1. Introduction
Duchenne muscular dystrophy (DMD) is a severe X-linked genetic disorder characterized by the progressive degeneration of skeletal and cardiac muscles, primarily caused by mutations in the
DMD gene that result in the absence of dystrophin, a crucial protein involved in the maintenance of muscle membrane integrity. The lack of dystrophin leads to muscle fiber damage, progressive weakness, loss of ambulation, and ultimately respiratory and cardiac failure, severely impacting the quality of life and life expectancy of affected individuals [
1]. Current therapies, such as corticosteroids, offer limited benefits, highlighting the urgent need for innovative curative therapeutic strategies aimed at restoring dystrophin expression and improving muscle function.
Becker muscular dystrophy (BMD) is also associated with mutations in the DMD gene, leading to a significantly milder clinical presentation compared to DMD. In contrast with mutations found in DMD patients, which often disrupt the open reading frame, deletions found in BMD patients mostly preserve it, allowing for the production of a partially truncated but still functional dystrophin protein.
Antisense oligonucleotide (ASO)-based therapies, aiming at inducing exon skipping, have emerged as promising strategies for the treatment of DMD. Indeed, the therapeutic antisense-mediated exon-skipping approach for DMD aims to remove one or several exons from the mRNA by utilizing ASOs to block crucial splicing sites during the pre-mRNA splicing process. This technique results in an mRNA with a corrected reading frame, facilitating the expression of a dystrophin similar to that seen in BMD. The US FDA has already approved several ASO-based drugs for the treatment of DMD, including eteplirsen, golodirsen, viltolarsen, and casimersen, which target exons 51, 53, 53, and 45, respectively [
2]. A major challenge associated with ASO-mediated exon skipping is effectively delivering ASOs to the targeted tissues [
