Investigation of the Role of miR-1236-3p in Heat Tolerance of American Shad (Alosa sapidissima) by Targeted Regulation of hsp90b1
Abstract
1. Introduction
2. Results
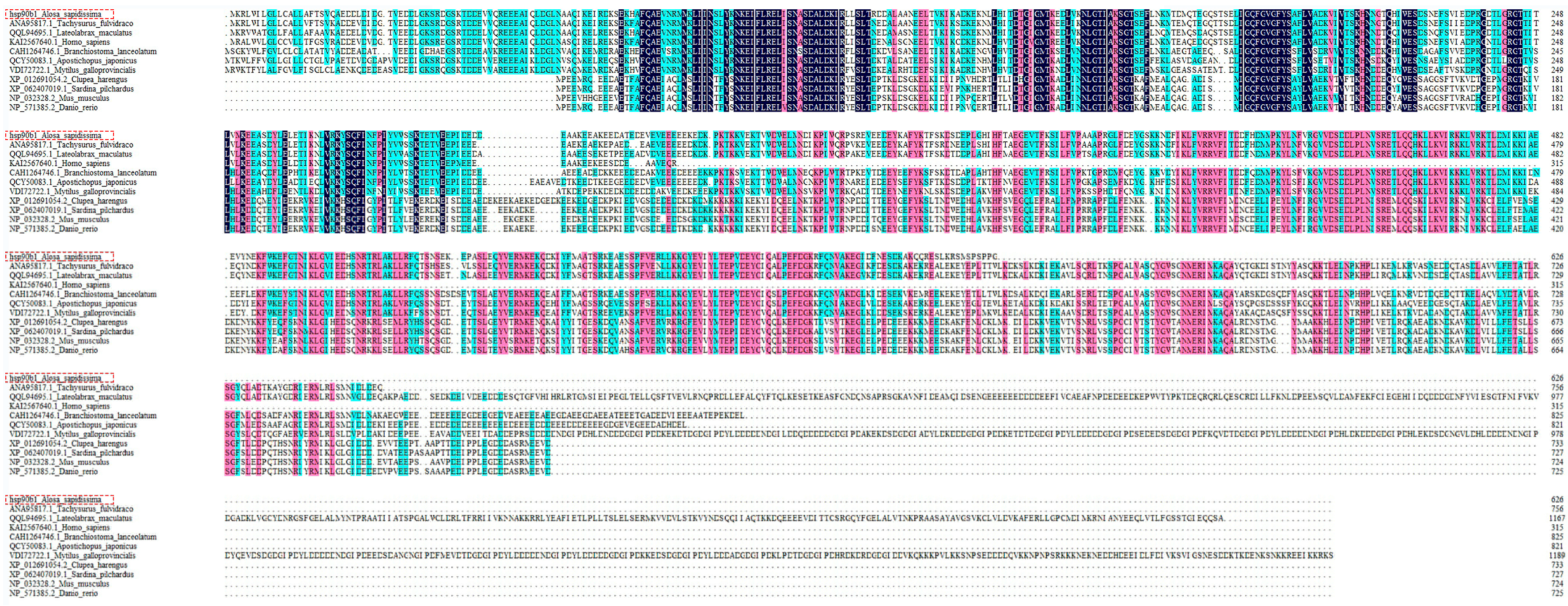
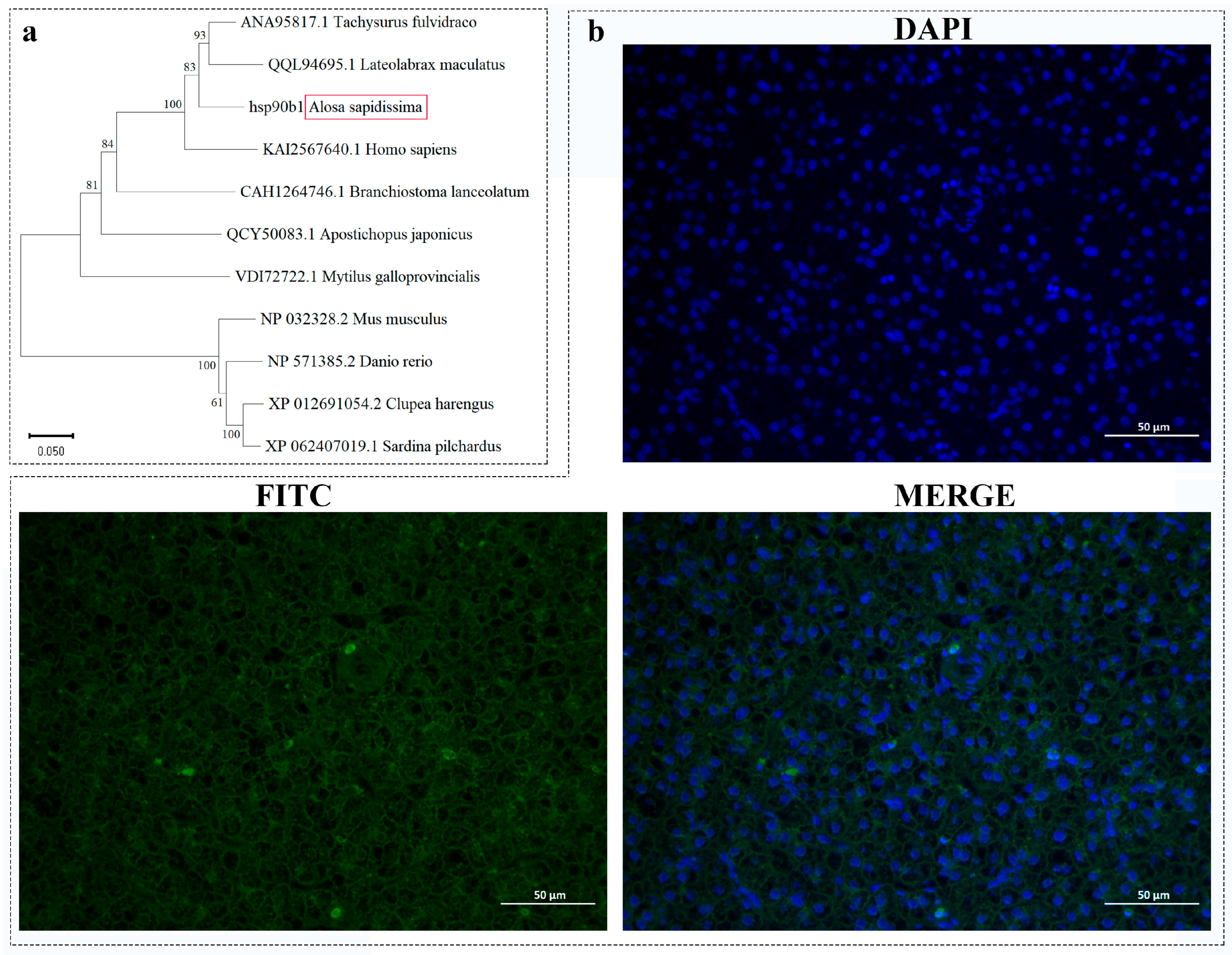
2.1. Cloning and Sequence Characterization of the hsp90b1 Gene
2.2. Association Analysis Between miR-1236-3p and hsp90b1
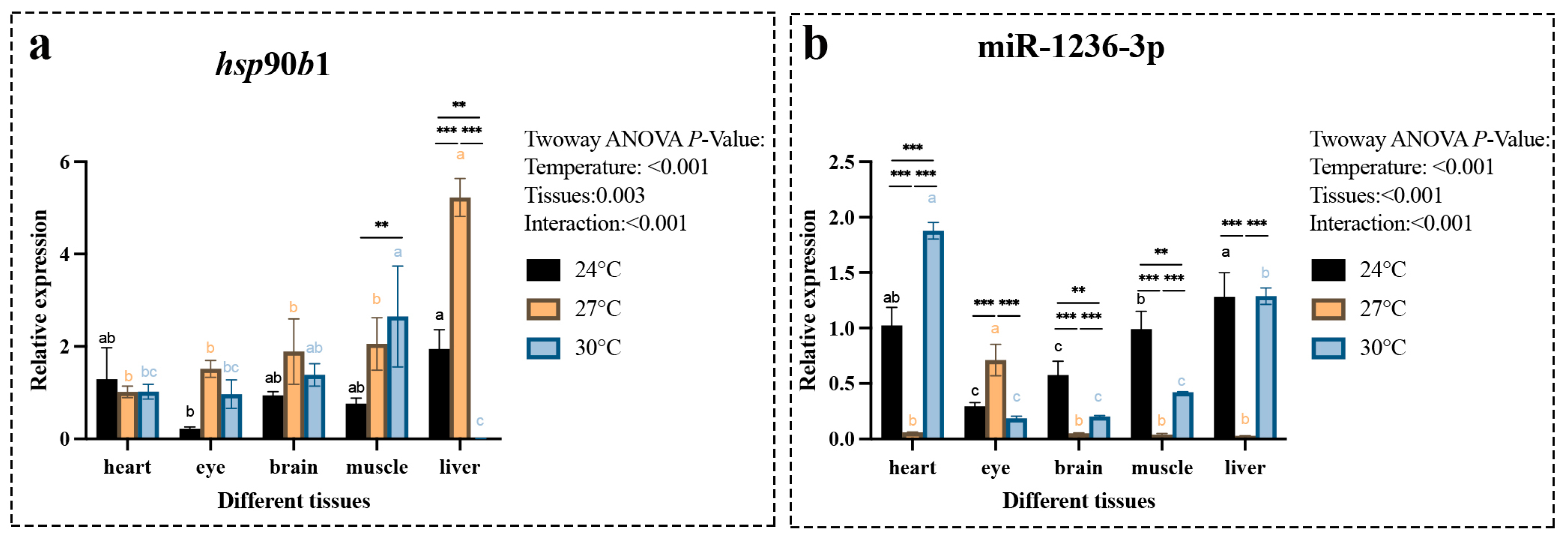
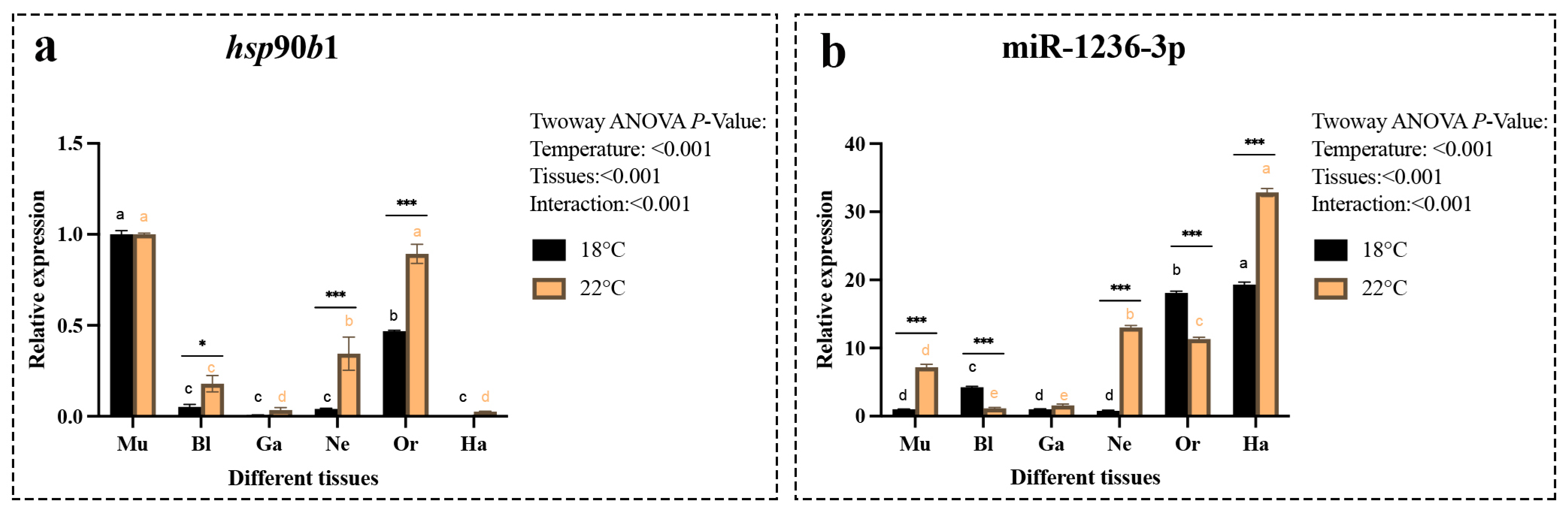
2.3. Spatiotemporal Expression Properties of the hsp90b1 and miR-1236-3p
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Experimental Fish and Sample Collection
4.3. Cloning and In Situ Hybridization (ISH) Analysis
4.4. Dual-Luciferase Reporter Assays
4.5. RNA Isolation and Quantitative PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HSP | Heat shock protein |
| miRNA | MicroRNAs |
| CDS | Coding sequence |
| UTR | Untranslated region |
| NJ | Neighbor joining |
| qRT-PCR | Real-time quantitative reverse transcription polymerase chain reaction |
References
- Yadav, N.K.; Patel, A.B.; Singh, S.K.; Mehta, N.K.; Anand, V.; Lal, J.; Dekari, D.; Devi, N.C. Climate change effects on aquaculture production and its sustainable management through climate-resilient adaptation strategies: A review. Environ. Sci. Pollut. Res. Int. 2024, 31, 31731–31751. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish. Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.; Kimera, F.; Sewilam, H. Anthropogenic temperature fluctuations and their effect on aquaculture: A comprehensive review. Aquac. Fish. 2022, 7, 223–243. [Google Scholar] [CrossRef]
- Soyano, K.; Mushirobira, Y. The Mechanism of Low-Temperature Tolerance in Fish. Adv. Exp. Med. Biol. 2018, 1081, 149–164. [Google Scholar]
