Therapeutic Potential of Metal-Based and PARP Inhibitor Chemotherapy for BRCA1-Associated Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Role of BRCA1 in BRCA1-Associated Triple-Negative Breast Cancer
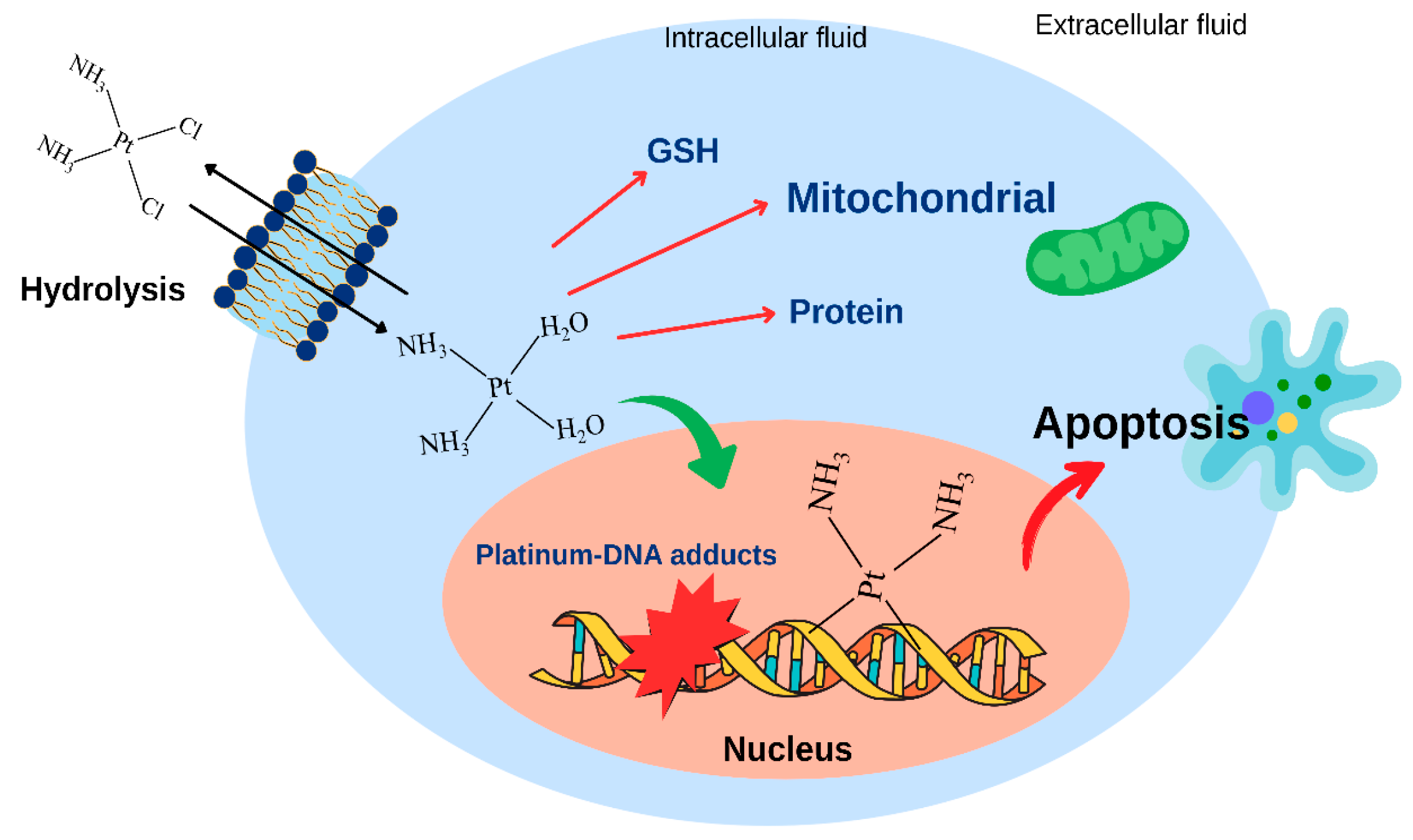
3. Platinum-Based Chemotherapy for BRCA1-Associated Triple-Negative Breast Cancer
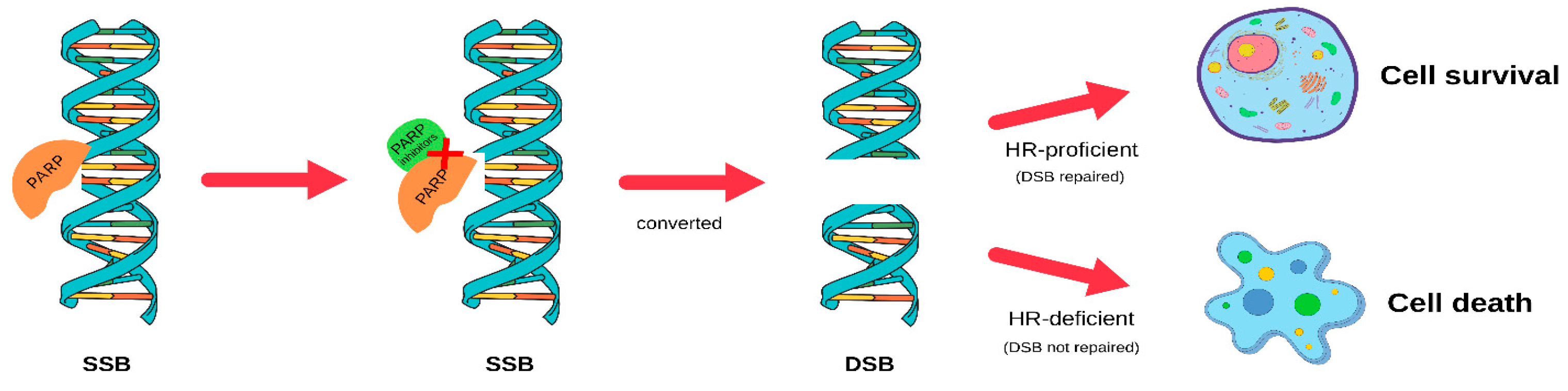
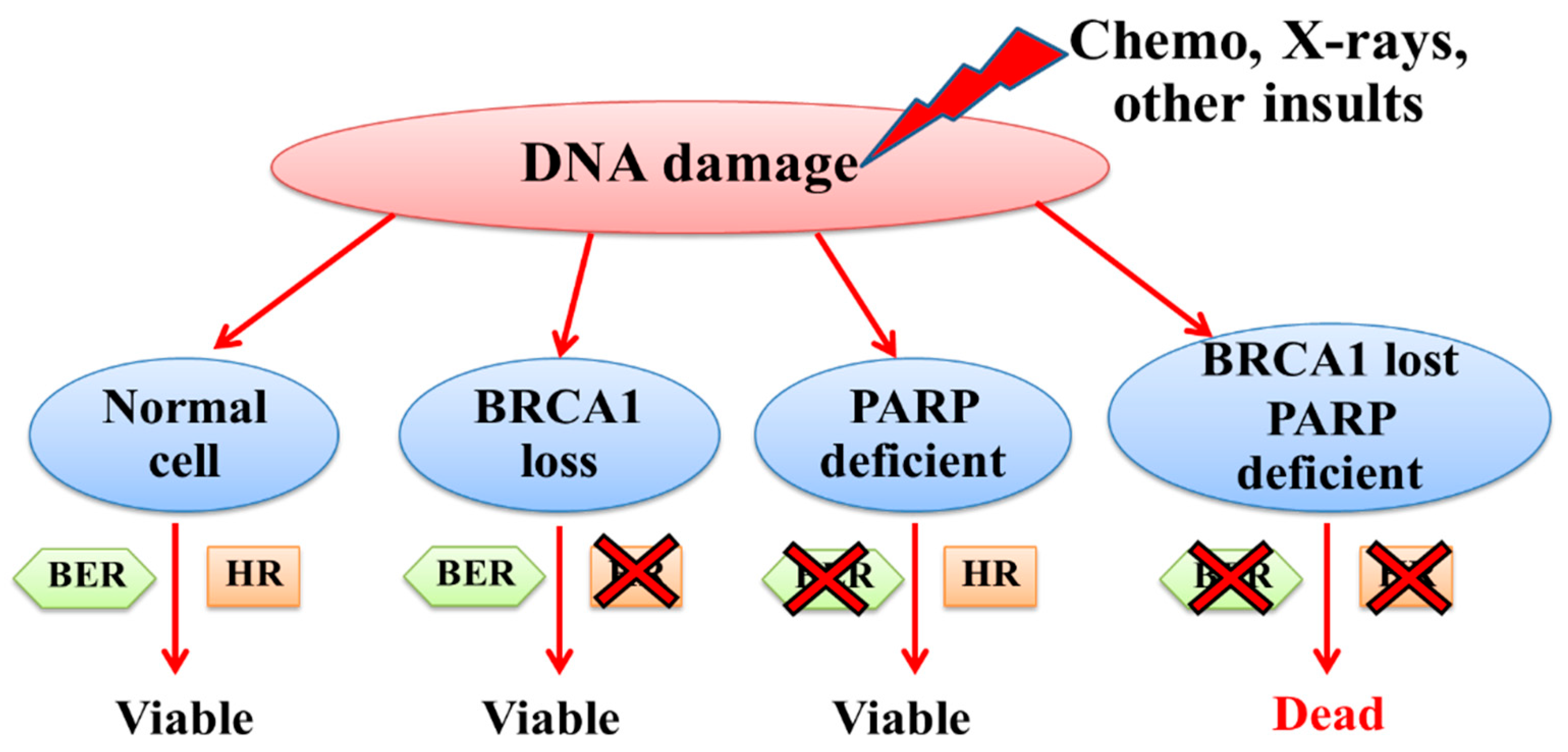
4. PARP Inhibitors for BRCA1-Associated Triple-Negative Breast Cancer
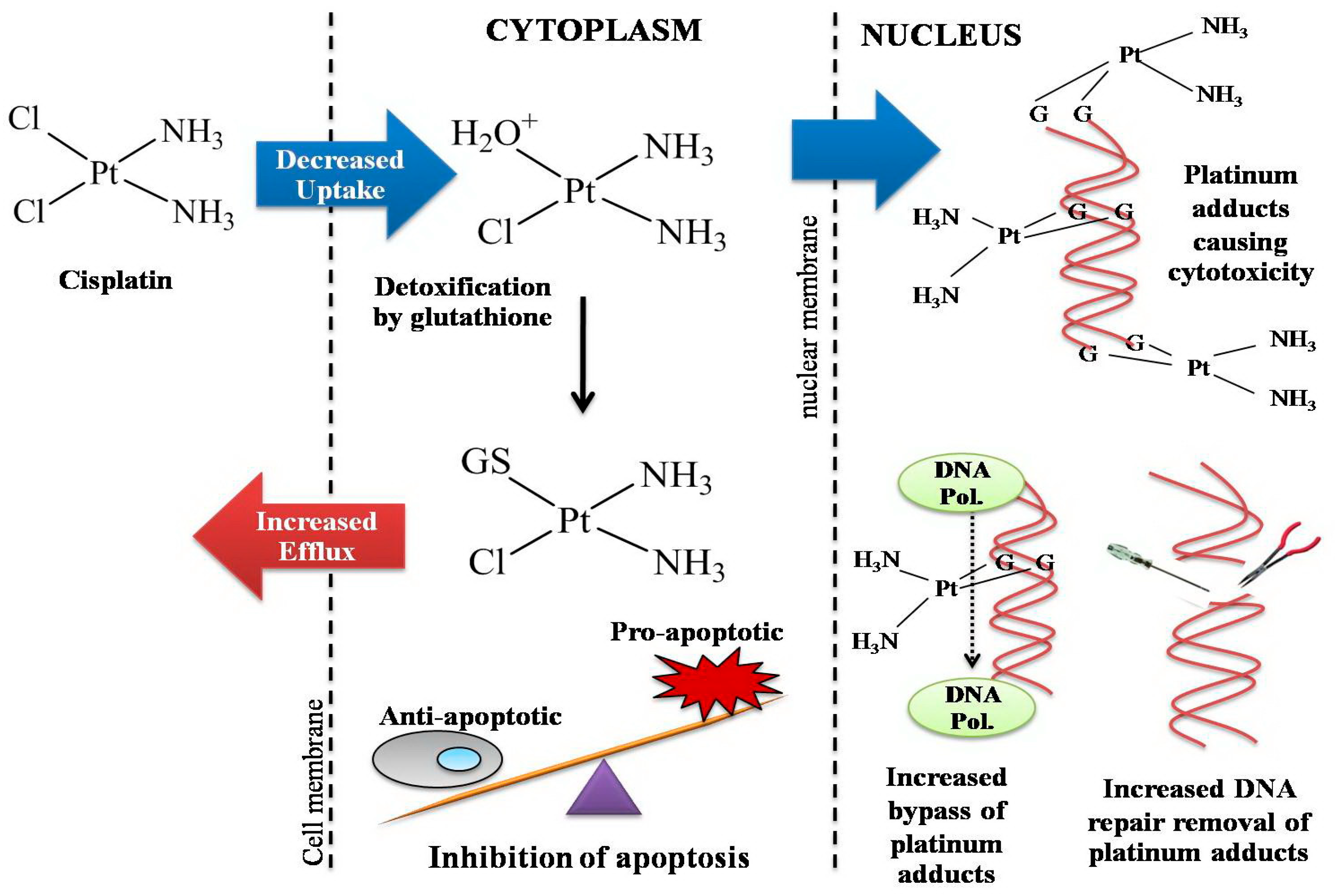
5. Cellular Resistance to Platinum Drugs
5.1. Decreased Drug Accumulation in Cisplatin Resistance
5.2. Increased Binding to Intracellular Thiol Molecules
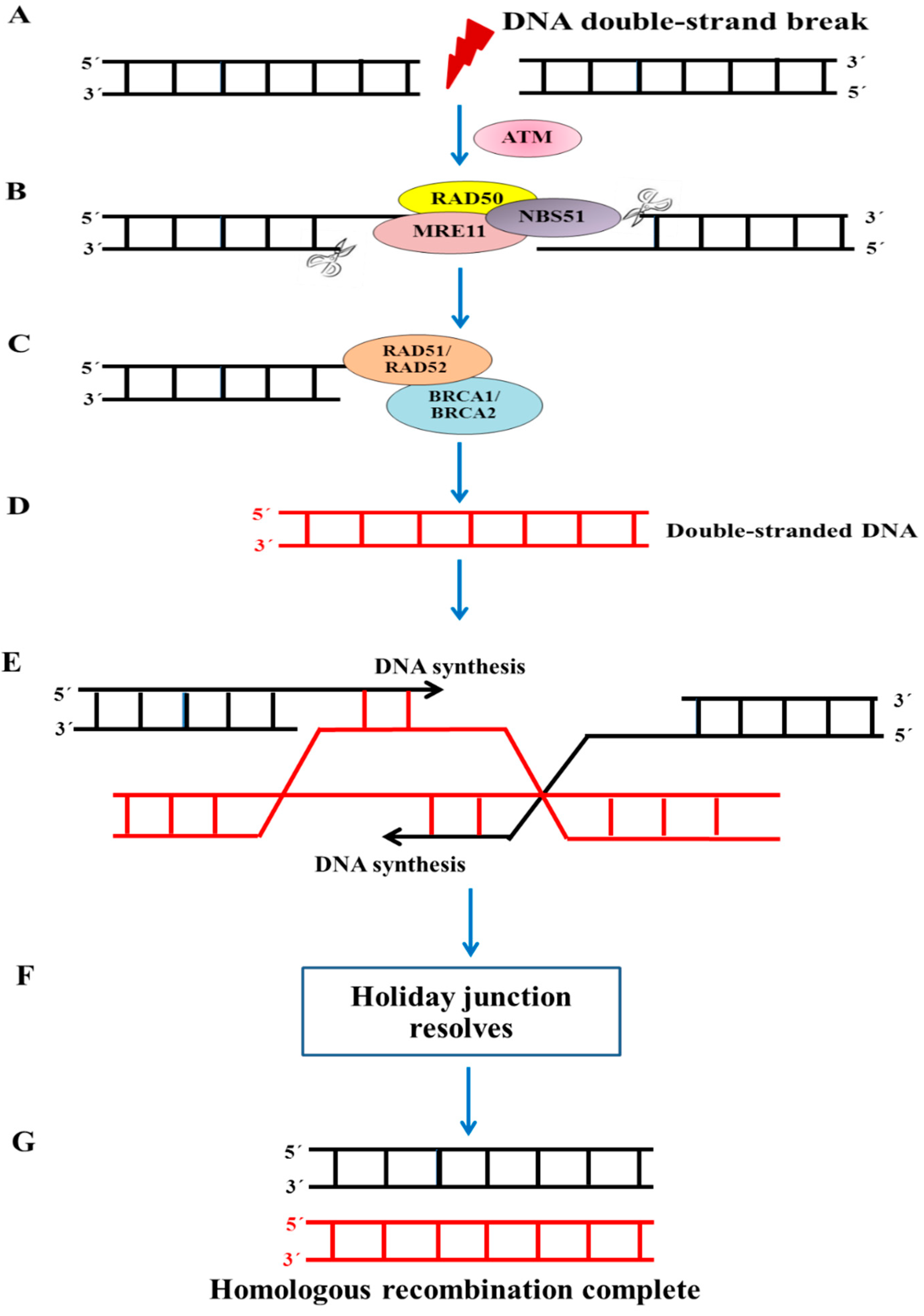
5.3. Increased DNA Repair
5.4. Epigenetics in Resistance to Cisplatin
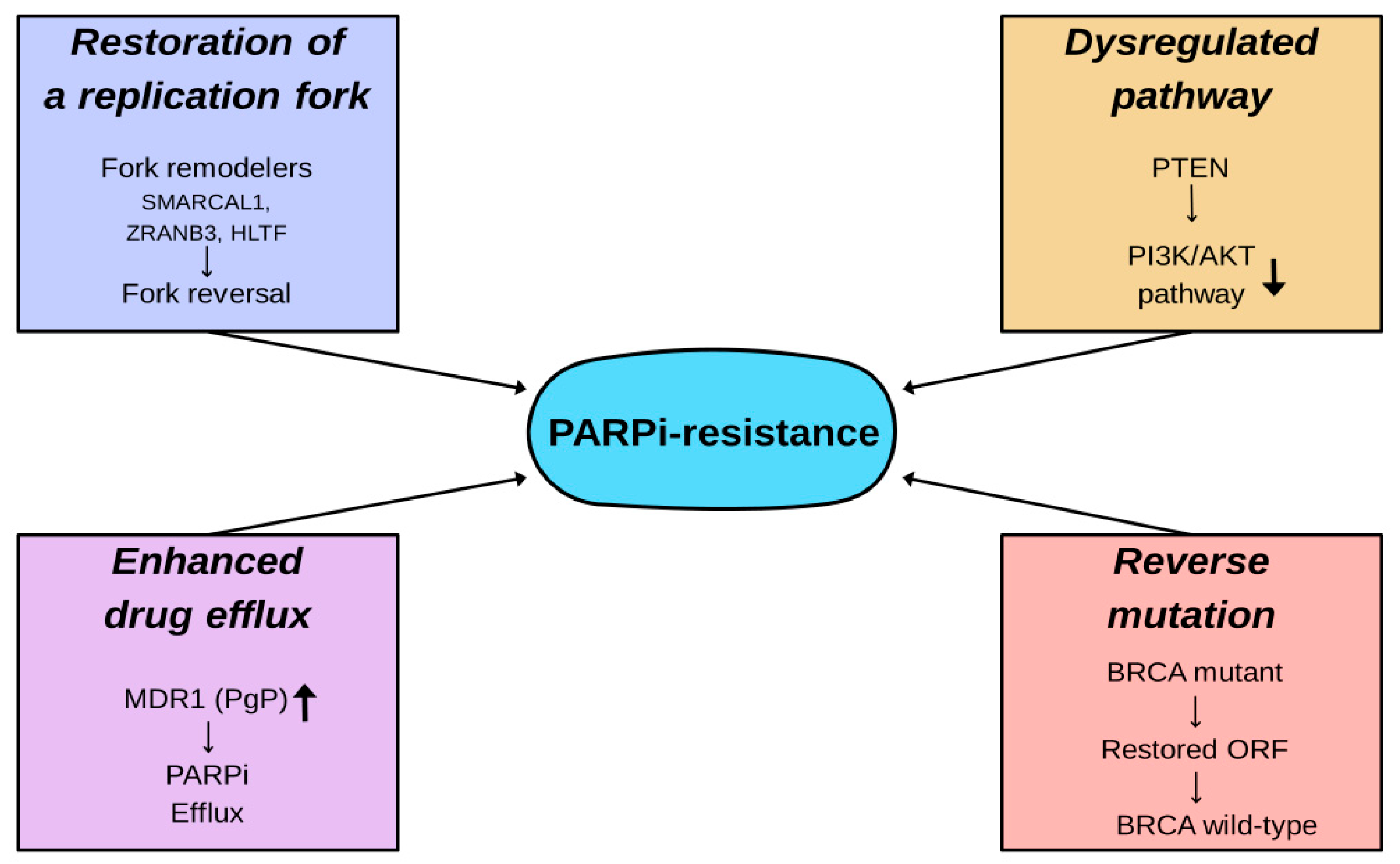
6. Cellular Resistance to PARP Inhibitors
6.1. Reverse Mutation
6.2. Restoration of Replication Fork Stability
6.3. Dysregulation Within Molecular Signaling Pathways
6.4. Enhanced Drug Efflux
7. Ruthenium-Based Chemotherapy for BRCA1-Associated Triple-Negative Breast Cancer
8. Synergistic Effects of Olaparib in Combination with Platinum/Ruthenium-Based Anticancer Agents in BRCA1-Associated Triple-Negative Breast Cancers
9. Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahin, T.K.; Rizzo, A.; Guven, D.C.; Aksoy, S. Post-progression treatment options after CDK4/6 inhibitors in hormone receptor-positive, HER2-negative metastatic breast cancer. Cancer Treat. Rev. 2025, 135, 102924. [Google Scholar] [CrossRef]
- Huppert, L.A.; Gumusay, O.; Idossa, D.; Rugo, H.S. Systemic therapy for hormone receptor positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J. Clin. 2023, 73, 480–515. [Google Scholar] [CrossRef]
- Rajan, A.; Nadhan, R.; Latha, N.R.; Krishnan, N.; Warrier, A.V.; Srinivas, P. Deregulated estrogen receptor signaling and DNA damage response in breast tumorigenesis. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188482. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Badve, S.; Dabbs, D.J.; Schnitt, S.J.; Baehner, F.L.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Basal-like and triple-negative breast cancers: A critical review with an emphasis on the implications for pathologists and oncologists. Mod. Pathol. 2011, 24, 157–167. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Pignol, J.-P.; Levine, M.N.; Julian, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Neven, P.; Casalnuovo, M.L.; Kim, S.-B.; Tokunaga, E.; Aftimos, P.; Saura, C.; O’Shaughnessy, J.; Harbeck, N.; Carey, L.A.; et al. Imlunestrant with or without Abemaciclib in Advanced Breast Cancer. N. Engl. J. Med. 2025, 392, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Cortés, J.; Bidard, F.-C.; Neven, P.; Garcia-Sáenz, J.; Aftimos, P.; O’Shaughnessy, J.; Lu, J.; Tonini, G.; Scartoni, S.; et al. Elacestrant in ER+, HER2- Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups. Clin. Cancer Res. 2024, 30, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.-A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the randomized phase III EMERALD trial. J. Clin. Oncol. 2022, 40, 3246–3256, Erratum in: J. Clin. Oncol. 2023, 41, 3962. [Google Scholar] [CrossRef]
- Shah, M.; Lingam, H.; Gao, X.; Gittleman, H.; Fiero, M.H.; Krol, D.; Biel, N.; Ricks, T.K.; Fu, W.; Hamed, S.; et al. US Food and Drug Administration approval summary: Elacestrant for estrogen receptor-positive, human epidermal growth factor receptor 2-negative, ESR1-mutated advanced or metastatic breast cancer. J. Clin. Oncol. 2024, 42, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, R.; Talker, E.; Chitra, J.; Kadam, R.; Gardi, N.; Ursekar, R.; Kadam, A.; Singh, A.; Sale, S.; Pandey, S.; et al. Genomic landscape of hormone therapy-resistant HR-positive, HER2-negative breast cancer. Breast Cancer Res. Treat. 2025, 213, 247–259. [Google Scholar] [CrossRef]
- Mason, S.R.E.; Willson, M.L.; Egger, S.J.; Beith, J.; Dear, R.F.; Goodwin, A. Platinum-based chemotherapy for early triple-negative breast cancer. Cochrane Database Syst. Rev. 2023, 9, CD014805. [Google Scholar]
- Ratanaphan, A. A DNA repair BRCA1 estrogen receptor and targeted therapy in breast cancer. Int. J. Mol. Sci. 2012, 13, 14898–14916. [Google Scholar] [CrossRef]
- Sabit, H.; Abouelnour, S.; Hassen, B.M.; Magdy, S.; Yasser, A.; Wadan, A.-H.S.; Abdel-Ghany, S.; Radwan, F.; Alqosaibi, A.I.; Hafiz, H.; et al. Anticancer Potential of Prebiotics: Targeting Estrogen Receptors and PI3K/AKT/mTOR in Breast Cancer. Biomedicines 2025, 13, 990. [Google Scholar] [CrossRef]
- Coakley, M.; Villacampa, G.; Sritharan, P.; Swift, C.; Dunne, K.; Kilburn, L.; Goddard, K.; Pipinikas, C.; Rojas, P.; Emmett, W.; et al. Comparison of circulating tumor DNA assays for molecular residual disease detection in early-stage triple-negative breast cancer. Clin. Cancer Res. 2024, 30, 895–903. [Google Scholar] [CrossRef]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Lee, A.H.S.; Robertson, J.F.; Ellis, I.O. Prognostic markers in triple-negative breast cancer. Cancer 2007, 109, 25–32. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.Y.K.; Papa, A. Signaling Pathways in Cancer: Therapeutic Targets Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Romieu, G.; Dieras, V.; Byrtek, M.; Duenne, A.A.; Miles, D. Abstract P6-12-03: Meta-Analysis of Patients with Triple-Negative Breast Cancer (TNBC) from Three Randomized Trials of First-Line Bevacizumab (BV) and Chemotherapy Treatment for Metastatic Breast Cancer (MBC). Cancer Res. 2010, 70 (Suppl. S24), P6-12-03. [Google Scholar]
- Liu, B.; Wu, L.; Liu, C.; Long, X.; Hu, S.; Zhang, L.; Liu, Z.; Liang, C. Baseline and Early Treatment MRI Model for Predicting Complete Pathologic Response to Neoadjuvant Chemoimmunotherapy in Patients With Triple-Negative Breast Cancer. AJR Am. J. Roentgenol. 2025, 25, 33178. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Alkassis, S.; Suresh, Y.; Lipsyc-Sharf, M.; Zhang, S.; Gianni, C.; Medford, A.; Bardia, A.; Ashouri, S.; Kapoor, N. Circulating tumor DNA detection of local recurrence in a patient with early stage triple-negative breast cancer. Breast Cancer Res. Treat. 2025, 213, 219–223. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Cheng, M.-Y.; Zhuang, X.; Zou, J.; Wei, D.; Lin, Y.-Y.; Zhang, Y.; Wang, K. Homologous recombination deficiency predicts the response to platinum-based neoadjuvant chemotherapy in early-stage triple-negative breast cancer patients: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2022, 14, 17588359221096253. [Google Scholar] [CrossRef]
- Masuda, N.; Bando, H.; Yamanaka, T.; Kadoya, T.; Takahashi, M.; Nagai, S.E.; Ohtani, S.; Aruga, T.; Suzuki, E.; Kikawa, Y.; et al. Eribulin-based neoadjuvant chemotherapy for triple-negative breast cancer patients stratified by homologous recombination deficiency status: A multicenter randomized phase II clinical trial. Breast Cancer Res. Treat. 2021, 188, 117–131. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, J.S.; Yost, S.E.; Li, S.M.; Frankel, P.H.; Ruel, C.; Schmolze, D.; Robinson, K.; Tang, A.; Martinez, N.; et al. Phase II Trial of Neoadjuvant Carboplatin and Nab-Paclitaxel in Patients with Triple-Negative Breast Cancer. Oncologist 2021, 26, e382–e393. [Google Scholar] [CrossRef]
