Genome-Wide Identification of the SnRK2 Gene Family and Its Response to Abiotic Stress in Populus euphratica
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of the Physicochemical Properties of PeSnRK2 Family Members
2.2. Analysis of Gene and Protein Structure of the PeSnRK2s
2.3. Prediction of Cis-Acting Elements in the Promoter Regions of PeSnRK2s
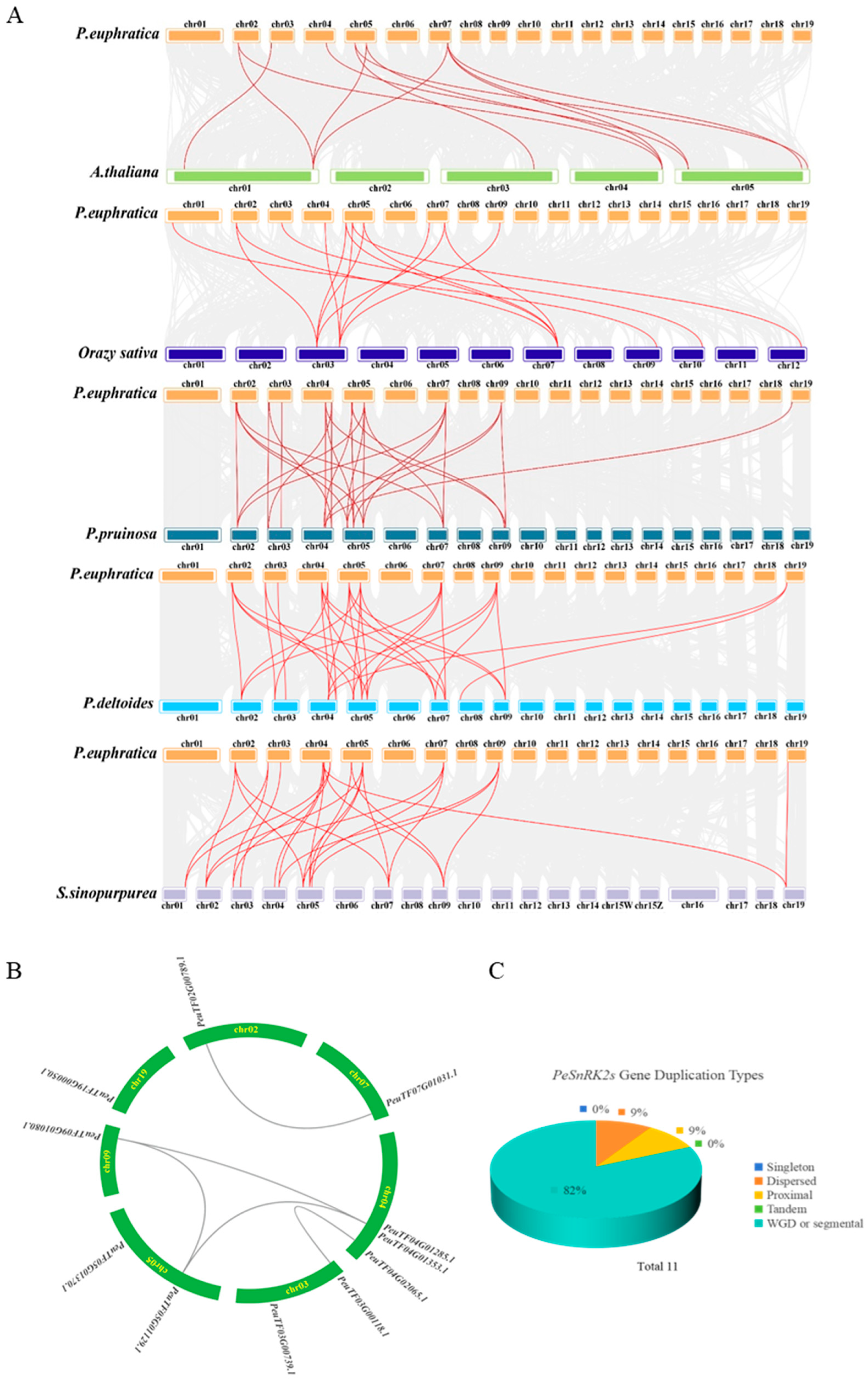
2.4. Collinearity Analysis of the SnRK2 Family in Multiple Species, PeSnRK2 Gene Duplication Types and Their Chromosomal Localization
2.5. Systematic Evolutionary Analysis of the SnRK2 Gene Family in P. euphratica, P. pruinosa, A. thaliana and O. sativa
2.6. Prediction of the Secondary Structure and Three-Dimensional Structure of the PeSnRK2 Protein
2.7. Expression Profiles of PeSnRK2 Genes in Response to Abiotic Stresses
2.8. Subcellular Localization of PeSnRK2s
2.9. Predicting Protein–Protein Interactions of the PeSnRK2.6
3. Discussion
3.1. Identification of 11 SnRK2 Family Members in P. euphratica
3.2. PeSnRK2 Family Members Respond to Abiotic Stress
3.3. PeSnRK2.6 Enhances Plant Drought Tolerance by Regulating Stomatal Movement
4. Materials and Methods
4.1. Identification and Physical and Chemical Property Analysis of Members of the PeSnRK2 Family
4.2. Genetic Structure and Conserved Domain Analysis of Members of the PeSnRK2 Family
4.3. Analysis of Cis-Acting Elements of PeSnRK2s
4.4. Multispecies Collinearity, Gene Duplication Types and Chromosome Localization of PeSnRK2s
4.5. Phylogenetic Analysis of PeSnRK2s
4.6. Prediction of the Secondary and Three-Dimensional Structures of PeSnRK2 Proteins
4.7. Transcriptome Sequencing and Data Analysis of PeSnRK2s
4.8. Subcellular Localization of PeSnRK2.6
4.9. Predicting Protein–Protein Interactions of PeSnRK2.6
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebrechorkos, S.H.; Sheffield, J.; Vicente-Serrano, S.M.; Funk, C.; Miralles, D.G.; Peng, J.; Dyer, E.; Talib, J.; Beck, H.E.; Singer, M.B.; et al. Warming accelerates global drought severity. Nature 2025, 642, 628–635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosso, L.; Cantamessa, S.; Bergante, S.; Biselli, C.; Fricano, A.; Chiarabaglio, P.M.; Gennaro, M.; Nervo, G.; Secchi, F.; Carra, A. Responses to Drought Stress in Poplar: What Do We Know and What Can We Learn? Life 2023, 13, 533. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeon, B.W.; Kim, J. Signaling peptides regulating abiotic stress responses in plants. Front. Plant Sci. 2021, 12, 704490. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621, Correction in J. Exp. Bot. 2020, 71, 1647. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.; Yang, N.; Cao, W.; Li, Y.; Peng, Y.; Wei, X.; Ma, B.; Ma, F.; Ruan, Y.; et al. Apple vacuolar sugar transporters regulated by MdDREB2A enhance drought resistance by promoting accumulation of soluble sugars and activating ABA signaling. Hortic. Res. 2024, 11, uhae251. [Google Scholar] [CrossRef] [PubMed]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Liu, X.D.; Waseem, M.; Guang-Qian, Y.; Alabdallah, N.M.; Jahan, M.S.; Fang, X.W. ABA activated SnRK2 kinases: An emerging role in plant growth and physiology. Plant Signal. Behav. 