3]. Significant research efforts are currently aimed at enhancing this delivery, particularly through the development of alternative chemical structures or various conjugates such as peptides or antibodies [
4]. Many of these novel compounds are presently undergoing, or are soon to undergo, clinical trial evaluations.
Among the alternative chemistries of ASOs that have been investigated, our team has worked on the tricyclo-DNA (tcDNA) [
5,
6] and shown that conjugating palmitic acid to tcDNA (palm-tcDNA) considerably improves its therapeutic efficacy and delivery to muscle tissues [
7,
8].
In addition to delivery issues, a lesser-known challenge impacting the efficacy of ASOs is the limited availability of target mRNA. Patients with DMD exhibit a high frequency of transcript imbalance, characterized by non-homogeneous expression of the
DMD transcript along its length, with the 5′ end being more highly expressed than the 3′ end. Although transcript imbalance in DMD was first described in 1995 [
9], its implications for mRNA-based therapies have been highlighted more recently [
10,
11]. Transcriptional studies have indicated that the levels of dystrophin mRNA are decreased in dystrophic muscles and that this is at least partially attributed to a chromatin structure less conducive to transcription in
mdx mice compared to wild-type mice [
11]. Previous research has demonstrated that valproic acid (VPA) influences gene expression by promoting histone acetylation, which can increase transcriptional activity and improve therapeutic outcomes in models of muscle diseases [
12]. Pharmacological inhibition of histone deacetylases has been demonstrated to mitigate fibrosis and enhance muscle regeneration in
mdx mice, primarily through the upregulation of follistatin. VPA, a branched-chain fatty acid approved by the FDA for the treatment of epilepsy and bipolar disorder, activates the Akt signaling pathway in neurons, thereby promoting their survival. VPA offers several advantages for skeletal muscle, such as improved sarcolemmal integrity, reduced contractures in the hind limbs, and diminished inflammation. Additionally, VPA facilitates hypertrophy and decreases apoptosis in myotubes by engaging the Akt/mTOR/p70S6K signaling cascade.
