In Silico and In Vitro Studies of Anti-Inflammatory, Anti-Oxidative Stress, and Anti-Apoptosis Effect of 7-Octenoic Acid Derived from Moringa oleifera Lam., on LPS-Induced Monocyte-Derived Macrophages (MDM)
Abstract
1. Introduction
2. Results
2.1. The Effect of 7-Octenoic Acid (7OCT) on Cell Viability
2.2. Prediction of Interactions Between 7OCT and Protein Target Using Molecular Docking Analysis
2.3. The Effect of 7OCT on the Expression of Pro-Inflammatory Cytokine Production in LPS-Stimulated Macrophages
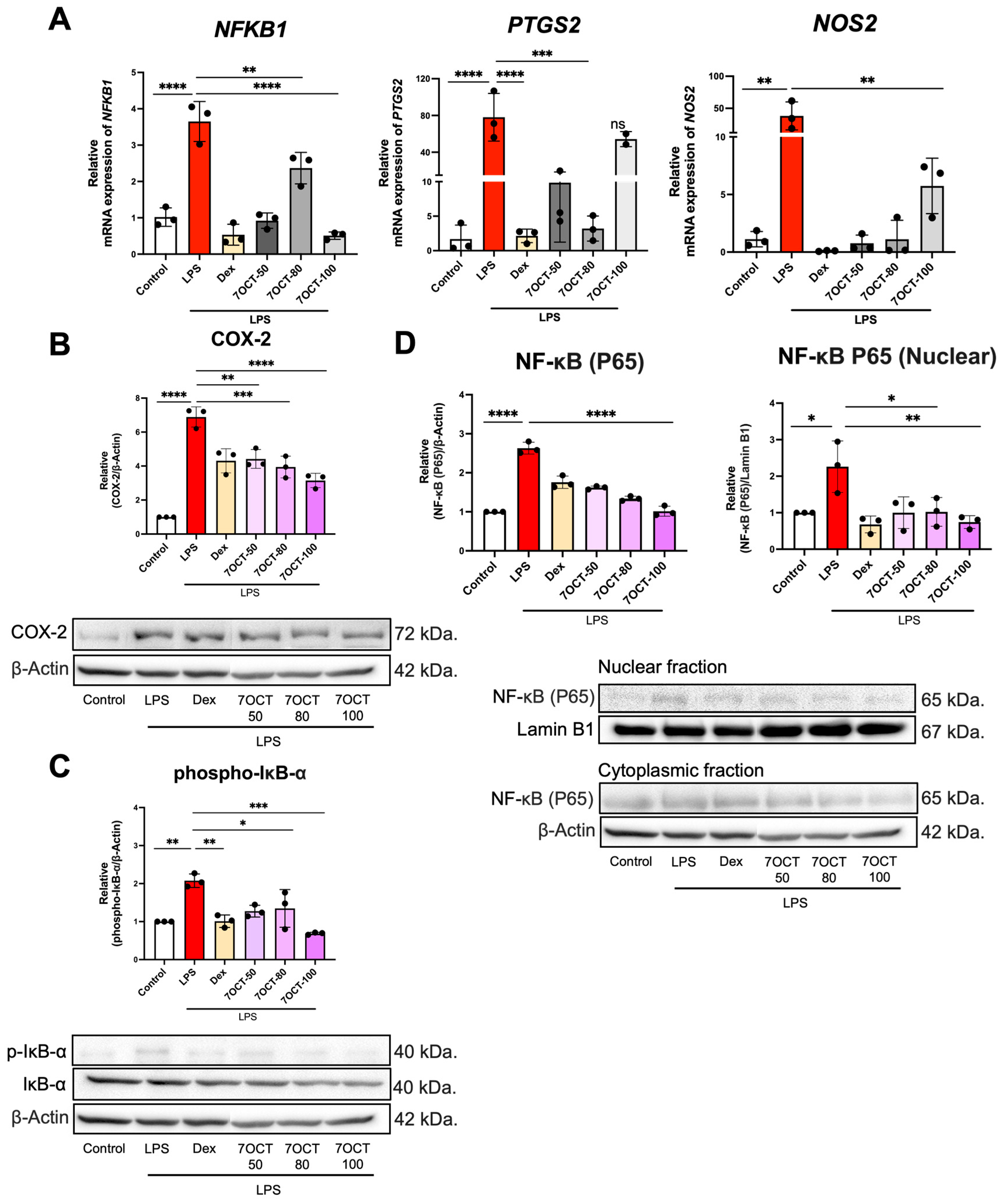
2.4. The Effect of 7OCT on the NF-κB Pathway in LPS-Stimulated Macrophages
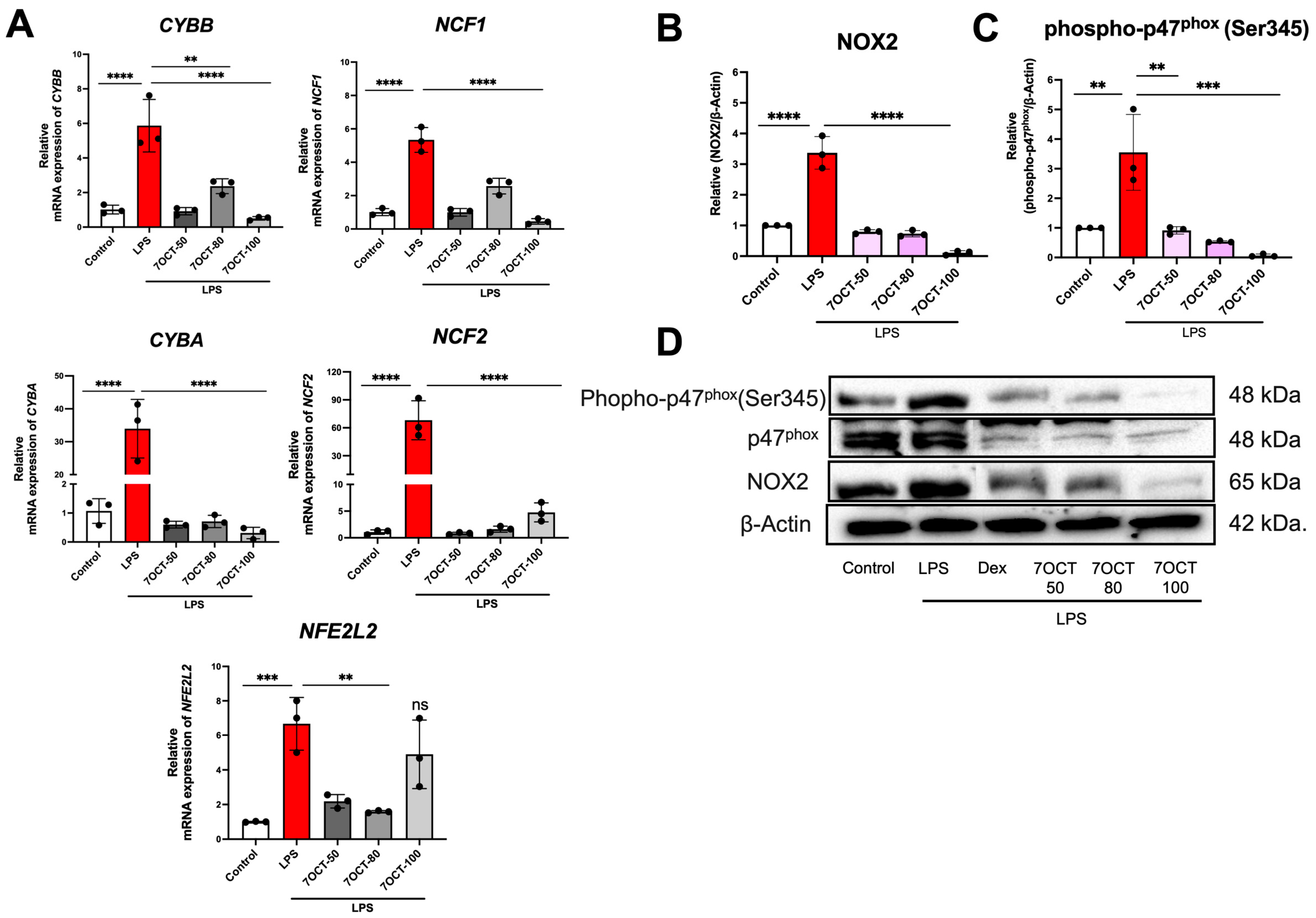
2.5. The Effect of 7OCT on NADPH Oxidase 2 (NOX2) Pathway in LPS-Stimulated Macrophages
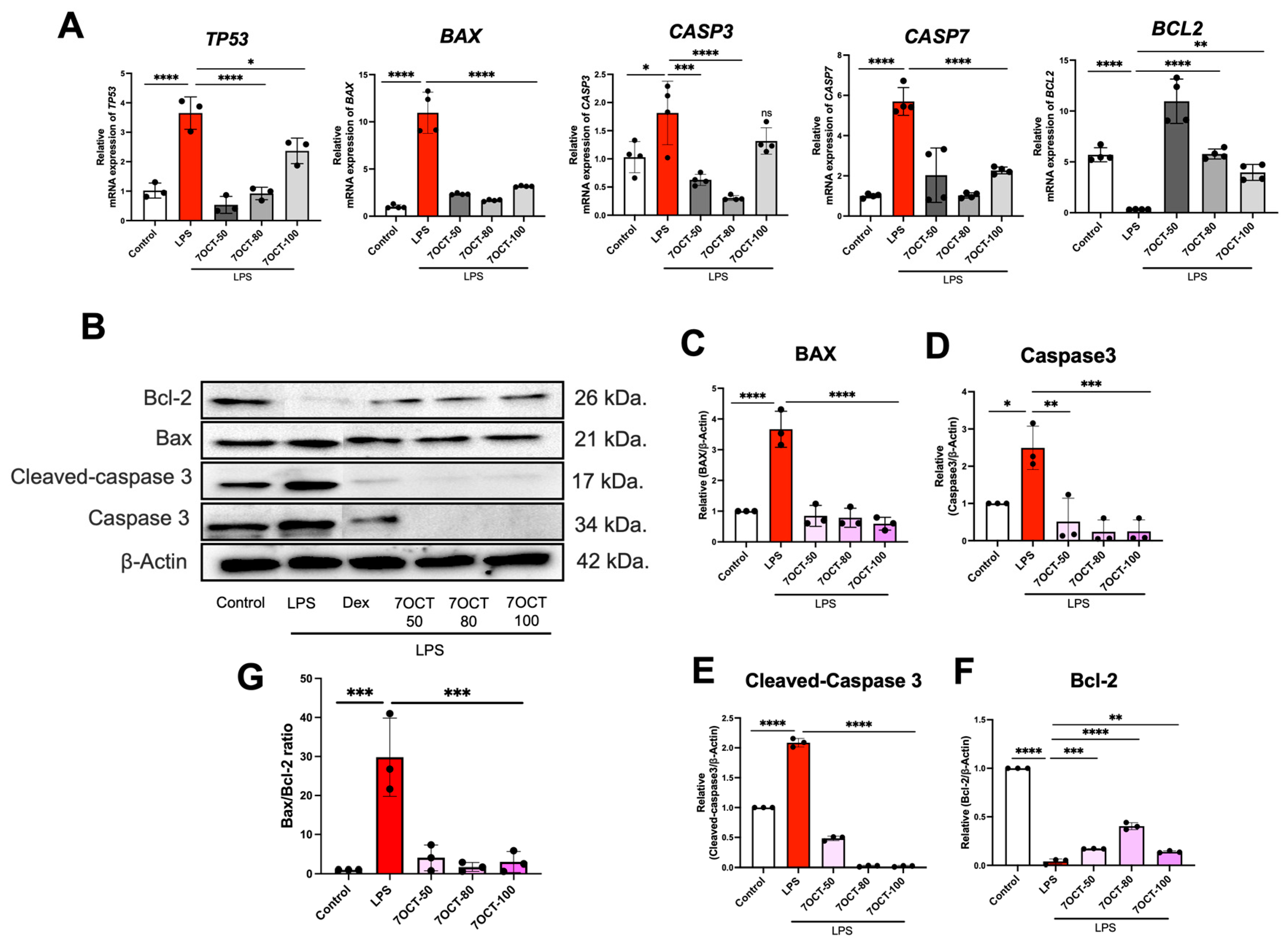
2.6. The Effect of 7OCT on Apoptosis in LPS-Stimulated Macrophages
3. Discussion
4. Materials and Methods
4.1. Cell Differentiation and Culture
4.2. Cytotoxicity Assessment by MTT Assay
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
4.5. Western Blot Analysis
4.6. Molecular Docking Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013, 34, 216–223. [Google Scholar] [CrossRef]
- Bao, Z.; Guan, S.; Cheng, C.; Wu, S.; Wong, S.H.; Kemeny, D.M.; Leung, B.P.; Wong, W.S.F. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathway. Am. J. Respir. Crit. Care Med. 2009, 179, 657–665. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; A Willment, J.