The Two-Pore Channel 2 in Human Physiology and Diseases: Functional Characterisation and Pharmacology
Abstract
1. The Two-Pore Channel 2
2. Methods
2.1. Heterologous Expression in Plant Vacuoles
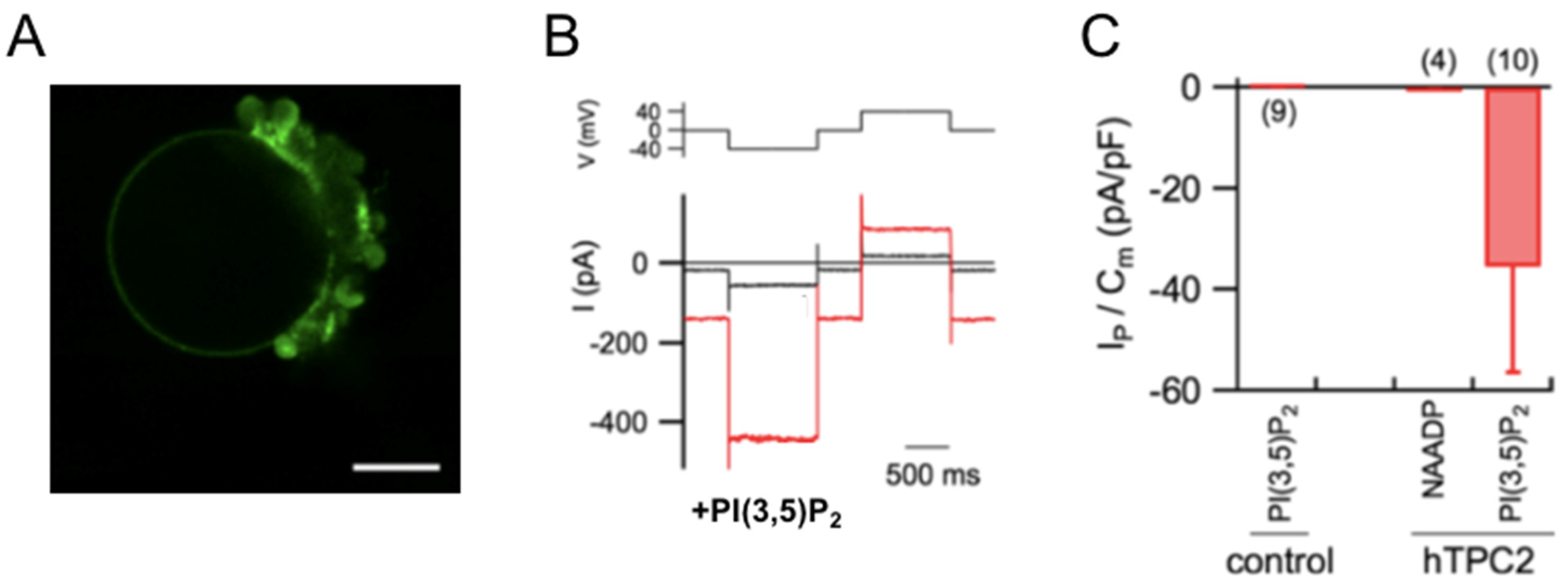
2.2. Computational Methods
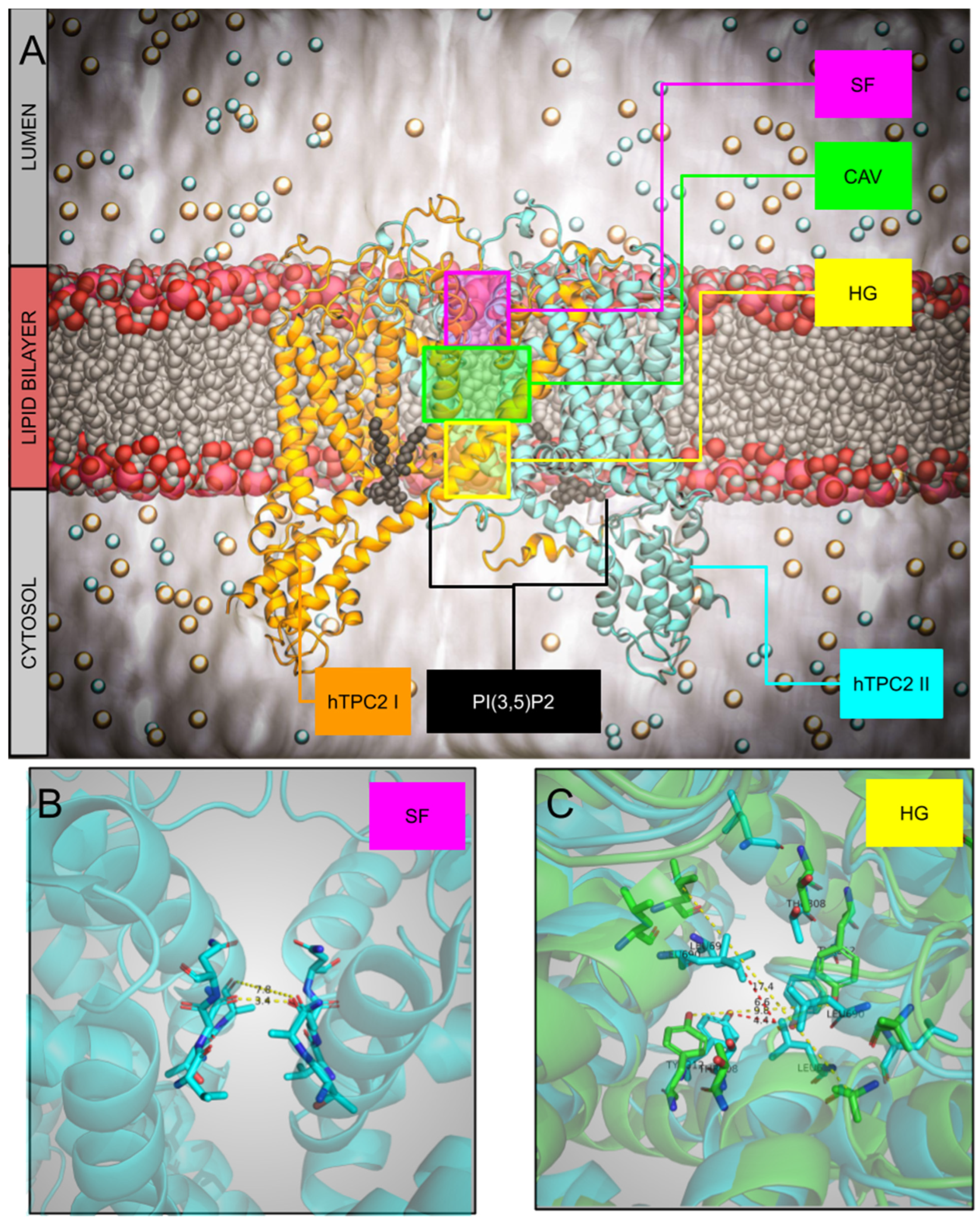
3. Structure
The Mechanism of Ions Permeation: The SF and the Role of Central Cavity on Kinetics
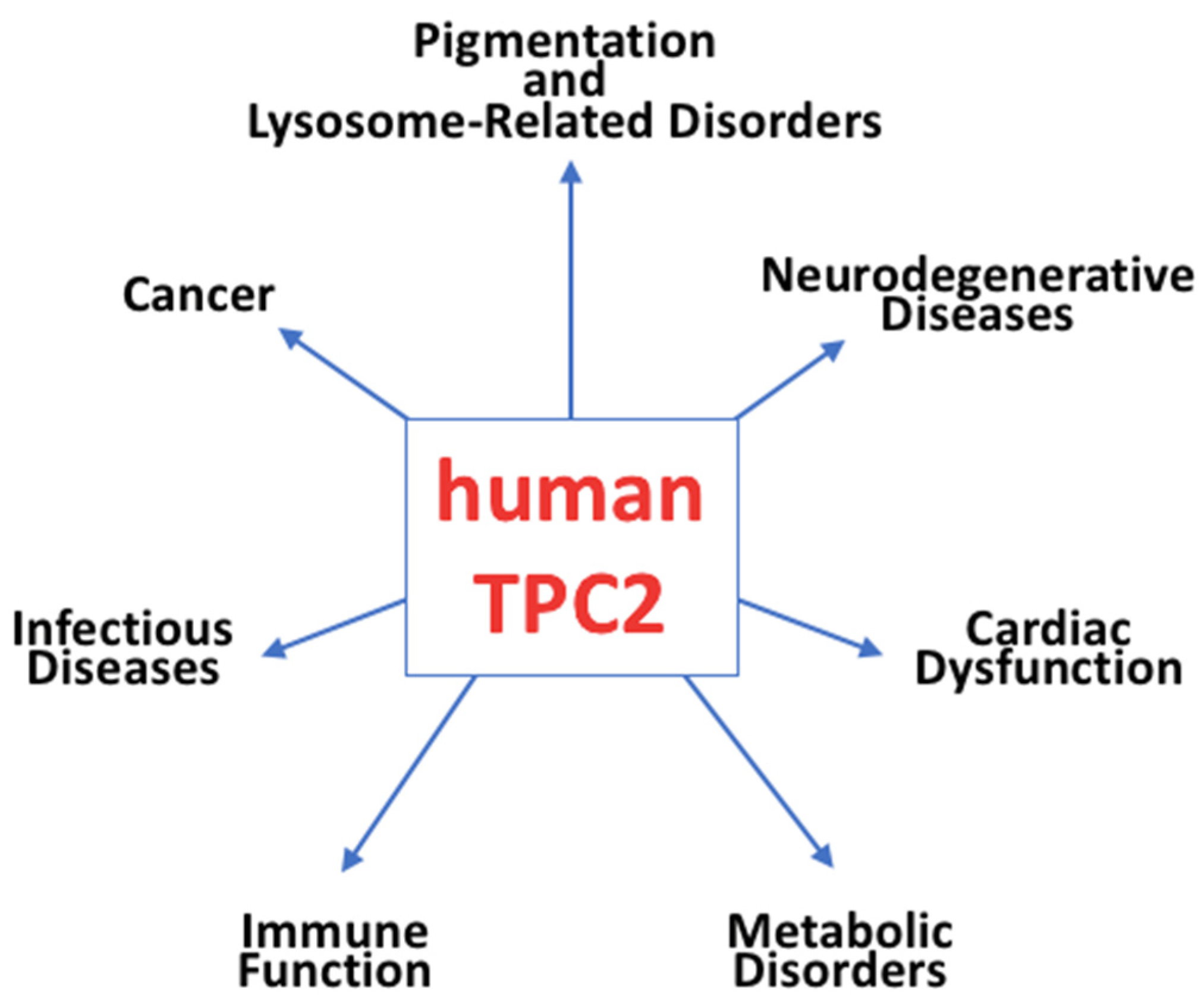
4. Role of TPC2 in Physiology and Human Diseases
4.1. Pigmentation and Lysosome-Related Disorders
4.2. Neurodegenerative Diseases
4.3. Cardiac Dysfunction
4.4. Metabolic Disorders
4.5. Immune Function
4.6. Viral Infections
SARS-CoV-2 Infection
4.7. Cancer
4.7.1. Melanoma
4.7.2. Neoangiogenesis and Naringenin Inhibition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ALS | amyotrophic lateral sclerosis |
| CAV | Central Cavity |
| EGFP | Enhanced Green Fluorescent Protein |
| EMT | epithelial–mesenchymal transition |
| GFP | Green Fluorescent Protein |
| HD | Huntington’s disease |
| HG | Hydrophobic Gate |
| LEL | Late Endosomes/Lysosomes |
| LRO | Lysosome-Related Organelle |
| LSD | lysosomal storage disorders |
| MD | Molecular Dynamics |
| ML | Machine Learning |
| NAADP | nicotinic acid adenine dinucleotide phosphate |
| PD | Parkinson’s Disease |
| PI(3,5)P2 | phosphatidylinositol 3,5-bisphosphate |
| SF | Selectivity Filter |
| SOCE | Store-Operated Calcium Entry |
| TPC2 | Two Pore Channel 2 |
| VSD | Voltage Sensing Domain |
References
- Hedrich, R.; Neher, E. Cytoplasmic Calcium Regulates Voltage-Dependent Ion Channels in Plant Vacuoles. Nature 1987, 329, 833. [Google Scholar] [CrossRef]
- Carpaneto, A.; Cantù, A.M.; Gambale, F. Effects of Cytoplasmic Mg2+ on Slowly Activating Channels in Isolated Vacuoles of Beta Vulgaris. Planta 2001, 213, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Carpaneto, A.; Gradogna, A. Modulation of Calcium and Potassium Permeation in Plant TPC Channels. Biophys. Chem. 2018, 236, 1–7. [Google Scholar] [CrossRef]
- Carpaneto, A.; Cantù, A.M.; Gambale, F. Redox Agents Regulate Ion Channel Activity in Vacuoles from Higher Plant Cells. FEBS Lett. 1999, 442, 129–132. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; De Angeli, A.; Ferraretto, C.; Paluzzi, S.; Gambale, F.; Carpaneto, A. Redox-Dependent Modulation of the Carrot SV Channel by Cytosolic pH. FEBS Lett. 2004, 576, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Starke, J.; Gambale, F.; Carpaneto, A. Modulation of Plant Ion Channels by Oxidizing and Reducing Agents. Arch. Biochem. Biophys. 2005, 434, 43–50. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Naso, A.; Carpaneto, A. A Perspective on the Slow Vacuolar Channel in Vacuoles from Higher Plant Cells. J. Chem. Inf. Model. 2005, 45, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Gutla, P.V.K.; Boccaccio, A.; De Angeli, A.; Gambale, F.; Carpaneto, A. Modulation of Plant TPC Channels by Polyunsaturated Fatty Acids. J. Exp. Bot. 2012, 63, 6187–6197. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Carpaneto, A.; Gambale, F. On the Interaction of Neomycin with the Slow Vacuolar Channel of Arabidopsis Thaliana. J. Gen. Physiol. 2006, 127, 329–340. [Google Scholar] [CrossRef]
- Paganetto, A.; Carpaneto, A.; Gambale, F. Ion Transport and Metal Sensitivity of Vacuolar Channels from the Roots of the Aquatic Plant Eichhornia Crassipes. Plant Cell Environ. 2001, 24, 1329–1336. [Google Scholar] [CrossRef]
- Carpaneto, A.; Cantu’, A.M.; Busch, H.; Gambale, F. Ion Channels in the Vacuoles of the Seagrass Posidonia Oceanica. FEBS Lett. 1997, 412, 236–240. [Google Scholar] [CrossRef]
- Peiter, E.; Maathuis, F.J.M.; Mills, L.N.; Knight, H.; Pelloux, J.; Hetherington, A.M.; Sanders, D. The Vacuolar Ca2+-Activated Channel TPC1 Regulates Germination and Stomatal Movement. Nature 2005, 434, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, A.; Scholz-Starke, J.; Hamamoto, S.; Larisch, N.; Festa, M.; Gutla, P.V.K.; Costa, A.; Dietrich, P.; Uozumi, N.; Carpaneto, A. The Phosphoinositide PI(3,5)P2 Mediates Activation of Mammalian but Not Plant TPC Proteins: Functional Expression of Endolysosomal Channels in Yeast and Plant Cells. Cell. Mol. Life Sci. 2014, 71, 4275–4283. [Google Scholar] [CrossRef]
