Abstract
Platinum-based drugs play a pivotal role in contemporary cancer treatment, but their therapeutic utility is often limited by acquired resistance. The diiodido analog, cis-[PtI2(NH3)2] is a promising derivative that has demonstrated the ability to overcome cisplatin resistance in vitro. To establish the molecular basis for this superior activity, we integrated experimental (NMR) spectroscopy with computational density functional theory (DFT) methods to precisely and comparatively understand the drug activation mechanisms. Comparative 14N NMR experiments elucidated the initial ligand substitution step, confirming halide displacement and a markedly higher tendency for ammonia release from cis-[PtI2(NH3)2], particularly when reacting with sulfur-containing amino acids. Complementary DFT calculations determined the substitution energy values, revealing that the superior leaving-group ability of iodide results in a thermodynamically more favorable activation. Conceptual DFT parameters (softness, hardness, and Fukui indices) further demonstrated that initial substitution induces a strong trans effect, leading to the electronic sensitization of the remaining iodide ligand. This strong agreement between computational predictions and experimental data establishes a coherent molecular activation mechanism for cis-[PtI2(NH3)2], demonstrating that iodide substitution promotes both thermodynamic and electronic activation of the platinum center, which is the key to its distinct pharmacological profile and ability to circumvent resistance.
1. Introduction
Platinum-based drugs represent one of the cornerstones of contemporary anticancer chemotherapy, with cisplatin being the paradigm and still one of the most widely used agents in clinical practice [1,2,3]. Despite its remarkable efficacy, the therapeutic utility of cisplatin is limited by intrinsic and acquired resistance, as well as by severe dose-dependent side effects [4,5,6]. While effective against various cancers, cisplatin is limited by severe adverse effects [7,8] and the widespread problem of intrinsic and acquired resistance [9]. To overcome these challenges, the search for better and more effective alternatives is essential, motivating research into new Pt complexes and other metallodrugs [10,11,12]. Also, starting from classical platinum drugs, the exploitation of different Pt oxidation states (e.g., Pt(IV)), is a reliable option because of the possibility of coupling additional ligands at the axial positions owing to the octahedral geometry. In this configuration, axial ligands are typically released under specific conditions (that are finely tunable) such as the reducing cellular environment [13,14]. In this context, provided the axial ligands are biologically active molecules, it is possible to exploit their delivery in combination of a Pt-based anticancer drug that undergoes reduction to cisplatin-like Pt(II) entity [15,16,17]. Additionally, several different transition metals are well exploitable for developing new and more efficient treatments [12,18,19,20]. For this reason, the development and mechanistic investigation of cisplatin analogs remain a highly active area of research, aiming both at improving pharmacological performance and at disclosing the molecular principles underlying platinum–biomolecule interactions [21,22,23].
In this context, the diiodido analog of cisplatin, cis-[PtI2(NH3)2] (hereafter cisPtI2, Figure 1), has long been neglected, mainly due to early and largely unsubstantiated claims of poor pharmacological activity [24]. Our recent systematic studies, however, have contributed to reappraising the chemical and biological profile of cisPtI2. In particular, we have shown that this compound displays cytotoxicity comparable to cisplatin against several tumor cell lines and, importantly, is able to overcome platinum resistance in vitro [25]. Furthermore, while the DNA platination pattern of cisPtI2 closely mirrors that of cisplatin, subtle yet significant differences in its solution behavior and reactivity appear to be responsible for its distinct biological effects, notably its ability to overcome platinum resistance [25,26].
Figure 1.
Chemical structure of cisplatin (left) and its iodide analog (right).
Previous studies highlighted how cisPtI2 differs from cisplatin in its interactions with proteins and model amino acid sidechains. Comparative experiments with lysozyme and cytochrome c revealed a divergent reactivity pattern, which was further rationalized by density functional theory (DFT) calculations on sulfur- and nitrogen-containing ligands. These studies demonstrated that while both cisplatin and cisPtI2 readily undergo halide substitution, the subsequent steps, including possible ammonia release, are markedly influenced by the nature of the incoming ligand [27]. Such findings pointed to the protein environment as a crucial determinant of the peculiar reactivity of cisPtI2 and of the nature of its adducts.
Building on these foundations, the present study aims to provide a deeper mechanistic understanding of the activation processes of cisplatin and cisPtI2 by combining complementary experimental and computational approaches. In particular, we employed 14N nuclear magnetic resonance (NMR) spectroscopy to monitor the earliest substitution steps, focusing on halide displacement and the potential release of coordinated ammonia. Complementary to this experimental work, DFT was used to calculate the Gibbs free energy of substitution—which assesses the thermodynamic stability of the Pt-biomolecule conjugates—along with conceptual DFT descriptors such as softness, hardness, and Fukui indices [28]. These calculations, performed using small-molecule models, correlate theoretical reactivity parameters with the experimentally observed ligand exchange behavior.
The integration of NMR spectroscopy with computational chemistry offers a coherent and complementary perspective on the ligand substitution processes of these platinum complexes. By elucidating the molecular basis of halide and ammonia release, this work contributes to a more precise definition of the activation pathways of cisplatin and cisPtI2. Ultimately, such knowledge is essential for rationalizing the distinct pharmacological properties of these complexes and for guiding the design of new platinum-based drugs with improved efficacy and resistance profiles.
