CAP-LAMP2b–Modified Stem Cells’ Extracellular Vesicles Hybrid with CRISPR-Cas9 Targeting ADAMTS4 to Reverse IL-1β–Induced Aggrecan Loss in Chondrocytes
Abstract
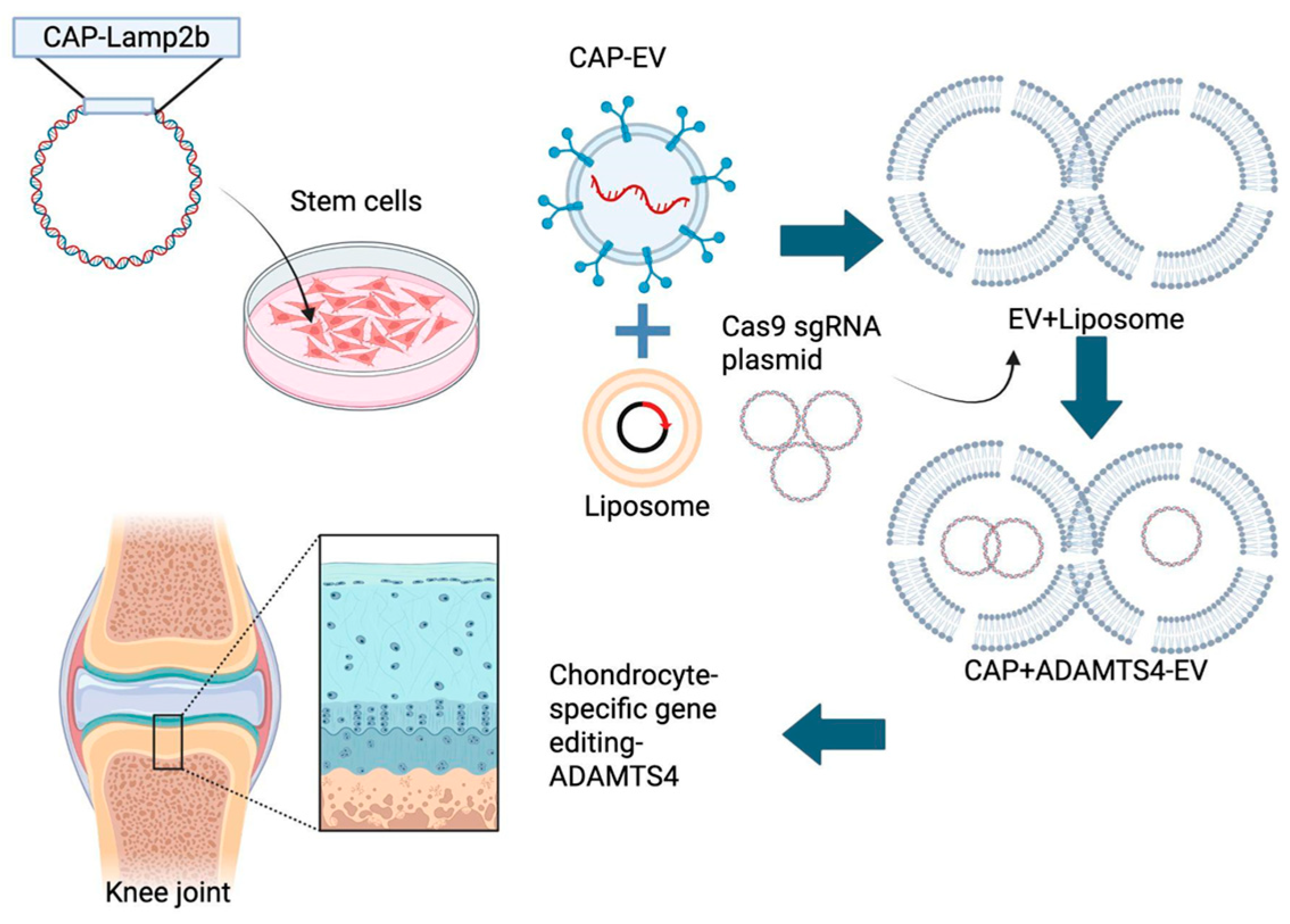
1. Introduction
2. Results
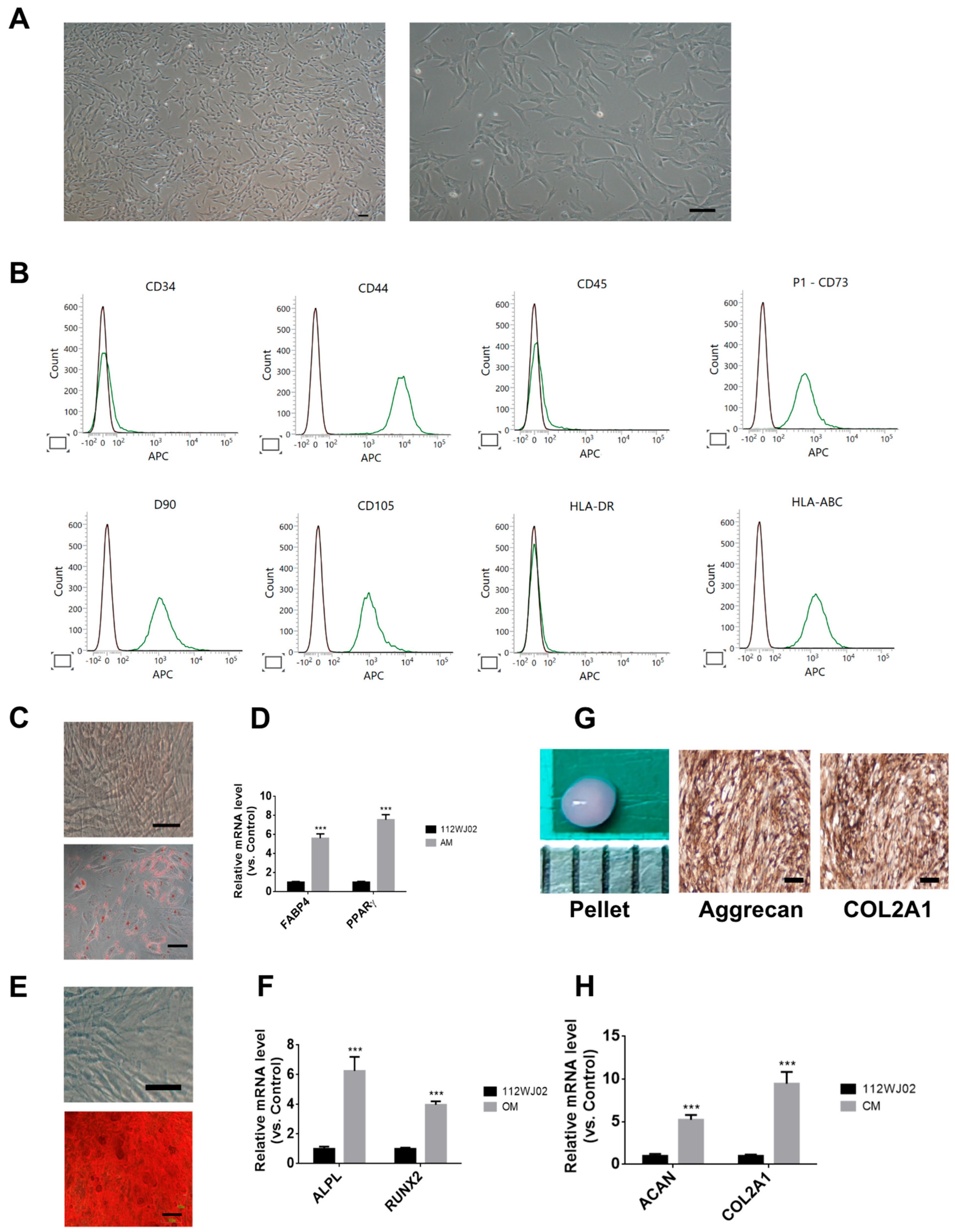
2.1. Characterization of Human Umbilical Cord-Derived Mesenchymal Stem Cells
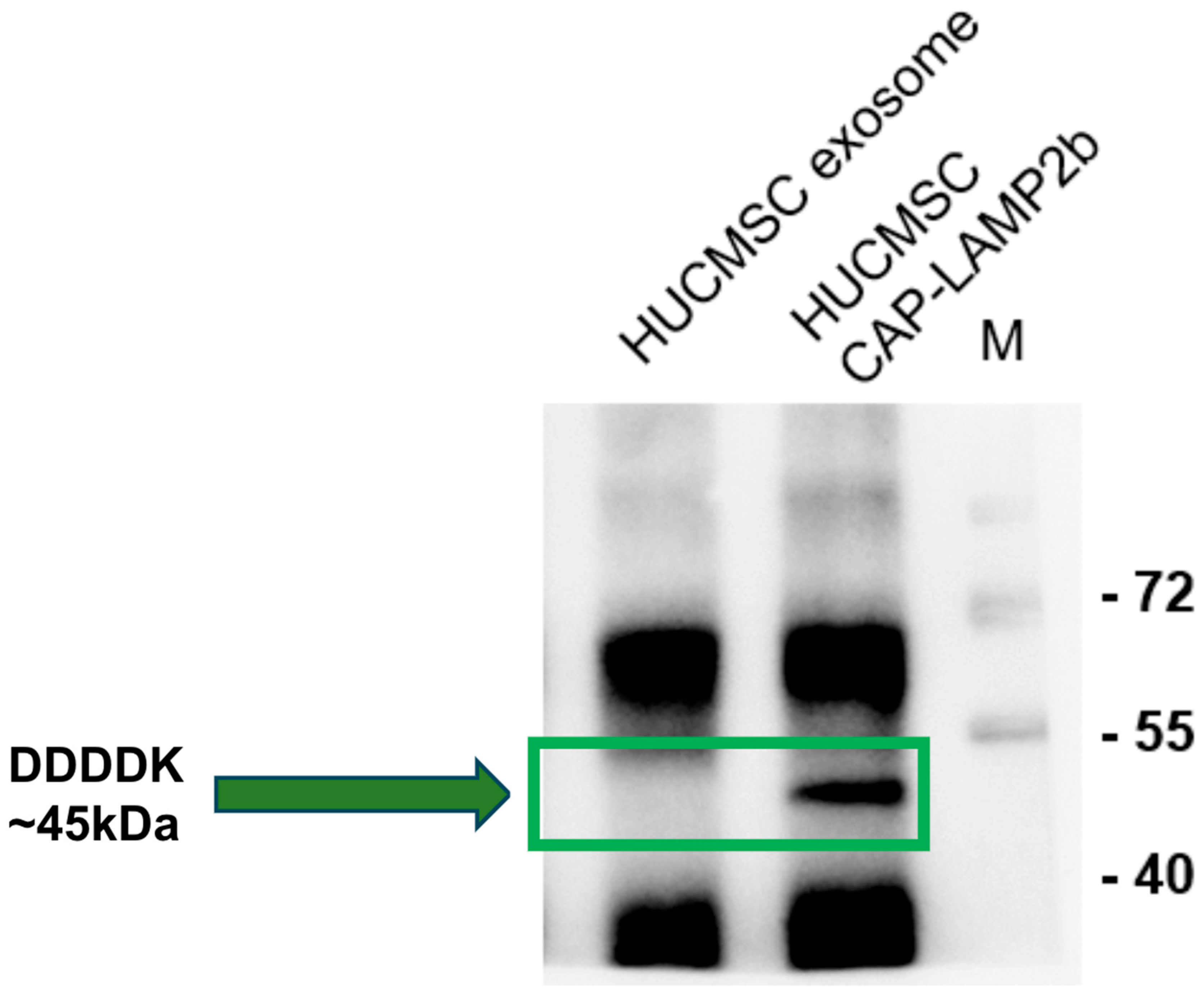
2.2. Detection of FLAG-Tagged CAP-LAMP2b Expression in hUCMSC-Derived Exosomes and Cells
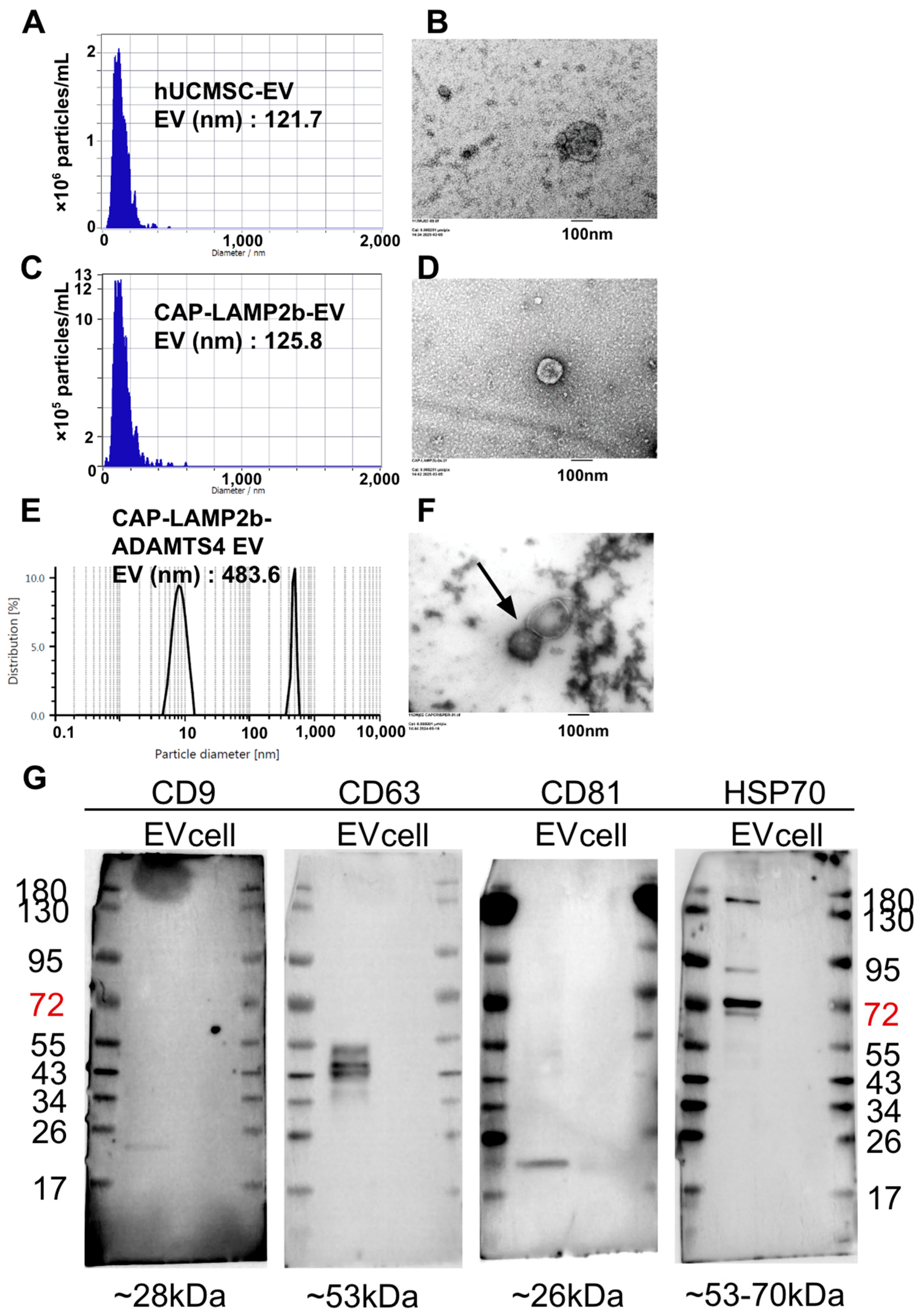
2.3. Characterization of hUCMSC-EVs, CAP-LAMP2b-EVs, and CAP-LAMP2b-ADAMTS4 EVs
2.4. Enhanced Cellular Uptake of CAP-Modified Hybrid EVs
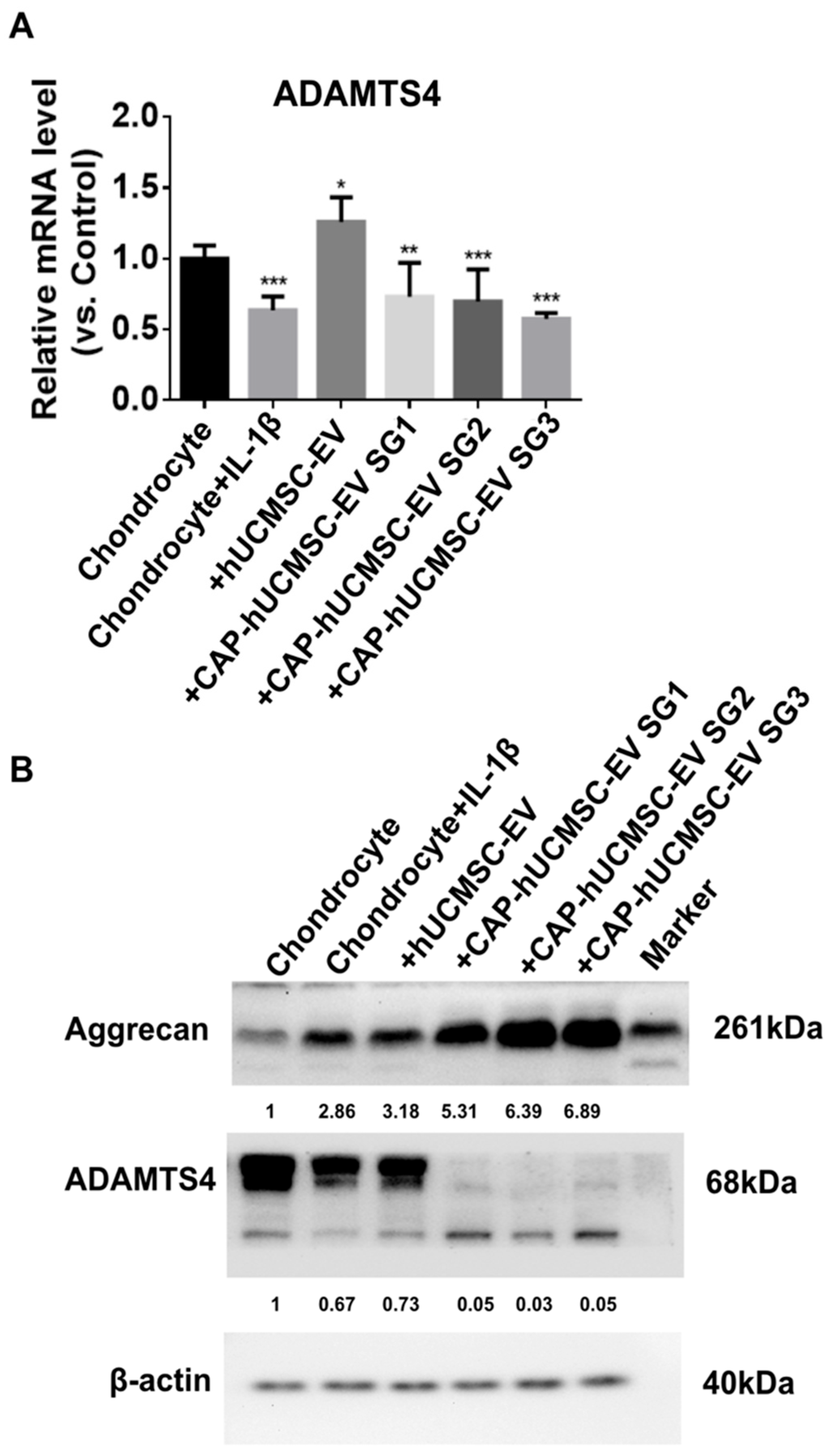
2.5. Western Blot Analysis of ADAMTS4 Protein Expression Following EV Treatment in IL-1β-Stimulated Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Culture of hUCMSCs
4.2. Flow Cytometry
4.3. Trilineage Differentiation
4.3.1. Adipogenesis
4.3.2. Osteogenesis
4.3.3. Chondrogenesis
4.4. Immunohistochemical Staining
4.5. Quantitative Real-Time PCR
4.6. CAP-Lamp2b Plasmid Development and Transfection
4.7. EV Isolation
4.8. Western Blot
4.9. Transmission Electron Microscope
4.10. Nanoparticle Tracking Analysis
4.11. CRISPR Cas9 Plasmid Construction and Transfection
4.12. Human Chondrocyte Culture
4.13. CAP Hybrid EV Retention Measurement in Chondrocytes
4.14. Induction of OA in Chondrocytes
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| hUCMSC | Human umbilical cord MSC |
