Abstract
The increasing prevalence of overweight and obesity not only in adults but also among children and adolescents has become one of the most alarming health problems worldwide. Metabolic disorders accompanying fat accumulation during pathological weight gain induce chronic low-grade inflammation, which, in a vicious cycle, increases the immune response through pro-inflammatory changes in the cytokine (adipokine) profile. Obesity decreases life expectancy, largely because obese individuals are at an increased risk of many medical complications, often referred to as metabolic syndrome, which refers to the co-occurrence of insulin resistance (IR), impaired glucose tolerance, type 2 diabetes (T2D), atherogenic dyslipidemia, hypertension, and premature ischemic heart disease. Metabotropic G protein-coupled receptors (GPCRs) constitute the most numerous and diverse group of cell surface transmembrane receptors in eukaryotes. Among the GPCRs, researchers are focusing on the connection of G protein-coupled receptor 120 (GPR120), also known as free fatty acid receptor 4 (FFAR4), with signaling pathways regulating the inflammatory response and insulin sensitivity. This review presents the current state of knowledge concerning the involvement of GPR120 in anti-inflammatory and metabolic signaling. Since both inflammation in adipose tissue and insulin resistance are key problems in obesity, there is a rationale for the development of novel, GPR120-based therapies for overweight and obese individuals. The main problems associated with introducing this type of treatment into clinical practice are also discussed.
1. Introduction
Abnormal chemical reactions occurring in the body may lead to a disruption of normal metabolic processes [1]. Metabolic disorders adversely affect the processing and distribution of nutrients in the body, including macronutrients such as proteins, fats, and carbohydrates, as well as micronutrients (mostly vitamins and minerals), which are equally important but are consumed in very small amounts [2,3,4]. Metabolic disorders in humans are an increasingly recognized health problem in modern civilization, and in developed countries, the disease of obesity has come to the forefront [5]. Over the past 25 to 30 years, the incidence of overweight and obesity has increased at an alarming rate, becoming a pandemic [6,7,8]. According to data from the World Obesity Organization, between 1975 and 2022, the incidence of obesity among women almost tripled (6.6% to 18.5%), and among men, it increased fourfold (3% to 14.0%) [9]. This increased incidence of obesity in adults is accompanied by a growing problem of obesity in children and adolescents. Compared with 2000–2011, in 2012–2023, a 1.5-fold increase in the incidence of obesity occurred among children and adolescents worldwide [10].
Excessive fat accumulation, which causes health risks, is reflected in the increased body mass index (BMI) values that define overweight (BMI > 25 kg/m2) and generalized obesity (BMI > 30 kg/m2), among which extreme obesity (BMI > 40 kg/m2) and central obesity can also be distinguished [11]. Chronic pathological weight gain in obese individuals is considered multifactorial with strong genetic and epigenetic components [12,13]. Owing to the genetic background, numerous cultural and environmental factors may affect mental characteristics and behavior, ultimately disrupting hormonal and metabolic homeostasis toward fat accumulation [14,15].
Obesity, in particular, is associated with a reduced life expectancy, largely because obese individuals are at an increased risk of many medical complications [16]. Well-known diseases comorbid with obesity include type 2 diabetes mellitus (T2D), hypertension, heart disease, stroke, metabolic syndrome (also called insulin resistance syndrome), nonalcoholic fatty liver disease (NAFLD), chronic kidney disease, some cancers (e.g., colorectal cancer, liver cancer, breast cancer, and prostate cancer), breathing problems, gout, osteoarthritis, sexual dysfunction, fertility disorders, and mental health problems [16,17,18,19,20,21,22,23].
A phenomenon that has not been appreciated until recently in the course of obesity is the persistence of a low-grade inflammatory response in fatty tissues [24,25]. In particular, excess adipose tissue (AT) macrophages present in the visceral AT of individuals with central obesity induce chronic inflammation, exacerbating insulin resistance (IR) by interfering with insulin signaling via insulin receptors [26]. Moreover, glucose intolerance in IR, which manifests as hyperglycemia, additionally stimulates the primary defensive inflammatory response. The numerous pro-inflammatory cytokines released in individuals with these conditions pose a real risk of deterioration of organ function [27,28].
Progress in understanding the pathophysiology of obesity therefore prompts the search for therapeutic effects on metabolic pathways through the identification of key receptors that mediate anti-inflammatory responses. G protein-coupled receptors (GPCRs), also known as seven transmembrane domain (7TM) receptors, are the largest and most diverse group of integral membrane (cell surface) metabotropic receptors in eukaryotes [29,30]. Among the GPCRs that are relatively widely expressed in many tissues of various human and rodent organs and adipose tissue, G protein-coupled receptor 120 (GPR120), also known as free fatty acid receptor (FFAR4), is of interest [31,32].
This review presents the current state of knowledge concerning the involvement of GPR120 in anti-inflammatory and metabolic signaling, which may provide a rationale for the development of novel, GPR120-based therapies for overweight and obese individuals. The main problems associated with introducing this type of treatment into clinical practice are also discussed.
2. G Protein-Coupled Receptor (GPCR) Family
The expression of GPCRs was described for the first time by an American molecular biologist and physician, Robert J. Lefkowitz, in the 1970s. Detailed studies of the structures and roles of GPCRs were then initiated by his colleague Brian K. Kobilka. For their achievements in the field of GPCR research, which were “crucial for understanding how G protein-coupled receptors function”, both outstanding researchers were awarded the Nobel Prize in Chemistry in 2012 [33].
The characteristic 7TM structure of a GPCR with six loops causes the detection of bound molecular ligands on the outer surface of the cell to activate intracellular responses. The three extracellular loops interact with ligands on the N-terminal extracellular side of the GPCR, whereas the three intracellular loops interact with G proteins on the C-terminal (intracellular) side of the receptor [29,30] (Figure 1).
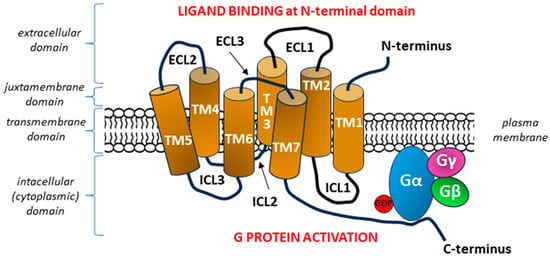
Figure 1.
General diagram of the structure of a G protein-coupled receptor (GPCR). The seven transmembrane heptahelical structures (TM1–TM7) are accompanied by three extracellular loops (ECL1–ECL3) on the N-terminal side of the molecule and three intracellular loops (ICL1–ICL3) on the side of C-terminal tail [29,30]. After binding the signal molecule (ligand) at the N-terminal domain, conformation changes occur in the transmembrane GPCR molecule, which enable ICLs to interact with the G protein within the C-terminus located in the intracellular domain, with subsequent activation of the G protein [34]. Each G protein is composed of three subunits α, β, and γ with a nucleotide-binding pocket located in the α subunit. In the inactive heterotrimeric state, guanosine diphosphate (GDP) is bound to the Gα subunit. The formation of the GPCR—G protein complex after GPCR stimulation begins with the release of GDP from its binding site on the G alpha subunit, which is equivalent to the activation of the G protein, as it allows the binding of guanosine triphosphate (GTP) and inducing the dissociation of the α subunit of the G protein from the βγ subunits [35,36].
G proteins form heterotrimeric complexes that consist of the α (39–52 kDa), β (35–36 kDa), and γ (7–10 kDa) subunits. G protein classes are defined based on the sequence and function of their Gα subunits, which are categorized into several subtypes in mammals: Gαi, Gαs, Gα12/13, Gαq, and transducin (GαTr). The observed differences between these classes result mainly from the fact that Gαi and Gαs regulate the activity of adenylyl cyclase, whereas Gα12/13, Gαq, and GαTr activate small GTPase family proteins and phospholipase C-beta (PLC-β), respectively [35,36].
Conformational changes in the GPCR after ligand binding activate the guanine nucleotide exchange factor (GEF) on the intracellular side, thus activating monomeric GTPase by stimulating the release of guanosine diphosphate (GDP), which then binds to guanosine triphosphate (GTP) [34]. Activation of the G protein, which has inherent GTPase activity, involves the exchange of GDP for GTP in the Gα subunit, which leads to the reversible dissociation of the Gα subunit from the tightly connected Gβ and Gγ subunits that form a separate functional unit. Therefore, the G protein complex functions through a cycle of dissociation of the Gα and Gβ/Gγ subunits, and it functions as a molecular binary switch [35,37]. Intracellular downstream signaling via Gα-GTP and Gβγ, as well as the targeting of functional proteins after GPCR activation, directly depend on the Gα subunit type (Gαi, Gαs, Gα12/13, or Gαq). The G protein remains active until the GTP bound to the Gα subunit is hydrolyzed to GDP [38]. The intracellular signaling cascades activated by GPCRs that lead to the cyclic adenosine 3,5-monophosphate (cAMP) response, calcium mobilization, or the phosphorylation of extracellular signal-regulated protein kinases 1/2 (pERK1/2) have been considered to be remarkably complex, similar to the biological processes they regulate [39,40,41].
Approximately 850 different genes encoding GPCR proteins have been detected in the human genome, accounting for approximately 4% of all human coding genes [42]. Thus, GPCRs, which constitute the most abundant superfamily of integral membrane proteins, represent the largest protein family encoded in the human genome [41,43,44].
Given the crucial role of cell membranes in cell life, the finding that the spectrum of signaling through GPCRs is extremely broad is not surprising, covering most physiological responses to neurotransmitters, hormones, autocrine and paracrine signaling molecules, and environmental stimuli [45,46,47]. Therefore, the group of endogenous GPCR ligands is very diverse, ranging from photons, ions, biogenic amines, amino acids, odorants, pheromones, carbohydrates, peptides, lipids, eicosanoids, chemokines, and neurotransmitters to proteins and hormones [41,48]. Nevertheless, for approximately 30% of nonolfactory human GPCRs, their natural ligands have not been identified, which is why they remain orphan receptors [49,50].
GPCR signaling disorders are associated with the occurrence of many different diseases, including but not limited to type 2 diabetes (T2D), hypo- and hyperthyroidism, obesity, Alzheimer’s disease (AD), cancer, pain, cardiac problems, asthma, depression, and fertility issues [35,51,52,53,54,55]. For example, disease-causing mutations have been reported in at least 55 GPCRs for more than 66 inherited monogenic diseases in humans [56,57].
Notably, of the 826 GPCR family proteins identified thus far in humans, according to various estimates, approximately 350 to 400 nonolfactory proteins are considered druggable, which, in 165 cases, has been validated in the development of targeted drugs [41,58,59]. Nearly 40% of all U.S. Food and Drug Administration (FDA)-approved drugs currently target GPCRs to achieve therapeutic goals, such as treating hypertension, chronic pain, diabetes and other endocrinopathies, cancer, or neurological diseases [41,59,60,61,62].
2.1. Classification of GPCRs
Many classifications have been developed for the superfamily of GPCRs, but in practice, the two most commonly used are as follows:
- -
- The classical A–F system, in which GPCRs are grouped into six classes based on sequence homology and functional similarity
A (or 1), rhodopsin-like receptors;
B (or 2), secretin receptor family;
C (or 3), metabotropic glutamate/pheromone receptors;
D (or 4), fungal mating pheromone receptors;
E (or 5), cyclic AMP receptors;
F (or 6), frizzled/smoothened receptors;
- -
- A newer alternative classification proposed for vertebrates, known by its acronym GRAFS, which stands for glutamate, rhodopsin, adhesion, Frizzled/Taste2, and Secretin. The GRAFS system corresponds to classical classes C, A, B2 (Secretin receptor family, long N-terminal), F, and B1 + 3 (other secretins) [63,64,65].
The most frequently represented class among GPCRs is class A (rhodopsin-like), which includes approximately 83% of GPCR genes and translates into 241 nonolfactory receptors of a total of 701 [66]. GPR120/FFAR4 belongs to this large family of rhodopsin-like receptors [67].
2.2. G Protein-Coupled Receptor 120 (GPR120)
The rhodopsin-like class of GPCRs includes, among others, receptors whose activators are fatty acids of various chain lengths. For example, short-chain fatty acids (SCFAs) bind to GPR41 and GPR43 [68,69], medium-chain fatty acids (MCFAs) bind to GPR84 [70], and LCFAs bind to GPR40 and GPR120/FFAR4 [71].
GPR120/FFAR4 is a sensor that detects saturated and unsaturated long-chain fatty acids (LCFAs) with 14–18 and 16–22 carbons, respectively. Therefore, GPR120 is a functional receptor for omega-3 fatty acids (Ω-3 FAs) and the related plant-derived molecule alpha-linolenic acid (ALA) [72].
When the results of studies on GPR120 conducted in animals are applied to the human body, important differences should be taken into account. For example, the amino acid sequences of mouse (BC053698) and human (BC101175) GPR120 exhibit an 82% match [73].
Compared with human GPR120, murine GPR120 has a stronger response to low doses of agonists, and dose–response analysis revealed a lower expression level of murine GPR120. Interestingly, when comparing human and mouse receptors, no differences in the half maximal effective concentration (EC50) were observed between the two GPR120s [73,74].
Only one GPR120 variant, composed of 361 amino acids, has been detected in rodents and cynomolgus monkeys, while the human GPR120 gene encodes two protein isoforms [75,76]. The chromosomal position of GPR120 in humans is 10q23.33, and the two splice variants that occur are known as short GPR120 (GPR120S) and long GPR120 (GPR120L), containing 361 and 377 amino acids, respectively [77]. The insertion of 16 amino acids between positions 231 and 247 in the third intracellular loop (ICL3) in GPR120L prevents G protein-dependent intracellular calcium and dynamic mass redistribution in the absence of a functional connection with the adaptor protein β-arrestin [76,78]. Human GPR120 appears to correlate more closely with the long form of this receptor, which is one of the first examples of a native β-arrestin-biased receptor [75]. The domain within ICL3 is crucial for binding to G proteins and interacting with β-arrestin during GPR120 activation. In the basal state, GPR120L is phosphorylated at a lower level than GPR120S is [79].
Determinants and localization of GPR120 synthesis. The rate of GPR120 receptor synthesis is influenced by genetic and epigenetic (environmental) factors [80]. Studies of the Danish population have shown that the gene polymorphism leading to the p.R270H variant is associated with a 70% decrease in GPR120 expression compared with that in people without this mutation [81]. The expression level of GPR120 also varies depending on the contents of its natural ligands [eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and ALA] in the diet and the location (cell type) in the body [82]. Compared with control rats fed soybean oil, rats fed a diet with an increased content of fish and/or linseed oil presented significantly higher GPR120 expression in the cells of the large intestine [83]. Studies in rats have also shown that the dietary administration of unsaturated Ω-3 FAs leads to an increase in GPR120 expression in the central nervous system (CNS) [84]. In rats, a high-fat diet led to increased expression of GPR120 in the cardiac muscle and extensor digitorum longus (skeletal muscle), whereas no changes in GPR120 expression were detected in the liver or soleus muscle (skeletal muscle) [85,86]. The effect of a high-fat diet on tissue-specific changes in GPR120 expression was also observed in mice. For example, GPR120 mRNA expression was already high at baseline in four different adipose tissue depots (subcutaneous, perimesenteric, epididymal, and periuterine) and then became upregulated after the consumption of a high-fat diet. The expression of the GPR120 mRNA was higher in adipocytes than in stromal–vascular (S-V) cells. However, in mice, a high-fat diet had no effect on GPR120 expression in perirenal adipose tissue, the nucleus accumbens (NAcs), or the mid-basal part of the hypothalamus [86,87].
In situ hybridization and immunohistochemistry revealed GPR120 expression in mouse gonadotropes of the anterior pituitary gland (adenohypophysis) but not in thyrotropes, somatotropes, lactotropes, corticotropes, melanotropes, or the posterior pituitary gland [88]. Moreover, 24 h of fasting induced the upregulation of GPR120 mRNA expression in the pituitary gland, which may indicate that GPR120 in pituitary gonadotropes is involved in controlling gonadotropin synthesis and secretion [88,89,90].
GPR120 signaling. Importantly, the activation of GPR120 and the subsequent metabolic effects related to the interaction of the receptor with the Gα protein via activated extracellular signal-regulated kinase (ERK) cascades and increases in the intracellular Ca2+ concentration seem to be potentially beneficial in treating obesity by promoting anti-inflammatory and insulin-sensitizing effects [91,92,93]. The β-arrestin2 (ARRβ2)–transforming growth factor-beta (TGFβ)-activated kinase 1 (TAK1)-binding protein 1 (TAB1) complex and the inhibition of the TAK1–c-Jun N-terminal kinase (JNK)–nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway play key roles in the anti-inflammatory mechanism induced by GPR120 stimulation [82,94,95]. The main signaling pathways associated with GPR120/FFAR4 stimulation are presented in detail in Figure 2.
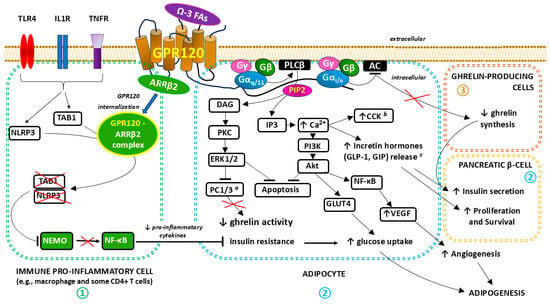
Figure 2.
The three main signal transduction pathways associated with GPR120/FFAR4 stimulation:  —ANTI-INFLAMMATORY EFFECTS via beta-arrestin 2 (ARRβ2) signaling in immune pro-inflammatory cells. After receptor stimulation, the adapter protein ARRβ2 is recruited, which, after forming a complex with GPR120, leads to the desensitization of GPR120 as well as other receptors [e.g., Toll-like receptor 4 (TLR4), interleukin 1 receptor (IL1R), and tumor necrosis factor receptor (TNFR)] to pro-inflammatory stimuli. The GPR120- ARRβ2 complex internalizes and interacts with TGF-β-activated kinase 1 (MAP3K7)-binding protein 1 (TAB1) and NLRP3 [nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR) family member 3] inflammasome, which leads to the inhibition of the subsequent inflammatory cascade. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB) essential modulator (NEMO) ubiquitination, which is crucial for the activation of the canonical NF-κB signaling pathway, does not occur. A decrease in the concentration of pro-inflammatory cytokines indirectly modulates adipose tissue cell functions by reducing insulin resistance and, consequently, stimulating glucose uptake [82,94,95,96,97,98].
