Putative Epigenetic Regulator microRNAs (epi-miRNAs) and Their Predicted Targets in High-Fat Diet-Induced Cardiac Dysfunction: An In Silico Analysis in Obese Rats
Abstract
1. Introduction
2. Results
2.1. Putative Epi-miRNAs in HFD-Induced Cardiac Dysfunction
2.2. Predicted Targets of Epi-miRNAs with Epigenetic Regulator Function
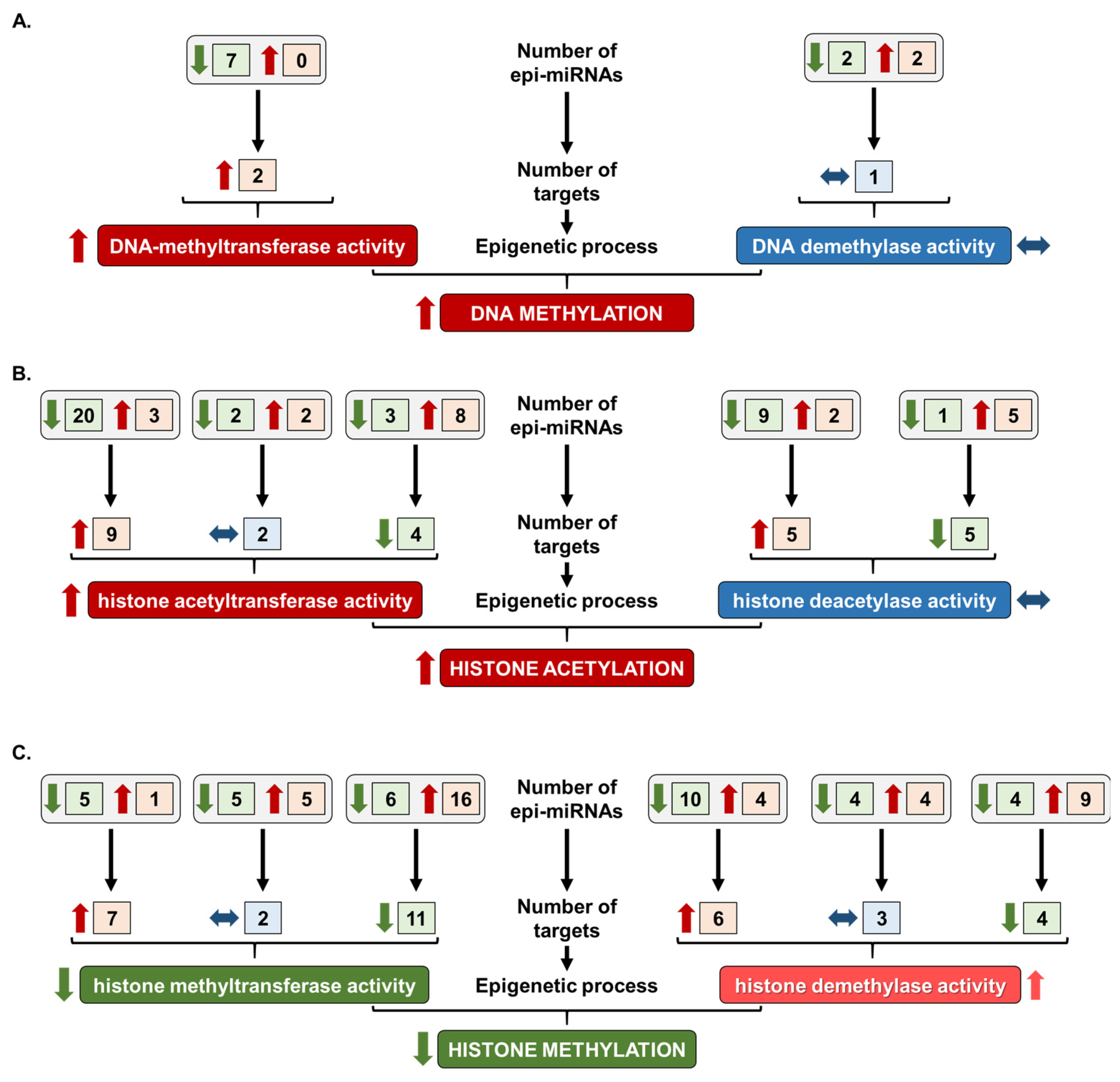
2.3. Prediction of Overall Epigenetic Changes in HFD-Induced Cardiac Dysfunction
3. Discussion
4. Materials and Methods
4.1. Cardiac miRNA Dataset of HFD-Fed Rats
4.2. Target Prediction
4.3. Establishment of Epigenetic Regulators Expressed in the Heart
4.4. Identification of Epi-miRNAs
4.5. Network Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Dnmt | DNA methyltransferase |
| epi-miRNA | Epigenetic Regulator microRNAs |
| Ezh2 | Enhancer of Zeste homolog 2 |
| Hat | Histone acetyltransferase |
| Hdac | Histone deacetylase |
| HFD | High-fat diet |
| miRNA | Micro RNA |
| Myh7 | β-myosin heavy chain |
References
- Katya, P.; Mark, E. Review on the update in obesity management: Epidemiology. BMJ Public Health 2024, 2, e000247. [Google Scholar]
- WHO. European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022.
