Abstract
Alternative splicing (AS) is a crucial post-transcriptional regulatory mechanism that enhances transcriptomic and proteomic diversity by generating multiple mRNA isoforms from a single gene. In plants, AS plays a central role in modulating growth, development, and stress responses. We summarize the prevalence and functional roles of AS in plant development and stress adaptation, highlighting mechanisms that link AS to hormone signaling, RNA surveillance, and epigenetic regulation. Polyploid crops, with their duplicated genomes, exhibit expanded AS complexity, contributing to phenotypic plasticity, stress tolerance, and adaptive evolution. Thus, this review synthesizes current knowledge on AS in plants, with a focus on three economically important polyploid crops—Brassica napus, Gossypium hirsutum, and Triticum aestivum. We also discuss how subgenome interactions shape diversity in polyploids and influence trait variation. Despite significant advances enabled by high-throughput sequencing, mechanistic studies that directly link specific AS events to phenotypic outcomes remain limited. Understanding how polyploidy reprograms AS and how isoform variation contributes to stress adaptation will be critical for harnessing AS in crop improvement.
1. Introduction
Plants, as sessile organisms, continuously adapt to fluctuating environmental conditions that threaten their growth, development, and survival [,,]. Unlike mobile organisms that can relocate to favorable environments, plants depend on complex molecular networks to perceive external cues and adjust their physiological and developmental processes accordingly [,]. A central component of this adaptive plasticity is post-transcriptional regulation, which dynamically modulates gene expression in response to both endogenous and exogenous stimuli [,,,,,,,,]. Among these mechanisms, alternative splicing (AS) plays a critical role in enhancing transcriptomic complexity by generating multiple mRNA isoforms from a single gene. This diversification fine-tunes protein function and enhances plant resilience to environmental stress [,,].
AS is executed through several well-defined modes that influence how pre-mRNAs are processed into mature transcripts. The five canonical AS types are intron retention (IR), exon skipping (ES), mutually exclusive exons (MXEs), alternative 5′ splice sites (A5SS), and alternative 3′ splice sites (A3SS) [,,,,] (Figure 1).
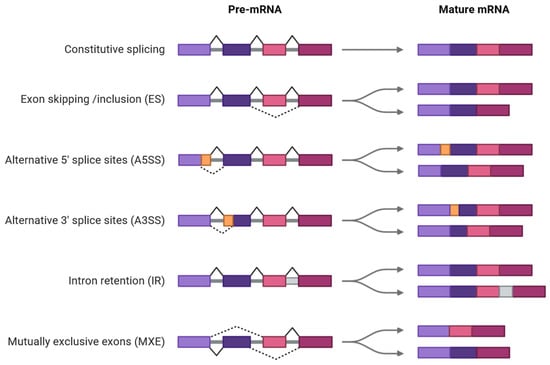
Figure 1.
Canonical modes of AS in plants. Solid and dotted lines in pre-mRNA indicate constitutive splicing and AS events, respectively. Diagrams were created in BioRender. Faiza Fatima. (2025). https://app.biorender.com/illustrations/68c05363ab47b2405e081788 (accessed on 12 September 2025).
Comparative analyses across eukaryotes have revealed clear lineage-specific patterns. In plants, IR is the dominant AS mode, accounting for approximately 30–60% of all events and closely associated with regulatory strategies such as non-sense mediated decay (NMD) coupling and stress-responsive transcriptome reprogramming [,]. By contrast, ES predominates in animals, contributing to tissue-specific proteome diversity and developmental plasticity [,,,]. A5SS and A3SS occur at intermediate frequencies in both, whereas MXE events are comparatively rare [,]. Additional sources of transcript diversity include alternative promoter (AP) usage and alternative polyadenylation (APA), which modify the 5′ and 3′ ends of transcripts. Both AP and APA events are widespread in plants, and their frequency increases under abiotic and biotic stresses [,,].
Polyploidy, which results from whole-genome duplication (WGD), has been a major force in the evolution of angiosperms and strongly influences transcriptomic regulation under stress [,,]. The presence of extra gene copies in polyploids facilitates subgenome divergence, relaxed selection, and expression buffering, all of which contribute to greater adaptive potential and splicing complexity [,]. AS further adds to this potential by increasing isoform diversity across duplicated loci. Compared to diploid plants, polyploids typically exhibit a higher frequency of IR, greater isoform divergence between homoeologs, and stronger subgenome-biased splicing, reflecting the regulatory flexibility conferred by genome duplication [,]. Evidence shows that AS divergence in polyploids is frequently tissue-specific and stress-responsive, providing an additional layer of post-polyploidization gene regulation [,,].
AS is common across plant species but varies with genetic background, developmental stage, and environmental conditions [,,]. While much work has characterized AS in Arabidopsis thaliana [], Oryza sativa [,], and Zea mays [], more recent studies have focused on polyploid crops such as Brassica napus [], Gossypium hirsutum [], and Triticum aestivum []. These crops are both economically important and genetically complex, making them valuable systems for investigating how AS influences stress adaptation and productivity [,].
This review synthesizes current knowledge of AS regulation in plants, with a focus on the polyploid crops B. napus, G. hirsutum, and T. aestivum. It summarizes recent insights into their AS landscapes, functional consequences, and evolutionary significance. By highlighting key advances and knowledge gaps, the review provides perspectives on how AS can be harnessed for crop improvement strategies.
2. Prevalence and Variability of AS Across Plant Species
AS is a crucial post-transcriptional mechanism that increases transcriptomic and proteomic diversity in plants. Although conserved across plant lineages, its prevalence and regulatory patterns differ markedly among species, tissues, developmental stages, and ploidy levels.
Comparative transcriptome studies have shown that 40–70% of multi-exon genes undergo AS in many model species such as A. thaliana, O. sativa, and Z. mays [,,]. Variation in these estimates largely reflects differences in gene architecture, regulatory motifs, sequencing technologies, and read depth [,,].
Tissue-specific regulation of AS enables plants to fine-tune gene function across diverse organs and developmental contexts. For example, in Rosa roxburghii, RNA-seq analysis across eight tissues revealed 8586 AS genes and over 49,000 AS events, with hundreds of isoforms restricted to leaves, flowers, and fruits []. Many of these tissue-specific AS genes (e.g., 4CL, DFR, ANR, MYB, and bHLH) were directly associated with flavonoid synthesis, underscoring the link between AS isoform diversity and organ-specific metabolism. Similarly, in Nicotiana tabacum, a transcriptome atlas covering 13 tissues identified 4585 tissue-specific transcripts, most of which arose from AS []. Trichomes, flowers, and roots exhibited particularly high numbers of unique isoforms, many linked to secondary metabolism and stress responses, highlighting AS as a key driver of tissue-specialized functions. At the single-gene level, the study of Musa acuminata provides an illustrative example: AS of MaMYB16L generates two isoforms with opposing functions—the full-length variant represses starch-degradation genes and delays ripening, while the truncated isoform is preferentially expressed in fruit tissue, promoting softening and metabolic transition [].
AS frequency and isoform distribution shift dynamically throughout development. In a cultivated strawberry (Fragaria × ananassa), long-read sequencing revealed over 20,000 AS isoforms, highlighting the transcriptomic complexity of this octoploid genome []. More recently, Nanopore-based profiling across four fruit stages identified hundreds of isoform switches affecting hormone signaling and metabolism, underscoring developmental stage-specific regulation of AS []. These events were frequently associated with protein domain gain or loss and hormone signaling pathways, underscoring developmental stage-specific regulation. A similar developmental modulation of AS has been described in Brassica juncea, where the MADS-box gene BjuAGL18-1 produces two antagonistic isoforms. The full-length transcript (BjuAGL18-1L) represses flowering by forming complexes with AFR2–HDA9 and AGL15 to inhibit FUL and FT, while the truncated isoform (BjuAGL18-1S) counteracts this repression and promotes early flowering []. Their expression patterns are strongly influenced by environmental conditions, with BjuAGL18-1L induced under short days and BjuAGL18-1S favored under long days and higher temperatures, providing a clear example of how AS integrates developmental stage transitions with external cues to fine-tune flowering.
Polyploidy significantly increases AS abundance and complexity because genome duplication and functional diversification of homoeologous gene copies provide additional regulatory flexibility [,]. This increased complexity arises from several interacting mechanisms: relaxed purifying selection on duplicated genes allows splice-site mutations to accumulate []; divergence in promoter and chromatin landscapes between subgenomes alters co-transcriptional splicing decisions; and differential expression of spliceosomal components across subgenomes further amplifies isoform diversity []. Together, these processes create an expanded regulatory network that enables polyploids to fine-tune gene expression under stress [,]. For example, He et al. [] reconstructed full-length transcriptomes of interspecific Oryza hybrids with different ploidy levels, revealing that tetraploid and hexaploid hybrids exhibited higher AS diversity than their diploid counterparts. These differences were particularly evident in stress-response and developmental genes, highlighting the role of genome duplication in expanding regulatory capacity through AS []. A similar phenomenon was observed in B. napus, in which the number of isoforms and the number of genes involved in AS increased compared to its diploid progenitors []. In addition, in B. napus, subgenome-dominant expression and AS have been shown to jointly shape the immune response to Sclerotinia infection, with the C-subgenome contributing more to both expression and splicing regulation []. Likewise, in coffee, genome-wide analysis of the AP2/ERF transcription factor family showed that allotetraploid Coffea arabica contains nearly twice as many genes as its diploid progenitors C. canephora and C. eugenioides, largely due to post-polyploid tandem and segmental duplications []. Subgenome-specific biases were evident, with canephora-derived chromosomes enriched in disease-resistance genes and eugenioides-derived chromosomes retaining more temperature-tolerance genes, illustrating how polyploidy diversifies regulatory repertoires under stress.
These findings show that AS is a pervasive yet highly variable regulatory process. Its prevalence is shaped not only by intrinsic genomic features, but also by tissue identity, developmental stage, and ploidy level, each of which introduces additional layers of regulatory complexity.
3. The Role of AS in Plant Growth and Development
AS influences a wide range of developmental processes, but its functional roles have been most clearly demonstrated in flowering regulation, germination, hormone transport, and shoot architecture [,,]. This section summarizes representative cases in which specific splice isoforms, splicing factors, or splicing-related mutations have been mechanistically linked to developmental outcomes, providing clear evidence of AS function during plant growth.
Numerous studies have demonstrated that AS regulates flowering in many plant species (reviewed in []), by generating distinct isoforms of flowering time genes such as FLOWERING LOCUS T (FT) [] and FLOWERING LOCUS C (FLC) [], as well as transcription factors including the bZIP transcription factor FD [], MADS-box transcription factor FLOWERING LOCUS M (FM) [,], and CONSTANS (CO) [,]. For example, splicing factor 30 (SR30) regulates flowering primarily through FLC, while its own function is self-regulated by AS []. The balance between functional and NMD-targeted isoforms fine-tune SR30 abundance of SR30, thereby modulating flowering time.
