β-Casein A1 and A2 Genetic Variants and β-Casomorphin-7 in Raw Milk and Processed Milk Products
Abstract
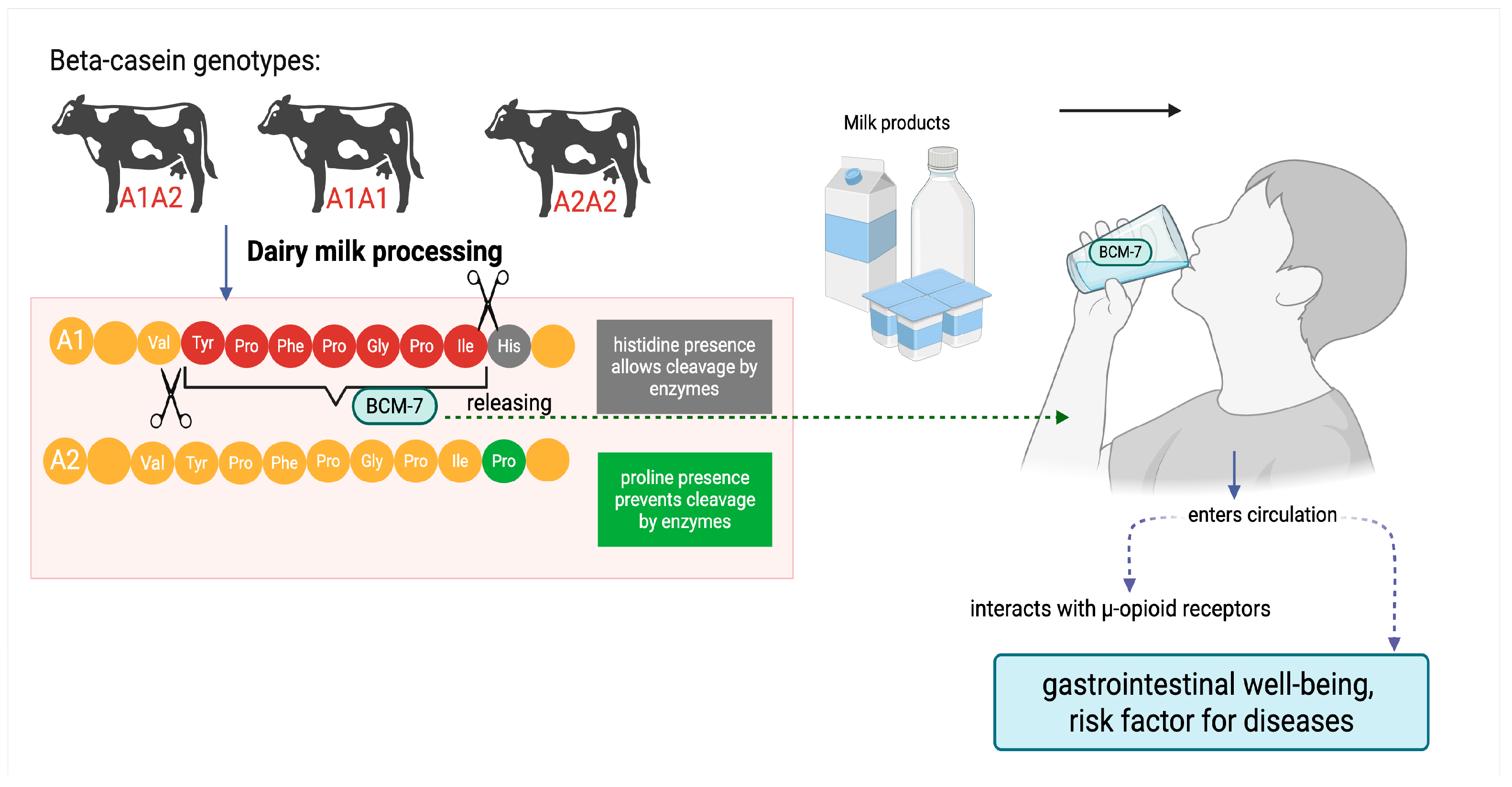
1. Introduction
2. Results
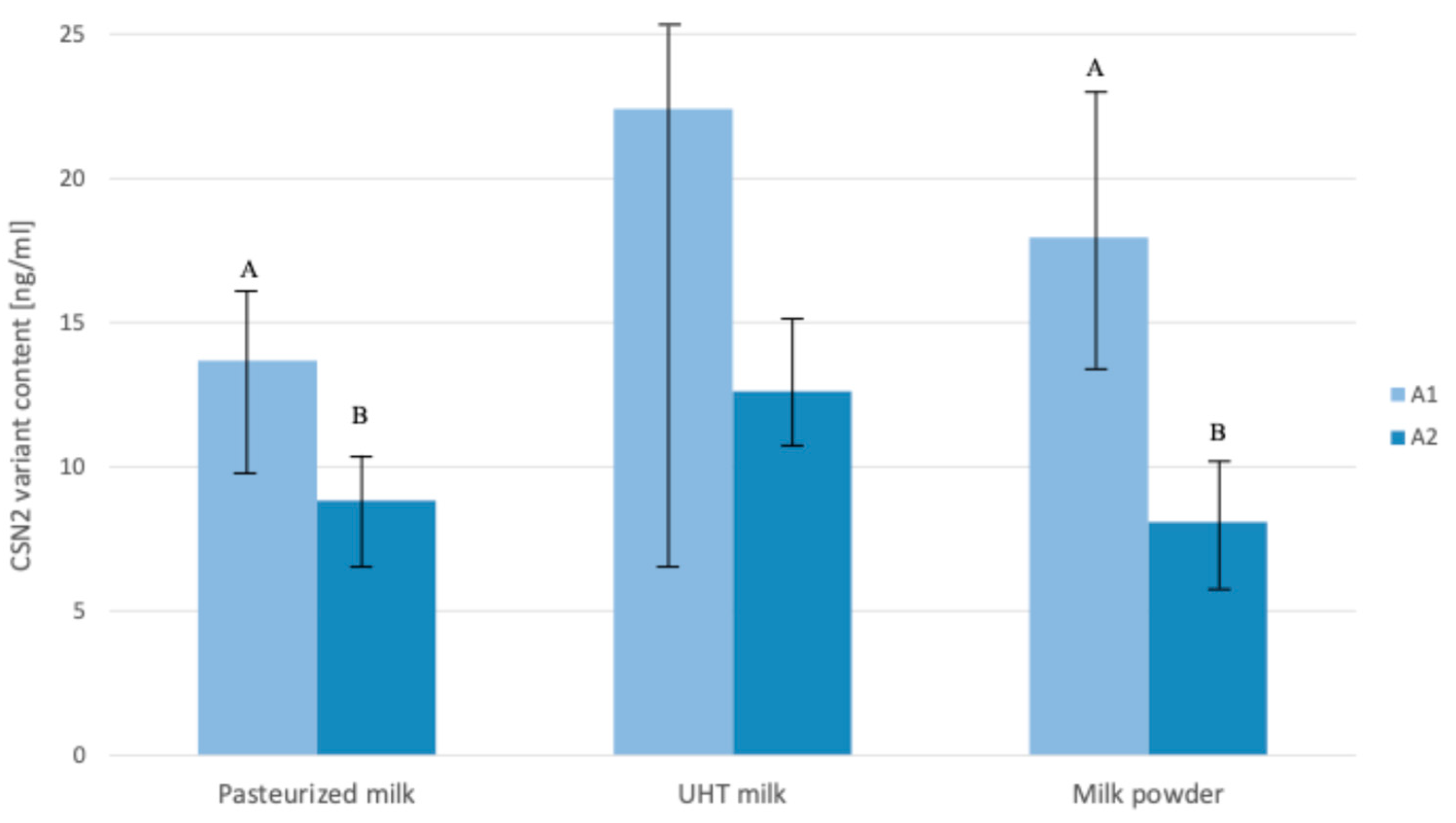
2.1. Content of β-Casein A1 and A2 Variants in Dairy Products
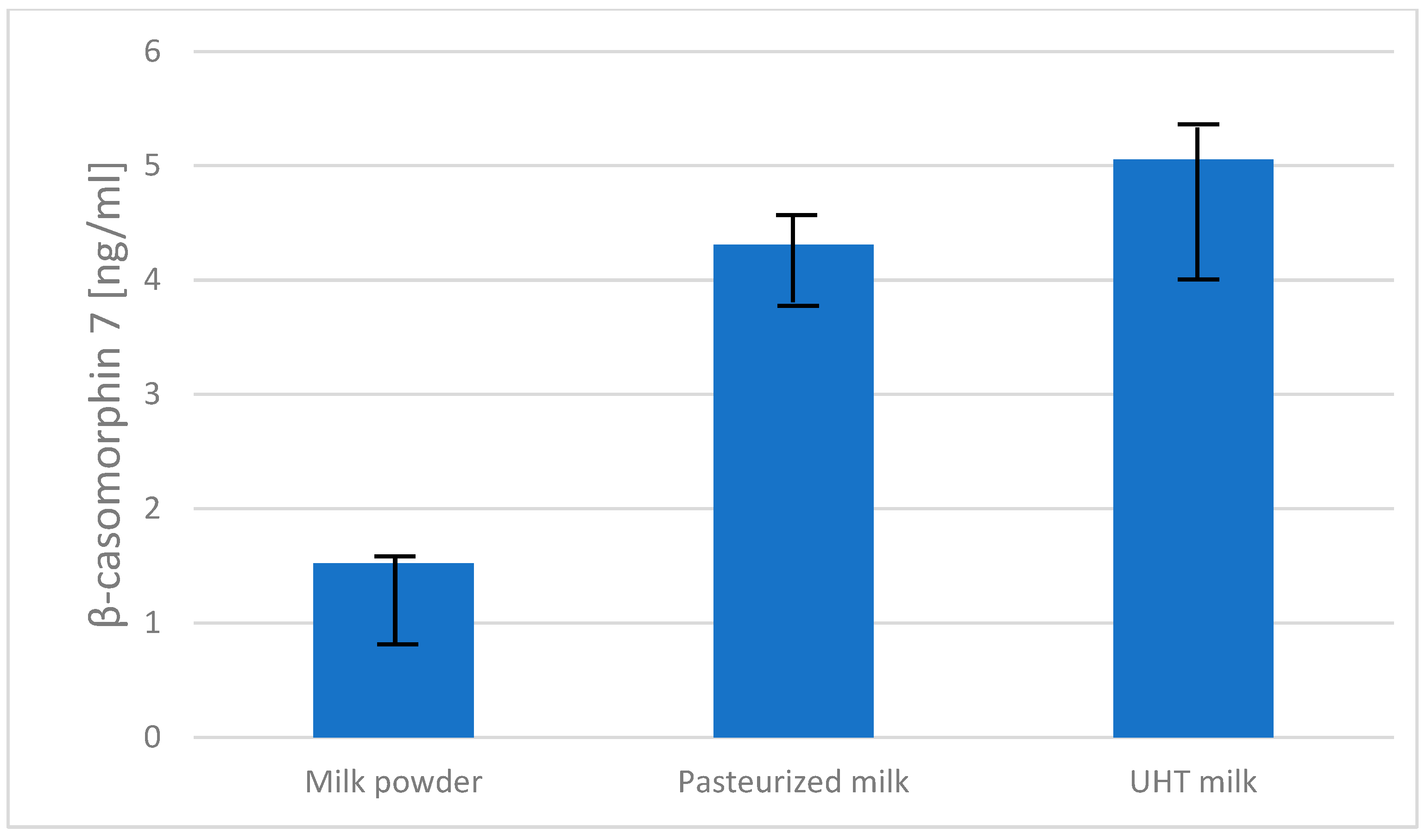
2.2. Content of β-Casomorphin-7 in Dairy Products
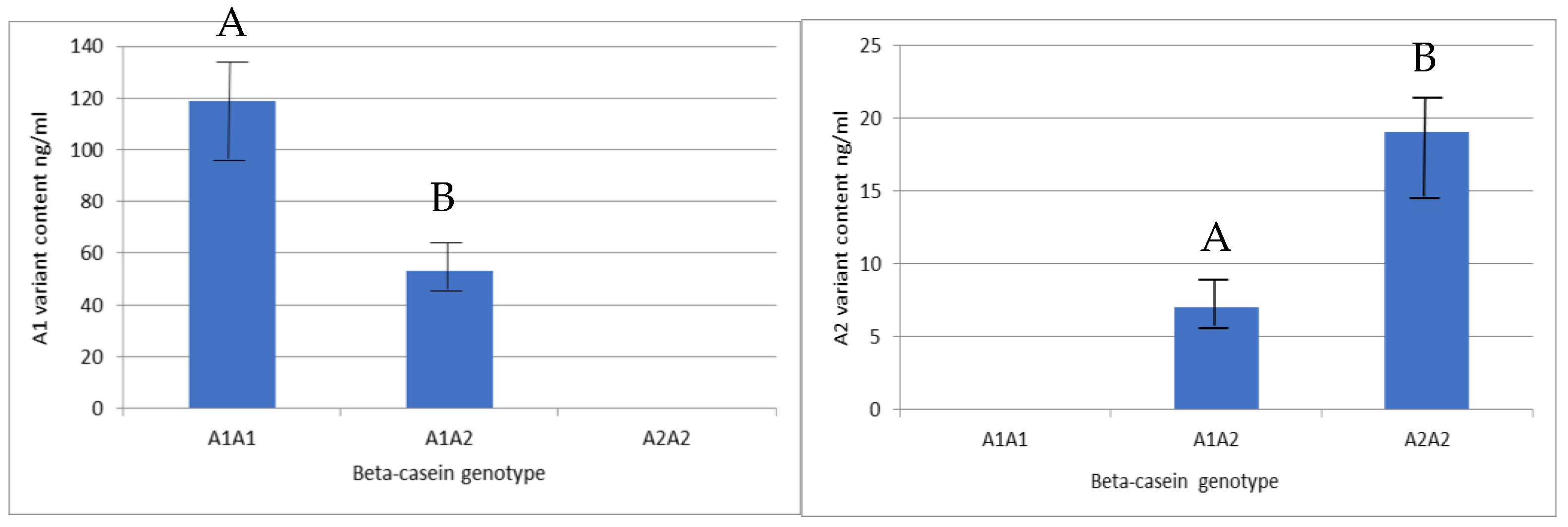
2.3. Content of β-Casein Variants in Raw Milk
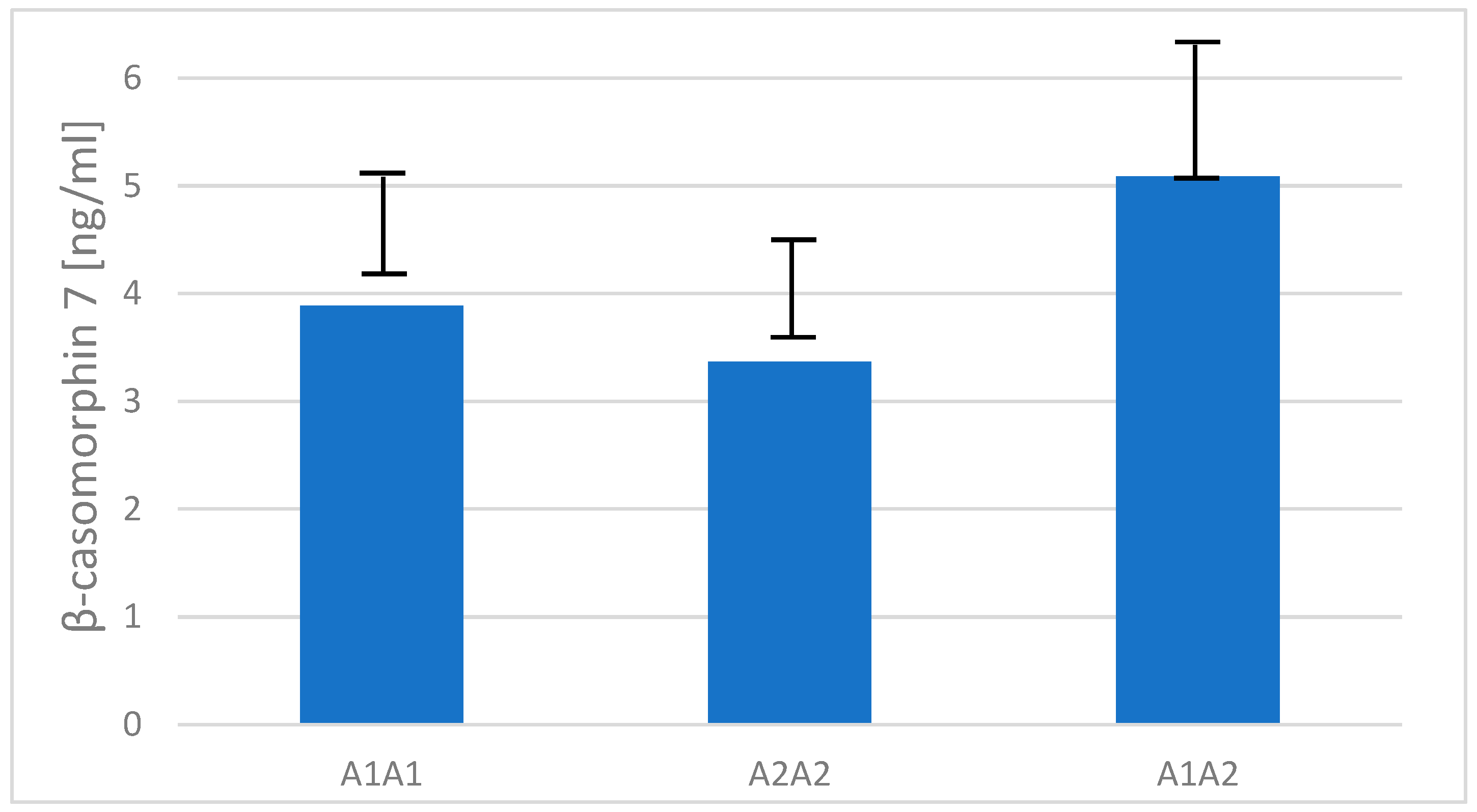
2.4. Content of β-Casomorphin-7 in Raw Milk
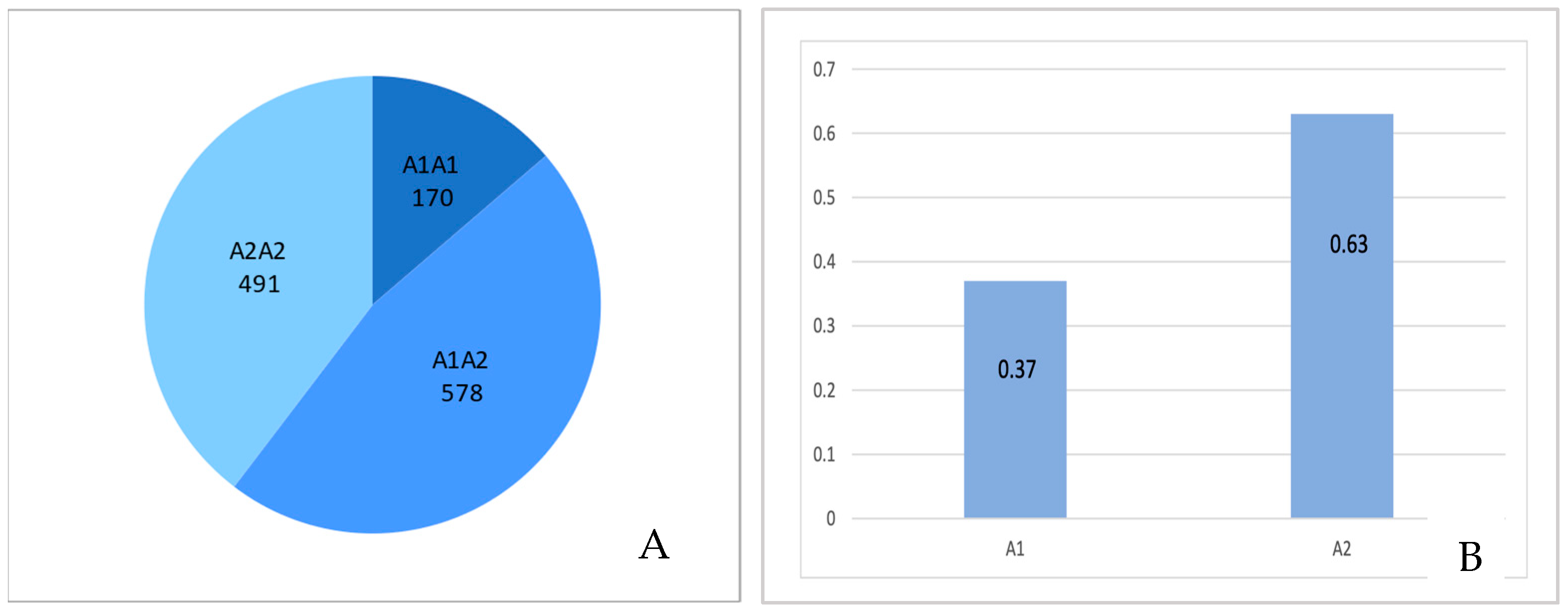
2.5. Frequency of Beta-Casein Genotypes and Alleles in Holstein-Friesian Cows
3. Discussion
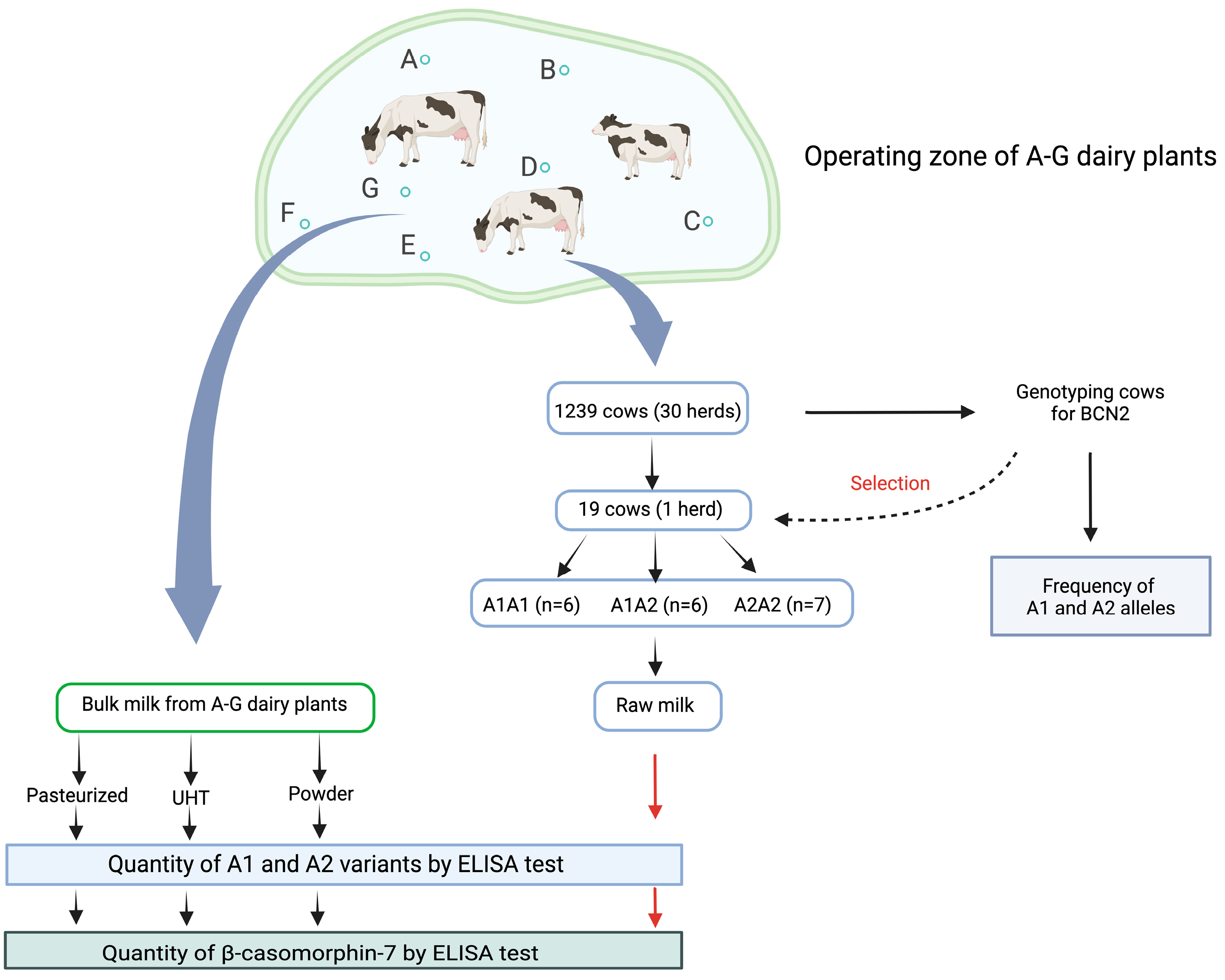
4. Materials and Methods
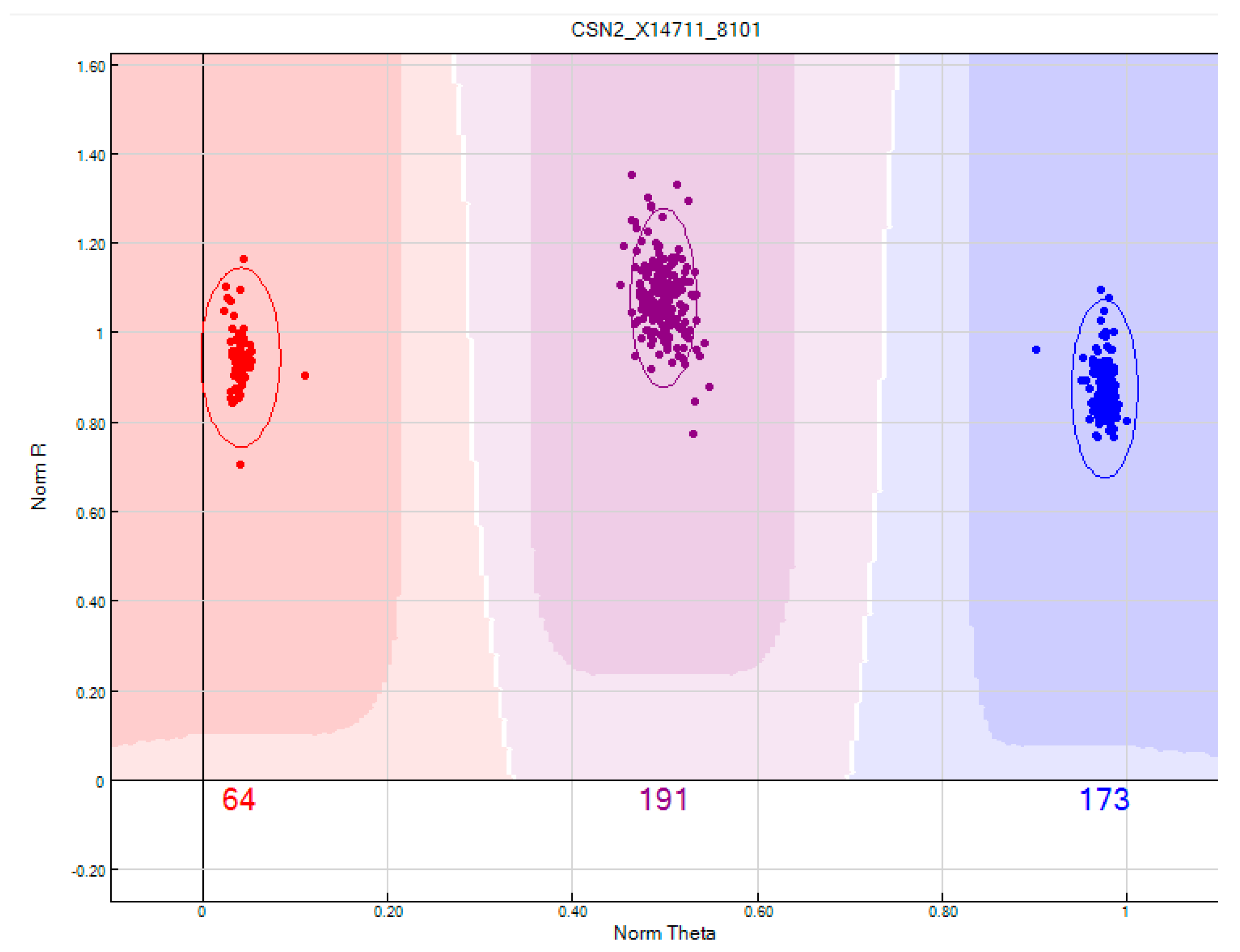
4.1. Genotyping of Beta-Casein Allele A1 and A2 in Cows
4.2. Milk Samples
4.2.1. Commercial Dairy Products
4.2.2. Raw Milk
4.3. Measuring the Content of β-Casein A1 and A2 Variants by ELISA
4.4. β-Casomorphin-7 Extraction and Measurement by ELISA
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSN2 | bovine β-casein |
| BCM-7 | β-casomorphin-7 |
| SNP | single nucleotide polymorphism |
References
- Mukesh, M.; Swami, S.; Bhakhri, G.; Chaudhary, V.; Sharma, V.; Goyal, N.; Vivek, P.; Dalal, V.; Mohanty, A.K.; Kataria, R.S.; et al. Demographic pattern of A1/A2 beta casein variants indicates conservation of A2 type haplotype across native cattle breeds (Bos indicus) of India. 3 Biotech 2022, 12, 167. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAOSTAT). FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/QL (accessed on 17 July 2025).