- Dahms, C.; Killen, S.S. Temperature change effects on marine fish range shifts: A meta-analysis of ecological and methodological predictors. Glob. Change Biol. 2023, 29, 4459–4479. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.H.; Park, J.W.; Lee, J.H.; Kim, J.H. High water temperature-mediated immune gene expression of olive flounder, Paralichthys olivaceus according to pre-stimulation at high temperatures. Environ. Toxicol. Pharmacol. 2023, 101, 104159. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, R.O.; Batista, R.O.; Corrêa, C.F.; Mattioni, B.; Filer, K.; Pettigrew, J.E.; Fracalossi, D.M. Dietary supplementation of Aurantiochytrium sp. meal, a docosahexaenoic-acid source, promotes growth of Nile tilapia at a suboptimal low temperature. Aquaculture 2019, 507, 500–509. [Google Scholar] [CrossRef]
- Fang, M.; Lei, Z.; Ruilin, M.; Jing, W.; Leqiang, D. High temperature stress induced oxidative stress, gut inflammation and disordered metabolome and microbiome in tsinling lenok trout. Ecotoxicol. Environ. Saf. 2023, 266, 115607. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Padmini, E.; Usha Rani, M. Impact of seasonal variation on HSP70 expression quantitated in stressed fish hepatocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 278–285. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, Z.; Ji, J.; Wang, T.; Yin, S.; Zhang, K. Characterization of HSP70 and HSP90 Gene Family in Takifugu fasciatus and Their Expression Profiles on Biotic and Abiotic Stresses Response. Genes 2024, 15, 1445. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Z.; Li, Y.; Zhu, W.; Feng, B.; Xu, W.; Fu, J.; Wei, P.; Luo, M.; Dong, Z. Integrated transcriptome and microRNA analysis reveals molecular responses to high-temperature stress in the liver of American shad (Alosa sapidissima). BMC Genom. 2024, 25, 656. [Google Scholar] [CrossRef] [PubMed]
- Higgs, D.M.; Plachta, D.T.; Rollo, A.K.; Singheiser, M.; Hasting, M.C.; Popper, A.N. Development of ultrasound detection in American shad (Alosa sapidissima). J. Exp. Biol. 2004, 207, 155–163. [Google Scholar] [CrossRef]