- Telli, M.L.; Hellyer, J.; Audeh, W.; Jensen, K.C.; Bose, S.; Timms, K.M.; Gutin, A.; Abkevich, V.; Peterson, R.N.; Neff, C.; et al. Homologous Recombination Deficiency (Hrd) Status Predicts Response to Standard Neoadjuvant Chemotherapy in Patients with Triple-Negative or Brca1/2 Mutation-Associated Breast Cancer. Breast Cancer Res. Treat. 2018, 168, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Stemmer, S.; Pego, A.; Schneeweiss, A. Abstract PD01-01: Cetuximab + cisplatin in estrogen receptor-negative, progesterone receptor-negative, HER2-negative (triple-negative) metastatic breast cancer: Results of the randomized phase II bali-1 trial. Cancer Res. 2011, 70, PD01-01. [Google Scholar] [CrossRef]
- Pavese, F.; Capoluongo, E.D.; Muratore, M.; Minucci, A.; Santonocito, C.; Fuso, P.; Concolino, P.; Di Stasio, E.; Carbognin, L.; Tiberi, G.; et al. BRCA Mutation Status in Triple-Negative Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Pivotal Role for Treatment Decision-Making. Cancers 2022, 14, 4571. [Google Scholar] [CrossRef]
- Choi, E.; Mun, G.-I.; Lee, J.; Lee, H.; Cho, J.; Lee, Y.-S. BRCA1 deficiency in triple-negative breast cancer: Protein stability as a basis for therapy. Biomed. Pharmacother. 2023, 158, 114090. [Google Scholar] [CrossRef]
- Xu, Y.; Diao, L.; Chen, Y.; Liu, Y.; Wang, C.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; et al. Promoter methylation of BRCA1 in triple-negative breast cancer predicts sensitivity to adjuvant chemotherapy. Ann. Oncol. 2013, 24, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Tokunaga, E.; Kitao, H.; Hitchins, M.; Inoue, Y.; Tanaka, K.; Hisamatsu, Y.; Taketani, K.; Akiyoshi, S.; Okada, S.; et al. Epigenetic inactivation of BRCA1 through promoter hypermethylation and its clinical importance in triple-negative breast cancer. Clin. Breast Cancer 2015, 15, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shan, L.; Wang, F.; Wang, J.; Wang, F.; Shen, G.; Liu, X.; Wang, B.; Yuan, Y.; Ying, J.; et al. Hypermethylation of BRCA1 gene: Implication for prognostic biomarker and therapeutic target in sporadic primary triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 150, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Veeck, J.; Ropero, S.; Setien, F.; Gonzalez-Suarez, E.; Osorio, A.; Benitez, J.; Herman, J.G.; Esteller, M. BRCA1 CpG island hypermethylation predicts sensitivity to poly(adenosine diphosphate)-ribose polymerase inhibitors. J. Clin. Oncol. 2010, 28, 563–564. [Google Scholar] [CrossRef]
- Glodzik, D.; Bosch, A.; Hartman, J.; Aine, M.; Vallon-Christersson, J.; Reutersward, C.; Karlsson, A.; Mitra, S.; Nimeus, E.; Holm, K.; et al. Comprehensive molecular comparison of BRCA1 hypermethylated and BRCA1 mutated triple negative breast cancers. Nat. Commun. 2020, 11, 3747. [Google Scholar] [CrossRef]
- Prajzendanc, K.; Domagała, P.; Hybiak, J.; Ryś, J.; Huzarski, T.; Szwiec, M.; Tomiczek-Szwiec, J.; Redelbach, W.; Sejda, A.; Gronwald, J.; et al. BRCA1 promoter methylation in peripheral blood is associated with the risk of triple-negative breast cancer. Int. J. Cancer 2020, 146, 1293–1298. [Google Scholar] [CrossRef]
- Gardi, N.; Chaubal, R.; Parab, P.; Pachakar, S.; Kulkarni, S.; Shet, T.; Joshi, S.; Kembhavi, Y.; Chandrani, P.; Quist, J.; et al. Natural history of germline BRCA1 mutated and BRCA wild-type triple-negative breast cancer. Cancer Res. Commun. 2024, 4, 404–417. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, Y.; Wu, F.; Li, J.; Liu, M.; Lv, W.; Li, C.; Wang, W.; Tan, Q.; Xue, X.; et al. Relationship between homologous recombination deficiency and clinical features of breast cancer based on genomic scar score. Breast 2023, 69, 392–400. [Google Scholar] [CrossRef]
- Creeden, J.F.; Nanavaty, N.S.; Einloth, K.R.; Gillman, C.E.; Stanbery, L.; Hamouda, D.M.; Dworkin, L.; Nemunaitis, J. Homologous recombination proficiency in ovarian and breast cancer patients. BMC Cancer 2021, 21, 1154. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-Homologous DNA End Joining and Alternative Pathways to Double-Strand Break Repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Stinson, B.M.; Loparo, J.J. Repair of DNA Double-Strand Breaks by the Non-homologous End Joining Pathway. Annu. Rev. Biochem. 2021, 90, 137–164. [Google Scholar] [CrossRef]
- Fu, X.; Tan, W.; Song, Q.; Pei, H.; Li, J. BRCA1 and Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2022, 10, 813457. [Google Scholar] [CrossRef]
- Moreno, N.N.; Olthof, A.M.; Svejstrup, J.Q. Transcription-Coupled Nucleotide Excision Repair and the Transcriptional Response to UV-Induced DNA Damage. Annu. Rev. Biochem. 2023, 92, 81–113. [Google Scholar] [CrossRef]
- Atipairin, A.; Ratanaphan, A. In vitro enhanced sensitivity to cisplatin in D67Y BRCA1 RING domain protein. Breast Cancer Basic Clin. Res. 2011, 5, 201–208. [Google Scholar] [CrossRef]
- Atipairin, A.; Canyuk, B.; Ratanaphan, A. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by the platinum-based anticancer drugs. Breast Cancer Res. Treat. 2011, 126, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Nhukeaw, T.; Temboot, P.; Hansongnern, K.; Ratanaphan, A. Cellular responses of BRCA1-defective and triple-negative breast cancer cells and in vitro BRCA1 interactions induced by metallo-intercalator ruthenium(II) complexes containing chloro-substituted phenylazopyridine. BMC Cancer 2014, 14, 73. [Google Scholar] [CrossRef]
- Akashi-Tanaka, S.; Watanabe, C.; Takamaru, T.; Kuwayama, T.; Ikeda, M.; Ohyama, H.; Mori, M.; Yoshida, R.; Hashimoto, R.; Terumasa, S. BRCAness Predicts Resistance to Taxane-Containing Regimens in Triple Negative Breast Cancer During Neoadjuvant Chemotherapy. Clin. Breast Cancer 2015, 15, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, S.J.; Mayer, E.L.; He, L.; Traina, T.A.; Carey, L.A.; Krag, K.J.; Rugo, H.S.; Liu, M.C.; Stearns, V.; Come, S.E.; et al. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J. Clin. Oncol. 2015, 33, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Bernges, F.; Holler, E. The reaction of platinum(II) complexes with DNA. Kinetics of intrastrand crosslink formation in vitro. Nucleic Acids Res. 1991, 19, 1483–1489. [Google Scholar] [CrossRef][Green Version]
- Niu, Q.; Zhang, T. Synergistic mechanism of olaparib and cisplatin on breast cancer elucidated by network pharmacology. Sci. Rep. 2025, 15, 14800. [Google Scholar] [CrossRef]
- Hongthong, K.; Nhukeaw, T.; Temboot, P.; Dyson, P.J.; Ratanaphan, A. Anticancer activity of RAPTA-EA1 in triple-negative BRCA1 proficient breast cancer cells: Single and combined treatment with the PARP inhibitor olaparib. Heliyon 2021, 7, e07749. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 316. [Google Scholar]
- Isakoff, S.J.; Goss, P.E.; Mayer, E.L.; Traina, T.A.; Carey, L.A.; Krag, K. TBCRC009: A multicenter phase II study of cisplatin or carboplatin for metastatic triple-negative breast cancer and evaluation of p63/p73 as a biomarker of response. J. Clin. Oncol. 2011, 29, 1025. [Google Scholar] [CrossRef]
