Abstract
Post-acute sequelae of COVID-19 have been associated with an elevated risk of thromboembolism and adverse cardiovascular events (CVEs). We aim to evaluate whether alterations in poorly studied hemostatic and endothelial proteins are associated with CVEs in patients previously admitted to the ICU and evaluated one year post-discharge. We carried out a cross-sectional study involving 63 COVID-19 patients previously admitted to the ICU one year post-discharge. Plasma levels of factor IX (coagulation factor), protein C, protein S (natural anticoagulant), and von Willebrand factor (VWF, an endothelial marker) were measured using a Luminex 200™ analyzer. Generalized linear models (GLMs) were used to assess the association of these coagulation proteins with CVEs and N-terminal pro-B-type natriuretic peptide (NT-proBNP). We found that lower levels of factor IX (p = 0.011), protein C (p = 0.028), and protein S (p = 0.008) were associated with CVEs one year after ICU discharge. Additionally, at the one-year follow-up, we found lower levels of factor IX (p = 0.002) and higher levels of VWF (p = 0.006) associated with higher levels of NT-proBNP, underscoring the involvement of both hemostatic imbalance and persistent endothelial dysfunction. Our findings revealed a gender-specific pattern of associations with NT-proBNP levels. These findings highlight the significant role of persistent hemostatic imbalance and endothelial dysfunction in the development of cardiovascular abnormalities among COVID-19 survivors discharged from the ICU.
1. Introduction
After the acute phase of COVID-19, around 30–80% of patients continue to have symptoms related to SARS-CoV-2 infection up to several months after recovery, which has been termed long COVID [1]. According to the World Health Organization, long COVID is defined as the continuation or development of symptoms 3 months after the initial SARS-CoV-2 infection and the persistence of these symptoms for at least 2 months.
Studies conducted on patients previously admitted to the ICU up to 12 months post-discharge have demonstrated long-term symptoms such as fatigue, dyspnea, pulmonary fibrosis, pathological findings on computed tomography, functional impairment, and even a worse health-related quality of life [2]. An elevated risk of thromboembolism and adverse cardiovascular events (CVEs) has also been linked to long COVID [3,4]. Furthermore, several reports focused on hospitalized patients after discharge have shown the persistence of cardiac symptoms 6–12 months after discharge, such as fatigue, dyspnea, chest pain, and palpitations [5].
NT-proBNP is widely used as a biomarker of heart failure and cardiac dysfunction [6]. Also, it has significant diagnostic and prognostic implications for asymptomatic and early-stage cardiovascular diseases [7,8]. Remarkably, it has been reported that NT-proBNP could independently predict the risk of in-hospital death in patients with severe COVID-19 [9].
SARS-CoV-2 infection is known to induce a prothrombotic state with a characteristic increase in D-dimer [10]. Patients with severe COVID-19 have been shown to have a longer prothrombin time (PT) and activated partial thromboplastin time (aPTT) with maintained high D-dimer levels [11]. D-dimer, coagulation factor VIII, and von Willebrand factor (VWF) levels have been associated with cardiovascular disease risk [12,13]. However, the relationships between other hemostatic and endothelial-related proteins and this condition remain unclear.
Despite the several abnormalities in the coagulation cascade that have been reported in individuals with long-COVID [14], the precise role of specific hemostatic and endothelial proteins, such as factor IX, protein C, protein S, and VWF, in the onset of long-term cardiovascular sequelae of COVID-19 remains unknown. Since VWF reflects endothelial activation, its assessment alongside natural anticoagulants may offer additional insight into vascular dysfunction not captured by conventional coagulation parameters. Furthermore, men and women exhibit different coagulation profiles [15], with women usually showing a higher coagulability status compared to men. However, gender-specific studies on the hemostatic and endothelial profiles of COVID-19 patients are lacking in the current literature, and to our knowledge, no studies have approached gender differences in patients who were admitted to the ICU.
