang of human soil-transmitted helminth infections. Int. J. Parasitol. 2021, 51, 1243–1253. [Google Scholar]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals—A Road Map for Neglected Tropical Diseases 2021–2030; WHO: Geneva, Switzerland, 2020; p. 55. [Google Scholar]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Grisi, L.; Leite, R.C.; Martins, J.R.; Barros, A.T.; Andreotti, R.; Cançado, P.H.; León, A.A.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 150–156. [Google Scholar] [CrossRef]
- Selzer, P.M.; Epe, C. Antiparasitics in animal health: Quo vadis? Trends Parasitol. 2021, 37, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, I.; Hobbs, R.; Slapeta, J. Parasites. Australasian Animal Parasites Inside & Out; The Australian Society for Parasitology Inc.: Cairns, Australia, 2015; pp. 25–305. [Google Scholar]
- Rinaldi, L.; Krücken, J.; Martinez-Valladares, M.; Pepe, P.; Maurelli, M.P.; de Queiroz, C.; Castilla Gómez de Agüero, V.; Wang, T.; Cringoli, G.; Charlier, J.; et al. Advances in diagnosis of gastrointestinal nematodes in livestock and companion animals. Adv. Parasitol. 2022, 118, 85–176. [Google Scholar] [CrossRef]
- Roeber, F.; Jex, A.R.; Gasser, R.B. Advances in the diagnosis of key gastrointestinal nematode infections of livestock, with an emphasis on small ruminants. Biotechnol. Adv. 2013, 31, 1135–1152. [Google Scholar] [CrossRef]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef]
- Kotze, A.C.; Prichard, R.K. Anthelmintic resistance in Haemonchus contortus: History, mechanisms and diagnosis. Adv. Parasitol. 2016, 93, 397–428. [Google Scholar] [CrossRef]
- Sargison, N.; Francis, E.; Davison, C.; Bronsvoort, B.M.; Handel, I.; Mazeri, S. Observations on the biology, epidemiology and economic relevance of rumen flukes (Paramphistomidae) in cattle kept in a temperate environment. Vet. Parasitol. 2016, 219, 7–16. [Google Scholar] [CrossRef]
- Claerebout, E.; Geldhof, P. Helminth vaccines in ruminants: From development to application. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 159–171. [Google Scholar] [CrossRef]
- Nisbet, A.J.; Meeusen, E.N.; González, J.F.; Piedrafita, D.M. Immunity to Haemonchus contortus and vaccine development. Adv. Parasitol. 2016, 93, 353–396. [Google Scholar] [CrossRef]
- Ehsan, M.; Hu, R.-S.; Liang, Q.-L.; Hou, J.-L.; Song, X.; Yan, R.; Zhu, X.Q.; Li, X. Advances in the development of anti-Haemonchus contortus vaccines: Challenges, opportunities, and perspectives. Vaccines 2020, 8, 555. [Google Scholar] [CrossRef]
- Green, P.E.; Forsyth, B.A.; Rowan, K.J.; Payne, G. The isolation of a field strain of Haemonchus contortus in Queensland showing multiple anthelmintic resistance. Aust. Vet. J. 1981, 57, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Le Jambre, L.F.; Martin, P.J.; Webb, R.F. Thiabendazole resistance in field populations of Haemonchus contortus. Aust. Vet. J. 1979, 55, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Le Jambre, L.F. Molecular variation in trichostrongylid nematodes from sheep and cattle. Acta Trop. 1993, 53, 331–343. [Google Scholar] [CrossRef]
- Mederos, A.E.; Ramos, Z.; Banchero, G.E. First report of monepantel Haemonchus contortus resistance on sheep farms in Uruguay. Parasit. Vectors 2014, 7, 598. [Google Scholar] [CrossRef]
- Rolfe, P.; Boray, J.; Fitzgibbon, C.; Parsons, G.; Kemsley, P.; Sangster, N. Closantel resistance in Haemonchus contortus from sheep. Aust. Vet. J. 1990, 67, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.; Bartley, D.J.; Sotiraki, S.; Martinez-Valladares, M.; Claerebout, E.; von Samson-Himmelstjerna, G.; Thamsborg, S.M.; Hoste, H.; Morgan, E.R.; Rinaldi, L. Anthelmintic resistance in ruminants: Challenges and solutions. Adv. Parasitol. 2022, 115, 171–227. [Google Scholar] [CrossRef]
- Hodgkinson, J.E.; Kaplan, R.M.; Kenyon, F.; Morgan, E.R.; Park, A.W.; Paterson, S.; Babayan, S.A.; Beesley, N.J.; Britton, C.; Chaudhry, U.; et al. Refugia and anthelmintic resistance: Concepts and challenges. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 51–57. [Google Scholar] [CrossRef]