- McSweeney, P.L.H.; McNamara, J.P. Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kamiński, S.; Cieślińska, A.; Kostyra, E. Polymorphism of bovine beta-casein and its potential effect on human health. J. Appl. Genet. 2007, 48, 189–198. [Google Scholar] [CrossRef]
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Invited review: Milk protein genetic variation in cattle: Impact on animal breeding and human nutrition. J. Dairy Sci. 2009, 92, 5335–5352. [Google Scholar] [CrossRef] [PubMed]
- Cieślińska, A.; Fiedorowicz, E.; Rozmus, D.; Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Kamiński, S. Does a Little Difference Make a Big Difference? Bovine β-Casein A1 and A2 Variants and Human Health—An Update. Int. J. Mol. Sci. 2022, 23, 15637. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cow’s milk—Sixth revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Bisutti, V.; Pegolo, S.; Giannuzzi, D.; Mota, L.F.M.; Vanzin, A.; Toscano, A.; Trevisi, E.; Ajmone Marsan, P.; Brasca, M.; Cecchinato, A. The β-casein (CSN2) A2 allelic variant alters milk protein profile and slightly worsens coagulation properties in Holstein cows. J. Dairy Sci. 2022, 105, 3794–3809. [Google Scholar] [CrossRef] [PubMed]
- Faggion, S.; Degano, L.; Carnier, P.; Bonfatti, V. β-Casein A2 affects milk renneting properties, cheese yield before and after ripening, and alters the texture of Caciotta cheese produced in field conditions. J. Dairy Sci. 2025, 108, 3199–3213. [Google Scholar] [CrossRef]
- Vigolo, V.; Visentin, E.; Ballancin, E.; Lopez-Villalobos, N.; Penasa, M.; De Marchi, M. β-Casein A1 and A2: Effects of polymorphism on the cheese-making process. J. Dairy Sci. 2023, 106, 5276–5287. [Google Scholar] [CrossRef]
