1. Introduction
Electrostatically driven interaction and complexation of globular proteins with biological and synthetic polyelectrolytes play a prominent role in nature as well as in many technological and biomedical applications [
1]. These assemblies, including immobilization of proteins, enzymes, and antibodies on solid supports, are of particular interest in the fields of biotechnology and medicine. However, when biomolecules adhere to solid surfaces, their biological function might be compromised.
As a promising alternative, immobilization of globular proteins in polyelectrolyte brushes (monolayers of charged macromolecules end-attached to planar substrates or surfaces of colloidal particles, and immersed in aqueous solutions [
2,
3,
4,
5,
6,
7]) has been actively explored over the last few decades [
8,
9,
10,
11] (see also ref. [
12] for a comprehensive review). It was found that when biomolecules are bound to polyelectrolyte brushes, their enzymatic activity is often preserved [
13,
14,
15,
16], which opens a fascinating perspective of using polyelectrolyte brushes in supported enzymatic catalysis.
The most important experimental findings that motivated and guided theoretical research on the interaction of globular proteins with polyelectrolyte (PE) brushes are (i) proteins can be absorbed by both polyanionic and polycationic brushes in the vicinity of the isoelectric point (IEP), i.e., when the charge of the protein globule is not only opposite but also of the same sign as that of the brush-forming chains. Only at sufficiently far away from the IEP proteins are repelled from the similarly charged brush; and (ii) an increase in the ionic strength of the solution suppresses protein uptake by a polyelectrolyte brush on both sides of the protein IEP. These findings unambiguously indicated that protein uptake by PE brushes is electrostatically driven on both sides of the IEP.
While the interaction of proteins with an oppositely charged brush is plausibly governed by Coulomb attraction, the mechanism of protein uptake by a similarly charged brush is more delicate and remains a matter of discussion in the literature. Two possible explanations of this effect have been proposed. According to the first explanation [
17,
18,
19], it is the inhomogeneity of the charge distribution on the protein surface that drives the absorption of proteins by the PE brush. Although the net charge of the protein on the “wrong side” of the IEP is of the same sign as that of the brush-forming chains, patches of charge of opposite sign on the globule surface drive the adsorption of PE chains on these patches, overcompensating for the penalty of polyelectrolyte repulsion from similarly charged patches. Evidently, this mechanism is operative provided that the distribution of anionic and cationic residues on the globule is essentially inhomogeneous.
An alternative mechanism [
20,
21] is based on the fact that the local pH inside the PE brush differs from that in the buffer solution due to the strong electrostatic field inside the brush. As a result, the net charge of the protein globule (controlled by the balance of ionization of pH-sensitive cationic and anionic residues) may change its sign inside the brush, so that the globule becomes charged oppositely to the brush and is driven inside by the Coulomb force. This second mechanism is more general and does not require any pre-assumption concerning the distribution of cationic and anionic residues on the globule surface. However, as it has been proven by a more accurate theoretical analysis [
22,
23,
24,
25], an inversion of the globule charge in the brush is an indispensable but not sufficient condition for the globule uptake by a similarly charged PE brush. As was demonstrated, the charge inversion leads to the appearance of a free energy barrier that may hinder protein uptake by the brush even when it is thermodynamically favorable.
Both experimental and theoretical studies reveal that the buffer is the key control parameter in the interaction of globular proteins and ampholytic protein-like nanocolloids with polyelectrolyte brushes. When the PE chains are negatively charged (polyacid brush), the electrostatic driving force for globule absorption is suppressed under strongly basic conditions (. It becomes operative in the vicinity of the IEP, , and is further promoted upon a decrease in . This trend is attributed to the progressively increasing net charge of the nanocolloid — from negative at to positive at — in the buffer solution and, with a positive offset, inside the negatively charged brush. The reverse trend is observed for positively charged (polybase) brushes.