- Li, L.; Zhu, H.J.; Yi, X.Y.; Nie, Z.J.; Zheng, Y.; Yang, X.W.; Xu, P.; Yu, Y.; Xu, G. Study of the Quality and Nutritional Value of Alosa sapidissima in the Postmortem Process. Fishes 2022, 7, 302. [Google Scholar] [CrossRef]
- Gao, X.Q.; Liu, Z.F.; Guan, C.T.; Huang, B.; Lei, J.L.; Li, J.; Guo, Z.-L.; Wang, Y.-H.; Hong, L. Developmental changes in digestive enzyme activity in American shad, Alosa sapidissima, during early ontogeny. Fish Physiol. Biochem. 2017, 43, 397–409. [Google Scholar] [CrossRef]
- Liang, Z.; Miao, L.; Liu, Y.; Luo, M.; Feng, B.; Zhu, W.; Gong, Y.; Shi, X.; Li, Y.; Jiang, C.; et al. Effect of temperature on the embryonic development of American shad (Alosa sapidissima). J. Fish. China 2025, 49, 089104. (In Chinese) [Google Scholar]
- Luo, M.; Zhu, W.; Liang, Z.; Feng, B.; Xie, X.; Li, Y.; Liu, Y.; Shi, X.; Fu, J.; Miao, L.; et al. High-temperature stress response: Insights into the molecular regulation of American shad (Alosa sapidissima) using a multi-omics approach. Sci. Total Environ. 2024, 916, 170329. [Google Scholar] [CrossRef]
- Luo, M.; Feng, B.; Zhu, W.; Liang, Z.; Xu, W.; Fu, J.; Miao, L.; Dong, Z. Proteomics and metabolomics analysis of American shad (Alosa sapidissima) liver responses to heat stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 296, 111686. [Google Scholar] [CrossRef] [PubMed]
- Tafrihi, M.; Hasheminasab, E. MiRNAs: Biology, Biogenesis, their Web-based Tools, and Databases. Microrna 2019, 8, 4–27. [Google Scholar] [CrossRef]
- Bizuayehu, T.T.; Babiak, I. MicroRNA in teleost fish. Genome Biol. Evol. 2014, 6, 1911–1937. [Google Scholar] [CrossRef]
- Dweep, H.; Showe, L.C.; Kossenkov, A.V. Functional Annotation of MicroRNAs Using Existing Resources. Methods Mol. Biol. 2022, 2257, 57–77. [Google Scholar]
