Unscheduled m6A Deposition in RNA via m6ATP Incorporation by DNA Polymerases
Abstract
1. Introduction
2. Results
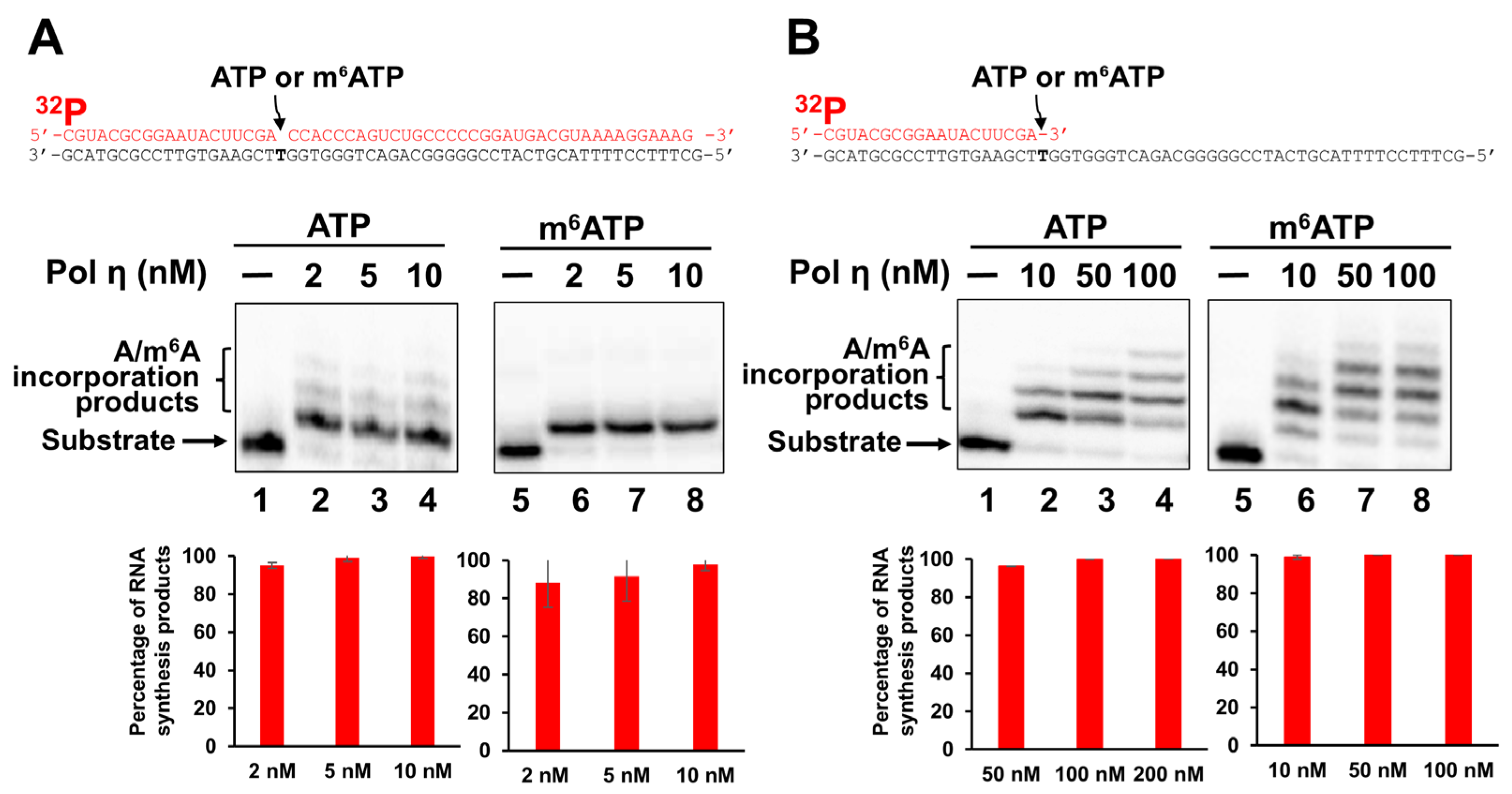
2.1. Pol β Can Incorporate m6ATP into RNA in the Presence of Mn2+
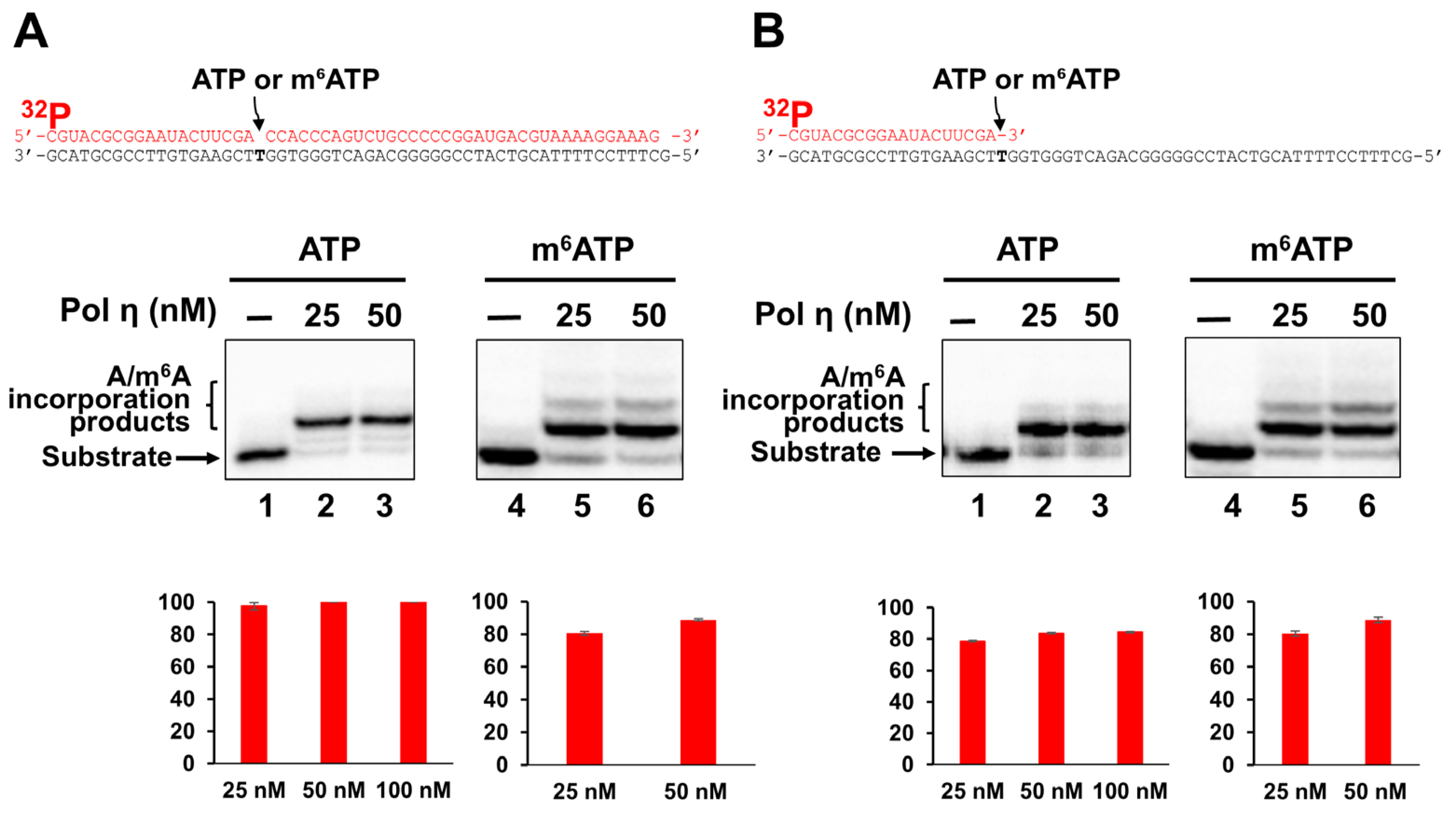
2.2. Incorporation of m6ATP into RNA by Pol η
2.3. The Catalytic Efficiency of m6ATP Incorporation by Pol β and Pol η
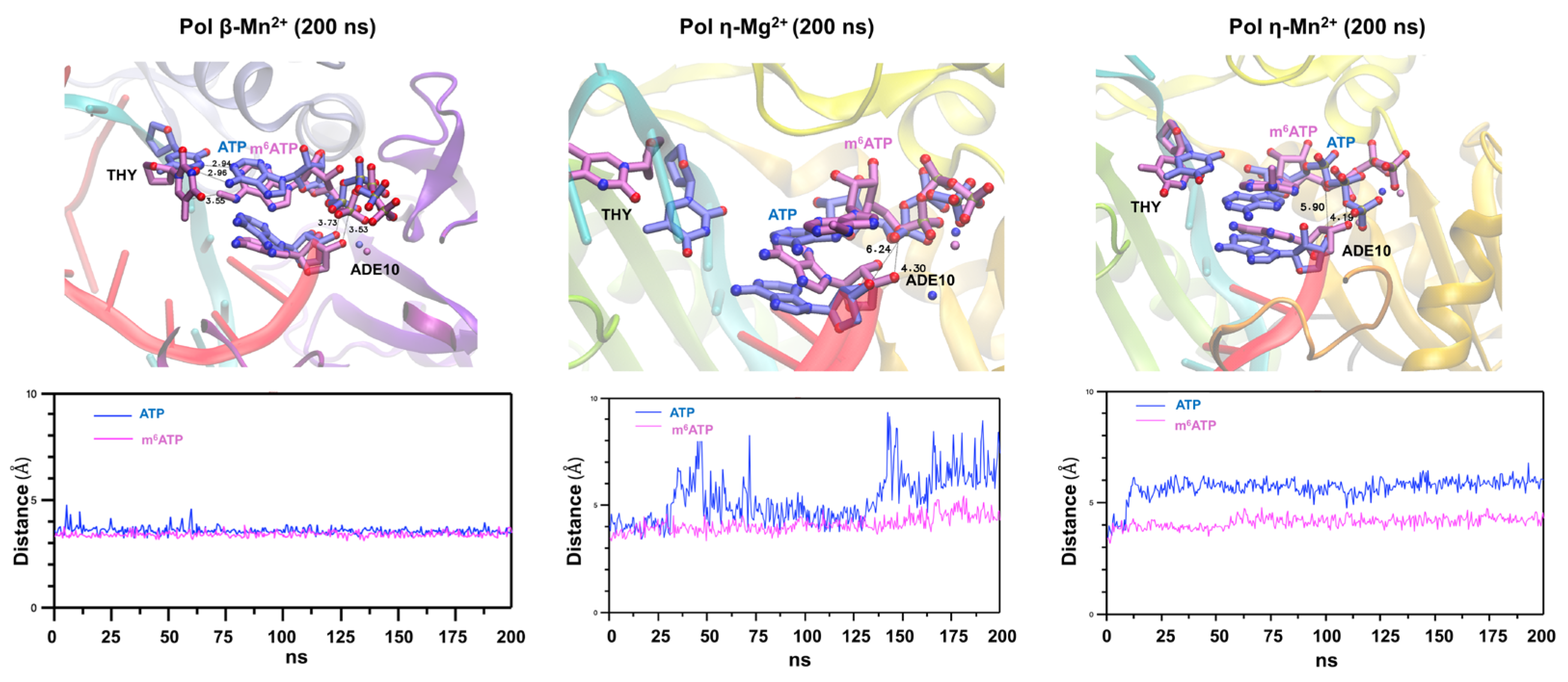
2.4. The Structural Basis of m6ATP RNA Incorporation by Pol β and Pol η in the Presence of Mg2+ and Mn2+
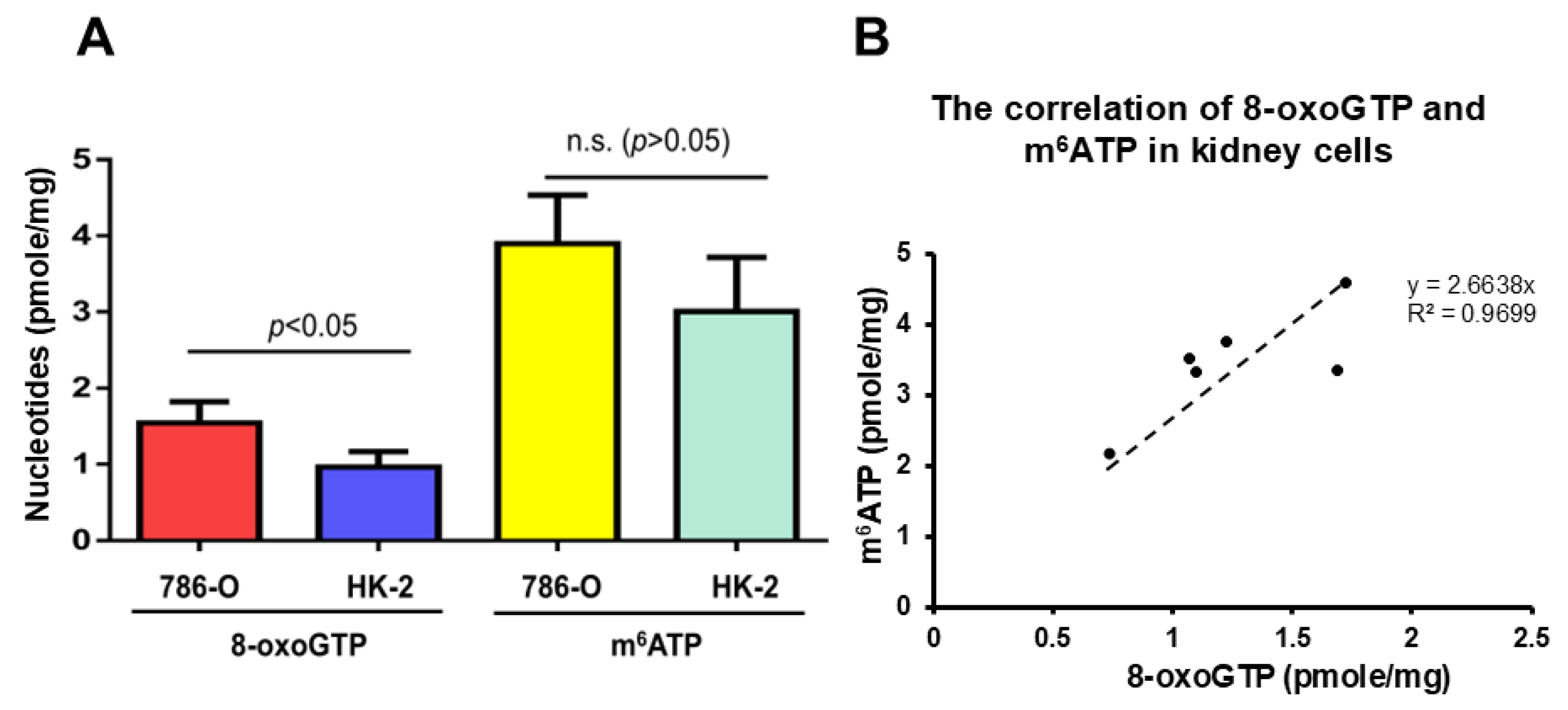
2.5. A Significant Amount of m6ATP Is Detected in Human Normal and Cancer Cells and Is Associated with Cellular Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. RNA-DNA Hybrid Oligonucleotide Substrates
4.3. Determination of RNA Synthesis Activity by DNA Polymerases
4.4. Determination of the Efficiency of ATP and m6ATP Incorporation in RNA by Pol β and Pol η Using Steady-State Kinetics
4.5. Molecular Dynamics Simulation of the Ternary Complexes of Pol β or Pol η-RNA-DNA Hybrid-NTPs
4.6. Cellular m6ATP Level Measurement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.A.; He, C. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Trends Genet. 2016, 32, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; Liu, M.; Ma, J.; Wu, L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 12626. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, Q.; Chen, X.; He, L.; Wang, D.; Su, R.; Xue, Y.; Sun, H.; Wang, H. Nuclear m6A reader YTHDC1 promotes muscle stem cell activation/proliferation by regulating mRNA splicing and nuclear export. eLife 2023, 12, e82703. [Google Scholar] [CrossRef]