—ANTI-INFLAMMATORY EFFECTS via beta-arrestin 2 (ARRβ2) signaling in immune pro-inflammatory cells. After receptor stimulation, the adapter protein ARRβ2 is recruited, which, after forming a complex with GPR120, leads to the desensitization of GPR120 as well as other receptors [e.g., Toll-like receptor 4 (TLR4), interleukin 1 receptor (IL1R), and tumor necrosis factor receptor (TNFR)] to pro-inflammatory stimuli. The GPR120- ARRβ2 complex internalizes and interacts with TGF-β-activated kinase 1 (MAP3K7)-binding protein 1 (TAB1) and NLRP3 [nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR) family member 3] inflammasome, which leads to the inhibition of the subsequent inflammatory cascade. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB) essential modulator (NEMO) ubiquitination, which is crucial for the activation of the canonical NF-κB signaling pathway, does not occur. A decrease in the concentration of pro-inflammatory cytokines indirectly modulates adipose tissue cell functions by reducing insulin resistance and, consequently, stimulating glucose uptake [82,94,95,96,97,98].  —METABOLIC EFFECTS via classical Gα q/11 signaling. In this pathway, omega-3-fatty acids (Ω-3 FAs), acting as a ligand and agonist of GPR120, activate the Gαq/11-linked phospholipase C-beta (PLCβ). After PLCβ activation, phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolyzes into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. IP3 binds to receptors, the conformational changes of which, after activation, lead to the mobilization of calcium ions (Ca2+) from intracellular stores. The following cytosolic Ca2+ influx stimulates the release of both cholecystokinin (CCK) and incretin hormones, including glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP, also known as glucose-dependent insulinotropic polypeptide). Incretin hormones support the survival and proliferation of pancreatic β-cells, which translates into increased insulin secretion. Moreover, the increase in Ca2+ concentration activates the phosphoinositide 3-kinase (PI3K) pathway including Akt, which stimulates downstream anabolic signaling, favoring cell growth (adipogenesis), proliferation, (angiogenesis), and survival. Upregulated glucose transporter type 4 (GLUT4) activity promotes increased glucose uptake, whereas augmented vascular endothelial growth factor (VEGF) expression induced by NF-кB enhances angiogenesis. Increased DAG synthesis activates protein kinase C (PKC), which results in the further activation of various intracellular signal transduction systems, including extracellular signal-regulated kinases ERK1 and ERK2 (ERK1/2). ERK1/2 has anti-apoptotic effects promoting cell growth and survival and indirectly reduces ghrelin activity by inhibiting proprotein convertase 1/3 (PC1/3), an enzyme necessary for the cleavage of pro-ghrelin into mature ghrelin in the Golgi apparatus [91,92,93,96,99,100,101,102,103,104].
—METABOLIC EFFECTS via classical Gα q/11 signaling. In this pathway, omega-3-fatty acids (Ω-3 FAs), acting as a ligand and agonist of GPR120, activate the Gαq/11-linked phospholipase C-beta (PLCβ). After PLCβ activation, phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolyzes into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. IP3 binds to receptors, the conformational changes of which, after activation, lead to the mobilization of calcium ions (Ca2+) from intracellular stores. The following cytosolic Ca2+ influx stimulates the release of both cholecystokinin (CCK) and incretin hormones, including glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP, also known as glucose-dependent insulinotropic polypeptide). Incretin hormones support the survival and proliferation of pancreatic β-cells, which translates into increased insulin secretion. Moreover, the increase in Ca2+ concentration activates the phosphoinositide 3-kinase (PI3K) pathway including Akt, which stimulates downstream anabolic signaling, favoring cell growth (adipogenesis), proliferation, (angiogenesis), and survival. Upregulated glucose transporter type 4 (GLUT4) activity promotes increased glucose uptake, whereas augmented vascular endothelial growth factor (VEGF) expression induced by NF-кB enhances angiogenesis. Increased DAG synthesis activates protein kinase C (PKC), which results in the further activation of various intracellular signal transduction systems, including extracellular signal-regulated kinases ERK1 and ERK2 (ERK1/2). ERK1/2 has anti-apoptotic effects promoting cell growth and survival and indirectly reduces ghrelin activity by inhibiting proprotein convertase 1/3 (PC1/3), an enzyme necessary for the cleavage of pro-ghrelin into mature ghrelin in the Golgi apparatus [91,92,93,96,99,100,101,102,103,104].  —METABOLIC EFFECTS via Gα i/o signaling. Gαi/o affects related signaling pathways through the inhibition of adenylate cyclase (AC) activity, influencing intracellular cyclic adenosine 3,5-monophosphate (cAMP) levels in ghrelin-producing cells with a subsequent decrease in ghrelin synthesis. In this mechanism, the inhibitory effect of ghrelin on insulin secretion in pancreatic islet β-cells is reduced [99,105]. a PC1/3 is synthesized and expressed in a variety of tissues such as the neuroendocrine cells in the arcuate and paraventricular nuclei of the hypothalamus, the enteroendocrine cells (gut), and in β cells of the pancreas. b CCK is produced in the intestinal epithelial endocrine I-cells of the small intestine (mainly in the duodenum) and various neurons in the gastrointestinal tract and central nervous system (CNS). c GLP-1 and GIP are produced in the intestinal epithelial endocrine L cells and K cells, respectively. Since 2003, when Fredriksson et al. discovered the GPR120/FFAR4 protein [43,106], interest in this receptor has consistently increased as further reports of the anti-inflammatory and metabolic effects of its agonists have appeared [99,107,108,109,110,111,112].
—METABOLIC EFFECTS via Gα i/o signaling. Gαi/o affects related signaling pathways through the inhibition of adenylate cyclase (AC) activity, influencing intracellular cyclic adenosine 3,5-monophosphate (cAMP) levels in ghrelin-producing cells with a subsequent decrease in ghrelin synthesis. In this mechanism, the inhibitory effect of ghrelin on insulin secretion in pancreatic islet β-cells is reduced [99,105]. a PC1/3 is synthesized and expressed in a variety of tissues such as the neuroendocrine cells in the arcuate and paraventricular nuclei of the hypothalamus, the enteroendocrine cells (gut), and in β cells of the pancreas. b CCK is produced in the intestinal epithelial endocrine I-cells of the small intestine (mainly in the duodenum) and various neurons in the gastrointestinal tract and central nervous system (CNS). c GLP-1 and GIP are produced in the intestinal epithelial endocrine L cells and K cells, respectively. Since 2003, when Fredriksson et al. discovered the GPR120/FFAR4 protein [43,106], interest in this receptor has consistently increased as further reports of the anti-inflammatory and metabolic effects of its agonists have appeared [99,107,108,109,110,111,112].
 —ANTI-INFLAMMATORY EFFECTS via beta-arrestin 2 (ARRβ2) signaling in immune pro-inflammatory cells. After receptor stimulation, the adapter protein ARRβ2 is recruited, which, after forming a complex with GPR120, leads to the desensitization of GPR120 as well as other receptors [e.g., Toll-like receptor 4 (TLR4), interleukin 1 receptor (IL1R), and tumor necrosis factor receptor (TNFR)] to pro-inflammatory stimuli. The GPR120- ARRβ2 complex internalizes and interacts with TGF-β-activated kinase 1 (MAP3K7)-binding protein 1 (TAB1) and NLRP3 [nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR) family member 3] inflammasome, which leads to the inhibition of the subsequent inflammatory cascade. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB) essential modulator (NEMO) ubiquitination, which is crucial for the activation of the canonical NF-κB signaling pathway, does not occur. A decrease in the concentration of pro-inflammatory cytokines indirectly modulates adipose tissue cell functions by reducing insulin resistance and, consequently, stimulating glucose uptake [82,94,95,96,97,98].
—ANTI-INFLAMMATORY EFFECTS via beta-arrestin 2 (ARRβ2) signaling in immune pro-inflammatory cells. After receptor stimulation, the adapter protein ARRβ2 is recruited, which, after forming a complex with GPR120, leads to the desensitization of GPR120 as well as other receptors [e.g., Toll-like receptor 4 (TLR4), interleukin 1 receptor (IL1R), and tumor necrosis factor receptor (TNFR)] to pro-inflammatory stimuli. The GPR120- ARRβ2 complex internalizes and interacts with TGF-β-activated kinase 1 (MAP3K7)-binding protein 1 (TAB1) and NLRP3 [nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR) family member 3] inflammasome, which leads to the inhibition of the subsequent inflammatory cascade. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB) essential modulator (NEMO) ubiquitination, which is crucial for the activation of the canonical NF-κB signaling pathway, does not occur. A decrease in the concentration of pro-inflammatory cytokines indirectly modulates adipose tissue cell functions by reducing insulin resistance and, consequently, stimulating glucose uptake [82,94,95,96,97,98].  —METABOLIC EFFECTS via classical Gα q/11 signaling. In this pathway, omega-3-fatty acids (Ω-3 FAs), acting as a ligand and agonist of GPR120, activate the Gαq/11-linked phospholipase C-beta (PLCβ). After PLCβ activation, phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolyzes into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. IP3 binds to receptors, the conformational changes of which, after activation, lead to the mobilization of calcium ions (Ca2+) from intracellular stores. The following cytosolic Ca2+ influx stimulates the release of both cholecystokinin (CCK) and incretin hormones, including glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP, also known as glucose-dependent insulinotropic polypeptide). Incretin hormones support the survival and proliferation of pancreatic β-cells, which translates into increased insulin secretion. Moreover, the increase in Ca2+ concentration activates the phosphoinositide 3-kinase (PI3K) pathway including Akt, which stimulates downstream anabolic signaling, favoring cell growth (adipogenesis), proliferation, (angiogenesis), and survival. Upregulated glucose transporter type 4 (GLUT4) activity promotes increased glucose uptake, whereas augmented vascular endothelial growth factor (VEGF) expression induced by NF-кB enhances angiogenesis. Increased DAG synthesis activates protein kinase C (PKC), which results in the further activation of various intracellular signal transduction systems, including extracellular signal-regulated kinases ERK1 and ERK2 (ERK1/2). ERK1/2 has anti-apoptotic effects promoting cell growth and survival and indirectly reduces ghrelin activity by inhibiting proprotein convertase 1/3 (PC1/3), an enzyme necessary for the cleavage of pro-ghrelin into mature ghrelin in the Golgi apparatus [91,92,93,96,99,100,101,102,103,104].
—METABOLIC EFFECTS via classical Gα q/11 signaling. In this pathway, omega-3-fatty acids (Ω-3 FAs), acting as a ligand and agonist of GPR120, activate the Gαq/11-linked phospholipase C-beta (PLCβ). After PLCβ activation, phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolyzes into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. IP3 binds to receptors, the conformational changes of which, after activation, lead to the mobilization of calcium ions (Ca2+) from intracellular stores. The following cytosolic Ca2+ influx stimulates the release of both cholecystokinin (CCK) and incretin hormones, including glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP, also known as glucose-dependent insulinotropic polypeptide). Incretin hormones support the survival and proliferation of pancreatic β-cells, which translates into increased insulin secretion. Moreover, the increase in Ca2+ concentration activates the phosphoinositide 3-kinase (PI3K) pathway including Akt, which stimulates downstream anabolic signaling, favoring cell growth (adipogenesis), proliferation, (angiogenesis), and survival. Upregulated glucose transporter type 4 (GLUT4) activity promotes increased glucose uptake, whereas augmented vascular endothelial growth factor (VEGF) expression induced by NF-кB enhances angiogenesis. Increased DAG synthesis activates protein kinase C (PKC), which results in the further activation of various intracellular signal transduction systems, including extracellular signal-regulated kinases ERK1 and ERK2 (ERK1/2). ERK1/2 has anti-apoptotic effects promoting cell growth and survival and indirectly reduces ghrelin activity by inhibiting proprotein convertase 1/3 (PC1/3), an enzyme necessary for the cleavage of pro-ghrelin into mature ghrelin in the Golgi apparatus [91,92,93,96,99,100,101,102,103,104].  —METABOLIC EFFECTS via Gα i/o signaling. Gαi/o affects related signaling pathways through the inhibition of adenylate cyclase (AC) activity, influencing intracellular cyclic adenosine 3,5-monophosphate (cAMP) levels in ghrelin-producing cells with a subsequent decrease in ghrelin synthesis. In this mechanism, the inhibitory effect of ghrelin on insulin secretion in pancreatic islet β-cells is reduced [99,105]. a PC1/3 is synthesized and expressed in a variety of tissues such as the neuroendocrine cells in the arcuate and paraventricular nuclei of the hypothalamus, the enteroendocrine cells (gut), and in β cells of the pancreas. b CCK is produced in the intestinal epithelial endocrine I-cells of the small intestine (mainly in the duodenum) and various neurons in the gastrointestinal tract and central nervous system (CNS). c GLP-1 and GIP are produced in the intestinal epithelial endocrine L cells and K cells, respectively. Since 2003, when Fredriksson et al. discovered the GPR120/FFAR4 protein [43,106], interest in this receptor has consistently increased as further reports of the anti-inflammatory and metabolic effects of its agonists have appeared [99,107,108,109,110,111,112].
—METABOLIC EFFECTS via Gα i/o signaling. Gαi/o affects related signaling pathways through the inhibition of adenylate cyclase (AC) activity, influencing intracellular cyclic adenosine 3,5-monophosphate (cAMP) levels in ghrelin-producing cells with a subsequent decrease in ghrelin synthesis. In this mechanism, the inhibitory effect of ghrelin on insulin secretion in pancreatic islet β-cells is reduced [99,105]. a PC1/3 is synthesized and expressed in a variety of tissues such as the neuroendocrine cells in the arcuate and paraventricular nuclei of the hypothalamus, the enteroendocrine cells (gut), and in β cells of the pancreas. b CCK is produced in the intestinal epithelial endocrine I-cells of the small intestine (mainly in the duodenum) and various neurons in the gastrointestinal tract and central nervous system (CNS). c GLP-1 and GIP are produced in the intestinal epithelial endocrine L cells and K cells, respectively. Since 2003, when Fredriksson et al. discovered the GPR120/FFAR4 protein [43,106], interest in this receptor has consistently increased as further reports of the anti-inflammatory and metabolic effects of its agonists have appeared [99,107,108,109,110,111,112].
3. Inflammatory Background of Metabolic Diseases
3.1. Inflammatory Response in Obesity
The appearance of a chronic inflammatory component in obesity is a direct derivative of IR. Under physiological conditions, insulin not only provides glycemic control but also has anti-inflammatory effects [113]. Under the influence of insulin, the nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR) family member 3 (NLRP3) inflammasome, which is extremely important for the innate immune system, is downregulated [114]. This immunomodulation significantly decreases the activation of caspase-1 and the secretion of the pro-inflammatory cytokines IL-1β/IL-18 in response to cell damage and bacterial infection. Accordingly, in vitro and in vivo studies have shown that the alleviation of inflammation symptoms after a pretreatment with insulin is accompanied by the significant inhibition of nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) activation along with a subsequent decrease in the expression of pro-inflammatory cytokines [115,116]. Chronic IR, which deprives insulin of its effective hypoglycemic and anti-inflammatory effects, leads to the persistence of low-grade inflammation, which is typically observed in obese individuals [117]. The paradox is that this process usually happens despite hyperinsulinemia. Moreover, a vicious cycle occurs in which excess visceral adipose tissue (AT) in obese individuals initiates a chronic low-grade inflammatory background that interferes with insulin signaling via insulin receptors (INSRs), thereby intensifying IR [28]. Moreover, hyperglycemia, which is a derivative of IR itself (without additional metabolic factors), stimulates a primarily defensive inflammatory response, which is associated with the release of numerous inflammatory cytokines, including interferon gamma (IFN-γ), tumor necrosis factor alpha (TNFα), interleukins (IL-1β, IL-6, and IL-8), and interleukin 1 receptor antagonist (IL-1RA), and poses a real threat to the proper functioning of organs [118]. This response is accompanied by a change in the profile and ratio of classically activated (M1) and alternatively activated (M2) macrophages, which are essential regulators of inflammation, in a pro-inflammatory direction [119]. This chronic state of inflammation, termed “meta-inflammation” and mediated by macrophages in AT (and also in the colon, liver, and muscles), involves interactions with components of the adaptive immune response through complex and not fully understood mechanisms [120,121,122]. Therefore, the disturbed balance of Th1/Th2 cytokine profiles resulting from the shift from Th2 cells to Th1 cells and additionally toward Th17 cells and cytotoxic T lymphocytes (CTLs) is not simply the result of the direct impact of IR on lymphocyte subpopulations [123].
In particular, low-grade systemic inflammation and increased reactive oxygen species (ROS) production (oxidative stress) can be observed in individuals with visceral obesity [124]. Visceral obesity, which is characterized by an increased mass of AT surrounding intra-abdominal organs, is also referred to as abdominal or central obesity [125]. Visceral obesity itself is an independent component of metabolic syndrome, the cluster of metabolic factors that also includes high blood pressure, impaired fasting glucose levels, high triglyceride levels, and low high-density lipoprotein (HDL) cholesterol levels [126]. Metabolic syndrome greatly increases the risks of developing diabetes, heart disease, stroke, or all three, and visceral fat accumulation appears to be an accurate predictor of metabolic syndrome [127,128,129]. In other words, visceral obesity may be a partial marker of a dysmetabolic condition that predisposes individuals to the occurrence of diabetogenic, atherogenic, prothrombotic, and pro-inflammatory events and their consequences (complications) [117,130,131]. Moreover, the disruption of numerous endocrine, metabolic, and immunological functions of AT, which is especially pronounced in individuals with visceral obesity, increases the risk of cancer development, including breast cancer, esophageal adenocarcinoma, and colorectal adenocarcinoma [132,133,134].
Notably, the inflammatory process accompanying excess AT is not limited to AT but is generalized (systemic) [135]. A large body of evidence indicates that the regional distribution of body fat, rather than overall obesity, is linked to systemic inflammation. Thus, increased visceral fat promotes generalized inflammation [136,137]. The inflammatory response, especially an increased tendency toward macrophage infiltration, spreads to other tissues, and modifications of the cell environment caused by excess FFAs and increases in the concentrations of growth factors and pro-inflammatory cytokines have been observed in the liver, pancreas, heart, and skeletal muscles [138,139]. For example, among the activities of enzymes and other molecules produced by visceral fat and released from macrophages, the adipocytokine visfatin/nicotinamide phosphoribosyl transferase (NAMPT) has been linked to several inflammatory conditions outside AT [117,140,141]. Unlike the intracellular form of Visfatin/NAMPT (iNAMPT), which plays a regulatory role in NAD+ biosynthesis and thereby affects many NAD-dependent proteins [e.g., sirtuins, poly ADP–ribose polymerases (PARPs), mono ADP–ribosyl transferases (MARTs), and ADP–ribosyl cyclases (CD38/157)], the extracellular form (eNAMPT) can upregulate pro-inflammatory cytokines and matrix metalloproteinases (MMPs) in various types of cells, with potentially important effects on glucose metabolism and atherosclerosis [142,143]. Serum visfatin/NAMPT levels are significantly higher in obese subjects than in control subjects [144].
The multifactorial pathomechanism of the development of IR and the inflammatory response in individuals with visceral obesity is summarized in Figure 3.