- Bonfatti, V.; Di Martino, G.; Cecchinato, A.; Degano, L.; Carnier, P. Effects of beta-kappa-casein (CSN2-CSN3) haplotypes, beta-lactoglobulin (BLG) genotypes, and detailed protein composition on coagulation properties of individual milk of Simmental cows. J. Dairy Sci. 2010, 93, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.A.; Bertelsen, H.P.; Jensen, H.B.; Gustavsson, F.; Glantz, M.; Månsson, H.L.; Larsen, L.B. The occurrence of noncoagulating milk and the association of bovine milk coagulation properties with genetic variants of the caseins in 3 Scandinavian dairy breeds. J. Dairy Sci. 2013, 96, 4830–4842. [Google Scholar] [CrossRef]
- Massella, E.; Piva, S.; Giacometti, F.; Liuzzo, G.; Zambrini, A.V.; Serraino, A. Evaluation of bovine beta casein polymorphism in two dairy farms located in northern Italy. Ital. J. Food Saf. 2017, 6, 6904. [Google Scholar] [CrossRef]
- Troch, T.; Lefébure, É.; Baeten, V.; Colinet, F.; Gengler, N.; Sindic, M. Cow milk coagulation: Process description, variation factors and evaluation methodologies. A review. Biotechnol. Agron. Soc. Environ. 2017, 21, 276–287. [Google Scholar] [CrossRef]
- Nadugala, B.H.; Pagel, C.N.; Raynes, J.K.; Ranadheera, C.S.; Logan, A. The effect of casein genetic variants, glycosylation and phosphorylation on bovine milk protein structure, technological properties, nutrition and product manufacture. Int. Dairy J. 2022, 133, 105440. [Google Scholar] [CrossRef]
