Abstract
Parasite control in aquaculture faces challenges primarily due to the drug resistance of traditional chemical treatments, as well as environmental pollution and toxicity. Aquaculture is among the fastest-growing food-producing sectors worldwide, yet parasite infections remain a significant challenge to productivity and sustainability. Emerging methods such as natural products, gene editing, immunotherapy, and auxiliary technologies like nanotechnology and biosensors are becoming alternative strategies for sustainable parasite control. These methods show significant potential, particularly in preventing drug resistance and reducing environmental impact. However, these approaches remain at an early research stage, with issues such as unstable efficacy, limited validation in field conditions and uncertain long-term safety hindering their translation into practice. This review synthesizes current advances, highlights these knowledge and application gaps, and outlines future directions for developing more reliable and sustainable parasite management strategies in aquaculture.
1. Introduction
According to data provided by Food and Agriculture Organization (FAO, 2024) [1], aquatic animal foods are an important source of high-quality protein, providing about 15% of the world population’s intake of animal proteins in 2021 and provided about 3.3 billion people with at least 20% of their average per capita intake of animal proteins. In 2022, global aquaculture production reached 130.9 million tonnes (≈USD 313 billion) and accounted for around 59% of total fisheries and aquaculture output. Focusing on aquatic animals, farmed production represented about 51% of the total (94.4 of 185.4 million tonnes) and supplied over 57% of aquatic animal foods for direct human consumption. This highlights the importance of aquatic foods as a key part of the global diet. However, parasite infections represent a significant issue in aquaculture, posing risks not only to food safety but also to the economic stability of industry. Many widely consumed fish are known hosts for certain parasites. For example, Bothriocephalus acheilognathi in grass carp (Ctenopharyngodon idella) can cause abdominal pain and hinder nutrient absorption in humans [2,3,4], while Dibothriocephalus dendriticum and Dibothriocephalus latus in trout and perch may lead to symptoms such as nausea, abdominal pain, vitamin B12 deficiency, diarrhea, and weight loss [5,6,7,8,9,10,11]. Globally, diseases caused by Diphyllobothriidea affect as many as 20 million people [7]. These fish-borne parasitic infections represent a significant food safety concern and are considered one of the major global foodborne zoonoses, with particularly high prevalence in some regions [12,13,14]. In aquaculture systems, several parasites have been documented to cause extremely high mortality and cost increases. For instance, Oreochromis niloticus in Upper Egypt showed an overall parasite prevalence of about 82% [15], while outbreaks of Ichthyophthirius multifiliis in freshwater aquaculture can cause severe mortality [16], with one study in India reporting up to 90% mortality in an affected farm [17]. In terms of economic impact, in marine systems, infestations of sea lice (Lepeophtheirus salmonis) impose a substantial economic burden on salmon aquaculture, with global control and production losses estimated at around £700 million annually and approximately £65 million per year in Scotland alone [18]. Farm-level assessments further indicate that sea lice management accounts for about 9% of the total farm-gate value in major producing countries [19]. Global annual losses from parasitic infections in farmed fish are estimated to range from approximately US$1.05 billion to US$9.58 billion, according to a report [20]. This represents roughly 0.3–3% of the total annual value of global aquaculture production, which may appear modest, yet it still translates into losses of hundreds of millions to nearly ten billion USD each year and should therefore be regarded as a significant concern for sustainable aquaculture development. And more recent evaluations indicate that the economic burden continues to rise, driven by increasing treatment costs and expanding parasite distribution in major aquaculture sectors [21,22,23].
In aquaculture, chemical treatments remain a primary approach for parasite control. For example, emamectin benzoate is used to treat L. salmonis infections and can reduce parasite loads by about 35% [24], hydrogen peroxide baths achieve approximately 74% delousing efficiency [24], and praziquantel shows nearly 100% therapeutic efficacy against fish tapeworms [25], and its removal rate for monogeneans often exceeds 80% [26]. These drugs can effectively reduce parasite loads in the short term, but long-term and repeated use has led to increased drug resistance and posed risks to the environment and food safety [27,28]. Residues of emamectin benzoate and fluazuron have been repeatedly detected in sediments and waters near Norwegian fish farms, with concentrations occasionally exceeding environmental quality standards [29]. Emamectin benzoate can persist in sediments for approximately 5–6 months (with a half-life of about 150–400 days), while fluazuron and teflubenzuron can still be detected 8–22 months after treatment cessation [29]. These chemicals can inhibit chitin synthesis in non-target crustaceans, causing molting disorders and developmental deformities in organisms such as shrimp and crabs, thereby posing long-term ecological risks [29]. In addition, chemical residues may also enter the food chain. A survey of farmed fish in China revealed that approximately 24–40% of edible fish samples from 19 provinces contained detectable antibiotic residues, reflecting the widespread use of chemical drugs and raising concerns about food safety [30]. Rising drug resistance and environmental concerns have driven a shift in L. salmonis control within the Norwegian salmon industry from chemical to mainly non-chemical methods in 2016 and 2017 [31]. Among these approaches, thermal and mechanical treatments can remove up to 70–80% of L. salmonis, but because they rely on temperature stimulation, brushing, and water jets, suboptimal operating conditions often lead to stress responses and skin lesions in salmon, with mortality rates of about 25–31% [31,32]. In contrast, freshwater and low-salinity baths are relatively mild osmotic treatments [33]. They are effective against common external parasites of marine fish, including L. salmonis [31], Neoparamoeba perurans [34], and Neobenedenia [35]. For Norwegian salmon, these treatments can remove 82–100% and about 95% of L. salmonis, respectively, although they are labor-intensive, consume large amounts of water, and may cause temporary osmotic stress [31]. These fish graze on visible, relatively large ectoparasites such as L. salmonis [36] and certain monogeneans [37], thereby helping to reduce parasite burdens. However, their mortality rates are high and are greatly influenced by temperature and rearing conditions. Overall, these non-chemical treatments help mitigate drug resistance and environmental contamination, but their applicability remains limited and, compared with chemical therapies, they may have more pronounced negative effects on fish growth and welfare [32,38].Given the limitations of chemical treatments, it is essential to explore alternative approaches for effective and sustainable parasite control in aquaculture. Promising alternatives include the use of natural plant extracts, which have shown potential for therapeutic applications with minimal environmental impact [39,40,41]. Predatory fungi offer another approach, targeting intestinal parasites within hosts without introducing synthetic chemicals into the ecosystem [42]. Advances in genetic technologies, particularly CRISPR, provide opportunities to enhance the innate resistance of aquaculture species against parasitic infections [43,44]. Furthermore, integrating these treatments with sensitive biosensors could enable real-time monitoring and timely interventions, optimizing control strategies [45].
This review summarizes current methods for parasite control and examines their potential applications in aquaculture, aiming to provide valuable insights that enhance food safety and sustainability in the aquaculture industry.
2. Natural-Based Approaches
2.1. Plant-Based Treatments
The use of herbal remedies in disease treatment has received increasing attention, particularly as an alternative approach to address microbial and parasitic infections, contributing to sustainable aquaculture practices [46,47]. Most of the plant extracts and secondary metabolites such as alkaloids, terpenoids and phenolic compounds are potent bioactive substances that can be used as antibiotics for disease treatment [40,48]. Herbal compounds, unlike chemical drugs, are typically mixtures of multiple active components. They often inhibit the physiological processes of parasites through synergistic effects, making it challenging for parasites to develop resistance to multiple compounds simultaneously and reducing the likelihood of resistance development [49]. Meanwhile, medicinal plants are more environmentally friendly, have lower side-effect profiles, are biodegradable, and do not accumulate as residues in animal tissues or the environment [50,51,52,53,54]. Moreover, plant-based medicines are often more cost-effective than chemical drugs, readily cultivable and accessible, making them extensively utilized in underdeveloped countries [47,55,56].
Many plant-based therapies have been proven effective for controlling parasites in aquaculture and can be administered through oral delivery or bath treatments [49]. Table 1 summarizes examples of treatments using natural drugs, including their active compounds, target parasites, effective concentrations, and therapeutic effects. These examples highlight the diversity and potential of herbal remedies in controlling aquaculture parasites. Table 1 shows that essential oils (EOs) are highly effective in killing Euclinostomum heterostomum. However, the authors noted that plant-derived EOs contain unstable compounds, which can potentially cause undesirable side effects in fish. They emphasized the importance of carefully distinguishing harmful compounds from beneficial ones during the refining process to achieve better results [57]. The treatment of Gyrodactylus spp. with saponins extracted from Dioscorea collettii var. hypoglauca (Table 1) was found to be effective only for internal parasites, showing no efficacy against external ones [58]. In laboratory studies, Allium sativum and Terminalia catappa were also highly effective in treating Trichodina sp. (Table 1). However, their effects were later found to be short-term, as the parasites reappeared after two weeks. Further research is needed to explore their potential for long-term control [59].
Moreover, the types and levels of bioactive substances in plants vary with their environment or health, resulting in differences in therapeutic effects [40]. Additionally, the low yield of active compounds in plants makes some natural drugs difficult to apply directly in aquaculture [60]. Although some studies suggest that herbal remedies have low toxicity to fish, research is still limited. Fish tolerance to these treatments also varies with age, species, and health status, making further studies essential to ensure their safe application in different fish species [49,61,62]. Herbal remedies are safer and more reliable than chemical pesticides. They have gradually replaced synthetic drugs and hormones as feed additives, but there is still significant potential for improvement [39]. To further their promising application in aquaculture, future research should prioritize long-term control mechanisms, toxicity evaluation, and production optimization, aiming to advance their widespread adoption in global sustainable aquaculture. And given the specificity of herbal treatments against parasites, efforts should also be directed toward the most common parasites in aquaculture and first address the most urgent challenges. Additionally, exploring common and easily accessible plants as herbal sources could help reduce costs and support the sustainable development of aquaculture.

Table 1.
Herbal Treatment of Aquaculture Parasites.
Table 1.
Herbal Treatment of Aquaculture Parasites.
| Parasites | Fish Species | Drugs | Usage | Effect | References |
|---|---|---|---|---|---|
| Euclinostomum heterostomum | Tilapia zillii | Verbesina alternifolia EOs | 24 h/600 mg/L | 100% anthelmintic | [63] |
| Mentha piperita EOs | 24 h/1000 mg/L | 50% anthelmintic | |||
| Dactylogyrus spp. | Poecilia reticulata Oreochromis niloticus | Lippia origanoides EOs | 5 min/100 mg/L | 100% anthelmintic | [63] |
| Lippia sidoides EOs | 5 min/100 mg/L | 100% anthelmintic | |||
| Gyrodactylus spp. | Poecilia reticulata Oreochromis niloticus | Methanol extract of Dioscorea collettii var. hypoglauca | 10 mg/L | 100% anthelmintic | [58] |
| Dioscin isolated from D. collettii var. hypoglauca | 2 h/0.6 mg/L | 100% anthelmintic (in vivo) | |||
| Neoechinorhynchus buttnerae | Tambaqui Colossoma macropomum | Oleoresins from Copaifera duckei | 24 h/0.1868 mg/mL | 100% anthelmintic | [62] |
| Oleoresins from Copaifera pubiflora | 24 h/0.1868 mg/mL | 100% anthelmintic | |||
| Oleoresins from Copaifera reticulata | 24 h/0.1868 mg/mL | 100% anthelmintic | |||
| Neobenedenia girellae | marine fishes (in vitro assay) | Pomegranate extract | 8 h/62.5 mg/L | 100% anthelmintic | [64] |
| Trichodina sp. | Oreochromis niloticus | Allium sativum | 2 d/800 ppm | 100% anthelmintic | [59] |
| Terminalia catappa | 2 d/800 ppm | 100% anthelmintic | |||
| Gyrodactylus | Oreochromis niloticus | crushed garlic cloves (Allium sativum) | 300 mg/L | 68% elimination of disease | [65] |
| garlic oil (Allium sativum) | 4 h/2, 2.5 and 3 ppt | 100% anthelmintic | |||
| Gyrodactyluskobayashii | Carassius auratus (model fish) | plant-derived plumbagin | 30–60 min/0.4–0.7 mg/L | 100% anthelmintic | [60] |
2.2. Microbial-Based Approaches
Probiotics
Probiotics are live microorganisms that provide health benefits and are widely used in animal production and human health protection [66,67,68]. Many probiotic products have been commercially used in aquaculture as feed additives and alternatives to antibiotics [68,69,70]. In aquaculture, where farmed animals closely interact with the microbial communities of their surrounding water, the concept of probiotics has been broadened and is sometimes described as microbial “water additives” [71]. Verschuere et al. [72] defined aquaculture probiotics as “live microbial adjuncts which have a beneficial effect on the host by modifying the host-associated or ambient microbial community, by ensuring improved use of the feed or enhancing its nutritional value, by enhancing the host response towards diseases, or by improving the quality of its environment.” In aquaculture applications, inactivated microorganisms or their cellular components have also been used as additives with probiotic-like functions to improve host health or the microbial balance of the rearing environment [73]. These preparations are now more accurately referred to as parabiotics and postbiotics. Parabiotics describe non-viable or inactivated microbial cells that still retain functional activity, whereas postbiotics refer to cell-free metabolites, secretions, or other microbial by-products that exert beneficial effects on the host [74]. Because both parabiotics and postbiotics are essentially composed of molecules rather than live organisms, they can successfully withstand adverse digestive and physiological conditions and may serve as suitable alternatives to traditional probiotics in aquaculture applications [74].
Probiotics include various types of bacteria, bacteriophages, microalgae, and yeasts [75], which can enhance fish resistance to pathogens, protect their health, or promote growth [76]. The use of probiotics to combat bacterial and viral infections in aquaculture is currently the most extensively studied [77,78]. Extensive research has proven that fish treated with probiotics have higher survival rates after pathogen exposure [79,80,81]. Feed additives and direct addition to water are the two main approaches to delivering aquatic probiotics [82,83]. Their modes of action are multifactorial, involving mechanisms such as competitive exclusion, metabolite inhibition, and immune modulation. Probiotics compete with pathogens for attachment sites and nutrients, preventing their colonization in the host. They also secrete metabolites such as antimicrobial peptides, lactic acid, and bacteriocins, which directly inhibit pathogen growth. Furthermore, probiotics enhance the resistance of the host to infections by stimulating the innate immune system through the activation of inflammatory cytokines or by boosting macrophage activity [76,77,84,85,86].
The use of probiotics in treating aquaculture parasites is less common, as they are less effective against ectoparasites and endoparasites compared to their use in treating bacterial pathogens [69,77,87]. The mechanisms by which probiotics treat parasitic infections remain largely unknown and may be similar to those used in resisting bacterial infections [77,87]. However, due to the size differences between parasites and microorganisms, competition for nutrients may only serve as a mechanism against smaller parasitic pathogens, such as Saprolegnia sp. [77,88]. In addition, probiotics indirectly reduce the risk of parasite transmission by regulating microbial communities in the water. This effect may involve depleting nutrients in the water, degrading pollutants, and adjusting pH levels to create an environment unfavorable for pathogen survival [77,82,83,89,90].