In a previous study, we showed that the combination of histone deacetylase inhibitors (HDACi) such as givinostat or VPA with exon-skipping therapies over a short period of time (4 weeks) significantly enhances dystrophin expression in
mdx mice, thereby underscoring the potential of this combined approach [
13]. In the present work, we aimed to further investigate the effects of VPA on ASO-mediated exon skipping and functional outcomes in
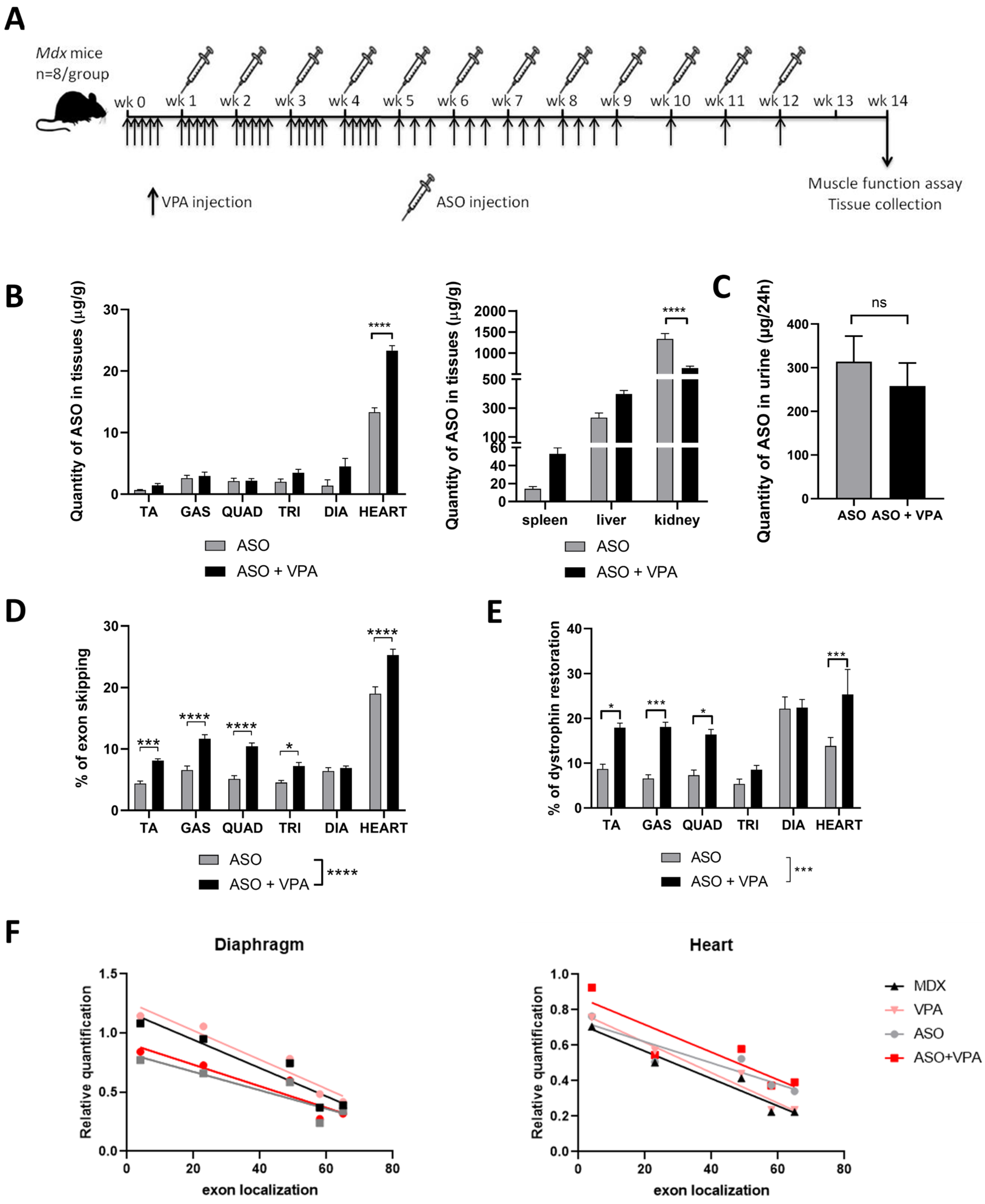
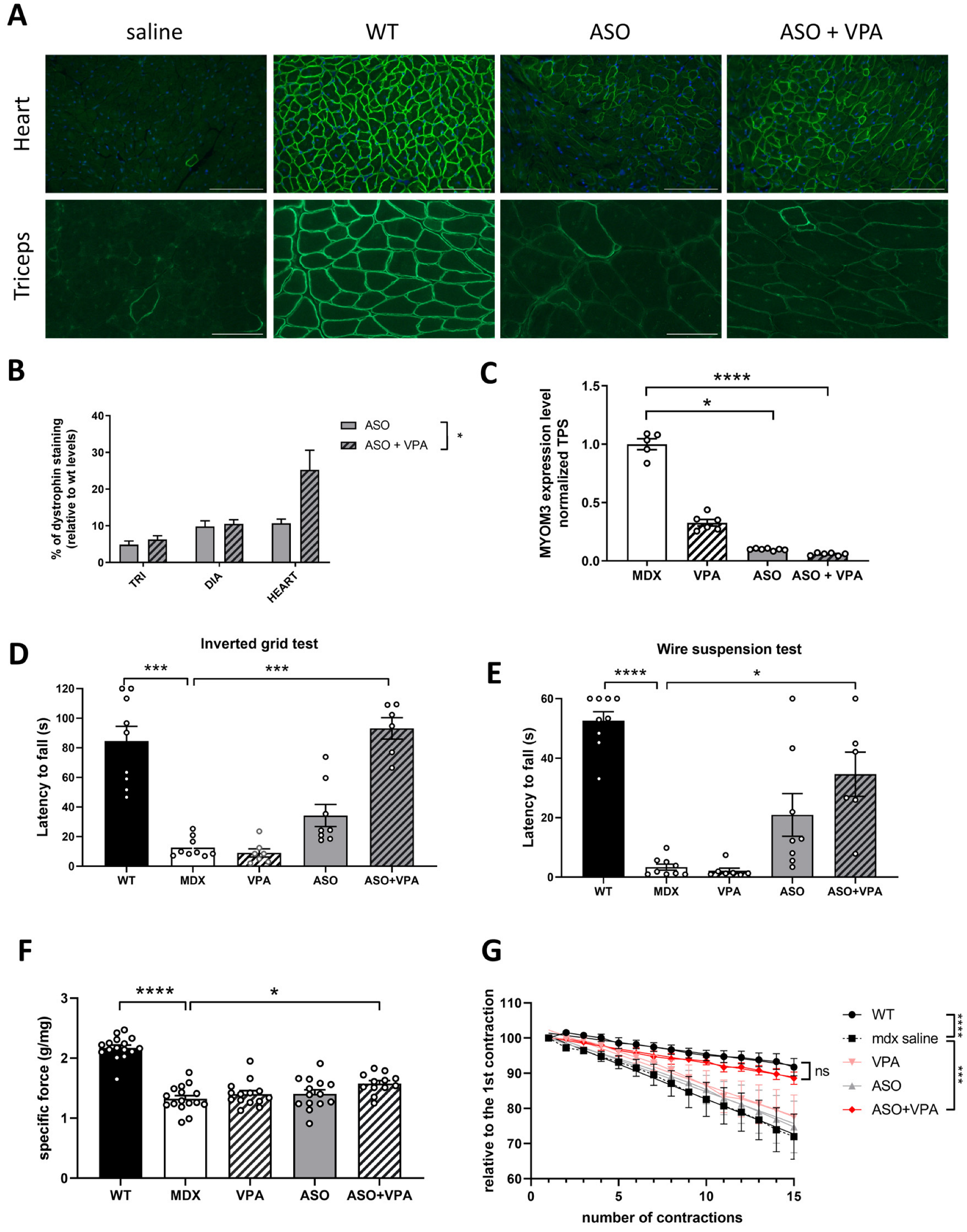
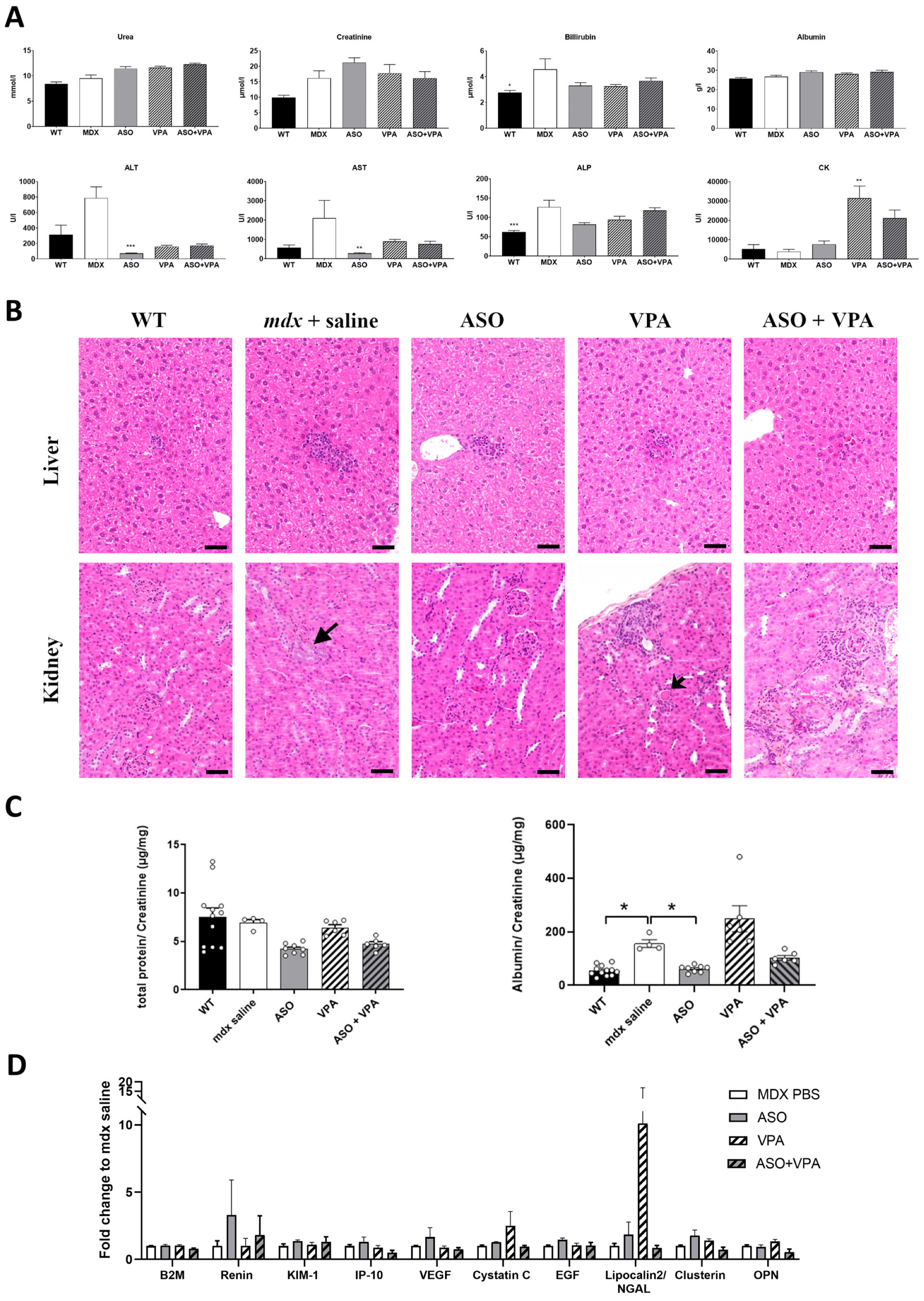
mdx mice over an extended treatment period of 12 weeks. We hypothesized that VPA would enhance ASO efficacy in promoting exon skipping and restoring higher dystrophin levels. Our findings demonstrate that VPA treatment significantly increases the accumulation of ASOs in skeletal and cardiac muscle tissues, resulting in elevated levels of exon skipping across various muscle types, particularly in the heart, where dystrophin restoration was found to be twice as high compared to ASO treatment alone. Additionally, assessment of the functional outcomes revealed remarkable improvements in muscle function, as evidenced by the enhanced performances in latency-to-fall tests and grip strength assessments. We also evaluated the safety profile of the combined treatment regimen, focusing on the potential hepatotoxicity and nephrotoxicity, which are common concerns associated with VPA administration. Our assessments indicate an overall favorable safety profile, despite some initial signs of nephrotoxicity and minimal adverse effects, suggesting that the dosing in VPA should be carefully adapted for the therapeutic benefits of the ASO+VPA combination to outweigh potential risks.