- Doyle, S.R.; Tracey, A.; Laing, R.; Holroyd, N.; Bartley, D.; Bazant, W.; Beasley, H.; Beech, R.; Britton, C.; Brooks, K.; et al. Genomic and transcriptomic variation defines the chromosome-scale assembly of Haemonchus contortus, a model gastrointestinal worm. Commun. Biol. 2020, 3, 656. [Google Scholar] [CrossRef]
- Gasser, R.B.; Schwarz, E.M.; Korhonen, P.K.; Young, N.D. Understanding Haemonchus contortus better through genomics and transcriptomics. Adv. Parasitol. 2016, 93, 519–567. [Google Scholar] [CrossRef]
- Laing, R.; Kikuchi, T.; Martinelli, A.; Tsai, I.J.; Beech, R.N.; Redman, E.; Holroyd, N.; Bartley, D.J.; Beasley, H.; Britton, C.; et al. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 2013, 14, R88. [Google Scholar] [CrossRef]
- Preston, S.; Jiao, Y.; Baell, J.B.; Keiser, J.; Crawford, S.; Koehler, A.V.; Wang, T.; Simpson, M.M.; Kaplan, R.M.; Cowley, K.J.; et al. Screening of the ‘Open Scaffolds’ collection from Compounds Australia identifies a new chemical entity with anthelmintic activities against different developmental stages of the barber’s pole worm and other parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 286–294. [Google Scholar] [CrossRef]
- Preston, S.; Korhonen, P.K.; Mouchiroud, L.; Cornaglia, M.; McGee, S.L.; Young, N.D.; Davis, R.A.; Crawford, S.; Nowell, C.; Ansell, B.R.E.; et al. Deguelin exerts potent nematocidal activity via the mitochondrial respiratory chain. FASEB J. 2017, 31, 4515–4532. [Google Scholar] [CrossRef]
- Schwarz, E.M.; Korhonen, P.K.; Campbell, B.E.; Young, N.D.; Jex, A.R.; Jabbar, A.; Hall, R.S.; Mondal, A.; Howe, A.C.; Pell, J.; et al. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013, 14, R89. [Google Scholar] [CrossRef]
- Taki, A.C.; Byrne, J.J.; Wang, T.; Sleebs, B.E.; Nguyen, N.; Hall, R.S.; Korhonen, P.K.; Chang, B.C.H.; Jackson, P.; Jabbar, A.; et al. High-throughput phenotypic assay to screen for anthelmintic activity on Haemonchus contortus. Pharmaceuticals 2021, 14, 616. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, G.; Ang, C.-S.; Korhonen, P.K.; Stroehlein, A.J.; Young, N.D.; Hofmann, A.; Chang, B.C.H.; Williamson, N.A.; Gasser, R.B. The developmental phosphoproteome of Haemonchus contortus. J. Proteom. 2020, 213, 103615. [Google Scholar] [CrossRef]
- Wang, T.; Ma, G.; Ang, C.-S.; Korhonen, P.K.; Koehler, A.V.; Young, N.D.; Nie, S.; Williamson, N.A.; Gasser, R.B. High throughput LC-MS/MS-based proteomic analysis of excretory-secretory products from short-term in vitro culture of Haemonchus contortus. J. Proteomics 2019, 204, 103375. [Google Scholar] [CrossRef]
- Wang, T.; Ma, G.; Ang, C.-S.; Korhonen, P.K.; Xu, R.; Nie, S.; Koehler, A.V.; Simpson, R.J.; Greening, D.W.; Reid, G.E.; et al. Somatic proteome of Haemonchus contortus. Int. J. Parasitol. 2019, 49, 311–320. [Google Scholar] [CrossRef]
- Wang, T.; Nie, S.; Ma, G.; Korhonen, P.K.; Koehler, A.V.; Ang, C.-S.; Reid, G.E.; Williamson, N.A.; Gasser, R.B. The developmental lipidome of Haemonchus contortus. Int. J. Parasitol. 2018, 48, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.P.D.; Taki, A.C.; Sleebs, B.E.; Hofmann, A.; Nguyen, N.; Preston, S.; Davis, R.A.; Jabbar, A.; Gasser, R.B. Advances in the discovery and development of anthelmintics by harnessing natural product scaffolds. Adv. Parasitol. 2021, 111, 203–251. [Google Scholar] [CrossRef]
- Jiao, Y.; Preston, S.; Hofmann, A.; Taki, A.; Baell, J.; Chang, B.C.H.; Jabbar, A.; Gasser, R.B. A perspective on the discovery of selected compounds with anthelmintic activity against the barber’s pole worm—Where to from here? Adv. Parasitol. 2020, 108, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Shanley, H.T.; Taki, A.C.; Byrne, J.J.; Jabbar, A.; Wells, T.N.C.; Samby, K.; Boag, P.R.; Nguyen, N.; Sleebs, B.E.; Gasser, R.B. A high-throughput phenotypic screen of the ‘Pandemic Response Box’ identifies a quinoline derivative with significant anthelmintic activity. Pharmaceuticals 2022, 15, 257. [Google Scholar] [CrossRef]
