Neurotransmitter Alterations in Prediabetes and Type 2 Diabetes Mellitus: A Narrative Review
Abstract
1. Introduction
2. Neurotransmitter Systems Involved in Metabolic Regulation
2.1. Dopamine
Central Dopamine Alongside Peripheral Dopamine Receptors and Insulin Sensitivity
2.2. Serotonin (5-HT)
2.2.1. Skeletal Muscle Signaling and β-Cell Autocrine Signaling
2.2.2. Peripheral Actions and Central Actions
2.2.3. Therapeutic Implications
2.3. Norepinephrine (NE)
2.3.1. Sympathetic Overactivity in Early Metabolic Disease and Insulin Sensitivity
2.3.2. Impact on Beta-Cell Function
2.4. Gamma-Aminobutyric Acid (GABA)
2.4.1. CNS and Hypothalamic Regulation
2.4.2. Clinical Evidence
2.4.3. Animal Evidence and Human Trials
2.5. Glutamate
2.5.1. Central Excitatory Imbalance and Pancreatic β-Cell Glutamate Toxicity
2.5.2. Systemic Biomarkers and Therapeutic Insight
3. Evidence of Neurotransmitter Alterations in Prediabetes
3.1. MRI/MRS Studies
3.2. CSF and Peripheral Neurochemical Markers
3.3. Behavioral and Cognitive Correlates
4. Neurotransmitter Changes in New-Onset T2D
4.1. Alterations in Neurotransmitter Levels and Receptor Expression
4.2. HPA Axis Overactivation
4.3. Effects on Mood, Cognition, and Eating Behavior
- Neurotransmitter imbalance in new-onset T2D involves increased excitatory (glutamate) tone and reduced inhibitory (GABAergic) control, contributing to cognitive decline and metabolic worsening;
- HPA axis dysregulation, with elevated cortisol, both reflects and exacerbates early T2D pathophysiology;
- Neurobehavioral consequences—including anxiety, depression, and disordered eating—are linked to impaired dopaminergic and serotonergic regulation, further undermining glycemic control.
5. Potential Mechanisms Linking Neurotransmitter Dysfunction and Glucose Metabolism
5.1. Brain Insulin Signaling and Neurotransmitter Regulation
5.2. Inflammatory Pathways
5.3. Microbiota–Gut–Brain Axis and Integrated Pathophysiological Feedback
6. Future Directions and Gaps
6.1. Longitudinal Human Studies
6.2. Multi-Omics and Neuroimaging Integration
- Employ longitudinal, sex-aware, and mechanistic designs;
- Integrate neuroimaging with multi-omics data;
- Build toward precision diagnostics and targeted neuro-metabolic therapies.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, X.; Tao, S.; Tong, N. Potential Therapeutic Targeting Neurotransmitter Receptors in Diabetes. Front. Endocrinol. 2022, 13, 884549. [Google Scholar] [CrossRef]
- Hu, S.; Ji, W.; Zhang, Y.; Zhu, W.; Sun, H.; Sun, Y. Risk factors for progression to type 2 diabetes in prediabetes: A systematic review and meta-analysis. BMC Public Health 2025, 25, 1220. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Banks, W.A.; Raber, J. Insulin Resistance in Peripheral Tissues and the Brain: A Tale of Two Sites. Biomedicines 2022, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Güemes, A.; Georgiou, P. Review of the role of the nervous system in glucose homoeostasis and future perspectives towards the management of diabetes. Bioelectron. Med. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.K.; Inglis, J.T.; Madden, K.M.; Boyd, L.A. Brain and Body: A Review of Central Nervous System Contributions to Movement Impairments in Diabetes. Diabetes 2019, 69, 3–11. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wen, Z.; Yang, Y.; Bu, T.; Bu, X.; Ni, Q. Cognitive dysfunction in diabetes: Abnormal glucose metabolic regulation in the brain. Front. Endocrinol. 2023, 14, 1192602. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Roh, E.; Song, D.K.; Kim, M.-S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef] [PubMed]
- Shpakov, A.O.; Derkach, K.V.; Berstein, L.M. Brain Signaling Systems in the Type 2 Diabetes and Metabolic Syndrome: Promising Target to Treat and Prevent These Diseases. Future Sci. OA 2015, 1, FSO25. [Google Scholar] [CrossRef]
- Gruber, J.; Hanssen, R.; Qubad, M.; Bouzouina, A.; Schack, V.; Sochor, H.; Schiweck, C.; Aichholzer, M.; Matura, S.; Slattery, D.A.; et al. Impact of insulin and insulin resistance on brain dopamine signalling and reward processing–An underexplored mechanism in the pathophysiology of depression? Neurosci. Biobehav. Rev. 2023, 149, 105179. [Google Scholar] [CrossRef] [PubMed]
- Vranic, M.; Ahmed, F.; Kristófi, R.; Hetty, S.; Mokhtari, D.; Svensson, M.K.; Eriksson, J.W.; Pereira, M.J. Subcutaneous adipose tissue dopamine D2 receptor is increased in prediabetes and T2D. Endocrine 2024, 83, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Freyberg, Z.; Codario, R.A. Biological mechanisms of dopamine D2-like receptor agonist therapy in diabetes. Front. Endocrinol. 2025, 16, 1532414. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, M.; Li, M.; Ding, J.; Ye, M.; Tan, Z.; Liang, S. Effects of the selective serotonin reuptake inhibitors citalopram and escitalopram on glucolipid metabolism: A systematic review. Front. Endocrinol. 2025, 16, 1578326. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. New opportunity for serotonin receptor agonists. Nat. Rev. Drug Discov. 2008, 7, 17. [Google Scholar] [CrossRef]
