Unlocking Novel Therapeutic Potential of Angiotensin II Receptor Blockers
Abstract
1. Introduction
1.1. Introducing Drug Repurposing
1.2. RAAS Physiology
1.3. Multi-Target Directed Ligand (MTDL) Approach
2. Establishing Drug Repurposing: The Example of Sildenafil
3. Repurposing Angiotensin II Receptor Blockers (ARBs)
3.1. Hypertension
3.2. Heart Failure
3.3. Chronic Kidney Disease
- Ang II acting via AT1R activates signaling cascades—MAPK (Mitogen-activated protein kinase)/ERK (Extracellular signal-regulated kinase), JNK (c-Jun N-terminal kinase), STAT (Signal transducer and activator of transcription), NF-κB (Nuclear factor kappa light chain enhancer of activated B cells), and Activator Protein (AP)-1—to drive fibrosis, inflammation, cell proliferation, and proteinuria in CKD. AT2 receptors counter these effects via inhibitory signaling [162];
- Ang II as a renal growth factor, stimulates proliferation of mesangial/tubular cells and fibroblasts, promoting extracellular matrix (ECM) accumulation and Transforming Growth Factor (TGF)-β induction. RAS blockade (ACE inhibitors, AT1R antagonists) prevents proteinuria, fibrosis, and inflammatory infiltration [162]; and
- On podocytes—a key filtration-cell type—Ang II causes cytoskeletal disruption, ROS production, and apoptosis, driving podocytopathy and glomerulosclerosis. RAS blockade protects structurally and functionally [163].
3.4. Acute Coronary Syndrome
- Systemic and local RAS activation drives remodeling and worsens outcomes post–MI (myocardial infarction) [168];
- Ang II activates NADPH (Nicotinamide Adenine Dinucleotide Phosphate) oxidase, causing oxidative injury and atherosclerosis [169];
- Ang II, AT1R, and ACE co-localize in plaques, promoting interleukin IL-6 release and instability [170];
- ARBs post-MI upregulate ACE2/Ang (1–7)/MAS (MAS proto-oncogene) and inhibit fibrosis [171]; and
- The ACE2/Ang (1–7)/MAS axis mitigates ischemia–reperfusion injury (IRI) via anti-inflammatory, antioxidant signaling [172].
3.5. Alzheimer’s Disease
- Ang II via AT1R increases amyloid-β (Aβ) by upregulating APP mRNA, β-secretase activity, and presenilin expression; it also promotes tau phosphorylation and reactive oxygen species (ROS) generation [184];
- AT1R activation contributes to neuroinflammation, oxidative stress, Aβ accumulation, all implicated in AD pathogenesis [185];
- Overactivation of the Ang II/AT1R axis leads to blood–brain barrier (BBB) disruption, and neurotoxicity [186],
- Brain aging shows an imbalance favoring renin/ACE1/Ang II/AT1R activation, contributing to cognitive decline and neuroinflammation [187];
- Ang II/AT1R-mediated vasoconstriction impairs neurovascular coupling, undermining cerebrovascular function [188]; and
- Hyperactivation of AT1Rs has been shown to induce NADPH oxidase activity that leads to ROS production, thereby prompting oxidative stress, a pathway activated by Aβ in AD [180].
3.6. Parkinson’s Disease
- Ang II induces dopaminergic neuron apoptosis via NADPH oxidase–mediated ROS [193];
- Overactivation of Ang II/AT1R exacerbates neurodegeneration in PD models [194];
- Brain RAS–dopamine dysregulation promotes neuroinflammation and degeneration [195]; and
- Local RAS in substantia nigra increases vulnerability to degeneration [195].
3.7. Anxiety
- Overactivation of the RAS—particularly through AT1R—drives HPA (Hypothalamic–Pituitary–Adrenal) axis hyperactivity, resulting in anxiety-like behaviors. In contrast, AT1R blockers exert anxiolytic effects by normalizing RAS and HPA activity [205],
- Ang II via AT1R is localized to stress-sensitive brain regions (e.g., hypothalamus) and has been shown to stimulate CRH (Corticotropin-releasing hormone) production, AVP (arginine vasopressin) release, and adrenal catecholamine output, thereby amplifying stress responses [206]; and
- Stress-induced high Ang II levels cause anxiogenesis via AT1R, and AT2R appears to mediate anxiolytic effects. This suggests that AT2R agonism may counterbalance AT1R-driven anxiety [207].
3.8. Cancer Glioma
- Glioblastoma cells express renin, angiotensinogen, renin receptor, ACE, AT1R, AT2R, and renin inhibition induces apoptosis [213];
- Losartan decreases glioma growth, angiogenic factors, increases apoptosis [214];
- In a rat glioblastoma (C6 glioma) model, Ang (1–7) inhibited the JNK (c-Jun N-terminal kinase) pathway, which is activated by GBM and known to disrupt tight junction proteins. Blocking JNK preserved endothelial junction integrity, reduced vascular leak, and limited tumor-induced edema [215].
3.9. Pathogenic Inflammation
3.10. Candidosis
3.11. Fibrosis
- Ang II acts through AT1R leading to TGF-β/Smad (Suppressor of Mothers against Decapentaplegic) activation, ROS, inflammation [225];
- Ang (1–7), acting through the MAS receptor, inhibits fibrosis, reduces inflammation, restores tissue integrity [226]; and
- In liver fibrosis, AT2R is upregulated and exerts antifibrotic effects by inhibiting the IRE1α-XBP1 (Inositol-Requiring Enzyme 1 alpha-X-Box Binding Protein 1) pathway [227].
3.12. Tissue Fibrosis in Systemic Sclerosis
3.13. Diabetic Peripheral Neuropathy
3.14. Inflammatory Bowel Diseases
3.15. Marfan Syndrome
- AT1R blockade (losartan) in MS mice prevents aneurysm, reverses pathology via TGF-β/Smad suppression [240];
- AT2R plays a pivotal role for full therapeutic effect; required for ERK inhibition [241];
- Losartan restored proper muscle regeneration in fibrillin-1–deficient mice by antagonizing TGF-β. This demonstrates that AT1R blockade alleviates systemic manifestations of MS (e.g., myopathy, lung architecture defects), not only vascular issues [242]; and
- Ang II/AT1R signaling activates ERK1/2 pathways and TGF-β/Smad, driving extracellular matrix degradation and aneurysm formation [243].
3.16. SARS-CoV-2
3.17. Rheumatoid Arthritis
3.18. Osteoarthritis
3.19. Opioid Addiction
4. Novel Synthetic AT1 Antagonists and Dual Inhibitors
| Structures of Bioactive Compounds | Biological Evaluation |
|---|---|
| Group A: small non-peptide molecules | |
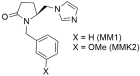 | Significant antihypertensive activity (MM1: 71% and MMK2: 80% when compared to losartan defined as 100% losartan) when injected to anesthetized rabbits made hypertensive by Ang II infusion. In vitro experiments showed that compounds MM1 and MMK2 exhibited negligible activity compared to the reference drug losartan [284,285]. |
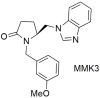 | Antihypertensive activity (MMK3: 48% when compared to losartan defined as 100% losartan) when injected to anesthetized rabbits made hypertensive by Ang II infusion. In vitro experiments showed that compound MMK3 exhibited negligible activity compared to the reference drug losartan [285]. |
| Group B: sartan derivatives | |
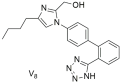 | In vitro binding studies; V8 exhibits affinity in the nanomolar range similar to losartan for the AT1 receptor (V8: IC50 = 53.8 ± 6.4 nM and losartan; IC50 = 16.4 ± 1.6 nM). V8 is a selective AT1 antagonist [286,287]. |
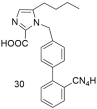 | Higher binding affinity of compound 30 compared to losartan (30: −logIC50 = 8.46; and losartan: −logIC50 = 8.25). Importance of carboxy group at the C-2 position [288]. |
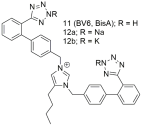 | Compounds 11 (also named BV6 or BisA), 12a and 12b showcase higher antagonistic activity (potency) when compared to losartan (11: −logIC50 = 9.46; 12a: −logIC50 = 9.04; 12b: −logIC50 = 8.54; and losartan: −logIC50 = 8.25). Compound’s 11 elevated docking score for the AT1 receptor is due to a greater number of hydrophobic interactions compared to losartan [222,288,289,290,291,292]. |
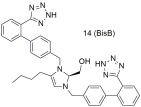 | Compound 14 (also named BisB) showcases higher antagonistic activity (potency) when compared to losartan (14: −logIC50 = 8.37; and losartan: −logIC50 = 8.25) [222,288,289,290,291,292]. |
| Group C: hybrid molecules | |
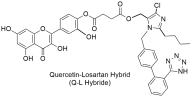 | The quercetin–losartan (Q-L) hybrid retains the binding potential of losartan to the AT1R (Q-L IC50: 140 ± 10 nM; losartan IC50: 10.3 ± 1.1 nM), exhibits ROS inhibition and antioxidant capacity similar to native quercetin, modifies the cell-cycle distribution in GBM cells, and inhibits cancer cell proliferation and angiogenesis in primary GBM cultures [293]. |
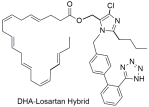 | DHA–losartan; potent inhibitor of multiple pathway-induced platelet aggregation, like P2Y12, PAR-1 (Protease-Activated Receptor-1), PAF (Platelet-Activating Factor), COX-1 (cyclooxygenase-1), and collagen receptors (collagen; losartan IC50: 112.9 μΜ; DHA IC50: 185.6 μΜ; and DHA–losartan hybrid IC50: 249.1 μΜ) [294]. |
| Group D: databases | |
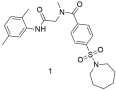 | Compound 1 shows good binding affinity for the AT1 receptor but not better than losartan (1: −logIC50 = 5.66 ± 0.14; and losartan: −logIC50 = 8.49 ± 0.18) [296]. |
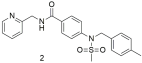 | Compound 2 shows good binding affinity for the AT1 receptor but no better than losartan (2: −logIC50 = 5.68 ± 0.26; and losartan: −logIC50 = 8.49 ± 0.18) [296]. |
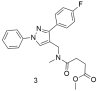 | Compound 3 shows the worst binding affinity for the AT1 receptor compared to 1 and 2 and no better than losartan (3: −logIC50 = 5.59 ± 0.33; and losartan: −logIC50 = 8.49 ± 0.18) [296]. |
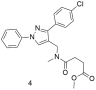 | Compound 4 has 10-fold higher binding affinity for the AT1 receptor compared to 1, 2, and 3 but is not better than losartan (4: −logIC50 = 6.70 ± 0.19; and losartan: −logIC50 = 8.49 ± 0.18) [296]. |
| Structures of Bioactive Compounds | Biological Evaluation |
|---|---|
| Group A; Sartan Derivatives | |
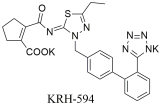 | In vitro binding studies; higher affinity of KRH-594 when compared to losartan and its active metabolite EXP3174 for the AT1 receptor [KRH-594: Ki = 0.39 ± 0.08 nM (n = 4), losartan; Ki = 14 ± 3.0 nM (n = 4) and Ki = 0.79 ± 0.18 nM (n = 3)] [299]. |
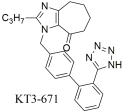 | In vitro binding studies; higher affinity of KT3–671 when compared to losartan and its active metabolite EXP3174 for the AT1 receptor [KT3–671: Ki = 0.71 ± 0.14 nM; losartan (DuP 753): Ki = 5.02 ± 1.63 nM (n = 4); and EXP3174: Ki = 0.32 ± 0.06] [301]. Strong affinity for this receptor in rat liver membranes. |
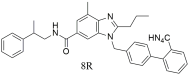 | In vitro binding studies; higher activity of 8R when compared to losartan for the AT1 receptor (8R: IC50 = 1.1 ± 0.5 nM and losartan: IC50 = 28.6 ± 2.0 nM). Promising candidate due to its strong antihypertensive efficacy and relatively low toxicity, as evidenced by plasma analyses, toxicology studies, and chronic oral testing [302]. |
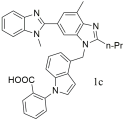 | Oral administration of compound 1c (IC50 = 0.36 ± 0.18 nM, Ki = 0.23 ± 0.17 nM) resulted in maximal decreases of 53 mmHg at 5 mg/kg and 64 mmHg at 10 mg/kg, with the antihypertensive effect persisting for over 24 h—surpassing the efficacy of both losartan (IC50 = 20.09 ± 0.11 nM, Ki = 13.06 ± 0.07 nM) and telmisartan (IC50 = 3.80 ± 0.22 nM, Ki = 2.75 ± 0.17 nM) [303]. |
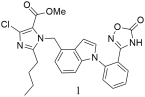 | Compound 1 (IC50 = 5.01 ± 1.67 nM, Ki = 3.63 ± 1.21 nM) shows good binding affinity for the AT1 receptor, comparable to losartan (IC50 = 10.51 ± 2.19 nM, Ki = 7.61 ± 1.59 nM), but weaker than irbesartan (IC50 = 1.30 ± 0.06 nM, Ki = 0.94 ± 0.04 nM). It reduced MBP by 30 mmHg, surpassing the effect of irbesartan, and had low acute toxicity [304]. |
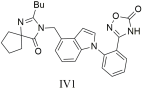 | Compound IV1 (IC50 = 7.7 ± 1.2 nM, Ki = 5.5 ± 0.6 nM) proved especially effective, showing greater potency than losartan (IC50 = 14.6 ± 1.6 nM, Ki = 10.5 ± 1.2 nM), highlighting its potential as a drug candidate [305]. |
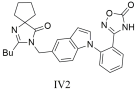 | Compound IV2 with IC50 = 8.0 ± 0.5 nM and Ki = 5.8 ± 0.4 nM showed greater potency than losartan (IC50 = 14.6 ± 1.6 nM, Ki = 10.5 ± 1.2 nM), highlighting its potential as a candidate for antihypertensive drug development [305]. |
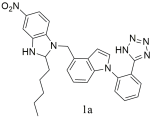 | Compound 1a has a higher affinity to bind with AT1 receptor (1a: IC 50 = 4.05 ± 2.11 nM, Ki = 2.93 ± 1.53 nM) compared to losartan: (IC50 = 12.23 ± 3.42 nM, Ki = 8.86 ± 2.49 nM) [306]. |
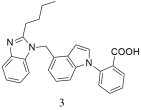 | Compound 3, displayed a high affinity for the angiotensin II type 1 receptor with IC50 value of 1.03 ± 0.26 nM and Ki value of 0.97 ± 0.43 nM (higher compared to losartan; IC50 = 3.54 ± 0.34 nM, Ki = 2.53 ± 1.12) [307]. |
5. Bisartans: Second-Generation Non-Peptide Mimetics of Ang II as Pan-Antiviral Drugs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
Abbreviations
| RAAS | Renin–Angiotensin–Aldosterone system (RAAS) |
| Ang I | Angiotensin I |
| Ang II | Angiotensin II |
| ACE | Angiotensin-converting enzyme |
| AT1R | Angiotensin II Type 1 receptor |
| AT2R | Angiotensin II Type 2 receptor |
| MTDLs | Multi-target Directed Ligands |
| HT | Hypertension |
| HF | Heart Failure |
| CKD | Chronic Kidney disease |