- Tutt, A.; Robson, M.; Garber, J.E.; Domchek, S.M.; Audeh, M.W.; Weitzel, J.N.; Friedlander, M.; Arun, B.; Loman, N.; Schmutzler, R.K.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet 2010, 376, 235–244. [Google Scholar]
- Rodler, E.; Sharma, P.; Barlow, W.E.; Gralow, J.R.; Puhalla, S.L.; Anders, C.K.; Goldstein, L.; Tripathy, D.; Brown-Glaberman, U.A.; Huynh, T.-T.; et al. Cisplatin with veliparib or placebo in metastatic triple-negative breast cancer and BRCA mutation-associated breast cancer (S1416): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2023, 24, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, B.H.; Yuan, P.; Cai, R.G. P5-19-04: Results of a randomized phase II study demonstrate benefit of platinum-based regimen in the first-line treatment of triple negative breast cancer (TNBC). Cancer Res. 2012, 71, P5-19-04. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Rugo, H.S.; Olopade, O.I.; DeMichele, A.; Yau, C.; Veer, L.J.V.T.; Buxton, M.B.; Hogarth, M.; Hylton, N.M.; Paoloni, M.; Perlmutter, J.; et al. Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N. Engl. J. Med. 2016, 375, 23–34. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; Von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Schwartzberg, L.; Danso, M.A.; Miller, K.D.; Rugo, H.S.; Neubauer, M.; Robert, N.; Hellerstedt, B.; Saleh, M.; Richards, P.; et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2014, 32, 3804–3807. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Dettwiler, S.; Beuzboc, P.; Alran, S.; Laurence, V.; Pierga, J.Y.; Fréneaux, P.; Sigal-Zafrani, B.; Diéras, V.; Vincent-Salomon, A.; et al. Pathologic response to short intensified taxane-free neoadjuvant chemotherapy in patients with proliferative operable breast cancer. Am. J. Clin. Oncol. 2012, 35, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sikov, W.M.; Polley, M.-Y.; Twohy, E.; Perou, C.M.; Singh, B.; Berry, D.A.; Tolaney, S.M.; Somlo, G.; Port, E.R.; Ma, C.X.; et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/- carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J. Clin. Oncol. 2019, 37, 591. [Google Scholar] [CrossRef]
- Abraham, J.E.; Pinilla, K.; Dayimu, A.; Grybowicz, L.; Demiris, N.; Harvey, C.; Drewett, L.M.; Lucey, R.; Fulton, A.; Roberts, A.N.; et al. The PARTNER trial of neoadjuvant olaparib with chemotherapy in triple-negative breast cancer. Nature 2024, 629, 1142–1148. [Google Scholar] [CrossRef]
- Sirohi, B.; Arnedos, M.; Popat, S.; Ashley, S.; Nerurkar, A.; Walsh, G.; Johnston, S.; Smith, I.E. Platinum-based chemotherapy in triple-negative breast cancer. Ann. Oncol. 2008, 19, 1847–1852. [Google Scholar] [CrossRef]
- Telli, M.L.; Jensen, K.C.; Vinayak, S.; Kurian, A.W.; Lipson, J.A.; Flaherty, P.J.; Timms, K.; Abkevich, V.; Schackmann, E.A.; Wapnir, I.L.; et al. Phase II Study of Gemcitabine, Carboplatin, and Iniparib As Neoadjuvant Therapy for Triple-Negative and BRCA1/2 Mutation-Associated Breast Cancer With Assessment of a Tumor-Based Measure of Genomic Instability: PrECOG 0105. J. Clin. Oncol. 2015, 33, 1895–1901. [Google Scholar] [CrossRef]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J. Clin. Oncol. 2020, 38, 4274–4282. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef]
- Wood, R.D.; Mitchell, M.; Sgouros, J.; Lindahl, T. Human DNA repair genes. Science 2001, 291, 1284–1289. [Google Scholar] [CrossRef]
- Fasching, P.A.; Link, T.; Hauke, J.; Seither, F.; Jackisch, C.; Klare, P.; Schmatloch, S.; Hanusch, C.; Huober, J.; Stefek, A.; et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Ann. Oncol. 2021, 32, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Schmatloch, S.; Hauke, J.; Rey, J.; Jackisch, C.; Klare, P.; Link, T.; Hanusch, C.; Huober, J.; Stefek, A.; et al. Neoadjuvant Paclitaxel/Olaparib in Comparison to Paclitaxel/Carboplatin in Patients with HER2-Negative Breast Cancer and HRD-Long-term Survival of the GeparOLA Study. Clin. Cancer Res. 2025, 31, 1596–1604. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef]
- Caldecott, K.W. Causes and consequences of DNA single-strand breaks. Trends Biochem. Sci. 2024, 49, 68–78. [Google Scholar] [CrossRef]
- Zandarashvili, L.; Langelier, M.-F.; Velagapudi, U.K.; Hancock, M.A.; Steffen, J.D.; Billur, R.; Hannan, Z.M.; Wicks, A.J.; Krastev, D.B.; Pettitt, S.J.; et al. Structural basis for allosteric PARP-1 retention on DNA breaks. Science 2020, 368, eaax6367. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.X.; Ma, N.; Wang, X.; Guo, M.; Jiang, Y.; Tian, Y.E. The Discovery of a Potent PARP1 Inhibitor Senaparib. Mol. Cancer Ther. 2025, 24, 47–55. [Google Scholar] [CrossRef]
- Rudolph, J.; Jung, K.; Luger, K. Inhibitors of PARP: Number crunching and structure gazing. Proc. Natl. Acad. Sci. USA 2022, 119, e2121979119. [Google Scholar] [CrossRef]
- Hunia, J.; Gawalski, K.; Szredzka, A.; Suskiewicz, M.J.; Nowis, D. The potential of PARP inhibitors in targeted cancer therapy and immunotherapy. Front. Mol. Biosci. 2022, 9, 1073797. [Google Scholar] [CrossRef]
- Loap, P.; Loirat, D.; Berger, F.; Ricci, F.; Vincent-Salomon, A.; Ezzili, C.; Mosseri, V.; Fourquet, A.; Ezzalfani, M.; Kirova, Y.; et al. Combination of Olaparib and Radiation Therapy for Triple Negative Breast Cancer: Preliminary Results of the Radioparp Phase 1 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 436–440. [Google Scholar] [CrossRef]
- Taylor, A.K.; Kosoff, D.; Emamekhoo, H.; Lang, J.M.; Kyriakopoulos, C.E. PARP inhibitors in metastatic prostate cancer. Front. Oncol. 2023, 13, 1159557. [Google Scholar] [CrossRef] [PubMed]
- Rouleau-Turcotte, E.; Pascal, J.M. ADP-ribose contributions to genome stability and PARP enzyme trapping on sites of DNA damage; paradigm shifts for a coming-of-age modification. J. Biol. Chem. 2023, 299, 105397. [Google Scholar] [CrossRef]
- Mateo, J.; Lord, C.J.; Serra, V.; Tutt, A.; Balmanña, J.; Castroviejo-Bermejo, M.; Cruz, C.; Oaknin, A.; Kaye, S.B.; de Bono, J.S. A decade of clinical development of PARP inhibitors in perspective. Ann. Oncol. 2019, 30, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Habaka, M.; Daly, G.R.; Shinyanbola, D.; Alabdulrahman, M.; McGrath, J.; Dowling, G.P.; Hehir, C.; Huang, H.Y.R.; Hill, A.D.K.; Varešlija, D.; et al. PARP Inhibitors in the Neoadjuvant Setting; A Comprehensive Overview of the Rationale for their Use, Past and Ongoing Clinical Trials. Curr. Oncol. Rep. 2025, 27, 533–551. [Google Scholar] [CrossRef]
- Li, Q.; Qian, W.; Zhang, Y.; Hu, L.; Chen, S.; Xia, Y. A new wave of innovations within the DNA damage response. Sig. Transduct. Target Ther. 2023, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Arisa, O.; Peer, C.J.; Fojo, A.; Figg, W.D. PARP inhibitors: A review of the pharmacology, pharmacokinetics, and pharmacogenetics. Semin. Oncol. 2024, 51, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Guo, Y.; Liu, Y.; Wang, H.; Gong, W.; Liu, Y.; Wang, X.; Gao, Y.; Yu, F.; Su, D.; et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia 2020, 22, 431–440. [Google Scholar] [CrossRef]
