The Significance of the Microenvironment in T/Nk-Cell Neoplasms
Abstract
1. Introduction
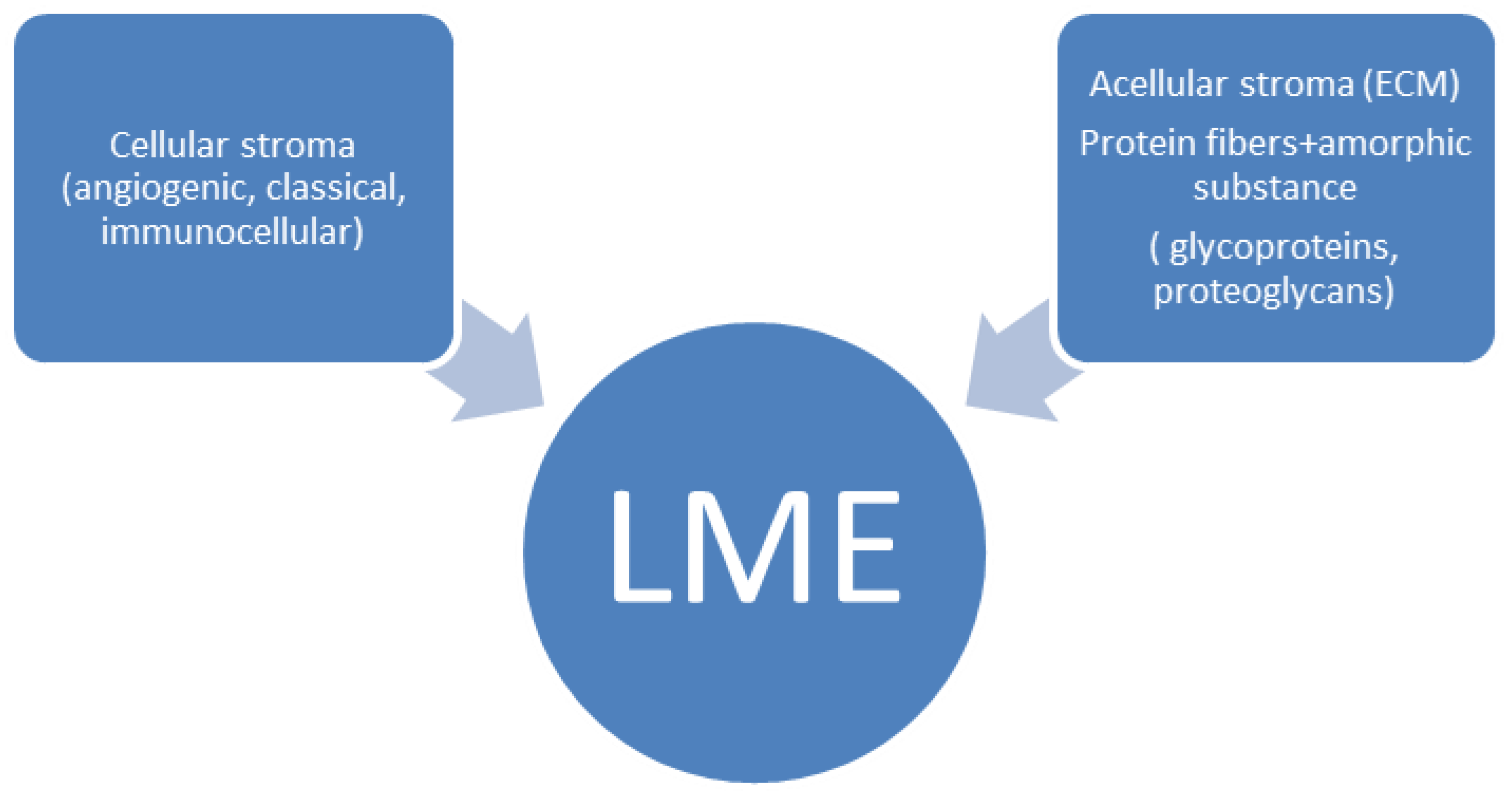
2. The Concept of the Microenvironment in T/NK-Cell Neoplasms
3. Cellular Stroma Composition in T/NK-Cell Neoplasms
3.1. Angiogenic Stroma (Angiogenesis-Neovascularisation)
3.2. Stromal Cells
3.3. Immuno-Cellular Stroma (Reactive T, NK and B Lymphocytes)
4. Acellular Stroma-Extracellular Matrix Composition in T/NK-Cell Neoplasms
4.1. Role of Extracellular Matrix Protein Fibers in T/NK-Cell Lymphomagenesis
4.2. Role of Extracellular Matrix Glycoproteins in T/NK-Cell Lymphomagenesis
4.3. Role of Proteoglycans in T/NK-Cell Lymphomagenesis
4.4. Role of MMPs and TIMPs in T/NK-Cell Lymphomagenesis
4.5. Role of Cytokines, Cytokine Receptors, Growth Factors in T/NK-Cell Lymphomagenesis
4.6. Role of Exosome or Lymphoma Cell Extracellular Vesicles in T/NK-Cell Lymphomagenesis
5. Role of the Biological Agencies (EBV and HTLV-1) in T/NK-Cell Lymphomagenesis
5.1. EBV as the T/NK-Cell Lymphoma Promoting Agent
5.2. HTLV-1 as the T/NK-Cell Lymphoma Promoting Agent
6. Inflammation as a Significant Factor in T/NK-Cell Neoplasms
6.1. Leukemic Entities
6.2. Nodal Lymphomas
6.3. Extranodal Lymphomas
6.4. Provisional/Childhood EBV-Driven Entities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petković, I.; Popović, A.; Džunić, M.; Pejčić, I. Nodal and extranodal peripheral T/NK-cell neoplasms: Current aspects. Acta Fac. Med. Naissensis 2020, 37, 99–120. [Google Scholar] [CrossRef]
- Montes-Mojarro, I.A.; Fend, F.; Quintanilla-Martinez, L. EBV and the Pathogenesis of NK/T Cell Lymphoma. Cancers 2021, 13, 1414. [Google Scholar] [CrossRef]
- Lin, G.W.; Xu, C.; Chen, K.; Huang, H.Q.; Chen, J.; Song, B.; Chan, J.K.C.; Li, W.; Liu, W.; Shih, L.Y.; et al. Genetic risk of extranodal natural killer T-cell lymphoma: A genome-wide association study in multiple populations. Lancet Oncol. 2020, 21, 306–316. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748, Erratum in Leukemia 2023, 37, 1944–1951. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253, Erratum in Blood 2023, 141, 437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Attygalle, A.D.; Karube, K.; Jeon, Y.K.; Cheuk, W.; Bhagat, G.; Chan, J.K.C.; Naresh, K.N. The fifth edition of the WHO classification of mature T cell, NK cell and stroma-derived neoplasms. J. Clin. Pathol. 2025, 78, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Ahmed, R.; Feldman, A.L. Updates in the Classification of T-cell Lymphomas and Lymphoproliferative Disorders. Curr. Hematol. Malig. Rep. 2023, 18, 252–263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asano, N.; Suzuki, R.; Kagami, Y.; Ishida, F.; Kitamura, K.; Fukutani, H.; Morishima, Y.; Takeuchi, K.; Nakamura, S. Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T-cell lymphoma, unspecified. Am. J. Surg. Pathol. 2005, 29, 1284–1293. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Jaffe, E.S.; Brousset, P.; Chan, J.K.; de Leval, L.; Gaulard, P. International Lymphoma Study Group. Cytotoxic T-cell and NK-cell lymphomas: Current questions and controversies. Am. J. Surg. Pathol. 2014, 38, e60–e71. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.M.; Warnke, R.A.; Hu, Q.; Gaulard, P.; Copie-Bergman, C.; Alkan, S.; Wang, H.-Y.; Cheng, J.X.; Bacon, C.M.; Delabie, J.; et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood 2013, 122, 3599–3606. [Google Scholar] [CrossRef] [PubMed]
- Petrella, T.; Maubec, E.; Cornillet-Lefebvre, P.; Willemze, R.; Pluot, M.; Durlach, A. Indolent CD8-positive lymphoid proliferation of the ear: A distinct primary cutaneous T-cell lymphoma. Am. J. Surg. Pathol. 2007, 31, 1887–1892. [Google Scholar] [CrossRef]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8 T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef]
- Lanier, L.L.; Testi, R.; Bindl, J.; Phillips, J.H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J. Exp. Med. 1989, 169, 2233–2238. [Google Scholar] [CrossRef]
- Chan, J.K.; Tsang, W.Y.; Ng, C.S. Clarification of CD3 immunoreactivity in nasal T/natural killer cell lymphomas: The neoplastic cells are often CD3 epsilon+. Blood 1996, 87, 839–841. [Google Scholar] [CrossRef]
- de Leval, L.; Rickman, D.S.; Thielen, C.; de Reynies, A.; Huang, Y.-L.; Delsol, G.; Lamant, L.; Leroy, K.; Brière, J.; Molina, T.; et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood 2007, 109, 4952–4963. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Weisenburger, D.D.; Greiner, T.C.; Vose, J.M.; McKeithan, T.; Kucuk, C.; Geng, H.; Deffenbacher, K.; Smith, L.; Dybkaer, K.; et al. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood 2010, 115, 1026–1036. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B. Extracellular matrix stiffness: Mechanisms in tumor progression and therapeutic potential in cancer. Exp. Hematol. Oncol. 2025, 14, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Jin, H.J.; Choi, M.R.; Lim, D.W.; Park, J.E.; Kim, Y.S.; Lim, S.B. Matrisomics: Beyond the extracellular matrix for unveiling tumor microenvironment. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189178. [Google Scholar] [CrossRef]
- Bangham, C.R.M. HTLV-1 persistence and the oncogenesis of adult T-cell leukemia/lymphoma. Blood 2023, 141, 2299–2306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribatti, D.; Tamma, R.; Annese, T.; Ingravallo, G.; Specchia, G. Macrophages and angiogenesis in human lymphomas. Clin. Exp. Med. 2024, 24, 26. [Google Scholar] [CrossRef]
- Wang, T.; Feldman, A.L.; Wada, D.A.; Lu, Y.; Polk, A.; Briski, R.; Ristow, K.; Habermann, T.M.; Thomas, D.; Ziesmer, S.C.; et al. GATA-3 expression identifies a high-risk subset of PTCL-NOS with distinct molecular and clinical features. Blood 2014, 123, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tse, E.; Kwong, Y.L. T-cell lymphoma: Microenvironment-related biomarkers. Semin. Cancer Biol. 2015, 34, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jaffe, E.S. How I Diagnose Angioimmunoblastic T-Cell Lymphoma. Am. J. Clin. Pathol 2021, 156, 1–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasca, S.; Jurj, A.; Matei, D. Angioimmunoblastic T-cell Lymphoma Microenvironment. Arch. Cancer Biol. Ther. 2020, 1, 11–13. [Google Scholar]
- Zhao, W.L.; Mourah, S.; Mounier, N.; Leboeuf, C.; Daneshpouy, M.E.; Legrès, L.; Meignin, V.; Oksenhendler, E.; Maignin, C.L.; Calvo, F.; et al. Vascular endothelial growth factor-A is expressed both on lymphoma cells and endothelial cells in angioimmunoblastic T-cell lymphoma and related to lymphoma progression. Lab. Investig. 2004, 84, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.M.; Sørensen, F.B.; Bendix, K.; Nielsen, J.L.; Funder, A.; Karkkainen, M.J.; Tainola, T.; Sørensen, A.B.; Pedersen, F.S.; D’Amore, F. Expression level, tissue distribution pattern, and prognostic impact of vascular endothelial growth factors VEGF and VEGF-C and their receptors Flt-1, KDR, and Flt-4 in different subtypes of non-Hodgkin lymphomas. Leuk. Lymphoma 2009, 50, 1647–1660. [Google Scholar] [CrossRef]
- Huang, W.; Cao, Z.; Zeng, L.; Guo, L.; Liu, X.; Lv, N.; Feng, X. nm23, TOP2A and VEGF expression: Potential prognostic biologic factors in peripheral T-cell lymphoma, not otherwise specified. Oncol. Lett. 2019, 18, 3803–3810. [Google Scholar] [CrossRef]
- Huang, Y.; de Reyniès, A.; de Leval, L.; Ghazi, B.; Martin-Garcia, N.; Travert, M.; Bosq, J.; Brière, J.; Petit, B.; Thomas, E.; et al. Gene expression profiling identifies emerging oncogenic pathways operating in extranodal NK/T-cell lymphoma, nasal type. Blood 2010, 115, 1226–1237. [Google Scholar] [CrossRef]
