Role of AMP-Activated Protein Kinase (AMPK) in Female Reproduction: A Review
Abstract
1. Introduction
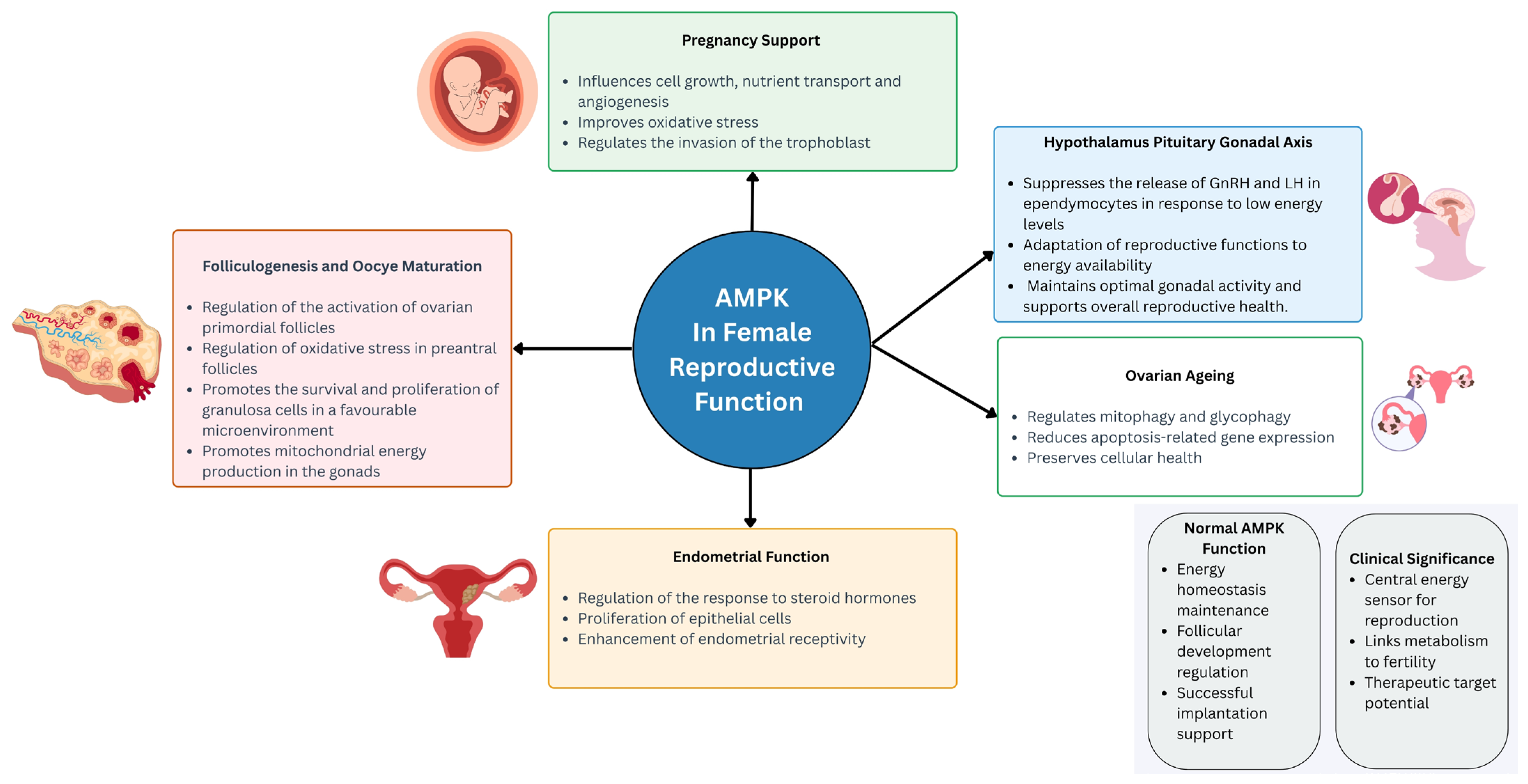
2. AMPK in the Female Reproductive System
2.1. AMPK in Female Reproductive Function
2.1.1. AMPK and Hypothalamus Pituitary Gonadal Axis
2.1.2. AMPK in Folliculogenesis and Oocyte Maturation
2.1.3. AMPK in Pregnancy
2.1.4. AMPK in Ovarian Ageing
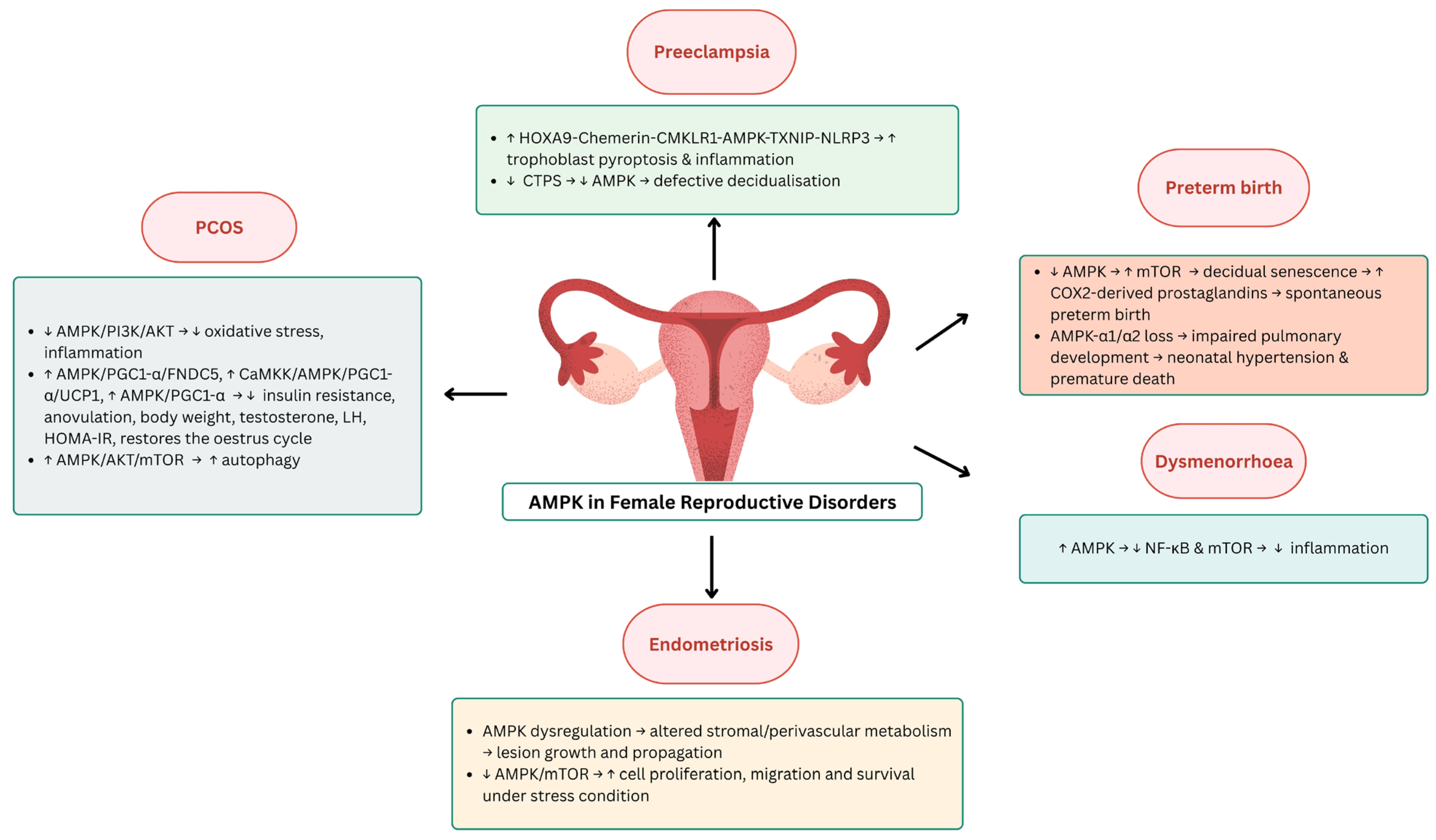
2.2. AMPK in Female Reproductive Diseases
2.2.1. AMPK in Pre-Eclampsia
2.2.2. AMPK in Premature Birth
2.2.3. AMPK in Polycystic Ovary Syndrome (PCOS)
2.2.4. AMPK in Endometriosis
2.2.5. AMPK in Dysmenorrhea
3. Limitations and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, X.E.; Xu, H.E.; Melcher, K. Structure and Physiological Regulation of AMPK. Int. J. Mol. Sci. 2018, 19, 3534. [Google Scholar] [CrossRef] [PubMed]
- Grahame Hardie, D. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Paz, M.; Cotán, D.; Garrido-Maraver, J.; Oropesa-Ávila, M.; de la Mata, M.; Delgado-Pavón, A.; de Lavera, I.; Alcocer-Gómez, E.; Álvarez-Córdoba, M.; Sánchez-Alcázar, J.A. AMPK Regulation of Cell Growth, Apoptosis, Autophagy, and Bioenergetics. In AMP-Activated Protein Kinase; Cordero, M.D., Viollet, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 45–71. [Google Scholar] [CrossRef]
- Guigas, B.; Viollet, B. Targeting AMPK: From Ancient Drugs to New Small-Molecule Activators. In AMP-Activated Protein Kinase; Cordero, M.D., Viollet, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 327–350. [Google Scholar] [CrossRef]
- Gu, X.; Yan, Y.; Novick, S.J.; Kovach, A.; Goswami, D.; Ke, J.; Tan, M.H.E.; Wang, L.; Li, X.; de Waal, P.W.; et al. Deconvoluting AMP-activated protein kinase (AMPK) adenine nucleotide binding and sensing. J. Biol. Chem. 2017, 292, 12653–12666. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Calabrese, M.F. Structure and Regulation of AMPK. In AMP-Activated Protein Kinase; Cordero, M.D., Viollet, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–22. [Google Scholar] [CrossRef]
- Baumgartner, C.; Yadav, A.K.; Chefetz, I. Chapter Ten—AMPK-like proteins and their function in female reproduction and gynecologic cancer. In Advances in Protein Chemistry and Structural Biology; Academic Press: Cambridge, MA, USA, 2023; Volume 134, pp. 245–270. [Google Scholar]
- Liang, M.; Li, J.W.; Luo, H.; Lulu, S.; Calbay, O.; Shenoy, A.; Tan, M.; Law, B.K.; Huang, S.; Xiao, T.S.; et al. Epithelial-Mesenchymal Transition Suppresses AMPK and Sensitizes Cancer Cells to Pyroptosis under Energy Stress. Cells 2022, 11, 2208. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, L.; Wang, F.; Yuan, S. Roles of AMP-Activated Protein Kinase (AMPK) in Mammalian Reproduction. Front. Cell Dev. Biol. 2020, 8, 593005. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.L. PRKA/AMPK: Integrating Energy Status with Fertility in Pituitary Gonadotrophs. Biol. Reprod. 2010, 84, 205–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McCallum, M.L.; Pru, C.A.; Smith, A.R.; Kelp, N.C.; Foretz, M.; Viollet, B.; Du, M.; Pru, J.K. A functional role for AMPK in female fertility and endometrial regeneration. Reproduction 2018, 156, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.M.; Pru, C.A.; Behura, S.K.; Cronrath, A.R.; McCallum, M.L.; Kelp, N.C.; Winuthayanon, W.; Spencer, T.E.; Pru, J.K. AMPK is required for uterine receptivity and normal responses to steroid hormones. Reproduction 2020, 159, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Minabe, S.; Deura, C.; Ikegami, K.; Goto, T.; Sanbo, M.; Hirabayashi, M.; Inoue, N.; Uenoyama, Y.; Maeda, K.