- Kamiński, S.; Brym, P.; Zabolewicz, T.; Oleński, K.; Sadowska, J. Unbalanced expression of β-casein variants A1 and A2 in Holstein-Friesian cows. Anim. Sci. Pap. Rep. 2024, 42, 401–414. [Google Scholar] [CrossRef]
- McLachlan, C.N. β-Casein A1, ischaemic heart disease mortality, and other illnesses. Med. Hypotheses 2001, 56, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Venkidasamy, B.; Thirupathi, P.; Chung, I.-M.; Subramanian, U. β-Casomorphin: A complete health perspective. Food Chem. 2021, 337, 127765. [Google Scholar] [CrossRef]
- Jeong, H.; Park, Y.S.; Yoon, S.S. A2 milk consumption and its health benefits: An update. Food Sci. Biotechnol. 2024, 33, 491–503. [Google Scholar]
- Allied Market Research. A2 Milk Market Size, Share, Trends|Growth Opportunities. 2025. Available online: https://www.alliedmarketresearch.com (accessed on 17 July 2025).
- Sanchez, M.P.; Fritz, S.; Patry, C.; Delacroix-Buchet, A.; Boichard, D. Frequencies of milk protein variants and haplotypes estimated from genotypes of over 1 million French cattle. J. Dairy Sci. 2020, 103, 9124–9141. [Google Scholar] [CrossRef]
- Sebastiani, C.; Arcangeli, C.; Ciullo, M.; Torricelli, M.; Cinti, G.; Fisichella, S.; Biagetti, M. Frequencies evaluation of β-casein gene polymorphisms in dairy cows reared in Central Italy. Animals 2020, 10, 252. [Google Scholar] [CrossRef]
- Kamiński, S.; Zabolewicz, T.; Oleński, K.; Babuchowski, A. Long-term changes in the frequency of β-casein, κ-casein and β-lactoglobulin alleles in Polish Holstein-Friesian cattle. J. Anim. Feed Sci. 2023, 32, 205–210. [Google Scholar] [CrossRef]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 