| ACS | Acute Coronary Syndrome |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| RA | Rheumatoid Arthritis |
| OA | Osteoarthritis |
| DN | Diabetic Nephropathy |
| DPN | Diabetic Peripheral Neuropathy |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| CS | Candidosis |
| FS | Fibrosis |
| TFSSc | Tissue Fibrosis in Systemic Sclerosis |
| RP | Raynaud’s phenomenon |
| GC | Glioma Cancer |
| EAE | Auto-immune encephalomyelitis |
| MS | Marfan Syndrome |
| MBP | Myelin Basic Protein |
| IBDs | Inflammatory Bowel diseases |
| TH17 | T helper 17 |
| Akt | Protein kinase B |
| ICU | Intensive Care Unit |
| AT | Anxiety |
| PI | Pathogenic Inflammation |
| YU | Yet Unknown diseases |
| UK | United Kingdom |
| PDE5 | Phosphodiesterase-5 |
| cGMP | Cyclic Guanosine Monophosphate |
| NO | Nitric Oxide |
| CAD | Coronary Artery disease |
| CRS | Charge Relay System |
| ARBs | Angiotensin II receptor blockers |
| CCBs | Calcium channel blockers |
| ARNIs | Angiotensin receptor neprilysin inhibitors |
| ESC | European Society of Cardiology |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HFmrEF | Heart Failure with Mid-Range Ejection Fraction |
| KDIGO | Kidney Disease: Improving Kidney Outcomes |
| APP | Amyloid precursor protein |
| Aβ | Amyloid-beta |
| BACE1 | Beta-site amyloid precursor protein-cleaving enzyme 1 inhibitors |
| CSF | Cerebrospinal fluid |
| AT4 | Angiotensin IV receptor |
| ACEIs | Angiotensin-converting enzyme inhibitors |
| Ang | Angiotensin |
| DA | Dopamine |
| ROS | Reactive Oxygen Species |
| STAI | State-Trait Anxiety Inventory |
| BBB | Blood–Brain Barrier |
| FDA | Food and Drug Administration |
| U87 | Uppsala 87 Malignant Glioma |
| PCR | Polymerase Chain Reaction |
| TNF-α | Tumor necrosis factor alpha |
| IL-6 | Interleukin-6 |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| proIL-1β | pro-interleukin-1β |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| ERK1/2 | Extracellular signal-regulated kinase 1 and 2 |
| JNK1/2 | c-Jun N-terminal kinase 1 and 2. to PKR, NEK7, and ASC. |
| AP-1 | Activator Protein-1 |
| LVEF | Left Ventricular Ejection Fraction |
| PKR | Protein kinase R |
| NEK7 | NIMA-related kinase 7 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| T2DM | Type 2 diabetes mellitus |
| FBN1 | Fibrillin-1 |
| Ang (1–7) | Angiotensin (1–7) |
| IL-1 | Interleukin-1 |
| MMPs | Matrix metalloproteinases |
| ECM | Extracellular matrix |
| TGF-β | Transforming Growth Factor-β |
| CVD | Cardiovascular disease |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| MAS | MAS proto-oncogene |
| IRI | Ischemia–Reperfusion Injury |
| CRH | Corticotropin-releasing hormone |
| AVP | Arginine Vasopressin |
| STAT3 | Signal transducer and activator of transcription 3 |
| MAPK | Mitogen-activated protein kinase |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| VEGF | Vascular endothelial growth factor |
| Caspase-3 | Cysteine-aspartic acid protease 3 |
| IC50 | Half-maximal inhibitory concentration |
| GBM | Glioblastoma multiforme |
| PBMC | Peripheral Blood Mononuclear Cells |
| MHC II | Major Histocompatibility Complex Class II molecule |
| Smad | Suppressor of Mothers against Decapentaplegic |
| IRE1α-XBP1 | Inositol-Requiring Enzyme 1 alpha-X-Box Binding Protein 1 |
| AKI | Acute Kidney Injury |
| DHA | Docosahexaenoic acid |
| P2Y12 | Purinergic Receptor P2Y, G-protein coupled 12 |
| PAR-1 | Protease-Activated Receptor-1 |
| PAF | Platelet-Activating Factor |
| COX-1 | Cyclooxygenase-1 |
| MD | Molecular Dynamics |
| GPCRs | G protein-coupled receptors |
| α1AR | α1-adrenergic receptor |
| α2AR | A2-adrenergic receptor |
| µ-(µOR) | μ-opioid receptor |
| ժOR | δ-opioid receptor |
References
- Pan, X.; Lin, X.; Cao, D.; Zeng, X.; Yu, P.S.; He, L.; Nussinov, R.; Cheng, F. Deep Learning for Drug Repurposing: Methods, Databases, and Applications. WIREs Comput. Mol. Sci. 2022, 12, e1597. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug Repurposing: A Promising Tool to Accelerate the Drug Discovery Process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Singh, T.U.; Parida, S.; Lingaraju, M.C.; Kesavan, M.; Kumar, D.; Singh, R.K. Drug Repurposing Approach to Fight COVID-19. Pharmacol. Rep. 2020, 72, 1479–1508. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Alagarsamy, V.; Solomon, V.R.; Jose, P.A.; Murugesan, S. Drug Repurposing: An Effective Tool in Modern Drug Discovery. Russ. J. Bioorg. Chem. 2023, 49, 157–166. [Google Scholar] [CrossRef]
- Masoudi-Sobhanzadeh, Y.; Omidi, Y.; Amanlou, M.; Masoudi-Nejad, A. Drug Databases and Their Contributions to Drug Repurposing. Genomics 2020, 112, 1087–1095. [Google Scholar] [CrossRef]
- Dotolo, S.; Marabotti, A.; Facchiano, A.; Tagliaferri, R. A Review on Drug Repurposing Applicable to COVID-19. Brief. Bioinform. 2021, 22, 726–741. [Google Scholar] [CrossRef]
- Parisi, D.; Adasme, M.F.; Sveshnikova, A.; Bolz, S.N.; Moreau, Y.; Schroeder, M. Drug Repositioning or Target Repositioning: A Structural Perspective of Drug-Target-Indication Relationship for Available Repurposed Drugs. Comput. Struct. Biotechnol. J. 2020, 18, 1043–1055. [Google Scholar] [CrossRef]
- Mishra, A.S.; Vasanthan, M.; Malliappan, S.P. Drug Repurposing: A Leading Strategy for New Threats and Targets. ACS Pharmacol. Transl. Sci. 2024, 7, 915–932. [Google Scholar] [CrossRef]
- Maggioni, A.P. Efficacy of Angiotensin Receptor Blockers in Cardiovascular Disease. Cardiovasc. Drugs Ther. 2006, 20, 295–308. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Latini, R. The Angiotensin-Receptor Blockers: From Antihypertensives to Cardiovascular All-Round Medications in 10 Years? Blood Press. 2002, 11, 328–338. [Google Scholar] [CrossRef]
- Qaradakhi, T.; Matsoukas, M.T.; Hayes, A.; Rybalka, E.; Caprnda, M.; Rimarova, K.; Sepsi, M.; Büsselberg, D.; Kruzliak, P.; Matsoukas, J.; et al. Alamandine Reverses Hyperhomocysteinemia-induced Vascular Dysfunction via PKA-dependent Mechanisms. Cardiovasc. Ther. 2017, 35, e12306. [Google Scholar] [CrossRef]
- Weber, M.A. The Angiotensin II Receptor Blockers: Opportunities across the Spectrum of Cardiovascular Disease. Rev. Cardiovasc. Med. 2002, 3, 183–191. [Google Scholar]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-Target-Directed Ligands To Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Brindisi, M.; Kessler, S.M.; Kumar, V.; Zwergel, C. Editorial: Multi-Target Directed Ligands for the Treatment of Cancer. Front. Oncol. 2022, 12, 980141. [Google Scholar] [CrossRef]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.; Pooe, O.J. Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des. Dev. Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef]
- Kumar, B.; Thakur, A.; Dwivedi, A.R.; Kumar, R.; Kumar, V. Multi-Target-Directed Ligands as an Effective Strategy for the Treatment of Alzheimer’s Disease. Curr. Med. Chem. 2022, 29, 1757–1803. [Google Scholar] [CrossRef]
- Ciociola, A.A.; Cohen, L.B.; Kulkarni, P.; Kefalas, C.; Buchman, A.; Burke, C.; Cain, T.; Connor, J.; Ehrenpreis, E.D.; Fang, J.; et al. How Drugs Are Developed and Approved by the FDA: Current Process and Future Directions. Am. J. Gastroenterol. 2014, 109, 620–623. [Google Scholar] [CrossRef]
- Tamimi, N.A.M.; Ellis, P. Drug Development: From Concept to Marketing! Nephron Clin. Pract. 2009, 113, c125–c131. [Google Scholar] [CrossRef]
- Tonkens, R. An Overview of the Drug Development Process. Physician Exec. 2005, 31, 48–52. [Google Scholar]
- Brodniewicz, T.; Grynkiewicz, G. Preclinical Drug Development. Acta Pol. Pharm. 2010, 67, 578–585. [Google Scholar]
- Singh, G. Preclinical Drug Development. In Pharmaceutical Medicine and Translational Clinical Research; Elsevier: London, UK, 2018; pp. 47–63. ISBN 978-0-12-802103-3. [Google Scholar]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Korth-Bradley, J.M. Regulatory Framework for Drug Development in Rare Diseases. J. Clin. Pharmacol. 2022, 62, S15–S26. [Google Scholar] [CrossRef]
- Fetro, C.; Scherman, D. Drug Repurposing in Rare Diseases: Myths and Reality. Therapies 2020, 75, 157–160. [Google Scholar] [CrossRef]
- Réda, C.; Vie, J.-J.; Wolkenhauer, O. Comprehensive Evaluation of Pure and Hybrid Collaborative Filtering in Drug Repurposing. Sci. Rep. 2025, 15, 2711. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Osterloh, I.H.; Grimminger, F. Sildenafil: From Angina to Erectile Dysfunction to Pulmonary Hypertension and Beyond. Nat. Rev. Drug Discov. 2006, 5, 689–702. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Bush, P.A.; Buga, G.M.; Wood, K.S.; Fukuto, J.M.; Rajfer, J. Nitric Oxide and Cyclic GMP For-mation upon Electrical Field Stimulation Cause Relaxation of Corpus Cavernosum Smooth Muscle. Biochem. Biophys. Res. Commun. 1990, 170, 843–850. [Google Scholar] [CrossRef]
- Baldaçara, L. Duloxetine: An Update. Res. Soc. Dev. 2024, 13, 1–9. [Google Scholar] [CrossRef]
- Goldstein, D.J. Duloxetine in the Treatment of Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2007, 3, 193–209. [Google Scholar] [CrossRef]
- Lunn, M.P.; Hughes, R.A.; Wiffen, P.J. Duloxetine for Treating Painful Neuropathy, Chronic Pain or Fibromyalgia. Cochrane Database Syst. Rev. 2014, 1, CD007115. [Google Scholar] [CrossRef]
- Khan, A.Y.; Macaluso, M. Duloxetine for the Treatment of Generalized Anxiety Disorder: A Review. Neuropsychiatr. Dis. Treat. 2009, 5, 23–31. [Google Scholar] [CrossRef]
- Gao, S.-H.; Huo, J.-B.; Pan, Q.-M.; Li, X.-W.; Chen, H.-Y.; Huang, J.-H. The Short-Term Effect and Safety of Duloxetine in Osteoarthritis: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e17541. [Google Scholar] [CrossRef]
- Mangır, N.; Uçar, M.; Gülpınar, Ö.; Özkürkçügil, C.; Demirkesen, O.; Tarcan, T. Duloxetine in the Treatment of Women with Urinary Incontinence: A Systematic Review and Meta-Analysis of Efficacy Data from Randomized Controlled Clinical Trials. J. Urol. Surg. 2023, 10, 1–8. [Google Scholar] [CrossRef]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. The Discovery of Fluoxetine Hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef]
- Romano, S.; Judge, R.; Dillon, J.; Shuler, C.; Sundell, K. The Role of Fluoxetine in the Treatment of Premenstrual Dysphoric Disorder. Clin. Ther. 1999, 21, 615–633. [Google Scholar] [CrossRef]
- Warner, C.B.; Ottman, A.A.; Brown, J.N. The Role of Atomoxetine for Parkinson Disease–Related Executive Dysfunction: A Systematic Review. J. Clin. Psychopharmacol. 2018, 38, 627–631. [Google Scholar] [CrossRef]
- Dell’Agnello, G.; Maschietto, D.; Bravaccio, C.; Calamoneri, F.; Masi, G.; Curatolo, P.; Besana, D.; Mancini, F.; Rossi, A.; Poole, L.; et al. Atomoxetine Hydrochloride in the Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder and Comorbid Oppositional Defiant Disorder: A Placebo-Controlled Italian Study. Eur. Neuropsychopharmacol. 2009, 19, 822–834. [Google Scholar] [CrossRef]
- Parkes, J.D.; Marsden, C.D.; Donaldson, I.; Galea-Debono, A.; Walters, J.; Kennedy, G.; Asselman, P. Bromocriptine Treatment in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1976, 39, 184–193. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Bromocriptine: A Sympatholytic, D2-Dopamine Agonist for the Treatment of Type 2 Diabetes. Diabetes Care 2011, 34, 789–794, Erratum in Diabetes Care 2011, 34, 1442. [Google Scholar] [CrossRef]
- Cho, K.R.; Jo, K.-I.; Shin, H.J. Bromocriptine Therapy for the Treatment of Invasive Prolactinoma: The Single Institute Experience. Brain Tumor Res. Treat. 2013, 1, 71–77. [Google Scholar] [CrossRef]
- Ciapparelli, A.; Dell’Osso, L.; Pini, S.; Chiavacci, M.C.; Fenzi, M.; Cassano, G.B. Clozapine for Treatment-Refractory Schizophrenia, Schizoaffective Disorder, and Psychotic Bipolar Disorder: A 24-Month Naturalistic Study. J. Clin. Psychiatry 2000, 61, 329–334. [Google Scholar] [CrossRef]
- Pu, Y.; Xu, F.; He, A.; Li, R.; Wang, X.; Zhou, L.; Sun, H.; Zhang, Y.; Xia, Y. Repurposing Chlorpromazine for the Treatment of Triple-Negative Breast Cancer Growth and Metastasis Based on Modulation of Mitochondria-Mediated Apoptosis and Autophagy/Mitophagy. Br. J. Cancer 2025, 132, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Licht, R.W. Lithium: Still a Major Option in the Management of Bipolar Disorder. CNS Neurosci. Ther. 2012, 18, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Jakobsson, E. Systems Biology Understanding of the Effects of Lithium on Cancer. Front. Oncol. 2019, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Chatupheeraphat, C.; Kaewsai, N.; Anuwongcharoen, N.; Phanus-umporn, C.; Pornsuwan, S.; Eiamphungporn, W. Penfluridol Synergizes with Colistin to Reverse Colistin Resistance in Gram-Negative Bacilli. Sci. Rep. 2025, 15, 16114. [Google Scholar] [CrossRef]
- Ali Ibrahim Mze, A.; Abdul Rahman, A. Repurposing the Antipsychotic Drug Penfluridol for Cancer Treatment (Review). Oncol. Rep. 2024, 52, 174. [Google Scholar] [CrossRef]
- Parker, S.G.; Raval, P.; Yeulet, S.; Eden, R.J. Tolerance to Peripheral, but Not Central, Effects of Ropinirole, a Selective Dopamine D2-like Receptor Agonist. Eur. J. Pharmacol. 1994, 265, 17–26. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, M. The Effect and Safety of Ropinirole in the Treatment of Parkinson Disease: A Systematic Review and Meta-Analysis. Medicine 2021, 100, e27653. [Google Scholar] [CrossRef]