| EV | Extracellular vesicle |
| MMP | Matrix metalloproteinase |
References
- GBD 2021 Osteoarthritis Collaborators. Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-Y.; Choi, S.; Yoo, S.J.; Lee, J.; Lim, C. Factors Associated with Osteoarthritis and Their Influence on Health-Related Quality of Life in Older Adults with Osteoarthritis: A Study Based on the 2020 Korea National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2023, 20, 6073. [Google Scholar] [CrossRef]
- Mort, J.S.; Geng, Y.; Fisher, W.D.; Roughley, P.J. Aggrecan Heterogeneity in Articular Cartilage from Patients with Osteoarthritis. BMC Musculoskelet. Disord. 2016, 17, 89. [Google Scholar] [CrossRef]
- Cuffaro, D.; Ciccone, L.; Rossello, A.; Nuti, E.; Santamaria, S. Targeting Aggrecanases for Osteoarthritis Therapy: From Zinc Chelation to Exosite Inhibition. J. Med. Chem. 2022, 65, 13505–13532. [Google Scholar] [CrossRef]
- Vincent, T.L.; Alliston, T.; Kapoor, M.; Loeser, R.F.; Troeberg, L.; Little, C.B. Osteoarthritis Pathophysiology: Therapeutic Target Discovery May Require a Multifaceted Approach. Clin. Geriatr. Med. 2022, 38, 193–219. [Google Scholar] [CrossRef]
- Ansori, A.N.; Antonius, Y.; Susilo, R.J.; Hayaza, S.; Kharisma, V.D.; Parikesit, A.A.; Zainul, R.; Jakhmola, V.; Saklani, T.; Rebezov, M.; et al. Application of CRISPR-Cas9 Genome Editing Technology in Various Fields: A Review. Narra J. 2023, 3, e184. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-L.; Li, H.-B.; Jin, Y. Application and Perspective of CRISPR/Cas9 Genome Editing Technology in Human Diseases Modeling and Gene Therapy. Front. Genet. 2024, 15, 1364742. [Google Scholar] [CrossRef]
