1. Introduction
During the COVID-19 pandemic, scientists focused on understanding the pathogenesis of SARS-CoV-2 infection during pregnancy and the potential routes of fetal/neonatal infection. Numerous studies have revealed parts of this complex pathogenesis, but the results have often been conflicting due to inconsistencies in terminology, numerous confusing factors, and potential biases. Still, scientific attention is focused on finding non-expensive, readily available tests that are acceptable for patients and can be performed in a short time to provide us with information on possible maternal or fetal/neonatal risks of COVID-19 complications.
Together with MERS and SARS-CoV, it is one of seven human-infecting viruses from the family of beta-type Coronaviridae [
1,
2]. SARS-CoV-2 is constituted of an enveloped RNA virus and four structural proteins [
3]. Each of these proteins, namely the spike (S) protein, nucleocapsid (N) protein, envelope (E) protein, and membrane (M) protein play distinct roles in pathogenesis [
4,
5].
SARS-CoV-2 has an affinity for binding to the human Angiotensin-converting enzyme 2 (ACE2) receptor through cleavage and conformational changes at the S1–S2 boundary and the receptor binding domain of the spike protein, which is also the most variable part of SARS-CoV-2 [
6]. When the virus encounters a potential host, it initially binds to ACE2 receptors with the help of the spike protein, and the membranes of the virus and the host cell fuse [
7,
8,
9]. These receptors are found on the kidney, endothelium, heart, macrophage, and pneumocyte cells, but are predominant on type 2 alveolar epithelial cells, making them highly sensitive to the virus’s effects [
10,
11,
12]. In addition to the ACE2 receptor, Type 2 Transmembrane Serine Protease (TMPRSS2) also plays a key role. This protease leads to the cleavage of the spike protein as well as a change in shape that facilitates the fusion of the virus’s and the host’s membranes, allowing the virus’s RNA to enter the host cell and initiate replication [
13]. The ACE2 receptor, crucial for regulating blood pressure, is also subject to changes during pregnancy. Numerous studies have shown an increased expression of ACE2 during pregnancy, especially in patients who consume tobacco, which affects the increased susceptibility of pregnant women to SARS-CoV-2 infection. An increased number of ACE2 receptors is detected in the tissues of the kidneys, placenta, and uterus [
14,
15,
16,
17].
The pathophysiological route of infection on the fetus can be direct, transmitted through the placenta, or indirect, creating unfavorable conditions for the development of the fetus because of inflammation, microthrombosis, and hypercoagulation. Based on these pathophysiological findings, we have investigated the influence of SARS-CoV-2 on placental tissue and oxidative stress in mothers and neonates in our latest study [
5]. Numerous studies have also examined macroscopic and microscopic pathohistological changes within the placental tissue in patients infected during pregnancy. Still, the results are incoherent due to equivocal terminology, classification of placenta lesions, and potential researcher biases [
18,
19,
20,
21,
22,
23,
24]. Fetal vascular malperfusion (FVM) was the most frequent finding in pregnant patients infected with SARS-CoV-2 [
25,
26]. In contrast, others have reported maternal vascular malperfusion (MVM) or inflammatory changes [
25,
26,
27,
28,
29,
30]. Placental tissue is rich in both maternal and fetal macrophages, which play an essential role during placentation, the immune response in pregnancy, and the initiation of the delivery mechanism [
31,
32]. Maternal macrophages express ACE2 receptors on their surface, potentially enabling binding of the SARS-CoV-2 virus and direct transmission to the fetus via the placenta during inflammation, especially during chorioamnionitis [
33,
34]. Hofbauer cells, which represent placental macrophages, are in the stroma of chorionic villi, localized near fetal vessels and trophoblasts, and possess classic macrophage/monocyte markers such as CD68+ [
35,
36]. In addition to Hofbauer’s cells, histopathological studies of the placentas of mothers infected with SARS-CoV-2 show that histiocytes are expressed at the decidual level [
37]. Histiocytic infiltration of the intervillous space is a characteristic feature of chronic histiocytic intervillitis, a rare pathological entity of unknown etiology often associated with poor perinatal outcomes [
38]. Chronic histiocytic intervillitis is characterized by maternal CD68+ cells infiltrating at least 5% of the intervillous space without other signs of infiltration, and the perinatal outcome is correlated with the degree of infiltration [
35,
39,
40,
41].
2. Results
2.1. Clinical Characteristics of Patients with COVID-19 Compared with the Control Group
This study included 50 pregnant women, 28 of whom were SARS-CoV-2 positive (COVID-19 group), and 22 in the SARS-CoV-2 negative (control) group. The average age of the participants was 30.61 ± 4.72 and 31.41 ± 4.65 in the COVID-19 and control groups, respectively. Of the total number of participants, 17.86% of pregnant women were infected during the first trimester, 42.86% during the second trimester, and 39.29% during the third trimester. The mean length of pregnancy was 39.19 ± 0.98 gestational weeks (gws) in the COVID-19 group and 39.42 ± 1.26 gws in the control group.
Subgroups were formed based on the severity of their symptoms; patients with mild COVID-19 were asymptomatic or experienced a cough, sore throat, fatigue, headache, loss of taste or smell, or low-grade fever. Patients with severe COVID-19 had a fever, tachypnea, respiratory distress symptoms, and lung infiltrates during pregnancy. A total of 41.38% of patients experienced mild symptoms, while 58.62% had severe symptoms of COVID-19. Within a group of patients with severe symptoms of COVID-19, three patients developed pneumonia and were dependent on oxygen therapy.
The mean length of pregnancy was similar in both groups: 39.19 ± 0.98 gws in the COVID-19 group and 39.42 ± 1.26 gws in the control group. Delivery was completed vaginally in 69% of cases and by cesarean section in 31% of cases within the SARS-CoV-2+ group, compared with 45% of vaginally completed deliveries and 55% of cesarean sections in the control group.
Statistically, there was no significant difference in the number of positively tested newborns between the groups (p = 0.37), in the manner of completion of labor (p = 0.29), in the sex of the newborn (p = 0.13), as well as in the meconial membranes and meconial amniotic fluid between the groups (p = 0.21 and p = 0.37).