2. Results and Discussion
2.1. Cisplatin vs. cisPtI2: NMR Investigation of Ligand Exchange with Histidine, Cysteine, and Methionine
The ligand substitution reactions of cisplatin and cisPtI2 with three representative amino acids (histidine, cysteine, and methionine) were investigated using NMR spectroscopy. Due to technical constraints and the risk of salt precipitation, the study was limited to the first substitution step, which is nevertheless the most relevant for understanding the distinct reactivity of these Pt complexes with small proteins, as previously reported [29,30,31]. All experiments were conducted in a 50% DMF/water mixture to ensure the solubility of all reagents throughout the reaction. The coordination behavior of the ammonia ligand, including its potential release from the platinum center, was monitored for both complexes by 14N NMR spectroscopy. 14N is a magnetically active nucleus (spin = 1) with a quadrupolar moment. Although this feature is often considered disadvantageous, we selected 14N because its faster magnetization recovery (T1 of 14N in ammonia is ~25 times shorter than that of 15N) enables the acquisition of thousands of scans within a relatively short time, yielding spectra with an excellent signal-to-noise ratio. However, the linewidth of 14N signals increases proportionally with lattice anisotropy around the observed nucleus, restricting the applicability of this technique mainly to highly symmetrical molecules. In the case of the two Pt complexes, the 14N NMR spectra typically displays a broad resonance attributable to platinum-coordinated ammonia. Upon the release of ammonia from the metal center, a sharp signal corresponding to the NH4+ cation emerges at −23 ppm, allowing a quantitative assessment of ammonia release by NMR spectroscopy (Table 1).

Table 1.
Quantitative determination (%) of released ammonia at 24 h of incubation.
As shown in Figures S1–S4, it is evident that the two complexes upon dissolution in the reference medium are stable over time and that no ammonia release occurs. The stability of the 14N NMR signal also suggests that no halide release occurs under the applied experimental conditions. This observation contrasts with our previous findings on halide release from the two Pt compounds under physiological-like conditions [25]. However, in our case, the different behavior is attributable to the relatively high percentage of DMF, which disfavors halide displacement, and to the relatively large concentration of the two complexes.
Upon addition of 1 equivalent of each amino acid to both cisplatin or cis-[PtI2(NH3)2], the 14N NMR spectra depicted in Figure 2 are obtained.
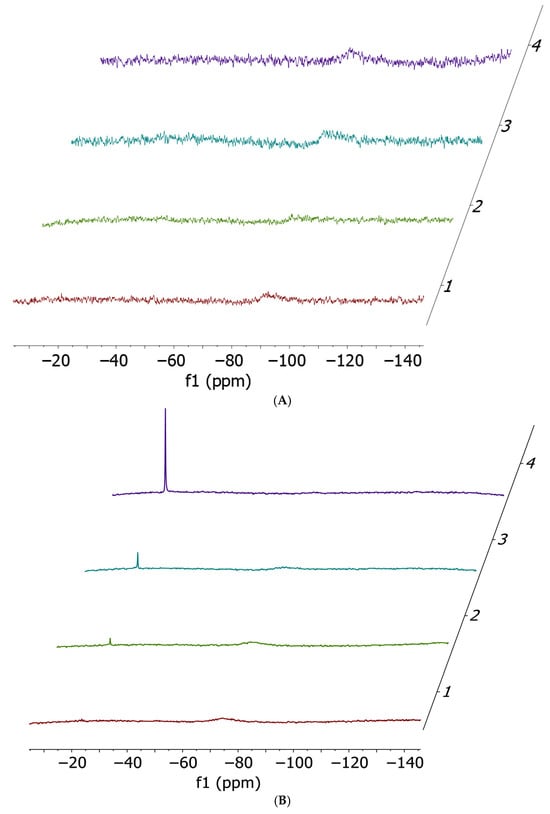
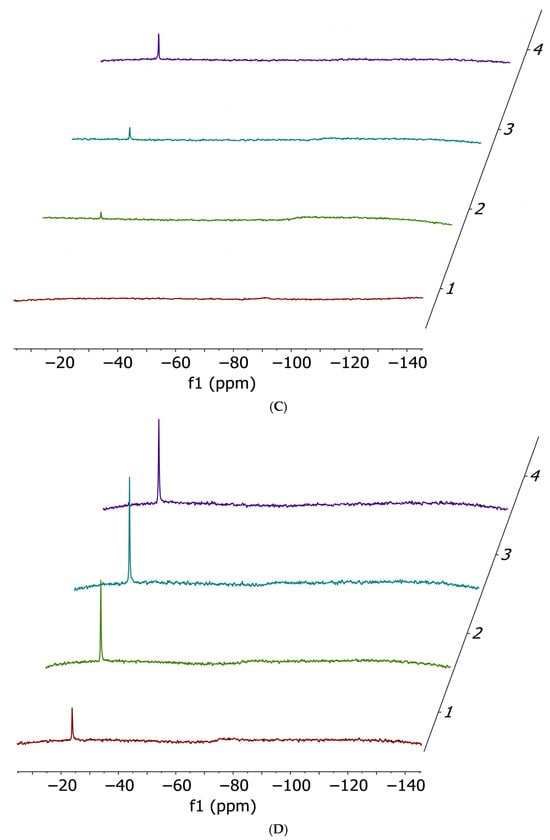
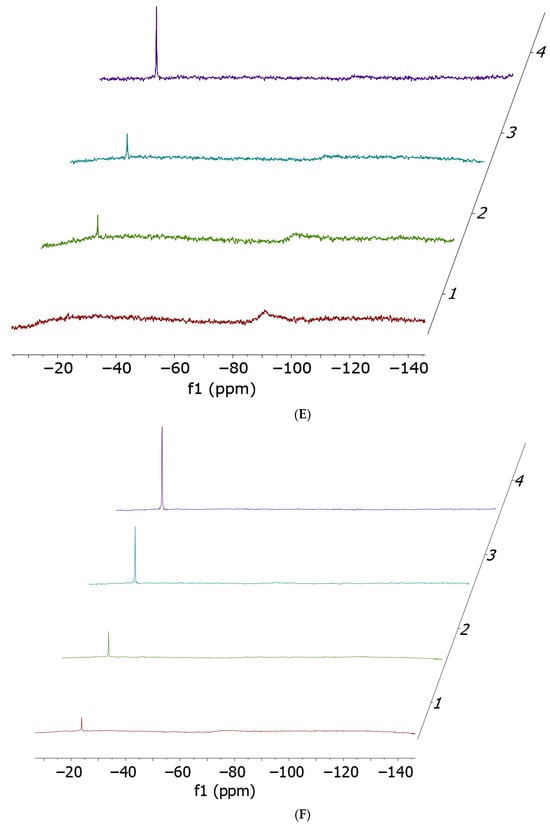
Figure 2.