- Gavarikar, S.; Craig, P.M. Developmental exposure to constant elevated temperatures and diel thermal variation alters microRNA expression and performance in zebrafish (Danio rerio). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2025, 279, 111122. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, F.; Kang, Y.; Liu, X. Gene ssa-miR-301a-3p improves rainbow trout (Oncorhynchus mykiss) resistance to heat stress by targeting hsp90b2. PeerJ 2022, 10, e13476. [Google Scholar] [CrossRef] [PubMed]
- Zaman, T.; Fahad, T.M.; Rana, M.; Hossain, M.S.; Mamun, A.; Haque, M.A.; Sarker, A.; Islam, S.; Haque, M.-U.L.; Naz, T.; et al. Endosulfan affects embryonic development synergistically under elevated ambient temperature. Environ. Sci. Pollut. Res. Int. 2023, 30, 73393–73404. [Google Scholar] [CrossRef]
- Lim, M.Y.; Manzon, R.G.; Somers, C.M.; Boreham, D.R.; Wilson, J.Y. Impacts of temperature, morpholine, and chronic radiation on the embryonic development of round whitefish (Prosopium cylindraceum). Environ. Toxicol. Chem. 2018, 37, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef]
- Miao, Q.X.; Si, X.Y.; Xie, Y.J.; Chen, L.; Tang, X.F.; Zhang, H.F. Acute heat stress alters the expression of genes and proteins associated with the unfolded protein response pathway in the liver of broilers. Br. Poult. Sci. 2022, 63, 125–132. [Google Scholar] [CrossRef]
- Guo, H.; Yang, Y.; Qiao, Y.; He, J.; Yao, W.; Zheng, W. Heat stress affects fetal brain and intestinal function associated with the alterations of placental barrier in late pregnant mouse. Ecotoxicol. Environ. Saf. 2021, 227, 112916. [Google Scholar] [CrossRef]
- Barreto Sánchez, A.L.; Wang, Q.; Thiam, M.; Zhang, J.; Zhang, Q.; Zhang, N.; Li, Q.; Wen, J.; Zhao, G. Liver Transcriptome Response to Heat Stress in Beijing You Chickens and Guang Ming Broilers. Genes 2022, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Kang, Y.; Li, L.; Zhao, G.; Sun, J.; Liu, Z. Proteome analysis of rainbow trout (Oncorhynchus mykiss) liver responses to chronic heat stress using DIA/SWATH. J. Proteom. 2021, 233, 104079. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Sui, M.; Cui, C.; Hu, Y.; Hou, X.; Xing, Q.; Huang, X.; Bao, Z. Identification and Characterization of HSP90 Gene Family Reveals Involvement of HSP90, GRP94 and Not TRAP1 in Heat Stress Response in Chlamys farreri. Genes 2021, 12, 1592. [Google Scholar] [CrossRef]
- Giménez-Andrés, M.; Čopič, A.; Antonny, B. The Many Faces of Amphipathic Helices. Biomolecules 2018, 8, 45. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, Y.; Zuo, P.; Miao, S.; Hu, B.; Kang, Y.; Liu, W.; Yang, Q.; Ren, H.; et al. α-Helix-Mediated Protein Adhesion. J. Am. Chem. Soc. 2023, 145, 17125–17135. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Hou, Y.P.; Liao, L.C. The Impact of STR Mutations on Kinship Identification. Fa Yi Xue Za Zhi 2024, 40, 484–491. [Google Scholar]
- Audouard, C.; Le Masson, F.; Charry, C.; Li, Z.; Christian, E.S. Oocyte-targeted deletion reveals that hsp90b1 is needed for the completion of first mitosis in mouse zygotes. PLoS ONE 2011, 6, e17109. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, C.; Cheng, L.; Zhong, C.; An, J.; Zou, M.; Liu, B.; Gao, X. Flavokawain C inhibits glucose metabolism and tumor angiogenesis in nasopharyngeal carcinoma by targeting the HSP90B1/STAT3/HK2 signaling axis. Cancer Cell Int. 2024, 24, 158. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X. A Pan-Cancer Analysis of Heat-Shock Protein 90 Beta1 (HSP90B1) in Human Tumours. Biomolecules 2022, 12, 1377. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Huang, Y.; He, S.; Yu, P.; Wu, L.; Peng., H. N6-methyladenosine modification in 18S rRNA promotes tumorigenesis and chemoresistance via HSF4b/HSP90B1/mutant p53 axis. Cell Chem. Biol. 2023, 30, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Y. Bioinformatics Analysis of Common Genetic and Molecular Traits and Association of Portal Hypertension with Pulmonary Hypertension. J. Healthc. Eng. 2022, 2022, 9237701. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.H.; Liu, S.Y.; Ren, Y.P. Evolution and function of heat shock protein 90 in economic shellfish: A review. Dev. Comp. Immunol. 2025, 170, 105456. [Google Scholar] [CrossRef]