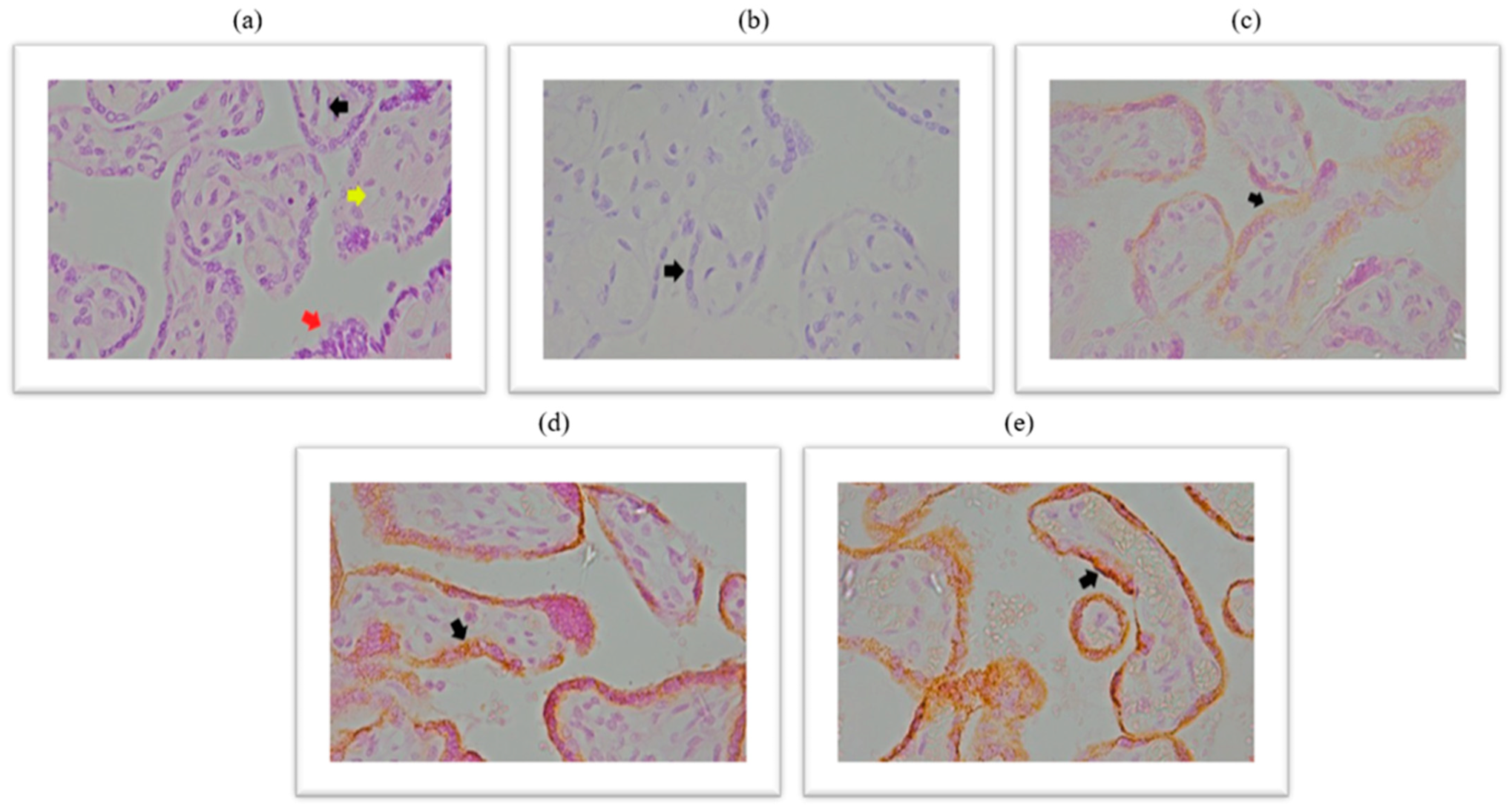
2.2. Immunohistochemical Analysis of the Expression of ACE2 Within the Placental Tissue of Patients Infected with SARS-CoV-2 During Pregnancy
Immunohistochemical analysis of placental ACE2 expression is documented in the micrographs shown in
Figure 1. HE stained chorionic villi with edematous stroma and dilatated blood vessels (
Figure 1a), a negative control micrograph staining without ACE2 antibody (
Figure 1b), and with different intensities of ACE2 protein immunopositivity are shown (
Figure 1c–e).
We performed a statistical analysis of the frequency distribution of the ACE2 immunoreactivity scores in the placental tissue of SARS-CoV-2+ patients and the control group. The results are shown graphically in
Figure 2.
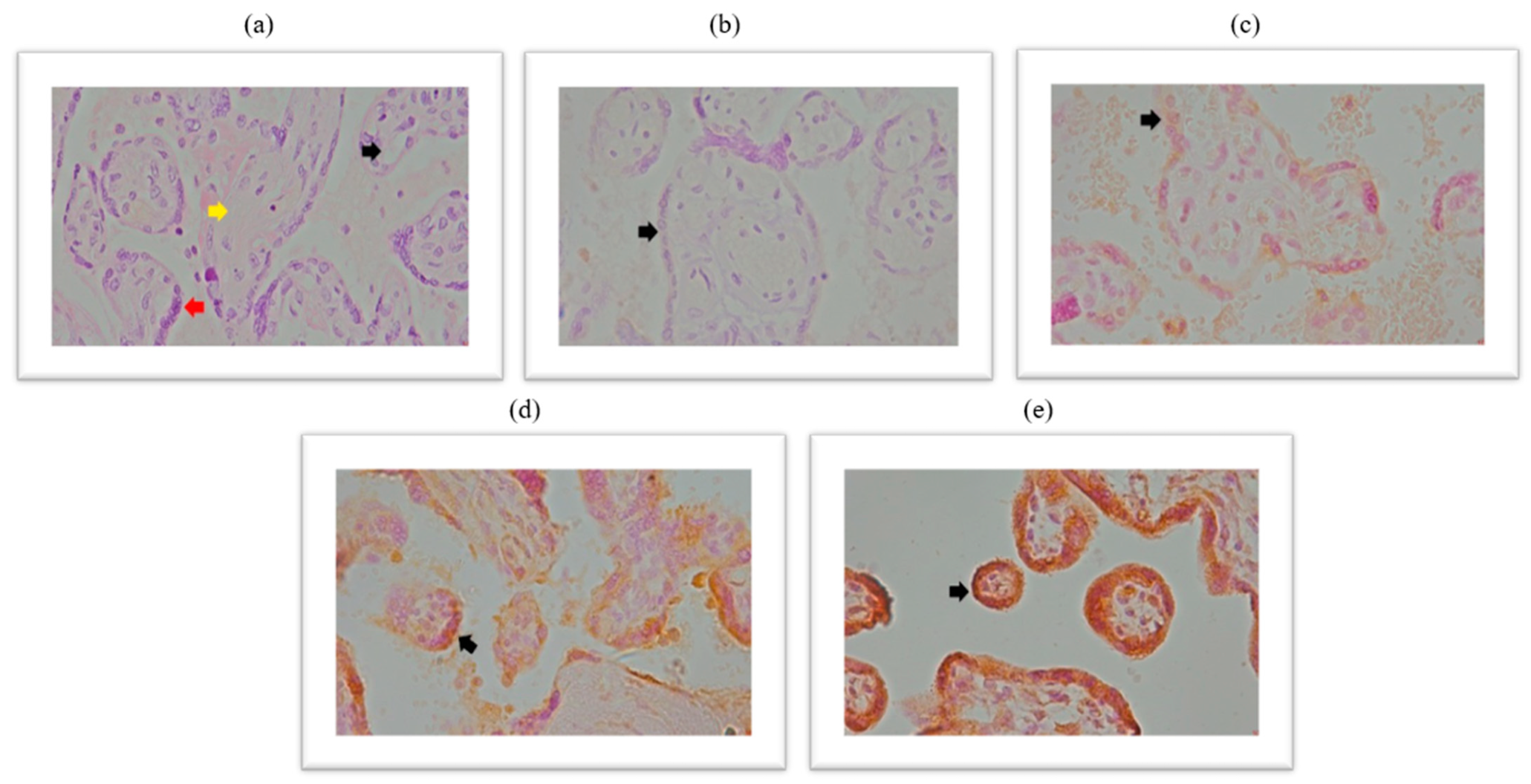
2.3. Expression of the Spike Protein Within the Placental Tissue of Patients Infected with SARS-CoV-2 During Pregnancy
Immunohistochemical analysis of the placental expression of the spike protein is documented in the micrographs shown in
Figure 3. HE stained placental tissue showing chorionic villi with edematous stroma and dilatated blood vessels (
Figure 3a) and a negative control micrograph staining without spike antibodies (
Figure 3b), together with different intensities of the spike protein immunopositivity are shown (
Figure 3c–e).