Although there is less research on treating parasitic infections with probiotics, it still holds great potential [77]. Nurhajati et al. demonstrated that Lactobacillus plantarum FNCC 226 has the ability to inhibit Saprolegnia parasitica A3 both in vivo and in vitro in catfish (Pangasius hypophthalmus) (Table 2) [88]. Bacillus subtilis not only serves as a probiotic additive to promote the growth of fish and shrimp, enhance immune responses, and improve disease resistance but also acts as an antigen delivery vehicle for parasite control (Table 2) [91,92,93]. Recombinant spores expressing Clonorchis sinensis paramyosin (CsPmy) (B. subtilis-CotC-CsPmy) were used to vaccinate grass carp (Ctenopharyngodon idella), significantly reducing the cercarial burden in the fish (Table 2) [85]. According to Pieters et al. [94], Aeromonas sobria GC2, at a concentration of 108 cells per gram of feed, was highly effective in treating Ichthyophthirius multifiliis on fish skin, reducing mortality from 98% to 0% (Table 2). In contrast, another probiotic, Brochotrix thermosphacta BA211, showed no effect [94]. Effective Microorganisms (EMs), as a probiotic formulation, have shown significant improvement in managing Trichodina infections in fish (Table 2). However, their effects last only 15 days, and high concentrations of EMs may cause toxicity, reducing their protective efficacy [95]. Ornamental fish and farmed fish face similar parasitic issues, such as common gill parasites (e.g., Myxobolus sp.) or skin parasites [96]. In a study on Cyprinus carpio infected with Myxobolus sp., a probiotic solution at a concentration of 0.55 mL/30 L effectively reduced gill tissue damage caused by the infection (Table 2). This approach could be extended to food fish suffering from similar infections [96].
The use of microbial probiotics for health maintenance, disease prevention, and control is now widely recognized as a new eco-friendly alternative for sustainable aquaculture [97]. However, the widespread commercialization of probiotics for parasite control in aquaculture still has a long way to go. The selection process for suitable probiotics is challenging, as qualified probiotics must lack plasmid-encoded antibiotic resistance genes, tolerate a wide range of pH levels, and pose no pathogenic risk to humans or animals [76,84]. It is also unclear how long probiotics can survive in the host and whether they can establish long-term colonization to provide sustained health benefits, requiring further research [98]. In many cases, improper probiotic supplementation can also lead to negative effects on fish growth, health and disease resistance [99,100]. Additionally, probiotic products currently sold in some countries are poorly regulated, often lacking specific labeling of species or strains, and suffer from serious quality issues [101]. Despite these challenges, probiotics remain a promising and environmentally friendly approach for parasite management in aquaculture. However, due to the larger size and complex life cycle of parasites, probiotic mechanisms that effectively target bacterial pathogens may not function in the same way against parasites. Further research is needed to elucidate these interactions, which could help refine treatment strategies and address challenges such as short-lived efficacy and potential toxicity at high concentrations. A deeper understanding of these mechanisms may also facilitate the development of more targeted and effective probiotic-based treatments. With continued research and development, overcoming these limitations could lead to more reliable and sustainable probiotic applications in aquaculture.

Table 2.
Probiotic Applications for Parasite Control in Aquaculture.
Table 2.
Probiotic Applications for Parasite Control in Aquaculture.
| Parasites | Fish Species | Probiotic | Probiotic Administration Route | References |
|---|---|---|---|---|
| Saprolegnia parasitica A3 | Pangasius hypophthalamus Sauvage | Lactobacillus plantarum FNCC 226 | Immersion | [88] |
| Clonorchis sinensis | Ctenopharyngodon idella | Bacillus subtilis | Diet | [85] |
| Ichthyophthirius multifiliis | Oncorhynchus mykiss | Aeromonas sobria GC2 | Diet | [94] |
| Trichodina | Oreochromis niloticus | Effective Microorganisms (EMs) | Diet | [95] |
| Myxobolus sp. | Cyprinus carpio | Bacillus spp., Lactobacillus sp. and Nitrosomonas sp. | Immersion | [96] |
3. Genetic and Molecular Therapeutic Approaches
Gene editing technology is a powerful tool for precise manipulation of the genome. Through gene knock-in, knock-out, or modulation of gene expression, we are able to modify specific genes in organisms to improve disease resistance, such as strengthening resistance genes in the host or silencing functional genes associated with pathogens [102].
3.1. RNA Interference (RNAi)
RNA interference (RNAi) is an innate immune mechanism based on double-stranded RNA (dsRNA) within cells [103,104,105]. It was first discovered by Fire et al. (1998), who demonstrated in Caenorhabditis elegans that dsRNA could specifically inhibit gene expression [106]. RNA interference (RNAi) initiates intracellular gene silencing pathways by introducing exogenous or endogenous double-stranded RNA (dsRNA). This technology has been extensively studied in aquaculture. It is considered a promising tool to combat diseases caused by viruses, parasites, and bacteria [105]. RNAi-induced gene silencing is transient, usually suppressing specific gene expression for a limited duration. Exogenous dsRNA is typically degraded or cleared from organisms within a few days to weeks. This reversible nature reduces long-term impacts on both the environment and organisms, making RNAi a promising and safe tool for gene editing [105,107,108,109].
Neobenedenia girellae is a significant parasite that poses a severe threat to marine aquaculture species such as amberjack (Seriola dumerili) and yellowtail (Seriola quinqueradiata). Currently, there are no effective control methods available. Ohashi et al. [110] successfully silenced two key genes, Ngvlg1 and Ngvlg2, associated with germ cell development, by immersing Neobenedenia girellae in a solution containing dsRNA. This resulted in a substantial reduction in the quantity and quality of germ cells in the parasite, significantly lowering egg hatchability. These findings offer promising prospects for developing sterilization-based methods to control parasitic infections [110]. In a study on the parasite Heterosporis saurida infecting lizardfish (Saurida undosquamis), Saleh et al. [111] used RNAi technology to silence two key genes—ATP/ADP antiporter 1 and methionine aminopeptidase II. This significantly reduced the number of spores and decreased initial infection levels by 40% and 60%, respectively. Combined silencing of both genes showed an even greater effect [111]. Salmon is an economically important fish species, and Myxobolus cerebralis is known to cause whirling disease in salmon. Sarker et al. [112] successfully disrupted the parasite’s lifecycle by silencing the MyxSP-1 gene in its intermediate host, Tubifex tubifex. Fish fry exposed to treated T. tubifex showed no signs of infection [112]. Lepeophtheirus salmonis is another significant parasite that affects salmon. Silencing its molting-associated chitinase gene (LsChi2) resulted in abnormal swimming behavior and functional impairments. This demonstrated significant gene regulation effects and highlighted the potential for developing novel therapeutic approaches against L. salmonis [113].
However, the application of RNAi in aquaculture still faces several challenges. In the study by Saleh et al. [111], the knockdown effects on parasite-related genes began to diminish after seven days, indicating the transient nature of RNAi suppression. Further optimization is needed to enhance its durability and practical application potential [111]. Eichner et al. [113] successfully impaired the locomotive function of parasites using RNAi technology. However, the effect was limited to specific developmental stages of the parasite and could not target the entire lifecycle [113]. Specificity and the potential for off-target effects are also challenges for RNAi. Designed dsRNA or siRNA may not perfectly complement the target gene, potentially binding to non-target mRNA and causing unintended gene silencing. This off-target effect, primarily linked to immune issues, can lead to cytotoxicity, disrupt normal cellular functions, and result in metabolic disorders or cell death [105,114]. Introduced dsRNA may be recognized by the immune system as a foreign molecule, triggering unintended inflammatory responses or cytotoxicity, which can harm host health [105,109]. Additionally, naked molecules face difficulties in penetrating cell membranes and are easily degraded by nucleases, making it essential to find effective delivery vectors [109]. Although dsRNA remains in the environment for a limited time, the application of this treatment through bathing or feeding still poses a risk of affecting non-target species in open-water systems. To enable the large-scale application of RNAi in aquaculture, future research should focus on enhancing the stability of dsRNA in the environment, reducing off-target effects, and exploring efficient delivery methods.
3.2. CRISPR/Cas9
CRISPR is a promising gene editing tool to modify disease-resistant genes in aquatic populations. It potentially reduces pathogen numbers and transmission rates, thereby improving the health of aquatic animals [115]. However, its research and application in aquaculture disease control are relatively limited compared to RNAi technology. There are few case studies for controlling parasitic infections.
This genetic modification is permanent, as it directly alters the organism’s genome, potentially resulting in the transmission of the modified genes to future generations [115]. If fish with altered genes escape into the natural environment, they could potentially impact the ecosystem [116]. Gene modification may bring fish specific advantages or disadvantages. For example, fish with enhanced growth hormone-related genes may gain a reproductive advantage in the wild due to their larger size and earlier maturation. However, their hybrid offspring have reduced adaptability to the wild, which results in higher mortality rates during the juvenile stage, leading to a gradual decrease in wild fish populations and disrupting the ecological balance [117,118,119]. This risk can be minimized by introducing sterility genes, which have been successfully tested in Atlantic salmon (Salmo salar) in Norway [120,121].
The acceptance of gene editing technology by the public is an important consideration as concerns about the safety of genetically modified foods prevails. This fast and targeted approach is quite different from the slower, more familiar process of selective breeding [115]. In the future, concerns regarding safety, environmental impact, and other issues need to be addressed through transparent and scientific evidence, along with more effective implementation strategies, to communicate with the public.
4. Immune-Based Approaches
4.1. Vaccine
Vaccination is an eco-friendly and sustainable disease control method with great potential for development in the aquaculture industry [122]. Vaccines can be classified into live, inactivated, and genetically engineered according to its preparation method [123]. Live vaccines use attenuated pathogens to mimic the natural infection process and induce an immune response. Inactivated vaccines use physical or chemical methods to inactivate pathogens while retaining their immunogenicity to stimulate the host’s immune system. Genetically engineered vaccines, including recombinant subunit vaccines, nucleic acid vaccines, protein-engineered vaccines, and others, are designed using modern biotechnologies to express pathogen-specific antigens and trigger protective immune responses [124]. Although live vaccines offer the best protection, they carry the risk of re-establishing virulence. Genetically engineered vaccines are costly to develop and generally provide more limited protection. As a result, in aquaculture, commercially available vaccines are primarily inactivated vaccines, which are safe and effective [124,125].
Currently, most research on aquaculture vaccines focuses on bacterial diseases, with fewer studies on parasitic infections [126]. Taking South Korea as an example, among the 29 commercially approved vaccines by the end of 2019, only 2 can prevent parasitic diseases [127]. However, existing research has provided valuable insights for the development of parasitic vaccines. Cryptocaryon irritans, a major parasite threatening marine aquaculture fish, can cause massive fish mortality and result in significant economic losses [128,129]. Josepriya et al. [129] developed a DNA vaccine combining the heat shock protein 70C (Hsp70C) adjuvant with immobilized antigen (iAg). Experiments showed that the vaccine significantly enhanced the immune response in fish, achieving a 100% relative protection rate after infection. The protective effect gradually declined after 1.5 months, but this study provides an important reference for parasitic vaccine development [129]. In another study, researchers showed that inactivated Cryptocaryon irritans vaccines significantly improved parasite resistance in orange-spotted grouper (Epinephelus coioides) by enhancing both specific and non-specific immune responses. In parasite challenge experiments, the survival rate in the high-dose inactivated vaccine group reached 80%, further confirming the critical role of vaccine dosage in immune protection [130]. Miamiensis avidus is also a harmful parasite to marine fish. Research has shown that inactivated vaccines encapsulated in chitosan microspheres (chitosan MS) provide up to 57.1% relative protection (RPS) in olive flounder (Paralichthys olivaceus) through oral immunization, significantly increasing the antibody levels against M. avidus in the fish [131,132].
Fish vaccines are safe for the ecosystem and have minimal side effects on the organisms [124,133]. However, there are still many challenges in commercial application. A major limitation in parasitic vaccine development is the difficulty in obtaining large quantities of high-quality parasites for antigen production. Many fish parasites have complex, poorly understood life cycles, making in vitro culturing a significant challenge [134]. This limitation constrains not only live and inactivated vaccine development but also antigen characterization and immune response studies. Although genetically engineered vaccines can be developed as alternatives, their protective efficacy is weaker compared to traditional vaccines. Additionally, there are currently no reports of successful polyvalent vaccines for fish parasites [20]. There is also insufficient research on host–parasite interactions and immune responses, which presents a challenge for vaccine development [20].
Next, administration of fish vaccination is also facing difficulties. Injection, bathing, and oral are the three common methods of vaccine delivery in aquaculture [135]. Injection is the most effective method, but it is labor-intensive and not the best approach for large-scale population vaccination in practical production [136,137]. Oral administration is considered the most feasible form of immunization for aquatic species and can be applied to various types of vaccines. However, vaccines are susceptible to degradation by enzymes in the digestive tract, so it is necessary to avoid delivering naked antigens. Encapsulating materials such as alginate, chitosan, liposomes, and other polymeric microspheres or biofilms should be explored. Alternatively, simpler and more cost-effective biological carriers, such as insects, probiotics, and plants, may be used [138,139,140,141]. Moreover, the number of fish receiving oral immunization and the vaccine dose are variable, making the outcome unpredictable. In some cases, the effectiveness is only half that of injectable vaccines [124]. Immersion is a unique vaccination method for aquatic species that can quickly and effectively activate a systemic immune response with relatively low labor intensity. However, to achieve optimal immune effects, it may be necessary to increase the dosage, add adjuvants, or administer multiple doses [142,143]. Finally, insufficient funding for vaccine development, along with the diverse approval processes and requirements, are major barriers to the commercialization of vaccines [126,144]. At present, many aquaculture vaccines are still in the clinical trial stage. However, with ongoing advancements in gene sequencing, antigen screening, and mucosal immunity research, along with the development of novel antigen expression and delivery technologies, aquaculture vaccines are expected to make significant breakthroughs in the future, providing a major boost to the aquaculture industry [124,144]. Future studies should focus on optimizing antigen production, such as using plant-based or insect cell expression systems to improve scalability and cost-effectiveness. Additionally, novel delivery approaches, including nanoparticle-based encapsulation and probiotic-mediated antigen transport, may enhance vaccine stability and efficacy. Addressing these challenges through interdisciplinary research could accelerate the development of effective parasitic vaccines and facilitate their large-scale application in aquaculture.
4.2. Immunostimulant
Immunostimulants are also a promising new treatment method. As an eco-friendly and safe strategy for parasite control, they have gradually become a hotspot for research and application. Immunostimulants work by activating the nonspecific immune system in fish, enhancing their overall immunity, and thus indirectly reducing parasite infections [145]. Moreover, for juvenile fish that are unsuitable for vaccination, immunostimulants can serve as a good alternative [145].