- Herencia-Ropero, A.; Llop-Guevara, A.; Staniszewska, A.D.; Domènech-Vivó, J.; García-Galea, E.; Moles-Fernández, A.; Pedretti, F.; Domènech, H.; Rodríguez, O.; Guzmán, M.; et al. The PARP1 selective inhibitor saruparib (AZD5305) elicits potent and durable antitumor activity in patient-derived BRCA1/2-associated cancer models. Genome Med. 2024, 16, 107. [Google Scholar] [CrossRef]
- Hobbs, E.A.; Litton, J.K.; Yap, T.A. Development of the PARP inhibitor talazoparib for the treatment of advanced BRCA1 and BRCA2 mutated breast cancer. Expert Opin. Pharmacother. 2021, 22, 1825–1837. [Google Scholar] [CrossRef]
- Gruber, J.J.; Afghahi, A.; Timms, K.; DeWees, A.; Gross, W.; Aushev, V.N.; Wu, H.-T.; Balcioglu, M.; Sethi, H.; Scott, D.; et al. A Phase II Study of Talazoparib Monotherapy in Patients with Wild-Type Brca1 and Brca2 with a Mutation in Other Homologous Recombination Genes. Nat. Cancer 2022, 3, 1181–1191. [Google Scholar] [CrossRef]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef]
- Xu, B.; Sun, T.; Shi, Y.; Cui, J.; Yin, Y.; Ouyang, Q.; Liu, Q.; Zhang, Q.; Chen, Y.; Wang, S.; et al. Pamiparib in patients with locally advanced or metastatic HER2-negative breast cancer with germline BRCA mutations: A phase II study. Breast Cancer Res. Treat. 2023, 197, 489–501. [Google Scholar] [CrossRef]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA Damage Response Pathways in Cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Krastev, D.B.; Li, S.; Sun, Y.; Wicks, A.J.; Hoslett, G.; Weekes, D.; Badder, L.M.; Knight, E.G.; Marlow, R.; Pardo, M.C.; et al. The Ubiquitin-Dependent ATPase P97 Removes Cytotoxic Trapped Parp1 from Chromatin. Nat. Cell Biol. 2022, 24, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Zatreanu, D.; Robinson, H.M.R.; Alkhatib, O.; Boursier, M.; Finch, H.; Geo, L.; Grande, D.; Grinkevich, V.; Heald, R.A.; Langdon, S.; et al. Poltheta Inhibitors Elicit BRCA-Gene Synthetic Lethality and Target PARP Inhibitor Resistance. Nat. Commun. 2021, 12, 3636. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, H.; Chen, P.; Tang, J.; Yang, S.; Nicot, C.; Guan, Z.; Li, X.; Tang, H. Clinical approaches to overcome PARP inhibitor resistance. Mol. Cancer 2025, 24, 156. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Song, C.; Wu, S.; Xie, J.; Zou, Y.; Xie, X.; Wu, T.; Yang, H.; Tang, H. PRMT1-Mediated PARP1 Methylation Drives Lung Metastasis and Chemoresistance via P65 Activation in Triple-Negative Breast Cancer. Research 2025, 8, 0854. [Google Scholar] [CrossRef]
- Eckstein, N. Platinum resistance in breast and ovarian cancer cell lines. J. Exp. Clin. Cancer Res. 2011, 30, 91. [Google Scholar] [CrossRef]
- Martin, L.P.; Hamilton, T.C.; Schilder, R.J. Platinum resistance: The role of DNA repair pathways. Clin. Cancer Res. 2008, 14, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Kartalou, M.; Essigmann, J.M. Recognition of cisplatin adducts by cellular proteins. Mutat. Res. 2001, 478, 1–21. [Google Scholar] [CrossRef]
- Gately, D.P.; Howell, S.B. Cellular accumulation of the anticancer agent cisplatin: A review. Br. J. Cancer 1993, 67, 1171–1176. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Zhou, B.; Cosco, D.; Gitschier, J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6836–6841. [Google Scholar] [CrossRef]
- Howell, S.B.; Safaei, R.; Larson, C.A.; Sailor, M.J. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol. 2010, 77, 887–894. [Google Scholar] [CrossRef]
- Kuo, M.T.; Fu, S.; Savaraj, N.; Chen, H.H. Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res. 2012, 72, 4616–4621. [Google Scholar] [CrossRef]
- Katano, K.; Safaei, R.; Samimi, G.; Holzer, A.; Rochdi, M.; Howell, S.B. The copper export pump ATP7B modulates the cellular pharmacology of carboplatin in ovarian carcinoma cells. Mol. Pharmacol. 2003, 64, 466–473. [Google Scholar] [CrossRef]
- Maxfield, A.B.; Heaton, D.N.; Winge, D.R. Cox17 is functional when tethered to the mitochondrial inner membrane. J. Biol. Chem. 2004, 279, 5072–5080. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, Q.; Wang, Z.; Xi, Z.; Xu, D.; Liu, Y. Cisplatin binds to human copper chaperone Cox17: The mechanistic implication of drug delivery to mitochondria. Chem. Commun. 2014, 50, 2667–2669. [Google Scholar] [CrossRef]
- Godwin, A.K.; Meister, A.; O’Dwyer, P.J.; Huang, C.S.; Hamilton, T.C.; Anderson, M.E. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc. Natl. Acad. Sci. USA 1992, 89, 3070–3074. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ali-Osman, F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J. Biol. Chem. 1993, 268, 20116–20125. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemeret, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts, T.G., Jr. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Sharma, R.A.; Dianov, G.L. Targeting base excision repair to improve cancer therapies. Mol. Aspects Med. 2007, 28, 345–374. [Google Scholar] [CrossRef][Green Version]
- Sugasawa, K.; Okamoto, T.; Shimizu, Y.; Masutani, C.; Iwai, S.; Hanaoka, F. A multistep damage recognition mechanism for global genomic nucleotide excision repair. Gene Dev. 2001, 15, 507–521. [Google Scholar] [CrossRef]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; He, G.; Venkatraman, E.S.; Spriggs, D.R. BRCA1 upregulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II). Cancer Res. 1998, 58, 1120–1123. [Google Scholar]
- Quinn, J.E.; Kennedy, R.E.; Mullan, P.B.; Gilmore, P.M.; Carty, M.; Johnston, P.G.; Harkinet, D.P. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003, 63, 6221–6228. [Google Scholar] [CrossRef] [PubMed]
- Swisher, E.M.; Sakai, W.; Karlan, B.Y.; Wurz, K.; Urban, N.; Taniguchi, T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008, 68, 2581–2586. [Google Scholar] [CrossRef]
- Sklar, M.D. Increased resistance to cis-diamminedichloroplatinum(II) in NIH 3T3 cells transformed by ras oncogenes. Cancer Res. 1988, 48, 793–797. [Google Scholar]
- O’Byrne, K.J.; Barr, M.P.; Gray, S.G. The role of epigenetics in resistance to cisplatin chemotherapy in lung cancer. Cancer 2011, 3, 1426–1453. [Google Scholar] [CrossRef] [PubMed]
- Menghi, F.; Banda, K.; Kumar, P.; Straub, R.; Dobrolecki, L.; Rodriguez, I.V.; Yost, S.E.; Chandok, H.; Radke, M.R.; Somlo, G.; et al. Genomic and Epigenomic BRCA Alterations Predict Adaptive Resistance and Response to Platinum-Based Therapy in Patients with Triple-Negative Breast and Ovarian Carcinomas. Sci. Transl. Med. 2022, 14, eabn1926. [Google Scholar] [CrossRef]
- Menghi, F.; Banda, K.; Kumar, P.; Straub, R.; Dobrolecki, L.E.; Rodriguez, I.; Yost, S.E.; Chandok, H.; Radke, M.; Somlo, G.; et al. Abstract PD5-01: PD5-01 Genomic and epigenomic BRCA alterations predict adaptive resistance and response to platinum-based therapy in triple negative breast cancer and ovarian carcinoma. Cancer Res. 2023, 83, PD5-01. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Z.; Guo, C. Insights into the DNA damage response and tumor drug resistance. Cancer Biol. Med. 2025, 22, 197–204. [Google Scholar] [CrossRef]