We have shown the distribution frequency of spike protein immunoreactivity scores within the placental tissue of SARS-CoV-2+ patients and the control group in
Figure 4 and
Figure 5.
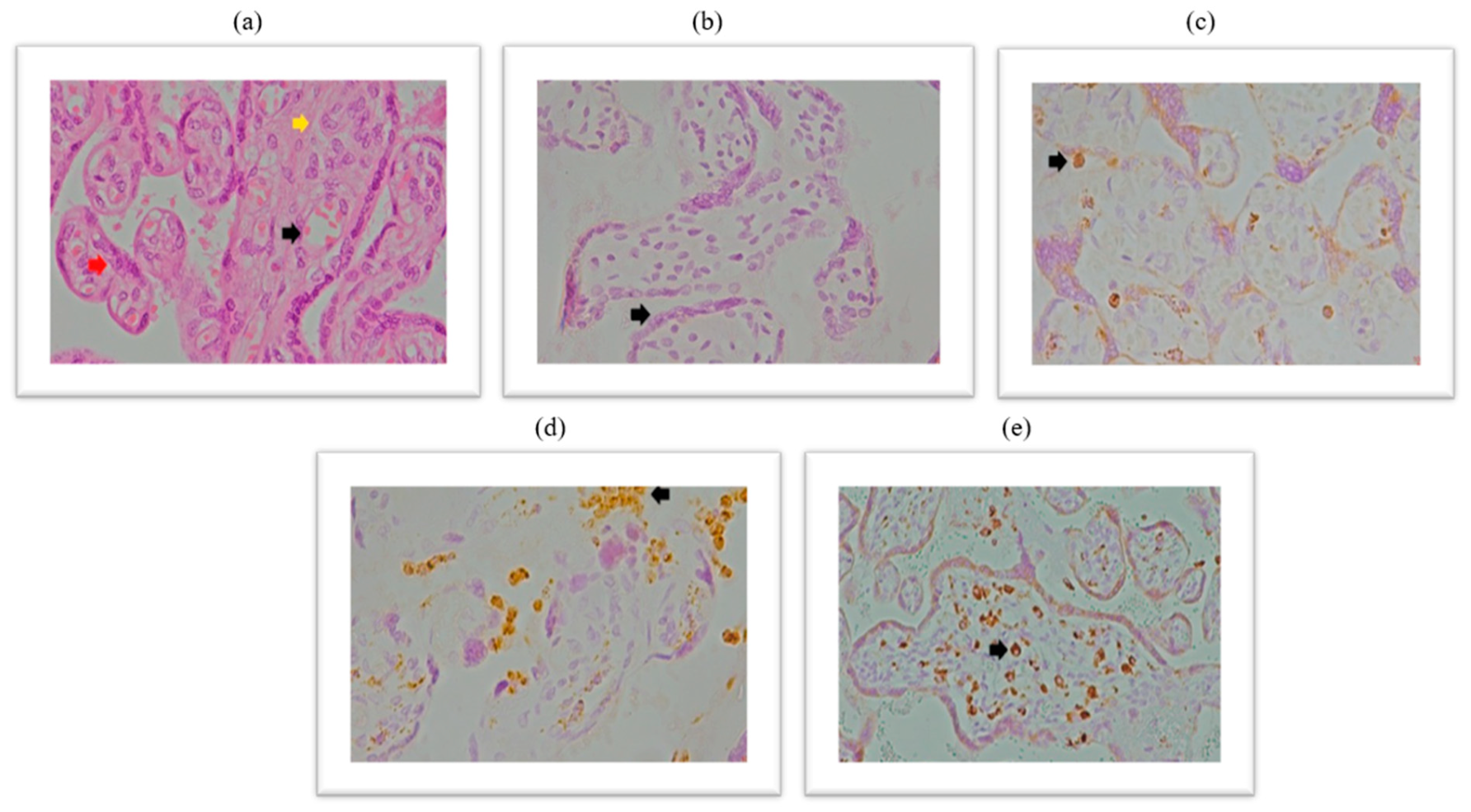
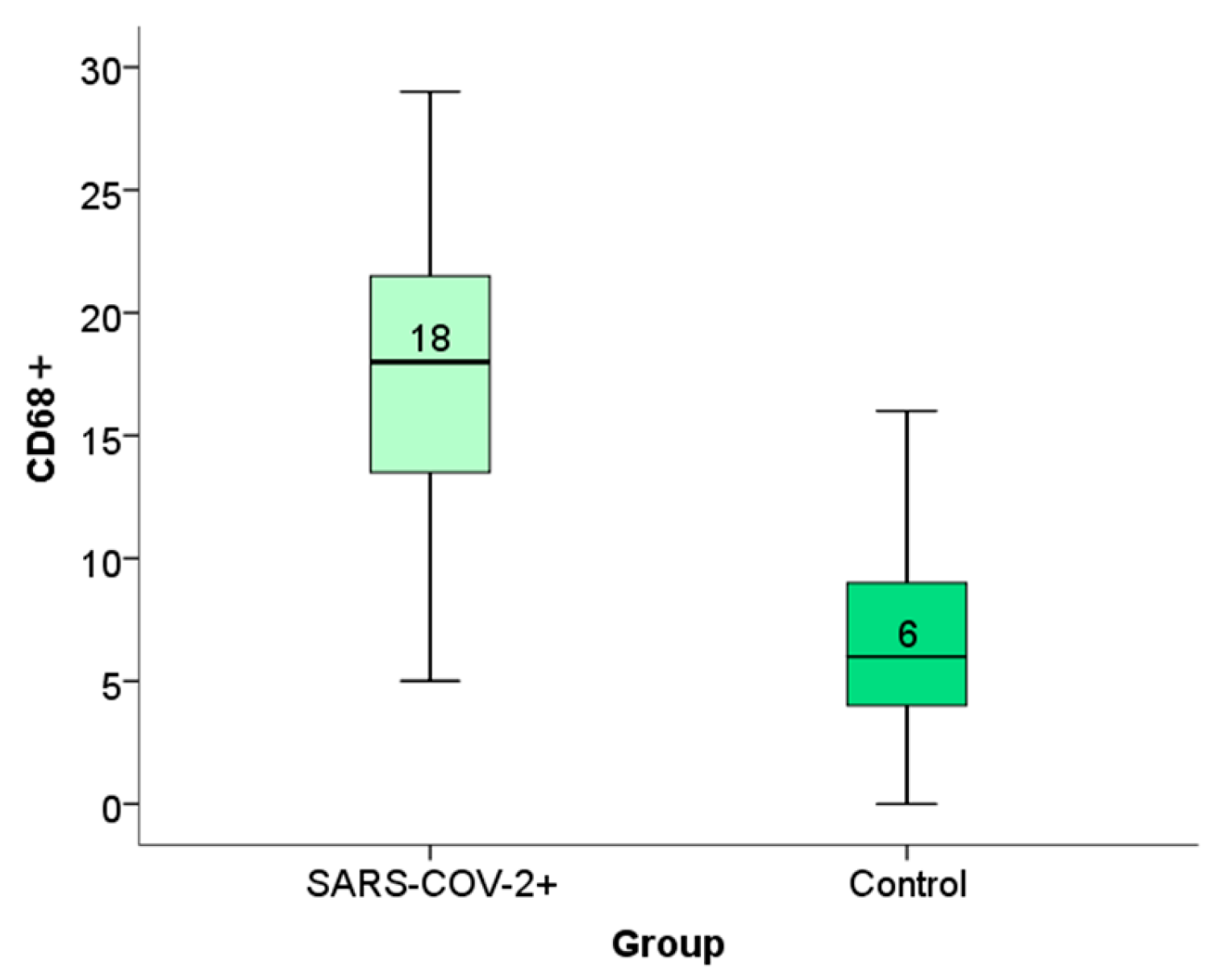
2.4. Expression of CD68+ Macrophages Within the Placental Tissue of Patients Infected with SARS-CoV-2 During Pregnancy
Immunohistochemical analysis of placental CD68+ expression is documented in the micrographs shown in
Figure 5. HE stained placental tissue showing chorionic villi with edematous stroma and dilatated blood vessels (
Figure 6a), and a negative control micrograph staining without spike antibody (
Figure 6b) is shown together with different levels of CD68+ immunopositivity (
Figure 6c–e).
We have shown the distribution frequency of CD68+ immunoreactivity scores within the placental tissue of SARS-CoV-2+ patients and the control group in
Figure 7 and
Figure 8.
Based on the results from immunopositivity scoring (
Table 1), we performed a statistical analysis to compare the expression of spike, ACE2, and CD68+ cells within the placental tissue of patients infected with SARS-CoV-2 during pregnancy with that of the control group.