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Pagan, L.U.; Gomes, M.J.; Gatto, M.; Mota, G.A.F.; Okoshi, K.; Okoshi, M.P. The role of oxidative stress in the aging heart. Antioxidants 2022, 11, 336. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Nauseef, W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004, 122, 277–291. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Singh, A.; Zarember, K.A.; Kuhns, D.B.; Gallin, J.I. Impaired priming and activation of the neutrophil NADPH oxidase in patients with IRAK4 or NEMO deficiency. J. Immunol. 2009, 182, 6410–6417. [Google Scholar] [CrossRef]
- Kowluru, A. Friendly, and not so friendly, roles of Rac1 in islet β-cell function: Lessons learnt from pharmacological and molecular biological approaches. Biochem. Pharmacol. 2011, 81, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Yamamoto, T.; Amemiya, T.; Kanamura, N.; Nishikawa, T.; Okamura, T.; Tanaka, A.; Sumitomo, S.; Shikimori, M. The functions of nerve growth factor and nerve growth factor receptor in wound healing. Oral Med. Pathol. 2012, 16, 51–65. [Google Scholar] [CrossRef][Green Version]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lane, D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Nadendla, E.K.; Tweedell, R.E.; Kasof, G.; Kanneganti, T.-D. Caspases: Structural and molecular mechanisms and functions in cell death, innate immunity, and disease. Cell Discov. 2025, 11, 42. [Google Scholar] [CrossRef]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Kao, C.