Thus, our objective was to evaluate whether alterations in understudied hemostatic and endothelial proteins are associated with the development of CVEs in patients previously admitted to the ICU one year after hospital discharge, considering potential gender-related differences.
2. Results
2.1. Clinical and Epidemiological Data
Clinical and epidemiological data are shown in Table 1 (extended information in Table S1). A total of 63 patients were included in the study. The median age on admission was 59.7 (interquartile range (IQR) = 53.2–68.8) years, with a median follow-up time of 15.4 months (IQR = 13.2–20.9). One year after discharge, 9.5% of patients developed a CVE. A significant difference between patients with and without a CVE was observed solely with the use of enoxaparin during hospitalization.

Table 1.
Main clinical and epidemiological characteristics of patients with COVID-19 included in the study.
2.2. Association Between Hemostatic and Endothelial Proteins and Cardiovascular Events
One year after discharge, patients who developed a CVE at any time after discharge showed lower levels of factor IX (p = 0.014), protein C (p < 0.001), and protein S (p = 0.002) (Figure 1). Similarly, after adjusting for relevant covariates using GLMs, factor IX [aOR = 0.96 (0.93–0.99), p = 0.011], protein C [aOR = 0.93 (0.87–0.99), p = 0.028], and protein S [aOR = 0.94 (0.90–0.98), p = 0.008] remained inversely associated with the presence of a CVE (Figure 1, Table S2). Conversely, no differences in aPTT, INR, and VWF were observed between groups. Unfortunately, due to the limited number of cases in the CVE group, subgroup analysis by gender was not performed.
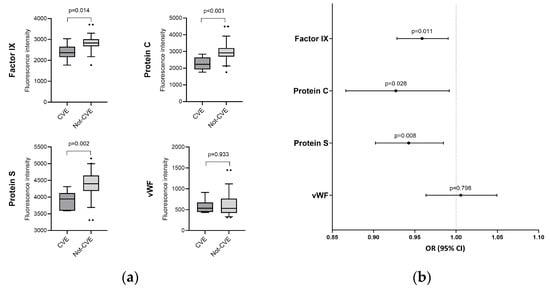
Figure 1.
Coagulation protein levels regarding the presence of CVE. (a) Boxplots depicting factor IX, protein C, protein S, and VWF levels in patients with (dark gray) and without (gray) cardiovascular events; (b) Visual representation of adjusted associations between coagulation protein levels after discharge and the occurrence of cardiovascular events. Statistics: Data were calculated using generalized linear models (GLMs) with a binomial distribution. Odds ratio, 95% confidence intervals (lower boundary: 2.5%, upper boundary: 97.5%), and p-value are shown. GLMs were adjusted for age, gender, body mass index (BMI), follow-up time after discharge, and whether the patient received anticoagulant therapy after discharge. Covariates were selected through a stepwise approach based on the Akaike Information Criterion (AIC). Abbreviations: OR, odds ratio; 95%CI, 95% of confidence interval; p, level of significance; VWF, von Willebrand factor; CVE: cardiovascular event.
Note that an additional analysis showed no associations between hemostatic and endothelial proteins on ICU admission and a CVE after discharge (Table 2).

Table 2.
Association of coagulation proteins on ICU admission with cardiovascular events one year after discharge.
2.3. Association Between Hemostatic and Endothelial Proteins and NT-ProBNP Levels by Gender
One year after discharge, lower levels of factor IX [aAMR = 0.99 (0.98–0.99), p = 0.002] and higher levels of VWF [aAMR = 1.01 (1.01–1.02), p = 0.006] were significantly associated with higher levels of NT-proBNP (Figure 2, Table S3). These associations remained for men after stratifying by gender [p = 0.018 (VWF) and p = 0.018 (factor IX)] (Figure 2, Table S4). Regarding females, factor IX [aAMR = 0.99 (0.97–0.99), p = 0.024], protein C [aOR = 0.98 (0.97–0.99), p = 0.022], and protein S [aAMR = 0.98 (0.97–0.99), p = 0.003] were also significantly associated with higher levels of NT-proBNP (Figure 2, Table S5). Additionally, no significant associations were found between hemostatic and endothelial biomarkers on ICU admission and NT-proBNP levels one year after discharge (Tables S6–S8).