2022, 17, 2071024. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Wang, X.Q.; Watson, M.B.; Assmann, S.M. Regulation of Abscisic Acid-Induced Stomatal Closure and Anion Channels by Guard Cell AAPK Kinase. Science 2000, 287, 300–303. [Google Scholar] [CrossRef]
- Umezawa, T.; Yoshida, R.; Maruyama, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. SRK2C, a SNF1-related protein kinase 2, improves drought tolerance by controlling stress-responsive gene expression in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 17306–17311. [Google Scholar] [CrossRef]
- Zhang, Z.; Ali, S.; Zhang, T.; Wang, W.; Xie, L. Identification, Evolutionary and Expression Analysis of PYL-PP2C-SnRK2s Gene Families in Soybean. Plants 2020, 9, 1356. [Google Scholar] [CrossRef]
- Fujii, H.; Verslues, P.E.; Zhu, J.K. Identification of Two Protein Kinases Required for Abscisic Acid Regulation of Seed Germination, Root Growth, and Gene Expression in Arabidopsis. Plant Cell 2007, 19, 485–494. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Z.; Ren, Z.; Zhi, L.; Yao, B.; Su, C.; Liu, L.; Li, X. SCFAtPP2-B11 modulates ABA signaling by facilitating SnRK2.3 degradation in Arabidopsis thaliana. PLOS Genet. 2017, 13, e1006947. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.K. In vitro reconstitution of an abscisic acid signaling pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Wang, X.J.; Ding, W.H.; Zhu, S.Y.; Zhao, R.; Zhang, Y.X.; Xin, Q.; Wang, X.F.; Zhang, D.P. Identification of an important site for function of the type 2C protein phosphatase ABI2 in abscisic acid signaling in Arabidopsis. J. Exp. Bot. 2011, 62, 5713–5725. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, E.M.; Chan, C.W.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK Superfamily of Protein Kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.; Kagaya, Y.; Hattori, T. Differential Activation of the Rice Sucrose Nonfermenting1–Related Protein Kinase2 Family by Hyperosmotic Stress and Abscisic Acid. Plant Cell 2004, 16, 1163–1177. [Google Scholar] [CrossRef]
- Umar, A.W.; Ahmad, N.; Hussain, H.; Zeeshan, M.; Xu, M. Deciphering auxin-regulated stomatal development: Novel insights into phytochrome-interacting factors, FOUR LIPS, and SNF1-related kinase interactions. Plant Sci. 2025, 362, 112815. [Google Scholar] [CrossRef]
- Soma, F.; Mogami, J.; Yoshida, T.; Abekura, M.; Takahashi, F.; Kidokoro, S.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-unresponsive SnRK2 protein kinases regulate mRNA decay under osmotic stress in plants. Nat. Plants 2017, 3, 16204. [Google Scholar] [CrossRef]