β-Casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Rossi, F.; Leni, G.; Antonelli, G.; Bordoni, A.; Bertuzzi, T.; Santoni, M.; Risso, D.; Canzoneri, F.; Menta, R.; et al. Estimating β-casomorphin-7 exposure from milk and dairy product consumption: A comprehensive assessment for the European population. Int. J. Food Sci. Nutr. 2025, 76, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Aschaffenburg, R. Inherited Casein Variants in Cow’s Milk. Nature 1961, 192, 431–432. [Google Scholar] [CrossRef]
- Ehrmann, S.; Bartenschlager, H.; Geldermann, H. Quantification of gene effects on single milk proteins in selected groups of dairy cows. J. Anim. Breed. Genet. 1997, 114, 121–132. [Google Scholar] [CrossRef]
- Cohn, R.D.; Scherer, S.W.; Hamosh, A. Genetics and Genomics in Medicine; Elsevier: Philadelphia, PA, USA, 2024. [Google Scholar]
- Davis, S.R.; Ward, H.E.; Kelly, V.; Palmer, D.; Ankersmit-Udy, A.E.; Lopdell, T.J.; Berry, S.D.; Littlejohn, M.D.; Tiplady, K.; Adams, L.F.; et al. Screening for phenotypic outliers identifies an unusually low concentration of a β-lactoglobulin B protein isoform in bovine milk caused by a synonymous SNP. Genet. Sel. Evol. 2022, 54, 22. [Google Scholar] [CrossRef]
- Kilikevicius, A.; Meister, G.; Corey, D.R. Reexamining assumptions about miRNA-guided gene silencing. Nucleic Acids Res. 2022, 50, 617–634. [Google Scholar] [CrossRef]
- Duarte-Vazquez, M.A.; Garcia-Ugalde, C.; Villegas-Gutierrez, L.M.; Garcia-Almendarez, B.E.; Rosado, J.L. Production of cow’s milk free from β-casein A1 and its application in the manufacturing of specialized foods for early infant nutrition. Foods 2017, 6, 50. [Google Scholar] [CrossRef]