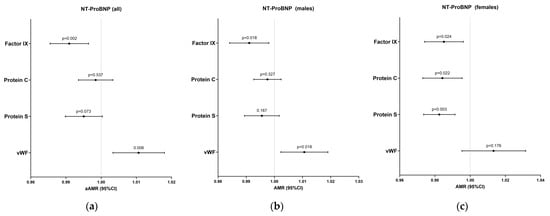
Figure 2.
Adjusted associations of coagulation proteins after discharge with NT-proBNP levels for all patients (a), males (b) and females (c). Statistics: Data were calculated using generalized linear models (GLMs) with a gamma distribution. AMR, 95% confidence intervals (lower boundary: 2.5%, upper boundary: 97.5%), and p-values are shown for both adjusted models. GLMs were adjusted for age, gender, body mass index (BMI), follow-up time after discharge, and whether the patient received anticoagulant therapy after discharge. Covariates were selected through a stepwise approach based on the Akaike Information Criterion (AIC). Abbreviations: AMR, arithmetic mean ratio; 95%CI, 95% confidence interval; p, level of significance; VWF, von Willebrand factor.
3. Discussion
Our findings reveal that patients who developed a cardiovascular event after hospital discharge due to COVID-19 exhibited a distinct hemostatic and endothelial protein profile one year post-discharge. We also identified gender-specific associations with NT-proBNP. To our knowledge, this is the first study to investigate the long-term association between coagulation and endothelial abnormalities and CVE in patients who survived being admitted to the ICU due to COVID-19.
It is well known that SARS-CoV-2 infection induces a procoagulant state, particularly in severe cases [16], during the acute phase. Coagulation abnormalities have been observed even several months after the acute phase, with a prothrombotic state persisting up to 18 months after hospital discharge [10]. These prolonged alterations in hemostatic balance suggest ongoing vascular dysfunction, potentially driven by persistent endothelial injury. Our results may reflect a procoagulant pattern characterized by lower levels of both natural anticoagulants (protein C and protein S) and coagulation factor IX in patients with a CVE. Although several studies have explored factor IX, protein C, and protein S during ICU admission [17,18,19], these proteins have been understudied in the long-term post-discharge period. Fan et al. [4] showed a procoagulant pattern characterized by higher levels of D-dimer and factor VIII levels, and significantly lower antithrombin levels approximately one year after recovery. However, the same study found no differences in protein C and protein S activities in 39 patients compared to the controls. These findings are not necessarily in conflict with ours, as our study focused exclusively on patients admitted to the ICU, while Fan et al. included only seven patients admitted to the ICU in their analysis [4]. Furthermore, our study compared patients admitted to the ICU who developed a CVE with those who did not, in contrast to Fan et al., who compared patients with healthy controls, irrespective of pre-existing cardiovascular pathologies. In addition, Garcia-Larragoiti et al. [20], in a follow-up study, reported higher plasma levels of factor IX in patients with long COVID compared to healthy controls. Unlike our study, which focused specifically on patients admitted to the ICU, this study included COVID-19 patients without specifying their disease severity. However, Garcia-Larragoiti et al. [20] considered not only CVEs, such as tachycardia or arrhythmia, but also other types of post-COVID-19 conditions. These differences may explain the discrepancies with our findings. Nonetheless, larger-scale studies are required to corroborate our findings.
