Dynamic Hydrogels: Adaptive Biomaterials for Engineering Tumor Microenvironment and Cancer Treatment
Abstract
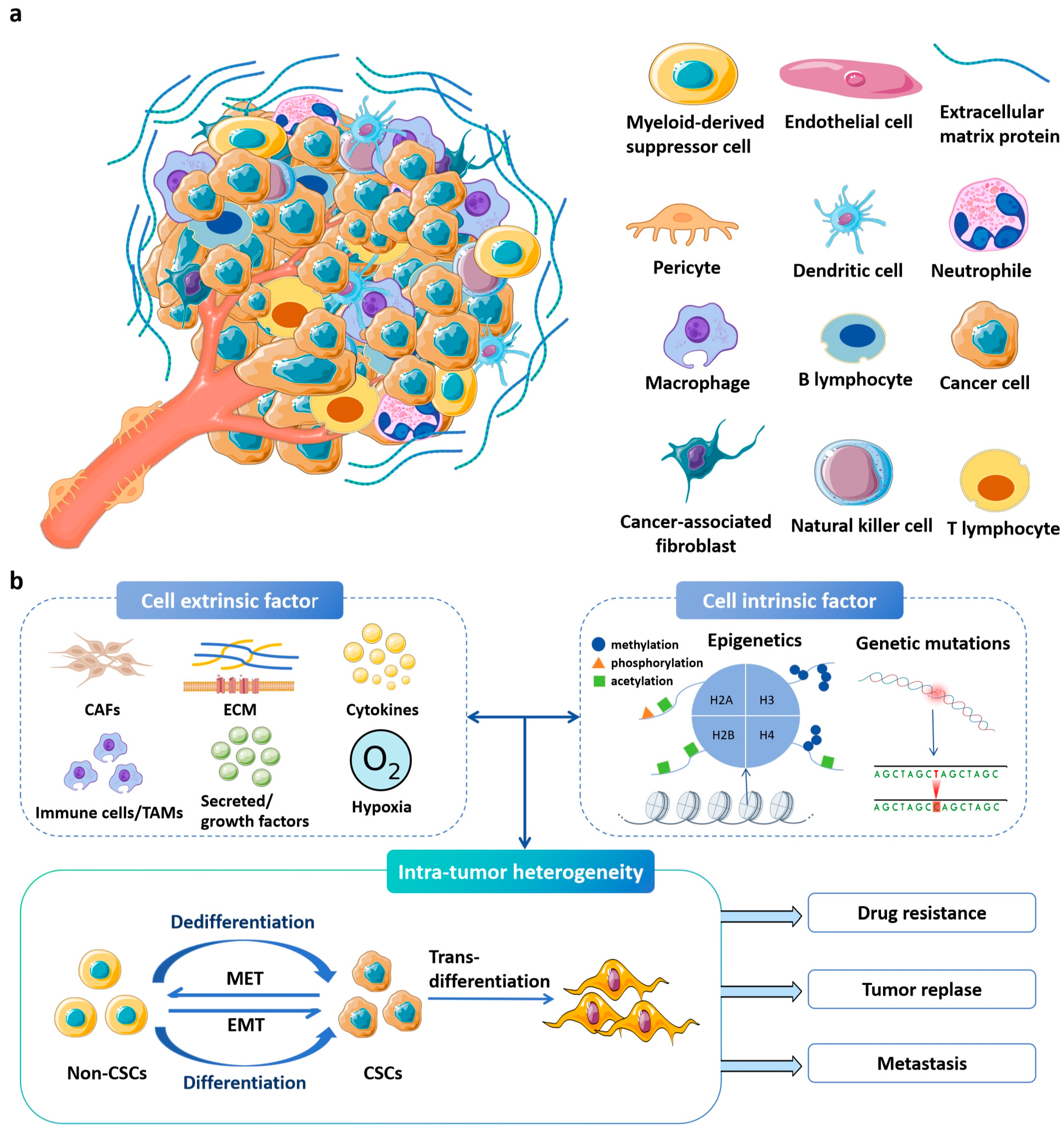
1. Introduction
2. Dynamic Hydrogels: Designs and Applications
2.1. Fundamentals of Dynamic Hydrogels for Recreating Tumor Microenvironment and Cancer Therapy
2.2. Design and Classification of Dynamic Hydrogels
2.2.1. Natural vs. Synthetic Hydrogels
2.2.2. Multi-Stimuli Responsive Hydrogel
| Performance Parameters | Natural Hydrogels | Synthetic Hydrogels | Multi-Stimuli Responsive Hydrogel | Reference |
|---|---|---|---|---|
| Mechanical stiffness range | 0.1–10 kPa (collagen); 0.1–1.5 kPa (agarose) | 0.1–150 kPa (PVA); 0.01–7360 kPa (PEG) | Dynamically Adjustable: Light-responsive (10 kPa → 2 kPa); pH-responsive (modulus change ± 50%) | [38] |
| Stimulus-response sensitivity | No inherent response | Requires functional modification | pH response: Swelling ratio 2.98–10, response time 60–300 s (PAAc); Temperature response: Swelling ratio 2.98–20, response time 300–540 s (PNIPAAm); Light response: Contraction rate 15%, response time < 120 s | [59] |
| Drug loading efficiency (EE%) | 65–90% (limited by low mechanical strength) | 70–98% (capable of designing high drug-loading structures) | Smart Controlled Release: pH-triggered release rate > 80%; Temperature-triggered sustained release cycle: 1–7 days | [60] |
| degradation cycle | Days–weeks (enzymatic degradation dominant) | Weeks–months (hydrolysis controllable) | Stimulus-dependent degradation: Photodegradation (hour-scale); Enzyme/pH synergistic degradation (day-scale) | [61] |
| Biocompatibility | High (low immunogenicity) | Moderate–High (potential residual toxic crosslinking agents) | Moderate (requires safety verification) | [62] |
2.2.3. Multi-Drug Loading Hydrogels
2.2.4. Immune-Activated Hydrogels
| Hydrogel Type | Stimulus/Mechanism | Application | Reference |
|---|---|---|---|
| MMP-Sensitive Hydrogel | Enzyme (MMP-2/9) | Drug release, immune recruitment | [1] |
| pH/GSH Hydrogel | pH, GSH | Drug delivery (adriamycin) | [20] |
| ALG-βCD Hydrogel | Host-guest, chemokine | T cell recruitment | [24] |
| Photocrosslinked Network | Schiff base, UV | Mechanical toughness | [25] |
| Pluronic F127 | Temperature | Localized therapy (breast cancer) | [50] |
| CS15-FTP-gel | hypoxia induction | maximize chemotherapeutic efficacy (AQ4 toxicity) | [53] |
| cerium-based nanocomposite hydrogel | Temperature/remove ROS | Skin wound healing and regeneration | [55] |
| PepABS-py | Exosome adsorption | Immunotherapy (PD-L1 neutralization) | [68] |
| Photoresponsive hydrogel | Specific wavelength illumination (UV/Vis/NIR) | soft robotics | [70] |
3. Multifunctional Applications in TME Engineering and Therapy
3.1. Precise Drug Delivery
| Technology | Hydrogel Type | Application | Outcome | Reference |
|---|---|---|---|---|
| pH/GSH dual response release | Adriamycin-peptide nanogel | Breast cancer treatment | Reduced toxicity, enhanced membrane permeability, prolonged drug retention time, and 65% improvement in efficacy. | [72] |
| Photothermal/photodynamic synergy | Polyaniline-indocyanine green hydrogel | tumor ablation | Tumor ablation efficiency of 90% in acidic TME. | [72] |
| Targeted molecular coupling | D-Lys6-GnRH peptide hydrogel | Precise targeting of tumor cells | A single injection of 250 nmol/kg AN-207 resulted in 100% tumor regression in MX-1 breast cancer mice. | [73] |
| Somatostatin receptor targeting | RC-121 coupled with AN-201 hydrogel | Precise targeting of tumor cells | AN-238 reduced tumor volume by 50% in NCI-N87 gastric cancer nude mice (p < 0.05). | [74] |
| Gold nanorods enhance ROS | AuNR/GO hybrid hydrogel | tumor ablation | ROS production increased threefold, with a survival rate of <15% for 4T1 cells. | [24,76] |
3.2. Bioprinting Technology for Tumor Modeling
3.3. Microfluidics-Hydrogel Coupling
3.4. Combined Therapy
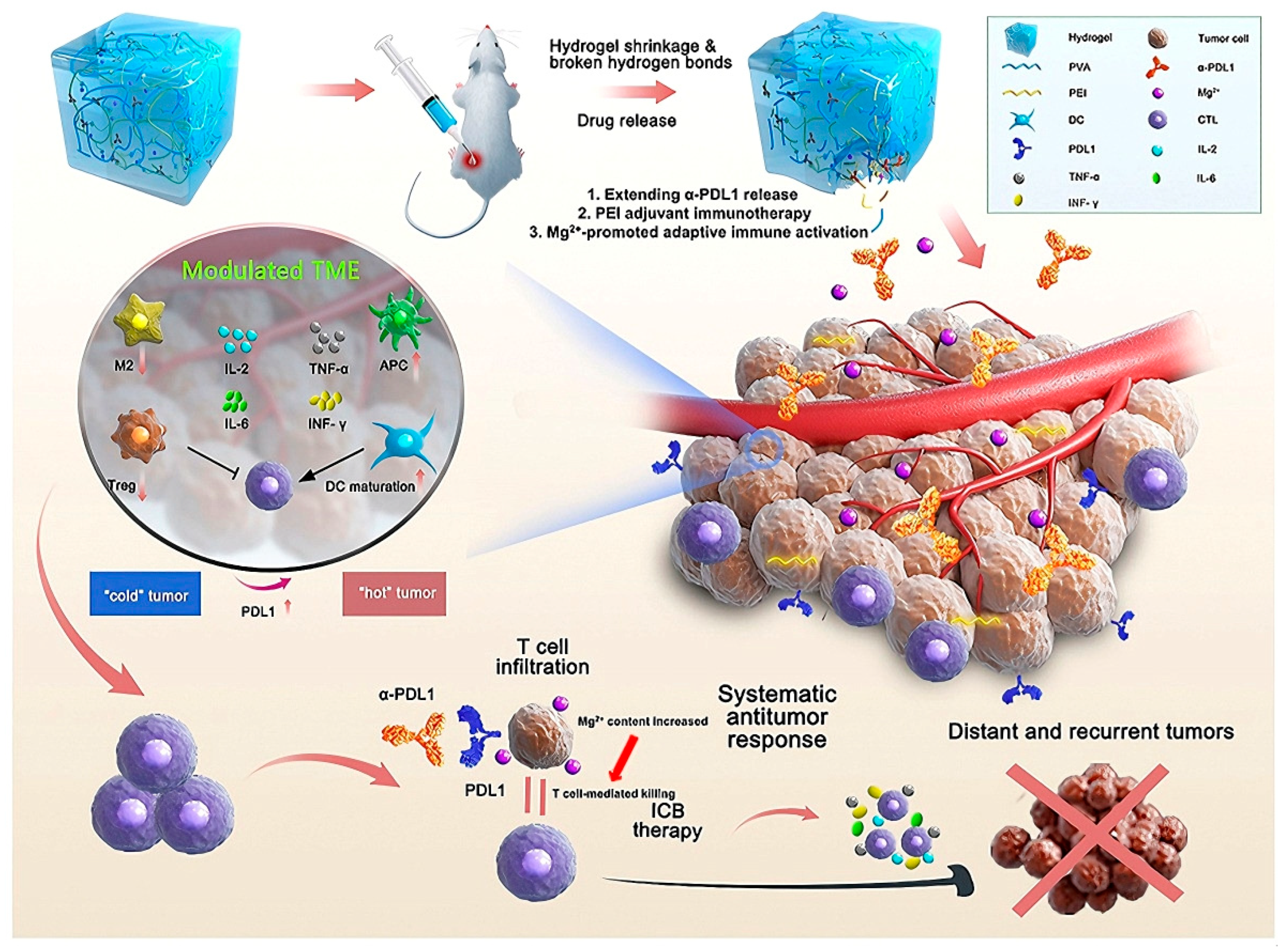
3.5. Immune Reprogramming
4. Challenges to Clinical Translation
4.1. Biocompatibility
4.2. Scaling Up
4.3. Regulatory Barriers
4.4. Model Limitations
5. Future Directions: Interdisciplinary Innovations
5.1. Biomimetic Design
5.2. Synergistic Effects of CRISPR and Hydrogels
5.3. AI-Driven Design