3. Discussion
Duchenne muscular dystrophy poses a major challenge in neuromuscular medicine notably due to the extensive size of the affected tissues, since skeletal and cardiac muscles represent a significant portion of the body. While ASO-mediated exon skipping is a promising therapy, its efficacy is hindered by challenges like poor ASO delivery but also the limited availability of dystrophin pre-mRNA, exacerbated by a 5′-3′ imbalance. Elevated H3K9me3 levels and increased modifications at the
Dmd locus in
mdx mice have suggested reduced transcription due to restrictive chromatin conformation [
11]. We thus propose that HDAC inhibitors could enhance histone acetylation, improve transcription, and increase pre-mRNA availability for exon skipping. Our study aimed to evaluate the combined effects of an ASO targeting the
Dmd exon 23 and the HDACi VPA on dystrophin restoration and functional outcomes in
mdx mice. Our findings reveal that the combination of ASO and VPA resulted in a substantial increase in dystrophin restoration across all muscle tissues examined (+70% on average), with particularly noteworthy effects observed in the heart, where dystrophin levels were restored to approximately two times greater than those achieved with ASO treatment alone. This enhancement highlights the potential of the ASO and VPA combination to increase dystrophin levels across different muscle types, which is crucial for effective treatment. These results confirm the preliminary results obtained during a short-term (4 weeks) study [
13] but more importantly demonstrate a significant improvement in functional outcomes with the combined therapy while the ASO therapy alone often did not reach statistically significant differences. We intentionally selected a lower dose of ASO than used in previous studies on tcDNA-ASO [
7] to (i) allow room for improvement with combined VPA treatment and (ii) simulate potential clinical scenarios where ASO therapies, such as FDA-approved PMOs, achieve dystrophin restoration levels insufficient for meaningful clinical outcomes. For instance, the approval of PMO-ASO drugs for DMD (eteplirsen, golodirsen, viltolarsen, and casimersen) was based on safety and modest increases in dystrophin expression in muscle biopsies, reaching levels of up to ~5.9% after exon 53 skipping [
19]. Although long-term eteplirsen treatment has now shown some benefits, such as delayed loss of ambulation and slower pulmonary decline compared to natural history cohorts [
20], the overall efficacy of these exon-skipping strategies remains limited. This underscores the potential value of approaches like HDACi co-treatment to enhance therapeutic outcomes.
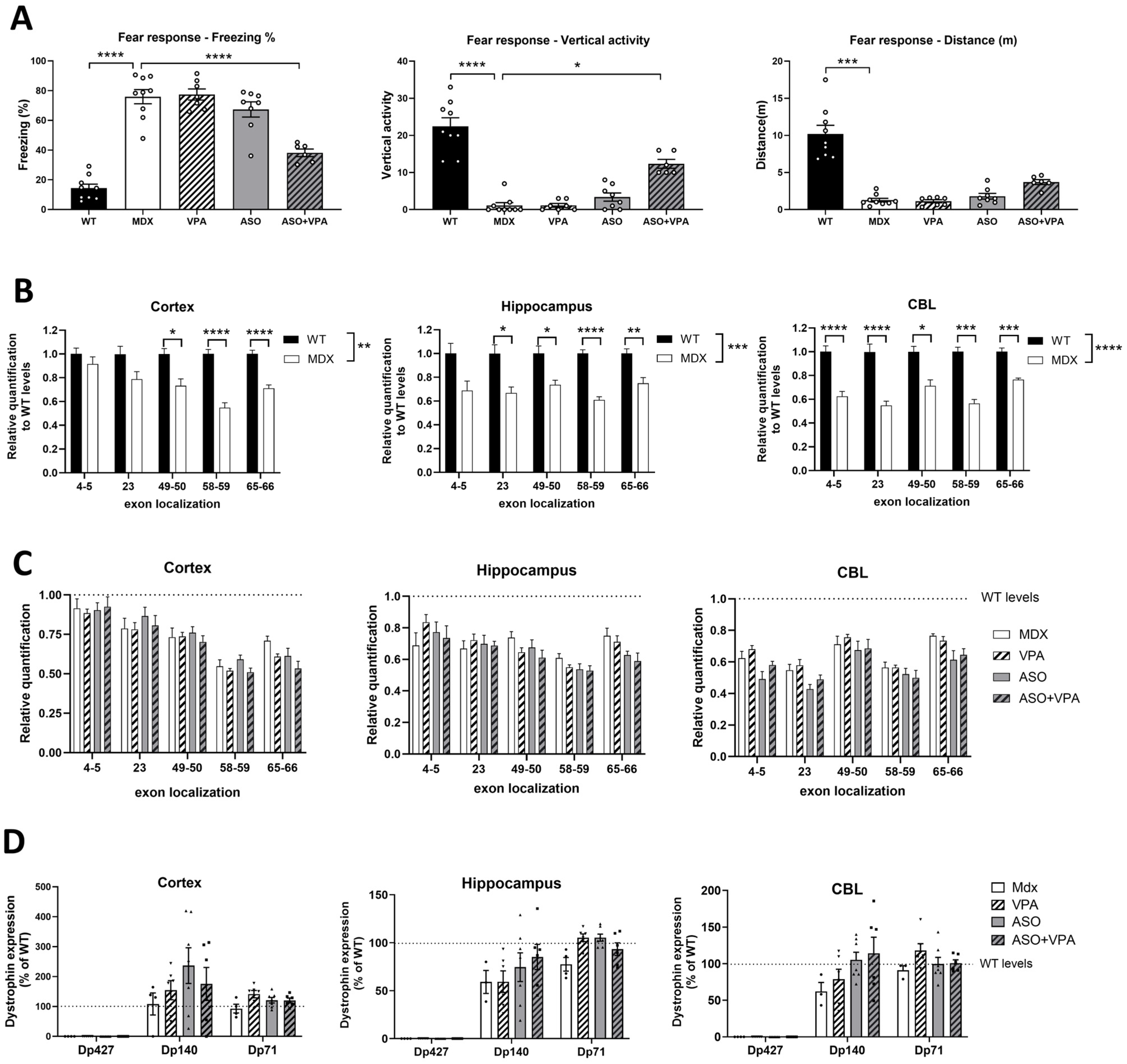
Yet, when we assessed the transcript imbalance after the various treatments, we did not observe a correction of the 5′-3′ difference in transcript levels and detected at best a tendency for higher overall levels of Dmd transcripts across all exon junctions in heart. This suggests that higher dystrophin restoration and improved functional outcomes are not resulting from a correction of the imbalance as we initially hypothesized. In fact, the positive impact of VPA co-treatment is already detected at the exon-skipping levels, although to a lesser extent than protein level (+50% on average across muscles).
Pharmacological blockade of histone deacetylases has previously been reported to reduce fibrosis and promote compensatory regeneration in
mdx skeletal muscle notably through the upregulation of follistatin [
12,
21,
22]. However, we did not observe the upregulation of follistatin here in mice treated with VPA (with or without ASO) suggesting a different mechanism underlying the increased dystrophin expression and improved functional benefits.