- van Galen, K.A.; Ter Horst, K.W.; Serlie, M.J. Serotonin, food intake, and obesity. Obes. Rev. 2021, 22, e13210. [Google Scholar] [CrossRef] [PubMed]
- Phadke, D.; Beller, J.P.; Tribble, C. The Disparate Effects of Epinephrine and Norepinephrine on Hyperglycemia in Cardiovascular Surgery. Heart Surg. Forum 2018, 21, E522–E526. [Google Scholar] [CrossRef]
- Hagan, D.W.; Ferreira, S.M.; Santos, G.J.; Phelps, E.A. The role of GABA in islet function. Front. Endocrinol. 2022, 13, 972115. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, Q.; Prud’homme, G.J. GABAergic system in the endocrine pancreas: A new target for diabetes treatment. Diabetes Metab. Syndr. Obes. 2015, 8, 79–87. [Google Scholar] [CrossRef]
- Jin, Z.; Korol, S.V. GABA signalling in human pancreatic islets. Front. Endocrinol. 2023, 14, 1059110. [Google Scholar] [CrossRef]
- Kanaani, J.; Cianciaruso, C.; Phelps, E.A.; Pasquier, M.; Brioudes, E.; Billestrup, N.; Baekkeskov, S. Compartmentalization of GABA Synthesis by GAD67 Differs between Pancreatic Beta Cells and Neurons. PLoS ONE 2015, 10, e0117130. [Google Scholar] [CrossRef]
- Grasso, P. Harnessing the Power of Leptin: The Biochemical Link Connecting Obesity, Diabetes, and Cognitive Decline. Front. Aging Neurosci. 2022, 14, 861350. [Google Scholar] [CrossRef] [PubMed]
- Vijayashankar, U.; Ramashetty, R.; Rajeshekara, M.; Vishwanath, N.; Yadav, A.K.; Prashant, A.; Lokeshwaraiah, R. Leptin and ghrelin dynamics: Unraveling their influence on food intake, energy balance, and the pathophysiology of type 2 diabetes mellitus. J. Diabetes Metab. Disord. 2024, 23, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Rausch, J.; Horne, K.E.; Marquez, L. The Effects of Adipose Tissue Dysregulation on Type 2 Diabetes Mellitus. Biomedicines 2025, 13, 1770. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Dodd, G.T. Hypothalamic neuronal-glial crosstalk in metabolic disease. NPJ Metab. Health Dis. 2024, 2, 27. [Google Scholar] [CrossRef]
- Bhusal, A.; Rahman, M.H.; Suk, K. Hypothalamic inflammation in metabolic disorders and aging. Cell. Mol. Life Sci. 2021, 79, 32. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Zhou, C.X.; Li, H.M.; Cheng, M.; Chen, D.; Li, Z.Y.; Feng, B.; Song, J. Correlation between cerebral neurotransmitters levels by proton magnetic resonance spectroscopy and HbA1c in patients with type 2 diabetes. World J. Diabetes 2024, 15, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Thielen, J.-W.; Gancheva, S.; Hong, D.; Rohani Rankouhi, S.; Chen, B.; Apostolopoulou, M.; Anadol-Schmitz, E.; Roden, M.; Norris, D.G.; Tendolkar, I. Higher GABA concentration in the medial prefrontal cortex of Type 2 diabetes patients is associated with episodic memory dysfunction. Hum. Brain Mapp. 2019, 40, 4287–4295. [Google Scholar] [CrossRef]
- d’Almeida, O.C.; Violante, I.R.; Quendera, B.; Moreno, C.; Gomes, L.; Castelo-Branco, M. The neurometabolic profiles of GABA and Glutamate as revealed by proton magnetic resonance spectroscopy in type 1 and type 2 diabetes. PLoS ONE 2020, 15, e0240907. [Google Scholar] [CrossRef]
- Al-Sayyar, A.; Hammad, M.; Williams, M.; Al-Onaizi, M.; Abubaker, J.; Alzaid, F. Neurotransmitters in Type 2 Diabetes and the Control of Systemic and Central Energy Balance. Metabolites 2023, 13, 384. [Google Scholar] [CrossRef]
- Freyberg, Z.; Gittes, G.K. Roles of Pancreatic Islet Catecholamine Neurotransmitters in Glycemic Control and in Antipsychotic Drug–Induced Dysglycemia. Diabetes 2022, 72, 3–15. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Halverson, T. Role of Peripheral and Central Insulin Resistance in Neuropsychiatric Disorders. J. Clin. Med. 2024, 13, 6607. [Google Scholar] [CrossRef]
- Heni, M. The insulin resistant brain: Impact on whole-body metabolism and body fat distribution. Diabetologia 2024, 67, 1181–1191. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; De Simone, G.; De Prisco, M.; Barone, A.; Napoli, R.; Beguinot, F.; Billeci, M.; Fornaro, M. Insulin effects on core neurotransmitter pathways involved in schizophrenia neurobiology: A meta-analysis of preclinical studies. Implications for the treatment. Mol. Psychiatry 2023, 28, 2811–2825. [Google Scholar] [CrossRef]
- van Bussel, F.C.G.; Backes, W.H.; Hofman, P.A.M.; Puts, N.A.J.; Edden, R.A.E.; van Boxtel, M.P.J.; Schram, M.T.; Stehouwer, C.D.A.; Wildberger, J.E.; Jansen, J.F.A. Increased GABA concentrations in type 2 diabetes mellitus are related to lower cognitive functioning. Medicine 2016, 95, e4803. [Google Scholar] [CrossRef]