- Du, H.; Zou, N.Y.; Zuo, H.L.; Zhang, X.Y.; Zhu, S.C. YTHDF3 mediates HNF1alpha regulation of cervical cancer radio-resistance by promoting RAD51D translation in an m6A-dependent manner. FEBS J. 2023, 290, 1920–1935. [Google Scholar] [CrossRef]
- Lee, K.P.; Liu, K.; Kim, E.Y.; Medina-Puche, L.; Dong, H.; Di, M.; Singh, R.M.; Li, M.; Qi, S.; Meng, Z.; et al. The m6A reader ECT1 drives mRNA sequestration to dampen salicylic acid-dependent stress responses in Arabidopsis. Plant Cell 2024, 36, 746–763. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, Q.; Song, P.; Tian, E.; Yang, J.; Jia, G. The m6A reader ECT8 is an abiotic stress sensor that accelerates mRNA decay in Arabidopsis. Plant Cell 2024, 36, 2908–2926. [Google Scholar] [CrossRef]
- Yin, H.; Ju, Z.; Zheng, M.; Zhang, X.; Zuo, W.; Wang, Y.; Ding, X.; Zhang, X.; Peng, Y.; Li, J.; et al. Loss of the m6A methyltransferase METTL3 in monocyte-derived macrophages ameliorates Alzheimer’s disease pathology in mice. PLoS Biol. 2023, 21, e3002017. [Google Scholar] [CrossRef]
- Wang, J.N.; Wang, F.; Ke, J.; Li, Z.; Xu, C.H.; Yang, Q.; Chen, X.; He, X.Y.; He, Y.; Suo, X.G.; et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci. Transl. Med. 2022, 14, eabk2709. [Google Scholar] [CrossRef]
- Zhou, C.; She, X.; Gu, C.; Hu, Y.; Ma, M.; Qiu, Q.; Sun, T.; Xu, X.; Chen, H.; Zheng, Z. FTO fuels diabetes-induced vascular endothelial dysfunction associated with inflammation by erasing m6A methylation of TNIP1. J. Clin. Investig. 2023, 133, e160517. [Google Scholar] [CrossRef]