- Fuerer, C.; Jenni, R.; Cardinaux, L.; Andetsion, F.; Wagniere, S.; Mpulin, J.; Affolter, M. Protein fingerprinting and quantification of β-casein variants using UPLC–HRMS. J. Dairy Sci. 2020, 103, 1193–1207. [Google Scholar] [CrossRef]
- Cieślińska, A.; Kostyra, E.; Kostyra, H.; Oleński, K.; Fiedorowicz, E.; Kamiński, S. Milk from cows of different β-casein genotypes as a source of β-casomorphin-7. Int. J. Food Sci. Nutr. 2012, 63, 426–430. [Google Scholar] [CrossRef]
- Krishna, T.C.; Najda, A.; Bains, A.; Tosif, M.M.; Papliński, R.; Kapłan, M.; Chawla, P. Influence of ultra-heat treatment on properties of milk proteins. Polymers 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.S.; Montoya, C.A.; Maes, E.; Hefer, C.; Cruz, R.A.P.A.; Roy, N.C.; McNabb, W.C. Effect of Heat Treatment on Protein Self-Digestion in Ruminants’ Milk. Foods. 2023, 2, 3511. [Google Scholar] [CrossRef]
- Asledottir, T.; Le, T.T.; Poulsen, N.A.; Devold, T.G.; Larsen, L.B.; Vegarud, G.E. Release of β-casomorphin-7 from bovine milk of different β-casein variants after ex vivo gastrointestinal digestion. Int. Dairy J. 2018, 81, 8–11. [Google Scholar]
- Edwards, T.S.; Dawson, K.L.; Keenan, J.I.; Day, A.S. A simple method to generate β-casomorphin-7 by in vitro digestion of casein from bovine milk. J. Funct. Foods 2021, 85, 104631. [Google Scholar] [CrossRef]
- de Vasconcelos, M.L.; Oliveira, L.M.F.; Hill, J.P.; Vidal, A.M.C. Difficulties in establishing the adverse effects of β-casomorphin-7 released from β-casein variants—A review. Foods 2023, 12, 3151. [Google Scholar]
