Abstract
Reactive molecules, including oxygen and nitrogen species, serve dual roles in human physiology. While they function as essential signaling molecules under normal physiological conditions, they contribute to cellular dysfunction and damage when produced in excess by normal metabolism or in response to stressors. Oxidative/nitrosative stress is a pathological state, resulting from the overproduction of reactive species exceeding the antioxidant capacity of the body, which is implicated in several chronic human diseases. Antioxidant therapies aimed at restoring redox balance and preventing oxidative/nitrosative stress have demonstrated efficacy in preclinical models. However, their clinical applications have met with inconsistent success owing to efficacy, safety, and bioavailability concerns. This summative review analyzes the role of reactive species in human pathophysiology, the mechanisms of action of antioxidant protection, and the challenges that hinder their translation into effective clinical therapies in order to evaluate potential emerging strategies such as targeted delivery systems, precision medicine, and synergistic therapeutic approaches, among others, to overcome current limitations. By integrating recent advances, this review highlights the value of targeting reactive species in the prevention and management of chronic diseases.
1. Introduction
Normal cellular metabolism produces several reactive oxygen and nitrogen species as byproducts. These reactive molecules are critical for cellular redox homeostasis and as signaling molecules in physiological processes, including immune response, gene regulation, and apoptosis. However, when produced in excess or inadequately neutralized by the body’s endogenous antioxidant systems, these species cause oxidative and nitrosative stress, leading to cellular dysfunction and damage Thomas, et al. [1]. This state of cellular redox imbalance, termed oxidative/nitrosative stress, is well known for the incidence of pathologies including cancers, cardiovascular, metabolic, and neurodegenerative diseases. All these pathological conditions share common features of redox imbalance, mitochondrial dysfunction, and chronic inflammation, which are exacerbated by environmental and lifestyle factors that increase cellular reactive species production in humans.
The human body possesses an arsenal of antioxidant systems that work in concert to ensure cellular redox balance [2]. When the body’s endogenous capacity is overwhelmed by the excessive accumulation of reactive species, exogenous antioxidants can provide supplementary protection to prevent oxidative/nitrosative stress and cellular damage. A plethora of preclinical studies demonstrate that exogenous antioxidants can effectively neutralize reactive species, regenerate endogenous enzymes, and restore cellular redox balance, thereby reducing tissue damage and inflammation. Despite promising results, exogenous antioxidant interventions have met with limited success in clinical practice. In other instances, their therapeutic applications have resulted in adverse health effects in subjects [3]. Consequently, in view of the role reactive species play in human health, the development of novel innovative antioxidant therapies to address these shortcomings is the subject of widespread interest.
This review examines the relevance of reactive species in human pathophysiology, the mechanistic basis of antioxidant action, and key limitations impeding the clinical translation of antioxidant therapeutics. It also evaluates the strengths and limitations of current therapeutic approaches aimed at enhancing antioxidant efficacy and safety in preclinical and clinical models, including targeted delivery systems, nanotechnology-based formulations, and synergistic combination therapies. By integrating current research findings, this review provides a clearer comprehension of antioxidant therapeutics to guide the future development of innovative strategies for managing reactive species-driven chronic diseases.
2. Bioactive Reactive Species
There are numerous reactive molecules that feature in human pathophysiology [4]. Among these, reactive oxygen species (ROS) specifically refer to highly reactive oxygen-derived molecules, including radicals such as superoxide (O2•−) and hydroxyl (•OH), among others, and non-radicals such as hydrogen peroxide (H2O2), singlet oxygen (1O2), and ozone (O3), all of which exist as oxygen-containing molecules (Table 1). Similarly, reactive molecules derived from nitrogen, including radicals such as nitric oxide (•NO) and nitrogen dioxide (•NO2), along with non-radicals such as peroxynitrite (ONOO−), nitrous acid (HNO2), and dinitrogen tetroxide (N2O4), among others, which contain nitrogen, correspond to reactive nitrogen species (RNS) [5]. The terms oxidative stress and nitrosative stress describe pathological conditions characterized by the overproduction of ROS and RNS, respectively, beyond cellular antioxidant capacity. However, while most RNS also contain oxygen, the reactivity and biological targets of RNS differ from ROS, and the distinction will be made throughout this text, whenever applicable, for simplicity. Additionally, non-free radical reactive molecules generally exhibit lower reactivity compared to their radical counterparts, with the former transformed to free radicals under stress conditions [6]. Table 1 lists the common bioactive reactive molecules, including ROS and RNS, associated with human health.

Table 1.
Common bioactive reactive species associated with human health and diseases.
2.1. Production of Reactive Oxygen and Nitrogen Species
Human cells naturally generate ROS and RNS during normal metabolism and immune defense. Additionally, various external stimuli can trigger their production. This section outlines the key sources of bioactive ROS and RNS implicated in human physiology and pathology.
2.1.1. Reactive Oxygen Species (ROS)
Endogenous Sources
Reactive oxygen species production occurs endogenously via mitochondrial, enzymatic, and non-enzymatic pathways (Figure 1). The mitochondria represent the principal sites of cellular ROS generation during oxidative phosphorylation [7]. Electron leakage at Complexes I and III of the electron transport chain (ETC) furnishes electrons to partially reduce molecular oxygen to O2•− in the mitochondria [8]. These species are short-lived, and the enzyme superoxide dismutase (SOD) transforms highly toxic O2•− to H2O2 and O2, which are less harmful. Hydrogen peroxide, when untreated by cellular antioxidants, transforms to highly reactive •OH via Fe2+-catalyzed Fenton and Haber–Weiss reactions [6]. Mitochondrial ROS production is tightly regulated, balancing its generation and elimination to maintain cellular homeostasis. This regulation involves various factors, including mitochondrial membrane potential, enzymatic systems, and signaling pathways, in balancing signaling roles with the prevention of oxidative damage [9].
Beyond mitochondrial sources, several extra-mitochondrial enzymes are critical contributors to ROS production, particularly in immune and inflammatory responses (Figure 1). Xanthine oxidase, an essential purine metabolic enzyme, generates O2•− and H2O2 during the oxidation of hypoxanthine and xanthine to uric acid [10]. Under ischemia––reperfusion conditions, the conversion of xanthine dehydrogenase to xanthine oxidase amplifies ROS generation, contributing to endothelial injury and tissue damage in cardiovascular and renal pathologies [11]. Similarly, NADPH oxidases (NOX), particularly in phagocytic cells, mediate ROS production during respiratory bursts via NADPH oxidase-catalyzed oxygen reduction [12]. The resulting O2•− and derived oxidants play essential roles in microbial killing and inflammatory signaling. Impaired NOX function, as observed in chronic granulomatous disease, leads to recurrent infections, underscoring its physiological importance [13]. Cytochrome P450 enzymes (notably CYP2E1) also contribute to ROS formation via catalytic uncoupling during xenobiotic metabolism, with elevated activity linked to oxidative liver injury in alcohol-associated and drug-induced hepatotoxicity [14]. Furthermore, nitric oxide synthases (NOS), when uncoupled due to deficiencies in cofactors such as tetrahydrobiopterin or substrates like L-arginine, shift from NO• production to O2•− generation. This uncoupling exacerbates oxidative stress and is implicated in endothelial dysfunction, hypertension, and atherosclerosis [15]. Together, these enzymatic systems represent spatially distinct but mechanistically interconnected sources of ROS. Their tightly regulated activity is essential for redox signaling and host defense, while their dysregulation leads to multiple oxidative stress-related diseases.
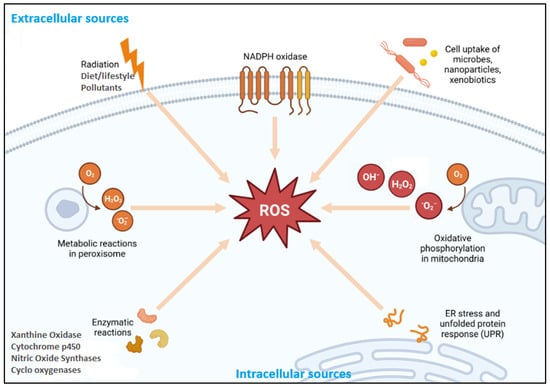
Figure 1.
Schematic illustration depicts the various endogenous and exogenous sources contributing to the generation of reactive oxygen species (ROS) within a cell. Key endogenous sources include oxidative phosphorylation in mitochondria; enzymatic reactions involving xanthine oxidase, cytochrome P450, nitric oxide synthases, and cyclooxygenases; and NADPH oxidase activity, metabolic reactions in peroxisomes, and endoplasmic reticulum (ER) stress with unfolded protein response (UPR). Exogenous contributors include radiation, lifestyle and dietary factors, environmental pollutants, and the cellular uptake of microbes, nanoparticles, and xenobiotics. These processes lead to the formation of ROS such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−), which can affect cellular homeostasis and contribute to oxidative stress. Modified from [16].
Beyond enzymatic pathways, non-enzymatic and organelle-associated processes significantly contribute to intracellular ROS generation (Figure 1). Peroxisomes produce H2O2 during fatty acid β-oxidation via flavin-dependent oxidases that transfer electrons to molecular oxygen. Under high metabolic demand or catalase (CAT) insufficiency, excess H2O2 may accumulate, contributing to oxidative liver injury and peroxisomal disorders [17]. The endoplasmic reticulum (ER) is another ROS source, particularly during oxidative protein folding. Enzymes such as protein disulfide isomerase (PDI) and ER oxidoreductin 1 (Ero1) catalyze disulfide bond formation, transferring electrons to oxygen and generating H2O2 as a byproduct [18]. Under ER stress, induced by misfolded proteins or calcium dysregulation, the prolonged activation of the unfolded protein response (UPR) enhances ROS production, linking ER dysfunction to metabolic and neurodegenerative diseases [19]. Immune cells, including neutrophils and macrophages, also release ROS, including O2•−, H2O2, and HOCl, during respiratory bursts aimed at pathogen destruction [20]. Although essential for host defense, excessive ROS release can damage surrounding tissues, driving chronic inflammation. Under physiological conditions, antioxidant systems maintain redox balance. However, the dysregulation of these systems contributes to oxidative stress and disease pathogenesis.
Exogenous Sources
Exogenous factors significantly influence cellular redox balance by either directly generating ROS or enhancing endogenous ROS production through various molecular mechanisms (Figure 1). For instance, ultraviolet (UV) radiation, particularly UVA and UVB, induces the formation of 1O2, O2•−, and •OH through the excitation of endogenous chromophores and the initiation of lipid peroxidation cascades [21]. UV exposure also enhances ROS production during the nucleotide excision repair and base excision repair of UV-damaged DNA, contributing to mutagenesis and photoaging [22]. Ionizing radiation, including X-rays, γ-rays, and neutron bursts, interacts with water to generate highly reactive •OH and H2O2 within cells via radiolysis, resulting in the breakdown of DNA, proteins, and lipids [23].
Environmental pollutants are major external inducers of ROS (Figure 1). Tropospheric ozone, cigarette smoke, and particulate matter (PM2.5) contain or stimulate the formation of free radicals that oxidize cellular components and impair antioxidant defense mechanisms [24,25]. Heavy metals such as cadmium, arsenic, and mercury promote ROS formation by interfering with mitochondrial respiration and depleting endogenous glutathione levels, leading to oxidative damage and inflammation [26]. Additionally, bacterial pathogens and their endotoxins, particularly lipopolysaccharides (LPSs), activate Toll-like receptor 4 (TLR4) and nuclear factor-κB (NF-κB) signaling pathways, upregulating inducible nitric oxide synthase (iNOS) and NOX enzymes and thereby increasing ROS and RNS during the inflammatory response [27].
Dietary and lifestyle factors are increasingly recognized as modifiable contributors to ROS burden (Figure 1). Diets high in oxidized polyunsaturated fats and advanced glycation end-products (AGEs) stimulate ROS through receptor-mediated pathways and mitochondrial overload [28]. Chronic alcohol consumption induces hepatic ROS generation primarily through the upregulation of cytochrome P450 2E1 (CYP2E1), exacerbating oxidative stress and hepatocellular injury [29]. Furthermore, pharmaceutical agents such as non-steroidal anti-inflammatory drugs (NSAIDs), chemotherapeutics (e.g., doxorubicin), and immunosuppressants undergo redox cycling or mitochondrial metabolism, leading to ROS production and antioxidant depletion with prolonged use [30,31].
Collectively, these diverse exogenous stressors contribute to a heightened oxidative load that can overwhelm endogenous antioxidants and drive the pathogenesis of various ROS-related diseases and chronic inflammatory disorders.
2.1.2. Reactive Nitrogen Species (RNS)
Endogenous Sources
The endogenous production of RNS occurs primarily through tightly regulated enzymatic pathways that are crucial for physiological signaling but can contribute to pathological processes under the conditions of oxidative stress (Figure 2). The principal RNS, NO•, is synthesized by NOS enzymes, which catalyze the oxidation of L-arginine to produce NO• and citrulline [32]. There are three isoforms, neuronal NOS (nNOS/NOS1) and endothelial NOS (eNOS/NOS3), that are constitutively expressed and calcium-dependent and mediate critical functions such as neurotransmission and vasodilation, while inducible NOS (iNOS/NOS2) is transcriptionally upregulated as a result of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and microbial products such as lipopolysaccharides (LPSs), generating sustained and high-output NO• during immune responses [33]. Under physiological conditions, NO• promotes vascular homeostasis, inhibits platelet aggregation, and regulates leukocyte adhesion. However, in oxidative environments, NO• combines with O2•− to produce ONOO−, a potent RNS that nitrates proteins, oxidizes lipids, and damages DNA, thereby contributing to nitrosative stress and tissue injury [34,35].
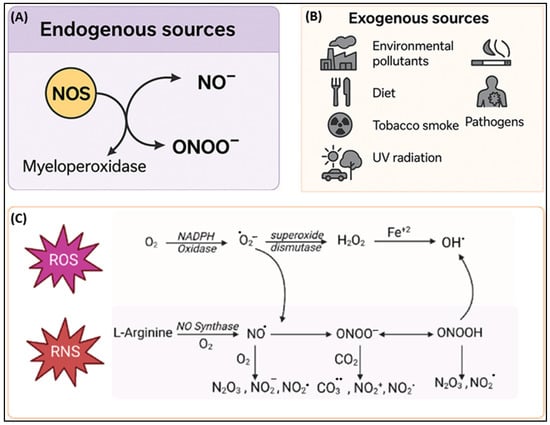
Figure 2.
Canonical schematic outlines the sources and biochemical pathways of reactive oxygen species (ROS) and reactive nitrogen species (RNS). (A) Endogenous RNS are generated via nitric oxide synthase (NOS), producing nitric oxide (NO−) and peroxynitrite (ONOO−) through interaction with myeloperoxidase. (B) Exogenous sources, such as environmental pollutants, diet, tobacco smoke, pathogens, and UV radiation, also contribute to RNS production. (C) The diagram details the interplay between ROS and RNS, highlighting the enzymatic generation of superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (OH−), and downstream nitrogen species from L-arginine through NO synthesis and emphasizing their interconnected redox biology. Modified from [36].
Other nitrogen species, such as nitrogen dioxide (•NO2) along with dinitrogen trioxide (N2O3), arise from secondary reactions involving NO• and other oxidants [37]. For example, myeloperoxidase, produced by activated neutrophils during inflammation, catalyzes the reaction of nitrite (NO2−) with H2O2 to produce •NO2, contributing to protein nitration and cellular dysfunction in inflammatory conditions like rheumatoid arthritis and asthma [38,39,40]. Moreover, sources such as xanthine oxidase and the mitochondrial ETC, though primarily known for ROS generation, contribute indirectly to RNS production by supplying O2•−, which combines with NO• to produce ONOO−, thereby linking oxidative and nitrosative stress pathways [41]. The balance between NO• signaling and its conversion into cytotoxic species is a key determinant of redox homeostasis and disease progression.
Exogenous Sources
The exogenous sources of RNS arise primarily from environmental exposures and dietary inputs, contributing significantly to systemic nitrosative stress (Figure 2). The inhalation of airborne pollutants, such as cigarette smoke, vehicle exhaust, and industrial emissions, introduces reactive nitrogen oxides (NOₓ), including •NO2 and NO•, which readily penetrate lung tissues and initiate inflammatory cascades in the respiratory tract [42]. Ozone (O3) and NOx pollutants further interact with endogenous molecules to generate secondary oxidants, compounding respiratory and systemic oxidative stress [43].
Dietary intake is another critical route of exogenous RNS exposure. Processed meats and preserved foods often contain nitrites (NO2−) and nitrates (NO3−) as additives. Under the acidic conditions of the stomach, nitrites are reduced to NO• and other nitrogenous intermediates, such as dinitrogen trioxide (N2O3), which can nitrosate amines to produce genotoxic N-nitroso compounds (NOCs). The presence of heme iron in red meats accelerates this process and has been implicated in colorectal cancer development via lipid and protein nitration [44,45].