Ammonia release 14N NMR spectra performed at t0 (red), 2 h (green), 5 h (cyan) and 24 h (violet). Cis-[PtCl2(NH3)2] 17 mM (A) and cisPtI2 34 mM (B) incubated with 1 equivalent of histidine; cis-[PtCl2(NH3)2] 17 mM (C) and cisPtI2 34 mM (D) incubated with 1 equivalent of methionine; cis-[PtCl2(NH3)2] 17 mM (E) and cisPtI2 34 mM (F) incubated with 1 equivalent of cysteine. All spectra have been acquired in a solvent mixture of 250 µL of DMF-d7 and 250 µL of Buffer Acetate 100 mM pH 6.9.
14N NMR experiments confirmed the occurrence of ammonia release (−23 ppm) in almost all cases, with the only exception being the incubation of cisplatin with histidine. Quantitatively, the preferential ammonia release from cisPtI2 (−75 ppm, broad) with respect to cisplatin (−91 ppm, broad) is clearly demonstrated by the significantly higher NH4+ peak heights.
2.2. Computational Assessment
The present study employs a purely thermodynamic approach, calculating the Gibbs free energy of substitution (GFE) for the reaction in which reactants and products are infinitely separated. This methodology differs significantly from the pseudounimolecular approach used in our previous work on cisplatin and cisPtI2 [27], which focused exclusively on the reaction profile including reactant-adduct, transition-state, and product-adduct species. While the prior work provided insight into kinetic barriers, the current free energy values reflect the inherent thermodynamic driving force for the overall substitution process, providing important insight into the relative stability of the resulting Pt-biomolecule conjugates at equilibrium. To accurately reflect the experimental conditions (DMF/acetate buffer), all presented data in the main text (Table 2, Table 3, Table 4 and Table 5) and the discussion below were calculated using the implicit N,N-dimethylformamide (DMF) solvent model. Complete results calculated in water are provided in the Supporting Information (Tables S1 and S2).

Table 2.
Gibbs free energies of substitution (GFE in kcal/mol) and conceptual DFT global descriptors (, , , ) for cisplatin and monosubstituted analogs. In DMF.

Table 3.
Gibbs free energies of substitution (GFE in kcal/mol) and conceptual DFT global descriptors (μ, , , ) for cisPtI2 and monosubstituted analogs.

Table 4.
Calculated Fukui Indices ( and ) for atomic centers in cisplatin and its monosubstituted analogs. In DMF.

Table 5.
Calculated Fukui indices ( and ) for atomic centers in iodoplatin and its monosubstituted analogs. In DMF.
A comprehensive understanding of the complex’s reactivity requires the integration of both kinetic (rate) and thermodynamic (stability) data, an approach critical for a complete mechanistic picture, as emphasized also in [32]. Indeed, the kinetic data from our previous study [12] strongly corroborates the thermodynamic trends presented here. Specifically, the superior leaving-group ability of I−, which leads to favorable GFE values, is quantitatively confirmed by significantly lower activation free energies for halide substitution in cisPtI2 compared to cisplatin. Furthermore, the enhanced propensity for NH3 labilization, which is the defining feature of cisPtI2’s unique pharmacological profile, is confirmed kinetically by significantly reduced kinetic barriers for ammine substitution that are 4–6 kcal/mol lower than those for cisplatin. This significant difference in kinetic barriers is a direct consequence of the strong trans-effect of iodide and provides the mechanistic basis for the compound’s rapid activation, a phenomenon consistent with the findings of Sicilia et al. [32] that link the increased soft character, leaving, and bridging abilities of iodide to the unique cytotoxic profile.
A systematic comparison of the substitution reaction GFE energies calculated in DMF (Table 2 and Table 3) immediately highlights a substantial thermodynamic advantage for iodoplatin over cisplatin. For all three neutral, biologically relevant nucleophile models—thiol (CH3SH), dimethyl sulfur (CH3SCH3), and imidazole (imi)—substitution is thermodynamically more favorable for the iodo-complexes. This thermodynamic advantage stems directly from the intrinsically superior leaving group ability of the iodide (I−) ligand compared to chloride (Cl−). This fundamental difference shifts the substitution reaction to become significantly less endothermic or more exothermic in the iodoplatin series. For instance, substitution by the neutral thiol model is highly endothermic for cisplatin (GFE = +7.5 kcal/mol in DMF), yet for iodoplatin, the reaction is only mildly endothermic (GFE = +2.1 kcal/mol), representing an approximate 5.4 kcal/mol thermodynamic driving force differential. Crucially, the overall order of substitution favorability remains consistent for both parent compounds: imidazole ≫ dimethyl sulfur > neutral thiol. The most thermodynamically favorable substitution observed among the neutral nucleophiles is the replacement of iodide by imidazole (GFE = −7.6 kcal/mol), reflecting the strong binding ability of the imidazole nitrogen to Pt(II). This robust thermodynamic preference for N-donor sites supports and quantitatively extends observations learned from the kinetic analysis in the iodoplatin study [27].