Based on the results of the Man–Whitney test, we observed a statistically significant difference in the expression of spike proteins and CD68+ macrophages between the SARS-CoV-2+ group and the control group. (
p < 0.05) (
Table 2).
The statistical assay used in this analysis—Chi-square test—showed that there were no significant differences in the expression of ACE2, spike proteins, and CD68+ macrophages within the placental tissue of patients infected with SARS-CoV-2 virus during pregnancy compared with the trimester in which the infection occurred (tested for significance level
p < 0.05 (
Table 3).
The result of the statistical test used in this analysis (Mann–Whitney test) suggests that there are no statistically significant differences in the expression of ACE2 receptors, spike proteins, and CD68+ macrophages within the placental tissue of patients infected with SARS-CoV-2 virus during pregnancy compared with the severity of the maternal clinical picture (for the significance level
p < 0.05) (
Table 3).
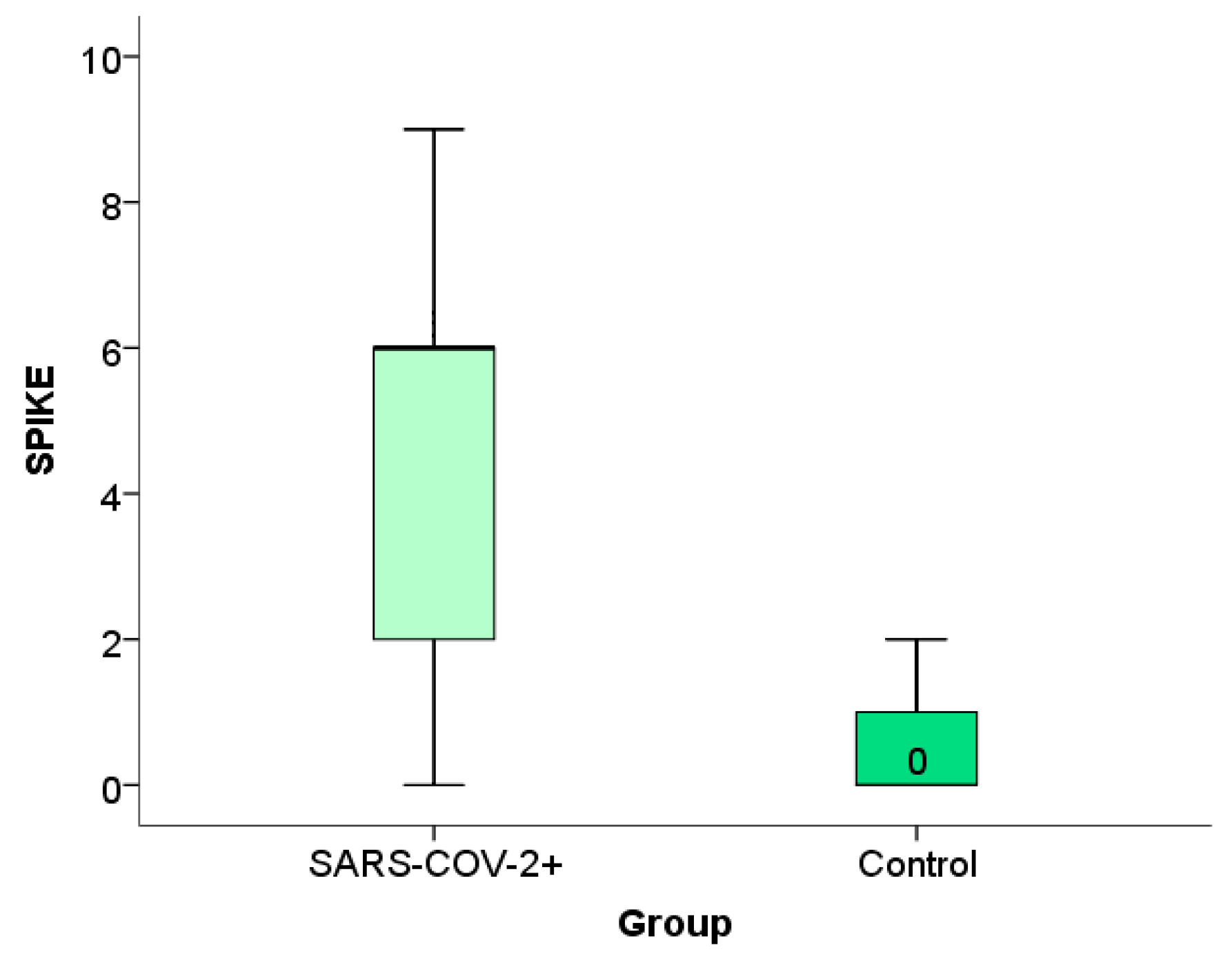
2.5. Examination of the Association of Perinatal Outcome with the Moment of Disease
For the analysis of categorical variables regarding the gestational week when SARS-CoV-2 infection occurred during pregnancy, the Mann–Whitney U test was employed, and a box plot (rectangular graph) was used to visually represent statistically significant results.