- Szymańska, K.P.; Polkowska-Kowalczyk, L.; Lichocka, M.; Maszkowska, J.; Dobrowolska, G. SNF1-Related Protein Kinases SnRK2.4 and SnRK2.10 Modulate ROS Homeostasis in Plant Response to Salt Stress. Int. J. Mol. Sci. 2019, 20, 143. [Google Scholar] [CrossRef]
- Julkowska, M.M.; McLoughlin, F.; Galvan-Ampudia, C.S.; Rankenberg, J.M.; Kawa, D.; Klimecka, M.; Haring, M.A.; Munnik, T.; Kooijman, E.E.; Testerink, C. Identification and functional characterization of the Arabidopsis Snf1-related protein kinase SnRK2.4 phosphatidic acid-binding domain. Plant Cell Environ. 2015, 38, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, Y.J.; Seo, P.J.; Kim, J.H.; Sim, H.J.; Kim, S.G.; Park, C.M. Systemic Immunity Requires SnRK2.8-Mediated Nuclear Import of NPR1 in Arabidopsis. Plant Cell 2015, 27, 3425–3438. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xue, L.; Batelli, G.; Lee, S.; Hou, Y.J.; Van Oosten, M.J.; Zhang, H.; Tao, W.A.; Zhu, J.K. Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc. Natl. Acad. Sci. USA 2013, 110, 11205–11210. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, X.; Crosley, R.A.; Greenwalt, S.A.; Sun, Y.; Blakeslee, B.; Wang, L.; Ni, L.; Sopko, M.S.; Yao, C.; et al. The Protein Kinase SnRK2.6 Mediates the Regulation of Sucrose Metabolism and Plant Growth in Arabidopsis. Plant Physiol. 2010, 153, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Hobo, T.; Ichimura, K.; Mizoguchi, T.; Takahashi, F.; Aronso, J.; Ecker, J.R.; Shinozaki, K. ABA-Activated SnRK2 Protein Kinase is Required for Dehydration Stress Signaling in Arabidopsis. Plant Cell Physiol. 2002, 43, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 Protein Kinase Mediates the Regulation of Stomatal Aperture by Abscisic Acid and Acts Upstream of Reactive Oxygen Species Production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef]
- Han, Y.; Dang, R.; Li, J.; Jiang, J.; Zhang, N.; Jia, M.; Wei, L.; Li, Z.; Li, B.; Jia, W. SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE2.6, an Ortholog of OPEN STOMATA1, Is a Negative Regulator of Strawberry Fruit Development and Ripening. Plant Physiol. 2015, 167, 915–930. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, J.; Duan, W.; Wang, Z.; Song, X.; Hou, X. Molecular evolution, characterization, and expression analysis of SnRK2 gene family in Pak-choi (Brassica rapa ssp. chinensis). Front. Plant Sci. 2015, 6, 879. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, X.; Yang, Z.; Zhang, C.; Zhao, G.; Chen, E.; Liu, J.; Zhang, X.; Li, F. Genome-wide identification and characterization of SnRK2 gene family in cotton (Gossypium hirsutum L.). BMC Genet. 2017, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Mao, J.; Yang, H.; Khan, A.; Fan, A.; Liu, S.; Zhang, J.; Wang, D.; Gao, H.; Zhang, J. Sucrose non-ferment 1 related protein kinase 2 (SnRK2) genes could mediate the stress responses in potato (Solanum tuberosum L.). BMC Genet. 2017, 18, 41. [Google Scholar] [CrossRef]