Interestingly, nitrate-rich vegetables (e.g., spinach, arugula, beets) also contribute to endogenous RNS generation, but their health impact is modulated by co-occurring antioxidants like vitamin C and polyphenols, which inhibit nitrosation reactions [46]. Pathological states such as inflammation or infection can further amplify both endogenous and exogenous RNS production, tipping redox homeostasis toward nitrosative stress. Understanding these environmental and dietary contributions is vital for developing targeted antioxidant strategies and reducing disease risk linked to nitrosative imbalance.
3. Reactive Species in the Pathophysiology of Human Health
Reactive species play dual roles in cellular biology. Under physiological conditions, they serve as critical secondary messengers in redox-regulated signaling pathways, facilitating normal cellular processes. However, when production overwhelms antioxidant defenses, they promote oxidative and nitrosative stress, which leads to cellular dysfunction and disease pathogenesis. Understanding the precise roles and molecular mechanisms by which reactive species contribute to both physiological signaling and pathological damage is essential for elucidating their impact on human health.
3.1. ROS in Cellular Signaling and Oxidative Stress
3.1.1. Physiological Roles of ROS
Reactive oxygen species, including O2•−, H2O2, and •OH, are traditionally regarded as deleterious byproducts of aerobic metabolism. However, substantial evidence supports their critical function as secondary messengers in redox signaling (Figure 3). At physiological concentrations, ROS regulate diverse intracellular signaling pathways via the reversible oxidation of cysteine residues in key signaling proteins, such as kinases, phosphatases, and transcription factors [47]. These modifications influence multiple cellular processes, including proliferation, differentiation, metabolism, apoptosis, immune responses, and inflammation.
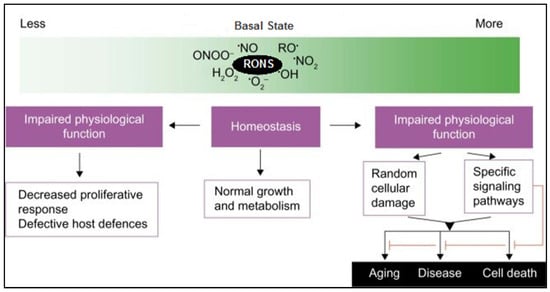
Figure 3.
Schematic shows the dual role of reactive oxygen and nitrogen species (RONS) in cellular physiology. At basal levels, RONS support homeostasis, enabling normal growth and metabolism. However, both insufficient and excessive RONS levels lead to impaired physiological function. Low RONS can reduce proliferation and weaken host defenses, while high RONS cause random cellular damage or activate signaling pathways, contributing to aging, disease, and cell death. Modified from [48].
Among various ROS, H2O2 is particularly important in signal transduction owing to its relative stability, membrane permeability, and selective reactivity [49]. Transient and spatially regulated ROS generation, principally via enzymatic sources such as NADPH oxidases (NOXs), is activated by cytokines, growth factors, and stress. NOX-derived ROS are critical for controlling endothelial function, immune cell activation, and tissue remodeling processes such as angiogenesis [50]. Key redox-sensitive signaling pathways modulated by ROS correspond to the mitogen-activated protein kinase (MAPK) cascade and phosphoinositide 3-kinase (PI3K)/Akt pathway, along with the Janus kinase/signal transducer and activator of the transcription (JAK/STAT) axis [51]. Transcription factors, such as NF-κB, Nrf2, HIF-1α, FOXO proteins, STAT3, and AP-1, orchestrate complex gene expression programs in response to oxidative cues [7,52].
For instance, NF-κB is activated by ROS through the phosphorylation of IκBα by the IκB kinase (IKK) complex, leading to its degradation and the nuclear transference of the NF-κB dimers p65/p50 [53]. This promotes the transcription of pro-inflammatory genes, such as TNF-α, IL-6, and IL-1β; COX-2; iNOS; chemokines; and adhesion molecules to control inflammatory responses [54]. In contrast, Nrf2 functions as a master controller of antioxidant defenses. Under physiological conditions, Nrf2 is sequestered by Kelch-like ECH-associated protein 1 (Keap1) [55]. Upon oxidative stress, ROS oxidize critical cysteine residues in Keap1, releasing Nrf2 to translocate to the nucleus, where it induces the transcription of cytoprotective antioxidant genes, such as NAD(P)H:quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), and glutathione S-transferases (GSTs), among others, to protect against oxidative damage [56,57,58].
Other redox-regulated transcription factors contribute to cellular adaptation. AP-1 (composed of c-Jun and c-Fos) regulates genes linked to cell growth and inflammation [59]. STAT3 supports survival, proliferation, and fibrosis in response to redox and inflammatory stress, particularly in the context of chronic disease and cancer [60]. HIF-1α integrates hypoxic and oxidative signals to promote angiogenesis and metabolic reprogramming. FOXO transcription factors are modulated by ROS-dependent post-translational modifications, and control genes modulate cell cycle arrest, apoptosis, and oxidative stress resistance [61,62].
3.1.2. Pathological Roles of ROS
While low-to-moderate ROS levels mediate beneficial signaling effects, excessive ROS production, when antioxidant systems are overwhelmed, results in oxidative stress and cellular injury (Figure 3). Membrane lipids, especially polyunsaturated fatty acids, are vulnerable to ROS-induced peroxidation, generating reactive aldehydes, including 4-hydroxynonenal and malondialdehyde [63]. Although low levels of 4-hydroxynonenal may activate protective Nrf2 responses, higher concentrations denature proteins and nucleic acids, disrupting enzymatic activity and inducing apoptosis [64,65]. Similarly, proteins undergo oxidative modifications including carbonylation, disulfide cross-linking, and methionine or cysteine oxidation, often leading to functional impairment or proteasomal degradation [49]. ROS also attacks DNA, with damage manifesting as strand breaks, abasic sites, and oxidized bases, including 8-oxo-2′-deoxyguanosine, which contributes to mutagenesis and carcinogenesis if not resolved [66].
These findings underscore the duality of ROS as both essential secondary messengers and damaging agents of oxidative stress. Thus, the maintenance of redox homeostasis is essential for cellular integrity since oxidative stress contributes to numerous inflammation- and oxidative stress-related diseases.
3.2. RNS in Cellular Signaling and Nitrosative Stress
3.2.1. Physiological Roles of RNS
Reactive nitrogen species, including NO•, ONOO−, and •NO2, are important mediators of cellular signaling with both physiological and pathological consequences. Like ROS, RNS participate in redox-dependent post-translational modifications that influence signaling pathways and gene expression (Figure 3).
Under physiological conditions, RNS are tightly regulated and play essential roles in multiple systems. Nitric oxide is biosynthesized from L-arginine under the control of the NOS isoforms eNOS, nNOS, and iNOS [32]. In the vasculature, eNOS-derived NO• activates soluble guanylate cyclase, elevating cyclic GMP levels to induce vasodilation, inhibit platelet aggregation, and maintain endothelial integrity and cardiovascular health [67]. In the nervous system, nNOS-derived NO• acts as a neurotransmitter, modulating synaptic proteins and transcription factors through reversible S-nitrosylation, thereby influencing synaptic plasticity, neurovascular coupling, and memory formation [68]. Transcription factors modulated by nNOS-derived NO• include NF-κB, AP-1, and cAMP response element-binding proteins, among others, through direct S-nitrosylation or indirect redox signaling. In the immune system, controlled iNOS activity in macrophages and other immune cells generates NO• as part of the antimicrobial defense, contributing to the clearance of pathogens [69].
RNS also regulate transcription by S-nitrosylating key signaling proteins, such as IKK and AP-1, thereby modulating inflammatory gene expression. Additionally, RNS can modify Keap1, leading to the release and nuclear translocation of Nrf2, and activate antioxidant gene expression. RNS further modulate the action of other transcription factors, including HIF-1α and STAT3, along with p53, integrating redox and nitrosative signals in cellular decision-making [70,71].
3.2.2. Pathological Roles of RNS
When RNS production becomes excessive or dysregulated, as seen in chronic inflammation or oxidative stress, they contribute to nitrosative stress and cellular damage (Figure 3). High levels of NO•, particularly from iNOS, and the formation of ONOO− lead to irreversible modifications of proteins, lipids, and DNA. One significant pathological modification is tyrosine nitration, primarily mediated by ONOO−, which alters protein structure and function, disrupts signaling pathways, and impairs enzyme function and cellular homeostasis [72,73]. Tyrosine nitration has been cited in the incidence of cardiovascular, neurodegenerative, and inflammatory diseases [74,75].
Furthermore, while reversible under normal conditions, dysregulated S-nitrosylation can disturb cellular homeostasis and contribute to disease. For instance, the nitrosylation of caspases hinders apoptosis, while modifications of metabolic enzymes impair energy production [76,77]. Persistent nitrosative stress also affects epigenetic regulation by altering DNA methylation and histone structures, further impacting gene expression and cellular function and contributing to genetic mutations and carcinogenesis if left unrepaired [78]. Thus, while RNS are integral to normal cellular physiology, their overproduction disrupts redox-sensitive transcriptional networks and contributes to disease development.
3.3. Synergistic Effects of Reactive Oxygen and Nitrogen Species
ROS and RNS frequently act concertedly to exacerbate cellular damage. For instance, the interaction between NO• and O2•− not only generates ONOO− but also consumes bioavailable NO•, impairing vasodilation and promoting endothelial dysfunction, a key event in atherosclerosis and hypertension [79]. Furthermore, combined oxidative and nitrosative stress can inhibit DNA repair enzymes such as poly(ADP-ribose) polymerases and 8-oxoguanine glycosylase, perpetuating genomic instability and accelerating disease progression [80].
Table 2 highlights how oxidative, along with nitrosative stress, drives the pathogenesis of various chronic diseases [81]. Their widespread impact underscores the value of targeting redox imbalances in the prevention and treatment of these pathologies. Understanding the spatial and temporal dynamics of ROS and RNS production, as well as their dual physiological and pathological roles, is critical for developing therapeutic strategies to restore redox homeostasis. This includes interventions that enhance endogenous antioxidant systems or pharmacologically scavenge reactive species to mitigate disease-associated damage and restore cellular redox balance. The next section discusses the major antioxidant systems involved in protecting against oxidative and nitrosative damage.

Table 2.
Chronic human diseases associated with persistent oxidative stress and nitrosative stress.
4. Antioxidant Modulation of Oxidative and Nitrosative Stress
Oxidative and nitrosative stress perturbs redox signaling and damages vital biomolecules, including lipids, proteins, and nucleic acids. These disruptions significantly contribute to the development of a wide spectrum of human diseases. Antioxidants constitute a broad class of molecules that mitigate these effects by neutralizing reactive species, including ROS and RNS; maintaining redox homeostasis; and preventing macromolecular damage.
4.1. Types of Antioxidant Systems
The body’s antioxidant defense network encompasses both endogenous and exogenous systems, each playing complementary roles in cellular protection, signaling modulation, and stress adaptation.
4.1.1. Endogenous Antioxidants
Enzymatic Antioxidants
Endogenous enzymatic antioxidants are the primary protection against oxidative and nitrosative stress. The key antioxidants include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and paraoxonase-2 (PON2) [109]. These enzymes facilitate the detoxification of ROS and RNS through highly regulated catalytic reactions, preserving redox balance and preventing macromolecular damage. For comprehensive discussions of antioxidant systems, readers are directed to these reviews [110,111].
Superoxide Dismutases: SODs are metalloenzymes responsible for the dismutation of O2•− to H2O2 and oxygen [112]. Three isoforms exist, corresponding to cytosolic Cu/Zn-SOD (SOD1), mitochondrial Mn-SOD (SOD2), and extracellular SOD (SOD3) [113]. SOD2 is particularly essential in mitochondrial protection, as mitochondria represent primary sites for ROS production. The deficiency or inactivation of SOD2 has been implicated in mitochondrial dysfunction and increased susceptibility to oxidative stress in models of neurodegeneration and cardiomyopathy [114,115].
Catalase (CAT): Catalase, predominantly located in peroxisomes, rapidly decomposes H2O2 into water and oxygen [116]. Its activity prevents the accumulation of H2O2, which could otherwise undergo Fenton reactions to produce the highly reactive •OH. High CAT activity is observed in hepatic tissues, highlighting its importance in detoxification processes and hepatocellular protection [108].
Glutathione Peroxidases: GPxs constitute a family of selenium-dependent enzymes which reduce lipid hydroperoxides and H2O2 using reduced glutathione (GSH) as a substrate [117]. GPx1 is ubiquitously expressed and critical in cardiovascular and neuronal antioxidant defense, while GPx4 specifically protects membrane lipids from peroxidation and is essential for embryonic development and ferroptosis suppression [118].
Paraoxonase-2: PON2 is an abundant membrane-associated enzyme with lactonase activity, and it is involved in hydrolyzing lipid peroxides and protecting against oxidative stress-induced apoptosis [119]. Interestingly, while PON2 exerts protective effects in oxidative and inflammatory conditions such as atherosclerosis and neuroinflammation, emerging evidence suggests its aberrant overexpression in several malignancies, including glioblastoma, ovarian, and prostate cancers, where it contributes to tumor progression, metabolic reprogramming, and resistance to chemotherapeutics [120,121].
These enzymatic defenses are particularly abundant in organs with high metabolic rates and oxygen needs, including liver, brain, and kidneys, reflecting their vital role in maintaining tissue homeostasis under oxidative pressure.
Non-Enzymatic Antioxidants
These antioxidants are free radical scavengers, metal chelators, and redox modulators. They include glutathione, uric acid, bilirubin, and coenzyme Q10 (CoQ10) among others, which either directly neutralize ROS/RNS or regenerate oxidized enzymatic antioxidants, reinforcing cellular antioxidant capacity.
Glutathione: GSH is the most abundant intracellular antioxidant [122]. It directly neutralizes ROS, detoxifies electrophilic xenobiotics, and serves as a cofactor for GPxs and glutathione S-transferases [123]. Additionally, GSH contributes to the regeneration of oxidized vitamin C and E, thereby preserving the antioxidant network. GSH depletion is a biomarker of oxidative stress in diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and type 2 diabetes [124,125].
Uric Acid: Uric acid functions as a potent scavenger of singlet oxygen and peroxynitrite in the plasma [126]. It represents the final product of purine catabolism. Although high uric acid is linked to gout and cardiovascular risk, at physiological levels, it contributes significantly to the plasma antioxidant capacity, particularly under ischemic or inflammatory conditions [127].
Bilirubin: As a heme degradation product, bilirubin has emerged as a cytoprotective antioxidant. It can neutralize ROS and inhibit lipid peroxidation, particularly in neuronal and hepatic tissues [128]. Interestingly, low physiological levels of bilirubin are known to lessen risks of cardiovascular diseases, while mild hyperbilirubinemia, such as in Gilbert’s syndrome, confers protection against oxidative stress-related pathologies [129].
Coenzyme Q10 (CoQ10): Coenzyme Q10, an oleophilic antioxidant, resides in the inner mitochondrial membrane, where it participates in electron transport and prevents lipid peroxidation [130,131]. It also regenerates vitamin E from its oxidized form and modulates mitochondrial permeability transition. CoQ10 deficiency has been linked to heart failure, neurodegeneration, and aging-related decline, and supplementation has shown promise in reducing oxidative biomarkers in clinical settings [132].
These non-enzymatic antioxidants operate in synergy with enzymatic systems, and their levels are tightly regulated through biosynthesis, recycling, and dietary intake. An imbalance in these components not only heightens susceptibility to oxidative/nitrosative damage but also alters redox-sensitive gene expression profiles that are critical for inflammation, immunity, and apoptosis.
4.1.2. Exogenous Antioxidants
Exogenous antioxidants are derived from dietary sources, and they are essential for maintaining redox homeostasis, particularly when endogenous antioxidant defenses are insufficient, as seen during aging, metabolic stress, environmental toxin exposure, and chronic inflammation [116]. These dietary antioxidants include vitamins, polyphenols, carotenoids, and trace minerals, all of which contribute to oxidative balance either by directly scavenging ROS and RNS or upregulating endogenous antioxidant defense pathways.
Vitamins as Antioxidants
Vitamin C (Ascorbic Acid): This is a hydrophilic antioxidant that functions in the aqueous compartments of cells [133]. It directly neutralizes •OH, O2•−, and peroxyl (ROO•) radicals, and it is involved in regenerating the oxidized forms of other antioxidants, including vitamin E and glutathione [134]. Additionally, it is a cofactor for a number of dioxygenase enzymes pivotal for collagen synthesis, immune function, and epigenetic regulation through histone demethylation. High vitamin C intake is linked to a lowered incidence of cardiovascular disease and cognitive decline, largely due to its capacity to lower endothelial oxidative stress and preserve nitric oxide bioavailability [135].