In Arabidopsis, the spliceosomal component SmEb modulates abscisic acid (ABA) sensitivity during germination by maintaining the proper splicing balance of HAB1, a negative regulator of ABA signaling. Loss of SmEb disrupts this balance, resulting in increased ABA sensitivity and delayed cotyledon greening []. Similarly, in barley, the cap-binding complex (CBC), composed of CBP20 and CBP80, regulates ABA-responsive AS during germination. Double mutants lacking CBC subunits exhibit altered splicing of ABA-related genes and reduced ABA sensitivity, suggesting that the CBC functions upstream of ABA signaling through AS control [].
Auxin-mediated development is also regulated through AS of PIN7 in Arabidopsis. Two isoforms, PIN7a and PIN7b, have opposite effects on auxin transport and developmental processes such as apical hook formation and hypocotyl bending. Their co-expression and physical interaction indicate that a precise isoform balance is essential for proper tropic responses [].
In Gossypium arboreum, a single-nucleotide mutation in the TFL1 gene alters the canonical 5′ splice site, producing a truncated isoform that disrupts shoot apical meristem maintenance. This mutation leads to determinate growth and terminal flower formation. Transcriptomic analysis of the mutant revealed altered expression of auxin and sugar transporters, suggesting that TFL1-mediated AS integrates hormonal and metabolic signals during shoot development [].
In polyploid species, several studies have investigated the roles of AS in the development of cotton and wheat. As a major textile crop, AS profiles of cotton were examined during fiber development in G. hirsutum [] and G. barbadense []. Earlier work demonstrated that AS of MADS-box transcription factors in G. hirsutum fiber cells generates multiple isoforms with distinct expression patterns and possible regulatory functions [], supporting the hypothesis that AS contributes to tissue-specific differentiation and secondary cell wall biosynthesis in cotton fibers.
In wheat, several recent studies have revealed how alternative splicing (AS) fine-tunes developmental processes and yield formation. The TaPP2C-a5 gene produces two splice isoforms, TaPP2C-a5L and TaPP2C-a5S, with opposing effects on abscisic acid (ABA) signaling and seed dormancy: the Triticeae-specific short isoform enhances dormancy and ABA sensitivity, whereas the long isoform promotes germination []. An intron-located single-nucleotide variation in TaGS5-3D alters splicing efficiency, leading to intron-retained transcripts that increase grain size and yield []. Similarly, TaNAK1 generates two isoforms, TaNAK1.1 and TaNAK1.2, which exert opposite regulatory effects on flowering and plant architecture—TaNAK1.2 accelerates flowering and increases plant height and seed yield, whereas TaNAK1.1 delays flowering and restricts growth []. Another AP2-like gene, TaTOE1-B1, produces three splice variants, of which TaTOE1-B1-3 acts as a floral inhibitor by repressing FT and SOC1 expression, thus delaying the transition to flowering []. Together, these studies demonstrate that AS functions as a dynamic post-transcriptional mechanism that coordinates hormone signaling, flowering, and yield traits in polyploid wheat.
Collectively, these examples show that AS can modulate signaling pathways (e.g., ABA and auxin transport) or generate variable isoforms that drive distinct developmental outcomes, highlighting its critical role in shaping plant growth and development.
4. Abiotic Stress-Induced Alternative Splicing Variants
Abiotic stresses such as drought, salinity, and extreme temperatures profoundly alter plant transcriptomes. AS acts as a rapid and flexible regulatory layer, frequently targeting transcription factors, RNA-binding proteins, and signaling components to modulate hormone pathways, ion transport, and metabolic responses. Many isoform shifts occur independently of overall transcript abundance, underscoring AS as a distinct mechanism of stress adaptation. This section highlights how drought, salinity, and temperature reshape splicing landscapes and influence plant resilience in model and major crop species such as Arabidopsis, maize, rice, and so on. These examples illustrate general regulatory principles that are also conserved or amplified in the three focal polyploid crops described in Section 5.
4.1. Drought Stress
Drought is a major abiotic stress that limits plant growth and productivity, and AS has emerged as a critical mechanism for enhancing drought tolerance by modulating stress-responsive gene expression [,].
In maize, the clade B phosphatase gene ZmPP2C26 undergoes alternative first exon splicing to produce isoforms with distinct activities, both of which negatively regulate drought tolerance []. Mutant lines lacking ZmPP2C26 exhibit improved photosynthesis and water retention, linking AS directly to drought sensitivity. Epigenetic regulation also influences splicing outcomes, as DNA-methylation changes near splice junctions affect drought-induced AS in genes related to photosynthesis and stress signaling [].
Similar AS-mediated regulation has been observed in soybean, where thousands of drought-responsive events involve transcription factors (e.g., MYB, NAC, bZIP) and splicing factors (e.g., SR45a, U2AF), many of which display stage-specific or inverse expression relative to their parent genes [].
In barley, ABA-dependent splice variants of HvABI5 and serine/arginine-rich (SR) proteins modulate hormone-responsive pathways, while an isoform of HvLHCA4.2-S enhances ROS scavenging and stomatal closure, improving drought tolerance in transgenic plants [,].
Rice studies have highlighted a role for AS in drought memory, identifying nearly 2000 memory-associated AS events, enriched in RNA-binding proteins and transcription factors, that contribute to stress imprinting and recall []. Also, OsbHLH59 produces two isoforms, OsbHLH59.1 and OsbHLH59.2, the latter of which is induced by ABA and enhances drought tolerance [].
These studies demonstrate that drought-induced AS frequently targets transcription factors, ABA signaling components, and stress-protective genes. However, functional validation remains limited to a few genes and species, underscoring the need for broader mechanistic studies in crops.
4.2. Salinity Stress
Salinity stress leads to large-scale transcriptomic reprogramming, and AS is increasingly recognized as an important regulator of salt-tolerance pathways [,]. Several studies have identified specific splice variants, splicing factors, and natural splice site polymorphisms that contribute to salt stress responses.
In Arabidopsis, Salt-Responsive Alternatively Spliced gene 1 (SRAS1), which encodes a RING-type E3 ubiquitin ligase, produces two splice variants, SRAS1.1 and SRAS1.2, that display contrasting responses to salt stress []. Under salt stress conditions, the alternative splicing event favors increased accumulation of SRAS1.1 and a reduced level of SRAS1.2.
In Populus trichocarpa, the SR-rich factor PtSC27 is strongly induced under saline conditions []. Overexpression in Arabidopsis altered splicing patterns of ABA-responsive genes, suggesting a role in fine-tuning hormone-regulated isoforms during salt adaptation.
Long-read sequencing in rice identified extensive early AS responses to salinity, dominated by IR. Stress-responsive transcription factors, ion transporters, and SR proteins (e.g., RS33, RS2Z36) displayed genotype-specific AS events, implying a feedback loop that modulates splicing efficiency in tolerant genotypes []. Complementing this, Genome-wide association studies (GWAS)–AS integration revealed 764 genotype-specific splicing events, including variants in OsRAD23 and OsNUC1 linked to Na+ accumulation and salt-tolerance diversity []. Moreover, in Gossypium species, 47 sodium/hydrogen antiporter (NHX) genes with long introns were identified, indicating high potential for AS-mediated diversification of ion transporters [].
Polyploidization may further expand AS diversity under salt stress. Evidence from legumes shows that autopolyploidization reprograms post-transcriptional regulation [], while polyploid citrus exhibits epigenetic remodeling of ethylene-biosynthesis genes, potentially enhancing stress resilience through expanded isoform variability []. Salinity-induced AS predominantly affects transcription factors, ABA-responsive regulators, and ion-transport genes. Polyploid species may further exploit their increased gene copy number to expand AS diversity and enhance salt tolerance.
4.3. Temperature Stress
Temperature extremes strongly influence plant development and productivity, and numerous studies have revealed that AS is a key regulatory layer in both cold and heat stress responses. These studies particularly implicate transcription factors, heat shock proteins, and splicing regulators as major AS targets.
In A. thaliana, the spliceosomal component SmEb is essential for proper splicing of HAB1 and other stress-related genes under chilling stress. SmEb-deficient mutants show chlorophyll degradation, abnormal development, and heightened cold sensitivity, linking splicing fidelity to stress adaptation []. A related study revealed that CBF transcription factors promote condensation of splicing regulators (RS40, RSZ32) into nuclear speckles, enhancing cold-responsive AS; mutations in these components impair isoform switching and reduce cold tolerance [].
Cold-induced AS has also been documented in poplar (P. trichocarpa, P. ussuriensis), where >14,000 events—mainly IR—affect numerous transcription factors (PtrNAC72, PtrCBF1, PtrDREB2A) and splicing regulators, indicating feedback control of the splicing machinery itself [].
Heat stress likewise triggers AS of transcription factors and chaperone regulators. In maize, ZmHSF12, heat shock transcription factor, encodes two alternative spliced transcripts, ZmHSF12-1 and ZmHSF12-2, which regulate the balance of plant growth and heat tolerance []. In Arabidopsis, RDM16, a pre-mRNA splicing factor, produces two isoforms—RDL (long) and RDS (short) []. The RDS facilitates the condensation of RDL, synergistically strengthening their function in heat tolerance. Heat stress also modulates spliceosome activity, for example, the dephosphorylation of spliceosome subunits by PP2A B’η modulates the splicing pattern, leading to the production of different protein isoforms in response to heat stress [].
Other mechanisms include exitron splicing—cryptic introns located within annotated exons of MBD4L in Arabidopsis, which alters nuclear localization of the resulting isoforms []. In waxy maize, salicylic acid pretreatment reduced the frequency of heat-induced AS events and restored the normal expression patterns of key heat-responsive genes, thereby enhancing thermotolerance [].
Overall, both cold and heat stress induce widespread AS changes, particularly in transcription factors, splicing regulators, and heat-shock proteins. Many of these genes form feedback loops in which the splicing machinery itself undergoes stress responsive AS, amplifying transcriptome flexibility.
4.4. Molecular Pathways Linked to AS Under Stress
Environmental stresses such as drought, salinity, and high temperature extensively reprogram AS patterns at the genome-wide level. These changes are not random but are regulated through interconnected pathways involving splicing factors, hormone signaling, RNA surveillance, and chromatin dynamics (Figure 2A–D). Although the core spliceosome (U1, U2, U4/U6, and U5 snRNPs) remains intact, stress conditions influence splice-site selection by modulating the activity, expression, and isoform diversity of associated regulators—particularly SR proteins and heterogeneous nuclear ribonucleoproteins (hnRNPs) (Figure 2A) [,,].