- Werz, O.; Stettler, H.; Theurer, C.; Seibel, J. The 125th Anniversary of Aspirin—The Story Continues. Pharmaceuticals 2024, 17, 437. [Google Scholar] [CrossRef]
- Undas, A.; Brummel-Ziedins, K.E.; Mann, K.G. Antithrombotic Properties of Aspirin and Resistance to Aspirin: Beyond Strictly Antiplatelet Actions. Blood 2007, 109, 2285–2292. [Google Scholar] [CrossRef]
- Clemett, D.; Goa, K.L. Celecoxib: A Review of Its Use in Osteoarthritis, Rheumatoid Arthritis and Acute Pain. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef]
- Lynch, P.M.; Ayers, G.D.; Hawk, E.; Richmond, E.; Eagle, C.; Woloj, M.; Church, J.; Hasson, H.; Patterson, S.; Half, E.; et al. The Safety and Efficacy of Celecoxib in Children With Familial Adenomatous Polyposis. Am. J. Gastroenterol. 2010, 105, 1437–1443. [Google Scholar] [CrossRef]
- Edwards, J.E.; Moore, R.A. Finasteride in the Treatment of Clinical Benign Prostatic Hyperplasia: A Systematic Review of Randomised Trials. BMC Urol. 2002, 2, 14. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Talukder, M.; Bamimore, M.A. Finasteride for Hair Loss: A Review. J. Dermatol. Treat. 2022, 33, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; McClellan, K.J. Losartan: A Review of Its Use, with Special Focus on Elderly Patients. Drugs Aging 2000, 16, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Thriene, K.; Mittapalli, V.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan Ameliorates Dystrophic Epidermolysis Bullosa and Uncovers New Disease Mechanisms. EMBO Mol. Med. 2015, 7, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, W.; Chung, H.; Sinha, S.; Bui-Marinos, M.P.; Arora, R.; Jaffer, A.; Corcoran, J.A.; Biernaskie, J.; Chun, J. Attenuation of SARS-CoV-2 Infection by Losartan in Human Kidney Organoids. iScience 2022, 25, 103818. [Google Scholar] [CrossRef]
- Mehta, P.K. Severe Hypertension: Treatment with Minoxidil. JAMA 1975, 233, 249. [Google Scholar] [CrossRef]
- Randolph, M.; Tosti, A. Oral Minoxidil Treatment for Hair Loss: A Review of Efficacy and Safety. J. Am. Acad. Dermatol. 2021, 84, 737–746. [Google Scholar] [CrossRef]
- Hicks, C.; Gulick, R.M. Raltegravir: The First HIV Type 1 Integrase Inhibitor. Clin Infect. Dis. 2009, 48, 931–939. [Google Scholar] [CrossRef]
- Alburquerque-González, B.; Bernabé-García, Á.; Bernabé-García, M.; Ruiz-Sanz, J.; López-Calderón, F.F.; Gonnelli, L.; Banci, L.; Peña-García, J.; Luque, I.; Nicolás, F.J.; et al. The FDA-Approved Antiviral Raltegravir Inhibits Fascin1-Dependent Invasion of Colorectal Tumor Cells In Vitro and In Vivo. Cancers 2021, 13, 861. [Google Scholar] [CrossRef]
- Broder, S. The Development of Antiretroviral Therapy and Its Impact on the HIV-1/AIDS Pandemic. Antivir. Res. 2010, 85, 1–18. [Google Scholar] [CrossRef]
- Langtry, H.D.; Campoli-Richards, D.M. Zidovudine: A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 1989, 37, 408–450. [Google Scholar] [CrossRef]
- Yamashita, M. Auranofin: Past to Present, and Repurposing. Int. Immunopharmacol. 2021, 101, 108272. [Google Scholar] [CrossRef]
- Pessetto, Z.Y.; Weir, S.J.; Sethi, G.; Broward, M.A.; Godwin, A.K. Drug Repurposing for Gastrointestinal Stromal Tumor. Mol. Cancer Ther. 2013, 12, 1299–1309. [Google Scholar] [CrossRef]
- Merino, M.; Kasamon, Y.; Li, H.; Ma, L.; Leong, R.; Zhou, J.; Reaman, G.; Chambers, W.; Richardson, N.; Theoret, M.; et al. FDA Approval Summary: Crizotinib for Pediatric and Young Adult Patients with Relapsed or Refractory Systemic Anaplastic Large Cell Lymphoma. Pediatr. Blood Cancer 2022, 69, e29602. [Google Scholar] [CrossRef]
- Roberts, P.J. Clinical Use of Crizotinib for the Treatment of Non-Small Cell Lung Cancer. Biologics 2013, 7, 91–101. [Google Scholar] [CrossRef]
- Sacha, T. Imatinib in Chronic Myeloid Leukemia: An Overview. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014007. [Google Scholar] [CrossRef]
- Lopes, L.F.; Bacchi, C.E. Imatinib Treatment for Gastrointestinal Stromal Tumour (GIST). J. Cell. Mol. Med. 2010, 14, 42–50. [Google Scholar] [CrossRef]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a Key Chemotherapeutic Drug for Metastatic Colorectal Cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]
- Brown, M.B.; Blair, H.A. Liposomal Irinotecan: A Review as First-Line Therapy in Metastatic Pancreatic Adenocarcinoma. Drugs 2025, 85, 255–262. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Therapeutic Profile in the Treatment of Type 2 Diabetes. Diabetes Obes. Metab. 2024, 26, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Van Eijck, C.W.F.; Vadgama, D.; Van Eijck, C.H.J.; Wilmink, J.W.; for the Dutch Pancreatic Cancer Group (DPCG). Metformin Boosts Antitumor Immunity and Improves Prognosis in Upfront Resected Pancreatic Cancer: An Observational Study. JNCI J. Natl. Cancer Inst. 2024, 116, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Martinez-Outschoorn, U.E.; Schilder, R.J.; Kim, C.H.; Richard, S.D.; Rosenblum, N.G.; Johnson, J.M. Metformin as a Therapeutic Target in Endometrial Cancers. Front. Oncol. 2018, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Higurashi, T.; Nakajima, A. Metformin and Colorectal Cancer. Front. Endocrinol. 2018, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-C.; An, R.; Jiang, Y.-Q.; Yang, J. Effects and Mechanisms of Metformin on the Proliferation of Esophageal Cancer Cells In Vitro and In Vivo. Cancer Res. Treat. 2017, 49, 778–789. [Google Scholar] [CrossRef]
- Wallet, M.A.; Reist, C.M.; Williams, J.C.; Appelberg, S.; Guiulfo, G.L.; Gardner, B.; Sleasman, J.W.; Goodenow, M.M. The HIV-1 Protease Inhibitor Nelfinavir Activates PP2 and Inhibits MAPK Signaling in Macrophages: A Pathway to Reduce Inflammation. J. Leukoc. Biol. 2012, 92, 795–805. [Google Scholar] [CrossRef]
- Subeha, M.R.; Telleria, C.M. The Anti-Cancer Properties of the HIV Protease Inhibitor Nelfinavir. Cancers 2020, 12, 3437. [Google Scholar] [CrossRef]
- D’Amelio, P.; Isaia, G.C. The Use of Raloxifene in Osteoporosis Treatment. Expert Opin. Pharmacother. 2013, 14, 949–956. [Google Scholar] [CrossRef]
- Moen, M.D.; Keating, G.M. Raloxifene: A Review of Its Use in the Prevention of Invasive Breast Cancer. Drugs 2008, 68, 2059–2083. [Google Scholar] [CrossRef]
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab—The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 163. [Google Scholar] [CrossRef]
- Cohen, M.D.; Keystone, E. Rituximab for Rheumatoid Arthritis. Rheumatol. Ther. 2015, 2, 99–111. [Google Scholar] [CrossRef]
- Rizzo, M.; Porta, C. Sunitinib in the Treatment of Renal Cell Carcinoma: An Update on Recent Evidence. Ther. Adv. Urol. 2017, 9, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Margalef, N.; Garcia-Del-Muro, X. Sunitinib in the Treatment of Gastrointestinal Stromal Tumor: Patient Selection and Perspectives. Onco Targets Ther. 2016, 9, 7573–7582. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513, Correction in N. Engl. J. Med. 2011, 364, 1082. [Google Scholar] [CrossRef] [PubMed]
- Camejo, N.; Castillo, C.; Alonso, R.; Correa, F.; Rivero, E.; Mezquita, C.; Rosich, A.; Dellacasa, F.; Silveira, L.; Delgado, L. Effectiveness of Trastuzumab for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer in a Real-Life Setting: One Decade of Experience Under National Treatment Coverage Regulations. JCO Glob. Oncol. 2020, 6, 217–223. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Younis, T. Trastuzumab for HER2-Positive Metastatic Breast Cancer: Clinical and Economic Considerations. Clin. Med. Insights Oncol. 2012, 6, 179–187. [Google Scholar] [CrossRef]
- Gunturu, K.S.; Woo, Y.; Beaubier, N.; Remotti, H.E.; Saif, M.W. Gastric Cancer and Trastuzumab: First Biologic Therapy in Gastric Cancer. Ther. Adv. Med. Oncol. 2013, 5, 143–151. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Trastuzumab for Early-Stage, HER2-Positive Breast Cancer: A Meta-Analysis of 13 864 Women in Seven Randomised Trials. Lancet Oncol. 2021, 22, 1139–1150. [Google Scholar] [CrossRef]
- Kwitkowski, V.E.; Prowell, T.M.; Ibrahim, A.; Farrell, A.T.; Justice, R.; Mitchell, S.S.; Sridhara, R.; Pazdur, R. FDA Approval Summary: Temsirolimus as Treatment for Advanced Renal Cell Carcinoma. Oncologist 2010, 15, 428–435. [Google Scholar] [CrossRef]
- Chang, H.-W.; Wu, M.-J.; Lin, Z.-M.; Wang, C.-Y.; Cheng, S.-Y.; Lin, Y.-K.; Chow, Y.-H.; Ch’ang, H.-J.; Chang, V.H.S. Therapeutic Effect of Repurposed Temsirolimus in Lung Adenocarcinoma Model. Front. Pharmacol. 2018, 9, 778. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It Only Takes One to Do Many Jobs: Amphotericin B as Antifungal and Immunomodulatory Drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Balasegaram, M.; Ritmeijer, K.; Lima, M.A.; Burza, S.; Ortiz Genovese, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal Amphotericin B as a Treatment for Human Leishmaniasis. Expert Opin. Emerg. Drugs 2012, 17, 493–510. [Google Scholar] [CrossRef]
- Hirabayashi, K.E.; Kalin-Hajdu, E.; Brodie, F.L.; Kersten, R.C.; Russell, M.S.; Vagefi, M.R. Retrobulbar Injection of Amphotericin B for Orbital Mucormycosis. Ophthalmic Plast. Reconstr. Surg. 2017, 33, e94–e97. [Google Scholar] [CrossRef]
- Liu, G.; Yin, Y.; Zhang, L.; He, D.; Yang, L. Efficacy of Dapoxetine in the Treatment of Patients With Lifelong Premature Ejaculation as an Alternative to Sertraline Therapy. Sex. Med. 2022, 10, 100473-1. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, J.; Zhu, G.; Huang, Y.; Zhang, K.; Xiao, X.; He, W.; Yuan, J.; Gao, X. Dapoxetine, a Selective Serotonin Reuptake Inhibitor, Suppresses Zika Virus Infection In Vitro. Molecules 2023, 28, 8142. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.-L.; Tan, H.-H. Use of Everolimus in Liver Transplantation. World J. Hepatol. 2017, 9, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Medici, B.; Caffari, E.; Maculan, Y.; Benatti, S.; Piacentini, F.; Dominici, M.; Gelsomino, F. Everolimus in the Treatment of Neuroendocrine Tumors: Lights and Shadows. Biomedicines 2025, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Coppin, C. Everolimus: The First Approved Product for Patients with Advanced Renal Cell Cancer after Sunitinib and/or Sorafenib. Biologics 2010, 4, 91–101. [Google Scholar] [CrossRef]
- Krueger, D.A.; Care, M.M.; Holland, K.; Agricola, K.; Tudor, C.; Mangeshkar, P.; Wilson, K.A.; Byars, A.; Sahmoud, T.; Franz, D.N. Everolimus for Subependymal Giant-Cell Astrocytomas in Tuberous Sclerosis. N. Engl. J. Med. 2010, 363, 1801–1811. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an Anti-Influenza Drug against Life-Threatening RNA Virus Infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Boretti, A. Favipiravir Use for SARS-CoV-2 Infection. Pharmacol. Rep. 2020, 72, 1542–1552. [Google Scholar] [CrossRef]
- Crump, A. Ivermectin: Enigmatic Multifaceted ‘Wonder’ Drug Continues to Surprise and Exceed Expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef]
- Formiga, F.R.; Leblanc, R.; De Souza Rebouças, J.; Farias, L.P.; De Oliveira, R.N.; Pena, L. Ivermectin: An Award-Winning Drug with Expected Antiviral Activity against COVID-19. J. Control. Release 2021, 329, 758–761. [Google Scholar] [CrossRef]
- Heel, R.C.; Brogden, R.N.; Carmine, A.; Morley, P.A.; Speight, T.M.; Avery, G.S. Ketoconazole: A Review of Its Therapeutic Efficacy in Superficial and Systemic Fungal Infections. Drugs 1982, 23, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Loli, P.; Berselli, M.E.; Tagliaferri, M. Use of Ketoconazole in the Treatment of Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 1986, 63, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.H.; Darwish, H.; Darwish, I.A.; Al-Ghusn, A.I. Remdesivir. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: London, UK, 2023; Volume 48, pp. 71–108. ISBN 978-0-443-19382-8. [Google Scholar]
- Jeon, H.J.; Lee, H.-E.; Yang, J. Safety and Efficacy of Rapamune® (Sirolimus) in Kidney Transplant Recipients: Results of a Prospective Post-Marketing Surveillance Study in Korea. BMC Nephrol. 2018, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Mejia, P.; Treviño-Villarreal, J.H.; Reynolds, J.S.; De Niz, M.; Thompson, A.; Marti, M.; Mitchell, J.R. A Single Rapamycin Dose Protects against Late-Stage Experimental Cerebral Malaria via Modulation of Host Immunity, Endothelial Activation and Parasite Sequestration. Malar. J. 2017, 16, 455. [Google Scholar] [CrossRef]
- Okafor, M.C. Thalidomide for Erythema Nodosum Leprosum and Other Applications. Pharmacotherapy 2003, 23, 481–493. [Google Scholar] [CrossRef]
- Latif, T.; Chauhan, N.; Khan, R.; Moran, A.; Usmani, S.Z. Thalidomide and Its Analogues in the Treatment of Multiple Myeloma. Exp. Hematol. Oncol. 2012, 1, 27. [Google Scholar] [CrossRef]
- Jenneck, C.; Novak, N. The Safety and Efficacy of Alefacept in the Treatment of Chronic Plaque Psoriasis. Ther. Clin. Risk Manag. 2007, 3, 411–420. [Google Scholar]