- Ebong, I.A.; Goff, D.C.; Rodriguez, C.J.; Chen, H.; Bertoni, A.G. Mechanisms of heart failure in obesity. Obes. Res. Clin. Pract. 2014, 8, e540–e548. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramírez, H.C.; Galicia-Moreno, M.; García-Bañuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular mechanisms of obesity-linked cardiac dysfunction: An up-date on current knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef]
- Mahajan, R.; Lau, D.H.; Sanders, P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc. Med. 2015, 25, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Preguiça, I.; Alves, A.; Nunes, S.; Fernandes, R.; Gomes, P.; Viana, S.D.; Reis, F. Diet-induced rodent models of obesity-related metabolic disorders—A guide to a translational perspective. Obes. Rev. 2020, 21, e13081. [Google Scholar] [CrossRef]
- Guedes, E.C.; França, G.S.; Lino, C.A.; Koyama, F.C.; Moreira, L.d.N.; Alexandre, J.G.; Barreto-Chaves, M.L.M.; Galante, P.A.F.; Diniz, G.P. Microrna expression signature is altered in the cardiac remodeling induced by high fat diets. J. Cell. Physiol. 2016, 231, 1771–1783. [Google Scholar] [CrossRef]
- Ternacle, J.; Wan, F.; Sawaki, D.; Surenaud, M.; Pini, M.; Mercedes, R.; Ernande, L.; Audureau, E.; Dubois-Rande, J.-L.; Adnot, S.; et al. Short-term high-fat diet compromises myocardial function: A radial strain rate imaging study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1283–1291. [Google Scholar] [CrossRef]
- Zou, T.; Zhu, M.; Ma, Y.-C.; Xiao, F.; Yu, X.; Xu, L.; Ma, L.-Q.; Yang, J.; Dong, J.-Z. Microrna-410-5p exacerbates high-fat diet-induced cardiac remodeling in mice in an endocrine fashion. Sci. Rep. 2018, 8, 8780. [Google Scholar] [CrossRef]
- Ciccarone, F.; Castelli, S.; Ioannilli, L.; Ciriolo, M.R. High dietary fat intake affects DNA methylation/hydroxymethylation in mouse heart: Epigenetic hints for obesity-related cardiac dysfunction. Mol. Nutr. Food Res. 2019, 63, 1800970. [Google Scholar] [CrossRef]
- Upadhyaya, B.; Larsen, T.; Barwari, S.; Louwagie, E.J.; Baack, M.L.; Dey, M. Prenatal exposure to a maternal high-fat diet affects histone modification of cardiometabolic genes in newborn rats. Nutrients 2017, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Blin, G.; Liand, M.; Mauduit, C.; Chehade, H.; Benahmed, M.; Simeoni, U.; Siddeek, B. Maternal exposure to high-fat diet induces long-term derepressive chromatin marks in the heart. Nutrients 2020, 12, 181. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microrna biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, R.M.W.; Calore, M. Micrornas in cardiac diseases. Cells 2019, 8, 737. [Google Scholar] [CrossRef]
- D’Amato, A.; Prosperi, S.; Severino, P.; Myftari, V.; Correale, M.; Perrone Filardi, P.; Badagliacca, R.; Fedele, F.; Vizza, C.D.; Palazzuoli, A. Microrna and heart failure: A novel promising diagnostic and therapeutic tool. J. Clin. Med. 2024, 13, 7560. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Elliott, E.K.; Haupt, L.M.; Griffiths, L.R. Regulatory mechanisms of epigenetic mirna relationships in human cancer and potential as therapeutic targets. Cancers 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, M.-A.; Panoutsopoulou, K.; Pilala, K.-M.; Scorilas, A.; Avgeris, M. Epi-mirnas: Modern mediators of methylation status in human cancers. WIREs RNA 2023, 14, e1735. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, R.M.W.; Calore, M. Epigenetics and micrornas in cardiovascular diseases. Genomics 2021, 113, 540–551. [Google Scholar] [CrossRef]
- Coppola, A.; Romito, A.; Borel, C.; Gehrig, C.; Gagnebin, M.; Falconnet, E.; Izzo, A.; Altucci, L.; Banfi, S.; Antonarakis, S.E.; et al. Cardiomyogenesis is controlled by the mir-99a/let-7c cluster and epigenetic modifications. Stem Cell Res. 2014, 12, 323–337. [Google Scholar] [CrossRef]
- Liu, R.; Gu, J.; Jiang, P.; Zheng, Y.; Liu, X.; Jiang, X.; Huang, E.; Xiong, S.; Xu, F.; Liu, G.; et al. Dnmt1–microrna126 epigenetic circuit contributes to esophageal squamous cell carcinoma growth via adam9–egfr–akt signaling. Clin. Cancer Res. 2015, 21, 854–863. [Google Scholar] [CrossRef]
- Han, S.; Lin, F.; Ruan, Y.; Zhao, S.; Yuan, R.; Ning, J.; Jiang, K.; Xie, J.; Li, H.; Li, C.; et al. Mir-132-3p promotes the cisplatin-induced apoptosis and inflammatory response of renal tubular epithelial cells by targeting sirt1 via the nf-κb pathway. Int. Immunopharmacol. 2021, 99, 108022. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Y.; Lv, X.-W.; Dai, R.-X.; Yang, X.-H.; Kong, B.-H. Lncrna tug1 mediates ischemic myocardial injury by targeting mir-132-3p/hdac3 axis. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H332–H344. [Google Scholar] [CrossRef]