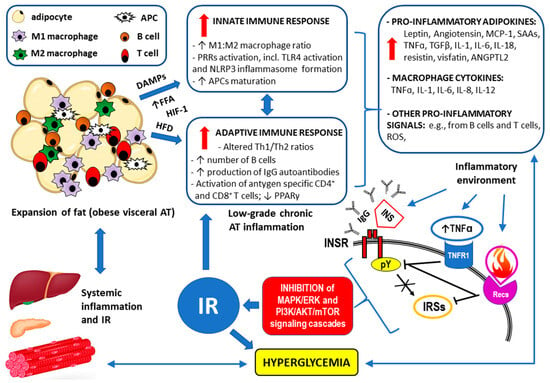
Figure 3.
Summary of the mechanisms by which visceral obesity is a triggering factor for both systemic inflammation and insulin resistance (IR). Adopted from [28]. Strongly enlarged adipocytes in the obese visceral adipose tissue (AT) fail to maintain longer metabolic homeostasis because the lipid overload leads to endoplasmic reticulum stress, increased expression of the inflammation regulator NF-kB, and the production of inflammation-inducing signals [135]. This chronic metabolism-induced inflammation or meta-inflammation in obese AT activates the resident immune cells, including macrophages, B cells, T cells, and antigen-presenting cells (APCs) [145]. A high-fat diet (HFD), an increased flux of free fatty acids (FFAs) accumulating damage-associated molecular patterns (DAMPs) from necrotic AT, and hypoxia-induced factor 1 (HIF-1) trigger both innate and adaptive immune responses [135]. The resulting low-grade chronic AT inflammation manifests as significantly elevated levels of the pro-inflammatory adipokines and cytokines as well as through the overproduction of reactive oxygen species (ROS) [117,126,130,146,147,148,149,150]. Such an inflammatory environment interferes with insulin signaling via the insulin receptor (INSR) through antibodies/autoantibodies against both insulin (INS) and INSR [151,152]; tumor necrosis factor receptor (TNFR1) through the inhibition of tyrosine autophosphorylation (pY) within the INSR and/or serine/threonine phosphorylation (pS) of the insulin receptor substrates (IRSs) [152,153,154,155]; and receptors for other pro-inflammatory cytokines (Recs) through abnormal, similar to that observed for TNFR1 signaling, phosphorylation of the INSR and IRSs [153,154,156]. Disrupted downstream signaling via the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathways eventually lead to IR. The resulting hyperglycemia may increase the inflammatory response by itself and lead to systemic inflammation [157,158,159]. The remaining abbreviations: ANGPTL2—angiopoietin-like 2 protein; IL-1, IL-6, IL-8, IL-12, IL-18—the respective interleukins; MCP-1—monocyte chemoattractant protein-1 (also known as CCL2); NLRP3 inflammasome—leucine-rich repeat (LRR)-containing proteins (NLR) family member 3 inflammasome; SAAs—sulfur amino acids; TGFβ—transforming growth factor β.
3.2. Anti-Inflammatory and Metabolic Effects of GPR120 Signaling in the Context of Overweight and Obesity
In overweight and obese individuals, significant homeostasis disorders are observed at the level of adipose tissue, cells lining the digestive tract that produce gastrointestinal hormones, and the endocrine function of the pancreas [99,160,161,162]. The vast majority of cells present in all these locations express GPR120. Therefore, it is important to determine whether obesity-induced changes in the functional GPR120 signaling pathways that may cause metabolism-induced inflammation (meta-inflammation) are justified [97,163,164]. This cause-and-effect relationship takes a new dimension after considering reports that the GPR120 genetic variant p.Arg270His is detected more often in obese people and that this genetic variant is functionally associated with obesity in humans [165].
In the context of such comprehensive disturbances of GPR120 signaling in overweight and obesity, the importance of proper nutrition should be taken into account [166]. In the diet, in addition to individually selected caloric restrictions in order to reduce body weight, attention should be paid to food quality, including healthy (monounsaturated and polyunsaturated) fats and sufficient micronutrient content [2,3,4,167]. For example, coconut oil products containing lauric acid, whose weight-controlling effects are well known, may have a beneficial effect on metabolism by providing effective ligands for GPR84 (MCFAs), GPR40 (LCFAs), and GPR120 (LCFAs) [168,169,170]. Additionally, actions aimed at reducing the environmental contamination of food with various toxins with a potential impact on GPRs signaling may be important [1,171,172].
3.2.1. GPR120 in Adipose Tissue
The importance of AT in the body extends far beyond its role in maintaining energy homeostasis through energy storage and release, insulation from cold and heat, or cushioning around soft organs. Adipocytes represent a unique population of specialized cells involved in endocrine, nervous, and immune functions, which translate into the regulation of glucose and cholesterol levels; the maintenance of insulin sensitivity; the metabolism of sex hormones; the regulation of hunger and satiety; and, in cooperation with resident immune cells, the regulation of the immune/inflammatory response via secreted adipokines [166,173,174,175].
Two types of adipose tissue have been identified in mammals: white adipose tissue (WAT), which is the body’s main energy storage, and brown adipose tissue (BAT), which provides resistance to cold and obesity through adaptive heat production, known as nonshivering thermogenesis. BAT is a homogeneous tissue, and cells are scattered within WAT and are independent of the developmental origin; these cells are called “brown-in-white” (brite) or beige cells [176]. These brite (beige) cells are also called “inducible brown adipocytes” because they can be induced by cold and a broad spectrum of pharmacological substances [177]. In mammals, brown and beige adipocytes constitute two populations of thermogenic adipocytes with different thermogenic pathways. Thermogenic adipocytes express high levels of uncoupling protein 1 (UCP1) to dissipate energy in the form of heat by uncoupling the mitochondrial proton gradient from mitochondrial respiration. Regardless of the mechanism of thermogenesis involving UCP1, which is considered the main mechanism for BAT and crucial for systemic energy homeostasis, the existence of UCP1-independent thermogenic pathways in thermogenic adipocytes related to creatine–substrate cycling and Ca2+ cycling has been confirmed [178,179,180].
The development of BAT and/or brite cell induction methods may constitute a breakthrough in the treatment of obesity, as some studies have shown that BMI and body fat percentage both have significant negative correlations with BAT, whereas the resting metabolic rate has a significant positive correlation [181,182]. However, some research results limit the positive correlation of BAT volume with whole-body adiposity only to the population of young men, without confirming such a correlation in women [183].
Both WAT and BAT/brite cells express GPR120. High GPR120 expression has been detected in mature 3T3-L1 cells, a cell line often used in the study of white adipocytes [184,185,186]. Interestingly, GPR120 cannot be detected in preadipocytes, but an incubation in MDI differentiation medium containing insulin, isobutylmethylxanthine, and dexamethasone induces and increases GPR120 expression in vitro [185]. These findings and the observation that GPR120 knockdown leads to the inhibition of adipogenesis suggest the involvement of GPR120 as an adipogenic receptor in the process of adipocyte differentiation [87,187,188]. During the differentiation of 3T3-L1 adipocytes, GPR120 signaling promotes adipogenesis by increasing both peroxisome proliferator-activated receptor gamma (PPARγ) expression via Ca2+ influx and the phosphorylation of ERK1/2 [189].
Similarly to studies of rodents, experiments in humans revealed significantly higher expression of GPR120 in the WAT of obese individuals than in that of lean individuals [187]. This result may suggest that high lipid intake in rodents and humans induces increased expression of this receptor. In turn, increased fat oxidation in BAT observed after exposure to cold is correlated with upregulated GPR120 expression [190]. Moreover, the activation of GPR120 in BAT can promote the browning of white fat in mice [189]. The activation of the lipid sensor GPR120 is accompanied by increased secretion of fibroblast growth factor-21 (FGF-21) by BAT and brite cells, the concentration of which increases in the blood. Consistently, knockout of the FGF-21 gene causes a severe impairment of WAT browning [191].
Notably, GPR120 signaling in WAT is associated with anti-inflammatory and insulin-sensitizing effects [93]. In particular, the anti-inflammatory effects of Ω-3 FAs in WAT mediated by GPR120 are potentially therapeutically important in obesity, where low-grade chronic inflammation is a measure of adipocyte dysfunction [93,192]. This typical inflammatory state in obese individuals may most likely be a consequence of impaired signaling via GPR120 in BAT/brite cells, leading to a reduction in the BAT content as a result of the conversion of brown adipocytes to white-like unilocular cells [190,193]. Such BAT whitening can be induced by many factors, including a high ambient temperature, leptin receptor deficiency, impaired β-adrenergic signaling, and lipase deficiency, each of which is capable of inducing macrophage infiltration, brown adipocyte death, and crown-like structure (CLS) formation—a histologic marker of local inflammation with damaged or necrotic adipocytes surrounded by macrophages. A gene expression analysis revealed that BAT whitening in triglyceride lipase-deficient mice was associated with a strong inflammatory response and activation of the NLRP3 inflammasome, which consequently led to increased susceptibility of enlarged brown adipocytes to death [193]. A continuation of the above studies is necessary because the results of other experiments using GPR120-knockout mice may indicate that GPR120 signaling is not required or is not solely responsible for the anti-inflammatory and insulin-sensitizing effects mediated by Ω-3 FAs [194,195].
In summary, in adipose tissue, GPR120 plays key roles in adipogenesis, energy metabolism, and inflammation [185].
3.2.2. GPR120 and Gastrointestinal Hormones
GPR120 is highly expressed in human and murine intestines and is associated with the fatty acid-induced secretion of cholecystokinin and the incretin glucagon-like peptide (GLP)-1 [196,197]. This increased expression of GPR120 in the intestinal epithelium suggests that the secretion of gastrointestinal hormones (or gut hormones) may be regulated by this receptor [97]. Disturbances in this regulation in relation to cholecystokinin (CCK) and glucagon-like peptide-1 (GLP-1), hormones that regulate appetite and food intake, promote weight loss, and improve hyperglycemia, may be important in the context of the pathomechanism of obesity [198,199,200,201,202,203]. Moreover, oil-induced secretion of another major incretin, gastric inhibitory polypeptide (GIP; also known as glucose-dependent insulinotropic polypeptide), from K cells located in the upper small intestine, is mediated by GPR120 through CCK [204,205,206,207]. As an anabolic hormone, GIP plays primary roles in lipid metabolism and the development of obesity by stimulating lipogenesis and blocking lipolysis. Chronic consumption of a high-fat diet leads to functional hyperplasia of K cells, accompanied by an increase in GIP concentrations. The results of recent studies indicate that the inhibition of the GPR120 signaling pathway in the intestine ameliorates IR and nonalcoholic steatohepatitis in mice maintained on a high-fat diet. This effect is accompanied by a reduction in GIP and CCK secretion [208,209]. However, some controversy exists, as reduced expression of GPR120/FFAR4 has been documented in cases of extreme obesity and IR in children. Researchers have speculated that this decrease in GPR120 expression, which is associated with extreme obesity and parameters indicative of obesity comorbidities in children, may be due in part to the presence of the C allele of the rs11187533 GPR120 single-nucleotide polymorphism (SNP) [210]. GPR120 gene variants associated with obesity have been identified in humans and dogs (p.Arg270His and p.Pro199Thr, respectively) [165,187].
3.2.3. GPR120 and the Endocrine Function of the Pancreas
In addition to incretin hormones and glycemia regulation in the gut via the stimulation of insulin secretion after nutrient intake, GPR120 signaling affects the pancreas directly. Significant GPR120 expression was detected in the endo pancreas. Inhibitory GPR120 signaling in pancreatic islet δ cells contributes to both insulin and glucagon secretion in part by mitigating somatostatin (SRIF—somatotropin release inhibiting factor, also known as GHIH—growth hormone-inhibiting hormone) release, a powerful paracrine inhibitor of both insulin and glucagon release from islet β-cells and α-cells, respectively [211,212,213]. Consequently, GPR120 activation promotes glucose-stimulated insulin secretion (GSIS), enhances arginine-induced glucagon secretion, and inhibits glucose-stimulated somatostatin secretion (GSSS) [109,212,214].
Given that δ cells are electrically excitable, glucose can stimulate action potential firing and SRIF secretion through both metabolic and nonmetabolic effects [213,215]. In addition, membrane potential-independent pathways of glucose-induced SRIF secretion, involving cAMP-dependent stimulation of calcium-induced calcium release (CICR) and exocytosis of SRIF, are of greater quantitative importance [216].
The stimulation of insulin and glucagon secretion following GPR120 activation in δ cells constitutes an opposing mechanism to the SRIF secretion-stimulating effect of urocortin 3 (UCN3). UCN3 produced and released by pancreatic β cells activates the corticotropin-releasing factor type-2 (CRF2) receptor (CRF2R) and downstream pathways mediated by different G protein or arrestin subtypes in δ cells, resulting in SRIF gene upregulation along with the subsequent inhibition of both insulin and glucagon release, which is a part of an important feedback circuit for glucose homeostasis [217,218].
Further studies have shown that although GPR120 is preferentially expressed in pancreatic δ cells, its expression is not selective within the pancreatic islets of Langerhans, as it also affects β and α cells [164,212]. GPR120 activation in β cells reduces their dysfunction and susceptibility to apoptosis, which has a significant effect on islet hormone secretion [164,219]. For example, GPR120 signaling protects against lipotoxicity-induced pancreatic β-cell dysfunction through the regulation of pancreatic duodenal homeobox-1 (PDX1) expression and the inhibition of islet inflammation [164]. Studies examining the protective effects of polyunsaturated fatty acids (PUFAs) on β-cells have shown that docosahexaenoic acid attenuates palmitate-induced apoptosis by increasing autophagy through the GPR120/mTOR axis [220].
In obese individuals both with and without diabetes, the insulinotropic effects of GPR120 agonists are altered. Under these conditions, impaired GPR120 expression in β-cells was observed, accompanied by increased peroxisome proliferator-activated receptor γ (PPARγ) expression [221]. Promising results from the latest research on obesity and IR indicate the therapeutic potential of GPR120 activation, especially in combination with incretin (GLP-1), in enhancing the activity of dipeptidyl peptidase-IV (DPP-IV) inhibitors, which effectively stimulate the proliferation of β-cells [222,223,224,225].
4. Targeting GPR120 Signaling as a Promising Therapeutic Approach in Obesity: The Need for New Ligands
The use of GPR120 agonists may be beneficial in the treatment of metabolic diseases, especially those involving IR and impaired glucose tolerance, as well as chronic low-grade inflammation, which involves mainly adipose tissue in obese individuals [99,226,227,228]. Moreover, GPR120 agonists/antagonists can be used as specific anticancer therapies [229,230]. The above actions cannot be achieved selectively after the stimulation/inhibition of GPR120, but the dominant effect observed may be a result of the expression of GPR120 in a particular tissue and the lack/severity of pathological changes (e.g., intensity of the inflammatory response or progression of cancer) [32,99,231]. However, for most GPR120 agonists, separating these effects would be inappropriate, as overweight and obesity are usually accompanied by IR, T2D, and an increased inflammatory response [99,232].
The in vivo application of the anti-inflammatory and insulin-sensitizing effects associated with GPR120 stimulation, which are promising tools for the prevention and treatment of obesity, is very difficult [233]. This difficulty is caused not only by interspecies differences between the sequences encoded by the GPR120 gene in humans and laboratory animals (primarily rodents) but also by the existence of two splice variants in humans: short GPR120 (GPR120S) and long GPR120 (GPR120L) [73,75,77,79]. First, natural ligands detected by GPR120 in the form of long-chain FFAs, both saturated (14–18 carbon atoms) and unsaturated (6–22 carbon atoms), can be easily and quickly transformed (including being broken down) in vivo into other biologically active compounds [234,235]. This conversion may lead to a state in which the resulting compounds do not interact with GCPRs for LCFAs (GPR40/FFAR1 and GPR120/FFAR4) but exert biological effects through receptors for SCFAs (GPR41 and GPR43) and MCFAs (GPR84) [68,69,70,71]. Controlling the LCFA conversion process in vivo is a major challenge, which makes the results of studies on the effects of fatty acids difficult to interpret unambiguously. Moreover, as already mentioned, GPR120 expression depends on the cell type and the dietary contents of EPA, DHA, and ALA [82,236]. Therefore, the identification/isolation of new selective ligands existing in nature and, above all, the development of synthetic GPR120 ligands become crucial for a detailed and selective examination of the signaling pathways of this receptor, especially in the context of therapeutic possibilities [72,233,237,238].
With respect to the selectivity of the actions of such synthetic ligands, although GPR120 and GPR40 are physiologically activated by the same class of fatty acids (LCFAs), their structural relatedness is distant. For example, the amino acid residues involved in endogenous ligand recognition by GPR120 are similar to sphingosine-1-phosphate receptor-1 (S1P1), which is a target of the lipid signaling molecule sphingosine-1-phosphate (S1P), whereas the basis of recognition of fatty acids by GPR40 is similar to that of the SCFA receptors GPR41 and GPR43 [71].
Under in vivo conditions, researchers have not completely excluded the possibility that the anti-inflammatory effect of EPA or the increase in body weight loss in DHA-treated mice results from the activation of other molecular targets, including the transcription factors peroxisome proliferator-activated receptor alpha (PPARα) and retinoid X receptor alpha (RXRα) [72]. The conversion of fatty acids to specialized pro-resolving mediators (SPMs), such as lipoxins, resolvins, protectins, and maresins, may also be important [239]. The list of endogenous GPR120 ligands is also subject to modification. For example, a lipodomic analysis of mice overexpressing the glucose transporter 4 (GLUT4) in adipocytes revealed structures of branched fatty acid esters of hydroxy fatty acids, including palmitic acid-9-hydroxystearic acid (9-PAHSA), which act as GPR120 agonists. In adipocytes, 9-PAHSA augmented insulin-stimulated glucose uptake through GPR120 [240]. Moreover, by being involved in the antiobesity mechanism of WAT browning and increasing thermogenic activity, 9-PAHSA may become a key compound in understanding the pathogenesis of obesity. The concomitant anti-inflammatory effect of 9-PAHSA is due to the inhibition of the LPS/NF-κB pathway [241].
4.1. Non-LCFA GPR120 Agonists Derived from Compounds of Natural Origin
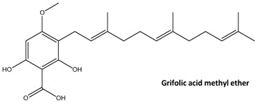
The screening of 80 natural compounds of plant origin, particularly lipids with an acidic structure, allowed the isolation of a series of grifolin derivatives, which were characterized by the ability to activate GPR120 [242]. Compounds such as grifolic acid and grifolic acid methyl ether activated ERK in cells expressing GPR120 (Flp-in GPR120). However, the potencies of these two compounds are significantly lower than that of linolenic acid (LA), which positions them as selective partial GPR120 agonists [74]. In addition, grifolic acid and grifolic acid methyl ether can act as antagonists of GPR120 by inhibiting both LA-induced ERK phosphorylation and LA-induced [Ca2+]i in a dose-dependent manner [242,243]. Grifolic acid, which acts via GPR120, has been shown to promote GLP-1 secretion from intestinal secretin tumor cell line 1 (STC-1) [244]. In studies analyzing the usefulness of grifolic acid in a specific therapy, assays using the macrophage-like RAW264.7 cell line revealed that grifolic acid reduced cell viability in a dose- and time-dependent manner. The apoptotic death of RAW264.7 macrophages was caused by a reduction in the mitochondrial membrane potential (MiMP) and the inhibition of ATP production via a GPR120-independent mechanism [245]. Although unrelated to the topic of this review, the ability of grifolin to induce apoptosis, cell cycle arrest, autophagy, and senescence in human cancer cell lines has attracted the attention of researchers searching for anticancer drugs [246]. An exception of uncertain significance may be apoptosis, which, under conditions of preserved homeostasis, occurs without an inflammatory reaction because the activation of caspases ensures that inflammatory pathways are disabled. However, under specific, altered conditions accompanying obesity, apoptosis and the apoptotic machinery can be rewired into a process that is inflammatory [247,248].