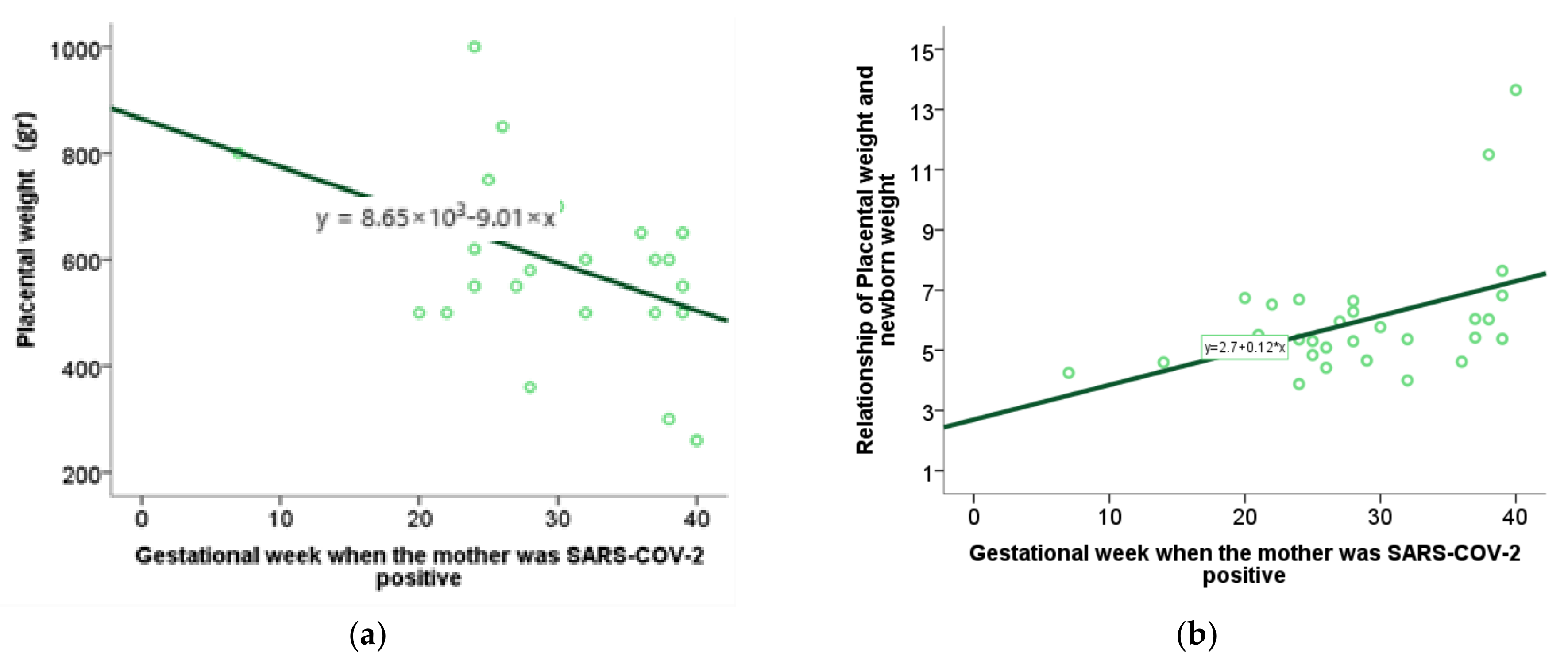
For the analysis of numerical variables related to the gestational week in which the infection occurred, the method of correlation and regression was applied. Specifically, the value of the Spearman correlation coefficient was interpreted, and a scatterplot was used to graphically represent the statistically significant results. By testing the characteristics of the perinatal outcome using the Spearman range correlation test with the moment at which SARS-CoV-2 infection occurred during pregnancy, we found an association between placental weight and the week of gestation in which the infection occurred, as well as a relationship between placental weight and newborn weight and week of gestation in which the infection occurred (
Table 4,
Figure 9a,b). Other examined features did not show a statistically significant correlation.
2.6. Investigation of the Association Between Spike Protein Expression in the Placental Tissue of Infected Patients and the Level of Maternal and Fetal Oxidative Stress Biomarkers
We have been examining the association between biomarkers of oxidative stress and antioxidant protection in mothers and newborns from our previous study [
5] and the expression of the spike protein within placental tissue. A positive correlation was observed between maternal serum TBARS levels and the degree of spike protein’s immunoreactivity within placental tissue. (
p = 0.04,
r = 0.29) (
Table 5).
2.7. Assessment of the Association of Spike Protein and CD68+ Expression with Perinatal Outcome
Using Spearman’s rank coefficient test, we examined the association between perinatal outcomes from our previous study and spike expression within placental tissue, with a significance level of
p < 0.05. The newborns’ stay in intensive care positively correlates with the spike protein’s expression level in placental tissue (
Figure 10) (
p = 0.02,
r = 0.33)
. The correlation and regression methods were applied to the analysis of IHH numerical variables scores, i.e., the value of the Spearman correlation coefficient. A scatter diagram was used to visually present statistically significant results. Through the Spearman correlation test, we examined the association between the expression of spike proteins within the placental tissue of patients infected with SARS-CoV-2 and the expression of CD68+ macrophages and the expression of ACE2 receptors within the same tissue. We found a positive correlation between the expression of spike proteins and the expression of CD68+ macrophages (
p = 0.00,
r = 0.60) (
Figure 11). In contrast, no statistically significant correlation was observed between ACE2 levels and spike protein levels within placental tissue. (
p = 0.10) (
Figure 10a,b)
2.8. Analysis of the Association Between ACE2 Receptor Expression Within Placental Tissue and Blood Biomarkers of Oxidative Stress in Mothers and Newborns After SARS-CoV-2
A statistical analysis was performed to investigate the association between ACE2 expressions in placental tissue and biomarkers of oxidative stress in mothers and newborns using the Spearman rank correlation coefficient, with a significance level set at p < 0.05.