- Buatig, R.; Clegg, M.; Michael, N.; Oruna-Concha, M.J. Quantification of β-casomorphin-7 in commercially available filtered and pasteurized cow’s milk. Biol. Life Sci. Forum 2023, 26, 125. [Google Scholar]
- Kay, S.S.; Delgado, S.; Mittal, J.; Eshraghi, R.S.; Mittal, R.; Eshraghi, A.A. Beneficial Effects of Milk Having A2 β-Casein Protein: Myth or Reality? J. Nutr. 2021, 151, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Farhat, L.B.; Selmi, H.; Toth, V.; Hoarau, A.; Suli, A.; Labas, K.S.; Hadj Ahmed, M.; Marzouki, M.N.; Neifar, M.; Abdelkafi, S.; et al. A2 milk: The impact of A1 and A2 beta-casein on human health. Curr. Protein Pept. Sci. 2025, 26, 751–760. [Google Scholar]
- Santillo, A.; d’Angelo, F.; Lamberti, C.; Giuffrida, M.G.; Romaniello, F.; Albenzio, M. Distribution of β-casein variants and effects on milk composition in Podolian cows reared in Gargano Promontory (Southern Italy). J. Dairy Sci. 2025, 108, 5570–5579. [Google Scholar] [CrossRef]
- Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Krawczuk, S.; Kostyra, E.; Kostyra, H.; Iwan, M. Contents of agonistic and antagonistic opioid peptides in different cheese varieties. Int. Dairy J. 2009, 19, 258–263. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Busetti, F.; Johnson, S.K.; Solah, V.A. Identification and quantification of native beta-casomorphins in Australian milk by LC–MS/MS and LC–HRMS. J. Food Compos. Anal. 2015, 44, 102–110. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Solah, V.A.; Johnson, S.K.; Nguyen, H.A.; Nguyen, T.L.D.; Tran, T.L.H.; Busetti, F. Identification and quantification of beta-casomorphin peptides naturally yielded in raw milk by liquid chromatography-tandem mass spectrometry. LWT 2019, 111, 465–469. [Google Scholar] [CrossRef]
