Abstract
The [1,2]-phospha-Brook rearrangement serves as a powerful synthetic strategy that enables efficient carbonyl umpolung through phosphoryl group migration, providing direct access to α-hydroxyphosphoryl compounds—a privileged class of synthons with broad applications in organophosphorus chemistry, medicinal chemistry, and materials science. This review provides a comprehensive overview of recent progress in synthetic methodologies, possible mechanisms, and asymmetric transformations, highlighting key breakthroughs and future directions in this rapidly evolving field.
1. Introduction
The Brook rearrangement, also known as the intramolecular 1,2-silicon-based migration reaction, was first reported by Brook in 1958 []. This reaction typically involves an intramolecular 1,2-anionic migration of a silyl group in an α-silyl carbinol intermediate, which is generated from a silicon reagent and a carbonyl substrate. The process proceeds through the formation of a cyclic silicon species, facilitated by catalysts or bases, ultimately yielding the corresponding silyl ether as the product (Figure 1a) []. The [1,2]-phospha-Brook rearrangement represents a variant of the Brook rearrangement, characterized by the substitution of the original silicon atom with a phosphorus atom as the migratory center. This transformation specifically involves the rearrangement reaction of α-hydroxyphosphonates, where the phosphonyl group migrates from a carbon atom to an oxygen atom, generating α-phosphonyloxy ester as the product (Figure 1b). The [1,2]-phospha-Brook rearrangement serves as a valuable synthetic tool for the efficient preparation of biologically active α-phosphoryloxy esters, which find extensive applications in medicine, materials science, pesticide development, and the total synthesis of natural products. Notably, the α-phosphoryloxy structure readily generates carbon anions that can participate in coupling reactions to form new C-C bonds, thereby providing a versatile platform for constructing complex molecular architectures, particularly compounds with multiple chiral centers.
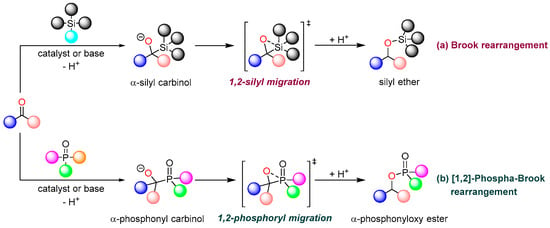
Figure 1.
Brook rearrangement and [1,2]-phospha-Brook rearrangement. (a) Brook rearrangement. (b) [1,2]-phospha-Brook rearrangement.
Terada and coworkers [,] have reviewed the [1,2]-phospha-Brook rearrangement for generating anionic nucleophiles in addition reactions, while Singh [] has focused on stereoselective or enantioselective transformations of various structural units. However, a comprehensive classification based on carbonyl substrate types remains unexplored. This review summarizes the progress of [1,2]-phospha-Brook rearrangement over the past two decades. The content is organized into several sections based on the types of carbonyl substrates involved in the reaction, including aldehydes, ketones, ketoesters, ketoamides, vinyl ketones, alkynyl ketones, and α-hydroxyphosphates.
2. Aldehyde Rearrangement
Although the use of simple aldehyde substrates in Pudovik reactions has been extensively documented, their application in [1,2]-phospha-Brook rearrangement reactions remains relatively unexplored. This limited exploration can be attributed to the significantly higher activation energy barrier associated with the [1,2]-phospha-Brook rearrangement compared to the Pudovik reaction, which necessitates more rigorous reaction conditions. These demanding thermodynamic requirements have substantially hindered progress in this research area. It is noteworthy that while aldehyde substrates have occasionally been employed in certain [1,2]-phospha-Brook reaction studies, they have primarily served supplementary roles within experimental frameworks rather than being the primary focus of investigation.
2.1. Aldehyde Rearrangement with Phosphodiesters
In 2005, Kaïm et al. [] reported a pioneering discovery wherein phosphodiesters reacted with aromatic aldehydes in DMF (N,N-dimethylformamide) mediated by the organic base DBU (1,8-diazabicyclo [5.4.0]undec-7-ene), affording α-phosphoryloxy ester derivatives 1 (Figure 2). This breakthrough emerged against the backdrop of previous limitations in [1,2]-phospha-Brook rearrangements, where such transformations with carbonyl compounds were predominantly confined to specialized substrates featuring anion stabilizing α-substituents including α-dicarbonyl compounds, perfluoroalkyl aldehydes/ketones, benzophenones, cyclopentadienones, and typically required low-temperature conditions. Notably, this work represents the inaugural documented [1,2]-phospha-Brook rearrangement employing simple aldehydes as coupling partners, achieving remarkable synthetic efficiency (up to 92% yield) across a diverse set of 10 derivatives. These findings established crucial mechanistic and synthetic precedents for subsequent advancements in aldehyde-involved [1,2]-phospha-Brook rearrangement chemistry.
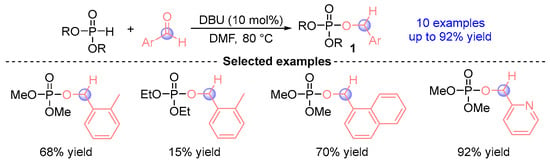
Figure 2.
DBU-catalyzed [1,2]-phospha-Brook rearrangement reported by Kaïm.
In 2015, Manab [] demonstrated that diethyl phosphite under nBuLi (n-butyllithium) catalysis could undergo efficient rearrangement with aldehydes under solvent-free conditions, establishing a robust synthetic protocol. Building upon this foundation, Ghosal’s research group [] subsequently optimized the methodology in 2019, achieving remarkable synthetic efficiency (up to 92% yield) across structurally distinct α-phosphoryloxy ester derivatives 2 (Figure 3A). This innovative approach enabled the streamlined synthesis of two valuable compound classes: (i) allyl phosphate esters exhibiting notable bioactivity and broad synthetic utility, and (ii) electron-rich ketones bearing strategically positioned electron-donating substituents. Mechanistic investigations were systematically conducted to elucidate the reaction pathway (Figure 3B). The proposed catalytic cycle initiates with Li⁺-mediated generation of intermediate Int-1 from diethyl phosphite, followed by a Pudovik addition with carbonyl compounds to form Int-2. This α-hydroxyphosphonate intermediate undergoes sequential transformation through a metastable ternary transition state (Int-3) prior to final reorganization into product 2 via Int-4. Notably, comprehensive reaction monitoring revealed substrate-dependent behavior: α-hydroxyphosphonates (Int-2) were isolable in aldehyde-based reactions but proved elusive with ketone substrates. To decipher this substrate dichotomy, DFT (density functional theory) calculations unveiled critical energy landscape features. Crucially, the formation of Int-3 from benzaldehyde-derived Int-2 exhibited a substantial 10 kcal/mol energy barrier elevation compared to benzophenone analogs, accounting for the slower Int-2→Int-4 conversion kinetics in aldehyde systems. This kinetic disparity rationalizes the experimentally observed isolability of α-hydroxyphosphonates exclusively in aldehyde reactions, while explaining the inherent predisposition of ketone substrates toward facile [1,2]-phospha-Brook rearrangement to directly yield α-phosphoryloxy esters.
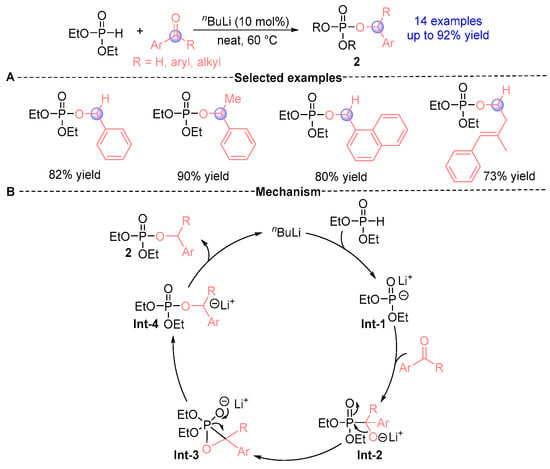
Figure 3.
nBuLi-catalyzed [1,2]-phospha-Brook rearrangement reported by Manab. (A) Selected examples for 2a. (B) Plausible mechanism.
Recently, Wu’s group [] reported a palladium-catalyzed cyclization reaction leveraging [1,2]-phospha-Brook rearrangement to achieve base-modulated chemodivergent synthesis of 2H-isoindole-1-carboxamide 4 and 2H-isoindole-1-carbonitrile 5 derivatives (Figure 4A). This methodology exhibits three hallmark characteristics: (i) dual isocyanide incorporation, (ii) concurrent C-C/C-N bond formation, and (iii) ambient-temperature operability. Mechanistic investigations (Figure 4B) demonstrate the catalytic cycle commences with base-mediated [1,2]-phospha-Brook rearrangement between o-bromobenzaldehyde and diethyl phosphite, yielding phosphoryl intermediate Int-5. Subsequent oxidative addition of the C-Br bond to Pd(0) generates organopalladium species Int-6, which undergoes first isocyanide 3 migratory insertion to afford iminopalladium(II) bromide Int-7. This intermediate progresses through intramolecular cyclization/isomerization to form metallocycle Int-8, followed by secondary isocyanide insertion producing Pd(II) intermediate Int-9. Divergent termination pathways emerge through either: (i) aqueous nucleophilic capture of Int-9 yielding carboxamide 4, or (ii) β-carbon elimination en route to carbonitrile 5, while accomplishing catalytic regeneration of Pd(0) for sustained turnover.
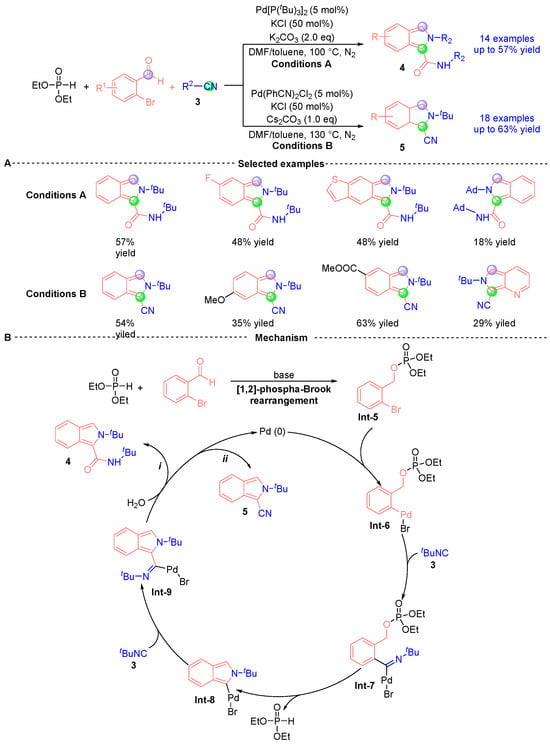
Figure 4.
Pd-catalyzed [1,2]-phospha-Brook rearrangement reported by Wu. (A) Selected examples for 4 and 5. (B) Plausible mechanism.
2.2. Aldehyde Rearrangement with Phosphodiester Precursors
In 2010, Petr et al. [] developed a pioneering trifluoromethylation protocol through [1,2]-phospha-Brook rearrangement (Figure 5), producing α-CF3-phosphoryloxy esters 7 via coupling diethyl trifluoromethylphosphate 6 with aromatic aldehydes under alkoxide base mediation (tBuOK (potassium tert-butoxide)/PhOK (potassium phenoxide)). This work established a catalytic platform for electrophilic trifluoromethylation, demonstrating remarkable functional group compatibility while unlocking innovative trifluoromethylation strategies for diverse electrophilic substrates.
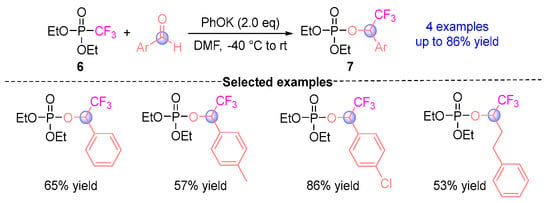
Figure 5.