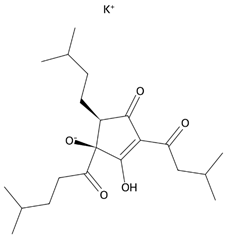
The common hop or hops (Humulus lupulus L.) became the source of KDT501, the potassium salt of the n-(isobutyl) congener of a tetrahydroiso-α-acid, also known as an isohumulone drug [249]. This PPARγ and GPR120 agonist was tested in a small group (n = 9) of obese patients, and thus far, KDT501 is the only GPR120 agonist that has entered phase II clinical trials. After considering the short duration of the study, the small study group, and the lack of selectivity of KDT501, its effectiveness in treating IR, obesity, or metabolic syndrome can be confirmed, as in all cases, a reduction in the levels of systemic inflammation markers and improved postmeal plasma triglyceride levels were observed [250]. Moreover, KDT501 post-transcriptionally induced adiponectin secretion from subcutaneous WAT and increased the expression of thermogenic and lipolytic genes in response to cold exposure [251].
Teadenol A, a polyphenol (1-benzopyran) isolated from Japanese postfermented tea (i.e., produced by microbial fermentation), acts as a novel GPR120 ligand that binds directly and induces ERK1/2 phosphorylation and intracellular Ca2+ store activation with cytoplasmic Ca2+ influx [252]. In a human embryonic kidney (HEK) cell line (293T), these effects were completely dependent on GPR120 expression and correlated with teadenol A-induced GPR120 upregulation. These effects were abolished by the use of the selective GPR120 antagonist AH-7614 (Compound 39). In addition, teadenol A increased the secretion of GLP-1 from intestinal endocrine STC-1 cells, which indicates its indirect effects on metabolic homeostasis, as GLP-1 improves glycemic control and stimulates satiety (suppresses appetite), leading to reductions in food intake and body weight [252].
The characteristics of the substances of natural origin listed above, including the possible therapeutic use of their GPR120 agonist activities in the context of metabolic changes accompanying obesity, are summarized in Table 1.

Table 1.
Examples of naturally occurring GPR120 agonists with potentially beneficial effects in obesity-related metabolic disorders.
4.2. GPR120 Synthetic Agonists
The use of new techniques for computer modeling of the spatial structure of ligands for GPR120 has expanded the potential group of compounds functioning as agonists of this receptor to a previously unavailable scale. For example, in the search for peptides that bind to GPR120, a virtual library was created in which 531,441 low-polarity hexapeptides were collected [224]. The screening of these more than 500,000 peptides, which involved computational work starting with the narrow filtering of hexapeptides based on their chemical similarity to known GPR120 agonists, provided valuable mechanistic insights into GPR120 activation and further extended the knowledge of GPR120 stereochemistry, which is crucial for agonistic ligand recognition. The best hits were tested through docking studies, molecular dynamics, and umbrella sampling simulations, which identified G[I,L]FGGG [the binding affinity (ΔG) of GIFGGG: −6 kCal/mol] as a promising GPR120 agonist sequence. Additionally, D-amino acids may increase the peptide–GPR120 interaction (ΔG of GdIFGGG: −13 kCal/mol) [224]. In the development of predictive models for better real-time monitoring of GPR120–ligand interactions at the nanomolar level in the context of designing new synthetic agonists, the use of fluorescence is an important aid. The use of ligands with fluorescent properties and the high viscosity and low polarity of the ligand binding area, which also show environmental sensitivity, enables the detection of small-molecule sulfonamide agonists for GPR120 [267].
The current classic approach to developing selective, potent GPR120 agonists involves the use of existing crystal structures of other GPCRs [72,225,243,268]. In this way, the key to the activity of the agonist toward GPR120 is the formation of hydrogen bonds between the carboxylic acid residue of the ligand and the guanidine of Arg99 in GPR120 [228]. However, the greatest problem was ensuring high selectivity of the synthetic ligand, especially when GPR40/FFAR1 is coexpressed.
The first synthetic GPR120 agonist was GW9508, although it was originally developed as a highly selective GPR40/FFAR1 orthosteric agonist [269]. GW9508 shows ~100-fold selectivity for GPR40 over GPR120. This selectivity corresponds to the pEC50 values for GW9508, which, in relation to GPR40 and GPR120, are 7.32 and 5.46, respectively [269]. However, GW9508 also activated GPR120 at relatively high concentrations. The weak affinity and lack of selectivity of GW9508 for GPR120 limits the use of this compound in studies of GPR120 stimulation. In the absence of other synthetic ligands, GW9508 is used as a GPR120 antagonist in cell systems in which GPR40 is not detected because of GPR40 knockout (e.g., the RAW 264.7 monocyte-/macrophage-like cell line) [93,196]. GW9508 is inactive against other GPCRs, kinases, proteases, integrins, and PPARs. Its potential therapeutic effects (e.g., anti-atherosclerotic and anti-inflammatory effects) involve promoting insulin secretion depending on the glucose concentration and opening of ATP-sensitive potassium (KATP) channels [270].
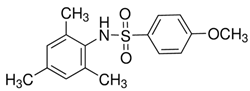
Another trend in the search for GPR120 agonists has been attempts to modify PPAR-γ agonists with N-carbamoyl-beta-D-glucopyranosylamine (NCG). NCG21, a PPAR-γ agonist derivative, shows high (albeit incomplete) selectivity for GPR120 over GPR40 [225]. The problem of very limited selectivity remains; therefore, NCG21 can be used with cell lines and tissues expressing GPR120 in the absence of GPR40 expression to study the role of GPR120 in physiological processes [243]. NCG21, the NCG compound with the lowest calculated hydrogen bonding energy, was determined to be a very strong extracellular signal-regulated kinase (ERK) activator in a cloned GPR120 system. In addition, in murine enteroendocrine STC-1 cells endogenously expressing GPR120, NCG21 potently activated ERK, intracellular calcium responses, and GLP-1 secretion. In the mouse colon, an increase in plasma GLP-1 levels was positively correlated with intracolonic NCG21 administration [243,271].
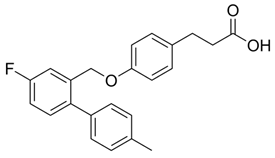
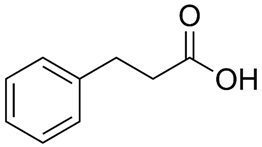
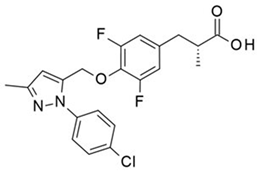
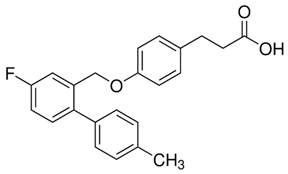
Extensive research by Shimpukade et al. [268] on the relationship between the spatial structures and biological activities of new compounds led to the identification of TUG-891, a derivative of the GPR40 agonist GW9508. Unlike GW9508, TUG-891 proved to be the first GPR120 agonist with high activity and 1000-fold selectivity over human GPR40 in assays based on the induced interactions between the receptor and β-arrestin 2 [268]. TUG-891 has high potency against both human and mouse GPR120 and is a more selective and potent agonist of human GPR120 than ALA, GW9508, or NCG21 [272]. Initially, TUG-891 was widely used in research on the physiological function of GPR120. More extensive studies, however, revealed that the selectivity between human GPR120 and GPR40 was significantly less pronounced in mouse orthologs and variable when G protein-mediated increases in Ca2+ influx versus receptor interactions with arrestin were measured [233]. This difference in the selectivity of TUG-891 between humans and mice is likely not due to the decreased potency of mouse GPR120 but rather to the increased potency of mouse GPR40. Therefore, in practice, TUG-891 should be treated as a strong and selective GPR agonist in studies using human cells and tissues, whereas in the equivalent rodent tissues, TUG-891 should be viewed as a dual GPR120 and GPR40 agonist [233,272]. Hence, the potential utility of TUG-891 in animal studies may be restricted. Additionally, limitations in the use of TUG-891 result from its poor metabolic stability and high lipophilicity (cLogP = 5.88) due to the susceptibility of phenylpropanoic acid to β-oxidation and the presence of a biphenyl structure in the molecule with high lipophilicity [273]. Derivative compounds have been successfully developed to eliminate susceptibility to β-oxidation and the associated loss of GPR120 agonistic activity by TUG-891 [274].
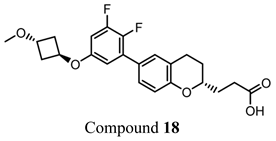
The importance of GPR120 in the regulation of glucose homeostasis, especially through its effect on insulin sensitivity, was proven in studies of highly selective GPR120 agonists in rodents: a phenylpropanoic acid (Compound 29)-based series and a chromane propionic acid (Compound 18)-based series. Compared with GPR40, Compound 29 derivatives showed at least 300-fold greater selectivity for both human and mouse GPR120 [275]. Additionally, Compound 18 derivatives showed significantly increased GPR120 selectivity, which exceeded GPR40 selectivity by 40–130 times across human, mouse, and rat orthologs [276].
Merck (Darmstadt, Germany) patented a highly selective, nontoxic, cell-permeable, and reversible GPR120 superagonist (EC50~0.35 µM) called Compound A (cpdA), which is available in oral form. Compound A is approximately 50-fold more effective than DHA in stimulating GPR120-related responses [277]. The affinity of cpdA for GPR40/FFAR1 and thus the degree of activation of this receptor were negligible. Compared with ω3-FA administration, treatment with cpdA in vitro and in vivo caused concentration-dependent anti-inflammatory and insulin-sensitizing effects [82]. These effects were observed in high-fat-diet-fed obese mice when cpdA was administered at a dose of 30 mg/kg [187,277]. The anti-inflammatory effect was observed in macrophages, where cpdA strongly inhibited LPS-induced TAK1, IKKβ, and JNK phosphorylation and reduced IκB degradation [274]. Using cpdA, researchers have also confirmed the beneficial immunomodulatory effects of DHA on the treatment of atopic dermatitis, which involves an increase in the Foxp3+ Treg population after GPR120 stimulation [278].
Metabolex 36 (3-(3,5-difluoro-4-((3-methyl-1-phenyl-1H-pyrazol-5-yl)methoxy)phenyl)-2-methylpropanoic acid) was developed by the company Metabolex (currently Cymabay Therapeutics) as a potent, selective agonist of human and mouse GPR120. The selectivity of Metabolex 36 toward GPR120 was confirmed by the lack of GPR40/FFAR1 activation, as determined via a calcium mobilization assay [279]. The dynamic mass redistribution (DMR) response and cAMP production were induced by Metabolex 36 in GPR120-overexpressing cells. However, in mouse islets, this compound inhibits cAMP production, and in a murine neuroendocrine cell line (STC-1), Metabolex 36 increases GLP-1 secretion. In studies of lean mice, Metabolex 36 (30 mg/kg) improves oral glucose tolerance and increases insulin secretion during the intravenous glucose tolerance test (IVGTT). This GPR120 agonist inhibits glucose-induced somatostatin secretion in a dose-dependent manner when murine pancreatic islets are incubated under either maximal stimulating conditions (16.6 mmol/L) or with intermediate glucose concentrations (8 mmol/L). Maximal inhibition was achieved with 30 μmol/L of the ligand. Under both basal conditions and in the presence of a stimulating glucose concentration (16.6 mmol/L), Metabolex 36 has no effect on insulin secretion [211].
Developed later than Metabolex 36, the compound AZ13581837 was a selective human and mouse GPR120 agonist and was able to induce an approximately 100-fold greater DMR response in CHO-hGPR120/mGPR120 cells (EC50 values of 5.2 nM and 4.3 nM, respectively) than Metabolex 36 [279]. AZ13581837 selectively induces a calcium response in CHO-hGPR120 cells with an EC50 of 120 nM and induces GPR120-dependent calcium mobilization and cAMP production in GPR120-overexpressing cells. Like Metabolex 36, AZ13581837 (10 µM) inhibits cAMP production in mouse islets and increases GLP-1 secretion from STC-1 cells. When administered at a dose of 18 mg/kg, AZ13581837 improves oral glucose tolerance and increases insulin secretion in lean mice, according to IVGTTs [212,280].
Another synthetic agonist, GPR120 agonist III (3-(4-((4-fluoro-4′-methyl-(1,1′-biphenyl)-2-yl)methoxy)-phenyl)propanoic acid), is fully selective for GPR120 (pEC50 = 7.62) and has negligible activity toward GPR40 [277]. Under the influence of GPR120 agonist III, a concentration-dependent increase in IP3 production was detected in human and mouse cells expressing GPR120, as well as a concentration-dependent response in which β-arrestin-2 was recruited (EC50s~0.35 μM). The significant anti-inflammatory effect of GPR120 agonist III is manifested by the inhibition of the LPS-induced phosphorylation of TAK1, IKKβ, and JNK and the blockade of IκB degradation [281]. To date, the increase in insulin sensitivity induced by GPR120 III agonist at increased glucose infusion rates, the increased rate of insulin-stimulated glucose disposal, and the increased ability of insulin to inhibit hepatic gluconeogenesis have been documented only in WT mice. The use of GPR120 agonist III may have a beneficial effect on hepatic lipid metabolism because it leads to decreased hepatic steatosis and decreased liver triglycerides and DAG levels, along with a reduced saturated FFA content [282].
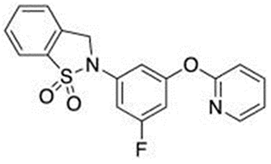
In light of the research that led to the development of GSK137647A, GSK137657A, and TUG-1197 (also known as Compound 34), the presence of a carboxylate that directly interacts with Arg99 of GPR120 is not a sine qua non condition for agonist properties. Unlike most synthetic agonists with a structural similarity to free fatty acids, GSK137647A, GSK137657A, and TUG-1197 are potent nonacidic sulfonamide-containing GPR120 agonists [233,267,283,284]. TUG-1197 has no detectable activity toward GPR40, while GSK137647A shows over 50 times greater selectivity for GPR120 than GPR40, and this selectivity is maintained across species [233]. The finding that the selectivity of GSK137647A toward GPR120 is related to its phenyl ring-conjugated structure was the starting point for the development of new types of agonists based on the core structure of GSK137647A [267]. For example, the sulfonamide agonist GSK137657A has high selectivity and activity in promoting insulin secretion [267]. The lack of the acidic nature of the above compounds did not prevent easy binding within the same orthosteric binding pocket as the carboxylate-containing agonists that resemble synthetic fatty acids [74,233]. The effects of GSK137647A and TUG-1197, among others, on the hypothalamus of obese individuals were tested to assess the impact of GPR120 stimulation on energy homeostasis and GPR120-induced anti-inflammatory effects [285,286]. Even if the poor pharmacokinetic and pharmacodynamic properties of GSK137647A and TUG-1197 are considered, the preliminary results are promising in terms of the possibility of identifying new pharmacological targets for metabolic diseases. However, despite extensive in vitro and in vivo animal studies, none of these compounds, and none of the currently known synthetic selective GPR120 agonists, have entered regular clinical trials with a large number of patients [274].
The characteristics of the selected synthetic agonists listed in this section, including the potential therapeutic use of their GPR120 activities in the context of the metabolic changes accompanying obesity, are summarized in Table 2.

Table 2.
General characteristics of selected synthetic GPR120 agonists showing potentially beneficial effects in obesity-related metabolic disorders.
5. In Summary: Challenges in the Therapeutic Use of GPR120 Agonists and Future Strategies
Table 3 summarizes the fundamental difficulties in developing and implementing GPR120 agonist-based treatments for metabolic disorders in obesity and indicates likely future strategies in this area. The limitations of naturally occurring and synthetic agonists are often similar, while future prospects differ significantly in favor of synthetic ligands.

Table 3.
General limitations in the therapeutic use of GPR120/FFAR4 agonists in obesity-related metabolic disorders and future strategies to overcome them.
6. Concluding Remarks
Recent studies have shown that FFAs not only are sources of energy in the body as nutritional components but also serve as ligands for a group of orphan GPCRs called free fatty acid receptors (FFARs), which activate numerous signaling pathways. Among FFARs, special attention is given to those activated by long-chain saturated and unsaturated FFAs, i.e., GPR40/FFAR1 and GPR120/FFAR4, especially the latter.
Undoubtedly, signaling through GPR120/FFAR4 significantly affects the body’s metabolism and plays a key role in maintaining energy homeostasis in both humans and rodents. Glucoregulatory and insulin-sensitizing/insulin-releasing activities have come to the forefront. They become particularly important in obesity, where metabolic disorders are characterized by IR and glucose intolerance, with an increased incidence of diabetes. Moreover, the high expression of GPR120 in adipocytes, pancreatic islet cells, and enteroendocrine L and K cells of the gastrointestinal tract enables the definition of therapeutic goals that can be achieved using GPR120 agonists. Examples include stimulating thermogenesis in BAT and/or the browning of WAT, promoting direct beneficial effects on pancreatic β-cell health (e.g., anti-inflammatory and immunomodulatory effects), and modulating the secretion of hormones from the gastrointestinal tract and pancreas, including GLP-1/GIP secretory pathways. The expected reduction in chronic low-grade inflammation in adipose tissue associated with GPR120 stimulation should additionally work toward restoring normal metabolism.
Owing to the detailed knowledge of the structure, particularly the 3D conformation of GPR120, the relatively small pool of naturally occurring GPR120 agonists is constantly expanding through the almost unlimited possibilities of developing synthetic ligands. A properly designed structure of such a ligand is likely to direct its action in a more specific way, e.g., with a dominant anti-inflammatory effect, stronger stimulation of thermogenesis, or the promotion of the secretion of incretin hormones and insulin. However, the biological properties of these new GPR120 agonists and/or antagonists must be comprehensively tested in terms of potency, selectivity, or dominant effects under specific clinical conditions as well as in terms of side effects and safety in humans. Current knowledge of their effects on humans is still insufficient, and large-scale clinical trials using synthetic GPR120 agonists have not been conducted. Interspecies differences mean that the results of studies using rodents should be interpreted with caution, but interindividual differences in humans may also be important.