- Zaidi, A.; Meng, Q. Can We Repurpose FDA-Approved Alefacept to Diminish the HIV Reservoir? Immunother. Open Acc. 2016, 1, 1000104. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Chang, A.R.; Brotman, D.J.; Inker, L.A.; Grams, M.E.; Shin, J. Baclofen and the Risk of Fall and Fracture in Older Adults: A Real-world Cohort Study. J. Am. Geriatr. Soc. 2024, 72, 91–101. [Google Scholar] [CrossRef]
- de Beaurepaire, R.; Sinclair, J.M.A.; Heydtmann, M.; Addolorato, G.; Aubin, H.-J.; Beraha, E.M.; Caputo, F.; Chick, J.D.; de La Selle, P.; Franchitto, N.; et al. The Use of Baclofen as a Treatment for Alcohol Use Disorder: A Clinical Practice Perspective. Front. Psychiatry 2018, 9, 708. [Google Scholar] [CrossRef]
- Feldman, S.R.; Yentzer, B.A. Topical Clobetasol Propionate in the Treatment of Psoriasis: A Review of Newer Formulations. Am. J. Clin. Dermatol. 2009, 10, 397–406. [Google Scholar] [CrossRef]
- Azhar, S.D.; Shahid, N.; Sadiq, A.; Khan, A.W.; Sultan Dar, M.; Fadlalla Ahmed, T.K. Clobetasol Propionate for Post-Cataract Surgery Pain and Inflammation. Ann. Med. Surg. 2024, 86, 6395–6398. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.A.; Ogrodnik, M.A.; Plave, A.; Mao-Draayer, Y. Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis. Front. Neurol. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Burlando, M.; Campione, E.; Cuccia, A.; Malara, G.; Naldi, L.; Prignano, F.; Zichichi, L. Real-World Use of Dimethyl Fumarate in Patients with Plaque Psoriasis: A Delphi-Based Expert Consensus. Dermatol. Rep. 2023, 15, 9613. [Google Scholar] [CrossRef]
- McGuire, V.A.; Ruiz-Zorrilla Diez, T.; Emmerich, C.H.; Strickson, S.; Ritorto, M.S.; Sutavani, R.V.; Weiβ, A.; Houslay, K.F.; Knebel, A.; Meakin, P.J.; et al. Dimethyl Fumarate Blocks Pro-Inflammatory Cytokine Production via Inhibition of TLR Induced M1 and K63 Ubiquitin Chain Formation. Sci. Rep. 2016, 6, 31159. [Google Scholar] [CrossRef]
- Hayat, A.; Haria, D.; Salifu, M.O. Erythropoietin Stimulating Agents in the Management of Anemia of Chronic Kidney Disease. Patient Prefer. Adherence 2008, 2, 195–200. [Google Scholar] [CrossRef]
- Skrifvars, M.B.; Luethi, N.; Bailey, M.; French, C.; Nichol, A.; Trapani, T.; McArthur, C.; Arabi, Y.M.; Bendel, S.; Cooper, D.J.; et al. The Effect of Recombinant Erythropoietin on Long-Term Outcome after Moderate-to-Severe Traumatic Brain Injury. Intensive Care Med. 2023, 49, 831–839. [Google Scholar] [CrossRef]
- Dowlut-McElroy, T.; Shankar, R.K. The Care of Adolescents and Young Adults with Turner Syndrome: A Pediatric and Adolescent Gynecology Perspective. J. Pediatr. Adolesc. Gynecol. 2022, 35, 429–434. [Google Scholar] [CrossRef]
- Likis, F.E. CONTRACEPTIVE APPLICATIONS OF ESTROGEN. J. Midwifery Women’s Health 2002, 47, 139–156. [Google Scholar] [CrossRef]
- Franks, S.; Layton, A.; Glasier, A. Cyproterone Acetate/Ethinyl Estradiol for Acne and Hirsutism: Time to Revise Prescribing Policy. Hum. Reprod. 2007, 23, 231–232. [Google Scholar] [CrossRef]
- Pelletier, D.; Hafler, D.A. Fingolimod for Multiple Sclerosis. N. Engl. J. Med. 2012, 366, 339–347. [Google Scholar] [CrossRef]
- Leßmann, V.; Kartalou, G.-I.; Endres, T.; Pawlitzki, M.; Gottmann, K. Repurposing Drugs against Alzheimer’s Disease: Can the Anti-Multiple Sclerosis Drug Fingolimod (FTY720) Effectively Tackle Inflammation Processes in AD? J. Neural Transm. 2023, 130, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Patmanathan, S.N.; Yap, L.F.; Murray, P.G.; Paterson, I.C. The Antineoplastic Properties of FTY720: Evidence for the Repurposing of Fingolimod. J. Cell. Mol. Med. 2015, 19, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Gesualdo, C.; Balta, C.; Platania, C.B.M.; Trotta, M.C.; Herman, H.; Gharbia, S.; Rosu, M.; Petrillo, F.; Giunta, S.; Della Corte, A.; et al. Fingolimod and Diabetic Retinopathy: A Drug Repurposing Study. Front. Pharmacol. 2021, 12, 718902. [Google Scholar] [CrossRef] [PubMed]
- Iepsen, E.W.; Torekov, S.S.; Holst, J.J. Liraglutide for Type 2 Diabetes and Obesity: A 2015 Update. Expert Rev. Cardiovasc. Ther. 2015, 13, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Edison, P.; Femminella, G.D.; Ritchie, C.W.; Holmes, C.; Walker, Z.; Ridha, B.H.; Raza, S.; Livingston, N.R.; Nowell, J.; Busza, G.; et al. Evaluation of Liraglutide in the Treatment of Alzheimer’s Disease. Alzheimer’s Dement. 2021, 17, e057848. [Google Scholar] [CrossRef]
- Sadowska, A.M. N.-Acetylcysteine Mucolysis in the Management of Chronic Obstructive Pulmonary Disease. Ther. Adv. Respir. Dis. 2012, 6, 127–135. [Google Scholar] [CrossRef]
- Carollo, M.; Carollo, N.; Montan, G. The Promise of N-Acetylcysteine in the Treatment of Obsessive-Compulsive Disorder. CNS Neurosci. Ther. 2024, 30, e14653. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Bell, R.F.; Straube, S.; Wiffen, P.J.; Aldington, D.; Moore, R.A. Pregabalin for Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2019, 1, CD007076. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.S.; Ajel, K.; Masdrakis, V.G.; Nowak, M.; Rafiq, R. Pregabalin for the Treatment of Generalized Anxiety Disorder: An Update. Nephrol. Dial. Transplant. 2013, 9, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.; Huffman, M.D.; Macedo, A.F.; Moore, T.H.M.; Burke, M.; Davey Smith, G.; Ward, K.; Ebrahim, S. Statins for the Primary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2013, 2013, CD004816. [Google Scholar] [CrossRef]
- Tripathi, S.; Gupta, E.; Galande, S. Statins as Anti-Tumor Agents: A Paradigm for Repurposed Drugs. Cancer Rep. 2024, 7, e2078. [Google Scholar] [CrossRef]
- Bhat, A.; Dalvi, H.; Jain, H.; Rangaraj, N.; Singh, S.B.; Srivastava, S. Perspective Insights of Repurposing the Pleiotropic Efficacy of Statins in Neurodegenerative Disorders: An Expository Appraisal. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100012. [Google Scholar] [CrossRef]
- Boyle, A.K.; Rinaldi, S.F.; Rossi, A.G.; Saunders, P.T.K.; Norman, J.E. Repurposing Simvastatin as a Therapy for Preterm Labor: Evidence from Preclinical Models. FASEB J. 2019, 33, 2743–2758. [Google Scholar] [CrossRef]
- Moglad, E.; Elekhnawy, E.; Alanazi, N.; Al-Fakhrany, O.M. Repurposing Simvastatin for Treatment of Klebsiella Pneumoniae Infections: In Vitro and in Vivo Study. Biofouling 2024, 40, 801–815. [Google Scholar] [CrossRef]
- Lyseng-Williamson, K.A.; Yang, L.P.H. Topiramate: A Review of Its Use in the Treatment of Epilepsy. Drugs 2007, 67, 2231–2256. [Google Scholar] [CrossRef]
- Wajid, I.; Vega, A.; Thornhill, K.; Jenkins, J.; Merriman, C.; Chandler, D.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Topiramate (Topamax): Evolving Role in Weight Reduction Management: A Narrative Review. Life 2023, 13, 1845. [Google Scholar] [CrossRef]
- Park, K. A Review of Computational Drug Repurposing. Transl. Clin. Pharmacol. 2019, 27, 59. [Google Scholar] [CrossRef]
- Natsheh, I.Y.; Alsaleh, M.M.; Alkhawaldeh, A.K.; Albadawi, D.K.; Darwish, M.M.; Shammout, M.J.A. The Dark Side of Drug Repurposing. From Clinical Trial Challenges to Antimicrobial Resistance: Analysis Based on Three Major Fields. Drug Target Insights 2024, 18, 8–19. [Google Scholar] [CrossRef]
- Pandit, J.N.; Kumari, R.; Kumari, M.; Mp, A.R.; Yadav, A.; Arava, S. Rare Fatal Effect of Combined Use of Sildenafil and Alcohol Leading to Cerebrovascular Accident. J. Forensic Leg. Med. 2023, 95, 102504. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Cusick, A.S.; Goyal, A.; Patel, P. ACE Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Moore, G.J.; Matsoukas, J.M. Angiotensin as a Model for Hormone—Receptor Interactions. Biosci. Rep. 1985, 5, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.M.; Hondrelis, J.; Keramida, M.; Mavromoustakos, T.; Makriyannis, A.; Yamdagni, R.; Wu, Q.; Moore, G.J. Role of the NH2-Terminal Domain of Angiotensin II (ANG II) and [Sar1]Angiotensin II on Conformation and Activity. NMR Evidence for Aromatic Ring Clustering and Peptide Backbone Folding Compared with [Des-1,2,3]Angiotensin II. J. Biol. Chem. 1994, 269, 5303–5312. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.M.; Goghari, M.H.; Scanlon, M.N.; Franklin, K.J.; Moore, G.J. Synthesis and Biological Activities of Analogs of Angiotensins II and III Containing O-Methyltyrosine and D-Tryptophan. J. Med. Chem. 1985, 28, 780–783. [Google Scholar] [CrossRef]
- Matsoukas, J.; Cordopatis, P.; Belte, U.; Goghari, M.H.; Ganter, R.C.; Franklin, K.J.; Moore, G.J. Importance of the N-Terminal Domain of the Type II Angiotensin Antagonist Sarmesin for Receptor Blockade. J. Med. Chem. 1988, 31, 1418–1421. [Google Scholar] [CrossRef]
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef]
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global Burden of Cardiovascular Diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, 32, 1001–1015. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2025, 151, e41–e660, Correction in Circulation 2025, 151, e1096. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hu, H.-Y.; Chou, Y.-J.; Huang, N.; Chou, Y.-C.; Li, C.-P. High Blood Pressure and All-Cause and Cardiovascular Disease Mortalities in Community-Dwelling Older Adults. Medicine 2015, 94, e2160. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood Pressure Lowering for Prevention of Cardiovascular Disease and Death: A Systematic Review and Meta-Analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal Trends and Patterns in Heart Failure Incidence: A Population-Based Study of 4 Million Individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Ghionzoli, N.; Gentile, F.; Del Franco, A.M.; Castiglione, V.; Aimo, A.; Giannoni, A.; Burchielli, S.; Cameli, M.; Emdin, M.; Vergaro, G. Current and Emerging Drug Targets in Heart Failure Treatment. Heart Fail. Rev. 2022, 27, 1119–1136. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO). CKD Work Group KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Mezzano, S.A.; Ruiz-Ortega, M.; Egido, J. Angiotensin II and Renal Fibrosis. Hypertension 2001, 38, 635–638. [Google Scholar] [CrossRef]
- Durvasula, R.V.; Shankland, S.J. The Renin-Angiotensin System in Glomerular Podocytes: Mediator of Glomerulosclerosis and Link to Hypertensive Nephropathy. Curr. Sci. Inc. 2006, 8, 132–138. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788, Erratum in Lancet 2018, 392, 2170; Erratum in Lancet 2019, 393, e44. [Google Scholar] [CrossRef]
- Roth, G.A.; Nguyen, G.; Forouzanfar, M.H.; Mokdad, A.H.; Naghavi, M.; Murray, C.J.L. Estimates of Global and Regional Premature Cardiovascular Mortality in 2025. Circulation 2015, 132, 1270–1282. [Google Scholar] [CrossRef]
- Naghavi, M.; Makela, S.; Foreman, K.; O’Brien, J.; Pourmalek, F.; Lozano, R. Algorithms for Enhancing Public Health Utility of National Causes-of-Death Data. Popul Health Metrics 2010, 8, 9. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Dargie, H.J.; Byrne, J. Pathophysiological Aspects of the Renin-Angiotensin-Aldosterone System in Acute Myocardial Infarction. Eur. J. Cardiovasc. Prev. Rehabil. 1995, 2, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Schieffer, B.; Schieffer, E.; Hilfiker-Kleiner, D.; Hilfiker, A.; Kovanen, P.T.; Kaartinen, M.; Nussberger, J.; Harringer, W.; Drexler, H. Expression of Angiotensin II and Interleukin 6 in Human Coronary Atherosclerotic Plaques: Potential Implications for Inflammation and Plaque Instability. Circulation 2000, 101, 1372–1378. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Guo, L.; Zhang, Y.; Li, H.; Han, S.; Shen, D. The ACE2-Ang (1–7)-Mas Receptor Axis Attenuates Cardiac Remodeling and Fibrosis in Post-Myocardial Infarction. Mol. Med. Rep. 2017, 16, 1973–1981. [Google Scholar] [CrossRef]
- Xie, J.-X.; Hu, J.; Cheng, J.; Liu, C.; Wei, X. The Function of the ACE2/Ang(1–7)/Mas Receptor Axis of the Renin-Angiotensin System in Myocardial Ischemia Reperfusion Injury. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1852–1859. [Google Scholar] [CrossRef]
- Evans-Lacko, S.; Aguzzoli, E.; Read, S.; Comas-Herrera, A.; Farina, N. World Alzheimer Report 2024: Global Changes in Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2024. [Google Scholar]
- Chatzipieris, F.P.; Kokkalis, A.; Georgiou, N.; Petsas, E.; Apostolou, E.V.; Vougioukalakis, G.C.; Mavromoustakos, T. New Prospects in the Inhibition of Monoamine Oxidase-B (MAO-B) Utilizing Propargylamine Derivatives for the Treatment of Alzheimer’s Disease: A Review. ACS Omega 2025, 10, 26208–26232. [Google Scholar] [CrossRef]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical Trials of New Drugs for Alzheimer Disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef]
- Lyu, D.; Lyu, X.; Huang, L.; Fang, B. Effects of Three Kinds of Anti-Amyloid-β Drugs on Clinical, Biomarker, Neuroimaging Outcomes and Safety Indexes: A Systematic Review and Meta-Analysis of Phase II/III Clinical Trials in Alzheimer’s Disease. Ageing Res. Rev. 2023, 88, 101959. [Google Scholar] [CrossRef]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral Blood Flow Regulation and Neurovascular Dysfunction in Alzheimer Disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Royea, J.; Hamel, E. Brain Angiotensin II and Angiotensin IV Receptors as Potential Alzheimer’s Disease Therapeutic Targets. GeroScience 2020, 42, 1237–1256. [Google Scholar] [CrossRef] [PubMed]