PhOK-mediated [1,2]-phospha-Brook rearrangement reported by Petr.
2.3. Aldehyde Rearrangement with Secondary Phosphine Oxides
Yang and coworkers [] established in 2022 a Lewis acid-catalyzed paradigm for [1,2]-phospha-Brook rearrangements, employing Lewis acid Cu(OTf)2 to mediate reactions between secondary phosphine oxides (SPOs) and aldehydes (Figure 6). This catalytic system generated 38 pyridine-derived α-phosphoryloxy architectures alongside their dimeric bisphosphoryloxy derivatives 8 with unprecedented 94% efficiency. The methodology demonstrated excellent functional tolerance across diverse phosphine oxide donors and pyridine-based electrophiles, thereby significantly expanding the synthetic versatility of [1,2]-phospha-Brook rearrangement in organophosphorus chemistry.
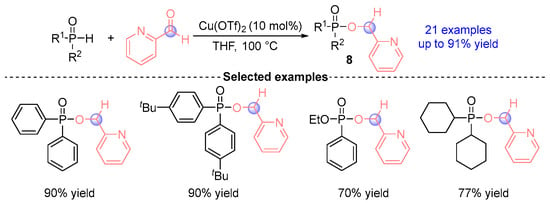
Figure 6.
Cu(OTf)2-catalyzed [1,2]-phospha-Brook rearrangement reported by Yang.
2.4. Aldehyde Rearrangement with SPO-Modified Precursors
In 2022, Terda [] disclosed the [1,2]-phospha-Brook reaction of SPO-modified precursor 9 (alkynyl, bromoalkyl, N-Boc amino, and boron-based phosphonates) catalyzed by Brønsted base P2-tBu with aldehydes, successfully obtaining 19 corresponding functionalized phosphonates 10 (Figure 7). The methodology established a novel molecular editing strategy for post-synthetic functionalization of carbonyl-containing scaffolds, particularly enabling precise functional group installation at ketone or formyl anchoring sites.
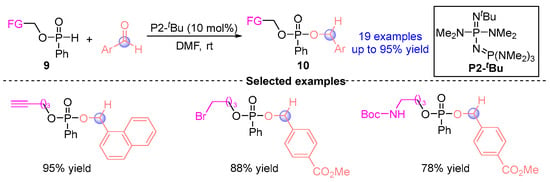
Figure 7.
P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement reported by Terda.
3. Ketone Rearrangement
Comparative analysis reveals distinct activation profiles between carbonyl substrates in [1,2]-phospha-Brook rearrangements. Ketone derivatives exhibit reduced activation barriers relative to aldehyde counterparts, enabling transformations to proceed with enhanced kinetic facility. Notably, electron-deficient ketones bearing strong electron-withdrawing substituents demonstrate superior reaction efficiency through transition state stabilization. Within this mechanistic framework, acyl phosphonates are categorized as privileged substrates due to their reactive phosphoryl centers. These compounds fundamentally undergo [1,2]-phospha-Brook rearrangement to generate stabilized carbanion intermediates that engage in downstream derivatization. Significantly, this substrate class displays a unique propensity for intramolecular rearrangement pathways distinct from classical intermolecular variants.
3.1. Ketone Rearrangement with Phosphodiester Precursors
In 2005, Eymur [] disclosed that under the catalytic effect of KCN, acylphosphonic acid ester 11 could react with aldehydes to obtain cross benzobenzoin product 12 with outstanding yield and regioselectivity (Figure 8A). The proposed catalytic cycle (Figure 8B) initiates with cyanide-mediated nucleophilic activation of compound 11, generating cyanophosphate alkoxide intermediate Int-10. Subsequent [1,2]-phospha-Brook rearrangement produces stabilized acyl anion equivalent Int-11, which engages in carbonyl addition to form tetrahedral adduct Int-12. Phosphorylation of this intermediate yields Int-13, ultimately undergoing protonolysis to release product 12. Relative to conventional acylsilane-mediated protocols, this mechanistically distinct strategy demonstrates enhanced atom economy and accelerated reaction kinetics, establishing an operationally simple platform for catalytic carbanion generation.
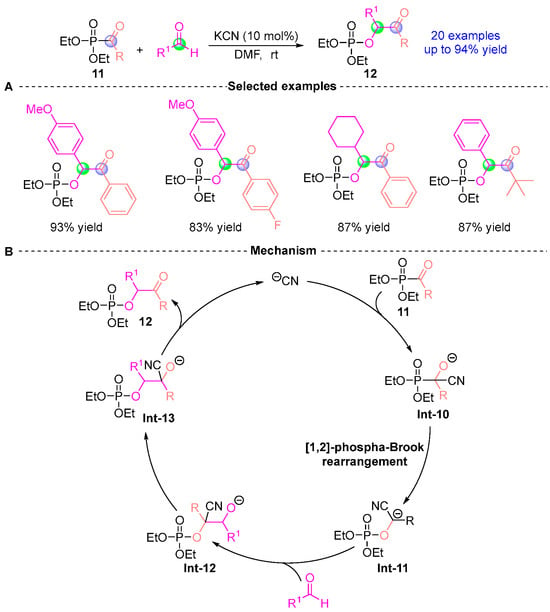
Figure 8.
KCN-catalyzed [1,2]-phospha-Brook rearrangement reported by Eymur. (A) Selected examples for 12. (B) Plausible mechanism.
Building upon this foundation, Johnson [] developed an enhanced catalytic system employing crown ether-modified KCN to mediate reactions between acyl phosphonates and aldehydes, producing α-phosphoryloxy esters 14 in up to 93% yields (Figure 9A). Remarkably, these products underwent chemoselective P–O bond cleavage in aqueous amine solutions to afford chiral benzoic acid derivatives, demonstrating exceptional configurational stability. Subsequently, Saglam’s team [] engineered a cyanide-catalyzed protocol in 2007 for a one-pot synthesis of cyano-functionalized α-phosphoryloxy esters 16 (Figure 9B). These compounds served as versatile synthons for constructing diverse bioactive scaffolds, including privileged structures in medicinal chemistry.
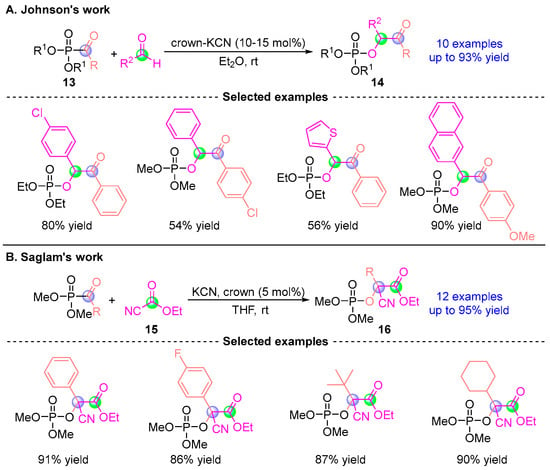
Figure 9.
KCN/crown-catalyzed [1,2]-phospha-Brook rearrangement reported by Johnson and Saglam. (A) Johnson’s crown-KCN catalytic method. (B) Saglam’s crown/KCN catalytic method.
3.2. Ketone Rearrangement with Phosphodiesters
Consecutive studies by Serebryakova [] in 2009 and Makhaeva [] in 2010 established catalytic protocols for synthesizing α-phosphoryloxy trifluoromethyl esters 18 via [1,2]-phospha-Brook rearrangements (Figure 10). Employing Et3N mediated reactions between trifluoromethyl ketones 17 and phosphodiesters under thermal conditions mechanistic investigations revealed initial Pudovik addition yielding α-hydroxyphosphonates, followed by thermal activation driving rearrangement to thermodynamically favored α-phosphoryloxy products. The pharmacological evaluation demonstrated compound 18’s pronounced selectivity as AChE (acetylcholinesterase)and BChE (butyrylcholinesterase) inhibitor with phenyl-substituted derivatives exhibiting reversible CaE (carboxylesterases) inhibition. Notably, electronic activation parameters confirmed electron-withdrawing α-substituents as critical structural determinants.
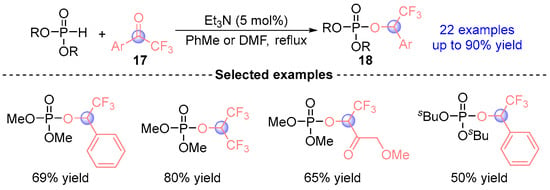
Figure 10.
Et3N-catalyzed [1,2]-phospha-Brook rearrangement reported by Serebryakova and Makhaeva.
Zhao et al. [] pioneered the first chiral auxiliary-mediated [1,2]-phospha-Brook rearrangement system, employing menthol-derived H-phosphonate (Menthyl-PHO) 19 with ketone substrates (Figure 11A). While demonstrating broad compatibility with aryl aldehydes, the methodology exhibits notable substrate scope constraints for ketones, yielding merely 10 derivatives 20. Mechanistic analysis revealed complete configuration retention at the phosphorus center (>99:1 drp) but limited stereochemical induction at the α-carbon (highest reach to 61:46 drc), suggesting partial erosion of chiral information transfer during the rearrangement process. This seminal work delineates a groundbreaking intermolecular mechanism for [1,2]-Phospha-Brook rearrangement, representing a paradigm shift from classical intramolecular pathways (Figure 11B). The catalytic cycle commences with base-induced deprotonation of 20’ to generate oxyanion Int-14, which orchestrates nucleophilic displacement at phosphorus in a second substrate molecule, forming dimeric intermediate Int-15. Subsequent cyclization via O→P transesterification produces chair-configured Int-16, where stereochemical inversion occurs through Berry pseudorotation (BPR) to axial Int-17. Concerted bond reorganization cleaves Cα-P2 yield Int-18 followed by Cα-P1 dissociation Int-19, ultimately delivering product 20 via protonation. Crucially, phosphorus configuration remains preserved despite complete stereochemical scrambling at Cα, demonstrating unprecedented mechanistic divergence from conventional systems.
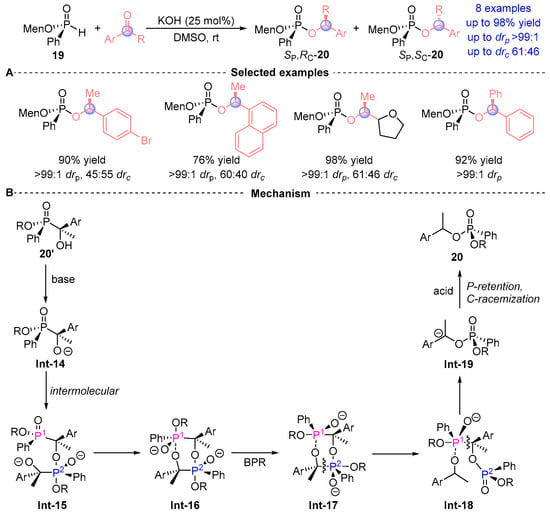
Figure 11.
KOH-catalyzed [1,2]-phospha-Brook rearrangement reported by Zhao. (A) Selected examples for 20. (B) Plausible mechanism.
4. Ketoester Rearrangement
Ketoesters constitute privileged synthons in contemporary organic synthesis, finding extensive application as pivotal intermediates for pharmaceutical development and complex molecule construction. Owing to their superior reactivity compared to simple ketones, these compounds exhibit exceptional utility in [1,2]-phospha-Brook rearrangements.
