Cytokine Profiles of Bronchoalveolar Lavage in Patients with Interstitial Lung Diseases and Non-Allergic Asthma
Abstract
1. Introduction
2. Results
2.1. Study Participants
2.2. Pulmonary Function and BAL Cellularity Differ Significantly Between Included ILDs and OLDs
2.3. Lymphocyte Immunophenotyping in BAL Showed a Significant Difference in CD4 and CD8 Lymphocytes
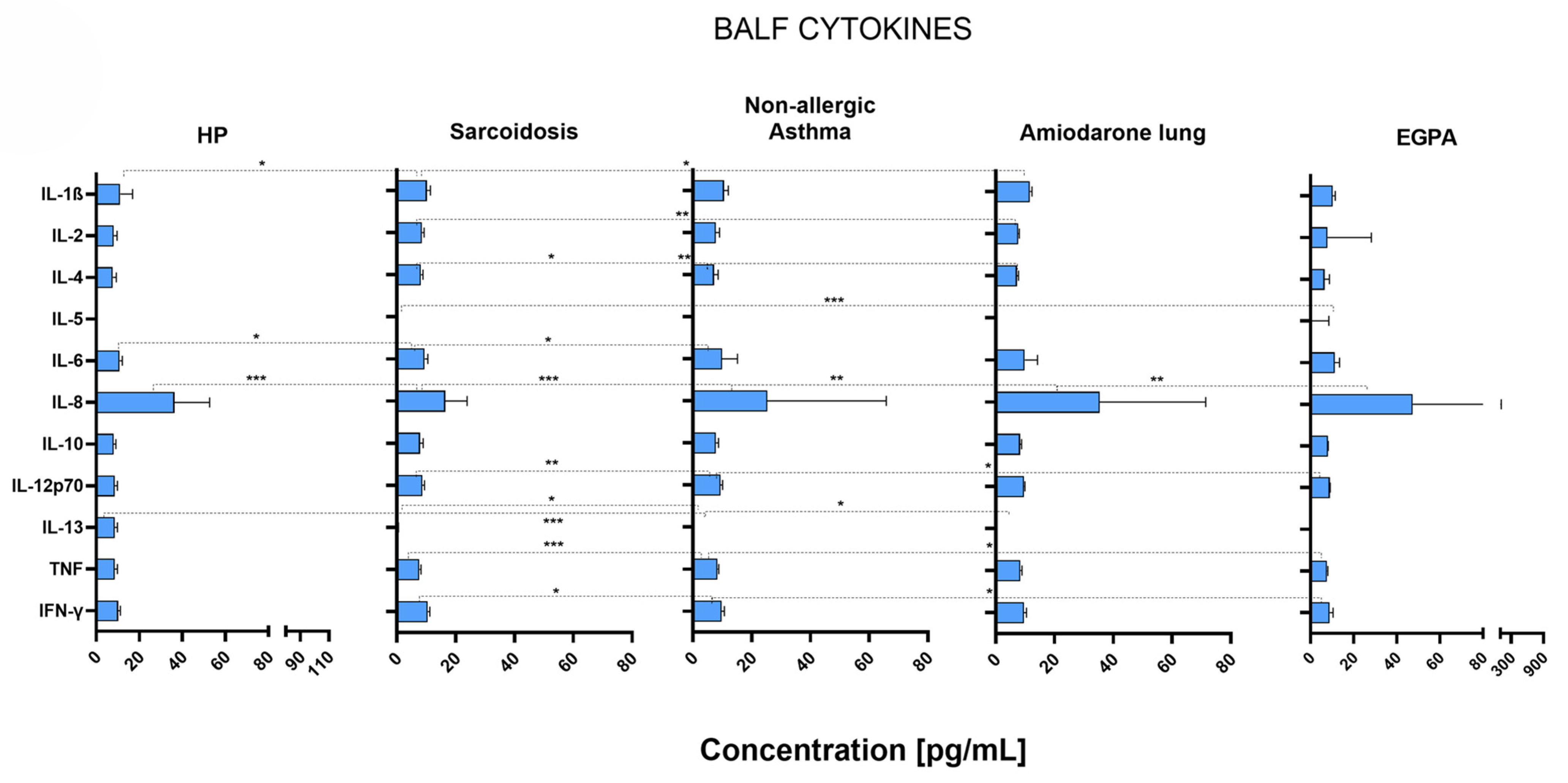
2.4. Different Disease-Specific Cytokine Patterns in the Lungs of Patients with ILDs and OLDs
2.5. VEGF and Angiogenin Were Inversely Increased in the Lungs of Patients with Different ILDs and OLDs
2.6. Complement Anaphylatoxins and Chemokines Were Increased or Decreased in Different ILDs and OLDs
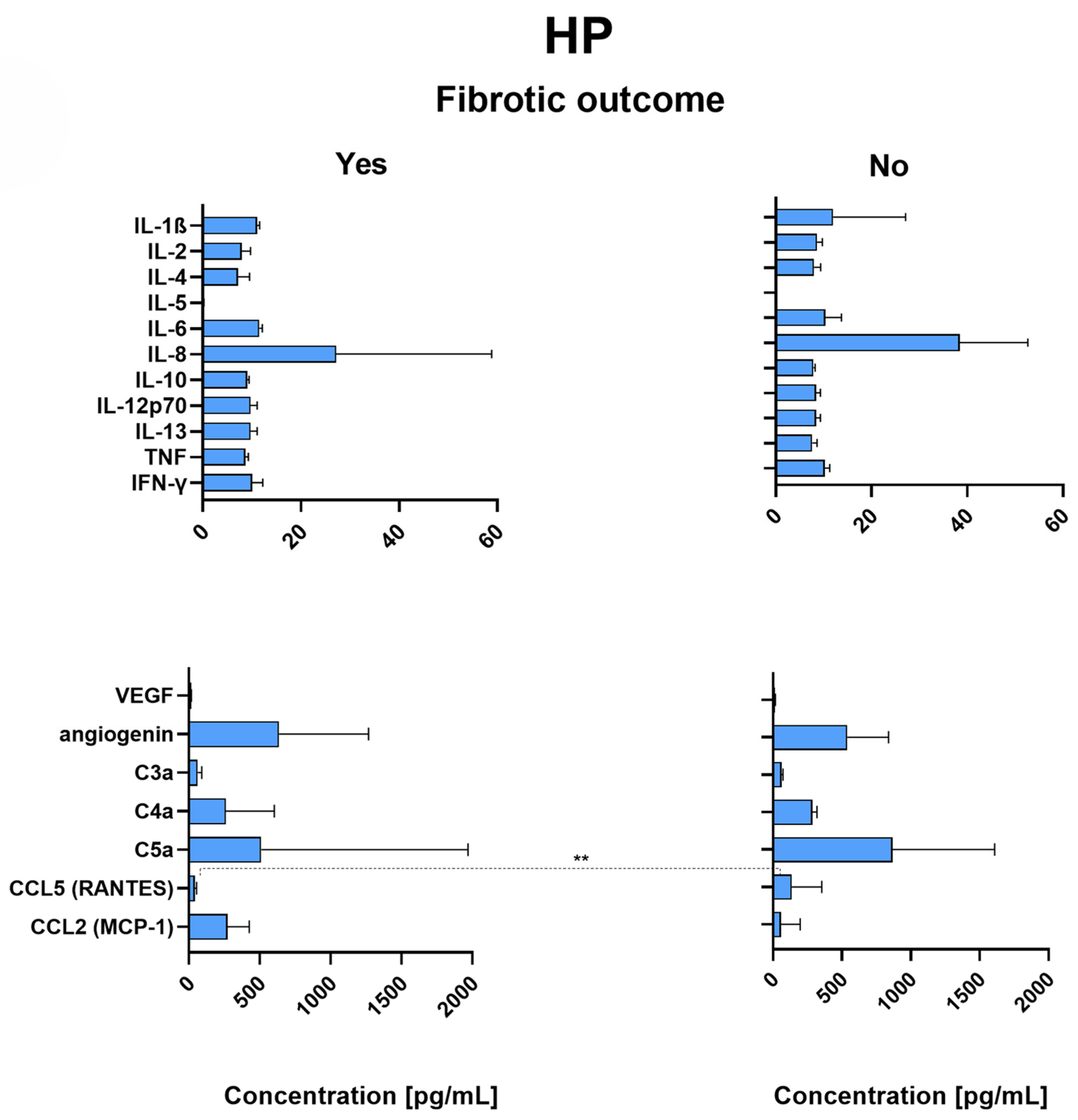
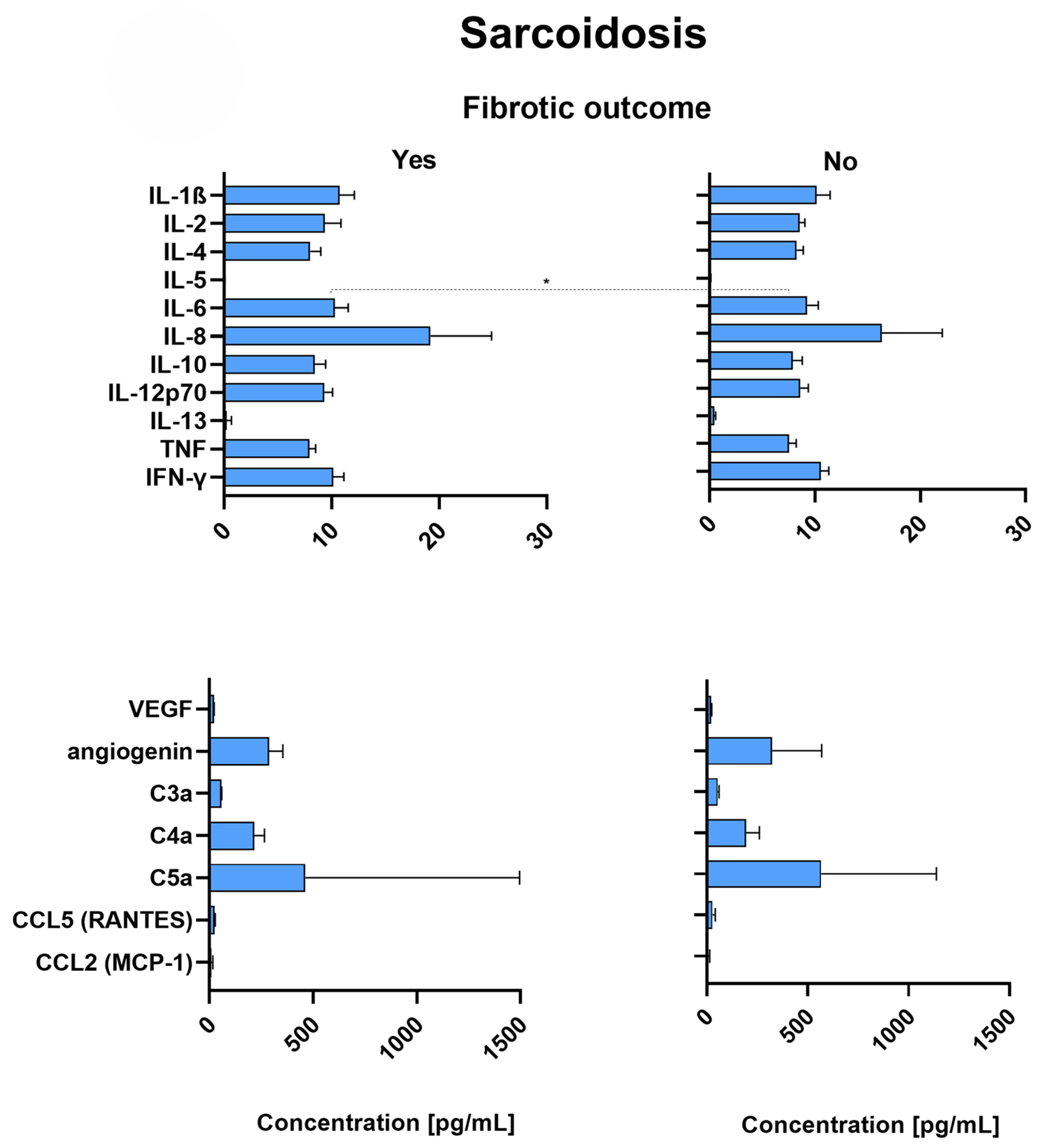
2.7. Patients with Fibrotic Outcome
2.8. RANTES Is Significantly Decreased in the Lungs of HP Patients with Fibrotic Outcome
2.9. Sarcoidosis Patients with Fibrotic Outcome Have Significantly Increased Levels of IL-6
3. Discussion
4. Materials and Methods
4.1. Research Design and Protocol
4.2. Methods
4.2.1. BAL Procedure
4.2.2. Measurements of Cytokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α, IFN-γ), Factors Related to Angiogenesis (VEGF, Angiogenin), Anaphylatoxins (C3a, C4a, C5a) and Chemokines CCL5 (RANTES), and CCL2 (MCP-1) in the Supernatant of Bronchoalveolar Lavage
4.2.3. Flow Cytometric Analysis of Lymphocytes in Bronchoalveolar Lavage
4.2.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ILD | interstitial lung disease |
| OLD | obstructive lung disease |
| HP | hypersensitivity pneumonitis |
| EGPA | eosinophilic granulomatosis with polyangiitis |
| BAL | bronchoalveolar lavage |
| BALF | bronchoalveolar lavage fluid |
| VC | vital capacity |
| FEV1 | forced expiratory volume in 1 s |
| NK | natural killer |
| NKT | natural killer T |
| IL | interleukin |
| TNF-α | tumor necrosis factor α |
| IFN-γ | interferon gamma |
| VEGF | vascular endothelial growth factor |
