New Role of Protein Misfolding Corrector in the ER Stress-Inflammation Axis: Possible Therapeutic Indication in Neuronal and Epithelial Tumor Cells
Abstract
1. Introduction
2. Results
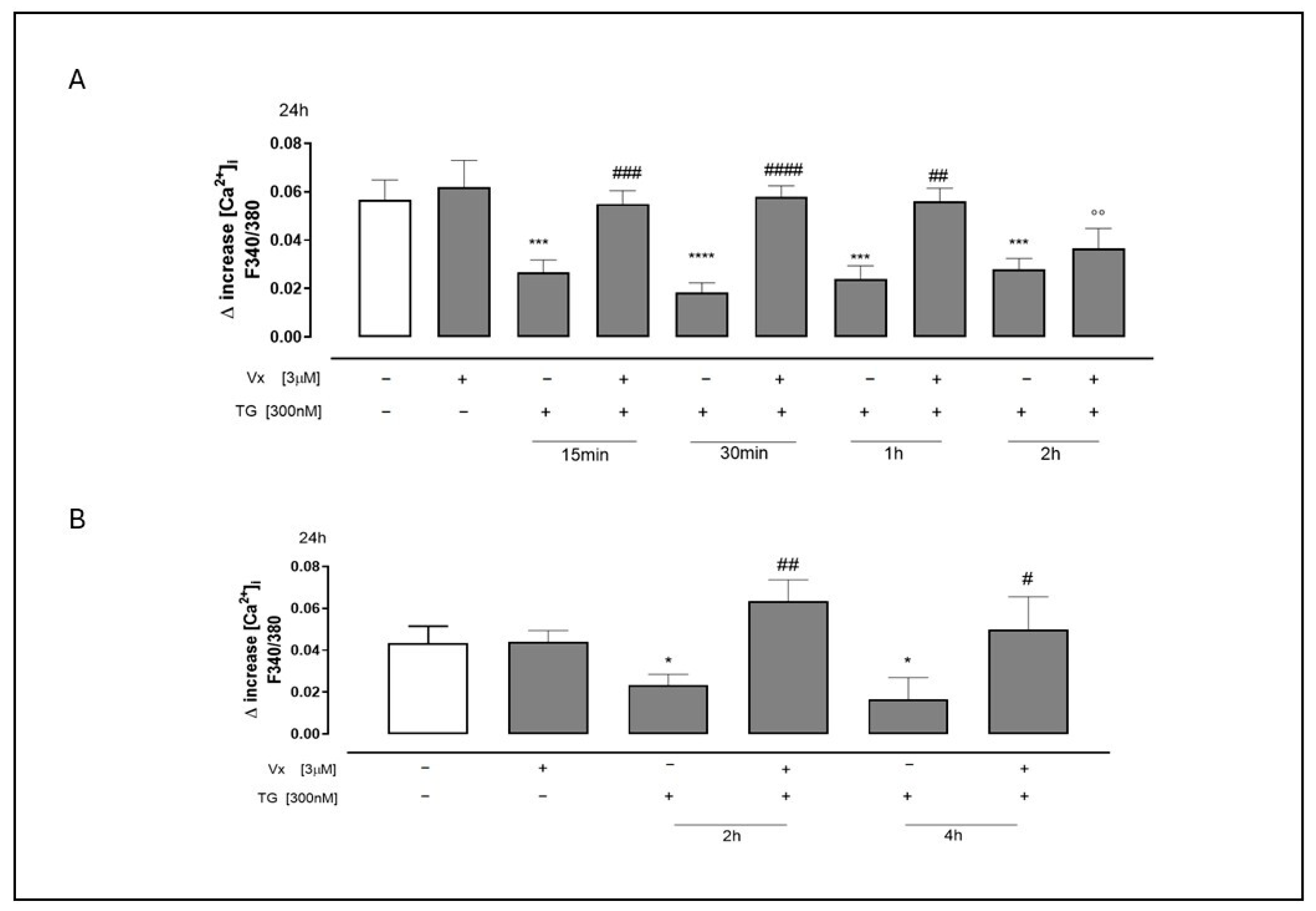
2.1. Vx445 Corrector Counteracts Calcium Homeostasis Dysregulation in ER in Response to Stress
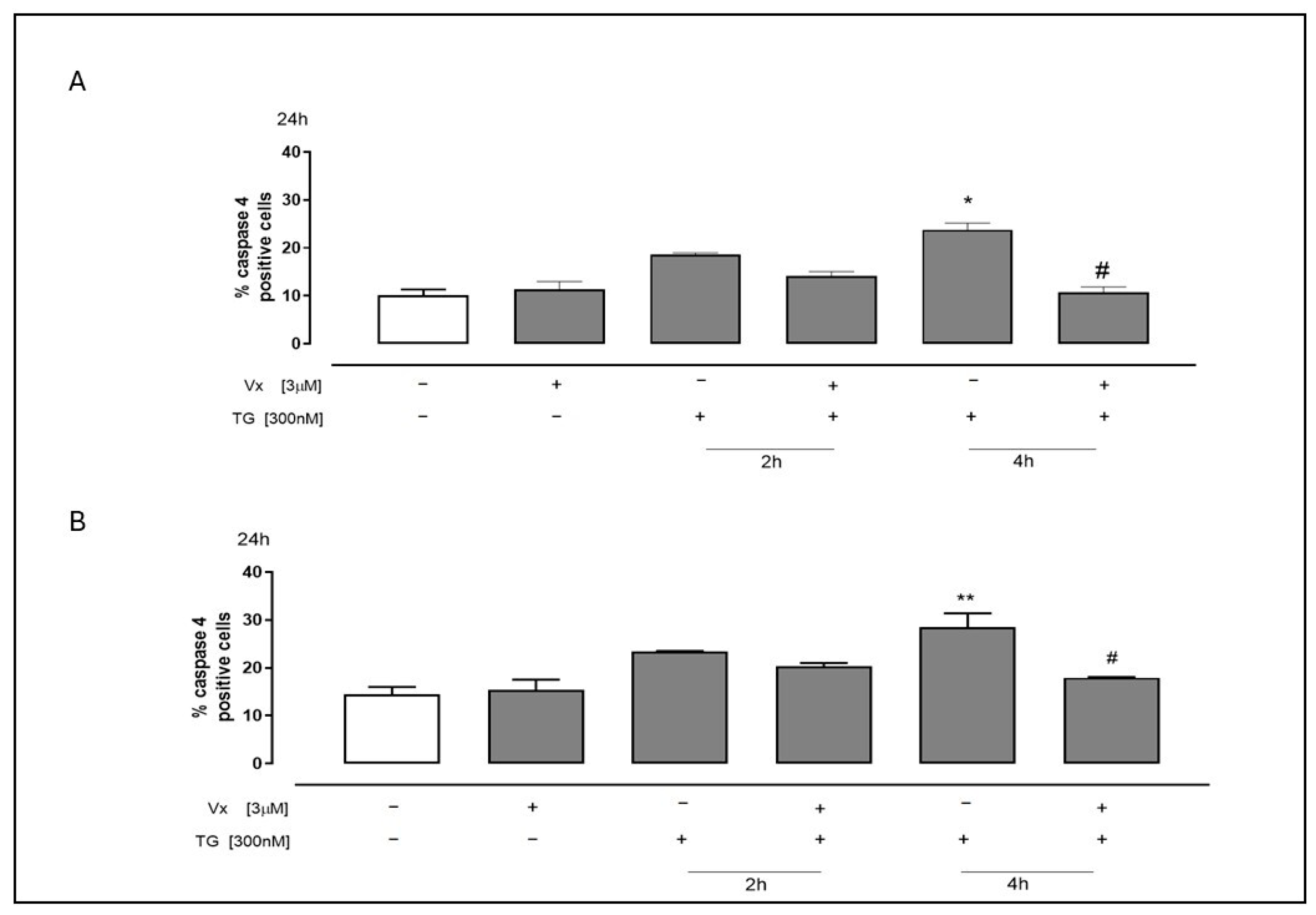
2.2. The Corrector Interferes in the Cleavage of Procaspase 4
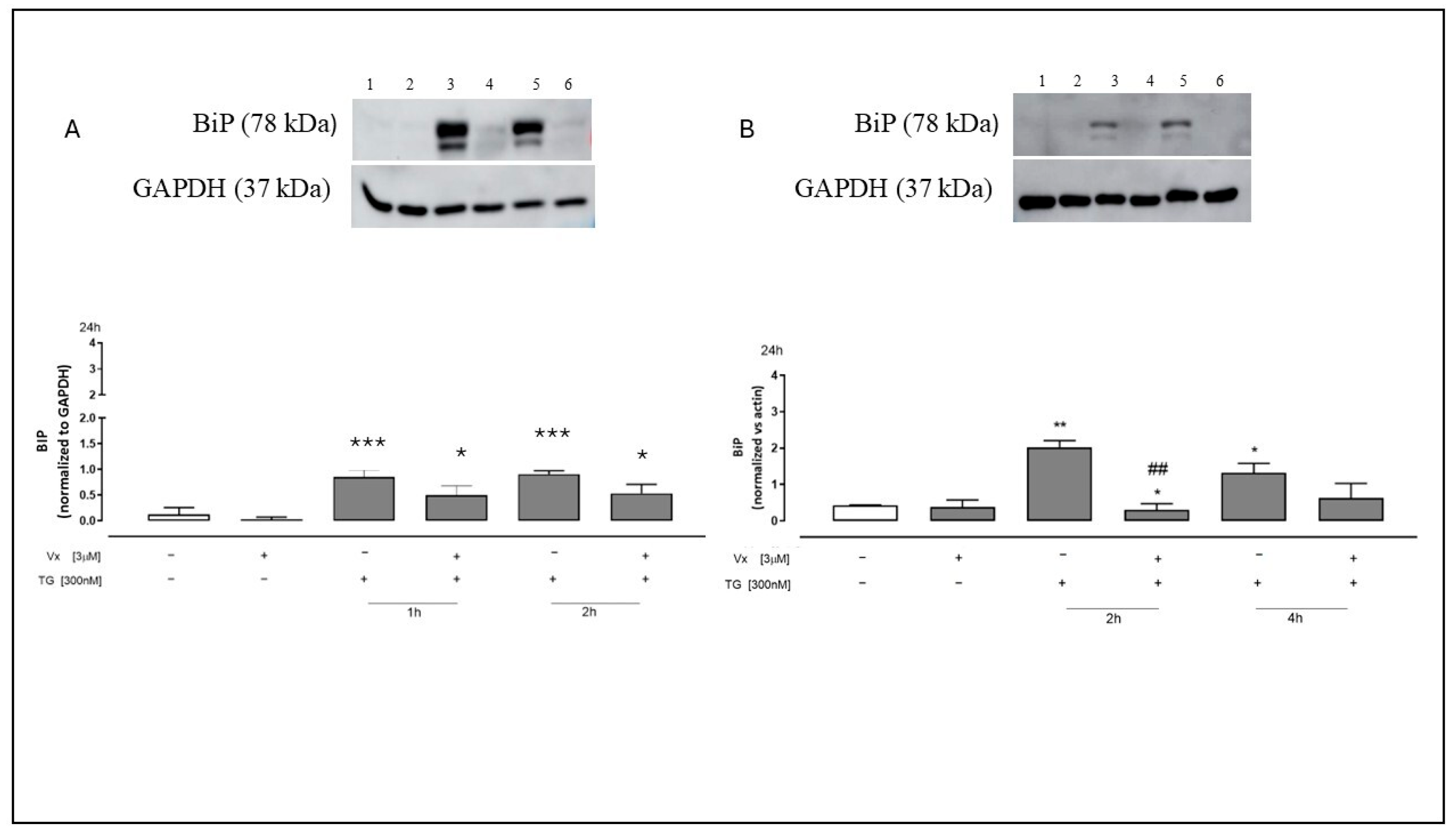
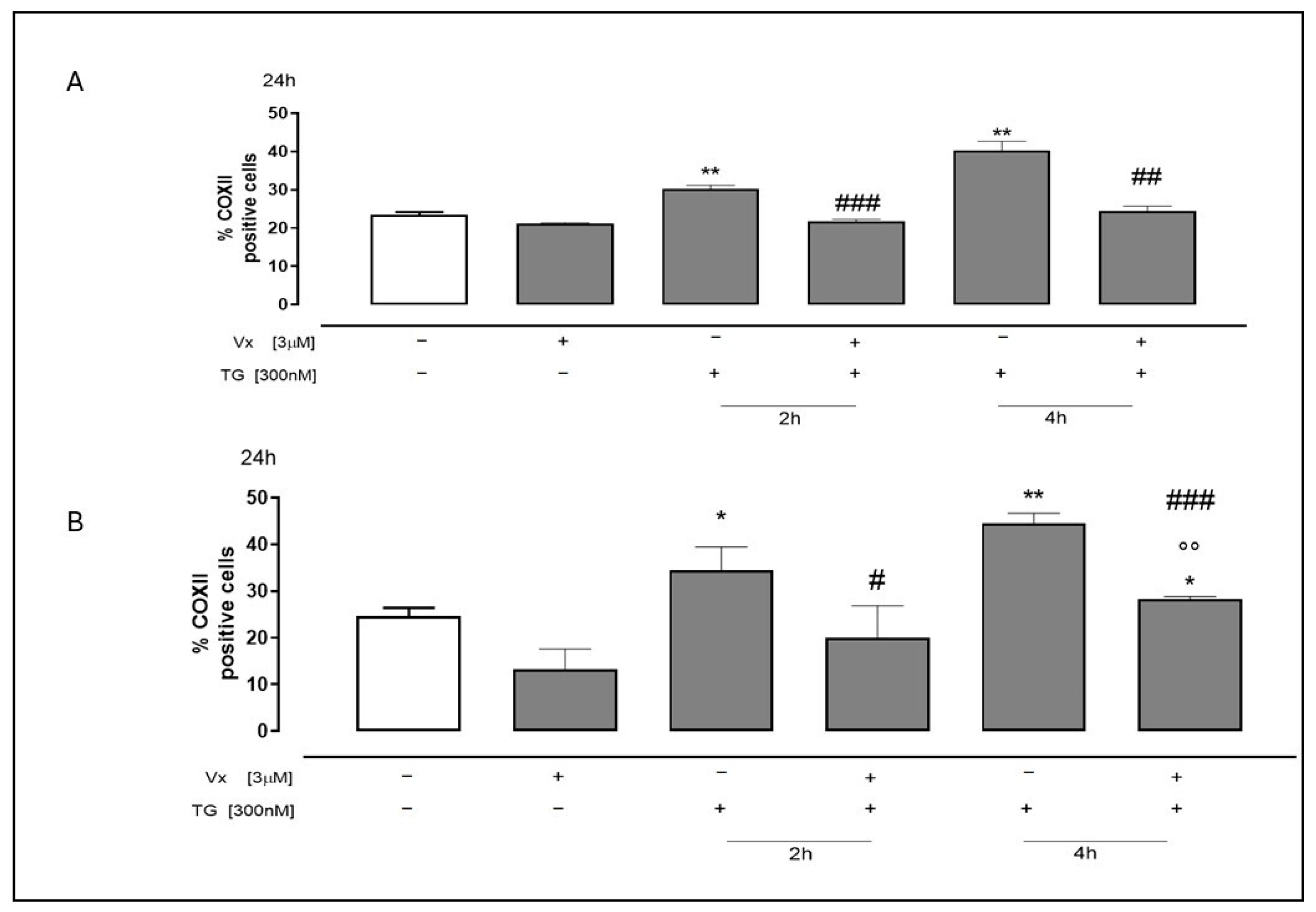
2.3. Vx-445 Reduces GRP78/BiP Overexpression Induced by ER Stress
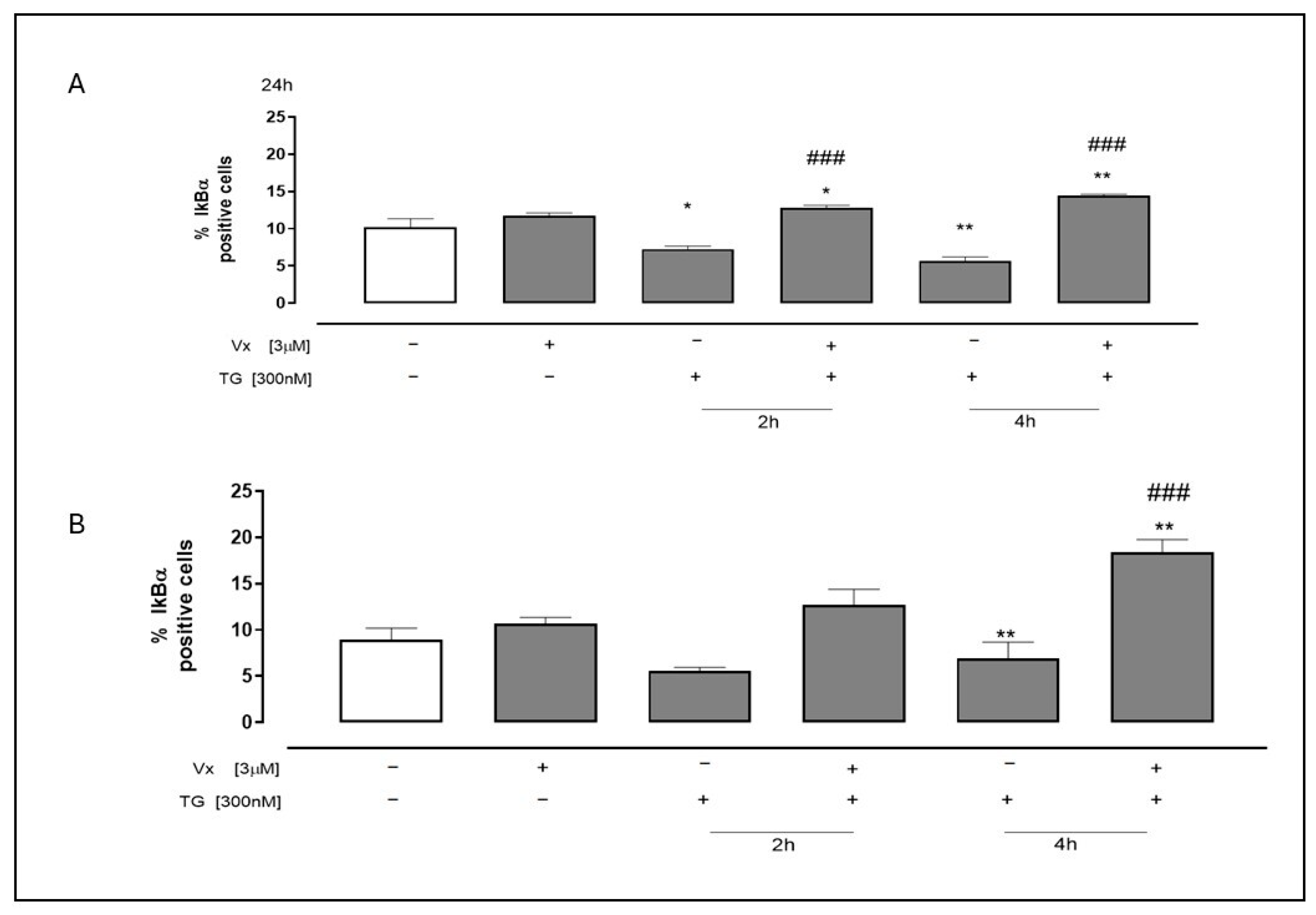
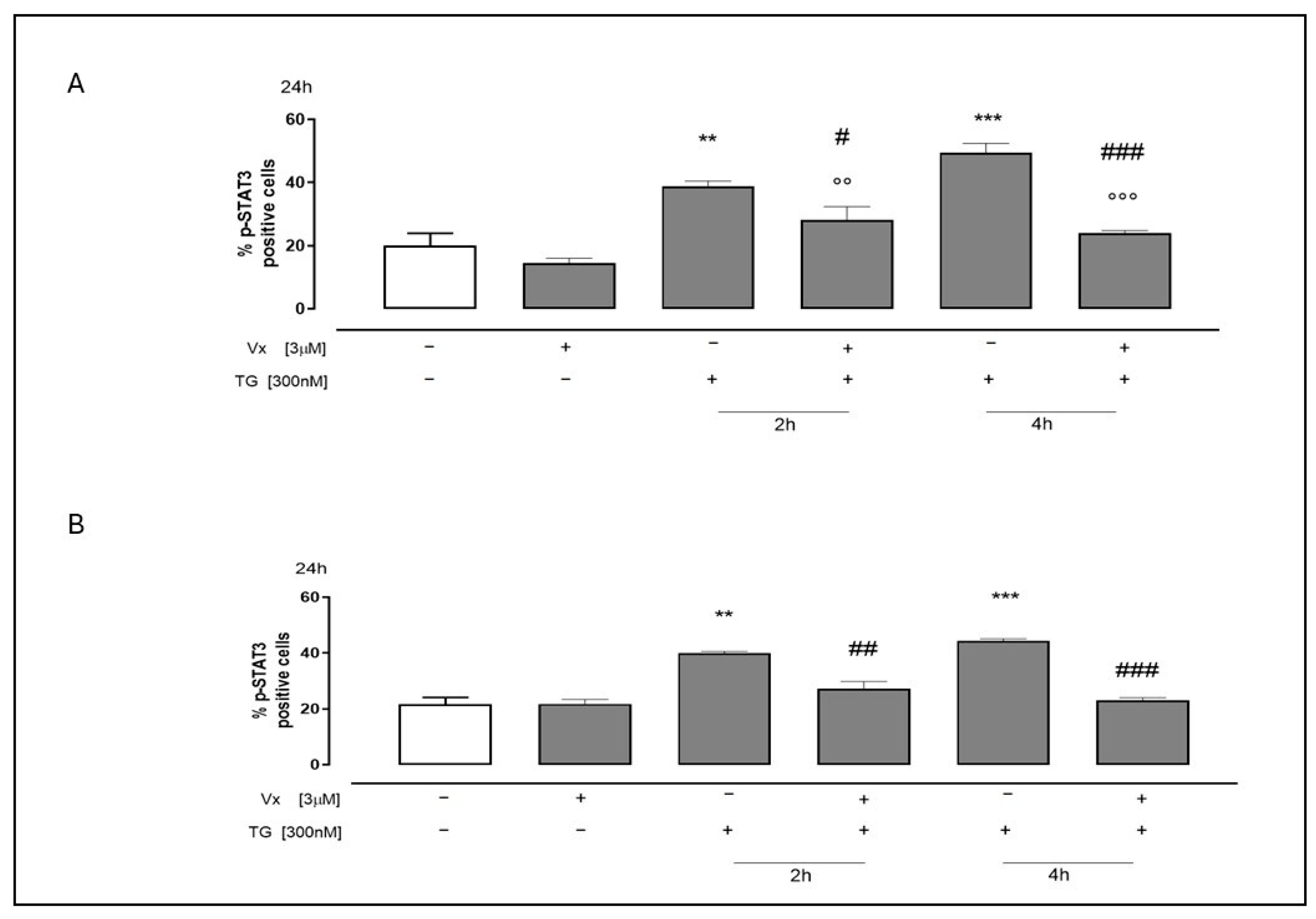
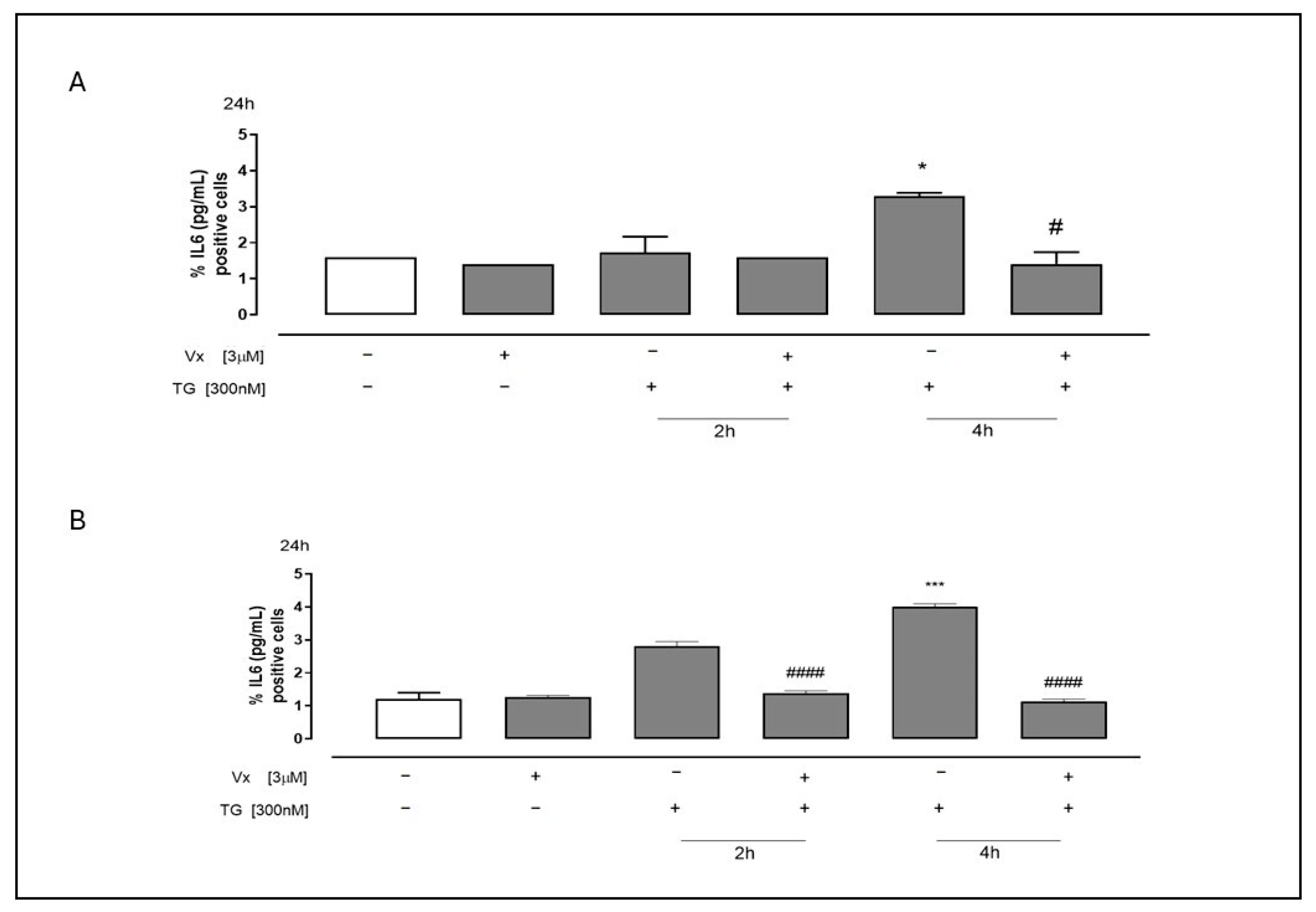
2.4. Vx-445 Attenuates Inflammatory Signaling Through NF-kB and STAT3 Pathways
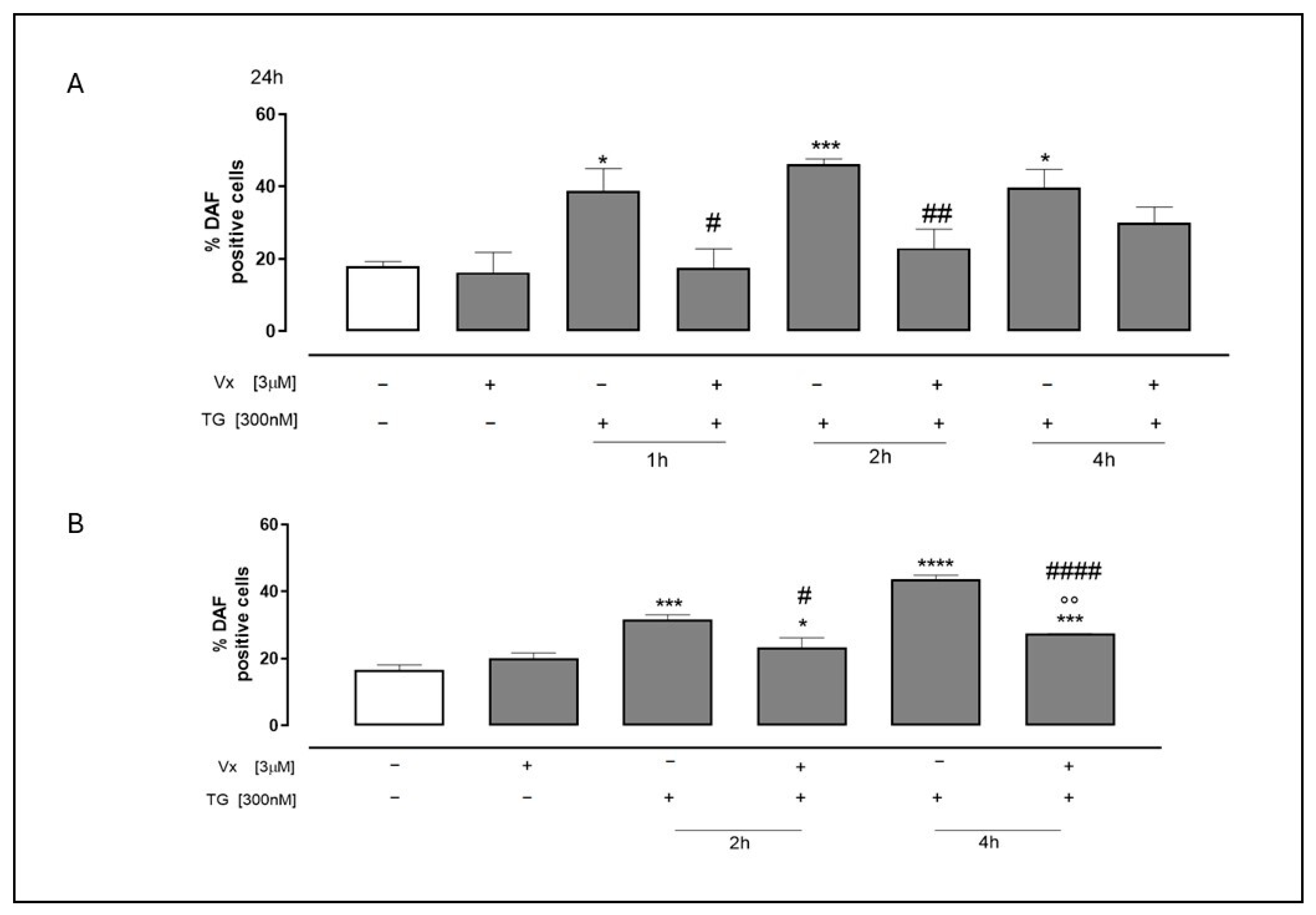
2.5. Vx-445 Interferes in NO Release Under ER Stress
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Experimental Protocol
4.4. Protein Extraction and Western Blot Analysis
4.5. Flow Cytometry Assay
4.6. Analysis of Reticular Calcium Homeostasis
4.7. IL-6 Measurement
4.8. Detection of Nitric Oxide Levels
