To obtain multitarget compounds, a logical sequence was followed for analyzing basic features, designing, and screening the ligands with the best profiles. The workflow began with the QSAR model and proceeded to molecular docking analyses. Next, new ligands were designed by incorporating the modifications suggested by the model and their interactions with the three receptors. These ligands were screened based on both their molecular interaction profiles and the biological activity predicted by the QSAR model. The top-performing ligands were then evaluated through 100 ns molecular dynamics simulations and ADME/Tox studies.
2.5. Combining Molecular Docking with QSAR to Design New Anticancer Molecules
After completing the QSAR analysis, we decided to take a closer look at how our designed compounds might interact with real biological targets. To do this, we carried out molecular docking studies against three key proteins involved in hormone-related cancers: the progesterone receptor, the HER2 tyrosine kinase domain, and estrogen receptor-α. These proteins were chosen because of their well-established roles in cancer progression and treatment. As a benchmark, we used three known antagonists—Mifepristone, Lapatinib, and Raloxifene—which are clinically validated inhibitors for each respective target.
By combining QSAR modeling with molecular docking, we were able to bridge the gap between statistical prediction and structural insight. QSAR helped us identify which molecular features are most likely to contribute to anticancer activity, while docking allowed us to visualize how these compounds might bind to their targets. This combination not only strengthens the reliability of our predictions but also gives us a clearer picture of the types of interactions—like hydrogen bonds or hydrophobic contacts—that are essential for strong and selective binding.
This integrated approach has been gaining traction in drug discovery because it offers a more efficient and informed way to design new molecules. Instead of relying solely on trial-and-error or high-throughput screening, we can now prioritize compounds with a higher chance of success based on both their predicted activity and their structural compatibility with the target. Recent studies have shown that this strategy can significantly improve hit rates and lead optimization in anticancer drug development [
34,
35].
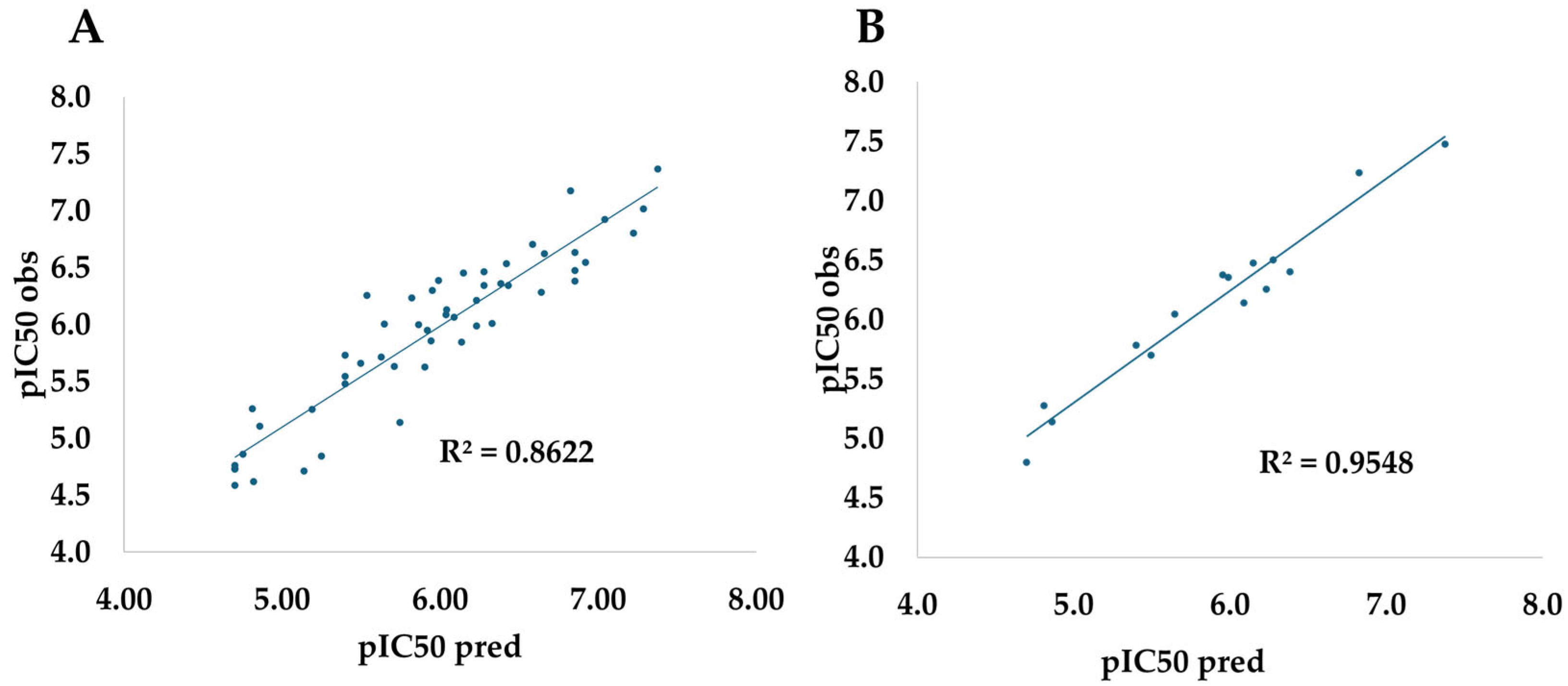
For this reason, to jointly assure the reproducibility of these methods, a redocking protocol was carried out using the ligands against the target, respectively. The graphic results are shown in
Figure 2. As revealed in this figure, the redocking protocol can reproduce closely the experimental values, as shown for the small rmsd in each case (<2 Å). Once the redocking protocol is validated, all data were docked against three targets and compared with the inhibitor compounds. The results are shown in
Table 4. Compounds with equal or higher values were considered as the baseline for further modifications.
The molecular docking results provide a valuable layer of validation for the QSAR model, offering a structural explanation for the electronic and topological descriptors that were statistically linked to anticancer activity. By analyzing the interactions between the designed steroidal compounds and the three selected targets (summarized in
Table 5 and shown in
Figure 2 and
Figure 3), we observed a consistent pattern of hydrophobic and π-type interactions, particularly with residues such as leucine, methionine, and phenylalanine. These findings align closely with the QSAR descriptors C and H, which were positively correlated with activity and are associated with molecular features that enhance polarizability and electronic adaptability.