- Kuber, B.; Fadnavis, M.; Chatterjee, B. Role of Angiotensin Receptor Blockers in the Context of Alzheimer’s Disease. Fundam. Clin. Pharmacol. 2023, 37, 429–445. [Google Scholar] [CrossRef]
- Santos, C.R.D.; Grigorova, Y.N.; McDevitt, R.A.; Long, J.M.; Cezayirli, D.; Zernetkina, V.; Wei, W.; Haghkar, M.; Morrell, C.H.; Juhasz, O.; et al. Treatment with Losartan, an AT1 Receptor Blocker, Improves Cognitive and Cardiovascular Function in a Dahl Salt-sensitive Rat Model of Age-associated Vascular Dementia. Alzheimer’s Dement. 2022, 18, e062715. [Google Scholar] [CrossRef]
- Hajjar, I.; Okafor, M.; Wan, L.; Yang, Z.; Nye, J.A.; Bohsali, A.; Shaw, L.M.; Levey, A.I.; Lah, J.J.; Calhoun, V.D.; et al. Safety and Biomarker Effects of Candesartan in Non-Hypertensive Adults with Prodromal Alzheimer’s Disease. Brain Commun. 2022, 4, fcac270. [Google Scholar] [CrossRef]
- Gebre, A.K.; Altaye, B.M.; Atey, T.M.; Tuem, K.B.; Berhe, D.F. Targeting Renin–Angiotensin System Against Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Ababei, D.-C.; Bild, V.; Macadan, I.; Vasincu, A.; Rusu, R.-N.; Blaj, M.; Stanciu, G.D.; Lefter, R.-M.; Bild, W. Therapeutic Implications of Renin-Angiotensin System Modulators in Alzheimer’s Dementia. Pharmaceutics 2023, 15, 2290. [Google Scholar] [CrossRef]
- Cosarderelioglu, C.; Nidadavolu, L.S.; George, C.J.; Oh, E.S.; Bennett, D.A.; Walston, J.D.; Abadir, P.M. Brain Renin–Angiotensin System at the Intersect of Physical and Cognitive Frailty. Front. Neurosci. 2020, 14, 586314. [Google Scholar] [CrossRef]
- Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Carrera-González, M.P.; Martínez-Martos, J.M. Physiopathology of the Brain Renin-Angiotensin System. Life 2025, 15, 1333. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Q.; Yuan, S.; Wang, J.; Li, F.; Gao, H.; Chen, X.; Yang, R.; Xu, J. AT1 Receptors: Their Actions from Hypertension to Cognitive Impairment. Cardiovasc. Toxicol. 2022, 22, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Contaldi, E.; Magistrelli, L.; Milner, A.; Cosentino, M.; Marino, F.; Comi, C. Potential Protective Role of ACE-Inhibitors and AT1 Receptor Blockers against Levodopa-Induced Dyskinesias: A Retrospective Case-Control Study. Neural Regen. Res. 2021, 16, 2475. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Otero-Losada, M.; Toblli, J.E.; Capani, F. Renin-Angiotensin System as a Potential Target for New Therapeutic Approaches in Parkinson’s Disease. Expert Opin. Investig. Drugs 2017, 26, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Udovin, L.; Otero-Losada, M.; Bordet, S.; Chevalier, G.; Quarracino, C.; Capani, F.; Pérez-Lloret, S. Effects of Angiotensin Type 1 Receptor Antagonists on Parkinson’s Disease Progression: An Exploratory Study in the PPMI Database. Park. Relat. Disord. 2021, 86, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-R.; Jiang, T.; Tian, Y.-Y.; Gao, Q.; Li, Z.; Pan, Y.; Wu, L.; Lu, J.; Zhang, Y.-D. Angiotensin II Triggers Apoptosis Via Enhancement of NADPH Oxidase-Dependent Oxidative Stress in a Dopaminergic Neuronal Cell Line. Neurochem. Res. 2015, 40, 854–863. [Google Scholar] [CrossRef]
- Kobiec, T.; Otero-Losada, M.; Chevalier, G.; Udovin, L.; Bordet, S.; Menéndez-Maissonave, C.; Capani, F.; Pérez-Lloret, S. The Renin–Angiotensin System Modulates Dopaminergic Neurotransmission: A New Player on the Scene. Front. Synaptic Neurosci. 2021, 13, 638519. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Labandeira, C.M.; Guerra, M.J.; Rodriguez-Perez, A.I. The Role of the Brain Renin-Angiotensin System in Parkinson’s Disease. Transl. Neurodegener. 2024, 13, 22. [Google Scholar] [CrossRef]
- Rothlin, R.P.; Pelorosso, F.G.; Duarte, M.; Nicolosi, L.; Ignacio, F.C.; Salgado, M.V.; Vetulli, H. Telmisartan and Losartan: The Marked Differences between Their Chemical and Pharmacological Properties May Explain the Difference in Therapeutic Efficacy in Hospitalized Patients with COVID-19. Pharmacol. Res. Perspect. 2023, 11, e01083. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, M.A.; El-Assal, M.I.A.; Tadros, M.I.; El-Gazayerly, O.N. Olmesartan Medoxomil-Loaded Mixed Micelles: Preparation, Characterization and in-Vitro Evaluation. Future J. Pharm. Sci. 2017, 3, 90–94. [Google Scholar] [CrossRef]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Pharmacodynamic Study of Eprosartan Mesylate-Loaded Transfersomes Carbopol® Gel under Dermaroller® on Rats with Methyl Prednisolone Acetate-Induced Hypertension. Biomed. Pharmacother. 2017, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Darwish, H.W.; Bakheit, A.H.; Al-Kahtani, H.M.; Alanazi, Z. Irbesartan (a Comprehensive Profile). In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: London, UK, 2021; Volume 46, pp. 185–272. ISBN 978-0-12-824127-1. [Google Scholar]
- Gebhart, M.; Elvert, C.A.; Schoepf, A.M.; Scheiber, A.; Schwaiger, S.; Karg, C.A.; Gust, R.; Salcher, S. Key Determinants of the Chemo-Sensitizing Activity of Novel Telmisartan Derivatives. Eur. J. Med. Chem. Rep. 2025, 15, 100281. [Google Scholar] [CrossRef]
- Anwar, W.; Dawaba, H.; Afouna, M.; Samy, A.; Rashed, M.; Abdelaziz, A. Enhancing the Oral Bioavailability of Candesartan Cilexetil Loaded Nanostructured Lipid Carriers: In Vitro Characterization and Absorption in Rats after Oral Administration. Pharmaceutics 2020, 12, 1047. [Google Scholar] [CrossRef]
- Glodzik, L.; Santisteban, M.M. Blood-Brain Barrier Crossing Renin-Angiotensin System Drugs: Considerations for Dementia and Cognitive Decline. Hypertension 2021, 78, 644–646. [Google Scholar] [CrossRef]
- Ho, J.K.; Moriarty, F.; Manly, J.J.; Larson, E.B.; Evans, D.A.; Rajan, K.B.; Hudak, E.M.; Hassan, L.; Liu, E.; Sato, N.; et al. Blood-Brain Barrier Crossing Renin-Angiotensin Drugs and Cognition in the Elderly: A Meta-Analysis. Hypertension 2021, 78, 629–643. [Google Scholar] [CrossRef]
- Bordet, S.; Grasso, L.; Udovin, L.; Chevalier, G.; Otero-Losada, M.; Capani, F.; Perez-Lloret, S. An Open-Label, Non-randomized, Drug-Repurposing Study to Explore the Clinical Effects of Angiotensin II Type 1 (AT1) Receptor Antagonists on Anxiety and Depression in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2025, 12, 653–658. [Google Scholar] [CrossRef]
- Balthazar, L.; Lages, Y.V.M.; Romano, V.C.; Landeira-Fernandez, J.; Krahe, T.E. The Association between the Renin-Angiotensin System and the Hypothalamic-Pituitary-Adrenal Axis in Anxiety Disorders: A Systematic Review of Animal Studies. Psychoneuroendocrinology 2021, 132, 105354. [Google Scholar] [CrossRef]
- Raasch, W.; Wittmershaus, C.; Dendorfer, A.; Voges, I.; Pahlke, F.; Dodt, C.; Dominiak, P.; Jöhren, O. Angiotensin II Inhibition Reduces Stress Sensitivity of Hypothalamo-Pituitary-Adrenal Axis in Spontaneously Hypertensive Rats. Endocrinology 2006, 147, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Armando, I.; Carranza, A.; Nishimura, Y.; Hoe, K.L.; Barontini, M.; Saavedra, J.M. The Role of Angiotensin II AT1 Receptors in the Sympathoadrenal Response to Stress. In Catecholamine Research; Nagatsu, T., Nabeshima, T., McCarty, R., Goldstein, D.S., Eds.; Advances in Behavioral Biology; Springer: Boston, MA, USA, 2002; Volume 53, pp. 313–316. ISBN 978-1-4419-3388-1. [Google Scholar]
- Konain, K.; Faheem, M.; Ullah, K.; Ayub, S.; Ahmed, J.; Huma, Z.; Javed, A.; Khan, T.; Hussain, D.; Khan, I.N. Biomarker-Guided Drug Repurposing and Molecular Validation of Angiotensin-2 Receptor Type-1 in Brain Tumor. Precis. Med. Commun. 2023, 3, 27–42. [Google Scholar] [CrossRef]
- O’Rawe, M.; Wickremesekera, A.C.; Pandey, R.; Young, D.; Sim, D.; FitzJohn, T.; Burgess, C.; Kaye, A.H.; Tan, S.T. Treatment of Glioblastoma with Re-Purposed Renin-Angiotensin System Modulators: Results of a Phase I Clinical Trial. J. Clin. Neurosci. 2022, 95, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, H.; Chasapis, C.T.; Kelaidonis, K.; Ligielli, I.; Moore, G.J.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; Mavromoustakos, T.; Matsoukas, J.M. Understanding the Driving Forces That Trigger Mutations in SARS-CoV-2: Mutational Energetics and the Role of Arginine Blockers in COVID-19 Therapy. Viruses 2022, 14, 1029. [Google Scholar] [CrossRef] [PubMed]
- Hajji, N.; Garcia-Revilla, J.; Soto, M.S.; Perryman, R.; Symington, J.; Quarles, C.C.; Healey, D.R.; Guo, Y.; Orta-Vázquez, M.L.; Mateos-Cordero, S.; et al. Arginine Deprivation Alters Microglial Polarity and Synergizes with Radiation to Eradicate Non-Arginine-Auxotrophic Glioblastoma Tumors. J. Clin. Investig. 2022, 132, e142137. [Google Scholar] [CrossRef]
- Perryman, R.; Renziehausen, A.; Shaye, H.; Kostagianni, A.D.; Tsiailanis, A.D.; Thorne, T.; Chatziathanasiadou, M.V.; Sivolapenko, G.B.; El Mubarak, M.A.; Han, G.W.; et al. Inhibition of the Angiotensin II Type 2 Receptor AT2R Is a Novel Therapeutic Strategy for Glioblastoma. Proc. Natl. Acad. Sci. USA 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L. The Other Angiotensin II Receptor: AT2R as a Therapeutic Target. J. Med. Chem. 2020, 63, 1978–1995. [Google Scholar] [CrossRef]
- Arrieta, O.; Pineda-Olvera, B.; Guevara-Salazar, P.; Hernández-Pedro, N.; Morales-Espinosa, D.; Cerón-Lizarraga, T.L.; González-De La Rosa, C.H.; Rembao, D.; Segura-Pacheco, B.; Sotelo, J. Expression of AT1 and AT2 Angiotensin Receptors in Astrocytomas Is Associated with Poor Prognosis. Br. J. Cancer 2008, 99, 160–166. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xie, J.; Liang, B.; Wu, J. Suppression of Angiotensin-(1–7) on the Disruption of Blood-Brain Barrier in Rat of Brain Glioma. Pathol. Oncol. Res. 2019, 25, 429–435. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Li, L.-H.; Hsiao, Y.-Y.; Wong, W.-T.; Chiu, H.-W.; Hsu, H.-T.; Peng, Y.-J.; Ho, C.-L.; Chernikov, O.V.; Cheng, S.-M.; et al. Repositioning of the Angiotensin II Receptor Antagonist Candesartan as an Anti-Inflammatory Agent With NLRP3 Inflammasome Inhibitory Activity. Front. Immunol. 2022, 13, 870627. [Google Scholar] [CrossRef]
- Awad, K.; Zaki, M.M.; Mohammed, M.; Lewek, J.; Lavie, C.J.; Banach, M. Effect of the Renin-Angiotensin System Inhibitors on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mayo Clin. Proc. 2022, 97, 1808–1823. [Google Scholar] [CrossRef]
- Stegbauer, J.; Lee, D.-H.; Seubert, S.; Ellrichmann, G.; Manzel, A.; Kvakan, H.; Muller, D.N.; Gaupp, S.; Rump, L.C.; Gold, R.; et al. Role of the Renin-Angiotensin System in Autoimmune Inflammation of the Central Nervous System. Proc. Natl. Acad. Sci. USA 2009, 106, 14942–14947. [Google Scholar] [CrossRef] [PubMed]
- Haliga, R.E.; Cojocaru, E.; Sîrbu, O.; Hrițcu, I.; Alexa, R.E.; Haliga, I.B.; Șorodoc, V.; Coman, A.E. Immunomodulatory Effects of RAAS Inhibitors: Beyond Hypertension and Heart Failure. Biomedicines 2025, 13, 1779. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Chatzantoni, K.; Matsoukas, M.-T.; Tselios, T.; Deraos, S.; Katsara, M.; Papathanasopoulos, P.; Vynios, D.; Apostolopoulos, V.; Mouzaki, A.; et al. Citrullination of Linear and Cyclic Altered Peptide Ligands from Myelin Basic Protein (MBP87−99) Epitope Elicits a Th1 Polarized Response by T Cells Isolated from Multiple Sclerosis Patients: Implications in Triggering Disease. J. Med. Chem. 2008, 51, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, E.; Mavromoustakos, T.; Platts, J.; Matsoukas, J.; Tselios, T. Structural Requirements for Binding of Myelin Basic Protein (MBP) Peptides to MHC II: Effects on Immune Regulation. Curr. Med. Chem. 2005, 12, 1521–1535. [Google Scholar] [CrossRef]
- Ridgway, H.; Moore, G.J.; Mavromoustakos, T.; Tsiodras, S.; Ligielli, I.; Kelaidonis, K.; Chasapis, C.T.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; et al. Discovery of a New Generation of Angiotensin Receptor Blocking Drugs: Receptor Mechanisms and in Silico Binding to Enzymes Relevant to SARS-CoV-2. Comput. Struct. Biotechnol. J. 2022, 20, 2091–2111. [Google Scholar] [CrossRef]
- Lara, V.S.; Silva, R.A.D.; Ferrari, T.P.; Santos, C.F.D.; Oliveira, S.H.P.D. Losartan Plays a Fungistatic and Fungicidal Activity Against Candida Albicans Biofilms: Drug Repurposing for Localized Candidosis. ASSAY Drug Dev. Technol. 2023, 21, 157–165. [Google Scholar] [CrossRef]
- Sumners, C.; Peluso, A.A.; Haugaard, A.H.; Bertelsen, J.B.; Steckelings, U.M. Anti-fibrotic Mechanisms of Angiotensin AT2-receptor Stimulation. Acta Physiol. 2019, 227, e13280. [Google Scholar] [CrossRef]
- Murphy, A.M.; Wong, A.L.; Bezuhly, M. Modulation of Angiotensin II Signaling in the Prevention of Fibrosis. Fibrogenesis Tissue Repair 2015, 8, 7. [Google Scholar] [CrossRef]
- Chappell, M.C.; Al Zayadneh, E.M. Angiotensin-(1–7) and the Regulation of Anti-Fibrotic Signaling Pathways. J. Cell Signal 2017, 2, 134. [Google Scholar] [CrossRef]
- An, Y.; Xu, C.; Liu, W.; Jiang, J.; Ye, P.; Yang, M.; Zhu, W.; Yu, J.; Yu, M.; Sun, W.; et al. Angiotensin II Type-2 Receptor Attenuates Liver Fibrosis Progression by Suppressing IRE1α-XBP1 Pathway. Cell. Signal. 2024, 113, 110935. [Google Scholar] [CrossRef]
