Agrocybe cylindracea Polysaccharides Ameliorate DSS-Induced Colitis by Restoring Intestinal Barrier Function and Reprogramming Immune Homeostasis via the Gut–Liver Axis
Abstract
1. Introduction
2. Results
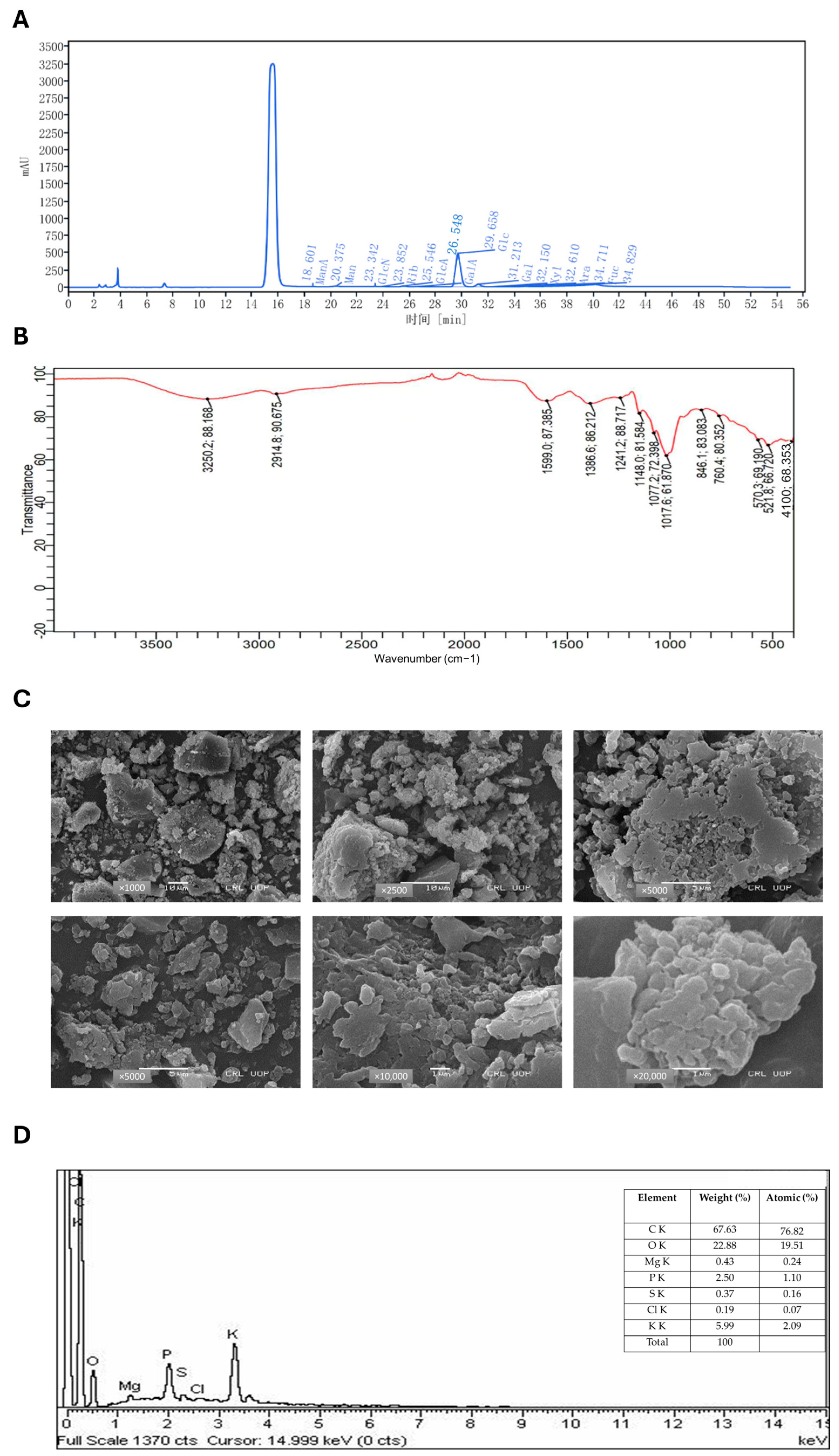
2.1. Chemical Characterization of ACP by HPLC