-L.; Liu, C.-M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Cicio, A.; Serio, R.; Zizzo, M.G. Anti-inflammatory potential of Brassicaceae-derived phytochemicals: In vitro and in vivo evidence for a putative role in the prevention and treatment of IBD. Nutrients 2022, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Srivastav, A.K. Phytochemical extraction and characterization of the leaves of Andrographis paniculata for its anti-bacterial, anti-oxidant, anti-pyretic and anti-diabetic activity. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15176–15184. [Google Scholar] [CrossRef]
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013, 136, 237–244. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Vishwakarma, S.; Singh, M.P. Elemental analysis, phytochemical screening and evaluation of antioxidant, antibacterial and anticancer activity of Pleurotus ostreatus through in vitro and in silico approaches. Metabolites 2022, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, S.; Grabarska, A.; Kukula-Koch, W. The potential anticancer activity of phytoconstituents against gastric cancer—A review on in vitro, in vivo, and clinical studies. Int. J. Mol. Sci. 2020, 21, 8307. [Google Scholar] [CrossRef] [PubMed]
- Jongrungruangchok, S.; Bunrathep, S.; Songsak, T. Nutrients and minerals content of eleven different samples of Moringa oleifera cultivated in Thailand. J. Health Res. 2010, 24, 123–127. [Google Scholar]