The repeatability of metabolic effects on regulating glycemia and changes in adipose tissue may be influenced by the lack of selectivity of the action of a given agonist. Notably, the lack of selectivity for GPR120 alone does not determine the therapeutic usefulness of a given agonist because, for example, the metabolic effects of simultaneous GPR40 stimulation are largely similar to those observed after GPR120 stimulation and are generally beneficial for obese individuals. The key to the effectiveness of these FFAR agonists is their ability to activate adipose tissue by inducing changes in mitochondrial dynamics, resulting in increased O2 consumption, increased nutrient uptake, and a reduction in the fat mass. Just reducing fat mass should lead to a decrease in the intensity of the inflammatory reaction and improved insulin sensitivity. However, at the current stage, the development of drugs based on GPR120 agonists for the treatment of patients with obesity-related disorders requires conducting many large-scale clinical trials and solving the above-mentioned problems.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created. Instead, the data are quoted from the available cited literature.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
| 7TM receptors | seven transmembrane domain receptors, also known as G protein-coupled receptors (GPCRs) |
| 9-PAHSA | palmitic-acid-9-hydroxy-stearic-acid |
| Ω-3 FAs | omega-3-fatty acids |
| AD | Alzheimer’s disease |
| ALA | alpha-linolenic acid |
| ANGPTL2 | angiopoietin-like 2 protein |
| APCs | antigen-presenting cells |
| ARRβ2 | beta-arrestin 2 |
| AT | adipose tissue |
| ATP | adenosine triphosphate |
| BAT | brown adipose tissue |
| BMI | body mass index |
| cAMP | cyclic adenosine 3,5-monophosphate |
| CCK | cholecystokinin |
| CICR | calcium-induced calcium release |
| CNS | central nervous system |
| cpdA | compound A |
| CRF2 | corticotropin-releasing factor type-2 |
| CRF2R | corticotropin-releasing factor type-2 receptor |
| CTL | cytotoxic T lymphocyte |
| DAG | diacyl glycerol |
| DHA | docosahexaenoic acid |
| DIO | diet-induced obesity |
| DPP-IV | dipeptidyl peptidase-IV |
| EC50 | half maximal effective concentration |
| ECL1–ECL3 | three extracellular loops (from 1 to 3) forming the GPCR |
| eNAMPT | extracellular nicotinamide phosphoribosyl transferase |
| EPA | eicosapentaenoic acid |
| ERK1/2 | extracellular regulated protein kinases 1/2 |
| ERKs | extracellular signal-regulated kinases |
| FDA | U.S. Food and Drug Administration |
| FFAR1 | free fatty acid receptor 1, also known as GPR40 |
| FFAR4 | free fatty acid receptor 4, also known as GPR120 |
| FFAs | free fatty acids |
| FGF-21 | fibroblast growth factor-21 |
| GDP | guanosine diphosphate |
| GEF | guanine nucleotide exchange factor |
| GHIH | growth hormone-inhibiting hormone, also known as somatotropin release inhibiting factor (SRIF) |
| GIP | gastric inhibitory polypeptide, also known as glucose-dependent insulinotropic polypeptide |
| GLP-1 | glucagon-like peptide-1 |
| GLUT4 | glucose transporter type 4 |
| GPCRs | G protein-coupled receptors, also known as seven transmembrane domain (7TM) receptors |
| GPR40 | G protein-coupled receptor 40, also known as FFAR1 |
| GPR120 | G protein-coupled receptor 120, also known as FFAR4 |
| GPR120L | a long form of GPR120, |
| GPR120S | a short form of GPR120 |
| GRAFS | acronym defining classification of GPCRs that stands for glutamate, rhodopsin, adhesion, Frizzled/Taste2, and Secretin |
| GSIS | glucose-stimulated insulin secretion |
| GSSS | glucose-stimulated somatostatin secretion |
| GTP | guanosine triphosphate |
| HbA1c | hemoglobin A1C |
| HDL | high-density lipoprotein |
| HEC | human embryonic kidney |
| HFD | high-fat diet |
| HIF-1 | hypoxia-induced factor 1 |
| ICL1–ICL3 | three intracellular loops (from 1 to 3) forming the GPCR |
| IFN-γ | interferon gamma |
| IKKβ | nuclear factor kappa-B kinase |
| IL1R | interleukin 1 receptor |
| IL-1RA | interleukin 1 receptor antagonist |
| ILs | interleukins, including IL-1β, IL-6, IL-8), |
| iNAMPT | intracellular nicotinamide phosphoribosyl transferase |
| INSR | insulin receptor |
| IP3 | inositol 1,4,5-trisphosphate |
| IR | insulin resistance |
| IRSs | insulin receptor substrates |
| IVGTT | intravenous glucose tolerance test |
| JNK | c-Jun N-terminal kinase |
| KATP | ATP-sensitive potassium channels |
| LA | linolenic acid |
| LCFAs | long-chain fatty acids |
| LPS | lipopolysaccharide |
| MAP3K7 | TGF-β activated kinase 1 |
| M1, M2 macrophages | classically activated and alternatively activated macrophages, respectively |
| MARTs | mono ADP-ribosyl transferases |
| MCFAs | medium-chain fatty acids |
| MCP-1 | monocyte chemoattractant protein-1, also known as CCL2 |
| MMPs | matrix metalloproteinases |
| MiMP | mitochondrial membrane potential |
| NAc | nucleus accumben |
| N.A.D. | denotes no data or ambiguous data |
| NAFLD | nonalcoholic fatty liver disease |
| NAMPT | nicotinamide phosphoribosyl transferase |
| NCG | N-Carbamoyl-Beta-D-Glucopyranosylamine |
| NEMO | nuclear factor kappa-light-chain-enhancer of activated B cells (NF-кB) essential modulator |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 inflammasome | nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR)-containing protein (NLR) family member 3 inflammasome |
| PARPs | poly ADP-ribose polymerases |
| PC1/3 | proprotein convertase 1/3 |
| PDX1 | pancreatic duodenal homeobox-1 |
| pERK1/2 | phosphorylated extracellular regulated protein kinases 1/2 |
| PI3K | phosphoinositide 3-kinase |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PKC | protein kinase C |
| PLC-β | phospholipase C-beta |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| PUFAs | polyunsaturated fatty acids |
| RAW 264.7 | monocyte/macrophage-like cell line |
| ROS | reactive oxygen species |
| RXRα | retinoid X receptor alpha |
| SAAs | sulfur amino acids |
| S1P | sphingosine-1-phosphate |
| S1P1 | sphingosine-1-phosphate receptor-1 |
| SCFAs | short-chain fatty acids |
| SNP | single-nucleotide polymorphism |
| SPM | specialized pro-resolving mediator |
| SRIF | somatotropin release inhibiting factor, also known as growth hormone-inhibiting hormone (GHIH) |
| STC-1 | secretin tumor cell line 1 |
| S-V cells | stromal–vascular cells |
| T2D | type 2 diabetes mellitus |
| TAB1 | TAK1 binding protein 1 |
| TAK1 | TGFβ-activated kinase 1 |
| TGFβ | transforming growth factor-beta |
| Th1, Th2, Th17 | types of lymphocyte subpopulations |
| TLR4 | Toll-like receptor 4 |
| TM1–TM7 | seven transmembrane heptahelical structures (from 1 to 7) forming GPCR |
| TNFR | tumor necrosis factor receptor |
| UCN3 | urocortin 3 |
| UCP1 | uncoupling protein 1 |
| VEGF | vascular endothelial growth factor |
| WAT | white adipose tissue |
| ZDF | Zucker Diabetic Fatty (rats) |
References
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Tummolo, A.; Carella, R.; De Giovanni, D.; Paterno, G.; Simonetti, S.; Tolomeo, M.; Leone, P.; Barile, M. Micronutrient Deficiency in Inherited Metabolic Disorders Requiring Diet Regimen: A Brief Critical Review. Int. J. Mol. Sci. 2023, 24, 17024. [Google Scholar] [CrossRef]
- Nemer, M.; Osman, F.; Said, A. Dietary macro and micronutrients associated with MASLD: Analysis of a national US cohort database. Ann. Hepatol. 2024, 29, 101491. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Harjutsalo, V.; Thorn, L.M.; Freese, R.; Forsblom, C.; Mäkimattila, S.; Groop, P.H. The association between macronutrient intake and the metabolic syndrome and its components in type 1 diabetes. Br. J. Nutr. 2017, 117, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- World Obesity Federation. World Obesity Atlas 2024; World Obesity Federation: London, UK, 2024; Available online: https://data.worldobesity.org/publications/?cat=22 (accessed on 5 February 2025).
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Tirthani, E.; Said, M.S.; Rehman, A. Genetics and Obesity. [Updated 31 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK573068/ (accessed on 5 February 2025).
- Smith, E.N.L.; Chandanathil, M.; Millis, R.M. Epigenetic Mechanisms in Obesity: Broadening Our Understanding of the Disease. Cureus 2023, 15, e47875. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Svensson, S.I.A.; Rohde-Zimmermann, K.; Kovacs, P.; Böttcher, Y. Genetics and Epigenetics in Obesity: What Do We Know so Far? Curr. Obes. Rep. 2023, 12, 482–501. [Google Scholar] [CrossRef]
- Lee, A.; Cardel, M.; Donahoo, W.T. Social and Environmental Factors Influencing Obesity. [Updated 12 October 2019]. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Available online: https://www.ncbi.nlm.nih.gov/books/NBK278977/ (accessed on 10 February 2025).
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Haboubi, H.; Haboubi, N. Adult obesity complications: Challenges and clinical impact. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820934955. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Pinto, K.R.D.; Feckinghaus, C.M.; Hirakata, V.N. Obesity as a predictive factor for chronic kidney disease in adults: Systematic review and meta-analysis. Braz. J. Med. Biol. Res. 2021, 54, e10022. [Google Scholar] [CrossRef]
- Rajesh, Y.; Sarkar, D. Association of Adipose Tissue and Adipokines with Development of Obesity-Induced Liver Cancer. Int. J. Mol. Sci. 2021, 22, 2163. [Google Scholar] [CrossRef]
- Tzenios, N.; Tazanios, M.E.; Chahine, M. The impact of BMI on breast cancer—An updated systematic review and meta-analysis. Medicine 2024, 103, e36831. [Google Scholar] [CrossRef]
- Tzenios, N.; Tazanios, M.E.; Chahine, M. The impact of body mass index on prostate cancer: An updated systematic review and meta-analysis. Medicine 2022, 101, e30191. [Google Scholar] [CrossRef]
- Opio, J.; Wynne, K.; Attia, J.; Oldmeadow, C.; Hancock, S.; Kelly, B.; Inder, K.; McEvoy, M. Metabolic Health, Overweight or Obesity, and Depressive Symptoms among Older Australian Adults. Nutrients 2024, 16, 928. [Google Scholar] [CrossRef]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Guria, S.; Hoory, A.; Das, S.; Chattopadhyay, D.; Mukherjee, S. Adipose tissue macrophages and their role in obesity-associated insulin resistance: An overview of the complex dynamics at play. Biosci. Rep. 2023, 43, BSR20220200. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Szukiewicz, D. Molecular Mechanisms for the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity. Int. J. Mol. Sci. 2023, 24, 9818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G protein-coupled receptors (GPCRs): Advances in structures, mechanisms, and drug discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef]
- Liu, S.; Anderson, P.J.; Rajagopal, S.; Lefkowitz, R.J.; Rockman, H.A. G Protein-Coupled Receptors: A Century of Research and Discovery. Circ. Res. 2024, 135, 174–197. [Google Scholar] [CrossRef]
- Liu, H.D.; Wang, W.B.; Xu, Z.G.; Liu, C.H.; He, D.F.; Du, L.P.; Li, M.Y.; Yu, X.; Sun, J.P. FFA4 receptor (GPR120): A hot target for the development of anti-diabetic therapies. Eur. J. Pharmacol. 2015, 763 Pt B, 160–168. [Google Scholar] [CrossRef]
- Al Mahri, S.; Malik, S.S.; Al Ibrahim, M.; Haji, E.; Dairi, G.; Mohammad, S. Free Fatty Acid Receptors (FFARs) in Adipose: Physiological Role and Therapeutic Outlook. Cells 2022, 11, 750. [Google Scholar] [CrossRef]
- Lefkowitz, R.; Kobilka, B. Royal Swedish Academy of Sciences. The Nobel Prize in Chemistry 2012. Retrieved 10 October 2012. Available online: https://www.nobelprize.org/prizes/chemistry/2012/summary/ (accessed on 10 February 2025).
- Vithani, N.; Todd, T.D.; Singh, S.; Trent, T.; Blumer, K.J.; Bowman, G.R. G Protein Activation Occurs via a Largely Universal Mechanism. J. Phys. Chem. B 2024, 128, 3554–3562. [Google Scholar] [CrossRef]
- Afzal, M.S. G proteins: Binary switches in health and disease. Cent. Eur. J. Immunol. 2020, 45, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Kamato, D.; Thach, L.; Bernard, R.; Chan, V.; Zheng, W.; Kaur, H.; Brimble, M.; Osman, N.; Little, P.J. Structure, Function, Pharmacology, and Therapeutic Potential of the G Protein, Gα/q,11. Front. Cardiovasc. Med. 2015, 2, 14. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Dong, D.; Guo, L.; Dong, X.; Leng, J.; Zhao, B.; Guo, Y.D.; Zhang, N. Research Advances in Heterotrimeric G-Protein α Subunits and Uncanonical G-Protein Coupled Receptors in Plants. Int. J. Mol. Sci. 2021, 22, 8678. [Google Scholar] [CrossRef]
- Rehman, S.; Rahimi, N.; Dimri, M. Biochemistry, G Protein Coupled Receptors. [Updated 30 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK518966/ (accessed on 10 February 2025).