- Scanlon, K.J.; Kashani-Sabet, M.; Miyachi, H.; Sowers, L.C.; Rossi, J.J. Molecular basis of cisplatin resistance in human carcinomas: Model systems and patients. Anticancer Res. 1989, 9, 1310–1312. [Google Scholar]
- Dhar, R.; Basu, A. Costitutive activation of p70 S6 kinase is associated with intrinsic resistance to cisplatin. Int. J. Oncol. 2008, 32, 1133–1137. [Google Scholar] [PubMed][Green Version]
- Aebi, S.; Kurdi-Haidar, B.; Gordon, R.; Cenni, B.; Zheng, H.; Fink, D.; Christen, R.D.; Boland, C.R.; Koi, M.; Fishel, R.; et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Res. 1996, 56, 3087–3090. [Google Scholar][Green Version]
- Hicks, J.K.; Chute, C.L.; Paulsen, M.T.; Ragland, R.L.; Howlett, N.G.; Gueranger, Q.; Glover, T.W.; Canmanet, C.E. Differential roles for DNA polymerases eta, zeta, and REV1 in lesion bypass of intrastrand versus interstrand DNA cross-links. Mol. Cell. Biol. 2010, 30, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.K.; Harrell, M.I.; Oza, A.M.; Oaknin, A.; Ray-Coquard, I.; Tinker, A.V.; Helman, E.; Radke, M.R.; Say, C.; Vo, L.T. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2019, 9, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.G.; Sarkaria, J.N.; Kaufmann, S.H. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3406–3411. [Google Scholar] [CrossRef]
- Rondinelli, B.; Gogola, E.; Yucel, H.; Duarte, A.A.; van de Ven, M.; van der Sluijs, R.; Konstantinopoulos, P.A.; Jonkers, J.; Ceccaldi, R.; Rottenberg, S.; et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat. Cell Biol. 2017, 19, 1371–1378. [Google Scholar] [CrossRef]
- Taglialatela, A.; Alvarez, S.; Leuzzi, G.; Sannino, V.; Ranjha, L.; Huang, J.W.; Madubata, C.; Anand, R.; Levy, B.; Rabadan, R.; et al. Restoration of Replication Fork Stability in BRCA1- and BRCA2-Deficient Cells by Inactivation of SNF2-Family Fork Remodelers. Mol. Cell 2017, 68, 414–430.e418. [Google Scholar] [CrossRef]
- Matsumoto, K.; Matsumoto, Y.; Wada, J. PARylation-mediated post-transcriptional modifications in cancer immunity and immunotherapy. Front. Immunol. 2025, 16, 1537615. [Google Scholar] [CrossRef]
- Noordermeer, S.M.; van Attikum, H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019, 29, 820–834. [Google Scholar] [CrossRef]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Saal, L.H.; Gruvberger-Saal, S.K.; Persson, C.; Lovgren, K.; Jumppanen, M.; Staaf, J.; Jonsson, G.; Pires, M.M.; Maurer, M.; Holm, K.; et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat. Genet. 2008, 40, 102–107. [Google Scholar] [CrossRef]
- Mendes-Pereira, A.M.; Martin, S.A.; Brough, R.; McCarthy, A.; Taylor, J.R.; Kim, J.S.; Waldman, T.; Lord, C.J.; Ashworth, A. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 2009, 1, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Piombino, C.; Cortesi, L. Insights into the Possible Molecular Mechanisms of Resistance to PARP Inhibitors. Cancers 2022, 14, 2804. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef]
- Leitner, I.; Nemeth, J.; Feurstein, T.; Abrahim, A.; Matzneller, P.; Lagler, H.; Erker, T.; Langer, O.; Zeitlinger, M. The third-generation P-glycoprotein inhibitor tariquidar may overcome bacterial multidrug resistance by increasing intracellular drug concentration. J. Antimicrob. Chemother. 2011, 66, 834–839. [Google Scholar] [CrossRef]
- Christie, E.L.; Pattnaik, S.; Beach, S.J.; Copeland, A.; Rashoo, N.; Fereday, S.; Hendley, J.; Alsop, K.; Brady, S.L.; Lamb, G.; et al. Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nat. Commun. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Choi, Y.E.; Meghani, K.; Brault, M.-E.; Leclerc, L.; He, Y.J.; Day, T.A.; Elias, K.M.; Drapkin, R.; Weinstock, D.M.; Dao, F.; et al. Platinum and PARP Inhibitor Resistance Due to Overexpression of MicroRNA-622 in BRCA1-Mutant Ovarian Cancer. Cell Rep. 2016, 14, 429–439. [Google Scholar] [CrossRef]
- Wang, Y.; Krais, J.J.; Bernhardy, A.J.; Nicolas, E.; Cai, K.Q.; Harrell, M.I.; Kim, H.H.; George, E.; Swisher, E.M.; Simpkins, F.; et al. RING domain-deficient BRCA1 promotes PARP inhibitor and platinum resistance. J. Clin. Investig. 2016, 126, 3145–3157. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.P.; Moser, S.C.; Ganesan, S.; Jonkers, J. Understanding and Overcoming Resistance to PARP Inhibitors in Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 773–791. [Google Scholar] [CrossRef]
- Morganti, S.; Marra, A.; De Angelis, C.; Toss, A.; Licata, L.; Giugliano, F.; Salimbeni, B.T.; Giachetti, P.P.M.B.; Esposito, A.; Giordano, A.; et al. PARP Inhibitors for Breast Cancer Treatment: A Review. JAMA Oncol. 2024, 10, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Mukhtar, M.; Rahdar, A.; Barani, M.; Pandey, S.; Diez-Pascual, A.M. Active targeted nanoparticles for delivery of poly(ADP-ribose) polymerase (PARP) inhibitors: A preliminary review. Int. J. Mol. Sci. 2021, 22, 10319. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Sig. Transduct. Target Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Schluga, P.; Hartinger, C.G.; Egger, A.; Reisner, E.; Galanski, M.; Jakupec, M.A.; Keppler, B.K. Redox behavior of tumor-inhibiting ruthenium(III) complexes and effects of physiological reductants on their binding to GMP. Dalton Trans. 2006, 14, 1796. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Reisner, E.; Eichinger, A.; Pongratz, M.; Arion, V.B.; Galanski, M.; Hartinger, C.G.; Keppler, B.K. Redox-active antineoplastic ruthenium complexes with indazole: Correlation of in vitro potency and reduction potential. J. Med. Chem. 2005, 48, 2831–2837. [Google Scholar] [CrossRef]
- Ang, W.H.; Dyson, P.J. Classical and non-classical ruthenium-based anticancer drugs: Towards targeted chemotherapy. Eur. J. Inorg. Chem. 2006, 20, 4003–4018. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Zorbas-Seifried, S.; Jakupec, M.A.; Kynast, B.; Zorbas, H.; Keppler, B.K. From bench to bedside-preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A). J. Inorg. Biochem. 2006, 100, 891–904. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Jakupec, M.A.; Zorbas-Seifried, S.; Groessl, M.; Egger, A.; Berger, W.; Zorbas, H.; Dyson, P.J.; Keppler, B.K. KP1019, a new redox-active anticancer agent--preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008, 5, 2140–2155. [Google Scholar] [CrossRef]
- Clarke, M.J. Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 2003, 236, 209–233. [Google Scholar] [CrossRef]
- Sulyok, J.M.; Hann, S.; Hartinger, C.G.; Keppler, B.K.; Stingeder, G.; Koellensperger, G. Two dimensional separation schemes for investigation of the interaction of an anticancer ruthenium (III) compound with plasma proteins. J. Anal. At. Spectrom. 2005, 20, 856–863. [Google Scholar] [CrossRef]
- Ratanaphan, A.; Temboot, P.; Dyson, P.J. In vitro Ruthenation of Human Breast Cancer Suppressor Gene 1 (BRCA1) by the Antimetastasis Compound RAPTA-C and Its Analogue CarboRAPTA-C. Chem. Biodivers. 2010, 7, 1290–1302. [Google Scholar] [CrossRef]
- Ang, W.H.; Casini, A.; Sava, G.; Dyson, P.J. Organometallic ruthenium-based antitumor compounds with novel modes of action. J. Org. Chem. 2011, 695, 989–998. [Google Scholar] [CrossRef]