-i.; Tsukamura, H. Pharmacological and Morphological Evidence of AMPK-Mediated Energy Sensing in the Lower Brain Stem Ependymocytes to Control Reproduction in Female Rodents. Endocrinology 2015, 156, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Franssen, D.; Barroso, A.; Ruiz-Pino, F.; Vázquez, M.J.; García-Galiano, D.; Castellano, J.M.; Onieva, R.; Ruiz-Cruz, M.; Poutanen, M.; Gaytán, F.; et al. AMP-activated protein kinase (AMPK) signaling in GnRH neurons links energy status and reproduction. Metab. Clin. Exp. 2021, 115, 154460. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Bongrani, A.; Ramé, C.; Kurowska, P.; Błaszczyk, K.; Rak, A.; Ducluzeau, P.-H.; Froment, P.; Dupont, J. Energy sensors and reproductive hypothalamo-pituitary ovarian axis (HPO) in female mammals: Role of mTOR (mammalian target of rapamycin), AMPK (AMP-activated protein kinase) and SIRT1 (Sirtuin 1). Mol. Cell. Endocrinol. 2021, 521, 111113. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.K.; Briski, K.P. Hindbrain lactate regulates preoptic gonadotropin-releasing hormone (GnRH) neuron GnRH-I protein but not AMPK responses to hypoglycemia in the steroid-primed ovariectomized female rat. Neuroscience 2015, 298, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Santiquet, N.; Sasseville, M.; Laforest, M.; Guillemette, C.; Gilchrist, R.B.; Richard, F.J. Activation of 5′ adenosine monophosphate-activated protein kinase blocks cumulus cell expansion through inhibition of protein synthesis during in vitro maturation in Swine. Biol. Reprod. 2014, 91, 51. [Google Scholar] [CrossRef] [PubMed]
- Bowdridge, E.C.; Goravanahally, M.P.; Inskeep, E.K.; Flores, J.A. Activation of Adenosine Monophosphate-Activated Protein Kinase Is an Additional Mechanism that Participates in Mediating Inhibitory Actions of Prostaglandin F2Alpha in Mature, but Not Developing, Bovine Corpora Lutea. Biol. Reprod. 2015, 93, 7. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Arvisais, E.W.; Davis, J.S. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: Modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 2010, 151, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Plewes, M.R.; Przygrodzka, E.; Monaco, C.F.; Snider, A.P.; Keane, J.A.; Burns, P.D.; Wood, J.R.; Cupp, A.S.; Davis, J.S. Prostaglandin F2α regulates mitochondrial dynamics and mitophagy in the bovine corpus luteum. Life Sci. Alliance 2023, 6, e202301968. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.W.; Kawamura, K.; Cheng, Y.; Fauser, B.C.J.M. Intraovarian Control of Early Folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wei, Y.; Gao, X.; Song, Y.; Huang, Y.; Jiang, Q. Unveiling the Ovarian Cell Characteristics and Molecular Mechanism of Prolificacy in Goats via Single-Nucleus Transcriptomics Data Analysis. Curr. Issues Mol. Biol. 2024, 46, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, G.; Cimadomo, D.; Innocenti, F.; Soscia, D.; Vaiarelli, A.; Ubaldi, F.M.; Gennarelli, G.; Garagna, S.; Rienzi, L.; Zuccotti, M. Biomechanical forces and signals operating in the ovary during folliculogenesis and their dysregulation: Implications for fertility. Hum. Reprod. Update 2022, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.; Kumar, M.; Kalmbach, K. Oocyte competency is the key to embryo potential. Fertil. Steril. 2015, 103, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, Y.; Pan, X.; Wang, P.; Yuan, X.; Ma, B. Advances in Oocyte Maturation In Vivo and In Vitro in Mammals. Int. J. Mol. Sci. 2023, 24, 9059. [Google Scholar] [CrossRef] [PubMed]