- Shillo, P.; Sloan, G.; Selvarajah, D.; Greig, M.; Gandhi, R.; Anand, P.; Edden, R.A.; Wilkinson, I.D.; Tesfaye, S. Reduced Thalamic γ-Aminobutyric Acid (GABA) in Painless but Not Painful Diabetic Peripheral Neuropathy. Diabetes 2024, 73, 1317–1324. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.; Marques, D.; Barra, C.; Rosendo-Silva, D.; Costa, A.; Rodrigues, T.; Gasparini, P.; Melo, B.F.; Sacramento, J.F.; Seiça, R.; et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. Mol. Metab. 2021, 51, 101241. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- García-Tornadú, I.; Ornstein, A.M.; Chamson-Reig, A.; Wheeler, M.B.; Hill, D.J.; Arany, E.; Rubinstein, M.; Becu-Villalobos, D. Disruption of the Dopamine D2 Receptor Impairs Insulin Secretion and Causes Glucose Intolerance. Endocrinology 2010, 151, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; De Tullio, A.; Iovino, M.; Disoteo, O.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes. Biomedicines 2023, 11, 2993. [Google Scholar] [CrossRef]
- Lopez Vicchi, F.; Luque, G.M.; Brie, B.; Nogueira, J.P.; Garcia Tornadu, I.; Becu-Villalobos, D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol. Res. 2016, 109, 74–80. [Google Scholar] [CrossRef]
- Caravaggio, F.; Borlido, C.; Hahn, M.; Feng, Z.; Fervaha, G.; Gerretsen, P.; Nakajima, S.; Plitman, E.; Chung, J.K.; Iwata, Y.; et al. Reduced insulin sensitivity is related to less endogenous dopamine at D2/3 receptors in the ventral striatum of healthy nonobese humans. Int. J. Neuropsychopharmacol. 2015, 18, pyv014. [Google Scholar] [CrossRef]
- Wei, H.; Zapata, R.C.; Lopez-Valencia, M.; Aslanoglou, D.; Farino, Z.J.; Benner, V.; Osborn, O.; Freyberg, Z.; McCarthy, M.J. Dopamine D2 receptor signaling modulates pancreatic beta cell circadian rhythms. Psychoneuroendocrinology 2020, 113, 104551. [Google Scholar] [CrossRef]
- Ustione, A.; Piston, D.W.; Harris, P.E. Minireview: Dopaminergic regulation of insulin secretion from the pancreatic islet. Mol. Endocrinol. 2013, 27, 1198–1207. [Google Scholar] [CrossRef]
- Nonogaki, K. The Regulatory Role of the Central and Peripheral Serotonin Network on Feeding Signals in Metabolic Diseases. Int. J. Mol. Sci. 2022, 23, 1600. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.-M.; Park, S.; Kim, H. Serotonin as a New Therapeutic Target for Diabetes Mellitus and Obesity. Diabetes Metab. J. 2016, 40, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, X.; Zhou, H.; Zhou, J. The serotonergic system dysfunction in diabetes mellitus. Front. Cell. Neurosci. 2022, 16, 899069. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoairy, R.; Pedrini, M.T.; Khan, M.I.; Engl, J.; Tschoner, A.; Ebenbichler, C.; Gstraunthaler, G.; Salzmann, K.; Bakry, R.; Niederwanger, A. Serotonin improves glucose metabolism by Serotonylation of the small GTPase Rab4 in L6 skeletal muscle cells. Diabetol. Metab. Syndr. 2017, 9, 1. [Google Scholar] [CrossRef]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y.; et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef]
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Moon, J.H.; Kim, H. Serotonergic regulation of energy metabolism in peripheral tissues. J. Endocrinol. 2020, 245, R1–R10. [Google Scholar] [CrossRef]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology 2017, 158, 1049–1063. [Google Scholar] [CrossRef]
- Martin, A.M.; Yabut, J.M.; Choo, J.M.; Page, A.J.; Sun, E.W.; Jessup, C.F.; Wesselingh, S.L.; Khan, W.I.; Rogers, G.B.; Steinberg, G.R.; et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc. Natl. Acad. Sci. USA 2019, 116, 19802–19804. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Shin, S.; Kim, Y.; Kang, Y.; Cha, H.-N.; Park, S.-Y.; Park, S.; Oh, C.-M. Inhibition of serotonin-Htr2b signaling in skeletal muscle mitigates obesity-induced insulin resistance. Exp. Mol. Med. 2025, 57, 1177–1188. [Google Scholar] [CrossRef]
- Kong, C.C.; Cheng, J.D.; Wang, W. Neurotransmitters regulate β cells insulin secretion: A neglected factor. World J. Clin. Cases 2023, 11, 6670–6679. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Brouwers, B.; Liu, H.; Liu, H.; Lawler, K.; Mendes de Oliveira, E.; Lee, D.-K.; Yang, Y.; Cox, A.R.; Keogh, J.M.; et al. Human loss-of-function variants in the serotonin 2C receptor associated with obesity and maladaptive behavior. Nat. Med. 2022, 28, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Blasi, C. Obesity and Related Type 2 Diabetes: A Failure of the Autonomic Nervous System Controlling Gastrointestinal Function? Gastrointest. Disord. 2020, 2, 423–447. [Google Scholar] [CrossRef]
- Fox, J.H.; Boucher, M.N.; Abedrabbo, K.S.; Hare, B.D.; Grimmig, B.A.; Falls, W.A.; Hammack, S.E. Exercise reduces the anxiogenic effects of meta-chlorophenylpiperazine: The role of 5-HT2C receptors in the bed nucleus of the stria terminalis. Front. Synaptic Neurosci. 2023, 14, 1067420. [Google Scholar] [CrossRef]
- Wallace, T.J.; Zai, C.C.; Brandl, E.J.; Müller, D.J. Role of 5-HT(2C) receptor gene variants in antipsychotic-induced weight gain. Pharmgenomics Pers. Med. 2011, 4, 83–93. [Google Scholar] [CrossRef]