In the theory developed earlier [
22,
23,
24,
25], it was, however, assumed that the charge on the brush-forming chains does not depend on
, as it happens for strong (quenched) polyelectrolytes, like polystyrenesulfonate (PSS, anionic) or poly(diallyldimethylammonium chloride) (PDADMAC, cationic). As a result, the electrostatic field created by a quenched PE brush and acting on the ampholytic particle is also independent of
. In many experiments, however, PE brushes were formed by weak (
-sensitive) polyelectrolytes, like poly(acrylic) acid (PAA, anionic) [
26,
27,
28] or poly (2-aminoethyl methacrylate hydrochloride) (PAEMH, cationic) [
28,
29].
Evidently, if
of a pH-sensitive polyanionic/polycationic brush is significantly larger/smaller than
of the nanocolloid, the PE brush is virtually fully ionized in the vicinity of
. In this case, the interactions of globular proteins with such a brush are similar to those with a strong polyelectrolyte (permanently ionized) brush. The situation may become more delicate if
, that is, variation in
around the protein IEP is accompanied by a significant change in the ionization of the brush-forming PE chains. One should also bear in mind that due to cooperative intermolecular Coulomb interactions between brush-forming pH-sensitive PE chains, the effective
is lower (for polyacid) or higher (for polybase) than the “bare”
for an individual monomer unit in the buffer and depends on the brush architecture (chain length, grafting density) and the ionic strength of the solution [
30,
31].
The aim of the present theoretical study is to unravel the effect of variations in buffer
on the interactions and uptake of protein-like ampholytic nanocolloids by brushes formed by pH-sensitive (annealing) polyanionic chains with a bare ionization constant
. We aim to investigate how simultaneous variations in the ionization of nanocolloid cationic and anionic residues and of monomer units in the brush-forming chains manifest themselves in the pH-controlled shape of the insertion free energy profiles and thus in the switch from absorption to depletion scenarios. To reach these goals, we implement an analytical self-consistent field Poisson–Boltzmann framework [
31,
32,
33] combined with the numerical Scheutjens–Fleer calculations [
34]. The latter approach must be applied under conditions of suppressed ionization of PE chains, i.e., when the free energy contributions due to the electrostatic and the excluded volume intermolecular interactions are comparable.
2. Results and Discussion
In
Figure 1, the electrostatic potential profiles
are presented for an annealing (weak) anionic brush at different offsets
from the brush pK (
) and for a quenched (strong) PE brush with fractions
of permanently charged monomer units equal to the average degrees of ionization
of the weak PE brush at each respective value of
. Four offsets were considered:
and
at fixed
. Solid lines correspond to strong-stretching analytical theory predictions, while dashed lines represent numerical results obtained from the Scheutjens–Fleer self-consistent field (SF-SCF) method for the annealing brush. The dash-dot lines represent the electrostatic potential profiles for quenched PE brushes, as obtained from the strong-stretching analytical theory.
As demonstrated by
Figure 1, the thickness of the annealing polyanionic brush monotonically increases upon an increase in
, which is unambiguously attributed to an increase in the average degree of ionization
of the monomer units of the brush-forming chains that leads to an increase in the osmotic pressure inside the brush and stronger chain stretching. The local degree of ionization
is an increasing function of the distance
z from the grafting surface in accordance with Equation (
20). For the given choice of the brush parameters and salt concentration, the results demonstrate excellent agreement between the analytical theory (solid lines) and SF-SCF calculations (dashed lines) for the annealing brush with average degrees of ionization
between 0.1 and 0.4.
We remind readers that the analytical theory takes into account only electrostatic intermolecular interactions in the brush and assumes linear (Gaussian) elasticity of the brush-forming chains, while numerical SF-SCF modelling accounts for both electrostatic and excluded volume interactions and for the finite chains’ extensibility as well. Therefore, certain discrepancies between the results of the analytical theory and numerical modelling arise: (i) at
, when the brush is weakly swollen and the contributions of electrostatic and excluded volume intermolecular interactions are comparable; and (ii) at
, due to the non-linear elastic response of strongly stretched chains (underestimated in the analytical theory). Comparison of the electrostatic potential profiles generated by quenched and annealing PE brushes with the same
reveals that the quenched brush generates a stronger electrostatic field, which should promote the absorption of ampholytic nanocolloids. Remarkably, as one can see from
Figure 1, PE chains in the annealing brush with
are more extended than those in the quenched PE brush (the annealing PE brush thickness is larger) because the degree of ionization of monomer units increases as a function of
z, from the grafting surface
towards the edge of the brush
.