- Gradogna, A.; Scholz-Starke, J.; Gutla, P.V.K.; Carpaneto, A. Fluorescence Combined with Excised Patch: Measuring Calcium Currents in Plant Cation Channels. Plant J. 2009, 58, 175–182. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dong, X.-P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC Proteins Are Phosphoinositide- Activated Sodium-Selective Ion Channels in Endosomes and Lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.-T.; et al. NAADP Mobilizes Calcium from Acidic Organelles through Two-Pore Channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef]
- Galione, A. NAADP Receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035071. [Google Scholar] [CrossRef]
- Marchant, J.S.; Gunaratne, G.S.; Cai, X.; Slama, J.T.; Patel, S. NAADP-Binding Proteins Find Their Identity. Trends Biochem. Sci. 2022, 47, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Gerndt, S.; Chen, C.-C.; Chao, Y.-K.; Yuan, Y.; Burgstaller, S.; Scotto Rosato, A.; Krogsaeter, E.; Urban, N.; Jacob, K.; Nguyen, O.N.P.; et al. Agonist-Mediated Switching of Ion Selectivity in TPC2 Differentially Promotes Lysosomal Function. eLife 2020, 9. [Google Scholar] [CrossRef]
- Hu, M.; Feng, X.; Liu, Q.; Liu, S.; Huang, F.; Xu, H. The Ion Channels of Endomembranes. Physiol. Rev. 2024, 104, 1335–1385. [Google Scholar] [CrossRef]
- Klingl, Y.E.; Petrauskas, A.; Jaślan, D.; Grimm, C. TPCs: FROM PLANT TO HUMAN. Physiol. Rev. 2025, 105, 1695–1732. [Google Scholar] [CrossRef]
- Festa, M.; Minicozzi, V.; Boccaccio, A.; Lagostena, L.; Gradogna, A.; Qi, T.; Costa, A.; Larisch, N.; Hamamoto, S.; Pedrazzini, E.; et al. Current Methods to Unravel the Functional Properties of Lysosomal Ion Channels and Transporters. Cells 2022, 11, 921. [Google Scholar] [CrossRef]
- Nicastro, G.; Orsomando, G.; Ferrari, E.; Manconi, L.; Desario, F.; Amici, A.; Naso, A.; Carpaneto, A.; Pertinhez, T.A.; Ruggieri, S. Solution Structure of the Phytotoxic Protein PcF: The First Characterized Member of the Phytophthora PcF Toxin Family. Protein Sci. 2009, 18, 1786–1791. [Google Scholar] [CrossRef]
- Maierhofer, T.; Scherzer, S.; Carpaneto, A.; Müller, T.D.; Pardo, J.M.; Hänelt, I.; Geiger, D.; Hedrich, R. Arabidopsis HAK5 under Low K+ Availability Operates as PMF Powered High-Affinity K+ Transporter. Nat. Commun. 2024, 15, 8558. [Google Scholar] [CrossRef]
- Carpaneto, A.; Accardi, A.; Pisciotta, M.; Gambale, F. Chloride Channels Activated by Hypotonicity in N2A Neuroblastoma Cell Line. Exp. Brain Res. 1999, 124, 193–199. [Google Scholar] [CrossRef]
- Bregante, M.; Yang, Y.; Formentin, E.; Carpaneto, A.; Schroeder, J.I.; Gambale, F.; Lo Schiavo, F.; Costa, A. KDC1, a Carrot Shaker-like Potassium Channel, Reveals Its Role as a Silent Regulatory Subunit When Expressed in Plant Cells. Plant Mol. Biol. 2008, 66, 61–72. [Google Scholar] [CrossRef]
- Brailoiu, E.; Rahman, T.; Churamani, D.; Prole, D.L.; Brailoiu, G.C.; Hooper, R.; Taylor, C.W.; Patel, S. An NAADP-Gated Two-Pore Channel Targeted to the Plasma Membrane Uncouples Triggering from Amplifying Ca2+ Signals. J. Biol. Chem. 2010, 285, 38511–38516. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Ahuja, M.; Patel, S.; Brailoiu, E.; Muallem, S. Convergent Regulation of the Lysosomal Two-Pore Channel-2 by Mg2+, NAADP, PI(3,5)P2 and Multiple Protein Kinases. EMBO J. 2014, 33, 501–511. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Zeng, W.; Guo, J.; Chen, Q.; Bai, X.-C.; Jiang, Y. Structural Mechanisms of Phospholipid Activation of the Human TPC2 Channel. eLife 2019, 8, e45222. [Google Scholar] [CrossRef]