- Taki, A.C.; Wang, T.; Nguyen, N.N.; Ang, C.-S.; Leeming, M.G.; Nie, S.; Byrne, J.J.; Young, N.D.; Zheng, Y.; Ma, G.; et al. Thermal proteome profiling reveals Haemonchus orphan protein HCO_011565 as a target of the nematocidal small molecule UMW-868. Front. Pharmacol. 2022, 13, 1014804. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar] [CrossRef]
- Cherkasov, A.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J.; Gramatica, P.; Martin, Y.C.; Todeschini, R.; et al. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef]
- Tropsha, A. Best practices for QSAR model development, validation, and exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Cramer, R.D.; Patterson, D.E.; Bunce, J.D. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J. Am. Chem. Soc. 1988, 110, 5959–5967. [Google Scholar] [CrossRef]
- Hansch, C.; Fujita, T. p-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Kubinyi, H.; Kehrhahn, O.H. Quantitative structure-activity relationships. VI. Non-linear dependence of biological activity on hydrophobic character: Calculation procedures for bilinear model. Arzneimittelforschung 1978, 28, 598–601. [Google Scholar] [PubMed]
- Ajay, A.; Walters, W.P.; Murcko, M.A. Can we learn to distinguish between “drug-like” and “nondrug-like” molecules? J. Med. Chem. 1998, 41, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Burden, F.R.; Winkler, D.A. Robust QSAR models using Bayesian regularized neural networks. J. Med. Chem. 1999, 42, 3183–3187. [Google Scholar] [CrossRef]
- Alves, V.M.; Muratov, E.; Fourches, D.; Strickland, J.; Kleinstreuer, N.; Andrade, C.H.; Tropsha, A. Predicting chemically-induced skin reactions. Part I: QSAR models of skin sensitization and their application to identify potentially hazardous compounds. Toxicol. Appl. Pharmacol. 2015, 284, 262–272. [Google Scholar] [CrossRef]
- Svetnik, V.; Liaw, A.; Tong, C.; Culberson, J.C.; Sheridan, R.P.; Feuston, B.P. Random forest: A classification and regression tool for compound classification and QSAR modeling. J. Chem. Inf. Comput. Sci. 2003, 43, 1947–1958. [Google Scholar] [CrossRef]
- Du, H.; Wang, J.; Hu, Z.; Yao, X.; Zhang, X. Prediction of fungicidal activities of rice blast disease based on least-squares support vector machines and project pursuit regression. J. Agric. Food Chem. 2008, 56, 10785–10792. [Google Scholar] [CrossRef] [PubMed]
- Gaulton, A.; Bellis, L.J.; Bento, A.P.; Chambers, J.; Davies, M.; Hersey, A.; Light, Y.; McGlinchey, S.; Michalovich, D.; Al-Lazikani, B.; et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40, D1100–D1107. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Zhou, Z.; Han, L.; Karapetyan, K.; Dracheva, S.; Shoemaker, B.A.; et al. PubChem’s BioAssay database. Nucleic Acids Res. 2012, 40, D400–D412. [Google Scholar] [CrossRef]
- Braga, R.C.; Alves, V.M.; Silva, A.C.; Nascimento, M.N.; Silva, F.C.; Liao, L.M.; Andrade, C.H. Virtual screening strategies in medicinal chemistry: The state of the art and current challenges. Curr. Top. Med. Chem. 2014, 14, 1899–1912. [Google Scholar] [CrossRef]
- Melo-Filho, C.C.; Dantas, R.F.; Braga, R.C.; Neves, B.J.; Senger, M.R.; Valente, W.C.; Rezende-Neto, J.M.; Chaves, W.T.; Muratov, E.N.; Paveley, R.A.; et al. QSAR-driven discovery of novel chemical scaffolds active against Schistosoma mansoni. J. Chem. Inf. Model. 2016, 56, 1357–1372. [Google Scholar] [CrossRef]
- Neves, B.J.; Dantas, R.F.; Senger, M.R.; Melo-Filho, C.C.; Valente, W.C.; de Almeida, A.C.; Rezende-Neto, J.M.; Lima, E.F.; Paveley, R.; Furnham, N.; et al. Discovery of new anti-schistosomal hits by integration of QSAR-based virtual screening and high content screening. J. Med. Chem. 2016, 59, 7075–7088. [Google Scholar] [CrossRef]
- Hartung, T. Making big sense from big data in toxicology by read-across. ALTEX 2016, 33, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, J.; Kim, M.T.; Boison, A.; Sedykh, A.; Moran, K. Big data in chemical toxicity research: The use of high-throughput screening assays to identify potential toxicants. Chem. Res. Toxicol. 2014, 27, 1643–1651. [Google Scholar] [CrossRef]