- Ni, M.; Wang, Y.; Yang, J.; Ma, Q.; Pan, W.; Li, Y.; Xu, Q.; Lv, H.; Wang, Y. IL-33 aggravates extranodal NK/T cell lymphoma aggressiveness and angiogenesis by activating the Wnt/β-catenin signaling pathway. Mol. Cell Biochem. 2025, 480, 265–278. [Google Scholar] [CrossRef]
- Tamma, R.; Ingravallo, G.; Annese, T.; Gaudio, F.; Perrone, T.; Musto, P.; Specchia, G.; Ribatti, D. Tumor microenvironment and microvascular density in follicular lymphoma. J. Clin. Med. 2022, 11, 1257. [Google Scholar] [CrossRef]
- Mazur, G.; Woźniak, Z.; Wróbel, T.; Maj, J.; Kuliczkowski, K. Increased angiogenesis in cutaneous T-cell lymphomas. Pathol. Oncol. Res. 2004, 10, 34–36. [Google Scholar] [CrossRef]
- Ganjoo, K.; Hong, F.; Horning, S.J.; Gascoyne, R.D.; Natkunam, Y.; Swinnen, L.J.; Habermann, T.M.; Kahl, B.S.; Advani, R.H. Bevacizumab and cyclosphosphamide, doxorubicin, vincristine and prednisone in combination for patients with peripheral T-cell or natural killer cell neoplasms: An Eastern Cooperative Oncology Group study (E2404). Leuk. Lymphoma 2014, 55, 768–772. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T.; d’Amati, A.; Ingravallo, G.; Specchia, G. Vascular growth in lymphomas: Angiogenesis and alternative ways. Cancers 2023, 15, 3262. [Google Scholar] [CrossRef] [PubMed]
- Gloger, M.; Menzel, L.; Grau, M.; Vion, A.C.; Anagnostopoulos, I.; Zapukhlyak, M.; Gerlach, K.; Kammertöns, T.; Hehlgans, T.; Zschummel, M.; et al. Lymphoma Angiogenesis Is Orchestrated by Noncanonical Signaling Pathways. Cancer Res. 2020, 80, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Schijns, V.; Powell, D.J., Jr.; Pasukoniene, V.; Dobrovolskiene, N.; Michalek, J. Dendritic cells and their role in tumor immunosurveillance. Innate Immun. 2013, 19, 98–111. [Google Scholar] [CrossRef]
- Li, Y.Q.; Luo, C.L.; Jiang, J.X.; He, S.; Liu, Y.; Yan, W.X.; Xia, Y.; Cui, Q.; Huang, Y.; Lim, J.Q.; et al. Single-Cell Analysis Reveals Malignant Cells Reshape the Cellular Landscape and Foster an Immunosuppressive Microenvironment of Extranodal NK/T-Cell Lymphoma. Adv. Sci. 2023, 10, e2303913. [Google Scholar] [CrossRef]
- Wallet, M.A.; Sen, P.; Tisch, R. Immunoregulation of dendritic cells. Clin. Med. Res. 2005, 3, 166–175. [Google Scholar] [CrossRef]
- Raaijmakers, T.K.; Ansems, M. Microenvironmental derived factors modulating dendritic cell function and vaccine efficacy: The effect of prostanoid receptor and nuclear receptor ligands. Cancer Immunol. Immunother. 2018, 67, 1789–1796. [Google Scholar] [CrossRef]
- Tan, J.K.; O’Neill, H.C. Maturation requirements for dendritic cells in T cell stimulation leading to tolerance versus immunity. J. Leukoc. Biol. 2005, 78, 319–324. [Google Scholar] [CrossRef]
- Svajger, U.; Rozman, P. Tolerogenic dendritic cells: Molecular and cellular mechanisms in transplantation. J. Leukoc. Biol. 2014, 95, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, R.; Hu, J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci. 2011, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Curti, A.; Trabanelli, S.; Salvestrini, V.; Baccarani, M.; Lemoli, R.M. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: Focus on hematology. Blood 2009, 113, 2394–2401. [Google Scholar] [CrossRef]
- Idoyaga, J.; Moreno, J.; Bonifaz, L. Tumor cells prevent mouse dendritic cell maturation induced by TLR ligands. Cancer Immunol. Immunother. 2007, 56, 1237–1250. [Google Scholar] [CrossRef]
- Scheuerpflug, A.; Ahmetlić, F.; Bauer, V.; Riedel, T.; Röcken, M.; Mocikat, R. The role of dendritic cells for therapy of B-cell lymphoma with immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.Y.; Belabed, M.; Park, M.D.; Mattiuz, R.; Puleston, D.; Merad, M. Dendritic cell maturation in cancer. Nat. Rev. Cancer 2025, 25, 225–248. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Anandasabapathy, N. The role of dendritic cells in cancer and anti-tumor immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef]
- Kumar, D.; Xu, M.L. Microenvironment Cell Contribution to Lymphoma Immunity. Front. Oncol. 2018, 8, 288, Erratum in Front. Oncol. 2018, 8, 522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.E.; Dutta, B.; Tse, S.W.; Gupta, N.; Tan, C.F.; Low, J.K.; Yeoh, K.W.; Kon, O.L.; Tam, J.P.; Sze, S.K. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene 2019, 38, 5158–5173. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Xie, X.; Wang, Z.; Zhang, Y.; Wang, L. Tumor-associated macrophages in lymphoma: From mechanisms to therapy. Int. Immunopharmacol. 2022, 112, 109235. [Google Scholar] [CrossRef] [PubMed]
- Barbera-Guillem, E.; Nyhus, J.K.; Wolford, C.C.; Friece, C.R.; Sampsel, J.W. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002, 62, 7042–7049. [Google Scholar]
- Mishra, A.K.; Banday, S.; Bharadwaj, R.; Ali, A.; Rashid, R.; Kulshreshtha, A.; Malonia, S.K. Macrophages as a Potential Immunotherapeutic Target in Solid Cancers. Vaccines 2022, 11, 55. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, G. Macrophages in leukemia microenvironment. Blood Sci. 2019, 1, 29–33. [Google Scholar] [CrossRef]
- Bi, X.W.; Wang, H.; Zhang, W.W.; Wang, J.H.; Liu, W.J.; Xia, Z.J.; Huang, H.Q.; Jiang, W.Q.; Zhang, Y.J.; Wang, L. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J. Hematol. Oncol. 2016, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Moyano, A.; Ferressini, N.; De Matteo, E.; Preciado, M.V.; Chabay, P. PD-L1 is upregulated in CD163+ tonsillar macrophages from children undergoing EBV primary infection. Front. Immunol. 2022, 13, 940910. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Liu, J.; Zhu, F.; Wang, Z.; Wang, J. The prognostic value of tumour-associated macrophages in Non-Hodgkin’s lymphoma: A systematic review and meta-analysis. Scand. J. Immunol. 2020, 91, e12814. [Google Scholar] [CrossRef]
- Lin, Z.X.; Bai, B.; Cai, Q.C.; Cai, Q.Q.; Wang, X.X.; Wu, X.Y.; Huang, H.Q. High numbers of tumor-associated macrophages correlate with poor prognosis in patients with mature T- and natural killer cell lymphomas. Med. Oncol. 2012, 29, 3522–3528. [Google Scholar] [CrossRef]
- Bai, B.; Huang, H.Q.; Cai, Q.C.; Wang, X.X.; Cai, Q.; Gao, Y.; Zhao, W. The Activation Of M2 Polarized Tumor-Associated Macrophages in the Microenvironment of NK/T-Cell Lymphoma and the Characteristics of Cytokine Spectrum. Blood 2013, 122, 4274. [Google Scholar] [CrossRef]
- Gao, X.; Kady, N.; Wang, C.; Abdelrahman, S.; Gann, P.; Sverdlov, M.; Wolfe, A.; Brown, N.; Reneau, J.; Robida, A.M.; et al. Targeting Lymphoma-associated Macrophage Expansion via CSF1R/JAK Inhibition is a Therapeutic Vulnerability in Peripheral T-cell Lymphomas. Cancer Res. Commun. 2022, 2, 1727–1737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Hazrati, A.; Malekpour, K.; Khorramdelazad, H.; Rajaei, S.; Hashemi, S.M. Therapeutic and immunomodulatory potentials of mesenchymal stromal/stem cells and immune checkpoints related molecules. Biomark. Res. 2024, 12, 35. [Google Scholar] [CrossRef]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune cells-derived exosomes function as a double-edged sword: Role in disease progression and their therapeutic applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef]
- Peled, A.; Klein, S.; Beider, K.; Burger, J.A.; Abraham, M. Role of CXCL12 and CXCR4 in the pathogenesis of hematological malignancies. Cytokine 2018, 109, 11–16. [Google Scholar] [CrossRef]
- Ramuta, T.Z.; Kreft, M.E. Mesenchymal Stem/Stromal Cells May Decrease Success of Cancer Treatment by Inducing Resistance to Chemotherapy in Cancer Cells. Cancers 2022, 14, 3761. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sun, Z. Chemokines and their receptors in the esophageal carcinoma tumor microenvironment: Key factors for metastasis and progression. Front. Oncol. 2025, 15, 1523751. [Google Scholar] [CrossRef]