- Chiș, A.; Noubissi, P.A.; Pop, O.-L.; Mureșan, C.I.; Tagne, M.A.F.; Kamgang, R.; Fodor, A.; Sitar-Tăut, A.-V.; Cozma, A.; Orășan, O.H.; et al. Bioactive compounds in Moringa oleifera: Mechanisms of action, focus on their anti-inflammatory properties. Plants 2023, 13, 20. [Google Scholar] [CrossRef]
- Mohammad Shafie, N.; Shah, R.N.I.R.S.; Krishnan, P.; Haleem, N.A.; Tan, T.Y.C. Scoping review: Evaluation of Moringa oleifera (Lam.) for potential wound healing in In Vivo studies. Molecules 2022, 27, 5541. [Google Scholar] [CrossRef]
- Yousefirad, A.; Rastegari, A.A.; Shahanipour, K.; Monajemi, R. Evaluation of Proliferative inhibition effect of Moringa oleifera total extract on breast cancer: An in vitro and in vivo study. Iran. J. Sci. 2023, 47, 653–662. [Google Scholar] [CrossRef]
- Muthu, T.; Adusumalli, R.; Vemuri, S.K.; Devi, M.I.; Kumar, P.P.; Banala, R.R.; Reddy, A.V.G. Eco-biofabrication of silver nanoparticles from Azadirachta indica, Gymnema sylvestre, and Moringa oleifera for lung cancer treatment. J. Egypt. Natl. Cancer Inst. 2025, 37, 1. [Google Scholar] [CrossRef]
- de Barros, M.C.; de Souza Silva, V.C.; Silva, A.G.B.; Neto, J.D.C.S.; de Lima Maux, J.M.; Dos Santos, P.É.M.; Almeida Oliveira, W.d.; Barroso Coelho, L.C.B.; Fernandes, M.P.; de Oliveira, A.M.; et al. Antioxidant activity and chemoprotective effect of Moringa oleifera Lam. leaf infusion against methotrexate-induced damage in mice. Toxicol. Rep. 2025, 14, 102000. [Google Scholar] [CrossRef]