A direct comparison between the experimental 14N NMR findings (Table 1) and the GFE data (Table 3) reveals an apparent kinetic/thermodynamic discrepancy regarding cysteine reactivity with the cisPtI2 complex. Specifically, the reaction with cysteine is the most kinetically facile (100% NH3 release), yet the computational model utilizing the neutral thiol suggests the product is thermodynamically disfavored (GFE = +2.1 kcal/mol). This discrepancy is fully resolved by the inclusion of the thiolate anion (CH3S−), which models the deprotonated cysteine.
The substitution of iodide by the thiolate anion is highly exothermic (GFE = −24.4 kcal/mol in DMF). This strong thermodynamic favorability demonstrates that the true product observed at equilibrium is the highly stable Pt-thiolate conjugate. The mechanistic path thus involves a sequence where the initial binding step (substitution by either R-SH or the available R-S−) is followed by a rapid deprotonation of the coordinated thiol (Pt-SH → Pt-S−) due to the significant increase in thiol acidity upon coordination to Pt(II). The resulting Pt-thiolate bond then exerts a maximal trans-effect on the NH3 ligand, driving the rapid, subsequent release observed in the 14N NMR experiments. Therefore, the fast ammonia release is a kinetic consequence of forming the Pt-thiolate, which is the overwhelmingly favorable thermodynamic product.
The computational trends observed in DMF are robustly maintained in the aqueous solvent model, suggesting that the intrinsic reactivity differences are retained in the physiological environment (see SI and compare with Tables S1 and S2). The discrepancy between the kinetic observation (14N NMR monitoring subsequent NH3 release, driven by the strong trans-effect) and the initial substitution equilibrium (GFE of the neutral thiol adduct) is reconciled by the overwhelming stability of the final Pt-thiolate product. The experimental observation of maximal NH3 release from the cysteine adduct suggests that once the initial Pt-S bond forms, the resulting intermediate complex is significantly more prone to fast, subsequent NH3 release than the corresponding methionine or histidine adducts, a phenomenon that is kinetically favored despite the less favorable initial substitution equilibrium.
Iodoplatin exhibits a significantly lower chemical hardness ( = 0.147 in DMF) and a commensurately higher global softness ( = 3.391 in DMF) compared to cisplatin ( = 0.169 in DMF, = 2.957 in DMF). This electronic profile indicates that iodoplatin is an intrinsically softer molecule, which aligns with the principle of hard-soft acid-base (HSAB) theory and suggests a strong preference for interaction with soft, polarizable nucleophiles, such as the sulfur donors (CH3SH, CH3SCH3). This intrinsic electronic softness provides a direct, quantitative rationale for the experimentally observed enhanced reactivity: the HSAB alignment with soft sulfur donors (cysteine and methionine) predicted by this lower hardness is confirmed by the significantly greater NH3 release seen in the 14N NMR studies (Table 1). Furthermore, the significantly greater index value on the iodide atom compared to chloride directly correlates with its superior leaving-group ability, providing the electronic basis for the faster overall ligand displacement seen in the experimental analysis. The increased softness quantitatively supports the kinetic lability implied by the GFE data, confirming that iodoplatin is electronically primed for substitution. Furthermore, the chemical potential () shifts to more negative values upon substitution by sulfur ligands for both cisplatin and iodoplatin, suggesting the resulting cationic products are stronger electron acceptors. The electrophilicity index (), a measure of the complex’s capacity to acquire electrons, generally increases upon substitution, with the substituted iodoplatin complexes, [Pt(NH3)2I(L)]+, showing the highest values (up to = 0.087 for the CH3SH adduct in DMF). This heightened electrophilicity indicates that the positively charged substitution products are not inert, but rather highly reactive species with an increased propensity for subsequent binding events, such as coordination to a second nucleophile or cross-linking DNA.
The demonstrated intrinsic electronic softness of cisPtI2 () and the superior thermodynamic favorability of substitution by sulfur donors (Table 3) have critical pharmacokinetic implications. Glutathione (GSH), an abundant tripeptide containing a highly reactive soft thiol group, is the primary biological detoxification agent for platinum drugs [33]. Based on the HSAB principle, which predicts soft acids (like cisPtI2) will react faster with soft bases (like the GSH thiolate), the increased lability of cisPtI2 suggests it is intrinsically more susceptible to rapid inactivation by GSH than cisplatin. While this increased reactivity poses a challenge for maintaining therapeutic concentrations in vivo, this very lability is what drives the compound’s enhanced activation kinetics, especially in the low-GSH tumor microenvironment, where its therapeutic benefit is realized through rapid DNA binding and its ability to circumvent resistance mechanisms.
To understand the specific electronic reorganization driving the substitution, we analyzed the local reactivity using Fukui indices ( and ), which quantify the change in electron density at specific atomic sites upon gaining or losing an electron (Table 4 and Table 5, calculated in DMF; Tables S3 and S4 in water). The index describes local electrophilicity (susceptibility to nucleophilic attack at the Pt center), and describes local nucleophilicity (electron-donating ability, relevant for the halide leaving groups). The calculated Fukui indices confirm the electronic basis for the thermodynamic preference of ligand substitution in cisplatin and iodoplatin.