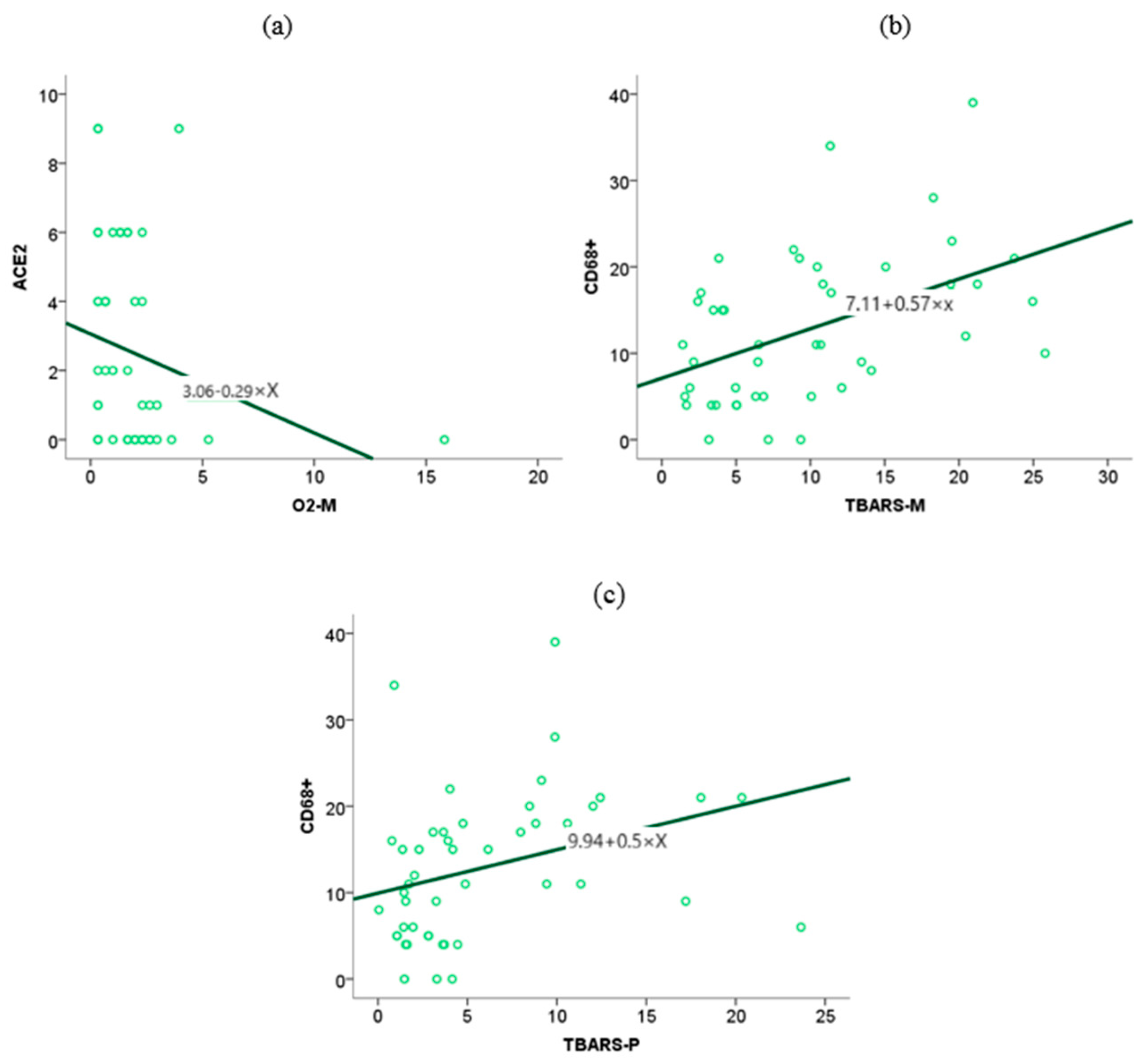
The level of ACE2 expression in placental tissue is associated with elevated concentrations of O2- anions in maternal blood, whereas other biomarkers of oxidative stress do not exhibit a similar association (
Figure 11).
The association between CD68+ macrophage expression in placental tissue and maternal and neonatal oxidative stress biomarkers, as identified in our previous study, was investigated using the Spearman rank correlation test. The test results showed an association between TBARS in maternal (
p = 0.00,
r = 0.48) and fetal serum (
p = 0.00,
r = 0.44) and the expression of CD68+ in placental tissue. (
Figure 11).