- Chakree, K.; Ovatlarnporn, C.; Dyson, P.J.; Ratanaphan, A. Altered DNA binding and amplification of human breast cancer suppressor gene BRCA1 induced by a novel antitumor compound, [Ru(η6-p-phenylethacrynate)Cl2(pta)]. Int. J. Mol. Sci. 2012, 13, 13183–13202. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kundu, S.; Bhattacharyya, A.; Hartinger, C.G.; Dyson, P.J. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J. Biol. Inorg. Chem. 2008, 13, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Klaimanee, E.; Temram, T.; Ratanaphan, A.; Saithong, S.; Sooksawat, D.; Samphao, A.; Yakiyama, Y.; Sakurai, H.; Konno, T.; Tantirungrotechai, Y.; et al. Iridium(III) coordination compounds based on organophosphorus ancillary ligands showing cytotoxicity against breast cancer cells and Fe(III) luminescent sensing. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 325, 125150. [Google Scholar] [CrossRef]
- Sojka, M.; Gamez, P. Exploring the toxicity of mononuclear piano-stool Ru(II) anticancer agents: A comprehensive literature review. Coord. Chem. Rev. 2025, 543, 216902. [Google Scholar] [CrossRef]
- Temram, T.; Klaimanee, E.; Saithong, S.; Amornpitoksuk, P.; Phongpaichit, S.; Ratanaphan, A.; Tantirungrotechai, Y.; Leesakul, N. Iridium(III) complexes based on cyanomethane and cyanamide ligands with luminescence quenching properties for Fe(III) sensing and biological activities. Polyhedron 2023, 243, 116540. [Google Scholar] [CrossRef]
- Leesakul, N.; Kullawanichaiyanan, K.; Mutić, S.; Guzsvány, V.; Nhukeaw, T.; Ratanaphan, A.; Saithong, S.; Konno, T.; Sirimahachai, U.; Promarak, V. A photoactive iridium(III) complex with 3-methyl-2-phenyl pyridine and 1,1-bis(diphenylphosphino)methane: Synthesis, structural characterization and cytotoxicity in breast cancer cells. J. Coord. Chem. 2021, 74, 1949585. [Google Scholar] [CrossRef]
- Bibi, R.; Zahid, M.; Rasool, F.; Tariq, M.; Hussain, A.; Asif, H.M.; Khan, M.A.; Shah, K.H.; Hussain, S.; Sirajuddin, M.; et al. Synthesis, spectroscopic, computational, molecular docking, antidiabetic (in vitro & in vivo) DNA and BSA interaction studies of ruthenium(II) carboxylate complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 330, 125630. [Google Scholar]
- Kim, W.K.; An, J.M.; Lim, Y.J.; Kim, K.; Kim, Y.H.; Kim, D. Recent advances in metallodrug: Coordination-induced synergy between clinically approved drugs and metal ions. Mater. Today Adv. 2025, 25, 100569. [Google Scholar] [CrossRef]
- Kozieł, S.; Wojtala, D.; Szmitka, M.; Lesiów, M.; Ziółkowska, A.; Sawka, J.; Carpio, E.D.; Crans, D.C.; Komarnicka, U.K. Half-Sandwich Organometallic Ir(III) and Ru(II) Compounds and their Interactions with Biomolecules. ChemPlusChem 2025, 90, e202400621. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A. Alternative of cisplatin—Introduction of rhodium analogues. J. Indian Chem. Soc. 2024, 101, 101389. [Google Scholar] [CrossRef]
- Bashir, M.; Mantoo, I.A.; Prasad, C.P.; Yousuf, I. New mono and dinuclear half-sandwiched organoruthenium(II) complexes: Effect of dinuclearity on the biomolecular interaction and cytotoxicity. Inorg. Chim. Acta 2024, 569, 122122. [Google Scholar] [CrossRef]
- Klaimanee, E.; Nhukeaw, T.; Saithong, S.; Ratanaphan, A.; Phongpaichit, S.; Tantirungrotechai, Y.; Leesakul, N. Half-sandwich ruthenium (II) p-cymene complexes based on organophosphorus ligands: Structure determination, computational investigation, in vitro antiproliferative effect in breast cancer cells and antimicrobial activity. Polyhedron 2021, 204, 115244. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Adon, T.; Dsouza, K.; Kumar, H.Y. Exploring the Future of Metal-Based Anticancer Agents: A Comprehensive Review of Ruthenium-Based Complexes. ChemistrySelect 2025, 10, e202404147. [Google Scholar] [CrossRef]
- Sahu, G.; Patra, S.A.; Lima, S.; Das, S.; Görls, H.; Plass, W.; Dinda, R. Ruthenium(II)-Dithiocarbazates as Anticancer Agents: Synthesis, Solution Behavior, and Mitochondria-Targeted Apoptotic Cell Death. Chem. Eur. J. 2023, 29, e202202694. [Google Scholar] [CrossRef]
- Katheria, S. Ruthenium Complexes as Potential Cancer Cell Growth Inhibitors for Targeted Chemotherapy. ChemistrySelect 2022, 7, e202201645. [Google Scholar] [CrossRef]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In Vitro and in Vivo Evaluation of Ruthenium(II)-Arene PTA Complexes. J. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef]
- Lorenzon, T.; Vescovo, M.; Maiullari, M.; Tonon, G.; Conceiçao, N.R.; Carabineiro, S.A.C.; Mahmoud, A.G.; Dietl, M.C.; Demitri, N.; Orian, L.; et al. Influence of the charge of 1,3,5-triaza-7-phosphaadamantane-based ligands on the anticancer activity of organopalladium complexes. RSC Adv. 2025, 15, 14058–14071. [Google Scholar] [CrossRef]
- Nhukeaw, T.; Hongthong, K.; Dyson, P.J.; Ratanaphan, A. Cellular responses of BRCA1-defective HCC1937 breast cancer cells induced by the antimetastasis ruthenium(II) arene compound RAPTA-T. Apoptosis 2019, 24, 612–622. [Google Scholar] [CrossRef]
- Babak, M.V.; Meier, S.M.; Huber, K.; Reynisson, J.; Legin, A.A.; Jakupec, M.; Roller, A.; Stukalov, A.; Gridling, M.; Bennett, K.L.; et al. Target profiling of an antimetastatic RAPTA agent by chemical proteomics: Relevance to the mode of action. Chem. Sci. 2015, 6, 2449–2456. [Google Scholar] [CrossRef]
- Ang, W.H.; Parker, L.J.; De Luca, A.; Juillerat-Jeanneret, L.; Morton, C.J.; Bello, M.L.; Parker, M.W.; Dyson, P.J. Rational design of an organometallic glutathione transferase inhibitor. Angew. Chem. Int. Ed. Engl. 2009, 48, 3854–3857. [Google Scholar] [CrossRef] [PubMed]
- Bernal, G.; Aquea, G.; Ramírez-Rivera, S. Metal-based molecules in the treatment of cancer: From bench to bedside. Oncol. Res. 2025, 33, 759–779. [Google Scholar] [CrossRef]
- Amato, A.D.; Mariconda, A.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Sinicropi, M.S.; Longo, P. Complexes of Ruthenium(II) as Promising Dual-Active Agents against Cancer and Viral Infections. Pharmaceuticals 2023, 16, 1729. [Google Scholar] [PubMed]
- Heric, A.; Dibranin, N.; Martic, L.; Hodzic, E.; Zahirovic, A.; Kozaric, A.K. Ruthenium-based complexes as antitumor agents. J. Health Sci. 2024, 14, 70–83. [Google Scholar]
- Levina, A.; Chetcuti, A.R.M.; Lay, P.A. Controversial role of transferrin in the transport of ruthenium anticancer drugs. Biomolecules 2022, 12, 1319. [Google Scholar] [CrossRef]
- Nayeem, N.; Sauma, S.; Ahad, A.; Rameau, R.; Kebadze, S.; Bazett, M.; Park, B.J.; Casaccia, P.; Prabha, S.; Hubbard, K.; et al. Insights into mechanisms and promising triple negative breast cancer therapeutic potential for a water-soluble ruthenium compound. ACS Pharmacol. Transl. Sci. 2024, 7, 1364–1376. [Google Scholar] [CrossRef]
- Adhireksan, Z.; Davey, G.E.; Campomanes, P.; Groessl, M.; Clavel, C.M.; Yu, H.; Nazarov, A.A.; Yeo, C.H.F.; Ang, W.H.; Dröge, P.; et al. Ligand substitutions between ruthenium-cymene compounds can control protein versus DNA targeting and anticancer activity. Nat. Commun. 2014, 5, 3462–3475. [Google Scholar] [CrossRef]