- Vollenhoven, B.; Hunt, S. Ovarian ageing and the impact on female fertility [version 1; peer review: 2 approved]. F1000Research 2018, 7, 1835. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Guo, S.; Cheng, Y.; Kim, J.H.; Feng, Y.; Feng, Y. Stimulation of ovarian follicle growth after AMPK inhibition. Reproduction 2017, 153, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, R.; Zou, J.; Ying, Y.; Luo, Z. Dual Roles of the AMP-Activated Protein Kinase Pathway in Angiogenesis. Cells 2019, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, Y.; Pang, W.; Li, C.; Xie, X.; Shyy, J.Y.; Zhu, Y. AMP-activated protein kinase promotes the differentiation of endothelial progenitor cells. Arter. Thromb. Vasc. Biol. 2008, 28, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hao, M.; Zhang, J.; Chen, Z.; Zhou, J.; Wang, C.; Zhang, H.; Wang, J. FSHR-mTOR-HIF1 signaling alleviates mouse follicles from AMPK-induced atresia. Cell Rep. 2023, 42, 113158. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.F.; Ernst, E.H.; Amoushahi, M.; Dueholm, M.; Ernst, E.; Lykke-Hartmann, K. Dorsomorphin inhibits AMPK, upregulates Wnt and Foxo genes and promotes the activation of dormant follicles. Commun. Biol. 2024, 7, 747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yue, W.; Zhu, M.J.; Sreejayan, N.; Du, M. AMP-activated protein kinase (AMPK) cross-talks with canonical Wnt signaling via phosphorylation of beta-catenin at Ser 552. Biochem. Biophys. Res. Commun. 2010, 395, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ji, S.Y.; Yang, J.L.; Li, X.X.; Zhang, J.; Zhang, Y.; Hu, Z.Y.; Liu, Y.X. Wnt/β-catenin signaling regulates follicular development by modulating the expression of Foxo3a signaling components. Mol. Cell. Endocrinol. 2014, 382, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Chen, X.; Wang, J.; Lv, B.; Zhang, J.; Ni, B.; Xue, Z. Mitochondria: The panacea to improve oocyte quality? Ann. Transl. Med. 2019, 7, 789. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.M.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhong, Y.; Li, Y.; Liu, S.; Pan, X. Astaxanthin Alleviates Oxidative Stress in Mouse Preantral Follicles and Enhances Follicular Development Through the AMPK Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 2241. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Nawaz, A.; Hecht, K.; Tobe, K. Astaxanthin as a Novel Mitochondrial Regulator: A New Aspect of Carotenoids, beyond Antioxidants. Nutrients 2021, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Z.; Xia, F.; Jia, R.; Wang, Y.; Bai, Y.; Wei, C.; Chen, Y.; Lu, M.; Shi, D.; et al. Beta-aminoisobutyric acid improves bovine oocyte maturation and subsequent embryonic development by promoting lipid catabolism. Theriogenology 2025, 234, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xue, Y.; Wang, H.; Ma, Z.; Hu, N.; Zhang, C.; Gao, Y.; Fan, R.; Hu, L.; Li, J.; et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation caused fertility decline in female mouse offspring. Ecotoxicol. Environ. Saf. 2025, 289, 117632. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Vasam, G.; Green, A.E.; Bainbridge, S.A.; Menzies, K.J. Placental Mitochondrial Function and Dysfunction in Preeclampsia. Int. J. Mol. Sci. 2023, 24, 4177. [Google Scholar] [CrossRef] [PubMed]
- Afrose, D.; Alfonso-Sánchez, S.; McClements, L. Targeting oxidative stress in preeclampsia. Hypertens. Pregnancy 2025, 44, 2445556. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, P.; Zhang, F.; Zhang, L.; Zheng, Y.; Hu, M.; Wang, L.; Han, T.-l.; Peng, C.; Wang, L.; et al. AMPK Hyper-Activation Alters Fatty Acids Metabolism and Impairs Invasiveness of Trophoblasts in Preeclampsia. Cell. Physiol. Biochem. 2018, 49, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Xiao, S.; Diao, L.; Lian, R.; Chen, C.; Zeng, Y.; Liu, S. Decreased AMPK/SIRT1/PDK4 induced by androgen excess inhibits human endometrial stromal cell decidualization in PCOS. Cell. Mol. Life Sci. 2024, 81, 324. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chaudhry, P.; Asselin, E. Bridging endometrial receptivity and implantation: Network of hormones, cytokines, and growth factors. J. Endocrinol. 