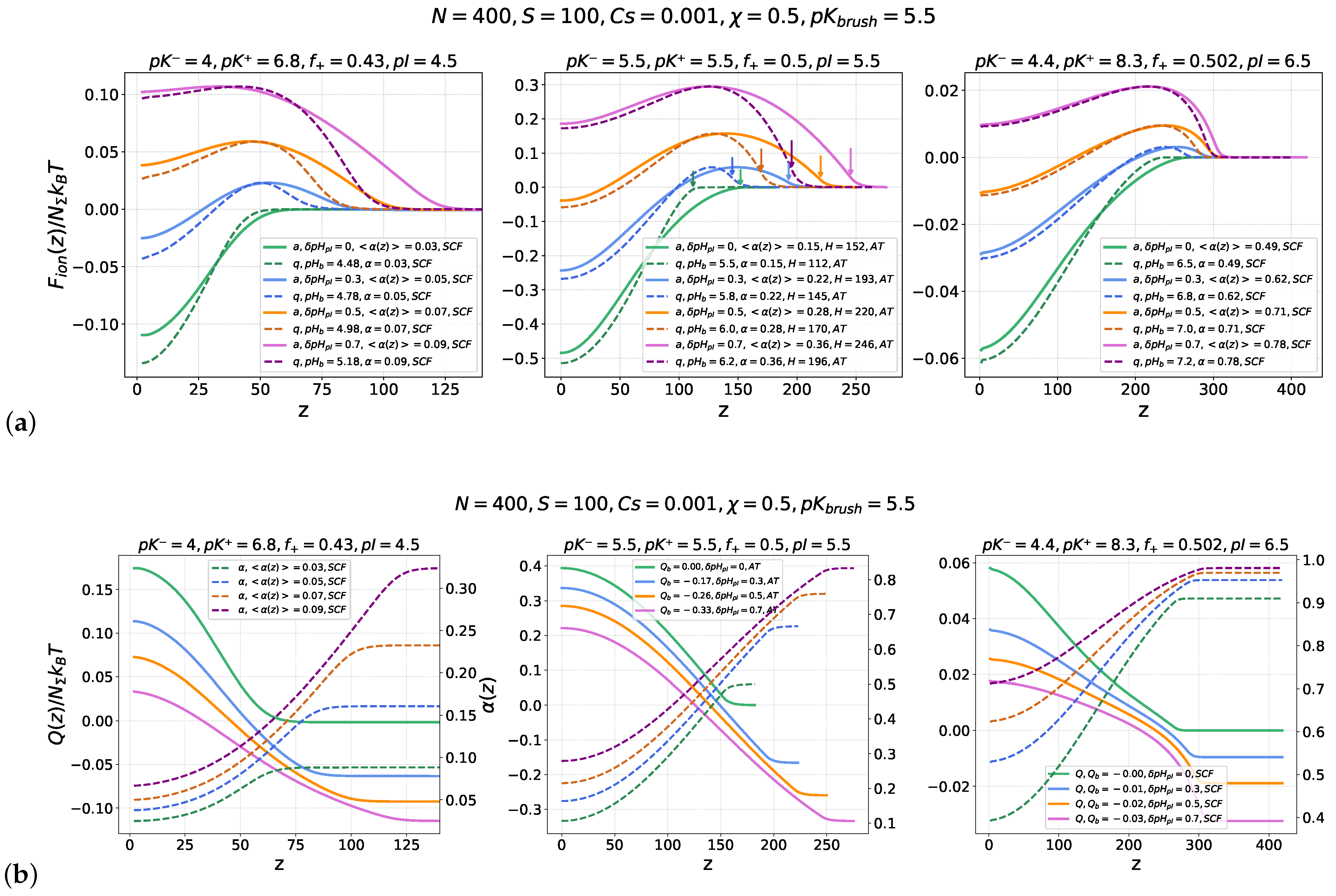
Figure 2 illustrates similarities and differences in the nanocolloid absorption scenario by annealing and quenched PE brushes on the “wrong side” of the IEP, i.e., when the brush and the nanocolloid in the buffer are similarly (negatively, in our case) charged. In
Figure 2a, the electrostatic parts
of the nanocolloid insertion free energy into an annealing (solid lines) or quenched (dashed lines) PE brushes with a similar average fraction of charged monomer units,
, are presented. The three panels, from left to right, correspond to different values of the nanocolloid isoelectric point
, representing nanocolloids with different fractions and ionization constants (
,
) of cationic and anionic groups. The ionization constant of the annealing brush,
, is the same in all the panels. Curves of different colors correspond to different offsets
of the buffer
from the respective IEPs of the nanocolloids. For each value of
, four values of
above the IEP, that is,
, corresponding to the zero or negative charge of the nanocolloid in the buffer, are considered.