| MCP-1 | monocyte chemoattractant protein-1 |
| HRCT | high-resolution computed tomography |
| TLC | total lung capacity |
| DLCO | diffusing capacity of the lungs for carbon monoxide |
| FVC | forced vital capacity |
| COPD | chronic obstructive pulmonary disease |
References
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef] [PubMed]
- Vasakova, M.; Selman, M.; Morell, F.; Sterclova, M.; Molina-Molina, M.; Raghu, G. Hypersensitivity Pneumonitis: Current Concepts of Pathogenesis and Potential Targets for Treatment. Am. J. Respir. Crit. Care Med. 2019, 200, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Buist, A.S. Similarities and differences between asthma and chronic obstructive pulmonary disease: Treatment and early outcomes. Eur. Respir. J. Suppl. 2003, 39, 30s–35s. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Paul, I. Insights on immune profile, pathogenesis and differential diagnosis of hypersensitivity pneumonitis and pulmonary sarcoidosis—A holistic review and bibliometric analysis. Respir. Investig. 2025, 63, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Tao, X.; Fan, Y.; Teng, Y. Sarcoidosis: Molecular mechanisms and therapeutic strategies. Mol. Biomed. 2025, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Woodruff, P.G.; Zhou, X. Advances in non-type 2 severe asthma: From molecular insights to novel treatment strategies. Eur. Respir. J. 2024, 64, 2300826. [Google Scholar] [CrossRef] [PubMed]
- Schwaiblmair, M.; Berghaus, T.; Haeckel, T.; Wagner, T.; Von Scheidt, W. Amiodarone-induced pulmonary toxicity: An under-recognized and severe adverse effect? Clin. Res. Cardiol. 2010, 99, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Emmi, G.; Bettiol, A.; Gelain, E.; Bajema, I.M.; Berti, A.; Burns, S.; Cid, M.C.; Tervaert, J.W.C.; Cottin, V.; Durante, E.; et al. Evidence-Based Guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat. Rev. Rheumatol. 