The presence of van der Waals, alkyl, and π–alkyl interactions supports the inclusion of hydrocarbon rings and alkyl chains, structural elements that contribute to the electronic descriptors captured by the QuBiLS-MAS model. Similarly, the observed π–π stacking and π–sigma interactions reinforce the importance of aromatic or unsaturated systems, which not only stabilize the ligand–receptor complex but also reflect the electronic delocalization patterns that the QSAR model identified as favorable.
Moreover, the docking studies highlighted the role of hydrogen bonding and salt bridge formation, particularly with polar and charged residues like aspartate and arginine. These interactions are consistent with the positive contribution of descriptor E (number of hydrogen bond donors) in the QSAR equation, confirming that functional groups capable of forming such bonds are critical for strong and specific binding.
Interestingly, interactions such as π–sulfur and halogen bonding, observed in complexes involving sulfur-containing or halogenated substituents, further validate the inclusion of these groups as a strategy to enhance binding affinity and specificity. In fact, their role in improving ligand–receptor interactions has been well documented before [
36]. These features are not only chemically intuitive but also statistically supported by the QSAR model, which penalized descriptors associated with rigid or sterically hindered structures (A, B, D, F, G).
All these analyses derived from the obtained model were considered in the design of new steroidal molecules, aiming to enhance their biological activity. Various structures were designed by introducing functional groups according to the guidelines established by the QSAR model. Additionally, these designs incorporated molecular fragments that enhance the interactions identified in the molecular docking studies conducted with inhibitors of the Progesterone-activated transcription factor nuclear receptor, Estrogen-activated transcription factor nuclear receptor, and tyrosine protein kinase erbB-2 receptor, which are key targets in the progression of breast cancer.
A total of 266 compounds were designed and subsequently optimized at the DFT level using the LanL2DZ basis set and the WB97XD functional (
Supplementary Material Table S2). These compounds were then evaluated using the QSAR model, leading to the selection of compounds with the highest predicted biological activity. These compounds were further analyzed through molecular docking simulations with the selected target proteins. A matrix was generated based on interaction energy values, and machine learning techniques were applied to refine the selection, (
Supplementary Material S3) ultimately identifying the seven most promising compounds (
Supplementary Material Figures S1–S3). Docking scores are reported against three breast cancer targets: progesterone receptor (PR, PDB 2W8Y), HER2 tyrosine kinase domain (PDB 7JXH), and estrogen-receptor-α ligand-binding domain (PDB 6VJD), together with pIC
50 values predicted by the QSAR model (pIC
50 = −log
10 IC
50, M). The dataset comprises the reference compound RM-581, two clinically used steroidal inhibitors (Exemestane and Formestane), and the seven designed ligands (Estero-253 to Estero-268) that achieved the highest docking scores and the best QSAR-predicted biological activity (
Table 6). The molecular 2D structures are shown below in
Figure 4.
2.6. ADME-TOX Properties
To assess the drug-likeness properties as well as the safety of the predicted compounds, the SMILES codes for the seven top-ranked designed compounds were used to determine their ADME-Tox values. For this goal, ProTox 3.0 software was used
https://tox.charite.de/protox3/, accessed 5 June 2025), and the results are shown in
Table 7. The in silico assessment of the steroid candidate analogs (
Table 8): E-253, E-254, E-255, E-261, E-264, E-265 and E-268, confirms that, aside from the occasional minor deviation, they all satisfy the classical drug-likeness criteria: molecular weights range from 441 to 476 g mol
−1, each molecule contains at most four rotatable bonds and no more than four hydrogen-bond acceptors. Lipophilicity spans from Log
p = 4.8 for E-264 to 6.1 for E-253 and E-261, a gradient that is mirrored in solubility; indeed, E-264 offers the best compromise (Log S = −5.50), whereas E-253 and E-261 show the lowest solubilities (≈−6.97). Five of the seven compounds (E-254, E-255, E-264, E-265, and E-268) are predicted to have high gastrointestinal absorption and thus emerge as promising oral candidates, in contrast to E-253 and E-261, whose high log
p values are associated with slightly limited absorption and therefore call for specialized formulation strategies.
From a toxicological standpoint, compounds E-253, E-255, and E-261 tested positive in the Ames assay, indicating potential mutagenic risk. This suggests that future structural optimization should consider reducing aromatic electron density or exploring prodrug strategies to mask reactive moieties during systemic circulation [
37]. In contrast, E-264 is flagged for hepatotoxicity, yet its favorable balance between lipophilicity and solubility suggests that this liability could be addressed through targeted modification, such as fine-tuning metabolic soft spots or introducing steric shields to reduce bioactivation [
38]. Importantly, none of the candidate steroidal compounds demonstrated the ability to cross the blood–brain barrier, a desirable trait for minimizing central nervous system side effects in non-CNS-targeted therapies [
39,
40]. Additionally, all compounds exhibited moderate synthetic accessibility scores (ranging from 4.31 to 4.52), indicating that they are within a practical range for chemical synthesis and scale-up.
Among the candidates, E-254 and E-265 emerged as the most promising prototypes, combining high predicted absorption, absence of major toxicological alerts, log p values near the optimal threshold of 5, and a favorable balance between solubility and synthetic feasibility. E-264 remains a viable lead, provided its hepatotoxicity can be mitigated through rational design. Meanwhile, E-268 offers a structurally interesting alternative with good acute safety indicators, though its long-term tolerability warrants further investigation.
When compared with the reference drugs, RM-581, exemestane, and formestane, the newly designed steroid compounds show several clear advantages. First, all candidates have molecular weights under 500 g/mol, which is beneficial for passive membrane permeability. In contrast, RM-581 exceeds this threshold, which may limit its absorption. Among the new compounds, E-254, E-265, and E-264 stand out for maintaining an ideal lipophilicity, with log p values close to 5. This balance supports good membrane permeability without compromising solubility. On the other hand, exemestane and formestane are more hydrophilic (Log p around 3.1–3.5), and although RM-581 has a Log p of 4.78, its high molecular weight and flexible structure reduce its overall drug-likeness.