- Odoemelam, C.S.; Percival, B.; Wallis, H.; Chang, M.W.; Ahmad, Z.; Scholey, D.; Burton, E.; Williams, I.H.; Kamerlin, C.L.; Wilson, P.B. G-Protein coupled receptors: Structure and function in drug discovery. RSC Adv. 2020, 10, 36337–36348. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure and function-based drug discovery. Signal Transduct. Target. Ther. 2021, 6, 7. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef]
- Wess, J. Designer GPCRs as Novel Tools to Identify Metabolically Important Signaling Pathways. Front. Endocrinol. 2021, 12, 706957. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. [Google Scholar] [CrossRef]
- Edward Zhou, X.; Melcher, K.; Eric Xu, H. Structural biology of G protein-coupled receptor signaling complexes. Protein Sci. 2019, 28, 487–501. [Google Scholar] [CrossRef]
- Cheng, L.; Xia, F.; Li, Z.; Shen, C.; Yang, Z.; Hou, H.; Sun, S.; Feng, Y.; Yong, X.; Tian, X.; et al. Structure, function and drug discovery of GPCR signaling. Mol. Biomed. 2023, 4, 46. [Google Scholar] [CrossRef]
- Laschet, C.; Dupuis, N.; Hanson, J. The G protein-coupled receptors deorphanization landscape. Biochem. Pharmacol. 2018, 153, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20, G protein-coupled receptors. Br. J. Pharmacol. 2019, 176 (Suppl. S1), S21–S141. [Google Scholar] [CrossRef]
- Santhanam, B.; Sluter, M.; Babu, M.M. Exploring GPCR signaling pathway networks as cancer therapeutic targets. Cell Genom. 2024, 4, 100560. [Google Scholar] [CrossRef] [PubMed]
- Arora, C.; Matic, M.; Bisceglia, L.; Di Chiaro, P.; De Oliveira Rosa, N.; Carli, F.; Clubb, L.; Nemati Fard, L.A.; Kargas, G.; Diaferia, G.R.; et al. The landscape of cancer-rewired GPCR signaling axes. Cell Genom. 2024, 4, 100557. [Google Scholar] [CrossRef]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735, Erratum in Circ. Res. 2018, 123, e34. https://doi.org/10.1161/RES.0000000000000235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, Y.; Jiang, Z.; Qing, H. G Protein-Coupled Receptors (GPCRs) in Alzheimer’s Disease: A Focus on BACE1 Related GPCRs. Front. Aging Neurosci. 2016, 8, 58. [Google Scholar] [CrossRef]
- Wong, T.S.; Li, G.; Li, S.; Gao, W.; Chen, G.; Gan, S.; Zhang, M.; Li, H.; Wu, S.; Du, Y. G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders. Signal Transduct. Target. Ther. 2023, 8, 177. [Google Scholar] [CrossRef]
- Schöneberg, T.; Liebscher, I. Mutations in G Protein-Coupled Receptors: Mechanisms, Pathophysiology and Potential Therapeutic Approaches. Pharmacol. Rev. 2021, 73, 89–119. [Google Scholar] [CrossRef]
- Thompson, M.D.; Percy, M.E.; Cole, D.E.C.; Bichet, D.G.; Hauser, A.S.; Gorvin, C.M. G protein-coupled receptor (GPCR) gene variants and human genetic disease. Crit. Rev. Clin. Lab. Sci. 2024, 61, 317–346. [Google Scholar] [CrossRef] [PubMed]
- Secundo, L.; Snitz, K.; Weissler, K.; Pinchover, L.; Shoenfeld, Y.; Loewenthal, R.; Agmon-Levin, N.; Frumin, I.; Bar-Zvi, D.; Shushan, S.; et al. Individual olfactory perception reveals meaningful nonolfactory genetic information. Proc. Natl. Acad. Sci. USA 2015, 112, 8750–8755. [Google Scholar] [CrossRef]
- Shimada, I.; Ueda, T.; Kofuku, Y.; Eddy, M.T.; Wüthrich, K. GPCR drug discovery: Integrating solution NMR data with crystal and cryo-EM structures. Nat. Rev. Drug Discov. 2019, 18, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Saikia, S.; Bordoloi, M.; Sarmah, R. Established and In-trial GPCR Families in Clinical Trials: A Review for Target Selection. Curr. Drug Targets 2019, 20, 522–539. [Google Scholar] [CrossRef]
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR Drug Targets. Cell 2018, 172, 41–54.e19. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Scholz, N.; Langenhan, T.; Schöneberg, T. Revisiting the classification of adhesion GPCRs. Ann. N. Y. Acad. Sci. 2019, 1456, 80–95. [Google Scholar] [CrossRef]
- Krishnan, A.; Nijmeijer, S.; de Graaf, C.; Schiöth, H.B. Classification, Nomenclature, and Structural Aspects of Adhesion GPCRs. Handb. Exp. Pharmacol. 2016, 234, 15–41. [Google Scholar] [CrossRef]
- Wittlake, A.; Prömel, S.; Schöneberg, T. The Evolutionary History of Vertebrate Adhesion GPCRs and Its Implication on Their Classification. Int. J. Mol. Sci. 2021, 22, 11803. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Wu, M.; Guo, Y.; Guo, W.; Zhong, L.; Cai, X.; Dai, A.; Jang, W.; Shakhnovich, E.I.; et al. Common activation mechanism of class A GPCRs. eLife 2019, 8, e50279. [Google Scholar] [CrossRef]
- Stuttgen, G.M.; Sahoo, D. FFAR4, A New Player in Cardiometabolic Disease? Endocrinology 2021, 162, bqab111. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Gu, Z.; Heid, B.; Akers, R.M.; Jiang, H. Identification and characterization of the bovine G protein-coupled receptor GPR41 and GPR43 genes. J. Dairy Sci. 2009, 92, 2696–2705. [Google Scholar] [CrossRef]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation - Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef]
- Luscombe, V.B.; Lucy, D.; Bataille, C.J.R.; Russell, A.J.; Greaves, D.R. 20 Years an Orphan: Is GPR84 a Plausible Medium-Chain Fatty Acid-Sensing Receptor? DNA Cell Biol. 2020, 39, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.; Alvarez-Curto, E.; Watterson, K.R.; Ulven, T.; Hudson, B.D. Characterizing pharmacological ligands to study the long-chain fatty acid receptors GPR40/FFA1 and GPR120/FFA4. Br. J. Pharmacol. 2015, 172, 3254–3265. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Kim, N.J.; Im, D.S. Development of Free Fatty Acid Receptor 4 (FFA4/GPR120) Agonists in Health Science. Biomol. Ther. 2021, 29, 22–30. [Google Scholar] [CrossRef]
- Galindo, M.M.; Voigt, N.; Stein, J.; van Lengerich, J.; Raguse, J.D.; Hofmann, T.; Meyerhof, W.; Behrens, M. G protein-coupled receptors in human fat taste perception. Chem. Senses 2012, 37, 123–139. [Google Scholar] [CrossRef]
- Carullo, G.; Mazzotta, S.; Vega-Holm, M.; Iglesias-Guerra, F.; Vega-Pérez, J.M.; Aiello, F.; Brizzi, A. GPR120/FFAR4 Pharmacology: Focus on Agonists in Type 2 Diabetes Mellitus Drug Discovery. J. Med. Chem. 2021, 64, 4312–4332. [Google Scholar] [CrossRef]
- Moore, K.; Zhang, Q.; Murgolo, N.; Hosted, T.; Duffy, R. Cloning, expression, and pharmacological characterization of the GPR120 free fatty acid receptor from cynomolgus monkey: Comparison with human GPR120 splice variants. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 419–426. [Google Scholar] [CrossRef]
- Watson, S.J.; Brown, A.J.; Holliday, N.D. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol. Pharmacol. 2012, 81, 631–642. [Google Scholar] [CrossRef]
- Zhang, X.; Guseinov, A.A.; Jenkins, L.; Li, K.; Tikhonova, I.G.; Milligan, G.; Zhang, C. Structural basis for the ligand recognition and signaling of free fatty acid receptors. Sci. Adv. 2024, 10, eadj2384. [Google Scholar] [CrossRef] [PubMed]
- Sadler, F.; Ma, N.; Ritt, M.; Sharma, Y.; Vaidehi, N.; Sivaramakrishnan, S. Autoregulation of GPCR signalling through the third intracellular loop. Nature 2023, 615, 734–741. [Google Scholar] [CrossRef]
- Burns, R.N.; Moniri, N.H. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem. Biophys. Res. Commun. 2010, 396, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Vestmar, M.A.; Andersson, E.A.; Christensen, C.R.; Hauge, M.; Glümer, C.; Linneberg, A.; Witte, D.R.; Jørgensen, M.E.; Christensen, C.; Brandslund, I.; et al. Functional and genetic epidemiological characterisation of the FFAR4 (GPR120) p.R270H variant in the Danish population. J. Med. Genet. 2016, 53, 616–623. [Google Scholar] [CrossRef]
- Oh, D.Y.; Walenta, E. Omega-3 Fatty Acids and FFAR4. Front. Endocrinol. 2014, 5, 115. [Google Scholar] [CrossRef]
- Cheshmehkani, A.; Senatorov, I.S.; Kandi, P.; Singh, M.; Britt, A.; Hayslett, R.; Moniri, N.H. Fish oil and flax seed oil supplemented diets increase FFAR4 expression in the rat colon. Inflamm. Res. 2015, 64, 809–815. [Google Scholar] [CrossRef]
- Yin, J.; Li, H.; Meng, C.; Chen, D.; Chen, Z.; Wang, Y.; Wang, Z.; Chen, G. Inhibitory effects of omega-3 fatty acids on early brain injury after subarachnoid hemorrhage in rats: Possible involvement of G protein-coupled receptor 120/β-arrestin2/TGF-β activated kinase-1 binding protein-1 signaling pathway. Int. J. Biochem. Cell Biol. 2016, 75, 11–22. [Google Scholar] [CrossRef]
- Cornall, L.M.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Diet-induced obesity up-regulates the abundance of GPR43 and GPR120 in a tissue specific manner. Cell. Physiol. Biochem. 2011, 28, 949–958. [Google Scholar] [CrossRef]
- Karakuła-Juchnowicz, H.; Róg, J.; Juchnowicz, D.; Morylowska-Topolska, J. GPR120, Mechanism of action, role and potential for medical applications. Postępy Hig. Med. Doświadczalnej 2017, 71, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, C.; Hong, Y.H.; Iga, T.; Hishikawa, D.; Suzuki, Y.; Song, S.H.; Choi, K.C.; Adachi, T.; Hirasawa, A.; Tsujimoto, G.; et al. The regulation of adipogenesis through GPR120. Biochem. Biophys. Res. Commun. 2007, 354, 591–597. [Google Scholar] [CrossRef]
- Moriyama, R.; Deura, C.; Imoto, S.; Nose, K.; Fukushima, N. Expression of the long-chain fatty acid receptor GPR120 in the gonadotropes of the mouse anterior pituitary gland. Histochem. Cell Biol. 2015, 143, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Noda, K.; Miwa, M.; Minabe, S.; Hagiwara, T.; Hirasawa, A.; Matsuyama, S.; Moriyama, R. Colocalization of GPR120 and anterior pituitary hormone-producing cells in female Japanese Black cattle. J. Reprod. Dev. 2020, 66, 135–141. [Google Scholar] [CrossRef]
- Deura, C.; Kimura, Y.; Nonoyama, T.; Moriyama, R. Gpr120 mRNA expression in gonadotropes in the mouse pituitary gland is regulated by free fatty acids. J. Reprod. Dev. 2020, 66, 249–254. [Google Scholar] [CrossRef]
- Duah, M.; Zhang, K.; Liang, Y.; Ayarick, V.A.; Xu, K.; Pan, B. Immune regulation of poly unsaturated fatty acids and free fatty acid receptor 4. J. Nutr. Biochem. 2023, 112, 109222. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, K.P.; Park, S.J.; Kang, S.; Huang, J.; Lee, J.M.; Sato, K.; Chung, H.Y.; Okajima, F.; Im, D.S. Omega-3 fatty acids induce Ca(2+) mobilization responses in human colon epithelial cell lines endogenously expressing FFA4. Acta Pharmacol. Sin. 2015, 36, 813–820. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Kasonga, A.E.; Kruger, M.C.; Coetzee, M. Free fatty acid receptor 4-β-arrestin 2 pathway mediates the effects of different classes of unsaturated fatty acids in osteoclasts and osteoblasts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 281–289. [Google Scholar] [CrossRef]
- Alharbi, A.G.; Tobin, A.B.; Milligan, G. How Arrestins and GRKs Regulate the Function of Long Chain Fatty Acid Receptors. Int. J. Mol. Sci. 2022, 23, 12237. [Google Scholar] [CrossRef]
- Schulte, G.; Fredholm, B.B. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 2003, 15, 813–827. [Google Scholar] [CrossRef]
- Anbazhagan, A.N.; Priyamvada, S.; Gujral, T.; Bhattacharyya, S.; Alrefai, W.A.; Dudeja, P.K.; Borthakur, A. A novel anti-inflammatory role of GPR120 in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2016, 310, C612–C621. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.; Coin, I. Biochemical insights into structure and function of arrestins. FEBS J. 2021, 288, 2529–2549. [Google Scholar] [CrossRef]
- Zhao, Y.F. Free fatty acid receptors in the endocrine regulation of glucose metabolism: Insight from gastrointestinal-pancreatic-adipose interactions. Front. Endocrinol. 2022, 13, 956277. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Thompson, D.G.; Warhurst, G.; Case, R.M.; Benson, R.S. Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J. Physiol. 2000, 528 Pt 1, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef] [PubMed]
- Danciu, T.E.; Adam, R.M.; Naruse, K.; Freeman, M.R.; Hauschka, P.V. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003, 536, 193–197. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, W.; Yin, Y.; Zhang, W. Ghrelin Based Therapy of Metabolic Diseases. Curr. Med. Chem. 2021, 28, 2565–2576. [Google Scholar] [CrossRef]
- Bang, A.S.; Soule, S.G.; Yandle, T.G.; Richards, A.M.; Pemberton, C.J. Characterisation of proghrelin peptides in mammalian tissue and plasma. J. Endocrinol. 2007, 192, 313–323. [Google Scholar] [CrossRef]
- Simonds, W.F. G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 1999, 20, 66–73. [Google Scholar] [CrossRef]
- Fredriksson, R.; Höglund, P.J.; Gloriam, D.E.; Lagerström, M.C.; Schiöth, H.B. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003, 554, 381–388. [Google Scholar] [CrossRef] [PubMed]
- da Silva Batista, E.; Nakandakari, S.C.B.R.; Ramos da Silva, A.S.; Pauli, J.R.; Pereira de Moura, L.; Ropelle, E.R.; Camargo, E.A.; Cintra, D.E. Omega-3 pleiad: The multipoint anti-inflammatory strategy. Crit. Rev. Food Sci. Nutr. 2024, 64, 4817–4832. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lei, X.T.; Huang, Q.; Wang, T.; Sun, H.B.; Wang, H.Y. A novel GPR120-selective agonist promotes insulin secretion and improves chronic inflammation. Life Sci. 2021, 269, 119029. [Google Scholar] [CrossRef]
- Suckow, A.T.; Polidori, D.; Yan, W.; Chon, S.; Ma, J.Y.; Leonard, J.; Briscoe, C.P. Alteration of the glucagon axis in GPR120 (FFAR4) knockout mice: A role for GPR120 in glucagon secretion. J. Biol. Chem. 2014, 289, 15751–15763. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, Z. Recent Advance in Regulatory Effect of GRP120 on Bone Metabolism. Aging Dis. 2023, 14, 1714–1727. [Google Scholar] [CrossRef]
- Zhang, X.; Macielag, M.J. GPR120 agonists for the treatment of diabetes: A patent review (2014 present). Expert. Opin. Ther. Pat. 2020, 30, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Satapati, S.; Qian, Y.; Wu, M.S.; Petrov, A.; Dai, G.; Wang, S.P.; Zhu, Y.; Shen, X.; Muise, E.S.; Chen, Y.; et al. GPR120 suppresses adipose tissue lipolysis and synergizes with GPR40 in antidiabetic efficacy. J. Lipid Res. 2017, 58, 1561–1578. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Gao, F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabetes 2014, 5, 89–96. [Google Scholar] [CrossRef]
- Chang, Y.W.; Hung, L.C.; Chen, Y.C.; Wang, W.H.; Lin, C.Y.; Tzeng, H.H.; Suen, J.L.; Chen, Y.H. Insulin Reduces Inflammation by Regulating the Activation of the NLRP3 Inflammasome. Front. Immunol. 2021, 11, 587229. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, W.; Shi, J.; Shen, J.; Gao, T.; Zhang, J.; Xi, F.; Li, J.; Li, N. Insulin alleviates the inflammatory response and oxidative stress injury in cerebral tissues in septic rats. J. Inflamm. 2014, 11, 18. [Google Scholar] [CrossRef]
- Huang, C.T.; Lue, J.H.; Cheng, T.H.; Tsai, Y.J. Glycemic control with insulin attenuates sepsis-associated encephalopathy by inhibiting glial activation via the suppression of the nuclear factor kappa B and mitogen-activated protein kinase signaling pathways in septic rats. Brain Res. 2020, 1738, 146822. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Matuschik, L.; Riabov, V.; Schmuttermaier, C.; Sevastyanova, T.; Weiss, C.; Klüter, H.; Kzhyshkowska, J. Hyperglycemia Induces Inflammatory Response of Human Macrophages to CD163-Mediated Scavenging of Hemoglobin-Haptoglobin Complexes. Int. J. Mol. Sci. 2022, 23, 1385. [Google Scholar] [CrossRef]
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Klüter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017, 222, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; et al. The interaction of innate immune and adaptive immune system. MedComm (2020) 2024, 5, e714. [Google Scholar] [CrossRef]
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, R.M.; Kim, C.S.; Kim, S.K. Immunological Profiling of Obesity. J. Lifestyle Med. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef]
- Rowan, C.R.; McManus, J.; Boland, K.; O’Toole, A. Visceral adiposity and inflammatory bowel disease. Int. J. Colorectal Dis. 2021, 36, 2305–2319. [Google Scholar] [CrossRef]
- Santillana, N.; Astudillo-Guerrero, C.; D’Espessailles, A.; Cruz, G. White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements. Nutrients 2023, 15, 1722. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, J.; Min, S.; Kang, O.; Kwon, H.; Oh, S.W. Impact of Visceral Obesity on the Risk of Incident Metabolic Syndrome in Metabolically Healthy Normal Weight and Overweight Groups: A Longitudinal Cohort Study in Korea. Korean J. Fam. Med. 2020, 41, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mongraw-Chaffin, M.; Hairston, K.G.; Hanley, A.J.G.; Tooze, J.A.; Norris, J.M.; Palmer, N.D.; Bowden, D.W.; Lorenzo, C.; Chen, Y.I.; Wagenknecht, L.E. Association of Visceral Adipose Tissue and Insulin Resistance with Incident Metabolic Syndrome Independent of Obesity Status: The IRAS Family Study. Obesity 2021, 29, 1195–1202. [Google Scholar] [CrossRef]
- Navaneeth, G.C.; Hiremath, R.; Poojary, S.R.; Kini, D.V.; Chittaragi, K.B. Computed tomographic abdominal fat volume estimation—A handy tool to predict the risk of metabolic syndrome. Pol. J. Radiol. 2023, 88, e379–e388. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Kabelitz, D.; Kalantar, K. An update on immune dysregulation in obesity-related insulin resistance. Scand. J. Immunol. 2019, 89, e12747. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Yuan, M.; Wang, Y.; Wang, Y.; Bao, W.; Zeng, S.; Zhang, D.; Liu, P.; Sun, L. Association Between Visceral Obesity and Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Retrospective Study. Diabetes Metab. Syndr. Obes. 2024, 17, 2869–2880. [Google Scholar] [CrossRef]
- Doyle, S.L.; Donohoe, C.L.; Lysaght, J.; Reynolds, J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc. Nutr. Soc. 2012, 71, 181–189. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Chen, Y.; Liu, T.; Liu, C.; Lin, S.; Xie, H.; Ma, X.; Wang, Z.; Shi, J.; et al. Metabolic obesity phenotypes and the risk of cancer: A prospective study of the Kailuan cohort. Front. Endocrinol. 2024, 15, 1333488. [Google Scholar] [CrossRef]