2.2. FTIR Spectroscopy
2.3. SEM Microscopy
2.4. EDX Spectroscopy
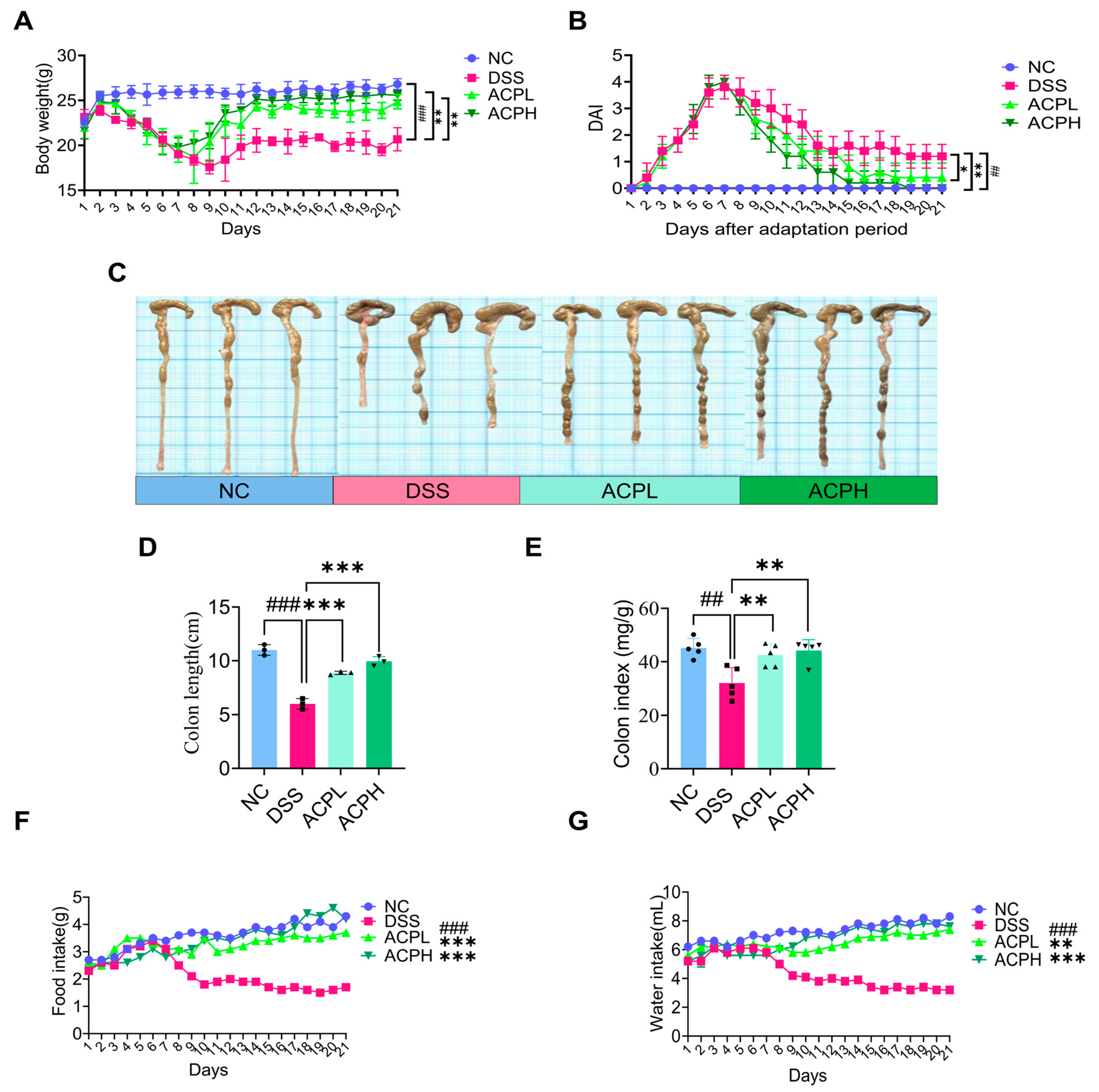
2.5. ACP Attenuates Clinical Parameters and DAI Score
2.6. ACP Ameliorates Colitis-Associated Morphological Changes
2.7. ACP Attenuates Colitis-Associated Histopathological Damage