The profiles of the position-dependent nanocolloid charge
and local brush ionization degree
for the same values of
and
are shown in
Figure 2b, which illustrates a monotonous increase in
and a concomitant decrease in
upon approaching the grafting surface.
All three panels in
Figure 2a,b demonstrate similar qualitative features in the evolution of
and
curves upon an increase in
up from the nanocolloid’s IEP for both annealing and quenched PE brushes: At
, the free energy
monotonously decreases upon a decrease in
z, from zero at
to a negative value attained in the edge minimum located at
, which points to thermodynamically favorable absorption of nanocolloids in the brush. Simultaneously, the nanocolloid charge monotonously increases from zero far away from the brush to a positive value inside the brush. The edge minimum of
at the grafting surface,
, is preserved also in the vicinity of the IEP,
, when the nanocolloid is negatively charged in the buffer,
, but acquires a positive net charge inside the brush. Additionally, a maximum in
emerges in the proximity of the brush edge. The position of the maximum coincides with the nanocolloid charge inversion point,
, as shown in
Figure 2b. The height of the maximum increases, whereas the depth of the minimum decreases upon an increase in
. Negative values of
indicate the presence of a thermodynamic driving force for the nanocolloid absorption in the brush, whereas positive values of
in the minimum indicate its metastable character, i.e., inversion of the nanocolloid charge upon insertion into the brush does not imply that the colloid absorption by the brush is thermodynamically favorable. These trends in the nanocolloid interaction with a PE brush as a function of
were previously discussed in [
23,
25].
The depth of the freeenergy
minimum at
is systematically larger for a quenched than for an annealing PE brush for the same
and the same
, which points to more efficient nanocolloid absorption by a strong than by a weak PE brush on the “wrong side” of the IEP. As one can see in
Figure 2a, due to the larger thickness of the annealing PE brush than that of the quenched PE brush with the same
, the freeenergy maximum at the brush periphery is systematically wider in the annealing PE brush than in the quenched PE brush, whereas the magnitude of the maximum for given
and
is the same in quenched and annealing brushes, as follows from Equations (
4)–(
10).
The right panels in
Figure 2a,b correspond to the case
, when the brush is already strongly charged,
at
(i.e. at
), and its charge further increases upon an increase in
, reaching
at
. As a result of the high ionization degree, the brush-forming PE chains are stretched beyond the linear elasticity regime, and therefore, we use the numerical SF-SCF scheme to calculate the electrostatic potential in the brush. Because in a strongly ionized annealing PE brush at
, the local degree of ionization
weakly varies across the brush, the insertion freeenergy
profiles at
fairly coincide for quenched and annealing PE brushes with
. Remarkably, for the chosen values of
and
, the nanocolloid remains very weakly charged in the vicinity of its isoelectric point due to the broad buffering region between
and
, as previously discussed in [
25]. As a result, the magnitude of variation of
is small, and the driving force for the nanocolloid absorption is weak.