- Wang, S.; Nie, J.; Xu, K.; Liu, Y.; Tong, W.; Li, A.; Zuo, W.; Liu, Z.; Yang, F. YY1 is regulated by ALKBH5-mediated m6A modification and promotes autophagy and cancer progression through targeting ATG4B. Aging 2023, 15, 9590–9613. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, S.; Zhang, C.; Jin, Y.; Xu, G.; Zhou, L.; Ding, G.; Pang, T.; Jia, S.; Cao, L. ZC3H13-mediated N6-methyladenosine modification of PHF10 is impaired by fisetin which inhibits the DNA damage response in pancreatic cancer. Cancer Lett. 2022, 530, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Grozhik, A.V.; Olarerin-George, A.O.; Meydan, C.; Mason, C.E.; Jaffrey, S.R. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 2015, 12, 767–772. [Google Scholar] [CrossRef]
- Fu, Y.; Dominissini, D.; Rechavi, G.; He, C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet. 2014, 15, 293–306. [Google Scholar] [CrossRef]
- Flamand, M.N.; Tegowski, M.; Meyer, K.D. The Proteins of mRNA Modification: Writers, Readers, and Erasers. Annu. Rev. Biochem. 2023, 92, 145–173. [Google Scholar] [CrossRef]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.B.; Jaffrey, S.R. 5′ UTR m(6)A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef]
- Brown, J.A.; Suo, Z. Unlocking the sugar “steric gate” of DNA polymerases. Biochemistry 2011, 50, 1135–1142. [Google Scholar] [CrossRef]
- Gao, G.; Orlova, M.; Georgiadis, M.M.; Hendrickson, W.A.; Goff, S.P. Conferring RNA polymerase activity to a DNA polymerase: A single residue in reverse transcriptase controls substrate selection. Proc. Natl. Acad. Sci. USA 1997, 94, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Trincao, J.; Johnson, R.E.; Escalante, C.R.; Prakash, S.; Prakash, L.; Aggarwal, A.K. Structure of the catalytic core of S. cerevisiae DNA polymerase eta: Implications for translesion DNA synthesis. Mol. Cell 2001, 8, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Prakash, S.; Prakash, L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science 1999, 283, 1001–1004. [Google Scholar] [CrossRef]