- Shaju, A.; Thomas, A.; Roselin, P. Antimicrobial Activity of Azadirachta indica and Moringa oleifera on oral biofilm forming bacteria—A comparative study. In Environmental Biology: Advanced Research and Multidisciplinary Applications; JPS Scientific Publications: Olaipadi, India, 2025; p. 121. [Google Scholar]
- Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In vitro bioassay-guided identification of anticancer properties from Moringa oleifera Lam. leaf against the MDA-MB-231 cell line. Pharmaceuticals 2020, 13, 464. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Zhang, Y.; Zhang, Y.; Tang, H.; Yue, H.; Zhang, L.; Song, D. Macrophages in the inflammatory response to endotoxic shock. Immun. Inflamm. Dis. 2024, 12, e70027. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia responses to pro-inflammatory stimuli (LPS, IFNγ + TNFα) and reprogramming by resolving cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Chai, Y.; Wang, Y.; Zhang, J.; Chen, X. Astrocyte-mediated inflammatory responses in traumatic brain injury: Mechanisms and potential interventions. Front. Immunol. 2025, 16, 1584577. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.L.; Carmody, R.J. The regulation of NF-κB subunits by phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Jannati, S.; Patel, A.; Patnaik, R.; Banerjee, Y. Oleocanthal as a Multifunctional Anti-Cancer Agent: Mechanistic Insights, Advanced Delivery Strategies, and Synergies for Precision Oncology. Int. J. Mol. Sci. 2025, 26, 5521. [Google Scholar] [CrossRef]