Vitamin E (Tocopherols and Tocotrienols): This comprises an oleophilic family of antioxidants, having α-tocopherol as the most biologically active form [136]. It is uniquely suited to protect polyunsaturated fatty acids in cellular membranes from peroxidation by intercepting lipid radicals and terminating lipid peroxidation [137]. Vitamin E also modulates redox-sensitive signaling pathways such as protein kinase C, NF-κB, and transforming growth factor-β, contributing to its anti-inflammatory and immunomodulatory functions [138]. Clinical studies report that optimal vitamin E intake reduces the markers of oxidative stress and improves outcomes in pathologies including non-alcoholic fatty liver disease and AD [139].
Vitamin A and Carotenoids: Carotenoids, including β-carotene, lycopene, lutein, and zeaxanthin, are lipophilic pigments with potent antioxidant and photoprotective properties [140]. β-carotene serves as a precursor to retinol (vitamin A) and contributes to mucosal immunity and epithelial integrity [141,142]. Lycopene, abundant in tomatoes and watermelon, is particularly effective in quenching 1O2 and inhibiting lipid peroxidation [143]. Lutein and zeaxanthin are concentrated in the retina and shield ocular tissues from blue light-induced oxidative damage, significantly lowering the incidence of age-related macular degeneration [144,145,146].
Polyphenols as Antioxidants
Polyphenols constitute a structurally diverse class of phytochemicals that contain phenol units. They exhibit strong antioxidant and anti-inflammatory effects by regulating redox-sensitive signaling pathways [147,148]. They are subdivided into flavonoids (e.g., quercetin, catechins), phenolic acids (e.g., caffeic acid), stilbenes (e.g., resveratrol), and lignans [149].
For instance, quercetin, found in onions, apples, and tea, has strong radical-scavenging activity and inhibits lipid peroxidation, xanthine oxidase, and NADPH oxidase [150]. It suppresses pro-inflammatory cytokines including TNF-α and IL-6, partly through the control of the NF-κB pathway [151]. Similarly, resveratrol, abundant in red wine and grapes, activates Sirtuin 1 (SIRT1) and Nrf2 pathways, enhances mitochondrial biogenesis, and promotes autophagic flux, all of which confer antioxidant and anti-aging benefits [152]. Epigallocatechin gallate (EGCG) from green tea scavenges ROS, suppresses inflammatory gene expression, and inhibits matrix metalloproteinases implicated in tissue degradation [153]. These polyphenols often exhibit hormetic effects, whereby low-to-moderate doses trigger the transcription of Nrf2 to express endogenous antioxidant responses, contributing to long-term cytoprotection [154].
Essential Trace Minerals as Antioxidant Cofactors
Trace minerals, including selenium, zinc, and manganese, play essential roles in maintaining redox homeostasis by supporting catalytic activity and the structural integrity of key antioxidant enzymes. Selenium (Se), a critical cofactor for glutathione peroxidases (GPx) and thioredoxin reductases (TrxR), is vital for peroxide detoxification and redox signaling [155]. Selenium deficiency contributes to reduced immune function and diseases such as Keshan cardiomyopathy, highlighting its physiological importance [156]. Zinc (Zn) contributes structurally to Cu/Zn-superoxide dismutase (SOD1), stabilizes cellular membranes to reduce lipid peroxidation, and modulates redox signaling by downregulating NADPH oxidase activity [157]. Additionally, zinc supports the functioning of DNA repair enzymes, thereby preserving genomic integrity under oxidative stress [158]. Manganese (Mn) is indispensable for the activity of Mn-superoxide dismutase (SOD2), the primary mitochondrial antioxidant enzyme responsible for neutralizing ROS and protecting mitochondrial DNA and proteins. Mn-SOD is especially vital in metabolically active tissues with high oxidative needs, including the brain and heart [159]. Beyond their enzymatic roles, these trace minerals also regulate redox-sensitive transcriptional and translational processes that govern cellular stress responses and adaptation to oxidative environments.
Dietary Patterns and Antioxidant Synergy
The health benefits of exogenous antioxidants are best realized in the context of whole dietary patterns rather than isolated supplementation. This is due to synergistic interactions among various nutrients, bioavailability differences, and modulatory effects on gut microbiota and metabolic pathways [160]. The Mediterranean diet contains extra virgin olive oil, fruits, vegetables, legumes, nuts, and moderate red wine, and it has been extensively studied for its antioxidant potential [161]. This diet enhances systemic antioxidant capacity (e.g., increased plasma total antioxidant status, reduced oxidized low-density lipoproteins, lowers C-reactive proteins) and is linked with the reduced incidence of cardiovascular, metabolic, and neurodegenerative diseases [162]. Antioxidant-dense foods, such as berries (anthocyanins), dark chocolate (flavanols), green tea (catechins), turmeric (curcumin), and pomegranate (ellagitannins), have demonstrated the ability to reduce oxidative biomarkers such as malondialdehyde and F2-isoprostanes in both observational and interventional studies [163,164]. These dietary elements also support gut redox signaling and microbiota composition, thereby exerting indirect antioxidant effects by regulating microbial metabolites, including short-chain fatty acids and polyphenol derivatives, that regulate innate inflammation and oxidative stress [165].
Taken together, exogenous antioxidants obtained through diet provide critical reinforcement to endogenous redox systems, particularly under pathological or age-associated oxidative stress. A diverse and balanced diet incorporating multiple sources of antioxidant nutrients confers synergistic protection against oxidative and nitrosative damage, thereby contributing to health maintenance, disease prevention, and the promotion of longevity.
4.2. Mechanisms of Action of Antioxidants
The generation and detoxification of ROS and RNS by antioxidants are tightly regulated at the transcriptional level through redox-sensitive signaling pathways. Central to this regulation is Nrf2, a transcription factor that modulates cellular redox balance by promoting the secretion of several cytoprotective genes [166]. Once activated, Nrf2 migrates to the nucleus and binds to antioxidant response elements in the promoters of target genes, driving the transcription of key detoxification and antioxidant enzymes, such as GSTs, HO-1, glutamate–cysteine ligase, NQO1, SOD, GPx, and CAT, and TrxR [167,168,169]. Importantly, the activity of Nrf2 is context-dependent. While transient activation confers protection against oxidative damage and inflammation, persistent activation has been associated with tumorigenesis, metastasis, and chemoresistance due to the enhanced cellular survival and suppression of pro-oxidant signaling [170,171]. The dysregulation of Nrf2 signaling is reported to promote chronic diseases, including cancer, neurodegeneration, and metabolic disorders [172], emphasizing the need for the precise modulation of this pathway in antioxidant-based therapies.
In addition to transcriptional control, antioxidants also control gene expression via epigenetic means. These include DNA methylation and histone modifications that influence chromatin structure and gene accessibility [173]. Polyphenols such as curcumin and resveratrol reverse aberrant epigenetic patterns, restoring normal gene activity associated with oxidation and inflammatory response and cell survival [174]. Beyond gene regulation, antioxidants exert protective effects through several interrelated biochemical mechanisms. A primary mechanism involves the direct scavenging of ROS/RNS, thereby neutralizing free radicals and preventing oxidative modifications of cellular macromolecules [175]. Lipid-soluble antioxidants like vitamin E terminate lipid peroxidation chain reactions by transferring hydrogen atoms to lipid peroxyl radicals, thus maintaining membrane integrity, fluidity, and signal transduction [176,177]. Similarly, water-soluble antioxidants, including vitamin C, neutralize hydroxyl and peroxyl radicals and regenerate reduced antioxidants to their active forms.
Another essential mechanism involves antioxidant recycling, which enhances the efficiency of the redox defense system. Vitamin C regenerates oxidized vitamin E, while coenzyme Q restores other lipid-phase antioxidants, forming a cooperative network that sustains antioxidant capacity under stress conditions [178,179]. This synergy highlights the interdependence of antioxidant systems in maintaining cellular redox balance. Antioxidants also bolster endogenous enzymatic antioxidant defenses, including SOD, GPx, and CAT. These enzymes promote the detoxification of O2•− and H2O2 to less reactive species. Their activity depends on essential cofactors such as selenium (for GPx) and manganese or copper/zinc (for SOD) [180]. Notably, certain dietary antioxidants such as flavonoids and phenolic acids can upregulate the gene expression or activity of these enzymes, thereby reinforcing intrinsic defense mechanisms [181,182].
In parallel, many antioxidants exhibit anti-inflammatory properties by modulating redox-sensitive signaling pathways. For instance, ROS generated during immune responses can activate NF-κB, which regulates the pro-inflammatory cytokines (TNF-α, IL-1, and IL-6) and adhesion molecules. Polyphenolic antioxidants such as EGCG and curcumin inhibit NF-κB activation, thereby attenuating inflammatory cascades and offering therapeutic potential in chronic inflammatory diseases [50,182].
Table 3 summarizes key endogenous and exogenous antioxidants, their representative examples, sites of action, and mechanisms of antioxidant activity. Collectively, the combined actions of transcriptional regulation, enzymatic modulation, radical scavenging, and inflammatory suppression constitute a multifaceted antioxidant defense system. The dynamic interplay between endogenous and exogenous antioxidants provides a comprehensive framework for maintaining redox homeostasis and mitigating oxidative and nitrosative stress-related pathologies. Elucidating these pathways is fundamental to advancing targeted antioxidant strategies in clinical settings.

Table 3.
Summary of major endogenous and exogenous antioxidants and their mechanisms of action.
5. Application of Antioxidant-Based Therapies
Building on their mechanistic versatility, antioxidant-based therapies have been widely explored for their potential in mitigating oxidative and nitrosative stress in human pathologies. These include cardiovascular and neurodegenerative disorders, cancer, diabetes, inflammatory conditions, ocular and skin diseases, reproductive dysfunction, and impaired physical performance [81,183]. By neutralizing excessive reactive oxygen and nitrogen species, antioxidants help preserve cellular integrity, attenuate inflammation, and restore redox homeostasis, thereby contributing to disease prevention and symptom alleviation.
For instance, in skin health and antiaging, the aging process is accelerated by oxidative stress, which causes cumulative cellular and tissue damage. Research evidence demonstrates that antioxidants can extend lifespan in model organisms; for example, compounds such as spermidine and resveratrol stimulate autophagy, thereby protecting cells from oxidative injury [184]. In dermatology, both endogenous and exogenous antioxidants play critical roles in counteracting aging skin and environmental damage, particularly damage caused by UV radiation. UV exposure significantly increases the production of ROS, which accelerates skin aging, wrinkle formation, and photoaging. The skin’s intrinsic antioxidant defense system, including enzymatic antioxidants such as SOD, CAT, GPx, and PON2, works to neutralize ROS and maintain redox homeostasis. Notably, PON2 expression is relatively low in benign actinic keratosis but markedly elevated in squamous cell carcinoma, highlighting its complex role in skin oxidative stress and carcinogenesis linked to aging and UV exposure [185]. Alongside these endogenous systems, exogenous antioxidants such as vitamins C and E, coenzyme Q10, and polyphenols from green tea, berries, and other plant extracts help scavenge ROS and reduce UV-induced skin damage [186,187]. Strengthening both intrinsic and extrinsic antioxidant defenses remains a promising approach to support skin health and mitigate visible signs of aging. Table 4 provides a detailed overview of therapeutic applications, mechanisms, and relevant antioxidant compounds across several disease domains.

Table 4.
Therapeutic applications of antioxidants in human disease management: key outcomes and limitations.
While preclinical studies consistently highlight protective benefits of antioxidants in various models of disease, translation of these benefits into clinical practice has yielded mixed outcomes [305]. This discrepancy underscores the need to critically examine the limitations and controversies surrounding antioxidant therapy. Despite promising experimental data, human trials have produced inconsistent results, raising concerns about clinical efficacy, optimal dosing strategies, pharmacokinetics, safety, and the inherent biological complexity of antioxidant interventions as outlined below. Additionally, the dualistic role of ROS and RNS, as both harmful byproducts and essential signaling molecules, complicates the rationale for broad-spectrum antioxidant use [306].
5.1. Efficacy and Safety Concerns
One of the most persistent hurdles in the field of antioxidant therapy is the difficulty in achieving both effective and safe outcomes [306]. Several antioxidants, including vitamin C, vitamin E, and polyphenols, face significant pharmacokinetic barriers that limit their clinical utility. For example, vitamin C absorption is saturable, and any excess is rapidly excreted, making it difficult to sustain therapeutic plasma concentrations [307,308]. Similarly, polyphenols, despite their abundance in fruits and teas, are extensively metabolizes in the liver and gut, resulting in low systemic bioavailability along with limited tissue penetration [309,310].
Concerns about safety are equally pressing. High-dose supplementation of certain antioxidants can lead to unintended and sometimes serious adverse effects. Vitamin E, for instance, is linked to heightened incidence of hemorrhagic stroke and all-cause mortality when consumed in excessive amounts [311]. Selenium, another widely used antioxidant, can be toxic at high doses, causing selenosis, a condition characterized by gastrointestinal disturbances, hair loss, and neurological symptoms [312]. Such adverse outcomes have been observed in large-scale clinical studies, including the SELECT trial, which reported increased risks of prostate cancer and diabetes with high-dose vitamin E and selenium supplementation (NCT00006392).
Individual variability further complicates the picture. Genetic differences in antioxidant enzyme systems, pre-existing health conditions, and interactions with other medications all influence how patients respond to antioxidant therapy. This diversity underscores the necessity for personalized dosing and vigilant monitoring instead of a universal one-size-fits-all approach [313].
5.2. Inconsistent Clinical Trial Outcomes
The translation of antioxidant therapy from promising epidemiological and preclinical findings to successful clinical outcomes has proven elusive. While observational studies and laboratory experiments propose that diets containing antioxidants decrease the incidence of chronic diseases, large-scale randomized clinical trials have largely failed to confirm these benefits when antioxidants are administered as supplements [306]. For example, early studies indicated that high intake of vitamins C and E might protect against atherosclerotic cardiovascular disease, yet subsequent trials involving thousands of participants did not demonstrate a reduction in cardiovascular events or mortality with supplementation [314,315,316].
Several factors help explain these inconsistencies. There is a fundamental difference between consuming antioxidants as part of whole foods and as isolated supplements. The food matrix, which includes fiber, other micronutrients, and non-antioxidant bioactives, may produce synergistic effects that are not replicated by single-agent supplementation [317]. Furthermore, the timing, dosage, and duration of antioxidant therapy are important factors. High-dose supplementation over short periods may not reverse decades of accumulated oxidative damage, and in some cases, such as β-carotene in smokers, supplementation has actually heightened the incidence of lung cancer [318]. These reports highlight the situational and sometimes adverse effects of antioxidants, challenging the notion of a universal benefit.
Additionally, many clinical trials may not have been of sufficient duration to detect meaningful benefits, particularly for chronic diseases that develop over many years. The choice of antioxidant compounds in trials has often been dictated by availability rather than demonstrated efficacy, further complicating the interpretation of results.
5.3. Controversies in Cancer Therapy
The administration of antioxidants as adjuvants during cancer therapy remains in the field [319]. Chemotherapy and radiation therapy impart their cytotoxic effects, in part, by generating ROS that damage tumor cell DNA. The rationale for antioxidant supplementation is to protect normal tissues from collateral oxidative injury, thereby reducing side effects and improving patient well-being. However, this protective effect is not selective; antioxidants may also shield malignant cells from therapy-induced oxidative damage, potentially diminishing the effectiveness of cancer treatments [320].
Systematic reviews and meta-analyses have brought this dilemma into sharp relief. Some studies suggest that antioxidants, including vitamin A, β-carotene, and CoQ10, reduce treatment toxicity and improve patient outcomes [321,322], yet others find no benefit or even report harm [323,324]. For instance, vitamin A supplementation has been linked to higher risks of death and recurrence in some cancer cohorts, while β-carotene supplementation exacerbated lung cancer and mortality incidence among smokers [325]. A meta-analysis found that antioxidant use during chemotherapy and radiation was associated with increased mortality rates and reduced chances of cancer-free survival among breast cancer survivors. These conflicting results have led many oncologists to advise against the administration of antioxidant supplements during active cancer treatment until more definitive evidence is available [319].
5.4. Pro-Oxidant Effects and Redox Imbalance
A paradoxical and often overlooked challenge in antioxidant therapy is the potential for certain antioxidants to act as pro-oxidants under specific circumstances. The redox environment within cells is delicately balanced, and excessive supplementation can disrupt this equilibrium. High doses of vitamin C and E, for example, may not only fail to quench reactive oxygen species but may actually promote oxidative stress by interfering with physiological redox signaling or by participating in redox cycling reactions [306,314,326]. This is important in the context of exercise, where ROS serve as essential signaling molecules that mediate beneficial adaptations such as mitochondrial biogenesis. Suppressing these signals with antioxidant supplements can impair the body’s adaptive response to exercise [327,328].
Moreover, endogenous antioxidant enzymes, including SOD and CAT, are far more efficient and specific than exogenous small-molecule antioxidants. This raises important questions about the ability of dietary supplements to meaningfully influence redox status in vivo, especially in individuals with robust endogenous defenses [306,329].