- Jia, S.; Liang, R.; Chen, J.; Liao, S.; Lin, J.; Li, W. Emerging Technology Has a Brilliant Future: The CRISPR-Cas System for Senescence, Inflammation, and Cartilage Repair in Osteoarthritis. Cell. Mol. Biol. Lett. 2024, 29, 64. [Google Scholar] [CrossRef]
- Wu, K.-C.; Yang, H.-I.; Chang, Y.-H.; Chiang, R.Y.-S.; Ding, D.-C. Extracellular Vesicles Derived from Human Umbilical Mesenchymal Stem Cells Transfected with miR-7704 Improved Damaged Cartilage and Reduced Matrix Metallopeptidase 13. Cells 2025, 14, 82. [Google Scholar] [CrossRef]
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, J.; Qiu, X.; Wang, C.; Peng, J.; Zhang, C.; Zhang, M.; Lu, H.; Chen, W. LAMP2A, LAMP2B and LAMP2C: Similar Structures, Divergent Roles. Autophagy 2023, 19, 2837–2852. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, Q.; Yan, X.; Ding, B.; Chen, D.; Cheng, L. Chondrocyte Affinity Peptide Modified PAMAM Conjugate as a Nanoplatform for Targeting and Retention in Cartilage. Nanomedicine 2018, 13, 749–767. [Google Scholar] [CrossRef]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2022, 11, e2100639. [Google Scholar] [CrossRef]