- Dal-Pra, S.; Hodgkinson, C.P.; Mirotsou, M.; Kirste, I.; Dzau, V.J. Demethylation of h3k27 is essential for the induction of direct cardiac reprogramming by mir combo. Circ. Res. 2017, 120, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Chavali, V.; Tyagi, N.; Tyagi, S.C.; Mishra, P.K. Mir-133 as an epigenetic regulator of diabetic heart failure. FASEB J. 2012, 26, 1057.1022. [Google Scholar] [CrossRef]
- Thienpont, B.; Aronsen, J.M.; Robinson, E.L.; Okkenhaug, H.; Loche, E.; Ferrini, A.; Brien, P.; Alkass, K.; Tomasso, A.; Agrawal, A.; et al. The h3k9 dimethyltransferases ehmt1/2 protect against pathological cardiac hypertrophy. J. Clin. Investig. 2017, 127, 335–348. [Google Scholar] [CrossRef]
- Crippa, S.; Nemir, M.; Ounzain, S.; Ibberson, M.; Berthonneche, C.; Sarre, A.; Boisset, G.; Maison, D.; Harshman, K.; Xenarios, I.; et al. Comparative transcriptome profiling of the injured zebrafish and mouse hearts identifies mirna-dependent repair pathways. Cardiovasc. Res. 2016, 110, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Naik, D.; Kalle, A.M. Microrna-mediated epigenetic regulation of hdac8 and hdac6: Functional significance in cervical cancer. Noncoding RNA Res. 2024, 9, 732–743. [Google Scholar] [CrossRef]
- Pal, S.; Baiocchi, R.A.; Byrd, J.C.; Grever, M.R.; Jacob, S.T.; Sif, S. Low levels of mir-92b/96 induce prmt5 translation and h3r8/h4r3 methylation in mantle cell lymphoma. EMBO J. 2007, 26, 3558–3569. [Google Scholar] [CrossRef]
- Xu, Q.; Song, Y.; Lu, L. Overexpression of let-7d explains down-regulated kdm3a and eno2 in the pathogenesis of preeclampsia. J. Cell Mol. Med. 2021, 25, 8127–8139. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, W.; Wang, S.E.; Baltimore, D. Dual mechanisms of posttranscriptional regulation of tet2 by let-7 microrna in macrophages. Proc. Natl. Acad. Sci. USA 2019, 116, 12416–12421. [Google Scholar] [CrossRef]
- Liu, C.; Teng, Z.-Q.; McQuate, A.L.; Jobe, E.M.; Christ, C.C.; von Hoyningen-Huene, S.J.; Reyes, M.D.; Polich, E.D.; Xing, Y.; Li, Y.; et al. An epigenetic feedback regulatory loop involving microrna-195 and mbd1 governs neural stem cell differentiation. PLoS ONE 2013, 8, e51436. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lin, X.; Shan, S.K.; Li, F.; Xu, F.; Zhong, J.Y.; Guo, B.; Zheng, M.H.; Wang, Y.; Mo, Z.H.; et al. The suppression of mir-199a-3p by promoter methylation contributes to papillary thyroid carcinoma aggressiveness by targeting rap2a and dnmt3a. Front. Cell Dev. Biol. 2020, 8, 594528. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-C.; Sun, J.; Xu, J.-C.; Zhou, Z.-Y.; Zhang, Y.-F. Down-regulated microrna-199a-3p enhances osteogenic differentiation of bone marrow mesenchymal stem cells by targeting kdm3a in ovariectomized rats. Biochem. J. 2021, 478, 721–734. [Google Scholar] [CrossRef]
- Yan, M.; Chen, C.; Gong, W.; Yin, Z.; Zhou, L.; Chaugai, S.; Wang, D.W. Mir-21-3p regulates cardiac hypertrophic response by targeting histone deacetylase-8. Cardiovasc. Res. 2014, 105, 340–352. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, L.; Cheng, R.; Wu, M.; Bai, X.; Fan, L.; Liu, Y. Mir-29c-3p acts as a tumor promoter by regulating β-catenin signaling through suppressing dnmt3a, tet1 and hbp1 in ovarian carcinoma. Cell. Signal. 2024, 113, 110936. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ji, X.B.; Wang, L.H.; Xia, Z.K.; Xie, Y.X.; Liu, W.J.; Qiu, J.G.; Jiang, B.H.; Liu, L.Z. Mirna-30e downregulation increases cancer cell proliferation, invasion and tumor growth through targeting rps6kb1. Aging 2021, 13, 24037–24049. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Meng, X.; Ying, X.; Zuo, Y.; Liu, R.; Pan, Z.; Kang, T.; Huang, W. Microrna-342 inhibits colorectal cancer cell proliferation and invasion by directly targeting DNA methyltransferase 1. Carcinogenesis 2011, 32, 1033–1042. [Google Scholar] [CrossRef]
- Martino, T.A.; Young, M.E. Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. J. Biol. Rhythm. 2015, 30, 183–205. [Google Scholar] [CrossRef]
- Durgan, D.J.; Tsai, J.-Y.; Grenett, M.H.; Pat, B.M.; Ratcliffe, W.F.; Villegas-Montoya, C.; Garvey, M.E.; Nagendran, J.; Dyck, J.R.B.; Bray, M.S.; et al. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol. Int. 2011, 28, 187–203. [Google Scholar] [CrossRef]
- Peliciari-Garcia, R.A.; Goel, M.; Aristorenas, J.A.; Shah, K.; He, L.; Yang, Q.; Shalev, A.; Bailey, S.M.; Prabhu, S.D.; Chatham, J.C.; et al. Altered myocardial metabolic adaptation to increased fatty acid availability in cardiomyocyte-specific clock mutant mice. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 1579–1595. [Google Scholar] [CrossRef]