From a safety perspective, only E-264 and RM-581 raised hepatotoxicity concerns. Interestingly, the clinical inhibitors did not trigger this alert, although formestane showed the weakest toxicological profile overall, being classified in the lowest safety category (class 6). In terms of synthetic feasibility, the new steroid candidates also performed well, with synthetic accessibility scores averaging around 4.4, lower (and thus more favorable) than exemestane (5.03) and especially RM-581 (5.92), suggesting they are easier and more cost-effective to produce. Finally, none of the new steroid compounds are predicted to cross the blood–brain barrier, which is a desirable trait for drugs not intended to act on the central nervous system. This reduces the risk of neurological side effects, a notable advantage over exemestane and formestane, which do have the potential to enter the brain.
Following the confirmation that all designed compounds met acceptable ADME-Tox criteria, we proceeded to investigate their molecular interactions with the selected protein targets. This analysis was conducted using PyMOL and Discovery Studio Visualizer, which allowed for detailed visualization of the binding modes and interaction profiles. The resulting 2D interaction diagrams, presented in
Table 8,
Table 9 and
Table 10, illustrate the key contacts formed between each compound and its respective target, offering valuable insights into the structural features that contribute to binding affinity and specificity.
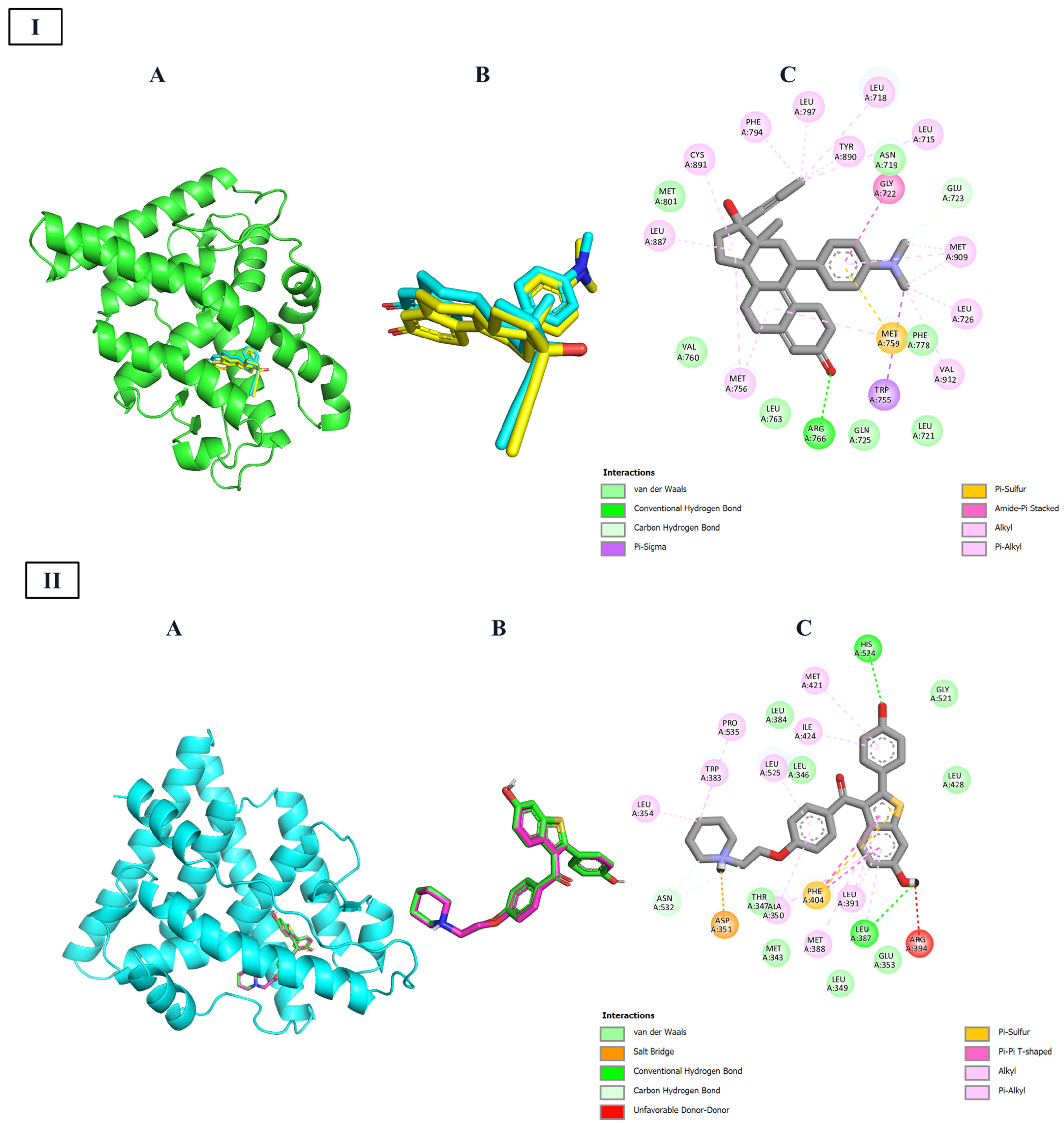
Docking simulations against the progesterone receptor ligand-binding domain (PDB ID: 2W8Y,
Table 8) revealed that all seven designed steroidal compounds consistently occupied the same hydrophobic pocket. This cavity is primarily defined by residues such as Leu715, Leu718, Leu721, Leu763, Val760, Met756, Met759, Phe778, and Met801/Met909. Within this site, the ligands formed a stable network of van der Waals, alkyl, and π–alkyl interactions, which contributed significantly to binding affinity. Each compound also exhibited unique polar and π-stacking interactions superimposed on this shared hydrophobic framework. For instance, E-253 formed π–sulfur and π–alkyl contacts without steric clashes. E-254 maintained a similar interaction pattern but introduced dual π–sulfur interactions and a hydrogen bond with Arg766, slightly improving its binding energy. E-255 added hydrogen bonds with Gln725 and Arg766, along with a π–sulfur contact, though a minor clash at Phe778 was observed. E-261 preserved the hydrophobic core and established hydrogen bonds with Arg766 and Gln725, plus a π–sulfur interaction, despite mild steric interference. E-264 demonstrated enhanced aromatic interactions, forming dual π–π stacking contacts with Phe778 and Tyr890, a halogen bond with Met909, and a hydrogen bond with Arg766—though accompanied by steric hindrance near Gln725. E-265 retained the core interaction profile and added hydrogen bonds and a π–sulfur contact, resulting in an intermediate binding profile. Lastly, E-268 relied on halogen bonding and π–π interactions to maintain affinity, despite lacking conventional hydrogen bonds.