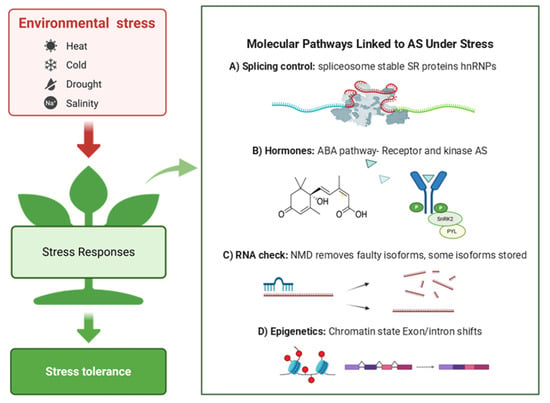
Figure 2.
Conceptual model of stress responsive AS in plants. Environmental stressors (heat, cold, drought, salinity) are perceived and transduced to elicit cellular responses that promote stress tolerance (left). The (right) panel summarize four major molecular pathways that modulate AS during stress: (A) splicing control via spliceosome dynamics mediated by SR proteins and hnRNPs; (B) hormone signaling exemplified by the ABA pathway, where ABA-bound PYR/PYL receptors inhibit PP2C phosphatases, activating SnRK2 kinases and downstream AS regulators; (C) RNA surveillance by NMD, which degrades aberrant isoforms while some isoforms are sequestered/stored; (D) epigenetic regulation in which chromatin-state changes exon–intron selection. Images were created with BioRender. Faiza Fatima. (2025). https://app.biorender.com/illustrations/68c05363ab47b2405e081788 (accessed on 12 September 2025).
Several SR proteins, such as SR30, SR45, and RSZp22, are themselves regulated by AS under stress. For example, heat stress in Arabidopsis triggers AS of SR34b and SF1, which subsequently modulates the splicing of heat-responsive genes including HSFA2 and HSP21 [,]. This feedback regulation forms a dynamic loop in which splicing factors undergo AS to fine-tune downstream gene expression, enabling plants to rapidly adapt to temperature fluctuations.
Hormone signaling is another major regulator of stress-responsive AS. ABA pathway is strongly influenced by the AS of key components such as PYL receptors, SnRK2 kinases, and downstream transcription factors. These events often generate truncated or nonfunctional isoforms that alter ABA sensitivity (Figure 2B), leading to either enhanced or reduced stress responses depending on the environmental context [,,].
AS is also tightly linked to NMD, which degrades transcripts containing premature stop codons. Many stress-induced isoforms are predicted NMD targets; however, some escape degradation by remaining in the nucleus, potentially acting as reservoirs for rapid translation during recovery (Figure 2C) [,,]. This coupling between AS and NMD provides an additional layer of gene expression control in fluctuating environments.
Epigenetic modifications and chromatin remodeling also influence AS under stress. Changes in DNA methylation, histone modifications, and RNA polymerase II elongation rate can affect co-transcriptional splicing decisions. Stress-induced chromatin state changes have been shown to shift exon–intron boundaries and increase the frequency of IR or ES events (Figure 2D) [,].
Importantly, transcriptome analyses reveal that AS and gene-expression levels often act independently, affecting distinct gene sets. This uncoupling allows plants to produce functionally distinct isoforms without changing overall transcript abundance, offering a rapid means of stress adaptation [,].
Finally, advances in long-read sequencing (e.g., Iso-Seq) and splicing-aware CRISPR technologies are accelerating the discovery and functional validation of stress-responsive isoforms. These tools are expected to facilitate the targeted manipulation of AS pathways to develop stress-resilient crop varieties [].
5. Alternative Splicing in Polyploids
This section provides a comparative synthesis of AS patterns across the three focal polyploid crops, including predominant event types, representative genes responsive to biotic or abiotic stresses, and functional outcomes. As polyploids often exhibit enhanced stress modulation compared to their diploid progenitors, they serve as ideal systems for investigating how duplicated genomes interact with AS to expand regulatory capacity under stress. In the following subsections, we summarize recent advances in B. napus, G. hirsutum, and T. aestivum—which are widely studied due to their economic importance, complex genome organization, and well-developed genomic resources.
5.1. Brassica napus
Brassica napus is an economically important allotetraploid oilseed crop (AACC) formed by hybridization between B. rapa (A-genome) and B. oleracea (C-genome) approximately 7500 years ago []. AS is a key post-transcriptional mechanism that contributes to transcriptomic diversity, stress adaptation, and phenotypic variation in this species.
High-resolution isoform studies using PacBio Iso-Seq and Illumina RNA-seq (Beijing Genomics Institute, Wuhan, China) identified 147,698 isoforms and 222,000 AS events, of which 69% were tissue-specific []. Approximately 31,500 events altered open-reading frames or protein domains, indicating potential functional diversification.
Comparative analyses of natural and resynthesized B. napus lines showed that 26–30% of duplicated genes exhibit AS divergence relative to their diploid parents, with many AS losses or shifts converging across independently resynthesized lines []. Notably, natural B. napus exhibited more extensive AS responses than resynthesized lines, reinforcing the idea that genomic interactions and subgenome dominance—where resynthesized B. napus plants showed a greater subgenome bias under infection, with the C-subgenome contributing disproportionately to the defense response—co-evolve with AS regulation []. Complementing these findings, Sun et al. (2024) directly compared B. napus with its diploid progenitors (B. rapa and B. oleracea) across four tissues (stems, leaves, flowers, and siliques) []. They showed that B. napus harbors a greater number of AS events than either progenitor during allopolyploidization. These splicing changes were associated with DNA methylation dynamics and cis/trans regulatory divergence, indicating that polyploidization expands isoform diversity while reshaping epigenetic control. Although many duplicated genes remain conserved, a substantial fraction undergoes neofunctionalization or specialization, highlighting the interplay between AS divergence, epigenetic remodeling, and subgenome interactions [].
Extending these observations to environmental regulation, long-read sequencing revealed that temperature stress in B. napus alters both the frequency and diversity of AS events. Heat stress increased the number of AS events and the proportion of isoforms predicted to undergo NMD, indicating heightened post-transcriptional regulation []. Conversely, cold stress reduced isoform diversity per gene. The study also revealed subgenome-biased splicing, with CT-homeologs producing more isoforms than AT-homeologs across conditions [].
AS is strongly associated with both biotic and abiotic stress responses. IR is the predominant AS type and is especially enriched under Sclerotinia sclerotiorum [,] and Leptosphaeria maculans [] infections, where it affects genes encoding splicing factors, transcriptional regulators, and defense-related proteins. Similarly, abiotic stresses such as boron deficiency [], heat, cold, drought, salinity, and dehydration [,] induce AS in genes involved in RNA binding, signal transduction, and stress-responsive pathways. In general, both biotic and abiotic stress increase the diversity of mRNA transcripts by inducing more AS events. Moreover, functional studies in B. napus have demonstrated that specific isoforms contribute to stress adaptation, linking AS variation to enhanced immune responses and abiotic stress tolerance [,,,]. A recent study revealed that BnABF4L, an ABA pathway regulator, undergoes A3SS-mediated alternative splicing to produce three isoforms (V1–V3) []. Stress conditions selectively repress V1 translation but favor V2 and V3, which together modulate stress responses.
Beyond stress responses, AS contributes to developmental variation. Splicing differences in flavonoid-biosynthesis genes are linked to seed-coat color [], and modifications in spliceosome components affect secondary dormancy [].
Subgenome-level divergence in AS has been widely observed. Under cold, heat, and drought stress, the A-subgenome tends to dominate gene expression, whereas the C-subgenome exhibits greater AS activity across more than 27,000 gene pairs []. AS frequency was negatively correlated with gene expression, and cold stress triggered the most extensive splicing changes, with cold–drought overlap being greater than cold–heat overlap []. A transcriptome-wide analysis of multiple abiotic stress (cold, dehydration, salt, and ABA) responses in B. napus identified 357 differentially alternatively spliced (DAS) genes, predominantly IR events. Weighted gene co-expression network analysis (WGCNA) revealed 23 modules associated with stress responses, highlighting hub genes such as RBP45C (RNA-binding protein regulating AS), LHY (circadian clock regulator), MYB59 (MYB transcription factor linked to cell cycle and stress responses), SCL30A (GRAS-family splicing factor), SR40 (serine/arginine-rich splicing regulator), MAJ23.10 (putative RNA-binding protein), and DWF4 (brassinosteroid biosynthesis enzyme) [].
Overall, studies in B. napus consistently demonstrate that AS is highly dynamic, stress-responsive, and shaped by subgenome interactions. The interplay of expression dominance and AS divergence highlights the role of polyploidy in expanding transcriptome complexity and adaptive potential.
5.2. Gossypium hirsutum
The genus Gossypium is a well-established model for studying polyploid genome evolution. Allotetraploid cotton species, including G. hirsutum (AD1), originated 1–2 million years ago from a hybridization event between an A-genome-like African diploid and a D-genome-like American diploid [,]. Since polyploidization, G. hirsutum has undergone asymmetric subgenome domestication and artificial selection for more than 4000–5000 years [,]. Survey-based analyses have shown that approximately 27–30% of multi-exon genes in allotetraploid cotton undergo AS, with IR as the most frequent event type, establishing AS as a pervasive regulatory layer in this crop [].
Comparative genome sequencing of diploid and tetraploid cotton species has revealed asymmetric evolution and biased gene expression between the At (A-subgenome) and Dt (D-subgenome) components [,,,,]. However, until recently, the lack of well-annotated transcript isoform datasets limited detailed analysis of AS divergence between homoeologous genes [,].
Abiotic stress studies further reveal that splicing plasticity is integral to cotton adaptation. Integrated transcriptomic and proteomic profiling demonstrated that salt tolerance is mediated through AS-dependent regulation of ion transport and protective pathways []. More recently, combined transcriptomic and proteomic analysis under salt and drought stress showed widespread splicing reprogramming dominated by IR, with several isoforms validated as regulators of stress response pathways [].
Biotic interactions also induce extensive isoform switching. Herbivory and pest challenge triggered differential splicing of defense-related genes, including transcription factors and hormone-responsive regulators, highlighting AS as a key component of cotton defense networks []. AS additionally influences developmental and metabolic traits. Isoform switching in gossypol biosynthesis genes contributes to gland morphogenesis, linking AS to the regulation of secondary metabolism and tissue specialization in cotton [].
More recent work has emphasized the stage- and tissue-specific nature of AS during fiber development. Approximately 23.3% of multi-exon genes undergo AS during this process, with events being more common in ovules and early fiber initiation stages than in mature fibers []. Interestingly, IR was not the dominant AS form during these stages, suggesting that splicing regulation is developmentally modulated. Gene-body methylation and splicing factor activity were implicated as regulators of these patterns, revealing an epigenetic component in cotton fiber transcriptome regulation [].