- Hamdani, N.; Costantino, S.; Mügge, A.; Lebeche, D.; Tschöpe, C.; Thum, T.; Paneni, F. Leveraging clinical epigenetics in heart failure with preserved ejection fraction: A call for individualized therapies. Eur. Heart J. 2021, 42, 1940–1958. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Tang, W.H.W. Epigenetics in cardiac hypertrophy and heart failure. JACC Basic Transl. Sci. 2019, 4, 976–993. [Google Scholar] [CrossRef]
- Tao, H.; Yang, J.-J.; Chen, Z.-W.; Xu, S.-S.; Zhou, X.; Zhan, H.-Y.; Shi, K.-H. Dnmt3a silencing rassf1a promotes cardiac fibrosis through upregulation of erk1/2. Toxicology 2014, 323, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Robinson, J.; Wang-Hu, J.; Jiang, L.; Freeman, D.A.; Rivkees, S.A.; Wendler, C.C. Camp induces hypertrophy and alters DNA methylation in hl-1 cardiomyocytes. Am. J. Physiol. Cell Physiol. 2015, 309, C425–C436. [Google Scholar] [CrossRef] [PubMed]
- Yanazume, T.; Hasegawa, K.; Morimoto, T.; Kawamura, T.; Wada, H.; Matsumori, A.; Kawase, Y.; Hirai, M.; Kita, T. Cardiac p300 is involved in myocyte growth with decompensated heart failure. Mol. Cell. Biol. 2003, 23, 3593–3606. [Google Scholar] [CrossRef]
- Madsen, A.; Krause, J.; Höppner, G.; Hirt, M.N.; Tan, W.L.W.; Lim, I.; Hansen, A.; Nikolaev, V.O.; Foo, R.S.Y.; Eschenhagen, T.; et al. Hypertrophic signaling compensates for contractile and metabolic consequences of DNA methyltransferase 3a loss in human cardiomyocytes. J. Mol. Cell. Cardiol. 2021, 154, 115–123. [Google Scholar] [CrossRef]
- Stenzig, J.; Schneeberger, Y.; Löser, A.; Peters, B.S.; Schaefer, A.; Zhao, R.-R.; Ng, S.L.; Höppner, G.; Geertz, B.; Hirt, M.N.; et al. Pharmacological inhibition of DNA methylation attenuates pressure overload-induced cardiac hypertrophy in rats. J. Mol. Cell. Cardiol. 2018, 120, 53–63. [Google Scholar] [CrossRef]
- Watson, C.J.; Horgan, S.; Neary, R.; Glezeva, N.; Tea, I.; Corrigan, N.; McDonald, K.; Ledwidge, M.; Baugh, J. Epigenetic therapy for the treatment of hypertension-induced cardiac hypertrophy and fibrosis. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 127–137. [Google Scholar] [CrossRef]
- Xiao, D.; Dasgupta, C.; Chen, M.; Zhang, K.; Buchholz, J.; Xu, Z.; Zhang, L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc. Res. 2014, 101, 373–382. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. Ezh2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef]
- Delgado-Olguín, P.; Huang, Y.; Li, X.; Christodoulou, D.; Seidman, C.E.; Seidman, J.G.; Tarakhovsky, A.; Bruneau, B.G. Epigenetic repression of cardiac progenitor gene expression by ezh2 is required for postnatal cardiac homeostasis. Nat. Genet. 2012, 44, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-J.; Tran, T.A.T.; Wang, M.; Ranek, M.J.; Kokkonen-Simon, K.M.; Gao, J.; Luo, X.; Tan, W.; Kyrychenko, V.; Liao, L.; et al. Histone lysine dimethyl-demethylase kdm3a controls pathological cardiac hypertrophy and fibrosis. Nat. Commun. 2018, 9, 5230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Liu, G.; Guo, X.; Liu, X.; Chen, J. Kdm3a inhibition ameliorates hyperglycemia-mediated myocardial injury by epigenetic modulation of nuclear factor kappa-b/p65. Front. Cardiovasc. Med. 2022, 9, 870999. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Xiao, B.; Neppl, R.L.; Kallin, E.M.; Li, J.; Chen, T.; Wang, D.-Z.; Xiao, X.; Zhang, Y. Dot1l regulates dystrophin expression and is critical for cardiac function. Genes Dev. 2011, 25, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Sunagawa, Y.; Fujita, M.; Hasegawa, K. Novel heart failure therapy targeting transcriptional pathway in cardiomyocytes by a natural compound, curcumin. Circ. J. 2010, 74, 1059–1066. [Google Scholar] [CrossRef]
- Rai, R.; Sun, T.; Ramirez, V.; Lux, E.; Eren, M.; Vaughan, D.E.; Ghosh, A.K. Acetyltransferase p300 inhibitor reverses hypertension-induced cardiac fibrosis. J. Cell. Mol. Med. 2019, 23, 3026–3031. [Google Scholar] [CrossRef]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microrna-132 in patients with heart failure: Results of a first-in-human phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2020, 42, 178–188. [Google Scholar] [CrossRef]
- Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Bär, C.; Borchert, T.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Cdr132l improves systolic and diastolic function in a large animal model of chronic heart failure. Eur. Heart J. 2020, 42, 192–201. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X. Prediction of functional microrna targets by integrative modeling of microrna binding and target expression data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. Mirdb: An online database for prediction of functional microrna targets. Nucleic Acids Res. 2019, 48, D127–D131. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. Mirwalk: An online resource for prediction of microrna binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef] [PubMed]