NT-proBNP is a widely recognized biomarker in the assessment of cardiovascular diseases, particularly heart failure [21]. Longitudinal studies showed that the NT-proBNP concentration may remain elevated up to 12 months after infection [22] and that patients with long COVID had significantly higher levels of NT-proBNP compared with asymptomatic patients [23]. In this setting, when investigating the association between hemostatic and endothelial proteins and NT-proBNP, we found that both lower levels of factor IX and increased levels of VWF were linked to higher NT-proBNP concentration one year after discharge. This finding further supports the role of endothelial dysregulation in the cardiovascular complications observed with long COVID. Persistent endothelial activation could be one of the mechanisms underlying the prothrombotic state and elevated cardiovascular risk in this population, although further mechanistic studies are needed to confirm this link.
COVID-19 can trigger a massive inflammatory response with the release of cytokines and chemokines, called a cytokine storm, especially in patients requiring ICU admission [24]. Due to this extensive inflammatory response, endothelial cells release multimers of VWF [25]. Previous studies have confirmed that higher plasma levels of VWF or VWF antigen (VWF:Ag) have been associated with a more severe clinical presentation in the acute phase [26,27] and may remain elevated several months after infection [4]. In addition, some molecules, including VWF, have been shown to be trapped inside microclots of long COVID patients compared to the controls [28]. Likewise, Danielle et al. [26], in a long-term follow-up study, showed that 42.9% (n = 12) of patients with altered VWF levels after 6 months experienced fatigue, 32.1% (n = 9) experienced dyspnea, 17.9% (n = 5) experienced chest pain, and 10.7% (n = 3) reported palpitations, symptoms that could reflect damage to the cardiovascular system. Similarly, Bellone et al. [29] found higher levels of VWF in a small study of 10 gynecological patients with long COVID compared to the controls. These findings are consistent with our results, supporting the association between VWF and CVEs in recovered patients previously admitted to the ICU and reinforcing the concept that VWF plays a pivotal role in the vascular complications observed in the post-COVID-19 period.
Regarding gender, our study showed that lower levels of factor IX, protein C, and protein S were associated with higher levels of NT-proBNP in females, while in males, lower factor IX and higher VWF levels were linked to higher NT-proBNP concentrations. Huang et al. [30] reported that female gender was a risk factor for developing post-acute sequelae of COVID-19 in a longitudinal cohort study (n = 2469). Furthermore, Bai et al. [31] demonstrated that the female gender was associated with the development of post-acute sequelae of COVID-19 at 3 months. The most frequent symptoms described in that study were fatigue and exertional dyspnea, which could be indicative of damage to the cardiovascular system. Consistent with previous evidence, our study suggests that males and females have distinct coagulation profiles, which may impact the development of COVID-19-derived CVEs. These gender-specific findings underlie biological differences in hemostatic and vascular recovery pathways following severe COVID-19.
We observed that the medians of NT-proBNP concentrations (91 pg/mL for the CVE group and 50 pg/mL for the non-CVE group) were within the range of normal values (<125 pg/mL), which would exclude the diagnosis of heart failure [32]. However, Reddy et al. have recently shown that the sensitivity for ruling out heart failure with preserved ejection fraction is improved by an NT-proBNP threshold of 50 pg/mL. They also suggest that accounting for BMI reduces misclassification with the current thresholds [33]. In our analysis, BMI was considered by adjusting the models accordingly. However, the observed associations between higher levels of NT-proBNP and hemostatic/endothelial proteins should be interpreted with caution, highlighting the need for further research.
Regarding additional comparisons between hemostatic and endothelial proteins on ICU admission and CVEs/NT-proBNP levels one-year post-discharge, no associations were found. These findings allowed us to rule out the possibility that baseline alterations in coagulation or endothelial profile were related to higher NT-proBNP levels or CVE development one year later.
Additionally, several considerations should be taken into account for correct data interpretation. Firstly, the limited sample size could have limited the statistical power to detect additional associations. The single-center design and observational nature of the study may also have limited the generalizability of the findings. Secondly, a comparison between baseline hemostatic and endothelial protein levels and baseline NT-proBNP levels on ICU admission would have provided a more comprehensive evaluation of the basal status of patients; however, NT-proBNP data on ICU admission were not available. Thirdly, the coagulation parameters were measured as total protein levels, which do not capture functional activity and therefore limit the ability to assess their biological activity or clinical significance. Additionally, it is important to bear in mind the dual role of NT-proBNP as a marker of both cardiovascular disease and inflammation [22]. Whether these hemostatic and endothelial alterations persist over time or eventually normalize remains an open question, warranting longitudinal studies with extended follow-up.