Despite these advances, direct comparisons of AS landscapes between G. hirsutum and its diploid progenitors remain scarce, with most insights coming from analyses of subgenome-biased splicing within the allotetraploid itself. These studies demonstrate that AS in G. hirsutum is a critical layer of transcriptome regulation shaped by polyploid genome architecture, developmental context, and epigenetic control. Isoform divergence between homeologs and spatiotemporal AS patterns underscore the functional complexity introduced by polyploidy in cotton.
5.3. Triticum aestivum
Bread wheat (T. aestivum) is an allohexaploid species (2n = 6x = 42) that originated approximately 8000 years ago through hybridization between the cultivated allotetraploid T. turgidum (AABB) and the diploid goatgrass Aegilops tauschii (DD), resulting in the AABBDD genome configuration [,]. This polyploidization has contributed to the structural complexity and plasticity of the wheat genome, shaping its adaptability under both natural and artificial selection [].
AS is a major post-transcriptional regulatory mechanism in wheat, especially under abiotic stress conditions. A transcriptome analysis of Najran wheat under 200 mM NaCl revealed that 22.5% of root-expressed genes and 23.1% of shoot-expressed genes underwent AS []. Unlike B. napus and G. hirsutum, where IR predominates, A3SS was most common in wheat, while MXE was least frequent. Salt stress also induced tissue-specific regulation, with 82% of AS events differing between roots and shoots. Functional annotation showed that root AS events were enriched in cytoskeleton-related processes, whereas shoot AS events were associated with metabolism and signal transduction []. Similar patterns were observed in comparative analyses of salt-tolerant and salt-sensitive cultivars, where more than 9000 differentially expressed genes and extensive AS reprogramming were detected. Interestingly, AS isoforms of SR protein genes were more prevalent than changes in their overall expression, underscoring that splicing regulation plays a central role in salt adaptation [].
Heat stress also triggers AS of transcription factors and chaperone regulators. TaHsfA2-7, heat shock transcription factor, produces a truncated isoform (TaHsfA2-7-AS) that accumulates in the nucleus and enhances heat tolerance during flowering and grain-filling, with TaHsfA1 directly regulating both isoforms []. Another heat shock transcription factor, TaHSFA6e, generates two isoforms under heat stress, with the extended TaHSFA6e-III more strongly activating TaHSP70 genes and promoting stress-granule disassembly during recovery [].
Consistent with these findings, a genome-wide AS analysis comparing the salt-sensitive cultivar Chinese Spring and the salt-tolerant cultivar Qing Mai 6 identified 11,141 genes with salt-responsive AS events []. Chinese Spring exhibited more events (21,203) than Qing Mai 6 (19,742), suggesting salt stress induces more AS events. AS events were distributed across the A, B, and D subgenomes (7235; 8143; 7407 events, respectively), with AS activity peaking 48 h after stress onset.
AS is also stress-specific: drought (DS), heat (HS), and combined heat–drought (HD) treatments induced AS in 200, 3576, and 4056 genes, respectively. The B-subgenome showed the highest AS activity across all conditions. Notably, only 12% of DS-responsive AS genes overlapped with differentially expressed genes, compared to ~40% under HS and HD, indicating that AS plays a particularly important role in post-transcriptional regulation under heat and combined stress []. Studies of thermosensitive genic male-sterile (TGMS) lines further illustrate this connection, where nearly 8000 differentials AS events were identified in developing pollen. These events disrupted modules related to cytoskeleton dynamics, calcium transport, and vesicle trafficking, directly linking AS to fertility regulation under stress [].
Beyond stress responses, AS contributes to wheat development and polyploid genome evolution. Isoform profiling during embryogenesis revealed extensive splicing dynamics, with IR most frequent and homoeolog-specific divergence evident across subgenomes, indicating that AS underlies both developmental regulation and evolutionary diversification []. Likewise, analyses of newly formed synthetic hexaploid wheat demonstrated that homoeologous exchanges reshape both gene expression and AS landscapes, showing how structural genomic changes introduce new layers of splicing variation []. Comparative analyses of wild and domesticated wheat further revealed that both domestication and polyploidization reduced AS frequency, largely through loss of IR events and downregulation of splicing regulators []. This observation contrasts with B. napus, in which more AS events were detected than in either diploid progenitor []. The difference may stem from contrasting experimental contexts, namely wheat seedlings under non-stress conditions versus mature Brassica leaves experiencing biotic stress. Many homoeolog triplets exhibited partitioned AS patterns, highlighting the combined role of subgenome interaction and selection in shaping the wheat AS landscape [].
These studies reveal that AS in hexaploid wheat is both stress- and subgenome-dependent, with distinct isoform profiles across tissues, genotypes, and stress types. The dynamic nature of AS highlights its potential for isoform-level breeding strategies to enhance wheat stress tolerance.
Across the three polyploid crops, AS shows both conserved and lineage-specific patterns. Heat and drought are the most extensively studied stresses, with IR prevailing in B. napus and G. hirsutum, whereas T. aestivum exhibits more ES [] and A3SS []. Despite these differences, stress-induced splicing of transcription factors, RNA-binding proteins, and hormone-responsive genes is a unifying theme. Cold- and salinity-related AS remain less explored, particularly in T. aestivum and G. hirsutum, indicating future research needs. In addition, given that most current reports are descriptive, detailed functional studies of genes producing AS isoforms are needed to determine how AS regulates gene expression and contributes to stress resilience in polyploids. A conceptual summary of splicing divergence following polyploidization across these three species is illustrated in Figure 3, emphasizing the deviation between expected additive expression and the observed complexity resulting from subgenome interactions and stress-responsive regulation.
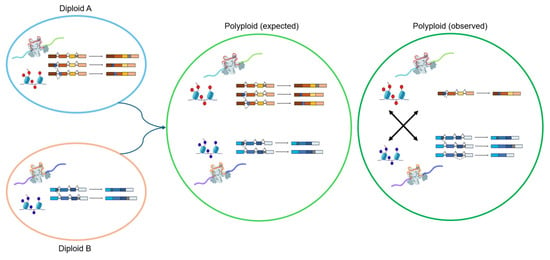
Figure 3.
Conceptual model illustrating AS divergence between polyploid and its diploid progenitors. Diploid A and Diploid B represent parental lineages contributing to polyploid formation. Polyploid (expected) depicts theoretical additive expression and splicing outcomes assuming simple parental legacy. In contrast, polyploid (observed) reflects empirical findings across B. napus, G. hirsutum, and T. aestivum, where subgenome interactions, epigenetic remodeling, and cis/trans regulatory divergence reshape AS landscapes. These processes result in asymmetric subgenome activity, isoform switching, and altered AS isoform frequencies under stress and during developmental stages, leading to enhanced transcriptional plasticity and stress adaptation compared to diploid progenitors.
5.4. Other Polyploid Species
Beyond these major crops, AS profiles have also been characterized in other polyploid systems such as soybean [], Camelina sativa [], Camellia oleifera [], peanut [], and cotton relatives (G. barbadense) [], all showing a predominance of IR. However, most of these studies have primarily cataloged AS events rather than elucidating their regulatory significance. Further research is needed to determine whether the mechanisms summarized here—subgenome-biased splicing, stress-responsive isoform switching, and epigenetic modulation—represent conserved features across diverse polyploid lineages.
6. Future Directions and Implications
Research over the past decade has greatly expanded our understanding of AS as a key post-transcriptional regulatory layer in plants. High-throughput short- and long-read sequencing studies have revealed that polyploid crops such as B. napus, G. hirsutum, and T. aestivum exhibit extensive AS diversity shaped by subgenome interactions, epigenetic regulation, and environmental signals [,,]. Despite these advances, most findings in those three species remain descriptive. For instance, while heat- and drought-responsive isoforms have been cataloged in B. napus and T. aestivum, and tissue-specific AS characterized in cotton fibers, their direct physiological or yield-related functions are still unclear.
Although transcriptomic surveys consistently reveal widespread AS in plants, the extent to which it translates into functional protein diversity is unresolved. AS isoforms can alter protein properties, including tissue-specific expression, subcellular localization, domain composition, or interaction partners [,]. For instance, splice variants may localize to distinct organelles (e.g., mitochondria vs. chloroplasts), act as dominant-negative regulators of transcription factors, or engage in cooperative loops that fine-tune signaling pathways [,]. Beyond localization, AS can modulate protein functionality by altering catalytic or binding domains, post-translational modification sites, or protein stability [,,]. Isoform-specific differences in phosphorylation, ubiquitination, or acetylation can redirect signaling fluxes or determine degradation rates, linking AS directly to proteostasis and stress adaptation []. Recent proteomic and interactomic analyses have also shown that AS frequently rewires protein–protein interaction networks, revealing isoform-dependent partners that contribute to tissue-specific and stress-responsive functions [,]. These mechanisms underscore that AS not only increases transcriptome complexity but also provides a versatile toolkit for expanding proteomic outputs, with consequences for stress adaptation, development, and evolutionary innovation [,].
Future research should prioritize experimental validation of isoform functions, using CRISPR-based splicing modulation, isoform-specific overexpression, and gene-editing approaches to link AS events to cold, heat, drought, and salinity resilience as well as yield stability. Integrating AS datasets with epigenomic, proteomic, and metabolic profiles will be essential to uncover how AS interacts with other regulatory layers. Moreover, the development of isoform-aware molecular markers could enable breeders to incorporate favorable splice variants into breeding programs, complementing traditional gene-based selection strategies [].
Recent work also links AS regulation to plant immune responses. The fungal pathogen Exserohilum turcicum secretes the effector EtEC81, which interacts with the maize splicing regulator ZmEIP1 to reprogram defense-related splicing and enhance immune signaling []. Such pathogen-driven manipulation of host AS demonstrates that splicing regulation extends beyond abiotic stress adaptation to encompass biotic stress and host–pathogen co-evolution. To translate these insights into breeding practice, future programs could integrate isoform-specific quantitative trait loci (iso-QTL) mapping and develop high-throughput assays to detect favorable splice variants in elite germplasm. In parallel, CRISPR/Cas-based genome and splice-site editing can be used to fine-tune intron–exon architecture or modulate splicing factors, creating cultivars with optimized stress-responsive isoform profiles.
As climate change intensifies abiotic and biotic stresses, harnessing AS in key polyploid crops, such as B. napus, G. hirsutum, and T. aestivum—offers a promising but under-explored path for improving resilience and productivity. Their duplicated genomes provide natural flexibility for fine-tuning splicing patterns through breeding and genome editing, making them ideal models for developing AS-informed improvement strategies. Future studies should aim to link transcript isoform diversity to adaptive traits and productivity. Strengthening collaborations between molecular biologists, breeders, and computational scientists will be critical to translating AS knowledge into practical applications for sustainable agriculture.