- van der Meel, R.; Fens, M.H.A.M.; Vader, P.; van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular Vesicles as Drug Delivery Systems: Lessons from the Liposome Field. J. Control. Release 2014, 195, 72–85. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Coffey, R.J. Extracellular Vesicles and Nanoparticles at a Glance. J. Cell Sci. 2024, 137, jcs260201. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Yoshida, K.; Takechi-Haraya, Y.; Izutsu, K.-I. Physicochemical Characterization of Liposomes That Mimic the Lipid Composition of Exosomes for Effective Intracellular Trafficking. Langmuir 2020, 36, 12735–12744. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Fehér, B.; Kitka, D.; Wacha, A.; Bóta, A.; Berényi, S.; Pipich, V.; Fraikin, J.-L. Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona. Colloids Surf. B Biointerfaces 2020, 192, 111053. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An Emerging Focus on Lipids in Extracellular Vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Piffoux, M.; Silva, A.K.A.; Gazeau, F.; Tareste, D. Generation of Hybrid Extracellular Vesicles by Fusion with Functionalized Liposomes. Methods Mol. Biol. 2022, 2473, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Arca, V.; Costa, R.R.; Carvalho, A.M.; Taboada, P.; Reis, R.L.; Prieto, G.; Pashkuleva, I. Liposomes Embedded in Layer by Layer Constructs as Simplistic Extracellular Vesicles Transfer Model. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111813. [Google Scholar] [CrossRef]