- Shangguan, L.; Li, X.; Wang, Z.; Luo, Z. Transforming growth factor-β1 induces bone marrow-derived mesenchymal stem cells to differentiate into cancer-associated fibroblasts. Zhonghua Zhong Liu Za Zhi 2015, 37, 804–809. [Google Scholar]
- Ahn, S.Y. The Role of MSCs in the Tumor Microenvironment and Tumor Progression. Anticancer. Res. 2020, 40, 3039–3047. [Google Scholar] [CrossRef]
- Monu, N.R.; Frey, A.B. Myeloid-derived suppressor cells and anti-tumor T cells: A complex relationship. Immunol. Investig. 2012, 41, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.L.; Ye, S.B.; Ouyang, L.Y.; Chen, Y.S.; He, J.; Huang, H.Q.; Zeng, Y.X.; Zhang, X.S.; Li, J. Myeloid-derived suppressor cells inhibit T cell proliferation in human extranodal NK/T cell lymphoma: A novel prognostic indicator. Cancer Immunol. Immunother. 2015, 64, 1587–1599. [Google Scholar] [CrossRef]
- Papafragkos, I.; Markaki, E.; Kalpadakis, C.; Verginis, P. Decoding the Myeloid-Derived Suppressor Cells in Lymphoid Malignancies. J. Clin. Med. 2021, 10, 3462. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, S.; Naselli, G.; Harrison, L.C.; Boyd, A.W.; Cebon, J.; Jack, I. Cytokine production in response to Epstein-Barr virus infection of peripheral blood mononuclear cells in vitro. Immunol. Cell Biol. 1993, 71 Pt 4, 259–264. [Google Scholar] [CrossRef]
- Rodríguez, P.C.; Ochoa, A.C. Arginine Regulation by Myeloid Derived Suppressor Cells and Tolerance in Cancer: Mechanisms and Therapeutic Perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Zea, A.H.; Culotta, K.S.; Zabaleta, J.; Ochoa, J.B.; Ochoa, A.C. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J. Biol. Chem. 2002, 277, 21123–21129. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. L-Arginine Availability Regulates T-Lymphocyte Cell-Cycle Progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Bronte, V.; Visintin, A.; Spitzer, J.H.; Apolloni, E.; Serafini, P.; Zanovello, P.; Segal, D.M. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 2002, 168, 689–695. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Corzo, C.A.; Cotter, M.J.; Cheng, P.; Cheng, F.; Kusmartsev, S.; Sotomayor, E.; Padhya, T.; McCaffrey, T.V.; McCaffrey, J.C.; Gabrilovich, D.I. Mechanism Regulating Reactive Oxygen Species in Tumor-Induced Myeloid-Derived Suppressor Cells. J. Immunol. 2009, 182, 5693–5701. [Google Scholar] [CrossRef]
- Huang, B.; Pan, P.Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.H. Gr-1+CD115+ Immature Myeloid Suppressor Cells Mediate the Development of Tumor-Induced T Regulatory Cells and T-Cell Anergy in Tumor-Bearing Host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef]
- Cioccarelli, C.; Molon, B. MDSCs and T cells in solid tumors and non-Hodgkin lymphomas: An immunosuppressive speech. Clin. Exp. Immunol. 2022, 208, 147–157. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ansell, S.M.; Mondello, P. Unravelling the role of cancer-associated fibroblasts in B cell lymphoma. Front. Immunol. 2024, 15, 1451791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Küçük, C.; Jiang, B.; Hu, X.; Zhang, W.; Chan, J.K.; Xiao, W.; Lack, N.; Alkan, C.; Williams, J.C.; Avery, K.N.; et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat. Commun. 2015, 6, 6025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, T.L.; Nairismägi, M.L.; Laurensia, Y.; Lim, J.Q.; Tan, J.; Li, Z.M.; Pang, W.L.; Kizhakeyil, A.; Wijaya, G.C.; Huang, D.C.; et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018, 132, 1146–1158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jo, J.C.; Kim, M.; Choi, Y.; Kim, H.J.; Kim, J.E.; Chae, S.W.; Kim, H.; Cha, H.J. Expression of programmed cell death 1 and programmed cell death ligand 1 in extranodal NK/T-cell lymphoma, nasal type. Ann. Hematol. 2017, 96, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, J.; Kim, S.J.; Park, W.Y.; Kim, J.; Woo, J.; Kim, G.; Yoon, S.E.; Ko, Y.H.; Kim, W.S. Immune subtyping of extranodal NK/T-cell lymphoma: A new biomarker and an immune shift during disease progression. Mod. Pathol. 2020, 33, 603–615. [Google Scholar] [CrossRef]
- Du, Y.; Cai, Y.; Lv, Y.; Zhang, L.; Yang, H.; Liu, Q.; Hong, M.; Teng, Y.; Tang, W.; Ma, R.; et al. Single-cell RNA sequencing unveils the communications between malignant T and myeloid cells contributing to tumor growth and immunosuppression in cutaneous T-cell lymphoma. Cancer Lett. 2022, 551, 215972. [Google Scholar] [CrossRef] [PubMed]
- Krejsgaard, T.; Lindahl, L.M.; Mongan, N.P.; Wasik, M.A.; Litvinov, I.V.; Iversen, L.; Langhoff, E.; Woetmann, A.; Odum, N. Malignant inflammation in cutaneous T-cell lymphoma-a hostile takeover. Semin. Immunopathol. 2017, 39, 269–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maharaj, K.; Uriepero, A.; Sahakian, E.; Pinilla-Ibarz, J. Regulatory T cells (Tregs) in lymphoid malignancies and the impact of novel therapies. Front. Immunol. 2022, 13, 943354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Ke, X.Y. The four types of Tregs in malignant lymphomas. J. Hematol. Oncol. 2011, 4, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaulard, P.; de Leval, L. Follicular helper T cells: Implications in neoplastic hematopathology. Semin. Diagn. Pathol. 2011, 28, 202–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Mel, S.; Li, J.B.; Abid, M.B.; Tang, T.P.L.; Tay, H.M.; Ting, W.C.; Poon, L.M.; Chung, T.H.; Mow, B.; Tso, A.; et al. The Utility of Flow Cytometry in Differentiating NK/T Cell Lymphoma from Indolent and Reactive NK Cell Proliferations. Cytom. Part B 2018, 94B, 159–168. [Google Scholar] [CrossRef]
- Lin, C.W.; Lee, W.H.; Chang, C.L.; Yang, J.Y.; Hsu, S.M. Restricted killer cell immunoglobulin-like receptor repertoire without T-cell receptor gamma rearrangement supports a true natural killer-cell lineage in a subset of sinonasal lymphomas. Am. J. Pathol. 2001, 159, 1671–1679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheffschick, A.; Nenonen, J.; Xiang, M.; Winther, A.H.; Ehrström, M.; Wahren-Herlenius, M.; Eidsmo, L.; Brauner, H. Skin infiltrating NK cells in cutaneous T-cell lymphoma are increased in number and display phenotypic alterations partially driven by the tumor. Front. Immunol. 2023, 14, 1168684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barreira da Silva, R.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858. [Google Scholar] [CrossRef] [PubMed]
- de Mel, S.; Hue, S.S.; Jeyasekharan, A.D.; Chng, W.J.; Ng, S.B. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J. Hematol. Oncol. 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tse, E.; Zhao, W.L.; Xiong, J.; Kwong, Y.L. How we treat NK/T-cell lymphomas. J. Hematol. Oncol. 2022, 15, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwong, Y.L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.M.; Mow, B.; Khong, P.L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, M.; Yan, J.; Li, L.; Fu, X.; Zhang, X.; Chang, Y.; Sun, Z.; Yu, H.; et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J. Hematol. Oncol. 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Liu, M.; Li, W.; Song, Y. Immune cells in the B-cell lymphoma microenvironment: From basic research to clinical applications. Chin. Med. J. 2024, 137, 776–790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, R.; Nie, M.; Long, W. The role of B cells in cancer development. Front. Oncol. 2022, 12, 958756. [Google Scholar] [CrossRef]