Author Contributions
Conceptualization, F.F., M.-J.Y.; writing—original draft preparation and review and editing, F.F., M.-J.Y.; supervision, M.-J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Khaipho-Burch, M.; Cooper, M.; Crossa, J.; de Leon, N.; Holland, J.; Lewis, R.; McCouch, S.; Murray, S.C.; Rabbi, I.; Ronald, P. Genetic modification can improve crop yields—But stop overselling it. Nature 2023, 621, 470–473. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Dinneny, J.R. Developmental responses to water and salinity in root systems. Annu. Rev. Cell Dev. Biol. 2019, 35, 239–257. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Lv, P.; Wang, W.; Wang, J.; He, Y.; Song, Z.; Cai, D. Full-length transcriptome reconstruction reveals genetic differences in hybrids of Oryza sativa and Oryza punctata with different ploidy and genome compositions. BMC Plant Biol. 2022, 22, 131. [Google Scholar] [CrossRef]
- Alhabsi, A.; Ling, Y.; Crespi, M.; Reddy, A.S.; Mahfouz, M. Alternative splicing dynamics in plant adaptive responses to stress. Annu. Rev. Plant Biol. 2025, 76, 687–717. [Google Scholar] [CrossRef] [PubMed]
- Dikaya, V.; El Arbi, N.; Rojas-Murcia, N.; Nardeli, S.M.; Goretti, D.; Schmid, M. Insights into the role of alternative splicing in plant temperature response. J. Exp. Bot. 2021, 72, 7384–7403. [Google Scholar] [CrossRef] [PubMed]
- Godinho, D.P.; Yanez, R.J.; Duque, P. Pathogen-responsive alternative splicing in plant immunity. Trends Plant Sci. 2025, 30, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-X.; Guo, Q.-H.; Xu, W.-B.; Liu, P.; Yan, K. Rapid regulation of alternative splicing in response to environmental stresses. Front. Plant Sci. 2022, 13, 832177. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Xu, X.; Zhou, W.; Wu, L. Alternative splicing: An efficient regulatory approach towards plant developmental plasticity. Wiley Interdiscip. Rev. RNA 2023, 14, e1758. [Google Scholar] [CrossRef]
- Punzo, P.; Grillo, S.; Batelli, G. Alternative splicing in plant abiotic stress responses. Biochem. Soc. Trans. 2020, 48, 2117–2126. [Google Scholar] [CrossRef]
- Rosenkranz, R.R.; Ullrich, S.; Löchli, K.; Simm, S.; Fragkostefanakis, S. Relevance and regulation of alternative splicing in plant heat stress response: Current understanding and future directions. Front. Plant Sci. 2022, 13, 911277. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Gene regulation at transcriptional and post-transcriptional levels to combat salt stress in plants. Physiol. Plant. 2021, 173, 1556–1572. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Mo, R.; Cui, D.; Cheng, W.; Wang, H.; Qin, J.; Liu, Z. Alternative splicing in the regulatory circuit of plant temperature response. Int. J. Mol. Sci. 2023, 24, 3878. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Staiger, D.; Brown, J.W. Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell 2013, 25, 3640–3656. [Google Scholar] [CrossRef] [PubMed]
- Sybilska, E.; Daszkowska-Golec, A. A complex signaling trio in seed germination: Auxin-JA-ABA. Trends Plant Sci. 2023, 28, 873–875. [Google Scholar] [CrossRef]
- Liu, G.; Wang, H.; Gao, H.; Yu, S.; Liu, C.; Wang, Y.; Sun, Y.; Zhang, D. Alternative Splicing of Functional Genes in Plant Growth, Development, and Stress Responses. Int. J. Mol. Sci. 2025, 26, 5864. [Google Scholar] [CrossRef]
- Mandadi, K.K.; Petrillo, E.; Dubrovina, A.S.; Kiselev, K.V. Regulation of alternative splicing in plant stress responses. Front. Plant Sci. 2023, 13, 1120961. [Google Scholar] [CrossRef]
- Liu, Y.; Do, S.; Huynh, H.; Li, J.-X.; Liu, Y.-G.; Du, Z.-Y.; Chen, M.-X. Importance of pre-mRNA splicing and its study tools in plants. Adv. Biotechnol. 2024, 2, 4. [Google Scholar] [CrossRef]
- Szakonyi, D.; Duque, P. Alternative splicing as a regulator of early plant development. Front. Plant Sci. 2018, 9, 1174. [Google Scholar] [CrossRef]
- Marasco, L.E.; Kornblihtt, A.R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. 2023, 24, 242–254. [Google Scholar] [CrossRef]
- Du, Y.; Cao, L.; Wang, S.; Guo, L.; Tan, L.; Liu, H.; Feng, Y.; Wu, W. Differences in alternative splicing and their potential underlying factors between animals and plants. J. Adv. Res. 2024, 64, 83–98. [Google Scholar] [CrossRef]
- de la Fuente, R.; Díaz-Villanueva, W.; Arnau, V.; Moya, A. Evolutionary trends of alternative splicing. eLife 2024, 13, RP94802. [Google Scholar] [CrossRef]
- Chaudhary, S.; Khokhar, W.; Jabre, I.; Reddy, A.S.; Byrne, L.J.; Wilson, C.M.; Syed, N.H. Alternative splicing and protein diversity: Plants versus animals. Front. Plant Sci. 2019, 10, 708. [Google Scholar] [CrossRef]
- McCue, K.; Burge, C.B. An interpretable model of pre-mRNA splicing for animal and plant genes. Sci. Adv. 2024, 10, eadn1547. [Google Scholar] [CrossRef]
- Wright, C.J.; Smith, C.W.; Jiggins, C.D. Alternative splicing as a source of phenotypic diversity. Nat. Rev. Genet. 2022, 23, 697–710. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.-H. Alternative splicing for improving abiotic stress tolerance and agronomic traits in crop plants. J. Plant Biol. 2020, 63, 409–420. [Google Scholar] [CrossRef]
- Islam, M.M.; Deepo, D.M.; Nasif, S.O.; Siddique, A.B.; Hassan, O.; Siddique, A.B.; Paul, N.C. Cytogenetics and consequences of polyploidization on different biotic-abiotic stress tolerance and the potential mechanisms involved. Plants 2022, 11, 2684. [Google Scholar] [CrossRef]
- Yu, H.; Bi, X.; Li, Z.; Fu, X.; Li, Y.; Li, Y.; Yang, Y.; Liu, D.; Li, G.; Dong, W. Transcriptomic Analysis of Alternative Splicing Events during Different Fruit Ripening Stages of Coffea arabica L. Genes 2024, 15, 459. [Google Scholar] [CrossRef]
- Tossi, V.E.; Martínez Tosar, L.J.; Laino, L.E.; Iannicelli, J.; Regalado, J.J.; Escandón, A.S.; Baroli, I.; Causin, H.F.; Pitta-Álvarez, S.I. Impact of polyploidy on plant tolerance to abiotic and biotic stresses. Front. Plant Sci. 2022, 13, 869423. [Google Scholar] [CrossRef]
- Gao, P.; Quilichini, T.D.; Zhai, C.; Qin, L.; Nilsen, K.T.; Li, Q.; Sharpe, A.G.; Kochian, L.V.; Zou, J.; Reddy, A.S. Alternative splicing dynamics and evolutionary divergence during embryogenesis in wheat species. Plant Biotechnol. J. 2021, 19, 1624–1643. [Google Scholar] [CrossRef]
- de Jong, G.W.; Adams, K.L. Subgenome-dominant expression and alternative splicing in response to Sclerotinia infection in polyploid Brassica napus and progenitors. Plant J. 2023, 114, 142–158. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Quiroz, L.F.; Reddy, A.S.; Spillane, C.; Ortiz, R. Alternative splicing variation: Accessing and exploiting in crop improvement programs. Int. J. Mol. Sci. 2023, 24, 15205. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Ashman, T.-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26, Erratum in Plant Cell 2021, 33, 2899. [Google Scholar] [CrossRef]
- Marquardt, S.; Petrillo, E.; Manavella, P.A. Cotranscriptional RNA processing and modification in plants. Plant Cell 2023, 35, 1654–1670. [Google Scholar] [CrossRef]
- Tognacca, R.S.; Rodríguez, F.S.; Aballay, F.E.; Cartagena, C.M.; Servi, L.; Petrillo, E. Alternative splicing in plants: Current knowledge and future directions for assessing the biological relevance of splice variants. J. Exp. Bot. 2023, 74, 2251–2272. [Google Scholar] [CrossRef]
- Martín, G.; Márquez, Y.; Mantica, F.; Duque, P.; Irimia, M. Alternative splicing landscapes in Arabidopsis thaliana across tissues and stress conditions highlight major functional differences with animals. Genome Biol. 2021, 22, 35. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, W.; Zhu, D.; Zhang, B.; Xu, Q.; Shi, C.; He, H.; Dai, X.; Li, Y.; He, W. Population-level exploration of alternative splicing and its unique role in controlling agronomic traits of rice. Plant Cell 2024, 36, 4372–4387. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, Q.; Zhang, J.; Zhang, S.; Weng, J.; Di, H.; Zhang, L.; Li, X.; Liang, Y.; Dong, L. Genome-wide profiling of alternative splicing and gene fusion during Rice black-streaked dwarf virus stress in maize (Zea mays L.). Genes 2022, 13, 456. [Google Scholar] [CrossRef]
- Zhu, R.; Yue, C.; Wu, S.; Wu, M.; Xu, Z.; Liu, X.; Wang, R.; Wang, M. Alternative Splicing of BnABF4L Mediates Response to Abiotic Stresses in Rapeseed (Brassica napus L.). Biotechnol. Biofuels Bioprod. 2025, 18, 51. [Google Scholar] [CrossRef]
- Bao, Y.; Yin, X.; Song, X.; Liu, L.; Liu, Y.; Meng, S. Genome-wide identification of novel alternative splicing events and related isoforms linked to cotton domestication and fiber domestication. Gene 2025, 963, 149601. [Google Scholar] [CrossRef]
- Alyahya, N.; Taybi, T. Transcriptome-wide characterization of alternative splicing regulation in Najran wheat (Triticum aestivum) under salt stress. Curr. Plant Biol. 2024, 38, 100334. [Google Scholar] [CrossRef]
- Marquez, Y.; Brown, J.W.; Simpson, C.; Barta, A.; Kalyna, M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in Arabidopsis. Genome Res. 2012, 22, 1184–1195. [Google Scholar] [CrossRef]