- Conde, K.; Fang, S.; Xu, Y. Unraveling the serotonin saga: From discovery to weight regulation and beyond-a comprehensive scientific review. Cell Biosci. 2023, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Wyler, S.C.; Lord, C.C.; Lee, S.; Elmquist, J.K.; Liu, C. Serotonergic Control of Metabolic Homeostasis. Front. Cell. Neurosci. 2017, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, Z.; Faber, C.L.; Schwartz, M.W. Central Nervous System Control of Glucose Homeostasis: A Therapeutic Target for Type 2 Diabetes? Annu. Rev. Pharmacol. Toxicol. 2022, 62, 55–84. [Google Scholar] [CrossRef]
- Toczyska, K.; Haq, N.; Lyu, Z.; Bewick, G.; Zhao, M.; Rosa, H.; Starikova, J.; Liu, B.; Persaud, S.J. The selective serotonin reuptake inhibitors, sertraline and paroxetine, improve islet beta-cell mass and function in vitro. Diabetes Obes. Metab. 2024, 26, 3606–3617. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, N.S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Ragab, A.E.; Alenazi, A.A.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Antidepressants and type 2 diabetes: Highways to knowns and unknowns. Diabetol. Metab. Syndr. 2023, 15, 179. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Shen, C.; Shao, F. Efficacy of pharmacotherapies for bulimia nervosa: A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2023, 24, 72. [Google Scholar] [CrossRef]
- Namkung, J.; Shong, K.E.; Kim, H.; Oh, C.-M.; Park, S.; Kim, H. Inhibition of Serotonin Synthesis Induces Negative Hepatic Lipid Balance. Diabetes Metab. J. 2018, 42, 233–243. [Google Scholar] [CrossRef]
- Sun, E.W.L.; Martin, A.M.; Young, R.L.; Keating, D.J. The Regulation of Peripheral Metabolism by Gut-Derived Hormones. Front. Endocrinol. 2019, 9, 754. [Google Scholar] [CrossRef]
- Penesova, A.; Radikova, Z.; Cizmarova, E.; Kvetňanský, R.; Blazicek, P.; Vlcek, M.; Koska, J.; Vigas, M. The Role of Norepinephrine and Insulin Resistance in an Early Stage of Hypertension. Ann. N. Y. Acad. Sci. 2008, 1148, 490–494. [Google Scholar] [CrossRef]
- Marangou, A.G.; Alford, F.P.; Ward, G.; Liskaser, F.; Aitken, P.M.; Weber, K.M.; Boston, R.C.; Best, J.D. Hormonal effects of norepinephrine on acute glucose disposal in humans: A minimal model analysis. Metabolism 1988, 37, 885–891. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Grima, M.T.; Sari, C.I.; Eikelis, N.; Lambert, E.A.; Nestel, P.J.; Esler, M.D.; Dixon, J.B.; Chopra, R.; Tilbrook, A.J.; et al. Neuroadrenergic dysfunction along the diabetes continuum: A comparative study in obese metabolic syndrome subjects. Diabetes 2012, 61, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Khoury, N.; McGill, J.B. Reduction in insulin sensitivity following administration of the clinically used low-dose pressor, norepinephrine. Diabetes Metab. Res. Rev. 2011, 27, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Hyun, U.; Sohn, J.-W. Autonomic control of energy balance and glucose homeostasis. Exp. Mol. Med. 2022, 54, 370–376. [Google Scholar] [CrossRef]
- Privitera, M.; von Ziegler, L.M.; Floriou-Servou, A.; Duss, S.N.; Zhang, R.; Waag, R.; Leimbacher, S.; Sturman, O.; Roessler, F.K.; Heylen, A.; et al. Noradrenaline release from the locus coeruleus shapes stress-induced hippocampal gene expression. eLife 2024, 12, RP88559. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kielar, D.; Minokoshi, Y.; Shimazu, T. Noradrenaline increases glucose transport into brown adipocytes in culture by a mechanism different from that of insulin. Biochem. J. 1996, 314 Pt 2, 485–490. [Google Scholar] [CrossRef]
- Sakamoto, K.; Butera, M.A.; Zhou, C.; Maurizi, G.; Chen, B.; Ling, L.; Shawkat, A.; Patlolla, L.; Thakker, K.; Calle, V.; et al. Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity. Cell Metab. 2025, 37, 121–137.e126. [Google Scholar] [CrossRef]
- McGuinness, O.P.; Shau, V.; Benson, E.M.; Lewis, M.; Snowden, R.T.; Greene, J.E.; Neal, D.W.; Cherrington, A.D. Role of epinephrine and norepinephrine in the metabolic response to stress hormone infusion in the conscious dog. Am. J. Physiol.-Endocrinol. Metab. 1997, 273, E674–E681. [Google Scholar] [CrossRef]
- Esler, M.; Rumantir, M.; Wiesner, G.; Kaye, D.; Hastings, J.; Lambert, G. Sympathetic nervous system and insulin resistance: From obesity to diabetes. Am. J. Hypertens. 2001, 14, 304s–309s. [Google Scholar] [CrossRef]
- Flaa, A.; Aksnes, T.A.; Kjeldsen, S.E.; Eide, I.; Rostrup, M. Increased sympathetic reactivity may predict insulin resistance: An 18-year follow-up study. Metabolism 2008, 57, 1422–1427. [Google Scholar] [CrossRef]
- Morton, G.J.; Muta, K.; Kaiyala, K.J.; Rojas, J.M.; Scarlett, J.M.; Matsen, M.E.; Nelson, J.T.; Acharya, N.K.; Piccinini, F.; Stefanovski, D.; et al. Evidence That the Sympathetic Nervous System Elicits Rapid, Coordinated, and Reciprocal Adjustments of Insulin Secretion and Insulin Sensitivity During Cold Exposure. Diabetes 2017, 66, 823–834. [Google Scholar] [CrossRef]