5.5. Biological Complexity and Limitations in Trial Design
The biological landscape of oxidative stress is characterized by intricate feedback loops, redundancy, and compartmentalization. Diseases such as hypertension, neurodegeneration, and cancer involve multiple sources of ROS and diverse cellular targets, making it unlikely that a single antioxidant could provide universal benefit. Patient heterogeneity, including genetic background, baseline antioxidant status, and the occurrence of comorbidities, further complicates the interpretation of trial results [306,330,331].
Another major limitation is the lack of reliable, disease-specific biomarkers of oxidative stress. Commonly used markers, including malondialdehyde or F2-isoprostanes, offer only a crude estimate of systemic oxidative burden and may not reflect tissue-specific pathology or the dynamic nature of redox changes [332]. This makes it challenging to select appropriate patient populations, monitor therapeutic responses, and tailor interventions effectively.
Trial design itself poses additional challenges. Many studies have not adequately accounted for confounding factors such as dietary patterns, lifestyle, or genetic predispositions. Moreover, the endpoints chosen in trials may not be sensitive enough to detect subtle but clinically meaningful changes in oxidative stress or disease progression [306].
Persistent gaps between antioxidant mechanisms and clinical outcomes reflect deeper complexities in redox biology and disease pathology. Addressing these challenges requires a shift in how antioxidant interventions are conceptualized and applied within therapeutic contexts. The next section explores emerging strategies designed to overcome these barriers and enhance the clinical utility of antioxidant-based therapies.
6. Emerging Advances in Antioxidant Research: Innovative Solutions to Clinical Limitations
Despite extensive evidence demonstrating the therapeutic potential of antioxidants across a wide range of diseases, significant limitations continue to hinder their clinical efficacy. Challenges such as poor bioavailability, rapid metabolic degradation, lack of target specificity, and unpredictable redox behavior have contributed to inconsistent outcomes in randomized controlled trials and meta-analyses. In response to these issues, a variety of innovative approaches have emerged in recent years to optimize antioxidant delivery, enhance therapeutic specificity, and individualize treatment strategies [333]. These advances represent a paradigm shift in antioxidant therapy and are described below in detail.
6.1. Nanotechnology-Driven Antioxidant Delivery
Nanotechnology has significantly advanced the delivery of antioxidant compounds by addressing key pharmacokinetic and pharmacodynamic barriers. Nano-formulations, including nanoparticles, liposomes, dendrimers, and solid lipid carriers, offer protection against premature degradation, increase aqueous solubility, and enable targeted or sustained release at specific tissue sites [334]. For a comprehensive review of the application of nanotechnology in antioxidant therapies, the reader is directed to the following reviews: [335,336].
For example, curcumin-loaded polymeric nanoparticles have demonstrated superior bioavailability along with neuroprotective efficacy in animal models of AD by reducing β-amyloid aggregation and improving cognitive outcomes [337,338]. Similarly, clinical studies confirmed the benefits of nanocarriers to enhance the pharmacological profile of natural antioxidants. A recent randomized controlled trial involving type 2 diabetic patients showed that nano-encapsulated resveratrol and curcumin significantly improved insulin sensitivity and reduced oxidative biomarkers compared to their free forms [339]. In oncology, tocotrienol-loaded nanocarriers have selectively targeted tumor cells and induced apoptosis without affecting normal tissue in preclinical breast and pancreatic cancer models [340,341]. Meta-analyses also support the efficacy of nanotechnology-based antioxidant delivery in improving tissue uptake and therapeutic outcomes across cardiovascular and neurological diseases [342].
6.2. Synergistic and Combination Therapies
The complexity of oxidative stress-related pathologies often requires therapeutic interventions that extend beyond monotherapy. Consequently, synergistic regimens combining antioxidants with pharmaceuticals, immunomodulators, or nutraceuticals have gained considerable attention [343]. These combination approaches not only enhance efficacy through complementary mechanisms but also mimic the endogenous antioxidant network. For instance, a meta-analysis encompassing 19 randomized controlled trials demonstrated that combining antioxidants such as vitamin C, vitamin E, and Se, along with N-acetylcysteine, with standard chemotherapeutics reduced treatment-induced toxicity while preserving anticancer efficacy [344]. In inflammatory diseases such as rheumatoid arthritis, co-administration of vitamin E along with omega-3 fatty acids resulted in greater reductions in pro-inflammatory cytokines and oxidative stress markers than either compound alone [345]. Similarly, EGCG, a green tea polyphenol, synergistically enhances the efficacy of NSAIDs in experimental models of inflammatory bowel disease by reducing mucosal damage and suppressing oxidative and nitrosative stress [346,347]. These reports emphasize the value of combination therapies to target multiple disease pathways and achieve superior clinical outcomes.
6.3. Personalized and Precision Antioxidant Medicine
The inter-individual variability in redox homeostasis, disease etiology, and response to antioxidant supplementation has stimulated interest in personalized medicine approaches. Advances in genomics, metabolomics, and microbiome science now enable more refined and targeted antioxidant interventions. Genetic polymorphisms in antioxidant enzymes, including superoxide dismutase 2 (SOD2 Val16Ala) and glutathione peroxidase 1 (GPX1), are known to modulate susceptibility to oxidative damage and influence disease outcomes [348,349].
Emerging clinical studies are using genetic screening to tailor antioxidant therapy. For example, individuals harboring the SOD2 Ala/Ala genotype have shown enhanced responses to mitochondria-targeted antioxidants such as MitoQ, with improved endothelial function and reduced vascular oxidative stress [350]. Moreover, patient-specific interventions based on metabolomic and microbiome profiles have demonstrated efficacy in metabolic syndrome. Targeted supplementation with ellagitannin-rich polyphenols led to improved insulin sensitivity and lipid profiles in a metabolically stratified cohort [351,352]. Precision dosing algorithms that account for age, sex, comorbidities, genetic variants, and metabolic status are currently under development to further refine antioxidant pharmacotherapy. Ongoing clinical trials are evaluating redox-based tumor signatures as predictive biomarkers for tailored antioxidant co-administration in cancer therapy (e.g., NCT03529110) [353,354].
6.4. Enzyme-Mimetic Antioxidants
To overcome the stoichiometric limitations of classical antioxidants, researchers have developed small-molecule mimetics of endogenous antioxidant enzymes, including SOD, CAT, and GPx [306]. These mimetics offer catalytic activity, target specificity, and sustained action.
One notable example is GC4419 (avasopasem manganese), a SOD mimetic that significantly reduced the severity and duration of oral mucositis in subjects receiving radiotherapy for head and neck cancer in recent phase III clinical trials [355,356]. Another example is Ebselen, a selenium-containing glutathione peroxidase mimic, which has demonstrated neuroprotective effects in clinical trials targeting bipolar disorder and noise-induced hearing loss [357,358,359]. Preclinical models of stroke, myocardial infarction, and radiation injury have also shown that these compounds provide broad-spectrum cytoprotection by restoring redox homeostasis and modulating pro-inflammatory pathways [360].
6.5. Redox Biomarkers and Real-Time Monitoring Tools
A key advancement in improving antioxidant therapy lies in the ability to assess oxidative stress levels in real time [361,362]. The integration of redox biomarkers into clinical and translational research has facilitated risk stratification, therapeutic monitoring, and early detection of treatment failure. Biomarkers including 8-hydroxy-2′-deoxyguanosine, F2-isoprostanes, malondialdehyde, and glutathione disulfide-to-glutathione ratios are being increasingly utilized in clinical trials as surrogate endpoints of oxidative stress [363,364]. Moreover, emerging technologies such as biosensors and wearable diagnostic platforms are being designed to provide continuous, non-invasive measurements of redox biomarkers. These innovations hold promise in enabling dynamic dose adjustment and timely intervention, ultimately enhancing the efficacy and safety of antioxidant-based therapies [365].
6.6. Future Outlook
Collectively, these innovations signify a critical turning point in antioxidant therapeutics. The integration of nanotechnology, synergistic treatment modalities, personalized medicine, enzyme-mimetic compounds, and real-time biomonitoring addresses many of the longstanding challenges that have impeded clinical translation. These approaches not only improve antioxidant pharmacokinetics and pharmacodynamics but also align therapeutic strategies with individual patient biology. With progress in understanding the complexity of redox biology and the development of advanced delivery and monitoring systems, antioxidant therapy is poised to evolve from a generalized supplement-based model into a targeted, evidence-based, and clinically actionable paradigm. Future studies incorporating multi-omics technologies, digital health tools, and large-scale longitudinal trials will be essential in validating these advances and translating them into routine clinical practice.
7. Conclusions
Oxidative and nitrosative stress features predominantly in pathogenesis of numerous chronic diseases, underscoring the therapeutic potential of antioxidant-based strategies. However, clinical outcomes have been inconsistent, reflecting challenges related to bioavailability, individual variability, and the complex dual roles of reactive species in human pathophysiology. Looking forward, the future of antioxidant therapy lies in an integrated approach: discovery of novel bioactive compounds with precise redox-modulating activity, advancement of nanotechnology-based delivery platforms to enhance tissue targeting and stability, and adoption of precision medicine to tailor interventions to individual genetic and metabolic profiles. These innovations offer a promising path toward safer, more effective, and personalized therapies for managing oxidative stress-related diseases.
Author Contributions
C.F.M. contributed to the conceptualization and prepared the original draft. D.S. edited the manuscript, along with E.F., A.A.S., L.A. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
There are no conflicts of interest declared by the authors.
Abbreviations
The following abbreviations are used in this manuscript:
| GPx | Glutathione peroxidase; |
| SOD | Superoxide dismutase; |
| COX-2 | Cyclooxygenase-2; |
| CAT | Catalase; |
| EGCG | Epigallocatechin-3-gallate; |
| DNA | Deoxyribonucleic acid; |
| Nrf2 | Nuclear factor erythroid 2-related factor 2; |
| TNF-α | Tumor necrosis factor-alpha; |
| IL-6 | Interleukin-6; |
| NF-κB | Nuclear factor kappa B; |
| GSTs | Glutathione S-transferases; |
| HO-1 | Heme oxygenase-1; |
| NQO1 | NAD(P)H:quinone oxidoreductase 1; |
| TrxR | Thioredoxin reductase; |
| SIRT1 | Sirtuin 1; |
| CoQ10 | Coenzyme Q10; |
| GSH | Glutathione; |
| PON2 | Paraoxonase-2; |
| HIF-1α | Hypoxia-inducible factor 1-alpha; |
| STAT3 | Signal transducer and activator of transcription 3; |
| FOXO | Forkhead box O; |
| p53 | Tumor protein 53; |
| IKK | IκB kinase; |
| AP-1 | Activator protein 1; |
| Keap1 | Kelch-like ECH-associated protein 1. |
References
- Thomas, C.; Wurzer, L.; Malle, E.; Ristow, M.; Madreiter-Sokolowski, C.T. Modulation of reactive oxygen species homeostasis as a pleiotropic effect of commonly used drugs. Front. Aging 2022, 3, 905261. [Google Scholar] [CrossRef]
- Jakubek, P.; Parchem, K.; Wieckowski, M.R.; Bartoszek, A. The interplay between endogenous and foodborne pro-oxidants and antioxidants in shaping redox homeostasis. Int. J. Mol. Sci. 2024, 25, 7827. [Google Scholar] [CrossRef]
- Pham, T.-P.-T.; Le, T.-H.-T.; Pham, H.-T.-X.; Tran, T.-T.; Pham, V.-T.; Mafruhah, O.R.; Ha, H.-A. Comparative efficacy of antioxidant therapies for sepsis and septic shock in the intensive care unit: A frequentist network meta-analysis. Heliyon 2024, 10, e31447. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Perfetti, T.A.; King, J.A. Indirect oxidative stress from pulmonary inflammation exceeds direct oxidative stress from chemical damage to mitochondria. Toxicol. Res. Appl. 2019, 3, 2397847319842845. [Google Scholar] [CrossRef]
- Zhao, F.; Li, B.; Yang, W.; Ge, T.; Cui, R. Brain–immune interaction mechanisms: Implications for cognitive dysfunction in psychiatric disorders. Cell Prolif. 2022, 55, e13295. [Google Scholar] [CrossRef] [PubMed]
- Hernansanz-Agustín, P.; Enríquez, J.A. Generation of reactive oxygen species by mitochondria. Antioxidants 2021, 10, 415. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide anion chemistry—Its role at the core of the innate immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ghosh, K.K.; Chakrabortty, S.; Gulyás, B.; Padmanabhan, P.; Ball, W.B. Mitochondrial reactive oxygen species in infection and immunity. Biomolecules 2024, 14, 670. [Google Scholar] [CrossRef]
- Han, C.; Wu, Y.; Rong, J.; Xia, Q.; Du, D. Unveiling the Emerging Role of Xanthine Oxidase in Acute Pancreatitis: Beyond Reactive Oxygen Species. Antioxidants 2025, 14, 95. [Google Scholar] [CrossRef]
- Tang, S.-P.; Mao, X.-L.; Chen, Y.-H.; Yan, L.-L.; Ye, L.-P.; Li, S.-W. Reactive oxygen species induce fatty liver and ischemia-reperfusion injury by promoting inflammation and cell death. Front. Immunol. 2022, 13, 870239. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chen, J.; Zhao, J.; Hu, S.-S.; Zhang, S.-Q.; Mi, X.-Q.; Shi, X.; Cao, X.-H.; Li, Z. Xanthohumol induces ROS through NADPH oxidase, causes cell cycle arrest and apoptosis. Oxidative Med. Cell. Longev. 2021, 2021, 9877170. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Cross-talk between NADPH oxidase and mitochondria: Role in ROS signaling and angiogenesis. Cells 2020, 9, 1849. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Hartman, J.H.; Fromenty, B. Role of mitochondrial cytochrome P450 2E1 in healthy and diseased liver. Cells 2022, 11, 288. [Google Scholar] [CrossRef]
- Gonzalez, M.; Clayton, S.; Wauson, E.; Christian, D.; Tran, Q.-K. Promotion of nitric oxide production: Mechanisms, strategies, and possibilities. Front. Physiol. 2025, 16, 1545044. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwang, J.; Park, C.; Park, R. Redox system and ROS-related disorders in peroxisomes. Free Radic. Res. 2024, 58, 662–675. [Google Scholar] [CrossRef]
- Brahma, M.K.; Gilglioni, E.H.; Zhou, L.; Trépo, E.; Chen, P.; Gurzov, E.N. Oxidative stress in obesity-associated hepatocellular carcinoma: Sources, signaling and therapeutic challenges. Oncogene 2021, 40, 5155–5167. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Berardo, C.; Maghraby, E.; Mauri, A.; Messa, L.; Esposito, L.; Casili, G.; Ottolenghi, S.; Bonaventura, E.; Cuzzocrea, S. Redox imbalance in neurological disorders in adults and children. Antioxidants 2023, 12, 965. [Google Scholar] [CrossRef]
- Shoham, S.; Pintel, N.; Avni, D. Oxidative Stress, Gut Bacteria, and Microalgae: A Holistic Approach to Manage Inflammatory Bowel Diseases. Antioxidants 2025, 14, 697. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and oxidative stress: An overview of basic concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Talapko, J.; Talapko, D.; Katalinić, D.; Kotris, I.; Erić, I.; Belić, D.; Vasilj Mihaljević, M.; Vasilj, A.; Erić, S.; Flam, J. Health Effects of Ionizing Radiation on the Human Body. Medicina 2024, 60, 653. [Google Scholar] [CrossRef]