- Mei, W.; Boatwright, L.; Feng, G.; Schnable, J.C.; Barbazuk, W.B. Evolutionarily conserved alternative splicing across monocots. Genetics 2017, 207, 465–480. [Google Scholar] [CrossRef]
- Mei, W.; Liu, S.; Schnable, J.C.; Yeh, C.-T.; Springer, N.M.; Schnable, P.S.; Barbazuk, W.B. A comprehensive analysis of alternative splicing in paleopolyploid maize. Front. Plant Sci. 2017, 8, 694. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Gong, W. Genome-wide identification and comparative analysis of alternative splicing across four legume species. Planta 2019, 249, 1133–1142. [Google Scholar] [CrossRef]
- Misra, C.S.; Sousa, A.G.; Barros, P.M.; Kermanov, A.; Becker, J.D. Cell-type-specific alternative splicing in the Arabidopsis germline. Plant Physiol. 2023, 192, 85–101. [Google Scholar] [CrossRef]
- Abulfaraj, A.A.; Alshareef, S.A. Concordant gene expression and alternative splicing regulation under abiotic stresses in Arabidopsis. Genes 2024, 15, 675. [Google Scholar] [CrossRef]
- An, Y.; Wu, J.; Chen, Y.; Shize, L. Comprehensive analysis of alternative splicing in Rosa roxburghii Tratt revealed its role in flavonoid synthesis. Front. Plant Sci. 2025, 16, 1627126. [Google Scholar] [CrossRef]
- Yu, S.; Wan, J.; Xu, T.; Zhang, J.; Cao, L.; Liu, J.; Liu, H.; Ren, X.; Yang, Z. A gene expression atlas of Nicotiana tabacum across various tissues at transcript resolution. Front. Plant Sci. 2025, 16, 1500654. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, D.; Li, Z.; Liang, H.; Deng, R.; Su, X.; Jiang, Y.; Duan, X. Alternative splicing of MaMYB16L regulates starch degradation in banana fruit during ripening. J. Integr. Plant Biol. 2021, 63, 1341–1352. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, H.; Huang, T.; Shen, X.; Xia, J.; Pang, F.; Wang, J.; Zhao, M. The complexity of the Fragaria x ananassa (octoploid) transcriptome by single-molecule long-read sequencing. Hortic. Res. 2019, 6, 46, Erratum in Hortic. Res. 2019, 6, 63. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, X.; Tang, W.; Deng, Q.; Wang, Y.; Lin, Y.; He, W.; Zhang, Y.; Li, M.; Luo, Y.; et al. Transcriptomic Complexity in Strawberry Fruit Development and Maturation Revealed by Nanopore Sequencing. Front. Plant Sci. 2022, 13, 872054. [Google Scholar] [CrossRef]
- Deng, Q.; Lu, H.; Liu, D.; Huang, Y.; Feng, J.; Wei, D.; Wang, Z.; Tang, Q. Modulation of flowering by an alternatively spliced AGL18-1 transcript in Brassica juncea. Crop J. 2025, 13, 456–467. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wei, X.; Datta, T.; Wei, F.; Xie, Z. Polyploidization: A biological force that enhances stress resistance. Int. J. Mol. Sci. 2024, 25, 1957. [Google Scholar] [CrossRef]
- Scarrow, M.; Wang, Y.; Sun, G. Molecular regulatory mechanisms underlying the adaptability of polyploid plants. Biol. Rev. 2021, 96, 394–407. [Google Scholar] [CrossRef]
- Li, M.; Hu, M.; Xiao, Y.; Wu, X.; Wang, J. The activation of gene expression and alternative splicing in the formation and evolution of allopolyploid Brassica napus. Hortic. Res. 2022, 9, uhab075. [Google Scholar] [CrossRef]
- Park, S.; Ahn, E.; Zhang, D.; Meinhardt, L.W. Comparative genome-wide characterization and evolutionary insights into the AP2/ERF gene family in three Coffea species (C. canephora, C. eugenioides, and C. arabica). BMC Genom. 2025, 26, 653. [Google Scholar]
- Lam, P.Y.; Wang, L.; Lo, C.; Zhu, F.-Y. Alternative splicing and its roles in plant metabolism. Int. J. Mol. Sci. 2022, 23, 7355. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Guo, C.; He, Y.; Li, Z.; Ning, G.; Shi, X.; Bao, M. The FLOWERING LOCUS T orthologous gene of Platanus acerifolia is expressed as alternatively spliced forms with distinct spatial and temporal patterns. Plant Biol. 2011, 13, 809–820. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Li, Z.-M.; Mei, L.; Yao, J.-L.; Hu, C.-G. PtFLC homolog from trifoliate orange (Poncirus trifoliata) is regulated by alternative splicing and experiences seasonal fluctuation in expression level. Planta 2009, 229, 847–859. [Google Scholar] [CrossRef]
- Ye, L.X.; Wu, Y.M.; Zhang, J.X.; Zhang, J.X.; Zhou, H.; Zeng, R.F.; Zheng, W.X.; Qiu, M.Q.; Zhou, J.J.; Xie, Z.Z. A bZIP transcription factor (CiFD) regulates drought- and low-temperature-induced flowering by alternative splicing in citrus. J. Integr. Plant Biol. 2023, 65, 674–691. [Google Scholar] [CrossRef]
- Capovilla, G.; Symeonidi, E.; Wu, R.; Schmid, M. Contribution of major FLM isoforms to temperature-dependent flowering in Arabidopsis thaliana. J. Exp. Bot. 2017, 68, 5117–5127. [Google Scholar] [CrossRef]
- Jin, S.; Kim, S.Y.; Susila, H.; Nasim, Z.; Youn, G.; Ahn, J.H. FLOWERING LOCUS M isoforms differentially affect the subcellular localization and stability of SHORT VEGETATIVE PHASE to regulate temperature-responsive flowering in Arabidopsis. Mol. Plant 2022, 15, 1696–1709. [Google Scholar] [CrossRef]
- Ma, H.; Pei, J.; Zhuo, J.; Tang, Q.; Hou, D.; Lin, X. The CONSTANS-LIKE gene PeCOL13 regulates flowering through intron-retained alternative splicing in Phyllostachys edulis. Int. J. Biol. Macromol. 2024, 274, 133393. [Google Scholar] [CrossRef]
- Gil, K.E.; Park, M.J.; Lee, H.J.; Park, Y.J.; Han, S.H.; Kwon, Y.J.; Seo, P.J.; Jung, J.H.; Park, C.M. Alternative splicing provides a proactive mechanism for the diurnal CONSTANS dynamics in Arabidopsis photoperiodic flowering. Plant J. 2017, 89, 128–140. [Google Scholar] [CrossRef]
- Zhang, D.; Li, M.; Aslam, M.M.; Huang, M.; Chen, M.-X.; Liu, Y.-G.; Zhang, J. The role of Arabidopsis Splicing Factor 30 in floral transition and the implications of its alternative splicing. Plant Physiol. 2025, 198, kiaf335. [Google Scholar] [CrossRef]
- Hong, Y.; Yao, J.; Shi, H.; Chen, Y.; Zhu, J.-K.; Wang, Z. The Arabidopsis spliceosomal protein SmEb modulates ABA responses by maintaining proper alternative splicing of HAB1. Stress Biol. 2021, 1, 4. [Google Scholar] [CrossRef]
- Sybilska, E.; Collin, A.; Sadat Haddadi, B.; Mur, L.A.; Beckmann, M.; Guo, W.; Simpson, C.G.; Daszkowska-Golec, A. The cap-binding complex modulates ABA-responsive transcript splicing during germination in barley (Hordeum vulgare). Sci. Rep. 2024, 14, 18278. [Google Scholar] [CrossRef]
- Kashkan, I.; Hrtyan, M.; Retzer, K.; Humpolíčková, J.; Jayasree, A.; Filepová, R.; Vondráková, Z.; Simon, S.; Rombaut, D.; Jacobs, T.B. Mutually opposing activity of PIN7 splicing isoforms is required for auxin-mediated tropic responses in Arabidopsis thaliana. New Phytol. 2022, 233, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Miao, P.; Qin, W.; Hu, W.; Wei, Z.; Ding, W.; Zhang, H.; Wang, Z. A novel single nucleotide mutation of TFL1 alters the plant architecture of Gossypium arboreum through changing the pre-mRNA splicing. Plant Cell Rep. 2024, 43, 26. [Google Scholar] [CrossRef]
- Zheng, J.; Wen, S.; Yu, Z.; Luo, K.; Rong, J.; Ding, M. Alternative splicing during fiber development in G. hirsutum. Int. J. Mol. Sci. 2023, 24, 11812. [Google Scholar] [CrossRef]
- Wang, M.; Wang, P.; Liang, F.; Ye, Z.; Li, J.; Shen, C.; Pei, L.; Wang, F.; Hu, J.; Tu, L. A global survey of alternative splicing in allopolyploid cotton: Landscape, complexity and regulation. New Phytol. 2018, 217, 163–178. [Google Scholar] [CrossRef]
- Lightfoot, D.J.; Malone, K.M.; Timmis, J.N.; Orford, S.J. Evidence for alternative splicing of MADS-box transcripts in developing cotton fibre cells. Mol. Genet. Genom. 2008, 279, 75–85. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, X.; Wu, Y.n.; Wang, R.; Zhang, Y.; Shi, F.; Zhao, H.; Yu, P.; Wang, Y.; Chen, M. TaPP2C-a5 fine-tunes wheat seed dormancy and germination with a Triticeae-specific, alternatively spliced transcript. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, H.; Xiao, Y.; Wang, C.; Zhang, J.; Shi, X.; Xie, S.; Wang, C.; Li, T.; Deng, P. An intron-located single nucleotide variation of TaGS5-3D is related to wheat grain size through accumulating intron retention transcripts. Theor. Appl. Genet. 2023, 136, 193. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Hu, K.; Zheng, H.; Zhang, S.; Liu, X.; Ma, M.; Zhao, H. Two alternative splicing variants of a wheat gene TaNAK1, TaNAK1. 1 and TaNAK1. 2, differentially regulate flowering time and plant architecture leading to differences in seed yield of transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 1014176. [Google Scholar] [CrossRef]
- Song, T.; Yu, Y.; Zhang, M.; Zhou, H.; Zhang, S.; Yu, M.; Zhou, J.; Cheng, J.; Xiang, J.; Yang, S. A wheat TaTOE1-B1 transcript TaTOE1-B1-3 can delay the flowering time of transgenic Arabidopsis. Int. J. Mol. Sci. 2021, 22, 12645. [Google Scholar] [CrossRef] [PubMed]