4.1. Ketoester Rearrangement with Phosphodiesters
Terada et al. [] demonstrated in 2015 a Brønsted base-catalyzed annulation strategy utilizing phosphazene base P2-tBu to synthesize functionalized phenanthrenes 22 through sequential [1,2]-phospha-Brook/[3,3]-sigmatropic rearrangements (Figure 12A). This transformation proceeds through: (i) base-mediated Pudovik addition between phosphodiester and ketoester yield Int-20, (ii) [1,2]-phospha-Brook rearrangement generating stabilized carbanion intermediate Int-21, (iii) intramolecular cyclization forming Int-22 with chair-like transition state, and (iv) [3,3]-sigmatropic reorganization delivering phenanthrene derivatives 22 (Figure 12B).
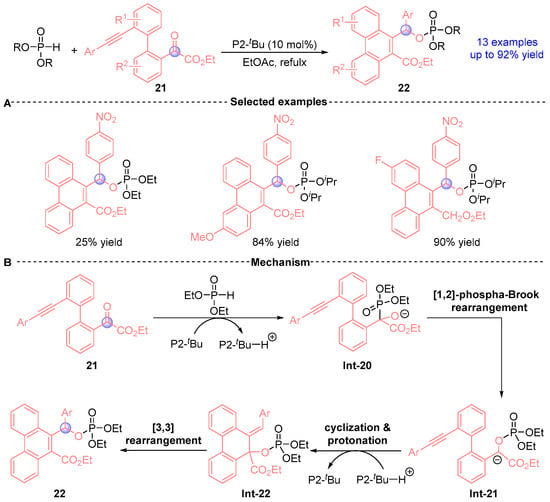
Figure 12.
P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada. (A) Selected examples for 22. (B) Plausible mechanism.
In 2023, Zi’s group [] demonstrates a catalytic O-phosphination strategy for α-dicarbonyl derivatives 24 through sequential Kukhtin–Ramirez adduct formation and P(NMe₂)₃-mediated ambiphilic activation (Figure 13). This versatile platform accommodates diverse P(O)–H nucleophiles (diarylphosphine oxides, arylphosphinates, phosphinates) with high yields across >30 substrates, achieving gram-scale synthesis. The operational simplicity, paired with water tolerance and air stability, establishes this methodology as a robust platform for late-stage phosphorylation of bioactive carbonyls.
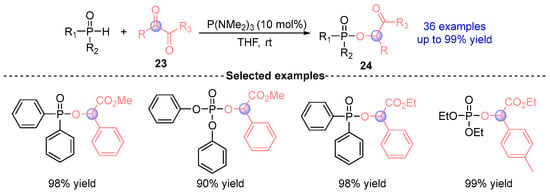
Figure 13.
P(NMe2)3-catalyzed [1,2]-phospha-Brook rearrangement reported by Zi.
Nakamura et al. [] achieved a milestone by developing the first stereodivergent synthesis of α-phosphoryloxy esters 26 through quinine or quinidine catalyzed [1,2]-phospha-Brook rearrangements in 2011. Employing a chiral Lewis base catalysis system (quinine/Na2CO3) with phosphodiesters and α-ketoesters 25, this protocol delivered 26 derivatives up to 99% yield with up to 92% ee (Figure 14). Stereochemical control arises from the precise manipulation of alkaloid substituents, enabling predictable configuration inversion at Cα. Mechanistic studies revealed that enantioselectivity originates from enantiotopic face discrimination during enol protonation.
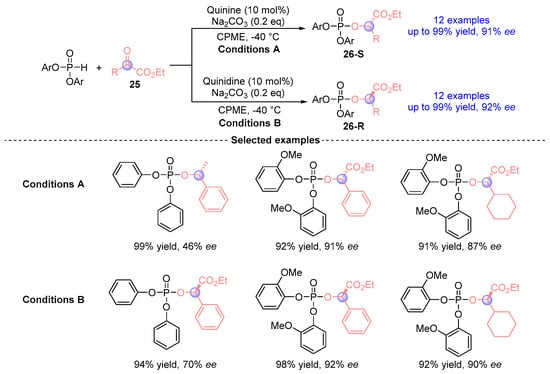
Figure 14.
Quinine or quinidine-catalyzed [1,2]-phospha-Brook rearrangement reported by Nakamur.
4.2. Ketoester Rearrangement with Three Components
In 2016, Xu [] disclosed a catalyst-free stereodivergent synthesis through LiHMDS (lithium hexamethyldisilazide)) or NaHMDS (sodium hexamethyldisilazide) mediated three-component cascades of α-ketoesters 27, phosphites, and imines (28–29). This transformation orchestrates: (i) [1,2]-phospha-Brook rearrangement, (ii) stereoselective Mannich addition (up to 20:1 dr), and (iii) aza-Darzens cyclization, delivering syn-α-hydroxy-β-amino acids 30 and trans-aziridines 31 through switchable pathways. Mechanistic analysis revealed N-protecting group-dependent stereocontrol: sulfonyl imines direct (Z)-enol Int-23 to open Int-24, which then evolves into Int-25, followed by 3-exo cyclization to yield trans-aziridine 31. In contrast, bulky N-aryl groups promote the formation of (E)-enol Int-26 via retro-Mannich/tautomerization cascades, ultimately leading to product 30 (Figure 15).
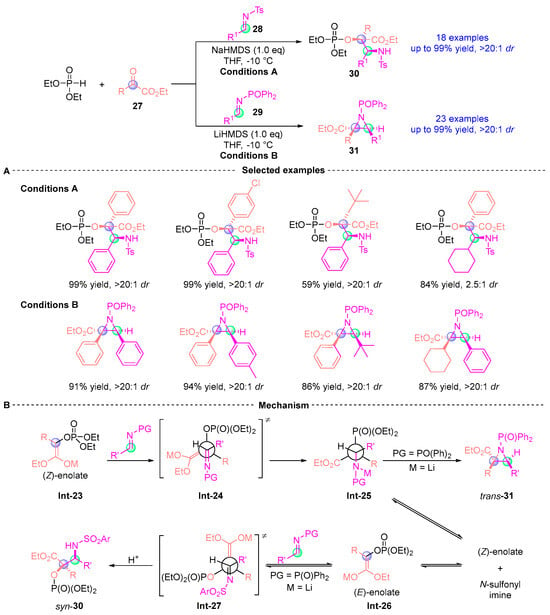
Figure 15.
Li(Na)HMDS-mediated [1,2]-phospha-Brook rearrangement reported by Xu. (A) Selected examples for 30 and 31. (B) Plausible mechanism.
Building upon their earlier work, Xu [] reported in 2017 an efficient and highly selective method for the asymmetric synthesis of chiral cyclopropane compounds via [1,2]-phospha-Brook rearrangement (Figure 16). This three-component coupling reaction employs dimethyl phosphite, ketoester 32, and α,β-unsaturated ketones 33 as starting materials. Under LiHMDS catalysis, the reaction proceeds through a stereoselective pathway to yield highly functionalized cyclopropane compound 34 with excellent diastereoselectivity (dr > 20:1) and high yield (up to 90%).
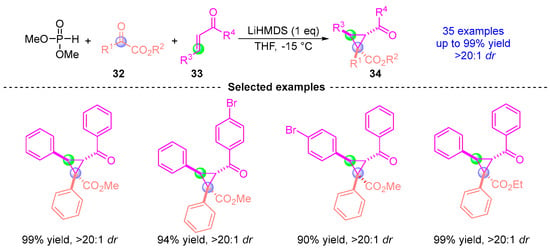
Figure 16.
LiHMDS-promoted [1,2]-phospha-Brook rearrangement reported by Xu.
In 2016, Terada’s group [] developed a three-component asymmetric coupling reaction involving α-ketoester 35, diethyl phosphite, and either imine or nitroalkene 36 (Figure 17). This catalytic system, based on the same principle, proceeds through a cascade of Pudovik addition and [1,2]-phospha-Brook rearrangement under the catalysis of NaOtBu/LiHMDS, generating an enol-phosphate intermediate. Subsequently, imines or nitroalkenes undergo stereoselective nucleophilic addition with this intermediate to afford the final products. This efficient methodology yields a series of α-amino derivatives 37 with excellent yield (up to 95%) and high stereoselectivity (up to 86:14 dr).
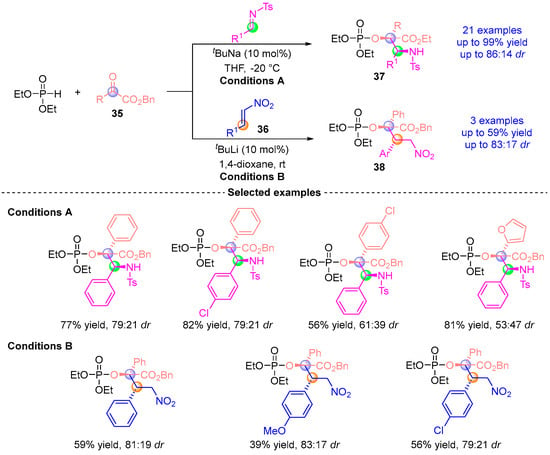
Figure 17.
tBuNa(Li)-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
Inspired by the previous work, Terada [] reported the synthesis of a series of α-phosphonyloxy ester compounds (43, 44) via a three-component coupling reaction involving silyl phosphites 39, α-ketoesters 40, and electron-deficient fluorinated aromatic hydrocarbons (41, 42), catalyzed by P2-tBu (Figure 18A). This methodology exhibits excellent substrate scope and regioselectivity, accommodating a wide range of aliphatic and aromatic α-ketoesters without the need for expensive transition metal catalysts. The method enables the introduction of highly electron-deficient aromatic groups, such as polyfluoroaromatic moieties, offering new insights into the development of aromatic nucleophilic reactions. Additionally, the authors proposed a plausible mechanism for the reaction (Figure 18B). Under base or fluoride ion activation, the silyl phosphite 39, α-ketoester 40, and fluorinated aromatic hydrocarbon 41 undergo sequential Pudovik addition and [1,2]-phospha-Brook rearrangement to form intermediate Int-29, which subsequently proceeds through an SNAr reaction to yield the final product 43.
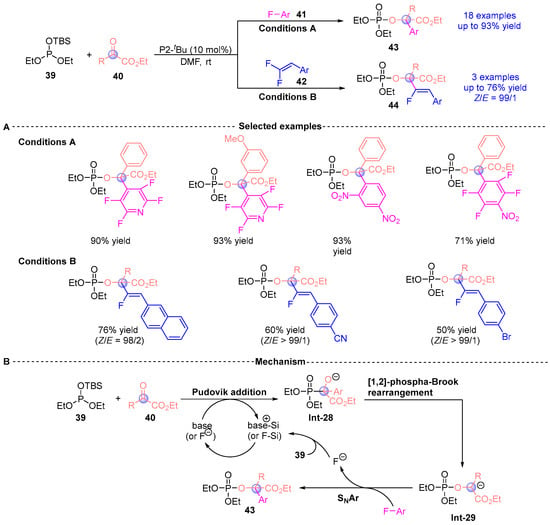
Figure 18.
P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada. (A) Selected examples for 43 and 44. (B) Plausible mechanism.
In 2016, Johnson et al. [] reported a three-component asymmetric coupling reaction involving dimethyl phosphite, benzylidenepyruvate 45, and aldehydes, catalyzed by chiral iminophosphorane (Figure 19). This catalytic system orchestrates a cascade of Pudovik addition and [1,2]-phospha-Brook rearrangement between dimethyl phosphite and benzylidenepyruvate 45 to form enol ester intermediates. Subsequently, these intermediates undergo stereoselective nucleophilic addition with aldehydes, delivering chiral central compound 46 with excellent chemical yield (up to 87%) and high stereoselectivity (up to 97% ee, dr > 20:1).