- Rumyantsev, K.A.; Polyakova, V.V.; Sorokina, I.V.; Feoktistova, P.S.; Khatkov, I.E.; Bodunova, N.A.; Zhukova, L.G. The Gut Microbiota Impacts Gastrointestinal Cancers through Obesity, Diabetes, and Chronic Inflammation. Life 2024, 14, 1219. [Google Scholar] [CrossRef]
- Kolb, H. Obese visceral fat tissue inflammation: From protective to detrimental? BMC Med. 2022, 20, 494. [Google Scholar] [CrossRef]
- Yu, J.Y.; Choi, W.J.; Lee, H.S.; Lee, J.W. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: An observational study. Medicine 2019, 98, e14740. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, Y.; He, S.; Tan, X.; Zhang, Y.; Zhang, X.; Wang, L.; Zheng, W. Macrophages, Chronic Inflammation, and Insulin Resistance. Cells 2022, 11, 3001. [Google Scholar] [CrossRef]
- Graßmann, S.; Wirsching, J.; Eichelmann, F.; Aleksandrova, K. Association Between Peripheral Adipokines and Inflammation Markers: A Systematic Review and Meta-Analysis. Obesity 2017, 25, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, A.; Stangret, A.; Wątroba, M.; Płatek, A.E.; Skoda, M.; Cendrowski, K.; Sawicki, W.; Szukiewicz, D. Can adipokine visfatin be a novel marker of pregnancy-related disorders in women with obesity? Obes. Rev. 2020, 21, e13022. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Saddi-Rosa, P.; Oliveira, C.S.; Giuffrida, F.M.; Reis, A.F. Visfatin, glucose metabolism and vascular disease: A review of evidence. Diabetol. Metab. Syndr. 2010, 2, 21. [Google Scholar] [CrossRef]
- Dakroub, A.; ANasser, S.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Role of visfatin in obesity-induced insulin resistance. World J. Clin. Cases 2022, 10, 10840–10851. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Investig. 2003, 112, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Pitharouli, M.C.; Hagenaars, S.P.; Glanville, K.P.; Coleman, J.R.I.; Hotopf, M.; Lewis, C.M.; Pariante, C.M. Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank. Am. J. Psychiatry 2021, 178, 522–529. [Google Scholar] [CrossRef]
- Parkitny, L.; McAuley, J.H.; Di Pietro, F.; Stanton, T.R.; O’Connell, N.E.; Marinus, J.; van Hilten, J.J.; Moseley, G.L. Inflammation in complex regional pain syndrome: A systematic review and meta-analysis. Neurology 2013, 80, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Rodelo, C.; Arellano-Plancarte, A.; Hernandez-Aranda, J.; Landa-Galvan, H.V.; Parra-Mercado, G.K.; Moreno-Licona, N.J.; Hernandez-Gonzalez, K.D.; Catt, K.J.; Villalobos-Molina, R.; Olivares-Reyes, J.A. Angiotensin II Inhibits Insulin Receptor Signaling in Adipose Cells. Int. J. Mol. Sci. 2022, 23, 6048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.K.; Jang, Y.J.; Park, H.S.; Kim, J.H.; Hong, J.P.; Lee, Y.J.; Heo, Y.S. Enhanced ANGPTL2 expression in adipose tissues and its association with insulin resistance in obese women. Sci. Rep. 2018, 8, 13976. [Google Scholar] [CrossRef]
- Corbin, J.A.; Bhaskar, V.; Goldfine, I.D.; Issafras, H.; Bedinger, D.H.; Lau, A.; Michelson, K.; Gross, L.M.; Maddux, B.A.; Kuan, H.F.; et al. Inhibition of insulin receptor function by a human, allosteric monoclonal antibody: A potential new approach for the treatment of hyperinsulinemic hypoglycemia. MAbs 2014, 6, 262–272. [Google Scholar] [CrossRef]
- Willard, D.L.; Stevenson, M.; Steenkamp, D. Type B insulin resistance syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 318–323. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Pei, J.; Wang, B.; Wang, D. Current Studies on Molecular Mechanisms of Insulin Resistance. J. Diabetes Res. 2022, 2022, 1863429. [Google Scholar] [CrossRef]
- Lorenzo, M.; Fernández-Veledo, S.; Vila-Bedmar, R.; Garcia-Guerra, L.; De Alvaro, C.; Nieto-Vazquez, I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J. Anim. Sci. 2008, 86 (Suppl. S14), E94–E104. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.M.; Pennings, N. Insulin Resistance. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed]
- Auguet, T.; Guiu-Jurado, E.; Berlanga, A.; Terra, X.; Martinez, S.; Porras, J.A.; Ceausu, A.; Sabench, F.; Hernandez, M.; Aguilar, C.; et al. Downregulation of lipogenesis and fatty acid oxidation in the subcutaneous adipose tissue of morbidly obese women. Obesity 2014, 22, 2032–2038. [Google Scholar] [CrossRef]
- Zhou, W.; Ramachandran, D.; Mansouri, A.; Dailey, M.J. Glucose stimulates intestinal epithelial crypt proliferation by modulating cellular energy metabolism. J. Cell Physiol. 2018, 233, 3465–3475. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Duca, F.A.; Swartz, T.D.; Sakar, Y.; Covasa, M. Decreased intestinal nutrient response in diet-induced obese rats: Role of gut peptides and nutrient receptors. Int. J. Obes. 2013, 37, 375–381. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, T.; Zhang, D.; Leung, P.S. GPR120 protects lipotoxicity-induced pancreatic β-cell dysfunction through regulation of PDX1 expression and inhibition of islet inflammation. Clin. Sci. 2019, 133, 101–116. [Google Scholar] [CrossRef]
- Miyabe, M.; Gin, A.; Onozawa, E.; Daimon, M.; Yamada, H.; Oda, H.; Mori, A.; Momota, Y.; Azakami, D.; Yamamoto, I.; et al. Genetic variants of the unsaturated fatty acid receptor GPR120 relating to obesity in dogs. J. Vet. Med. Sci. 2015, 77, 1201–1206. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Stojko, M.; Spychał, A.; Nikel, K.; Kołodziej, R.; Zalejska-Fiolka, J. The Impact of Diet on Lipoprotein(a) Levels. Life 2024, 14, 1403. [Google Scholar] [CrossRef]
- Wallace, T.C. Health Effects of Coconut Oil-A Narrative Review of Current Evidence. J. Am. Coll. Nutr. 2019, 38, 97–107. [Google Scholar] [CrossRef]
- Panchal, S.K.; Carnahan, S.; Brown, L. Coconut Products Improve Signs of Diet-Induced Metabolic Syndrome in Rats. Plant Foods Hum. Nutr. 2017, 72, 418–424. [Google Scholar] [CrossRef]
- Araújo de Vasconcelos, M.H.; Tavares, R.L.; Dutra, M.L.D.V.; Batista, K.S.; D’Oliveira, A.B.; Pinheiro, R.O.; Pereira, R.A.; Lima, M.D.S.; Salvadori, M.G.D.S.S.; de Souza, E.L.; et al. Extra virgin coconut oil (Cocos nucifera L.) intake shows neurobehavioural and intestinal health effects in obesity-induced rats. Food Funct. 2023, 14, 6455–6469. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Qadri, S.; Luukkonen, P.K.; Ragnarsdottir, O.; McGlinchey, A.; Jäntti, S.; Juuti, A.; Arola, J.; Schlezinger, J.J.; Webster, T.F.; et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fang, R.; Wang, H.; Xu, D.X.; Yang, J.; Huang, X.; Cozzolino, D.; Fang, M.; Huang, Y. A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ. Int. 2022, 158, 106941. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Cho, S.H.; Yoon, J.C. Adipose Tissue and Metabolic Health. Diabetes Metab. J. 2023, 47, 595–611. [Google Scholar] [CrossRef]
- Teixeira, L.; Whitmire, J.K.; Bourgeois, C. Editorial: The role of adipose tissue and resident immune cells in infections. Front. Immunol. 2024, 15, 1360262. [Google Scholar] [CrossRef]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2014, 3, 4–9. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, R.; Pfeifer, A. Fat tissues, the brite and the dark sides. Pflugers Arch. 2016, 468, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Kazak, L.; Chouchani, E.T.; Bogaczyńska, M.G.; Paranjpe, I.; Wainwright, G.L.; Bétourné, A.; Kajimura, S.; Spiegelman, B.M.; Kirichok, Y. Mitochondrial Patch Clamp of Beige Adipocytes Reveals UCP1-Positive and UCP1-Negative Cells Both Exhibiting Futile Creatine Cycling. Cell Metab. 2017, 25, 811–822.e4. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508, Erratum in N. Engl. J. Med. 2009, 360, 1917. https://doi.org/10.1056/NEJMoa0808718. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Xiang, J.; Zhou, J.; Cao, H.; Che, Q.; Bai, Y.; Guo, J.; Su, Z. Non-shivering Thermogenesis Signalling Regulation and Potential Therapeutic Applications of Brown Adipose Tissue. Int. J. Biol. Sci. 2021, 17, 2853–2870. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Delgado, G.; Martinez-Tellez, B.; Acosta, F.M.; Virtue, S.; Vidal-Puig, A.; Gil, A.; Llamas-Elvira, J.M.; Ruiz, J.R. Brown Adipose Tissue Volume and Fat Content Are Positively Associated With Whole-Body Adiposity in Young Men-Not in Women. Diabetes 2021, 70, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Chiadak, J.D.; Arsenijevic, T.; Verstrepen, K.; Gregoire, F.; Bolaky, N.; Delforge, V.; Flamand, V.; Perret, J.; Delporte, C. Forskolin Inhibits Lipopolysaccharide-Induced Modulation of MCP-1 and GPR120 in 3T3-L1 Adipocytes through an Inhibition of NFκB. Mediat. Inflamm. 2016, 2016, 1431789. [Google Scholar] [CrossRef]
- Song, T.; Yang, Y.; Zhou, Y.; Wei, H.; Peng, J. GPR120, a critical role in adipogenesis, inflammation, and energy metabolism in adipose tissue. Cell Mol. Life Sci. 2017, 74, 2723–2733. [Google Scholar] [CrossRef]
- Otto, T.C.; Lane, M.D. Adipose development: From stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 229–242. [Google Scholar] [CrossRef]
- Ichimura, A.; Hirasawa, A.; Poulain-Godefroy, O.; Bonnefond, A.; Hara, T.; Yengo, L.; Kimura, I.; Leloire, A.; Liu, N.; Iida, K.; et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012, 483, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hara, T.; Hirasawa, A. Regulation of Energy Homeostasis via GPR120. Front. Endocrinol. 2014, 5, 111. [Google Scholar] [CrossRef]
- Song, T.; Zhou, Y.; Peng, J.; Tao, Y.X.; Yang, Y.; Xu, T.; Peng, J.; Ren, J.; Xiang, Q.; Wei, H. GPR120 promotes adipogenesis through intracellular calcium and extracellular signal-regulated kinase 1/2 signal pathway. Mol. Cell Endocrinol. 2016, 434, 1–13. [Google Scholar] [CrossRef]
- Christian, M. Elucidation of the roles of brown and brite fat genes: GPR120 is a modulator of brown adipose tissue function. Exp. Physiol. 2020, 105, 1201–1205. [Google Scholar] [CrossRef]
- Quesada-López, T.; Cereijo, R.; Turatsinze, J.V.; Planavila, A.; Cairó, M.; Gavaldà-Navarro, A.; Peyrou, M.; Moure, R.; Iglesias, R.; Giralt, M.; et al. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat. Commun. 2016, 7, 13479. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Umemoto, T.; Kakei, M.; Momomura, S.I.; Kawakami, M.; Ishikawa, S.E.; Hara, K. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr. Metab. 2017, 14, 33. [Google Scholar] [CrossRef]
- Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; Zechner, R.; Cinti, S. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794. [Google Scholar] [CrossRef]
- Pærregaard, S.I.; Agerholm, M.; Serup, A.K.; Ma, T.; Kiens, B.; Madsen, L.; Kristiansen, K.; Jensen, B.A. FFAR4 (GPR120) Signaling Is Not Required for Anti-Inflammatory and Insulin-Sensitizing Effects of Omega-3 Fatty Acids. Mediat. Inflamm. 2016, 2016, 1536047. [Google Scholar] [CrossRef] [PubMed]
- Sveiven, S.N.; Anesko, K.; Morgan, J.; Nair, M.G.; Nordgren, T.M. Lipid-Sensing Receptor FFAR4 Modulates Pulmonary Epithelial Homeostasis following Immunogenic Exposures Independently of the FFAR4 Ligand Docosahexaenoic Acid (DHA). Int. J. Mol. Sci. 2023, 24, 7072. [Google Scholar] [CrossRef]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Katsuma, S.; Hatae, N.; Yano, T.; Ruike, Y.; Kimura, M.; Hirasawa, A.; Tsujimoto, G. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J. Biol. Chem. 2005, 280, 19507–19515. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Katsuma, S.; Adachi, T.; Koshimizu, T.A.; Hirasawa, A.; Tsujimoto, G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 377, 523–527. [Google Scholar] [CrossRef]
- Popoviciu, M.S.; Păduraru, L.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef]
- McKillop, A.M.; Miskelly, M.G.; Moran, B.M.; Flatt, P.R. Incretins play an important role in FFA4/GPR120 regulation of glucose metabolism by GW-9508. Life Sci. 2023, 318, 121475. [Google Scholar] [CrossRef] [PubMed]
- Loona, D.P.S.; Das, B.; Kaur, R.; Kumar, R.; Yadav, A.K. Free Fatty Acid Receptors (FFARs): Emerging Therapeutic Targets for the Management of Diabetes Mellitus. Curr. Med. Chem. 2023, 30, 3404–3440. [Google Scholar] [CrossRef]
- Patil, M.; Casari, I.; Warne, L.N.; Falasca, M. G protein-coupled receptors driven intestinal glucagon-like peptide-1 reprogramming for obesity: Hope or hype? Biomed. Pharmacother. 2024, 172, 116245. [Google Scholar] [CrossRef]
- Sankoda, A.; Harada, N.; Iwasaki, K.; Yamane, S.; Murata, Y.; Shibue, K.; Thewjitcharoen, Y.; Suzuki, K.; Harada, T.; Kanemaru, Y.; et al. Long-Chain Free Fatty Acid Receptor GPR120 Mediates Oil-Induced GIP Secretion Through CCK in Male Mice. Endocrinology 2017, 158, 1172–1180. [Google Scholar] [CrossRef]
- Sankoda, A.; Harada, N.; Kato, T.; Ikeguchi, E.; Iwasaki, K.; Yamane, S.; Murata, Y.; Hirasawa, A.; Inagaki, N. Free fatty acid receptors, G protein-coupled receptor 120 and G protein-coupled receptor 40, are essential for oil-induced gastric inhibitory polypeptide secretion. J. Diabetes Investig. 2019, 10, 1430–1437. [Google Scholar] [CrossRef]
- Iwasaki, K.; Harada, N.; Sasaki, K.; Yamane, S.; Iida, K.; Suzuki, K.; Hamasaki, A.; Nasteska, D.; Shibue, K.; Joo, E.; et al. Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 2015, 156, 837–846. [Google Scholar] [CrossRef]
- Yamane, S.; Harada, N.; Inagaki, N. Mechanisms of fat-induced gastric inhibitory polypeptide/glucose-dependent insulinotropic polypeptide secretion from K cells. J. Diabetes Investig. 2016, 7 (Suppl. S1), 20–26. [Google Scholar] [CrossRef]
- Yasuda, T.; Harada, N.; Hatoko, T.; Ichimura, A.; Ikeguchi-Ogura, E.; Murata, Y.; Wada, N.; Kiyobayashi, S.; Yamane, S.; Hirasawa, A.; et al. Inhibition of GPR120 signaling in intestine ameliorates insulin resistance and fatty liver under high-fat diet feeding. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E449–E460. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Alejos, A.; Carrasco-Luna, J.; Carrasco-García, Á.; Codoñer-Franch, P. Reduced Free Fatty Acid Receptor 4 Gene Expression is Associated With Extreme Obesity and Insulin Resistance in Children. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.M.; Dhayal, S.; Brocklehurst, K.J.; Lenaghan, C.; Sörhede Winzell, M.; Hammar, M.; Xu, X.; Smith, D.M.; Morgan, N.G. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia 2014, 57, 1182–1191. [Google Scholar] [CrossRef]
- Croze, M.L.; Flisher, M.F.; Guillaume, A.; Tremblay, C.; Noguchi, G.M.; Granziera, S.; Vivot, K.; Castillo, V.C.; Campbell, S.A.; Ghislain, J.; et al. Free fatty acid receptor 4 inhibitory signaling in delta cells regulates islet hormone secretion in mice. Mol. Metab. 2021, 45, 101166. [Google Scholar] [CrossRef]
- Rorsman, P.; Huising, M.O. The somatostatin-secreting pancreatic δ-cell in health and disease. Nat. Rev. Endocrinol. 2018, 14, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.M.; Abdel-Wahab, Y.H.; Flatt, P.R.; McKillop, A.M. Evaluation of the insulin-releasing and glucose-lowering effects of GPR120 activation in pancreatic β-cells. Diabetes Obes. Metab. 2014, 16, 1128–1139. [Google Scholar] [CrossRef]
- Vergari, E.; Denwood, G.; Salehi, A.; Zhang, Q.; Adam, J.; Alrifaiy, A.; Wernstedt Asterholm, I.; Benrick, A.; Chibalina, M.V.; Eliasson, L.; et al. Somatostatin secretion by Na+-dependent Ca2+-induced Ca2+ release in pancreatic delta-cells. Nat. Metab. 2020, 2, 32–40. [Google Scholar] [CrossRef]
- Denwood, G.; Tarasov, A.; Salehi, A.; Vergari, E.; Ramracheya, R.; Takahashi, H.; Nikolaev, V.O.; Seino, S.; Gribble, F.; Reimann, F.; et al. Glucose stimulates somatostatin secretion in pancreatic δ-cells by cAMP-dependent intracellular Ca2+ release. J. Gen. Physiol. 2019, 151, 1094–1115. [Google Scholar] [CrossRef]
- Greenhill, C. Urocortin 3 function in glucose metabolism. Nat. Rev. Endocrinol. 2022, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Gao, M.X.; Qi, H.B.; An, W.T.; Lin, J.Y.; Ning, S.L.; Yang, F.; Xiao, P.; Cheng, J.; Pan, W.; et al. Differential contributions of G protein- or arrestin subtype-mediated signalling underlie urocortin 3-induced somatostatin secretion in pancreatic δ cells. Br. J. Pharmacol. 2024, 181, 2600–2621. [Google Scholar] [CrossRef]
- Du, Y.Q.; Sha, X.Y.; Cheng, J.; Wang, J.; Lin, J.Y.; An, W.T.; Pan, W.; Zhang, L.J.; Tao, X.N.; Xu, Y.F.; et al. Endogenous Lipid-GPR120 Signaling Modulates Pancreatic Islet Homeostasis to Different Extents. Diabetes 2022, 71, 1454–1471. [Google Scholar] [CrossRef]
- Hong, S.W.; Lee, J.; Moon, S.J.; Kwon, H.; Park, S.E.; Rhee, E.J.; Lee, W.Y. Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells. Endocrinol. Metab. 2024, 39, 353–363. [Google Scholar] [CrossRef]
- Zhang, D.; So, W.Y.; Wang, Y.; Wu, S.Y.; Cheng, Q.; Leung, P.S. Insulinotropic effects of GPR120 agonists are altered in obese diabetic and obese non-diabetic states. Clin. Sci. 2017, 131, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, A.I.; Corbett, R.C.; Flatt, P.R.; McKillop, A.M. Positive interplay between FFAR4/GPR120, DPP-IV inhibition and GLP-1 in beta cell proliferation and glucose homeostasis in obese high fat fed mice. Peptides 2024, 177, 171218. [Google Scholar] [CrossRef]
- Ichimura, A.; Hasegawa, S.; Kasubuchi, M.; Kimura, I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front. Pharmacol. 2014, 5, 236. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, L.; Perugino, F.; Magnaghi, F.; Dall’Asta, C.; Galaverna, G.; Dellafiora, L. Free fatty acid receptors beyond fatty acids: A computational journey to explore peptides as possible binders of GPR120. Curr. Res. Food Sci. 2024, 8, 100710. [Google Scholar] [CrossRef]