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, J.S.M.; Linxweiler, J.; Radosa, J.C.; Linxweiler, M.; Zimmermann, R. The endoplasmic reticulum membrane protein Sec62 as potential therapeutic target in SEC62 overexpressing tumors. Front. Physiol. 2022, 13, 1014271. [Google Scholar] [CrossRef]
- Sicking, M.; Lang, S.; Bochen, F.; Roos, A.; Drenth, J.P.H.; Zakaria, M.; Zimmermann, R.; Linxweiler, M. Complexity and specificity of Sec61 channelopathies: Human diseases affecting gating of the Sec61 complex. Cells 2021, 10, 1036. [Google Scholar] [CrossRef]
- Zhu, G.; Lee, A.S. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J. Cell. Physiol. 2015, 230, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Collins, H.E.; Young, M.E.; Zhang, J.; Wende, A.R.; Darley-Usmar, V.M.; Chatham, J.C. The Identification of a Novel Calcium-Dependent Link Between NAD+ and Glucose Deprivation-Induced Increases in Protein O-GlcNAcylation and ER Stress. Front. Mol. Biosci. 2021, 8, 780865. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Z.; Hussain, T.; Zhao, D.; Yang, L. A central role for calcineurin in protein misfolding neurodegenerative diseases. Cell Mol. Life Sci. 2017, 74, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, J.; Sun, S.; Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling; excitability; and synaptic plasticity defects in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 45, 561–580. [Google Scholar] [CrossRef]
- Haeri, M.; Knox, B.E. Endoplasmic Reticulum Stress and Unfolded Protein Response Pathways: Potential for Treating Age-related Retinal Degeneration. J. Ophthalmic Vis. Res. 2012, 7, 45–59. [Google Scholar]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Credle, J.J.; Forcelli, P.A.; Delannoy, M.; Oaks, A.W.; Permaul, E.; Berry, D.L.; Duka, V.; Wills, J.; Sidhu, A. α-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson’s disease. Neurobiol. Dis. 2015, 76, 112–125. [Google Scholar] [CrossRef]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.Z.; Wang, T.; Chen, J.J.; Xie, X.Y.; Hu, H.; Yu, F.; Liu, H.L.; Jiang, X.Y.; Fan, H.D. Molecular signal networks and regulating mechanisms of the unfolded protein response. J. Zhejiang Univ. Sci. B. 2017, 18, 1–14. [Google Scholar] [CrossRef]
- Bian, Z.M.; Elner, S.G.; Elner, V.M. Dual involvement of caspase 4 in inflammatory and ER stress-induced apoptotic responses in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 6006–6014. [Google Scholar] [CrossRef]
- Hitomi, J.; Katayama, T.; Eguchi, Y.; Kudo, T.; Taniguchi, M.; Koyama, Y.; Manabe, T.; Yamagishi, S.; Bando, Y.; Imaizumi, K.; et al. Involvement of caspase 4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J. Cell Biol. 2004, 165, 347–356. [Google Scholar] [CrossRef]
- Lakshmanan, U.; Porter, A.G. Caspase 4 interacts with TNF receptor-associated factor 6 and mediates lipopolysaccharide-induced NF-kappaB-dependent production of IL-8 and CC chemokine ligand 4 (macrophage-inflammatory protein-1). J. Immunol. 2007, 179, 8480–8490. [Google Scholar] [CrossRef] [PubMed]
- Sonninen, T.M.; Goldsteins, G.; Laham-Karam, N.; Koistinaho, J.; Lehtonen, Š. Proteostasis Disturbances and Inflammation in Neurodegenerative Diseases. Cells 2020, 9, 2183. [Google Scholar] [CrossRef] [PubMed]
- Meares, G.P.; Liu, Y.; Rajbhandari, R.; Qin, H.; Nozell, S.E.; Mobley, J.A.; Corbett, J.A.; Benveniste, E.N. PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol. Cell. Biol. 2014, 34, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef]