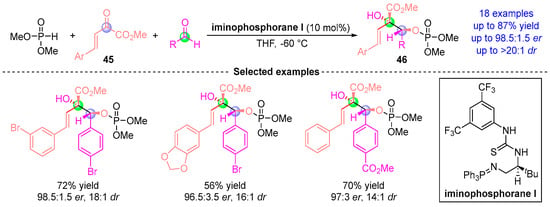
Figure 19.
Iminophosphorane I-catalyzed [1,2]-phospha-Brook rearrangement reported by Johnson.
In 2021, Sigh [] developed a one-pot catalytic asymmetric synthesis of phosphonate-derived coumarins via a LiHMDS-mediated [1,2]-phospha-Brook/Michael/cyclization cascade (Figure 20). This strategy involves: (i) stereoselective [1,2]-phospha-Brook rearrangement of α-ketoester 47 to generate an enol-phosphate intermediate, (ii) aza-Michael addition with o-quinone methide 48, and (iii) rate-determining intramolecular lactonization, affording coumarins 49 with exceptional diastereoselectivity (dr > 19:1) and high yields (up to 95%). Notably, this work represents the first catalytic asymmetric synthesis of phosphate-derived coumarins through the direct coupling of phosphoryloxyacetic acid derivatives with ortho-quinone methides.
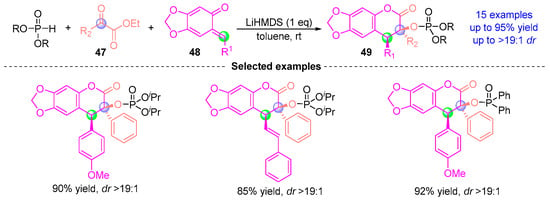
Figure 20.
LiHMDS-mediated [1,2]-phospha-Brook rearrangement reported by Sigh.
In 2023, Deng [] developed a Pd-catalyzed allylic alkylation reaction that synthesizes a series of phosphoketoesters 52 via a three-component tandem reaction involving α-ketoester 50, β-ketoacid 51, and dialkyl phosphite (Figure 21A). This protocol is operationally simple and proceeds under mild conditions, exhibiting excellent chemical and regioselectivity with a broad substrate scope. A total of 50 phosphoketoesters with diverse potential biological activities were successfully synthesized with yields up to 99%. Additionally, the authors conducted preliminary studies on the asymmetric synthesis of this reaction and found that using chiral bisphosphine ligands resulted in the target product with a yield of 71% and an ee value of 30%, laying the foundation for future asymmetric catalysis. The proposed mechanism (Figure 21B) involves the following sequence: Diethyl phosphite is first protonated by LiHMDS to form an intermediate, which undergoes Pudovik addition with α-keto acid 50 to generate Int-30. Subsequently, Int-30 undergoes a [1,2]-Phospha-Brook rearrangement to reverse the carbonyl polarity and generate the nucleophilic intermediate Int-31. The in situ generated Pd(0)-ligand complex then participates in oxidative addition with Int-31 to form the π-allylpalladium intermediate Int-32. Finally, Int-32 delivers a nucleophilic attack on Int-31, yielding the target product 52 while regenerating the palladium catalyst to perpetuate the catalytic cycle.
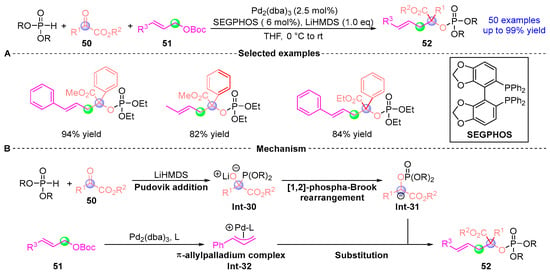
Figure 21.
Pd-catalyzed [1,2]-phospha-Brook rearrangement reported by Deng. (A) Selected examples for 52. (B) Plausible mechanism.
Very recently, the Yamaguchi group [] developed a deoxygenative functionalization strategy for aromatic dicarbonyl compounds 53 through sequential treatment with DBU, TMSOTf (trimethylsilyl triflate), diphenylphosphine oxide, and nucleophilic reagents 54 to afford functionalized products 55 (Figure 22). This methodology establishes a sequential [1,2]-phospha-Brook rearrangement/benzylic substitution protocol for converting aromatic aldehydes into deoxygenative products. Notably, the reaction proceeds efficiently in the absence of TMSOTf when employing highly nucleophilic reagents. Furthermore, Yamaguchi has extended this approach to enable transformations of diaryl ketones into heterofunctionalized and carbon-functionalized diaryl methanes [].
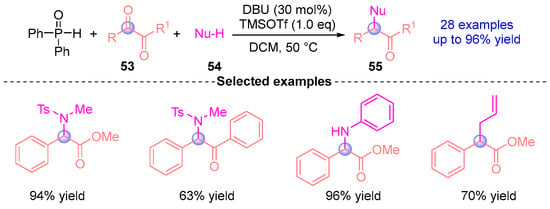
Figure 22.
DBU-catalyzed [1,2]-phospha-Brook rearrangement reported by Yamaguchi.
5. Ketoamide Rearrangement
Ketoamide derivatives demonstrate versatile reactivity profiles owing to their unique combination of nucleophilic and electrophilic sites, complemented by the structural diversity of amide substituents. This distinctive structural characteristic establishes them as privileged substrates for diverse applications, including asymmetric organocatalysis, multifunctional molecular construction, pharmacophore development, and natural product derivatization. Investigating their reactivity patterns in [1,2]-phospha-Brook rearrangements, particularly with respect to stereoelectronic effects and chemodivergent pathways, carries significant implications for the advancement of phosphorus-containing molecular architectures.
5.1. Ketoamide Rearrangement with Phosphodiesters
In 2011, Wang’s group [] achieved the first catalytic asymmetric reaction between diphenyl phosphite and indigo derivatives using a quinine-based catalyst, affording α-phosphoryloxy ester compounds 57 containing an indigo core (Figure 23). This pioneering methodology demonstrated excellent yield and good stereoselectivity in constructing chiral phosphorus-containing heterocycles.
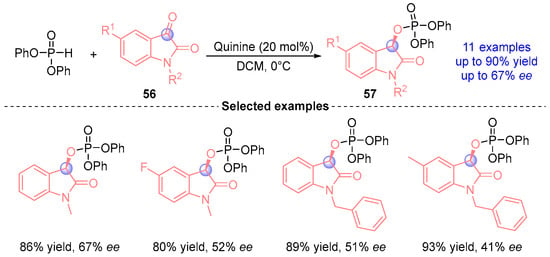
Figure 23.
Quinine-catalyzed [1,2]-phospha-Brook rearrangement reported by Wang.
In 2014, Terada’s group [] developed a catalytic cyclization strategy employing alkynyl α-ketoanilines through [1,2]-phospha-Brook rearrangement, demonstrating broad substrate scope and high efficiency (Figure 24A). The methodology utilizes P2-tBu as a catalytic base, where ketoamide 58 undergoes a cascade process through Pudovik addition followed by [1,2]-phospha-Brook rearrangement to generate intermediate Int-34. This intermediate subsequently undergoes intramolecular alkyne addition to afford product 59, as outlined in Figure 24B.
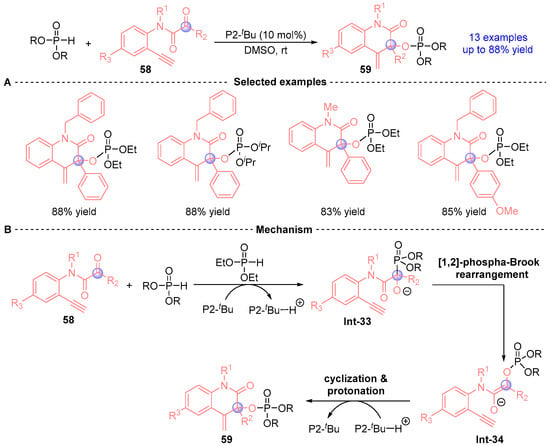
Figure 24.
P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada. (A) Selected examples for 59. (B) Plausible mechanism.
In 2020, Wu [] reported a DBU-catalyzed [1,2]-phospha-Brook rearrangement between α-ketoamides (60, 61) and phosphites under solvent-free conditions (Figure 25). This rapid protocol operates efficiently at room temperature with remarkably short reaction times (2–5 min), delivering 46 structurally diverse analogs (62, 63) in excellent yields (up to 96%). The methodology exhibits broad substrate compatibility with various phosphite derivatives and α-ketoamide substrates. Notably, the process demonstrates scalability to molar quantities while maintaining high efficiency (>91% yield within 5 min).
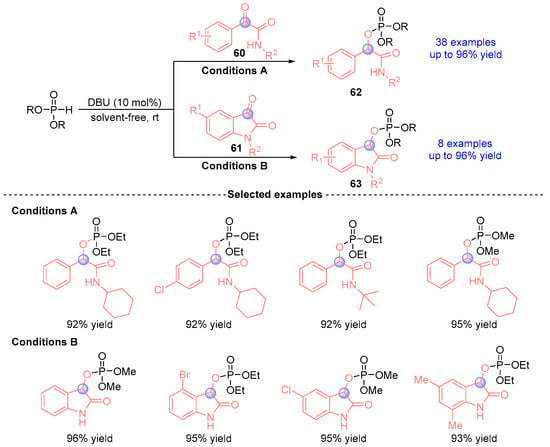
Figure 25.
DBU-catalyzed [1,2]-phospha-Brook rearrangement reported by Wu.
5.2. Ketoamide Rearrangement with Three Components
In 2016, Xu’s group [] developed an efficient asymmetric synthesis strategy for chiral epoxy ternary compounds through a [1,2]-phospha-Brook rearrangement-mediated three-component reaction (Figure 26). This catalytic system employed N-tert-butylsulfonyl α-ketoimine ester 64, dimethyl phosphite, and aromatic aldehydes as substrates under LiHMDS mediation. The reaction demonstrated remarkable stereochemical control, delivering 17 structurally diverse chiral epoxy products 65 in yields up to 93% with excellent diastereoselectivity (>20:1 dr). This methodology established a practical approach for constructing highly functionalized epoxy architectures through precise manipulation of the [1,2]-phospha-Brook rearrangement pathway.
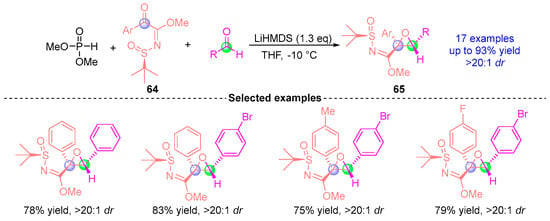
Figure 26.
LiHMDS-mediated [1,2]-phospha-Brook rearrangement reported by Xu.
Subsequently, Xu’s group [] in 2017 extended their methodology to achieve an asymmetric [1,2]-phospha-Brook rearrangement involving indigo carmine-derived substrates, α,β-unsaturated ketones, and dimethyl phosphite (Figure 27). Employing KHMDS (potassium hexamethyldisilazide) as the catalytic base, this three-component reaction enabled the stereocontrolled synthesis of highly functionalized cyclopropane derivative 66, which was obtained in 99% yield with excellent diastereoselectivity (>20:1 dr). The protocol demonstrated broad substrate compatibility while retaining mechanistic parallels to the group’s 2016 system, with diastereoselectivity primarily governed by substrate electronic effects and precise temperature modulation.
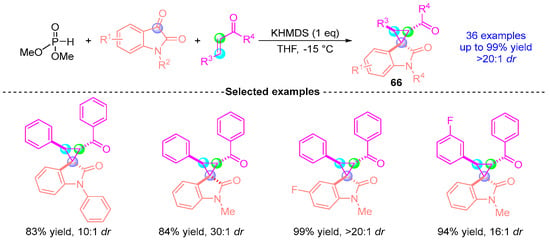
Figure 27.