2023, 19, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Thomson, N.C.; Chaudhuri, R. Omalizumab: Clinical use for the management of asthma. Clin. Med. Insights Circ. Respir. Pulm. Med. 2012, 6, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, A.; Urban, M.L.; Dagna, L.; Cottin, V.; Franceschini, F.; Del Giacco, S.; Schiavon, F.; Neumann, T.; Lopalco, G.; Novikov, P.; et al. Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis: A European Multicenter Observational Study. Arthritis Rheumatol. 2022, 74, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Sweiss, N.J.; Lower, E.E.; Mirsaeidi, M.; Dudek, S.; Garcia, J.G.; Perkins, D.; Finn, P.W.; Baughman, R.P. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur. Respir. J. 2014, 43, 1525–1528. [Google Scholar] [CrossRef] [PubMed]
- Cinetto, F.; Compagno, N.; Scarpa, R.; Malipiero, G.; Agostini, C. Rituximab in refractory sarcoidosis: A single centre experience. Clin. Mol. Allergy 2015, 13, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamphuis, L.S.; Lam-Tse, W.-K.; Dik, W.A.; van Daele, P.L.; van Biezen, P.; Kwekkeboom, D.J.; Kuijpers, R.W.; Hooijkaas, H.; van Laar, J.A.; Bastiaans, J.; et al. Efficacy of Adalimumab in Chronically Active and Symptomatic Patients with Sarcoidosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, H.A.; van der Burg, L.M.; Vorselaars, A.D.; Drent, M.; van Moorsel, C.H.; Rijkers, G.T.; Deneer, V.H.; Grutters, J.C. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir. Med. 2016, 115, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Sakkat, A.; Cox, G.; Khalidi, N.; Larche, M.; Beattie, K.; Renzoni, E.A.; Morar, N.; Kouranos, V.; Kolb, M.; Hambly, N. Infliximab therapy in refractory sarcoidosis: A multicenter real-world analysis. Respir. Res. 2022, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Doty, J.D.; Mazur, J.E.; Judson, M.A. Treatment of Sarcoidosis with Infliximab. Chest 2005, 127, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Lota, H.K.; Keir, G.J.; Hansell, D.M.; Nicholson, A.G.; Maher, T.M.; Wells, A.U.; A Renzoni, E. Novel use of rituximab in hypersensitivity pneumonitis refractory to conventional treatment. Thorax 2013, 68, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.M.; Anzueto, A.R.; Peters, J.I. Effectiveness and safety of mycophenolate mofetil in idiopathic pulmonary fibrosis. PLoS ONE 2017, 12, e0176312. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Troy, L.; Lee, C.T.; Sperling, A.; Strek, M.; Glaspole, I. Hypersensitivity pneumonitis: Current concepts in pathogenesis, diagnosis, and treatment. Allergy 2022, 77, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of hypersensitivity pneumonitis in adults: An official ATS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef] [PubMed]
- Shanthikumar, S.; Gubbels, L.; Davies, K.; Walker, H.; Wong, A.T.C.; Levi, E.; Saffery, R.; Ranganathan, S.; Neeland, M.R. Highly multiplexed cytokine analysis of bronchoalveolar lavage and plasma reveals age-related dynamics and correlates of inflammation in children. Mucosal Immunol. 2024, 18, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Sobiecka, M.; Szturmowicz, M.; Lewandowska, K.B.; Barańska, I.; Zimna, K.; Łyżwa, E.; Dybowska, M.; Langfort, R.; Radwan-Röhrenschef, P.; Roży, A.; et al. Bronchoalveolar Lavage Cell Count and Lymphocytosis Are the Important Discriminators between Fibrotic Hypersensitivity Pneumonitis and Idiopathic Pulmonary Fibrosis. Diagnostics 2023, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Díez, S.; Cruz, M.J.; de Homdedeu, M.; Ojanguren, I.; Romero-Mesones, C.; Sansano, I.; Muñoz, X. Immunopathological Mechanisms of Bird-Related Hypersensitivity Pneumonitis. Int. J. Mol. Sci. 2023, 24, 2884. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palomares, L.; Martín-Juan, J.; Gómez-Izquierdo, L.; Cayuela-Domínguez, A.; Rodríguez-Becerra, E.; Rodríguez-Panadero, F. Bronchoalveolar Lavage Findings in Patients with Diffuse Interstitial Lung Disease: Prospective Study of a Cohort of 562 Patients. Arch. Bronconeumol. (Engl. Ed.) 2009, 45, 115–121. [Google Scholar] [CrossRef]