- Ekins, S. The next era: Deep learning in pharmaceutical research. Pharm. Res. 2016, 33, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Ramsundar, B.; Liu, B.; Wu, Z.; Verras, A.; Tudor, M.; Sheridan, R.P.; Pande, V. Is multitask deep learning practical for pharma? J. Chem. Inf. Model. 2017, 57, 2068–2076. [Google Scholar] [CrossRef]
- Goh, G.B.; Hodas, N.O.; Vishnu, A. Deep learning for computational chemistry. J. Comput. Chem. 2017, 15, 1291–1307. [Google Scholar] [CrossRef]
- Ma, J.; Sheridan, R.P.; Liaw, A.; Dahl, G.E.; Svetnik, V. Deep neural nets as a method for quantitative structure-activity relationships. J. Chem. Inf. Model. 2015, 55, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.; Irwin, J.J. ZINC 15—Ligand discovery for everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef]
- Preston, S.; Jiao, Y.; Jabbar, A.; McGee, S.L.; Laleu, B.; Willis, P.; Wells, T.N.C.; Gasser, R.B. Screening of the ‘Pathogen Box’ identifies an approved pesticide with major anthelmintic activity against the barber’s pole worm. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 329–334. [Google Scholar] [CrossRef]
- Preston, S.; Jabbar, A.; Nowell, C.; Joachim, A.; Ruttkowski, B.; Baell, J.; Cardno, T.; Korhonen, P.K.; Piedrafita, D.; Ansell, B.R.; et al. Low-cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015, 45, 333–343. [Google Scholar] [CrossRef]
- Taki, A.C.; Hall, R.S.; Gasser, R.B.; Hofmann, A. Bioactivity of small-molecule compounds against Haemonchus contortus. Zenodo 2024. [Google Scholar] [CrossRef]
- Le, T.G.; Kundu, A.; Ghoshal, A.; Nguyen, N.H.; Preston, S.; Jiao, Y.; Ruan, B.; Xue, L.; Huang, F.; Keiser, J.; et al. Optimization of novel 1-methyl-1H-pyrazole-5-carboxamides leads to high potency larval development inhibitors of the barber’s pole worm. J. Med. Chem. 2018, 61, 10875–10894. [Google Scholar] [CrossRef] [PubMed]
- Le, T.G.; Kundu, A.; Ghoshal, A.; Nguyen, N.H.; Preston, S.; Jiao, Y.; Ruan, B.; Xue, L.; Huang, F.; Keiser, J.; et al. Structure-activity relationship studies of tolfenpyrad reveal subnanomolar inhibitors of Haemonchus contortus development. J. Med. Chem. 2019, 62, 1036–1053. [Google Scholar] [CrossRef] [PubMed]
- Ruan, B.; Zhang, Y.; Tadesse, S.; Preston, S.; Taki, A.C.; Jabbar, A.; Hofmann, A.; Jiao, Y.; Garcia-Bustos, J.; Harjani, J.; et al. Synthesis and structure-activity relationship study of pyrrolidine-oxadiazoles as anthelmintics against Haemonchus contortus. Eur. J. Med. Chem. 2020, 190, 112100. [Google Scholar] [CrossRef]
- Le, T.G.; Kundu, A.; Ghoshal, A.; Nguyen, N.H.; Preston, S.; Jiao, Y.; Ruan, B.; Xue, L.; Huang, F.; Keiser, J.; et al. Novel 1-methyl-1H-pyrazole-5-carboxamide derivatives with potent anthelmintic activity. J. Med. Chem. 2019, 62, 3367–3380. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Patra, M.; Rangasamy, L.; Konatschnig, S.; Blacque, O.; Jabbar, A.; Mac, P.; Jorgensen, E.M.; Gasser, R.B.; Gasser, G. Organometallic derivatization of the nematocidal drug monepantel leads to promising antiparasitic drug candidates. Chemistry 2016, 22, 16602–16612. [Google Scholar] [CrossRef]
- Jiao, Y.; Preston, S.; Garcia-Bustos, J.F.; Baell, J.B.; Ventura, S.; Le, T.; McNamara, N.; Nguyen, N.; Botteon, A.; Skinner, C.; et al. Tetrahydroquinoxalines induce a lethal evisceration phenotype in Haemonchus contortus in vitro. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 59–71. [Google Scholar] [CrossRef]
- Preston, S.; Luo, J.; Zhang, Y.; Jabbar, A.; Crawford, S.; Baell, J.; Hofmann, A.; Hu, M.; Zhou, H.B.; Gasser, R.B. Selenophene and thiophene-core estrogen receptor ligands that inhibit motility and development of parasitic stages of Haemonchus contortus. Parasit. Vectors 2016, 9, 346. [Google Scholar] [CrossRef]
- Nixon, S.A.; Saez, N.J.; Herzig, V.; King, G.F.; Kotze, A.C. The antitrypanosomal diarylamidines, diminazene and pentamidine, show anthelmintic activity against Haemonchus contortus in vitro. Vet. Parasitol. 2019, 270, 40–46. [Google Scholar] [CrossRef]