While these limitations may affect the broader applicability of our results, our study offers meaningful insights into persistent hemostatic and endothelial alterations following severe COVID-19, which may serve as a valuable foundation for future multicenter and longitudinal investigations.
4. Materials and Methods
4.1. Design and Study Population
A cross-sectional study was conducted on COVID-19 patients admitted to the ICU at Hospital Universitario del Tajo and Hospital Universitario Infanta Cristina between August 2020 and March 2021. The inclusion criteria were as follows: (i) ICU admission related to COVID-19; (ii) assessment of a new CVE (including arrhythmia, stroke, venous thromboembolic disease, or pulmonary thromboembolism) at least one-year post-discharge; (iii) availability of a plasma sample at least one year after discharge; and (iv) availability of clinical data and/or NT-proBNP at least one year after discharge. Patients with pre-existing cardiovascular diseases were excluded. The study protocol was approved by the Ethics Committees of the Institute of Health Carlos III (protocol CEI PI 28_2021-v3) on 24 May 2021, from the Hospital Universitario del Tajo and Hospital Infanta Cristina. Written informed consent was obtained from all patients or their authorized surrogates.
4.2. Clinical Data and Samples
An extensive questionnaire was completed both on ICU admission and approximately one year after discharge, in which the clinical and biochemical variables of interest were recorded, including the most relevant symptoms. Data were collected using an electronic case report form (eCRF) built using REDCap electronic data capture tools.
All patients received treatment with enoxaparin during their ICU stay. Patients in the prophylactic-dose group received 40 mg/24 h (<30 mg/24 h if they had renal insufficiency and 60 mg/24 h if their weight was greater than 150 kg). Those in the therapeutic-dose group received 1 mg/kg/12 h of enoxaparin.
Peripheral blood samples were collected in EDTA tubes one year after hospital discharge. The samples were centrifuged the same day of the extraction, and plasma was stored at −80 °C until it was transferred to the National Center for Microbiology for subsequent analysis.
4.3. Outcome
The main outcome was the presence of a new CVE one year after hospital discharge, including arrhythmia, stroke, venous thromboembolic disease (VTED), and/or pulmonary embolism. The secondary outcome was the association with NT-proBNP levels.
4.4. Measurement of Hemostatic and Endothelial Markers
Plasma concentrations of factor IX, protein C, protein S, and VWF were measured using the Human Coagulation 4-PLEX ProcartaPlex Panel (Invitrogen, Waltham, MA, USA) on a Luminex 200™ analyzer (Luminex Corporation, Austin, TX, USA), following the manufacturer’s protocol. These proteins were selected based on their involvement in coagulation pathways and endothelial-related processes. This multiplex immunoassay quantifies total protein levels rather than their functional activity. The raw fluorescence intensity (FI) values were measured and used (arbitrary units, a.u.) as previously described [34]. The aPTT and international normalized ratio (INR) were measured with the analyzer Siemens CS-2500 System (manufactured by Sysmex Corporation, Kobe, Japan; distributed by Siemens Healthineers, Erlangen, Germany).
4.5. Statistical Analysis
For the descriptive study, the Mann–Whitney U test was used to test differences in continuous variables between groups, and the chi-square test and Fisher’s exact test were used for categorical variables when appropriate.
The association between coagulation proteins (independent variables) and CVEs (dependent variable) was analyzed using both unadjusted and adjusted GLMs with binomial distribution. For the adjusted models, covariates were selected through a stepwise approach based on the Akaike Information Criterion (AIC). The covariates used were age, gender, body mass index (BMI), follow-up time post-discharge, and whether the patient received anticoagulant therapy after hospital discharge. Likewise, the association between coagulation proteins (independent variables) and NT-proBNP levels (dependent variable) was assessed using unadjusted and adjusted GLMs with a gamma distribution, employing the same stepwise covariate selection method indicated above.