- Valensi, P. Autonomic nervous system activity changes in patients with hypertension and overweight: Role and therapeutic implications. Cardiovasc. Diabetol. 2021, 20, 170. [Google Scholar] [CrossRef]
- Shetty, S.; Bhoraskar, A.; Mohan, J.; Chafekar, D.; Tripathi, K.; Sivalingam, M.; Desai, B.; Gude, D.; Sridhar, G.; Varghese, C.; et al. Selective imidazoline receptor agonists: Redefining role of centrally acting agents in role of centrally acting agents in management of hypertension. Int. J. Adv. Med. 2019, 6, 1688. [Google Scholar] [CrossRef]
- Straub, S.G.; Sharp, G.W. Evolving insights regarding mechanisms for the inhibition of insulin release by norepinephrine and heterotrimeric G proteins. Am. J. Physiol. Cell Physiol. 2012, 302, C1687–C1698. [Google Scholar] [CrossRef]
- Yajima, H.; Komatsu, M.; Sato, Y.; Yamada, S.; Yamauchi, K.; Sharp, G.W.; Aizawa, T.; Hashizume, K. Norepinephrine inhibits glucose-stimulated, Ca2+-independent insulin release independently from its action on adenylyl cyclase. Endocr. J. 2001, 48, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, H.; Limesand, S.W.; Chen, X. Pancreatic Islets Exhibit Dysregulated Adaptation of Insulin Secretion after Chronic Epinephrine Exposure. Curr. Issues Mol. Biol. 2021, 43, 240–250. [Google Scholar] [CrossRef]
- Heusser, K.; Tank, J.; Diedrich, A.; Fischer, A.; Heise, T.; Jordan, J. Limited evidence for sympathetic neural overactivation in older patients with type 2 diabetes mellitus. Front. Neurosci. 2022, 16, 1107752. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef]
- Drygała, S.; Radzikowski, M.; Maciejczyk, M. β-blockers and metabolic modulation: Unraveling the complex interplay with glucose metabolism, inflammation and oxidative stress. Front. Pharmacol. 2024, 15, 1489657. [Google Scholar] [CrossRef] [PubMed]
- Mara Ferreira, S.; Stis, A.E.; Widener, A.; Cuaycal, A.E.; Phelps, E. 352-OR: GABA Modulates Pancreatic Beta-Cell Function through Ca2+ Signaling. Diabetes 2025, 74, 352. [Google Scholar] [CrossRef]
- Rezazadeh, H.; Sharifi, M.R.; Soltani, N. Insulin resistance and the role of gamma-aminobutyric acid. J. Res. Med. Sci. 2021, 26, 39. [Google Scholar] [CrossRef]
- Martin, A.; Mick, G.J.; Choat, H.M.; Lunsford, A.A.; Tse, H.M.; McGwin, G.G.; McCormick, K.L. A randomized trial of oral gamma aminobutyric acid (GABA) or the combination of GABA with glutamic acid decarboxylase (GAD) on pancreatic islet endocrine function in children with newly diagnosed type 1 diabetes. Nat. Commun. 2022, 13, 7928. [Google Scholar] [CrossRef]
- Barakat, H.; Aljutaily, T. Role of γ-Aminobutyric Acid (GABA) as an Inhibitory Neurotransmitter in Diabetes Management: Mechanisms and Therapeutic Implications. Biomolecules 2025, 15, 399. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Y.; Li, J.; Xiong, Y.; Zhang, X.; Wan, Y.; Zheng, R.; Zhang, C. Hypothalamic GABAergic neurons: Their roles in health and metabolic diseases. Front. Endocrinol. 2025, 16, 1551741. [Google Scholar] [CrossRef]
- Yoon, N.A.; Diano, S. Hypothalamic glucose-sensing mechanisms. Diabetologia 2021, 64, 985–993. [Google Scholar] [CrossRef]
- Korol, S.V.; Jin, Z.; Jin, Y.; Bhandage, A.K.; Tengholm, A.; Gandasi, N.R.; Barg, S.; Espes, D.; Carlsson, P.O.; Laver, D.; et al. Functional Characterization of Native, High-Affinity GABA(A) Receptors in Human Pancreatic β Cells. eBioMedicine 2018, 30, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.E.; Ghimire, S.; Bruggink, S.M.; Miller, K.E.; Weninger, S.N.; Kronenfeld, J.M.; Yoshino, J.; Klein, S.; Duca, F.A.; Renquist, B.J. A critical role of hepatic GABA in the metabolic dysfunction and hyperphagia of obesity. Cell Rep. 2021, 35, 109301. [Google Scholar] [CrossRef] [PubMed]
- de Bie, T.H.; Witkamp, R.F.; Balvers, M.G.; Jongsma, M.A. Effects of γ-aminobutyric acid supplementation on glucose control in adults with prediabetes: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2023, 118, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Dastgerdi, A.; Sharifi, M.; Soltani, N. GABA administration improves liver function and insulin resistance in offspring of type 2 diabetic rats. Sci. Rep. 2021, 11, 23155. [Google Scholar] [CrossRef]
- Antoni, F.A. The Case for Clinical Trials with Novel GABAergic Drugs in Diabetes Mellitus and Obesity. Life 2022, 12, 322. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.S.; Dizon, M.P.; Yaw, C.K.; Kaufman, D.L. Oral treatment with γ-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef]
- Palazzo, E.; Marabese, I.; Ricciardi, F.; Guida, F.; Luongo, L.; Maione, S. The influence of glutamate receptors on insulin release and diabetic neuropathy. Pharmacol. Ther. 2024, 263, 108724. [Google Scholar] [CrossRef]
- Cabrera, O.; Jacques-Silva, M.C.; Speier, S.; Yang, S.N.; Köhler, M.; Fachado, A.; Vieira, E.; Zierath, J.R.; Kibbey, R.; Berman, D.M.; et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 2008, 7, 545–554. [Google Scholar] [CrossRef]