The higher levels of dystrophin in skeletal and cardiac muscles likely play a crucial role in improving muscle integrity and function, which contributes directly to physical performance. The stabilization of muscle membranes during contraction due to increased dystrophin levels can lead to improved force generation and reduced muscle damage, facilitating better overall muscle performance. In addition to its role as an HDAC inhibitor that modulates protein levels, VPA also affects ion channel function in healthy individuals [
23,
24,
25]. Therefore, VPA may reduce susceptibility to contraction-induced functional loss by maintaining muscle excitability through ion channels, as the decrease in force following eccentric contractions is also linked to impaired membrane excitability [
26,
27]. Recent studies have indeed demonstrated that the force drop following eccentric contractions is significantly reduced after 7 days of VPA treatment in
mdx mice [
28].
The positive impact of the VPA co-treatment may be attributed to its multiple biological effects on skeletal muscles including decreased fibrosis, damage and inflammation as well as increased sarcolemmal integrity [
16]. Given that VPA treatment alone has previously been associated with such outcomes, it makes sense that combining it with ASO therapy, which restores dystrophin expression and produces similar effects, would result in a synergistic benefit.
Moreover, HDACi were previously shown to stimulate myogenesis in various cell types, including pluripotent stem cells [
29] and fibro-adipogenic progenitors (FAPs) [
30]. Additional studies have revealed that exposure to HDACi promotes the formation and release of pro-regenerative and antifibrotic extra-cellular vesicles (EVs) from FAPs of DMD muscles [
31]. Thus, the modified microenvironment induced by HDACi may enhance overall muscle tissue health, which in turn could facilitate improved uptake of ASOs and/or promote the recovery of dystrophin protein induced by ASOs.
When evaluating the
Dmd transcript imbalance in this study, we found no correction following ASO treatment, consistent with findings by Spitali et al., who observed no improvement in transcript imbalance rates after PMO-ASO treatment in
mdx mice [
10]. However, these results contrast with recent work by Rossi and colleagues [
32], who demonstrated for the first time that golodirsen (a PMO-ASO targeting exon 53) restored transcript imbalance in muscle cells from DMD patients. Specifically, golodirsen-treated cultures expressed significantly higher levels of
DMD transcripts compared to untreated patient cultures, though the imbalance was not fully corrected to levels seen in healthy controls. This discrepancy may stem from differences in the biological context of the models used: their in vitro cellular system versus our in vivo model. In addition to the inherent complexity differences between in vivo and in vitro systems, the exon-skipping levels observed in patient cells were much higher than those measured in vivo, likely facilitated by the use of the transfection agent EndoPorter, which bypasses ASO delivery challenges. Consequently, the lower skipping levels achieved in vivo in
mdx mice may explain the lack of transcript imbalance correction in our system, although the levels observed in our study, ranging from 7% to 25%, are already considerably higher than those typically reported in clinical trials. Another contributing factor could be the distinct mutations and their localizations: the
mdx mouse model harbors a nonsense mutation in
Dmd exon 23, whereas the DMD patient cells had more distal deletions spanning
DMD exons 45–52.
Despite these differences, both studies underscore the importance of considering the 5′-3′ transcript imbalance phenomenon in ASO therapy for DMD. Overlooking this phenomenon could lead to underestimations of skipping efficiency and transcript expression, as well as inaccurate predictions of dystrophin restoration in humans, regardless of the ASO backbone used.