- Cheng, T.; Kosgei, B.K.; Soko, G.F.; Meena, S.S.; Li, T.; Cao, Q.; Zhao, Z.; Cheng, S.K.S.; Liu, Q.; Wang, F.; et al. Using Functionalized Liposomes to Harvest Extracellular Vesicles of Similar Characteristics in Dermal Interstitial Fluid. Anal. Chem. 2023, 95, 17968–17973. [Google Scholar] [CrossRef]
- Li, T.; Peng, J.; Li, Q.; Shu, Y.; Zhu, P.; Hao, L. The Mechanism and Role of ADAMTS Protein Family in Osteoarthritis. Biomolecules 2022, 12, 959. [Google Scholar] [CrossRef]
- Rose, K.W.J.; Taye, N.; Karoulias, S.Z.; Hubmacher, D. Regulation of ADAMTS Proteases. Front. Mol. Biosci. 2021, 8, 701959. [Google Scholar] [CrossRef]
- Cilek, M.Z.; de Vega, S.; Shiozawa, J.; Yoshinaga, C.; Miyamae, Y.; Chijiiwa, M.; Mochizuki, S.; Ito, M.; Kaneko, H.; Kaneko, K.; et al. Synergistic Upregulation of ADAMTS4 (aggrecanase-1) by Cytokines and Its Suppression in Knee Osteoarthritic Synovial Fibroblasts. Lab. Investig. 2022, 102, 102–111. [Google Scholar] [CrossRef]
- Clarissa, E.M.; Karmacharya, M.; Choi, H.; Kumar, S.; Cho, Y.-K. Nature Inspired Delivery Vehicles for CRISPR-Based Genome Editing. Small 2025, e2409353. [Google Scholar] [CrossRef]
- Glass, Z.; Lee, M.; Li, Y.; Xu, Q. Engineering the Delivery System for CRISPR-Based Genome Editing. Trends Biotechnol. 2018, 36, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gupta, D.; Xie, J.; Van Wonterghem, E.; Van Hoecke, L.; Hean, J.; Niu, Z.; Ghaeidamini, M.; Wiklander, O.P.B.; Zheng, W.; et al. Engineering of Extracellular Vesicles for Efficient Intracellular Delivery of Multimodal Therapeutics Including Genome Editors. Nat. Commun. 2025, 16, 4028. [Google Scholar] [CrossRef]