- Hood, J.D.; Banks, R.M.; Brewer, M.D.; Fish, J.P.; Manger, B.R.; Poulton, M.E. A novel series of milbemycin antibiotics from Streptomyces strain E225—I. Discovery, fermentation and anthelmintic activity. J. Antibiot. 1989, 42, 1593–1598. [Google Scholar] [CrossRef]
- Witola, W.H.; Matthews, K.; McHugh, M. In vitro anthelmintic efficacy of inhibitors of phosphoethanolamine methyltransferases in Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 44–53. [Google Scholar] [CrossRef] [PubMed]
- White, W.H.; Gutierrez, J.A.; Naylor, S.A.; Cook, C.A.; Gonzalez, I.C.; Wisehart, M.A.; Smith, C.K., II; Thompson, W.A. In vitro and in vivo characterization of p-amino-phenethyl-m-trifluoromethylphenyl piperazine (PAPP), a novel serotonergic agonist with anthelmintic activity against Haemonchus contortus, Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Parasitol. 2007, 146, 58–65. [Google Scholar] [CrossRef]
- Kaminsky, R.; Bapst, B.; Stein, P.A.; Strehlau, G.A.; Allan, B.A.; Hosking, B.C.; Rolfe, P.F.; Sager, H. Differences in efficacy of monepantel, derquantel and abamectin against multi-resistant nematodes of sheep. Parasitol. Res. 2011, 109, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Neilson, F.; Biddle, A.; Mckinnon, R. Moxidectin-resistant Haemonchus contortus in sheep in northern New South Wales. Aust. Vet. J. 2003, 81, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.P.D.; Preston, S.; Jabbar, A.; Garcia-Bustos, J.; Taki, A.C.; Addison, R.S.; Hayes, S.; Beattie, K.D.; McGee, S.L.; Martin, S.D.; et al. Identification of fromiamycalin and halaminol A from Australian marine sponge extracts with anthelmintic activity against Haemonchus contortus. Mar. Drugs 2019, 17, 598. [Google Scholar] [CrossRef]
- Waruiru, R.M. Efficacy of closantel, albendazole and levamisole on an ivermectin-resistant strain of Haemonchus contortus in sheep. Vet. Parasitol. 1997, 73, 65–71. [Google Scholar] [CrossRef]
- Page, K.W. The anthelmintic effect of phenothiazine administered in small daily doses to lambs bearing heavy infestations of Haemonchus contortus. J. Comp. Pathol. Ther. 1949, 59, 70–80. [Google Scholar] [CrossRef]
- Bosco, A.; Kießler, J.; Amadesi, A.; Varady, M.; Hinney, B.; Ianniello, D.; Maurelli, M.P.; Cringoli, G.; Rinaldi, L. The threat of reduced efficacy of anthelmintics against gastrointestinal nematodes in sheep from an area considered anthelmintic resistance-free. Parasit. Vectors 2020, 13, 457. [Google Scholar] [CrossRef]
- Hadipour, H.; Liu, C.; Davis, R.; Cardona, S.T.; Hu, P. Deep clustering of small molecules at large-scale via variational autoencoder embedding and K-means. BMC Bioinform. 2022, 23, 132. [Google Scholar] [CrossRef]
- Harder, A.; von Samson-Himmelstjerna, G. Activity of the cyclic depsipeptide emodepside (BAY 44-4400) against larval and adult stages of nematodes in rodents and the influence on worm survival. Parasitol. Res. 2001, 87, 924–928. [Google Scholar] [CrossRef]
- Xiao, S.-H.; Hui-Ming, W.; Tanner, M.; Utzinger, J.; Chong, W. Tribendimidine: A promising, safe, and broad-spectrum anthelmintic agent from China. Acta Trop. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Kaminsky, R.; Ducray, P.; Jung, M.; Clover, R.; Rufener, L.; Bouvier, J.; Weber, S.S.; Wenger, A.; Wieland-Berghausen, S.; Goebel, T.; et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature 2008, 452, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Little, P.R.; Hodge, A.; Maeder, S.J.; Wirtherle, N.C.; Nicholas, D.R.; Cox, G.G.; Conder, G.A. Efficacy of a combined oral formulation of derquantel–abamectin against the adult and larval stages of nematodes in sheep, including anthelmintic-resistant strains. Vet. Parasitol. 2011, 181, 180–193. [Google Scholar] [CrossRef]
- Nixon, S.A.; Welz, C.; Woods, D.J.; Costa-Junior, L.; Zamanian, M.; Martin, R.J. Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 8–16. [Google Scholar] [CrossRef]