- Suzuki, T.; Igari, S.; Hirasawa, A.; Hata, M.; Ishiguro, M.; Fujieda, H.; Itoh, Y.; Hirano, T.; Nakagawa, H.; Ogura, M.; et al. Identification of G protein-coupled receptor 120-selective agonists derived from PPARgamma agonists. J. Med. Chem. 2008, 51, 7640–7644. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Funk, C.D. Cyclooxygenase-2 induction in macrophages is modulated by docosahexaenoic acid via interactions with free fatty acid receptor 4 (FFA4). FASEB J. 2013, 27, 4987–4997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Leung, P.S. Potential roles of GPR120 and its agonists in the management of diabetes. Drug Des. Dev. Ther. 2014, 8, 1013–1027. [Google Scholar] [CrossRef]
- Pal, A.; Curtin, J.F.; Kinsella, G.K. In silico and in vitro screening for potential anticancer candidates targeting GPR120. Bioorg. Med. Chem. Lett. 2021, 31, 127672. [Google Scholar] [CrossRef]
- Rubbino, F.; Garlatti, V.; Garzarelli, V.; Massimino, L.; Spanò, S.; Iadarola, P.; Cagnone, M.; Giera, M.; Heijink, M.; Guglielmetti, S.; et al. GPR120 prevents colorectal adenocarcinoma progression by sustaining the mucosal barrier integrity. Sci. Rep. 2022, 12, 381. [Google Scholar] [CrossRef]
- He, Q.; Zhu, S.; Lin, M.; Yang, Q.; Wei, L.; Zhang, J.; Jiang, X.; Zhu, D.; Lu, X.; Chen, Y.Q. Increased GPR120 level is associated with gestational diabetes mellitus. Biochem. Biophys. Res. Commun. 2019, 512, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Pompili, S.; Cappariello, A.; Vetuschi, A.; Sferra, R. G-Protein-Coupled Receptor 120 Agonist Mitigates Steatotic and Fibrotic Features Triggered in Obese Mice by the Administration of a High-Fat and High-Carbohydrate Diet. ACS Omega 2024, 9, 31899–31909. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.; Alvarez-Curto, E.; Hudson, B.D.; Prihandoko, R.; Tobin, A.B. FFA4/GPR120, Pharmacology and Therapeutic Opportunities. Trends Pharmacol. Sci. 2017, 38, 809–821. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Murff, H.J.; Edwards, T.L. Endogenous Production of Long-Chain Polyunsaturated Fatty Acids and Metabolic Disease Risk. Curr. Cardiovasc. Risk Rep. 2014, 8, 418. [Google Scholar] [CrossRef]
- Rodriguez-Pacheco, F.; Garcia-Serrano, S.; Garcia-Escobar, E.; Gutierrez-Repiso, C.; Garcia-Arnes, J.; Valdes, S.; Gonzalo, M.; Soriguer, F.; Moreno-Ruiz, F.J.; Rodriguez-Cañete, A.; et al. Effects of obesity/fatty acids on the expression of GPR120. Mol. Nutr. Food Res. 2014, 58, 1852–1860, Erratum in Mol. Nutr. Food Res. 2015, 59, 2338. https://doi.org/10.1002/mnfr.201570115. [Google Scholar] [CrossRef]
- Holliday, N.D.; Watson, S.J.; Brown, A.J. Drug discovery opportunities and challenges at g protein coupled receptors for long chain free Fatty acids. Front. Endocrinol. 2012, 2, 112. [Google Scholar] [CrossRef]
- Hansen, S.V.; Ulven, T. Pharmacological Tool Compounds for the Free Fatty Acid Receptor 4 (FFA4/GPR120). Handb. Exp. Pharmacol. 2017, 236, 33–56. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Aspects Med. 2018, 64, 1–17. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- Wang, Y.M.; Liu, H.X.; Fang, N.Y. 9-PAHSA promotes browning of white fat via activating G-protein-coupled receptor 120 and inhibiting lipopolysaccharide/NF-kappa B pathway. Biochem. Biophys. Res. Commun. 2018, 506, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Hirasawa, A.; Sun, Q.; Sadakane, K.; Itsubo, C.; Iga, T.; Adachi, T.; Koshimizu, T.A.; Hashimoto, T.; Asakawa, Y.; et al. Novel selective ligands for free fatty acid receptors GPR120 and GPR40. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 380, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hirasawa, A.; Hara, T.; Kimura, I.; Adachi, T.; Awaji, T.; Ishiguro, M.; Suzuki, T.; Miyata, N.; Tsujimoto, G. Structure-activity relationships of GPR120 agonists based on a docking simulation. Mol. Pharmacol. 2010, 78, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Hara, T.; Katsuma, S.; Adachi, T.; Tsujimoto, G. Free fatty acid receptors and drug discovery. Biol. Pharm. Bull. 2008, 31, 1847–1851. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Yan, A.; Zhu, J.; Liu, K.; Chen, D.; Xie, R.; Xu, X.; Su, X. Grifolic acid induces mitochondrial membrane potential loss and cell death of RAW264.7 macrophages. Mol. Med. Rep. 2018, 17, 3281–3287. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Allam, A.; Zeouk, I.; Taha, D.; Zengin, G.; Goh, B.H.; Catauro, M.; Montesano, D.; El Omari, N. Pharmacological Effects of Grifolin: Focusing on Anticancer Mechanisms. Molecules 2022, 27, 284. [Google Scholar] [CrossRef]
- Wilson, C.H.; Kumar, S. Caspases in metabolic disease and their therapeutic potential. Cell Death Differ. 2018, 25, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Riley, J.S. When cell death goes wrong: Inflammatory outcomes of failed apoptosis and mitotic cell death. Cell Death Differ. 2023, 30, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Konda, V.R.; Desai, A.; Darland, G.; Grayson, N.; Bland, J.S. KDT501, a derivative from hops, normalizes glucose metabolism and body weight in rodent models of diabetes. PLoS ONE 2014, 9, e87848. [Google Scholar] [CrossRef]
- Kern, P.A.; Finlin, B.S.; Ross, D.; Boyechko, T.; Zhu, B.; Grayson, N.; Sims, R.; Bland, J.S. Effects of KDT501 on Metabolic Parameters in Insulin-Resistant Prediabetic Humans. J. Endocr. Soc. 2017, 1, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Zhu, B.; Kok, B.P.; Godio, C.; Westgate, P.M.; Grayson, N.; Sims, R.; Bland, J.S.; Saez, E.; Kern, P.A. The Influence of a KDT501, a Novel Isohumulone, on Adipocyte Function in Humans. Front. Endocrinol. 2017, 8, 255. [Google Scholar] [CrossRef]
- Nagasawa, T.; Ishimaru, K.; Higashiyama, S.; Hama, Y.; Mitsutake, S. Teadenol A in microbial fermented tea acts as a novel ligand on GPR120 to increase GLP-1 secretion. Food Funct. 2020, 11, 10534–10541. [Google Scholar] [CrossRef]
- Wilches, I.; Jiménez-Castillo, P.; Cuzco, N.; Clos, M.V.; Jiménez-Altayó, F.; Peñaherrera, E.; Jerves-Andrade, L.; Tobar, V.; Vander Heyden, Y.; Leon-Tamariz, F.; et al. Anti-inflammatory and sedative activities of Peperomia galioides: In vivo studies in mice. Nat. Prod. Res. 2021, 35, 1657–1661. [Google Scholar] [CrossRef]
- Ye, C.; Jin, M.; Li, R.; Sun, J.; Wang, R.; Wang, J.; Li, S.; Zhou, W.; Li, G. Phytochemical and chemotaxonomic study on the leaves of Rhododendron dauricum L. Biochem. Syst. Ecol. 2020, 90, 104038. [Google Scholar] [CrossRef]
- Wang, Y.; Alkhalidy, H.; Liu, D. The Emerging Role of Polyphenols in the Management of Type 2 Diabetes. Molecules 2021, 26, 703. [Google Scholar] [CrossRef]
- Nukata, M.; Hashimoto, T.; Yamamoto, I.; Iwasaki, N.; Tanaka, M.; Asakawa, Y. Neogrifolin derivatives possessing anti-oxidative activity from the mushroom Albatrellus ovinus. Phytochemistry 2002, 59, 731–737. [Google Scholar] [CrossRef]
- Grabovyi, G.A.; Mohr, J.T. Total Synthesis of Grifolin, Grifolic Acid, LL-Z1272α, LL-Z1272β, and Ilicicolinic Acid A. Org. Lett. 2016, 18, 5010–5013. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Laermans, J.; Iwakura, H.; Tack, J.; Depoortere, I. Sensing of fatty acids for octanoylation of ghrelin involves a gustatory G-protein. PLoS ONE 2012, 7, e40168. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A. An insight into the multifunctional role of ghrelin and structure activity relationship studies of ghrelin receptor ligands with clinical trials. Eur. J. Med. Chem. 2022, 235, 114308. [Google Scholar] [CrossRef]
- Usui, A.; Nakamura, A.; Era, M.; Matsuo, Y.; Tanaka, T.; Ishimaru, K. A New Flavonoid from Camellia sinensis Fermented Tea. Nat. Prod. Commun. 2016, 11, 1281–1282. [Google Scholar] [CrossRef]
- Wulandari, R.A.; Amano, M.; Yanagita, T.; Tanaka, T.; Kouno, I.; Kawamura, D.; Ishimaru, K. New phenolic compounds from Camellia sinensis L. leaves fermented with Aspergillus sp. J. Nat. Med. 2011, 65, 594–597. [Google Scholar] [CrossRef]
- Katanasaka, Y.; Sunagawa, Y.; Miyazaki, Y.; Funamoto, M.; Shimizu, S.; Shimizu, K.; Yamakage, H.; Satoh-Asahara, N.; Toyama, K.; Sabashi, T.; et al. Ameliorating prediabetic subject status via fermented tea supplementation: A randomized, double-blind, parallel-group comparison study. J. Funct. Foods. 2022, 97, 105257. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Klobučar, I.; Habisch, H.; Klobučar, L.; Trbušić, M.; Pregartner, G.; Berghold, A.; Kostner, G.M.; Scharnagl, H.; Madl, T.; Frank, S.; et al. Serum Levels of Adiponectin Are Strongly Associated with Lipoprotein Subclasses in Healthy Volunteers but Not in Patients with Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 5050. [Google Scholar] [CrossRef]
- Ma, S.; Li, Z.; Yang, Y.; Zhang, L.; Li, M.; Du, L. Fluorescent Ligand-Based Discovery of Small-Molecule Sulfonamide Agonists for GPR120. Front. Chem. 2022, 10, 816014. [Google Scholar] [CrossRef] [PubMed]
- Shimpukade, B.; Hudson, B.D.; Hovgaard, C.K.; Milligan, G.; Ulven, T. Discovery of a potent and selective GPR120 agonist. J. Med. Chem. 2012, 55, 4511–4515. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.P.; Peat, A.J.; McKeown, S.C.; Corbett, D.F.; Goetz, A.S.; Littleton, T.R.; McCoy, D.C.; Kenakin, T.P.; Andrews, J.L.; Ammala, C.; et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40, identification of agonist and antagonist small molecules. Br. J. Pharmacol. 2006, 148, 619–628. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Lopes-Virella, M.F.; Huang, Y. GPR40/GPR120 Agonist GW9508 Improves Metabolic Syndrome-Exacerbated Periodontitis in Mice. Int. J. Mol. Sci. 2024, 25, 9622. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.; Shimpukade, B.; Ulven, T.; Hudson, B.D. Complex Pharmacology of Free Fatty Acid Receptors. Chem. Rev. 2017, 117, 67–110. [Google Scholar] [CrossRef]
- Hudson, B.D.; Shimpukade, B.; Mackenzie, A.E.; Butcher, A.J.; Pediani, J.D.; Christiansen, E.; Heathcote, H.; Tobin, A.B.; Ulven, T.; Milligan, G. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol. Pharmacol. 2013, 84, 710–725. [Google Scholar] [CrossRef]
- Ji, G.; Guo, Q.; Xue, Q.; Kong, R.; Wang, S.; Lei, K.; Liu, R.; Wang, X. Novel GPR120 Agonists with Improved Pharmacokinetic Profiles for the Treatment of Type 2 Diabetes. Molecules 2021, 26, 6907. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wei, S.; Wang, M.; Xu, Y.; Hu, W.; Gao, Z.; Liu, R.; Wang, S.; Ji, G. Discovery of Novel and Selective G-Protein Coupled Receptor 120 (GPR120) Agonists for the Treatment of Type 2 Diabetes Mellitus. Molecules 2022, 27, 9018. [Google Scholar] [CrossRef]
- Adams, G.L.; Velazquez, F.; Jayne, C.; Shah, U.; Miao, S.; Ashley, E.R.; Madeira, M.; Akiyama, T.E.; Di Salvo, J.; Suzuki, T.; et al. Discovery of Chromane Propionic Acid Analogues as Selective Agonists of GPR120 with in Vivo Activity in Rodents. ACS Med. Chem. Lett. 2016, 8, 96–101. [Google Scholar] [CrossRef]
- Sparks, S.M.; Aquino, C.; Banker, P.; Collins, J.L.; Cowan, D.; Diaz, C.; Dock, S.T.; Hertzog, D.L.; Liang, X.; Swiger, E.D.; et al. Exploration of phenylpropanoic acids as agonists of the free fatty acid receptor 4 (FFA4): Identification of an orally efficacious FFA4 agonist. Bioorganic Med. Chem. Lett. 2017, 27, 1278–1283. [Google Scholar] [CrossRef]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Park, S.J.; Koh, J.M.; Im, D.S. Free fatty acid receptor 4 (FFA4) activation ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis by increasing regulatory T cells in mice. Acta Pharmacol. Sin. 2020, 41, 1337–1347. [Google Scholar] [CrossRef]
- Sundström, L.; Myhre, S.; Sundqvist, M.; Ahnmark, A.; McCoull, W.; Raubo, P.; Groombridge, S.D.; Polla, M.; Nyström, A.C.; Kristensson, L.; et al. The acute glucose lowering effect of specific GPR120 activation in mice is mainly driven by glucagon-like peptide 1. PLoS ONE 2017, 12, e0189060. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, A.G.; Miskelly, M.G.; Flatt, P.R.; McKillop, A.M. Pharmacological potential of novel agonists for FFAR4 on islet and enteroendocrine cell function and glucose homeostasis. Eur. J. Pharm. Sci. 2020, 142, 105104. [Google Scholar] [CrossRef]
- Cox, J.M.; Chu, H.D.; Chelliah, M.V.; Debenham, J.S.; Eagen, K.; Lan, P.; Lombardo, M.; London, C.; Plotkin, M.A.; Shah, U.; et al. Design, Synthesis, and Evaluation of Novel and Selective G-protein Coupled Receptor 120 (GPR120) Spirocyclic Agonists. ACS Med. Chem. Lett. 2016, 8, 49–54. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Ruan, L. G-Protein-Coupled Receptors 120 Agonist III Improves Hepatic Inflammation and ER Stress in Steatohepatitis. Dig. Dis. Sci. 2021, 66, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Watterson, K.R.; Hansen, S.V.F.; Hudson, B.D.; Alvarez-Curto, E.; Raihan, S.Z.; Azevedo, C.M.G.; Martin, G.; Dunlop, J.; Yarwood, S.J.; Ulven, T.; et al. Probe-Dependent Negative Allosteric Modulators of the Long-Chain Free Fatty Acid Receptor FFA4. Mol. Pharmacol. 2017, 91, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.M.; Watterson, K.R.; Wargent, E.T.; Hansen, S.V.; Hudson, B.D.; Kępczyńska, M.A.; Dunlop, J.; Shimpukade, B.; Christiansen, E.; Milligan, G.; et al. Non-Acidic Free Fatty Acid Receptor 4 Agonists with Antidiabetic Activity. J. Med. Chem. 2016, 59, 8868–8878. [Google Scholar] [CrossRef]
- Dragano, N.R.V.; Solon, C.; Ramalho, A.F.; de Moura, R.F.; Razolli, D.S.; Christiansen, E.; Azevedo, C.; Ulven, T.; Velloso, L.A. Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. J. Neuroinflamm. 2017, 14, 91. [Google Scholar] [CrossRef]
- Tian, M.; Wu, Z.; Heng, J.; Chen, F.; Guan, W.; Zhang, S. Novel advances in understanding fatty acid-binding G protein-coupled receptors and their roles in controlling energy balance. Nutr. Rev. 2022, 80, 187–199. [Google Scholar] [CrossRef]
- Gong, Z.; Yoshimura, M.; Aizawa, S.; Kurotani, R.; Zigman, J.M.; Sakai, T.; Sakata, I. G protein-coupled receptor 120 signaling regulates ghrelin secretion in vivo and in vitro. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E28–E35. [Google Scholar] [CrossRef] [PubMed]
- Graciano, M.F.; Valle, M.M.; Curi, R.; Carpinelli, A.R. Evidence for the involvement of GPR40 and NADPH oxidase in palmitic acid-induced superoxide production and insulin secretion. Islets 2013, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Raptis, D.A.; Limani, P.; Jang, J.H.; Ungethüm, U.; Tschuor, C.; Graf, R.; Humar, B.; Clavien, P.A. GPR120 on Kupffer cells mediates hepatoprotective effects of ω3-fatty acids. J. Hepatol. 2014, 60, 625–632. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lin, Y.P.; Sun, C.K.; Huang, T.H.; Yip, H.K.; Chen, Y.T. Extracorporeal shockwave against inflammation mediated by GPR120 receptor in cyclophosphamide-induced rat cystitis model. Mol. Med. 2018, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Olmo, I.; Teuber, S.; Larrazabal, C.; Alarcon, P.; Raipane, F.; Burgos, R.A.; Hidalgo, M.A. Docosahexaenoic acid and TUG-891 activate free fatty acid-4 receptor in bovine neutrophils. Vet. Immunol. Immunopathol. 2019, 209, 53–60. [Google Scholar] [CrossRef]
- Gozal, D.; Qiao, Z.; Almendros, I.; Zheng, J.; Khalyfa, A.; Shimpukade, B.; Ulven, T. Treatment with TUG891, a free fatty acid receptor 4 agonist, restores adipose tissue metabolic dysfunction following chronic sleep fragmentation in mice. Int. J. Obes. 2016, 40, 1143–1149. [Google Scholar] [CrossRef]
- Schilperoort, M.; van Dam, A.D.; Hoeke, G.; Shabalina, I.G.; Okolo, A.; Hanyaloglu, A.C.; Dib, L.H.; Mol, I.M.; Caengprasath, N.; Chan, Y.W.; et al. The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat. EMBO Mol. Med. 2018, 10, e8047. [Google Scholar] [CrossRef]
- Murtaza, B.; Hichami, A.; Khan, A.S.; Shimpukade, B.; Ulven, T.; Ozdener, M.H.; Khan, N.A. Novel GPR120 agonist TUG891 modulates fat taste perception and preference and activates tongue-brain-gut axis in mice. J. Lipid Res. 2020, 61, 133–142. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, C.; Sui, Z.; Macielag, M.; Wang, Y.; Yan, W.; Suckow, A.; Hua, H.; Bell, A.; Haug, P.; et al. Discovery of an Isothiazole-Based Phenylpropanoic Acid GPR120 Agonist as a Development Candidate for Type 2 Diabetes. ACS Med. Chem. Lett. 2017, 8, 947–952. [Google Scholar] [CrossRef]
- Ma, J.; Novack, A.; Nashashibi, I.; Pham, P.; Rabbat, C.J.; Song, J.; Shi, D.F.; Zhao, Z.; Choi, Y.J.; Chen, X. Aryl GPR120 Receptor Agonists and Uses Thereof. International Patent WO 2010/048207, 29 April 2010. [Google Scholar]
- Wang, C.; Liu, Y.; Pan, Y.; Jin, H. Effect of GSK-137647A, the first non-carboxylic FFA4 agonist, on the osteogenic and adipogenic differentiation of bone mesenchymal stem cells in db/db mice. J. Pharm. Pharmacol. 2020, 72, 461–469. [Google Scholar] [CrossRef]
- Salaga, M.; Bartoszek, A.; Binienda, A.; Krajewska, J.B.; Fabisiak, A.; Mosińska, P.; Dziedziczak, K.; Niewinna, K.; Talar, M.; Tarasiuk, A.; et al. Activation of Free Fatty Acid Receptor 4 Affects Intestinal Inflammation and Improves Colon Permeability in Mice. Nutrients 2021, 13, 2716. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.U.; Ohmori, K.; Hashimoto, T.; Kamitori, K.; Yamaguchi, F.; Noma, T.; Igarashi, J.; Tsuboi, K.; Tokuda, M.; Nishiyama, A.; et al. GPR120 in adipocytes has differential roles in the production of pro-inflammatory adipocytokines. Biochem. Biophys. Res. Commun. 2017, 486, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, M.; Bender, E.; Schamberger, J.; Eitner, F. Pharmacology of Free Fatty Acid Receptors and Their Allosteric Modulators. Int. J. Mol. Sci. 2021, 22, 1763. [Google Scholar] [CrossRef]
- Im, H.; Park, J.H.; Im, S.; Han, J.; Kim, K.; Lee, Y.H. Regulatory roles of G-protein coupled receptors in adipose tissue metabolism and their therapeutic potential. Arch. Pharm. Res. 2021, 44, 133–145. [Google Scholar] [CrossRef]
- Zhang, S.; Roth, B.L. Sensing unsaturated fatty acids: Insights from GPR120 signaling. Cell Res. 2023, 33, 657–658. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).