- Wu, B.; Ong, M.S.; Groessl, M.; Adhireksan, Z.; Hartinger, C.G.; Dyson, P.J.; Davey, C.A. A ruthenium antimetastasis agent forms specific histone protein adducts in the nucleosome core. Chem. Eur. J. 2011, 17, 3562–3566. [Google Scholar] [CrossRef]
- Adhireksan, Z.; Palermo, G.; Riedel, T.; Ma, Z.; Muhammad, R.; Rothlisberger, U.; Dyson, P.J.; Davey, C.A. Allosteric crosstalk in chromatin can mediate drug-drug synergy. Nat. Commun. 2017, 8, 14806–14817. [Google Scholar] [CrossRef]
- Berndsen, R.H.; Weiss, A.; Abdul, U.K.; Wong, T.J.; Meraldi, P.; Griffioen, A.W.; Dyson, P.J.; Nowak-Sliwinska, P. Combination of ruthenium(II)-arene complex [Ru(η6-p-cymene)Cl2(pta)] (RAPTA-C) and the epidermal growth factor receptor inhibitor erlotinib results in efficient angiostatic and antitumor activity. Sci. Rep. 2017, 7, 43005. [Google Scholar] [CrossRef]
- Swaminathan, S.; Karvembu, R. Dichloro Ru(II)-p-cymene-1,3,5-triaza-7-phosphaadamantane(RAPTA-C): A Case Study. ACS Pharmacol. Transl. Sci. 2023, 6, 982–996. [Google Scholar] [CrossRef]
- Romagnolo, A.P.G.; Romagnolo, D.F.; Selmin, O. BRCA1 as Target for Breast Cancer Prevention and Therapy. Anti-Cancer Agents Med. Chem. 2015, 15, 4–14. [Google Scholar] [CrossRef]
- Hongthong, K.; Ratanaphan, A. BRCA1-associated triple-negative breast cancer and potential treatment for ruthenium-based compounds. Curr. Cancer Drug Targets 2016, 16, 606–617. [Google Scholar] [CrossRef]
- Ratanaphan, A.; Nhukeaw, T.; Hongthong, K.; Dyson, P.J. Differential Cytotoxicity, Cellular Uptake, Apoptosis and Inhibition of BRCA1 Expression of BRCA1-Defective and Sporadic Breast Cancer Cells Induced by an Anticancer Ruthenium (II)-Arene Compound, RAPTA-EA1. Anti-Cancer Agents Med. Chem. 2017, 17, 212–220. [Google Scholar] [CrossRef]
- Temboot, P.; Lee, R.F.S.; Menin, L.; Dyson, P.J.; Ratanaphan, A. Biochemical and biophysical characterization of ruthenation of BRCA1 RING protein by RAPTA complexes and its E3 ubiquitin ligase activity. Biochem. Biophys. Res. Commun. 2017, 488, 355–361. [Google Scholar] [CrossRef]
- Toss, A.; Cristofanilli, M. Molecular characterization and targeted therapeutic approaches in breast cancer. Breast Cancer Res. 2015, 17, 60. [Google Scholar] [CrossRef]
- Carvalho, E.; Canberk, S.; Schmitt, F.; Vale, N. Molecular Subtypes and Mechanisms of Breast Cancer: Precision Medicine Approaches for Targeted Therapies. Cancers 2025, 17, 1102. [Google Scholar] [CrossRef]
- Yusoh, N.A.; Ahmad, H.; Gill, M.R. Combining PARP Inhibition with Platinum, Ruthenium or Gold Complexes for Cancer Therapy. ChemMedChem 2020, 15, 2121–2135. [Google Scholar] [CrossRef]
- Pilie, P.; Gay, C.M.; Byers, L.A.; O’Connor, M.J.; Yap, T.A. PARP inhibitors: Extending benefit beyond BRCA mutant cancers. Clin. Cancer Res. 2019, 25, 3759–3771. [Google Scholar] [CrossRef]
- Thein, K.Z.; Thawani, R.; Kummar, S. Combining Poly (ADP-Ribose) Polymerase (PARP) Inhibitors with Chemotherapeutic Agents: Promise and Challenges. Cancer Treat Res. 2023, 186, 143–170. [Google Scholar]
- Balmaña, J.; Tung, N.M.; Isakoff, S.J.; Graña, B.; Ryan, P.D.; Saura, C.; Lowe, E.S.; Frewer, P.; Winer, E.; Baselga, J.; et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann. Oncol. 2014, 25, 1656–1663. [Google Scholar] [CrossRef]
- Bouwman, P.; Jonkers, J. Molecular pathways: How can BRCA-mutated tumors become resistant to PARP inhibitors? Clin. Cancer Res. 2014, 20, 540–547. [Google Scholar] [CrossRef]
- Pan, J.-N.; Lei, L.; Ye, W.-W.; Wang, X.-J.; Cao, W.-M. BRCA1 Reversion Mutation Confers Resistance to Olaparib and Camrelizumab in a Patient with Breast Cancer Liver Metastasis. J. Breast Cancer 2021, 24, 474–480. [Google Scholar] [CrossRef]
- Waks, A.G.; Cohen, O.; Kochupurakkal, B.; Kim, D.; Dunn, C.E.; Buendia, J.B.; Wander, S.; Helvie, K.; Lloyd, M.R.; Marini, L.; et al. Reversion and non-reversion mechanisms of resistance to PARP inhibitor or platinum chemotherapy in BRCA1/2-mutant metastatic breast cancer. Ann. Oncol. 2020, 31, 590–598. [Google Scholar] [CrossRef]
- Yusoh, N.A.; Leong, S.W.; Chia, S.L.; Harun, S.N.; Abdul Rahman, M.B.; Vallis, K.A.; Gill, M.R.; Ahmad, H. Metallointercalator [Ru(dppz)2(PIP)]2+ Renders BRCA Wild-Type Triple-Negative Breast Cancer Cells Hypersensitive to PARP Inhibition. ACS Chem. Biol. 2020, 15, 378–387. [Google Scholar] [CrossRef]
- Fong, P.C.; Yap, T.A.; Boss, D.S.; Carden, C.P.; Mergui-Roelvink, M.; Gourley, C.; De Greve, J.; Lubinski, J.; Shanley, S.; Messiou, C.; et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 2010, 28, 2512–2519. [Google Scholar] [CrossRef]
- Pandya, K.; Scher, A.; Omene, C.; Ganesan, S.; Kumar, S.; Ohri, N.; Potdevin, L.; Haffty, B.; Toppmeyer, D.L.; George, M.A. Clinical efficacy of PARP inhibitors in breast cancer. Breast Cancer Res. Treat. 2023, 200, 15–22. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Bai, Y.; Aodeng, G.; Ga, L.; Hai, W.; Ai, J. Research progress of metal anticancer drugs. Pharmaceutics 2023, 15, 2750. [Google Scholar] [CrossRef]
- Pandy, J.G.P.; Balolong-Garcia, J.C.; Valerie, M.; Ordinario, B.C.; Victoria, F.; Que, F. Triple negative breast cancer and platinum-based systemic treatment: A meta-analysis and systematic review. BMC Cancer 2019, 19, 1065. [Google Scholar] [CrossRef]
- Singh, D.D.; Parveen, A.; Yadav, D.K. Role of PARP in TNBC: Mechanism of inhibition, clinical applications, and resistance. Biomedicines 2021, 9, 1512. [Google Scholar] [CrossRef]
- Golbaghi, G.; Castonguay, A. Rationally designed ruthenium complexes for breast cancer therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef]
- Ji, S.; Chen, L.; Yu, Y.; Chen, X.; Wei, L.; Gou, L.; Shi, C.; Zhuang, S. A comprehensive comparison of PARP inhibitors as maintenance therapy in platinum-sensitive recurrent ovarian cancer: A systematic review and network meta-analysis. J. Ovarian Res. 2025, 18, 18. [Google Scholar] [CrossRef]
- Yusoh, N.A.; Ahmad, H.; Vallis, K.A.; Gill, M.R. Advances in platinum-based cancer therapy: Overcoming platinum resistance through rational combinatorial strategies. Med. Oncol. 2025, 42, 262. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Kim, D.; Nam, H.J. PARP inhibitors: Clinical limitations and recent attempts to overcome them. Int. J. Mol. Sci. 2022, 23, 8412. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, L.; Xing, Z.; Wang, F.; Cheng, X. Update on combination strategies of PARP inhibitors. Cancer Control 2024, 31, 10732748241298329. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, J. Therapeutic advances and application of PARP inhibitors in breast cancer. Transl. Oncol. 2025, 57, 102410. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP inhibitors: Clinical relevance, mechanisms of action and tumor resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Litton, J.K.; Broglio, K.R.; Meric-Bernstam, F.; Rakkhit, R.; Cardoso, F.; Peintinger, F.; Hanrahan, E.O.; Sahin, A.; Guray, M.; et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J. Clin. Oncol. 2009, 27, 5700–5706. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, D.; Le, Q.; Wang, Y.; Wang, W. Ruthenium-based antitumor drugs and delivery systems from monotherapy to combination therapy. Nanoscale 2022, 14, 16339. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, C.Y.; Nam, T.G. Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives. Drug Des. Dev. Ther. 2022, 18, 5375–5392. [Google Scholar] [CrossRef]