- Corey, K.E.; Shah, N.; Misdraji, J.; Abu Dayyeh, B.K.; Zheng, H.; Bhan, A.K.; Chung, R.T. The Effect of Angiotensin-blocking Agents on Liver Fibrosis in Patients with Hepatitis C. Liver Int. 2009, 29, 748–753. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Takagi, K.; Hara, M.; Fukasawa, C.; Sugiura, T.; Nishimagi, E.; Harigai, M.; Kamatani, N. Angiotensin II in the Lesional Skin of Systemic Sclerosis Patients Contributes to Tissue Fibrosis via Angiotensin II Type 1 Receptors. Arthritis Rheum. 2004, 50, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Dziadzio, M.; Denton, C.P.; Smith, R.; Howell, K.; Blann, A.; Bowers, E.; Black, C.M. Losartan Therapy for Raynaud’s Phenomenon and Scleroderma: Clinical and Biochemical Findings in a Fifteen-Week, Randomized, Parallel-Group, Controlled Trial. Arthritis Rheum. 1999, 42, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Iwane, S.; Nemoto, W.; Miyamoto, T.; Hayashi, T.; Tanaka, M.; Uchitani, K.; Muranaka, T.; Fujitani, M.; Koizumi, Y.; Hirata, A.; et al. Clinical and Preclinical Evidence That Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Prevent Diabetic Peripheral Neuropathy. Sci. Rep. 2024, 14, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Nemoto, W.; Nakagawasai, O.; Yamagata, R.; Tadano, T.; Tan-No, K. Involvement of Spinal Angiotensin II System in Streptozotocin-Induced Diabetic Neuropathic Pain in Mice. Mol. Pharmacol. 2016, 90, 205–213. [Google Scholar] [CrossRef]
- Maxfield, E.K.; Cameron, N.E.; Cotter, M.A.; Dines, K.C. Angiotensin II Receptor Blockade Improves Nerve Function, Modulates Nerve Blood Flow and Stimulates Endoneurial Angiogenesis in Streptozotocin-Diabetic Ratsand Nerve Function. Diabetologia 1993, 36, 1230–1237. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-Induced Production of Mitochondrial Reactive Oxygen Species: Potential Mechanisms and Relevance for Cardiovascular Disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- Salmenkari, H.; Korpela, R.; Vapaatalo, H. Renin–Angiotensin System in Intestinal Inflammation—Angiotensin Inhibitors to Treat Inflammatory Bowel Diseases? Basic Clin. Pharmacol. Toxicol. 2021, 129, 161–172. [Google Scholar] [CrossRef]
- He, L.; Du, J.; Chen, Y.; Liu, C.; Zhou, M.; Adhikari, S.; Rubin, D.T.; Pekow, J.; Li, Y.C. Renin-Angiotensin System Promotes Colonic Inflammation by Inducing TH17 Activation via JAK2/STAT Pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G774–G784. [Google Scholar] [CrossRef]
- Khajah, M.A.; Fateel, M.M.; Ananthalakshmi, K.V.; Luqmani, Y.A. Anti-Inflammatory Action of Angiotensin 1–7 in Experimental Colitis. PLoS ONE 2016, 11, e0150861. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.D.; Wagner, T.; Gulotta, G.; Liao, C.; Li, Y.C.; Bissonnette, M.; Pekow, J. Impact of Angiotensin II Signaling Blockade on Clinical Outcomes in Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2019, 64, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, A.; Spata, E.; Emberson, J.; Davies, K.; Halls, H.; Holland, L.; Wilson, K.; Reith, C.; Child, A.H.; Clayton, T.; et al. Angiotensin Receptor Blockers and β Blockers in Marfan Syndrome: An Individual Patient Data Meta-Analysis of Randomised Trials. Lancet 2022, 400, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 Antagonist, Prevents Aortic Aneurysm in a Mouse Model of Marfan Syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef]
- Habashi, J.P.; Doyle, J.J.; Holm, T.M.; Aziz, H.; Schoenhoff, F.; Bedja, D.; Chen, Y.; Modiri, A.N.; Judge, D.P.; Dietz, H.C. Angiotensin II Type 2 Receptor Signaling Attenuates Aortic Aneurysm in Mice Through ERK Antagonism. Science 2011, 332, 361–365. [Google Scholar] [CrossRef]
- Cohn, R.D.; Van Erp, C.; Habashi, J.P.; Soleimani, A.A.; Klein, E.C.; Lisi, M.T.; Gamradt, M.; Ap Rhys, C.M.; Holm, T.M.; Loeys, B.L.; et al. Angiotensin II Type 1 Receptor Blockade Attenuates TGF-β–Induced Failure of Muscle Regeneration in Multiple Myopathic States. Nat. Med. 2007, 13, 204–210. [Google Scholar] [CrossRef]
- Asano, K.; Cantalupo, A.; Sedes, L.; Ramirez, F. Pathophysiology and Therapeutics of Thoracic Aortic Aneurysm in Marfan Syndrome. Biomolecules 2022, 12, 128. [Google Scholar] [CrossRef]
- Matsoukas, J.; Apostolopoulos, V.; Zulli, A.; Moore, G.; Kelaidonis, K.; Moschovou, K.; Mavromoustakos, T. From Angiotensin II to Cyclic Peptides and Angiotensin Receptor Blockers (ARBs): Perspectives of ARBs in COVID-19 Therapy. Molecules 2021, 26, 618. [Google Scholar] [CrossRef]
- Jardine, M.J.; Kotwal, S.S.; Bassi, A.; Hockham, C.; Jones, M.; Wilcox, A.; Pollock, C.; Burrell, L.M.; McGree, J.; Rathore, V.; et al. Angiotensin Receptor Blockers for the Treatment of COVID-19: Pragmatic, Adaptive, Multicentre, Phase 3, Randomised Controlled Trial. BMJ 2022, 379, e072175. [Google Scholar] [CrossRef]
- Martins, A.L.V.; Annoni, F.; Da Silva, F.A.; Bolais-Ramos, L.; De Oliveira, G.C.; Ribeiro, R.C.; Diniz, M.M.L.; Silva, T.G.F.; Pinheiro, B.D.; Rodrigues, N.A.; et al. Angiotensin-(1–7) Infusion in COVID-19 Patients Admitted to the ICU: A Seamless Phase 1–2 Randomized Clinical Trial. Ann. Intensive Care 2024, 14, 139. [Google Scholar] [CrossRef]
- Moreira, F.R.C.; De Oliveira, T.A.; Ramos, N.E.; Abreu, M.A.D.; Simões E Silva, A.C. The Role of Renin Angiotensin System in the Pathophysiology of Rheumatoid Arthritis. Mol. Biol. Rep. 2021, 48, 6619–6629. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, H.; Choudhary, G.; Prakash, A.; Medhi, B.; Chatterjee, D.; Saini, U.C.; Kaur, J.; Verma, I.; Sharma, S. Natural Angiotensin II Type 1 Receptor Inhibitors: Virtual Screening and in Vitro Evaluation of Beta-1,2,3,4,6-Penta-O-Galloyl-d-Glucopyranose, Icarrin, and Sesamin for Osteoarthritis Therapy. Int. J. Biol. Macromol. 2025, 309, 142184. [Google Scholar] [CrossRef]
- Guo, Z.; Di, J.; Zhang, Z.; Chen, S.; Mao, X.; Wang, Z.; Yan, Z.; Li, X.; Tian, Z.; Mu, C.; et al. Antihypertensive Drug-Associated Adverse Events in Osteoarthritis: A Study of a Large Real-World Sample Based on the FAERS Database. Front. Pharmacol. 2024, 15, 1404427. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health; Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services: Rockville, MD, USA, 2020. [Google Scholar]
- Ignaszewski, M.J. The Epidemiology of Drug Abuse. J. Clin. Pharmacol. 2021, 61, S10–S17. [Google Scholar] [CrossRef]
- Ridgway, H.; Moore, G.J.; Gadanec, L.K.; Matsoukas, J.M. Docking Simulations of G-Protein Coupled Receptors Uncover Crossover Binding Patterns of Diverse Ligands to Angiotensin, Alpha-Adrenergic and Opioid Receptors: Implications for Cardiovascular Disease and Addiction. Biomolecules 2025, 15, 855. [Google Scholar] [CrossRef]
- Colombo, G.L.; Caruggi, M.; Ottolini, C.; Maggioni, A.P. Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) and Resource Utilization and Costs in Italy. Vasc. Health Risk Manag. 2008, 4, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Latini, R.; Masson, S.; Anand, I.; Judd, D.; Maggioni, A.P.; Chiang, Y.-T.; Bevilacqua, M.; Salio, M.; Cardano, P.; Dunselman, P.H.J.M.; et al. Effects of Valsartan on Circulating Brain Natriuretic Peptide and Norepinephrine in Symptomatic Chronic Heart Failure: The Valsartan Heart Failure Trial (Val-HeFT). Circulation 2002, 106, 2454–2458. [Google Scholar] [CrossRef]
- Sharma, M. The RENAAL Study Investigation. Clin. Diabetes 2002, 20, 19–20. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed]
- The ONTARGET Investigators. Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; McMurray, J.J.V.; Velazquez, E.J.; Rouleau, J.-L.; Køber, L.; Maggioni, A.P.; Solomon, S.D.; Swedberg, K.; Van De Werf, F.; White, H.; et al. Valsartan, Captopril, or Both in Myocardial Infarction Complicated by Heart Failure, Left Ventricular Dysfunction, or Both. N. Engl. J. Med. 2003, 349, 1893–1906. [Google Scholar] [CrossRef]
- Dickstein, K.; Kjekshus, J. Effects of Losartan and Captopril on Mortality and Morbidity in High-Risk Patients after Acute Myocardial Infarction: The OPTIMAAL Randomised Trial. Lancet 2002, 360, 752–760. [Google Scholar] [CrossRef]
- Suzuki, H.; Kusuyama, T.; Omori, Y.; Soda, T.; Tsunoda, F.; Sato, T.; Shoji, M.; Iso, Y.; Kondo, T.; Koba, S.; et al. Inhibitory Effect of Candesartan Cilexetil on Left Ventricular Remodeling After Myocardial Infarction. Int. Heart J. 2006, 47, 715–725. [Google Scholar] [CrossRef]
- Akshayraj Vanrajbhai, C.; Thorat, V.M.; Patel, V.S.; Patil, K.P.; Chawla, L.L. Evaluation of Anxiolytic Activity of Angiotensin Receptor Blockers Using Actophotometer Test in Wistar Rats. Cureus 2024, 16, e69798. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Chatterjee, S.; Perez, E.M.; Gritsch, S.; Roberge, S.; Duquette, M.; Chen, I.X.; Naxerova, K.; Kumar, A.S.; Ghosh, M.; et al. Losartan Controls Immune Checkpoint Blocker-Induced Edema and Improves Survival in Glioblastoma Mouse Models. Proc. Natl. Acad. Sci. USA 2023, 120, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Gad, A.I.; El-Sayed, M.A.; El-Ganiny, A.M. Impeding Virulence of Candida Albicans by Candesartan and Domperidone. Curr. Microbiol. 2021, 78, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Couluris, M.; Kinder, B.W.; Xu, P.; Gross-King, M.; Krischer, J.; Panos, R.J. Treatment of Idiopathic Pulmonary Fibrosis with Losartan: A Pilot Project. Lung 2012, 190, 523–527. [Google Scholar] [CrossRef]
- Attia, Y.M.; Elalkamy, E.F.; Hammam, O.A.; Mahmoud, S.S.; El-Khatib, A.S. Telmisartan, an AT1 Receptor Blocker and a PPAR Gamma Activator, Alleviates Liver Fibrosis Induced Experimentally by Schistosoma Mansoni Infection. Parasit. Vectors 2013, 6, 199. [Google Scholar] [CrossRef]
- Mostafa, T.M.; El-Azab, G.A.; Badra, G.A.; Abdelwahed, A.S.; Elsayed, A.A. Effect of Candesartan and Ramipril on Liver Fibrosis in Patients with Chronic Hepatitis C Viral Infection: A Randomized Controlled Prospective Study. Curr. Ther. Res. Clin. Exp. 2021, 95, 100654. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Q.; Ding, Z.; Zhang, X.; Li, Y.; Zang, Y.; Zhang, G. Silibinin Augments the Antifibrotic Effect of Valsartan Through Inactivation of TGF-Β1 Signaling in Kidney. Drug Des. Devel Ther. 2020, 14, 603–611. [Google Scholar] [CrossRef]
- Cavusoglu, T.; Karadeniz, T.; Cagiltay, E.; Karadeniz, M.; Yigitturk, G.; Acikgoz, E.; Uyanikgil, Y.; Ates, U.; Tuglu, M.; Erbas, O. The Protective Effect of Losartan on Diabetic Neuropathy in a Diabetic Rat Model. Exp. Clin. Endocrinol. Diabetes 2015, 123, 479–484. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Ahmed, M.M.; Arrejaie, A.S.; Aleisa, A.M.; AlSharari, S.D. Telmisartan Inhibits Hyperalgesia and Inflammatory Progression in a Diabetic Neuropathic Pain Model of Wistar Rats. Neurosciences 2015, 20, 115–123. [Google Scholar] [CrossRef]
- Wengrower, D.; Zanninelli, G.; Latella, G.; Necozione, S.; Metanes, I.; Israeli, E.; Lysy, J.; Pines, M.; Papo, O.; Goldin, E. Losartan Reduces Trinitrobenzene Sulphonic Acid-Induced Colorectal Fibrosis in Rats. Can. J. Gastroenterol. 2012, 26, 33–39. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan Attenuates Colon Inflammation, Oxidative Perturbations and Apoptosis in a Rat Model of Experimental Inflammatory Bowel Disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef] [PubMed]
- Okawada, M.; Miyasaka, E.A.; Teitelbaum, D.H. Blockade of Angiotensin II Type 1a Receptor in a DSS Model of Colitis Results in Significant Expansion of Foxp3 Regulatory T Cells. J. Am. Coll. Surg. 2010, 211, S12. [Google Scholar] [CrossRef]
- Santiago, O.I.; Rivera, E.; Ferder, L.; Appleyard, C.B. An Angiotensin II Receptor Antagonist Reduces Inflammatory Parameters in Two Models of Colitis. Regul. Pept. 2008, 146, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Groenink, M.; Den Hartog, A.W.; Franken, R.; Radonic, T.; De Waard, V.; Timmermans, J.; Scholte, A.J.; Van Den Berg, M.P.; Spijkerboer, A.M.; Marquering, H.A.; et al. Losartan Reduces Aortic Dilatation Rate in Adults with Marfan Syndrome: A Randomized Controlled Trial. Eur. Heart J. 2013, 34, 3491–3500. [Google Scholar] [CrossRef]
- Muiño-Mosquera, L.; De Backer, J. Angiotensin-II Receptor Blockade in Marfan Syndrome. Lancet 2019, 394, 2206–2207. [Google Scholar] [CrossRef]
- Takagi, H.; Yamamoto, H.; Iwata, K.; Goto, S.; Umemoto, T. An Evidence-Based Hypothesis for Beneficial Effects of Telmisartan on Marfan Syndrome. Int. J. Cardiol. 2012, 158, 101–102. [Google Scholar] [CrossRef]
- Duarte, M.; Pelorosso, F.; Nicolosi, L.N.; Victoria Salgado, M.; Vetulli, H.; Aquieri, A.; Azzato, F.; Castro, M.; Coyle, J.; Davolos, I.; et al. Telmisartan for Treatment of COVID-19 Patients: An Open Multicenter Randomized Clinical Trial. eClinicalMedicine 2021, 37, 100962. [Google Scholar] [CrossRef]