- Festa, M.; Lagostena, L.; Carpaneto, A. Using the Plant Vacuole as a Biological System to Investigate the Functional Properties of Exogenous Channels and Transporters. Biochim. Biophys. Acta 2016, 1858, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Gradogna, A.; Carpaneto, A. Electrophysiology and Fluorescence to Investigate Cation Channels and Transporters in Isolated Plant Vacuoles. Stress Biol. 2022, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Gradogna, A.; Carpaneto, A. The Plant Vacuole as Heterologous System to Characterize the Functional Properties of TPC Channels. Handb. Exp. Pharmacol. 2023, 278, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Furini, S.; Maragliano, L.; Masetti, M. Editorial: Recent Advancements in Modeling and Simulations of Ion Channels. Front. Mol. Biosci. 2022, 9, 1098216. [Google Scholar] [CrossRef]
- Milenkovic, S.; Wang, J.; Acosta-Gutierrez, S.; Winterhalter, M.; Ceccarelli, M.; Bodrenko, I.V. How the Physical Properties of Bacterial Porins Match Environmental Conditions. Phys. Chem. Chem. Phys. 2023, 25, 12712–12722. [Google Scholar] [CrossRef]
- Milenkovic, S.; Bodrenko, I.V.; Lagostena, L.; Gradogna, A.; Serra, G.; Bosin, A.; Carpaneto, A.; Ceccarelli, M. The Mechanism and Energetics of a Ligand-Controlled Hydrophobic Gate in a Mammalian Two Pore Channel. Phys. Chem. Chem. Phys. 2020, 22, 15664–15674. [Google Scholar] [CrossRef]
- Milenkovic, S.; Bodrenko, I.V.; Carpaneto, A.; Ceccarelli, M. The Key Role of the Central Cavity in Sodium Transport through Ligand-Gated Two-Pore Channels. Phys. Chem. Chem. Phys. 2021, 23, 18461–18474. [Google Scholar] [CrossRef]
- Zaki, A.-M.; Çınaroğlu, S.S.; Rahman, T.; Patel, S.; Biggin, P.C. Plasticity of the Selectivity Filter Is Essential for Permeation in Lysosomal TPC2 Channels. Proc. Natl. Acad. Sci. USA 2024, 121, e2320153121. [Google Scholar] [CrossRef]
- Şahin, A.T.; Zachariae, U. In Silico Characterization of the Gating and Selectivity Mechanism of the Human TPC2 Cation Channel. J. Gen. Physiol. 2025, 157, e202313506. [Google Scholar] [CrossRef]
- Bodrenko, I.V.; Milenkovic, S.; Ceccarelli, M. Diffusion of Molecules through Nanopores under Confinement: Time-Scale Bridging and Crowding Effects via Markov State Model. Biomol. Concepts 2022, 13, 207–219. [Google Scholar] [CrossRef]
- Benkerrou, D.; Minicozzi, V.; Gradogna, A.; Milenkovic, S.; Bodrenko, I.V.; Festa, M.; Lagostena, L.; Cornara, L.; D’Amore, A.; Ceccarelli, M.; et al. A Perspective on the Modulation of Plant and Animal Two Pore Channels (TPCs) by the Flavonoid Naringenin. Biophys. Chem. 2019, 254, 106246. [Google Scholar] [CrossRef] [PubMed]
- Minicozzi, V.; Qi, T.; Gradogna, A.; Pozzolini, M.; Milenkovic, S.; Filippini, A.; Ceccarelli, M.; Carpaneto, A. A Commentary on the Inhibition of Human TPC2 Channel by the Natural Flavonoid Naringenin: Methods, Experiments, and Ideas. Biomol. Concepts 2023, 14, 20220036. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, S.; Bondar, A.-N. Motions of the SecA Protein Motor Bound to Signal Peptide: Insights from Molecular Dynamics Simulations. Biochim. Biophys. Acta Biomembr. 2018, 1860, 416–427. [Google Scholar] [CrossRef]
- Moynié, L.; Milenkovic, S.; Mislin, G.L.A.; Gasser, V.; Malloci, G.; Baco, E.; McCaughan, R.P.; Page, M.G.P.; Schalk, I.J.; Ceccarelli, M.; et al. The Complex of Ferric-Enterobactin with Its Transporter from Pseudomonas Aeruginosa Suggests a Two-Site Model. Nat. Commun. 2019, 10, 3673. [Google Scholar] [CrossRef]
- Moynié, L.; Hoegy, F.; Milenkovic, S.; Munier, M.; Paulen, A.; Gasser, V.; Faucon, A.L.; Zill, N.; Naismith, J.H.; Ceccarelli, M.; et al. Hijacking of the Enterobactin Pathway by a Synthetic Catechol Vector Designed for Oxazolidinone Antibiotic Delivery in Pseudomonas Aeruginosa. ACS Infect. Dis. 2022, 8, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, S.; Boi, S.; Scorciapino, M.A.; Bodrenko, I.V.; Ceccarelli, M. Machine Learning Prediction of Small Molecule Accumulation in Escherichia Coli Enhanced with Descriptor Statistics. J. Chem. Theory Comput. 2024, 20, 6695–6705. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. The VGL-Chanome: A Protein Superfamily Specialized for Electrical Signaling and Ionic Homeostasis. Sci. STKE 2004, 2004, re15. [Google Scholar] [CrossRef]
- Rahman, T.; Cai, X.; Brailoiu, G.C.; Abood, M.E.; Brailoiu, E.; Patel, S. Two-Pore Channels Provide Insight into the Evolution of Voltage-Gated Ca2+ and Na+ Channels. Sci. Signal. 2014, 7, ra109. [Google Scholar] [CrossRef]