On the other hand,
Table 9 presents the 2D interaction for the seven steroidal ligands with the highest predicted affinity toward the HER2 tyrosine kinase domain (PDB ID: 7JXH). In all complexes, the hydrophobic tetracyclic core of the ligands consistently fits into a conserved pocket defined by Leu785, Leu800, and Met801, forming a stable network of van der Waals, alkyl, and π–alkyl interactions that dominate the binding energy landscape. Estero-253 reinforces this hydrophobic anchoring with a T-shaped π–π stacking interaction with Phe864 and a conventional hydrogen bond to the carboxylate of Asp863, effectively stabilizing its terminal orientation. Estero-254 maintains the same hydrophobic profile but introduces an additional π–anion interaction with Asp863 and a C–H···O hydrogen bond, which likely accounts for its slightly improved binding energy. Estero-255 combines the core hydrophobic contact with a weak hydrogen bond to the ε-ammonium group of Lys753 and shows no steric clashes, consistent with its low fluctuation during molecular dynamics simulations.
In the case of Estero-264, the extended aromatic substituent enables dual π–π stacking with Phe864 and Tyr835. A halogen bond between its fluorine atom and Phe864, along with electrostatic interaction with Lys753, further enhances its binding affinity. Finally, Estero-265 forms two classical hydrogen bonds with Glu770 and Asp863 and establishes a halogen contact with Tyr835. However, a donor–donor clash with Asp863 may slightly reduce its binding efficiency.
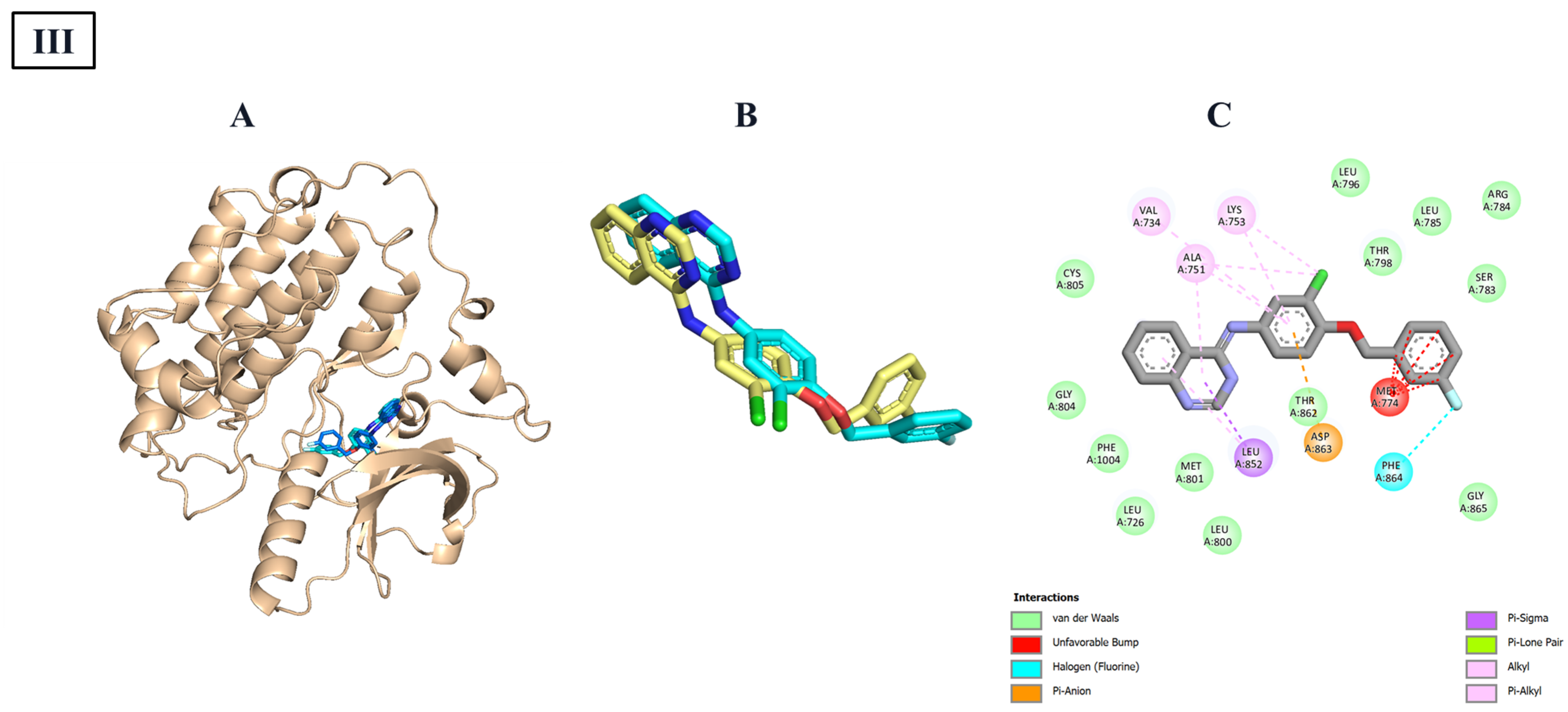
Finally,
Table 10 shown the Docking simulations of the seven designed steroidal compounds into the ligand-binding domain of estrogen receptor-α (ER-α, PDB ID: 6VJD).