- Volpp, B.D.; Nauseef, W.M.; Clark, R.A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 1988, 242, 1295–1297. [Google Scholar] [CrossRef]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
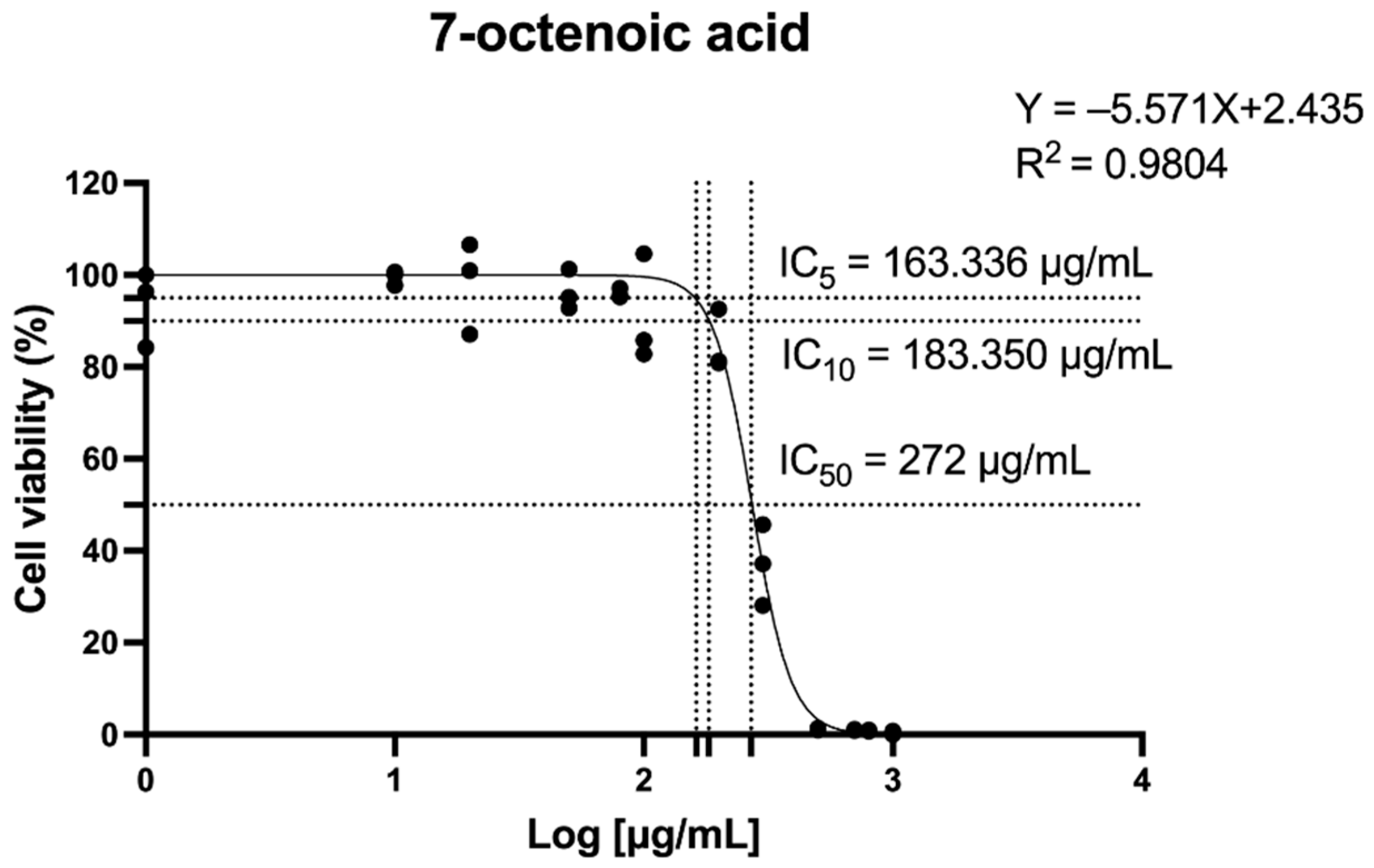
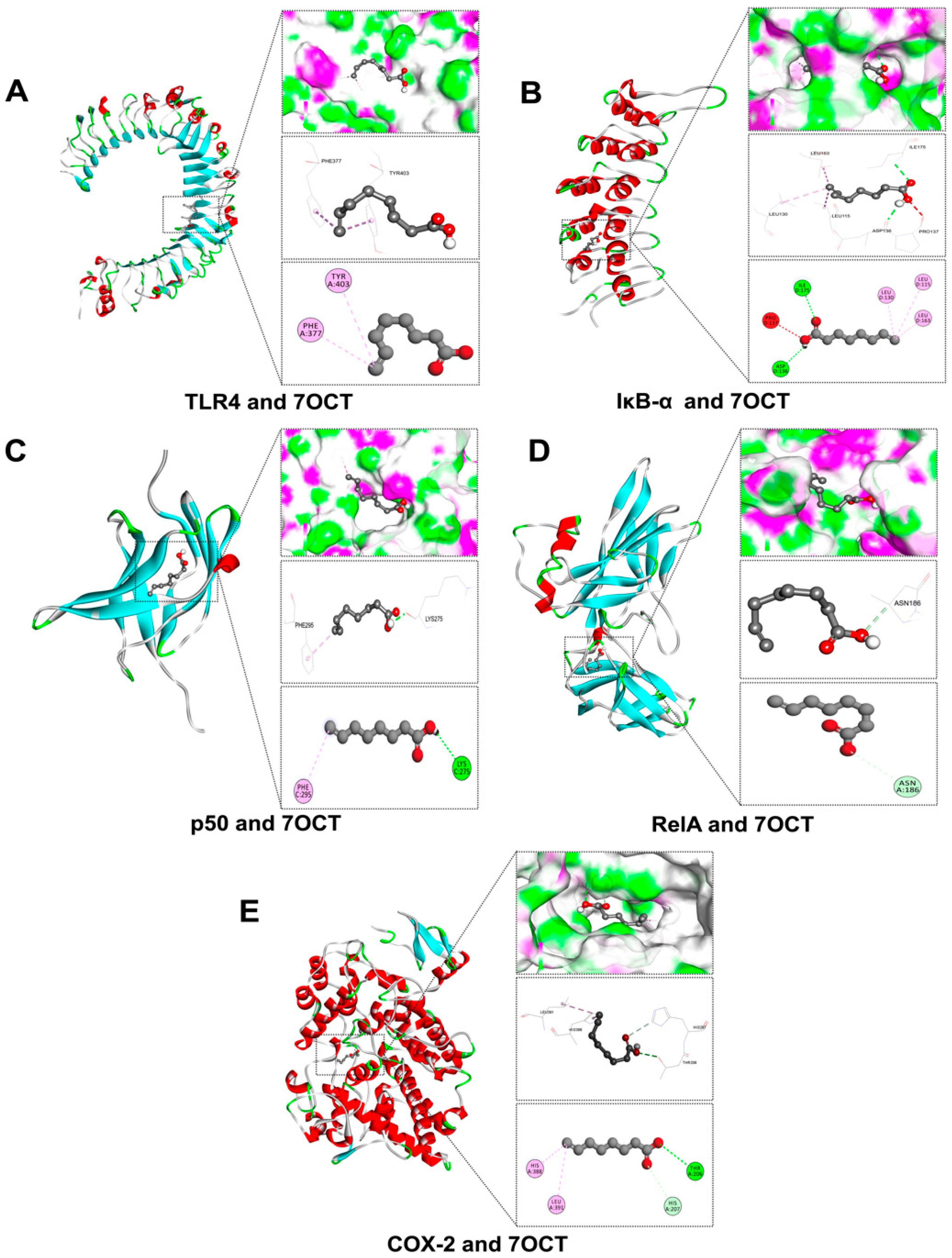
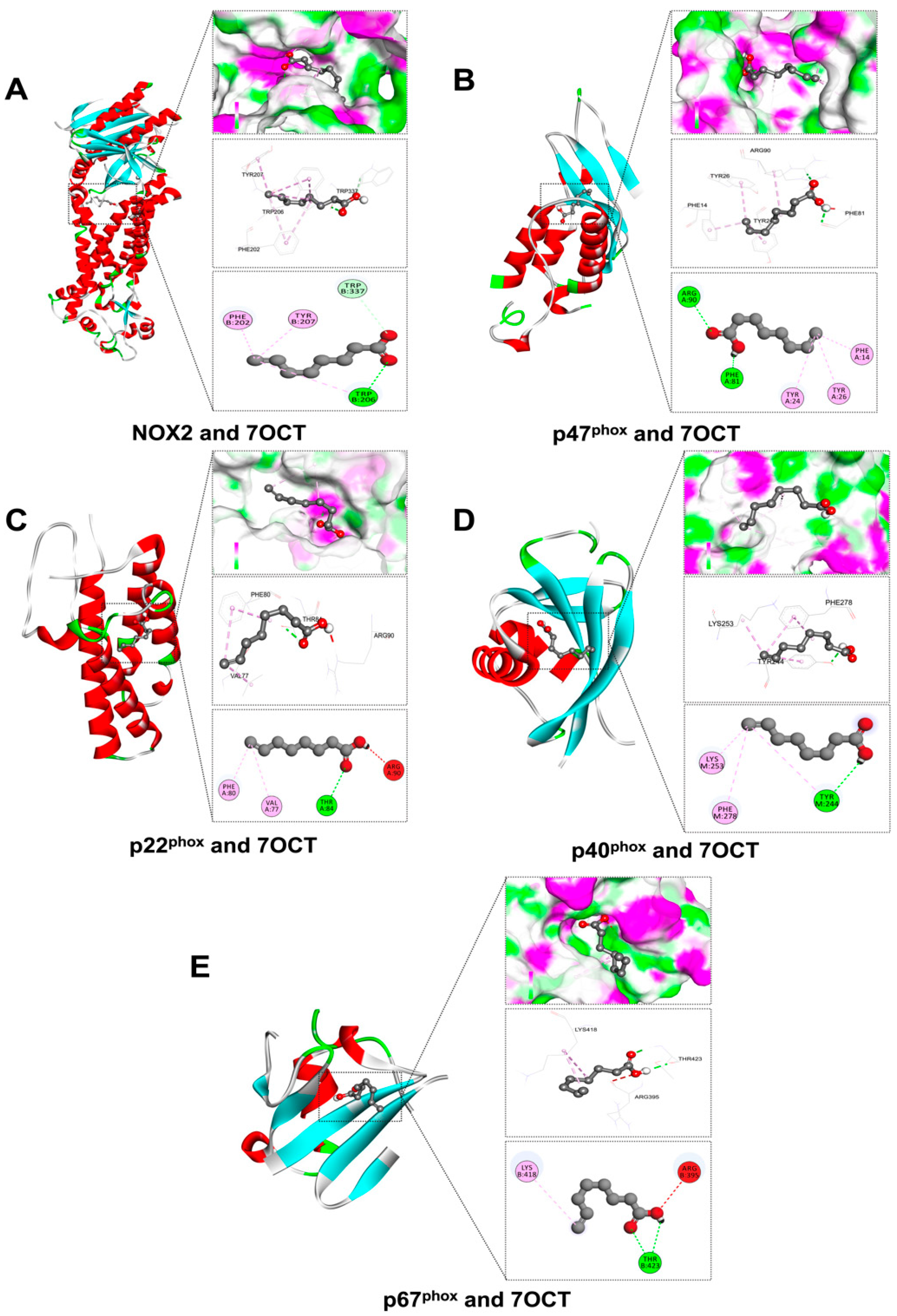
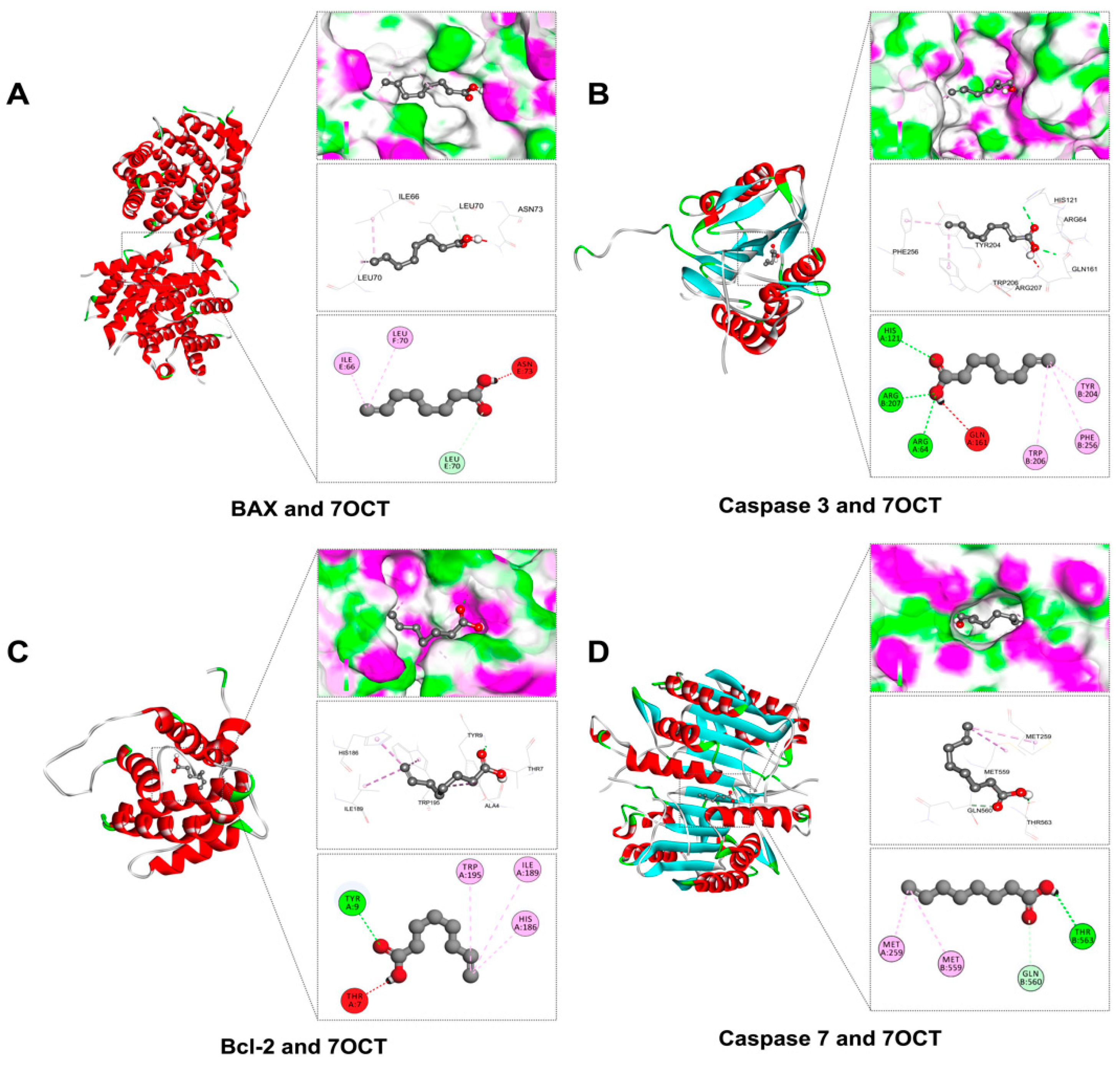

| Amino Acid Residues | Docking Score (Kcal/mol) | Protein Name | Compound |
|---|---|---|---|
| HIS207, HIS388, LEU391, THR206 | −5.5 | Cyclooxygenase-2 (COX-2) | 7-Octenoic acid |
| ASN186 | −5.0 | v-rel avian reticuloendotheliosis viral oncogene homolog A (RelA) | |
| ASP136, ILE175, LEU115, LEU130, LEU163, PRO137 | −4.6 | Inhibitor of nuclear factor kappa-B alpha (IκB-α) | |
| LYS275, PHE295 | −4.5 | Nuclear factor NF-κB p50 Subunit (p50) | |
| PHE377, TYR403 | −3.7 | Toll-like receptor 4 (TLR4) | |
| PHE202, TRP207, TRP337, TYR207 | −5.9 | NADPH oxidase 2 (NOX2) | |
| ARG90, PHE14, PHE81, TYR24, TYR26 | −5.4 | Neutrophil cytosolic factor 1 (p47phox) | |
| ARG90, PHE80, THR84, VAL77 | −4.9 | Cytochrome b-245 alpha chain (p22phox) | |
| LYS253, PHE27, TYR244 | −4.2 | Neutrophil cytosolic factor 4 (p40phox) | |
| ARG395, LYS418, THR423 | −4.0 | Neutrophil cytosolic factor 2 (p67phox) | |
| ASN73, ILE66, IEU70 | −5.0 | BCL2-associated X, apoptosis regulator (BAX) | |
| ARG64, ARG207, GLN161, HIS121, PHE256, TRP206, TYR204 | −4.8 | Caspase 3 | |