The left panels in
Figure 2a,b correspond to the opposite case of
, when the annealing PE brush is almost uncharged in the vicinity of the nanocolloid’s IEP (
). Therefore, contributions of both ionic and excluded volume interactions between brush-forming chains are essential in determining the grafted chains’ conformations and resulting electrostatic potential profiles, which motivated us to use the numerical SF-SCF approach in this case as well. Since at
, the brush is only weakly charged and the magnitude of the positive charge acquired by the nanocolloid inside the brush is small (see the left panel in
Figure 2b), the driving force for the nanocolloid absorption by the brush is weak. As
increases above the IEP, both the colloid and then (at even larger
) the brush acquire negative charge, leading to mutual repulsion and eventual suppression of the driving force for absorption. Here, the difference between
curves for the nanocolloid inserted into quenched or annealing PE brushes is most pronounced in the peripheral regions of the brushes,
, with a noticeably wider free energy barrier in the latter case.
The most interesting scenario of the nanocolloid–brush interaction occurs at
, as illustrated by the middle panels in
Figure 2a,b. In this case, both the nanocolloid and the brush rapidly change their charge in response to small
shifts with respect to
, resulting in the largest magnitude of variation in the electrostatic free energy
and the most pronounced deviations between
curves for quenched and annealing PE brushes with the same
. In particular, this difference is manifested in a significantly wider freeenergy maximum in the case of the annealing PE brush compared to the quenched one. In the
range considered here, the chains in the PE brushes are moderately stretched (far below the finite extensibility limit), which enabled us to derive the electrostatic potential and insertion free energy analytically (not shown results of the SF-SCF numerical modelling perfectly match the analytical results).
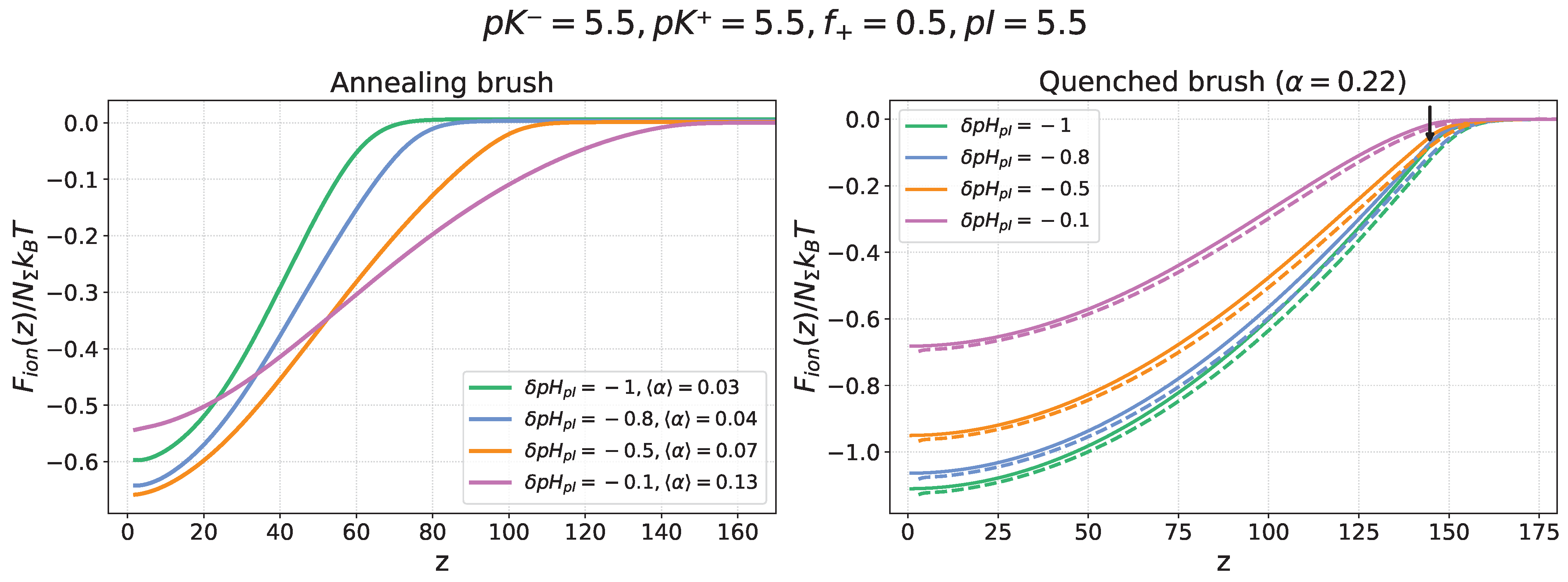
Figure 3 presents the electrostatic freeenergy profiles
of a nanocolloid in an annealing PE brush (left panel) with a varied,
-dependent average degree of ionization
and a quenched PE brush (right panel) with a fixed degree of ionization
. The ionization constant of the monomer units in the annealing PE brush is set to
, matching the nanocolloid’s isoelectric point,
. The freeenergy profiles are shown for several values of the
offset
, corresponding to acidic conditions (
) when the nanocolloid is positively charged, while the brush is always charged negatively.