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; Di Lisa, F.; Schulz, R.; Münzel, T. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress–Implications for cardiovascular and neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 696, 108662. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Park, J.-M.; Kim, J.-H.; Lee, M.-Y. Cigarette smoke-induced reactive oxygen species formation: A concise review. Antioxidants 2023, 12, 1732. [Google Scholar] [CrossRef]
- Teschke, R. Aluminum, arsenic, beryllium, cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum, nickel, platinum, thallium, titanium, vanadium, and zinc: Molecular aspects in experimental liver injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Song, X.; Huang, Q.; Lin, S.; Deng, S.; Qi, M.; Yang, Y.; Lu, Q.; Zhao, D. TLR4 overexpression aggravates bacterial lipopolysaccharide-induced apoptosis via excessive autophagy and NF-κB/MAPK signaling in transgenic mammal models. Cells 2023, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Youn, D.Y.; Xiaoli, A.M.; Kwon, H.; Yang, F.; Pessin, J.E. The subunit assembly state of the Mediator complex is nutrient-regulated and is dysregulated in a genetic model of insulin resistance and obesity. J. Biol. Chem. 2019, 294, 9076–9083. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Dash, U.C.; Nayak, V.; Navani, H.S.; Samal, R.R.; Agrawal, P.; Singh, A.K.; Majhi, S.; Mogare, D.G.; Duttaroy, A.K.; Jena, A.B. Understanding the molecular bridges between the drugs and immune cell. Pharmacol. Ther. 2025, 267, 108805. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Lushchak, O. Interplay between reactive oxygen and nitrogen species in living organisms. Chem.-Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef] [PubMed]
- Iova, O.-M.; Marin, G.-E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric oxide/nitric oxide synthase system in the pathogenesis of neurodegenerative disorders—An overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef]
- Casella, A.C.; Prolo, C.; Pereyra, J.; Ríos, N.; Piacenza, L.; Radi, R.; Álvarez, M.N. Superoxide, nitric oxide and peroxynitrite production by macrophages under different physiological oxygen tensions. Free Radic. Biol. Med. 2024, 212, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Lee, J.S. Advances in the Regulation of Inflammatory Mediators in Nitric Oxide Synthase: Implications for Disease Modulation and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1204. [Google Scholar] [CrossRef]
- Beigloo, F.; Khoshkar-Vandi, S.A.; Pourmand, E.; Heydari, M.; Molaabasi, F.; Gharib, N.; Zare, Y.; Rhee, K.Y.; Park, S.-J. Electrochemical micro-and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain. Nanotechnol. Rev. 2023, 12, 20230124. [Google Scholar] [CrossRef]
- Crochemore, C.; Cimmaruta, C.; Fernández-Molina, C.; Ricchetti, M. Reactive species in progeroid syndromes and aging-related processes. Antioxid. Redox Signal. 2022, 37, 208–228. [Google Scholar] [CrossRef]
- Khelfi, A. Reactive Species. In Biomarkers of Oxidative Stress: Basics and Measurement of Oxidative Stress; Springer: Berlin/Heidelberg, Germany, 2024; pp. 25–68. [Google Scholar]
- Hawkins, C.L.; Davies, M.J. Role of myeloperoxidase and oxidant formation in the extracellular environment in inflammation-induced tissue damage. Free Radic. Biol. Med. 2021, 172, 633–651. [Google Scholar] [CrossRef]
- da Fonseca, C.A.R.; Paltian, J.J.; da Motta, K.P.; Martins, C.C.v.o.; Kazmierczak, J.C.; Schumacher, R.F.; Soares, M.P.; Luchese, C.; Wilhelm, E.A. Aging is a Risk Factor for Rheumatoid Arthritis in Rats: Therapeutic Potential of 4-(Phenylselanyl)-2H-chromen-2-one. ACS Omega 2025, 10, 25990–26005. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef]
- Joshi, B.; Singh, S.; Sharma, P.; Mohapatra, T.; Kumar, P. Effect of Cigarette Smoking on Selected Antioxidant Enzymes and Oxidative Stress Biomarkers. J. Clin. Diagn. Res. 2020, 14, 19. [Google Scholar] [CrossRef]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Maciejczyk, M. The antioxidant barrier, oxidative/nitrosative stress, and protein glycation in allergy: From basic research to clinical practice. Front. Immunol. 2024, 15, 1440313. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Kononiuk, A. Nitrates/nitrites in food—Risk for nitrosative stress and benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- de Andrade Júnior, F.P.; Sobreira de Cabral, A.L.; Dantas de Araújo, J.M.; Cordeiro, L.V.; de Barros Cândido, M.; Pontes da Silva, A.; de Medeiros Lima, B.T.; Braga Dantas, B. Food nitrates and nitrites as possible causes of cancer: A review. Rev. Colomb. De Cienc. Químico-Farm. 2021, 50, 269–291. [Google Scholar] [CrossRef]
- Olas, B. The cardioprotective role of nitrate-rich vegetables. Foods 2024, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Iliadis, S.; Papanikolaou, N.A. Reactive Oxygen Species Mechanisms that Regulate Protein–Protein Interactions in Cancer. Int. J. Mol. Sci. 2024, 25, 9255. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S.; Lindner, A.B. The good, the bad, and the ugly of ROS: New insights on aging and aging-related diseases from eukaryotic and prokaryotic model organisms. Oxidative Med. Cell. Longev. 2018, 2018, 1941285. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible mechanisms of oxidative stress-induced skin cellular senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Naina Mohamed, I. Interdependence of anti-inflammatory and antioxidant properties of squalene–implication for cardiovascular health. Life 2021, 11, 103. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Song, K.; Tu, B.; Lin, L.-C.; Sun, H.; Zhou, Y.; Li, R.; Shi, Y.; Yang, J.-J.; Zhang, Y. Crosstalk between oxidative stress and epigenetic marks: New roles and therapeutic implications in cardiac fibrosis. Redox Biol. 2023, 65, 102820. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, E.; Sorrentino, C. Oxidative stress and age-related tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Keap1-Nrf2 pathway: From mechanism to medical applications. In Oxidative Stress; Elsevier: Amsterdam, The Netherlands, 2020; pp. 125–147. [Google Scholar]
- Zhang, D.D. Thirty years of NRF2: Advances and therapeutic challenges. Nat. Rev. Drug Discov. 2025, 24, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xiao, Y.; Miao, J.; Zhang, X.; Liu, M.; Zhu, L.; Liu, H.; Shen, X.; Wang, J.; Xie, B. Oxidative Stress and Inflammation: Drivers of Tumorigenesis and Therapeutic Opportunities. Antioxidants 2025, 14, 735. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 transcription factors as regulators of immune responses in cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef]
- Lai, J.-Q.; Zhao, L.-L.; Hong, C.; Zou, Q.-M.; Su, J.-X.; Li, S.-J.; Zhou, X.-F.; Li, Z.-S.; Deng, B.; Cao, J. Baicalein triggers ferroptosis in colorectal cancer cells via blocking the JAK2/STAT3/GPX4 axis. Acta Pharmacol. Sin. 2024, 45, 1715–1726. [Google Scholar] [CrossRef]
- Bae, T.; Hallis, S.P.; Kwak, M.-K. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef]
- Kim, M.E.; Kim, D.H.; Lee, J.S. FoxO transcription factors: Applicability as a novel immune cell regulators and therapeutic targets in oxidative stress-related diseases. Int. J. Mol. Sci. 2022, 23, 11877. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A.; Britton, R.S. Free radical damage and lipid peroxidation. In Hepatotoxicology; CRC Press: Boca Raton, FL, USA, 2020; pp. 401–436. [Google Scholar]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Coda, A.R.D.; Trecca, M.I.; Lo Buglio, A.; Serviddio, G.; Vendemiale, G. Redox Imbalance in Inflammation: The Interplay of Oxidative and Reductive Stress. Antioxidants 2025, 14, 656. [Google Scholar] [CrossRef]
- Al-Taie, A.; Sancar, M.; Izzettin, F.V. 8-Hydroxydeoxyguanosine: A valuable predictor of oxidative DNA damage in cancer and diabetes mellitus. In Cancer; Elsevier: Amsterdam, The Netherlands, 2021; pp. 179–187. [Google Scholar]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.S.; Cordon, B.; Ma, B.; Xie, J. Nitric oxide: Physiological functions, delivery, and biomedical applications. Adv. Sci. 2023, 10, 2303259. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Laranjinha, J. Nitric oxide pathways in neurovascular coupling under normal and stress conditions in the brain: Strategies to rescue aberrant coupling and improve cerebral blood flow. Front. Physiol. 2021, 12, 729201. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The role of reactive species on innate immunity. Vaccines 2022, 10, 1735. [Google Scholar] [CrossRef]
- Halabitska, I.; Oksenych, V.; Kamyshnyi, O. Exploring the efficacy of alpha-lipoic acid in Comorbid Osteoarthritis and Type 2 diabetes Mellitus. Nutrients 2024, 16, 3349. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Q.; Chen, F.; Pang, J.; Pan, C.; Xu, F.; Chen, Y. Fundamental mechanisms of the cell death caused by nitrosative stress. Front. Cell Dev. Biol. 2021, 9, 742483. [Google Scholar] [CrossRef]
- Prolo, C.; Piacenza, L.; Radi, R. Peroxynitrite: A multifaceted oxidizing and nitrating metabolite. Curr. Opin. Chem. Biol. 2024, 80, 102459. [Google Scholar] [CrossRef]
- Griswold-Prenner, I.; Kashyap, A.K.; Mazhar, S.; Hall, Z.W.; Fazelinia, H.; Ischiropoulos, H. Unveiling the human nitroproteome: Protein tyrosine nitration in cell signaling and cancer. J. Biol. Chem. 2023, 299, 105038. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Oh, C.-k.; Zhang, X.; Lipton, S.A. Protein S-nitrosylation and oxidation contribute to protein misfolding in neurodegeneration. Free Radic. Biol. Med. 2021, 172, 562–577. [Google Scholar] [CrossRef]
- Guil-Luna, S.; Sanchez-Montero, M.T.; Rodríguez-Ariza, A. S-Nitrosylation at the intersection of metabolism and autophagy: Implications for cancer. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 189012. [Google Scholar] [CrossRef]
- Sharma, V.; Fernando, V.; Letson, J.; Walia, Y.; Zheng, X.; Fackelman, D.; Furuta, S. S-Nitrosylation in tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 4600. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Lastra, J.M.P.d.l.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical insights into oxidative and nitrative modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemèle, E.J.; Fulton, D.J.; Fukai, T. Interplay between reactive oxygen/reactive nitrogen species and metabolism in vascular biology and disease. Antioxid. Redox Signal. 2021, 34, 1319–1354. [Google Scholar] [CrossRef]
- Jurkovicova, D.; Neophytou, C.M.; Gašparović, A.Č.; Gonçalves, A.C. DNA damage response in cancer therapy and resistance: Challenges and opportunities. Int. J. Mol. Sci. 2022, 23, 14672. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The role of oxidative stress in atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative stress and hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Shah, A.K.; Bhullar, S.K.; Elimban, V.; Dhalla, N.S. Oxidative Stress as A Mechanism for Functional Alterations in Cardiac Hypertrophy and Heart Failure. Antioxidants 2021, 10, 931. [Google Scholar] [CrossRef]
- Zheng, J.; Winderickx, J.; Franssens, V.; Liu, B. A mitochondria-associated oxidative stress perspective on Huntington’s disease. Front. Mol. Neurosci. 2018, 11, 329. [Google Scholar] [CrossRef]
- Gil-Mohapel, J.; S. Brocardo, P.; R. Christie, B. The role of oxidative stress in Huntington’s disease: Are antioxidants good therapeutic candidates? Curr. Drug Targets 2014, 15, 454–468. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.; Kolesnikova, L.a.; Kolesnikov, S. Oxidative stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Martínez-Nava, G.A.; Clavijo-Cornejo, D.; Fernández-Torres, J.; Sánchez-Sánchez, R. Rheumatoid arthritis and oxidative stress. Cell. Mol. Biol. 2022, 68, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Saunders, R.M.; Biddle, M.; Amrani, Y.; Brightling, C.E. Stressed out-The role of oxidative stress in airway smooth muscle dysfunction in asthma and COPD. Free Radic. Biol. Med. 2022, 185, 97–119. [Google Scholar] [CrossRef]
- Lewis, B.W.; Ford, M.L.; Rogers, L.K.; Britt, R.D., Jr. Oxidative stress promotes corticosteroid insensitivity in asthma and COPD. Antioxidants 2021, 10, 1335. [Google Scholar] [CrossRef]
- Nucera, F.; Mumby, S.; Paudel, K.R.; Dharwal, V.; Stefano, A.D.; Casolaro, V.; Hansbro, P.M.; Adcock, I.M.; Caramori, G. Role of oxidative stress in the pathogenesis of COPD. Minerva Medica 2022, 113, 370–404. [Google Scholar] [CrossRef] [PubMed]
- Piko, N.; Bevc, S.; Hojs, R.; Ekart, R. The role of oxidative stress in kidney injury. Antioxidants 2023, 12, 1772. [Google Scholar] [CrossRef]
- Jabeen, S.; Noor, S. Oxidative stress and chronic kidney disease. Fundam. Princ. Oxidative Stress Metab. Reprod. 2024, 151–165. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-related macular degeneration: Role of oxidative stress and blood vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Ahmed, T.; Suzumura, A.; Terasaki, H. Oxidative stress-induced cellular senescence in aging retina and age-related macular degeneration. Antioxidants 2022, 11, 2189. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative stress in non-alcoholic fatty liver disease. An updated mini review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Joffre, J.; Hellman, J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef] [PubMed]
- Harshithkumar, R.; Shah, P.; Jadaun, P.; Mukherjee, A. ROS chronicles in HIV infection: Genesis of oxidative stress, associated pathologies, and therapeutic strategies. Curr. Issues Mol. Biol. 2024, 46, 8852–8873. [Google Scholar] [CrossRef]
- Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Kvedariene, V.; Vaskovic, M.; Semyte, J.B. Role of Oxidative Stress and Antioxidants in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2025, 26, 4210. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef] [PubMed]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A comprehensive review of free radicals, oxidative stress, and antioxidants: Overview, clinical applications, global perspectives, future directions, and mechanisms of antioxidant activity of flavonoid compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Khan, A.; Gothalwal, R.; Kotnis, A. Reactive Oxygen Species: Generation and Signaling Pathways. In The Role of Reactive Oxygen Species in Human Health and Disease; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 1–42. [Google Scholar]
- Janssen, W.J.; Nozik-Grayck, E. Power of place: Intravascular superoxide dismutase for prevention of acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2017, 56, 147–149. [Google Scholar] [CrossRef]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial superoxide dismutase: What the established, the intriguing, and the novel reveal about a key cellular redox switch. Antioxid. Redox Signal. 2020, 32, 701–714. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress—A causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kelley, J.; Ye, Y.; Ye, Z.-W.; Townsend, D.M.; Zhang, J.; Wu, Y. REDOX Imbalance and Oxidative Stress in the Intervertebral Disc: The Effect of Mechanical Stress and Cigarette Smoking on ER Stress and Mitochondrial Dysfunction. Cells 2025, 14, 613. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Stolwijk, J.M.; Falls-Hubert, K.C.; Searby, C.C.; Wagner, B.A.; Buettner, G.R. Simultaneous detection of the enzyme activities of GPx1 and GPx4 guide optimization of selenium in cell biological experiments. Redox Biol. 2020, 32, 101518. [Google Scholar] [CrossRef]
- Parween, F.; Gupta, R.D. Insights into the role of paraoxonase 2 in human pathophysiology. J. Biosci. 2022, 47, 4. [Google Scholar] [CrossRef]
- Campagna, R.; Serritelli, E.N.; Salvolini, E.; Schiavoni, V.; Cecati, M.; Sartini, D.; Pozzi, V.; Emanuelli, M. Contribution of the Paraoxonase-2 Enzyme to Cancer Cell Metabolism and Phenotypes. Biomolecules 2024, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Fumarola, S.; Cecati, M.; Sartini, D.; Ferretti, G.; Milanese, G.; Galosi, A.B.; Pozzi, V.; Campagna, R.; Morresi, C.; Emanuelli, M. Bladder cancer chemosensitivity is affected by paraoxonase-2 expression. Antioxidants 2020, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Meza, H.; Vilchis-Landeros, M.M.; Vázquez-Carrada, M.; Uribe-Ramírez, D.; Matuz-Mares, D. Cellular compartmentalization, glutathione transport and its relevance in some pathologies. Antioxidants 2023, 12, 834. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Tram, N.K.; McLean, R.M.; Swindle-Reilly, K.E. Glutathione improves the antioxidant activity of vitamin C in human lens and retinal epithelial cells: Implications for vitreous substitutes. Curr. Eye Res. 2021, 46, 470–481. [Google Scholar] [CrossRef]