β-Glucans are one of the most widely studied immunostimulants in aquaculture, primarily derived from organisms such as yeast, fungi, and algae [145,146]. It can bind to receptors on the surface of various immune cells, triggering an immune response [147,148], and enhance the host’s resistance to pathogens such as parasites, fungi, and bacteria [149,150]. Microsporidia are widely distributed in marine, freshwater, and estuarine areas, posing a significant threat to aquaculture [151]. Research by Guselle et al. [152] demonstrated that after intraperitoneal injection of glucan in rainbow trout (Oncorhynchus mykiss), the number of Loma salmonae (Microsporidia) decreased by 90%, showing the potential of glucan as an immunostimulant in parasite control. Furthermore, it was found that the immune effect of glucan is time-dependent. Administering the treatment 21 days before parasite exposure produced the best results, significantly reducing gill hyperplasia, whereas treatment after infection proved to be less effective. The effect of β-glucan is highly dependent on the timing of administration [152]. In a study on Mesanophrys sp. infection in swimming crabs (Portunus trituberculatus), it was found that β-1,3-glucan, as an immunostimulant, significantly increased the enzymatic activity of hemocytes, including phenoloxidase, lysozyme, and superoxide dismutase (SOD). By regulating the expression of immune-related genes, it effectively inhibited the growth of the parasite Mesanophrys sp. and exhibited a dose-dependent immune protection effect [153].
Microalgae have recently been recognized as sustainable sources of immunostimulants in aquaculture. Representative genera include Spirulina, Chlorella, Haematococcus, and Porphyridium, which provide a range of bioactive compounds such as sulfated polysaccharides (SPs), polyunsaturated fatty acids (EPA and DHA), carotenoids (astaxanthin), and phycobiliproteins (phycocyanin) [154,155,156]. Among these compounds, polysaccharides enhance macrophage phagocytic activity and promote the expression of pro-inflammatory cytokine genes, activating the innate immune response [157]. Astaxanthin improves stress tolerance and immune performance in fish. Acting as a strong antioxidant, it scavenges reactive oxygen species and regulates redox-sensitive signaling pathways, helping to maintain tissue homeostasis and overall fish health [158,159]. In shrimp, a 30-day supplementation with 1.0% (w/w) sulfated polysaccharides from Porphyridium cruentum increased total hemocyte count and phenoloxidase activity in Litopenaeus vannamei. Survival after Vibrio challenge reached nearly 90%, reflecting stronger nonspecific defense and resistance to infection [160]. Feeding L. vannamei a diet containing 1% Chlorella vulgaris hot water extract (CVE) also enhanced enzyme activity, improved antioxidant status, and increased tolerance to ammonia stress, indicating protection under stress–infection conditions [161]. For fish, Spirulina platensis at 10 g/kg feed improved feed conversion efficiency in Nile tilapia (Oreochromis niloticus) and provided 22.2% relative protection against Edwardsiella tarda infection [162]. Astaxanthin from Haematococcus pluvialis at 25–75 mg/kg feed strengthened the intestinal barrier and boosted antioxidant and immune status in rainbow trout (Oncorhynchus mykiss), whereas higher doses inhibited growth, highlighting the need for an optimal dosage range [163]. Most aquaculture studies examining immunostimulants and pathogen resistance have focused on bacterial or viral infections, and parasite challenge evidence remains limited. Still, marine-derived sulfated polysaccharides have shown clear inhibitory or antagonistic effects against a range of marine parasites, combined with low toxicity and immunomodulatory activity. These findings support the potential use of algal polysaccharides in feed to enhance resistance to parasitic infections [164].
In addition, animal-derived substances such as chitosan and lactoferrin, and plant-derived compounds such as polyphenols, flavonoids, and saponins mentioned before, can also serve as immunostimulants. Under certain conditions, they stimulate the host’s immune system, thereby enhancing fish resistance to pathogens [146]. However, their practical application presents several challenges. Careful selection of immunostimulants and precise dosage control are essential to achieving optimal results, as excessive use may lead to immunosuppression and other adverse effects [165]. Although pre-infection administration of immunostimulants offers optimal protection, the unpredictable nature of disease outbreaks in aquaculture makes precise timing difficult. Additionally, individual variations in immune responses complicate dosage standardization [166]. Given the potential risks of immunosuppression and immune dysregulation, long-term use is not a viable strategy [167,168]. Therefore, immunostimulants should be considered a short-term immune-enhancing strategy rather than a long-term solution. Future research should focus on developing predictive disease monitoring systems to optimize their application by integrating biomarker-based immune assessments with environmental risk factors. Additionally, combining immunostimulants with complementary disease control methods, such as vaccination or functional feeds, may enhance their effectiveness and reduce the need for continuous administration.
Table 3 provides a comparative summary of the advantages and disadvantages of key parasite control techniques in aquaculture.

Table 3.
Overview of Aquaculture Parasite Treatment Methods.
5. Environmental Control Strategies
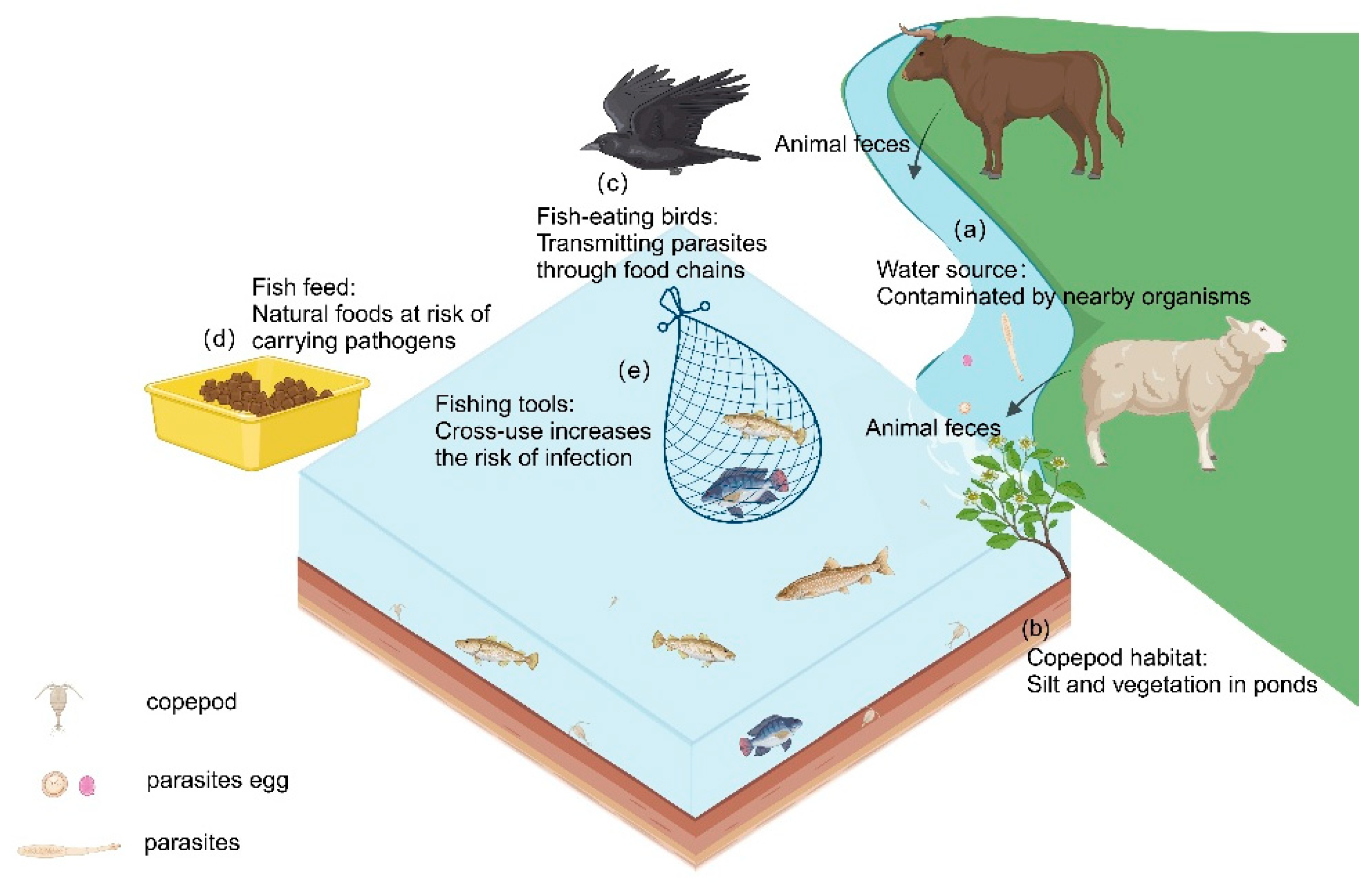
Parasites mainly enter aquaculture systems through the environment. Common sources include contaminated water, fish and birds carrying pathogens, and pollutants such as animal feces (Figure 1) [169]. These sources of pollution spread rapidly through the ecological chain, allowing parasites to propagate within aquaculture systems and increasing management complexity. Compared to post-infection treatments, optimizing the environment to interrupt transmission pathways is a more effective approach. Environmental management not only reduces infection risks at the source but also decreases dependence on drugs, helping to avoid resistance issues and environmental pollution. Therefore, implementing scientific environmental management measures at different stages of aquaculture can effectively block the transmission routes of parasites, thus reducing the risk of infection.

Figure 1.
Potential influences on parasitic infections in aquaculture systems. Inadequate selection of water sources for fisheries and the presence of contamination (a). Clearing pond silt and vegetation before culturing to reduce copepods, one of the intermediate hosts of the parasite (b). Avoid establishing fisheries where fish-eating birds are present to prevent the transfer of parasites between fishponds (c). Use pelleted fish feed instead of natural foods such as small fish and shrimp (d). Ensure clean equipment for mid-term fishpond maintenance and harvesting (e). (Created with BioRender.com).
5.1. Water Source Management
The water source is the core of the aquaculture system, and its quality directly determines the risk of parasitic infections. Contaminated water often becomes a primary transmission route as it carries parasite eggs or larvae [170]. Therefore, when selecting a water supply, priority should be given to relatively clean sources. Filtration and sterilization systems, such as ozone treatment or ultraviolet (UV) sterilization, can be implemented to ensure safety [171,172], and studies have shown that these methods can effectively reduce Cryptocaryon irritans infections in aquaculture farms [173].
5.2. Pond Cleaning and Environmental Safety
The sediment and aquatic plants in ponds provide a suitable habitat for parasites and their intermediate hosts. Regular cleaning helps eliminate these habitats, reducing the number of parasites and their intermediate hosts [169,174,175]. Thorough pond drying and chemical disinfection before stocking fish fry can effectively reduce the presence of intermediate hosts, such as copepods and snails, thus lowering the overall risk of parasite transmission [176]. In addition, preventing environmental pollution around the farm is crucial, especially to limit the activity of pathogen carriers such as birds. Installing isolation nets or bird deterrents can effectively reduce the risk of parasites being transmitted from wildlife to the aquaculture system [169].
5.3. Density and Feeding Management
Properly regulating stocking density is an important measure for disease management. High stocking densities increase the frequency of fish interactions, promoting the spread of parasites. In a study on parasite infections in farmed koi (Cyprinus carpio), it was found that higher stocking densities were associated with higher rates of parasitic infections [177]. Moreover, high stocking densities can lead to excessive oxygen consumption and increased water pollution, further adding stress to the fish [171,178]. Reducing stocking density and providing a more comfortable growing environment can significantly lower the risk of parasitic infections, while also improving fish growth rates and overall health. Additionally, feed management is equally important. Avoiding natural feeds that may contain parasite eggs, such as small fish and shrimp, and prioritizing processed pellet feeds can effectively disrupt the food chain transmission of parasites.
5.4. Water Quality Maintenance
Stable water quality is a critical foundation for controlling parasitic infections. Pollutants in the water and fluctuations in water quality parameters, such as insufficient dissolved oxygen, high ammonia levels, or abnormal pH, can weaken fish immunity and create favorable conditions for parasite reproduction [179]. Therefore, aquaculture farmers should maintain water quality within a healthy range through appropriate water exchange frequency, oxygenation measures, and pollution control. Research by Pan et al. (2024) has shown that ponds with shorter water exchange intervals and more frequent water quality monitoring and adjustments tend to have lower parasitic disease prevalence [180]. Modern technologies, such as biosensors, can be used to regularly monitor water quality parameters, providing data support for timely adjustments in management strategies. These methods help reduce environmental stress, improve fish health, and further decrease the risk of parasite transmission.
5.5. Tool Management and Wastewater Treatment
During the entire aquaculture process, the cross-use of tools such as fishing nets and fish baskets poses a potential route for parasite transmission. If these tools are not properly cleaned and disinfected, they may carry parasite eggs or pathogens and spread them between different aquaculture ponds. Therefore, it is recommended to implement strict cleaning protocols for tools in daily management, using high-temperature treatments or appropriate chemical disinfectants to ensure complete disinfection before use, thereby interrupting transmission pathways.
In addition, aquaculture wastewater often contains fish excrement, undigested feed residues, and potentially parasite eggs. To ensure environmental safety and prevent further spread of parasites, basic sedimentation and filtration treatments should be carried out before discharging the wastewater. These measures not only help protect the surrounding ecosystem but also improve the overall environmental hygiene of the aquaculture farm.
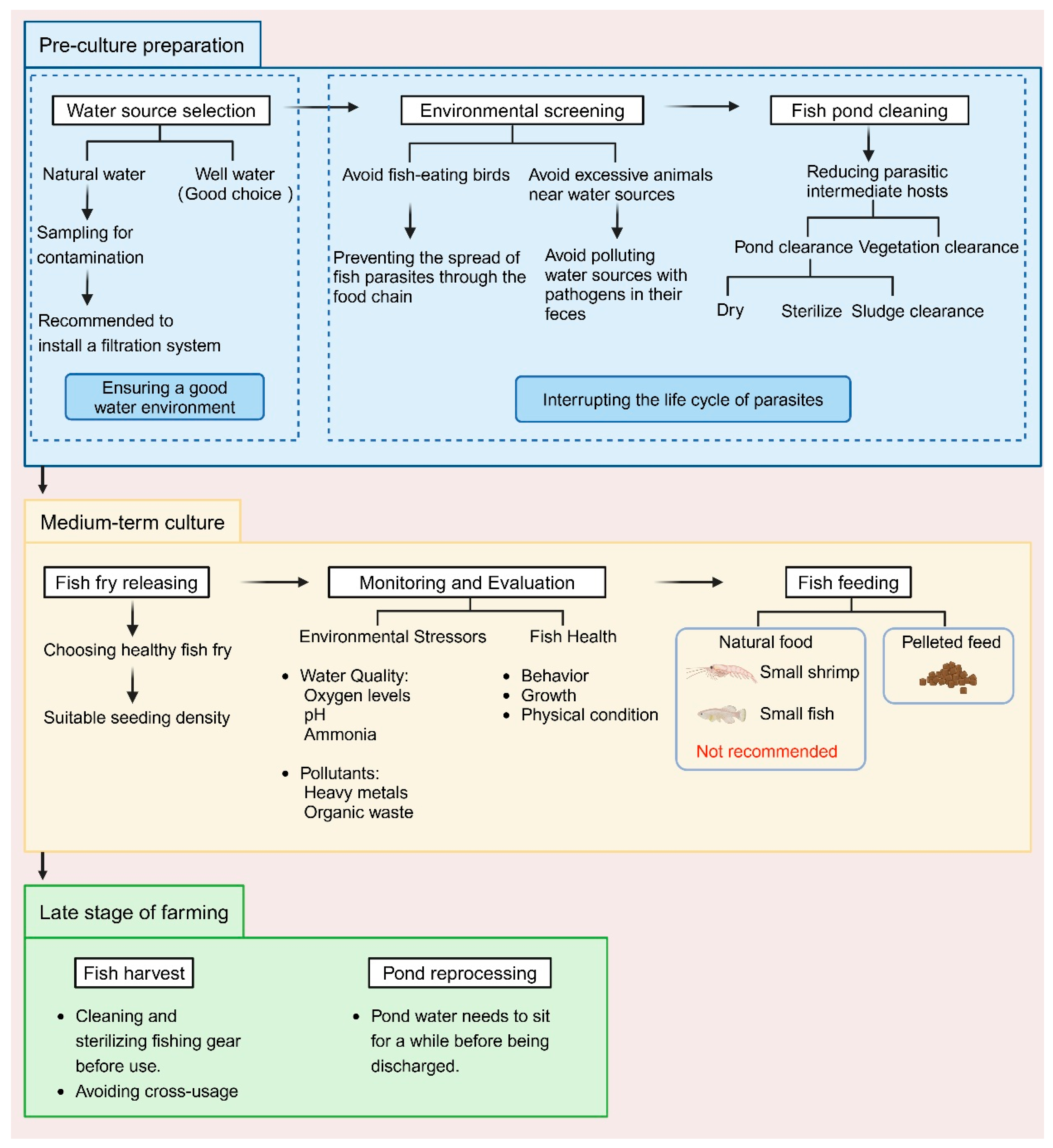
In conclusion, scientific environmental management is a key strategy for controlling parasitic infections and should be implemented throughout the entire aquaculture process. From the initial stages of water source selection and pond cleaning to scientific feeding, stocking density control, tool management, and water quality maintenance during the farming process, and finally to wastewater treatment at the end of the farming cycle, the collaborative efforts of each stage effectively interrupt the transmission pathways of parasites (Figure 2). These systematic measures enable aquaculture farms to reduce the risk of parasitic infections, minimize the use of medications, improve farming efficiency, and promote the sustainable development of aquaculture.