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TME | tumor microenvironment |
| ECM | extracellular matrix |
| CAFs | Cancer-associated fibroblasts |
| MMP | matrix metalloproteinases |
| LOX | lysyl oxidase |
| Treg | regulatory T cells |
| IL-2 | interleukin-2 |
| MDSCs | myeloid-derived suppressor cells |
| Arg-1 | arginase-1 |
| iNOS | inducible nitric oxide synthase |
| CSCs | cancer stem cells |
| CRC | colorectal cancer |
| EMT | epithelial-to-mesenchymal transition |
| MET | mesenchymal-to-epithelial transition |
| ECs | endothelial-like cells |
| anti-PD-1 | anti-programmed death-1 |
| ALG-βCD | in situ formed βCD-modified alginate |
| Ad | adamantane |
| VEGF | vascular endothelial growth factor |
| HA | hyaluronic acid |
| PEG | Polyethylene glycol |
| Ad-aPD1 | adamantane-modified anti-PD-1 antibodies |
| NLCs | nanostructured lipid carriers |
| PFCs | perfluorocarbons |
| PDT | photodynamic therapy |
| TPL | triptolide |
| CA IX | carbonic anhydrase IX |
| PepABS-py | Self-assembled short peptide |
| GSH | glutathione |
| GnRH/LHRH | Gonadotropin-releasing hormone/Luteinizing Hormone-Releasing Hormone |
| ROS | Reactive Oxygen Species |
| GelMA | Gelatin acryloyl |
| CAD | computer-aided design |
| PTT | photothermal therapy |
| IRE1α | Inhibition of inositol-requiring enzyme 1 |
| FlaB | flagellar protein B |
| VEGFR | vascular endothelial growth factor receptors |
| ICB | immune checkpoint blockade |
| DC | dendritic cell |
| TLR | Toll-like receptor |
| TAMs | tumor-associated macrophages |
| α-PDL1/PEIGel | The PVA-PEI hydrogel |
| CAR-T | Chimeric Antigen Receptor T-Cell |
| GMP | Good Manufacturing Practice |
| AFM | Atomic force microscopy |
| HCC | hepatocellular carcinoma |
| VAE | variational autoencoder |
| AUC | area under the curve |
| POLYGON | Polypharmacology Generative Optimization Network |
References
- Talib, W.H.; Alsayed, A.R.; Farhan, F.; Al Kury, L.T. Resveratrol and Tumor Microenvironment: Mechanistic Basis and Therapeutic Targets. Molecules 2020, 25, 4282. [Google Scholar] [CrossRef]
- Kim, S.J.; Khadka, D.; Seo, J.H. Interplay between Solid Tumors and Tumor Microenvironment. Front. Immunol. 2022, 13, 882718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longhurst, C.M.; Jennings, L.K. Integrin-mediated signal transduction. Cell. Mol. Life Sci. 1998, 54, 514–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roy, T.; Dutta, S.; Ghosh, S.; Sthanam, L.K.; Sen, S. CD44/Integrin β1 Association Drives Fast Motility on Hyaluronic Acid Substrates. J. Cell. Physiol. 2025, 240, e70001. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, M.; De Gregorio, V.; Iorio, A.L.; Giunti, L.; Guidi, M.; De Martino, M.; Genitori, L.; Sardi, I. Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int. J. Mol. Sci. 2018, 19, 2879. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, D.; Hachey, S.J.; Hughes, C.C.W. Improving tumor microenvironment assessment in chip systems through next-generation technology integration. Front. Bioeng. Biotechnol. 2024, 12, 1462293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piñeiro Fernández, J.; Luddy, K.A.; Harmon, C.; O’Farrelly, C. Hepatic Tumor Microenvironments and Effects on NK Cell Phenotype and Function. Int. J. Mol. Sci. 2019, 20, 4131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tao, S.; Liang, S.; Zeng, T.; Yin, D. Epigenetic modification-related mechanisms of hepatocellular carcinoma resistance to immune checkpoint inhibition. Front. Immunol. 2023, 13, 1043667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Sun, Q.; Li, Q.; Li, S.; Li, X. Dynamic Hydrogels with Viscoelasticity and Tunable Stiffness for the Regulation of Cell Behavior and Fate. Materials 2023, 16, 5161. [Google Scholar] [CrossRef]
