Induced Mammary Epithelial Cell-Derived Extracellular Vesicles Promote the Repair of Skin Trauma
Abstract
1. Introduction
2. Results
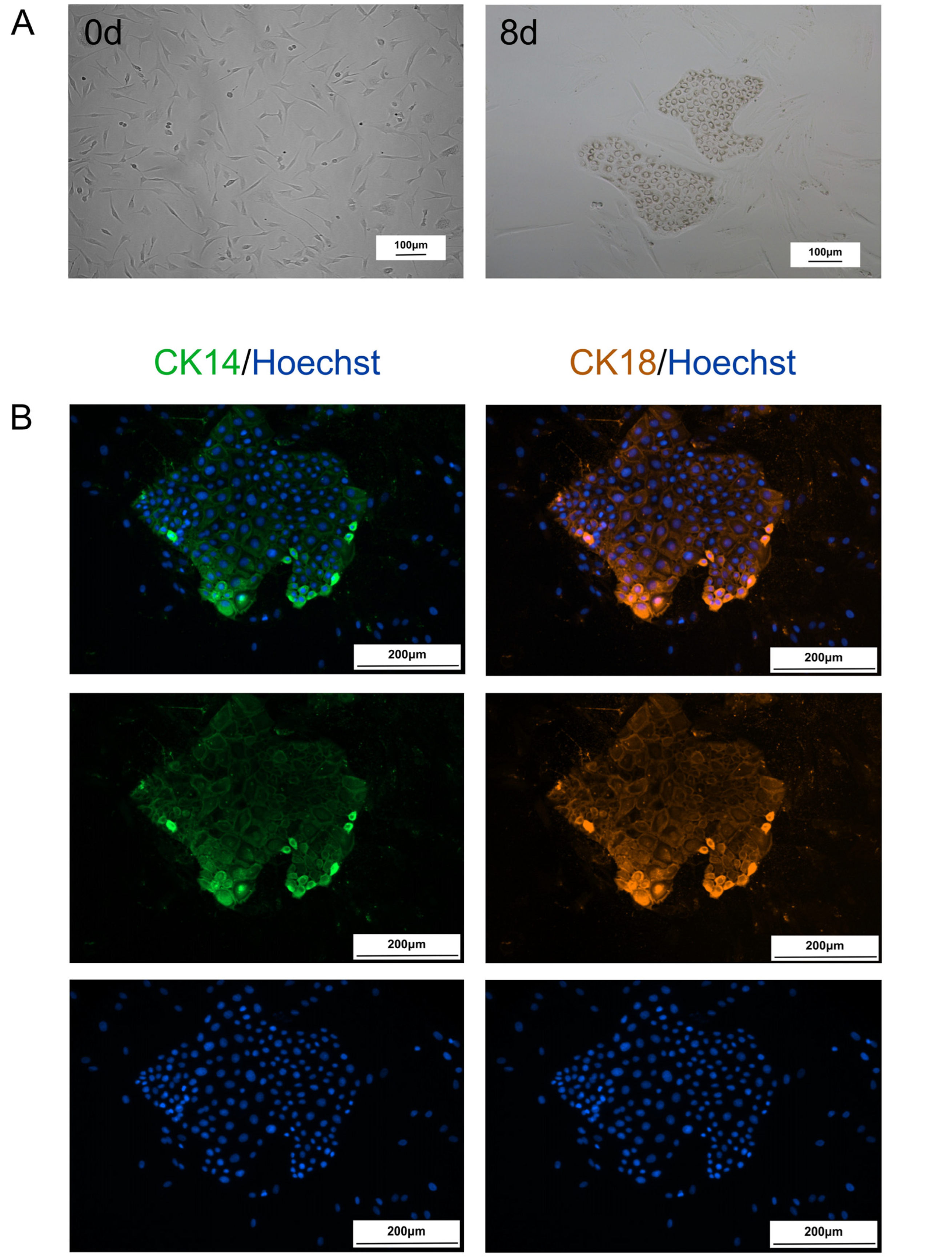
2.1. Fibroblasts Are Transformed into CiMECs Through the Action of RepSox
2.2. Extraction and Characterization of CiMECs-EVs
2.3. Preparation of the CMECG
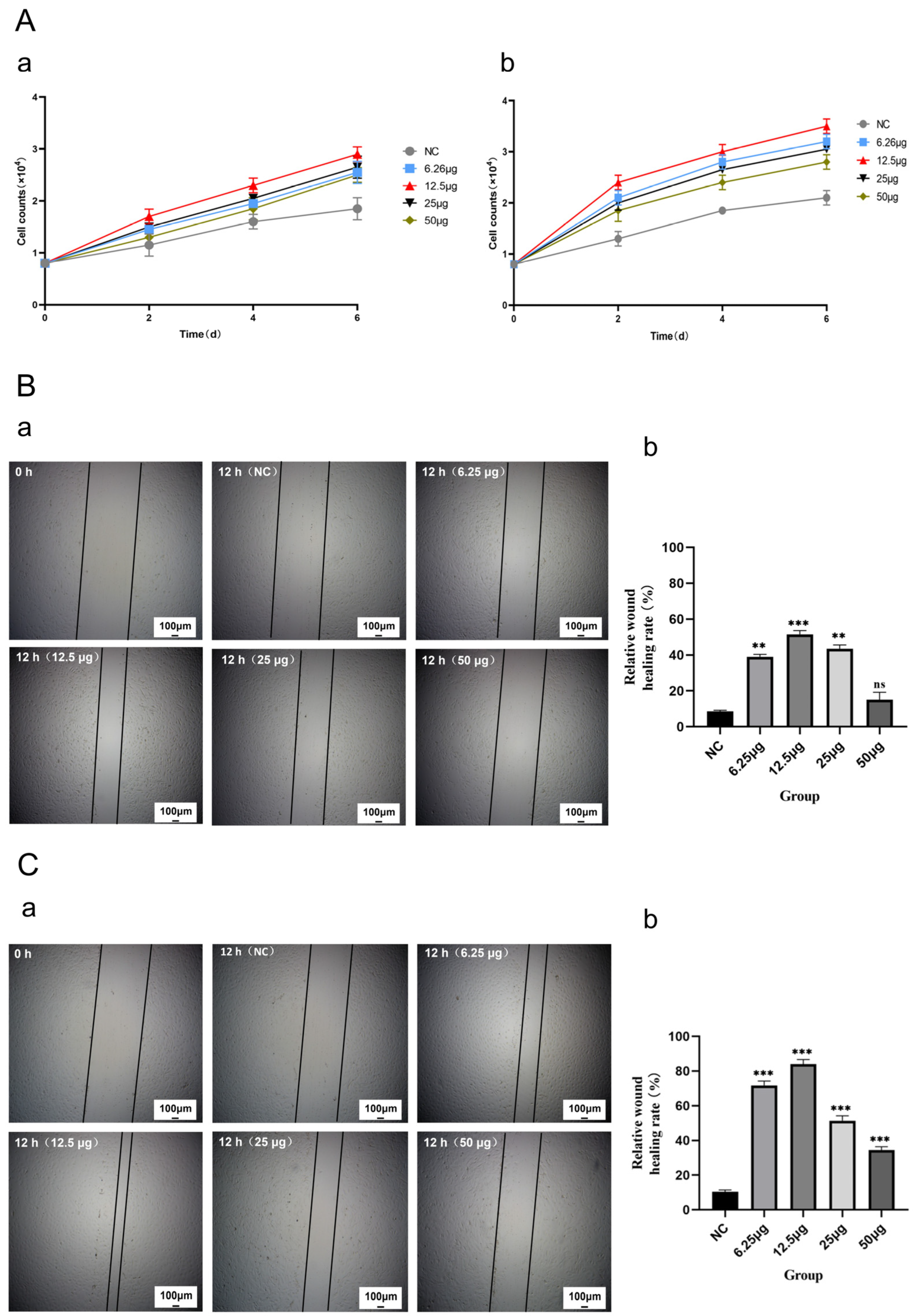
2.4. CMECG Can Promote the Proliferation of Fibroblasts/MECs
2.5. CMECG Can Promote the Migration of Fibroblasts/MECs
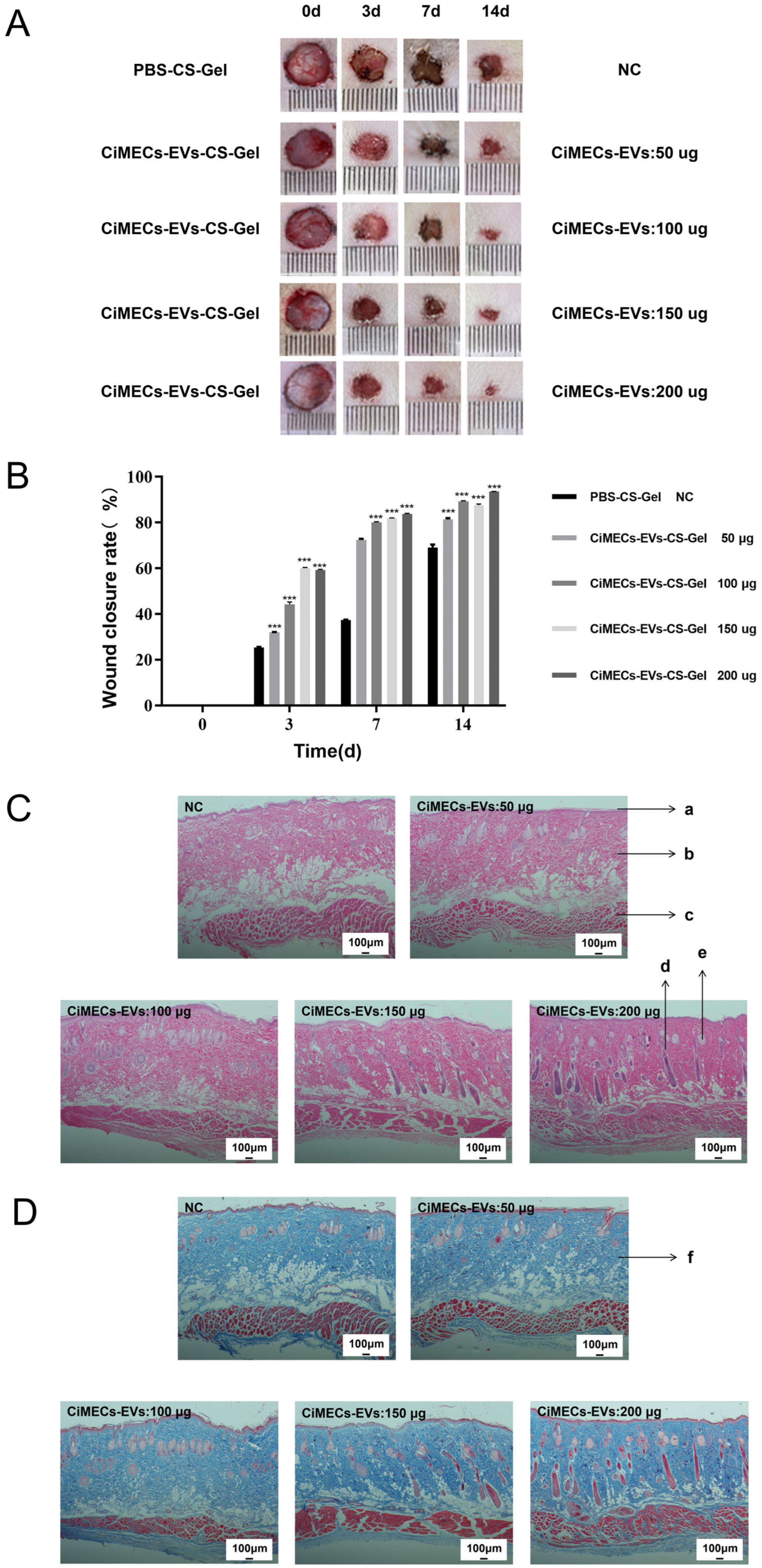
2.6. CMECG Can Accelerate the Repair of Wounded Skin in Rats
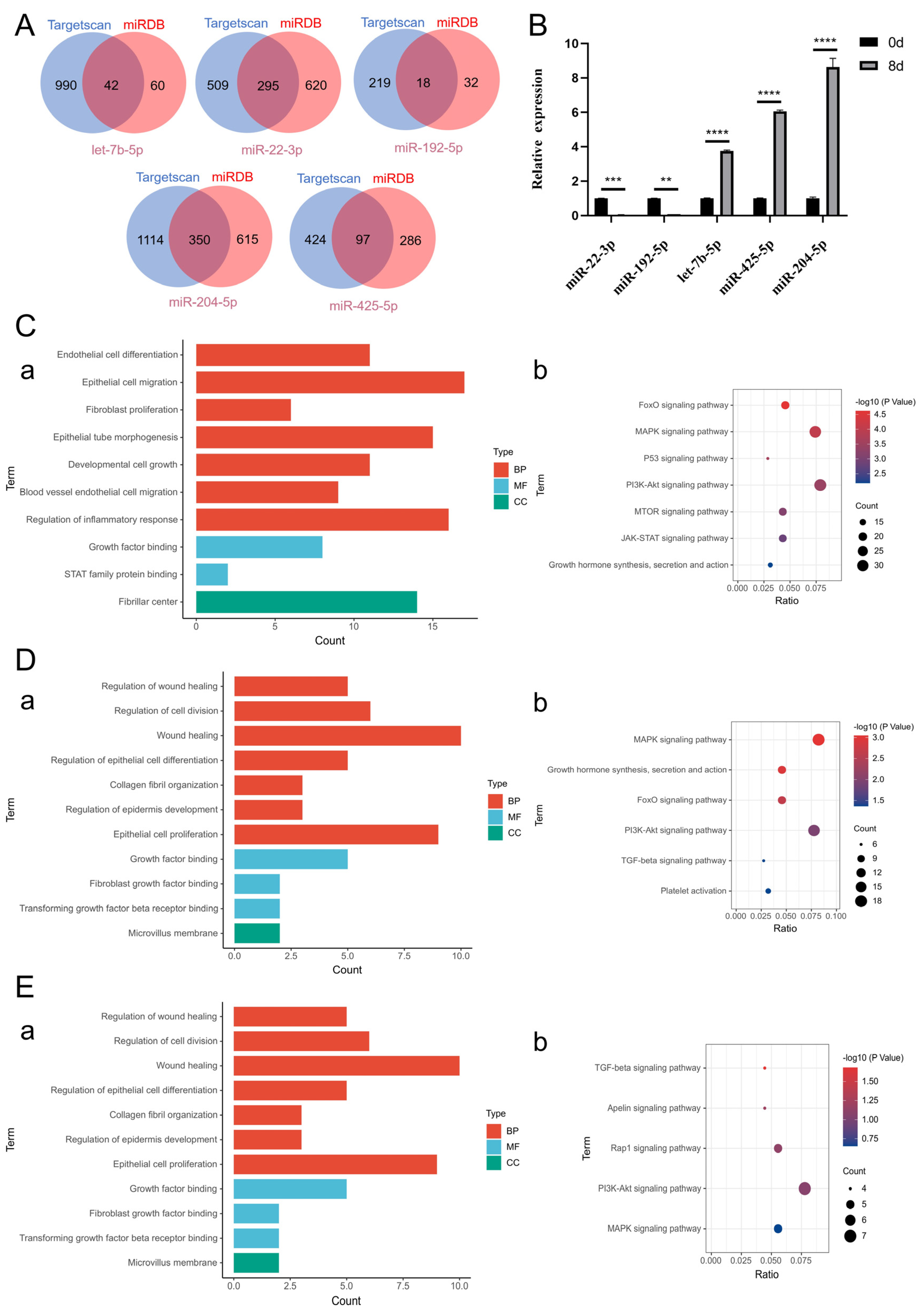
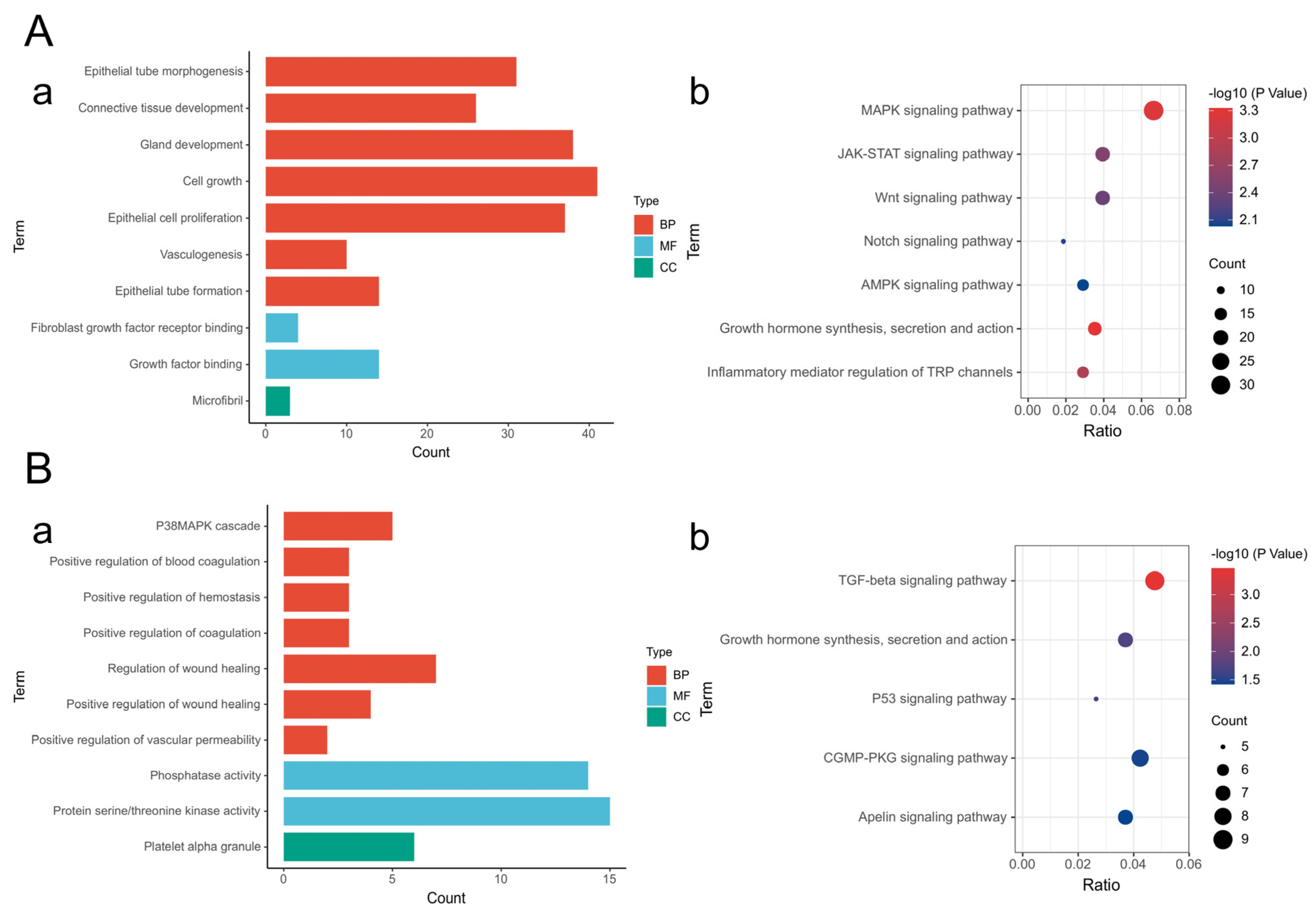
2.7. Mechanistic Study of CiMECs-EVs in Skin Wound Repair
3. Discussion
4. Materials and Methods
- Cell culture
- Induction of CiMECs
- IF staining
- EV extraction
- Morphology of the EVs observed via transmission electron microscopy
- Analysis of EV particle size via a nanoparticle tracking analyzer (NTA)
- Protein markers of EVs detected by Western blot
- Preparation of the CMECG
- Retention effect of the CMECG
- Release effect of CMECG
- The ability of CMECG to promote cell proliferation
- CMECG promoted cell migration ability
- Preparation of the rat skin wound model
- Assessment of wound healing ability
- Histological evaluation
- HE staining
- Masson staining
- miRNA sequencing
- Differential miRNA target gene prediction
- Target gene functional enrichment and pathway enrichment analysis
- qPCR detection
- Quantification and statistical analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; He, Q.; Yang, M.; Qiao, Q.; Chen, J.; Song, J.; Zang, N.; Hu, H.; Xia, L.; Xiang, Y.; et al. Glycoengineered extracellular vesicles released from antibacterial hydrogel facilitate diabetic wound healing by promoting angiogenesis. J. Extracell. Vesicles 2024, 13, e70013. [Google Scholar] [CrossRef]