- Lu, Y.; Godbout, K.; Lamothe, G.; Tremblay, J.P. CRISPR-Cas9 Delivery Strategies with Engineered Extracellular Vesicles. Mol. Ther. Nucleic Acids 2023, 34, 102040. [Google Scholar] [CrossRef] [PubMed]
- Vlashi, R.; Zhang, X.; Li, H.; Chen, G. Potential Therapeutic Strategies for Osteoarthritis via CRISPR/Cas9 Mediated Gene Editing. Rev. Endocr. Metab. Disord. 2024, 25, 339–367, Erratum in Rev. Endocr. Metab. Disord. 2024, 25, 451–452. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, J.; Fan, Y.; Li, J.; You, T.; He, S.; Xiao, G.; Chen, D. Exploration of CRISPR/Cas9-Based Gene Editing as Therapy for Osteoarthritis. Ann. Rheum. Dis. 2019, 78, 676–682. [Google Scholar] [CrossRef]
- Choi, Y.-R.; Collins, K.H.; Lee, J.-W.; Kang, H.-J.; Guilak, F. Genome Engineering for Osteoarthritis: From Designer Cells to Disease-Modifying Drugs. Tissue Eng. Regen. Med. 2019, 16, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, X.; Kang, R.; Cui, K.; Ou, J.; Zhang, X.; Liang, P. Current Therapies for Osteoarthritis and Prospects of CRISPR-Based Genome, Epigenome, and RNA Editing in Osteoarthritis Treatment. J. Genet. Genom. 2024, 51, 159–183. [Google Scholar] [CrossRef] [PubMed]