- Verta, J.-P.; Jacobs, A. The role of alternative splicing in adaptation and evolution. Trends Ecol. Evol. 2022, 37, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, W.; Peng, Y.; Cao, Y.; Qu, J.; Sun, F.; Yang, Q.; Lu, Y.; Zhang, X.; Zheng, L.; et al. ZmPP2C26 Alternative Splicing Variants Negatively Regulate Drought Tolerance in Maize. Front. Plant Sci. 2022, 13, 851531. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Pu, X.; Lv, H.; Liu, Y.; Ma, H.; Wu, F.; Wang, Q.; Feng, X.; Liu, T.; et al. Maize DNA Methylation in Response to Drought Stress Is Involved in Target Gene Expression and Alternative Splicing. Int. J. Mol. Sci. 2021, 22, 8285. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Hwang, H.; Bang, G.; Ha, J.; Park, Y.; Kim, J.Y. Understanding the molecular mechanisms of drought tolerance in wild soybean (Glycine soja) through multi-omics-based alternative splicing predictions. Environ. Exp. Bot. 2024, 225, 105872. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, T.; Zhou, Y.; Al-Saud, N.B.S.; Cheng, B.; Admas, T.; Zhang, W.; Pan, R. The alternative splicing of HvLHCA4.2 enhances drought tolerance in barley by regulating ROS scavenging and stomatal closure. Int. J. Biol. Macromol. 2025, 307 Pt 4, 142384. [Google Scholar] [CrossRef] [PubMed]
- Collin, A.; Matkowski, H.; Sybilska, E.; Biantari, A.; Krol, O.; Daszkowska-Golec, A. ABA-induced alternative splicing drives transcriptomic reprogramming for drought tolerance in barley. BMC Plant Biol. 2025, 25, 445. [Google Scholar] [CrossRef]
- Yang, H.; Li, P.; Jin, G.; Gui, D.; Liu, L.; Zhang, C. Temporal regulation of alternative splicing events in rice memory under drought stress. Plant Divers. 2022, 44, 116–125. [Google Scholar] [CrossRef]
- Ning, M.; Li, Q.; Wang, Y.; Li, Q.; Tao, Y.; Zhang, F.; Hu, F.; Huang, L. Alternative splicing drives the functional diversification of a bHLH transcription factor in the control of growth and drought tolerance in rice. Sci. Bull. 2025, 70, 153–156. [Google Scholar] [CrossRef]
- Laloum, T.; Martín, G.; Duque, P. Alternative splicing control of abiotic stress responses. Trends Plant Sci. 2018, 23, 140–150. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.-H.; Guo, Q.-H.; Liu, P.; Li, Y.; Wu, C.-A.; Yang, G.-D.; Huang, J.-G.; Zhang, S.-Z.; Zheng, C.-C. Salt responsive alternative splicing of a RING finger E3 ligase modulates the salt stress tolerance by fine-tuning the balance of COP9 signalosome subunit 5A. PLoS Genet. 2021, 17, e1009898. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, K.; He, T.; Wang, H.; Jiang, C.; Yan, H.; Xiang, Y. Systematic analysis of the serine/arginine-rich protein splicing factors (SRs) and focus on salt tolerance of PtSC27 in Populus trichocarpa. Plant Physiol. Biochem. 2022, 173, 97–109. [Google Scholar] [CrossRef]
- Jian, G.; Mo, Y.; Hu, Y.; Huang, Y.; Ren, L.; Zhang, Y.; Hu, H.; Zhou, S.; Liu, G.; Guo, J. Variety-specific transcriptional and alternative splicing regulations modulate salt tolerance in rice from early stage of stress. Rice 2022, 15, 56. [Google Scholar] [CrossRef]
- Yu, H.; Du, Q.; Campbell, M.; Yu, B.; Walia, H.; Zhang, C. Genome-wide discovery of natural variation in pre-mRNA splicing and prioritising causal alternative splicing to salt stress response in rice. New Phytol. 2021, 230, 1273–1287. [Google Scholar] [CrossRef]
- Long, L.; Zhao, J.-R.; Guo, D.-D.; Ma, X.-N.; Xu, F.-C.; Yang, W.-W.; Gao, W. Identification of NHXs in Gossypium species and the positive role of GhNHX1 in salt tolerance. BMC Plant Biol. 2020, 20, 147. [Google Scholar] [CrossRef]
- Mangena, P. Cell mutagenic autopolyploidy enhances salinity stress tolerance in leguminous crops. Cells 2023, 12, 2082. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Wang, T.T.; Duan, Y.Y.; Ren, J.; Gao, H.; Fan, Y.J.; Xia, Q.M.; Cao, H.X.; Xie, K.D. Polyploidization leads to salt stress resilience via ethylene signaling in citrus plants. New Phytol. 2025, 246, 176–191. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, Y.; Yao, J.; Huang, H.; Qian, B.; Liu, X.; Chen, Y.; Pang, J.; Zhan, X.; Zhu, J.K. Modulation of plant development and chilling stress responses by alternative splicing events under control of the spliceosome protein SmEb in Arabidopsis. Plant Cell Environ. 2022, 45, 2762–2779. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Song, Y.; Wu, S.; Peng, Y.; Ming, Y.; Li, Z.; Zhang, X.; Song, W.; Su, Z.; Gong, Z. Regulation of alternative splicing by CBF-mediated protein condensation in plant response to cold stress. Nat. Plants 2025, 11, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lv, W.; Shao, L.; Fu, Y.; Liu, H.; Yang, C.; Chen, A.; Xie, X.; Wang, Z.; Li, C. PacBio and Illumina RNA sequencing identify alternative splicing events in response to cold stress in two poplar species. Front. Plant Sci. 2021, 12, 737004. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, C.; Yang, L.; Liu, Y.; Wang, G.; Li, S.; Dirk, L.M.; Downie, A.B.; Zhao, T. Two alternatively spliced variants of ZmHSF12 regulate the balance of plant growth and heat tolerance in maize and Arabidopsis. Plant J. 2025, 123, e70372. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Wang, T.; Tao, Z.; Huang, S.; Lin, N.; Zhao, Y.; Wang, C.; Li, P. Cooperative condensation of RNA-DIRECTED DNA METHYLATION 16 splicing isoforms enhances heat tolerance in Arabidopsis. Nat. Commun. 2025, 16, 433. [Google Scholar] [CrossRef]
- Jo, S.H.; Park, H.J.; Jung, H.; Lee, G.S.; Moon, J.H.; Kim, H.-S.; Lee, H.-J.; Jung, C.; Cho, H.S. PROTEIN PHOSPHATASE 2A B′ η drives spliceosome subunit dephosphorylation to mediate alternative splicing following heat stress. Plant Cell 2025, 37, koaf117. [Google Scholar] [CrossRef]
- Cecchini, N.M.; Torres, J.R.; López, I.L.; Cobo, S.; Nota, F.; Alvarez, M.E. Alternative splicing of an exitron determines the subnuclear localization of the Arabidopsis DNA glycosylase MBD4L under heat stress. Plant J. 2022, 110, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Z.; Qu, L.; Hu, Y.; Lu, D. Transcriptomic and alternative splicing analyses provide insights into the roles of exogenous salicylic acid ameliorating waxy maize seedling growth under heat stress. BMC Plant Biol. 2022, 22, 432. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P. Variation in protein gene and intron content among land plant mitogenomes. Mitochondrion 2020, 53, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Vosseberg, J.; Schinkel, M.; Gremmen, S.; Snel, B. The spread of the first introns in proto-eukaryotic paralogs. Commun. Biol. 2022, 5, 476. [Google Scholar] [CrossRef]
- Kováčová, T.; Souček, P.; Hujová, P.; Freiberger, T.; Grodecká, L. Splicing enhancers at intron–exon borders participate in acceptor splice sites recognition. Int. J. Mol. Sci. 2020, 21, 6553. [Google Scholar] [CrossRef]
- Ling, Y.; Mahfouz, M.M.; Zhou, S. Pre-mRNA alternative splicing as a modulator for heat stress response in plants. Trends Plant Sci. 2021, 26, 1153–1170. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Cumbie, J.S.; Dharmawardhana, P.; Jaiswal, P.; Chang, J.H.; Palusa, S.G.; Reddy, A.; Megraw, M.; Mockler, T.C. Environmental stresses modulate abundance and timing of alternatively spliced circadian transcripts in Arabidopsis. Mol. Plant 2015, 8, 207–227. [Google Scholar] [CrossRef]
- Sybilska, E.; Daszkowska-Golec, A. Alternative splicing in ABA signaling during seed germination. Front. Plant Sci. 2023, 14, 1144990. [Google Scholar] [CrossRef]
- Raxwal, V.K.; Riha, K. The biological functions of nonsense-mediated mRNA decay in plants: RNA quality control and beyond. Biochem. Soc. Trans. 2023, 51, 31–39. [Google Scholar] [CrossRef]
- Ganie, S.A.; Reddy, A.S. Stress-induced changes in alternative splicing landscape in rice: Functional significance of splice isoforms in stress tolerance. Biology 2021, 10, 309. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Yao, S.; Liang, F.; Gill, R.A.; Huang, J.; Cheng, X.; Liu, Y.; Tong, C.; Liu, S. A global survey of the transcriptome of allopolyploid Brassica napus based on single-molecule long-read isoform sequencing and Illumina-based RNA sequencing data. Plant J. 2020, 103, 843–857. [Google Scholar] [CrossRef]
- Zhou, R.; Moshgabadi, N.; Adams, K.L. Extensive changes to alternative splicing patterns following allopolyploidy in natural and resynthesized polyploids. Proc. Natl. Acad. Sci. USA 2011, 108, 16122–16127. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, M.; Wang, J. Characteristics of duplicated gene expression and DNA methylation regulation in different tissues of allopolyploid Brassica napus. BMC Plant Biol. 2024, 24, 518. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.E. The Effects of Abiotic Stress on Isoform Composition in Polyploid Brassica napus. MS. Thesis, University of British Columbia, Vancouver, BC, Canada, 2021. [Google Scholar]
- Ma, J.-Q.; Xu, W.; Xu, F.; Lin, A.; Sun, W.; Jiang, H.-H.; Lu, K.; Li, J.-N.; Wei, L.-J. Differential alternative splicing genes and isoform regulation networks of rapeseed (Brassica napus L.) infected with Sclerotinia sclerotiorum. Genes 2020, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-Q.; Wei, L.-J.; Lin, A.; Zhang, C.; Sun, W.; Yang, B.; Lu, K.; Li, J.-N. The alternative splicing landscape of Brassica napus infected with Leptosphaeria maculans. Genes 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, W.; Wang, S.; Zhang, X.; Coules, A.; Ding, G.; Xu, F.; Ren, J.; Lu, C.; Shi, L. Differential alternative splicing genes in response to boron deficiency in Brassica napus. Genes 2019, 10, 224. [Google Scholar] [CrossRef]