Overall, our study demonstrates that the combined treatment of ASOs and VPA not only significantly enhances dystrophin restoration across various muscle tissues but also leads to notable improvements in functional outcomes. Future studies should aim to evaluate the impact of VPA co-treatment on cardiac function, particularly given the observed improvements in dystrophin restoration in the heart, to fully elucidate the therapeutic potential of this combined approach. The substantial increase in dystrophin levels in skeletal muscles and the heart, averaging +70%, highlights the efficacy of this combination therapy. However, while the safety profile was generally favorable, early signs of nephrotoxicity and minimal adverse effects observed through detailed histopathology underscore the need for careful dose optimization to ensure the therapeutic benefits outweigh potential risks. To mitigate the observed nephrotoxicity, future studies should explore dose optimization strategies, including lower or fractionated dosing of VPA, which may reduce renal stress while maintaining therapeutic efficacy. Adjusting dosing schedules, such as intermittent dosing or using combination treatments with protective agents, may also reduce cumulative toxicity. These approaches will be crucial in refining the therapeutic potential of HDACi and ASO co-treatments for DMD. Moreover, this study focused on a single ASO targeting exon 23, and further studies are needed to determine whether VPA enhances exon-skipping efficiency across other ASO sequences and in different DMD mutations. While VPA is a widely used HDAC inhibitor, it has broad epigenetic effects, and potential off-target actions cannot be excluded. Future investigations should explore alternative HDAC inhibitors with greater specificity or improved safety profiles to optimize therapeutic outcomes while minimizing unintended effects.
Evaluating the combination treatment in larger animal models, such as canine models of DMD, would also provide valuable translational insights into its efficacy and safety in a more clinically relevant setting. Furthermore, given the broad impact of HDAC inhibitors on gene regulation, it would be interesting to investigate whether VPA or other HDAC inhibitors could enhance the efficacy of additional gene therapies for DMD, such as gene replacement with microdystrophin.
While our initial hypothesis regarding the correction of the 5′-3′ transcript imbalance was not validated, the observed improvements in dystrophin restoration and functional outcomes suggest that alternative mechanisms, such as enhanced myogenesis or maintenance of muscle excitability, may underlie the beneficial effects of VPA. Further investigations are warranted to unravel the molecular mechanisms underlying the synergistic effects of HDAC inhibitors like VPA in this combined approach and to refine strategies that maximize clinical benefits for DMD patients and potentially other neuromuscular disorders. In summary, our findings provide compelling evidence supporting VPA as an effective adjuvant to ASO therapy, paving the way for future studies into HDAC inhibitors in exon-skipping therapies and offering new hope for improving treatments for individuals affected by DMD.
4. Materials and Methods
4.1. Antisense Oligonucleotides and Animal Experiments
Animal procedures were carried out in compliance with both national and European regulations and were approved by the French government (Ministère de l’Enseignement Supérieur et de la Recherche, Autorisation APAFiS #6518). Mdx (C57BL/10ScSc-Dmdmdx/J) mice were bred in our animal facility at the Plateforme 2Care, UFR des Sciences de la Santé, Université de Versailles Saint-Quentin, and were maintained under a standard 12 h light/dark cycle with ad libitum access to food and water. Mice were weaned at 4–5 weeks postnatally, with 2–5 mice housed per cage. TcDNA-ASO targeting the donor splice site of exon 23 of the mouse dystrophin pre-mRNA [
6] was synthesized by SQY Therapeutics (Montigny-le-Bretonneux, France). Palmitic acid was conjugated to the 5′ end of tcDNA-PO using a C6-amino linker and a phosphorothioate bond, as previously described [
7].
Groups of 8–10-week-old
mdx mice were injected intravenously with 30 mg/kg/wk of the tcDNA-ASO (one intravenous injection per week under general anesthesia using 2% isoflurane) together with valproic acid (Santa Cruz Biotechnology, Dallas, TX, USA, dissolved in PBS, used at a final concentration of 500 mg/kg/day) or saline for 12 weeks (n = 8 mice per group). Valproic acid was administered intraperitoneally 5 times per week for the first month, as previously described [
16] and, due to the long duration of this study, mice were then treated with VPA 3 times per week during the second month, and once per week during the final month. Age-matched
mdx groups receiving an equivalent volume of sterile saline were used as controls, and C57BL/10 mice were included as wild-type controls.
Animals were euthanized 2 weeks after the final ASO injection. Muscles and brain tissues were harvested, snap-frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C for subsequent analysis.
4.2. Serum and Urine Analysis
Blood samples were collected at the end of the treatment for myomesin-3 (MYOM-3) and biochemical analysis. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, creatinine, urea, and albumin were analyzed by the pathology laboratory at the Mary Lyon Centre, Medical Research Council, Harwell, Oxfordshire, UK.
Urine samples were collected over a 24 h period using metabolic cages, and the concentrations of creatinine and total protein were measured, following previously established protocols [
33].
4.3. Histopathological Profile of Liver and Kidneys
To evaluate the safety of valproic acid, liver and kidney tissues were collected at the end of the 12-week study protocol (two weeks after the final dose), fixed in 10% neutral buffered formalin, and embedded in paraffin. Four-micron thick sections were prepared and stained with hematoxylin–eosin–saffron (HES) for standard histopathological analysis. The evaluation was conducted by a veterinary pathologist who was blinded to the treatment groups.