- Partridge, F.A.; Forman, R.; Bataille, C.J.R.; Wynne, G.M.; Nick, M.; Russell, A.J.; Else, K.J.; Sattelle, D.B. Anthelmintic drug discovery: Target identification, screening methods, and the role of open science. Beilstein J. Org. Chem. 2020, 16, 1203–1224. [Google Scholar] [CrossRef] [PubMed]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Preston, S.; Gasser, R.B. Working towards new drugs against parasitic worms in a public-development partnership. Trends Parasitol. 2018, 34, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, R.; Graef, K.M.; Dent, J. WIPO Re:Search: Accelerating anthelmintic development through cross-sector partnerships. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 220–225. [Google Scholar] [CrossRef][Green Version]
- Cross, M.; York, M.; Długosz, E.; Straub, J.H.; Biberacher, S.; Herath, H.M.P.D.; Logan, S.A.; Kim, J.S.; Gasser, R.B.; Ryan, J.H.; et al. A suicide inhibitor of nematode trehalose-6-phosphate phosphatases. Sci. Rep. 2019, 9, 16165. [Google Scholar] [CrossRef]
- Knox, J.; Joly, N.; Linossi, E.M.; Carmona-Negrón, J.A.; Jura, N.; Pintard, L.; Zuercher, W.; Roy, P.J. A survey of the kinome pharmacopeia reveals multiple scaffolds and targets for the development of novel anthelmintics. Sci. Rep. 2021, 11, 9161. [Google Scholar] [CrossRef]
- Tyagi, R.; Elfawal, M.A.; Wildman, S.A.; Helander, J.; Bulman, C.A.; Sakanari, J.; Rosa, B.A.; Brindley, P.J.; Janetka, J.W.; Aroian, R.V.; et al. Identification of small molecule enzyme inhibitors as broad-spectrum anthelmintics. Sci. Rep. 2019, 9, 9085. [Google Scholar] [CrossRef]
- Zorn, K.M.; Sun, S.; McConnon, C.L.; Ma, K.; Chen, E.K.; Foil, D.H.; Lane, T.R.; Liu, L.J.; El-Sakkary, N.; Skinner, D.E.; et al. A machine learning strategy for drug discovery identifies anti-schistosomal small molecules. ACS Infect. Dis. 2021, 7, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Filho, J.T.; Neves, B.J.; Cajas, R.A.; Moraes, J.; Andrade, C.H. Artificial intelligence-guided approach for efficient virtual screening of hits against Schistosoma mansoni. Future Med. Chem. 2023, 15, 2033–2050. [Google Scholar] [CrossRef]
- Coyne, M.J.; Smith, G. The mortality and fecundity of Haemonchus contortus in parasite-naive and parasite-exposed sheep following single experimental infections. Int. J. Parasitol. 1992, 22, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.W. Size of inoculum dose regulates in part worm burdens, fecundity, and lengths in ovine Haemonchus contortus infections. J. Parasitol. 1988, 74, 975–978. [Google Scholar] [CrossRef]
- King, A.M.; Yang, X.-F.; Wang, Y.; Dustrude, E.T.; Barbosa, C.; Due, M.R.; Piekarz, A.D.; Wilson, S.M.; White, F.A.; Salomé, C.; et al. Identification of the benzyloxyphenyl pharmacophore: A structural unit that promotes sodium channel slow inactivation. ACS Chem. Neurosci. 2012, 3, 1037–1049. [Google Scholar] [CrossRef]
- Lee, H.; Gold, A.S.; Yang, X.-F.; Khanna, R.; Kohn, H. Benzyloxybenzylammonium chlorides: Simple amine salts that display anticonvulsant activity. Bioorg. Med. Chem. 2013, 21, 7655–7662. [Google Scholar] [CrossRef] [PubMed]
- Salomé, C.; Salomé-Grosjean, E.; Stables, J.P.; Kohn, H. Merging the structural motifs of functionalized amino acids and α-aminoamides: Compounds with significant anticonvulsant activities. J. Med. Chem. 2010, 53, 3756–3771. [Google Scholar] [CrossRef]
- Yeon, S.K.; Choi, J.W.; Park, J.-H.; Lee, Y.R.; Kim, H.J.; Shin, S.J.; Jang, B.K.; Kim, S.; Bahn, Y.S.; Han, G.; et al. Synthesis and evaluation of biaryl derivatives for structural characterization of selective monoamine oxidase B inhibitors toward Parkinson’s disease therapy. Bioorg. Med. Chem. 2018, 26, 232–244. [Google Scholar] [CrossRef]
- Wasan, H.; Singh, D.; Kh, R. Safinamide in neurological disorders and beyond: Evidence from preclinical and clinical studies. Brain Res. Bull. 2021, 168, 165–177. [Google Scholar] [CrossRef]
- Kaji, M.D.; Noonan, J.D.; Geary, T.G.; Beech, R.N. Structural mechanism underlying the differential effects of ivermectin and moxidectin on the C. elegans glutamate-gated chloride channel GLC-2. Biomed. Pharmacother. 2022, 145, 112380. [Google Scholar] [CrossRef]
- Rayes, D.; Rosa, M.J.; Bartos, M.; Bouzat, C. Molecular basis of the differential sensitivity of nematode and mammalian muscle to the anthelmintic agent levamisole. J. Biol. Chem. 2004, 279, 36372–36381. [Google Scholar] [CrossRef]