- Yang, L.; Yang, L.; Zhao, C.; Liu, J.; Tong, C.; Zhang, Y.; Cheng, X.; Jiang, H.; Shen, J.; Xie, M. Differential alternative splicing genes and isoform co-expression networks of Brassica napus under multiple abiotic stresses. Front. Plant Sci. 2022, 13, 1009998. [Google Scholar] [CrossRef]
- Lee, J.S.; Adams, K.L. Global insights into duplicated gene expression and alternative splicing in polyploid Brassica napus under heat, cold, and drought stress. Plant Genome 2020, 13, e20057. [Google Scholar] [CrossRef]
- Lin, A.; Ma, J.; Xu, F.; Xu, W.; Jiang, H.; Zhang, H.; Qu, C.; Wei, L.; Li, J. Differences in alternative splicing between yellow and black-seeded rapeseed. Plants 2020, 9, 977. [Google Scholar] [CrossRef]
- Liu, L.; Wu, D.; Gu, Y.; Liu, F.; Liu, B.; Mao, F.; Yi, X.; Tang, T.; Zhao, X. Comprehensive profiling of alternative splicing landscape during secondary dormancy in oilseed rape (Brassica napus L.). Mol. Breed. 2022, 42, 44. [Google Scholar] [CrossRef]
- Senchina, D.S.; Alvarez, I.; Cronn, R.C.; Liu, B.; Rong, J.; Noyes, R.D.; Paterson, A.H.; Wing, R.A.; Wilkins, T.A.; Wendel, J.F. Rate variation among nuclear genes and the age of polyploidy in Gossypium. Mol. Biol. Evol. 2003, 20, 633–643. [Google Scholar] [CrossRef]
- Wendel, J.F.; Cronn, R.C. Polyploidy and the evolutionary history of cotton. Adv. Agron. 2003, 87, 139–186. [Google Scholar]
- Wang, M.; Tu, L.; Lin, M.; Lin, Z.; Wang, P.; Yang, Q.; Ye, Z.; Shen, C.; Li, J.; Zhang, L. Asymmetric subgenome selection and cis-regulatory divergence during cotton domestication. Nat. Genet. 2017, 49, 579–587. [Google Scholar] [CrossRef]
- Viot, C.R.; Wendel, J.F. Evolution of the cotton genus Gossypium and its domestication in the americas: A review. Crit. Rev. Plant Sci. 2023, 42, 1–33. [Google Scholar] [CrossRef]
- Min, X.J. A survey of alternative splicing in allotetraploid cotton (Gossypium hirsutum L.). Comput. Mol. Biol. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Li, F.; Ye, W.; Wang, J.; Song, G.; Yue, Z.; Cong, L.; Shang, H.; Zhu, S. The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet. 2012, 44, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, G.; Lu, C.; Xiao, G.; Zou, C.; Kohel, R.J.; Ma, Z.; Shang, H.; Ma, X.; Wu, J. Genome sequence of cultivated Upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 2015, 33, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, B.; Zheng, H.; Hu, Y.; Lu, G.; Yang, C.; Zhang, L. Gossypium barbadense genome sequence provides insight into the evolution of extra-long staple fiber and specialized metabolites. Sci. Rep. 2015, 5, 14139. [Google Scholar] [CrossRef]
- Yuan, D.; Tang, Z.; Wang, M.; Gao, W.; Tu, L.; Jin, X.; Chen, L.; He, Y.; Zhang, L.; Zhu, L. The genome sequence of Sea-Island cotton (Gossypium barbadense) provides insights into the allopolyploidization and development of superior spinnable fibres. Sci. Rep. 2015, 5, 17662. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Gong, W.; Xu, F.; Pan, Z.; Jia, Y.; Geng, X.; Du, X. Integration of proteomic and transcriptomic profiles reveals multiple levels of genetic regulation of salt tolerance in cotton. BMC Plant Biol. 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, C.; Zhu, W.; Yan, G.; Chen, X.; Qiu, P.; Ruzimurod, B.; Ye, W.; Qaraevna, B.Z.; Yin, Z. Molecular mechanism that underlies cotton response to salt and drought stress revealed by complementary transcriptomic and iTRAQ analyses. Environ. Exp. Bot. 2023, 209, 105288. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Chen, Q.-Y.; Wang, D.-D.; Mu, Y.-P.; Wang, M.-Y.; Huang, J.-R.; Mao, Y.-B. Differential transcription and alternative splicing in cotton underly specialized defense responses against pests. Front. Plant Sci. 2020, 11, 573131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Song, Y.; Sun, Z.; Zhang, J.; Wu, H.; Yang, Y.; Wang, Z.; He, D. Comparative transcriptome analysis reveals genes associated with the gossypol synthesis and gland morphogenesis in Gossypium hirsutum. Genes 2022, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Marcussen, T.; Sandve, S.R.; Heier, L.; Spannagl, M.; Pfeifer, M.; International Wheat Genome Sequencing Consortium; Jakobsen, K.S.; Wulff, B.B.; Steuernagel, B.; Mayer, K.F. Ancient hybridizations among the ancestral genomes of bread wheat. Science 2014, 345, 1250092. [Google Scholar] [CrossRef]
- Cheng, L.; Bao, Z.; Kong, Q.; Lassois, L.; Stein, N.; Huang, S.; Zhou, Q. Genome analyses and breeding of polyploid crops. Nat. Plants 2025, 11, 1714–1728. [Google Scholar] [CrossRef]
- Li, J.; Gao, X.; Chen, X.; Fan, Z.; Zhang, Y.; Wang, Z.; Shi, J.; Wang, C.; Zhang, H.; Wang, L. Comparative transcriptome responses of leaf and root tissues to salt stress in wheat strains with different salinity tolerances. Front. Genet. 2023, 14, 1015599. [Google Scholar] [CrossRef]
- Ma, Z.; Li, M.; Zhang, H.; Zhao, B.; Liu, Z.; Duan, S.; Meng, X.; Li, G.; Guo, X. Alternative splicing of TaHsfA2-7 is involved in the improvement of thermotolerance in wheat. Int. J. Mol. Sci. 2023, 24, 1014. [Google Scholar] [CrossRef]
- Wen, J.; Qin, Z.; Sun, L.; Zhang, Y.; Wang, D.; Peng, H.; Yao, Y.; Hu, Z.; Ni, Z.; Sun, Q. Alternative splicing of TaHSFA6e modulates heat shock protein–mediated translational regulation in response to heat stress in wheat. New Phytol. 2023, 239, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yu, K.; Han, L.; Li, X.; Wang, H.; Liu, Y.; Zhang, Y. Global profiling of alternative splicing landscape responsive to salt stress in wheat (Triticum aestivum L.). Plant Growth Regul. 2020, 92, 107–116. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, J.; Tian, X.; Xu, S.; Wang, Y.; Li, H.; Wang, X.; Peng, H.; Yao, Y.; Hu, Z. Global profiling of alternative splicing landscape responsive to drought, heat and their combination in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2018, 16, 714–726. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Liu, Z.; Guo, H.; Zhang, F.; Guo, L.; Yuan, S.; Duan, W.; Li, Y.; Tan, Z. Global survey of alternative splicing and gene modules associated with fertility regulation in a thermosensitive genic male sterile wheat. J. Exp. Bot. 2022, 73, 2157–2174. [Google Scholar] [CrossRef]
- Zhang, Z.; Xun, H.; Lv, R.; Gou, X.; Ma, X.; Li, J.; Zhao, J.; Li, N.; Gong, L.; Liu, B. Effects of homoeologous exchange on gene expression and alternative splicing in a newly formed allotetraploid wheat. Plant J. 2022, 111, 1267–1282. [Google Scholar] [CrossRef]
- Yu, K.; Feng, M.; Yang, G.; Sun, L.; Qin, Z.; Cao, J.; Wen, J.; Li, H.; Zhou, Y.; Chen, X. Changes in alternative splicing in response to domestication and polyploidization in wheat. Plant Physiol. 2020, 184, 1955–1968. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, Z.; Wang, Z.; Li, W.; Fang, C.; Wu, M.; Ma, Y.; Liu, T.; Kong, L.-A.; Peng, D.-L. Global dissection of alternative splicing in paleopolyploid soybean. Plant Cell 2014, 26, 996–1008. [Google Scholar] [CrossRef]
- Kagale, S.; Nixon, J.; Khedikar, Y.; Pasha, A.; Provart, N.J.; Clarke, W.E.; Bollina, V.; Robinson, S.J.; Coutu, C.; Hegedus, D.D. The developmental transcriptome atlas of the biofuel crop Camelina sativa. Plant J. 2016, 88, 879–894. [Google Scholar] [CrossRef]
- Gong, W.; Song, Q.; Ji, K.; Gong, S.; Wang, L.; Chen, L.; Zhang, J.; Yuan, D. Full-length transcriptome from Camellia oleifera seed provides insight into the transcript variants involved in oil biosynthesis. J. Agric. Food Chem. 2020, 68, 14670–14683. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, H.; Zhuang, Y.; Chen, K.; Zhang, C.; Cai, T.; Yang, Q.; Fu, H.; Chen, X.; Chitkineni, A. Multiple strategies, including 6mA methylation, affecting plant alternative splicing in allopolyploid peanut. Plant Biotechnol. J. 2024, 22, 1681–1702. [Google Scholar] [CrossRef] [PubMed]
- Kashkan, I.; Timofeyenko, K.; Růžička, K. How alternative splicing changes the properties of plant proteins. Quant. Plant Biol. 2022, 3, e14. [Google Scholar] [CrossRef] [PubMed]
- Reixachs-Solé, M.; Eyras, E. Uncovering the impacts of alternative splicing on the proteome with current omics techniques. Wiley Interdiscip. Rev. RNA 2022, 13, e1707. [Google Scholar] [CrossRef]
- Kjer-Hansen, P.; Weatheritt, R.J. The function of alternative splicing in the proteome: Rewiring protein interactomes to put old functions into new contexts. Nat. Struct. Mol. Biol. 2023, 30, 1844–1856. [Google Scholar] [CrossRef]
- Manuel, J.M.; Guilloy, N.; Khatir, I.; Roucou, X.; Laurent, B. Re-evaluating the impact of alternative RNA splicing on proteomic diversity. Front. Genet. 2023, 14, 1089053. [Google Scholar] [CrossRef]
- Petrillo, E.; Kalyna, M.; Mandadi, K.K.; Tu, S.-L.; Simpson, C.G. Alternative splicing regulation in plants. Front. Plant Sci. 2020, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, X.; Ning, N.; Wu, H.; Mei, J.; Gu, X.; Ruan, H.; Zhang, M.; Li, Z.; Ma, S. The Exserohilum turcicum effector EtEC81 reprograms alternative splicing in maize and activates immunity. Cell Rep. 2025, 44, 115501. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).