KHMDS-promoted [1,2]-phospha-Brook rearrangement reported by Xu.
In 2016, Terada and coworkers [] developed a streamlined protocol for synthesizing 3-arylindole derivatives from indigo carmine-derived substrates (Figure 28). The process begins with an iPr2NEt-catalyzed [1,2]-phospha-Brook rearrangement, generating a C3-phosphorylated indole oxide intermediate. Subsequent palladium-catalyzed cross-coupling with arylboron reagent 67 enabled one-pot construction of 3-aryloxyindole derivatives 68. Mechanistically, the transformation proceeds through α-phosphoryloxy ester intermediates, followed by sequential phosphonyl group elimination and aryl coupling to assemble unique molecular architectures, establishing a novel platform for complex heterocycle synthesis.
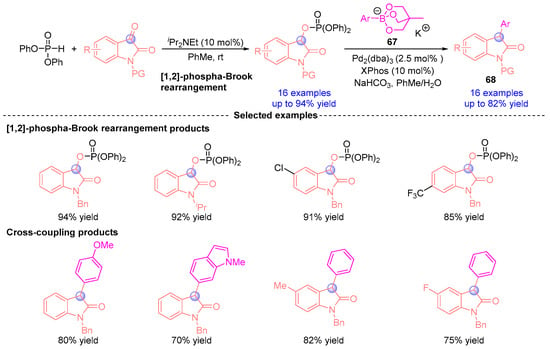
Figure 28.
iPr2NEt-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
In 2015, Ooi [] demonstrated a stereoselective three-component assembly of phosphodiesters, indigo-derived substrates, and aromatic aldehydes (Figure 29A). This catalytic asymmetric process delivered structurally diverse indigo-containing chiral compounds 69 with exceptional efficiency (up to 99% yield, 99:1 er, and >20:1 dr). Mechanistically, the transformation initiates with a Pudovik addition/[1,2]-phospha-Brook rearrangement cascade between indigo derivatives and phosphodiesters, generating chiral α-phosphoryloxy intermediate Int-35. Subsequent deprotonation forms a carbanion species that undergoes phosphoimine-catalyzed nucleophilic addition with aromatic aldehydes to yield intermediate Int-36. Phosphate group migration then produces Int-37, ultimately furnishing the target indigo-embedded chiral architectures through sequential bond reorganization (Figure 29B). This work established a powerful strategy for constructing complex indigo-based chiral systems via orchestrated phosphorus-mediated transformations.
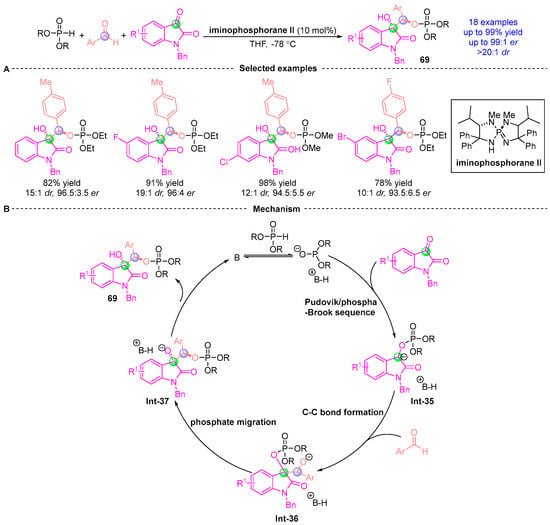
Figure 29.
Iminophosphorane II-catalyzed [1,2]-phospha-Brook rearrangement reported by Oil. (A) Selected examples for 69. (B) Plausible mechanism.
Based on previous work experience, Johnson et al. [] 2017 advanced the catalytic asymmetric coupling of indigo carmine-derived substrates with nitroalkenes and phosphodiesters (Figure 30). Employing a chiral quinine-derived thiourea catalyst with LDA (lithium diisopropylamide) in THF at −75 °C, this system generated 18 β-nitroindigo derivatives 70 through stereodivergent synthesis. The protocol achieved 89% yield with 83.5:16.5 dr, demonstrating precise stereochemical control predominantly governed by the thiourea catalyst’s chiral microenvironment. This work significantly expanded the synthetic utility of indigo frameworks in asymmetric transformations while maintaining operational simplicity under cryogenic conditions.
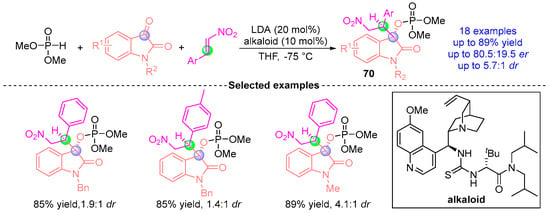
Figure 30.
LDA/alkaloid-catalyzed [1,2]-phospha-Brook rearrangement reported by Johnson.
In 2023, Feng [] achieved a stereodivergent synthesis of 3,3-disubstituted indolone derivatives 72 bearing two chiral centers through a chiral diazo/scandium-catalyzed cascade process combining [1,2]-phospha-Brook rearrangement and phospha-Michael addition (Figure 31A). This operationally simple protocol demonstrated remarkable stereocontrol (up to 99% yield, 99% ee, and 95:5 dr) under mild conditions with broad substrate generality, accommodating diverse indigo carmine derivatives, and γ-carbonyl butenoates bearing aryl, alkyl, or ester substituents. Mechanistic studies revealed the critical importance of sequential substrate addition and chiral Lewis acid mediation. The initial combination of hypophosphite and indigo substrates generates the α-phosphoryloxy intermediate through Pudovik addition/[1,2]-phospha-Brook rearrangement, which subsequently undergoes catalyst-controlled phospha-Michael addition with enone esters. Concurrent mixing of all three substrates proved ineffective for product formation. The proposed mechanism (Figure 31B) involves chiral L-PiEt2Me/Sc complex activation of the indigo substrate, followed by DMAP (4-dimethylaminopyridine) mediated generation of enol ester intermediate Int-40 via sequential Pudovik addition and phospha-Brook rearrangement with diethyl phosphite. Stereoselective Re-face attack of Int-40, directed by the diazo/scandium catalyst, forms Michael adduct Int-41. Final protonation yields the doubly stereogenic product 72 with defined (S,R)-configuration.
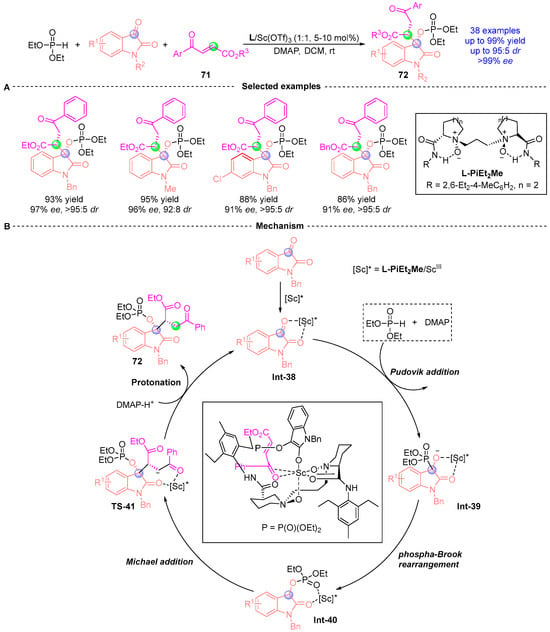
Figure 31.
L-PiEt2Me/Sc-catalyzed [1,2]-phospha-Brook rearrangement reported by Feng. (A) Selected examples for 72. (B) Plausible mechanism.
Very recently, Sigh and coworkers [] disclosed a catalytic asymmetric cascade sequence combining Pudovik addition, [1,2]-phospha-Brook cyclization, and Michael reaction using a bifunctional aminocatalytic system (Figure 32). This three-component strategy effectively converts indigo carmine derivatives, dialkyl phosphites, and α,β-unsaturated ketones 73 into indole derivatives 74 bearing vicinal stereocenters. The catalytic system operates through a dual activation mechanism: imine-mediated enol activation synergizes with Brønsted base-promoted carbanion stabilization. Remarkably, this additive-free protocol demonstrates broad functional group tolerance under mild conditions while achieving excellent chemoselectivity and stereocontrol (up to 98:2 er, 99:1 dr). The method’s synthetic potential is further highlighted by its scalability and versatile downstream transformations of the phospho-indole products.
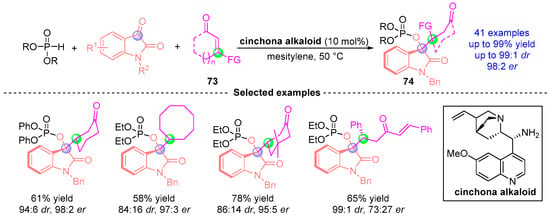
Figure 32.
Cinchona alkaloid-catalyzed [1,2]-phospha-Brook rearrangement reported by Sigh.
6. Ketene Rearrangement
The [1,2]-phospha-Brook rearrangement enables the efficient transformation of enones into enol-phosphate-versatile synthons that play pivotal roles in drug discovery, functional material fabrication, and biomolecular engineering.
In 2012, Xiao [] developed a TMG (tetramethylguanidine)-catalyzed [1,2]-phospha-Brook rearrangement protocol for synthesizing enol phosphates 76 from phosphodiesters and carbonyl esters 75 (Figure 33). This practical method delivered 17 structurally diverse enol-phosphate derivatives with up to 85% yield, highlighting operational simplicity and compatibility with commercially available hypophosphite reagents.
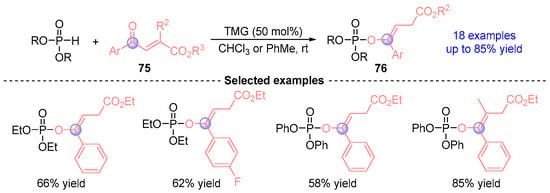
Figure 33.
TMG-catalyzed [1,2]-phospha-Brook rearrangement reported by Xiao.
In 2017, Liu [] established a catalyst-dependent divergent synthesis using α,β-unsaturated trifluoromethyl ketones 77, and diethyl phosphite (Figure 34A). DABCO (1,4-diazabicyclo[2.2.2]octane) catalysis induced selective Pudovik addition under mild conditions to furnish α-hydroxyphosphonate Int-42, while DBU promoted sequential Pudovik addition/[1,2]-phospha-Brook rearrangement, ultimately delivering α-phosphoryloxy ester 76 via protonation of intermediate Int-43. The electron-deficient trifluoromethyl group critically stabilizes transition state Int-44 during the rearrangement phase (Figure 34B), demonstrating strategic exploitation of electronic effects to control reaction trajectories.
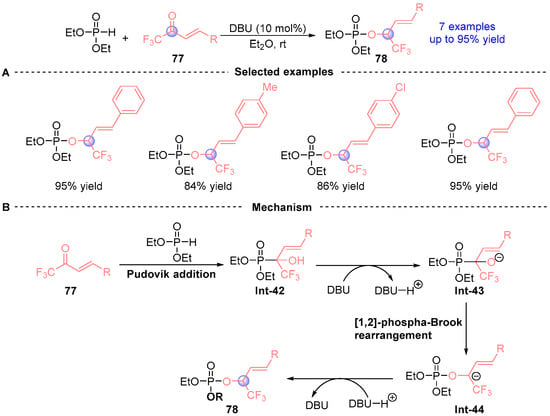
Figure 34.
DBU-catalyzed [1,2]-phospha-Brook rearrangement reported by Liu. (A) Selected examples for 78. (B) Plausible mechanism.
In 2024, Kanai [] established a Cs2CO3-promoted 1,2-phospha-Brook rearrangement based on tyrosine-derived spiro-lactones 79 and phosphite diesters to give a wide range of peptide substrates containing diverse nucleophilic amino acid residues, including serine and threonine (Figure 35). Particularly noteworthy is its successful implementation across various secondary structures and complex peptide sequences, addressing longstanding challenges in selective post-translational modification chemistry.