- Long, T.; Xu, B.; Hu, Y.; Wang, Y.; Mao, C.; Wang, Y.; Zhang, J.; Liu, H.; Huang, H.; Liu, Y.; et al. Genome-wide identification of ZmSnRK2 genes and functional analysis of ZmSnRK2.10 in ABA signaling pathway in maize (Zea mays L.). BMC Plant Biol. 2021, 21, 309. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Mao, X.; Jing, R.; Jia, H. Differential Activation of the Wheat SnRK2 Family by Abiotic Stresses. Front. Plant Sci. 2016, 7, 420. [Google Scholar] [CrossRef]
- Li, C.; Nong, Q.; Xie, J.; Wang, Z.; Liang, Q.; Solanki, M.K.; Malviya, M.K.; Liu, X.; Li, Y. Molecular Characterization and Co-expression Analysis of the SnRK2 Gene Family in Sugarcane (Saccharum officinarum L.). Sci. Rep. 2017, 7, 17659. [Google Scholar] [CrossRef]
- Tuskan, G.A.; Difazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef]
- Qiu, Q.; Ma, T.; Hu, Q.; Liu, B.; Wu, Y.; Zhou, H.; Wang, Q.; Wang, J.; Liu, J. Genome-scale transcriptome analysis of the desert poplar, Populus euphratica. Tree Physiol. 2011, 31, 452–461. [Google Scholar] [CrossRef]
- Li, Q.; Shen, C.; Zhang, Y.; Zhou, Y.; Niu, M.; Wang, H.L.; Lian, C.; Tian, Q.; Mao, W.; Wang, X.; et al. PePYL4 enhances drought tolerance by modulating water-use efficiency and ROS scavenging in Populus. Tree Physiol. 2023, 43, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, H.G.; Wang, J.; Wang, H.L.; He, F.; Su, Y.; Zhang, Y.; Feng, C.H.; Niu, M.; Li, Z.; et al. ABF3 enhances drought tolerance via promoting ABA-induced stomatal closure by directly regulating ADF5 in Populus euphratica. J. Exp. Bot. 2020, 71, 7270–7285. [Google Scholar] [CrossRef] [PubMed]
- Nekrutenko, A.; Makova, K.D.; Li, W.H. The K(A)/K(S) ratio test for assessing the protein-coding potential of genomic regions: An empirical and simulation study. Genome Res. 2002, 12, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Li, C.; Ma, J.; Liu, J.; Zhu, X.; Li, J.; He, F.; Yang, C. Genome-Wide Identification and Expression Profile Analysis of the SnRK2 Gene Family in Nicotiana tabacum. Biochem. Genet. 2022, 60, 1511–1526. [Google Scholar] [CrossRef]
- Ghillebert, R.; Swinnen, E.; Wen, J.; Vandesteene, L.; Ramon, M.; Norga, K.; Rolland, F.; Winderickx, J. The AMPK/SNF1/SnRK1 fuel gauge and energy regulator: Structure, function and regulation. FEBS J. 2011, 278, 3978–3990. [Google Scholar] [CrossRef]
- Kulik, A.; Wawer, I.; Krzywińska, E.; Bucholc, M.; Dobrowolska, G. SnRK2 Protein Kinases—Key Regulators of Plant Response to Abiotic Stresses. OMICS A J. Integr. Biol. 2011, 15, 859–872. [Google Scholar] [CrossRef]
- Fujii, H.; Zhu, J.K. Osmotic stress signaling via protein kinases. Cell. Mol. Life Sci. 2012, 69, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Khan, M.J.; Awan, H.M.; Akhtar, M.N.; Bibi, N.; Sughra, K.; Khan, M.R.; Ahmad, R.; Ibrahim, M.; Hussain, J.; et al. Genome-wide identification and expression analysis of SnRK2 gene family in mungbean (Vigna radiata) in response to drought stress. Crop Pasture Sci. 2020, 71, 469–476. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Su, H.; Liu, X.; Wang, Y.; Hong, G. Genome-wide analysis of PYL-PP2C-SnRK2s family in Camellia sinensis. Bioengineered 2020, 11, 103–115. [Google Scholar] [CrossRef]