- Vedi, M.; Smith, J.R.; Thomas Hayman, G.; Tutaj, M.; Brodie, K.C.; De Pons, J.L.; Demos, W.M.; Gibson, A.C.; Kaldunski, M.L.; Lamers, L.; et al. 2022 updates to the rat genome database: A findable, accessible, interoperable, and reusable (fair) resource. Genetics 2023, 224, iyad042. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The string database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2024, 53, D730–D737. [Google Scholar] [CrossRef]
- Ren, J. Intermittent hypoxia BMSCs-derived exosomal miR-31-5p promotes lung adenocarcinoma development via WDR5-induced epithelial mesenchymal transition. Sleep Breath. 2023, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tan, X.; Lin, J.; Yuan, L.; Chen, J.; Qiu, L.; Huang, W. Minicircle-oriP-miR-31 as a Novel EBNA1-Specific miRNA Therapy Approach for Nasopharyngeal Carcinoma. Hum. Gene Ther. 2016, 28, 415–427. [Google Scholar] [CrossRef]
- Cai, M.-Z.; Wen, S.-Y.; Wang, X.-J.; Liu, Y.; Liang, H. MYC Regulates PHF8, Which Promotes the Progression of Gastric Cancer by Suppressing miR-22-3p. Technol. Cancer Res. Treat. 2020, 19, 1533033820967472. [Google Scholar] [CrossRef]
- Shao, P.; Liu, Q.; Maina, P.K.; Cui, J.; Bair, T.B.; Li, T.; Umesalma, S.; Zhang, W.; Qi, H.H. Histone demethylase PHF8 promotes epithelial to mesenchymal transition and breast tumorigenesis. Nucleic Acids Res. 2017, 45, 1687–1702. [Google Scholar] [CrossRef]
- Alvarez-Saavedra, M.; Antoun, G.; Yanagiya, A.; Oliva-Hernandez, R.; Cornejo-Palma, D.; Perez-Iratxeta, C.; Sonenberg, N.; Cheng, H.-Y.M. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum. Mol. Genet. 2011, 20, 731–751. [Google Scholar] [CrossRef]
- Liu, F.; Sang, M.; Meng, L.; Gu, L.; Liu, S.; Li, J.; Geng, C. miR-92b promotes autophagy and suppresses viability and invasion in breast cancer by targeting EZH2. Int. J. Oncol. 2018, 53, 1505–1515. [Google Scholar] [CrossRef]
- Gao, S.; Li, J.; Song, L.; Wu, J.; Huang, W. Influenza A virus-induced downregulation of miR-26a contributes to reduced IFNα/β production. Virol. Sin. 2017, 32, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, C.; Wang, X.; Bai, J.; He, S.; Zhang, J.; Xin, W.; Li, Y.; Jiang, Y.; Li, J.; et al. MicroRNA-874-5p regulates autophagy and proliferation in pulmonary artery smooth muscle cells by targeting Sirtuin 3. Eur. J. Pharmacol. 2020, 888, 173485. [Google Scholar] [CrossRef] [PubMed]
- Latreille, M.; Hausser, J.; Stützer, I.; Zhang, Q.; Hastoy, B.; Gargani, S.; Kerr-Conte, J.; Pattou, F.; Zavolan, M.; Esguerra, J.L.; et al. MicroRNA-7a regulates pancreatic β cell function. J. Clin. Investig. 2014, 124, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lin, Y.; Guo, W.; Chen, L. BMSC-Derived Exosomes Carrying miR-26a-5p Ameliorate Spinal Cord Injury via Negatively Regulating EZH2 and Activating the BDNF-TrkB-CREB Signaling. Mol. Neurobiol. 2024, 61, 8156–8174. [Google Scholar] [CrossRef]
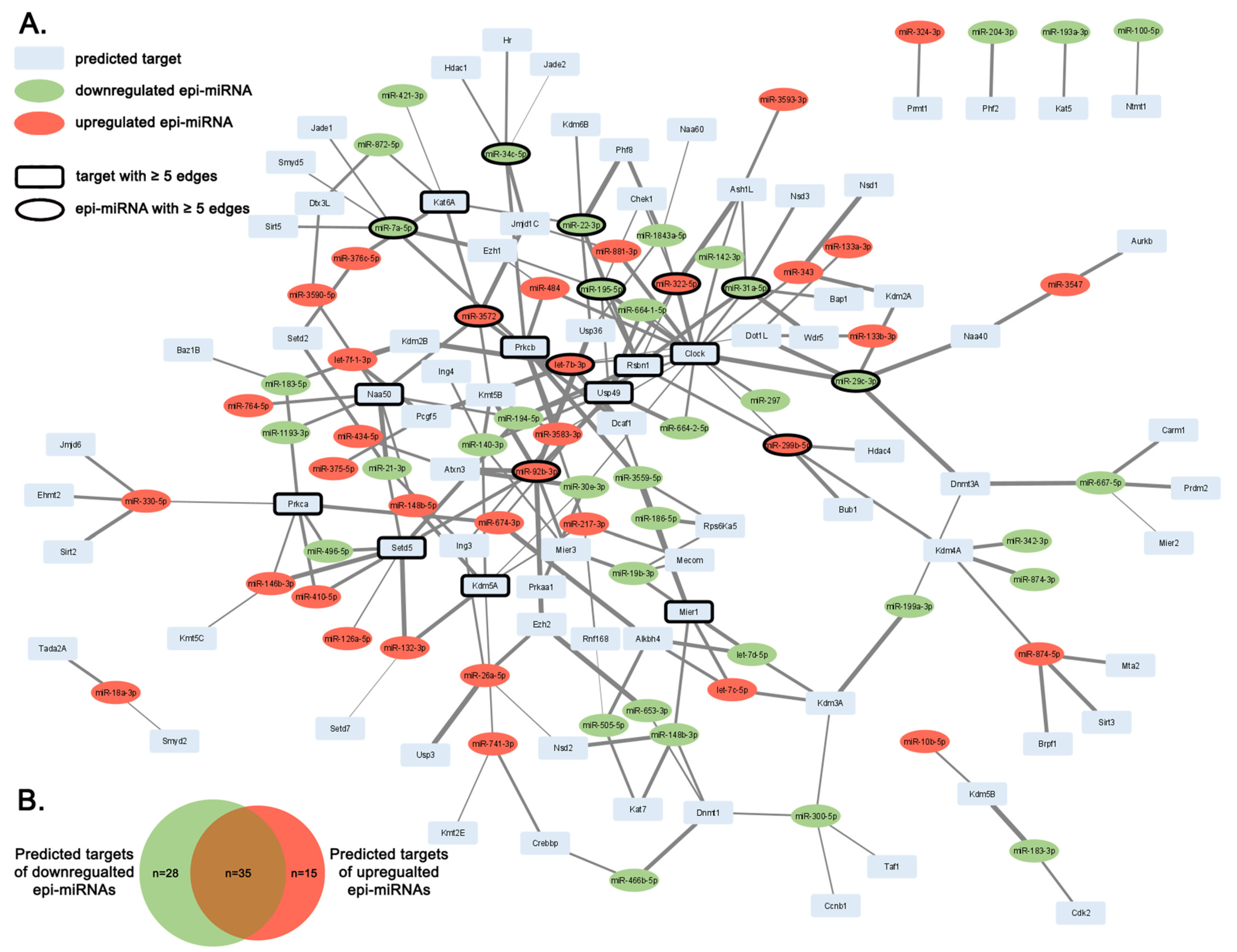

| Upregulated Cardiac miRNAs in Response to HFD [10] and Their Predicted Epigenetic Regulator Targets | ||||
| miRNA Name | Predicted mRNA Target Symbol | Cumulative Prediction Score | Identified Previously as Epi-miRNA | |
| Y/N | Target (Species and Tissue) | |||
| rno-let-7b-3p | Dcaf1, Kdm2b, Pcgf5, Clock, Dot1l | 1.95, 1.91, 1.89, 1.59, 1.48 | N | - |
| rno-let-7c-5p | Kdm3a, Mier1, Alkbh4 | 1.73, 1.73, 1.71 | Y | Ezh2 (mouse embryonic stem cells) [20] |
| rno-let-7f-1-3p | Kdm2b, Pcgf5 | 1.91, 1.81 | N | - |
| rno-miR-10b-5p | Kdm5b | 1.61 | N | - |
| rno-miR-126a-5p | Setd5 | 1.54 | Y | Dnmt1 (human esophageal squamous cell) [21] |
| rno-miR-132-3p | Setd5, Kdm5a, Setd7 | 1.87, 1.80, 1.46 | Y | Sirt1 (human proximal renal tubular epithelial cells) [22] Hdac3 (mouse neonatal cardiomyocytes) [23] |
| rno-miR-133a-3p | Dot1l | 1.63 | Y | Ezh2 (mouse neonatal cardiac fibroblasts) [24] |
| rno-miR-133b-3p | Dot1l | 1.71 | Y | Dnmt3a (mouse HL-1 cardiomyocytes) [25] |
| rno-miR-146b-3p | Setd5, Prkca, Kmt5c | 1.86, 1.61, 1.55 | N | - |
| rno-miR-148b-5p | Kdm5a, Naa50 | 1.78, 1.64 | N | - |
| rno-miR-18a-3p | Tada2a, Smyd2 | 1.69, 1.51 | N | - |
| rno-miR-217-3p | Mecom, Prkcb, Rnf168, Kdm5a | 1.70, 1.66, 1.58, 1.57 | Y | Ehmt1, Ehmt2 (rat neonatal cardiomyocytes) [26] |
| rno-miR-26a-5p | Usp3, Ezh2, Ing3, Nsd2 | 1.92, 1.78, 1.62, 1.50 | Y | Ezh2 (rat neonatal cardiomyocytes) [27] |