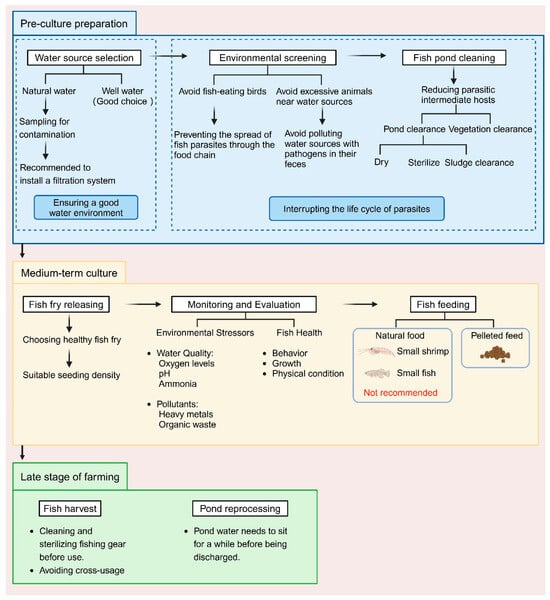
Figure 2.
Parasite preventive measures during the process of aquaculture (Created with BioRender.com).
6. Application of Modern Auxiliary Technologies
In aquaculture, modern auxiliary technologies have provided innovative ways to improve parasite control and overall farm management. Among them, nanotechnology and biosensor-based systems address different aspects of disease prevention and control. Nanoparticles can enhance therapeutic efficacy by improving drug delivery or acting directly against parasites, while biosensors allow rapid and sensitive detection of pathogens for timely intervention. Together, these technologies strengthen disease surveillance and treatment capacity, contributing to more effective parasite management and the sustainable development of aquaculture.
6.1. Nanoparticles
Particles ranging from 1 to 100 nanometers in size are referred to as nanoparticles (NPs), which can be classified into various types, including Metal Nanoparticles (MNPs), Carbon-Based Nanoparticles (CBNs), Lipid Nanoparticles (LNPs), and Polymeric Nanoparticles (PNPs) [181,182]. Its small size allows it to easily cross various biological barriers, while its high surface-to-volume ratio provides more sites for interaction with compounds [183]. These characteristics enable nanotechnology to demonstrate wide application potential in various fields, including food, healthcare, agriculture, and industry [184,185]. One of the most widespread applications is as a delivery carrier for therapeutic molecules, such as drugs or vaccines [186]. As nanotechnology gains attention in various fields, its potential in aquaculture has also been increasingly recognized, demonstrating unique value in optimizing vaccine delivery and pathogen control.
In aquaculture drug delivery, nanoparticles have gradually become a focus of research due to their unique advantages in drug protection and delivery. They can protect drugs or vaccines during the delivery process, preventing degradation caused by environmental pH changes and gastrointestinal digestive enzymes, thereby ensuring the stability of active molecules [187,188]. In addition, certain nanoparticles also possess good biocompatibility and biodegradability, allowing them to break down into harmless products within the body and be eliminated through natural metabolic processes [189]. For nanoparticles with slower degradation rates, they can gradually release drugs or antigens, extending the duration of action and enhancing the immune response [190]. Based on these advantages, the application of nanoparticles in aquaculture shows great potential.
Among the various types of nanoparticles, chitosan nanoparticles (CNPs) and poly (lactic-co-glycolic acid) (PLGA) nanoparticles are the most widely studied carriers. They combine the advantages mentioned above and are extensively used in the control of bacterial and viral diseases in aquaculture [191,192]. PLGA nanoparticles have shown significant advantages in treating aquatic animal diseases (Table 4). CNPs and LNPs also perform excellently in vaccine delivery and gene editing applications (Table 4). By encapsulating double-stranded RNA (dsRNA), nanoparticles enable precise targeting and silencing of pathogen genes, thereby preventing disease transmission. In addition, RNAi drugs delivered by LNPs have been approved by the U.S. Food and Drug Administration (FDA), further demonstrating their potential for clinical application [193].
While nanoparticles are best known for their role as drug delivery carriers in aquaculture disease treatment, recent findings have revealed that certain nanoparticles can also be directly applied to treat parasitic infections. This direct therapeutic potential has attracted significant attention, demonstrating their potential as effective tools for pest and parasite management (Table 4) [194]. Silver nanoparticles (AgNPs) [195] are the most widely studied and show broad antiparasitic activity against protozoan, monogenean, and crustacean parasites (Table 4). Recent studies have also reported the successful use of other nanoparticles, such as chitosan and iron oxide formulations, against a variety of fish parasites (Table 4). Although the formulations and exposure conditions differ, these studies consistently report similar outcomes—rapid parasite mortality, inhibition of reproduction, or detachment from host tissues following nanoparticle exposure. Microscopic observations have revealed severe deformation and rupture of parasite surfaces, while molecular analyses in Argulus have shown the upregulation of ion-channel–related genes, suggesting neuromuscular disruption. Together, these findings suggest that nanoparticles may exert antiparasitic activity mainly through physical and oxidative damage to parasite surfaces and by interfering with ionic or metabolic homeostasis. These effects highlight their potential as eco-friendly agents for managing both ecto- and endoparasitic infections in aquaculture. Apart from their therapeutic effects, NPs also have significant applications in the rapid diagnosis of pathogens. For example, an electrochemical DNA sensor based on AuNPs can be used to detect the fish pathogen Aphanomyces invadans [189]. This technology offers higher sensitivity and faster detection compared to traditional methods, providing valuable time for the early detection and treatment of parasitic diseases [196]. This topic will be further discussed in the following section on biosensors.
Although NPs has made significant progress in aquaculture, most research has focused on bacterial and viral diseases, with less emphasis on parasitic disease control [197,198]. Existing studies have demonstrated that some NPs have direct insecticidal effects, but their specific mechanisms of action during the multi-stage lifecycle of parasites remain unclear [194]. In addition, the potential impact of NPs on aquatic organisms and the environment needs further evaluation. For example, studies have shown that certain metal NPs, due to their non-biodegradable nature, may induce inflammation, oxidative stress, and DNA damage by penetrating cell membranes [199,200,201]. At the same time, the bioaccumulation of NPs in fish tissues may pose a risk of transmission along the food chain, potentially leading to adverse effects on ecosystems and human health [170,202]. As treatment methods for parasites continue to develop, research on the application of NPs in parasite control is expected to increase. With their unique advantages in precise delivery and targeted therapy, NPs are a highly promising approach in the field of parasitic treatment.

Table 4.
Applications of Nanoparticles (NPs) in Aquaculture.
Table 4.
Applications of Nanoparticles (NPs) in Aquaculture.
| Type of Nanoparticles (NPs) | Application | Key Findings | References |
|---|---|---|---|
| Poly (lactic-co-glycolic acid) (PLGA) | Encapsulation of rifampicin to treat Mycobacterium marinum infections in zebrafish embryos (Danio rerio) | More effective than rifampicin alone; significantly improves embryo survival rates | [203] |
| Chitosan nanoparticles (CNPs) | Delivery of dsRNA to control Yellow Head Virus (YHV) and White Spot Syndrome Virus (WSSV) | Precisely targets pathogen genes; significantly improves shrimp survival rates | [186] |
| Lipid nanoparticles (LNPs) | Delivery of VP28 vaccine to combat White Spot Disease (WSD) in shrimp | Superior immune protection compared to non-encapsulated vaccine | [188] |
| Chitosan, silver, selenium NPs | Anthelmintic effects on Clinostomum spp. and Prohemistomum vivax in Nile tilapia (Oreochromis niloticus) | All three showed insecticidal effects, with CNPs exhibiting the best performance | [194] |
| Ag, ZnO, Au NPs | Treatment of Ichthyophthirius multifiliis (theronts and tomonts) in rainbow trout (Oncorhynchus mykiss) | AgNPs (10 ng mL−1) caused 100% theront mortality within 2 h and inhibited tomont reproduction; reduced infectivity in vivo | [204] |
| Silver NPs | Treatment of Argulus siamensis (adult and copepodid stages) in freshwater fish (Labeo rohita) | 100% mortality at 25–50 ppm in vitro; strong antiparasitic efficacy | [205] |
| Iron oxide NPs | Control of Argulus siamensis infestation in Labeo rohita | In vivo bath (2.25 mg mL−1, 4 days) removed 100% parasites with moderate fish safety | [206] |
| Chitosan–silver nanocomposites | Treatment of Lernaea cyprinacea infection in goldfish (Carassius auratus) | 5.5 ppm for 24 h dislodged all parasites and promoted wound healing | [207] |
| Silver NPs | Anthelmintic treatment of Cichlidogyrus spp. (Monogenea; adults and eggs) in freshwater fish | 36 µg L−1 for 1 h achieved 100% mortality: tegumental disruption under SEM | [208] |
6.2. Biosensors
Biosensors are analytical devices that convert the physical or chemical signals of biomolecules into optical or electrical signals, enabling rapid, accurate, and real-time detection and quantification of biological substances [209]. By real-time monitoring of pathogens, stress biomarkers, and environmental pollutants, biosensors provide an efficient solution for the early diagnosis and integrated control of parasites [210]. The high sensitivity and specificity of biosensors greatly enhance detection efficiency. Additionally, their multifunctional capabilities not only support pathogen monitoring but also aid in assessing and optimizing the farming environment, enabling farmers to manage diseases more effectively [211].
Biosensors have achieved significant success in detecting microbial pathogens in aquaculture, particularly for common bacterial pathogens such as Vibrio, Aeromonas hydrophila, and Flavobacterium columnare [212]. Surface Plasmon Resonance (SPR) sensors and Optical Fiber Immunosensors (OFIs) have been successfully used to detect Escherichia coli, with a sensitivity of up to 94 CFU/mL [213,214]. Moreover, AuNPs can serve as key components in biosensors. In loop-mediated isothermal amplification (LAMP) technology, AuNPs enhance pathogen DNA detection efficiency by amplifying fluorescence or colorimetric signals. This method not only offers high sensitivity but also enables rapid diagnostics, and has been successfully applied for the quick detection of fish furunculosis infections [215]. Furthermore, electrochemical DNA biosensors based on AuNPs, coupled with DNA reporter probes, can be used to detect Aphanomyces invadans in fish. Their detection sensitivity is significantly higher than traditional PCR, with a lower detection limit of 0.5 fM for linear target DNA and 1 fM for PCR products [196].
Although most research has focused on bacteria, the potential application of biosensors in parasite treatment is also worth noting. Potentiometric biosensors have been successfully used for parasite detection [216,217]. This method combines Immunomagnetic Separation (IMS) with Enzyme-Linked Immunosorbent Assay (ELISA), enabling highly sensitive parasite diagnosis by measuring potential changes. The detection limit reaches as low as 500 cells [218,219]. The study by Santos-de-Souza et al. [220] also highlighted the potential of SPR in the rapid diagnosis of parasites. Leishmania amazonensis, at different stages of its lifecycle, releases an important protein marker—Cyspep (Cysteine Protease B C-terminal peptide). The study utilized Surface Plasmon Resonance (SPR) technology, where specific antibodies fixed on the sensor chip surface bind with Cyspep, to develop a sensor for real-time monitoring of this marker [220]. The method previously mentioned, combining the visual colorimetric nano-gold hybridization probe with isothermal amplification technology, can also be used for parasite detection. For example, in the case of Toxoplasma gondii, even as little as 1 fg of DNA in the sample can be detected [221]. Compared to traditional methods such as parasite culture and immunostaining, biosensors significantly improve detection speed and sensitivity, providing an efficient and accurate technological approach for the rapid diagnosis of parasites.
Biosensors also play a crucial role in monitoring environmental stress and fish stress biomarkers, providing significant support for the prevention and control of parasites and other diseases. Pollutants in the aquaculture environment, such as heavy metals (lead, mercury), organic compounds (phenols), as well as microplastics and nanoparticles, can accumulate and adversely affect fish health, even serving as vectors for the transmission of parasitic pathogens [210]. Biosensors based on Surface-Enhanced Raman Scattering (SERS) technology can efficiently detect the distribution and concentration of microplastics and pollutants in water, providing technical support for pollution source identification and pollution control [211]. By the real-time monitoring of water pollution levels, sensors can effectively provide early warnings of potential risks, reducing the occurrence of fish diseases caused by environmental pollution.
Fish are prone to stress under conditions such as high-density farming, temperature fluctuations, or deteriorating water quality. Stress can weaken their immune system, increasing their susceptibility to parasites and other pathogens. Cortisol, a stress hormone, is a key marker of fish stress, and its concentration changes can reflect the health status of the fish [211,222]. SPR combined with nanotechnology can sensitively detect cortisol concentrations as low as 0.001 ng/mL, enabling non-invasive real-time monitoring [223]. This provides aquaculture farmers with a quick way to assess fish health and adjust environmental conditions, such as reducing stocking density or improving water quality, thereby effectively preventing diseases triggered by stress. Although most biosensors are still in the research phase [224], with ongoing advancements in technology, they are expected to become early warning systems in aquaculture, helping farmers quickly identify potential health issues in fish and take timely intervention measures.
7. Conclusions
Parasite control remains a critical challenge in aquaculture. While traditional methods like chemical treatments have been effective, they are becoming less viable due to issues such as drug resistance, environmental pollution, and toxicity. As a result, new strategies are emerging, including treatments based on natural products, gene editing, and immunotherapy, as well as supportive technologies like nanotechnology and biosensors, which offer sustainable alternatives for parasite control in aquaculture.