- Gali, V.K.; Balint, E.; Serbyn, N.; Frittmann, O.; Stutz, F.; Unk, I. Translesion synthesis DNA polymerase eta exhibits a specific RNA extension activity and a transcription-associated function. Sci. Rep. 2017, 7, 13055. [Google Scholar] [CrossRef]
- Balint, E.; Unk, I. Selective Metal Ion Utilization Contributes to the Transformation of the Activity of Yeast Polymerase eta from DNA Polymerization toward RNA Polymerization. Int. J. Mol. Sci. 2020, 21, 8248. [Google Scholar] [CrossRef]
- Balint, E.; Unk, I. Manganese Is a Strong Specific Activator of the RNA Synthetic Activity of Human Poleta. Int. J. Mol. Sci. 2021, 23, 230. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, A.K.; Wang, J.; Konigsberg, W.H. Different Divalent Cations Alter the Kinetics and Fidelity of DNA Polymerases. J. Biol. Chem. 2016, 291, 20869–20875. [Google Scholar] [CrossRef]
- Tholey, G.; Ledig, M.; Mandel, P.; Sargentini, L.; Frivold, A.H.; Leroy, M.; Grippo, A.A.; Wedler, F.C. Concentrations of physiologically important metal ions in glial cells cultured from chick cerebral cortex. Neurochem. Res. 1988, 13, 45–50. [Google Scholar] [CrossRef]
- Tholey, G.; Ledig, M.; Kopp, P.; Sargentini-Maier, L.; Leroy, M.; Grippo, A.A.; Wedler, F.C. Levels and sub-cellular distribution of physiologically important metal ions in neuronal cells cultured from chick embryo cerebral cortex. Neurochem. Res. 1988, 13, 1163–1167. [Google Scholar] [CrossRef]