Analysis of the leaving group character, represented by , reveals that for the parent compounds [Pt(NH3)2X2], the negative value of is significantly greater on the iodide atoms (X = I, = −0.287) than on the chloride atoms (X = Cl, = −0.104). This 176% larger magnitude of on the iodide atom signifies a correspondingly greater capacity to donate electron density (be lost as I−), confirming its superior leaving group ability in the initial substitution step.
In terms of the central Pt atom’s reactivity, the Pt center in cisplatin exhibits a more negative value (−0.410) than that in iodoplatin (−0.300), suggesting the Pt atom in cisplatin is, unexpectedly, a slightly stronger electron acceptor in its initial state. However, the overall reaction outcome is decisively dominated by the facile release of the iodide ligand, as confirmed by the favorable GFE data.
Upon monosubstitution to form [Pt(NH3)2X(L)]+, a notable electronic and mechanistic consequence is observed: the value on the remaining halide (X) is significantly enhanced in magnitude, particularly for the remaining iodide atom (X = I) when a neutral sulfur donor is trans-positioned. The on I drops to values as low as −0.637 when the nucleophile is CH3SH. This 122% increase in the magnitude of the index on the remaining iodide signifies a powerful electronic activation, essentially demonstrating an augmented trans-effect from the newly bound neutral sulfur ligand. In contrast, while the Pt-thiolate complex is the thermodynamic product (GFE = −24.4 kcal/mol), the value on the remaining iodide is significantly lower (−0.089), suggesting that this highly stable product is less prone to subsequent I− release than the neutral S-donor adducts. However, the thiolate’s strong trans-effect is electronically confirmed by the significant positive observed on one of the coordinated NH3 ligands (up to +0.022 in [Pt(NH3)2I(CH3S−)]), which is consistent with the rapid NH3 labilization observed experimentally. This potent local electronic effect provides a robust molecular explanation for the distinct reactivity pattern of iodoplatin with proteins observed in initial experimental studies (as discussed in [27]), where the substitution leads to a product that is not just thermodynamically stable but is also electronically primed for subsequent, rapid binding events, a characteristic essential for achieving potent biological activity.
Overall, by integrating an unconventional NMR-based approach with conceptual DFT descriptors, we sought to directly probe the reactivity of the complexes and to reinforce previous evidence indicating that a significant difference indeed exists in the so-called in the interaction with amino acids and proteins, between cisplatin and its iodide analog. The claim that differences in the binding of biological substrates -and specifically proteins and amino acids- originate from previous publications. In 2015 we reported that very subtle differences exist in the metalation mode of DNA, based on the comparative analysis of ctDNA adducts formed by cisplatin and its iodide analog [25]. In contrast, remarkable differences occur in the protein metalation process, where the iodide analog preferentially releases the amine ligand instead of the halides. This represents a significant mechanistic divergence that may critically affect the pharmacological profile. Indeed, this behavior correlates with experimental evidence showing that the iodide analog can circumvent platinum resistance in colorectal cancer models. The distinct protein metalation pathway associated with the iodide analog was also structurally confirmed [34].
Taken together, these findings outline a highly promising biological behavior for cisPtI2 and strongly support its further evaluation in suitable preclinical settings. In addition, we believe that the data offer meaningful insights into the presumed mechanism of action of cisplatin and its derivatives. Considering that the DNA lesions induced by cisPtI2 are fewer in number and comparable in nature to those generated by cisplatin, the notion that its cytotoxic activity may involve pathways other than direct DNA damage gains further credibility. Within this perspective, the profoundly different pattern of protein metalation displayed by the iodide analog stands out as a plausible determinant of its capacity to bypass platinum resistance.
3. Materials and Methods
Synthesis of compounds and 14N NMR experiments. Cisplatin was purchased from Merck (Via Monte Rosa, 93 20149 Milano Italy) and used without further purification, cisPtI2 was synthesized as previously reported by some of us [25]. NMR spectra were recorded on a Bruker Avance III 400 spectrometer equipped with a Bruker Ultrashield 400 Plus (Viale V. Lancetti, 43, 20158 Milano, Italy) superconducting magnet (resonating frequencies: 400.13 and 28.89 MHz for 1H and 14N, respectively) and a 5 mm PABBO BB-1H/D Z-GRD Z108618/0049 probe (Viale V. Lancetti, 43, 20158 Milano, Italy). All experiments were run at room temperature (25 ± 2 °C) with a standard 1D sequence with inverse gated decoupling (zgig). All spectra were recorded as a mean of 2000 scans, with recycle delay of 2 s. All samples used in 14N NMR experiments were prepared in a solvent mixture of 250 µL of DMF-d7 and 250 µL of Buffer Acetate 100 mM pH 6.9. Quantitative determination of ammonia release at 24h was performed comparing absolute integrals of NH4+ signals with respect to a 100 mM NH4Br standard solution. The comparison was performed through nmrq function of Topspin 4.0.1 software.