Table 10 reveals a consistent binding mode across the series. In all cases, the tetracyclic steroid core was stably positioned within a hydrophobic pocket defined by residues Leu346, Leu349, Leu384, Leu428, Leu525, Met343, Met388, Met421, Ile424, and Pro535. This region formed a dense network of van der Waals, alkyl, and π–alkyl interactions, which dominated the binding energy profile for each complex. E-253 complemented this hydrophobic anchoring with a π–anion interaction involving Asp351, a π–sulfur contact with Met388, and a C–H···O hydrogen bond to His524. E-254 preserved the same core interactions and added a T-shaped π–π stacking interaction with Phe404 and a π–sulfur contact with Met421, resulting in a modest increase in binding affinity. E-255 introduced two classical hydrogen bonds with Arg394 and His524, along with a π–σ interaction with Met388. Although a minor acceptor–acceptor clash with Leu525 was observed, it did not disrupt the ligand’s position within the pocket. E-264, featuring an extended aromatic substituent, formed three simultaneous π–π stacking interactions with Phe404, Tyr526, and Trp383, and established a hydrogen bond with Arg394. These interactions compensated for its larger molecular size. E-261 maintained the core hydrophobic interactions and added a π–σ contact with Met388 and a hydrogen bond to His524, though minor steric clashes with Leu384 and Leu428 were detected. E-265 formed a π–sulfur interaction with Met343 and hydrogen bonds with Arg394 and His524, yielding an interaction profile intermediate in complexity between E-255 and E-253. Lastly, E-268 relied on a fluorine-centered halogen bond with Leu525, a π–σ interaction with Met388, and π–π stacking with Trp383 to maintain strong binding affinity, despite lacking classical hydrogen bonds.
Once molecular docking identifies potential binding poses between a ligand and its target, it becomes essential to evaluate the stability and realism of these complexes over time. To achieve this, molecular dynamics (MD) simulations are employed. Unlike docking, which provides a static snapshot, MD allows us to observe how the protein–ligand complex behaves in a dynamic, solvated environment—closer to physiological conditions. This step helps confirm whether the predicted interactions are stable, whether the ligand remains in the binding pocket, and how the complex responds to thermal fluctuations, thus offering a more reliable assessment of binding affinity and structural compatibility.
After confirming that all designed compounds met acceptable ADME-Tox criteria, we analyzed their molecular interactions with the selected protein targets. This study was performed using PyMOL and Discovery Studio Visualizer, tools that allowed detailed visualization of binding modes and interaction profiles. Comprehensive tables summarizing every interaction type for each ligand–protein pair (
Table 8,
Table 9 and
Table 10) were prepared, and the full set of 2D interaction images is provided in the
Supplementary Material (Figures S1–S3).
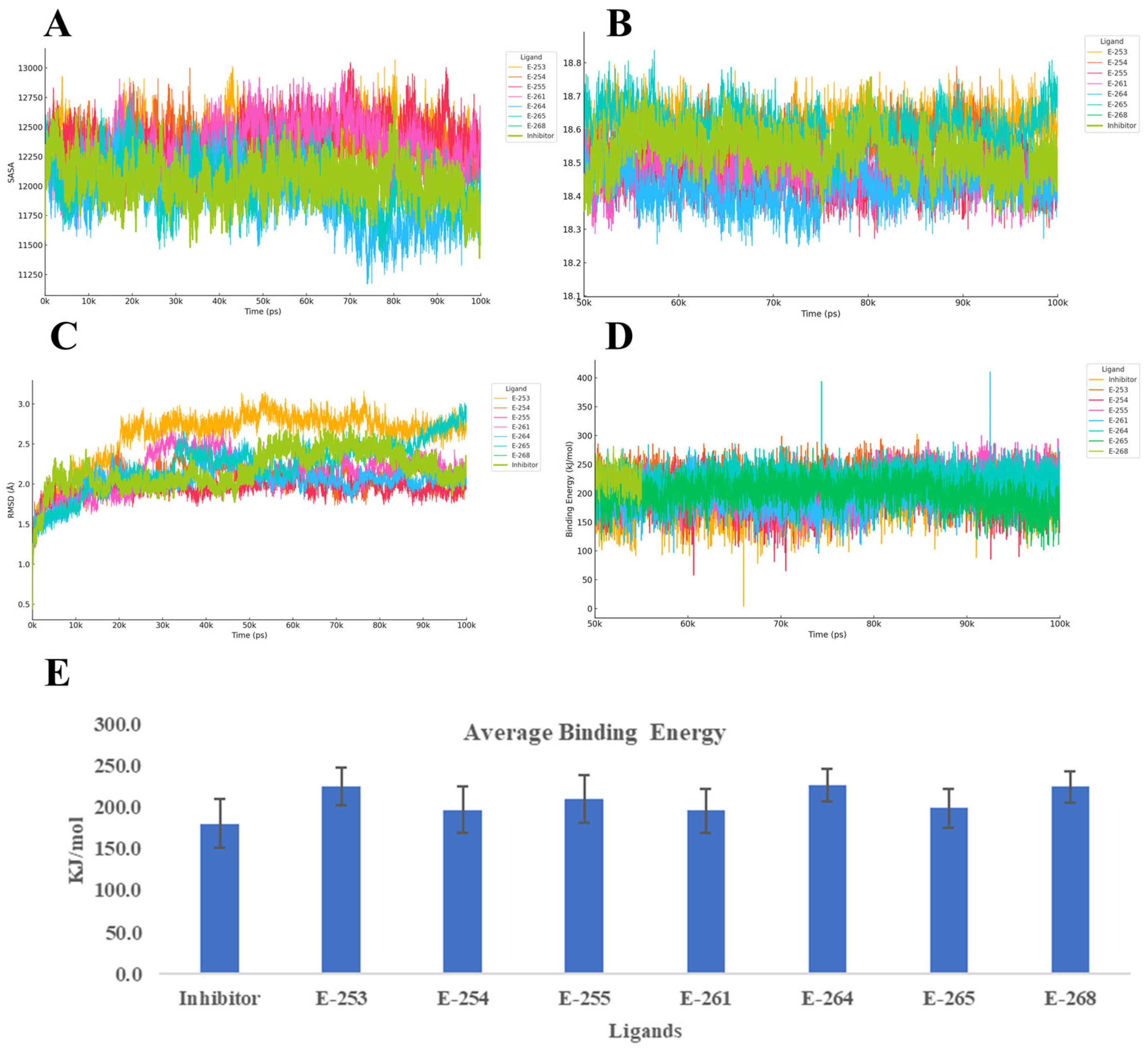
To evaluate the dynamic stability and binding behavior of the seven designed steroidal analogs with the progesterone receptor (PR, PDB ID: 2W8Y), 100 ns MD simulations were performed using YASARA, with the co-crystallized inhibitor serving as a reference. The analysis focused on four key parameters: Cα RMSD, radius of gyration (Rg), secondary structure evolution, and YASARA’s Binding Energy score proprietary metric, where higher values indicate stronger interactions (
Figure 5).