| HIS186, ILE189, TRP195, THR7, TYR9 | −4.5 | B-cell lymphoma 2 (Bcl-2) | |
| GLN560, MET259, ME559, THR563 | −4.2 | Caspase 7 |
| No | Primer | Description | Forward Primer (5′-3′) | Reward Primer (5′-3′) |
|---|---|---|---|---|
| 1 | NFKB1 | Nuclear Factor Kappa B Subunit 1 | AACAGAGAGGATTTCGTTTCCG | TTTGACCTGAGGGTAAGACTTCT |
| 2 | PTGS2 | Prostaglandin-Endoperoxide Synthase 2 | CTGGCGCTCGACCATACAG | CGCACTTATACTGGTCAAATCCC |
| 3 | NOS2 | Nitric Oxide Synthase 2 | CAGGGTGTTGCCCAAACTG | GGCTGCGTTCTTCTTTGCT |
| 5 | CYBB | Cytochrome b-245 Beta Chain | GCTGGTGGGTCATCAGGAAA | TGAGCAGCACGCACTGGA |
| 6 | NCF1 | Neutrophil Cytosolic Factor 1 | CCTGCAACTACCTTGAACCAGTT | GCCCTGACTTTTGCAGGTACA |
| 7 | NCF2 | Neutrophil Cytosolic Factor 2 | CCTGCAACTACCTTGAACCAGTT | GGACTGCGGAGAGCTTTCC |
| 8 | TP53 | Tumor Protein p53 | GTGGTAATCTACTGGGACGGA | CTTTCTTGCGGAGATTCTCTTC |
| 9 | BAX | BCL2-Associated X, Apoptosis Regulator | GGTTGTCGCCCTTTTCTA | CGGAGGAAGTCCAATGTC |
| 10 | CASP3 | Caspase 3 | GCTATTGTAGGCGGTTGT | TGTTTCCCTGAGGTTTGC |
| 11 | CASP7 | Caspase 7 | ACTGCTCTTGTGCCAAGATG | CATGGCTTAAGAGGATGCAG |
| 12 | BCL2 | B-Cell Lymphoma 2 | GATGTGATGCCTCTGCGAAG | CATGCTGATGTCTCTGGAATCT |
| 13 | GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | GGTGGTCTCCTCTGACTTCAACA | GTTGCTGTAGCCAAATTCGTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srimuang, K.; Buakaew, W.; Thongsri, Y.; Daowtak, K.; Potup, P.; Ferrante, A.; Usuwanthim, K. In Silico and In Vitro Studies of Anti-Inflammatory, Anti-Oxidative Stress, and Anti-Apoptosis Effect of 7-Octenoic Acid Derived from Moringa oleifera Lam., on LPS-Induced Monocyte-Derived Macrophages (MDM). Int. J. Mol. Sci. 2025, 26, 8911. https://doi.org/10.3390/ijms26188911