- Bej, R.; Haag, R. Mucus-Inspired Dynamic Hydrogels: Synthesis and Future Perspectives. J. Am. Chem. Soc. 2022, 144, 20137–20152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pal, S.; Medatwal, N.; Kumar, S.; Kar, A.; Komalla, V.; Yavvari, P.S.; Mishra, D.; Rizvi, Z.A.; Nandan, S.; Malakar, D.; et al. A Localized Chimeric Hydrogel Therapy Combats Tumor Progression through Alteration of Sphingolipid Metabolism. ACS Cent. Sci. 2019, 5, 1648–1662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, S.H.D.; Yin, B.; Li, Z.; Yuan, W.; Zhang, Q.; Xie, X.; Tan, Y.; Wong, N.; Zhang, K.; Bian, L. Mechanical manipulation of cancer cell tumorigenicity via heat shock protein signaling. Sci. Adv. 2023, 9, eadg9593. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, F.; Zhang, J.; Sun, X.; Yan, Y.; Wang, Y.; Ouyang, J.; Zhang, J.; Honore, T.; Ge, J.; et al. Study on Development of Composite Hydrogels With Tunable Structures and Properties for Tumor-on-a-Chip Research. Front. Bioeng. Biotechnol. 2020, 8, 611796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Briggs, F.; Browne, D.; Asuri, P. Role of Polymer Concentration and Crosslinking Density on Release Rates of Small Molecule Drugs. Int. J. Mol. Sci. 2022, 23, 4118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Wang, J.; Zhang, X.; Yu, S.; Wen, D.; Hu, Q.; Ye, Y.; Bomba, H.; Hu, X.; Liu, Z.; et al. In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018, 10, eaan3682. [Google Scholar] [CrossRef]
- Meng, J.; Yang, X.; Huang, J.; Tuo, Z.; Hu, Y.; Liao, Z.; Tian, Y.; Deng, S.; Deng, Y.; Zhou, Z.; et al. Ferroptosis-Enhanced Immunotherapy with an Injectable Dextran-Chitosan Hydrogel for the Treatment of Malignant Ascites in Hepatocellular Carcinoma. Adv. Sci. 2023, 10, e2300517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Fang, M.; Zhang, J.; Wang, J.; Song, Y.; Shi, J.; Li, W.; Wu, G.; Ren, J.; Wang, Z.; et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology 2015, 5, e1074374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, J.; Zhao, G.; Yu, J.; Xu, P.; Yan, J.; Jin, Z.; Chen, S.; Wang, Y.; Zhang, L.W.; Wang, Y. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnol. 2022, 20, 372. [Google Scholar] [CrossRef]
- Fan, S.; Liu, Q.; Dong, J.; Ai, X.; Li, J.; Huang, W.; Sun, T. In situ forming an injectable hyaluronic acid hydrogel for drug delivery and synergistic tumor therapy. Heliyon 2024, 10, e32135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Liu, M.; Cai, C.; Ye, C.; Guo, T.; Yang, K.; Xiao, H.; Tang, X.; Liu, H. Recent progress of hydrogel-based local drug delivery systems for postoperative radiotherapy. Front. Oncol. 2023, 13, 1027254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guedes, G.; Wang, S.; Fontana, F.; Figueiredo, P.; Lindén, J.; Correia, A.; Pinto, R.J.B.; Hietala, S.; Sousa, F.L.; Santos, H.A. Dual-Crosslinked Dynamic Hydrogel Incorporating {Mo154} with pH and NIR Responsiveness for Chemo-Photothermal Therapy. Adv. Mater. 2021, 33, e2007761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Li, Q.; Chen, X.; Nie, X.; Xue, F.; Xu, W.; Luan, Y. An Injectable Hydrogel to Modulate T Cells for Cancer Immunotherapy. Small 2022, 18, e2202663. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.A.; Martinez-Lozano, E.; Sheridan, R.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. Hydrogels for the management of second-degree burns: Currently available options and future promise. Burns Trauma 2022, 10, tkac047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, P. Preparation and Performance Study of Injectable Supramolecular Hydrogels Based on Host-Guest Interactions. Ph.D. Thesis, Southeast University, Nanjing, China, 2022. [Google Scholar]
- Tang, G.; Zhou, B.; Li, F.; Wang, W.; Liu, Y.; Wang, X.; Liu, C.; Ye, X. Advances of Naturally Derived and Synthetic Hydrogels for Intervertebral Disk Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137, Erratum in Polymers 2017, 9, 225. https://doi.org/10.3390/polym9060225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Xiao, F.; Zhang, L.; Li, W.; Wang, B.; Weng, Z.; Liu, Y.; Chen, G. Research progress of hydrogels as delivery systems and scaffolds in the treatment of secondary spinal cord injury. Front. Bioeng. Biotechnol. 2023, 11, 1111882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chamkouri, H.; Chamkouri, M. A Review of Hydrogels, Their Properties and Applications in Medicine. Am. J. Biomed. Sci. Res. 2021, 11, AJBSR.MS.ID.001682. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minhas, M.U.; Ahmad, M.; Anwar, J.; Khan, S. Synthesis and Characterization of Biodegradable Hydrogels for Oral Delivery of 5-Fluorouracil Targeted to Colon: Screening with Preliminary In Vivo Studies. Adv. Polym. Technol. 2018, 37, 221–229. [Google Scholar] [CrossRef]