Dashed lines in the right panel for the quenched PE brush represent SF-SCF numerical modelling results, which show good agreement with the results of analytical theory (solid lines). For the annealing PE brush (left panel), the freeenergy profiles were obtained by using the electrostatic potential , calculated by the SF-SCF numerical method, because the low degree of brush ionization under these conditions requires simultaneous accounting of electrostatic and excluded volume interactions between brush-forming chains.
In the case of the quenched brush, a decrease in below the IEP leads to progressive protonation of the nanocolloid and an increase in its net positive charge, which enhances electrostatic attraction to the permanently negatively charged PE brush and results in an increasing depth of the freeenergy minimum inside the brush.
The effect of
on the insertion freeenergy
profiles in the case of an annealing PE brush is more complex. As
decreases, the brush becomes less ionized and, as a result, produces a weaker electrostatic field. In spite of the concomitant increase in the positive charge of the nanocolloid, the balance of these two opposite trends may result in a weakening of the electrostatic driving force for absorption at sufficiently low
. This trend is illustrated in
Figure 3 (left panel), where the depth of the free energy minimum,
, increases upon a decrease in
from
to
but then decreases upon further lowering of
.
Up to now, we have considered only the ionic contribution,
, to the insertion free energy, with a focus on its dependence on
, and on defining the conditions when the electrostatic driving force for the nanocolloid absorption by the brush emerges. However, even if
provides the driving force for the absorption, it can be counterbalanced by the osmotic contribution to the free energy, defined by Equation (
11). The osmotic term in the free energy arises due to the difference in osmotic pressure of mobile ions in the brush interior and in the bulk of the solution, complemented by excluded volume repulsions between monomer units of the brush-forming chains. The osmotic term is always positive and produces the force expelling the nanocolloid from the brush. For a quenched PE brush, this term is
-independent, whereas for an annealing PE brush, both the concentration of mobile ions and that of monomer units in the brush depend on its degree of ionization and thus on
.
Figure 4 shows the profiles of the total insertion free energy
of a nanocolloid into the annealing PE brush, which includes both electrostatic and osmotic contributions, at three different values of
and different offsets
of the buffer
above the IEP of the nanocolloid. The sets of parameters used are the same as in
Figure 2, not volume. The ratio
of the nanocolloid volume to the number of ionizable groups is set to
.
Comparison of
Figure 2 and
Figure 4 shows that accounting for the osmotic force expelling the nanocolloid from the brush leads to a systematic shift of the
curves with respect to the
curves upwards, while the shape of the curves in the left and central panels is preserved because of the low or moderate ionization of the brush-forming chains in the vicinity of the nanocolloid IEP and the small excess osmotic pressure in the brush.
For the same values of , the addition of a positive term to the results in a decrease in the depth of the edge minimum at , or, at larger , to the disappearance of the minimum, while the height of the maximum increases. It is worth mentioning that the position of the total freeenergy maximum does not exactly match the point of the nanocolloid charge inversion.
In contrast, in the right panel, corresponding to , the brush is substantially ionized at , which produces strong excess osmotic pressure inside the brush. This overcompensates the electrostatic driving force for the nanocolloid absorption even in the proximity of the IEP: the total free energy monotonously increases upon approaching the grafting surface, and the nanocolloids are expelled from the brush.