- Dilmac, S.; Ozpolat, B. Mechanisms of PARP-Inhibitor-Resistance in BRCA-Mutated Breast Cancer and New Therapeutic Approaches. Cancers 2023, 15, 3642. [Google Scholar] [CrossRef]
- Jain, A.; Barge, A.; Parris, C.N. Combination strategies with PARP inhibitors in BRCA-mutated triple-negative breast cancer: Overcoming resistance mechanisms. Oncogene 2025, 44, 193–207, Erratum in: Oncogene 2025, 44, 1063. [Google Scholar] [CrossRef]
- Han, Y.; Li, C.W.; Hsu, J.M.; Hsu, J.L.; Chan, L.C.; Tan, X.; He, G.J. Metformin reverses PARP inhibitors-induced epithelial-mesenchymal transition and PD-L1 upregulation in triple-negative breast cancer. Am. J. Cancer Res. 2019, 9, 800–815. [Google Scholar]
- Quereda, V.; Bayle, S.; Vena, F.; Frydman, S.M.; Monastyrskyi, A.; Roush, W.R.; Duckett, D.R. Therapeutic Targeting of CDK12/CDK13 in Triple-Negative Breast Cancer. Cancer Cell 2019, 36, 545–558.e547. [Google Scholar] [CrossRef]
- Do, K.T.; Kochupurakkal, B.; Kelland, S.; de Jonge, A.; Hedglin, J.; Powers, A.; Quinn, N.; Gannon, C.; Vuong, L.; Parmar, K.; et al. Phase 1 Combination Study of the CHK1 Inhibitor Prexasertib and the PARP Inhibitor Olaparib in High-grade Serous Ovarian Cancer and Other Solid Tumors. Clin. Cancer Res. 2021, 27, 4710–4716. [Google Scholar] [CrossRef] [PubMed]
- Valiente, C.M.; Amir, E. Combining PARP inhibitors and platinum-based chemotherapy in metastatic triple negative and/or BRCA-associated breast cancer. Transl. Cancer Res. 2023, 12, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, G.; Roberto, M.; Verrico, M.; Vici, P.; Tomao, S.; Tomao, F. Emerging Role of PARP Inhibitors in Metastatic Triple Negative Breast Cancer. Current Scenario and Future Perspectives. Front. Oncol. 2021, 11, 769280. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Usmani, S.S.; Subbarayan, R.; Saini, R.; Pandey, P.K. Hypoxia: Syndicating triple negative breast cancer against various therapeutic regimens. Front. Oncol. 2023, 13, 1199105. [Google Scholar] [CrossRef]



| Chemotherapy | TNBC (n) | Setting | Outcomes | References |
|---|---|---|---|---|
| Cisplatin | 86 | Metastatic | RR 37% | [55] |
| Olaparib | 27 | Metastatic | pCR 41% | [56] |
| Cisplatin/veliparib | 162 | Neoadjuvant | pCR 74% | [57] |
| Cisplatin/docetaxel | 27 | Metastatic | ORR 59% | [58] |
| Carboplatin/docetaxel | 28 | Neoadjuvant | pCR 86% | [59] |
| Carboplatin/veliparib | 634 | Neoadjuvant | pCR 47% | [60] |
| Carboplatin/veliparib | 107 | Neoadjuvant | pCR 61% | [61] |
| Gemcitabine/carboplatin/iniparib | 258 | Metastatic | ORR 34% | [62] |
| Paclitaxel/carboplatin | 24 | Neoadjuvant | pCR 33% | [63] |
| Paclitaxel/doxorubicin/cyclophosphamide/carboplatin | 60 | Neoadjuvant | pCR 51% | [64] |
| Eribulin/carboplatin | 22 | Neoadjuvant | pCR 40% | [26] |
| Paclitaxel/carboplatin/olaparib | 559 | Neoadjuvant | pCR 51% | [65] |
| Mitomycin C/vinblastine/cisplatin | 34 | Metastatic | ORR 41% | [66] |
| Gemcitabine /carboplatin/iniparib | 80 | Neoadjuvant | ORR 36% | [67] |
| Ruthenium Complexes | Phase/ Status | Mechanism of Action/Clinical Challenges | Ref. |
|---|---|---|---|
| NAMI-A | Phase II |
| [177,178] |
| KP-1019 | Phase I |
| [177,178,179] |
| KP-1339 | Phase I |
| [177,178] |
| TLD1433 | Phase Ib |
| [177,178,180] |
| BOLD-100 | Phase I |
| [177,178,180] |
| RM175 | Preclinical |
| [177] |
| RAED-C | Preclinical |
| [177,181,182,183] |
| RAPTA-C | Preclinical |
| [177,181,182,183,184] |
| Class | Primary Mechanism | Toxicity | Efficacy |
|---|---|---|---|
| Platinum complexes | Platinum complexes exert cytotoxicity by forming DNA crosslinks that disrupt replication and transcription, leading to the accumulation of unrepaired DNA lesions, cell cycle arrest, and apoptosis in TNBC [203] |
|
|
| PARP inhibitors | PARP inhibitors indicate antitumor effects in TNBC by blocking the repair of single-strand DNA breaks (SSBs), leading to the accumulation of double-strand breaks (DSBs) during replication. In BRCA1/2 mutant or HR-deficient TNBC, these lesions cannot be effectively repaired, resulting in synthetic lethality [205] |
|
|
| Ruthenium complexes | Ru(III) complexes are involved in TNBC by accumulating in mitochondria, causing mitochondrial dysfunction, ROS generation, and membrane depolarization, which leads to DNA damage and cell death. Additionally, ruthenium inhibits the protein expression of macrophage colony-stimulating factor (M-CSF), which is relevant to the PI3K/AKT/mTOR pathway, thereby reducing migration, invasion, and angiogenesis of cancer cells [181] |
|
|
| Class | Mechanism | Advantages | Limitations | Clinical Status |
|---|---|---|---|---|
| Platinum drugs | The cytotoxicity of platinum complexes arises from the covalent binding of platinum atoms to the N7 position of purine bases in DNA, forming platinum adducts that generate intrastrand and interstrand crosslinks. This blocks replication and transcription, inhibits DNA synthesis, induces cell cycle arrest, and ultimately triggers apoptosis in cancer cells [207] | Widely used for the treatment of cancer
| High toxicity
|
|
| PARP inhibitors | PARP inhibitors induce synthetic lethality by inhibiting PARP1/2 catalytic activity, preventing the repair of single-strand DNA breaks (SSBs) and leading to their accumulation. This results in the formation of double-strand breaks (DSBs). In HR-deficient tumors (e.g., BRCA1/2 mutants), DSBs cannot be efficiently repaired, ultimately leading to cell death (apoptosis) [95] |
[214] | ||
| Ruthenium complexes | Ru(II) complexes inhibit tumor growth and metastasis by entering the nucleus, binding to DNA, inducing DNA damage, and causing cell cycle arrest. Additionally, ruthenium can localize to mitochondria, leading to mitochondrial dysfunction, increased ROS generation, and apoptosis of cancer cells [215] |
| Ru(II) complexes are not FDA-approved and are still under clinical evaluation in humans
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratanaphan, A. Therapeutic Potential of Metal-Based and PARP Inhibitor Chemotherapy for BRCA1-Associated Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 9881. https://doi.org/10.3390/ijms26209881
Ratanaphan A. Therapeutic Potential of Metal-Based and PARP Inhibitor Chemotherapy for BRCA1-Associated Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2025; 26(20):9881. https://doi.org/10.3390/ijms26209881
Chicago/Turabian StyleRatanaphan, Adisorn. 2025. "Therapeutic Potential of Metal-Based and PARP Inhibitor Chemotherapy for BRCA1-Associated Triple-Negative Breast Cancer" International Journal of Molecular Sciences 26, no. 20: 9881. https://doi.org/10.3390/ijms26209881
APA StyleRatanaphan, A. (2025). Therapeutic Potential of Metal-Based and PARP Inhibitor Chemotherapy for BRCA1-Associated Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 26(20), 9881. https://doi.org/10.3390/ijms26209881