2011, 210, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Sun, Y.; Gao, H.; Wu, H.; Wang, Z. Carbon disulfide induces embryo loss by perturbing the expression of the mTOR signalling pathway in uterine tissue in mice. Chem. Biol. Interact. 2019, 300, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.A.; Tee, A.R. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem. Soc. Trans. 2013, 41, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Wu, C.P.; Chen, S.F. Differential Changes in Akt and AMPK Phosphorylation Regulating mTOR Activity in the Placentas of Pregnancies Complicated by Fetal Growth Restriction and Gestational Diabetes Mellitus with Large-For-Gestational Age Infants. Front. Med. 2021, 8, 788969. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gan, B.; Zheng, S.; Zhao, X.; Jin, L.; Wei, J. AMPK-mTOR pathway modulates glycolysis reprogramming in unexplained recurrent spontaneous abortion. BMC Pregnancy Childbirth 2024, 24, 840. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, H.; Liu, M.; Yuan, Y.; Wang, Z.; Chen, Y.; Wei, J.; Su, F.; Zhang, J. Treg/Th17 Cell Imbalance and IL-6 Profile in Patients with Unexplained Recurrent Spontaneous Abortion. Reprod. Sci. 2017, 24, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, Y.; Ma, T.; Jing, L. Repurposing Metformin in hematologic tumor: State of art. Curr. Probl. Cancer 2023, 47, 100972. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Morentin, P.B.; Lage, R.; González-García, I.; Ruíz-Pino, F.; Martins, L.; Fernández-Mallo, D.; Gallego, R.; Fernø, J.; Señarís, R.; Saha, A.K.; et al. Pregnancy Induces Resistance to the Anorectic Effect of Hypothalamic Malonyl-CoA and the Thermogenic Effect of Hypothalamic AMPK Inhibition in Female Rats. Endocrinology 2015, 156, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.B.; Sampaio, O.G.M.; Câmara, F.E.A.; Schneider, A.; de Ávila, B.M.; Prosczek, J.; Masternak, M.M.; Campos, A.R. Ovarian aging in humans: Potential strategies for extending reproductive lifespan. GeroScience 2023, 45, 2121–2133. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Guo, C.; Yang, Z.; Wu, Y.; Zhang, C. Follicle-Stimulating Hormone Alleviates Ovarian Aging by Modulating Mitophagy- and Glycophagy-Based Energy Metabolism in Hens. Cells 2022, 11, 3270. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, C.; La, B.; Cao, M.; Ning, S.; Zhou, J.; Yan, Z.; Li, C.; Cui, Y.; Ma, X.; et al. Human amnion-derived mesenchymal stem cells improved the reproductive function of age-related diminished ovarian reserve in mice through Ampk/FoxO3a signaling pathway. Stem Cell Res. Ther. 2021, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, X.; Chen, Y.; Yuan, Q.; Yang, Z.; Mi, Y.; Zhang, C. Nobiletin Ameliorates Aging of Chicken Ovarian Prehierarchical Follicles by Suppressing Oxidative Stress and Promoting Autophagy. Cells 2024, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular Trends in the Rates of Preeclampsia, Eclampsia, and Gestational Hypertension, United States, 1987–2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Duley, L. The Global Impact of Pre-eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Keyes, K.M.; Wapner, R.J. Pre-eclampsia rates in the United States, 1980–2010: Age-period-cohort analysis. BMJ 2013, 347, f6564. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.-Z.; Ye, J.-H.; Yang, X.-Z.; Xie, Y. HOXA9-induced chemerin signals through CMKLR1/AMPK/TXNIP/NLRP3 pathway to induce pyroptosis of trophoblasts and aggravate preeclampsia. Exp. Cell Res. 2021, 408, 112802. [Google Scholar] [CrossRef] [PubMed]