- Saha, J.; Chatterjee, C.; Sengupta, A.; Gupta, K.; Gupta, B. Genome-wide analysis and evolutionary study of sucrose non-fermenting 1-related protein kinase 2 (SnRK2) gene family members in Arabidopsis and Oryza. Comput. Biol. Chem. 2014, 49, 59–70. [Google Scholar] [CrossRef]
- Xu, M.R.; Huang, L.Y.; Zhang, F.; Zhu, L.H.; Zhou, Y.L.; Li, Z.K. Genome-wide phylogenetic analysis of stress-activated protein kinase genes in rice (OsSAPKs) and expression profiling in response to Xanthomonas oryzae pv. oryzicola infection. Plant Mol. Biol. Rep. 2013, 31, 877–885. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Xu, C.; Wang, P.; Sun, W.; Yin, T.; Zhuge, Q. Heterologous overexpression of the Arabidopsis SnRK2.8 gene enhances drought and salt tolerance in Populus × euramericana cv ‘Nanlin895’. Plant Biotechnol. Rep. 2019, 13, 245–261. [Google Scholar] [CrossRef]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant. 2012, 147, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, J.; Hu, F.; Qin, C.; Xu, X.; Hu, K. The SnRK2 family in pepper (Capsicum annuum L.): Genome-wide identification and expression analyses during fruit development and under abiotic stress. Genes Genom. 2020, 42, 1117–1130. [Google Scholar] [CrossRef]
- Yoo, M.; Ma, T.; Zhu, N.; Liu, L.; Harmon, A.C.; Wang, Q.; Chen, S. Genome-wide identification and homeolog-specific expression analysis of the SnRK2 genes in Brassica napus guard cells. Plant Mol. Biol. 2016, 91, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lu, F.; Feng, W.; Liu, Y.; Cao, Y.; Li, W.; Fu, F.; Yu, H. Genome-Wide Identification and Expression Analyses of AnSnRK2 Gene Family under Osmotic Stress in Ammopiptanthus nanus. Plants 2021, 10, 882. [Google Scholar] [CrossRef]
- Hussain, Q.; Zheng, M.; Chang, W.; Ashraf, M.F.; Khan, R.; Asim, M.; Riaz, M.W.; Alwahibi, M.S.; Elshikh, M.S.; Zhang, R.; et al. Genome-Wide Identification and Expression Analysis of SnRK2 Gene Family in Dormant Vegetative Buds of Liriodendron chinense in Response to Abscisic Acid, Chilling, and Photoperiod. Genes 2022, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.L.; Li, Y.Y.; Zhang, H.J.; Zhang, H.J.; Liu, X.L.; Wang, J.Y.; Chang, X.P.; Mao, X.G.; Jing, R.L. TaSnRK2.4 is a vital regulator in control of thousand-kernel weight and response to abiotic stress in wheat. J. Integr. Agric. 2021, 20, 46–54. [Google Scholar] [CrossRef]
- Hong, Y.; Ahmad, N.; Zhang, J.; Lv, Y.; Zhang, X.; Ma, X.; Xiuming, L.; Na, Y. Genome-wide analysis and transcriptional reprogrammings of MYB superfamily revealed positive insights into abiotic stress responses and anthocyanin accumulation in Carthamus tinctorius L . Mol. Genet. Genom. 2022, 297, 125–145. [Google Scholar] [CrossRef]