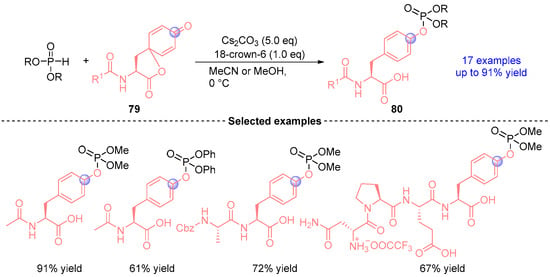
Figure 35.
Cs2CO3-mediated [1,2]-phospha-Brook rearrangement reported by Kanai.
7. Alkyne Ketone Rearrangement
The [1,2]-phospha-Brook rearrangement of ynones has emerged as a powerful strategy for generating allenyl intermediates that facilitate stereocontrolled intramolecular cyclization to access five-membered heterocyclic architectures. Notably, Feng and Wang recently extended this paradigm to asymmetric synthesis, achieving the first catalytic construction of axially chiral allenes through precise control of rearrangement dynamics, thereby opening new avenues in chiral molecule synthesis.
In 2016, Terada's team [] developed a catalytic cascade strategy for synthesizing 2-amino-3-phosphoryloxyfuran derivatives through sequential [1,2]-phospha-Brook rearrangement and gold-mediated cyclization (Figure 36A). The process initiates with α-alkynyl ketamide 81 undergoing P1-tBu-catalyzed Pudovik addition/[1,2]-phospho-Brook rearrangement with phosphodiesters to form bicyclic intermediate Int-46. Subsequent protonation generates Int-47, which undergoes gold-catalyzed stereoselective intramolecular cyclization to furnish phosphorylated furan 82 (Figure 36B). Notably, thermal activation of 82 in the absence of gold induces intramolecular Diels-Alder cyclization, selectively producing six-membered diene architectures.
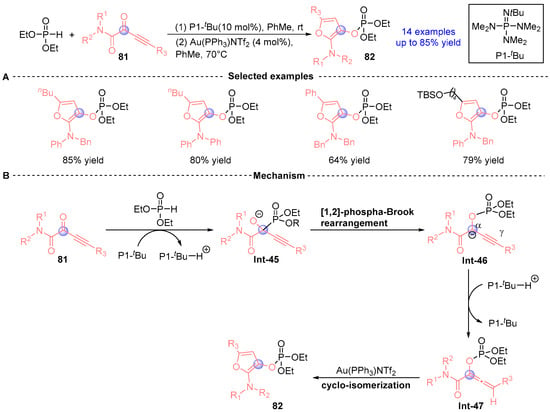
Figure 36.
P1-tBu/Au-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada. (A) Selected examples for 82. (B) Plausible mechanism.
Based on previous work, Terada [] advanced their [1,2]-phospha-Brook methodology by developing a transition metal-free protocol for indole synthesis through P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement (Figure 37). This operationally straightforward method efficiently constructed indole-pyrimidine derivatives 84 with good yields, demonstrating broad functional group compatibility. Furthermore, the system exhibited remarkable versatility nickel mediated arylative dephosphorylation of the phosphorylated intermediates with Grignard reagents enabled selective C-arylation, establishing a dual functionalization strategy for modular indole architecture construction. This cascade approach significantly expands the synthetic toolbox for heterocyclic compound diversification without requiring precious metal catalysts.
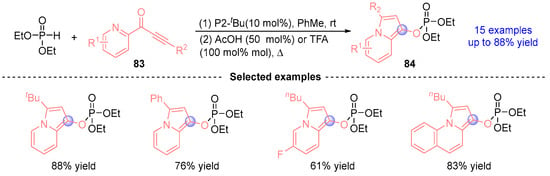
Figure 37.
P2-tBu/acid-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
In 2022, Feng [] achieved a breakthrough in asymmetric catalysis using their proprietary N,N′-dioxide/Sc(III) complex to mediate enantioselective Pudovik addition/[1,2]-phospha-Brook rearrangement cascades between α-alkyl-acyl ketone amides and diarylphosphine oxides (Figure 38A). This catalytic system delivered 36 axially chiral alkenes 85 with exceptional efficiency (up to 97% yield) and stereocontrol (up to 96% ee) while demonstrating broad substrate scope and functional group tolerance. The synthetic utility was further highlighted by converting products into complex bridged polycyclic architectures through dearomative cyclization pathways. Mechanistic studies combining experimental and computational methods (Figure 38B) revealed a three-stage process: (i) Scandium-activated ketone amide undergoes Pudovik addition to form tetrahedral intermediate Int-49; (ii) Configuration-inverting [1,2]-phospha-Brook rearrangement generates phospho-enolate Int-50; and (iii) Water-mediated Int-51 protonation completes the catalytic cycle. The work elucidated critical ligand field effects and counterion/water participation in controlling regio- and stereoselectivity, establishing fundamental principles for designing asymmetric [1,2]-phospha-Brook rearrangements.
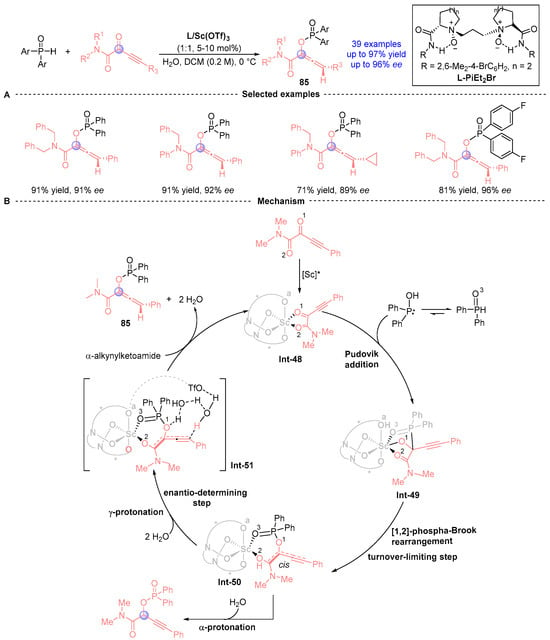
Figure 38.
L-PiEt2Me/Sc-catalyzed [1,2]-phospha-Brook rearrangement reported by Feng. (A) Selected examples for 85. (B) Plausible mechanism.
Wang [] established a biomimetic catalytic cascade employing peptide-mimic phosphonium salt as the catalyst for the asymmetric synthesis of axially chiral alkenylphosphines (Figure 39). This transition metal-free platform synergistically combines Pudovik addition and [1,2]-phospha-Brook rearrangement of alkynyl ketones 86 with phosphine oxides, delivering 41 stereodefined phosphoalkene derivatives 87 with exceptional efficiency and enantiocontrol. The methodology’s bioinspired design enables precise stereochemical regulation while maintaining broad functional group compatibility, representing a significant advance in organocatalytic phosphorus chemistry.
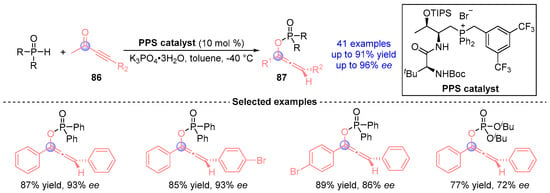
Figure 39.
PPS-catalyzed [1,2]-phospha-Brook rearrangement reported by Wang.
8. Multicomponent Rearrangement
While mechanistic consensus exists regarding the α-hydroxyphosphonate intermediate pathway in [1,2]-phospha-Brook rearrangement, experimental verification using prepared α-hydroxyphosphonates remains limited. This knowledge gap arises from the reaction’s inherent efficiency and the process readily initiates through in situ generation of reactive intermediates from phosphodiesters and carbon nucleophiles, circumventing the requirement for discrete intermediate isolation. This inherent reactivity profile not only demonstrates the transformation’s operational simplicity but also exemplifies its atom-economical nature, underscoring its value in contemporary synthetic methodologies.
Hammerschmidt’s team pioneered stereochemical studies of [1,2]-phospha-Brook rearrangements through sequential investigations. Their 2015 work [] demonstrated configuration retention in trichlorophosphoryl scaffold 88 under triethylamine mediation, cleanly generating dichloroallyl phosphate 89 while preserving stereochemical integrity (Figure 40). This established a viable route to optically pure enol phosphates through stereospecific transformations. Building on this foundation, then they [] developed a chiral resolution strategy to obtain enantiopure α-hydroxyphosphate 90. Sequential methylation with methyl thiofluorosulfate and P1-tBu-mediated rearrangement achieved complete stereochemical transfer, delivering enol phosphate 91 as a single stereoisomer. These systematic investigations fundamentally advanced the understanding of stereochemical fidelity in phospho-Brook processes.
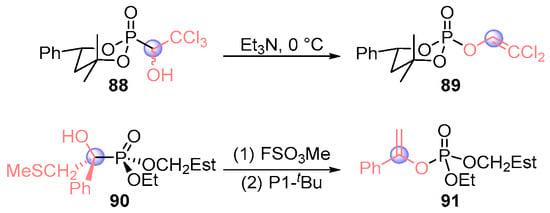
Figure 40.
Et3N or P1-tBu-mediated [1,2]-phospha-Brook rearrangement reported by Hammerschmidt.
In 2012, Johnson et al. [] developed an asymmetric [1,2]-phospha-Brook rearrangement strategy using α-hydroxy phosphate 92 and aldehydes to construct bis-chiral α-phosphoryloxy derivatives 93 with precise stereochemical control (Figure 41A). This catalytic system demonstrated broad substrate scope across alkyl, vinyl, aryl, and heteroaromatic aldehydes, delivering nine derivatives with excellent diastereocontrol (up to 97:3 dr) and yields (up to 91%). The phosphoryl group’s strategic lability enabled subsequent p-toluenesulfonic acid-mediated deprotection, establishing a versatile platform for chiral scaffold diversification. Mechanistic analysis (Figure 41B) revealed a concerted process: (i) Catalyst-mediated rearrangement of 92 generates oxaphosphetane intermediate Int-52; (ii) Stereoselective aldehyde addition forms Int-53; (iii) Phosphoryl migration through Int-54 precedes stereospecific protonation to finalize 93. This sequence highlights the methodology’s dual capacity for stereochemical induction and functional group manipulation.
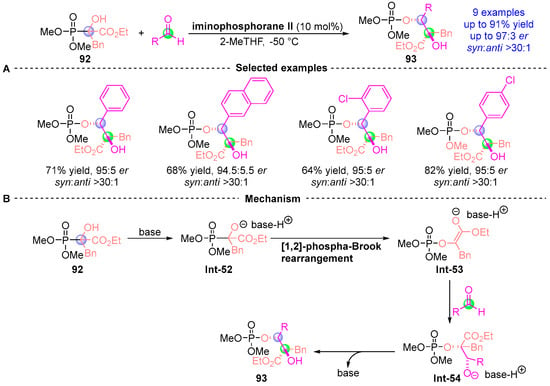
Figure 41.
Iminophosphorane II-catalyzed [1,2]-phospha-Brook rearrangement reported by Johnson. (A) Selected examples for 93. (B) Plausible mechanism.
In 2017, Terada [] pioneered a catalytic homologation strategy leveraging [1,2]-phospha-Brook rearrangement to generate allylic anions from phosphate ester 94 and α-hydroxyallyl-functionalized chalcone 95 (Figure 42). This transition metal-free protocol enabled stereoselective C–C bond formation, delivering chiral compounds 96 with excellent yields and stereocontrol. The methodology capitalizes on the inherent nucleophilicity of [1,2]-phospho-Brook intermediates while maintaining precise stereochemical fidelity, exemplifying a novel approach to constructing complex allylic architectures through anion relay chemistry.