- Vasakova, M.; Sterclova, M.; Kolesar, L.; Slavcev, A.; Pohunek, P.; Sulc, J.; Skibova, J.; Striz, I. Bronchoalveolar Lavage Fluid Cellular Characteristics, Functional Parameters and Cytokine and Chemokine Levels in Interstitial Lung Diseases. Scand. J. Immunol. 2009, 69, 268–274. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Carleo, A.; Cameli, P.; Bergantini, L.; Perrone, A.; Vietri, L.; Lanzarone, N.; Vagaggini, C.; Sestini, P.; Bargagli, E. BAL biomarkers’ panel for differential diagnosis of interstitial lung diseases. Clin. Exp. Med. 2020, 20, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; D’Alessandro, M.; Cameli, P.; Otranto, A.; Finco, T.; Curatola, G.; Sestini, P.; Bargagli, E. Prognostic role of NK cell percentages in bronchoalveolar lavage from patients with different fibrotic interstitial lung diseases. Clin. Immunol. 2021, 230, 108827. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, M.; Gangi, S.; Soccio, P.; Cantó, E.; Osuna-Gómez, R.; Bergantini, L.; Cameli, P.; Fabbri, G.; Croce, S.; Scioscia, G.; et al. The Effects of Interstitial Lung Diseases on Alveolar Extracellular Vesicles Profile: A Multicenter Study. Int. J. Mol. Sci. 2023, 24, 4071. [Google Scholar] [CrossRef] [PubMed]
- Rzepka-Wrona, P.; Skoczyński, S.; Barczyk, A. Are There Differences in Inflammatory and Fibrotic Pathways between IPAF, CTD-ILDs, and IIPs? A Single-Center Pilot Study. Int. J. Mol. Sci. 2022, 23, 15205. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Tanaka, K.; Fujita, T.; Umezawa, H.; Amano, H.; Yoshioka, K.; Naito, Y.; Hatano, M.; Kimura, S.; Tatsumi, K.; et al. Bidirectional role of IL-6 signal in pathogenesis of lung fibrosis. Respir. Res. 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, S.W.; Park, C.S.; Kim BIl Kang, H.; Koh, Y.Y. Bronchoalveolar lavage cytokine profiles in acute asthma and acute bronchiolitis. J. Allergy Clin. Immunol. 2003, 112, 64–71. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Dubey, S. Eosinophilic granulomatosis with polyangiitis: A review. Autoimmun. Rev. 2023, 22, 103219. [Google Scholar] [CrossRef] [PubMed]