- Shivakoti, I.; Kibria, G.; Cep, R.; Pradhan, B.B.; Sharma, A. Laser Surface Texturing for Biomedical Applications: A Review. Coatings 2021, 11, 124. [Google Scholar] [CrossRef]
- Leslie-Barbick, J.E.; Shen, C.; Chen, C.; West, J.L. Micron-scale spatially patterned, covalently immobilized vascular endothelial growth factor on hydrogels accelerates endothelial tubulogenesis and increases cellular angiogenic responses. Tissue Eng. Part A 2011, 17, 221–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Miranda, M.L.; Xu, D.; Ben Issa, A.A.; Johnston, D.A.; Browne, M.; Cook, R.B.; Sengers, B.G.; Evans, N.D. Geometric constraint of mechanosensing by modification of hydrogel thickness prevents stiffness-induced differentiation in bone marrow stromal cells. J. R. Soc. Interface 2024, 21, 20240485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, M.; Wu, J.; Zhang, C.; Deng, X.; Dhinakar, A.; Huang, W.; Qian, H.; Ge, L. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017, 51, 294–303. [Google Scholar] [CrossRef]
- Kharkar Prathamesh, M. Design of multimodal degradable hydrogels for controlled therapeutic delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Xin, H.; Yu, H.; Wang, H.; Ma, D.; Cao, X.; Wang, Y. MicroRNA-activated hydrogel scaffold generated by 3D printing accelerates bone regeneration. Bioact. Mater. 2021, 10, 1–4. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2021, 13, 100186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, J.; Yuan, P.; Wu, B.; Liu, Y.; Hu, C. Advances in the Research and Application of Smart-Responsive Hydrogels in Disease Treatment. Gels 2023, 9, 662. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Acciaretti, F.; Vesentini, S.; Cipolla, L. Fabrication Strategies Towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights. Chem. Asian J. 2022, 17, e202200797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, Y.; Li, J.; Hu, Y.; Gao, F.; Pak-Heng Leung, G.; Geng, F.; Fu, C.; Zhang, J. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on “two strikes” effects. Acta Pharm. Sin. B 2020, 10, 2227–2245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, Q.; Chen, J.; Yan, J.; Cai, S.; Xiong, H.; Liu, Y.; Peng, D.; Mo, M.; Liu, Z. Tumor microenvironment responsive drug delivery systems. Asian J. Pharm. Sci. 2020, 15, 416–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef]
- Chen, S.X.; Zhang, J.; Xue, F.; Liu, W.; Kuang, Y.; Gu, B.; Song, S.; Chen, H. In situ forming oxygen/ROS-responsive niche-like hydrogel enabling gelation-triggered chemotherapy and inhibition of metastasis. Bioact. Mater. 2023, 21, 86–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, A.; Gebremeskel, S.; Lichty, B.D.; Johnston, B. Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression. J. Immunother. Cancer. 2022, 10, e003923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, X.; Luo, M.; Wang, M.; Niu, W.; Wang, Y.; Lei, B. Injectable self-healing ceria-based nanocomposite hydrogel with ROS-scavenging activity for skin wound repair. Regen. Biomater. 2021, 9, rbab074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karoyo, A.H.; Wilson, L.D. A Review on the Design and Hydration Properties of Natural Polymer-Based Hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Tomatsu, I.; Kros, A. Light controlled protein release from a supramolecular hydrogel. Chem. Commun. 2010, 46, 4094–4096. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Yoshida, T.; Tanaka, R. Chapter 28-Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Cartilage Tissue Engineering; Academic Press: Cambridge, MA, USA, 2024; pp. 467–486. ISBN 9780323905978. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Ligorio, C.; Hoyland, J.A.; Saiani, A. Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration. Gels 2022, 8, 211. [Google Scholar] [CrossRef]