- Pecoraro, M.; Serra, A.; Pascale, M.; Franceschelli, S. Vx-809, a CFTR Corrector, Acts through a General Mechanism of Protein Folding and on the Inflammatory Process. Int. J. Mol. Sci. 2023, 24, 4252. [Google Scholar] [CrossRef]
- Pecoraro, M.; Serra, A.; Pascale, M.; Franceschelli, S. The ER Stress Induced in Human Neuroblastoma Cells Can Be Reverted by Lumacaftor; a CFTR Corrector. Curr. Issues Mol. Biol. 2024, 46, 9342–9358. [Google Scholar] [CrossRef]
- Shahzadi, C.; Di Serafino, A.; Aruffo, E.; Mascitelli, A.; Di Carlo, P. A549 as an In Vitro Model to Evaluate the Impact of Microplastics in the Air. Biology 2023, 12, 1243. [Google Scholar] [CrossRef]
- Veit, G.; Vaccarin, C.; Lukacs, G.L. Elexacaftor co-potentiates the activity of F508del and gating mutants of CFTR. J. Cyst. Fibros. 2021, 20, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, I.X.; Herrmann, A.; Leon, J.; Jeyarajan, S.; Arunagiri, A.; Arvan, P.; Gilon, P.; Satin, L.S. ER stress increases expression of intracellular calcium channel RyR1 to modify Ca2+ homeostasis in pancreatic beta cells. J. Biol. Chem. 2023, 299, 105065. [Google Scholar] [CrossRef] [PubMed]