Across the simulations, Estero-264 and Estero-255 formed the most stable complexes, with RMSD values around 1.5 Å and slightly more compact receptor conformations (Rg ≈ 18.46–18.47 Å) compared with the reference. These were followed by Estero-254 and Estero-261, which showed similar stability to the inhibitor (RMSD ≈ 1.6–1.7 Å). In contrast, Estero-253 exceeded 2 Å RMSD, suggesting conformational adaptation of the receptor to accommodate the ligand. Secondary structure analysis confirmed that the top-performing compounds preserved α-helical content, while Estero-265 and Estero-268 induced modest coil formation and, in the latter case, a net 3% helix loss. All candidates outperformed the inhibitor in terms of Binding Energy, with Estero-264 (226.8 ± 19.8 kcal/mol) and Estero-253 (226.2 ± 22.6 kcal/mol) showing the highest scores. Estero-268 and Estero-255 followed closely, while Estero-265, Estero-254, and Estero-261 still exceeded the reference by 15–18 kcal/mol within the method’s uncertainty but are indicative of improved affinity.
Taken together, the combination of low RMSD, reduced Rg, and high Binding Energy identifies Estero-264 and Estero-255 as the most robust and tightly bound complexes. Estero-254, Estero-261, and Estero-265 also show a favorable balance between structural stability and binding strength. Notably, Estero-265 exhibited the narrowest energy distribution, suggesting a consistently stable interaction network throughout the simulation. Even additional experiments are necessary, these results suggest that the designed steroids likely act as competitive inhibitors, stabilizing the receptor in a compact, low-energy conformation through persistent hydrophobic and polar interactions. The reduced flexibility (low RMSD), compactness (low Rg), and enhanced binding energy collectively support their potential to effectively block natural ligand access to the receptor’s active site [
41].
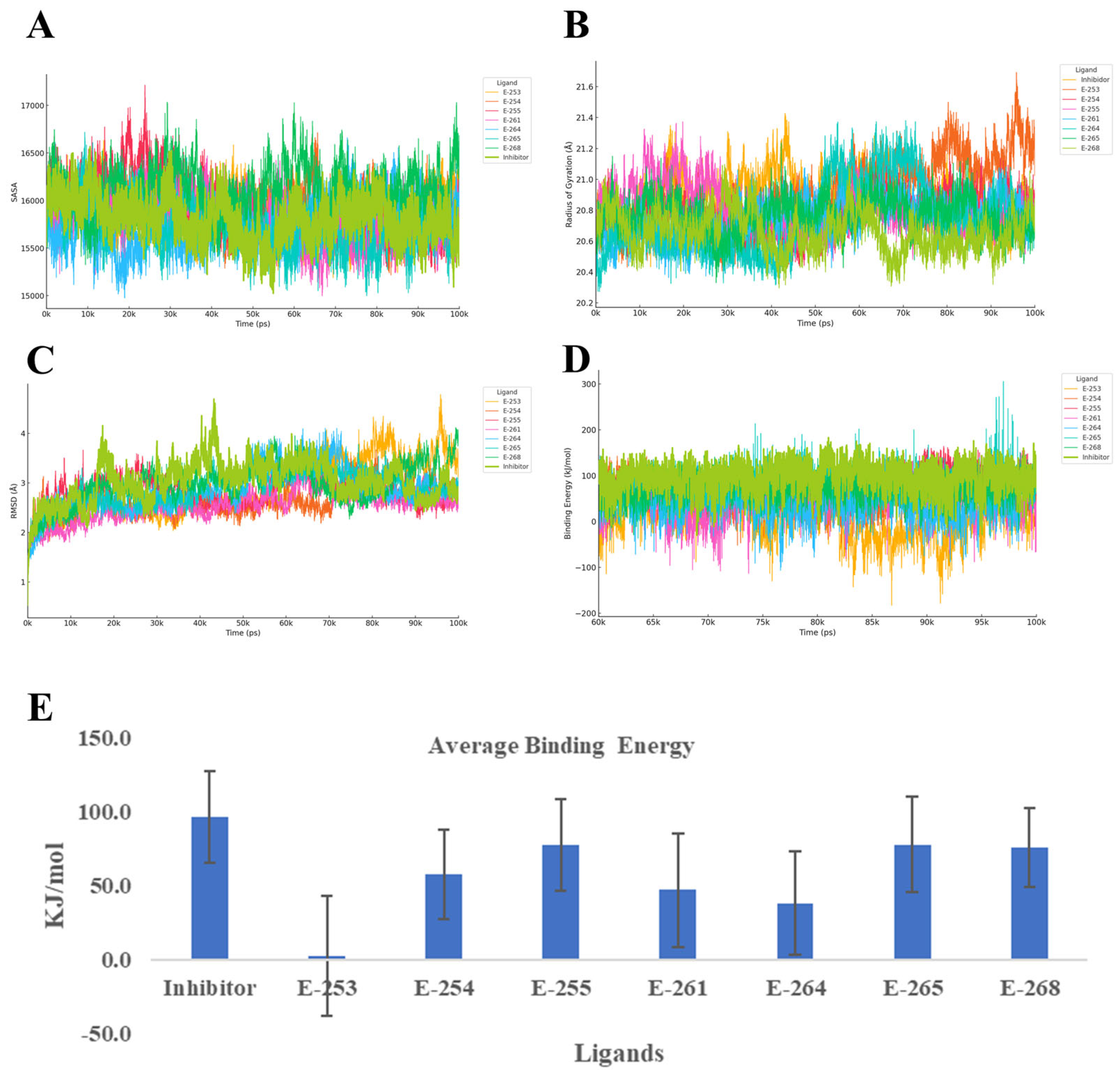
To better understand how the designed steroidal compounds interact with the HER2 tyrosine kinase domain (PDB ID: 7JXH), we ran 100-nanosecond molecular dynamics simulations using YASARA, using the co-crystallized inhibitor as a benchmark. We focused on four key indicators of complex behavior: Cα RMSD, radius of gyration (Rg), secondary structure changes, and YASARA’s Binding Energy score (
Figure 6). Throughout the simulations, all compounds maintained the structural integrity of the HER2 domain. Estero-261 and Estero-254 formed the most stable complexes, with RMSD values around 2.1–2.2 Å—more stable than the reference inhibitor (2.65 Å). The Rg values remained consistent (~20.8 Å), indicating no significant unfolding or collapse of the protein. Most compounds preserved the receptor’s secondary structure, with only Estero-268 (+4.8%) and Estero-264 (+2.7%) showing modest increases in coil content, but without signs of destabilization.