- Kirsch, S.A.; Kugemann, A.; Carpaneto, A.; Böckmann, R.A.; Dietrich, P. Phosphatidylinositol-3,5-Bisphosphate Lipid-Binding-Induced Activation of the Human Two-Pore Channel 2. Cell. Mol. Life Sci. 2018, 75, 3803–3815. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Li, P.; Calvo, R.; Southall, N.; Hu, X.; Bryant-Genevier, M.; Feng, X.; Geng, Q.; Gao, C.; et al. Agonist-Specific Voltage-Dependent Gating of Lysosomal Two-Pore Na+ Channels. eLife 2019, 8, e51423. [Google Scholar] [CrossRef] [PubMed]
- Lagostena, L.; Festa, M.; Pusch, M.; Carpaneto, A. The Human Two-Pore Channel 1 Is Modulated by Cytosolic and Luminal Calcium. Sci. Rep. 2017, 7, 43900. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.-C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Ebola Virus. Two-Pore Channels Control Ebola Virus Host Cell Entry and Are Drug Targets for Disease Treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef]
- Chi, G.; Jaślan, D.; Kudrina, V.; Böck, J.; Li, H.; Pike, A.C.W.; Rautenberg, S.; Krogsaeter, E.; Bohstedt, T.; Wang, D.; et al. Structural Basis for Inhibition of the Lysosomal Two-Pore Channel TPC2 by a Small Molecule Antagonist. Structure 2024, 32, 1137–1149.e4. [Google Scholar] [CrossRef]
- Kintzer, A.F.; Stroud, R.M. Structure, Inhibition and Regulation of Two-Pore Channel TPC1 from Arabidopsis Thaliana. Nature 2016, 531, 258–262. [Google Scholar] [CrossRef]
- Guo, J.; Zeng, W.; Chen, Q.; Lee, C.; Chen, L.; Yang, Y.; Cang, C.; Ren, D.; Jiang, Y. Structure of the Voltage-Gated Two-Pore Channel TPC1 from Arabidopsis Thaliana. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef]
- Guardiani, C.; Cecconi, F.; Chiodo, L.; Cottone, G.; Malgaretti, P.; Maragliano, L.; Barabash, M.L.; Camisasca, G.; Ceccarelli, M.; Corry, B.; et al. Computational Methods and Theory for Ion Channel Research. Adv. Phys. X 2022, 7, 2080587. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals for the Lysosome: A Control Center for Cellular Clearance and Energy Metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Morgan, A.J.; Martucci, L.L.; Davis, L.C.; Galione, A. Two-Pore Channels: Going with the Flows. Biochem. Soc. Trans. 2022, 50, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.A.; Uderhardt, S.; Saric, A.; Collins, R.F.; Buckley, C.M.; Mylvaganam, S.; Boroumand, P.; Plumb, J.; Germain, R.N.; Ren, D.; et al. Lipid-Gated Monovalent Ion Fluxes Regulate Endocytic Traffic and Support Immune Surveillance. Science 2020, 367, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, S.R.; Barreda, D.; Wu, J.Z.; Ye, G.; Yusuf, B.; Ren, D.; Freeman, S.A. Two-Pore Channels Regulate Endomembrane Tension to Enable Remodeling and Resolution of Phagolysosomes. Proc. Natl. Acad. Sci. USA 2024, 121, e2309465121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jaślan, D.; Rahman, T.; Bolsover, S.R.; Arige, V.; Larry E Wagner, I.I.; Abrahamian, C.; Tang, R.; Keller, M.; Hartmann, J.; et al. Segregated Cation Flux by TPC2 Biases Ca2+ Signaling through Lysosomes. Nat. Commun. 2022, 13, 4481. [Google Scholar] [CrossRef]
- Grimm, C.; Butz, E.; Chen, C.-C.; Wahl-Schott, C.; Biel, M. From Mucolipidosis Type IV to Ebola: TRPML and Two-Pore Channels at the Crossroads of Endo-Lysosomal Trafficking and Disease. Cell Calcium 2017, 67, 148–155. [Google Scholar] [CrossRef]
- Cang, C.; Zhou, Y.; Navarro, B.; Seo, Y.-J.; Aranda, K.; Shi, L.; Battaglia-Hsu, S.; Nissim, I.; Clapham, D.E.; Ren, D. mTOR Regulates Lysosomal ATP-Sensitive Two-Pore Na(+) Channels to Adapt to Metabolic State. Cell 2013, 152, 778–790. [Google Scholar] [CrossRef]
- Sun, W.; Yue, J. TPC2 Mediates Autophagy Progression and Extracellular Vesicle Secretion in Cancer Cells. Exp. Cell Res. 2018, 370, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Böck, J.; Krogsaeter, E.; Passon, M.; Chao, Y.-K.; Sharma, S.; Grallert, H.; Peters, A.; Grimm, C. Human Genome Diversity Data Reveal That L564P Is the Predominant TPC2 Variant and a Prerequisite for the Blond Hair Associated M484L Gain-of-Function Effect. PLoS Genet. 2021, 17, e1009236. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kilpatrick, B.S. Two-Pore Channels and Disease. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1678–1686. [Google Scholar] [CrossRef]