Although novel therapeutic and auxiliary methods show potential for parasite control in aquaculture, most research still primarily focuses on bacterial pathogens, with relatively less attention given to parasites. Due to the limited knowledge of most fish parasites’ life cycles, the biological characteristics and treatment responses at different stages are not yet fully understood, making it difficult for current treatment methods to achieve comprehensive and effective control, as well as posing significant challenges in research. To overcome this challenge, future research needs to further investigate the various stages of the parasite life cycle, uncover their biological mechanisms, and provide a foundation for developing more precise and effective treatment strategies. At the same time, although emerging technologies such as gene editing and immunotherapy show great potential, their long-term effectiveness, feasibility for widespread application, and environmental impact still need further validation. Combining these new methods with environmental management measures, such as water quality control and stocking density regulation, will enhance the overall effectiveness of parasite management and drive the aquaculture industry toward more efficient and sustainable development.
Author Contributions
J.Y. wrote this review paper. S.B. reviewed and edited the paper. A.R. contributed to improving the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was Funded by KETSA Grant_NO. GA006-2021, CATEGORY: J-PROJECT COST CENTRE: 0000021070-INS.SAINS BIO&GEN-WBS:UM.0000307/HGA.GV.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| FAO | Food and Agriculture Organization |
| EOs | Essential oils |
| RNAi | RNA interference |
| dsRNA | Double-stranded RNA |
| Hsp70C | Heat shock protein 70C |
| iAg | Immobilized antigen |
| RPS | Relative protection |
| SOD | Superoxide dismutase |
| UV | Ultraviolet |
| NPs | Nanoparticles |
| MNPs | Metal Nanoparticles |
| CBNs | Carbon-Based Nanoparticles |
| LNPs | Lipid Nanoparticles |
| PNPs | Polymeric Nanoparticles |
| CNPs | Chitosan nanoparticles |
| PLGA | Poly (lactic-co-glycolic acid) |
| FDA | Food and Drug Administration |
| YHV | Yellow Head Virus |
| WSSV | White Spot Syndrome Virus |
| WSD | White Spot Disease |
| AuNPs | Gold nanoparticles |
| SPR | Surface Plasmon Resonance |
| OFIs | Optical Fiber Immunosensors |
| LAMP | In loop-mediated isothermal amplification |
| PCR | Polymerase Chain Reaction |
| IMS | Immunomagnetic Separation |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| Cyspep | Cysteine Protease B C-terminal peptide |
| SERS | Surface-Enhanced Raman Scattering |
References
- FAO. The State of World Fisheries and Aquaculture 2024–Blue Transformation in Action; FAO: Rome, Italy, 2024; p. 264. [Google Scholar]
- Kline, S.J.; Archdeacon, T.P.; Bonar, S.A. Effects of Praziquantel on Eggs of the Asian Tapeworm Bothriocephalus acheilognathi. North. Am. J. Aquac. 2011, 71, 380–383. [Google Scholar] [CrossRef]
- Mohammed, R.A.; Shehata, S.; Ghanem, M.H.; Abdelhadi, Y.M.; Radwan, M.K. Prevalence of Cestode Parasites of Some freshwater Fishes Cultivated In EL-Abbasa Fish Farm, Egypt. Egypt. Acad. J. Biol. Sci. B Zool. 2018, 10, 61–69. [Google Scholar] [CrossRef]
- Yera, H.; Kuchta, R.; Brabec, J.; Peyron, F.; Dupouy-Camet, J. First identification of eggs of the Asian fish tapeworm Bothriocephalus acheilognathi (Cestoda: Bothriocephalidea) in human stool. Parasitol. Int. 2013, 62, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A. Parasites causing disease in wild and cultured fish in Newfoundland. Icel. Agric. Sci. 2009, 22, 29–35. [Google Scholar]
- Rahkonen, R.; Aalto, J.; Koski, P.; Särkkä, J.; Juntunen, K. Cestode larvae Diphyllobothrium dendriticum as a cause of heart disease leading to mortality in hatchery-reared sea trout and brown trout. Dis. Aquat. Org. 1996, 25, 15–22. [Google Scholar] [CrossRef]
- Scholz, T.; Garcia, H.H.; Kuchta, R.; Wicht, B. Update on the human broad tapeworm (genus diphyllobothrium), including clinical relevance. Clin. Microbiol. Rev. 2009, 22, 146–160. [Google Scholar] [CrossRef]
- Kuchta, R.; Esteban, J.-G.; Brabec, J.; Scholz, T. Misidentification of Diphyllobothrium species related to global fish trade, Europe. Emerg. Infect. Dis. 2014, 20, 1955. [Google Scholar] [CrossRef]
- Fan, C.-K.; Barčák, D.; Scholz, T.; Sonko, P.; Orosová, M.; Su, K.-E.; Chang, C.-C.; Lee, Y.-J.; Kuchta, R.; Oros, M. Human diphyllobothriosis in Taiwan: A review of cases and molecular evidence of Dibothriocephalus nihonkaiensis. Food Waterborne Parasitol. 2023, 33, e00213. [Google Scholar] [CrossRef]
- Aunamika, S.J.; Roy, B.C.; Islam, Z.; Ahmed, N.; Rahman, M.K.; Biswas, H.; Hasan, M.M.; Taluker, M.H. Prevalence of fish-borne zoonotic parasites and their molecular identification in Bhola district of Bangladesh. Bangladesh J. Fish. 2021, 33, 283–294. [Google Scholar] [CrossRef]
- Yoshida, K.; Saito, M.; Sumiyoshi, A.; Makino, H.; Tashiro, M.; Ichikawa-Seki, M. Dibothriocephalus nihonkaiensis infection accompanied with vitamin B(12) deficiency: A case report. J. Infect. Chemother. 2025, 31, 102815. [Google Scholar] [CrossRef]
- Van De, N.; Waikagul, J.; Dalsgaard, A.; Chai, J.-Y.; Sohn, W.-M.; Murrell, K.D. Fishborne zoonotic intestinal trematodes, Vietnam. Emerg. Infect. Dis. 2007, 13, 1828. [Google Scholar] [CrossRef]
- Chai, J.-Y.; Murrell, K.D.; Lymbery, A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005, 35, 1233–1254. [Google Scholar] [CrossRef]
- Cong, W.; Elsheikha, H.M. Focus: Zoonotic Disease: Biology, epidemiology, clinical features, diagnosis, and treatment of selected fish-borne parasitic zoonoses. Yale J. Biol. Med. 2021, 94, 297. [Google Scholar]
- Abd, E.S.M.; Gareh, A.; Mohamed, H.I.; Alrashdi, B.M.; Dyab, A.K.; El-Khadragy, M.F.; Khairy Elbarbary, N.; Fouad, A.M.; El-Gohary, F.A.; Elmahallawy, E.K.; et al. Prevalence and Morphological Investigation of Parasitic Infection in Freshwater Fish (Nile Tilapia) from Upper Egypt. Animals 2023, 13, 1088. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.-L.; Fu, Y.-W.; Guo, X.-C.; Guo, S.-G.; Huang, S.-L.; Zhang, Q.-Z. Immune responses of Ctenopharyngodon idella juveniles against the ciliated parasite’s reinfection induced by Ichthyophthirius multifiliis theronts at the threshold density. Aquaculture 2025, 607, 742638. [Google Scholar] [CrossRef]
- Kumar, V.; Das, B.K.; Swain, H.S.; Chowdhury, H.; Roy, S.; Bera, A.K.; Das, R.; Parida, S.N.; Dhar, S.; Jana, A.K.; et al. Outbreak of Ichthyophthirius multifiliis associated with Aeromonas hydrophila in Pangasianodon hypophthalmus: The role of turmeric oil in enhancing immunity and inducing resistance against co-infection. Front. Immunol. 2022, 13, 956478. [Google Scholar] [CrossRef] [PubMed]
- Boerlage, A.S.; Shrestha, S.; Leinonen, I.; Jansen, M.D.; Revie, C.W.; Reeves, A.; Toma, L. Sea lice management measures for farmed Atlantic salmon (Salmo salar) in Scotland: Costs and effectiveness. Aquaculture 2024, 580, 740274. [Google Scholar] [CrossRef]
- Abolofia, J.; Asche, F.; Wilen, J.E. The cost of lice: Quantifying the impacts of parasitic sea lice on farmed salmon. Mar. Resour. Econ. 2017, 32, 329–349. [Google Scholar] [CrossRef]
- Shivam, S.; El-Matbouli, M.; Kumar, G. Development of Fish Parasite Vaccines in the OMICs Era: Progress and Opportunities. Vaccines 2021, 9, 179. [Google Scholar] [CrossRef]
- Madsen, H.; Stauffer, J.R., Jr. Aquaculture of Animal Species: Their Eukaryotic Parasites and the Control of Parasitic Infections. Biology 2024, 13, 41. [Google Scholar] [CrossRef]
- Maezono, M.; Nielsen, R.; Buchmann, K.; Nielsen, M. The Current State of Knowledge of the Economic Impact of Diseases in Global Aquaculture. Rev. Aquac. 2025, 17, e70039. [Google Scholar] [CrossRef]
- Shinn, A.; Avenant-Oldewage, A.; Bondad-Reantaso, M.; Cruz-Laufer, J.; García-Vásquez, A.; Hernández-Orts, J.; Kuchta, R.; Longshaw, M.; Metselaar, M.; Pariselle, A. A global review of problematic and pathogenic parasites of farmed tilapia. Rev. Aquac. 2023, 15, 92–153. [Google Scholar] [CrossRef]
- Aldrin, M.; Huseby, R.B.; Stige, L.C.; Helgesen, K.O. Estimated effectiveness of treatments against salmon lice in marine salmonid farming. Aquaculture 2023, 575, 739749. [Google Scholar] [CrossRef]
- Mitchell, A.; Darwish, A. Efficacy of 6-, 12-, and 24-h Praziquantel Bath Treatments against Asian Tapeworms Bothriocephalus acheilognathi in Grass Carp. North. Am. J. Aquac. 2009, 71, 30–34. [Google Scholar] [CrossRef]
- Rigos, G.; Kogiannou, D.; Vasilaki, A.; Kotsiri, M. Evaluation of Praziquantel Efficacy against Zeuxapta seriolae Infections in Greater Amberjack, Seriola dumerili. Appl. Sci. 2021, 11, 4656. [Google Scholar] [CrossRef]
- Scholz, T. Parasites in cultured and feral fish. Vet. Parasitol. 1999, 84, 317–335. [Google Scholar] [CrossRef]
- Geitung, L.; Wright, D.W.; Stien, L.H.; Oppedal, F.; Karlsbakk, E. Tapeworm (Eubothrium sp.) infestation in sea caged Atlantic salmon decreased by lice barrier snorkels during a commercial-scale study. Aquaculture 2021, 541, 736774. [Google Scholar] [CrossRef]
- Langford, K.H.; Oxnevad, S.; Schoyen, M.; Thomas, K.V. Do antiparasitic medicines used in aquaculture pose a risk to the Norwegian aquatic environment? Environ. Sci. Technol. 2014, 48, 7774–7780. [Google Scholar] [CrossRef]
- Tang, Y.; Lou, X.; Yang, G.; Tian, L.; Wang, Y.; Huang, X. Occurrence and human health risk assessment of antibiotics in cultured fish from 19 provinces in China. Front. Cell Infect. Microbiol. 2022, 12, 964283. [Google Scholar] [CrossRef]
- Overton, K.; Dempster, T.; Oppedal, F.; Kristiansen, T.S.; Gismervik, K.; Stien, L.H. Salmon lice treatments and salmon mortality in Norwegian aquaculture: A review. Rev. Aquac. 2019, 11, 1398–1417. [Google Scholar] [CrossRef]
- Bui, S.; Madaro, A.; Nilsson, J.; Fjelldal, P.G.; Iversen, M.H.; Brinchman, M.F.; Venås, B.; Schrøder, M.B.; Stien, L.H. Warm water treatment increased mortality risk in salmon. Vet. Anim. Sci. 2022, 17, 100265. [Google Scholar] [CrossRef]
- Thompson, C.; Madaro, A.; Oppedal, F.; Stien, L.H.; Bui, S. Delousing Efficacy and Physiological Impacts on Atlantic Salmon of Freshwater and Hyposaline Bath Treatments. 2023. Available online: https://www.hi.no/templates/reporteditor/report-pdf?id=67938&00685741 (accessed on 30 October 2025).