In terms of binding strength, Estero-255, Estero-265, and Estero-268 showed the highest affinity, with Binding Energy scores between 75–78 kcal/mol—approaching the reference inhibitor’s 96.4 ± 31.3 kcal/mol. Interestingly, Estero-254 and Estero-261, despite their excellent structural stability, showed moderate binding energies (47–57 kcal/mol), suggesting that while they fit well geometrically, they may benefit from additional polar interactions to improve electrostatic complementarity. On the other hand, Estero-264 and especially Estero-253 (~2 kcal/mol) showed weak binding, likely due to poor contact formation or excessive flexibility.
When considering both stability and affinity, Estero-255 and Estero-265 stand out as the most promising HER2 inhibitors. They combine strong binding with structural stability and minimal disruption to the protein’s secondary structure. Estero-254 and Estero-261 offer rigid scaffolds that could be optimized further, while Estero-264 and Estero-268 may require structural refinement to improve their interaction profiles. Estero-253, due to its very low binding energy, appears unsuitable for this target. Finally, the binding profiles suggest that Estero-255 and Estero-265 likely act as ATP-competitive inhibitors. Their ability to maintain low RMSD and stable Rg values, along with high binding energy, indicates that they effectively occupy the ATP-binding cleft and stabilize the kinase in an inactive conformation. This mechanism aligns with the established mode of action for HER2-targeted tyrosine kinase inhibitors, which block ATP access and prevent downstream phosphorylation events critical for cancer cell proliferation [
42].
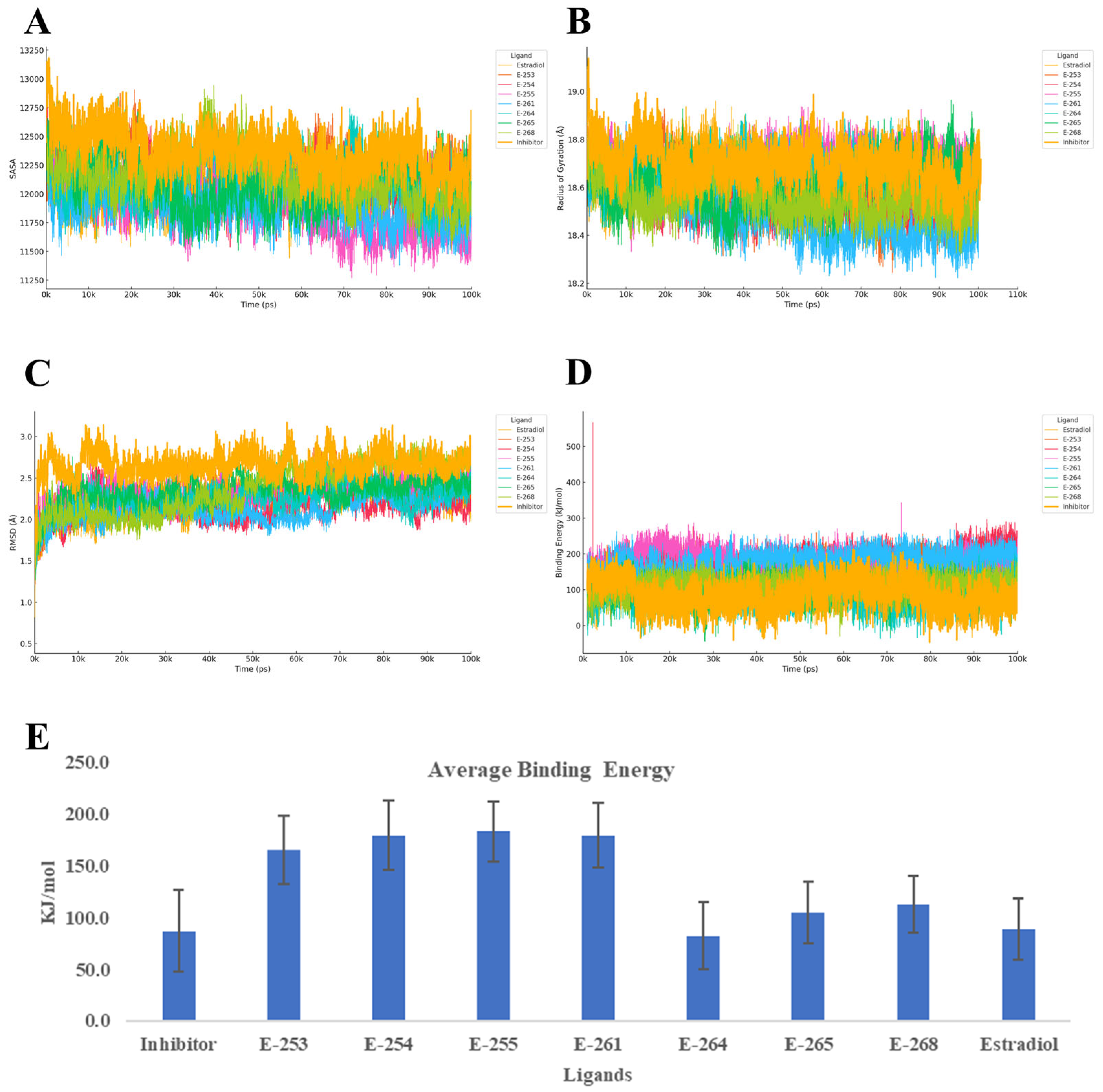
Finally, the 100-nanosecond molecular dynamics (MD) simulations of the estrogen receptor alpha ligand-binding domain (ER-α, PDB ID: 6VJD) in complex with the designed steroid analogs demonstrate favorable structural and energetic profiles (
Figure 7). All compounds preserved the receptor’s global fold, while several analogs exhibited superior binding characteristics compared with both estradiol and the co-crystallized inhibitor.