Furthermore, we performed additional GLMs to examine the relationship between coagulation protein levels on ICU admission and CVE/NT-proBNP levels at one year to exclude the possibility that alterations in coagulation factors on admission are related to CVEs or NT-proBNP levels at the follow-up visit.
A statistically significant association was considered with a p-value < 0.05. Statistical software R (v 4.3.0) (www.r-project.org was used for all statistical analyses).
5. Conclusions
In conclusion, our data show a distinct hemostatic and endothelial protein profile in survivors of COVID-19 admitted to the ICU who developed a CVE one year after discharge, characterized by reduced levels of natural anticoagulants and factor IX. Gender-specific associations with NT-proBNP were identified: lower levels of these factors correlated with higher NT-proBNP levels in women, while in men, elevated NT-proBNP levels were linked to lower factor IX and increased VWF levels. This novel investigation, focusing on ICU survivors, highlights the persistent impact of severe COVID-19 infection on coagulation and its potential contribution to long-term cardiovascular risk, underscoring the need for continued monitoring in this high-risk population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26146854/s1.
Author Contributions
Conceptualization, M.Á.J.-S. and A.F.-R.; Data curation, R.B.-L. and A.V.-B.; Formal analysis, R.B.-L., A.V.-B., M.Á.J.-S. and A.F.-R.; Investigation, Ó.M.-G., R.B., E.M., P.M.-G., M.J.M.P., J.M.d.P., M.H.-G., Ó.B.-K. and M.A.A.F.; Funding acquisition, R.B., M.Á.J.-S. and A.F.-R.; Supervision, Ó.M.-G., R.B., M.Á.J.-S., A.F.-R. and S.R.; Visualization, R.B.-L., A.V.-B., M.Á.J.-S. and A.F.-R.; Writing—original draft, R.B.-L., A.V.-B., M.Á.J.-S. and A.F.-R.; Writing—review and editing, R.B.-L., M.Á.J.-S., S.R., Ó.M.-G., R.B. and A.F.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación Universidad Alfonso X el Sabio (FUAX)–Santander, grant number 1.013.005; Instituto de Salud Carlos III, grant number MPY224/20; CIBER—Consorcio Centro de Investigación Biomédica en Red (CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea—NextGenerationEU, grant number CB21/13/00044. The APC was funded by Instituto de Salud Carlos III.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committees of the Institute of Health Carlos III, Hospital Universitario del Tajo, and Hospital Infanta Cristina (protocol CEI PI 28_2021-v3, 24 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This study would not have been possible without the collaboration of all the patients, their families, medical and nursing staff, and data managers who have taken part in the project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AIC | Akaike Information Criterion |
| ACE | Adverse cardiovascular events |
| aAMR | Adjusted arithmetic mean ratio |
| aPTT | Partial thromboplastin time |
| BMI | Body mass index |
| GLM | Generalized linear models |
| ICU | Intensive care unit |
| INR | International normalized ratio |
| IQR | Interquartile range |
| OR | Odds ratio |
| PT | Prothrombin time |
| VTED | Venous thromboembolic disease |
| VWF | von Willebrand factor |
| VWF:Ag | VWF antigen |
References
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef] [PubMed]