Computational details. Our computations were based upon density functional theory (DFT), a proven methodology for accurately modeling the geometry and reaction characteristics of transition metal compounds [35,36,37], including those containing platinum [38,39]. All calculations were carried out employing the Gaussian 16 quantum chemistry package [40]. We utilized the range-corrected density functional ωB97X-D [41] throughout, selecting it for its known precision in determining both molecular structures and electronic energies [42]. Geometries of all molecules were optimized, and their initial electronic and solvation energies calculated, using the ωB97X-D functional paired with the def2SVP basis set [43,44] in DMF and in water. To account for the surrounding solvent environment, the polarizable continuum model (PCM) in its integral equation formalism variant (IEFPCM) [45] was applied. This specific model was chosen because it has recently demonstrated significantly reduced errors for the aqueous solvation free energies of both neutral and ionic species compared to alternative continuum models [46]. For the solvation energy of the chloride ion in water, the experimental value of −74.5 kcal/mol was employed [47]. This decision was made to account for the unique effects related to the protic nature of water that the implicit PCM approach may not fully assess, ensuring the most accurate representation of the chloride ion in an aqueous environment for the solvent model comparison. To ensure the consistency and validity of the comparison with the robust experimental data, it is noted that while our DFT calculations employed an implicit solvation model, the primary mechanistic findings—namely the superior I− leaving group and the strong trans effect—are governed by intrinsic electronic factors and the large thermodynamic difference (~2–5 kcal/mol), making the overall reaction ordering and the validated experimental trends highly reliable and robust against minor variations in the mixed solvent environment [48]. Following geometry optimization, frequency calculations were performed to confirm the stability of the optimized structures and to obtain the necessary Zero-Point Energy (ZPE) and thermal corrections for calculating thermodynamic properties. The final, high-level single-point electronic and solvation energies were then computed on the optimized geometries using the ωB97X-D functional in conjunction with the larger def2TZVP basis set [43,44].
To comprehensively characterize the electronic reactivity of the compounds, we employed conceptual DFT (cDFT) [49,50]. The global reactivity parameters—specifically the chemical potential (), hardness (), softness (), and electrophilicity ()—were derived from the energies of the highest occupied molecular orbital (EHOMO) and the lowest unoccupied molecular orbital (ELUMO), as determined at the ωB97X-D/def2SVP level of theory in water. These parameters are reported in Hartree (Ha), with the exception of softness (), which is in Ha–1. The parameters were calculated using the following established formulas: the chemical potential μ was approximated as ; the hardness η was approximated as ; the softness S was defined as ; and the electrophilicity ω was calculated as .
The local reactivity at each atomic center was assessed by calculating the dimensionless Fukui indices () [51]. These indices were determined from the Mulliken charges () for a given atom , which were calculated at the same ωB97X-D/def2SVP level of theory in water. The formulas used for the Fukui indices for nucleophilic () and electrophilic () attack were: and , respectively, where , , and denote the number of electrons in the neutral, anionic, and cationic species.
4. Conclusions
The combined experimental and computational investigation presented here provides new mechanistic insights into the activation pathways of cisplatin and its iodide analog, cisPtI2. 14N NMR results revealed a markedly higher tendency of the iodide derivative to undergo ammonia release, particularly in the presence of sulfur-containing amino acids. Complementary DFT calculations demonstrated that the enhanced reactivity of cisPtI2 arises from its lower chemical hardness and the superior leaving-group ability of iodide, resulting in significantly more favorable substitution thermodynamics. Conceptual DFT descriptors and Fukui function analysis confirmed this, highlighting the intrinsic electronic softness of the iodinated species and the strong trans effect induced by the newly bound nucleophile on the remaining iodide ligand. Together, these findings outline a consistent picture in which iodide substitution promotes both thermodynamic and electronic activation of the platinum center, ultimately explaining the distinct reactivity and potent biological activity, including its ability to overcome cisplatin resistance, compared to cisplatin. The present results contribute to a deeper understanding of halide-dependent activation mechanisms and may guide the rational design of next-generation platinum-based anticancer agents with improved performance and resistance profiles.
Supplementary Materials
The supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262412138/s1.
Author Contributions
Conceptualization, T.M. and D.C.; methodology, D.C., T.M., A.P. and D.L.M.; investigation, R.D.L., E.B., M.P., D.C., L.C., L.F., D.P. and A.M.; data curation, T.M., A.M., I.T. and D.C.; writing—original draft preparation, T.M., D.C. and I.T.; writing—review and editing, T.M., I.T., A.Z., D.L.M., L.C., L.F., A.M., A.Z., T.M., A.N. and P.F. All authors have read and agreed to the published version of the manuscript.
Funding
T.M. thanks the financial support from Ministero Italiano dell’Università della Ricerca (MUR) under the program PRIN 2022-Progetti di Rilevante Interesse Nazionale, project code: 2022ALJRPL “Biocompatible nanostructures for the chemotherapy treatment of prostate cancer” and from University of Pisa, SPARK-Proof of Concept 2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding authors.