- Petković, I.; Stojnev, S.; Popović, A.; Krstić, M.; Pejčić, I. EBV Negative Angioimmunoblastic T-Cell Lymphoma with Sequential Development of Diffuse Large B-Cell Lymphoma in Course of Progression. Indian J. Hematol. Blood Transfus. 2021, 37, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, D.S.; Jeon, M.J.; Yu, E.S.; Choi, C.W.; Ko, Y.H. Peripheral T cell lymphoma of the nasopharynx with expansion of EBV-positive B cells masquerading as an extranodal NK/T cell lymphoma, nasal type. Virchows. Arch. 2022, 481, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Theurich, S.; Schlaak, M.; Steguweit, H.; Heukamp, L.C.; Wennhold, K.; Kurschat, P.; Rabenhorst, A.; Hartmann, K.; Schlösser, H.; Shimabukuro-Vornhagen, A.; et al. Targeting Tumor-Infiltrating B Cells in Cutaneous T-Cell Lymphoma. J. Clin. Oncol. 2016, 34, e110–e116. [Google Scholar] [CrossRef]
- Chen, M.M.; Zeng, G.P.; Li, J.; Fu, J.H.; Long, Y.Y.; Pan, J.Y.; Wei, L.Z.; Guan, J.Y.; Lin, Y.X.; You, H.; et al. High infiltration of CD20+ B lymphocytes in extranodal natural killer/T-cell lymphoma is associated with better prognosis. Br. J. Haematol. 2020, 191, e116–e120. [Google Scholar] [CrossRef]
- Shojaie, S.; Leibel, S.; Post, M. 5—The Extracellular Matrix in Development, Fetal and Neonatal Physiology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 49–54.e2. [Google Scholar]
- James, M.; Crawford, A.; Burt, D. Anatomy, Pathophysiology and Basic Mechanisms of Disease. MacSween’s Pathology of the Liver, 6th ed.; Churchill Livingstone: London, UK, 2012; pp. 1–77. ISBN 9780702033988. [Google Scholar]
- Wang, K.; Meng, X.; Guo, Z. Elastin Structure, Synthesis, Regulatory Mechanism and Relationship With Cardiovascular Diseases. Front. Cell Dev. Biol. 2021, 30, 596702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spada, S.; Tocci, A.; Di Modugno, F.; Nisticò, P. Fibronectin as a multiregulatory molecule crucial in tumor matrisome: From structural and functional features to clinical practice in oncology. J. Exp. Clin. Cancer Res. 2021, 40, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagarrigue, F.; Dupuis-Coronas, S.; Ramel, D.; Delsol, G.; Tronchère, H.; Payrastre, B.; Gaits-Iacovoni, F. Matrix metalloproteinase-9 is upregulated in nucleophosmin-anaplastic lymphoma kinase-positive anaplastic lymphomas and activated at the cell surface by the chaperone heat shock protein 90 to promote cell invasion. Cancer Res. 2010, 70, 6978–6987. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Libert, C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leucoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef]
- Rømer, A.M.A.; Thorseth, M.L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rygiel, T.P.; Stolte, E.H.; de Ruiter, T.; van de Weijer, M.L.; Meyaard, L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR. Mol. Immunol. 2011, 49, 402–406. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prakash, J.; Shaked, Y. The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics. Cancer Discov. 2024, 14, 1375–1388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.; Li, H.; Jian, Q.-J.; Zhang, W.; Zhao, S.; Wang, K.; Liu, W. Comparative Analysis of Clinicopathological Features and Genetic Landscape of Relapsed/Refractory and Effectively Treated Extranodal Nasal NK/T Cell Lymphoma. Blood 2021, 138 (Suppl. 1), 1384. [Google Scholar] [CrossRef]
- Petkovic, I.; Stojnev, S.; Popovic, A.; Krstic, M.; Pejcic, I. Extranodal NK/T-cell lymphoma, nasal type overlapping aggressive NK-cell leukemia in course of progression. UHOD-Uluslar. Hematol. 2020, 30, 248–251. [Google Scholar] [CrossRef]
- Wang, J.P.; Hielscher, A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J. Cancer 2017, 8, 674–682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schliemann, C.; Palumbo, A.; Zuberbühler, K.; Villa, A.; Kaspar, M.; Trachsel, E.; Klapper, W.; Menssen, H.D.; Neri, D. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL. Blood 2009, 113, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Hiserodt, J.C.; Laybourn, K.A.; Varani, J. Laminin inhibits the recognition of tumor target cells by murine natural killer (NK) and natural cytotoxic (NC) lymphocytes. Am. J. Pathol. 1985, 121, 148. [Google Scholar] [PubMed] [PubMed Central]
- Siegel, S.; Wagner, A.; Kabelitz, D.; Marget, M.; Coggin, J., Jr.; Barsoum, A.; Rohrer, J.; Schmitz, N.; Zeis, M. Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood 2003, 102, 4416–4423. [Google Scholar] [CrossRef] [PubMed]
- Pesapane, A.; Ragno, P.; Selleri, C.; Montuori, N. Recent Advances in the Function of the 67 kDa Laminin Receptor and its Targeting for Personalized Therapy in Cancer. Curr. Pharm. Des. 2017, 23, 4745–4757. [Google Scholar] [CrossRef] [PubMed]
- Brellier, F.; Chiquet-Ehrismann, R. How do tenascins influence the birth and life of a malignant cell? J. Cell Mol. Med. 2012, 16, 32–40. [Google Scholar] [CrossRef]
- Gritti, G.; Gianatti, A.; Petronzelli, F.; Boschini, C.; Rossi, R.; Trezzi, R.; Rossi, A.; Barbui, A.M.; Rambaldi, A. Tenascin-C Is Highly Expressed in T-Cell Non-Hodgkin Lymphomas and Represents an Attractive Target for Radioimmunotherapy. Blood 2016, 128, 4141. [Google Scholar] [CrossRef]
- Jahkola, T.; Toivonen, T.; Nordling, S.; von Smitten, K.; Virtanen, I. Expression of tenascin-C in intraductal carcinoma of human breast: Relationship to invasion. Eur. J. Cancer 1998, 34, 1687–1692. [Google Scholar] [CrossRef]
- Ghert, M.A.; Qi, W.N.; Erickson, H.P.; Block, J.A.; Scully, S.P. Tenascin-C splice variant adhesive/anti-adhesive effects on chondrosarcoma cell attachment to fibronectin. Cell Struct. Funct. 2001, 26, 179–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zagzag, D.; Capo, V. Angiogenesis in the central nervous system: A role for vascular endothelial growth factor/vascular permeability factor and tenascin-C. Common molecular effectors in cerebral neoplastic and non-neoplastic “angiogenic diseases”. Histol. Histopathol. 2002, 17, 301–321. [Google Scholar] [PubMed]
- Puente Navazo, M.D.; Valmori, D.; Ruegg, C. The alternatively spliced domain TnFnIII A1A2 of the extracellular matrix protein tenascin-C suppresses activation-induced T lymphocyte proliferation and cytokine production. J. Immunol. 2001, 167, 6431–6440. [Google Scholar] [CrossRef]
- Cardesa-Salzmann, T.M.; Colomo, L.; Gutierrez, G.; Chan, W.C.; Weisenburger, D.; Climent, F.; Gonzalez-Barca, E.; Mercadal, S.; Arenillas, L.; Serrano, S.; et al. High microvessel density determines a poor outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus chemotherapy. Haematologica 2011, 96, 996–1001. [Google Scholar] [CrossRef]
- Gritti, G.; Gianatti, A.; Petronzelli, F.; De Santis, R.; Pavoni, C.; Rossi, R.L.; Cattaneo, L.; Spagnoli, L.G.; Ferrari, S.; Rossi, A.; et al. Evaluation of tenascin-C by tenatumomab in T-cell non-Hodgkin lymphomas identifies a new target for radioimmunotherapy. Oncotarget 2018, 9, 9766–9775, Erratum in Oncotarget 2018, 9, 17256. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, B.; Husebekk, A.; Svineng, G.; Rekdal, Ø.; Yanagishita, M.; Kolset, S.O.; Uhlin-Hansen, L. The proteoglycan repertoire of lymphoid cells. Glycoconj. J. 2012, 29, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S.; Shiue, L.H.; Duvic, M.; Pandya, A.; Cruz, P.D.; Ariizumi, K., Jr. Sezary syndrome cells overexpress syndecan-4 bearing distinct heparan sulfate moieties that suppress T-cell activation by binding DC-HIL and trapping TGF-beta on the cell surface. Blood 2011, 117, 3382–3390. [Google Scholar] [CrossRef]