- Pesci, A.; Ricchiuti, E.; Ruggiero, R.; De Micheli, A. Bronchoalveolar lavage in idiopathic pulmonary fibrosis: What does it tell us? Respir. Med. 2010, 104, S70–S73. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | HP (n = 12) | Sarcoidosis (n = 56) | Non-Allergic Asthma (n = 14) | Amiodarone Lung (n = 5) | EGPA (n = 5) | p Value * |
|---|---|---|---|---|---|---|
| Age [years], median (range) | 68.5 (49–81) | 44.5 (25–79) | 43.5 (19–71) | 74 (65–79) | 50 (21–80) | <0.0001 |
| Sex, no. (%) | 0.5666 | |||||
| Male | 8 (66.7%) | 29 (51.8%) | 5 (35.7%) | 2 (40.0%) | 2 (40.0%) | |
| Female | 4 (33.3%) | 27 (48.2%) | 9 (64.3%) | 3 (60.0%) | 3 (60.0%) | |
| Smoking | 0.6500 | |||||
| Yes | 0 | 5 | 0 | 0 | 0 | |
| No | 10 | 43 | 11 | 5 | 4 | |
| Ex | 1 | 6 | 3 | 0 | 0 | |
| Pulmonary function, median (range) | ||||||
| VC | 3400 (600–5350) | 4255 (1800–7000) | 3085 (1640–5200) | 2400 (1600–3800) | 3060 (2500–4150) | 0.0126 |
| VC% | 97 (57–132) | 94 (63–123) | 85 (53–127) | 80 (26–93) | 71 (67–94) | 0.0481 |
| FEV1 | 2010 (1100–4000) | 3335 (1020–5150) | 2325 (750–4510) | 1780 (1300–2050) | 1850 (950–2320) | 0.0003 |
| FEV1% | 88 (37–130) | 90 (45–118) | 83 (30–113) | 81 (71–93) | 47 (37–90) | 0.0273 |
| Tiff% | 70 (42–86) | 76 (51–95) | 75 (43–96) | 76 (71–81) | 56 (38–75) | 0.0262 |
| BAL data, median (range) | ||||||
| Cells/ql | 140 (40–520) | 110 (40–420) | 90 (20–1240) | 100 (30–300) | 100 (50–320) | 0.6347 |
| Viability cells | 76.5 (28–92) | 78 (50–95) | 81.5 (50–96) | 84 (50–89) | 78 (71–95) | 0.7843 |
| Epithelial cells | 3 (0–22) | 3 (0–21) | 2 (1–8) | 1.5 (0–3) | 3 (0–8) | 0.5340 |
| Macrophages | 37.5 (14–87) | 68.5 (21–94) | 85 (8–95) | 85.5 (49–91) | 61 (47–83) | 0.0043 |
| Lymphocytes | 38.5 (2–84) | 29.5 (5–76) | 5.5 (2–29) | 7.5 (2–41) | 10 (3–14) | <0.0001 |
| Neutrophils | 5.5 (1–22) | 2 (1–8) | 5 (1–11) | 6.5 (4–7) | 2 (1–3) | 0.0002 |