- Jones, D.S., 2nd; Nardozzi, J.D.; Sackton, K.L.; Ahmad, G.; Christensen, E.; Ringgaard, L.; Chang, D.K.; Jaehger, D.E.; Konakondla, J.V.; Wiinberg, M.; et al. Cell surface-tethered IL-12 repolarizes the tumor immune microenvironment to enhance the efficacy of adoptive T cell therapy. Sci Adv. 2022, 8, eabi8075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chao, X.; Zhang, Y.; Zheng, C.; Huang, Q.; Lu, J.; Pulver, E.M.; Houthuijzen, J.; Hutten, S.; Luo, R.; He, J.; et al. Metastasis of breast cancer to bones alters the tumor immune microenvironment. Eur. J. Med. Res. 2023, 28, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumbhar, P.R.; Kumar, P.; Lasure, A.; Velayutham, R.; Mandal, D. An updated landscape on nanotechnology-based drug delivery, immunotherapy, vaccinations, imaging, and biomarker detections for cancers: Recent trends and future directions with clinical success. Discov. Nano 2023, 18, 156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, D.; Chen, J.; Gao, L.; Chen, X.; Lin, L.; Wei, X.; Liu, Y.; Cheng, H. Injectable Photothermal PDA/Chitosan/β-Glycerophosphate Thermosensitive Hydrogels for Antibacterial and Wound Healing Promotion. Macromol. Biosci. 2024, 24, e2400080. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Wu, Y.; Ghaemmaghami, A.M.; Sun, H.; Mata, A. Rational design of hydrogels for immunomodulation. Regen. Biomater. 2022, 9, rbac009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dorishetty, P.; Balu, R.; Athukoralalage, S.S.; Greaves, T.L.; Mata, J.; De Campo, L.; Saha, N.; Zannettino, A.C.; Dutta, N.K.; Choudhury, N.R. Tunable Biomimetic Hydrogels from Silk Fibroin and Nanocellulose. ACS Sustain. Chem. Eng. 2020, 8, 2375–2389. [Google Scholar] [CrossRef]
- Xu, F.; Luo, S.; Lu, P.; Cai, C.; Li, W.; Li, C. Composition, functions, and applications of exosomal membrane proteins. Front. Immunol. 2024, 15, 1408415. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, S.; Ma, H.; Li, C.; Huang, Z. Photoresponsive hydrogel-based soft robot: A review. Mater. Today Bio 2023, 20, 100657. [Google Scholar] [CrossRef]
- Xie, W. Tumor Microenvironment-Activated Nanostructures Enhance MRI Capability and Nanocatalytic Activity for Highly Tumor-Specific Multimodal Theranostics. Small 2023, 20, e2306446. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The pancreatic cancer microbiome promotes oncogenesis by inducing innate and adaptive immune suppression. Cancer Discov. 2018, 8, 403–416, Erratum in Cancer Discov. 2020, 10, 1988. https://doi.org/10.1158/2159-8290.CD-20-1573. [Google Scholar] [CrossRef]
- Guo, H.; Lu, J.; Hathaway, H.; Royce, M.E.; Prossnitz, E.R.; Miao, Y. Synthesis and evaluation of novel gonadotropin-releasing hormone receptor-targeting peptides. Bioconjug Chem. 2011, 22, 1682–1689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.Y.; Zhang, N.Z. Progress in somatostatin analogues and gastrointestinal tumors. J. Xuzhou Med. Univ. 2004, 6, 601–604. [Google Scholar]
- Wang, L.; Chen, Y.; Han, Z.; Wang, E.; Zhang, J.; Wang, B.; Yang, X. Tumor Microenvironment Activated Multifunctional Nanoparticles for Precisely Controlled Tumor Photothermal and Photodynamic Therapy. J. Anal. Test. 2023, 7, 215–226. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Li, B.; Li, X. Study on Photothermal/Photodynamic Synergistic Therapy of Mouse Breast Cancer Cells Using Gold Nanorods. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2021, 52, 247–251. [Google Scholar] [CrossRef]
- Wu, H.; Chen, P.; Zhan, X.; Lin, K.; Hu, T.; Xiao, A.; Liang, J.; Huang, Y.; Huang, Y.; Guan, B.O. Marriage of a Dual-Plasmonic Interface and Optical Microfiber for NIR-II Cancer Theranostics. Adv. Mater. 2024, 36, e2310571. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.Y.; Zhu, J.; Gao, Y.Q.; Jiang, M.; Yin, H. Narrative review of 3D bioprinting for the construction of in vitro tumor models: Present and prospects. Transl. Cancer Res. 2025, 14, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, J.M.; Park, I.A.; Lee, S.H.; Kim, W.H.; Bae, M.S.; Koo, H.R.; Yi, A.; Kim, S.J.; Cho, N.; Moon, W.K. Stiffness of tumors measured by shear-wave elastography correlated with subtypes of breast cancer. Eur. Radiol. 2013, 23, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bock, N.; Forouz, F.; Hipwood, L.; Clegg, J.; Jeffery, P.; Gough, M.; van Wyngaard, T.; Pyke, C.; Adams, M.N.; Bray, L.J.; et al. GelMA, Click-Chemistry Gelatin and Bioprinted Polyethylene Glycol-Based Hydrogels as 3D Ex Vivo Drug Testing Platforms for Patient-Derived Breast Cancer Organoids. Pharmaceutics 2023, 15, 261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Zhang, B.; Cui, Y.; Song, H.; Shang, D. In vitro co-culture models for studying organoid-macrophage interactions: The golden technology of cancer immunotherapy. Am. J. Cancer Res. 2024, 14, 3222–3240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wang, Z.; Hu, Q.; Luo, H.; Lu, B.; Gao, Y.; Qiao, Z.; Zhou, Y.; Fang, Y.; Gu, J.; et al. 3D Bioprinted GelMA-Nanoclay Hydrogels Induce Colorectal Cancer Stem Cells Through Activating Wnt/β-Catenin Signaling. Small 2022, 18, e2200364. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Xiong, A.; Nguyen, K.; Masek, M.; No, D.Y.; Elazar, M.; Shteyer, E.; Winters, M.A.; Voedisch, A.; Shaw, K.; et al. Long-term culture of human liver tissue with advanced hepatic functions. JCI Insight 2017, 2, e90853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, A.S.M.; Berg, J.; Roehrs, V.; Wu, D.; Hackethal, J.; Braeuning, A.; Woelken, L.; Rauh, C.; Kurreck, J. Xeno-Free 3D Bioprinted Liver Model for Hepatotoxicity Assessment. Int. J. Mol. Sci. 2024, 25, 1811. [Google Scholar] [CrossRef] [PubMed]
- Yousry, C.; Ahmed, I.S.; Amin, M.M.; El Gazayerly, O.N. Superhydrophobic Substrates for Ultrahigh Encapsulation of Hydrophilic Drug into Controlled-Release Polyelectrolyte Complex Beads: Statistical Optimization and In Vivo Evaluation. Pharmaceutics 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, A.; Zhang, S.; Yang, R.; Sui, C. Enhancing the mechanical strength of 3D printed GelMA for soft tissue engineering applications. Mater. Today Bio 2023, 24, 100939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shukla, A.K.; Yoon, S.; Oh, S.O.; Lee, D.; Ahn, M.; Kim, B.S. Advancement in Cancer Vasculogenesis Modeling through 3D Bioprinting Technology. Biomimetics 2024, 9, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, E.W.; Beebe, D.J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 2010, 39, 1036–1048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Cordero, J.L.; Maerkl, S.J. Microfluidic systems for cancer diagnostics. Curr. Opin. Biotechnol. 2020, 65, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sakolish, C.M.; Esch, M.B.; Hickman, J.J.; Shuler, M.L.; Mahler, G.J. Modeling Barrier Tissues In Vitro: Methods, Achievements, and Challenges. eBioMedicine 2016, 5, 30–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinho, D.; Santos, D.; Vila, A.; Carvalho, S. Establishment of Colorectal Cancer Organoids in Microfluidic-Based System. Micromachines 2021, 12, 497. [Google Scholar] [CrossRef]
- Wang, D.; Maharjan, S.; Kuang, X.; Wang, Z.; Mille, L.S.; Tao, M.; Yu, P.; Cao, X.; Lian, L.; Lv, L.; et al. Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci Adv. 2022, 8, eabq6900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Hartman, C.L.; Li, L.; Albert, C.J.; Si, F.; Gao, A.; Huang, L.; Zhao, Y.; Lin, W.; Hsueh, E.C.; et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci. Transl. Med. 2021, 13, eaaz6314. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Zhu, D. Research progress on reshaping the immunosuppressive tumor microenvironment to enhance immunotherapy. Int. J. Biomed. Eng. 2024, 47, 388–394. [Google Scholar] [CrossRef]
- Wang, Q.; Quan, X.; Shu, G. Research Progress on Photothermal Therapy and Its Combination Therapy for Hepatocellular Carcinoma. Int. J. Biomed. Eng. 2022, 45, 547–552. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, L.; Abdullah, M.; Shen, X.; He, Y.; Liu, J.; Chen, X. High resolution observation of a noble-metal/ZnO-QDs/rGO ternary system through an ultra-thin SiNx window using in situ liquid cell scanning electron microscopy. New J. Chem. 2024, 48, 14538–14547. [Google Scholar] [CrossRef]
- Qin, W.; Ma, Z.; Bai, G.; Qin, W.; Li, L.; Hao, D.; Wang, Y.; Yan, J.; Han, X.; Niu, W.; et al. Neurovascularization inhibiting dual responsive hydrogel for alleviating the progression of osteoarthritis. Nat. Commun. 2025, 16, 1390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, S.M.; Awasthi, D.; Jeong, J.; Sandoval, T.A.; Chae, C.S.; Ramos, Y.; Tan, C.; Falco, M.M.; McBain, I.T.; Mishra, B.; et al. Transgelin 2 guards T cell lipid metabolic programming and anti-tumor function. Res. Sq. 2023, rs.3, rs-3683989, Update in Nature 2024, 635, 1010–1018. https://doi.org/10.1038/s41586-024-08071-y. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, eaak9537. [Google Scholar] [CrossRef]