- Fujii, K.; Karpova, M.B.; Asagoe, K.; Georgiev, O.; Dummer, R.; Urosevic-Maiwald, M. Versican upregulation in Sézary cells alters growth, motility and resistance to chemotherapy. Leukemia 2015, 29, 2024–2032. [Google Scholar] [CrossRef]
- Borghini, N.; Lazzaretti, M.; Lunghi, P.; Malpeli, G.; Barbi, S.; Perris, R. A translational perspective of the malignant hematopoietic proteoglycome. Cell Biosci. 2025, 15, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deb, G.; Cicala, A.; Papadas, A.; Asimakopoulos, F. Matrix proteoglycans in tumor inflammation and immunity. Am. J. Physiol. Cell Physiol. 2022, 323, C678–C693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakata, K.; Someya, M.; Omatsu, M.; Asanuma, H.; Hasegawa, T.; Ichimiya, S.; Hareyama, M.; Himi, T. The enhanced expression of the matrix metalloproteinase 9 in nasal NK/T-cell lymphoma. BMC Cancer 2007, 7, 229, Erratum in BMC Cancer 2018, 8, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harabuchi, Y.; Takahara, M.; Kishibe, K.; Nagato, T.; Kumai, T. Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type: Basic Science and Clinical Progress. Front. Pediatr. 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, M.M.; Chan, J.K.; Lau, W.H.; Ngan, R.K.; Foo, W.W. Early stage nasal NK/T-cell lymphoma: Clinical outcome, prognostic factors, and the effect of treatment modality. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, L.; Sun, J.; Xiao, Q.; Feng, Z.; Li, J. Expression and Significance of MMP-26, TIMP-4 and MMP-9 in Extranodal Natural Killer (NK)/T-cell Lymphoma, Nasal type (ENKTCL) Lymphoma Cells. Cancer Res. Prev. Treat. Cancer 2014, 41, 269–273. [Google Scholar]
- Meneses-García, A.; Betancourt, A.M.; Abarca, J.H.; Montes, A.B.; Roa, L.S.; Ruíz-Godoy, L. Expression of the metalloproteases MMP-1, MMP-2, MMP-3, MMP-9, MMP-11, TIMP-1 and TIMP-2 in angiocentric midfacial lymphomas. World J. Surg. Oncol. 2008, 6, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Xu, B.; Wang, T. Intimate cross-talk between cancer cells and the tumor microenvironment of B-cell lymphomas: The key role of exosomes. Tumour Biol. 2017, 39, 1010428317706227. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.; Salehi, F.; Rostami, S.; Hadiloo, K.; Hashemi, M.; Baridjavadi, Z.; Ahangari, F.; Karami, N.; Samani, F.; Tahmasebi, S.; et al. Harnessing the power of exosomes for diagnosis, prognosis, and treatment of hematological malignancies. Stem Cell Res. Ther. 2025, 16, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Huang, J.; Chen, W.; Li, G.; Li, Z.; Lei, J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp. Mol. Med. 2022, 54, 1390–1400. [Google Scholar] [CrossRef]
- Navarro-Tableros, V.; Gomez, Y.; Camussi, G.; Brizzi, M.F. Extracellular Vesicles: New Players in Lymphomas. Int. J. Mol. Sci. 2018, 20, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyiadzis, M.; Whiteside, T.L. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia 2017, 31, 1259–1268. [Google Scholar] [CrossRef]
- Chen, W.; Xie, Y.; Li, F.; Wen, P.; Wang, L. EBV + B cell-derived exosomes promote EBV-associated T/NK-cell lymphoproliferative disease immune evasion by STAT3/IL-10/PD-L1 pathway. Immunol. Res. 2024, 72, 1327–1336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rutherford, S.C.; Fachel, A.A.; Li, S.; Sawh, S.; Muley, A.; Ishii, J.; Saxena, A.; Dominguez, P.M.; Caldas Lopes, E.; Agirre, X.; et al. Extracellular vesicles in DLBCL provide abundant clues to aberrant transcriptional programming and genomic alterations. Blood 2018, 132, e13–e23. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhong, M.; Zeng, S.; Wang, L.; Liu, P.; Xiao, X.; Liu, Y. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics 2018, 11, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, T.; Zhou, X. The role of exosomal shuttle RNA (esRNA) in lymphoma. Crit. Rev. Oncol. Hematol. 2019, 137, 27–34. [Google Scholar] [CrossRef]
- Kataoka, K.; Miyoshi, H.; Sakata, S.; Dobashi, A.; Couronne, L.; Kogure, Y.; Sato, Y.; Nishida, K.; Gion, Y.; Shiraishi, Y.; et al. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia 2019, 33, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wei, P.; Guo, Y.; Shi, D.; Yu, B.H.; Su, Y.F.; Li, X.Q.; Zhou, X.Y. Clinical significance of circulating exosomal PD-L1 and soluble PD-L1 in extranodal NK/T-cell lymphoma, nasal-type. Am. J. Cancer Res. 2020, 10, 4498. [Google Scholar] [PubMed] [PubMed Central]
- Smith, N.A.; Coleman, C.B.; Gewurz, B.E.; Rochford, R. CD21 (Complement Receptor 2) Is the Receptor for Epstein-Barr Virus Entry into T Cells. J. Virol. 2020, 94, e00428-20. [Google Scholar] [CrossRef]
- Kimura, H. EBV in T-/NK-cell tumorigenesis. Adv. Exp. Med. Biol. 2018, 1045, 459–475. [Google Scholar]
- Kimura, H.; de Leval, L.; Cai, Q.; Kim, W.S. EBV-associated NK and T-cell lymphoid neoplasms. Curr. Opin. Oncol. 2022, 34, 422–431. [Google Scholar] [CrossRef]
- Xiong, J.; Dai, Y.T.; Wang, W.F.; Zhang, H.; Wang, C.F.; Yin, T.; Cheng, S.; Zhong, H.J.; Yu, S.H.; Jiang, L.; et al. GPCR signaling contributes to immune characteristics of microenvironment and process of EBV-induced lymphomagenesis. Sci. Bull. 2023, 68, 2607–2619. [Google Scholar] [CrossRef]
- Proietti, F.A.; Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Murphy, E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005, 24, 6058–6068. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.U.; Proietti, F.A.; Ribas, J.G.; Araújo, M.G.; Pinheiro, S.R.; Guedes, A.C.; Carneiro-Proietti, A.B. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin. Microbiol. Rev. 2010, 23, 577–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohanty, S.; Harhaj, E.W. Mechanisms of Oncogenesis by HTLV-1 Tax. Pathogens 2020, 9, 543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Currer, R.; Van Duyne, R.; Jaworski, E.; Guendel, I.; Sampey, G.; Das, R.; Narayanan, A.; Kashanchi, F. HTLV tax: A fascinating multifunctional co-regulator of viral and cellular pathways. Front. Microbiol. 2012, 3, 406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeuchi, M.; Miyoshi, H.; Ohshima, K. Tumor microenvironment of adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematop. 2021, 61, 202–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakahata, S.; Enriquez-Vera, D.; Jahan, M.I.; Sugata, K.; Satou, Y. Understanding the Immunopathology of HTLV-1-Associated Adult T-Cell Leukemia/Lymphoma: A Comprehensive Review. Biomolecules 2023, 13, 1543. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Asano, N.; Miyoshi, H.; Kato, T.; Shimono, J.; Yoshida, N.; Kurita, D.; Sasaki, Y.; Kawamoto, K.; Ohshima, K.; Seto, M. Expression pattern of immunosurveillance-related antigen in adult T cell leukaemia/lymphoma. Histopathology 2018, 72, 945–954. [Google Scholar] [CrossRef]
- Holling, T.M.; Schooten, E.; Langerak, A.W.; van den Elsen, P.J. Regulation of MHC class II expression in human T-cell malignancies. Blood 2004, 103, 1438–1444. [Google Scholar] [CrossRef]
- Shirono, K.; Hattori, T.; Hata, H.; Nishimura, H.; Takatsuki, K. Profiles of expression of activated cell antigens on peripheral blood and lymph node cells from different clinical stages of adult T-cell leukemia. Blood 1989, 73, 1664–1671. [Google Scholar] [CrossRef]
- Takeuchi, M.; Miyoshi, H.; Asano, N.; Yoshida, N.; Yamada, K.; Yanagida, E. Human leukocyte antigen class II expression is a good prognostic factor in adult T-cell leukemia/lymphoma. Haematologica 2019, 104, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Niino, D.; Saito, Y.; Ohnishi, K.; Horlad, H.; Ohshima, K.; Takeya, M. Clinical significance of CD163⁺ tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci. 2013, 104, 945–951. [Google Scholar] [CrossRef]