- Raghuram, A.C.; Yu, R.P.; Lo, A.Y.; Sung, C.J.; Bircan, M.; Thompson, H.J.; Wong, A.K. Role of stem cell therapies in treating chronic wounds: A systematic review. World J. Stem Cells 2020, 12, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Vardakostas, D.; Moustogiannis, A.; Garoufalia, Z.; Karatza, E.; Philippou, A.; Kouraklis, G.; Koutsilieris, M.; Mantas, D. Expression of Tissue Remodeling- and Inflammation-Related Factors During the Wound-Healing Process in Humans. J. Pers. Med. 2025, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhao, H.; Wang, Y.; Cen, Y.; Zhang, Z. Mechanisms and applications of adipose-derived stem cell-extracellular vesicles in the inflammation of wound healing. Front. Immunol. 2023, 14, 1214757. [Google Scholar] [CrossRef]
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of Severe COVID-19 with Human Umbilical Cord Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- D’aUdigier, C.; Cochain, C.; Rossi, E.; Guérin, C.L.; Bièche, I.; Blandinières, A.; Marsac, B.; Silvestre, J.-S.; Gaussem, P.; Smadja, D.M. Thrombin receptor PAR-1 activation on endothelial progenitor cells enhances chemotaxis-associated genes expression and leukocyte recruitment by a COX-2-dependent mechanism. Angiogenesis 2015, 18, 347–359. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221, Correction in Stem Cell Res. Ther. 2021, 12, 490. [Google Scholar] [CrossRef]
- Carney, R.P.; Mizenko, R.R.; Bozkurt, B.T.; Lowe, N.; Henson, T.; Arizzi, A.; Wang, A.; Tan, C.; George, S.C. Harnessing extracellular vesicle heterogeneity for diagnostic and therapeutic applications. Nat. Nanotechnol. 2025, 20, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Lim, S.K. Overcoming challenges in MSC-sEV therapeutics: Insights and advances after a decade of research. Cytotherapy 2025, 27, 843–848. [Google Scholar] [CrossRef]