- Stroehlein, A.J.; Young, N.D.; Korhonen, P.K.; Jabbar, A.; Hofmann, A.; Sternberg, P.W.; Gasser, R.B. The Haemonchus contortus kinome—A resource for fundamental molecular investigations and drug discovery. Parasit. Vectors 2015, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Shanley, H.T.; Taki, A.C.; Nguyen, N.; Wang, T.; Byrne, J.J.; Ang, C.-S.; Leeming, M.G.; Nie, S.; Williamson, N.; Zheng, Y.; et al. Structure-activity relationship and target investigation of 2-aryl quinolines with nematocidal activity. Int. J. Parasitol. Drugs Drug Resist. 2024, 24, 100522. [Google Scholar] [CrossRef] [PubMed]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-scale machine learning on heterogeneous distributed systems. arXiv 2015, arXiv:1603.04467. [Google Scholar] [CrossRef]
- Chollet, F. Keras. 2015. Available online: https://keras.io (accessed on 26 March 2025).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Srivastava, N.; Hinton, G.; Krizhevsky, A.; Sutskever, I.; Salakhutdinov, R. Dropout: A simple way to prevent neural networks from overfitting. J. Mach. Learn. Res. 2014, 15, 1929–1958. [Google Scholar]
- Moriwaki, H.; Tian, Y.-S.; Kawashita, N.; Takagi, T. Mordred: A molecular descriptor calculator. J. Cheminform. 2018, 10, 4. [Google Scholar] [CrossRef]
- Morgan, H.L. The generation of a unique machine description for chemical structures—A technique developed at Chemical Abstracts Service. J. Chem. Doc. 1965, 5, 107–113. [Google Scholar] [CrossRef]
- Kingma, D.P.; Welling, M. Auto-encoding variational Bayes. arXiv 2013. [Google Scholar] [CrossRef]
- Sutskever, I.; Martens, J.; Dahl, G.; Hinton, G. On the importance of initialization and momentum in deep learning. In Proceedings of the 30th International Conference on Machine Learning, Atlanta, GA, USA, 16–21 June 2013; pp. III-1139–III-1147. [Google Scholar]
- Paszke, A.; Gross, S.; Massa, F.; Lerer, A.; Bradbury, J.; Chanan, G.; Killeen, T.; Lin, Z.; Gimelshein, N.; Antiga, L.; et al. PyTorch: An imperative style, high-performance deep learning library. In Proceedings of the 33rd International Conference on Neural Information Processing Systems, Red Hook, NY, USA, 8 December 2019; pp. 8026–8037. [Google Scholar]
- van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Levenshtein, V. Binary codes capable of correcting deletions, insertions, and reversals. Sov. Phys. Dokl. 1965, 10, 707–710. [Google Scholar]
- Amani, P.; Sneyd, T.; Preston, S.; Young, N.D.; Mason, L.; Bailey, U.-M.; Baell, J.; Camp, D.; Gasser, R.B.; Gorse, A.D.; et al. A practical Java tool for small-molecule compound appraisal. J. Cheminform. 2015, 7, 28. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.R.; Tan, C.; Jarman, K.E.; Lowes, K.N.; Curtis, J.M.; Nguyen, W.; Di Rago, A.E.; Bullen, H.E.; Prinz, B.; Duffy, S.; et al. Optimization of 2-anilino 4-amino substituted quinazolines into potent antimalarial agents with oral in vivo activity. J. Med. Chem. 2017, 60, 1171–1188. [Google Scholar] [CrossRef]
| Activity Label | Wiggle Index | Viability | Reduction | EC50 | MIC75 |
|---|---|---|---|---|---|
| active | x < 0.25 | x < 20% | x > 80% | x < 50 µM | x < 1 µg/mL |
| weakly active | 0.25 ≤ x < 0.5 | 20% ≤ x < 50% | 80% ≥ x > 50% | 50 µM ≤ x < 100 µM | 1 µg/mL ≤ x < 10 µg/mL |
| none | 0.5 ≤ x | 50% ≤ x | 50% ≥ x | 100 µM ≤ x | 10 µg/mL ≤ x |
| Library/Compound Family | N | Reference |
|---|---|---|
| Open Scaffolds | 14,464 | [26] |
| Pathogen Box | 400 | [61] |
| 1-methyl-1H-pyrazole-5-carboxamides | 64 | [64] |
| Tolfenpyrad derivatives | 57 | [65] |
| pyrrolidine-oxadiazoles | 57 | [66] |
| 1-methyl-1H-pyrazole-5-carboxamides | 30 | [67] |
| Monepantel derivatives | 28 | [68] |
| Tetrahydroquinoxalines | 1 | [69] |
| Selenophenes, thiophenes | 12 | [70] |
| Diarylamidines | 6 | [71] |
| Milbemycine derivatives | 4 | [72] |
| Anthelmintics (rafoxanide, naphthalophos, nitroxynil, disophenol) | 4 | [16] |
| Phosphoethanolamine methyltransferases inhibitors | 3 | [73] |
| p-amino-phenethyl-m-trifluoromethylphenyl piperazine | 3 | [74] |