Among the tested ligands, Estero-261 and Estero-254 were particularly effective. These analogs significantly reduced the Cα RMSD (~1.5 Å), indicating enhanced structural stability, and slightly decreased the radius of gyration (Rg), suggesting a more compact and potentially less flexible receptor conformation. These structural effects were accompanied by a marked increase in binding energy, nearly doubling the values observed for estradiol and the reference inhibitor. Such stabilization of the ligand-binding domain (LBD) is known to interfere with the dynamic positioning of helix 12 (H12), a critical element for coactivator recruitment and transcriptional activation [
43]. Estero-255 also demonstrated a substantial gain in binding energy, with only a marginal increase in backbone flexibility, while Estero-253 presented a balanced profile—moderate RMSD and Rg values coupled with high affinity—suggesting that further rigidification of its steroidal scaffold could enhance its inhibitory potential. Conversely, Estero-264 did not exhibit significant improvements in any of the evaluated parameters. Its RMSD and Rg values were more variable, and its binding energy remained comparable to the controls, indicating limited potential without further structural optimization.
Overall, these findings support a competitive inhibition mechanism, wherein the top-performing analogs occupy the ligand-binding pocket with higher affinity than estradiol, thereby preventing receptor activation. The observed reduction in RMSD and compaction in Rg suggest that these ligands stabilize the receptor in an inactive conformation, potentially obstructing coactivator binding [
44]. This interpretation is further supported by the enhanced binding energies, which are predictive of stronger ligand–receptor interactions and inhibitory potency [
45,
46].
2.7. Multitarget Molecular Dynamics Analysis of Steroid Analogs in Triple-Positive Breast Cancer
The 100-nanosecond molecular dynamics simulations conducted across the three principal targets in triple-positive breast cancer—progesterone receptor (PR, PDB ID: 2W8Y), HER2 tyrosine kinase (PDB ID: 7JXH), and estrogen receptor alpha (ER-α, PDB ID: 6VJD) enabled a comprehensive evaluation of seven designed steroid analogs (Estero-253, -254, -255, -261, -264, -265, and -268). This unified approach allowed for the identification of ligands with consistent performance across multiple receptor classes, a key criterion in the development of multitarget therapeutics.
Analysis of structural stability parameters, including root mean square deviation (RMSD), radius of gyration (Rg), and secondary structure retention, revealed that Estero-254 and Estero-261 consistently exhibited the lowest RMSD values and most compact Rg profiles across all three targets. These findings suggest a pre-organized chemical scaffold that minimizes the conformational rearrangement required upon binding, thereby enhancing complex stability. Estero-255 also demonstrated favorable rigidity in PR and ER-α, with acceptable RMSD values in HER2, while Estero-264 maintained structural integrity only in PR. In contrast, Estero-268 and Estero-253 induced significant helical perturbations and exhibited high flexibility, compromising their overall stability.
Energetic profiling further reinforced this hierarchy. Estero-261 emerged as the only compound to achieve top-tier binding energy scores across all three targets, doubling the reference inhibitor’s score at ER-α, outperforming it at PR, and approaching it within ~20 kcal/mol at HER2. Estero-254 mirrored this profile with negligible deviation. Estero-255 also reached peak affinity at PR and ER-α, and although its binding energy at HER2 (~78 kcal/mol) was more modest, it still surpassed the reference compound. Estero-265 showed strong affinity at HER2 but lacked sufficient electrostatic interaction at the nuclear receptors, while Estero-253, -264, and -268 were deprioritized due to weak binding or instability at two or more targets.
Detailed RMSD analysis confirmed that Estero-261 and Estero-265 provided the most stable complexes in PR (1.50 Å and 1.54 Å, respectively), while Estero-261 and Estero-253 were most stable in ER-α and HER2, with RMSD values below 1.80 Å. Rg analysis indicated that Estero-255 and Estero-261 induced the most compact conformations in the nuclear receptors, suggesting enhanced conformational stability. Total energy calculations supported these findings: Estero-255 and Estero-253 showed the most negative values in PR, Estero-265 and Estero-264 in ER-α, and Estero-268 and Estero-255 in HER2. Electrostatic (Coulomb) and van der Waals interaction analyses highlighted Estero-255 as a strong binder in PR and ER-α, while Estero-253 also demonstrated favorable short-range interactions across all targets. Hydrogen-bonding analysis, a critical determinant of complex specificity and stability, revealed that Estero-255 and Estero-253 formed the highest number of internal hydrogen bonds in PR, reinforcing their structural integrity. Conversely, Estero-265 and Estero-268 showed greater solvent interaction in ER-α and HER2, which may contribute to their solubility and bioavailability in physiological environments.
These results highlight the strong potential of our steroid-based compounds to act as multitarget inhibitors in triple-positive breast cancer. What stands out is how consistently Estero-255 and Estero-265 perform across three very different types of receptors, two nuclear hormone receptors (PR and ER-α) and a membrane-bound tyrosine kinase (HER2). This kind of versatility suggests that these molecules are not only structurally stable but also adaptable enough to engage with diverse binding environments without losing affinity or precision. That’s a rare and valuable trait in drug design.
Estero-255 shows a well-rounded profile, combining strong binding, compact structure, and stable interactions across all targets. Estero-265, while slightly more selective, shows excellent performance in ER-α, which is especially relevant for hormone-driven cancers. These findings support the idea that a multitarget approach, rather than focusing on a single receptor, could offer a more effective strategy for tackling the complexity of breast cancer. With further optimization and experimental validation, these compounds could serve as promising leads for the next generation of targeted therapies [
47,
48].