| rno-miR-299b-5p | Bub1, Hdac4, Kdm4a, Rsbn1, Clock | 1.79, 1.71, 1.70, 1.69, 1.55 | N | - |
| rno-miR-322-5p | Ash1l, Clock, Usp49, Chek1, Rsbn1 | 1.92, 1.85, 1.68, 1.60, 1.56 | N | - |
| rno-miR-324-3p | Prmt1 | 1.65 | Y | Hdac6 (human HeLa cells) [28] |
| rno-miR-330-5p | Sirt2, Ehmt2, Jmjd6, Prkca | 1.77, 1.71, 1.70, 1.54 | N | - |
| rno-miR-343 | Nsd1, Kdm2a, Clock | 1.89, 1.80, 1.64 | N | - |
| rno-miR-3547 | Naa40, Aurkb | 1.80, 1.76 | N | - |
| rno-miR-3572 | Jmjd1c, Dcaf1, Naa50, Kat6a, Kmt5b | 1.86, 1.78, 1.76, 1.74, 1.66 | N | - |
| rno-miR-3583-3p | Prkcb, Kmt5b, Ing3, Clock | 1.93, 1.65, 1.60, 1.53 | N | - |
| rno-miR-3590-5p | Naa50, Dtx3l | 1.69, 1.67 | N | - |
| rno-miR-3593-3p | Ash1l | 1.70 | N | - |
| rno-miR-375-5p | Pcgf5 | 1.69 | N | - |
| rno-miR-376c-5p | Kat6a, Setd2 | 1.77, 1.74 | N | - |
| rno-miR-410-5p | Setd5, Prkca | 1.74, 1.73 | N | - |
| rno-miR-434-5p | Atxn3 | 1.68 | N | - |
| rno-miR-484 | Prkcb, Clock, Ezh1 | 1.77, 1.73, 1.54 | N | - |
| rno-miR-674-3p | Alkbh4, Prkca, Usp49 | 1.88, 1.78, 1.61 | N | - |
| rno-miR-741-3p | Crebbp, Kdm5a, Kmt2e | 1.68, 1.56, 1.53 | N | - |
| rno-miR-764-5p | Naa50 | 1.71 | N | - |
| rno-miR-874-5p | Sirt3, Brpf1, Mta2, Kdm4a | 1.81, 1.79, 1.75, 1.66 | N | - |
| rno-miR-881-3p | Clock, Jmjd1c | 1.82, 1.60 | N | - |
| rno-miR-92b-3p | Usp36, Ezh2, Kmt5b, Atxn3, Rsbn1, Mier3, Setd5 | 1.94, 1.90, 1.89, 1.88, 1.85, 1.75, 1.74 | Y | Prmt5 (human B-lymphocytes) [29] |
| Downregulated Cardiac miRNAs in Response to HFD [10] and Their Predicted Epigenetic Regulator Targets | ||||
| miRNA Name | Predicted mRNA Target Symbol | Cumulative Prediction Score | Identified previously as epi-miRNA | |
| Y/N | Target (Species and Tissue) | |||
| let-7d-5p | Alkbh4, Mier1, Kdm3a | 1.79, 1.79, 1.71 | Y | Kdm3a (human placental BeWo cells) [11]; Tet2 (mouse macrophages) [30,31] |
| rno-miR-100-5p | Ntmt1 | 1.60 | N | - |
| rno-miR-1193-3p | Naa50 | 1.69 | N | - |
| rno-miR-140-3p | Kmt5b, Usp49, Kdm5a, Ing4, Mier3 | 1.76, 1.68, 1.66, 1.63, 1.62 | N | - |
| rno-miR-142-3p | Clock, Ash1l | 1.67, 1.64 | N | - |
| rno-miR-148b-3p | Nsd2, Kat7, Mier1, Dnmt1 | 1.79, 1.76, 1.68, 1.65 | N | - |
| rno-miR-183-3p | Kdm5b, Cdk2 | 1.91, 1.65 | N | - |
| rno-miR-183-5p | Kdm2b, Prkca, Baz1b | 1.73, 1.71, 1.61 | N | - |
| rno-miR-1843a-5p | Phf8, Clock, Usp36, Naa60 | 1.81, 1.61, 1.59, 1.51 | N | - |
| rno-miR-186-5p | Rps6ka5, Mier1, Dcaf1 | 1.78, 1.77, 1.67 | N | - |
| rno-miR-193a-3p | Kat5 | 1.66 | N | - |
| rno-miR-194-5p | Setd5, Rsbn1, Naa50, Clock | 1.81, 1.79, 1.65, 1.63 | N | - |
| rno-miR-195-5p | Clock, Rsbn1, Usp49, Ezh1, Chek1 | 1.85, 1.66, 1.62, 1.61, 1.54 | Y | Mbd1 (mouse neural stem cells) [32] |
| rno-miR-199a-3p | Kdm3a, Dnmt3a | 1.87, 1.57 | Y | Dnmt3a, Rap2a (human papillary thyroid cancer cells) [33]; Kdm3a (rat mesenchymal stem cells) [34] |
| rno-miR-19b-3p | Mier3, Mier1, Mecom, Rps6ka5 | 1.75, 1.72, 1.64, 1.61 | N | - |
| rno-miR-204-3p | Phf2 | 1.74 | N | - |
| rno-miR-21-3p | Naa50, Setd2, Ing3, Setd5 | 1.89, 1.76, 1.69, 1.68 | Y | Hdac8 (human embryonic kidney 293 cells) [35] |
| rno-miR-22-3p | Phf8, Rsbn1, Usp36, Kdm6b, Kat6a | 1.92, 1.81, 1.67, 1.64, 1.62 | N | - |
| rno-miR-297 | Clock | 1.68 | N | - |
| rno-miR-29c-3p | Clock, Dnmt3a, Dot1l, Naa40, Kdm2a | 1.89, 1.88, 1.85, 1.85, 1.76 | Y | Dnmt3a, Tet1 (human ovarian carcinoma) [36] |
| rno-miR-300-5p | Dnmt1, Kdm3a, Taf1, Ccnb1 | 1.59, 1.58, 1.55, 1.55 | N | - |
| rno-miR-30e-3p | Atxn3, Prkaa1, Clock, Kdm5a | 1.86, 1.75, 1.54, 1.51 | Y | Rps6kb1 (human esophageal cancer cells) [37] |
| rno-miR-31a-5p | Wdr5, Rsbn1, Nsd3, Bap1, Clock, Ash1l | 1.88, 1.79, 1.74, 1.69, 1.65, 1.62 | N | - |
| rno-miR-342-3p | Kdm4a | 1.76 | Y | Dnmt1 (human colorectal cancer SW480 cells) [38] |
| rno-miR-34c-5p | Jmjd1c, Prkcb, Hr, Hdac1, Jade2 | 1.77, 1.74, 1.65, 1.61, 1.45 | N | - |
| rno-miR-3559-5p | Prkcb, Mier1, Rps6ka5 | 1.88, 1.87, 1.66 | N | - |
| rno-miR-421-3p | Kat6a | 1.51 | N | - |
| rno-miR-466b-5p | Dnmt1, Crebbp | 1.81, 1.62 | N | - |
| rno-miR-496-5p | Setd5, Prkca | 1.74, 1.69 | N | - |
| rno-miR-505-5p | Alkbh4, Kat7, Rnf168 | 1.74, 1.68, 1.45 | N | - |
| rno-miR-653-3p | Ezh2, Dnmt1 | 1.91, 1.58 | N | - |
| rno-miR-664-1-5p | Usp49, Clock | 1.81, 1.56 | N | - |
| rno-miR-664-2-5p | Usp49, Clock | 1.81, 1.64 | N | - |
| rno-miR-667-5p | Dnmt3a, Prdm2, Carm1, Mier2 | 1.85, 1.78, 1.76, 1.49 | N | - |
| rno-miR-7a-5p | Ezh1, Prkcb, Sirt5, Jade1, Smyd5 | 1.82, 1.76, 1.65, 1.59, 1.55 | N | - |
| rno-miR-872-5p | Dtx3l, Kat6a | 1.69, 1.62 | N | - |
| rno-miR-874-3p | Kdm4a | 1.81 | N | - |