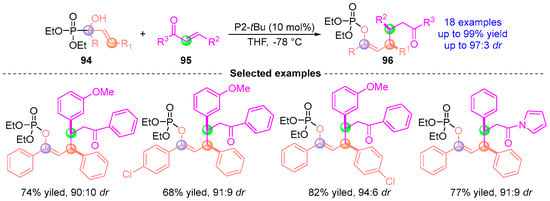
Figure 42.
P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
In 2020, Terada’s group [] developed a tBuOK-catalyzed cascade synthesis of tetrasubstituted furans through [1,2]-phospha-Brook rearrangement/cyclization sequences (Figure 43). The one-pot protocol initiates with α-hydroxy phosphate 97 undergoing base-mediated rearrangement to generate a reactive carbanion intermediate. This species subsequently engages aldehydes in NIS (N-iodosuccinimide) promoted [3 + 2] annulation, delivering 2,4,5-trisubstituted 3-iodofurans 98 that serve as versatile precursors to fully substituted furan architectures. Demonstrating exceptional functional group tolerance and step economy, this methodology establishes a robust platform for constructing complex heterocyclic systems through synergistic anion generation and electrophilic trapping strategies.
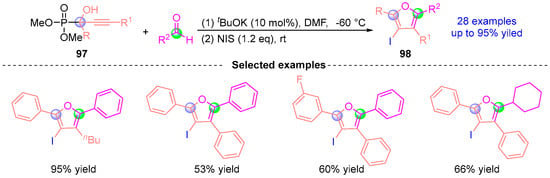
Figure 43.
tBuOK-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
Based on previous work, Terada [] achieved a breakthrough in heterocycle synthesis through a programmable two-phase assembly of thieno[3,2-b]furan architectures (Figure 44). This platform synergistically merges [1,2]-phospha-Brook rearrangement-driven [3 + 2] cyclofunctionalization with Brønsted base enabled annulative closure, enabling efficient construction of highly substituted 2,3,5,6-tetrasubstituted thieno[3,2-b]furans targets 100 traditionally inaccessible through conventional methods. The methodology demonstrates exceptional generality across diverse substrates while maintaining precise regiocontrol, with successful adaptation to seleno[3,2-b]furan systems further validating its versatility as a heterocyclic construction platform.
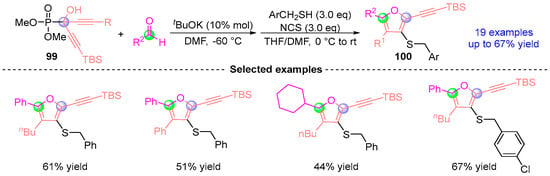
Figure 44.
tBuOK-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
Concurrently, they [] devised a polarity-reversal strategy for tertiary alcohol synthesis through Brønsted base-mediated umpolung functionalization (Figure 45). This innovative approach leverages α-hydroxyphosphonates 101 (derived from aryl ketones) to engage electrophiles like phenyl vinyl sulfone 102 via [1,2]-phospha-Brook rearrangement, forging tertiary alkyl-containing phosphates 103 with precise stereochemical control. Subsequent hydrolysis of these bench-stable intermediates provides streamlined access to sterically congested benzylic tertiary alcohol structures historically challenging to construct through classical nucleophilic pathways. The methodology’s synthetic power stems from its ability to invert conventional reactivity paradigms while maintaining operational simplicity, establishing a novel disconnection for three-dimensional molecular architectures.
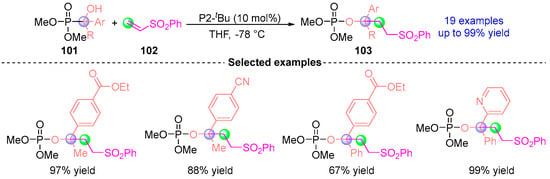
Figure 45.
P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
Recently, Terada [] also demonstrated the synthetic versatility of Brønsted base-catalyzed [1,2]-phospha-Brook rearrangement through an innovative intermolecular coupling platform (Figure 46). Employing phosphazene superbase P2-tBu, the methodology generates bench-stable benzofuran-anchored diarylmethyl anions 104 via rearrangement-induced deprotonation. These configurationally defined carbanions exhibit broad electrophile compatibility, engaging α,β-unsaturated ketones, and related partners to deliver structurally complex diarylalkanes 105 with excellent yield (up to 99%) and precise stereochemical governance (up to 99:1 dr).
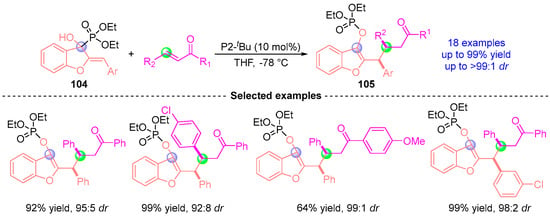
Figure 46.
P2-tBu-catalyzed [1,2]-phospha-Brook rearrangement reported by Terada.
9. Conclusions
In summary, the [1,2]-phospha-Brook rearrangement has emerged as a powerful paradigm for generating configurationally defined anionic nucleophiles through carbonyl group activation. Over two decades of development, this transformation has evolved into a versatile platform compatible with diverse carbonyl substrates including aldehydes, ketones, ketoesters, ketimines, alkynones, and α-hydroxyphosphates. What is more, the inherent capacity to generate stabilized carbanion intermediates enables efficient assembly of versatile motifs through subsequent regioselective functionalization even in both diastereo- and enantioselective pathways. In the future, the [1,2]-phospha-Brook rearrangement might focus on the development of novel catalytic systems, stereodivergent synthetic strategies, and mechanism-driven innovations in catalytic modes.
Author Contributions
Conceptualization, N.L., Q.W. and Y.H.; writing—original draft preparation, N.L. and J.L.; writing—review, and editing J.L. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations were used in this manuscript:
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| BPR | Berry pseudorotation |
| CaE | Carboxylesterases |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| DBU | 1,8-Diazabicyclo [5.4.0]undec-7-ene |
| DMAP | 4-Dimethylaminopyridine |
| DMF | N,N-Dimethylformamide |
| DFT | Density functional theory |
| KHMDS | Potassium Hexamethyldisilazide |
| LDA | Lithium Diisopropylamide |
| LiHMDS | Lithium Hexamethyldisilazide |
| NaHMDS | Sodium Hexamethyldisilazide |
| NIS | N-Iodosuccinimide |
| PhOK | Potassium phenoxide |
| SPO | Secondary phosphine oxides |
| TMG | Tetramethylguanidine |
| TMSOTf | Trimethylsilyl triflate |
| nBuLi | n-butyllithium |
| tBuOK | potassium tert-butoxide |
References
- Brook, A.G. Isomerism of Some α-Hydroxysilanes to Silyl Ethers. J. Am. Chem. Soc. 1958, 80, 1886–1889. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Dong, Y.; Zhang, N.; Zhang, C. Transition Metal Promoted Brook Rearrangement and Its Related Reactions. Tetrahedron 2024, 168, 134351. [Google Scholar] [CrossRef]
- Kondoh, A.; Terada, M. Development of Molecular Transformations on the Basis of Catalytic Generation of Anionic Species by Organosuperbase. Bull. Chem. Soc. Jpn. 2021, 94, 339–356. [Google Scholar] [CrossRef]
- Kondoh, A.; Terada, M. [1,2]-Phospha-Brook Rearrangement as Tool for Generation of Anionic Nucleophiles in Addition Reactions under Bronsted Base Catalysis. Asian. J. Org. Chem. 2023, 12, e202300003. [Google Scholar]
- Kaur, R.; Singh, R.P. Stereoselective Reductive Coupling Reactions Utilizing 1,2-Phospha-Brook Rearrangement: A Powerful Umpolung Approach. J. Org. Chem. 2023, 88, 10325–10338. [Google Scholar]
- Kaïm, L.E.; Gaultier, L.; Grimaud, L.; Santos, D.A. Formation of New Phosphates from Aldehydes by a DBU-Catalysed Phospha-Brook Rearrangement in a Polar Solvent. Synlett 2005, 15, 2335–2336. [Google Scholar]
- Pallikonda, G.; Santosh, R.; Ghosal, S.; Chakravarty, M. n-BuLi-Triggered Phospha-Brook Rearrangement: Efficient Synthesis of Organophosphates from Ketones and Aldehydes. Tetrahedron Lett. 2015, 56, 3796–3798. [Google Scholar]
- Ranga, S.; Chakravarty, M.; Chatterjee, T.; Ghosal, S. Mechanistic Insights into n-BuLi Mediated Phospha-Brook Rearrangement. New J. Chem. 2019, 43, 9886–9890. [Google Scholar]
- Wang, B.; Gao, Q.; Jiang, H.; Wu, W. [1,2]-Phospha-Brook Rearrangement-Initiated Palladium-Catalyzed Cyclization Reaction of Isocyanides and o-Bromobenzaldehydes: Access to 2H-Isoindole-1-carboxamides and 2H-Isoindole-1-carbonitriles. Chin. J. Chem. 2025, 43, 620–626. [Google Scholar]
- Cherkupally, P.; Beier, P. Alkoxide-Induced Nucleophilic Trifluoromethylation using Diethyl Trifluoromethylphosphonate. Tetrahedron Lett. 2010, 51, 252–255. [Google Scholar]
- Yang, J.; Qian, D.-W.; Yang, S.-D. Lewis acid-Catalyzed Pudovik Reaction–Phospha-Brook Rearrangement Sequence to Access Phosphoric Esters. Beilstein. J. Org. Chem. 2022, 18, 1188–1194. [Google Scholar] [CrossRef]
- Kondoh, A.; Terada, M. Brønsted Base-Catalyzed 1,2-Addition/[1,2]-Phospha-Brook Rearrangement Sequence Providing Functionalized Phosphonates. Org. Biomol. Chem. 2022, 20, 2863–2866. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.S.; Reis, Ö.; İǧdir, A.Ç.; Esiringü, İ.; Eymur, S. Generation of Acyl anion Equivalents from Acylphosphonates via Phosphonate-Phosphate Rearrangement: A highly Practical Method for Cross-Benzoin Reaction. J. Org. Chem. 2005, 70, 10584–10587. [Google Scholar] [PubMed]
- Bausch, C.C.; Johnson, J.S. Cyanide-Catalyzed Additions of Acyl Phosphonates to Aldehydes: A New Acyl Donor for Benzoin-Type Reactions. Adv. Synth. Catal. 2005, 347, 1207–1211. [Google Scholar] [CrossRef]
- Demir, A.S.; Reis, B.; Reis, Ö.; Eymuer, S.; Goellue, M.; Tural, S.; Saglam, G. Cyanide Ion Promoted Addition of Acyl Phosphonates to Ethyl Cyanoformate: Synthesis of Tertiary Carbinols via Tandem Carbon−Carbon Bond Formations. J. Org. Chem. 2007, 72, 7439–7442. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Aksinenko, A.Y.; Sokolov, V.B.; Serebryakova, O.G.; Richardson, R.J. Synthesis of Organophosphates with Fluorine-Containing Leaving Groups as Serine Esterase Inhibitors with Potential for Alzheimer Disease Therapeutics. Bioorg. Med. Chem. Lett. 2009, 19, 5528–5530. [Google Scholar] [CrossRef] [PubMed]