- Chao, Y.-K.; Schludi, V.; Chen, C.-C.; Butz, E.; Nguyen, O.N.P.; Müller, M.; Krüger, J.; Kammerbauer, C.; Ben-Johny, M.; Vollmar, A.M.; et al. TPC2 Polymorphisms Associated with a Hair Pigmentation Phenotype in Humans Result in Gain of Channel Function by Independent Mechanisms. Proc. Natl. Acad. Sci. USA 2017, 114, E8595–E8602. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Boyle, J.A.; Di Pietro, S.M. TPC2 Mediates New Mechanisms of Platelet Dense Granule Membrane Dynamics through Regulation of Ca2+ Release. Mol. Biol. Cell 2015, 26, 3263–3274. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.L.; Boyle, J.A.; Aradi, A.E.; Christian, K.A.; Di Pietro, S.M. TPC2 Controls Pigmentation by Regulating Melanosome pH and Size. Proc. Natl. Acad. Sci. USA 2016, 113, 5622–5627. [Google Scholar] [CrossRef]
- Bellono, N.W.; Escobar, I.E.; Oancea, E. A Melanosomal Two-Pore Sodium Channel Regulates Pigmentation. Sci. Rep. 2016, 6, 26570. [Google Scholar] [CrossRef]
- Davis, L.C.; Morgan, A.J.; Chen, J.-L.; Snead, C.M.; Bloor-Young, D.; Shenderov, E.; Stanton-Humphreys, M.N.; Conway, S.J.; Churchill, G.C.; Parrington, J.; et al. NAADP Activates Two-Pore Channels on T Cell Cytolytic Granules to Stimulate Exocytosis and Killing. Curr. Biol. 2012, 22, 2331–2337. [Google Scholar] [CrossRef]
- Tedeschi, V.; Sapienza, S.; Ciancio, R.; Canzoniero, L.M.T.; Pannaccione, A.; Secondo, A. Lysosomal Channels as New Molecular Targets in the Pharmacological Therapy of Neurodegenerative Diseases via Autophagy Regulation. Curr. Neuropharmacol. 2025, 23, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Brailoiu, G.C.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of Lysosomal Morphology by Pathogenic LRRK2 Is Corrected by TPC2 Inhibition. J. Cell. Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef]
- Gregori, M.; Pereira, G.J.S.; Allen, R.; West, N.; Chau, K.-Y.; Cai, X.; Bostock, M.P.; Bolsover, S.R.; Keller, M.; Lee, C.-Y.; et al. Lysosomal TPC2 Channels Disrupt Ca2+ Entry and Dopaminergic Function in Models of LRRK2-Parkinson’s Disease. J. Cell Biol. 2025, 224, e202412055. [Google Scholar] [CrossRef]
- Pereira, C.A.d.S.; Medaglia, N.d.C.; Ureshino, R.P.; Bincoletto, C.; Antonioli, M.; Fimia, G.M.; Piacentini, M.; Pereira, G.J.d.S.; Erustes, A.G.; Smaili, S.S. NAADP-Evoked Ca2+ Signaling Leads to Mutant Huntingtin Aggregation and Autophagy Impairment in Murine Astrocytes. Int. J. Mol. Sci. 2023, 24, 5593. [Google Scholar] [CrossRef]
- Martucci, L.L.; Launay, J.-M.; Kawakami, N.; Sicard, C.; Desvignes, N.; Dakouane-Giudicelli, M.; Spix, B.; Têtu, M.; Gilmaire, F.-O.; Paulcan, S.; et al. Endolysosomal TPCs Regulate Social Behavior by Controlling Oxytocin Secretion. Proc. Natl. Acad. Sci. USA 2023, 120, e2213682120. [Google Scholar] [CrossRef]
- Prat Castro, S.; Kudrina, V.; Jaślan, D.; Böck, J.; Scotto Rosato, A.; Grimm, C. Neurodegenerative Lysosomal Storage Disorders: TPC2 Comes to the Rescue! Cells 2022, 11, 2807. [Google Scholar] [CrossRef]
- García-Rúa, V.; Otero, M.F.; Lear, P.V.; Rodríguez-Penas, D.; Feijóo-Bandín, S.; Noguera-Moreno, T.; Calaza, M.; Álvarez-Barredo, M.; Mosquera-Leal, A.; Parrington, J.; et al. Increased Expression of Fatty-Acid and Calcium Metabolism Genes in Failing Human Heart. PLoS ONE 2012, 7, e37505. [Google Scholar] [CrossRef]
- de Zélicourt, A.; Fayssoil, A.; Mansart, A.; Zarrouki, F.; Karoui, A.; Piquereau, J.; Lefebvre, F.; Gerbaud, P.; Mika, D.; Dakouane-Giudicelli, M.; et al. Two-Pore Channels (TPCs) Acts as a Hub for Excitation-Contraction Coupling, Metabolism and Cardiac Hypertrophy Signalling. Cell Calcium 2024, 117, 102839. [Google Scholar] [CrossRef]
- Arredouani, A.; Ruas, M.; Collins, S.C.; Parkesh, R.; Clough, F.; Pillinger, T.; Coltart, G.; Rietdorf, K.; Royle, A.; Johnson, P.; et al. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) and Endolysosomal Two-Pore Channels Modulate Membrane Excitability and Stimulus-Secretion Coupling in Mouse Pancreatic β Cells. J. Biol. Chem. 2015, 290, 21376–21392. [Google Scholar] [CrossRef] [PubMed]