- van de Wakker, S.I.; Bauzá-Martinez, J.; Ríos Arceo, C.; Manjikian, H.; Snijders Blok, C.J.B.; Roefs, M.T.; Willms, E.; Maas, R.G.C.; Pronker, M.F.; de Jong, O.G.; et al. Size Matters: Functional Differences of Small Extracellular Vesicle Subpopulations in Cardiac Repair Responses. J. Extracell. Vesicles 2024, 13, e12396. [Google Scholar] [CrossRef]
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large Extracellular Vesicles: Size Matters in Tumor Progression. Cytokine Growth Factor Rev. 2020, 51, 69–74. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Ranjbaran, H.; Abediankenari, S.; Mohammadi, M.; Jafari, N.; Khalilian, A.; Rahmani, Z.; Momeninezhad Amiri, M.; Ebrahimi, P. Wharton’s Jelly Derived-Mesenchymal Stem Cells: Isolation and Characterization. Acta Med. Iran. 2018, 56, 28–33. [Google Scholar]
- Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018, 5, 1700611. [Google Scholar] [CrossRef] [PubMed]
- Al-Modawi, R.N.; Brinchmann, J.E.; Karlsen, T.A. Multi-Pathway Protective Effects of MicroRNAs on Human Chondrocytes in an in Vitro Model of Osteoarthritis. Mol. Ther. Nucleic Acids 2019, 17, 776–790. [Google Scholar] [CrossRef]

| Gene Name | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Product Size (bp) |
|---|---|---|---|
| PPARγ | AGCCTCATGAAGAGCCTTCCA | TCCGGAAGAAACCCTTGCA | 120 |
| FABP4 | ATGGGATGGAAAATCAACCA | GTGGAAGTGACGCCTTTCAT | 87 |
| ALPL | CCACGTCTTCACATTTGGTG | GCAGTGAAGGGCTTCTTGTC | 96 |
| RUNX2 | CGGAATGCCTCTGCTGTTAT | TTCCCGAGGTCCATCTACTG | 174 |
| Aggrecan | GAGATGGAG GGTGAGGTC | ACGCTGCCTCGGGCTTC | 443 |
| COL2A1 | GGACTTTTCTCCCCTCTCT | GACCCGAAGGTCTTACAGGA | 104 |