| Eosinophils | 1.5 (1–43) | 1.5 (1–4) | 4.5 (1–78) | 2 (1–3) | 12 (4–31) | 0.0052 |
| CD3 | 84 (69–95) | 91 (74–98) | 85 (76–94) | 92 | Not measured | 0.1373 |
| CD4 | 52 (29–91) | 75 (39–89) | 50.5 (19–82) | 57 | Not measured | 0.0459 |
| CD8 | 24 (8–61) | 13 (4–38) | 35 (12–58) | 32 | Not measured | 0.0115 |
| CD19 | 0 (0–4) | 0 (0–4) | 0.5 (0–1) | 2 | Not measured | 0.4972 |
| NK | 3 (1–23) | 2 (0–5) | 2 (1–3) | 4 | Not measured | 0.3755 |
| NKT | 3 (2–13) | 3 (1–13) | 3.5 (2–5) | 3 | Not measured | 0.9581 |
| CD4/8 | 2.24 (0.48–11.38) | 6.45 (1.03–22) | 3.58 (0.33–6.83) | 1.78 | Not measured | 0.0099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greif Lenarčič, D.; Bidovec Stojković, U.; Kristanc, P.; Kopač, P.; Malovrh, M.M.; Kern, I.; Osolnik, K.; Korošec, P. Cytokine Profiles of Bronchoalveolar Lavage in Patients with Interstitial Lung Diseases and Non-Allergic Asthma. Int. J. Mol. Sci. 2025, 26, 6831. https://doi.org/10.3390/ijms26146831
Greif Lenarčič D, Bidovec Stojković U, Kristanc P, Kopač P, Malovrh MM, Kern I, Osolnik K, Korošec P. Cytokine Profiles of Bronchoalveolar Lavage in Patients with Interstitial Lung Diseases and Non-Allergic Asthma. International Journal of Molecular Sciences. 2025; 26(14):6831. https://doi.org/10.3390/ijms26146831
Chicago/Turabian StyleGreif Lenarčič, Dana, Urska Bidovec Stojković, Pia Kristanc, Peter Kopač, Mateja Marc Malovrh, Izidor Kern, Katarina Osolnik, and Peter Korošec. 2025. "Cytokine Profiles of Bronchoalveolar Lavage in Patients with Interstitial Lung Diseases and Non-Allergic Asthma" International Journal of Molecular Sciences 26, no. 14: 6831. https://doi.org/10.3390/ijms26146831
APA StyleGreif Lenarčič, D., Bidovec Stojković, U., Kristanc, P., Kopač, P., Malovrh, M. M., Kern, I., Osolnik, K., & Korošec, P. (2025). Cytokine Profiles of Bronchoalveolar Lavage in Patients with Interstitial Lung Diseases and Non-Allergic Asthma. International Journal of Molecular Sciences, 26(14), 6831. https://doi.org/10.3390/ijms26146831