- Cane, M.C.; Parrington, J.; Rorsman, P.; Galione, A.; Rutter, G.A. The Two Pore Channel TPC2 Is Dispensable in Pancreatic β-Cells for Normal Ca2+ Dynamics and Insulin Secretion. Cell Calcium 2016, 59, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Zhang, Q.; Salehi, A.; Willems, M.; Knudsen, J.G.; Ringgaard, A.K.; Chapman, C.E.; Gonzalez-Alvarez, A.; Surdo, N.C.; Zaccolo, M.; et al. Adrenaline Stimulates Glucagon Secretion by Tpc2-Dependent Ca2+ Mobilization From Acidic Stores in Pancreatic α-Cells. Diabetes 2018, 67, 1128–1139. [Google Scholar] [CrossRef]
- Grimm, C.; Holdt, L.M.; Chen, C.-C.; Hassan, S.; Müller, C.; Jörs, S.; Cuny, H.; Kissing, S.; Schröder, B.; Butz, E.; et al. High Susceptibility to Fatty Liver Disease in Two-Pore Channel 2-Deficient Mice. Nat. Commun. 2014, 5, 4699. [Google Scholar] [CrossRef] [PubMed]
- Lear, P.V.; González-Touceda, D.; Porteiro Couto, B.; Viaño, P.; Guymer, V.; Remzova, E.; Tunn, R.; Chalasani, A.; García-Caballero, T.; Hargreaves, I.P.; et al. Absence of Intracellular Ion Channels TPC1 and TPC2 Leads to Mature-Onset Obesity in Male Mice, Due to Impaired Lipid Availability for Thermogenesis in Brown Adipose Tissue. Endocrinology 2015, 156, 975–986. [Google Scholar] [CrossRef]
- Chen, C.-C.; Krogsaeter, E.; Grimm, C. Two-Pore and TRP Cation Channels in Endolysosomal Osmo-/Mechanosensation and Volume Regulation. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118921. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.C.; Morgan, A.J.; Galione, A. NAADP-Regulated Two-Pore Channels Drive Phagocytosis through Endo-Lysosomal Ca2+ Nanodomains, Calcineurin and Dynamin. EMBO J. 2020, 39, e104058. [Google Scholar] [CrossRef]
- Maekawa, M.; Natsume, R.; Arita, M. Functional Significance of Ion Channels during Macropinosome Resolution in Immune Cells. Front. Physiol. 2022, 13, 1037758. [Google Scholar] [CrossRef]
- Ouologuem, L.; Bartel, K. Endolysosomal Transient Receptor Potential Mucolipins and Two-Pore Channels: Implications for Cancer Immunity. Front. Immunol. 2024, 15, 1389194. [Google Scholar] [CrossRef]
- Steiner, P.; Arlt, E.; Boekhoff, I.; Gudermann, T.; Zierler, S. TPC Functions in the Immune System. Handb. Exp. Pharmacol. 2023, 278, 71–92. [Google Scholar] [CrossRef]
- Gunaratne, G.S.; Yang, Y.; Li, F.; Walseth, T.F.; Marchant, J.S. NAADP-Dependent Ca2+ Signaling Regulates Middle East Respiratory Syndrome-Coronavirus Pseudovirus Translocation through the Endolysosomal System. Cell Calcium 2018, 75, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.-K.; Chang, S.-Y.; Grimm, C. Endo-Lysosomal Cation Channels and Infectious Diseases. Rev. Physiol. Biochem. Pharmacol. 2023, 185, 259–276. [Google Scholar] [CrossRef]
- Filippini, A.; D’Amore, A.; Palombi, F.; Carpaneto, A. Could the Inhibition of Endo-Lysosomal Two-Pore Channels (TPCs) by the Natural Flavonoid Naringenin Represent an Option to Fight SARS-CoV-2 Infection? Front. Microbiol. 2020, 11, 970. [Google Scholar] [CrossRef]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin Is a Powerful Inhibitor of SARS-CoV-2 Infection in Vitro. Pharmacol. Res. 2020, 105255. [Google Scholar] [CrossRef]
- D’Amore, A.; Gradogna, A.; Palombi, F.; Minicozzi, V.; Ceccarelli, M.; Carpaneto, A.; Filippini, A. The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection. Cells 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhong, M.; Wu, H.; Su, W.; Wang, Y.; Li, P. Potential Beneficial Effects of Naringin and Naringenin on Long COVID-A Review of the Literature. Microorganisms 2024, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Madeswaran, A.; Brahmasundari, S.; Midhuna, P.G. In Silico Molecular Docking Studies of Certain Commercially Available Flavonoids as Effective Antiviral Agents against Spike Glycoprotein of SARS-CoV-2. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6741–6744. [Google Scholar] [CrossRef]