- Socha, M.W.; Malinowski, B.; Puk, O.; Dubiel, M.; Wiciński, M. The NLRP3 Inflammasome Role in the Pathogenesis of Pregnancy Induced Hypertension and Preeclampsia. Cells 2020, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, N.; Ding, Y.; Zhang, Y.; Li, Q.; Flores, J.; Haghighiabyaneh, M.; Doycheva, D.; Tang, J.; Zhang, J.H. Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav. Immun. 2018, 70, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, N.; Ding, Y.; Doycheva, D.M.; Zhang, Y.; Li, Q.; Flores, J.; Haghighiabyaneh, M.; Tang, J.; Zhang, J.H. Chemerin reverses neurological impairments and ameliorates neuronal apoptosis through ChemR23/CAMKK2/AMPK pathway in neonatal hypoxic-ischemic encephalopathy. Cell Death Dis. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, N.; Tola, E.; Temel Yuksel, I.; Aslan Cetin, B.; Turhan, U.; Topcu, G.; Dag, I. Maternal serum AMP-activated protein kinase levels in mild and severe preeclampsia. J. Matern. Fetal Neonatal Med. 2019, 32, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, N.; Yan, X.; Zhu, Y.; Zhang, C. Energy deficiency caused by CTPS downregulation in decidua may contribute to pre-eclampsia by impairing decidualization. J. Cell. Physiol. 2021, 236, 6520–6533. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zeng, Y.; Li, L.; Wang, J.; Peng, D.; Zhang, T.; Zhang, H.; An, X. Electroacupuncture Upregulated Ghrelin in Rats with Functional Dyspepsia via AMPK/TSC2/Rheb-Mediated mTOR Inhibition. Dig. Dis. Sci. 2020, 65, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Tekeba, B.; Techane, M.A.; Workneh, B.S.; Zegeye, A.F.; Gonete, A.T.; Ahmed, M.A.; Wassie, Y.A.; Wassie, M.; Kassie, A.T.; Ali, M.S.; et al. Determinants of preterm birth among reproductive age women in sub-Saharan Africa: Evidence from the most recent Demographic and Health Survey data-2019–2022. PLoS ONE 2024, 19, e0305810. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, A.; Itakura, A.; Koya, D.; Kanasaki, K. AMP-Activated Protein (AMPK) in Pathophysiology of Pregnancy Complications. Int. J. Mol. Sci. 2018, 19, 3076. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Cha, J.; Yuan, J.; Haraguchi, H.; Bartos, A.; Leishman, E.; Viollet, B.; Bradshaw, H.B.; Hirota, Y.; Dey, S.K. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J. Clin. Investig. 2016, 126, 2941–2954. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Lappas, M. Activation of AMPK in human fetal membranes alleviates infection-induced expression of pro-inflammatory and pro-labour mediators. Placenta 2015, 36, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, K.; Palmsten, K.; Sugimoto, H.; Ahmad, S.; Hamano, Y.; Xie, L.; Parry, S.; Augustin, H.G.; Gattone, V.H.; Folkman, J.; et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008, 453, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.A.; Albers, R.E.; Doliboa, S.R.; Hughes, M.; Wyatt, C.N.; Natale, D.R.; Brown, T.L. AMPK knockdown in placental trophoblast cells results in altered morphology and function. Stem Cells Dev. 2014, 23, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Moral-Sanz, J.; Lewis, S.A.; MacMillan, S.; Meloni, M.; McClafferty, H.; Viollet, B.; Foretz, M.; del-Pozo, J.; Mark Evans, A. AMPK deficiency in smooth muscles causes persistent pulmonary hypertension of the new-born and premature death. Nat. Commun. 2022, 13, 5034. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, A.; Szabó, I. Endocrine characteristics of polycystic ovary syndrome (PCOS). Indian J. Exp. Biol. 2003, 41, 694–700. [Google Scholar] [PubMed]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.J.M.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L. Current concepts of polycystic ovary syndrome pathogenesis. Curr. Opin. Pediatr. 2020, 32, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.A.M.; Ibrahim, S.F.; Ugusman, A.; Mokhtar, M.H. Kelulut Honey Ameliorates Oestrus Cycle, Hormonal Profiles, and Oxidative Stress in Letrozole-Induced Polycystic Ovary Syndrome Rats. Antioxidants 2022, 11, 1879. [Google Scholar] [CrossRef] [PubMed]