- Balint, E.; Unk, I. For the Better or for the Worse? The Effect of Manganese on the Activity of Eukaryotic DNA Polymerases. Int. J. Mol. Sci. 2023, 25, 363. [Google Scholar] [CrossRef]
- Vashishtha, A.K.; Konigsberg, W.H. The effect of different divalent cations on the kinetics and fidelity of Bacillus stearothermophilus DNA polymerase. AIMS Biophys. 2018, 5, 125–143. [Google Scholar] [CrossRef]
- Sawyer, D.L.; Sweasy, J.B. DNA Polymerase beta in the Context of Cancer. Crit. Rev. Oncog. 2022, 27, 17–33. [Google Scholar] [CrossRef]
- Beard, W.A.; Wilson, S.H. Structure and mechanism of DNA polymerase Beta. Chem. Rev. 2006, 106, 361–382. [Google Scholar]
- Pelletier, H.; Sawaya, M.R.; Wolfle, W.; Wilson, S.H.; Kraut, J. A structural basis for metal ion mutagenicity and nucleotide selectivity in human DNA polymerase beta. Biochemistry 1996, 35, 12762–12777. [Google Scholar] [CrossRef]
- Reed, A.J.; Vyas, R.; Raper, A.T.; Suo, Z. Structural Insights into the Post-Chemistry Steps of Nucleotide Incorporation Catalyzed by a DNA Polymerase. J. Am. Chem. Soc. 2017, 139, 465–471. [Google Scholar] [CrossRef]
- Weng, P.J.; Gao, Y.; Gregory, M.T.; Wang, P.; Wang, Y.; Yang, W. Bypassing a 8,5′-cyclo-2′-deoxyadenosine lesion by human DNA polymerase eta at atomic resolution. Proc. Natl. Acad. Sci. USA 2018, 115, 10660–10665. [Google Scholar] [CrossRef]
- Yuan, Y.; Stumpf, F.M.; Schlor, L.A.; Schmidt, O.P.; Saumer, P.; Huber, L.B.; Frese, M.; Hollmuller, E.; Scheffner, M.; Stengel, F.; et al. Chemoproteomic discovery of a human RNA ligase. Nat. Commun. 2023, 14, 842. [Google Scholar] [CrossRef] [PubMed]