- Jakubowicz, M.; Nowak, W.; Gałgański, Ł.; Babula-Skowrońska, D.; Kubiak, P. Expression profiling of the genes encoding ABA route components and the ACC oxidase isozymes in the senescing leaves of Populus tremula. Plant Physiol. 2020, 248, 153143. [Google Scholar] [CrossRef]
- Liang, S.; Lu, K.; Wu, Z.; Jiang, S.; Yu, Y.; Bi, C.; Xin, Q.; Wang, X.; Zhang, D. A link between magnesium-chelatase H subunit and sucrose nonfermenting 1 (SNF1)-related protein kinase SnRK2.6/OST1 in Arabidopsis guard cell signaling in response to abscisic acid. J. Exp. Bot. 2015, 66, 6355–6369. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, S.; Liu, X.; He, J.; Cheng, L.; Duan, M.; Liu, H.; Wang, W.; Yu, Y. Overexpression of CsSnRK2.5 increases tolerance to drought stress in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 150, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, M.; Shen, J.; Chen, D.; Zheng, Y.; Zhang, W. ZmOST1 mediates abscisic acid regulation of guard cell ion channels and drought stress responses. J. Integr. Plant Biol. 2018, 61, 478–491. [Google Scholar] [CrossRef]
- Horvat, B.; Shikakura, Y.; Ohtani, M.; Demura, T.; Kikuchi, A.; Watanabe, K.N.; Oguchi, T. Heterogeneous Expression of Arabidopsis Subclass II of SNF1-Related Kinase 2 Improves Drought Tolerance via Stomatal Regulation in Poplar. Life 2024, 14, 161. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zhang, J.; Ma, X.; Li, Y.; Li, M.; Wang, D.; Kang, M.; Wu, H.; Yang, Y.; et al. Improved genome assembly provides new insights into genome evolution in a desert poplar (Populus euphratica). Mol. Ecol. Resour. 2020, 20, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wu, H.; Chen, Y.; Li, X.; Hou, J.; Lu, J.; Wei, S.; Dai, X.; Olson, M.S.; Liu, J.; et al. Evidences for a role of two Y-specific genes in sex determination in Populus deltoides. Nat. Commun. 2020, 11, 5893. [Google Scholar] [CrossRef]
- Zhou, R.; Macaya-Sanz, D.; Carlson, C.H.; Schmutz, J.; Jenkins, J.W.; Kudrna, D.; Sharma, A.; Sandor, L.; Shu, S.; Barry, K.; et al. A willow sex chromosome reveals convergent evolution of complex palindromic repeats. Genome Biol. 2020, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, J.; Qu, W.; Han, X.; Qiu, C.; Gai, Z.; Zhai, J.; Qin, R.; Liu, H.; Wu, Z.; et al. Genome-wide analysis of R2R3-MYB transcription factors reveals their differential responses to drought stress and ABA treatment in desert poplar (Populus euphratica). Gene 2023, 855, 147124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Liu, Z.; Wang, X.; Li, X.; Shan, G. A simple and versatile paper-based electrochemiluminescence biosensing platform for hepatitis B virus surface antigen detection. Biochem. Eng. J. 2018, 129, 1–6. [Google Scholar] [CrossRef]