- Stanley, T.R.; Guisbert, K.S.K.; Perez, S.M.; Oneka, M.; Kernin, I.; Higgins, N.R.; Lobo, A.; Subasi, M.M.; Carroll, D.J.; Turingan, R.G.; et al. Stress response gene family expansions correlate with invasive potential in teleost fish. J. Exp. Biol. 2022, 225, jeb243263. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Abdel-Baki, A.S.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Silencing of heat shock protein 90 (hsp90): Effect on development and infectivity of Ichthyophthirius multifiliis. BMC Vet. Res. 2023, 19, 62. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Hu, Z.; Xu, W. MicroRNA-1236-3p inhibits human osteosarcoma growth. Oncol. Lett. 2020, 20, 367. [Google Scholar] [CrossRef] [PubMed]
- Trikić, M.Z.; Monk, P.; Roehl, H.; Partridge, L.J. Regulation of zebrafish hatching by tetraspanin cd63. PLoS ONE 2011, 6, e19683. [Google Scholar] [CrossRef]
- Tokuhiro, K.; Dean, J. Glycan-Independent Gamete Recognition Triggers Egg Zinc Sparks and ZP2 Cleavage to Prevent Polyspermy. Dev. Cell 2018, 46, 627–640. [Google Scholar] [CrossRef]
- Myers, S.M.; Mulligan, L.M. The RET receptor is linked to stress response pathways. Cancer Res. 2004, 64, 4453–4463. [Google Scholar] [CrossRef]
- Xiong, X.; Luo, S.; Wu, B.; Wang, J. Comparative Developmental Toxicity and Stress Protein Responses of Dimethyl Sulfoxide to Rare Minnow and Zebrafish Embryos/Larvae. Zebrafish 2017, 14, 60–68. [Google Scholar] [CrossRef]
- Liang, Z.; Feng, B.; Miao, L.; Zhu, W.; Lin, Y.; Luo, M.; Yang, S.; Wang, L.; Fu, J.; Chen, H.; et al. Combined Illumina and Pacbio sequencing technology on transcriptome analysis reveals several key regulations during the early development of American shad (Alosa sapidissima). Aquacult. Rep. 2022, 25, 101264. [Google Scholar] [CrossRef]