4.4. ASO Quantification by Fluorescent Hybridization Assay
Tissues were homogenized using the Precellys 24 (Bertin Instruments, Montigny le Bretonneux, France) with lysis buffer (100 mmol/L Tris–HCl, pH 8.5, 200 mmol/L NaCl, 5 mmol/L EDTA, 0.2% sodium dodecyl sulfate) containing 2 mg/mL of proteinase K (Thermo Fisher Scientific, Montigny le Bretonneux, France) at a final concentration of 50 mg of tissue per ml of buffer. The homogenates were then incubated overnight at 55 °C in a hybridization oven. Following a 15 min centrifugation at 7000×
g (Sorval ST 8R centrifuge, 75005719 rotor), the supernatant was collected for analysis. ASO quantification was carried out using a hybridization assay with a molecular beacon probe, as previously described [
34]. The concentration of tcDNA in tissues was determined by comparing the fluorescence signals to a standard curve generated from known quantities of tcDNA dissolved in tissue lysates from mock-injected animals.
4.5. RNA Analysis
Total RNA was extracted from snap-frozen muscle tissues using TRIzol reagent following the manufacturer’s protocol (Thermo Fisher Scientific, Montigny le Bretonneux, France. Exon 23 skipping was quantified using real-time quantitative PCR with TaqMan assays targeting the exon 23–24 and exon 22–24 junctions, as previously described [
7]. The copy numbers for the skipped product (exon 22–24) and the unskipped product (exon 23–24) were calculated using standards Ex20–26 and Ex20–26Delta23, respectively, which were gBlocks gene fragments from Integrated DNA Technology. Exon 23 skipping was expressed as a percentage of total
Dmd transcripts, calculated by adding the copy numbers of exon 22–23 and exon 22–24.
Exon 23 skipping was also visualized on gel after RT-PCR using a forward primer in exon 20 (ex20F: 5′-CCCAGTCTACCACCCTATCAGAGC-3′) and a reverse primer across the exon 22–24 junction (Ex22–24R: 5′-TTATGTGATTCTGTAATTTC-3′).
Quantification of Dmd transcripts at various exon-exon junctions was performed similarly, with probes targeting the ex4–5 (Mm.PT.58.41636025), ex49–50 (Mm.PT.58.6233636), ex58–59 (Mm.PT.58.43613256), ex65–66 (Mm.PT.58.42993407), and GAPDH (Mm.PT.39a.1) junctions (Integrated DNA Technology, Coralville, IA, USA). Absolute quantification was determined using corresponding standards (gBlocks gene fragments from Integrated DNA Technology).
Follistatin quantification was conducted by real-time quantitative PCR using TaqMan assays (Mm.PT.58.11399784 from Integrated DNA Technology, Coralville, IA, USA), with delta-delta CT analysis using GAPDH for normalization.
4.6. Western Blot in Muscle Tissues
Protein lysates were prepared from muscle sections collected during cryosectioning using the Precellys 24 (Bertin Instruments, France) in RIPA buffer (Thermo Fisher Scientific, Montigny le Bretonneux, France) supplemented with SDS powder (5% final concentration, Bio-Rad, Marnes-la-Coquette, France) and protease inhibitor cocktail (Thermo Fisher Scientific, Montigny le Bretonneux, France). The protein extracts were denatured by heating at 100 °C for 3 min, followed by centrifugation at 13,000 rpm for 10 min at 10 °C. The supernatants were collected, and the total protein concentration was quantified using the BCA Protein Assay Kit (Thermo Fisher Scientific, Montigny le Bretonneux, France). A total of 25 μg of protein was loaded onto NuPAGE 3–8% Tris-Acetate Protein gels (Invitrogen) according to the manufacturer’s instructions. Dystrophin was detected using the iBind™ Flex Western Device (Thermo Fisher Scientific, Montigny le Bretonneux, France) and probed with the primary monoclonal antibody NCL-DYS1 (Novocastra, Newcastle, UK, dilution 1/200) and hVin-1 (Sigma, dilution 1/4000). After incubation with a goat anti-mouse secondary antibody (IRDye 800CW goat anti-mouse IgG, Li-Cor, Bad Homburg, Germany, dilution 1/2000), protein bands were visualized with the Odyssey CLx system (Li-Cor, Bad Homburg, Germany). Quantification of dystrophin expression was performed using Empiria Studio 2.3 software (Li-Cor, Bad Homburg, Germany), with data normalized against a standard curve generated from pooled lysates of C57BL10 (wild-type) and mdx control tissues.
For myomesin-3 detection, mouse serum was diluted 1:20 and loaded onto 3–8% Criterion™ XT Tris-Acetate Protein Gel (Biorad, Marnes-la-Coquette, France) according to the manufacturer’s protocol. Myomesin-3 was detected by probing the membrane with a primary rabbit polyclonal antibody against MYOM3 (Proteintech, Manchester, UK), followed by a secondary goat anti-rabbit antibody (IRDye 800CW goat anti-rabbit IgG, Li-Cor, Bad Homburg, Germany). Bands were visualized with the Odyssey Imaging System (Biosciences, Lincoln, NE, USA). The intensity of the signals in treated samples was quantified and normalized to the PBS control group using Image Studio 2.1 software (Li-Cor, Bad Homburg, Germany).