- Biswas, P.; Dellanoce, C.; Vezzoli, A.; Mrakic-Sposta, S.; Malnati, M.; Beretta, A.; Accinni, R. Antioxidant activity with increased endogenous levels of vitamin C, E and A following dietary supplementation with a combination of glutathione and resveratrol precursors. Nutrients 2020, 12, 3224. [Google Scholar] [CrossRef]
- Pinz, M.P.; Medeiros, I.; Carvalho, L.A.d.C.; Meotti, F.C. Is uric acid a true antioxidant? Identification of uric acid oxidation products and their biological effects. Redox Rep. 2025, 30, 2498105. [Google Scholar] [CrossRef]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and its related diseases: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; Furfaro, A.L.; Mann, G.E. Heme oxygenase dependent bilirubin generation in vascular cells: A role in preventing endothelial dysfunction in local tissue microenvironment? Front. Physiol. 2020, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Hinds, T.D.; Stec, D.E.; Tiribelli, C. The physiology of bilirubin: Health and disease equilibrium. Trends Mol. Med. 2023, 29, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Dini, I. The potential of Dietary antioxidants. Antioxidants 2021, 10, 1752. [Google Scholar] [CrossRef]
- Dini, I. Contribution of nanoscience research in antioxidants delivery used in nutricosmetic sector. Antioxidants 2022, 11, 563. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F. Coenzyme Q10: Clinical applications in cardiovascular diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, X. Mitochondrial Reactive Oxygen Species (mROS) Generation and Cancer: Emerging Nanoparticle Therapeutic Approaches. Int. J. Nanomed. 2025, 6085–6119. [Google Scholar] [CrossRef]
- Fu, M.; Yoon, K.-S.; Ha, J.; Kang, I.; Choe, W. Crosstalk between antioxidants and adipogenesis: Mechanistic pathways and their roles in metabolic health. Antioxidants 2025, 14, 203. [Google Scholar] [CrossRef]
- Zhitkovich, A. Nuclear and cytoplasmic functions of vitamin C. Chem. Res. Toxicol. 2020, 33, 2515–2526. [Google Scholar] [CrossRef]
- Briggs, M.A. From foods to chemotherapeutics: The antioxidant potential of dietary phytochemicals. Processes 2022, 10, 1222. [Google Scholar] [CrossRef]
- Niki, E. Lipid oxidation that is, and is not, inhibited by vitamin E: Consideration about physiological functions of vitamin E. Free Radic. Biol. Med. 2021, 176, 1–15. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q. Vitamin E δ-tocotrienol inhibits TNF-α-stimulated NF-κB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J. Nutr. Biochem. 2019, 64, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maboud, M.; Menshawy, A.; Menshawy, E.; Emara, A.; Alshandidy, M.; Eid, M. The efficacy of vitamin E in reducing non-alcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Ther. Adv. Gastroenterol. 2020, 13, 1756284820974917. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Blanco, A.; Amengual, J. Immunometabolic effects of β-carotene and vitamin A in atherogenesis. Immunometabolism 2024, 6, e00051. [Google Scholar] [CrossRef]
- Farhana, A.; Khan, Y.S.; Alsrhani, A.; Manni, E.; Alameen, A.A.; Derafa, W.; Alhathlaul, N.; Atif, M.; Eltayeb, L.B. Antioxidant and Prooxidant Functions of Carotenoids in Human Health: Trigger Factors, Mechanism and Application. In Recent Advances in Phytochemical Research; IntechOpen: London, UK, 2025. [Google Scholar]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M. Lycopene: Food sources, biological activities, and human health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Curiel-Regueros, A.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F. Impact of vegan and vegetarian diets on neurological health: A critical review. Nutrients 2025, 17, 884. [Google Scholar] [CrossRef]
- Nguyen, D.; Thrimawithana, T.; Piva, T.J.; Grando, D.; Huynh, T. Benefits of plant carotenoids against age-related macular degeneration. J. Funct. Foods 2023, 106, 105597. [Google Scholar] [CrossRef]
- Bekavac, N.; Krog, K.; Stanić, A.; Šamec, D.; Šalić, A.; Benković, M.; Jurina, T.; Gajdoš Kljusurić, J.; Valinger, D.; Jurinjak Tušek, A. Valorization of Food Waste: Extracting Bioactive Compounds for Sustainable Health and Environmental Solutions. Antioxidants 2025, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Jenča, A.; Mills, D.K.; Ghasemi, H.; Saberian, E.; Karimi Forood, A.M.; Petrášová, A.; Jenčová, J.; Jabbari Velisdeh, Z.; Zare-Zardini, H. Herbal therapies for cancer treatment: A review of phytotherapeutic efficacy. Biol. Targets Ther. 2024, 18, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Kupczyński, R.; Gałęska, E.; Araujo, J.P.; Czerniawska-Piątkowska, E. Clinical application of bioextracts in supporting the reproductive system of animals and humans: Potential and Limitations. Evid.-Based Complement. Altern. Med. 2022, 2022, 4766409. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Solka, M.; Jóźwik, A.; Ksepka, N.; Matin, M.; Wang, D.; Zielińska, A.; Mohanasundaram, A.; Vejux, A.; Zarrouk, A. Anthocyanins-dietary natural products with a variety of bioactivities for the promotion of human and animal health. Anim. Sci. Pap. Rep. 2024, 42, 5–33. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Zhang, Z.-Y.; Wang, R.-X. Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front. Physiol. 2020, 11, 956. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Grinán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tang, P.; Lv, H. Targeting Oxidative Stress in Diabetic Retinopathy: Mechanisms, Pathology, and Novel Treatment Approaches. Front. Immunol. 2025, 16, 1571576. [Google Scholar] [CrossRef]
- Murakami, A. Impact of hormesis to deepen our understanding of the mechanisms underlying the bioactivities of polyphenols. Curr. Opin. Biotechnol. 2024, 86, 103074. [Google Scholar] [CrossRef]
- Simon, P.; Török, É.; Szalontai, K.; Kari, B.; Neuperger, P.; Zavala, N.; Kanizsai, I.; Puskás, L.G.; Török, S.; Szebeni, G.J. Nutritional Support of Chronic Obstructive Pulmonary Disease. Nutrients 2025, 17, 1149. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From biological functions to therapeutic potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Chen, B.; Dai, R.; Xi, Z.; Xu, H. Insights into manganese superoxide dismutase and human diseases. Int. J. Mol. Sci. 2022, 23, 15893. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. Interactions between dietary antioxidants, dietary Fiber and the gut microbiome: Their putative role in inflammation and Cancer. Int. J. Mol. Sci. 2024, 25, 8250. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Ammar, A.; Kerkeni, M.; Boujelbane, M.A.; Uyar, A.M.; Köbel, L.M.; Selvaraj, S.; Zare, R.; Heinrich, K.M.; Jahrami, H. Short-Term Beetroot Juice Supplementation Enhances Strength, Reduces Fatigue, and Promotes Recovery in Physically Active Individuals: A Randomized, Double-Blind, Crossover Trial. Nutrients 2025, 17, 1720. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean diet on chronic non-communicable diseases and longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef]
- Helm, M.M.; Alaba, T.; Klimis-Zacas, D.; Izuora, K.; Basu, A. Effect of Dietary Berry Supplementation on Antioxidant Biomarkers in Adults with Cardiometabolic Risks: A Systematic Review of Clinical Trials. Antioxidants 2023, 12, 1182. [Google Scholar] [CrossRef]
- García-Cordero, J.; Martinez, A.; Blanco-Valverde, C.; Pino, A.; Puertas-Martín, V.; San Román, R.; de Pascual-Teresa, S. Regular consumption of cocoa and red berries as a strategy to improve cardiovascular biomarkers via modulation of microbiota metabolism in healthy aging adults. Nutrients 2023, 15, 2299. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Schneider, E.; Clarke, G.; Cryan, J.F. Diet and the Microbiome–Gut–Brain Axis: Feeding Your Microbes for Mental Health Benefit. In Nutritional Psychiatry: A Primer for Clinicians; Dinan, T., Ed.; Cambridge University Press: New York, NY, USA, 2023; pp. 15–38. [Google Scholar]
- Seminotti, B.; Grings, M.; Tucci, P.; Leipnitz, G.; Saso, L. Nuclear factor erythroid-2-related factor 2 signaling in the neuropathophysiology of inherited metabolic disorders. Front. Cell. Neurosci. 2021, 15, 785057. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Galaction, A.I.; Onose, G.; Turnea, M.; Rotariu, M. The Janus Face of Oxidative Stress and Hydrogen Sulfide: Insights into Neurodegenerative Disease Pathogenesis. Antioxidants 2025, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Dera, A.A.; Al Fayi, M. CB5712809, a novel Keap1 inhibitor upregulates SQSTM1/p62 mediated Nrf2 activation to induce cell death in colon cancer cells. Discov. Oncol. 2025, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-T.; Wan, C.; Lin, J.-H.; Hammes, H.-P.; Zhang, C. Mitochondrial oxidative stress and cell death in podocytopathies. Biomolecules 2022, 12, 403. [Google Scholar] [CrossRef] [PubMed]
- Fantone, S.; Marzioni, D.; Tossetta, G. NRF2/KEAP1 signaling inhibitors in gynecologic cancers. Expert Rev. Anticancer. Ther. 2024, 24, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Cellular modulators of the NRF2/KEAP1 signaling pathway in prostate cancer. Front. Biosci. 2023, 28, 143. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant metabolism pathways in vitamins, polyphenols, and selenium: Parallels and divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef]
- Hashim, N.T.; Babiker, R.; Chaitanya, N.C.; Mohammed, R.; Priya, S.P.; Padmanabhan, V.; Ahmed, A.; Dasnadi, S.P.; Islam, M.S.; Gismalla, B.G. New Insights in Natural Bioactive Compounds for Periodontal Disease: Advanced Molecular Mechanisms and Therapeutic Potential. Molecules 2025, 30, 807. [Google Scholar] [CrossRef]
- Divyajanani, S.; Harithpriya, K.; Ganesan, K.; Ramkumar, K.M. Dietary polyphenols remodel DNA methylation patterns of NRF2 in chronic disease. Nutrients 2023, 15, 3347. [Google Scholar] [CrossRef]
- Požgajová, M.; Klongová, L.; Kovár, M.; Navrátilová, A. Cell Protection by Oxidative Stress Mitigation Using Substances with Bioactive Properties. In The Power of Antioxidants-Unleashing Nature’s Defense Against Oxidative Stress; IntechOpen: London, UK, 2024. [Google Scholar]
- Kilicarslan You, D.; Fuwad, A.; Lee, K.H.; Kim, H.K.; Kang, L.; Kim, S.M.; Jeon, T.-J. Evaluation of the Protective Role of Vitamin E against ROS-Driven Lipid Oxidation in Model Cell Membranes. Antioxidants 2024, 13, 1135. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The effect of β-carotene, tocopherols and ascorbic acid as anti-oxidant molecules on human and animal in vitro/in vivo studies: A review of research design and analytical techniques used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef]
- Reed, D.J. Interaction of vitamin E, ascorbic acid, and glutathione in protection against oxidative damage. In Vitamin E in Health and Disease; CRC Press: Boca Raton, FL, USA, 2023; pp. 269–282. [Google Scholar]
- Kükürt, A.; Gelen, V. Understanding vitamin C: Comprehensive examination of its biological significance and antioxidant properties. In Ascorbic Acid-Biochemistry and Functions; IntechOpen: London, UK, 2024. [Google Scholar]
- Shen, Y.; Huang, H.; Wang, Y.; Yang, R.; Ke, X. Antioxidant effects of Se-glutathione peroxidase in alcoholic liver disease. J. Trace Elem. Med. Biol. 2022, 74, 127048. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Hofer, S.J.; Daskalaki, I.; Bergmann, M.; Friščić, J.; Zimmermann, A.; Mueller, M.I.; Abdellatif, M.; Nicastro, R.; Masser, S.; Durand, S. Spermidine is essential for fasting-mediated autophagy and longevity. Nat. Cell Biol. 2024, 26, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Sartini, D.; Campagna, R.; Lucarini, G.; Pompei, V.; Salvolini, E.; Mattioli-Belmonte, M.; Molinelli, E.; Brisigotti, V.; Campanati, A.; Bacchetti, T. Differential immunohistochemical expression of paraoxonase-2 in actinic keratosis and squamous cell carcinoma. Hum. Cell 2021, 34, 1929–1931. [Google Scholar] [CrossRef]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol treats UVB-induced photoaging by anti-MMP expression, through anti-inflammatory, antioxidant, and antiapoptotic properties, and treats photoaging by upregulating VEGF-B expression. Oxidative Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant secondary metabolites against skin photodamage: Mexican plants, a potential source of uv-radiation protectant molecules. Plants 2022, 11, 220. [Google Scholar] [CrossRef]
- Zhou, L.; Long, J.; Sun, Y.; Chen, W.; Qiu, R.; Yuan, D. Resveratrol ameliorates atherosclerosis induced by high-fat diet and LPS in ApoE−/− mice and inhibits the activation of CD4+ T cells. Nutr. Metab. 2020, 17, 41. [Google Scholar] [CrossRef]
- Chassot, L.N.; Scolaro, B.; Roschel, G.G.; Cogliati, B.; Cavalcanti, M.F.; Abdalla, D.S.; Castro, I.A. Comparison between red wine and isolated trans-resveratrol on the prevention and regression of atherosclerosis in LDLr(−/−) mice. J. Nutr. Biochem. 2018, 61, 48–55. [Google Scholar] [CrossRef]
- Phie, J.; Krishna, S.M.; Moxon, J.V.; Omer, S.M.; Kinobe, R.; Golledge, J. Flavonols reduce aortic atherosclerosis lesion area in apolipoprotein E deficient mice: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0181832. [Google Scholar] [CrossRef]
- Madokoro, Y.; Kamikokuryo, C.; Niiyama, S.; Ito, T.; Hara, S.; Ichinose, H.; Kakihana, Y. Early ascorbic acid administration prevents vascular endothelial cell damage in septic mice. Front. Pharmacol. 2022, 13, 929448. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller III, E.R. Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef]
- Mohammadipoor, N.; Shafiee, F.; Rostami, A.; Kahrizi, M.S.; Soleimanpour, H.; Ghodsi, M.; Ansari, M.J.; Bokov, D.O.; Jannat, B.; Mosharkesh, E. Resveratrol supplementation efficiently improves endothelial health: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2022, 36, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Luo, J.; Zhu, Y.; An, P.; Luo, Y.; Xing, Q. The effect of antioxidant polyphenol supplementation on cardiometabolic risk factors: A systematic review and meta-analysis. Nutrients 2024, 16, 4206. [Google Scholar] [CrossRef] [PubMed]
- Lagou, V.; Greyling, A.; Ferruzzi, M.G.; Skene, S.S.; Dubost, J.; Demirkan, A.; Prokopenko, I.; Shlisky, J.; Rodriguez-Mateos, A.; Heiss, C. Impact of flavan-3-ols on blood pressure and endothelial function in diverse populations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2025, zwaf173. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar] [CrossRef]
- Myung, S. Efficacy of vitamin and antioxidant supplements in prevention. Evid. Val. Health 2016, 2, 144–151. [Google Scholar]
- Schürks, M.; Glynn, R.J.; Rist, P.M.; Tzourio, C.; Kurth, T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ 2010, 341, c5702. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borrelli, L.; Rozkalne, A.; Hyman, B.; Bacskai, B. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Asadbegi, M.; Komaki, H.; Faraji, N.; Taheri, M.; Safari, S.; Raoufi, S.; Kourosh-Arami, M.; Golipoor, Z.; Komaki, A. Effectiveness of coenzyme Q10 on learning and memory and synaptic plasticity impairment in an aged Aβ-induced rat model of Alzheimer’s disease: A behavioral, biochemical, and electrophysiological study. Psychopharmacology 2023, 240, 951–967. [Google Scholar] [CrossRef]
- Hosseini, L.; Majdi, A.; Sadigh-Eteghad, S.; Farajdokht, F.; Ziaee, M.; Aghsan, S.R.; Farzipour, M.; Mahmoudi, J. Coenzyme Q10 ameliorates aging-induced memory deficits via modulation of apoptosis, oxidative stress, and mitophagy in aged rats. Exp. Gerontol. 2022, 168, 111950. [Google Scholar] [CrossRef]
- Komaki, H.; Faraji, N.; Komaki, A.; Shahidi, S.; Etaee, F.; Raoufi, S.; Mirzaei, F. Investigation of protective effects of coenzyme Q10 on impaired synaptic plasticity in a male rat model of Alzheimer’s disease. Brain Res. Bull. 2019, 147, 14–21. [Google Scholar] [CrossRef]
- Shults, C.W.; Oakes, D.; Kieburtz, K.; Beal, M.F.; Haas, R.; Plumb, S.; Juncos, J.L.; Nutt, J.; Shoulson, I.; Carter, J. Effects of coenzyme Q10 in early Parkinson disease: Evidence of slowing of the functional decline. Arch. Neurol. 2002, 59, 1541–1550. [Google Scholar] [CrossRef]
- Müller, T.; Büttner, T.; Gholipour, A.-F.; Kuhn, W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci. Lett. 2003, 341, 201–204. [Google Scholar] [CrossRef]