2.8. ACP Restores Mucosal Barrier Function in Colitis
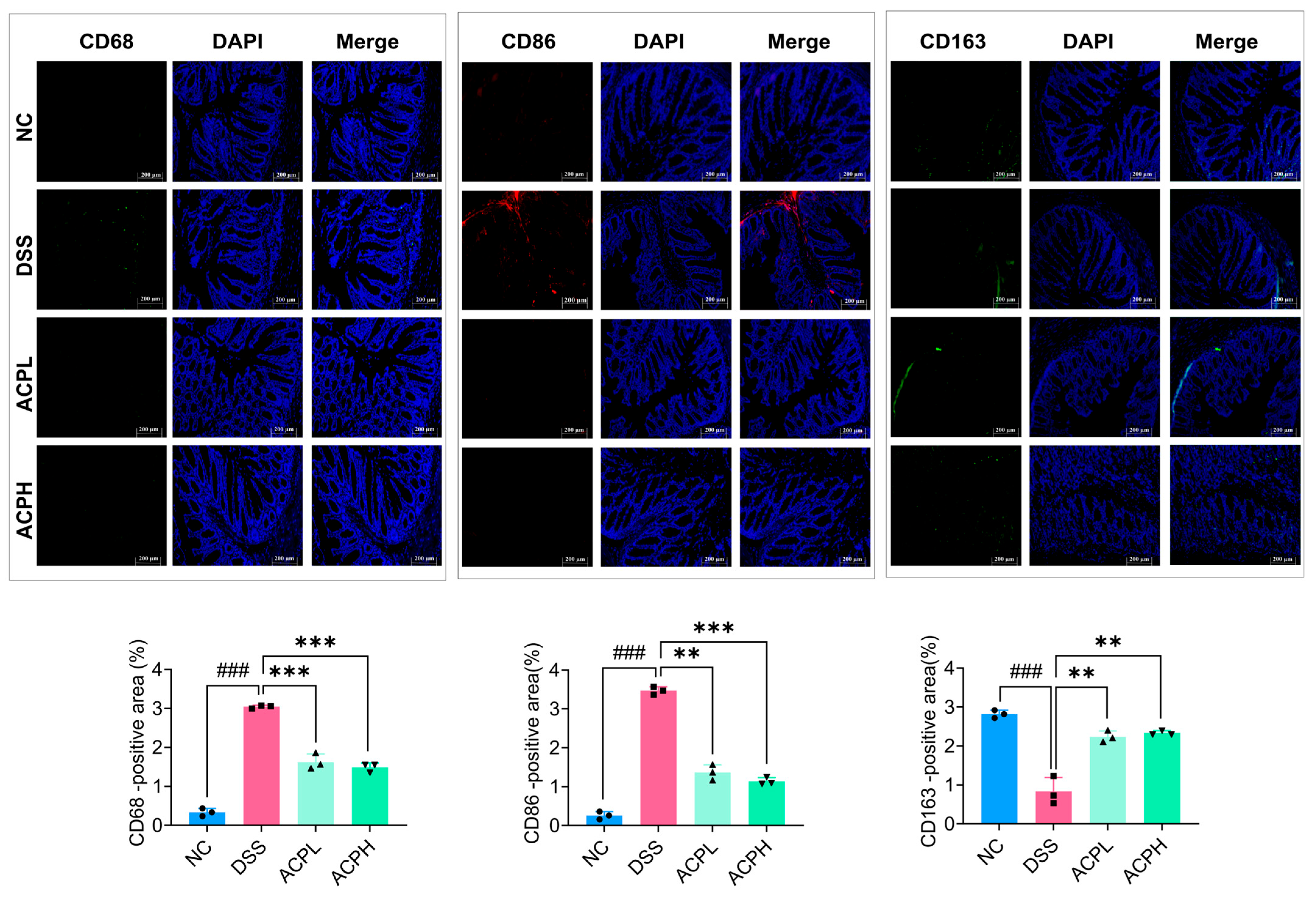
2.9. ACP Modulation of CD68, CD86, and CD163 in Colitis
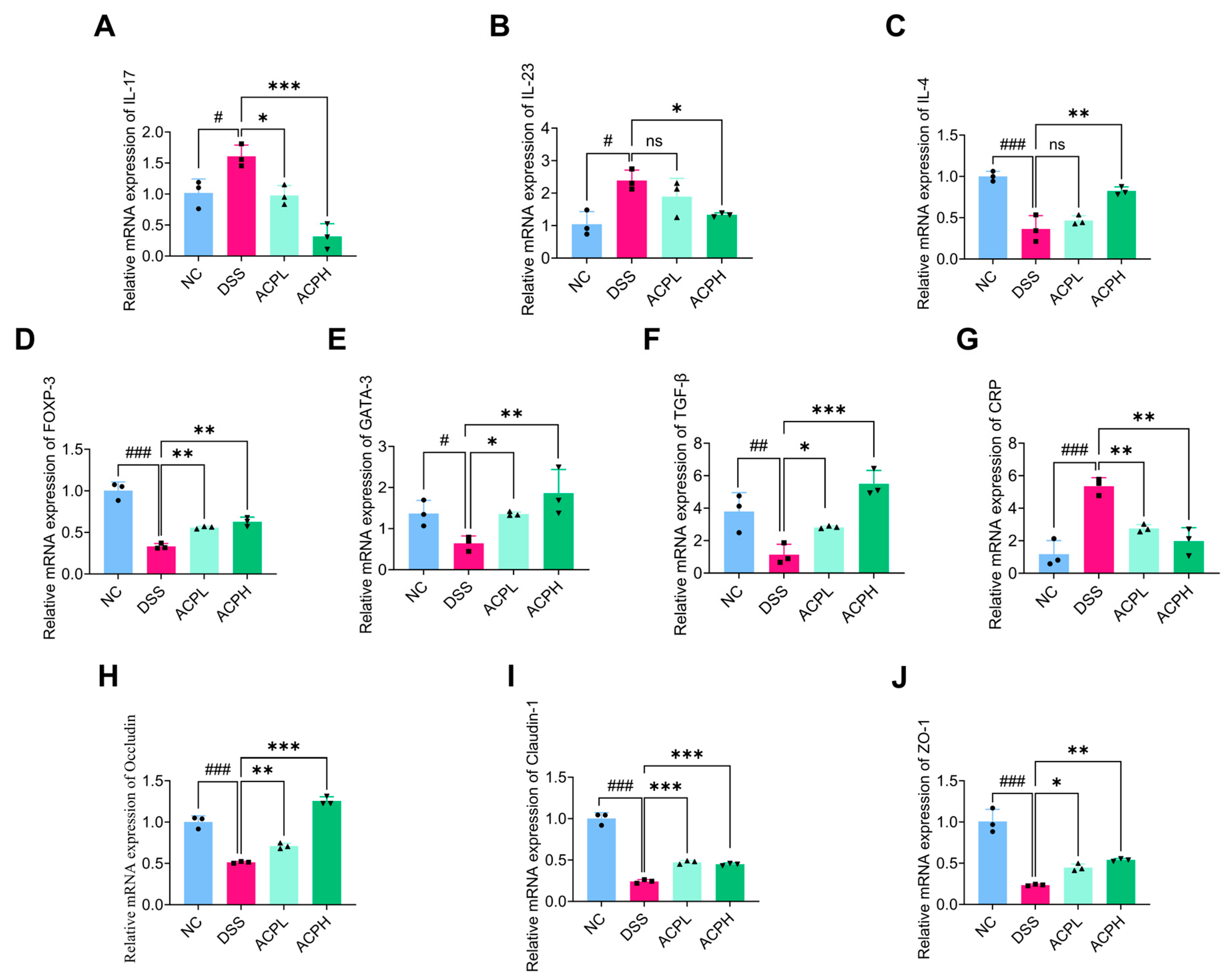
2.10. ACP Modulates Th-Cell Cytokine Balance in Colitis