- Aksinenko, A.Y.; Sokolov, V.B.; Goreva, T.V.; Makhaeva, G.F. Synthesis of O-phosphorylated 1-Substituted 2,2,2-Trifluoroethanols, Serine Hydrolase Inhibitors. Russ. Chem. Bull. 2010, 59, 102–106. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Y.-M.; Yao, L.; Ji, S.-Y.; Zheng, H.-X.; Wen, J.-H.; Xu, Q.; Zhao, C.-Q. Stereoselective O-Phosphorylation of Aldehydes and Ketones via Phospha-Brook Rearrangement: The Stereochemistry and Intermolecular Mechanism. New J. Chem. 2024, 48, 18520–18525. [Google Scholar] [CrossRef]
- Kondoh, A.; Aoki, T.; Terada, M. Synthesis of Phenanthrene Derivatives by Intramolecular Cyclization Utilizing the [1,2]-Phospha-Brook Rearrangement Catalyzed by a Brønsted Base. Chem. Eur. J. 2015, 21, 12577–12580. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, N.; Wu, Z.-G.; Wu, X.; Wang, M.; Huang, W.; Zi, Y. Sequential In Situ-Formed Kukhtin−Ramirez Adduct and P(NMe2)3-Catalyzed O-Phosphination of α-Dicarbonyls with P(O)−H. Org. Lett. 2023, 25, 7595–7600. [Google Scholar] [CrossRef]
- Hayashi, M.; Nakamura, S. Catalytic Enantioselective Protonation of α-Oxygenated Ester Enolates Prepared through Phospha-Brook Rearrangement. Angew. Chem. Int. Ed. 2011, 50, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, H.; Lu, C.-D.; Xu, Y.-J. Diethyl Phosphite Initiated Coupling of α-Ketoesters with Imines for Synthesis of α-Phosphonyloxy-β-Amino Acid Derivatives and Aziridine-2-Carboxylates. Org. Lett. 2016, 18, 880–883. [Google Scholar]
- Yin, D.; Liu, H.; Lu, C.-D.; Xu, Y.-J. Dialkyl Phosphite-Initiated Cyclopropanation of α,β-Unsaturated Ketones using α-Ketoesters or Isatin Derivatives. J. Org. Chem. 2017, 82, 3252–3261. [Google Scholar] [CrossRef]
- Kondoh, A.; Terada, M. Brønsted Base-Catalyzed Three-Component Coupling Reaction of α-Ketoesters, Imines, and Diethyl Phosphite Utilizing [1,2]-Phospha-Brook Rearrangement. Org. Biomol. Chem. 2016, 14, 4704–4711. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Aoki, T.; Terada, M. Organocatalytic Arylation of α-Ketoesters Based on Umpolung Strategy: Phosphazene-Catalyzed SNAr Reaction Utilizing [1,2]-Phospha-Brook Rearrangement. Chem.-Eur. J. 2018, 24, 13110–13113. [Google Scholar]
- Horwitz, M.A.; Zavesky, B.P.; Martinez-Alvarado, J.I.; Johnson, J.S. Asymmetric Organocatalytic Reductive Coupling Reactions between Benzylidene Pyruvates and Aldehydes. Org. Lett. 2016, 18, 36–39. [Google Scholar] [PubMed]
- Kaur, R.; Singh, D.; Singh, R.P. Stereoselective Synthesis of Dihydrocoumarins via [1,2]-PhosphaBrook Rearrangement in Three-Component Coupling Reaction of α-Ketoesters, o-Quinone Methides, and Dialkyl Phosphites. J. Org. Chem. 2021, 86, 15702–15711. [Google Scholar] [CrossRef]
- Zhang, J.; Su, J.-Y.; Liu, Y.-Z.; Li, H.; Wang, Q.; Deng, W.-P. Palladium-Catalyzed Allylic Alkylation Enabled by Ketone Umpolung via Pudovik Addition/[1,2]-Phospha-Brook Rearrangement. Sci. China Chem. 2023, 66, 2810–2816. [Google Scholar] [CrossRef]
- Sakihara, M.; Shimoyama, S.; Kurosawa, M.B.; Yamaguchi, J. Deoxygenative Functionalizations of Aromatic Dicarbonyls and Aldehydes. Bull. Chem. Soc. Jpn. 2024, 97, uoae078. [Google Scholar]
- Sakihara, M.; Kurosawa, M.B.; Watanabe, M.; Shimoyama, S.; Yamaguchi, J. Deoxygenative Hetero- and Carbofunctionalizations of Diarylketones. J. Org. Chem. 2024, 89, 8157–8167. [Google Scholar]
- Peng, L.; Wang, L.-L.; Bai, J.-F.; Jia, L.-N.; Yang, Q.-C.; Huang, Q.-C.; Xu, X.-Y.; Wang, L.-X. Highly Effective and Enantioselective Phospho-Aldol Reaction of Diphenyl Phosphite with N-Alkylated Isatins Catalyzed by Quinine. Tetrahedron Lett. 2011, 52, 1157–1160, Erratum in Tetrahedron Lett. 2011, 52, 6207–6209. [Google Scholar] [CrossRef]
- Kondoh, A.; Aoki, T.; Terada, M. Intramolecular Cyclization of Alkynyl α-Ketoanilide Utilizing 1,2 -Phospha-Brook Rearrangement Catalyzed by Phosphazene Base. Org. Lett. 2014, 16, 3528–3531. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, S.; He, F.; Zhang, Y.; Wu, Z.; Wu, J. DBU Catalyzed Phospho-Aldol-Brook Rearrangement for Rapid Preparation of α-Phosphates Amide in Solvent-Free Conditions. Catalysts 2020, 10, 1445. [Google Scholar] [CrossRef]
- Huang, W.; Liu, H.; Lu, C.-D.; Xu, Y.-J. Diastereoselective Synthesis of 2-Methoxyimidoyloxiranes Via Dimethyl Phosphite-Mediated Coupling of α-Keto N-Sulfinyl Imidates with Aldehydes. Chem. Commun. 2016, 52, 13592–13595. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Takei, A.; Terada, M. Novel Methodology for the Efficient Synthesis of 3-Aryloxindoles: [1,2]-Phospha-Brook Rearrangement–Palladium-Catalyzed Cross-Coupling Sequence. Synlett 2016, 27, 1848–1853. [Google Scholar] [CrossRef]
- Horwitz, M.A.; Tanaka, N.; Yokosaka, T.; Uraguchi, D.; Johnson, J.S.; Ooi, T. Enantioselective Reductive Multicomponent Coupling Reactions between Isatins and Aldehydes. Chem. Sci. 2015, 6, 6086–6090. [Google Scholar] [CrossRef]
- Motevalli, S.; Johnson, J.S. Phosphite-Mediated Reductive Cross-Coupling of Isatins and Nitrostyrenes. Synthesis 2017, 49, 2663–2676. [Google Scholar]
- Lin, Q.; Wang, S.; Weng, R.; Cao, W.; Feng, X. Chiral Lewis Acid-Catalyzed Asymmetric Multicomponent Michael Reaction through [1,2]-Phospha-Brook Rearrangement. Org. Lett. 2023, 25, 6262–6266. [Google Scholar] [CrossRef]
- Kaur, R.; Rautela, S.S.; Ali, M.; Singh, R.P. Deciphering Asymmetric Brønsted Base-Aminocatalytic Mode in Pudovik/1,2-Phospha-Brook Rearrangement/Michael Cascade Reaction. J. Org. Chem. 2024, 89, 14177–14182. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Chen, J.-R.; Lu, L.-Q.; Xiao, W.-J. An Efficient Synthesis of Enol Phosphates via Organic Base-Promoted Addition of Phosphites to 4-Oxo-Enoates. Tetrahedron 2012, 68, 6032–6037. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, M.; Liu, J.-T. Base-Controlled Reaction of α,β-Unsaturated Trifluoromethyl Ketone and Dialkyl Phosphite. Tetrahedron Lett. 2017, 58, 1871–1874. [Google Scholar]
- Fukuta, T.; Tatsumu, T.; Fujiyoshi, K.; Koyama, T.; Kawashima, S.A.; Mitsunuma, H.; Yamatsugu, K.; Kanai, M. Umpolung Phosphorylation of Tyrosine via 1,2-Phospha-Brook Rearrangement. Org. Lett. 2024, 26, 8827–8831. [Google Scholar] [CrossRef]
- Kondoh, A.; Ishikawa, S.; Aoki, T.; Terada, M. Synthesis of 2,3-Allenylamides Utilizing 1,2 -Phospha-Brook Rearrangement and Their Application to Gold-catalyzed Cycloisomerization Providing 2-Aminofuran Derivatives. Chem. Commun. 2016, 52, 12513–12516. [Google Scholar] [CrossRef]
- Kondoh, A.; Koda, K.; Kamata, Y.; Terada, M. Synthesis of Indolizine Derivatives Utilizing 1,2-Phospha-Brook Rearrangement/Cycloisomerization Sequence. Chem. Lett. 2017, 46, 1020–1023. [Google Scholar]
- Lin, Q.; Zheng, S.; Chen, L.; Wu, J.; Li, J.; Liu, P.; Dong, S.; Liu, X.; Peng, Q.; Feng, X. Catalytic Regio-and Enantioselective Protonation for the Synthesis of Chiral Allenes: Synergistic Effect of the Counterion and Water. Angew. Chem. Int. Ed. 2022, 61, e202203650. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Wang, F.; Zhang, Y.; Zheng, Z.; Wu, J.-H.; Ren, X.; Su, Z.; Chen, W.; Wang, T. Novel Stereo-Induction Pattern in Pudovik Addition/Phospha-Brook Rearrangement Towards Chiral Trisubstituted Allenes. Angew. Chem. Int. Ed. 2024, 63, e202403707. [Google Scholar]
- Pallitsch, K.; Roller, A.; Hammerschmidt, F. The Stereochemical Course of the α-Hydroxyphosphonate-Phosphate Rearrangement. Chem.-Eur. J. 2015, 21, 10200–10206. [Google Scholar]
- Prechelmacher, S.; Mereiter, K.; Hammerschmidt, F. The α-Hydroxyphosphonate-Phosphate Rearrangement of a Noncyclic Substrate-Some New Observations. Org. Biomol. Chem. 2018, 16, 3672–3680. [Google Scholar]
- Corbett, M.T.; Uraguchi, D.; Ooi, T.; Johnson, J.S. Base-Catalyzed Direct Aldolization of α-Alkyl-α-Hydroxy Trialkyl Phosphonoacetates. Angew. Chem. Int. Ed. 2012, 51, 4865–4869. [Google Scholar]
- Kondoh, A.; Aoki, T.; Terada, M. Generation and Application of Homoenolate Equivalents Utilizing [1,2]-Phospha-Brook Rearrangement under Brønsted Base Catalysis. Chem.-Eur. J. 2017, 23, 2769–2773. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Aita, K.; Ishikawa, S.; Terada, M. Synthesis of Tetrasubstituted Furans through One-Pot Formal [3+2] Cycloaddition Utilizing [1,2]-Phospha-Brook Rearrangement. Org. Lett. 2020, 22, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, A.; Aita, K.; Terada, M. Synthesis of 2,3,5,6-Tetrasubstituted Thieno [3,2-b]furans through Formal [3+2] Cycloaddition Utilizing [1,2]-Phospha-Brook Rearrangement/Brønsted Base-Mediated Cyclization Sequence. Adv. Synth. Catal. 2024, 366, 4723–4728. [Google Scholar]
- Kondoh, A.; Suzuki, H.; Hirozane, T.; Terada, M. Catalytic Generation of Benzyl Anions from Aryl Ketones Utilizing [1,2]-Phospha-Brook Rearrangement and Their Application to Synthesis of Tertiary Benzylic Alcohols. Chem. Eur. J. 2024, 30, e202402967. [Google Scholar]
- Kondoh, A.; Terada, M. Catalytic Generation and Intermolecular Addition of Diarylmethyl Anions Utilizing [1,2]-Phospha-Brook Rearrangement Under Brønsted Base Catalysis. Adv. Synth. Catal. 2024, 366, 1857–1862. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).