- Dennett, C.C.; Simon, J. The role of polycystic ovary syndrome in reproductive and metabolic health: Overview and approaches for treatment. Diabetes Spectr. 2015, 28, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Wahid, S.; Ramli, M.D.C.; Fazleen, N.E.; Naim, R.M.; Mokhtar, M.H. Exploring the Therapeutic Potential of Natural Products in Polycystic Ovarian Syndrome (PCOS): A Mini-Review of Lipid Profile, Blood Glucose, and Ovarian Histological Improvements. Life 2024, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, P.E.; Abo El Nasr, N.M.E.; Shabana, M.E.; Saleh, D.O. Fisetin mitigates letrozole-induced polycystic ovarian syndrome in rats: Crosstalk of AMPK/PI3K/AKT-mediated-Nrf2 antioxidant defense mechanism and the inflammasome NLRP3/NF-κB P65/IL-1β signaling pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 8077–8088. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mo, H.; Chen, W.; Li, L.; Xiao, Y.; Zhang, J.; Li, X.; Lu, Y. Role of the PI3K-Akt Signaling Pathway in the Pathogenesis of Polycystic Ovary Syndrome. Reprod. Sci. 2017, 24, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; D’Amico, R.; Fusco, R.; Peritore, A.F.; Genovese, T.; Interdonato, L.; Franco, G.; Arangia, A.; Gugliandolo, E.; Crupi, R.; et al. Discovering the Effects of Fisetin on NF-κB/NLRP-3/NRF-2 Molecular Pathways in a Mouse Model of Vascular Dementia Induced by Repeated Bilateral Carotid Occlusion. Biomedicines 2022, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Sui, C.; Guo, Y.; He, X. Woxuanzhongzhou formula improves DHEAS and high-fat diet-induced IR and anovulatory mice via AMPK/PGC1-α/Irisin pathway. J. Ovarian Res. 2025, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, S.; Xu, L.; Li, B.; Chen, N. Metformin-induced autophagy and irisin improves INS-1 cell function and survival in high-glucose environment via AMPK/SIRT1/PGC-1α signal pathway. Food Sci. Nutr. 2019, 7, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Chen, A.; Ye, Y.; Ren, Y.; Lu, J.; Xuan, F.; Zhou, W. Effect of berberine combined with metformin on autophagy in polycystic ovary syndrome by regulating AMPK/AKT/mTOR pathway. Mol. Reprod. Dev. 2024, 91, e23768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; He, M.; Zhu, Z.; Zhang, T.; Zheng, W.; Qin, S.; Gao, M.; Wang, W.; Chen, Z.; Han, J.; et al. P62 promotes FSH-induced antral follicle formation by directing degradation of ubiquitinated WT1. Cell. Mol. Life Sci. 2024, 81, 221. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, R.; Neto, A.C.; Rodrigues, A.R.; Gouveia, A.M.; Almeida, H.; Neves, D. Anti-oxidant effect of metformin through AMPK/SIRT1/PGC-1α/SIRT3– independent GPx1 expression in the heart of mice with endometriosis. Horm. Mol. Biol. Clin. Investig. 2022, 43, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Sarsenova, M.; Lawarde, A.; Pathare, A.D.S.; Saare, M.; Modhukur, V.; Soplepmann, P.; Terasmaa, A.; Käämbre, T.; Gemzell-Danielsson, K.; Lalitkumar, P.G.L.; et al. Endometriotic lesions exhibit distinct metabolic signature compared to paired eutopic endometrium at the single-cell level. Commun. Biol. 2024, 7, 1026. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, X.; Hou, S.; Wu, Z.; Xu, X.; Pang, C. Intracavitary physiotherapy combined with acupuncture mediated AMPK/mTOR signalling to improve endometrial receptivity in patients with thin endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 277, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Assaf, L.; Eid, A.A.; Nassif, J. Role of AMPK/mTOR, mitochondria, and ROS in the pathogenesis of endometriosis. Life Sci. 2022, 306, 120805. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.A.; Fogleman, C.D. Dysmenorrhea. Am. Fam. Physician 2021, 104, 164–170. [Google Scholar] [PubMed]