| Anthelmintics (derquantel, abamectin) | 2 | [75] |
| Anthelmintics (abamectin, benzimidazole) | 2 | [76] |
| Fromiamycalin, halaminol A | 2 | [77] |
| Anthelmintic (closantel) | 1 | [78] |
| Phenothiazine | 1 | [79] |
| Deguelin | 1 | [27] |
| Anthelmintic (eprinomectin) | 1 | [80] |
| Anthelmintic (albendazole, monepantel, morantel, moxidectin, thiabendazole) | 5 | in-house data |
| Total | 15,162 |
| Label | Training | Test | Total | |||
|---|---|---|---|---|---|---|
| # | Fraction | # | Fraction | # | Fraction | |
| None | 11,005 | 0.902 | 2643 | 0.894 | 13,648 | 0.900 |
| Weakly active | 1081 | 0.089 | 272 | 0.092 | 1353 | 0.089 |
| Active | 121 | 0.010 | 40 | 0.014 | 161 | 0.011 |
| (Combined active) | 1202 | 0.098 | 314 | 0.106 | 1516 | 0.100 |
| Total | 12,207 | 0.81 | 2955 | 0.19 | 15,162 | 1.0 |
| Tested Models | Best Model Performance on Test Set | |||||
|---|---|---|---|---|---|---|
| Series | No of Models | n_hidden | dim_hidden | dim_hidden | Precision ‘Active’ | Recall ‘Active’ |
| series_1001 | 39 | 1 | 5–195 | 100 | 0.816 | 0.756 |
| series_1002 | 19 | 2 | 5–95/5 | 75/5 | 0.825 | 0.805 |
| series_1003 | 9 | 2 | 100–900/10 | 500/10 | 0.821 | 0.780 |
| series_1004 | 9 | 2 | 100–900/50 | 600/50 | 0.811 | 0.732 |
| series_2001 | 39 | 1 | 5–195 | 185 | 0.789 | 0.732 |
| Predicted Bioactivity Against H. contortus | |||
|---|---|---|---|
| No of Compounds | Active | Weakly Active | None |
| 14,154,291 | 174,539 | 12,922 | 13,966,830 |
| 1.2% | 0.09% | 98.7% | |
| Compound | Structure | Larval Motility IC50 μM a (MI; %) | Larval Development IC50 μM a (MI; %) | Abnormal Phenotype b (Exhibited at 50 µM; %) |
|---|---|---|---|---|
| 1 | 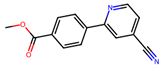 | 6.2 ± 0.3 (50) | >50 | Cur, Evi (20) |
| 2 | 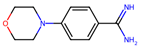 | 1.8 ± 1.4 (53) | >50 | nil |
| 3 | 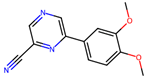 | 7.9 ± 1.9 (54) | 37.1 (100) | Evi (80) |
| 4 | 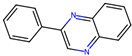 | 5.8 ± 4.1 (90) | >50 | nil |
| 5 | 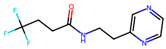 | 37.5 ± 2.5 (65) | >50 | nil |
| 6 | 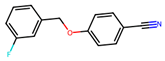 | 7.4 ± 9.4 (100) | 23.6 (100) | Evi (100) |
| 7 | 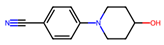 | 2.1 ± 3.5 (70) | >50 | nil |
| 8 | 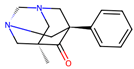 | 0.8 ± 1.6 (42) | >50 | nil |
| 9 | 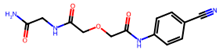 | 4.1 ± 21.4 (46) | >50 | nil |
| 10 | 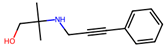 | 0.8 ± 1.2 (50) | >50 | nil |
| Monepantel | 0.2 ± 0.002 (100) | 0.4 (100) | Coi (100) | |
| Moxidectin | 0.8 ± 0.4 (75) | 45.0 (100) | nil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taki, A.C.; Kapp, L.; Hall, R.S.; Byrne, J.J.; Sleebs, B.E.; Chang, B.C.H.; Gasser, R.B.; Hofmann, A. Prediction and Prioritisation of Novel Anthelmintic Candidates from Public Databases Using Deep Learning and Available Bioactivity Data Sets. Int. J. Mol. Sci. 2025, 26, 3134. https://doi.org/10.3390/ijms26073134
Taki AC, Kapp L, Hall RS, Byrne JJ, Sleebs BE, Chang BCH, Gasser RB, Hofmann A. Prediction and Prioritisation of Novel Anthelmintic Candidates from Public Databases Using Deep Learning and Available Bioactivity Data Sets. International Journal of Molecular Sciences. 2025; 26(7):3134. https://doi.org/10.3390/ijms26073134
Chicago/Turabian StyleTaki, Aya C., Louis Kapp, Ross S. Hall, Joseph J. Byrne, Brad E. Sleebs, Bill C. H. Chang, Robin B. Gasser, and Andreas Hofmann. 2025. "Prediction and Prioritisation of Novel Anthelmintic Candidates from Public Databases Using Deep Learning and Available Bioactivity Data Sets" International Journal of Molecular Sciences 26, no. 7: 3134. https://doi.org/10.3390/ijms26073134
APA StyleTaki, A. C., Kapp, L., Hall, R. S., Byrne, J. J., Sleebs, B. E., Chang, B. C. H., Gasser, R. B., & Hofmann, A. (2025). Prediction and Prioritisation of Novel Anthelmintic Candidates from Public Databases Using Deep Learning and Available Bioactivity Data Sets. International Journal of Molecular Sciences, 26(7), 3134. https://doi.org/10.3390/ijms26073134