2.9. Investigation of the Association Between Pathohistological Findings in Placental Tissue of Patients Infected with SARS-CoV-2 and the Level of Maternal and Fetal Oxidative Stress Biomarkers
The Mann–Whitney U test was used to analyze oxidative stress parameters in relation to histopathological variables, utilizing data from our previous study [
5]. The statistically significant results were compared using a box plot (rectangular graph). We tested the correlation of significant histopathological findings of placental tissue of patients with SARS-CoV-2 with the level of biomarker oxidative stress of mothers and newborns and obtained the following results:
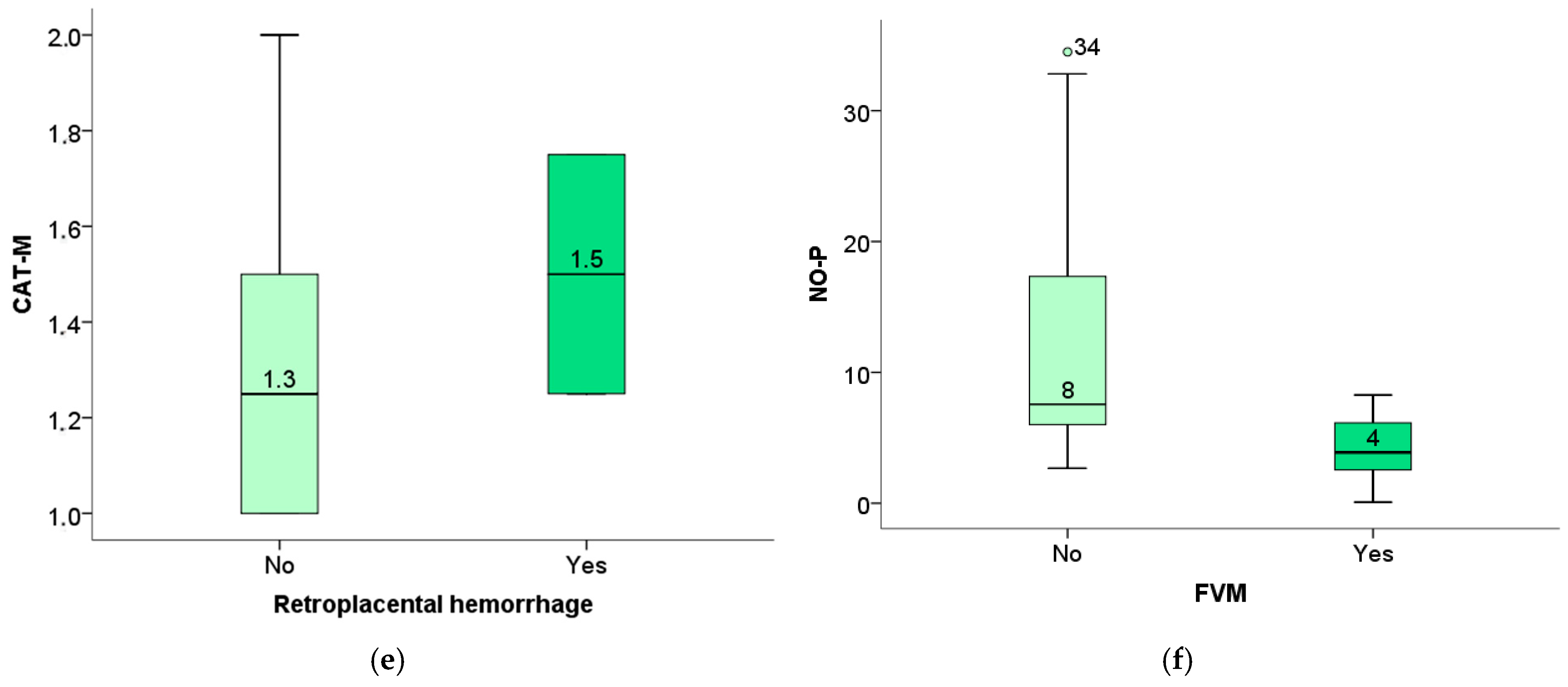
The level of TBARS of the newborn was significantly elevated in pregnant women with placental pathological findings of avascular villi (
Figure 12a), as well as obliteration of blood vessels (
Figure 12b). In contrast, the level of TBARS of the mother positively correlated with the findings of vascular ecstasy (
Figure 12c) as well as placental infarction (
Figure 12d).
The level of maternal catalase is positively correlated with the placental findings of retroplacental hemorrhage (
Figure 12e), and the level of the fetus’s NO positively correlated with the existence of FVM changes (
Figure 12f).
3. Discussion
The placenta is an organ that, through its protective mechanisms, prevents the propagation of the SARS-CoV-2 virus to the fetus. However, inflammatory or vascular lesions can weaken the placental barrier, thereby favoring direct transmission [
42]. There are several potential maternal–fetal routes of spreading infection. A fetal infection can occur within the uterus by direct transplacental transmission. Another possible way is an intrapartum infection (contact of the newborn with infected vaginal secretion or blood), and the third way is after birth through direct contact between mother and newborn or by breastfeeding [
43,
44]. Although placental lesions are not specific to SARS-CoV-2 infection, they can be a valuable clue in identifying patients who may develop long-term consequences that require special attention [
45,
46]. IHC analysis of the spike protein, ACE2 receptors, and CD68+ immunoreactivity can provide us with more specific information, which, together with morphological changes, could be essential in discovering a disruption of placental function and the possibility of transplacental transmission [
47]. Genetic disorders significantly impact placental changes and fetal/neonatal outcomes, making it more valuable to analyze these parameters if we had exclusion criteria for abnormal genetic results. Cell-free fetal DNA, as a non-invasive prenatal diagnostic tool for genetic disorders, is a valuable information resource and should be considered an inclusion criterion in future studies [
48].
Based on the severity of symptoms, 41.38% of patients had mild symptoms, while 58.62% had severe symptoms of COVID-19, of whom three patients were hospitalized for pneumonia treatment. Although elective admissions were suspended, there was no significant decrease in admissions to the obstetric clinic during the period of our study, similarly to Riemma’s study [
49]. Studies examining the impact of SARS-CoV-2 infection during pregnancy showed a positive correlation between the severity of COVID-19 symptoms and pronounced lesions in placental tissue, as well as an increase in oxidative stress during pregnancy [
50,
51]. The results of our study did not show a statistically significant difference in the frequency of pathohistological changes in placental tissue among the subgroups classified according to the severity of the clinical presentation. Our previous study found no statistically significant differences in biometry parameters between newborns from the SARS-CoV-2-positive and control groups; however, oxidative stress biomarkers were significantly increased in mothers and neonates from the SARS-CoV-2 groups [
5]. The mean age of participants in the SARS-CoV-2 group was not significantly different from that of the control group. Mothers’ age could potentially influence the oxidative stress level in neonates [
52]. This study provided additional information regarding the relationship between oxidative stress biomarkers and placental lesions, as well as spike, ACE2, and CD68+ immunoreactivity and perinatal outcome.
Decreased placental weight may indicate altered metabolic function and a potential risk to the fetus. Our study, consistent with previous studies, found no significant differences in placental weight and the P/TT (placenta-to-fetal-body weight) ratio between subjects in the SARS-CoV-2+ and control groups [
53]. Through additional analysis, we determined the association between the week of pregnancy during which the mother was infected and the altered weight of the placenta, as well as the ratio of placental weight to fetal body weight. The earlier the infection occurred in gestation, the lower the placental weight and the ratio of placental weight to fetal body weight, which may indicate a worse perinatal outcome [
54,
55,
56,
57]. Our study also found a positive correlation between placental lesions and oxidative stress biomarkers, highlighting the role of oxidative stress in the pathogenesis of placental lesions. Neonatal TBARS correlate with placental findings of avascular villi or occlusion of blood vessels, and maternal TBARS show a positive correlation with placental findings of avascular villi or occlusion of blood vessels, as well as with vascular extravasation and placental infarction. This finding highlights TBARS as a potentially viable biomarker in the prenatal diagnosis of potential perinatal or neonatal complications.