- Pedrosa, M.A.; Valenzuela, R.; Garrido-Gil, P.; Labandeira, C.M.; Navarro, G.; Franco, R.; Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I. Experimental Data Using Candesartan and Captopril Indicate No Double-Edged Sword Effect in COVID-19. Clin. Sci. 2021, 135, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Huang, W.; Zhang, P.; Guo, Y.; Körner, H.; Wu, H.; Wei, W. Losartan Suppresses the Inflammatory Response in Collagen-Induced Arthritis by Inhibiting the MAPK and NF-κB Pathways in B and T Cells. Inflammopharmacol 2019, 27, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, K.; Nagatani, K.; Komagata, Y.; Yamamoto, K. Angiotensin Receptor Blockers Suppress Antigen-specific T Cell Responses and Ameliorate Collagen-induced Arthritis in Mice. Arthritis Rheum. 2005, 52, 1920–1928. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, F.; Liu, Y.; Wang, J.; Lu, W.; Jiang, W.; Zhu, W. Losartan Protects against Osteoarthritis by Repressing the TGF-Β1 Signaling Pathway via Upregulation of PPARγ. J. Orthop. Transl. 2021, 29, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Karádi, D.Á.; Galambos, A.R.; Lakatos, P.P.; Apenberg, J.; Abbood, S.K.; Balogh, M.; Király, K.; Riba, P.; Essmat, N.; Szűcs, E.; et al. Telmisartan Is a Promising Agent for Managing Neuropathic Pain and Delaying Opioid Analgesic Tolerance in Rats. Int. J. Mol. Sci. 2023, 24, 7970. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, F.; Yao, J.; Su, S.; Zhao, Z. Angiotensin II Receptor Type 1 Blocker Candesartan Improves Morphine Tolerance by Reducing Morphine-induced Inflammatory Response and Cellular Activation of BV2 Cells via the PPARγ/AMPK Signaling Pathway. Mol. Med. Rep. 2022, 26, 318. [Google Scholar] [CrossRef]
- Moutevelis-Minakakis, P.; Gianni, M.; Stougiannou, H.; Zoumpoulakis, P.; Zoga, A.; Vlahakos, A.D.; Iliodromitis, E.; Mavromoustakos, T. Design and Synthesis of Novel Antihypertensive Drugs. Bioorganic Med. Chem. Lett. 2003, 13, 1737–1740. [Google Scholar] [CrossRef]
- Mavromoustakos, T.; Moutevelis-Minakakis, P.; Kokotos, C.G.; Kontogianni, P.; Politi, A.; Zoumpoulakis, P.; Findlay, J.; Cox, A.; Balmforth, A.; Zoga, A.; et al. Synthesis, Binding Studies and in Vivo Biological Evaluation of Novel Non-Peptide Antihypertensive Analogues. Bioorganic Med. Chem. 2006, 14, 4353–4360. [Google Scholar] [CrossRef]
- Zoumpoulakis, P.; Politi, A.; Grdadolnik, S.G.; Matsoukas, J.; Mavromoustakos, T. Structure Elucidation and Conformational Study of V8. J. Pharm. Biomed. Anal. 2006, 40, 1097–1104. [Google Scholar] [CrossRef]
- Agelis, G.; Roumelioti, P.; Resvani, A.; Durdagi, S.; Androutsou, M.-E.; Kelaidonis, K.; Vlahakos, D.; Mavromoustakos, T.; Matsoukas, J. An Efficient Synthesis of a Rationally Designed 1,5 Disubstituted Imidazole AT1 Angiotensin II Receptor Antagonist: Reorientation of Imidazole Pharmacophore Groups in Losartan Reserves High Receptor Affinity and Confirms Docking Studies. J. Comput. Aided Mol. Des. 2010, 24, 749–758. [Google Scholar] [CrossRef]
- Agelis, G.; Resvani, A.; Durdagi, S.; Spyridaki, K.; Tůmová, T.; Slaninová, J.; Giannopoulos, P.; Vlahakos, D.; Liapakis, G.; Mavromoustakos, T.; et al. The Discovery of New Potent Non-Peptide Angiotensin II AT1 Receptor Blockers: A Concise Synthesis, Molecular Docking Studies and Biological Evaluation of N-Substituted 5-Butylimidazole Derivatives. Eur. J. Med. Chem. 2012, 55, 358–374. [Google Scholar] [CrossRef]
- Agelis, G.; Resvani, A.; Ntountaniotis, D.; Chatzigeorgiou, P.; Koukoulitsa, C.; Androutsou, M.E.; Plotas, P.; Matsoukas, J.; Mavromoustakos, T.; Čendak, T.; et al. Interactions of the Potent Synthetic AT1 Antagonist Analog BV6 with Membrane Bilayers and Mesoporous Silicate Matrices. Biochim. Et. Biophys. Acta (BBA)—Biomembr. 2013, 1828, 1846–1855. [Google Scholar] [CrossRef]
- Moore, G.J.; Ridgway, H.; Kelaidonis, K.; Chasapis, C.T.; Ligielli, I.; Mavromoustakos, T.; Bojarska, J.; Matsoukas, J.M. Actions of Novel Angiotensin Receptor Blocking Drugs, Bisartans, Relevant for COVID-19 Therapy: Biased Agonism at Angiotensin Receptors and the Beneficial Effects of Neprilysin in the Renin Angiotensin System. Molecules 2022, 27, 4854. [Google Scholar] [CrossRef] [PubMed]
- Kelaidonis, K.; Ligielli, I.; Letsios, S.; Vidali, V.P.; Mavromoustakos, T.; Vassilaki, N.; Moore, G.J.; Hoffmann, W.; Węgrzyn, K.; Ridgway, H.; et al. Computational and Enzymatic Studies of Sartans in SARS-CoV-2 Spike RBD-ACE2 Binding: The Role of Tetrazole and Perspectives as Antihypertensive and COVID-19 Therapeutics. Int. J. Mol. Sci. 2023, 24, 8454. [Google Scholar] [CrossRef] [PubMed]
- Agelis, G.; Resvani, A.; Koukoulitsa, C.; Tůmová, T.; Slaninová, J.; Kalavrizioti, D.; Spyridaki, K.; Afantitis, A.; Melagraki, G.; Siafaka, A.; et al. Rational Design, Efficient Syntheses and Biological Evaluation of N, N′-Symmetrically Bis-Substituted Butylimidazole Analogs as a New Class of Potent Angiotensin II Receptor Blockers. Eur. J. Med. Chem. 2013, 62, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Tsiailanis, A.D.; Renziehausen, A.; Kiriakidi, S.; Vrettos, E.I.; Markopoulos, G.S.; Sayyad, N.; Hirmiz, B.; Aguilar, M.-I.; Del Borgo, M.P.; Kolettas, E.; et al. Enhancement of Glioblastoma Multiforme Therapy through a Novel Quercetin-Losartan Hybrid. Free Radic. Biol. Med. 2020, 160, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Tsiailanis, A.D.; Vrettos, E.I.; Choleva, M.; Kiriakidi, S.; Ganai, A.M.; Patha, T.K.; Karpoormath, R.; Mavromoustakos, T.; Fragopoulou, E.; Tzakos, A.G. Development of a DHA-Losartan Hybrid as a Potent Inhibitor of Multiple Pathway-Induced Platelet Aggregation. J. Biomol. Struct. Dyn. 2022, 40, 13889–13900. [Google Scholar] [CrossRef]
- Georgiou, N.; Gkalpinos, V.K.; Katsakos, S.D.; Vassiliou, S.; Tzakos, A.G.; Mavromoustakos, T. Rational Design and Synthesis of AT1R Antagonists. Molecules 2021, 26, 2927. [Google Scholar] [CrossRef]
- Kritsi, E.; Matsoukas, M.-T.; Potamitis, C.; Karageorgos, V.; Detsi, A.; Magafa, V.; Liapakis, G.; Mavromoustakos, T.; Zoumpoulakis, P. Exploring New Scaffolds for Angiotensin II Receptor Antagonism. Bioorganic Med. Chem. 2016, 24, 4444–4451. [Google Scholar] [CrossRef]
- Inada, Y.; Nakane, T.; Chiba, S. Binding of KRH-594, an Antagonist of the Angiotensin II Type 1 Receptor, to Cloned Human and Rat Angiotensin II Receptors. Fundam. Clin. Pharmacol. 2002, 16, 317–323. [Google Scholar] [CrossRef]
- Hirata, T.; Nomiyama, J.; Sakae, N.; Nishimura, K.; Yokomoto, M.; Inoue, S.; Tamura, K.; Okuhira, M.; Amano, H.; Nagao, Y. Acyliminothiadiazoline Derivatives: New, Highly Potent, and Orally Active Angiotensin II Receptor Antagonists. Bioorganic Med. Chem. Lett. 1996, 6, 1469–1474. [Google Scholar] [CrossRef]
- Tamura, K.; Okuhira, M.; Amano, H.; Inokuma, K.; Hirata, T.; Mikoshiba, I.; Hashimoto, K. Pharmacologic Profiles of KRH-594, a Novel Nonpeptide Angiotensin II-Receptor Antagonist. J. Cardiovasc. Pharmacol. 1997, 30, 607–615. [Google Scholar] [CrossRef]
- Kotobuki, P. (Kotobuki Research Laboratories, Kotobuki Seiyaku Company, Ltd., Kawagoe, Japan). Unpublished Data Held on File.
- Patterson, D.; Webster, J.; McInnes, G.; Brady, A.; MacDonald, T. The Effects of KT3-671, a New Angiotensin II (AT 1) Receptor Blocker in Mild to Moderate Hypertension. Br. J. Clin. Pharmacol. 2003, 56, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-F.; He, X.; Wang, M.; Xu, D.; Hao, L.-P.; Liang, A.-H.; Zhang, J.; Zhou, Z.-M. Discovery of Novel, Potent and Low-Toxicity Angiotensin II Receptor Type 1 (AT1) Blockers: Design, Synthesis and Biological Evaluation of 6-Substituted Aminocarbonyl Benzimidazoles with a Chiral Center. Eur. J. Med. Chem. 2015, 103, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Bao, X.; Ren, H.; Da, Y.; Wu, D.; Li, F.; Yan, Y.; Wang, L.; Chen, Z. N-Phenyl Indole Derivatives as AT1 Antagonists with Anti-Hypertension Activities: Design, Synthesis and Biological Evaluation. Eur. J. Med. Chem. 2016, 115, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Bao, X.; Ren, H.; Liao, P.; Zhu, W.; Yan, Y.; Wang, L.; Chen, Z. Design, Synthesis, and Pharmacological Evaluation of 5-Oxo-1,2,4-Oxadiazole Derivatives as AT1 Antagonists with Antihypertension Activities. Clin. Exp. Hypertens. 2016, 38, 435–442. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, X.; Zhang, L.; Lian, X.; Wu, Z. Design, Synthesis, and Biological Evaluation of New Oxadiazole Derivatives as Efficient Antihypertension Drugs. Chem. Res. Toxicol. 2025, 38, 145–150. [Google Scholar] [CrossRef]
- Tang, B.; Li, H.; Zhong, Z.; Wu, H.; Shen, H.; Hu, J.; Ma, J.; Wu, J.; Wang, Y. Pharmacological Evaluation on Antihypertensive Activity of a Novel AT1 Angiotensin II Receptor Antagonist. Curr. Sci. 2019, 116, 1987. [Google Scholar] [CrossRef]
- Zhu, W.; Da, Y.; Wu, D.; Zheng, H.; Zhu, L.; Wang, L.; Yan, Y.; Chen, Z. Design, Synthesis and Biological Evaluation of New 5-Nitro Benzimidazole Derivatives as AT1 Antagonists with Anti-Hypertension Activities. Bioorganic Med. Chem. 2014, 22, 2294–2302. [Google Scholar] [CrossRef]
- Ridgway, H.; Apostolopoulos, V.; Moore, G.J.; Gadanec, L.K.; Zulli, A.; Swiderski, J.; Tsiodras, S.; Kelaidonis, K.; Chasapis, C.T.; Matsoukas, J.M. Computational Evidence for Bisartan Arginine Blockers as Next-Generation Pan-Antiviral Therapeutics Targeting SARS-CoV-2, Influenza, and Respiratory Syncytial Viruses. Viruses 2024, 16, 1776. [Google Scholar] [CrossRef]
- Ridgway, H.; Ntallis, C.; Chasapis, C.T.; Kelaidonis, K.; Matsoukas, M.-T.; Plotas, P.; Apostolopoulos, V.; Moore, G.; Tsiodras, S.; Paraskevis, D.; et al. Molecular Epidemiology of SARS-CoV-2: The Dominant Role of Arginine in Mutations and Infectivity. Viruses 2023, 15, 309. [Google Scholar] [CrossRef]
- Ridgway, H.; Moore, G.J.; Gadanec, L.K.; Zulli, A.; Apostolopoulos, V.; Hoffmann, W.; Węgrzyn, K.; Vassilaki, N.; Mpekoulis, G.; Zouridakis, M.; et al. Novel Benzimidazole Angiotensin Receptor Blockers with Anti-SARS-CoV-2 Activity Equipotent to That of Nirmatrelvir: Computational and Enzymatic Studies. Expert. Opin. Ther. Targets 2024, 28, 437–459. [Google Scholar] [CrossRef]
- Matsoukas, J.M.; Panagiotopoulos, D.; Keramida, M.; Mavromoustakos, T.; Yamdagni, R.; Wu, Q.; Moore, G.J.; Saifeddine, M.; Hollenberg, M.D. Synthesis and Contractile Activities of Cyclic Thrombin Receptor-Derived Peptide Analogues with a Phe-Leu-Leu-Arg Motif: Importance of the Phe/Arg Relative Conformation and the Primary Amino Group for Activity. J. Med. Chem. 1996, 39, 3585–3591. [Google Scholar] [CrossRef]
- Maragoudakis, M.E.; Kraniti, N.; Giannopoulou, E.; Alexopoulos, K.; Matsoukas, J. Modulation of Angiogenesis and Progelatinase a by Thrombin Receptor Mimetics and Antagonists. Endothelium 2001, 8, 195–206. [Google Scholar] [CrossRef]
- Alexopoulos, K.; Fatseas, P.; Melissari, E.; Vlahakos, D.; Roumelioti, P.; Mavromoustakos, T.; Mihailescu, S.; Paredes-Carbajal, M.C.; Mascher, D.; Matsoukas, J. Design and Synthesis of Novel Biologically Active Thrombin Receptor Non-Peptide Mimetics Based on the Pharmacophoric Cluster Phe/Arg/NH2 of the Ser42-Phe-Leu-Leu-Arg46 Motif Sequence: Platelet Aggregation and Relaxant Activities. J. Med. Chem. 2004, 47, 3338–3352. [Google Scholar] [CrossRef]
- Polevaya, L.; Mavromoustakos, T.; Zoumboulakis, P.; Grdadolnik, S.G.; Roumelioti, P.; Giatas, N.; Mutule, I.; Keivish, T.; Vlahakos, D.V.; Iliodromitis, E.K.; et al. Synthesis and Study of a Cyclic Angiotensin II Antagonist Analogue Reveals the Role of Π*–Π* Interactions in the C-Terminal Aromatic Residue for Agonist Activity and Its Structure Resemblance with AT1 Non-Peptide Antagonists. Bioorganic Med. Chem. 2001, 9, 1639–1647. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, M.; Apostolopoulos, V.; Matsoukas, J.; Wolf, W.M.; Blaskovich, M.A.T.; Bojarska, J.; Ziora, Z.M. Tetrazoles: A Multi-Potent Motif in Drug Design. Eur. J. Med. Chem. 2024, 279, 116870. [Google Scholar] [CrossRef] [PubMed]
| Disease Area | Drug Name/Active Ingredients | Original Indication | New Indication(s) | New Indication(s) Status |
|---|---|---|---|---|
| Depression | Duloxetine hydrochloride | Major Depressive Disorder (MDD) [28,29] | Neuropathic pain [30], generalized anxiety disorder (GAD) [31], osteoarthritis [32], and stress incontinence [33] | Approved |
| Fluoxetine hydrochloride | Major Depressive Disorder (MDD) [34] | Premenstrual dysphoric disorder (PMDD) [35] | Approved | |
| Neurology | Atomoxetine hydrochloride | Parkinson’s disease (PD) [36] | Attention-deficit hyperactivity disorder (ADHD) [37] | Approved |
| Bromocriptine | Parkinson’s disease [38] | Diabetes mellitus [39], prolactinomas, and other pituitary adenomas [40] | Approved | |
| Chlorpromazine | Schizophrenia, bipolar disorder, and acute psychosis [41] | Breast cancer [42] | Investigational | |
| Lithium | Depression, bipolar disorder [43] | Cancer [44] | Investigational | |
| Penfluridol | Chronic schizophrenia, acute psychosis, and Tourette syndrome [45] | Cancer [46] | Investigational | |
| Ropinirole hydrochloride | Hypertension (HTN) [47] | Parkinson’s disease (PD) [48] | Approved | |
| Non-neurology | Aspirin | Analgesic, Antipyretic [49] | Antiplatelet [49], anti-thrombosis [50] | Approved |
| Celecoxib | Pain, inflammation, arthritis [51] | Familial adenomatous polyposis [52] | Approved | |
| Finasteride | Benign prostatic hyperplasia (BPH) [53] | Hair loss [54] | Approved | |
| Losartan | Hypertension [55] | Dystrophic epidermolysis bullosa (RDEB) [56] and COVID-19 [57] | Investigational | |