- Veillette, A.; Chen, J. SIRPα-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, E.; Miyoshi, H.; Takeuchi, M.; Yoshida, N.; Nakashima, K.; Yamada, K.; Umeno, T.; Shimasaki, Y.; Furuta, T.; Seto, M. Clinicopathological analysis of immunohistochemical expression of CD47 and SIRPα in adult T-cell leukemia/lymphoma. Hematol. Oncol. 2020, 38, 680–688. [Google Scholar] [CrossRef]
- Miyoshi, H.; Kiyasu, J.; Kato, T.; Yoshida, N.; Shimono, J.; Yokoyama, S.; Taniguchi, H.; Sasaki, Y.; Kurita, D.; Kawamoto, K.; et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 2016, 128, 1374–1381. [Google Scholar] [CrossRef]
- Azakami, K.; Sato, T.; Araya, N.; Utsunomiya, A.; Kubota, R.; Suzuki, K.; Hasegawa, D.; Izumi, T.; Fujita, H.; Aratani, S.; et al. Severe loss of invariant NKT cells exhibiting anti-HTLV-1 activity in patients with HTLV-1-associated disorders. Blood 2009, 114, 3208–3215. [Google Scholar] [CrossRef]
- Koya, J.; Saito, Y.; Kameda, T.; Kogure, Y.; Yuasa, M.; Nagasaki, J.; McClure, M.B.; Shingaki, S.; Tabata, M.; Tahira, Y.; et al. Single-Cell Analysis of the Multicellular Ecosystem in Viral Carcinogenesis by HTLV. Blood Cancer Discov. 2021, 2, 450–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Fu, B.-B.; Gale, R.P.; Liang, Y. NK-/T-cell lymphomas. Leukemia 2021, 35, 2460–2468. [Google Scholar] [CrossRef]
- Koo, G.C.; Tan, S.Y.; Tang, T.; Poon, S.L.; Allen, G.E.; Tan, L.; Chong, S.C.; Ong, W.S.; Tay, K.; Tao, M.; et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012, 2, 591–597. [Google Scholar] [CrossRef]
- Fochi, S.; Mutascio, S.; Bertazzoni, U.; Zipeto, D.; Romanelli, M.G. HTLV Deregulation of the NF-κB Pathway: An Update on Tax and Antisense Proteins Role. Front. Microbiol. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; McClain, K.; Allen, C.E.; Parikh, S.A.; Otrock, Z.; Rojas-Hernandez, C.; Blechacz, B.; Wang, S.; Minkov, M.; Jordan, M.B.; et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 2017, 123, 3229–3240. [Google Scholar] [CrossRef]
- Knauft, J.; Schenk, T.; Ernst, T.; Schnetzke, U.; Hochhaus, A.; La Rosée, P.; Birndt, S. Lymphoma-associated hemophagocytic lymphohistiocytosis (LA-HLH): A scoping review unveils clinical and diagnostic patterns of a lymphoma subgroup with poor prognosis. Leukemia 2024, 38, 235–249. [Google Scholar] [CrossRef]
- Semenzato, G.; Teramo, A.; Barilà, G.; Calabretto, G.; Rampazzo, E.; Buson, E.; Zambello, R. NK-type large granular lymphocyte leukemia comes of age. HemaSphere 2025, 9, e70161. [Google Scholar] [CrossRef] [PubMed]
- Calabretto, G.; Teramo, A.; Barilà, G.; Vicenzetto, C.; Gasparini, V.R.; Semenzato, G.; Zambello, R. Neutropenia and Large Granular Lymphocyte Leukemia: From Pathogenesis to Therapeutic Options. Cells 2021, 10, 2800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orland, M.; Ogbue, O.; Dima, D.; Omar, N.; Mustafa Ali, M.K. Large granular lymphocytic leukemia: Clinical features, molecular pathogenesis, diagnosis and treatment. Cancers 2024, 16, 1307. [Google Scholar] [CrossRef]
- Lamy, T.; Moignet, A.; Loughran, T.P., Jr. LGL leukemia: From pathogenesis to treatment. Blood 2017, 129, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Teramo, A.; Barilà, G.; Calabretto, G.; Vicenzetto, C.; Gasparini, V.R.; Semenzato, G.; Zambello, R. Insights Into Genetic Landscape of Large Granular Lymphocyte Leukemia. Front. Oncol. 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, R.; Shah, M.V.; Loughran, T.P., Jr. The root of many evils: Indolent large granular lymphocyte leukaemia and associated disorders. Hematol. Oncol. 2010, 28, 105–117. [Google Scholar] [CrossRef]
- Kiel, M.J.; Velusamy, T.; Rolland, D.; Sahasrabuddhe, A.A.; Chung, F.; Bailey, N.G.; Schrader, A.; Li, B.; Li, J.Z.; Ozel, A.B.; et al. Integrated genomic sequencing reveals mutational landscape of T-cell prolymphocytic leukemia. Blood 2014, 124, 1460–1472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wahnschaffe, L.; Braun, T.; Timonen, S.; Giri, A.K.; Schrader, A.; Wagle, P.; Almusa, H.; Johansson, P.; Bellanger, D.; López, C.; et al. JAK/STAT-Activating Genomic Alterations Are a Hallmark of T-PLL. Cancers 2019, 11, 1833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Hussein, S.; Medeiros, L.J.; Khoury, J.D. Aggressive NK Cell Leukemia: Current State of the Art. Cancers 2020, 12, 2900. [Google Scholar] [CrossRef]
- Zhao, A.; Yang, J.; Li, M.; Li, L.; Gan, X.; Wang, J.; Li, H.; Shen, K.; Yang, Y.; Niu, T. Epstein-Barr Virus-Positive Lymphoma-Associated Hemophagocytic Syndrome: A Retrospective, Single-Center Study of 51 Patients. Front. Immunol. 2022, 13, 882589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, T. Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1–infected T cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef]
- Sawada, L.; Nagano, Y.; Hasegawa, A.; Kanai, H.; Nogami, K.; Ito, S.; Sato, T.; Yamano, Y.; Tanaka, Y.; Masuda, T.; et al. IL-10-mediated signals act as a switch for lymphoproliferation in Human T-cell leukemia virus type-1 infection by activating the STAT3 and IRF4 pathways. PLoS Pathog. 2017, 13, e1006597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marques-Piubelli, M.L.; Amador, C.; Vega, F. Pathologic and molecular insights in nodal T-follicular helper cell lymphomas. Front. Oncol. 2023, 13, 1105651. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Sakata-Yanagimoto, M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia 2020, 34, 2592–2606. [Google Scholar] [CrossRef]
- Lage, L.A.d.P.C.; Culler, H.F.; Reichert, C.O.; da Siqueira, S.A.C.; Pereira, J. Angioimmunoblastic T-cell lymphoma and correlated neoplasms with TFH phenotype: From molecular mechanisms to therapeutic advances. Front. Oncol. 2023, 13, 1177590. [Google Scholar] [CrossRef]
- Amador, C.; Chan, W.C. Nodal peripheral T-cell lymphomas in the new classification systems. Cancer Biol. Med. 2024, 20, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Wright, G.; Wang, C.; Rosenwald, A.; Gascoyne, R.D.; Weisenburger, D.D.; Greiner, T.C.; Smith, L.; Guo, S.; Wilcox, R.A.; et al. Lymphoma Leukemia Molecular Profiling Project and the International Peripheral T-cell Lymphoma Project. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood 2014, 123, 2915–2923. [Google Scholar] [CrossRef]
- Miyawaki, K.; Sugio, T. Lymphoma Microenvironment in DLBCL and PTCL-NOS: The key to uncovering heterogeneity and the potential for stratification. J. Clin. Exp. Hematop. 2022, 62, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sugio, T.; Miyawaki, K.; Kato, K.; Sasaki, K.; Yamada, K.; Iqbal, J.; Miyamoto, T.; Ohshima, K.; Maeda, T.; Miyoshi, H.; et al. Microenvironmental immune cell signatures dictate clinical outcomes for PTCL-NOS. Blood Adv. 2018, 2, 2242–2252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiarle, R.; Simmons, W.J.; Cai, H.; Dhall, G.; Zamo, A.; Raz, R.; Karras, J.G.; Levy, D.E.; Inghirami, G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 2005, 11, 623–629. [Google Scholar] [CrossRef]
- Kasprzycka, M.; Marzec, M.; Liu, X.; Zhang, Q.; Wasik, M.A. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT. Proc. Natl. Acad. Sci. USA 2006, 103, 9964–9969. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Zhang, Q.; Goradia, A.; Raghunath, P.N.; Liu, X.; Paessler, M.; Wang, H.Y.; Wysocka, M.; Cheng, M.; Ruggeri, B.A.; et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 2008, 105, 20852–20857. [Google Scholar] [CrossRef]