Numerous studies revealed the complex role of ACE2 receptors in the pathogenesis of COVID-19 [
9,
12,
33,
34,
57,
58]. The enzymatic activity of the ACE2 receptor is increased during pregnancy to ensure adequate circulation to the placenta through the regulatory mechanism renin–angiotensin–aldosterone, by reducing peripheral blood pressure by converting angiotensin I and II to angiotensin (1–7) and angiotensin (1–9) [
5,
17,
33,
59,
60,
61]. Some studies have shown dynamic changes in the expression of this receptor throughout gestation in placental and fetal cells, making them susceptible to SARS-CoV-2 virus invasion [
54,
57]. This susceptibility is attributed to the presence and coregulation of TMPRSS2, which is necessary for direct transmission in addition to the ACE2 receptor [
17,
57]. Since this receptor is also the entry site of the SARS-CoV-2 virus into the cell, the virus’s binding leads to competitive inhibition of the receptor responsible for vasodilation and activation of the complement system, resulting in endothelial dysfunction and the formation of microthrombi in this prothrombotic environment [
33,
58,
62,
63]. ACE2 is not only a receptor responsible for cytokine production, immune response activation, and viral genome replication, but it is also involved in the regulation of two crucial hormonal systems, the RAS (Renin–angiotensin) and the KKS (Kinin–Kallikrein–Kininase) systems, during pregnancy [
14]. Decreased function of the ACE2 receptor is associated with the onset of preeclampsia and impaired fetal growth due to inadequate placentation and hemodynamic adaptation to pregnancy [
33,
64,
65,
66,
67]. Our study did not detect a statistically significant difference in the expression of ACE2 receptors in the placental tissue of patients infected with SARS-CoV-2 during pregnancy and the control group, nor between the subgroups formed according to the severity of the clinical presentation or the trimester in which the infection occurred but confirmed a positive correlation between the level of ACE2 expression in placental tissue and elevated concentration levels of O2-superoxide anion radicals in maternal blood. Our data supports the fact that patients with a pronounced expression of ACE2 receptors are more susceptible to oxidative stress and the potential complications of COVID-19. Heckt et al. found that SARS-CoV-2 does not result in specific macroscopic or histopathological changes in placental tissue and demonstrated the presence of ACE2 receptors on the membranous side of the syncytiotrophoblast of the chorionic villi as well as on the membranous side of the cytotrophoblast and extravillous trophoblast without expression in the placenta [
68,
69].
Under physiological conditions, the placenta serves as an effective barrier against the direct transmission of the SARS-CoV-2 virus [
42]. Numerous studies, including ours, have demonstrated the impact of SARS-CoV-2 on placental tissue and the fetus; however, the direct transmission of the virus remains a subject of scientific controversy. Data from the literature indicate that direct transmission was detected in only 4–8% of cases [
44,
70,
71,
72,
73,
74,
75].
Further, our research was focused on the immunohistochemical detection of the spike protein’s presence and localization within placental cells, revealing a statistically significant difference in spike protein expression between pregnant women infected with SARS-CoV-2 and the control group. Immunoreactivity was observed to the highest degree within cytotrophoblast cells, followed by syncytiotrophoblasts and intervillous mononuclear cells. According to experiences from studies dealing with other viral congenital infections, such as the ZIKA virus, we expected that placental lesions and immunoreactivity would be more pronounced in placentas infected during the first trimester [
76]. There were no statistically significant differences in the expression of spike within the placental tissue between the groups divided according to the trimester in which the infection occurred and the severity of the clinical presentation. Our study revealed no differences in immunoreactivity for ACE2, spike protein, or CD68+ expression in relation to the timing of infection. In addition, we found a positive correlation between immunoreactivity to the spike protein within placental tissue and maternal serum levels of TBARS, which indicate an elevated level of oxidative stress in mothers with identified placental SARS-CoV-2 infection. TBARS is important in identifying patients at high risk for complications in pregnancy and the neonatal period. Regarding perinatal outcome, the newborn’s stay in neonatal intensive care positively correlates with the spike protein’s expression level in placental tissue. IHC immunoreactivity analysis for spike antigen is significant in detecting newborns at risk of developing perinatal complications.
Still, the literature’s data on the detection of SARS-CoV-2 within placental tissue are inconsistent. Some studies detected the virus in 100% of the studied samples, while others denied its presence within placenta-infected samples [
68,
77,
78]. A study by Taglauer et al. detected the presence of the SARS-CoV-2 spike protein within villi cells and ACE2 in the outer layer of the syncytiotrophoblast [
77]. Other studies addressing similar issues have detected the presence of spike proteins within syncytiotrophoblast cells, with significantly lower expression in cytotrophoblasts, stromal cells, endothelial cells, and innervated mononuclear cells [
30,
79,
80]. In their study, Verma and colleagues showed that significantly higher levels of spike protein were detected within the placentas of preterm deliveries, in trophoblasts, and Hofbauer cells [
69]. The study conducted by Guo et al. also demonstrated increased immunoreactivity to the spike protein within the endothelial cells of placental samples from infected patients using the IHC method [
81]. From the above, it can be concluded that spike protein-induced release of cytokines and chemokines triggers the formation of cytokine storms, a key factor in the development of placental lesions and maternal and fetal complications. In contrast, elevated levels of tissue factor 3 contribute to thrombotic events and vascular placental lesions [
81]. Definitive evidence of placental infection is the presence of the active replication of the SARS-CoV-2 virus within the cells of the placenta. Indeed, the focus of future research on transmission must be to demonstrate active replication [
72]. In his study, Incognito concluded that the Delta variant was linked with unfavorable maternal and neonatal outcomes and more frequent placental detection of SARS-CoV-2 [
82].
Our study showed a statistically significant difference in CD68+ macrophage expression within placental tissue in patients infected with SARS-CoV-2 during pregnancy compared with the control group. No statistically significant differences were detected between the subgroups regarding the clinical presentation or the trimester in which the infection occurred. In addition to Hofbauer cells, our study also observed the presence of immunoreactivity in the intervillous space, indicating histiocytic intervillitis. Earlier immunohistochemical studies demonstrated intense staining with the CD68+ marker at the level of intervillous spaces, indicating the presence of histiocytic intervillitis in patients infected with the SARS-CoV-2 virus, which should be distinguished from the detected Hofbauer cells of fetal origin that are also CD68 positive [
83]. Chronic histiocytic intervillous is characterized by maternal CD68+ cells infiltrating at least 5% of the intervillous space without other signs of astonishment, and the perinatal outcome is correlated with the degree of infiltration [
35,
39,
40,
41]. Future studies should consider further differentiation of inflammatory cells using immunohistochemical markers to determine the presence of macrophages, monocytes, and neutrophils, as well as their activated states. In addition, we investigated the associations of CD68+ macrophage expression in placental tissue with biomarkers of maternal and neonatal oxidative stress. We have found a positive correlation between TBARS in both maternal and fetal serum and the expression of CD68+ biomarkers in placental tissue. These data lead to the conclusion that immunoreactivity testing for CD68+ macrophages within placental tissue significantly identifies newborns with possible early or delayed complications of oxidative stress and inflammation.
Future studies should focus on the co-expression of these two proteins and their impact on the outcome of pregnancy [
55] as well as on variants of the virus, considering differences in prognosis and outcomes. It is also essential to point out that data on vaccination were the exclusion criterion from our study because they were a possible confounding factor. Additionally, vaccination acceptance among pregnant women in Serbia was very low, likely due to a lack of adequate information, which is crucial for acceptance [
5,
84].