- Yoritaka, A.; Kawajiri, S.; Yamamoto, Y.; Nakahara, T.; Ando, M.; Hashimoto, K.; Nagase, M.; Saito, Y.; Hattori, N. Randomized, double-blind, placebo-controlled pilot trial of reduced coenzyme Q10 for Parkinson’s disease. Park. Relat. Disord. 2015, 21, 911–916. [Google Scholar] [CrossRef]
- Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S.; Ravina, B. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014, 71, 543–552. [Google Scholar] [PubMed]
- Farina, N.; Llewellyn, D.; Isaac, M.; Tabet, N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2017, 4, CD002854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, S.; Yu, Y.; Xu, G. (−)-Epigallocatechin-3-gallate suppresses prostate cancer cell growth via activating miR-520a-3p. Rev. Bras. De Farmacogn. 2020, 30, 528–536. [Google Scholar] [CrossRef]
- Mokhtari, H.; Yaghmaei, B.; Sirati-Sabet, M.; Jafari, N.; Mardomi, A.; Abediankenari, S.; Mahrooz, A. Epigallocatechin-3-gallate Enhances the Efficacy of MicroRNA-34a Mimic and MicroRNA-93 Inhibitor Co-transfection in Prostate Cancer Cell Line. Iran. J. Allergy Asthma Immunol. 2020, 19, 612–623. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, F.; Chen, J. Application and potential value of curcumin in prostate cancer: A meta-analysis based on animal models. Front. Pharmacol. 2024, 15, 1379389. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.; Malhotra Mukhtyar, R.; Theofanous, D.; Pepper, C.; Thomas, A.; Brown, K.; Khan, S. A systematic review assessing clinical utility of curcumin with a focus on cancer prevention. Mol. Nutr. Food Res. 2021, 65, 2000977. [Google Scholar] [CrossRef]
- Ahmad, T.; Khan, I.; Sultana, A.; Rizvi, M.M.A. A comparative study to evaluate the therapeutic effects of nutraceuticals in oral leukoplakia:-A randomized clinical trail. Natl. J. Maxillofac. Surg. 2023, 14, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.A.; Ramdas, K.; Dey, B.; Iyer, S.; Rajan, G.; Elango, K.K.; Suresh, A.; Ravindran, D.; Kumar, R.R.; Ramachandran, S. A randomized double-blind placebo-controlled phase IIB trial of curcumin in oral leukoplakia. Cancer Prev. Res. 2016, 9, 683–691. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Khosravi, M.A.; Seifert, R. Clinical trials on curcumin in relation to its bioavailability and effect on malignant diseases: Critical analysis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3477–3491. [Google Scholar] [CrossRef]
- Gutsche, L.C.; Dörfler, J.; Hübner, J. Curcumin as a complementary treatment in oncological therapy: A systematic review. Eur. J. Clin. Pharmacol. 2025, 81, 1–33. [Google Scholar] [CrossRef]
- Li, X.; Matsumoto, T.; Takuwa, M.; Saeed Ebrahim Shaiku Ali, M.; Hirabashi, T.; Kondo, H.; Fujino, H. Protective effects of astaxanthin supplementation against ultraviolet-induced photoaging in hairless mice. Biomedicines 2020, 8, 18. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef] [PubMed]
- Boyera, N.; Galey, I.; Bernard, B. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic acid (vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: Emerging combination therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapière, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef]
- Sauermann, K.; Jaspers, S.; Koop, U.; Wenck, H. Topically applied vitamin C increases the density of dermal papillae in aged human skin. BMC Dermatol. 2004, 4, 13. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-J.; Zhang, H.; Song, D.-Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.-M.; Shan, N.; Zhou, Z.-X.; Yang, P. Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Asghari, K.M.; Saleh, P.; Salekzamani, Y.; Dolatkhah, N.; Aghamohammadzadeh, N.; Hashemian, M. The effect of curcumin and high-content eicosapentaenoic acid supplementations in type 2 diabetes mellitus patients: A double-blinded randomized clinical trial. Nutr. Diabetes 2024, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Feng, B.; Tian, Z. The effect of curcumin on lipid profile and glycemic status of patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Evid. -Based Complement. Altern. Med. 2022, 2022, 8278744. [Google Scholar] [CrossRef]
- Khachik, F.; Bernstein, P.S.; Garland, D.L. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1802–1811. [Google Scholar]
- Bian, Q.; Gao, S.; Zhou, J.; Qin, J.; Taylor, A.; Johnson, E.J.; Tang, G.; Sparrow, J.R.; Gierhart, D.; Shang, F. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic. Biol. Med. 2012, 53, 1298–1307. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Domalpally, A.; Keenan, T.D.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; SanGiovanni, J.P. Long-term outcomes of adding lutein/zeaxanthin and ω-3 fatty acids to the AREDS supplements on age-related macular degeneration progression: AREDS2 report 28. JAMA Ophthalmol. 2022, 140, 692–698. [Google Scholar] [CrossRef]
- Li, S.-S.; Wang, H.-H.; Zhang, D. Efficacy of different nutrients in age-related macular degeneration: A systematic review and network meta-analysis. In Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2022; pp. 515–523. [Google Scholar]
- Group, A.-R.E.D.S.R. Lutein+ zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Ma, L.; Dou, H.-L.; Wu, Y.-Q.; Huang, Y.-M.; Huang, Y.-B.; Xu, X.-R.; Zou, Z.-Y.; Lin, X.-M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2023, 11, CD000254. [Google Scholar] [CrossRef]
- Group, A.-R.E.D.S.R.; Chew, E.Y.; SanGiovanni, J.P.; Ferris, F.L., III; Wong, W.T.; Agron, E.; Clemons, T.E.; Sperduto, R.; Danis, R.; Chandra, S.R. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013, 131, 843–850. [Google Scholar]
- Clichici, S.; Olteanu, D.; Nagy, A.-L.; Oros, A.; Filip, A.; Mircea, P.A. Silymarin inhibits the progression of fibrosis in the early stages of liver injury in CCl4-treated rats. J. Med. Food 2015, 18, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-A.; Chen, G.-M.; Liu, Y.; Chen, Y.-X.; Wu, H.-Y.; Chen, J.; Xiong, Y.-L.; Tian, C.; Wang, G.-Y.; Jia, B. Inhibitory effect of silymarin on CCl4-induced liver fibrosis by reducing Ly6Chi monocytes infiltration. Int. J. Clin. Exp. Pathol. 2017, 10, 11941. [Google Scholar]
- Gharbia, S.; Balta, C.; Herman, H.; Rosu, M.; Váradi, J.; Bácskay, I.; Vecsernyés, M.; Gyöngyösi, S.; Fenyvesi, F.; Voicu, S.N. Enhancement of silymarin anti-fibrotic effects by complexation with hydroxypropyl (HPBCD) and randomly methylated (RAMEB) β-cyclodextrins in a mouse model of liver fibrosis. Front. Pharmacol. 2018, 9, 883. [Google Scholar] [CrossRef]
- Chalasani, N.; Sanyal, A.; Kowdley, K.; Robuck, P.; Hoofnagle, J.; Kleiner, D. Pioglitazone vs. vitamin E vs. placebo for treatment of non-diabetic patients with nonalcoholic steatohepatitis (PIVENS). N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Vadarlis, A.; Antza, C.; Bakaloudi, D.R.; Doundoulakis, I.; Kalopitas, G.; Samara, M.; Dardavessis, T.; Maris, T.; Chourdakis, M. Systematic review with meta-analysis: The effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021, 36, 311–319. [Google Scholar] [CrossRef]
- Chee, N.M.Z.; Sinnanaidu, R.P.; Chan, W.K. Vitamin E improves serum markers and histology in adults with metabolic dysfunction-associated steatotic liver disease: Systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2024, 39, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, M.; Salehi, M.; Angaji, S.A.; Hashemi, M. Effects of coenzyme Q10 and diamond nanoparticles on ischemia-reperfusion-induced testicular damages in rats. Galen Med. J. 2022, 10, e2029. [Google Scholar] [CrossRef] [PubMed]
- Said, R.S.; Mohamed, H.A.; Kamal, M.M. Coenzyme Q10 mitigates ionizing radiation-induced testicular damage in rats through inhibition of oxidative stress and mitochondria-mediated apoptotic cell death. Toxicol. Appl. Pharmacol. 2019, 383, 114780. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-p.; Yang, X.-s.; Wu, T. The effect of antioxidants on sperm quality parameters and pregnancy rates for idiopathic male infertility: A network meta-analysis of randomized controlled trials. Front. Endocrinol. 2022, 13, 810242. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Qu, H.; Cao, Y.; Zhu, J.; Zhang, S.-z.; Wu, J.; Jiao, Y.-z. Effect of antioxidants on sperm quality parameters in subfertile men: A systematic review and network meta-analysis of randomized controlled trials. Adv. Nutr. 2022, 13, 586–594. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020, 8, CD007807. [Google Scholar] [CrossRef]
- Ntumy, M.; Maya, E.; Lizneva, D.; Adanu, R.; Azziz, R. The pressing need for standardization in epidemiologic studies of PCOS across the globe. Gynecol. Endocrinol. 2019, 35, 1–3. [Google Scholar] [CrossRef]
- Caro, A.A.; Bell, M.; Ejiofor, S.; Zurcher, G.; Petersen, D.R.; Ronis, M.J. N-acetylcysteine inhibits the up-regulation of mitochondrial biogenesis genes in livers from rats fed ethanol chronically. Alcohol. Clin. Exp. Res. 2014, 38, 2896–2906. [Google Scholar] [CrossRef]
- Kim, K.; Cort, T.A.; Kunz, E.M.; Moerschel, J.; Palzkill, V.R.; Dong, G.; Moparthy, C.N.; Anderson, E.M.; Fazzone, B.; O’Malley, K.A. N-acetylcysteine treatment attenuates hemodialysis access-related limb pathophysiology in mice with chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2023, 325, F271–F282. [Google Scholar] [CrossRef]
- Bolt, J.; Sandhu, S.; Mohammadi, A. Effect of coenzyme Q10 supplementation on sarcopenia, frailty, and falls: A scoping review. J. Nutr. Health Aging 2023, 27, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.; Laymon, M.; Khowailed, I.; Lee, H. Synergistic effects of continuous low level heat wraps and vitamins in improving balance and gait in adults. Int. J. Vitam. Nutr. Res. 2016, 86, 152–160. [Google Scholar] [CrossRef]
- Ganguly, A. Role of Jumpstart Nutrition®, a dietary supplement, to ameliorate calcium-to-Phosphorus ratio and parathyroid hormone of patients with osteoarthritis. Med. Sci. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; Holloway, C.; McArdle, F.; MacLaren, D.P. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br. J. Nutr. 2006, 95, 976–981. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G.; Lowe, T.E. Effects of dietary antioxidants on training and performance in female runners. Eur. J. Sport Sci. 2014, 14, 160–168. [Google Scholar] [CrossRef]
- Bryant, R.J.; Ryder, J.; Martino, P.; Kim, J.; Craig, B.W. Effects of vitamin E and C supplementation either alone or in combination on exercise-induced lipid peroxidation in trained cyclists. J. Strength Cond. Res. 2003, 17, 792–800. [Google Scholar]
- Paulsen, G.; Cumming, K.T.; Hamarsland, H.; Børsheim, E.; Berntsen, S.; Raastad, T. Can supplementation with vitamin C and E alter physiological adaptations to strength training? BMC Sports Sci. Med. Rehabil. 2014, 6, 28. [Google Scholar] [CrossRef]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Biensø, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; McAnulty, S.R.; McAnulty, L.S.; Morrow, J.D.; Ahmed, A.; Heward, C.B. Vitamin E and immunity after the Kona Triathlon World Championship. Med. Sci. Sports Exerc. 2004, 36, 1328–1335. [Google Scholar] [CrossRef]
- Laaksonen, R.; Fogelholm, M.; Himberg, J.-J.; Laakso, J.; Salorinne, Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 72, 95–100. [Google Scholar] [CrossRef]
- Parker, A.; Cuddihy, S.L.; Son, T.G.; Vissers, M.C.; Winterbourn, C.C. Roles of superoxide and myeloperoxidase in ascorbate oxidation in stimulated neutrophils and H2O2-treated HL60 cells. Free Radic. Biol. Med. 2011, 51, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, X.; Zhang, K.; Ye, Q. The effect of intravenous vitamin C on clinical outcomes in patients with sepsis or septic shock: A meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 964484. [Google Scholar] [CrossRef]
- Alissa, A.; Alrashed, M.A.; Alshaya, A.I.; Al Sulaiman, K.; Alharbi, S. Reevaluating vitamin C in sepsis and septic shock: A potential benefit in severe cases? Front. Med. 2024, 11, 1476242. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chiu, C.-T.; Lee, H.-S.; Lai, C.-C. The impact of vitamin C-containing treatment on the mortality of patients with sepsis: A systematic review and meta-analysis of randomized controlled trials. J. Infect. Public Health 2022, 15, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Miura, M.; Yanai, S.; Nishimune, H. Coenzyme Q10 supplementation improves the motor function of middle-aged mice by restoring the neuronal activity of the motor cortex. Sci. Rep. 2023, 13, 4323. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, N.; Hussain, H.K.; Rehman, Z.; Sabir, A.; Ashraf, W.; Ahmad, T.; Alqahtani, F.; Imran, I. Co-administration of coenzyme Q10 and curcumin mitigates cognitive deficits and exerts neuroprotective effects in aluminum chloride-induced Alzheimer’s disease in aged mice. Exp. Gerontol. 2025, 199, 112659. [Google Scholar] [CrossRef]
- Ahmadi-Soleimani, S.M.; Ghasemi, S.; Rahmani, M.A.; Gharaei, M.; Mohammadi Bezanaj, M.; Beheshti, F. Oral administration of coenzyme Q10 ameliorates memory impairment induced by nicotine-ethanol abstinence through restoration of biochemical changes in male rat hippocampal tissues. Sci. Rep. 2024, 14, 11413. [Google Scholar] [CrossRef]
- Rodríguez-Bies, E.; Tung, B.T.; Navas, P.; López-Lluch, G. Resveratrol primes the effects of physical activity in old mice. Br. J. Nutr. 2016, 116, 979–988. [Google Scholar] [CrossRef]
- Alehagen, U.; Alexander, J.; Aaseth, J. Supplementation with selenium and coenzyme Q10 reduces cardiovascular mortality in elderly with low selenium status. A secondary analysis of a randomised clinical trial. PLoS ONE 2016, 11, e0157541. [Google Scholar] [CrossRef]
- Dunning, B.J.; Bourgonje, A.R.; Bulthuis, M.L.; Alexander, J.; Aaseth, J.O.; Larsson, A.; van Goor, H.; Alehagen, U. Selenium and coenzyme Q10 improve the systemic redox status while reducing cardiovascular mortality in elderly population-based individuals. Free Radic. Biol. Med. 2023, 204, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Johansson, P.; Larsson, A. Supplemental selenium and coenzyme Q10 reduce glycation along with cardiovascular mortality in an elderly population with low selenium status–A four-year, prospective, randomised, double-blind placebo-controlled trial. J. Trace Elem. Med. Biol. 2020, 61, 126541. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Aaseth, J.O.; Schomburg, L.; Opstad, T.B.; Larsson, A.; Alexander, J. Selenium and Coenzyme Q10 Supplementation and Sex Differences in Cardiovascular Mortality Results from a Prospective Randomized Double-Blind Placebo-Controlled Trial in Elderly People Low in Selenium. Antioxidants 2025, 14, 685. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Kuscuoglu, N.; Wilson, J.; McCaffrey, G.; Stafford, G.; Chen, G.; Lantz, R.C.; Jolad, S.D. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2006, 54, 3452–3464. [Google Scholar] [CrossRef]
- Zhong, Y.; Tu, Y.; Ma, Q.; Chen, L.; Zhang, W.; Lu, X.; Yang, S.; Wang, Z.; Zhang, L. Corrigendum: Curcumin alleviates experimental colitis in mice by suppressing necroptosis of intestinal epithelial cells. Front. Pharmacol. 2023, 14, 1240661. [Google Scholar] [CrossRef]
- Yue, W.; Liu, Y.; Li, X.; Lv, L.; Huang, J.; Liu, J. Curcumin ameliorates dextran sulfate sodium-induced colitis in mice via regulation of autophagy and intestinal immunity. Turk. J. Gastroenterol. 2019, 30, 290. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and safety of curcumin and curcuma longa extract in the treatment of arthritis: A systematic review and meta-analysis of randomized controlled trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef]
- Li, C.; Chen, X.; Yao, J.; Zha, W.; Li, M.; Shen, J.; Jiang, H.; Tian, P. Curcumin modulated gut microbiota and alleviated renal fibrosis in 5/6 nephrectomy-induced chronic kidney disease rats. PLoS One 2025, 20, e0314029. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lang, X.; Cheng, D.; Yang, Z. Curcumin ameliorates chronic renal failure in 5/6 nephrectomized rats by regulation of the mTOR/HIF-1α/VEGF signaling pathway. Biol. Pharm. Bull. 2019, 42, 886–891. [Google Scholar] [CrossRef]
- Jun, M.; Venkataraman, V.; Razavian, M.; Cooper, B.; Zoungas, S.; Ninomiya, T.; Webster, A.C.; Perkovic, V. Antioxidants for chronic kidney disease. Cochrane Database Syst. Rev. 2012, 10, CD008176. [Google Scholar] [CrossRef]
- Chiu, A.-H.; Wang, C.-J.; Lin, Y.-L.; Wang, C.-L.; Chiang, T.-I. N-Acetylcysteine Alleviates the Progression of Chronic Kidney Disease: A Three-Year Cohort Study. Medicina 2023, 59, 1983. [Google Scholar] [CrossRef]