- Prashar, T.; De La Selle, F.; Hudak, K.A. Abasic RNA: Its formation and potential role in cellular stress response. RNA Biol. 2023, 20, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.L.; Zaher, H.S. How do cells cope with RNA damage and its consequences? J. Biol. Chem. 2019, 294, 15158–15171. [Google Scholar] [CrossRef]
- Cordes, J.; Zhao, S.; Engel, C.M.; Stingele, J. Cellular responses to RNA damage. Cell 2025, 188, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, M.; Liu, Y. RNA damage and its implications in genome stability. DNA Repair 2025, 147, 103821. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, P.S.; Hernandez, D.; Qu, F.; Olatunji, M.; Mamun, Y.; Chapagain, P.; Liu, Y. RNA-guided DNA base damage repair via DNA polymerase-mediated nick translation. Nucleic Acids Res. 2023, 51, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Musheev, M.U.; Baumgartner, A.; Krebs, L.; Niehrs, C. The origin of genomic N(6)-methyl-deoxyadenosine in mammalian cells. Nat. Chem. Biol. 2020, 16, 630–634. [Google Scholar] [CrossRef]
- Conti, B.A.; Novikov, L.; Tong, D.; Xiang, Q.; Vigil, S.; McLellan, T.J.; Nguyen, C.; De La Cruz, N.; Veettil, R.T.; Pradhan, P.; et al. N6-methyladenosine in DNA promotes genome stability. eLife 2025, 13, RP101626. [Google Scholar] [CrossRef] [PubMed]
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef]
- Solenov, E.I. Cell volume and sodium content in rat kidney collecting duct principal cells during hypotonic shock. J. Biophys. 2008, 2008, 420963. [Google Scholar] [CrossRef]
- Liu, Y.; Rodriguez, Y.; Ross, R.L.; Zhao, R.; Watts, J.A.; Grunseich, C.; Bruzel, A.; Li, D.; Burdick, J.T.; Prasad, R.; et al. RNA abasic sites in yeast and human cells. Proc. Natl. Acad. Sci. USA 2020, 117, 20689–20695. [Google Scholar] [CrossRef]