- Crescenzo, R.; Abate, F.; Lasorsa, E.; Tabbo’, F.; Gaudiano, M.; Chiesa, N.; Di Giacomo, F.; Spaccarotella, E.; Barbarossa, L.; Ercole, E.; et al. T-Cell Project: Prospective Collection of Data in Patients with Peripheral T-Cell Lymphoma and the AIRC 5xMille Consortium “Genetics-Driven Targeted Management of Lymphoid Malignancies”. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell 2015, 27, 516–532, Erratum in Cancer Cell 2015, 27, 516–532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boddicker, R.L.; Kip, N.S.; Xing, X.; Zeng, Y.; Yang, Z.Z.; Lee, J.H.; Almada, L.L.; Elsawa, S.F.; Knudson, R.A.; Law, M.E.; et al. The oncogenic transcription factor IRF4 is regulated by a novel CD30/NF-κB positive feedback loop in peripheral T-cell lymphoma. Blood 2015, 125, 3118–3127. [Google Scholar] [CrossRef]
- Virmani, P.; Jawed, S.; Myskowski, P.L.; Horwitz, S.; Skripnik Lucas, A.; Moskowitz, A.; Pulitzer, M.; Zain, J.; Rosen, S.T.; Querfeld, C. Long-term follow-up and management of small and medium-sized CD4+ T cell lymphoma and CD8+ lymphoid proliferations of acral sites: A multicenter experience. Int. J. Dermatol. 2016, 55, 1248–1254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujii, K. New Therapies and Immunological Findings in Cutaneous T-Cell Lymphoma. Front. Oncol. 2018, 8, 198. [Google Scholar] [CrossRef]
- Marques-Piubelli, M.L.; Miranda, R.N. What’s new in hematopathology 2023: Updates on mature T-cell neoplasms in the 5th edition of the WHO classification. J. Pathol. Transl. Med. 2023, 57, 246–249. [Google Scholar] [CrossRef]
- Stephan, C.; Grossman, M.E.; Magro, C.M. Primary cutaneous acral CD8-positive T-cell lymphoproliferative disorder: A clinical and histologic retrospective cohort study. Clin. Dermatol. 2023, 41, 666–679. [Google Scholar] [CrossRef]
- Al-Toma, A.; Verbeek, W.H.; Hadithi, M.; von Blomberg, B.M.; Mulder, C.J. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: Retrospective evaluation of single-centre experience. Gut 2007, 56, 1373–1378. [Google Scholar] [CrossRef]
- Cording, S.; Lhermitte, L.; Malamut, G.; Berrabah, S.; Trinquand, A.; Guegan, N.; Villarese, P.; Kaltenbach, S.; Meresse, B.; Khater, S.; et al. Oncogenetic landscape of lymphomagenesis in coeliac disease. Gut 2022, 71, 497–508. [Google Scholar] [CrossRef]
- Veloza, L.; Cavalieri, D.; Missiaglia, E.; Ledoux-Pilon, A.; Bisig, B.; Pereira, B.; Bonnet, C.; Poullot, E.; Quintanilla-Martinez, L.; Dubois, R.; et al. Monomorphic epitheliotropic intestinal T-cell lymphoma comprises morphologic and genomic heterogeneity impacting outcome. Haematologica 2023, 108, 181–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, D.; Morgan, E.A.; Berger, D.; Pinkus, G.S.; Ferry, J.A.; Zukerberg, L.R. NK-Cell Enteropathy and Similar Indolent Lymphoproliferative Disorders: A Case Series with Literature Review. Am. J. Clin. Pathol. 2019, 151, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Li, W. The 5th Edition of the World Health Organization Classification of Hematolymphoid Tumors. In Leukemia [Internet]; Li, W., Ed.; Exon Publications: Brisbane, AU, USA, 2022. [Google Scholar] [PubMed]
- Bao, C.; Zhou, D.; Zhu, L.; Qian, W.; Ye, X. Increased serum level of interleukin-6 correlates with negative prognostic factors in extranodal NK/T-cell lymphoma. Transl. Cancer Res. 2020, 9, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Wuxiao, Z.; Huang, H.; Jiang, W.; Li, Z.; Lu, Y.; Xia, Z. Increased serum levels of interleukin-10 predict poor prognosis in extranodal natural killer/T-cell lymphoma patients receiving asparaginase-based chemotherapy. Oncol. Targets Ther. 2015, 8, 2589–2599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.J.; Hong, M.; Do, I.G.; Lee, S.H.; Ryu, K.J.; Yoo, H.Y.; Hong, J.Y.; Ko, Y.H.; Kim, W.S. Serum survivin and vascular endothelial growth factor in extranodal NK/T-cell lymphoma, nasal type: Implications for a potential new prognostic indicator. Haematologica 2015, 100, e106–e109. [Google Scholar] [CrossRef]
- Glover, A.; Shannon-Lowe, C. From pathobiology to targeted treatment in Epstein-Barr virus–related T-cell and NK-cell lymphoproliferative diseases. Ann. Lymphoma 2021, 5, 31. [Google Scholar] [CrossRef]
- Cohen, J.I.; Manoli, I.; Dowdell, K.; Krogmann, T.A.; Tamura, D.; Radecki, P.; Bu, W.; Turk, S.P.; Liepshutz, K.; Hornung, R.L.; et al. Hydroa vacciniforme-like lymphoproliferative disorder: An EBV disease with a low risk of systemic illness in whites. Blood 2019, 133, 2753–2764. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Montes-Mojarro, I.A.; Fend, F.; Quintanilla-Martinez, L. Epstein–Barr Virus-Associated T and NK-Cell Lymphoproliferative Diseases. Front. Pediatr. 2019, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.; Nogami, A.; Imadome, K.; Kurata, M.; Murakami, N.; Fujiwara, S.; Miura, O. Sequential monitoring of serum IL-6, TNF-α, and IFN-γ levels in a CAEBV patient treated by plasma exchange and immunochemotherapy. Int. J. Hematol. 2012, 96, 669–673, Erratum in Int. J. Hematol. 2013, 97, 301. [Google Scholar] [CrossRef] [PubMed]
- Ohga, S.; Nomura, A.; Takada, H.; Tanaka, T.; Furuno, K.; Takahata, Y.; Kinukawa, N.; Fukushima, N.; Imai, S.; Hara, T. Dominant expression of interleukin-10 and transforming growth factor-beta genes in activated T-cells of chronic active Epstein-Barr virus infection. J. Med. Virol. 2004, 74, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Marsh, R.A.; Allen, C.E.; McClain, K.L. Epstein–Barr Virus and Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2017, 8, 1902. [Google Scholar] [CrossRef]
- Cvetković, Z.; Marković, O.; Marinković, G.; Pejić, S.; Vučić, V. Tumor Microenvironment, Inflammation, and Inflammatory Prognostic Indices in Diffuse Large B-Cell Lymphomas: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 5670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| WHO, 5th Edition (WHO-HAEM5) 2022 | International Consensus Classification (ICC) 2022 |
|---|---|
| Mature T-cell and NK-cell leukemias (primary leukemias) | |
| T-prolymphocytic leukemia (T-PLL) | T-cell prolymphocytic leukemia (T-PLL) |
| T-cell large granular lymphocytic leukemia (T-LGL) | T-cell large granular lymphocytic leukemia (T-LGL) |
| NK-cell large granular lymphocytic leukemia (NK-LGL) | Chronic LPD of NK-cells |
| Adult T-cell leukemia/lymphoma (ATLL) | Adult T-cell leukemia/lymphoma (ATLL) |
| Sézary syndrome (SSy) | Sézary syndrome (SSy) |
| Aggressive NK-cell leukemia (ANKL) | Aggressive NK-cell leukemia (ANKL) |
| Primary cutaneous T-cell lymphomas (CTCL) | |
| Primary cutaneous CD4+ small or medium T-cell LPD | Primary cutaneous small/medium CD4+ T-cell LPD |
| Primary cutaneous acral CD8+ LPD | Primary cutaneous acral CD8+ LPD |
| Mycosis fungoides (MF) | Mycosis fungoides (MF) |
| Primary cutaneous CD30+ T-cell LPD: Lymphomatoid papulosis (LyP) | Primary cutaneous CD30+ T-cell LPD: Lymphomatoid papulosis (LyP) |
| Primary cutaneous CD30+ T-cell LPD: Primary cutaneous anaplastic large cell lymphoma | Primary cutaneous CD30+ T-cell LPD: Primary cutaneous anaplastic large cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma | Subcutaneous panniculitis-like T-cell lymphoma |
| Primary cutaneous γ/δ T-cell lymphoma | Primary cutaneous γ/δ T-cell lymphoma |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma | Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma |
| Primary cutaneous peripheral T-cell lymphoma, NOS | Not included |
| Intestinal T-cell and NK-cell lymphoid proliferations and lymphomas (extranodal) | |
| Indolent T-cell lymphoma of the gastrointestinal tract | Indolent clonal T-cell LPD of the gastrointestinal tract |
| Enteropathy-associated T-cell lymphoma (EATL) | Enteropathy-associated T-cell lymphoma (EATL) |
| Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) | Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) |
| Intestinal T-cell lymphoma, NOS | Intestinal T-cell lymphoma, NOS |
| Hepatosplenic T-cell lymphoma (extranodal) | |
| Hepatosplenic T-cell lymphoma (HSTCL) | Hepatosplenic T-cell lymphoma (HSTCL) |
| Anaplastic large cell lymphoma (nodal) | |
| ALK+ anaplastic large cell lymphoma (ALCL, ALK+) | Anaplastic large cell lymphoma, ALK+ (ALCL, ALK+) |
| ALK- anaplastic large cell lymphoma (ALCL, ALK-) | Anaplastic large cell lymphoma, ALK- (ALCL, ALK-) |
| Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) | Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) |
| Nodal T-follicular helper (TFH) cell lymphoma | |
| Nodal TFH cell lymphoma, angioimmunoblastic-type (AITL) | TFH-cell lymphoma, angioimmunoblastic type (AITL) |
| Nodal TFH cell lymphoma, follicular-type | TFH-cell lymphoma, follicular type |
| Nodal TFH cell lymphoma, NOS | TFH-cell lymphoma, NOS |
| Other peripheral T-cell lymphomas | |
| Peripheral T-cell lymphoma, NOS (PTCL-NOS) | Peripheral T-cell lymphoma, NOS (PTCL-NOS) |
| EBV-positive NK/T-cell lymphomas | |
| EBV+ nodal T or NK-cell lymphoma | Primary nodal EBV+T/NK-cell lymphoma |
| Extranodal NK/T-cell lymphoma (ENKTCL) | Extranodal NK/T-cell lymphoma, nasal type (ENKTCL) |
| EBV-positive T and NK-cell lymphoid proliferations and lymphomas of childhood | |
| Severe mosquito bite allergy | Severe mosquito bite allergy |
| Hydroa vacciniforme LPD | Hydroa vacciniforme LPD |
| Systemic chronic active EBV disease | Chronic active EBV disease, systemic (T or NK-cell phenotype) |
| Systemic EBV+ T-cell lymphoma of childhood | Systemic EBV+ T-cell lymphoma of childhood |
| Class | Representative Molecules |
|---|---|
| Protein fibers | Collagens, Elastin |
| Glycoproteins | Fibronectin, Laminin, Tenascin |
| Proteoglycans | Serglycin, GAGs, Syndecan, Agrecan |
| Affiliated proteins | Annexins, Hemopexin, Galectins |
| ECM regulators | MMPs, TIMPs, Cathepsins, Serpins |
| Secreted proteins | Growth factors, Cytokines |
| WHO-HAEM5 | Cell of Origin | Immunophenotype | Specific LME |
|---|---|---|---|
| NODAL | |||
| PTCL-NOS | Variabile, mostly T-helper cell | CD4 > CD8, frequent antigen loss CD5, CD7, CD30+/−, CD56−/+, subset FTH features, cytotoxic granules+/− | Classical stromal cells but highly heterogenous composition |
| ALCL, ALK− | Cytotoxic T-cell | ALK−, CD30+, EMA+, CD25+, cytotoxic granules+, CD4+/−, CD3+/− | Reactive histiocytes, fibrosis, immune evasion via PD-L1 |
| ALCL, ALK+ | Cytotoxic T-cell | ALK+, CD30+, EMA+, CD25+, cytotoxic granules+, CD4+/−, CD3+/− | Inflammatory background, activated TME |
| BIA-ALCL | Undefined, Suggested T-cell of Th17/Th1 immune response | CD30+, ALK−, EMA+/−, variable T-cell markers | Fibrous capsule-related microenvironment, Th17 cytokines |
| AITL | Follicular helper T-cell (TFH) | Pan T+, CD4+, CD10+/−, bcl6+/−, CXCL13+, PD1+, ICOS+/−, SAP+/−, CCD5+/−, hyperplastic FDRC, EBV+ B blasts | Prominent angiogenesis (HEV), proliferating stromal cells, EBV+ B cells |
| Nodal TFH follicular-type | Similar to AITL | Expanded FDC meshworks, angiogenesis | |
| Nodal TFH NOS | Similar to AITL | Variable stromal and immune infiltration | |
| Other PTCLs | Variable | Depends on subtype | Heterogeneous |
| EBV+ nodal T/NK-cell lymphoma | Cytotoxic T or NK | EBV+, cytotoxic phenotype | EBV-driven microenvironment with high immune infiltration |
| EXTRANODAL | |||
| ENKTCL | NK, rarely cytotoxic T-cells | CD2+, CD56+, sCD3−, cCD3ε+, granzyme B+, TIA-1+, perforin+, EBV+, LMP1+ | Highly immune stroma, angiodestructive, necrosis |
| HSTL | Cytotoxic T-cell of the innate immune system | CD3+, CD56+, CD4−, CD8+, CD5−, TIA1+, granzyme M+, B−, perforin | Low level of stromal cells |
| MEITL | Intraepithelial T cells or NK, monomorphic, no preexisting enteropathy | CD3+, CD7+, CD5−, CD8+, CD56+, MATK+, HLA DQ2/DQ8 | Sparse stroma, epithelial interaction |
| EATL | Intraepithelial T cells (αβ), pleomorphic, preexisting enteropathy | CD3+, CD7+, CD5−, CD8−/+, CD56−, HLA DQ2/DQ8 | Inflammatory background, enteropathy-related |
| LEUKEMIC | |||
| T-PLL | Post-thymic T-cell | TdT−, CD1a−, CD2+, CD3+, CD7+, sCD3 week, CD52+, CD4/CD8 variable | Leukemic spread, minimal LME |
| T-LGL | Cytotoxic T-cell | CD3+, CD8+, CD57+, TIA1+ | Reactive marrow environment, immune dysregulation |
| NK-LGL | Cytotoxic NK-cell | CD2+, CD3−, CD56+, CD57+, CD16+ | Reactive marrow niche |
| ATLL | Peripheral CD4+ reg cells | Pan-T-cell, CD4+, CD25+, CD7− | HTLV-1 driven microenvironment, immune suppression |
| SSy | Central memory T-cell CD4+ | TH2 cytokine profile expression, CCR7/L-selectin+, CD27+, CD3+, CD4+, CD8−, CD7−, PD1+, bcl6+, CXCL13+ | Skin homing microenvironment, immune suppression |
| ANKL | Mature, cytotoxic NK-cells | CD2+, CD3−, CD3ε+, CD56+, CD57−, cytotoxic phenotype, CD16+ frequently, CD11b expressed in some cases | Hemophagocytic environment, cytokine storm prone |
| CUTANEOUS | |||
| Primary cutaneous CD4+ small or medium T-cell LPB | CD4+ T-cell | CD3+, CD4+, CD8−, CD30− | Reactive infiltrate, low stromal response |
| Primary cutaneous acral CD8+ LPD | CD8+ T-cell | CD3+, CD8+, CD4−, CD30− | Indolent, localized stroma |
| Mycosis fungoides (MF) | Mature CD4+ T-cell | CD2+, CD3+, CD4+, CD45RO+, TCRβ+, CD5+/−, CD7−, CD8−, CD45RA−, CD45 variable CD30 | Skin microenvironment with Langerhans cells, fibroblasts |
| Lymphomatoid papulosis (LyP) type A, B, C, D, E, w/6p25 | Activated T-cell (Reed-Sternberg like, cerbriform) | CD30+, CD4+, CD8−/+, variable | Inflammatory infiltrate, spontaneous regression |
| Primary cutaneous ALCL | Cytotoxic T-cell | CD30+, ALK− | Dense dermal infiltrate, reactive stroma |
| Subcutaneous panniculitis-like T-cell lymphoma | Cytotoxic T-cell (αβ) | CD3+, CD8+, TIA1+/βF+, granzyme B+, Ki67 elevated | Adipocyte-rich environment, macrophage infiltration |
| Primary cutaneous γ/δ T-cell lymphoma | γ/δ T-cell | CD3+, CD4−, CD8−, CD56+, TIA-1+, TCRγ+, Ki67 elevated | Ulcerating lesions, inflammatory milieu |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma | Cytotoxic CD8+ T-cell | CD3+, CD7+, CD8+, TIA-1+, CD45RA+, βF-1+, CD45RO−, CD56−, CD4−, EBER− | Epidermal infiltration, inflammatory background |
| Primary cutaneous peripheral T-cell lymphoma, NOS | Variable T-cell | Heterogeneous phenotype | Aggressive, heterogeneous stroma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petković, I.; Ritucci, M.; Stojković, A.; Stojnev, S.; Popović, A.; Conić, I.; Radić, M.; Džunić, M.; Krstić, M. The Significance of the Microenvironment in T/Nk-Cell Neoplasms. Int. J. Mol. Sci. 2025, 26, 11225. https://doi.org/10.3390/ijms262211225
Petković I, Ritucci M, Stojković A, Stojnev S, Popović A, Conić I, Radić M, Džunić M, Krstić M. The Significance of the Microenvironment in T/Nk-Cell Neoplasms. International Journal of Molecular Sciences. 2025; 26(22):11225. https://doi.org/10.3390/ijms262211225
Chicago/Turabian StylePetković, Ivan, Michele Ritucci, Ana Stojković, Slavica Stojnev, Aleksandar Popović, Irena Conić, Milica Radić, Miljana Džunić, and Miljan Krstić. 2025. "The Significance of the Microenvironment in T/Nk-Cell Neoplasms" International Journal of Molecular Sciences 26, no. 22: 11225. https://doi.org/10.3390/ijms262211225
APA StylePetković, I., Ritucci, M., Stojković, A., Stojnev, S., Popović, A., Conić, I., Radić, M., Džunić, M., & Krstić, M. (2025). The Significance of the Microenvironment in T/Nk-Cell Neoplasms. International Journal of Molecular Sciences, 26(22), 11225. https://doi.org/10.3390/ijms262211225