- Rasulo, F.A.; Piva, S.; Latronico, N. Long-term complications of COVID-19 in ICU survivors: What do we know? Minerva Anestesiol. 2022, 88, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Kaiser, R.; Stark, K. Thromboinflammation in long COVID-the elusive key to postinfection sequelae? J. Thromb. Haemost. 2023, 21, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.E.; Wong, S.W.; Sum, C.L.L.; Lim, G.H.; Leung, B.P.; Tan, C.W.; Ramanathan, K.; Dalan, R.; Cheung, C.; Lim, X.R.; et al. Hypercoagulability, endotheliopathy, and inflammation approximating 1 year after recovery: Assessing the long-term outcomes in COVID-19 patients. Am. J. Hematol. 2022, 97, 915–923. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Onodera, M.; Nakamura, M.; Tanaka, F.; Takahashi, T.; Makita, S.; Ishisone, T.; Ishibashi, Y.; Itai, K.; Onoda, T.; Ohsawa, M.; et al. Plasma B-type natriuretic peptide is useful for cardiovascular risk assessment in community-based diabetes subjects: Comparison with albuminuria. Int. Heart J. 2012, 53, 176–181. [Google Scholar] [CrossRef][Green Version]
- Welsh, P.; Hart, C.; Papacosta, O.; Preiss, D.; McConnachie, A.; Murray, H.; Ramsay, S.; Upton, M.; Watt, G.; Whincup, P.; et al. Prediction of Cardiovascular Disease Risk by Cardiac Biomarkers in 2 United Kingdom Cohort Studies: Does Utility Depend on Risk Thresholds For Treatment? Hypertension 2016, 67, 309–315. [Google Scholar] [CrossRef]
- Saricam, E.; Dursun, A.D.; Turkmen Sariyildiz, G.; Can, N.; Bozkurt, E.; Gonullu, U.; Basay, N.; Turkmen, M.; Denli, A.; Unlu, M. Laboratory and Imaging Evaluation of Cardiac Involvement in Patients with Post-Acute COVID-19. Int. J. Gen. Med. 2021, 14, 4977–4985. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Anguissola, M.; Pugliese, S.; Falco, M.; Menicanti, L. The Long Term Residual Effects of COVID-Associated Coagulopathy. Int. J. Mol. Sci. 2023, 24, 5514. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Thompson, A.; Aspelund, T.; Rumley, A.; Eiriksdottir, G.; Lowe, G.; Gudnason, V.; Angelantonio, E.D. Hemostatic factors and risk of coronary heart disease in general populations: New prospective study and updated meta-analyses. PLoS ONE 2013, 8, e55175. [Google Scholar] [CrossRef] [PubMed]
- Zakai, N.A.; Katz, R.; Jenny, N.S.; Psaty, B.M.; Reiner, A.P.; Schwartz, S.M.; Cushman, M. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: The Cardiovascular Health Study. J. Thromb. Haemost. 2007, 5, 1128–1135. [Google Scholar] [CrossRef]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, M.; Edmondson, S.J.; Rabensteiner, J.; Pruller, F.; Gary, T.; Zemann, W.; Wallner, J. Gender-specific differences in haemostatic parameters and their influence on blood loss in bimaxillary surgery. Clin. Oral Investig. 2022, 26, 3765–3779. [Google Scholar] [CrossRef]
- Violi, F.; Pastori, D.; Cangemi, R.; Pignatelli, P.; Loffredo, L. Hypercoagulation and Antithrombotic Treatment in Coronavirus 2019: A New Challenge. Thromb. Haemost. 2020, 120, 949–956. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, W.; Jiang, W.; Xiao, M.; Li, Y.; Tang, N.; Liu, Z.; Yan, X.; Zhao, Y.; Li, T.; et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J. Thromb. Thrombolysis 2020, 50, 580–586. [Google Scholar] [CrossRef]
- Gharib, A.; Nematollahi, Z.; Kazeminejad, B.; Najafi, G.; Pashapour, H.; Javadi, A.; Mollasharifi, T. Status of Inflammatory and Coagulation Factors in COVID-19 and Its Relation with the Disease Severity. Tanaffos 2023, 22, 389–394. [Google Scholar]