- Gutman, G.; Nunez, A.T.; Fisher, M. Dysmenorrhea in adolescents. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101186. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M.J. Primary Dysmenorrhea: Pathophysiology, Diagnosis, and Treatment Updates. Korean J. Fam. Med. 2022, 43, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Sato, H.; Goto, K.; Nishida, M.; Nasu, K. The inhibitory effect of AMP-activated protein kinase (AMPK) on chemokine and prostaglandin production in human endometrial stromal cells. Reprod. Biol. Endocrinol. 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
| Experimental Model | Intervention | Key Findings | AMPK-Related Pathway | Reference |
|---|---|---|---|---|
| Rat model | 2 µmol/8 µL, 200 µM AICAR infusion into 4th ventricle | ↓ LH pulsatility ↑ ependymocytes Ca2+ | AMPK | [16] |
| Ovariectomized, steroid-primed adult female Sprague–Dawley rats | 12.5 U/kg subcutaneous insulin injection 25 µM/2.0 µL/h continuous infusion of L-lactate into the caudal fourth ventricle | ↓ pAMPK, Fos protein profiles ↑ DbH protein | AMPK/GnRH | [19] |
| In vitro mouse ovaries | 0, 3, 10, 30 and 100 μM compound C treatment for 0, 1, 4, 8, 24 and 96 h | ↑ phosphorylation of ovarian mTOR, ribosomal protein S6, eIF4B ↓ TSC2 phosphorylation ↑ ovarian weights ↑ ovarian angiogenesis ↑ antral and preovulatory follicles ↑ Hif1a, Vegfa, Vegfr2 and Ctgf | AMPK/TSC2/mTOR/eIF4B/S6; AMPK/mTOR; AMPK/CTGF | [30] |
| In vitro juvenile mice ovaries | Dorsomorphin or metformin treatment | ↑ activation of primordial follicles | AMPK/Foxo/Wnt | [34] |
| In vitro mice preantral follicles | 2.5 nM astaxanthin treatment for 10 days | ↑ antrum formation and maturation rates ↑ area of follicle attachment ↑ estradiol ↓ follicular malondialdehyde ↑ GSH, SOD ↓ ROS ↑ p-AMPK, PGC-1α, NRF2, HO-1, CO1, CO2, CO3, ATP6, ATP8, TOM20, PINK1, Parkin, LC3-II, Bcl-2, StAR, P450scc ↑ mitochondrial membrane potential ↓ caspase 3, Bax, P53 | AMPK/PGC-1α, NRF2/HO-1; PINK1/Parkin/LC3-II; NRF1/TFAM; Bcl-2/Bax/P53/caspase 3; StAR/P450scc | [40,41] |
| In vitro maturation bovine oocyte | 10, 20, 50, 100, and 200 μmol/L BAIBA treatment | ↑ oocyte maturation ↑ CPT1A, CPT1B, CPT2 ↑ lipid metabolism ↓ lipid content ↑ mitochondrial membrane potential and active content | AMPK | [42] |
| Pregnant Kunming mice (F0 generation) | 30 mg/kg/day polystyrene nanoplastics via intragastric administration from 0.5 gestation day to 21 days postpartum | ↓ fertility of female F1 offspring ↑ rates of miscarriage and premature delivery ↓ litter size in the F0 generation ↓ primordial follicles ↑ growing follicles ↓ transzonal projections (TZPs) in the ovaries of adult F1 mice ↓ CAMKIIβ, Smad3 phosphorylation, E-cadherin ↑ oestrous phase duration ↓ diestrus phase duration ↓ serum levels of AMH and E2 in adult F1 progeny during proestrus ↓ body weight in offspring mice | AKT-FOXO3a | [43] |
| URSA mouse model (CBA/J × DBA/2) | 50 mg/kg/day subcutaneous metformin for 14 days 2 mg and 8 mg/kg/day intraperitoneal 2-DG for 14 days | ↓ rate of abortion ↓ cell degeneration and necrosis ↓ trophoblast inflammatory cell infiltration ↓ mTOR, GLUT1, and HK2 ↑ Foxp3 and IL-10, Tregs cells ↓ RORγt, IL-17, Th17 cells ↑ Treg/Th17 ratio | AMPK/mTOR | [53] |
| In vitro ageing chicken granulosa cells (GCs) | D-galactose (0, 12.5, 25, 50, 100, 200 mM) for 12 h or 24 h; FSH (0, 0.001, 0.01, 0.1, 1 IU/mL) for a further 24 h | Activates mitophagy Relieves mitochondrial oedema ↑ number of mitophagosomes ↑ mitochondrial light chain 3 (LC3) | AMPK-PI3K/AKT | [58] |