- Lee, J.C.; Ray, R.M.; Scott, T.A. Prospects and challenges of tissue-derived extracellular vesicles. Mol. Ther. 2024, 32, 2950–2978. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Y.; Wang, Y.; Gao, H.; Li, Y.; Zhang, C.; Chen, Q.; Chen, S.; Peng, Q. Multifunctional Regeneration Silicon-Loaded Chitosan Hydrogels for MRSA-Infected Diabetic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2303501. [Google Scholar] [CrossRef]
- Born, L.J.; McLoughlin, S.T.; Dutta, D.; Mahadik, B.; Jia, X.; Fisher, J.P.; Jay, S.M. Sustained released of bioactive mesenchymal stromal cell-derived extracellular vesicles from 3D-printed gelatin methacrylate hydrogels. J. Biomed. Mater. Res. 2022, 110 Pt A, 1190–1198. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Wang, J.; Kang, Y.; Wang, Z.; Cao, W.; Ye, J.; Gao, C. A tough, antibacterial and antioxidant hydrogel dressing accelerates wound healing and suppresses hypertrophic scar formation in infected wounds. Bioact. Mater. 2024, 34, 269–281. [Google Scholar] [CrossRef]
- Xu, H.; Huang, S.; Wang, J.; Lan, Y.; Feng, L.; Zhu, M.; Xiao, Y.; Cheng, B.; Xue, W.; Guo, R. Enhanced cutaneous wound healing by functional injectable thermosensitive chitosan-based hydrogel encapsulated human umbilical cord-mesenchymal stem cells. Int. J. Biol. Macromol. Struct. Funct. Interact. 2019, 137, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Y.; Li, Y.; Liu, W.; Yin, L.; Yin, S.; Ji, C.; Hu, Y.; Wang, Q.; Zhou, X.; et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury by regulating microRNA-146b expression. Biotechnol. Lett. 2020, 42, 669–679. [Google Scholar] [CrossRef]