- Cobre, A.F.; Maia Neto, M.; de Melo, E.B.; Fachi, M.M.; Ferreira, L.M.; Tonin, F.S.; Pontarolo, R. Naringenin-4’-Glucuronide as a New Drug Candidate against the COVID-19 Omicron Variant: A Study Based on Molecular Docking, Molecular Dynamics, MM/PBSA and MM/GBSA. J. Biomol. Struct. Dyn. 2024, 42, 5881–5894. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Therapeutic Strategies to Overcome Drug Resistance in Cancer Cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting Calcium Signaling in Cancer Therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef]
- Xu, M.; Seas, A.; Kiyani, M.; Ji, K.S.Y.; Bell, H.N. A Temporal Examination of Calcium Signaling in Cancer- from Tumorigenesis, to Immune Evasion, and Metastasis. Cell Biosci. 2018, 8, 25. [Google Scholar] [CrossRef]
- Barbonari, S.; D’Amore, A.; Palombi, F.; De Cesaris, P.; Parrington, J.; Riccioli, A.; Filippini, A. Relevance of Lysosomal Ca2+ Signalling Machinery in Cancer. Cell Calcium 2022, 102, 102539. [Google Scholar] [CrossRef]
- Alharbi, A.F.; Parrington, J. Endolysosomal Ca2+ Signaling in Cancer: The Role of TPC2, From Tumorigenesis to Metastasis. Front. Cell Dev. Biol. 2019, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, O.N.P.; Grimm, C.; Schneider, L.S.; Chao, Y.-K.; Atzberger, C.; Bartel, K.; Watermann, A.; Ulrich, M.; Mayr, D.; Wahl-Schott, C.; et al. Two-Pore Channel Function Is Crucial for the Migration of Invasive Cancer Cells. Cancer Res. 2017, 77, 1427–1438. [Google Scholar] [CrossRef]
- Favia, A.; Desideri, M.; Gambara, G.; D’Alessio, A.; Ruas, M.; Esposito, B.; Del Bufalo, D.; Parrington, J.; Ziparo, E.; Palombi, F.; et al. VEGF-Induced Neoangiogenesis Is Mediated by NAADP and Two-Pore Channel-2-Dependent Ca2+ Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E4706-4715. [Google Scholar] [CrossRef]
- Parrington, J.; Lear, P.; Hachem, A. Calcium Signals Regulated by NAADP and Two-Pore Channels--Their Role in Development, Differentiation and Cancer. Int. J. Dev. Biol. 2015, 59, 341–355. [Google Scholar] [CrossRef]
- Sperandio, L.P.; Lins, I.V.F.; Erustes, A.G.; Leão, A.H.F.F.; Antunes, F.; Morais, I.B.M.; Vieira, H.F.; de Campos, L.M.; Bincoletto, C.; Smaili, S.S.; et al. Blocking Autophagy by the Two-Pore Channels Antagonist Tetrandrine Improves Sorafenib-Induced Death of Hepatocellular Carcinoma Cells. Toxicol In Vitro 2023, 90, 105603. [Google Scholar] [CrossRef]
- Grimm, C.; Bartel, K.; Vollmar, A.M.; Biel, M. Endolysosomal Cation Channels and Cancer-A Link with Great Potential. Pharmaceuticals 2018, 11, 4. [Google Scholar] [CrossRef]
- Marconi, A.; Quadri, M.; Farnetani, F.; Ciardo, S.; Palazzo, E.; Lotti, R.; Cesinaro, A.M.; Fabbiani, L.; Vaschieri, C.; Puviani, M.; et al. In Vivo Melanoma Cell Morphology Reflects Molecular Signature and Tumor Aggressiveness. J. Invest. Dermatol. 2022, 142, 2205–2216.e6. [Google Scholar] [CrossRef]
- Eddy, K.; Shah, R.; Chen, S. Decoding Melanoma Development and Progression: Identification of Therapeutic Vulnerabilities. Front. Oncol. 2020, 10, 626129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Eraslan, Z.; Miller, D.; Taylor, I.; You, J.; Grondin, S.J.; Vega, M.; Manga, P.; Goff, P.S.; Sviderskaya, E.V.; et al. Two-Pore Channel 2 Is Required for Soluble Adenylyl Cyclase-Dependent Regulation of Melanosomal pH and Melanin Synthesis. Pigment. Cell Melanoma Res. 2024, 37, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Gerndt, S.; Chao, Y.-K.; Zisis, T.; Nguyen, O.N.P.; Gerwien, A.; Urban, N.; Müller, C.; Gegenfurtner, F.A.; Geisslinger, F.; et al. Gene Editing and Synthetically Accessible Inhibitors Reveal Role for TPC2 in HCC Cell Proliferation and Tumor Growth. Cell Chem. Biol. 2021, 28, 1119–1131.e27. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, A.; Hanbashi, A.A.; Di Agostino, S.; Palombi, F.; Sacconi, A.; Voruganti, A.; Taggi, M.; Canipari, R.; Blandino, G.; Parrington, J.; et al. Loss of Two-Pore Channel 2 (TPC2) Expression Increases the Metastatic Traits of Melanoma Cells by a Mechanism Involving the Hippo Signalling Pathway and Store-Operated Calcium Entry. Cancers 2020, 12, 2391. [Google Scholar] [CrossRef] [PubMed]
- Barbonari, S.; D’Amore, A.; Hanbashi, A.A.; Palombi, F.; Riccioli, A.; Parrington, J.; Filippini, A. Endolysosomal Two-Pore Channel 2 Plays Opposing Roles in Primary and Metastatic Malignant Melanoma Cells. Cell Biol. Int. 2024, 48, 521–540. [Google Scholar] [CrossRef]