- Marchetti, M.; Gomez-Rosas, P.; Russo, L.; Gamba, S.; Sanga, E.; Verzeroli, C.; Ambaglio, C.; Schieppati, F.; Restuccia, F.; Bonanomi, E.; et al. Fibrinolytic Proteins and Factor XIII as Predictors of Thrombotic and Hemorrhagic Complications in Hospitalized COVID-19 Patients. Front Cardiovasc. Med. 2022, 9, 896362. [Google Scholar] [CrossRef]
- Garcia-Larragoiti, N.; Cano-Mendez, A.; Jimenez-Vega, Y.; Trujillo, M.; Guzman-Cancino, P.; Ambriz-Murillo, Y.; Viveros-Sandoval, M.E. Inflammatory and Prothrombotic Biomarkers Contribute to the Persistence of Sequelae in Recovered COVID-19 Patients. Int. J. Mol. Sci. 2023, 24, 17468. [Google Scholar] [CrossRef]
- Force, T.; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar]
- Poyatos, P.; Luque, N.; Sabater, G.; Eizaguirre, S.; Bonnin, M.; Orriols, R.; Tura-Ceide, O. Endothelial dysfunction and cardiovascular risk in post-COVID-19 patients after 6- and 12-months SARS-CoV-2 infection. Infection 2024, 52, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- Niebauer, J.H.; Binder-Rodriguez, C.; Iscel, A.; Schedl, S.; Capelle, C.; Kahr, M.; Cadjo, S.; Schamilow, S.; Badr-Eslam, R.; Lichtenauer, M.; et al. Cardiopulmonary Long-Term Sequelae in Patients after Severe COVID-19 Disease. J. Clin. Med. 2023, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Caricchio, R.; Gallucci, M.; Dass, C.; Zhang, X.; Gallucci, S.; Fleece, D.; Bromberg, M.; Criner, G.J.; Temple University COVID-19 Research Group. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021, 80, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Campello, E.; Simion, C.; Simioni, P. Long-term hypercoagulability, endotheliopathy and inflammation following acute SARS-CoV-2 infection. Expert Rev. Hematol. 2023, 16, 1035–1048. [Google Scholar] [CrossRef]
- Danielle, R.C.S.; Debora, D.M.; Alessandra, N.L.P.; Alexia, S.S.Z.; Debora, M.C.R.; Elizabel, N.V.; Felipe, A.M.; Giulia, M.G.; Henrique, P.R.; Karen, R.M.B.; et al. Correlating COVID-19 severity with biomarker profiles and patient prognosis. Sci. Rep. 2024, 14, 22353. [Google Scholar] [CrossRef]
- Philippe, A.; Chocron, R.; Gendron, N.; Bory, O.; Beauvais, A.; Peron, N.; Khider, L.; Guerin, C.L.; Goudot, G.; Levasseur, F.; et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict COVID-19 in-hospital mortality. Angiogenesis 2021, 24, 505–517. [Google Scholar] [CrossRef]
- Kruger, A.; Vlok, M.; Turner, S.; Venter, C.; Laubscher, G.J.; Kell, D.B.; Pretorius, E. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc. Diabetol. 2022, 21, 190. [Google Scholar] [CrossRef]
- Bellone, S.; Siegel, E.R.; Scheim, D.E.; Santin, A.D. Increased von Willebrand and Factor VIII plasma levels in gynecologic patients with Post-Acute-COVID-Sequela (PASC)/Long COVID. Gynecol. Oncol. Rep. 2024, 51, 101324. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mule, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female gender is associated with long COVID syndrome: A prospective cohort study. Clin. Microbiol. Infect 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Reddy, Y.N.V.; Tada, A.; Obokata, M.; Carter, R.E.; Kaye, D.M.; Handoko, M.L.; Andersen, M.J.; Sharma, K.; Tedford, R.J.; Redfield, M.M.; et al. Evidence-Based Application of Natriuretic Peptides in the Evaluation of Chronic Heart Failure with Preserved Ejection Fraction in the Ambulatory Outpatient Setting. Circulation 2025, 151, 976–989. [Google Scholar] [CrossRef]
- Breen, E.J.; Polaskova, V.; Khan, A. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: Median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine 2015, 71, 188–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).