Srimuang K, Buakaew W, Thongsri Y, Daowtak K, Potup P, Ferrante A, Usuwanthim K. In Silico and In Vitro Studies of Anti-Inflammatory, Anti-Oxidative Stress, and Anti-Apoptosis Effect of 7-Octenoic Acid Derived from Moringa oleifera Lam., on LPS-Induced Monocyte-Derived Macrophages (MDM). International Journal of Molecular Sciences. 2025; 26(18):8911. https://doi.org/10.3390/ijms26188911
Chicago/Turabian StyleSrimuang, Kittipong, Watunyoo Buakaew, Yordhathai Thongsri, Krai Daowtak, Pachuen Potup, Antonio Ferrante, and Kanchana Usuwanthim. 2025. "In Silico and In Vitro Studies of Anti-Inflammatory, Anti-Oxidative Stress, and Anti-Apoptosis Effect of 7-Octenoic Acid Derived from Moringa oleifera Lam., on LPS-Induced Monocyte-Derived Macrophages (MDM)" International Journal of Molecular Sciences 26, no. 18: 8911. https://doi.org/10.3390/ijms26188911
APA StyleSrimuang, K., Buakaew, W., Thongsri, Y., Daowtak, K., Potup, P., Ferrante, A., & Usuwanthim, K. (2025). In Silico and In Vitro Studies of Anti-Inflammatory, Anti-Oxidative Stress, and Anti-Apoptosis Effect of 7-Octenoic Acid Derived from Moringa oleifera Lam., on LPS-Induced Monocyte-Derived Macrophages (MDM). International Journal of Molecular Sciences, 26(18), 8911. https://doi.org/10.3390/ijms26188911