- Tejaswini, T.; Keerthana, M.; Vidyavathi, M.; Kumar, R.V.S. Design and evaluation of atorvastatin-loaded chitosan-hydroxyapatite composite bioscaffolds for wound-healing activity. Future J. Pharm. Sci. 2020, 6, 111. [Google Scholar] [CrossRef]
- Altanerova, U.; Jakubechova, J.; Benejova, K.; Priscakova, P.; Pesta, M.; Pitule, P.; Topolcan, O.; Kausitz, J.; Zduriencikova, M.; Repiska, V.; et al. Prodrug suicide gene therapy for cancer targeted intracellular by mesenchymal stem cell exosomes. Int. J. Cancer 2019, 144, 897–908. [Google Scholar] [CrossRef]
- Chen, J.-S.; Chen, H.; Xu, C.-S.; Yang, J.; Jia, Y.-Y. Preparation of Cangyi nanoemulsion in situ gel and drug release mechanism of nasal mucosa in vitro. China J. Chin. Mater. Med. 2020, 45, 4211–4220. [Google Scholar]
- Bedelbaeva, K.; Cameron, B.; Latella, J.; Aslanukov, A.; Gourevitch, D.; Davuluri, R.; Heber-Katz, E. Epithelial-mesenchymal transition: An organizing principle of mammalian regeneration. Front. Cell Dev. Biol. 2023, 11, 16. [Google Scholar] [CrossRef]
- Matoušková, E.; Dudorkinová, D.; Krásná, L.; Veselý, P. Temporal in vitro expansion of the luminal lineage of human mammary epithelial cells achieved with the 3T3 feeder layer technique. Breast Cancer Res. Treat. 2000, 60, 241–249. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, G.; Qin, L.; Liu, Q.; Zhu, S.; Ye, S.; Li, X.; Wu, Y.; Hu, Y.; Liu, S.; et al. Restoring mammary gland structures and functions with autogenous cell therapy. Biomaterials 2021, 277, 121075. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.-J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Li, W.; Lu, Y.; Xu, X.; Hu, R.; Liu, H.; Wang, Y.; Xing, Q.; Wei, Z.; et al. Thermosensitive hydrogel as a sustained release carrier for mesenchymal stem cell-derived extracellular vesicles in the treatment of intrauterine adhesion. J. Nanobiotechnol. 2024, 22, 570. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef]