- Wang, L.M.; Bu, H.Y.; Song, F.B.; Zhu, W.B.; Fu, J.J.; Dong, Z.J. Characterization and functional analysis of slc7a11 gene, involved in skin color differentiation in the red tilapia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 236, 110529. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Luo, M.; Wang, L.; Yin, H.; Zhu, W.; Fu, J. MicroRNA-206 Regulation of Skin Pigmentation in Koi Carp (Cyprinus carpio L.). Front. Genet. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Liu, Z.; Kang, Y.; Ma, F.; Luo, Z.; Wang, J.; Huang, J. miR-434 and miR-242 have a potential role in heat stress response in rainbow trout (Oncorhynchus mykiss). J. Fish. Biol. 2021, 99, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Liu, Y.; Zhu, W.; Feng, B.; Xu, W.; Dong, Z. Investigation of the Role of miR-1236-3p in Heat Tolerance of American Shad (Alosa sapidissima) by Targeted Regulation of hsp90b1. Int. J. Mol. Sci. 2025, 26, 9908. https://doi.org/10.3390/ijms26209908
Luo M, Liu Y, Zhu W, Feng B, Xu W, Dong Z. Investigation of the Role of miR-1236-3p in Heat Tolerance of American Shad (Alosa sapidissima) by Targeted Regulation of hsp90b1. International Journal of Molecular Sciences. 2025; 26(20):9908. https://doi.org/10.3390/ijms26209908
Chicago/Turabian StyleLuo, Mingkun, Ying Liu, Wenbin Zhu, Bingbing Feng, Wei Xu, and Zaijie Dong. 2025. "Investigation of the Role of miR-1236-3p in Heat Tolerance of American Shad (Alosa sapidissima) by Targeted Regulation of hsp90b1" International Journal of Molecular Sciences 26, no. 20: 9908. https://doi.org/10.3390/ijms26209908
APA StyleLuo, M., Liu, Y., Zhu, W., Feng, B., Xu, W., & Dong, Z. (2025). Investigation of the Role of miR-1236-3p in Heat Tolerance of American Shad (Alosa sapidissima) by Targeted Regulation of hsp90b1. International Journal of Molecular Sciences, 26(20), 9908. https://doi.org/10.3390/ijms26209908