| Age-related diminished ovarian reserve mice | low dose (LD, 5.0 × 106 cells/kg), middle dose (MD, 7.5 × 106 cells/kg), and high dose (HD, 10.0 × 106 cells/kg) of human amnion-derived mesenchymal stem cells (hAMSCs) | ↑ ovarian function ↓ apoptosis of granulosa and stromal cells ↑ AMPK and the ratio of phosphorylated FoxO3a to total FoxO3a ↑ SOD2 | AMPK/FoxO3a | [59] |
| In vitro D-galactose-generated senescent SWFs granulosa cells | 1 to 100 μg/mL nobiletin treatment | Activates cell autophagy ↑ antioxidant capacity ↓ expression genes associated with cell apoptosis alleviates mitochondrial oedema | AMPK/SIRT1 | [60] |
| Experimental Model | Intervention | Key Findings | AMPK-Related Pathway | Reference |
|---|---|---|---|---|
| p-53 deficient mice model | 1 mg/kg of oral metformin on days 8, 10, and 12 30 mg/kg of oral resveratrol on days 8, 10, 12, and 14 | ↓ premature decidual senescence ↓ spontaneous and inflammation-induced preterm birth ↓ AMPK and mTORC1 signalling in decidual cells | AMPK/mTOR | [78] |
| Letrozole-induced PCOS rat model | 1.25 or 2.5 mg/kg/day of oral fisetin for 14 days | ↓ LH and FSH ↑ AMH ↑ Nrf2 ↓ NLRP3 | AMPK/PI3K/AKT; NLRP3/NF-κB p65/IL-1β | [89] |
| DHEA and HFD-induced PCOS-IR mice model | 270 mg/kg/day of gavage WXZZ for 2 weeks | ↓ body weight ↓ serum testosterone ↓ LH and LH/FSH ratio ↓ FINS ↓ HOMA-IR ↓ serum NEFA levels ↓ serum irisin levels ↓ lipid accumulation ↑ AMPK, PGC-1α, FNDC5, and irisin in gastrocnemius ↑ CaMKK, AMPK, PGC1-α, and UCP1 in subcutaneous fat | AMPK/PGC1-α/FDNC5; CaMKK/AMPK/UCP1 | [92] |
| DHEA-induced PCOS rat model | 150 mg/kg/day of gavage berberine, 300 mg/kg/day of gavage metformin or a combination of both for 30 days | ↓ body weight ↓ ovarian weight ↑ number of primordial and primary follicles ↓ number of secondary and atretic follicles Normalised the oestrous cycle Improved insulin resistance, androgen biosynthesis, oxidative stress and lipid metabolism disorders ↑ oestrogen ↓ autophagosomes in granulosa cells ↓ Beclin1 and LC3II/LC3I levels ↑ p62 | AMPK/AKT/mTOR | [94] |
| Surgically induced endometriosis female B6CBA/F1 mouse model | 50 mg/kg/day of oral metformin for 3 months | ↑ pAMPKα ↑ GPx1 ↑ miR-34a, miR-195, miR-155, and miR-421 | AMPK/SIRT1/PGC1-α/SIRT3 | [97] |
| Human endometrial stromal cells (ESCs) | AICAR treatment | ↓ inflammatory cytokines (IL-8 and MCP-1) ↓ prostaglandins (PGE2 and PGF2α) ↓ COX-2 ↓ phosphorylation of IκB, 4EBP-1, p70S6K and S6 ribosomal protein | AMPK/NF-κB/mTOR | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamar Bashah, N.A.; Hamid, A.A.; Adam, S.H.; Jaffar, F.H.F.; Abd Rahman, I.Z.; Mokhtar, M.H. Role of AMP-Activated Protein Kinase (AMPK) in Female Reproduction: A Review. Int. J. Mol. Sci. 2025, 26, 6833. https://doi.org/10.3390/ijms26146833
Kamar Bashah NA, Hamid AA, Adam SH, Jaffar FHF, Abd Rahman IZ, Mokhtar MH. Role of AMP-Activated Protein Kinase (AMPK) in Female Reproduction: A Review. International Journal of Molecular Sciences. 2025; 26(14):6833. https://doi.org/10.3390/ijms26146833
Chicago/Turabian StyleKamar Bashah, Nurul Ain, Adila A. Hamid, Siti Hajar Adam, Farah Hanan Fathihah Jaffar, Izzat Zulhilmi Abd Rahman, and Mohd Helmy Mokhtar. 2025. "Role of AMP-Activated Protein Kinase (AMPK) in Female Reproduction: A Review" International Journal of Molecular Sciences 26, no. 14: 6833. https://doi.org/10.3390/ijms26146833
APA StyleKamar Bashah, N. A., Hamid, A. A., Adam, S. H., Jaffar, F. H. F., Abd Rahman, I. Z., & Mokhtar, M. H. (2025). Role of AMP-Activated Protein Kinase (AMPK) in Female Reproduction: A Review. International Journal of Molecular Sciences, 26(14), 6833. https://doi.org/10.3390/ijms26146833