- Netcharoensirisuk, P.; Abrahamian, C.; Tang, R.; Chen, C.-C.; Rosato, A.S.; Beyers, W.; Chao, Y.-K.; Filippini, A.; Di Pietro, S.; Bartel, K.; et al. Flavonoids Increase Melanin Production and Reduce Proliferation, Migration and Invasion of Melanoma Cells by Blocking Endolysosomal/Melanosomal TPC2. Sci. Rep. 2021, 11, 8515. [Google Scholar] [CrossRef]
- Abrahamian, C.; Tang, R.; Deutsch, R.; Ouologuem, L.; Weiden, E.-M.; Kudrina, V.; Blenninger, J.; Rilling, J.; Feldmann, C.; Kuss, S.; et al. Rab7a Is an Enhancer of TPC2 Activity Regulating Melanoma Progression through Modulation of the GSK3β/β-Catenin/MITF-Axis. Nat. Commun. 2024, 15, 10008. [Google Scholar] [CrossRef]
- Hanbashi, A.; Alotaibi, M.; Sobeai, H.M.A.; Binobaid, L.; Alhazzani, K.; Jin, X.; Kamli, F.; Alhoshani, A.; Parrington, J. Loss of Two-Pore Channel 2 Function in Melanoma-Derived Tumours Reduces Tumour Growth in Vivo but Greatly Increases Tumour-Related Toxicity in the Organism. Cancer Cell Int. 2023, 23, 325. [Google Scholar] [CrossRef]
- He, L.-N.; Liu, Y.-J.; Jiang, J.-B.; Wang, D.-Y.; Li, Y.-L.; Zeng, S.-J.; Guo, Z.; Yao, P.-Y.; Lin, Z.-C.; Lv, S.-X.; et al. Tetrandrine Augments Melanoma Cell Immunogenicity via Dual Inhibition of Autophagic Flux and Proteasomal Activity Enhancing MHC-I Presentation. Acta Pharmacol. Sin. 2025, 46, 2056–2072. [Google Scholar] [CrossRef]
- Munaron, L. Intracellular Calcium, Endothelial Cells and Angiogenesis. Recent. Pat. Anticancer. Drug Discov. 2006, 1, 105–119. [Google Scholar] [CrossRef]
- Geisslinger, F.; Müller, M.; Chao, Y.-K.; Grimm, C.; Vollmar, A.M.; Bartel, K. Targeting TPC2 Sensitizes Acute Lymphoblastic Leukemia Cells to Chemotherapeutics by Impairing Lysosomal Function. Cell Death Dis. 2022, 13, 668. [Google Scholar] [CrossRef]
- Kpeli, G.W.; Conrad, K.M.; Bralower, W.; Byrne, C.E.; Boue, S.M.; Burow, M.E.; Mondrinos, M.J. Xenohormetic Phytochemicals Inhibit Neovascularization in Microphysiological Models of Vasculogenesis and Tumor Angiogenesis. Adv. Biol. 2024, 8, e2300480. [Google Scholar] [CrossRef] [PubMed]
- Pafumi, I.; Festa, M.; Papacci, F.; Lagostena, L.; Giunta, C.; Gutla, V.; Cornara, L.; Favia, A.; Palombi, F.; Gambale, F.; et al. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Sci. Rep. 2017, 7, 5121. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-T.; Tseng, Y.-T.; Lo, Y.-C.; Wu, S.-N. Ability of Naringenin, a Bioflavonoid, to Activate M-Type Potassium Current in Motor Neuron-like Cells and to Increase BKCa-Channel Activity in HEK293T Cells Transfected with α-hSlo Subunit. BMC Neurosci. 2014, 15, 135. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagostena, L.; Minicozzi, V.; Meucci, M.; Gradogna, A.; Milenkovic, S.; Palombi, F.; Ceccarelli, M.; Filippini, A.; Carpaneto, A. The Two-Pore Channel 2 in Human Physiology and Diseases: Functional Characterisation and Pharmacology. Int. J. Mol. Sci. 2025, 26, 9708. https://doi.org/10.3390/ijms26199708
Lagostena L, Minicozzi V, Meucci M, Gradogna A, Milenkovic S, Palombi F, Ceccarelli M, Filippini A, Carpaneto A. The Two-Pore Channel 2 in Human Physiology and Diseases: Functional Characterisation and Pharmacology. International Journal of Molecular Sciences. 2025; 26(19):9708. https://doi.org/10.3390/ijms26199708
Chicago/Turabian StyleLagostena, Laura, Velia Minicozzi, Martina Meucci, Antonella Gradogna, Stefan Milenkovic, Fioretta Palombi, Matteo Ceccarelli, Antonio Filippini, and Armando Carpaneto. 2025. "The Two-Pore Channel 2 in Human Physiology and Diseases: Functional Characterisation and Pharmacology" International Journal of Molecular Sciences 26, no. 19: 9708. https://doi.org/10.3390/ijms26199708
APA StyleLagostena, L., Minicozzi, V., Meucci, M., Gradogna, A., Milenkovic, S., Palombi, F., Ceccarelli, M., Filippini, A., & Carpaneto, A. (2025). The Two-Pore Channel 2 in Human Physiology and Diseases: Functional Characterisation and Pharmacology. International Journal of Molecular Sciences, 26(19), 9708. https://doi.org/10.3390/ijms26199708