4.7. Western Blot in Brain Tissues
Protein extracts were prepared from brain tissues using RIPA lysis and extraction buffers (Thermo Fisher Scientific, Rockford, IL, USA), supplemented with SDS powder (5% final concentration) (Bio-Rad, Marnes-la-Coquette, France). The total protein concentration was assessed using the BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). Samples were denatured by heating at 100 °C for 3 min, and 25 μg of protein was loaded onto NuPAGE 3–8% Tris-Acetate Protein gels (Thermo Fisher Scientific, Rockford, IL, USA), according to the manufacturer’s protocol. Dystrophin was detected by incubating the membrane with the AB154168 rabbit primary monoclonal antibody (AB154168, Abcam, Paris, France), and vinculin was used as an internal control with the hVin-1 primary antibody (Sigma, St. Louis, MI, USA). Secondary antibodies included IRDye 800CW goat anti-mouse IgG (Li-Cor, Bad Homburg, Germany) for vinculin detection and IRDye 700CW goat anti-rabbit IgG (Li-Cor, Bad Homburg, Germany) for dystrophin detection. Protein bands were visualized using the Odyssey CLx imaging system (Li-Cor, Bad Homburg, Germany). Densitometric analysis was performed using Empiria Studio software version 3.0 (Li-Cor, Bad Homburg, Germany), with quantification normalized to the vinculin internal control. A standard curve was created for each brain structure using a mixture of WT and mdx control lysates, representing known dystrophin percentages (0%, 5%, 10%, and 20% of corresponding WT tissues).
4.8. Immunohistochemistry Analysis
Ten-micron sections were prepared from triceps and heart tissues and examined for dystrophin expression using a rabbit polyclonal antibody against dystrophin (dilution 1:500; cat. number RB-9024-P, Thermo Fisher Scientific, Montigny le Bretonneux, France). Dystrophin was detected with donkey anti-rabbit IgG conjugated to Alexa 594 (dilution 1:400; Jackson Immuno Research, Cambridge, UK). Control sections, where the primary antibody was omitted, exhibited no specific staining. Images were captured at consistent locations and exposure times using a Zeiss Axio Imager, equipped with an Orkan camera (Hamamatsu) and AxioVision 4.7 software. Image analysis was performed using ImageJ 1.52 software.
4.9. Restraint-Induced Unconditioned Fear
Mice were gently restrained by holding the scruff and back skin between the thumb and index fingers, securing the tail between the third and little fingers, and positioning the animal upside down with its ventral side facing the experimenter. After 15 s of restraint, the mouse was placed into a novel cage with clean sawdust and monitored for 5 min under dim lighting using Any-maze 7.4 software (Stoelting). The unconditioned fear response to this stress was characterized by periods of tonic immobility (freezing), which were quantified during the 5 min recording session. Complete cessation of movement, except for respiration, was considered as freezing. The percentage of time spent freezing was calculated for group comparisons. The distance is calculated automatically by Any-maze software using tracking option and the vertical activity is measured manually by the experimenter considering every time the mouse is standing on their posterior paws.
4.10. Susceptibility to Contraction-Induced Loss of Function
Maximal forces before and after eccentric contractions were assessed by measuring the in situ contraction of the tibialis anterior (TA) muscle in response to nerve stimulation, as previously described [
7,
27]. The susceptibility of
mdx mice to contraction-induced functional loss was evaluated by measuring the immediate drop in isometric force following eccentric contractions. Fifteen eccentric contractions were applied, each separated by a 45 s rest period. Maximal isometric force was measured after each contraction and expressed as a percentage of the initial maximal force. Additionally, absolute maximal eccentric force was recorded during the first eccentric contraction, along with an index of muscle strain. Following these measurements, the mice were euthanized by cervical dislocation.
4.11. The Inverted Grid Test
Mice were individually placed on a horizontal wire grid (size typically 25 × 25 cm) which was then inverted to a vertical position at 50 cm height above an experimental cage with sawdust, causing the mouse to be upside down. The time the mouse was able to remain suspended on the grid or the time until it fell off was recorded. Mice were given a maximum of 2 min to remain on the grid. A failure to maintain grip within this time frame was considered as a loss of grip strength. The duration of time the mouse remained on the grid was used as an indicator of muscular strength and coordination. Differences between the groups were analyzed using one-way ANOVA with post hoc comparisons.
4.12. The Wire Suspension Test
Mice were positioned on a horizontal wire, and their ability to grip the wire was assessed by suspending them from the wire using their forelimbs and tail. The wire was suspended 50 cm above the surface. The time the mouse maintained its grip on the wire without falling was recorded. The test was conducted for a maximum of 3 min. If the mouse lost its grip and fell, the test was ended, and the time to fall was recorded.
The duration of suspension was used as an indicator of muscle strength and endurance. Statistical analysis, using one-way ANOVA, was conducted to compare the performance across different groups.
4.13. Statistical Analysis
All in vivo data were analyzed using GraphPad Prism10 software (San Diego, CA, USA) and are presented as means ± S.E.M. The “n” represents the number of mice per group. Group comparisons were conducted using one-way and two-way analyses of variance (ANOVA), with repeated measures when appropriate (e.g., comparing effects across different exon junctions or muscle tissues), followed by post hoc Dunnett’s or Sidak’s multiple comparisons tests as needed. For the comparison of overall treatment effects across different tissues or exon junctions, a two-way ANOVA was applied, and the p for the treatment effect is reported in the figure legend. The Kruskal–Wallis test was used for comparisons of groups that did not follow a normal distribution (assessed using the Shapiro–Wilk test).