- Li, F.; Ding, J.; Li, Z.; Rong, Y.; He, C.; Chen, X. ROS-responsive thermosensitive polypeptide hydrogels for localized drug delivery and improved tumor chemoimmunotherapy. Biomater. Sci. 2024, 12, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Halpin-Veszeleiova, K.; Mallouh, M.P.; Williamson, L.M.; Apro, A.C.; Botticello-Romero, N.R.; Bahr, C.; Shin, M.; Ward, K.M.; Rosenberg, L.; Ritov, V.B.; et al. Oxygen-carrying nanoemulsions and respiratory hyperoxia eliminate tumor hypoxia-induced immunosuppression. JCI Insight 2025, 10, e174675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Zhang, Z.; Du, H.; Chen, X.; Hu, N.; Yu, T.; Hou, M.; Yu, X. An enzyme-responsive hydrogel functionalized with mesoporous silica nanoparticles for co-delivery of cisplatin and shRNA to overcome chemotherapy resistance in non-small cell lung cancer. RSC Adv. 2025, 15, 23966–23977. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z.; Qin, X.; Zhang, M.; Du, Q.; Li, Z.; Luan, Y. A Checkpoint-Regulatable Immune Niche Created by Injectable Hydrogel for Tumor Therapy. Adv. Funct. Mater. 2021, 31, 2104630. [Google Scholar] [CrossRef]
- Peng, Y.; Liang, S.; Meng, Q.F.; Liu, D.; Ma, K.; Zhou, M.; Yun, K.; Rao, L.; Wang, Z. Engineered Bio-Based Hydrogels for Cancer Immunotherapy. Adv. Mater. 2024, 36, e2313188. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, D.; Wang, C.; Chen, Z.; Huang, C.; Yang, Y.; Xie, L.; Zhang, L.; Xu, L.; Zhang, M.R.; et al. PEIGel: A biocompatible and injectable scaffold with innate immune adjuvanticity for synergized local immunotherapy. Mater. Today Bio 2022, 15, 100297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Hu, Y.; Mei, H. Harmonizing the symphony of chimeric antigen receptor T cell immunotherapy with the elegance of biomaterials. Trends Biotechnol. 2025, 43, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Fürst, W.; Banerjee, A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann. Thorac. Surg. 2005, 79, 1522–1528, discussion 1529. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Yu, Y.; Liu, X.; Chai, Y.; Wang, X.; Wen, G. Recent advances of chitosan-based composite hydrogel materials in application of bone tissue engineering. Heliyon 2024, 10, e37431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, F.; Zhang, Q.; Yang, M.; Yin, B.; Wong, S.H. Recent advances in optical techniques for dynamically probing cellular mechanobiology. Biomed. Eng. Commun. 2024, 3, 13. [Google Scholar] [CrossRef]
- Hu, B.; Xin, Y.; Hu, G.; Li, K.; Tan, Y. Fluid shear stress enhances natural killer cell’s cytotoxicity toward circulating tumor cells through NKG2D-mediated mechanosensing. APL Bioeng. 2023, 7, 036108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the Clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, Q.; Fang, Z.; Gu, L.; Bian, L. Structurally Dynamic Hydrogels for Biomedical Applications: Pursuing a Fine Balance between Macroscopic Stability and Microscopic Dynamics. Chem. Rev. 2021, 121, 11149–11193. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Eylem, C.C.; Haciefendioglu, T.; Yildirim, E.; Sari, B.; Nemutlu, E.; Emregul, E.; Okesola, B.O.; Derkus, B. Mechanically robust hybrid hydrogels of photo-crosslinkable gelatin and laminin-mimetic peptide amphiphiles for neural induction. Biomater. Sci. 2021, 9, 8270–8284. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, X.; Jiang, H. Construction and Characterization of Adjustable Stiffness Hydrogels Based on Methacrylated Gelatin. Chin. J. Plast. Surg. 2024, 40, 1149–1156. [Google Scholar] [CrossRef]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.J. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- 2023 China Biopharmaceutical Industry Report. Available online: https://file.jgvogel.cn/126/upload/resources/file/436966.pdf (accessed on 19 April 2025).
- Global Premier Biologics Platforms to Enable and Expedite Innovations. Available online: https://www.wuxibiologics.com.cn/wp-content/uploads/pre210113.pdf (accessed on 19 April 2025).