| Predicted mRNA Target | Number of Epi-miRNAs Induced by HFD [10] That Target the Predicted mRNA | ||||||
| Symbol (Alternative Symbol) | Name | Gene ID | Expression Level in Heart | Annotated to Heart Disease (Evidence Code) | Total | Up-Regulated | Down-Regulated |
| DNA-methyltransferase activity (GO:0009008) | |||||||
| Dnmt1 | DNA methyltransferase 1 | 84350 | low | congestive heart failure (IEP), cardiomyopathy (ISO) | 4 | 0 | 4 |
| Dnmt3a | DNA methyltransferase 3 alpha | 444984 | low | congenital heart disease (IEP) | 3 | 0 | 3 |
| DNA demethylase activity (GO:0035514) | |||||||
| Alkbh4 | alkB homolog 4, lysine demethylase | 288587 | low | NO | 4 | 2 | 2 |
| histone acetyltransferase activity (GO:0004402) | |||||||
| Brpf1 | bromodomain and PHD finger containing, 1 | 679713 | low | cardiomyopathy (ISO) | 1 | 1 | 0 |
| Clock (Kat13d) | clock circadian regulator | 60447 | medium | NO | 17 | 7 | 10 |
| Crebbp (Kat3a) | CREB binding protein | 54244 | medium | cardiomyopathy (IEP) | 2 | 1 | 1 |
| Ing3 | inhibitor of growth family, member 3 | 312154 | low | NO | 3 | 2 | 1 |
| Ing4 | inhibitor of growth family, member 4 | 297597 | medium | NO | 1 | 0 | 1 |
| Jade1 | jade family PHD finger 1 | 310352 | medium | NO | 1 | 0 | 1 |
| Jade2 | jade family PHD finger 2 | 303113 | low | NO | 1 | 0 | 1 |
| Kat5 | lysine acetyltransferase 5 | 192218 | medium | NO | 1 | 0 | 1 |
| Kat6a | lysine acetyltransferase 6A | 306571 | medium | congenital heart disease (ISS) | 5 | 2 | 3 |
| Kat7 | lysine acetyltransferase 7 | 303470 | medium | NO | 2 | 0 | 2 |
| Naa40 (Nat11) | N(alpha)-acetyltransferase 40, NatD catalytic subunit | 361718 | medium | NO | 2 | 1 | 1 |
| Naa50 (Nat5, Nat13, Mak3) | N(alpha)-acetyltransferase 50, NatE catalytic subunit | 288108 | medium | NO | 7 | 4 | 3 |
| Naa60 (Hat4) | N(alpha)-acetyltransferase 60, NatF catalytic subunit | 363545 | medium | NO | 1 | 0 | 1 |
| Tada2a | transcriptional adaptor 2A | 360581 | low | NO | 1 | 1 | 0 |
| Taf1 (Kat4) | TATA-box binding protein associated factor 1 | 317256 | low | congenital heart disease (ISO) | 1 | 0 | 1 |
| histone deacetylase activity (GO:0004407) | |||||||
| Atxn3 | ataxin 3 | 60331 | low | NO | 3 | 2 | 1 |
| Hdac1 | histone deacetylase 1 | 297893 | medium | congestive heart failure (ISO) | 1 | 0 | 1 |
| Hdac4 | histone deacetylase 4 | 363287 | low | congestive heart failure (ISO) | 1 | 1 | 0 |
| Mier1 | MIER1 transcriptional regulator | 313418 | medium | NO | 6 | 1 | 5 |
| Mier2 | MIER family member 2 | 362841 | low | NO | 1 | 0 | 1 |
| Mier3 | MIER family member 3 | 310086 | low | NO | 3 | 1 | 2 |
| Mta2 | metastasis associated 1 family, member 2 | 361724 | medium | NO | 1 | 1 | 0 |
| Sirt2 | sirtuin 2 | 361532 | medium | NO | 1 | 1 | 0 |
| Sirt3 | sirtuin 3 | 293615 | medium | congestive heart failure (IEP) | 1 | 1 | 0 |
| Sirt5 | sirtuin 5 | 306840 | medium | NO | 1 | 0 | 1 |
| histone methyltransferase activity (GO:0042054) | |||||||
| Ash1l (Kmt2h) | ASH1 like histone lysine methyltransferase | 310638 | medium | NO | 4 | 2 | 2 |
| Carm1 | coactivator-associated arginine methyltransferase 1 | 363026 | medium | NO | 1 | 0 | 1 |
| Dot1l (Kmt4) | DOT1 like histone lysine methyltransferase | 362831 | low | cardiomyopathy (ISS) | 4 | 3 | 1 |
| Ehmt2 (Kmt1c) | euchromatic histone lysine methyltransferase 2 | 361798 | medium | NO | 1 | 1 | 0 |
| Ezh1 (Kmt6b) | enhancer of zeste 1 polycomb repressive complex 2 subunit | 303547 | medium | NO | 3 | 1 | 2 |
| Ezh2 (Kmt6a) | enhancer of zeste 2 polycomb repressive complex 2 subunit | 312299 | low | congestive heart failure (ISO) | 3 | 2 | 1 |
| Kmt2e | lysine methyltransferase 2E | 311968 | medium | NO | 1 | 1 | 0 |
| Kmt5b | lysine methyltransferase 5B | 361688 | medium | NO | 4 | 3 | 1 |
| Kmt5c | lysine methyltransferase 5C | 308345 | medium | NO | 1 | 1 | 0 |
| Mecom (Kmt8e) | MDS1 and EVI1 complex locus | 294924 | low | NO | 2 | 1 | 1 |
| Nsd1 (Kmt3b) | nuclear receptor binding SET domain protein 1 | 306764 | medium | congenital heart disease (ISO) | 1 | 1 | 0 |
| Nsd2 (Kmt3g) | nuclear receptor binding SET domain protein 2 | 680537 | low | cardiomyopathy (ISO) | 2 | 1 | 1 |
| Nsd3 (Kmt3f) | nuclear receptor binding SET domain protein 3 | 290831 | low | NO | 1 | 0 | 1 |
| Ntmt1 | N-terminal Xaa-Pro-Lys N-methyltransferase 1 | 362103 | medium | NO | 1 | 0 | 1 |
| Prdm2 (Kmt8, Kmt8a) | PR/SET domain 2 | 313678 | low | NO | 1 | 0 | 1 |
| Prmt1 | protein arginine methyltransferase 1 | 60421 | medium | NO | 1 | 1 | 0 |
| Setd2 (Kmt3a) | SET domain containing 2, histone lysine methyltransferase | 316013 | medium | NO | 2 | 1 | 1 |
| Setd5 | SET domain containing 5 | 297514 | medium | cardiomyopathy (ISO) | 8 | 5 | 3 |