- Jimi, S.; Saparov, A.; Takagi, S. Editorial: Cellular and Molecular Mechanisms at the Proliferation Stage in Wound Healing: From Scarring to Tissue Regeneration. Front. Cell Dev. Biol. 2021, 19, 659089. [Google Scholar] [CrossRef]
- Oh, E.J.; Gangadaran, P.; Rajendran, R.L.; Kim, H.M.; Oh, J.M.; Choi, K.Y.; Chung, H.Y.; Ahn, B.C. Extracellular vesicles derived from fibroblasts promote wound healing by optimizing fibroblast and endothelial cellular functions. Stem Cells 2021, 39, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicles have variable dose-dependent effects on cultured draining cells in the eye. J. Cell. Mol. Med. 2018, 22, 1992. [Google Scholar] [CrossRef]
- Bui, T.M.; Mascarenhas, L.A.; Sumagin, R. Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair. Tissue Barriers 2018, 6, e1431038. [Google Scholar] [CrossRef] [PubMed]
- Thai, V.L.; Ramos-Rodriguez, D.H.; Mesfin, M.; Leach, J.K. Hydrogel degradation promotes angiogenic and regenerative potential of cell spheroids for wound healing. Mater. Today Bio 2023, 22, 100769. [Google Scholar] [CrossRef]
- Song, Y.; You, Y.; Xu, X.; Lu, J.; Huang, X.; Zhang, J.; Zhu, L.; Hu, J.; Wu, X.; Xu, X.; et al. Adipose-Derived Mesenchymal Stem Cell-Derived Exosomes Biopotentiated Extracellular Matrix Hydrogels Accelerate Diabetic Wound Healing and Skin Regeneration. Adv. Sci. 2023, 10, e2304023. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Shi, T.; Yu, H.; Bi, J.; Chen, G. Epigallocatechin-3-gallate Augments Therapeutic Effects of Mesenchymal Stem Cells in Skin Wound Healing. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sen, C.K. MiRNA in innate immune responses: Novel players in wound inflammation. Physiol Genom. 2011, 43, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Skovronova, R.; Marabese, F.; Bussolati, B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells 2019, 8, 1240. [Google Scholar] [CrossRef]
- Fernandes, H.; Zonnari, A.; Abreu, R.; Aday, S.; Barão, M.; Albino, I.; Lino, M.; Branco, A.; Seabra, C.; Barata, T.; et al. Extracellular vesicles enriched with an endothelial cell pro-survival microRNA affects skin tissue regeneration. Mol. Ther.-Nucleic Acids 2022, 28, 307–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Liu, Y.; Chen, Z.; Li, X.; Tang, L.; Li, J.; Duan, M.; Zhang, G. Human Amniotic Fluid Stem Cell-Derived Exosomes as a Novel Cell-Free Therapy for Cutaneous Regeneration. Front. Cell Dev. Biol. 2021, 9, 685873. [Google Scholar] [CrossRef]
- Pea, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 18. [Google Scholar] [CrossRef]

| Main Instruments | Brand | Model | Origin |
|---|---|---|---|
| CO2 Incubator | Thermo | 371 | Waltham, MA, USA |
| Ultra-clean Workbench | Suzhou Jiade Purification | JB-CJ-1000FX | Suzhou, Jiangsu, China |
| Inverted Microscope | Nikon | Eclipse MA100N | Tokyo, Japan |
| Centrifuge | Eppendorf | 5702/R/RH | Hamburg, Germany |
| Constant Temperature Water Bath | Stuart | SWB1 | Stone, Staffordshire, UK |
| Gel Imaging System | Tianneng | 1600 | Shanghai, China |
| Inverted Fluorescence Microimaging System | Nikon | Eclipse Ti2 | Tokyo, Japan |
| Cell Imaging Multimode Microplate Reader | Bio Tek | Bio Tek Synergy Neo2 | Winooski, VT, USA |
| Refrigerator | Haier | DW-86L286 | Qingdao, Shandong, China |
| Ice Maker | Haier | HZB-12A | Qingdao, Shandong, China |