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tseng, H.C.; Xiong, W.; Badeti, S.; Yang, Y.; Ma, M.; Liu, T.; Ramos, C.A.; Dotti, G.; Fritzky, L.; Jiang, J.G.; et al. Efficacy of anti-CD147 chimeric antigen receptors targeting hepatocellular carcinoma. Nat. Commun. 2020, 11, 4810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fudge, N.; Keyvani, F.; Khatri, J.; Poudineh, M. Agarose-gel coating for improving the polydopamine-based pH sensor stability in continuous pH measurements. Sens. Diagn. 2024, 4, 136–146. [Google Scholar] [CrossRef]
- Yao, P.; Wang, X.; Wang, Q.; Dai, Q.; Peng, Y.; Yuan, Q.; Mou, N.; Lv, S.; Weng, B.; Wang, Y.; et al. Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy. Pharmaceutics 2023, 15, 1827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Q.; Li, J.; Abudousalamu, Z.; Sun, Y.; Xue, M.; Yao, L.; Chen, M. Advancing Ovarian Cancer Therapeutics: The Role of Targeted Drug Delivery Systems. Int. J. Nanomed. 2024, 19, 9351–9370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Gong, B.; Hu, W.; Liu, J.; Wang, Y.; Wang, Z.; Yang, X.; Wang, X.; Yang, H.; Yan, G. Cluster Missile-Inspired Dynamic Nanocomposite Hydrogel Precisely Mediates Robust Locoregional Tumor Theranostics. Adv. Funct. Mater. 2024, 35, 2418295. [Google Scholar] [CrossRef]
- Han, D.; Li, J.; Tan, W. CRISPR propels a smart hydrogel. Science 2019, 365, 754–755. [Google Scholar] [CrossRef]
- Alsaid, Y.; Wu, S.; Wu, D.; Du, Y.; Shi, L.; Khodambashi, R.; Rico, R.; Hua, M.; Yan, Y.; Zhao, Y.; et al. Tunable Sponge-Like Hierarchically Porous Hydrogels with Simultaneously Enhanced Diffusivity and Mechanical Properties. Adv. Mater. 2021, 33, e2008235. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Tang, R.; He, F.; Huang, Y.; Nie, Z.; Lei, C. Responsive MXene nanovehicles deliver CRISPR/Cas12a for boolean logic-controlled gene editing. Sci. China Chem. 2022, 65, 2318–2326. [Google Scholar] [CrossRef]
- Liao, H.; Hu, S.; Yang, H.; Wang, L.; Tanaka, S.; Takigawa, I.; Li, W.; Fan, H.; Gong, J.P. Data-driven de novo design of super-adhesive hydrogels. Nature 2025, 644, 89–95. [Google Scholar] [CrossRef]
- Munson, B.P.; Chen, M.; Bogosian, A.; Kreisberg, J.F.; Licon, K.; Abagyan, R.; Kuenzi, B.M.; Ideker, T. De novo generation of multi-target compounds using deep generative chemistry. Nat. Commun. 2024, 15, 3636. [Google Scholar] [CrossRef]
- Cardot, J.M.; Lukas, J.C.; Muniz, P. Time Scaling for In Vitro-In Vivo Correlation: The Inverse Release Function (IRF) Approach. AAPS J. 2018, 20, 95. [Google Scholar] [CrossRef] [PubMed]
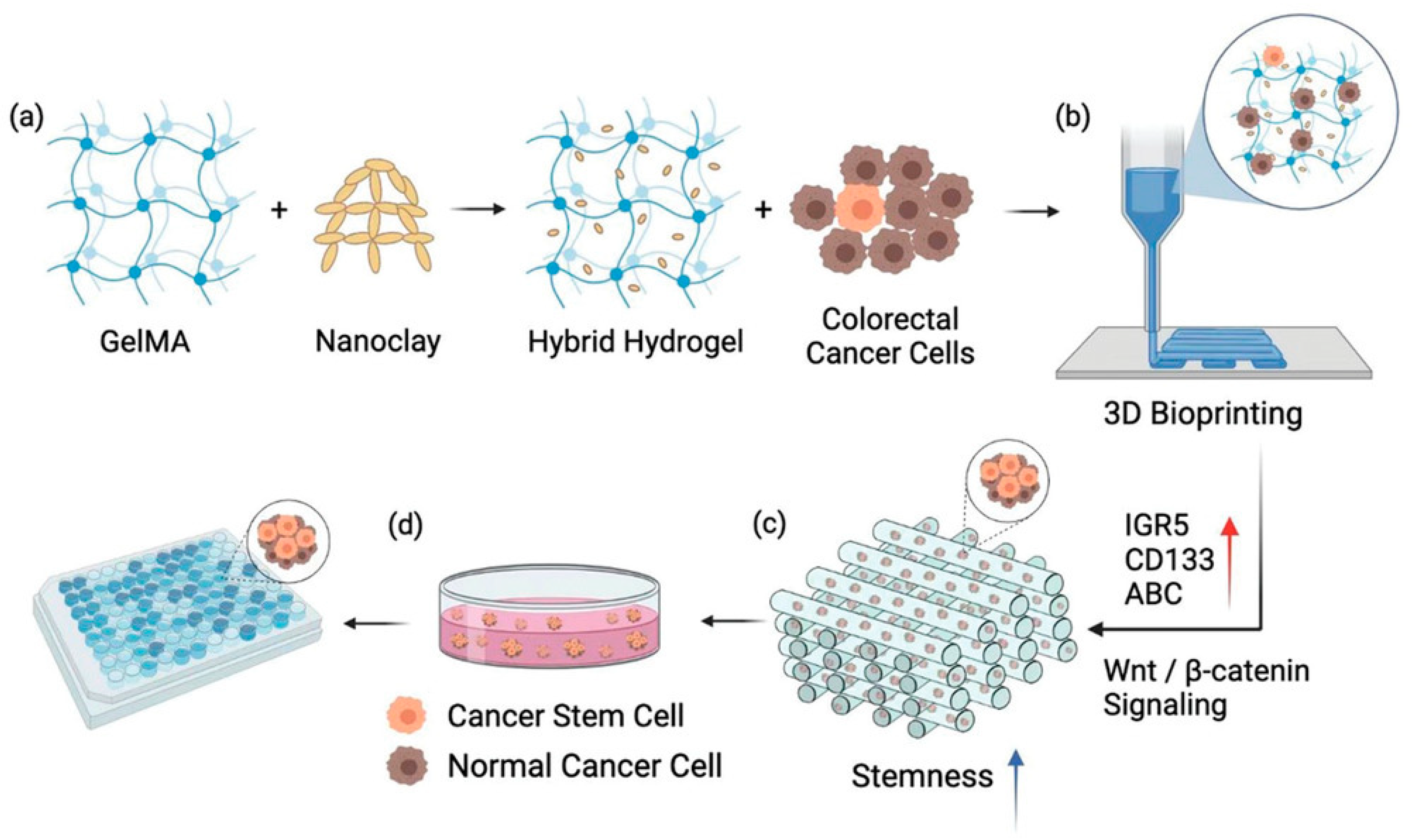
| Technology | Hydrogel Type | Application | Outcome | Reference |
|---|---|---|---|---|
| Bioprinting | GelMA | 3D tumor modeling | Replicates ECM stiffness | [79] |
| Bio-3D Printing | GelMA | Chemotherapy response prediction | 85% chemotherapy response accuracy | [81] |
| Immune-Tumor Co-culture | Immune cell-loaded bioinks | Simulate immune-tumor interactions | 70% fidelity to in vivo conditions | [82] |
| Vascular network modeling | Bioinks with cells/biomaterials | Tumor angiogenesis study | Enables creation of perfusable vascular networks resembling in vivo complexity | [89] |
| Microfluidics | Alginate-Gelatin | Angiogenesis simulation | 75% vascular structural similarity | [94] |
| Microfluidics-Organoid | GelMA | Metastatic invasion modeling | 90% accuracy with colorectal cancer organoids | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Xiao, Y.; Yin, B.; Wong, S.H.D. Dynamic Hydrogels: Adaptive Biomaterials for Engineering Tumor Microenvironment and Cancer Treatment. Int. J. Mol. Sci. 2025, 26, 9502. https://doi.org/10.3390/ijms26199502
Wu Y, Xiao Y, Yin B, Wong SHD. Dynamic Hydrogels: Adaptive Biomaterials for Engineering Tumor Microenvironment and Cancer Treatment. International Journal of Molecular Sciences. 2025; 26(19):9502. https://doi.org/10.3390/ijms26199502
Chicago/Turabian StyleWu, Yuting, Yifei Xiao, Bohan Yin, and Siu Hong Dexter Wong. 2025. "Dynamic Hydrogels: Adaptive Biomaterials for Engineering Tumor Microenvironment and Cancer Treatment" International Journal of Molecular Sciences 26, no. 19: 9502. https://doi.org/10.3390/ijms26199502
APA StyleWu, Y., Xiao, Y., Yin, B., & Wong, S. H. D. (2025). Dynamic Hydrogels: Adaptive Biomaterials for Engineering Tumor Microenvironment and Cancer Treatment. International Journal of Molecular Sciences, 26(19), 9502. https://doi.org/10.3390/ijms26199502