- Galli, A.; Moretti, S.; Dule, N.; Di Cairano, E.S.; Castagna, M.; Marciani, P.; Battaglia, C.; Bertuzzi, F.; Fiorina, P.; Pastore, I.; et al. Hyperglycemia impairs EAAT2 glutamate transporter trafficking and glutamate clearance in islets of Langerhans: Implications for type 2 diabetes pathogenesis and treatment. Am. J. Physiol. Endocrinol. Metab. 2024, 327, E27–E41. [Google Scholar] [CrossRef]
- Šterk, M.; Križančić Bombek, L.; Skelin Klemen, M.; Slak Rupnik, M.; Marhl, M.; Stožer, A.; Gosak, M. NMDA receptor inhibition increases, synchronizes, and stabilizes the collective pancreatic beta cell activity: Insights through multilayer network analysis. PLoS Comput. Biol. 2021, 17, e1009002. [Google Scholar] [CrossRef]
- Noguera Hurtado, H.; Gresch, A.; Düfer, M. NMDA receptors-regulatory function and pathophysiological significance for pancreatic beta cells. Biol. Chem. 2023, 404, 311–324. [Google Scholar] [CrossRef]
- Cyranka, M.; Monfeuga, T.; Vedovato, N.; Larabee, C.M.; Chandran, A.; Toledo, E.M.; de Wet, H. NMDA Receptor Antagonists Increase the Release of GLP-1 From Gut Endocrine Cells. Front. Pharmacol. 2022, 13, 861311. [Google Scholar] [CrossRef]
- Huang, X.-T.; Li, C.; Peng, X.-P.; Guo, J.; Yue, S.-J.; Liu, W.; Zhao, F.-Y.; Han, J.-Z.; Huang, Y.-H.; Yang, L.; et al. An excessive increase in glutamate contributes to glucose-toxicity in β-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci. Rep. 2017, 7, 44120. [Google Scholar] [CrossRef]
- Han, F.; Xu, C.; Hangfu, X.; Liu, Y.; Zhang, Y.; Sun, B.; Chen, L. Circulating glutamine/glutamate ratio is closely associated with type 2 diabetes and its associated complications. Front. Endocrinol. 2024, 15, 1422674. [Google Scholar] [CrossRef]
- Purkayastha, S.; Cai, D. Neuroinflammatory basis of metabolic syndrome. Mol. Metab. 2013, 2, 356–363. [Google Scholar] [CrossRef]
- Bennet, H.; Mollet, I.G.; Balhuizen, A.; Medina, A.; Nagorny, C.; Bagge, A.; Fadista, J.; Ottosson-Laakso, E.; Vikman, P.; Dekker-Nitert, M.; et al. Serotonin (5-HT) receptor 2b activation augments glucose-stimulated insulin secretion in human and mouse islets of Langerhans. Diabetologia 2016, 59, 744–754. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, Y.G.; Kim, K.; Osonoi, S.; Wang, S.; Saunders, D.C.; Wang, J.; Yang, K.; Kim, H.; Lee, J.; et al. Serotonin Regulates Adult β-Cell Mass by Stimulating Perinatal β-Cell Proliferation. Diabetes 2020, 69, 205–214. [Google Scholar] [CrossRef]
- Grundmann, R.; Rullmann, M.; Luthardt, J.; Zientek, F.; Becker, G.-A.; Patt, M.; Hankir, M.K.; Blüher, M.; Sabri, O.; Hesse, S. Higher HbA1c levels associate with lower hippocampal serotonin transporter availability in non-diabetic adults with obesity. Sci. Rep. 2020, 10, 21383. [Google Scholar] [CrossRef]
- Georgescu, T.; Lyons, D.; Heisler, L.K. Role of serotonin in body weight, insulin secretion and glycaemic control. J. Neuroendocrinol. 2021, 33, e12960. [Google Scholar] [CrossRef]
- Aslanoglou, D.; Bertera, S.; Sánchez-Soto, M.; Benjamin Free, R.; Lee, J.; Zong, W.; Xue, X.; Shrestha, S.; Brissova, M.; Logan, R.W.; et al. Dopamine regulates pancreatic glucagon and insulin secretion via adrenergic and dopaminergic receptors. Transl. Psychiatry 2021, 11, 59. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Koshelev, D.; Nedorubov, A.; Kosheleva, L.; Trukhan, V.; Rabinovitch, A.; Schiöth, H.B.; Levit, S. Triple drug therapy with GABA, sitagliptin, and omeprazole prevents type 1 diabetes onset and promotes its reversal in non-obese diabetic mice. Front. Endocrinol. 2022, 13, 1028114. [Google Scholar] [CrossRef]
- Lee, C.J.; Schnieders, J.H.; Rubakhin, S.S.; Patel, A.V.; Liu, C.; Naji, A.; Sweedler, J.V. d-Amino Acids and Classical Neurotransmitters in Healthy and Type 2 Diabetes-Affected Human Pancreatic Islets of Langerhans. Metabolites 2022, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Leclerc, M.; Evitts, K.; Brown, C.; Miller, W.; Hanson, A.J.; Banks, W.A.; Gibbons, L.; Domoto-Reilly, K.; Jayadev, S.; et al. Cerebrospinal fluid soluble insulin receptor levels in Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2024, 16, e12603. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Hou, T.H.; Yu, H.P.; Li, A.; Liu, F.C. Cerebrospinal fluid electrolytes and acid-base in diabetic patients. Transl. Neurosci. 2021, 12, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, X.; Liu, S.; Li, M. Changes in Cerebrospinal Fluid Tau and β-Amyloid Levels in Diabetic and Prediabetic Patients: A Meta-Analysis. Front. Aging Neurosci. 2018, 10, 271. [Google Scholar] [CrossRef]
- Ajilore, O.; Haroon, E.; Kumaran, S.; Darwin, C.; Binesh, N.; Mintz, J.; Miller, J.; Thomas, M.A.; Kumar, A. Measurement of Brain Metabolites in Patients with type 2 Diabetes and Major Depression Using Proton Magnetic Resonance Spectroscopy. Neuropsychopharmacology 2007, 32, 1224–1231. [Google Scholar] [CrossRef]