| ADAMTS4 | TCACTGACTTCCTGGACAATGGC | GGTCAGCATCATAGTCCTTGCC | 105 |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGA TGGTGATGGGATTTC | 172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-C.; Chang, Y.-H.; Chiang, R.Y.-S.; Ding, D.-C. CAP-LAMP2b–Modified Stem Cells’ Extracellular Vesicles Hybrid with CRISPR-Cas9 Targeting ADAMTS4 to Reverse IL-1β–Induced Aggrecan Loss in Chondrocytes. Int. J. Mol. Sci. 2025, 26, 9812. https://doi.org/10.3390/ijms26199812
Wu K-C, Chang Y-H, Chiang RY-S, Ding D-C. CAP-LAMP2b–Modified Stem Cells’ Extracellular Vesicles Hybrid with CRISPR-Cas9 Targeting ADAMTS4 to Reverse IL-1β–Induced Aggrecan Loss in Chondrocytes. International Journal of Molecular Sciences. 2025; 26(19):9812. https://doi.org/10.3390/ijms26199812
Chicago/Turabian StyleWu, Kun-Chi, Yu-Hsun Chang, Raymond Yuh-Shyan Chiang, and Dah-Ching Ding. 2025. "CAP-LAMP2b–Modified Stem Cells’ Extracellular Vesicles Hybrid with CRISPR-Cas9 Targeting ADAMTS4 to Reverse IL-1β–Induced Aggrecan Loss in Chondrocytes" International Journal of Molecular Sciences 26, no. 19: 9812. https://doi.org/10.3390/ijms26199812
APA StyleWu, K.-C., Chang, Y.-H., Chiang, R. Y.-S., & Ding, D.-C. (2025). CAP-LAMP2b–Modified Stem Cells’ Extracellular Vesicles Hybrid with CRISPR-Cas9 Targeting ADAMTS4 to Reverse IL-1β–Induced Aggrecan Loss in Chondrocytes. International Journal of Molecular Sciences, 26(19), 9812. https://doi.org/10.3390/ijms26199812