- Cheng, Y.; Hong, X.; Zhang, L.; Yang, W.; Zeng, Y.; Hou, Z.; Yang, Z.; Yang, D. Transcriptomic analysis provides insight into the regulation mechanism of silver ions (Ag+) and jasmonic acid methyl ester (MeJA) on secondary metabolism in the hairy roots of Salvia miltiorrhiza Bunge (Lamiaceae). Med. Plant Biol. 2023, 2, 3. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Gao, J.; Wang, P.; Hu, T.; Wang, Z.; Hou, Y.J.; Wan, Y.; Liu, W.; Xie, S.; et al. Arabidopsis Duodecuple Mutant of PYL ABA Receptors Reveals PYL Repression of ABA-Independent SnRK2 Activity. Cell Rep. 2018, 23, 3340–3351.e5. [Google Scholar] [CrossRef] [PubMed]








| Gene ID | Number of Amino Acids | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity (GRAVY) | Prediction of Subcellular Localization |
|---|---|---|---|---|---|---|---|
| PeuTF05G01370.1 | 581 | 65,986.13 | 9.57 | 35.69 | 82.03 | −0.508 | Cytoplasm |
| PeuTF04G01285.1 | 356 | 40,929.4 | 5.69 | 49.81 | 82.39 | −0.496 | Cytoskeleton |
| PeuTF04G01353.1 | 363 | 41,201.82 | 4.86 | 40.24 | 86.97 | −0.325 | Cytoplasm |
| PeuTF19G00050.1 | 361 | 41,608.17 | 5.66 | 50.77 | 83.99 | −0.516 | Cytoskeleton |
| PeuTF05G01129.1 | 362 | 41,140.95 | 4.86 | 40.85 | 86.13 | −0.277 | Chloroplast |
| PeuTF03G00118.1 | 357 | 41,088.73 | 6.09 | 46.02 | 78.94 | −0.527 | Cytoskeleton |
| PeuTF07G01031.1 | 340 | 38,669.79 | 5.20 | 44.09 | 85.74 | −0.370 | Cytoplasm |
| PeuTF03G00739.1 | 338 | 38,204.71 | 5.80 | 37.63 | 87.07 | −0.300 | Cytoskeleton |
| PeuTF02G00789.1 | 412 | 46,602.21 | 5.42 | 40.61 | 97.21 | −0.156 | Cytoplasm |
| PeuTF09G01080.1 | 362 | 40,992.69 | 4.76 | 40.71 | 89.67 | −0.246 | Cytoskeleton |
| PeuTF04G02065.1 | 363 | 41,612.34 | 5.61 | 47.11 | 84.05 | −0.499 | Cytoskeleton |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Li, J.; Song, T.; Miao, D.; Ning, Q.; Zhang, X.; Gai, Z.; Li, Z.; Jiao, P.; Wu, Z. Genome-Wide Identification of the SnRK2 Gene Family and Its Response to Abiotic Stress in Populus euphratica. Int. J. Mol. Sci. 2025, 26, 10750. https://doi.org/10.3390/ijms262110750
Jin H, Li J, Song T, Miao D, Ning Q, Zhang X, Gai Z, Li Z, Jiao P, Wu Z. Genome-Wide Identification of the SnRK2 Gene Family and Its Response to Abiotic Stress in Populus euphratica. International Journal of Molecular Sciences. 2025; 26(21):10750. https://doi.org/10.3390/ijms262110750
Chicago/Turabian StyleJin, Hongyan, Jing Li, Tongrui Song, Donghui Miao, Qi Ning, Xiao Zhang, Zhongshuai Gai, Zhijun Li, Peipei Jiao, and Zhihua Wu. 2025. "Genome-Wide Identification of the SnRK2 Gene Family and Its Response to Abiotic Stress in Populus euphratica" International Journal of Molecular Sciences 26, no. 21: 10750. https://doi.org/10.3390/ijms262110750
APA StyleJin, H., Li, J., Song, T., Miao, D., Ning, Q., Zhang, X., Gai, Z., Li, Z., Jiao, P., & Wu, Z. (2025). Genome-Wide Identification of the SnRK2 Gene Family and Its Response to Abiotic Stress in Populus euphratica. International Journal of Molecular Sciences, 26(21), 10750. https://doi.org/10.3390/ijms262110750