| Minoxidil | Hypertension (HTN) [58] | Hair loss [59] | Approved | |
| Raltegravir | HIV-1 integrase Inhibitor [60] | Colorectal cancer [61] | Investigational | |
| Sildenafil | Angina [26] | Erectile dysfunction (ED) and pulmonary arterial hypertension (PAH) [26] | Approved | |
| Zidovudine | Failed clinical trials for cancer [62] | Human immunodeficiency virus (HIV) [62,63] | Approved | |
| Cancer | Auranofin | Rheumatoid arthritis (RA) [64] | Gastrointestinal stromal tumor (GIST) [65] | Investigational |
| Crizotinib | Clinical trials for anaplastic large cell lymphoma (ALCL) [66] | Non-small cell lung cancer (NSCLC) [67] | Approved | |
| Imatinib | Chronic myeloid leukemia (CML) [68] | Gastrointestinal stromal tumors (GIST) [69] | Approved | |
| Irinotecan hydrochloride | Colorectal cancer [70] | Pancreatic cancer [71] | Approved | |
| Metformin hydrochloride | Type 2 diabetes (T2DM) [72] | Pancreatic cancer, endometrial cancer, colorectal cancer, and esophageal cancer [73,74,75,76] | Investigational | |
| Nelfinavir | Human immunodeficiency virus 1 (HIV-1) [77] | Colorectal cancer, lung cancer, cervical cancer, pancreatic cancer, ovarian cancer, metastatic cancer [78] | Investigational | |
| Raloxifene | Osteoporosis [79] | Breast cancer [80] | Approved | |
| Rituximab | Various cancers [81] | Rheumatoid arthritis [82] | Approved | |
| Sunitinib | Renal cell carcinoma (RCC) [83] and Gastrointestinal stromal tumor (GIST) [84] | Pancreatic neuroendocrine tumors (PNETs) [85] | Approved | |
| Trastuzumab | Human epidermal growth factor receptor 2 (HER2)-positive breast cancer [86] | Metastatic breast cancer, gastric cancer, and early breast cancer [87,88,89] | Approved | |
| Temsirolimus | Renal cell carcinoma [90] | Lung Adenocarcinoma [91] | Investigational | |
| Infectious | Apmphotericin B | Antifungal [92] | Leishmaniasis [93], Mucormycosis [94] | Approved |
| Dapoxetine | Premature ejaculation [95] | Zika virus infection [96] | Investigational | |
| Everolimus | Immunosuppressant [97] | Pancreatic neuroendocrine tumors (PNETs) [98], renal cell carcinoma (RCC) [99], and subependymal giant cell astrocytoma (SEGA) [100] | Approved | |
| Favipiravir | Influenza [101] | SARS-CoV-2 [102] | Investigational | |
| Ivermectin | Antiretroviral [103] | SARS-CoV-2 [104] | Investigational | |
| Ketoconazole | Fungal infections [105] | Cushing’s syndrome [106] | Approved | |
| Remdesivir | Antiviral [107] | SARS-CoV-2 [107] | Approved | |
| Sirolimus | Organ rejection in patients receiving renal transplants [108] | Malaria [109] | Investigational | |
| Thalidomide | Morning sickness (withdrawn) [67] | Erythema nodosum leprosum (Leprosy) [110] and Multiple Myeloma [111] | Approved | |
| Rare and orphan | Alefacept | Chronic plaque psoriasis [112] | Memory T cell-mediated autoimmune diseases, organ transplantation and type I diabetes (T1D) [113] | Investigational |
| Baclofen | Muscle relaxant [114] | Alcohol use disorder [115] | Approved | |
| Clobetasol | Psoriasis [116] | post-cataract surgery pain and inflammation [117] | Approved | |
| Dimethyl fumarate | Multiple sclerosis [118] | Psoriasis [119], anti-inflammatory [120] | Approved | |
| Erythropoietin | Anemia [121] | Traumatic brain injury [122] | Investigational | |
| Ethinyl estradiol | Turner syndrome [123] | Contraceptive applications [124], acne, hirsutism [125] | Approved | |
| Fingolimod | Multiple sclerosis (MS) [126] | Alzheimer’s disease [127], cancer [128], diabetic retinopathy [129] | Investigational | |
| Liraglutide | Diabetes [130] | Alzheimer’s disease (AD) [131] | Investigational | |
| N-acetyl cysteine | Mucolytic agent [132] | Obsessive–compulsive disorder [133] | Investigational | |
| Pregabalin | Neuropathic pain [134] | Generalized anxiety disorder [135] | Approved | |
| Simvastatin | Cardiovascular diseases [136] | Anti-tumor agents [137], neurodegenerative disorders [138], preterm labor [139] and Klebsiella pneumoniae infections [140] | Investigational | |
| Topiramate | Epilepsy [141] | Obesity [142] | Approved |
| Disease | RAAS Involvement | Preclinical/Clinical Support | Example ARB(s) |
|---|---|---|---|
| Hypertension | Antagonism with Ang II for the active site of the AT1 receptor; lower blood pressure | ARBs; approved drugs for hypertension | All sartans (losartan and its active metabolite EXP3174, olmesartan, eprosartan, irbesartan, telmisartan, candesartan) |
| Heart Failure | ARBs downstream action; blockade of Ang II from AT1 receptors; Ang II binding to AT2 receptors | ARBs approved for patients with HFrEF (Class I) and HFmrEF (Class IIa) who are intolerant to ACEIs and ARNI | Candesartan [253], valsartan [254] |
| Chronic Kidney Disease | Ang II through AT1R activates signaling cascades—MAPK/ERK, JNK, STAT, NF-κB, and AP-1; drives fibrosis, inflammation, cell proliferation; Ang II causes cytoskeletal disruption, ROS production, apoptosis, podocytopathy, and glomerulosclerosis | ARBs for patients with CKD and severely increased albuminuria (1B), moderately increased albuminuria (2C) and moderate-to-severe albuminuria and diabetes (1B) | Losartan [255], irbesartan [256], and telmisartan [257] |
| Acute Coronary Syndrome | RAS activation worsens outcomes post–MI; Ang II activates NADPH oxidase, causing oxidative injury and atherosclerosis; Ang II, AT1R, and ACE co-localize in plaques, promoting IL-6 release and instability of ARBs post-MI upregulation | ARBs for patients with intolerance of ACE inhibitors, after ACS with HF symptoms, LVEF < 40%, hypertension, and/or CKD | Valsartan [258], losartan [259], candesartan [260] |
| Alzheimer’s Disease | Ang II via AT1R increases Aβ through the upregulation of APP mRNA, β-secretase activity, and presenilin expression; promotes tau phosphorylation and ROS generation, neuroinflammation, oxidative stress, neurotoxicity | Candesartan: safe and reduces brain amyloid biomarkers, improves subcortical brain connectivity, and supports cognitive function in non-hypertensive individuals with prodromal Alzheimer’s disease | Telmisartan, candesartan, losartan, and irbesartan |
| Parkinson’s Disease | Regulates dopaminergic neurotransmission, blood flow, inflammatory responses; intricate interaction between Ang and DA; balance of D1, D2, AT1, AT2 receptors. | Animal models of PD; neuroprotective effects of AT1R antagonists, effects driven by decrease in ROS; perindopril enhanced the effects of levodopa without causing dyskinesias | Losartan, candesartan, and telmisartan |
| Anxiety | Overactivation of RAS through AT1R; HPA axis hyperactivity; anxiety-like behaviors; Ang II via AT1R stimulates CRH production, AVP release, adrenal catecholamine output, amplifying stress responses | Clinical study: ARB-treated patients had lower anxiety STAI scores than those on ACEIs or drug-free at baseline and during the follow-up | Losartan, telmisartan [261] |
| Cancer Glioma | Glioblastoma cells express renin, angiotensinogen, renin receptor, ACE, AT1R, AT2R, renin inhibition induces apoptosis, Ang (1–7) inhibited the JNK pathway; preserved endothelial junction integrity, reduced vascular leak, and limited tumor-induced edema. | Phase I clinical trial; patients with glioblastoma treated with combination of RAS modulators, treatment well tolerated; preserves quality of life/performance; may lengthen survival time | Losartan [262], telmisartan |
| Pathogenic Inflammation | Candesartan suppressed the NLRP3 inflammasome and pyroptosis in mac-rophages, reduced the expression of NLRP3 and proIL-1β by inhibiting NF-κB activation and decreasing phosphorylation of ERK1/2 and JNK1/2, lessened mitochondrial damage | A meta-analysis of randomized controlled trials found that ARBs significantly reduced levels of inflammatory markers such as CRP, IL-6, and TNF-α | Candesartan |
| Candidosis | Medications targeting the RAAS inhibit secreted aspartic proteases produced by Candida albicans | Preclinical studies; ARBs disrupt the metabolism and formation of Candida biofilms; decreased fungal viability at all concentrations | Losartan [223], candesartan [263] |
| Fibrosis | Ang II through AT1R, TGF-β/Smad activation; ROS, inflammation; Ang (1–7) through MAS receptor inhibits fibrosis, reduces inflammation, restores tissue integrity; liver fibrosis; AT2R upregulated; antifibrotic effects by inhibition of the IRE1α-XBP1 pathway | Ongoing clinical trials; ARBs in reducing fibrosis in sickle cell disease (NCT05012631), aortic stenosis (NCT04913870), AKI (NCT05272878), patients with hepatitis C and hypertension, ARB treatment, reduced fibrosis relative to untreated patients. | Losartan [264], telmisartan [265], candesartan [266], valsartan [267] |
| Tissue Fibrosis in Systemic Sclerosis | Ang II, fibrosis through ECM stimulation | Clinical study: compares the efficacy and tolerability of losartan, with nifedipine for the treatment of primary and secondary RP; tolerability of short-term treatment of RP with losartan | Losartan |
| Diabetic Peripheral Neuropathy | Spinal Ang II/AT1R signaling drives neuropathic pain via p38 MAPK; losartan blockade, Ang II–driven ROS via NADPH oxidase; broader neurotoxicity of Ang II/AT1R pathway | Clinical study: Bonferroni’s test indicated significantly later DPN development in the ARB and ACEI groups, beneficial to prevent DPN accompanying T2DM | Losartan [268], telmisartan [269] |
| Inflammatory Bowel Diseases | Ang II; activation of RAS; activation of JAK2/STAT1/3, elevated TH1/TH17 T-cell responses, IEC apoptosis, Ang (1–7)/MASR reduces signaling (p38/ERK/Akt) | Retrospective studies of IBD patients treated with ACEIs or ARBs; encouraging results, including milder disease progression, fewer hospitalizations, reduced corticosteroid use | Losartan [270], telmisartan [271], candesartan [272], valsartan [273] |
| Marfan Syndrome | AT1R blockade in MFS mice; prevents aneurysm, reverses pathology via TGF-β/Smad suppression, AT2R; pivotal role for full therapeutic effect; required for ERK inhibition | Individuals with Marfan syndrome who have not undergone aortic surgery; ARBs reduced the rate of aortic root Z score enlargement by roughly half, even in those also taking beta-blockers. | Losartan [274], irbesartan [275], telmisartan [276] |
| SARS-CoV-2 | ARBs increase levels of ACE2 more than other hypertension medications, ACE2 entry point for SARS-CoV-2 in the nasopharynx, lungs, and heart cells; ACE2 transforms harmful Ang II into the beneficial peptides Ang (1–7) and alamandine, helps maintain balance while simultaneously preventing SARS-CoV-2 from entering through ACE2. | Phase III clinical trial “CLARITY”; no evidence of benefit, based on disease severity score, for treatment with ARBs, seamless phase I and II study; intravenous infusion Angiotensin (1–7) in COVID-19 patients admitted to the ICU with severe pneumonia; in Phase II; no significant difference in OFD between groups (premature termination, further studies need to be performed) | Telmisartan [277], candesartan [278] |
| Rheumatoid Arthritis | Ang II increases IL-1, IL-6, TNF-α; role in the development of RA | Clinical research indicates that RAS inhibitors—especially ARBs, as well as ACE inhibitors and renin inhibitors—play a role in RA by primarily targeting inflammation and oxidative stress. | Losartan [279], olmesartan, candesartan, telmisartan [280] |
| Osteoarthritis | Joint tissues; AT1R stimulation by Ang II or inflammatory cytokines (IL-1β) triggers the release of pro-inflammatory substances and MMPs, speeding up cartilage degradation and wors-ening joint injury. | No clinical trials performed; one trial in patients with OA and hypertension; valsartan with strong osteoarthritis adverse reaction signals compared to irbesartan, cloxartan | Losartan [281] |
| Opioid Addiction | ARBs with higher affinities for AT1R demonstrate higher affinities for µORs and ժORs than opiate ligands, such as fentanyl and naltrexone | No ongoing clinical trials | Telmisartan [282], candesartan [283], valsartan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzipieris, F.P.; Mavromoustakou, K.; Matsoukas, J.M.; Mavromoustakos, T. Unlocking Novel Therapeutic Potential of Angiotensin II Receptor Blockers. Int. J. Mol. Sci. 2025, 26, 8819. https://doi.org/10.3390/ijms26188819
Chatzipieris FP, Mavromoustakou K, Matsoukas JM, Mavromoustakos T. Unlocking Novel Therapeutic Potential of Angiotensin II Receptor Blockers. International Journal of Molecular Sciences. 2025; 26(18):8819. https://doi.org/10.3390/ijms26188819
Chicago/Turabian StyleChatzipieris, Filippos Panteleimon, Kiriaki Mavromoustakou, John M. Matsoukas, and Thomas Mavromoustakos. 2025. "Unlocking Novel Therapeutic Potential of Angiotensin II Receptor Blockers" International Journal of Molecular Sciences 26, no. 18: 8819. https://doi.org/10.3390/ijms26188819
APA StyleChatzipieris, F. P., Mavromoustakou, K., Matsoukas, J. M., & Mavromoustakos, T. (2025). Unlocking Novel Therapeutic Potential of Angiotensin II Receptor Blockers. International Journal of Molecular Sciences, 26(18), 8819. https://doi.org/10.3390/ijms26188819