| Setd7 (Kmt7) | SET domain containing 7, histone lysine methyltransferase | 689954 | medium | NO | 1 | 1 | 0 |
| Smyd2 (Kmt3c) | SET and MYND domain containing 2 | 289372 | medium | NO | 1 | 1 | 0 |
| Smyd5 | SMYD family member 5 | 312503 | low | NO | 1 | 0 | 1 |
| Wdr5 | WD repeat domain 5 | 362093 | medium | congenital heart disease (ISO) | 1 | 0 | 1 |
| histone demethylase activity (GO:0032452) | |||||||
| Hr | HR, lysine demethylase and nuclear receptor corepressor | 60563 | low | congenital heart disease (ISO) | 1 | 0 | 1 |
| Jmjd1c (Kdm3c) | jumonji domain containing 1C | 171120 | medium | NO | 3 | 2 | 1 |
| Jmjd6 | jumonji domain containing 6, arginine demethylase and lysine hydroxylase | 360665 | medium | NO | 1 | 1 | 0 |
| Kdm2a | lysine demethylase 2A | 361700 | medium | congestive heart failure (ISO) | 2 | 1 | 1 |
| Kdm2b | lysine demethylase 2B | 304495 | low | NO | 3 | 2 | 1 |
| Kdm3a | lysine demethylase 3A | 312440 | medium | NO | 4 | 1 | 3 |
| Kdm4a | lysine demethylase 4A | 313539 | medium | cardiomegaly (ISO) | 4 | 2 | 2 |
| Kdm5a | lysine demethylase 5A | 312678 | medium | NO | 6 | 4 | 2 |
| Kdm5b | lysine demethylase 5B | 304809 | low | myocardial infarction (ISO) | 2 | 1 | 1 |
| Kdm6b | lysine demethylase 6B | 363630 | medium | NO | 1 | 0 | 1 |
| Phf2 (Kdm7c) | PHD finger protein 2 | 306814 | medium | NO | 1 | 0 | 1 |
| Phf8 (Kdm7b) | PHD finger protein 8 | 317425 | low | NO | 2 | 0 | 2 |
| Rsbn1 (Kdm9) | round spermatid basic protein 1 | 310749 | low | NO | 7 | 3 | 4 |
| histone ubiquitin ligase activity (GO:0140852) | |||||||
| Dtx3l | deltex E3 ubiquitin ligase 3L | 498089 | low | NO | 2 | 1 | 1 |
| Pcgf5 | polycomb group ring finger 5 | 681178 | medium | NO | 3 | 3 | 0 |
| Rnf168 | ring finger protein 168 | 690043 | low | NO | 2 | 1 | 1 |
| histone deubiquitinase activity (GO:0140934) | |||||||
| Bap1 | Brca1 associated protein 1 | 306257 | medium | cardiomyopathy (ISO) | 1 | 0 | 1 |
| Usp3 | ubiquitin specific peptidase 3 | 363084 | low | cardiomyopathy (ISO) | 1 | 1 | 0 |
| Usp36 | ubiquitin specific peptidase 36 | 303700 | low | NO | 3 | 1 | 2 |
| Usp49 | ubiquitin specific peptidase 49 | 316211 | low | NO | 6 | 2 | 4 |
| histone kinase activity (GO:0035173) | |||||||
| Aurkb | aurora kinase B | 114592 | low | NO | 1 | 1 | 0 |
| Baz1b | bromodomain adjacent to zinc finger domain, 1B | 368002 | medium | Williams–Beuren syndrome (ISO) | 1 | 0 | 1 |
| Bub1 | BUB1 mitotic checkpoint serine/threonine kinase | 296137 | low | NO | 1 | 1 | 0 |
| Ccnb1 | cyclin B1 | 25203 | low | NO | 1 | 0 | 1 |
| Cdk2 | cyclin dependent kinase 2 | 362817 | low | NO | 1 | 0 | 1 |
| Chek1 | checkpoint kinase 1 | 140583 | low | NO | 2 | 1 | 1 |
| Dcaf1 | DDB1 and CUL4 associated factor 1 | 315987 | low | NO | 3 | 2 | 1 |
| Prkaa1 (Ampka1) | protein kinase AMP-activated catalytic subunit alpha 1 | 65248 | low | NO | 1 | 0 | 1 |
| Prkca (Pkca, Pkcaalpha, Pkcα) | protein kinase C, alpha | 24680 | low | congestive heart failure (IEP), cardiomyopathy (ISO) | 6 | 4 | 2 |
| Prkcb (Pkcb, Pkcbeta, Pkcβ) | protein kinase C, beta | 25023 | low | congestive heart failure (IEP), cardiomyopathy (ISO) | 6 | 3 | 3 |
| Rps6ka5 (Msk1) | ribosomal protein S6 kinase A5 | 314384 | low | NO | 3 | 0 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipicz, M.; Biró, G.Z.; Szabó, M.R.; Zvara, Á.; Csont, T. Putative Epigenetic Regulator microRNAs (epi-miRNAs) and Their Predicted Targets in High-Fat Diet-Induced Cardiac Dysfunction: An In Silico Analysis in Obese Rats. Int. J. Mol. Sci. 2025, 26, 2247. https://doi.org/10.3390/ijms26052247
Pipicz M, Biró GZ, Szabó MR, Zvara Á, Csont T. Putative Epigenetic Regulator microRNAs (epi-miRNAs) and Their Predicted Targets in High-Fat Diet-Induced Cardiac Dysfunction: An In Silico Analysis in Obese Rats. International Journal of Molecular Sciences. 2025; 26(5):2247. https://doi.org/10.3390/ijms26052247
Chicago/Turabian StylePipicz, Márton, Gergő Zalán Biró, Márton Richárd Szabó, Ágnes Zvara, and Tamás Csont. 2025. "Putative Epigenetic Regulator microRNAs (epi-miRNAs) and Their Predicted Targets in High-Fat Diet-Induced Cardiac Dysfunction: An In Silico Analysis in Obese Rats" International Journal of Molecular Sciences 26, no. 5: 2247. https://doi.org/10.3390/ijms26052247
APA StylePipicz, M., Biró, G. Z., Szabó, M. R., Zvara, Á., & Csont, T. (2025). Putative Epigenetic Regulator microRNAs (epi-miRNAs) and Their Predicted Targets in High-Fat Diet-Induced Cardiac Dysfunction: An In Silico Analysis in Obese Rats. International Journal of Molecular Sciences, 26(5), 2247. https://doi.org/10.3390/ijms26052247