- Moorwood, C.; Barton, E.R. Caspase 12 ablation preserves muscle function in the mdx mouse. Hum. Molec. Genet. 2014, 23, 5325–5341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Li, T.; Li, Q.; Gu, Y.; Shu, Y.; Hu, K.; Chen, L.; Peng, X.; Peng, J.; Hao, L. Mechanism and Role of Endoplasmic Reticulum Stress in Osteosarcoma. Biomolecules 2022, 12, 1882. [Google Scholar] [CrossRef]
- Viennois, E.; Chen, F.; Merlin, D. NF-κB pathway in colitis-associated cancers. Transl. Gastrointest. Cancer. 2013, 2, 21–29. [Google Scholar]
- Solt, L.A.; May, M.J. The IkappaB kinase complex: Master regulator of NF-kappaB signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef]
- Perez, J.M.; Chirieleison, S.M.; Abbott, D.W. An IκB Kinase-Regulated Feedforward Circuit Prolongs Inflammation. Cell Rep. 2015, 12, 537–544. [Google Scholar] [CrossRef]
- Shen, N.; Wang, Z.; Wang, C.; Zhang, J.; Liu, C. Methane Alleviates Inflammation and Apoptosis of Dextran Sulfate Sodium-Induced Inflammatory Bowel Diseases by Inhibiting Toll-Like Receptor 4 (TLR4)/Myeloid Differentiation Factor 88 (MyD88)/Nuclear Translocation of Nuclear Factor-κB (NF-κB) and Endoplasmic Reticulum Stress Pathways in Mice. Med. Sci. Monit. 2020, 26, e922248. [Google Scholar]