- Laughlin, M.; Cooke, B.; Boutelle, K.; Savage, C.R.; Kravitz, A.; Small, D.; Arvanitakis, Z.; Martin, A.; Stoeckel, L.E. Neuroimaging and modulation in obesity and diabetes research: 10th anniversary meeting. Int. J. Obes. 2022, 46, 718–725. [Google Scholar] [CrossRef]
- Cuypers, K.; Marsman, A. Transcranial magnetic stimulation and magnetic resonance spectroscopy: Opportunities for a bimodal approach in human neuroscience. NeuroImage 2021, 224, 117394. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Ehlert, L.; Mullur, R.; Freeby, M.J.; Woo, M.A.; Kumar, R.; Choi, S. Regional Brain Gray Matter Changes in Patients with Type 2 Diabetes Mellitus. Sci. Rep. 2020, 10, 9925. [Google Scholar] [CrossRef]
- Mosili, P.; Mkhize, B.C.; Sibiya, N.H.; Ngubane, P.S.; Khathi, A. Review of the direct and indirect effects of hyperglycemia on the HPA axis in T2DM and the co-occurrence of depression. BMJ Open Diabetes Res. Care 2024, 12, e003218. [Google Scholar] [CrossRef]
- Chiodini, I.; Adda, G.; Scillitani, A.; Coletti, F.; Morelli, V.; Di Lembo, S.; Epaminonda, P.; Masserini, B.; Beck-Peccoz, P.; Orsi, E.; et al. Cortisol Secretion in Patients With Type 2 Diabetes: Relationship with chronic complications. Diabetes Care 2007, 30, 83–88. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Gil-Lozano, M.; Toba, L.; Fandiño, J.; Ogando, H.; González-Matías, L.C.; Mallo, F. Stressing diabetes? The hidden links between insulinotropic peptides and the HPA axis. J. Endocrinol. 2016, 230, R77–R94. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, J.; Jiang, W.; Wang, W.; Yang, X.; Liu, Y.; Jin, X.; Guo, Q.; Xu, Y.; Lu, B.; et al. Stronger association between morning serum cortisol level and diurnal time in range in type 2 diabetes? Diabetol. Metab. Syndr. 2024, 16, 290. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, C.J.; Hernandez, R.; Schneider, S.; Peters, A.; Hawkins, M.; Pyatak, E.A.; Gonzalez, J.S. Dynamic Relationships Among Continuous Glucose Metrics and Momentary Cognitive Performance in Diverse Adults With Type 1 Diabetes. Diabetes Care 2025, 48, 799–806. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Advancements Exploring Major Depressive Disorder: Insights on Oxidative Stress, Serotonin Metabolism, BDNF, HPA Axis Dysfunction, and Pharmacotherapy Advances. Int. J. Transl. Med. 2024, 4, 176–196. [Google Scholar] [CrossRef]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef]
- Blum, K.; Thanos, P.K.; Gold, M.S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 2014, 5, 919. [Google Scholar] [CrossRef]
- Naert, G.; Ixart, G.; Maurice, T.; Tapia-Arancibia, L.; Givalois, L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol. Cell Neurosci. 2011, 46, 55–66. [Google Scholar] [CrossRef]
- Lei, A.A.; Phang, V.W.X.; Lee, Y.Z.; Kow, A.S.F.; Tham, C.L.; Ho, Y.-C.; Lee, M.T. Chronic Stress-Associated Depressive Disorders: The Impact of HPA Axis Dysregulation and Neuroinflammation on the Hippocampus—A Mini Review. Int. J. Mol. Sci. 2025, 26, 2940. [Google Scholar] [CrossRef]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef]
- Diamanti, K.; Cavalli, M.; Pereira, M.J.; Pan, G.; Castillejo-López, C.; Kumar, C.; Mundt, F.; Komorowski, J.; Deshmukh, A.S.; Mann, M.; et al. Organ-specific metabolic pathways distinguish prediabetes, type 2 diabetes, and normal tissues. Cell Rep. Med. 2022, 3, 100763. [Google Scholar] [CrossRef]
- Blázquez, E.; Hurtado-Carneiro, V.; LeBaut-Ayuso, Y.; Velázquez, E.; García-García, L.; Gómez-Oliver, F.; Ruiz-Albusac, J.M.; Ávila, J.; Pozo, M.Á. Significance of Brain Glucose Hypometabolism, Altered Insulin Signal Transduction, and Insulin Resistance in Several Neurological Diseases. Front. Endocrinol. 2022, 13, 873301. [Google Scholar] [CrossRef]
- Pomytkin, I.; Pinelis, V. Brain Insulin Resistance: Focus on Insulin Receptor-Mitochondria Interactions. Life 2021, 11, 262. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, H.; Zhang, L.; Liu, Z.; Huang, Y.; Liu, Q.; Jin, L.; Zhu, M.; Zhang, L. Inflammation in diabetes complications: Molecular mechanisms and therapeutic interventions. MedComm (2020) 2024, 5, e516. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Park. Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Chan, L.C.; Borreginne, K.; Kale, R.P.; Hu, C.; Tye, S.J. A review of brain insulin signaling in mood disorders: From biomarker to clinical target. Neurosci. Biobehav. Rev. 2018, 92, 7–15. [Google Scholar] [CrossRef]
- Pomytkin, I.; Costa-Nunes, J.P.; Kasatkin, V.; Veniaminova, E.; Demchenko, A.; Lyundup, A.; Lesch, K.-P.; Ponomarev, E.D.; Strekalova, T. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci. Ther. 2018, 24, 763–774. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Mannerås-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Håkansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Bäckhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Herzog, C.; Pacheco, J.A.; Fujisaka, S.; Bullock, K.; Clish, C.B.; Kahn, C.R. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol. Psychiatry 2018, 23, 2287–2301. [Google Scholar] [CrossRef]