Acknowledgments
We acknowledge the CINECA award under the ISCRA initiative, for the availability of high performance computing resources and support. I.T. gratefully acknowledges the usage of HPC resources from Direction du Numérique—Centre de Calcul de l’Université de Bourgogne (DNUM CCUB).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M.P. Cisplatin in Cancer Treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef] [PubMed]
- Mariconda, A.; Ceramella, J.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Cisplatin, the Timeless Molecule. Inorganics 2025, 13, 246. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653, Erratum in Dalton Trans. 2018, 47, 7848.. [Google Scholar] [CrossRef]
- Elmorsy, E.A.; Saber, S.; Hamad, R.S.; Abdel-Reheim, M.A.; El-kott, A.F.; AlShehri, M.A.; Morsy, K.; Salama, S.A.; Youssef, M.E. Advances in Understanding Cisplatin-Induced Toxicity: Molecular Mechanisms and Protective Strategies. Eur. J. Pharm. Sci. 2024, 203, 106939. [Google Scholar] [CrossRef]
- Fu, R.; Zhao, B.; Chen, M.; Fu, X.; Zhang, Q.; Cui, Y.; Hu, X.; Zhou, W. Moving beyond Cisplatin Resistance: Mechanisms, Challenges, and Prospects for Overcoming Recurrence in Clinical Cancer Therapy. Med. Oncol. 2023, 41, 9. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; George, G.N.; Pickering, I.J.; Narendran, A.; Gailer, J. Tuning the Metabolism of the Anticancer Drug Cisplatin with Chemoprotective Agents to Improve Its Safety and Efficacy. Metallomics 2016, 8, 1170–1176. [Google Scholar] [CrossRef]
- Mitchell, E.; Pham, M.H.; Clay, A.; Sanghvi, R.; Williams, N.; Pietsch, S.; Hsu, J.I.; Øbro, N.F.; Jung, H.; Vedi, A.; et al. The Long-Term Effects of Chemotherapy on Normal Blood Cells. Nat. Genet. 2025, 57, 1684–1694, Erratum in Nat. Genet. 2025, 57, 2075.. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Casini, A.; Pöthig, A. Metals in Cancer Research: Beyond Platinum Metallodrugs. ACS Cent. Sci. 2024, 10, 242–250. [Google Scholar] [CrossRef]
- Wani, M.Y.; Malik, M.A. Non-Platinum Anticancer Agents. In Gold and Its Complexes in Anticancer Chemotherapy; Springer: Singapore, 2021; pp. 51–68. ISBN 978-981-336-313-7. [Google Scholar]
- Wang, Y.; Cao, B.; Wang, Q.; Zhong, S.; Fang, X.; Wang, J.; Chan, A.S.C.; Xiong, X.; Zou, T. Ligand Supplementation Restores the Cancer Therapy Efficacy of the Antirheumatic Drug Auranofin from Serum Inactivation. Nat. Commun. 2025, 16, 7347. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, Y.; Zhang, P.; Ma, R.; Zhang, W.; Liu, J.; Li, T.; Niu, H.; Cao, Y.; Hu, B.; et al. The Role of Platinum(IV)-Based Antitumor Drugs and the Anticancer Immune Response in Medicinal Inorganic Chemistry. A Systematic Review from 2017 to 2022. Eur. J. Med. Chem. 2022, 243, 114680. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. Pt(IV) Anticancer Prodrugs—A Tale of Mice and Men. ChemMedChem 2021, 16, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. Platinum(IV) Anticancer Agents; Are We En Route to the Holy Grail or to a Dead End? J. Inorg. Biochem. 2021, 217, 111353. [Google Scholar] [CrossRef]
- Gibson, D. Platinum(IV) Anticancer Prodrugs—Hypotheses and Facts. Dalton Trans. 2016, 45, 12983–12991. [Google Scholar] [CrossRef]
- Chen, S.; Yao, H.; Zhou, Q.; Tse, M.-K.; Gunawan, Y.F.; Zhu, G. Stability, Reduction, and Cytotoxicity of Platinum(IV) Anticancer Prodrugs Bearing Carbamate Axial Ligands: Comparison with Their Carboxylate Analogues. Inorg. Chem. 2020, 59, 11676–11687. [Google Scholar] [CrossRef]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An Approach against Invasion and Metastasis in Cancer Treatment. FEBS Open Bio 2022, 12, 880–899. [Google Scholar] [CrossRef]
- Kim, W.K.; An, J.M.; Lim, Y.J.; Kim, K.; Kim, Y.H.; Kim, D. Recent Advances in Metallodrug: Coordination-Induced Synergy between Clinically Approved Drugs and Metal Ions. Mater. Today Adv. 2025, 25, 100569. [Google Scholar] [CrossRef]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs According to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- Cirri, D.; Chiaverini, L.; Pratesi, A.; Marzo, T. Is the Next Cisplatin Already in Our Laboratory? Comments Inorg. Chem. 2023, 43, 465–478. [Google Scholar] [CrossRef]
- Akitsu, T.; Tsvetkova, D.; Yamamoto, Y.; Nakane, D.; Kostova, I. From Basics of Coordination Chemistry to Understanding Cisplatin-Analogue Pt Drugs. Curr. Pharm. Des. 2023, 29, 1747–1774. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Huang, Y.; Wang, Y.; Lu, D.; Sun, Q. Research Progress of Platinum-Based Complexes in Lung Cancer Treatment: Mechanisms, Applications, and Challenges. Int. J. Mol. Sci. 2025, 26, 7958. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, D.; Platella, C.; Riccardi, C.; Merlino, A.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. A First-in-Class and a Fished out Anticancer Platinum Compound: Cis-[PtCl2(NH3)2] and Cis-[PtI2(NH3)2] Compared for Their Reactivity towards DNA Model Systems. Dalton Trans. 2016, 45, 8587–8600. [Google Scholar] [CrossRef] [PubMed]