- Taylor, R.S.; Slinger, J.; Stratford, C.; Rigby, M.; Wynne, J.W. Evaluation of the Infectious Potential of Neoparamoeba perurans Following Freshwater Bathing Treatments. Microorganisms 2021, 9, 967. [Google Scholar] [CrossRef]
- Hoai, T.; Trinh, T.; Giang, N. The Effects of Short Freshwater Bath Treatments on the Susceptibility to Different Stages of Neobenedenia girellae Infecting Barramundi (Lates calcarifer). Vietnam. J. Agric. Sci. 2019, 2, 409–417. [Google Scholar] [CrossRef]
- Pincinato, R.B.M.; Oglend, A.; Smith, M.D.; Asche, F. Biological control of a parasite: The efficacy of cleaner fish in salmon farming. Ecol. Econ. 2025, 227, 108359. [Google Scholar] [CrossRef]
- Martins, M.L.; de Souza, A.P.; Lessa, J.G.M.; Brasil, E.M.; Magnotti, C.; Tsuzuki, M.Y.; Dutra, S.A.P. The cleaner fish Elacatinus figaro can control the monogenean Neobenedenia melleni, an ectoparasite of Lebranche mullet Mugil liza and does not decrease the hematological response. J. Parasit. Dis. 2025, 49, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Walde, C.S.; Stormoen, M.; Pettersen, J.M.; Persson, D.; Røsæg, M.V.; Bang Jensen, B. How delousing affects the short-term growth of Atlantic salmon (Salmo salar). Aquaculture 2022, 561, 738720. [Google Scholar] [CrossRef]
- Zhu, F. A review on the application of herbal medicines in the disease control of aquatic animals. Aquaculture 2020, 526, 735422. [Google Scholar] [CrossRef]
- Effendi, I.; Yoswaty, D.; Syawal, H.; Austin, B.; Lyndon, A.R.; Kurniawan, R.; Wahyuni, S.; Al-Harbi, A. The Use of Medicinal Herbs in Aquaculture Industry: A Review. In Current Aspects in Pharmaceutical Research and Development; Book Publisher International: Hooghly, India, 2022; Volume 7, pp. 7–20. [Google Scholar]
- Singh, A.; Mishra, A.; Chaudhary, R.; Kumar, V. Role of Herbal Plants in Prevention and Treatment of Parasitic Diseases. J. Sci. Res. 2020, 64, 50–58. [Google Scholar] [CrossRef]
- Canhao-Dias, M.; Paz-Silva, A.; Madeira de Carvalho, L.M. The efficacy of predatory fungi on the control of gastrointestinal parasites in domestic and wild animals—A systematic review. Vet. Parasitol. 2020, 283, 109173. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.A.; Robledo, D.; Sveen, L.; Daniels, R.R.; Krasnov, A.; Coates, A.; Jin, Y.H.; Barrett, L.T.; Lillehammer, M.; Kettunen, A.H.; et al. Applying genetic technologies to combat infectious diseases in aquaculture. Rev. Aquac. 2023, 15, 491–535. [Google Scholar] [CrossRef]
- Ferdous, M.A.; Islam, S.I.; Habib, N.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Shafie, A. CRISPR-Cas Genome Editing Technique for Fish Disease Management: Current Study and Future Perspective. Microorganisms 2022, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Pires, N.M.M.; Dong, T.; Yang, Z.; da Silva, L. Recent methods and biosensors for foodborne pathogen detection in fish: Progress and future prospects to sustainable aquaculture systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 1852–1876. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Dawood, M.A.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef]
- Pandey, G.; Madhuri, S.; Mandloi, A. Medicinal plants useful in fish diseases. Plant Arch. 2012, 12, 1–4. [Google Scholar]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 2011, 317, 1–15. [Google Scholar] [CrossRef]
- Doan, H.V.; Soltani, E.; Ingelbrecht, J.; Soltani, M. Medicinal Herbs and Plants: Potential Treatment of Monogenean Infections in Fish. Rev. Fish. Sci. Aquac. 2020, 28, 260–282. [Google Scholar] [CrossRef]
- Srichaiyo, N.; Tongsiri, S.; Hoseinifar, S.H.; Dawood, M.A.; Jaturasitha, S.; Esteban, M.Á.; Ringø, E.; Van Doan, H. The effects gotu kola (Centella asiatica) powder on growth performance, skin mucus, and serum immunity of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Rep. 2020, 16, 100239. [Google Scholar] [CrossRef]
- Shekarabi, S.P.H.; Omidi, A.H.; Dawood, M.A.; Adel, M.; Avazeh, A.; Heidari, F. Effect of Black Mulberry (Morus nigra) Powder on Growth Performance, Biochemical Parameters, Blood Carotenoid Concentration, and Fillet Color of Rainbow Trout. Ann. Anim. Sci. 2020, 20, 125–136. [Google Scholar] [CrossRef]
- Sadeghi, F.; Ahmadifar, E.; Shahriari Moghadam, M.; Ghiyasi, M.; Dawood, M.A.; Yilmaz, S. Lemon, Citrus aurantifolia, peel and Bacillus licheniformis protected common carp, Cyprinus carpio, from Aeromonas hydrophila infection by improving the humoral and skin mucosal immunity, and antioxidative responses. J. World Aquac. Soc. 2021, 52, 124–137. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Valentim, D.; Duarte, J.; Oliveira, A.; Cruz, R.; Carvalho, J.; Conceição, E.; Fernandes, C.; Tavares-Dias, M. Nanoemulsion from essential oil of Pterodon emarginatus (Fabaceae) shows in vitro efficacy against monogeneans of Colossoma macropomum (Pisces: Serrasalmidae). J. Fish Dis. 2018, 41, 443–449. [Google Scholar] [CrossRef]
- KP, A.K.M.; Haniffa, M. Evaluation of antibacterial activity of medicinal plants on fish pathogen Aeromonas hydrophila. J. Res. Biol. 2011, 1, 1–5. [Google Scholar]
- Pu, H.; Li, X.; Du, Q.; Cui, H.; Xu, Y. Research progress in the application of Chinese herbal medicines in aquaculture: A review. Engineering 2017, 3, 731–737. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El Basuini, M.F.; Zaineldin, A.I.; Yilmaz, S.; Hasan, M.T.; Ahmadifar, E.; El Asely, A.M.; Abdel-Latif, H.M.R.; Alagawany, M.; Abu-Elala, N.M.; et al. Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Dong, J.; Liu, Y.; Yang, Q.; Xu, N.; Yang, Y.; Ai, X. Antiparasitic efficacy of herbal extracts and active compound against Gyrodactylus kobayashii in Carassius auratus. Front. Vet. Sci. 2021, 8, 665072. [Google Scholar] [CrossRef] [PubMed]
- Chitmanat, C.; Tongdonmuan, K.; Nunsong, W. The use of crude extracts from traditional medicinal plants to eliminate Trichodina sp. in tilapia (Oreochromis niloticus) fingerlings. Songklanakarin J. Sci. Technol. 2005, 27, 359–364. [Google Scholar]
- Tu, X.; Duan, C.; Wu, S.; Fu, S.; Ye, J. Identification of plumbagin as an effective chemotherapeutic agent for treatment of Gyrodactylus infections. Aquaculture 2021, 535, 736372. [Google Scholar] [CrossRef]
- Malheiros, D.F.; Maciel, P.O.; Videira, M.N.; Tavares-Dias, M. Toxicity of the essential oil of Mentha piperita in Arapaima gigas (pirarucu) and antiparasitic effects on Dawestrema spp. (Monogenea). Aquaculture 2016, 455, 81–86. [Google Scholar] [CrossRef]
- de Seixas, A.T.; Gallani, S.U.; Noronha, L.d.S.; Silva, J.J.M.; Paschoal, J.A.R.; Bastos, J.K.; Valladão, G.M.R. Copaifera oleoresins as a novel natural product against acanthocephalan in aquaculture: Insights in the mode of action and toxicity. Aquac. Res. 2020, 51, 4681–4688. [Google Scholar] [CrossRef]
- Mahdy, O.A.; Abdel-Maogood, S.Z.; Mohammed, F.F.; Salem, M.A. Effect of Verbesina alternifolia and Mentha piperita oil extracts on newly excysted metacercaria of Euclinostomum heterostomum (Rudolphi, 1809) (Digenea: Clinostomatidae) from naturally infected kidneys of Tilapia zillii in Egypt. J. Egypt. Soc. Parasitol. 2017, 47, 513–521. [Google Scholar]
- Liu, H.R.; Liu, Y.M.; Hou, T.L.; Li, C.T.; Zhang, Q.Z. Antiparasitic Efficacy of Crude Plant Extracts and Compounds Purified from Plants against the Fish Monogenean Neobenedenia girellae. J. Aquat. Anim. Health 2021, 33, 155–161. [Google Scholar] [CrossRef]
- Abd El-Galil, M.A.; Aboelhadid, S.M. Trials for the control of trichodinosis and gyrodactylosis in hatchery reared Oreochromis niloticus fries by using garlic. Vet. Parasitol. 2012, 185, 57–63. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Division, N. Probiotics in Food. In FAO Food and Nutrition Paper; FAO/WHO: Rome, Italy, 2006; p. 56. [Google Scholar]
- Nwogu, N.A.; Olaji, E.D.; Eghomwanre, A. Application of probiotics in Nigeria aquaculture: Current status, challenges and prospects. Inter. Res. J. Microbiol. 2011, 2, 215–219. [Google Scholar]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ringø, E.; Shenavar Masouleh, A.; Esteban, M.Á. Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: A review. Rev. Aquac. 2016, 8, 89–102. [Google Scholar] [CrossRef]
- Moriarty, D. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 1998, 164, 351–358. [Google Scholar] [CrossRef]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, S.J.; Baker, R.T.; Bøgwald, J.; Castex, M.; Ringø, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Franco, W. Postbiotics and parabiotics derived from bacteria and yeast: Current trends and future perspectives. CyTA J. Food 2024, 22, 2425838. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Simón, R.; Docando, F.; Nuñez-Ortiz, N.; Tafalla, C.; Díaz-Rosales, P. Mechanisms Used by Probiotics to Confer Pathogen Resistance to Teleost Fish. Front. Immunol. 2021, 12, 653025. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S. Probiotics in Fish Aquaculture: The Cure Against Parasitic Diseases? Master’s Thesis, University of Groningen, Groningen, The Netherlands, 2019. [Google Scholar]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.-S. Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol. 2010, 29, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Sîrbu, E.; Dima, M.F.; Tenciu, M.; Cretu, M.; Coadă, M.T.; Țoțoiu, A.; Cristea, V.; Patriche, N. Effects of dietary supplementation with probiotics and prebiotics on growth, physiological condition, and resistance to pathogens challenge in Nile tilapia (Oreochromis niloticus). Fishes 2022, 7, 273. [Google Scholar] [CrossRef]
- Kong, Y.; Gao, C.; Du, X.; Zhao, J.; Li, M.; Shan, X.; Wang, G. Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on growth, immune response and disease resistance of snakehead fish (Channa argus). Fish. Shellfish Immunol. 2020, 102, 412–421. [Google Scholar] [CrossRef]
- Waiyamitra, P.; Zoral, M.A.; Saengtienchai, A.; Luengnaruemitchai, A.; Decamp, O.; Gorgoglione, B.; Surachetpong, W. Probiotics modulate tilapia resistance and immune response against tilapia lake virus infection. Pathogens 2020, 9, 919. [Google Scholar] [CrossRef]
- Nageswara, P.; Babu, D. Probiotics as an alternative therapy to minimize or avoid antibiotics use in aquaculture. Fish. Chimes 2006, 26, 112–114. [Google Scholar]
- Sahu, M.K.; Swarnakumar, N.; Sivakumar, K.; Thangaradjou, T.; Kannan, L. Probiotics in aquaculture: Importance and future perspectives. Indian. J. Microbiol. 2008, 48, 299–308. [Google Scholar] [CrossRef][Green Version]
- Banerjee, G.; Ray, A.K. The advancement of probiotics research and its application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef]
- Sun, H.; Shang, M.; Tang, Z.; Jiang, H.; Dong, H.; Zhou, X.; Lin, Z.; Shi, C.; Ren, P.; Zhao, L.; et al. Oral delivery of Bacillus subtilis spores expressing Clonorchis sinensis paramyosin protects grass carp from cercaria infection. Appl. Microbiol. Biotechnol. 2020, 104, 1633–1646. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.; Austin, B. Probiotics for disease control in aquaculture. Diagn. Control Dis. Fish Shellfish 2017, 189–222. [Google Scholar]
- Sihag, R.C.; Sharma, P. Probiotics: The new ecofriendly alternative measures of disease control for sustainable aquaculture. J. Fish. Aquat. Sci. 2012, 7, 72–103. [Google Scholar] [CrossRef]
- Nurhajati, J.; Aryantha, I.; Indah, D. The curative action of Lactobacillus plantarum FNCC 226 to Saprolegnia parasitica A3 on catfish (Pangasius hypophthalamus Sauvage). Int. Food Res. J. 2012, 19, 1723–1727. [Google Scholar]
- Garcia, L.d.O.; Becker, A.G.; Cunha, M.A.; Baldisserotto, B.; Copatti, C.E.; Kochhann, D. Effects of water pH and hardness on infection of silver catfish, Rhamdia quelen, fingerlings by Ichthyophthirius multifiliis. J. World Aquac. Soc. 2011, 42, 399–405. [Google Scholar] [CrossRef]
- C De, B.; Meena, D.; Behera, B.; Das, P.; Das Mohapatra, P.; Sharma, A. Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish. Physiol. Biochem. 2014, 40, 921–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Cai, Y.; Guo, X.; Cao, Z.; Zhang, Y.; Liu, S.; Yuan, W.; Zhu, W.; Zheng, Y. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish. Shellfish Immunol. 2017, 60, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Thy, H.T.T.; Tri, N.N.; Quy, O.M.; Fotedar, R.; Kannika, K.; Unajak, S.; Areechon, N. Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus). Fish. Shellfish Immunol. 2017, 60, 391–399. [Google Scholar]
- Sánchez-Ortiz, A.C.; Angulo, C.; Luna-González, A.; Álvarez-Ruiz, P.; Mazón-Suástegui, J.M.; Campa-Córdova, Á.I. Effect of mixed-Bacillus spp isolated from pustulose ark Anadara tuberculosa on growth, survival, viral prevalence and immune-related gene expression in shrimp Litopenaeus vannamei. Fish. Shellfish Immunol. 2016, 59, 95–102. [Google Scholar] [CrossRef]
- Pieters, N.; Brunt, J.; Austin, B.; Lyndon, A.R. Efficacy of in-feed probiotics against Aeromonas bestiarum and Ichthyophthirius multifiliis skin infections in rainbow trout (Oncorhynchus mykiss, Walbaum). J. Appl. Microbiol. 2008, 105, 723–732. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Bessat, M.; Fadel, A.; Elblehi, S. Responses of dietary supplementation of probiotic effective microorganisms (EMs) in Oreochromis niloticus on growth, hematological, intestinal histopathological, and antiparasitic activities. Aquac. Int. 2020, 28, 947–963. [Google Scholar] [CrossRef]
- Yanuhar, U.; Junirahma, N.; Susilowati, K.; Caesar, N.; Musa, M. Effects of Probiotic Treatment on Histopathology of Koi Carp (Cyprinus carpio) Infected by Myxobolus sp. Proc. J. Phys. Conf. Ser. 2019, 012051. [Google Scholar] [CrossRef]
- Abbas, F.; Hafeez-ur-Rehman, M.; Mubeen, N.; Bhatti, A.; Daniel, I. Parasites of Fish and Aquaculture and their Control. In Parasitism and Parasitic Control in Animals; CABI Publishing: Wallingford, UK, 2023; pp. 248–266. [Google Scholar]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2018, 77, 99–113. [Google Scholar] [CrossRef]
- Blewett, H.J.; Taylor, C.G. Dietary zinc deficiency in rodents: Effects on T-cell development, maturation and phenotypes. Nutrients 2012, 4, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Rigos, G.; Henry, M.; Alexis, M.; Kentouri, M. Effects of fish meal replacement by a soybean protein on growth, histology, selected immune and oxidative status markers of gilthead sea bream, Sparus aurata. J. World Aquac. Soc. 2015, 46, 115–128. [Google Scholar] [CrossRef]