- Kahali, S.; Das, S.K.; Kumar, R.; Gupta, K.; Kundu, R.; Bhattacharya, B.; Nath, A.; Venkatramani, R.; Datta, A. A water-soluble, cell-permeable Mn(ii) sensor enables visualization of manganese dynamics in live mammalian cells. Chem. Sci. 2024, 15, 10753–10769. [Google Scholar] [CrossRef]
- Tsegay, P.S.; Hernandez, D.; Brache, C.; Chatgilialoglu, C.; Krokidis, M.G.; Chapagain, P.; Liu, Y. Incorporation of 5′,8-cyclo-2′deoxyadenosines by DNA repair polymerases via base excision repair. DNA Repair 2022, 109, 103258. [Google Scholar] [CrossRef]
- Shimizu, M.; Gruz, P.; Kamiya, H.; Masutani, C.; Xu, Y.; Usui, Y.; Sugiyama, H.; Harashima, H.; Hanaoka, F.; Nohmi, T. Efficient and erroneous incorporation of oxidized DNA precursors by human DNA polymerase eta. Biochemistry 2007, 46, 5515–5522. [Google Scholar] [CrossRef]
- Beaver, J.M.; Lai, Y.; Xu, M.; Casin, A.H.; Laverde, E.E.; Liu, Y. AP endonuclease 1 prevents trinucleotide repeat expansion via a novel mechanism during base excision repair. Nucleic Acids Res. 2015, 43, 5948–5960. [Google Scholar] [CrossRef]
- Cavanaugh, N.A.; Beard, W.A.; Wilson, S.H. DNA polymerase beta ribonucleotide discrimination: Insertion, misinsertion, extension, and coding. J. Biol. Chem. 2010, 285, 24457–24465. [Google Scholar] [CrossRef]
- Su, Y.; Egli, M.; Guengerich, F.P. Mechanism of Ribonucleotide Incorporation by Human DNA Polymerase eta. J. Biol. Chem. 2016, 291, 3747–3756. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Zhao, Y.; Gregory, M.T.; Biertumpfel, C.; Hua, Y.J.; Hanaoka, F.; Yang, W. Mechanism of somatic hypermutation at the WA motif by human DNA polymerase eta. Proc. Natl. Acad. Sci. USA 2013, 110, 8146–8151. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L., 3rd; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; MacKerell, A.D., Jr. Force field development and simulations of intrinsically disordered proteins. Curr. Opin. Struct. Biol. 2018, 48, 40–48. [Google Scholar] [CrossRef]
- Nose, S.; Klein, M.L. Constant-temperature-constant-pressure molecular-dynamics calculations for molecular solids: Application to solid nitrogen at high pressure. Phys. Rev. B Condens. Matter 1986, 33, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Jiang, H.P.; Xiong, J.; Liu, F.L.; Ma, C.J.; Tang, X.L.; Yuan, B.F.; Feng, Y.Q. Modified nucleoside triphosphates exist in mammals. Chem. Sci. 2018, 9, 4160–4167. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, F.; Fuente, J.; Chapagain, P.; Liu, Y. Unscheduled m6A Deposition in RNA via m6ATP Incorporation by DNA Polymerases. Int. J. Mol. Sci. 2025, 26, 9263. https://doi.org/10.3390/ijms26199263
Qu F, Fuente J, Chapagain P, Liu Y. Unscheduled m6A Deposition in RNA via m6ATP Incorporation by DNA Polymerases. International Journal of Molecular Sciences. 2025; 26(19):9263. https://doi.org/10.3390/ijms26199263
Chicago/Turabian StyleQu, Fei, Jeanpierre Fuente, Prem Chapagain, and Yuan Liu. 2025. "Unscheduled m6A Deposition in RNA via m6ATP Incorporation by DNA Polymerases" International Journal of Molecular Sciences 26, no. 19: 9263. https://doi.org/10.3390/ijms26199263
APA StyleQu, F., Fuente, J., Chapagain, P., & Liu, Y. (2025). Unscheduled m6A Deposition in RNA via m6ATP Incorporation by DNA Polymerases. International Journal of Molecular Sciences, 26(19), 9263. https://doi.org/10.3390/ijms26199263