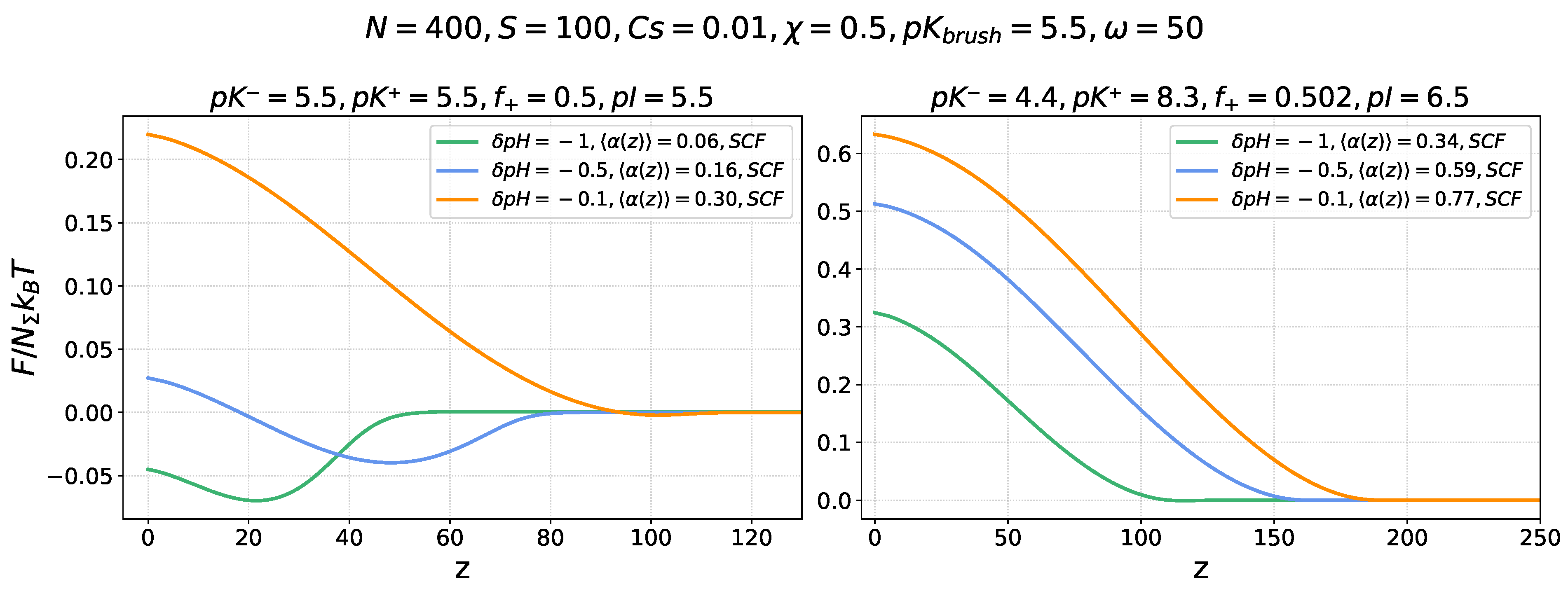
In
Figure 5, the profiles of the total insertion free energy
are shown for two values of
and different
offsets below the nanocolloid IEPs. In these cases, the nanocolloid is positively charged in the buffer, and the magnitude of its positive charge increases upon insertion into a negatively charged brush, providing an electrostatic driving force for the absorption (
is negative and monotonously decreases upon approaching the grafting surface). At
(right panel), the high osmotic pressure in the strongly ionized brush at
overperforms the electrostatic attraction and suppresses absorption. A decrease in
down to
leads to a lowering of the freeenergy profile and the appearance of a very shallow freeenergy minimum close to the brush edge due to the increasing positive charge of the nanocolloid. The same trend is even more pronounced at
, when the charge of the brush and, consequently, the osmotic pressure of counterions in the brush rapidly decrease upon lowering
below
: a shallow minimum in the
curve emerges in the brush periphery even at
. Upon a further decrease in
, this minimum becomes deeper and is displaced towards the central region of the brush. Hence, due to the interplay of electrostatic attraction and osmotic repulsion at
below the IEP, absorption and accumulation of positively charged nanocolloids occurs in the peripheral and central regions of the negatively charged PE brush, while the nanocolloids are expelled from the proximity of the grafting surface, where the local osmotic pressure is higher.