- Nimrat, S.; Vuthiphandchai, V. In vitro evaluation of commercial probiotic products used for marine shrimp cultivation in Thailand. Afr. J. Biotechnol. 2011, 10, 4643–4650. [Google Scholar]
- Elaswad, A.; Dunham, R. Disease reduction in aquaculture with genetic and genomic technology: Current and future approaches. Rev. Aquac. 2017, 10, 876–898. [Google Scholar] [CrossRef]
- Labreuche, Y.; Warr, G.W. Insights into the antiviral functions of the RNAi machinery in penaeid shrimp. Fish Shellfish Immunol. 2013, 34, 1002–1010. [Google Scholar] [CrossRef]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G. RNA Interference in Aquaculture: A Small Tool for Big Potential. J. Agric. Food Chem. 2021, 69, 4343–4355. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Lezer, Y.; Aflalo, E.D.; Manor, R.; Sharabi, O.; Abilevich, L.K.; Sagi, A. On the safety of RNAi usage in aquaculture: The case of all-male prawn stocks generated through manipulation of the insulin-like androgenic gland hormone. Aquaculture 2015, 435, 157–166. [Google Scholar] [CrossRef]
- Shpak, N.; Manor, R.; Aflalo, E.D.; Sagi, A. Three generations of cultured prawn without W chromosome. Aquaculture 2017, 467, 41–48. [Google Scholar] [CrossRef]
- Lima, P.C.; Harris, J.O.; Cook, M. Exploring RNAi as a therapeutic strategy for controlling disease in aquaculture. Fish. Shellfish Immunol. 2013, 34, 729–743. [Google Scholar] [CrossRef]
- Ohashi, H.; Umeda, N.; Hirazawa, N.; Ozaki, Y.; Miura, C.; Miura, T. Expression of vasa (vas)-related genes in germ cells and specific interference with gene functions by double-stranded RNA in the monogenean, Neobenedenia girellae. Int. J. Parasitol. 2007, 37, 515–523. [Google Scholar] [CrossRef]
- Saleh, M.; Kumar, G.; Abdel-Baki, A.A.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. In Vitro Gene Silencing of the Fish Microsporidian Heterosporis saurida by RNA Interference. Nucleic Acid. Ther. 2016, 26, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Menanteau-Ledouble, S.; Kotob, M.H.; El-Matbouli, M. A RNAi-based therapeutic proof of concept targets salmonid whirling disease in vivo. PLoS ONE 2017, 12, e0178687. [Google Scholar] [CrossRef] [PubMed]
- Eichner, C.; Nilsen, F.; Grotmol, S.; Dalvin, S. A method for stable gene knock-down by RNA interference in larvae of the salmon louse (Lepeophtheirus salmonis). Exp. Parasitol. 2014, 140, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Aquino-Jarquin, G. Current advances in overcoming obstacles of CRISPR/Cas9 off-target genome editing. Mol. Genet. Metab. 2021, 134, 77–86. [Google Scholar] [CrossRef]
- Robinson, N.A.; Østbye, T.K.K.; Kettunen, A.H.; Coates, A.; Barrett, L.T.; Robledo, D.; Dempster, T. A guide to assess the use of gene editing in aquaculture. Rev. Aquac. 2023, 16, 775–784. [Google Scholar] [CrossRef]
- Muir, W.M.; Howard, R.D. Possible ecological risks of transgenic organism release when transgenes affect mating success: Sexual selection and the Trojan gene hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 13853–13856. [Google Scholar] [CrossRef]
- Aikio, S.; Valosaari, K.-R.; Kaitala, V. Mating preference in the invasion of growth enhanced fish. Oikos 2008, 117, 406–414. [Google Scholar] [CrossRef]
- McDowell, N. Stream of escaped farm fish raises fears for wild salmon. Nature 2002, 416, 571. [Google Scholar] [CrossRef]
- McGinnity, P.; Stone, C.; Taggart, J.B.; Cooke, D.; Cotter, D.; Hynes, R.; McCamley, C.; Cross, T.; Ferguson, A. Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: Use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. ICES J. Mar. Sci. 1997, 54, 998–1008. [Google Scholar] [CrossRef]
- Kleppe, L.; Fjelldal, P.G.; Andersson, E.; Hansen, T.; Sanden, M.; Bruvik, A.; Skaftnesmo, K.O.; Furmanek, T.; Kjærner-Semb, E.; Crespo, D.; et al. Full production cycle performance of gene-edited, sterile Atlantic salmon-growth, smoltification, welfare indicators and fillet composition. Aquaculture 2022, 560, 738456. [Google Scholar] [CrossRef]
- Almeida, F.L.; Skaftnesmo, K.O.; Andersson, E.; Kleppe, L.; Edvardsen Norberg, B.; Fjelldal, P.G.; Hansen, T.J.; Schulz, R.W.; Wargelius, A. The Piwil1 N domain is required for germ cell survival in Atlantic salmon. Front. Cell Dev. Biol. 2022, 10, 977779. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yakovlev, I.A.; Van Eerde, A.; Su, J.; Clarke, J.L. Plant-produced vaccines: Future applications in aquaculture. Front. Plant Sci. 2021, 12, 718775. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in aquaculture vaccines against fish pathogens: Global status and current trends. Rev. Fish. Sci. Aquac. 2017, 25, 184–217. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.; Miao, L.; Chen, J. Current status and development prospects of aquatic vaccines. Front. Immunol. 2022, 13, 1040336. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Kumar, A.; Middha, S.K.; Menon, S.V.; Paital, B.; Gokarn, S.; Nelli, M.; Rajanikanth, R.B.; Chandra, H.M.; Mugunthan, S.P.; Kantwa, S.M.; et al. Current Challenges of Vaccination in Fish Health Management. Animals 2024, 14, 2692. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kwon, M.G.; Seo, J.S.; Hwang, S.D.; Jeong, J.M.; Lee, J.H.; Jeong, A.R.; Jee, B.Y. Current use and management of commercial fish vaccines in Korea. Fish Shellfish Immunol. 2020, 102, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, B.; Mo, Z.; Li, A.; Dan, X. Cryptocaryon irritans (Brown, 1951) is a serious threat to aquaculture of marine fish. Rev. Aquac. 2022, 14, 218–236. [Google Scholar] [CrossRef]
- Josepriya, T.A.; Chien, K.H.; Lin, H.Y.; Huang, H.N.; Wu, C.J.; Song, Y.L. Immobilization antigen vaccine adjuvanted by parasitic heat shock protein 70C confers high protection in fish against cryptocaryonosis. Fish Shellfish Immunol. 2015, 45, 517–527. [Google Scholar] [CrossRef]
- Dan, X.-M.; Zhang, T.-W.; Li, Y.-W.; Li, A.-X. Immune responses and immune-related gene expression profile in orange-spotted grouper after immunization with Cryptocaryon irritans vaccine. Fish Shellfish Immunol. 2013, 34, 885–891. [Google Scholar] [CrossRef]
- Shin, S.-M.; Kole, S.; Lee, J.; Choi, J.S.; Jung, S.-J. Formulation of chitosan microsphere-based oral vaccine against the scuticociliate parasite Miamiensis avidus: Evaluation of its protective immune potency in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2023, 142, 109159. [Google Scholar] [CrossRef]
- Kole, S.; Dar, S.A.; Shin, S.M.; Jeong, H.J.; Jung, S.J. Potential Efficacy of Chitosan-Poly (Lactide-Co-Glycolide)-Encapsulated Trivalent Immersion Vaccine in Olive Flounder (Paralichthys olivaceus) Against Viral Hemorrhagic Septicemia Virus, Streptococcus parauberis Serotype I, and Miamiensis avidus (Scuticociliate). Front. Immunol. 2021, 12, 761130. [Google Scholar] [CrossRef]
- Tamer, C.; Cavunt, A.; Durmaz, Y.; Ozan, E.; Kadi, H.; Kalayci, G.; Ozkan, B.; Isidan, H.; Albayrak, H. Inactivated infectious pancreatic necrosis virus (IPNV) vaccine and E.coli-expressed recombinant IPNV-VP2 subunit vaccine afford protection against IPNV challenge in rainbow trout. Fish Shellfish Immunol. 2021, 115, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Dzikowski, R.; Deitsch, K. Antigenic variation by protozoan parasites: Insights from Babesia bovis. Mol. Microbiol. 2006, 59, 364–366. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-López, F.; MacKenzie, S. Immunostimulant diets and oral vaccination in fish. Diagn. Control Dis. Fish Shellfish 2017, 147–184. [Google Scholar]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Liang, R.; Li, J.; Ruan, Y.; Zhu, S. The design and parameter optimization of automatic positioning mechanism for automatic injection system of fry vaccine. In Proceedings of the 2015 ASABE Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015; p. 1. [Google Scholar]
- Embregts, C.W.; Forlenza, M. Oral vaccination of fish: Lessons from humans and veterinary species. Dev. Comp. Immunol. 2016, 64, 118–137. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Sun, F.; Liu, Q.; Wang, Q.; Zhang, Y.; Liu, X. An oral vaccine based on chitosan/aluminum adjuvant induces both local and systemic immune responses in turbot (Scophthalmus maximus). Vaccine 2021, 39, 7477–7484. [Google Scholar] [CrossRef]
- Andresen, A.M.S.; Gjøen, T. Chitosan nanoparticle formulation attenuates poly (I: C) induced innate immune responses against inactivated virus vaccine in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100915. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.J.; Lee, Y.-K.; Cho, S. Oral Vaccine delivery for intestinal immunity—Biological basis, barriers, delivery system, and m cell targeting. Polymers 2018, 10, 948. [Google Scholar] [CrossRef]
- Chettri, J.K.; Jaafar, R.M.; Skov, J.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Booster immersion vaccination using diluted Yersinia ruckeri bacterin confers protection against ERM in rainbow trout. Aquaculture 2015, 440, 1–5. [Google Scholar] [CrossRef]
- Du, Y.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The influence of concentration of inactivated Edwardsiella tarda bacterin and immersion time on antigen uptake and expression of immune-related genes in Japanese flounder (Paralichthys olivaceus). Microb. Pathog. 2017, 103, 19–28. [Google Scholar] [CrossRef]
- Jose Priya, T.A.; Kappalli, S. Modern biotechnological strategies for vaccine development in aquaculture—Prospects and challenges. Vaccine 2022, 40, 5873–5881. [Google Scholar] [CrossRef] [PubMed]
- Ching, J.J.; Shuib, A.S.; Abdul Majid, N.; Mohd Taufek, N. Immunomodulatory activity of β-glucans in fish: Relationship between β-glucan administration parameters and immune response induced. Aquac. Res. 2020, 52, 1824–1845. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef]
- Machuca, C.; Méndez-Martínez, Y.; Reyes-Becerril, M.; Angulo, C. Yeast β-glucans as fish immunomodulators: A review. Animals 2022, 12, 2154. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Guardiola, F.A.; Sanchez, V.; Maldonado, M.; Angulo, C. Sterigmatomyces halophilus β-glucan improves the immune response and bacterial resistance in Pacific red snapper (Lutjanus peru) peripheral blood leucocytes: In vitro study. Fish Shellfish Immunol. 2018, 78, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Voloski, A.P.; de Figueiredo Soveral, L.; Dazzi, C.C.; Sutili, F.; Frandoloso, R.; Kreutz, L.C. β-Glucan improves wound healing in silver catfish (Rhamdia quelen). Fish. Shellfish Immunol. 2019, 93, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Villanueva, L.T.; Ascencio-Valle, F.; Macías-Rodríguez, M.E.; Tovar-Ramírez, D. Effects of dietary β-1,3/1,6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish. Physiol. Biochem. 2014, 40, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.L.; Speare, D.J. Review of the sequential development of Loma salmonae (Microsporidia) based on experimental infections of rainbow trout (Oncorhynchus mykiss) and Chinook salmon (O. tshawytscha). Folia Parasitol. 2005, 52, 63–68. [Google Scholar] [CrossRef]
- Guselle, N.J.; Markham, R.J.; Speare, D.J. Timing of intraperitoneal administration of beta-1,3/1,6 glucan to rainbow trout, Oncorhynchus mykiss (Walbaum), affects protection against the microsporidian Loma salmonae. J. Fish Dis. 2007, 30, 111–116. [Google Scholar] [CrossRef]
- Perveen, S.; Yang, L.; Zhou, S.; Feng, B.; Xie, X.; Zhou, Q.; Qian, D.; Wang, C.; Yin, F. beta-1,3-Glucan from Euglena gracilis as an immunostimulant mediates the antiparasitic effect against Mesanophrys sp. on hemocytes in marine swimming crab (Portunus trituberculatus). Fish Shellfish Immunol. 2021, 114, 28–35. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Rathi, D.-N.G.; Mustar, S.; Lee, J.C. Microalgae as an Eco-Friendly and Functional Ingredient for Sustainable Aquafeed. Aquac. J. 2025, 5, 14. [Google Scholar] [CrossRef]
- Ma, K.; Bao, Q.; Wu, Y.; Chen, S.; Zhao, S.; Wu, H.; Fan, J. Evaluation of Microalgae as Immunostimulants and Recombinant Vaccines for Diseases Prevention and Control in Aquaculture. Front. Bioeng. Biotechnol. 2020, 8, 2020. [Google Scholar] [CrossRef]
- Bahi, A.; Ramos-Vega, A.; Angulo, C.; Monreal-Escalante, E.; Guardiola, F.A. Microalgae with immunomodulatory effects on fish. Rev. Aquac. 2023, 15, 1522–1539. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Abirami, R.G.; Rajan, D.K. Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish Shellfish Immunol. 2019, 86, 1177–1193. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance. Animals 2023, 13, 3357. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Leng, X.; Zhang, C.; Han, Z.; Zhang, F. Effects of dietary astaxanthins on pigmentation of flesh and tissue antioxidation of rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2013, 21, 579–589. [Google Scholar] [CrossRef]
- Ozório, R.Á.; Lopes, R.G.; Vieira, F.d.N.; Bolívar-Ramírez, N.C.; Oliveira, C.Y.B.d.; Barracco, M.A.A.M.; Owatari, M.S.; Fracalossi, D.M.; Derner, R.B. Crude Polysaccharide Extract from the Microalga Porphyridium cruentum Improved Nonspecific Immune Responses and Resistance in Penaeus vannamei Exposed to Vibrio alginolyticus. Aquac. J. 2024, 4, 104–113. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, S.; Jia, X.; Zhou, W.; Sun, X.; Han, C.; Lu, Y. Effects of dietary hot water extracts of Chlorella vulgaris on muscle component, non-specific immunity, antioxidation, and resistance to non-ionic ammonia stress in Pacific white shrimp Litopenaeus vannamei. Front. Mar. Sci. 2024, 11, 2024. [Google Scholar] [CrossRef]
- Okasha, L.A.; Abdellatif, J.I.; Abd-Elmegeed, O.H.; Sherif, A.H. Overview on the role of dietary Spirulina platensis on immune responses against Edwardsiellosis among Oreochromis niloticus fish farms. BMC Vet. Res. 2024, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, F.; Zhu, L.; Zhan, L.; Numasawa, T.; Deng, J. Effects of dietary astaxanthin supplementation on growth performance, antioxidant status, immune response, and intestinal health of rainbow trout (Oncorhynchus mykiss). Anim. Nutr. 2024, 17, 387–396. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zaporozhets, T.S.; Andryukov, B.G.; Kryzhanovsky, S.P.; Ermakova, S.P.; Kuznetsova, T.A.; Voronova, A.N.; Shchelkanov, M.Y. Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts. Mar. Drugs 2021, 19, 637. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Fierro-Castro, C.; Mandiki, S.N.; Emile, W.; Tort, L.; Kestemont, P. Dietary β-glucans differentially modulate immune and stress-related gene expression in lymphoid organs from healthy and Aeromonas hydrophila-infected rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2017, 63, 285–296. [Google Scholar] [CrossRef]