- Martemucci, G.; Fracchiolla, G.; Muraglia, M.; Tardugno, R.; Dibenedetto, R.S.; D’Alessandro, A.G. Metabolic Syndrome: A Narrative Review from the Oxidative Stress to the Management of Related Diseases. Antioxidants 2023, 12, 2091. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Ferris, H.A.; Cai, W.; Kahn, C.R. Insulin Action in Brain Regulates Systemic Metabolism and Brain Function. Diabetes 2014, 63, 2232–2243. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the Brain: Its Pathophysiological Implications for States Related with Central Insulin Resistance, Type 2 Diabetes and Alzheimer’s Disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut–brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef]
- Vigil, P.; Meléndez, J.; Petkovic, G.; Del Río, J.P. The importance of estradiol for body weight regulation in women. Front. Endocrinol. 2022, 13, 951186. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Benkendorff, C.; Fritsche, L.; Prystupa, K.; Vosseler, A.; Gancheva, S.; Trenkamp, S.; Birkenfeld, A.L.; Preissl, H.; Roden, M.; et al. Brain insulin action on peripheral insulin sensitivity in women depends on menstrual cycle phase. Nat. Metab. 2023, 5, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
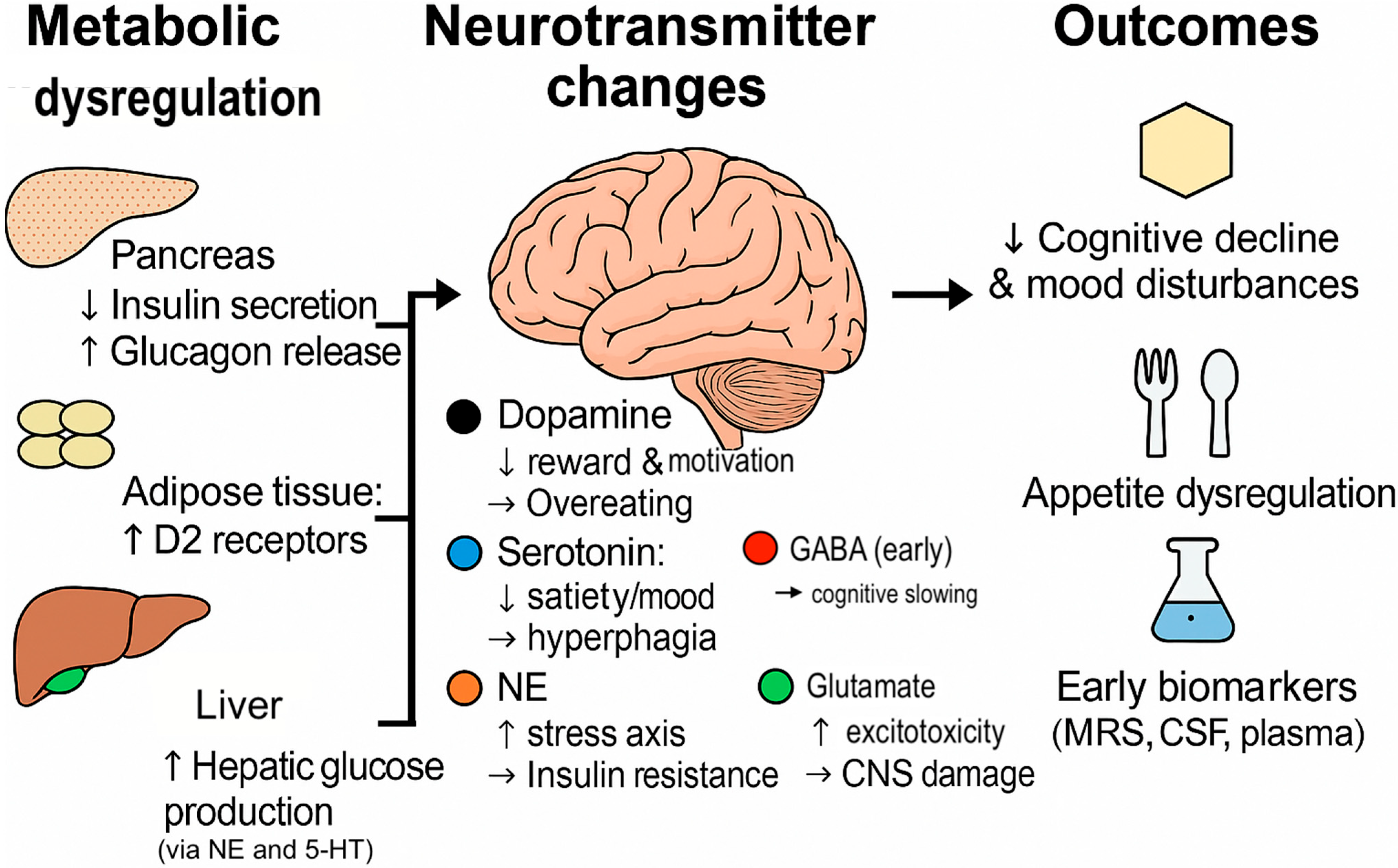
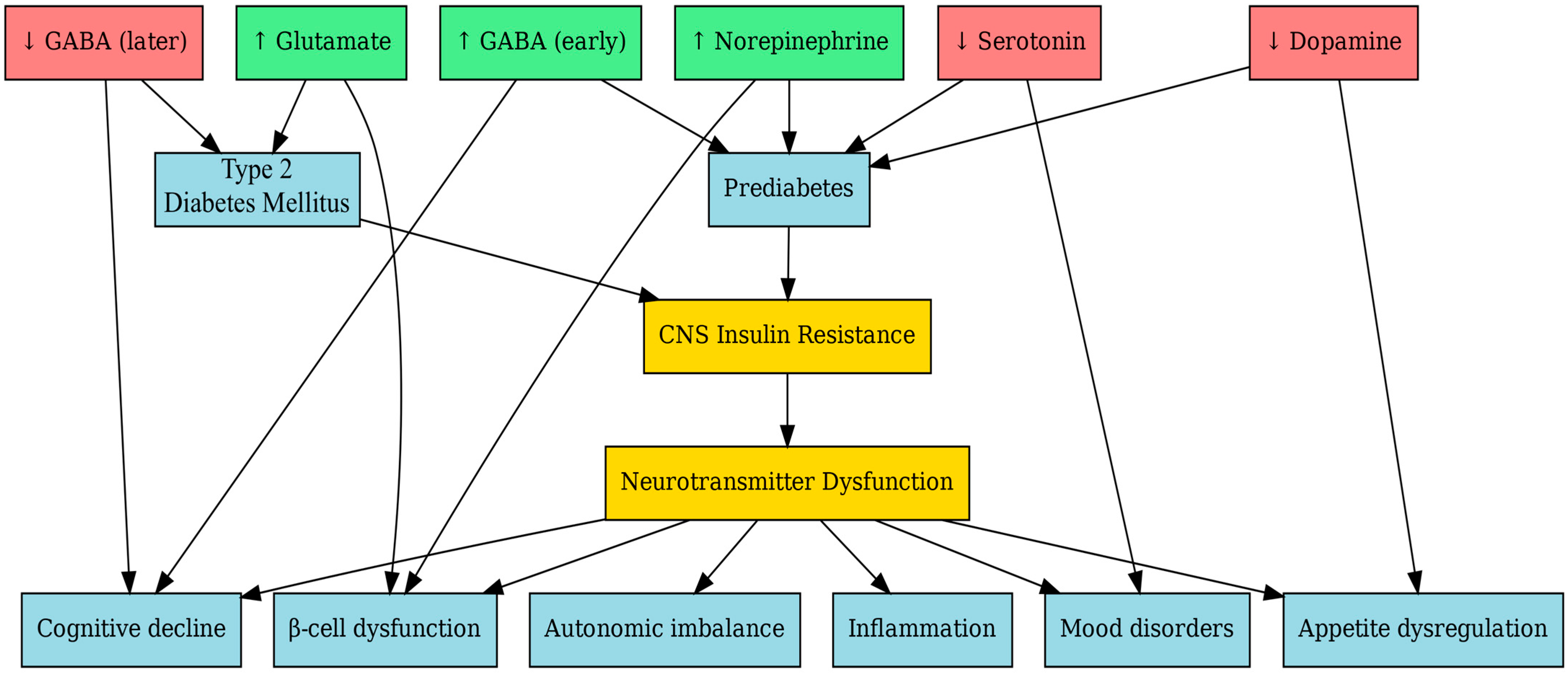
| Neurotransmitter | Source | Receptors | Effect on Insulin Secretion | Effect on Glucagon Secretion | Impact on β-Cell Health | CNS/Peripheral Mechanisms | Therapeutic Implications |
|---|---|---|---|---|---|---|---|
| Dopamine (DA) [12,13,40] | β-cells, CNS, adipose tissue | D2, D1/D2 heteromers | ↓ via D2R activation | ↑ via central mechanisms | ↓ induces apoptosis under stress | CNS reward disruption; adipose D2R upregulation | Bromocriptine, cabergoline, D2 agonists |
| Serotonin (5-HT) [52,110,111,112,113] | Enterochromaffin cells, β-cells | 5-HT1F, 2B, 3R | ↑ via serotonylation and 5-HT2B/3R | ↓ via 5-HT1F | ↑ proliferation, protects against metabolic stress | Regulates appetite, mood, satiety via 5-HT2C (CNS) | SSRIs (sertraline); microbiota modulation |
| Norepinephrine (NE) [83,114] | Locus coeruleus, SNS terminals | α2-adrenergic | ↓ via α2R on β-cells | ↑ via SNS tone | ↑ glucotoxicity stress; ↓ insulin signaling | SNS overactivity worsens IR, promotes HPA activation | Imidazoline receptor agonists, β-blockers |
| GABA [18,20,115] | β-cells, CNS, gut microbiota | GABA A, GABA B | ↑ pulsatile secretion; stabilizes release | ↓ via paracrine inhibition | ↑ proliferation, anti-inflammatory | Hypothalamic glucose sensing; hepatic GABA metabolism | GABA supplementation, vagal stimulation |
| Glutamate [101,116] | α-cells, CNS | NMDA, AMPA/ kainate | ↓ via NMDA excitotoxicity | ↑ via AMPA/kainate on α-cells | ↓ induces β-cell death | CNS excitotoxicity, ROS generation, neuroinflammation | NMDA antagonists (memantine) |
| Mechanism | Effect on Neurotransmitters | Consequent Metabolic Impact |
|---|---|---|
| Insulin resistance (central) | Dysregulated dopamine, glutamate, GABA | Increased appetite, hepatic glucose output |
| Inflammation | Monoamine/peptide imbalances | Impaired central regulation, peripheral IR |
| ROS and mitochondrial stress | Impaired synaptic function | Cognitive decline, further IR |
| Gut–brain signals | Disrupted vagal neurotransmission | HPA activation, glycemic dysregulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrițculesei, R.-V.; Boldeanu, L.; Dijmărescu, A.L.; Assani, M.-Z.; Boldeanu, M.V.; Siloși, I.; Vere, C.C. Neurotransmitter Alterations in Prediabetes and Type 2 Diabetes Mellitus: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7847. https://doi.org/10.3390/ijms26167847
Ahrițculesei R-V, Boldeanu L, Dijmărescu AL, Assani M-Z, Boldeanu MV, Siloși I, Vere CC. Neurotransmitter Alterations in Prediabetes and Type 2 Diabetes Mellitus: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(16):7847. https://doi.org/10.3390/ijms26167847
Chicago/Turabian StyleAhrițculesei, Roxana-Viorela, Lidia Boldeanu, Anda Lorena Dijmărescu, Mohamed-Zakaria Assani, Mihail Virgil Boldeanu, Isabela Siloși, and Cristin Constantin Vere. 2025. "Neurotransmitter Alterations in Prediabetes and Type 2 Diabetes Mellitus: A Narrative Review" International Journal of Molecular Sciences 26, no. 16: 7847. https://doi.org/10.3390/ijms26167847
APA StyleAhrițculesei, R.-V., Boldeanu, L., Dijmărescu, A. L., Assani, M.-Z., Boldeanu, M. V., Siloși, I., & Vere, C. C. (2025). Neurotransmitter Alterations in Prediabetes and Type 2 Diabetes Mellitus: A Narrative Review. International Journal of Molecular Sciences, 26(16), 7847. https://doi.org/10.3390/ijms26167847