| Pipette | Eppendorf | Research plus | Hamburg, Germany |
| Drying Oven | Lichen | DZF-6020AB | Shanghai, China |
| Autoclave | Panasonic | TOMY SX-500 | Osaka, Japan |
| Purification System | Millipore | Milli-Q integral | Burlington, MA, USA |
| Refrigerated Centrifuge | Eppendorf | 5910 Ri | Hamburg, Germany |
| Ultracentrifuge | BeckMan | Optima XE-100 | Brea, CA, USA |
| Transmission Electron Microscope | Hitachi | HT7800 | Tokyo, Japan |
| Magnetic Stirrer | Lichen | 78-1 | Shanghai, China |
| Vortex Mixer | Lichen | LC-Mixer-RD | Shanghai, China |
| Electron Microscope | FEI | Tecnai G2 20 | Hillsboro, OR, USA |
| Nanoparticle Tracking Analyzer | ZetaView | ZetaView PMX 110 | Berlin, Germany |
| Electric Constant Temperature Shaker | IKA | THZ-103B | Staufen, Germany |
| Microplate Reader | Biotek | Synergy H1 | Winooski, VT, USA |
| The miRNA Primers | ||
|---|---|---|
| Primer: let-7b-5p RT: GTCGTATCCAGTCAGGGTCCGAGGTATCGCACTGGATACGACAACCAC | This paper | N/A |
| Primer: let-7b-5p Forward: GCGCGTGAGGTAGTAGGTTGT | This paper | N/A |
| Primer: let-7b-5p Reverse: AGTGCAGGGTCCGAGGTATT | This paper | N/A |
| Primer: miR-22-3p RT: GTCGTATCAGTGCAGGGTCCGAGGTATCGCACTGGATACGACACGTT | This paper | N/A |
| Primer: miR-22-3p Forward: GCGAAGCTGCCAGTTGAAG | This paper | N/A |
| Primer: miR-22-3p Reverse: AGTGCAGGGTCCGAGGTATT | This paper | N/A |
| Primer: miR-192-5p RT: GTCGTATCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGCTGT | This paper | N/A |
| Primer: miR-192-5p Forward: GCGCGCTGACCTATGAATTG | This paper | N/A |
| Primer: miR-192-5p Reverse: AGTGCAGGGTCCGAGGTATT | This paper | N/A |
| Primer: miR-204-5p RT: GTCGTATCAGTGCAGGGTCCGAGGTATCGCACTGGATACGACAGGCAT | This paper | N/A |
| Primer: miR-204-5p Forward: CGCGTTCCTTTTGTCATCCT | This paper | N/A |