- Ye, M.; Lin, W.; Zheng, J.; Lin, S. N-acetylcysteine for chronic kidney disease: A systematic review and meta-analysis. Am. J. Transl. Res. 2021, 13, 2472. [Google Scholar] [PubMed]
- Ahmadi, F.; Abbaszadeh, M.; Razeghi, E.; Maziar, S.; Khoidaki, S.D.; Najafi, M.T.; Lessan-Pezeshki, M. Effectiveness of N-acetylcysteine for preserving residual renal function in patients undergoing maintenance hemodialysis: Multicenter randomized clinical trial. Clin. Exp. Nephrol. 2017, 21, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Colombijn, J.M.; Hooft, L.; Jun, M.; Webster, A.C.; Bots, M.L.; Verhaar, M.C.; Vernooij, R.W. Antioxidants for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2023, 11, CD008176. [Google Scholar] [CrossRef]
- Xu, L.; Cai, B.-q.; Zhu, Y.-J. Pathogenesis of cigarette smoke-induced chronic obstructive pulmonary disease and therapeutic effects of glucocorticoids and N-acetylcysteine in rats. Chin. Med. J. 2004, 117, 1611–1619. [Google Scholar]
- Cai, S.; Chen, P.; Zhang, C.; CHEN, J.B.; Wu, J. Oral N-acetylcysteine attenuates pulmonary emphysema and alveolar septal cell apoptosis in smoking-induced COPD in rats. Respirology 2009, 14, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wiegman, C.; Seiffert, J.M.; Zhu, J.; Clarke, C.; Chang, Y.; Bhavsar, P.; Adcock, I.; Zhang, J.; Zhou, X. Effects of N-acetylcysteine in ozone-induced chronic obstructive pulmonary disease model. PLoS ONE 2013, 8, e80782. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, F.; Kang, X.; Zhou, J.; Yao, Q.; Lin, Y.; Zhang, W. The antioxidant N-acetylcysteine promotes immune response and inhibits epithelial-mesenchymal transition to alleviate pulmonary fibrosis in chronic obstructive pulmonary disease by suppressing the VWF/p38 MAPK axis. Mol. Med. 2021, 27, 97. [Google Scholar] [CrossRef]
- Jiang, C.; Zou, J.; Lv, Q.; Yang, Y. Systematic review and meta-analysis of the efficacy of N-acetylcysteine in the treatment of acute exacerbation of chronic obstructive pulmonary disease. Ann. Palliat. Med. 2021, 10, 6564576–6566576. [Google Scholar] [CrossRef]
- Huang, C.; Kuo, S.; Lin, L.; Yang, Y. The efficacy of N-acetylcysteine in chronic obstructive pulmonary disease patients: A meta-analysis. Ther. Adv. Respir. Dis. 2023, 17, 17534666231158563. [Google Scholar] [CrossRef]
- De Backer, J.; Vos, W.; Van Holsbeke, C.; Vinchurkar, S.; Claes, R.; Parizel, P.M.; De Backer, W. Effect of high-dose N-acetylcysteine on airway geometry, inflammation, and oxidative stress in COPD patients. Int. J. Chronic Obstr. Pulm. Dis. 2013, 8, 569–579. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Geng, R.; Qiu, J.; He, X. Curcumin alleviates dextran sulfate sodium-induced colitis in mice through regulating gut microbiota. Mol. Nutr. Food Res. 2022, 66, 2100943. [Google Scholar] [CrossRef]
- Lang, A.; Salomon, N.; Wu, J.C.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.; Cheong, P.K.; Avidan, B.; Gamus, D. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449.e1441. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef]
- Shen, C.-L.; Yeh, J.K.; Cao, J.J.; Tatum, O.L.; Dagda, R.Y.; Wang, J.-S. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation-induced bone loss model. J. Nutr. Biochem. 2010, 21, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Smith, B.J.; Li, J.; Cao, J.J.; Song, X.; Newhardt, M.F.; Corry, K.A.; Tomison, M.D.; Tang, L.; Wang, J.-S. Effect of long-term green tea polyphenol supplementation on bone architecture, turnover, and mechanical properties in middle-aged ovariectomized rats. Calcif. Tissue Int. 2019, 104, 285–300. [Google Scholar] [CrossRef]
- Besora-Moreno, M.; Llauradó, E.; Valls, R.M.; Tarro, L.; Pedret, A.; Solà, R. Antioxidant-rich foods, antioxidant supplements, and sarcopenia in old-young adults ≥ 55 years old: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin. Nutr. 2022, 41, 2308–2324. [Google Scholar] [CrossRef] [PubMed]
- Nejadhosseinian, M.; Djalalinia, S.; Haerian, H.; Alikhani, M.; Mansour, A.; Mousavian, A.-H.; Mardani-Fard, H.A.; Kasaeian, A.; Faezi, S.T. The effects of antioxidants on knee osteoarthritis: A systematic review and meta-analysis. Front. Nutr. 2022, 9, 1026450. [Google Scholar] [CrossRef]
- Karimi, S.M.; Abbaspour, F.; Tabatabaei-Malazy, O.; Qolami, H.; Fayyaz, F.; Fana, S.E.; Rahimi, R.; Salari, P.; Larijani, B. Impact of Medicinal plants on Bone Health; a systematic review and meta-analysis of clinical studies. J. Agric. Food Res. 2024, 101545. [Google Scholar] [CrossRef]
- Austermann, K.; Baecker, N.; Zwart, S.R.; Fimmers, R.; Frippiat, J.-P.; Stehle, P.; Smith, S.M.; Heer, M. Antioxidant supplementation does not affect bone turnover markers during 60 days of 6 head-down tilt bed rest: Results from an exploratory randomized controlled trial. J. Nutr. 2021, 151, 1527–1538. [Google Scholar] [CrossRef]
- Li, Y.; Qi, H.; Huang, X.; Lu, G.; Pan, H. Exogenous and endogenous antioxidants in osteoporosis risk: Causal associations unveiled by Mendelian Randomization analysis. Front. Physiol. 2024, 15, 1411148. [Google Scholar] [CrossRef] [PubMed]
- Ashrafpour, S.; Ashrafpour, M. The double-edged sword of nutraceuticals: Comprehensive review of protective agents and their hidden risks. Front. Nutr. 2025, 12, 1524627. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. On the effect of vitamin C intake on human health: How to (mis) interprete the clinical evidence. Redox Biol. 2020, 34, 101532. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent advances in the conjugation approaches for enhancing the bioavailability of polyphenols. Food Hydrocoll. 2024, 146, 109221. [Google Scholar] [CrossRef]
- Owen, K.N.; Dewald, O. Vitamin E Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Bano, I.; Hassan, M.F.; Kieliszek, M. A Comprehensive Review of Selenium as a Key Regulator in Thyroid Health. Biol. Trace Elem. Res. 2025, 1–15. [Google Scholar] [CrossRef]
- Lian, W.; Yang, X.; Duan, Q.; Li, J.; Zhao, Y.; Yu, C.; He, T.; Sun, T.; Zhao, Y.; Wang, W. The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules 2024, 29, 2516. [Google Scholar] [CrossRef]
- Hasnain, B.I.; Mooradian, A.D. Recent trials of antioxidant therapy: What should we be telling our patients? Clevel. Clin. J. Med. 2004, 71, 327–334. [Google Scholar] [CrossRef]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008, 101, S14–S19. [Google Scholar] [CrossRef]
- Cook, N.R.; Albert, C.M.; Gaziano, J.M.; Zaharris, E.; MacFadyen, J.; Danielson, E.; Buring, J.E.; Manson, J.E. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the Women’s Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007, 167, 1610–1618. [Google Scholar] [CrossRef]
- Shahidi, F.; Pan, Y. Influence of food matrix and food processing on the chemical interaction and bioaccessibility of dietary phytochemicals: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6421–6445. [Google Scholar] [CrossRef] [PubMed]
- Middha, P.; Weinstein, S.J.; Männistö, S.; Albanes, D.; Mondul, A.M. β-carotene supplementation and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: The role of tar and nicotine. Nicotine Tob. Res. 2019, 21, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Woldeselassie, M.; Tamene, A. Therapeutic controversies over use of antioxidant supplements during cancer treatment: A scoping review. Front. Nutr. 2024, 11, 1480780. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Abrahamse, H. Redox potential of antioxidants in cancer progression and prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef]
- Calvani, M.; Pasha, A.; Favre, C. Nutraceutical boom in cancer: Inside the labyrinth of reactive oxygen species. Int. J. Mol. Sci. 2020, 21, 1936. [Google Scholar] [CrossRef]
- Talib, W.H.; Ahmed Jum’AH, D.A.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of vitamins A, C, D, E in cancer prevention and therapy: Therapeutic potentials and mechanisms of action. Front. Nutr. 2024, 10, 1281879. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Q.; Lu, X.; Li, W. Post-diagnosis use of antioxidant vitamin supplements and breast cancer prognosis: A systematic review and meta-analysis. Clin. Breast Cancer 2021, 21, 477–485. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Kordiak, J.; Bielec, F.; Jabłoński, S.; Pastuszak-Lewandoska, D. Role of beta-carotene in lung cancer primary chemoprevention: A systematic review with meta-analysis and meta-regression. Nutrients 2022, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef]
- Li, S.; Fasipe, B.; Laher, I. Potential harms of supplementation with high doses of antioxidants in athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, H.; Slabu, I.; Liehn, E.A.; Rusu, M. Vitamin C in cardiovascular disease: From molecular mechanisms to clinical evidence and therapeutic applications. Antioxidants 2025, 14, 506. [Google Scholar] [CrossRef]
- Yao, J.; Dai, X.; Yv, X.; Zheng, L.; Zheng, J.; Kuang, B.; Teng, W.; Yu, W.; Li, M.; Cao, H. The role of potential oxidative biomarkers in the Prognosis of Intracerebral Hemorrhage and the exploration antioxidants as possible Preventive and Treatment options. Front. Mol. Biosci. 2025, 12, 1541230. [Google Scholar] [CrossRef]
- Shen, Y.-J.; Huang, Y.-C.; Cheng, Y.-C. Advancements in Antioxidant-Based Therapeutics for Spinal Cord Injury: A Critical Review of Strategies and Combination Approaches. Antioxidants 2024, 14, 17. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Battaglini, M.; Marino, A.; Ciofani, G. Antioxidants and nanotechnology: Promises and limits of potentially disruptive approaches in the treatment of central nervous system diseases. Adv. Healthc. Mater. 2020, 9, 1901589. [Google Scholar] [CrossRef]
- Ammar, H.O.; Shamma, R.N.; Elbatanony, R.S.; Khater, B. Antioxidants in cancer therapy: Recent trends in application of nanotechnology for enhanced delivery. Sci. Pharm. 2020, 88, 5. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Dell’Agli, M. Anti-Inflammatory Activity of Plant Polyphenols 2.0. Biomedicines 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Palanisamy, C.P.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Srinivasan, G.P.; Panagal, M.; Jayaraman, S. Curcumin-loaded polymeric nanomaterials as a novel therapeutic strategy for Alzheimer’s disease: A comprehensive review. Ageing Res. Rev. 2024, 99, 102393. [Google Scholar] [CrossRef]
- Bateni, Z.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Goudarzi, R.; Sohrab, G. The effects of nano-curcumin supplementation on glycemic control, blood pressure, lipid profile, and insulin resistance in patients with the metabolic syndrome: A randomized, double-blind clinical trial. Phytother. Res. 2021, 35, 3945–3953. [Google Scholar] [CrossRef]
- Maniam, G.; Mai, C.-W.; Zulkefeli, M.; Dufès, C.; Tan, D.M.-Y.; Fu, J.-Y. Challenges and opportunities of nanotechnology as delivery platform for tocotrienols in cancer therapy. Front. Pharmacol. 2018, 9, 1358. [Google Scholar] [CrossRef]
- Chavda, V.P.; Nalla, L.V.; Balar, P.; Bezbaruah, R.; Apostolopoulos, V.; Singla, R.K.; Khadela, A.; Vora, L.; Uversky, V.N. Advanced phytochemical-based nanocarrier systems for the treatment of breast cancer. Cancers 2023, 15, 1023. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Fleming, E.; Luo, Y. Co-delivery of synergistic antioxidants from food sources for the prevention of oxidative stress. J. Agric. Food Res. 2021, 3, 100107. [Google Scholar] [CrossRef]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic efficacy: A systematic review of the evidence from randomized controlled trials. Cancer Treat. Rev. 2007, 33, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Sepidarkish, M.; Akbari-Fakhrabadi, M.; Daneshzad, E.; Yavari, M.; Rezaeinejad, M.; Morvaridzadeh, M.; Heshmati, J. Effect of omega-3 fatty acid plus vitamin E Co-Supplementation on oxidative stress parameters: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Boaru, D.L.; Fraile-Martinez, O.; De Leon-Oliva, D.; Garcia-Montero, C.; De Castro-Martinez, P.; Miranda-Gonzalez, A.; Saez, M.A.; Muñon-Zamarron, L.; Castillo-Ruiz, E.; Barrena-Blázquez, S. Harnessing the Anti-Inflammatory Properties of Polyphenols in the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Sci. 2024, 20, 5608. [Google Scholar] [CrossRef]
- Gao, X.; Feng, X.; Hou, T.; Huang, W.; Ma, Z.; Zhang, D. The roles of flavonoids in the treatment of inflammatory bowel disease and extraintestinal manifestations: A review. Food Biosci. 2024, 62, 105431. [Google Scholar] [CrossRef]
- Nikic, P.; Dragicevic, D.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Coric, V.; Jovanovic, D.; Bumbasirevic, U.; Pekmezovic, T.; Simic, T.; Dzamic, Z. Association between GPX1 and SOD2 genetic polymorphisms and overall survival in patients with metastatic urothelial bladder cancer: A single-center study in Serbia. J. BUON 2018, 23, 1130–1135. [Google Scholar]
- Nikic, P.; Dragicevic, D.; Jerotic, D.; Savic, S.; Djukic, T.; Stankovic, B.; Kovacevic, L.; Simic, T.; Matic, M. Polymorphisms of antioxidant enzymes SOD2 (rs4880) and GPX1 (rs1050450) are associated with bladder cancer risk or its aggressiveness. Medicina 2023, 59, 131. [Google Scholar] [CrossRef]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Looi, D.; Moorthy, M.; Chaiyakunapruk, N.; Palanisamy, U.D. Impact of ellagitannin-rich fruit consumption on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2022, 99, 105320. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.-S. The impact of ellagitannins and their metabolites through gut microbiome on the gut health and brain wellness within the gut–brain axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Tragni, V.; Primiano, G.; Tummolo, A.; Cafferati Beltrame, L.; La Piana, G.; Sgobba, M.N.; Cavalluzzi, M.M.; Paterno, G.; Gorgoglione, R.; Volpicella, M. Personalized medicine in mitochondrial health and disease: Molecular basis of therapeutic approaches based on nutritional supplements and their analogs. Molecules 2022, 27, 3494. [Google Scholar] [CrossRef] [PubMed]
- Tretter, V.; Hochreiter, B.; Zach, M.L.; Krenn, K.; Klein, K.U. Understanding Cellular Redox Homeostasis: A Challenge for Precision Medicine. Int. J. Mol. Sci. 2022, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Lee, C.M.; Saunders, D.; Curtis, A.E.; Dunlap, N.E.; Nangia, C.; Lee, A.S.; Kovoor, P.; Bar-Ad, V.; Pedadda, A.V., Jr. Two-year tumor outcomes of a phase 2B, randomized, double-blind trial of avasopasem manganese (GC4419) versus placebo to reduce severe oral mucositis owing to concurrent radiation therapy and cisplatin for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 416–421. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of turmeric and curcumin in prevention and treatment of chronic diseases: Lessons learned from clinical trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Kil, J.; Lobarinas, E.; Spankovich, C.; Griffiths, S.K.; Antonelli, P.J.; Lynch, E.D.; Le Prell, C.G. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017, 390, 969–979. [Google Scholar] [CrossRef]
- Sharpley, A.L.; Williams, C.; Holder, A.A.; Godlewska, B.R.; Singh, N.; Shanyinde, M.; MacDonald, O.; Cowen, P.J. A phase 2a randomised, double-blind, placebo-controlled, parallel-group, add-on clinical trial of ebselen (SPI-1005) as a novel treatment for mania or hypomania. Psychopharmacology 2020, 237, 3773–3782. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Dong, C.; Zhao, Y.; Zhou, J.; Yuan, C.; Zou, L. Mechanisms of ebselen as a therapeutic and its pharmacology applications. Future Med. Chem. 2020, 12, 2141–2160. [Google Scholar] [CrossRef]
- Zhou, S.; Cai, H.; He, X.; Tang, Z.; Lu, S. Enzyme-mimetic antioxidant nanomaterials for ROS scavenging: Design, classification, and biological applications. Coord. Chem. Rev. 2024, 500, 215536. [Google Scholar] [CrossRef]
- Zhang, S. Current development on wearable biosensors towards biomedical applications. J. Agric. Food Res. 2023, 11, 1264337. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Fan, R.; Zhang, Y.; Jia, Z.; Zhang, J.; Pan, H.; Wang, Q. Oxidative stress in the brain–lung crosstalk: Cellular and molecular perspectives. Front. Aging Neurosci. 2024, 16, 1389454. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Selles, A.J.; Nuñez-Musa, R.A.; Guillen-Marmolejos, R.A. Linking oxidative stress biomarkers to disease progression and antioxidant therapy in hypertension and diabetes mellitus. Front. Mol. Biosci. 2025, 12, 1611842. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).