- Bricknell, I.; Dalmo, R.A. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol. 2005, 19, 457–472. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, M.; Pereiro, P.; Reyes-Lopez, F.; Tort, L.; Figueras, A.; Novoa, B. Analysis of the Long-Lived Responses Induced by Immunostimulants and Their Effects on a Viral Infection in Zebrafish (Danio rerio). Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, H.; Teame, T.; Ran, C.; Yang, Y.; Yao, Y.; Ding, Q.; Liu, S.; Li, S.; Zhang, Z.; et al. Immune disorders induced by improper use of dietary immunostimulants in aquatic animals: Research progress and prospective solutions by targeting gut microbiota. Rev. Aquac. 2023, 16, 608–621. [Google Scholar] [CrossRef]
- Terech-Majewska, E. Improving disease prevention and treatment in controlled fish culture. Arch. Pol. Fish. 2016, 24, 115–165. [Google Scholar] [CrossRef]
- Luis, A.I.S.; Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F. Trends in aquaculture sciences: From now to use of nanotechnology for disease control. Rev. Aquac. 2019, 11, 119–132. [Google Scholar] [CrossRef]
- Sommerville, C. Advances in non-chemical methods for parasite prevention and control in fish. In Infectious Disease in Aquaculture; Woodhead Publishing: Sawston, UK, 2012; pp. 480–512. [Google Scholar]
- Scholz, T.; Kuchta, R.; Oros, M. Tapeworms as pathogens of fish: A review. J. Fish Dis. 2021, 44, 1883–1900. [Google Scholar] [CrossRef]
- Lu, G.-L.; Jiang, B.; Li, Z.-C.; Li, A.-X. Ultraviolet light and ozone controls Cryptocaryon irritans infection in factory aquaculture. Aquaculture 2022, 548, 737598. [Google Scholar] [CrossRef]
- Maina, K.; Mbuthia, P.; Waruiru, R.; Nzalawahe, J.; Murugami, J.; Njagi, L.; Mdegela, R.; Mavuti, S. Risk factors associated with parasites of farmed fish in Kiambu County, Kenya. Int. J. Fish. Aquat. Stud. 2017, 5, 217–223. [Google Scholar]
- Mathenge, C.G. Prevalence, Intensity and Pathological Lesions Associated with Helminth Infections in Farmed and Wild Fish in Upper Tana River Basin, Kenya. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2010. [Google Scholar]
- Thien, C.P.; Dalsgaard, A.; Thanh Nhan, N.; Olsen, A.; Murrell, K.D. Prevalence of zoonotic trematode parasites in fish fry and juveniles in fish farms of the Mekong Delta, Vietnam. Aquaculture 2009, 295, 1–5. [Google Scholar] [CrossRef]
- Elisafitri, M.; Satyantini, W.; Arief, M.; Sulmartiwi, L. Parasitic disease in Koi fish (Cyprinus carpio) in freshwater ponds with different densities in Sukabumi, West Java. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Hangzhou, China, 17–19 July 2021; p. 012050. [Google Scholar]
- Mwainge, V.M.; Ogwai, C.; Aura, C.M.; Mutie, A.; Ombwa, V.; Nyaboke, H.; Oyier, K.N.; Nyaundi, J. An overview of fish disease and parasite occurrence in the cage culture of Oreochromis niloticus: A case study in Lake Victoria, Kenya. Aquat. Ecosyst. Health Manag. 2021, 24, 43–55. [Google Scholar] [CrossRef]
- Shah, I.M.; Khurshid, I.; Maqbool, N.; Ahmad, F.; Ahmad, S.M. Unveiling the potential of parasites as proxy bioindicators for water quality assessment in river Jhelum Kashmir, India. Environ. Monit. Assess. 2024, 196, 1156. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Lv, J.; Li, X.; Li, Y.; Zhang, Y.; Wang, X.; Liang, H.; Wang, Y.; Xie, J.; Shi, C. Unlocking Eco-solutions: Analyzing parasitic disease resistance in Yellow River carp (Cyprinus carpio haematopterus) under different ecological treatments. Aquaculture 2024, 582, 740500. [Google Scholar] [CrossRef]
- Bowman, D.M. More than a decade on: Mapping today’s regulatory and policy landscapes following the publication of nanoscience and nanotechnologies: Opportunities and uncertainties. NanoEthics 2017, 11, 169–186. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, S.G.; Kang, J.W.; Kim, S.W.; Kwon, J.; Lee, S.B.; Jung, W.J.; Park, S.C. Applications of carbon nanotubes and polymeric micro-/nanoparticles in fish vaccine delivery: Progress and future perspectives. Rev. Aquac. 2021, 13, 1844–1863. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Dawit Moges, F.; Patel, P.; Parashar, S.K.S.; Das, B. Mechanistic insights into diverse nano-based strategies for aquaculture enhancement: A holistic review. Aquaculture 2020, 519, 734770. [Google Scholar] [CrossRef]
- Bhattacharyya, A. Nanoparticles from drug delivery to insect pest control. Akshar 2009, 1, 1–7. [Google Scholar]
- Jonjaroen, V.; Charoonnart, P.; Jitrakorn, S.; Payongsri, P.; Surarit, R.; Saksmerprome, V.; Niamsiri, N. Nanoparticles-based double-stranded RNA delivery as an antiviral agent in shrimp aquaculture. Rev. Aquac. 2024, 16, 1647–1673. [Google Scholar] [CrossRef]
- Muruganandam, M.; Chipps, S.; Ojasvi, P. On the advanced technologies to enhance fisheries production and management. Acta Sci. Agric. 2019, 3, 216–222. [Google Scholar] [CrossRef]
- Mavichak, R.; Takano, T.; Kondo, H.; Hirono, I.; Wada, S.; Hatai, K.; Inagawa, H.; Takahashi, Y.; Yoshimura, T.; Kiyono, H.; et al. The effect of liposome-coated recombinant protein VP28 against white spot syndrome virus in kuruma shrimp, Marsupenaeus japonicus. J. Fish Dis. 2010, 33, 69–74. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Kim, M.-G.; Park, J.Y.; Shon, Y.; Kim, G.; Shim, G.; Oh, Y.-K. Nanotechnology and vaccine development. Asian J. Pharm. Sci. 2014, 9, 227–235. [Google Scholar] [CrossRef]
- Okeke, E.S.; Chukwudozie, K.I.; Nyaruaba, R.; Ita, R.E.; Oladipo, A.; Ejeromedoghene, O.; Atakpa, E.O.; Agu, C.V.; Okoye, C.O. Antibiotic resistance in aquaculture and aquatic organisms: A review of current nanotechnology applications for sustainable management. Environ. Sci. Pollut. Res. Int. 2022, 29, 69241–69274. [Google Scholar] [CrossRef]
- Chalamcheria, V. Nano vaccines: New paradigm in aqua health sector. J. Aquac. Mar. Biol. 2015, 3, 00061. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Mahdy, O.A.; Salem, M.A.; Abdelsalam, M.; Attia, M.M. An innovative approach to control fish-borne zoonotic metacercarial infections in aquaculture by utilizing nanoparticles. Sci. Rep. 2024, 14, 25307. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Rai, S.; Yadav, R.B.; Sharma, S.; Dave, V. Green synthesis and characterization of silver nanoparticles by Allium cepa L. to produce silver nano-coated fabric and their antimicrobial evaluation. Appl. Organomet. Chem. 2018, 32, e4146. [Google Scholar] [CrossRef]
- Kuan, G.C.; Sheng, L.P.; Rijiravanich, P.; Marimuthu, K.; Ravichandran, M.; Yin, L.S.; Lertanantawong, B.; Surareungchai, W. Gold-nanoparticle based electrochemical DNA sensor for the detection of fish pathogen Aphanomyces invadans. Talanta 2013, 117, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Seethalakshmi, P.; Rajeev, R.; Kiran, G.S.; Selvin, J. Shrimp disease management for sustainable aquaculture: Innovations from nanotechnology and biotechnology. Aquac. Int. 2021, 29, 1591–1620. [Google Scholar] [CrossRef]
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; El-Sayed Ali, T. A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac. Int. 2021, 29, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Meghani, N.; Dave, S.; Kumar, A. Introduction to Nanofood. In Nano-Food Engineering; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Vicari, T.; Dagostim, A.C.; Klingelfus, T.; Galvan, G.L.; Monteiro, P.S.; da Silva Pereira, L.; de Assis, H.C.S.; Cestari, M.M. Co-exposure to titanium dioxide nanoparticles (NpTiO2) and lead at environmentally relevant concentrations in the Neotropical fish species Hoplias intermedius. Toxicol. Rep. 2018, 5, 1032–1043. [Google Scholar] [CrossRef]
- Sayes, C.M.; Marchione, A.A.; Reed, K.L.; Warheit, D.B. Comparative pulmonary toxicity assessments of C60 water suspensions in rats: Few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano Lett. 2007, 7, 2399–2406. [Google Scholar] [CrossRef]
- Khosravi-Katuli, K.; Prato, E.; Lofrano, G.; Guida, M.; Vale, G.; Libralato, G. Effects of nanoparticles in species of aquaculture interest. Environ. Sci. Pollut. Res. 2017, 24, 17326–17346. [Google Scholar] [CrossRef]
- Fenaroli, F.; Westmoreland, D.; Benjaminsen, J.; Kolstad, T.; Skjeldal, F.M.; Meijer, A.H.; van der Vaart, M.; Ulanova, L.; Roos, N.; Nystrom, B. Nanoparticles as drug delivery system against tuberculosis in zebrafish embryos: Direct visualization and treatment. ACS Nano 2014, 8, 7014–7026. [Google Scholar] [CrossRef]
- Saleh, M.; Abdel-Baki, A.-A.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Antiprotozoal effects of metal nanoparticles against Ichthyophthirius multifiliis. Parasitology 2017, 144, 1802–1810. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, S.; Brahmchari, R.K.; Singh, A.B.; Rajendran, K.V.; Shukla, S.P.; Sharma, R.; Raman, R.P. Anti-parasitic efficacy of green-synthesized silver nanoparticles on Argulus siamensis: An ectoparasite of fish and their effect on the expression of ion channel genes. Aquac. Int. 2024, 33, 83. [Google Scholar] [CrossRef]
- Brahmchari, R.K.; Kumari, P.; Kumar, S.; Prasad, K.P.; Srivastava, P.P.; Bedekar, M.K.; Raman, R.P. In vivo antiparasitic activity of biogenic Iron nanoparticles on ectoparasitic branchiuran, Argulus siamensis in Labeo rohita and effect on parasite ion channel genes. BMC Vet. Res. 2025, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elala, N.M.; Attia, M.M.; Abd-Elsalam, R.M. Chitosan-silver nanocomposites in goldfish aquaria: A new perspective in Lernaea cyprinacea control. Int. J. Biol. Macromol. 2018, 111, 614–622. [Google Scholar] [CrossRef]
- Pimentel-Acosta, C.A.; Morales-Serna, F.N.; Chávez-Sánchez, M.C.; Lara, H.H.; Pestryakov, A.; Bogdanchikova, N.; Fajer-Ávila, E.J. Efficacy of silver nanoparticles against the adults and eggs of monogenean parasites of fish. Parasitol. Res. 2019, 118, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Aguilar, M.R.; Pattiya Arachchillage, K.G.G.; Chandra, S.; Rangan, S.; Ghosal Gupta, S.; Artes Vivancos, J.M. Biosensors for Public Health and Environmental Monitoring: The Case for Sustainable Biosensing. ACS Sustain. Chem. Eng. 2024, 12, 10296–10312. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Krishnani, K.K. Emerging bioanalytical sensors for rapid and close-to-real-time detection of priority abiotic and biotic stressors in aquaculture and culture-based fisheries. Sci. Total Environ. 2022, 838, 156128. [Google Scholar] [CrossRef]
- Simone Soares, M.; Singh, R.; Kumar, S.; Jha, R.; Nedoma, J.; Martinek, R.; Marques, C. The role of smart optical biosensors and devices on predictive analytics for the future of aquaculture systems. Opt. Laser Technol. 2024, 177, 111049. [Google Scholar] [CrossRef]
- Islam, S.I.; Ahammad, F.; Mohammed, H. Cutting-edge technologies for detecting and controlling fish diseases: Current status, outlook, and challenges. J. World Aquac. Soc. 2024, 55, e13051. [Google Scholar] [CrossRef]
- Srinivasan, R.; Umesh, S.; Murali, S.; Asokan, S.; Siva Gorthi, S. Bare fiber Bragg grating immunosensor for real-time detection of Escherichia coli bacteria. J. Biophotonics 2017, 10, 224–230. [Google Scholar] [CrossRef]
- Kaushik, S.; Tiwari, U.K.; Pal, S.S.; Sinha, R.K. Rapid detection of Escherichia coli using fiber optic surface plasmon resonance immunosensor based on biofunctionalized Molybdenum disulfide (MoS2) nanosheets. Biosens. Bioelectron. 2019, 126, 501–509. [Google Scholar] [CrossRef]
- Saleh, M.; Soliman, H.; El-Matbouli, M. Gold nanoparticles as a potential tool for diagnosis of fish diseases. Vet. Infect. Biol. Mol. Diagn. High-Throughput Strateg. 2015, 1247, 245–252. [Google Scholar]
- Zhao, G.; Ding, J.; Yu, H.; Yin, T.; Qin, W. Potentiometric aptasensing of Vibrio alginolyticus based on DNA nanostructure-modified magnetic beads. Sensors 2016, 16, 2052. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Gray, D.W.; Tsai, M.-Y.; Shields, N.; Montrose, A.; Creedon, N.; Lovera, P.; O’Riordan, A.; Mooney, M.H.; Vogel, E.M. A potentiometric biosensor for rapid on-site disease diagnostics. Biosens. Bioelectron. 2016, 79, 669–678. [Google Scholar] [CrossRef]
- Pires, N.M.M.; Dong, T. A cascade-like silicon filter for improved recovery of oocysts from environmental waters. Environ. Technol. 2014, 35, 781–790. [Google Scholar] [CrossRef]
- Laczka, O.; Skillman, L.; Ditcham, W.G.; Hamdorf, B.; Wong, D.K.; Bergquist, P.; Sunna, A. Application of an ELISA-type screen printed electrode-based potentiometric assay to the detection of Cryptosporidium parvum oocysts. J. Microbiol. Methods 2013, 95, 182–185. [Google Scholar] [CrossRef]
- Santos-de-Souza, R.; Souza-Silva, F.; de Albuquerque-Melo, B.C.; Ribeiro-Guimaraes, M.L.; de Castro Cortes, L.M.; Pereira, B.A.S.; Silva-Almeida, M.; Cysne-Finkelstein, L.; de Oliveira Junior, F.O.R.; Pereira, M.C.S.; et al. Insights into the tracking of the cysteine proteinase B COOH-terminal polypeptide of Leishmania (Leishmania) amazonensis by surface plasmon resonance. Parasitol. Res. 2019, 118, 1249–1259. [Google Scholar] [CrossRef]
- Xue, Y.; Kong, Q.; Ding, H.; Xie, C.; Zheng, B.; Zhuo, X.; Ding, J.; Tong, Q.; Lou, D.; Lu, S.; et al. A novel loop-mediated isothermal amplification-lateral-flow-dipstick (LAMP-LFD) device for rapid detection of Toxoplasma gondii in the blood of stray cats and dogs. Parasite 2021, 28, 41. [Google Scholar] [CrossRef] [PubMed]
- Fanouraki, E.; Papandroulakis, N.; Ellis, T.; Mylonas, C.; Scott, A.; Pavlidis, M. Water cortisol is a reliable indicator of stress in European sea bass, Dicentrarchus labrax. Behaviour 2008, 145, 1267–1281. [Google Scholar] [CrossRef]
- Leitão, C.; Leal-Junior, A.; Almeida, A.R.; Pereira, S.O.; Costa, F.M.; Pinto, J.L.; Marques, C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 2021, 29, e00587. [Google Scholar] [CrossRef] [PubMed]
- Brijs, J.; Fore, M.; Grans, A.; Clark, T.D.; Axelsson, M.; Johansen, J.L. Bio-sensing technologies in aquaculture: How remote monitoring can bring us closer to our farm animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200218. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).