| Primer: miR-204-5p Reverse: AGTGCAGGGTCCGAGGTATT | This paper | N/A |
| Primer: miR-425-5p RT: GTCGTATCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACG | This paper | N/A |
| Primer: miR-425-5p Forward: GCGAATGACACGATCACTCC | This paper | N/A |
| Primer: miR-425-5p Reverse: AGTGCAGGGTCCGAGGTATT | This paper | N/A |
| Primer: U6 Forward: TCTGCTTTACTGCCGACCAG | This paper | N/A |
| Primer: U6 Reverse: CAGGCTGATGTGGAAGGAGG | This paper | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Zhang, D.; Wang, G.; Sun, L.; Wei, M.; Deng, S.; Chen, J.; Kallingappa, P.; Yuan, X.; Huang, B. Induced Mammary Epithelial Cell-Derived Extracellular Vesicles Promote the Repair of Skin Trauma. Int. J. Mol. Sci. 2025, 26, 9929. https://doi.org/10.3390/ijms26209929
Pan S, Zhang D, Wang G, Sun L, Wei M, Deng S, Chen J, Kallingappa P, Yuan X, Huang B. Induced Mammary Epithelial Cell-Derived Extracellular Vesicles Promote the Repair of Skin Trauma. International Journal of Molecular Sciences. 2025; 26(20):9929. https://doi.org/10.3390/ijms26209929
Chicago/Turabian StylePan, Siyao, Dandan Zhang, Guodong Wang, Longfei Sun, Mengzhen Wei, Shan Deng, Jianwei Chen, Prasanna Kallingappa, Xiang Yuan, and Ben Huang. 2025. "Induced Mammary Epithelial Cell-Derived Extracellular Vesicles Promote the Repair of Skin Trauma" International Journal of Molecular Sciences 26, no. 20: 9929. https://doi.org/10.3390/ijms26209929
APA StylePan, S., Zhang, D., Wang, G., Sun, L., Wei, M., Deng, S., Chen, J., Kallingappa, P., Yuan, X., & Huang, B. (2025). Induced Mammary Epithelial Cell-Derived Extracellular Vesicles Promote the Repair of Skin Trauma. International Journal of Molecular Sciences, 26(20), 9929. https://doi.org/10.3390/ijms26209929