- Smolenski, G.; Armstrong, K.; Trivedi, M.; Clarke, A. BCM-7 release from processed dairy products containing measured amounts of beta-casein variants. Int. Dairy J. 2025, 161, 106136. [Google Scholar] [CrossRef]
- Harwalkar, K.; Elliott, W.H. Method for peptide extraction from milk. J. Dairy Sci. 1971, 54, 8–11. [Google Scholar] [CrossRef]
- Cieślińska, A.; Kamiński, S.; Kostyra, E.; Sienkiewicz-Szłapka, E. Beta-casomorphin 7 in raw and hydrolyzed milk derived from cows of alternative β-casein genotypes. Milchwissenschaft 2007, 62, 125–127. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiński, S.; Cieślińska, A. β-Casein A1 and A2 Genetic Variants and β-Casomorphin-7 in Raw Milk and Processed Milk Products. Int. J. Mol. Sci. 2025, 26, 8612. https://doi.org/10.3390/ijms26178612
Kamiński S, Cieślińska A. β-Casein A1 and A2 Genetic Variants and β-Casomorphin-7 in Raw Milk and Processed Milk Products. International Journal of Molecular Sciences. 2025; 26(17):8612. https://doi.org/10.3390/ijms26178612
Chicago/Turabian StyleKamiński, Stanisław, and Anna Cieślińska. 2025. "β-Casein A1 and A2 Genetic Variants and β-Casomorphin-7 in Raw Milk and Processed Milk Products" International Journal of Molecular Sciences 26, no. 17: 8612. https://doi.org/10.3390/ijms26178612
APA StyleKamiński, S., & Cieślińska, A. (2025). β-Casein A1 and A2 Genetic Variants and β-Casomorphin-7 in Raw Milk and Processed Milk Products. International Journal of Molecular Sciences, 26(17), 8612. https://doi.org/10.3390/ijms26178612