- Lim, J.W.; Kim, H.; Kim, K.H. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab. Investig. 2001, 81, 349–360. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Linker, R.A.; Deng, J.; Kaltschmidt, C. Cyclooxygenase-2 is a neuronal target gene of NF-kappaB. BMC Mol. Biol. 2002, 3, 16. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Hartl, F.U. Protein Misfolding Diseases. Annu. Rev. Biochem. 2017, 86, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Panayi, G.S.; Corrigall, V.M. BiP regulates. autoimmune inflammation and tissue damage. Autoimmun. Rev. 2006, 5, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Kurekova, S.; Pavlikova, L.; Seres, M.; Bohacova, V.; Spaldova, J.; Breier, A.; Sulova, Z. Do wolframin, P-glycoprotein, and GRP78/BiP cooperate to alter the response of L1210 cells to endoplasmic reticulum stress or drug sensitivity? Cancer Cell Int. 2025, 25, 35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, M.; Serra, A.; Lamberti, M.J.; Pascale, M.; Franceschelli, S. New Role of Protein Misfolding Corrector in the ER Stress-Inflammation Axis: Possible Therapeutic Indication in Neuronal and Epithelial Tumor Cells. Int. J. Mol. Sci. 2025, 26, 10846. https://doi.org/10.3390/ijms262210846
Pecoraro M, Serra A, Lamberti MJ, Pascale M, Franceschelli S. New Role of Protein Misfolding Corrector in the ER Stress-Inflammation Axis: Possible Therapeutic Indication in Neuronal and Epithelial Tumor Cells. International Journal of Molecular Sciences. 2025; 26(22):10846. https://doi.org/10.3390/ijms262210846
Chicago/Turabian StylePecoraro, Michela, Adele Serra, Maria Julia Lamberti, Maria Pascale, and Silvia Franceschelli. 2025. "New Role of Protein Misfolding Corrector in the ER Stress-Inflammation Axis: Possible Therapeutic Indication in Neuronal and Epithelial Tumor Cells" International Journal of Molecular Sciences 26, no. 22: 10846. https://doi.org/10.3390/ijms262210846
APA StylePecoraro, M., Serra, A., Lamberti, M. J., Pascale, M., & Franceschelli, S. (2025). New Role of Protein Misfolding Corrector in the ER Stress-Inflammation Axis: Possible Therapeutic Indication in Neuronal and Epithelial Tumor Cells. International Journal of Molecular Sciences, 26(22), 10846. https://doi.org/10.3390/ijms262210846