- Marzo, T.; Pillozzi, S.; Hrabina, O.; Kasparkova, J.; Brabec, V.; Arcangeli, A.; Bartoli, G.; Severi, M.; Lunghi, A.; Totti, F.; et al. Cis-Pt I2(NH3)2: A Reappraisal. Dalton Trans. 2015, 44, 14896–14905. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Cama, M.; Pajuelo-Lozano, N.; Álvarez-Valdés, A.; Perez, I.S. New Findings in the Signaling Pathways of Cis and Trans Platinum Iodido Complexes’ Interaction with DNA of Cancer Cells. ACS Omega 2019, 4, 21855–21861. [Google Scholar] [CrossRef]
- Tolbatov, I.; Marzo, T.; Cirri, D.; Gabbiani, C.; Coletti, C.; Marrone, A.; Paciotti, R.; Messori, L.; Re, N. Reactions of Cisplatin and Cis-[PtI2(NH3)2] with Molecular Models of Relevant Protein Sidechains: A Comparative Analysis. J. Inorg. Biochem. 2020, 209, 111096. [Google Scholar] [CrossRef]
- Pucci, R.; Angilella, G.G.N. Density Functional Theory, Chemical Reactivity, and the Fukui Functions. Found. Chem. 2022, 24, 59–71. [Google Scholar] [CrossRef]
- Wang, J.; Tao, J.; Jia, S.; Wang, M.; Jiang, H.; Du, Z. The Protein-Binding Behavior of Platinum Anticancer Drugs in Blood Revealed by Mass Spectrometry. Pharmaceuticals 2021, 14, 104. [Google Scholar] [CrossRef]
- Zimmermann, T.; Chval, Z.; Burda, J.V. Cisplatin Interaction with Cysteine and Methionine in Aqueous Solution: Computational DFT/PCM Study. J. Phys. Chem. B 2009, 113, 3139–3150. [Google Scholar] [CrossRef]
- Corinti, D.; Paciotti, R.; Coletti, C.; Re, N.; Chiavarino, B.; Crestoni, M.E.; Fornarini, S. Elusive Intermediates in Cisplatin Reaction with Target Amino Acids: Platinum(II)-Cysteine Complexes Assayed by IR Ion Spectroscopy and DFT Calculations. J. Inorg. Biochem. 2022, 237, 112017. [Google Scholar] [CrossRef]
- Scoditti, S.; Vigna, V.; Dabbish, E.; Sicilia, E. Iodido Equatorial Ligands Influence on the Mechanism of Action of Pt(IV) and Pt(II) Anti-Cancer Complexes: A DFT Computational Study. J. Comput. Chem. 2021, 42, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.A.J.; Brouwer, J.; Reedijk, J. Glutathione Induces Cellular Resistance against Cationic Dinuclear Platinum Anticancer Drugs. J. Inorg. Biochem. 2002, 89, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Messori, L.; Marzo, T.; Gabbiani, C.; Valdes, A.A.; Quiroga, A.G.; Merlino, A. Peculiar Features in the Crystal Structure of the Adduct Formed between Cis-PtI2 (NH3)2 and Hen Egg White Lysozyme. Inorg. Chem. 2013, 52, 13827–13829. [Google Scholar] [CrossRef] [PubMed]
- Tolbatov, I.; Marzo, T.; Umari, P.; Mendola, D.L.; Marrone, A. Detailed Mechanism of a DNA/RNA Nucleobase Substituting Bridging Ligand in Diruthenium(II,III) and Dirhodium(II,II) Tetraacetato Paddlewheel Complexes: Protonation of the Leaving Acetate Is Crucial. Dalton Trans. 2025, 54, 662–673. [Google Scholar] [CrossRef]
- Tolbatov, I.; Umari, P.; Marrone, A. The Binding of Diruthenium (II,III) and Dirhodium (II,II) Paddlewheel Complexes at DNA/RNA Nucleobases: Computational Evidences of an Appreciable Selectivity toward the AU Base Pairs. J. Mol. Graph. Model. 2024, 131, 108806. [Google Scholar] [CrossRef]
- Tolbatov, I.; Marrone, A. Reactivity of N-Heterocyclic Carbene Half-Sandwich Ru-, Os-, Rh-, and Ir-Based Complexes with Cysteine and Selenocysteine: A Computational Study. Inorg. Chem. 2022, 61, 746–754. [Google Scholar] [CrossRef]
- Scoditti, S.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. Anticancer Activity, DNA Binding, and Photodynamic Properties of a N∧C∧N-Coordinated Pt(II) Complex. Inorg. Chem. 2021, 60, 10350–10360. [Google Scholar] [CrossRef]
- Tolbatov, I.; Cirri, D.; Tarchi, M.; Marzo, T.; Coletti, C.; Marrone, A.; Messori, L.; Re, N.; Massai, L. Reactions of Arsenoplatin-1 with Protein Targets: A Combined Experimental and Theoretical Study. Inorg. Chem. 2022, 61, 3240–3248. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets Tor Molecular Calculations; Springer: Berlin/Heidelberg, Germany, 2012; Volume 3. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Remya, K.; Suresh, C.H. Which Density Functional Is Close to CCSD Accuracy to Describe Geometry and Interaction Energy of Small Noncovalent Dimers? A Benchmark Study Using Gaussian09. J. Comput. Chem. 2013, 34, 1341–1353. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A New Definition of Cavities for the Computation of Solvation Free Energies by the Polarizable Continuum Model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Klamt, A.; Moya, C.; Palomar, J. A Comprehensive Comparison of the IEFPCM and SS(V)PE Continuum Solvation Methods with the COSMO Approach. J. Chem. Theory Comput. 2015, 11, 4220–4225. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; Cramer, C.J.; Truhlar, D.G. Single-Ion Solvation Free Energies and the Normal Hydrogen Electrode Potential in Methanol, Acetonitrile, and Dimethyl Sulfoxide. J. Phys. Chem. B 2007, 111, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Isaev, Y.I.; Makarov, D.M.; Khodov, I.A. Machine Learning Prediction of NMR Shifts for Rare and Transition Metal Complexes (45Sc, 49Ti, 89Y, 91Zr, 139La). J. Mol. Liq. 2025, 437, 128417. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chattaraj, P.K. Conceptual Density Functional Theory Based Electronic Structure Principles. Chem. Sci. 2021, 12, 6264–6279. [Google Scholar] [CrossRef]
- Yang, W.; Mortier, W.J. The Use of Global and Local Molecular Parameters for the Analysis of the Gas-Phase Basicity of Amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).