2.11. Effect of ACP on Immune Modulation Markers in Colitis
2.12. ACP Enhances Intestinal Barrier Function Through Tight Junction Regulation
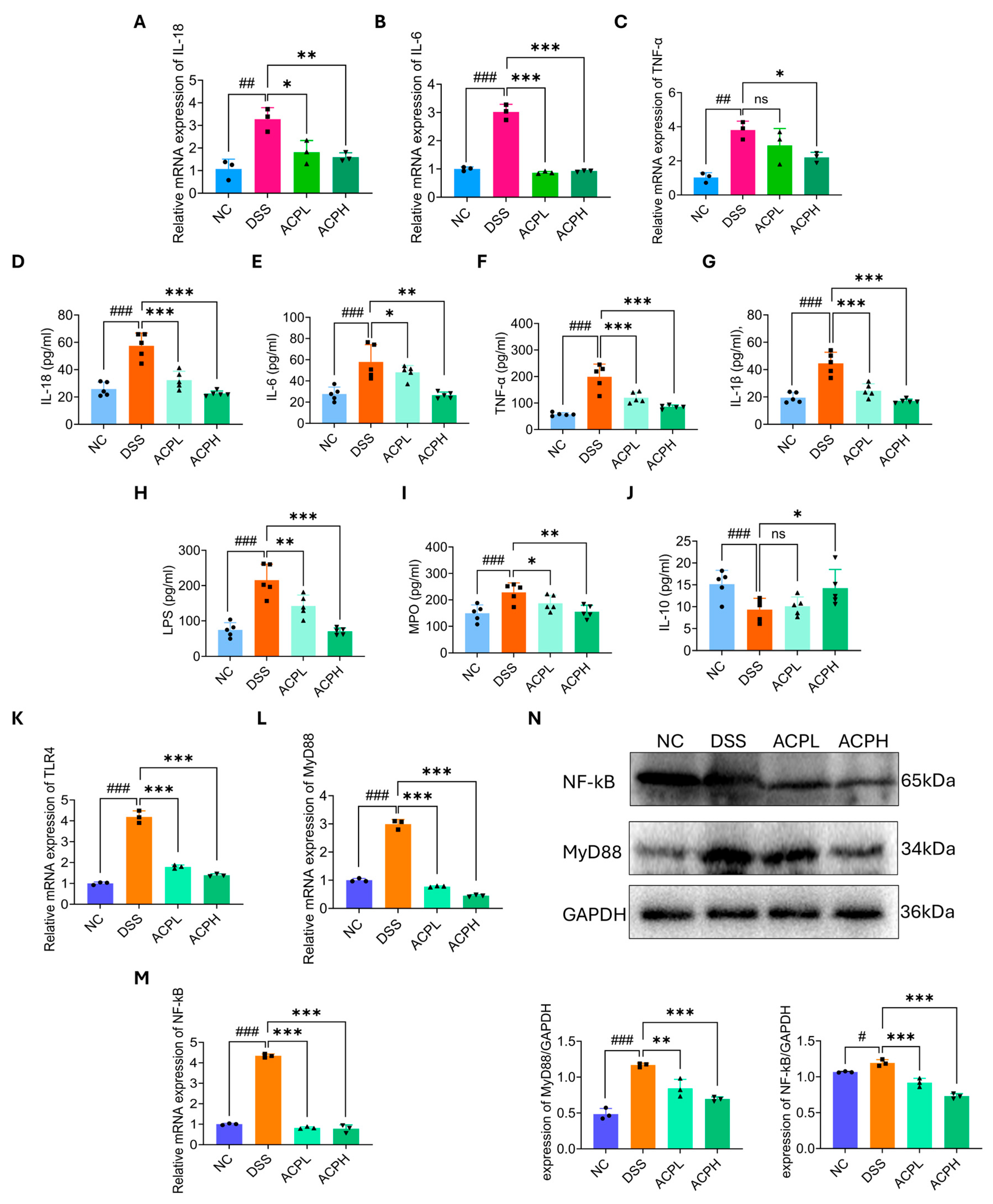
2.13. Hepatic Cytokine Modulation by ACP Treatment
2.14. Hepatic Anti-Inflammatory Effects of ACP in DSS-Induced Injury
2.15. ACP Modulates TLR4/MyD88/NF-κB Signaling
2.16. Microbial Diversity Analysis of ACP Treatment in Colitis Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of A. cylindracea Crude Polysaccharides (ACP)
4.3. Carbohydrate Content and Monosaccharide Composition
4.4. FTIR
4.5. SEM with EDX
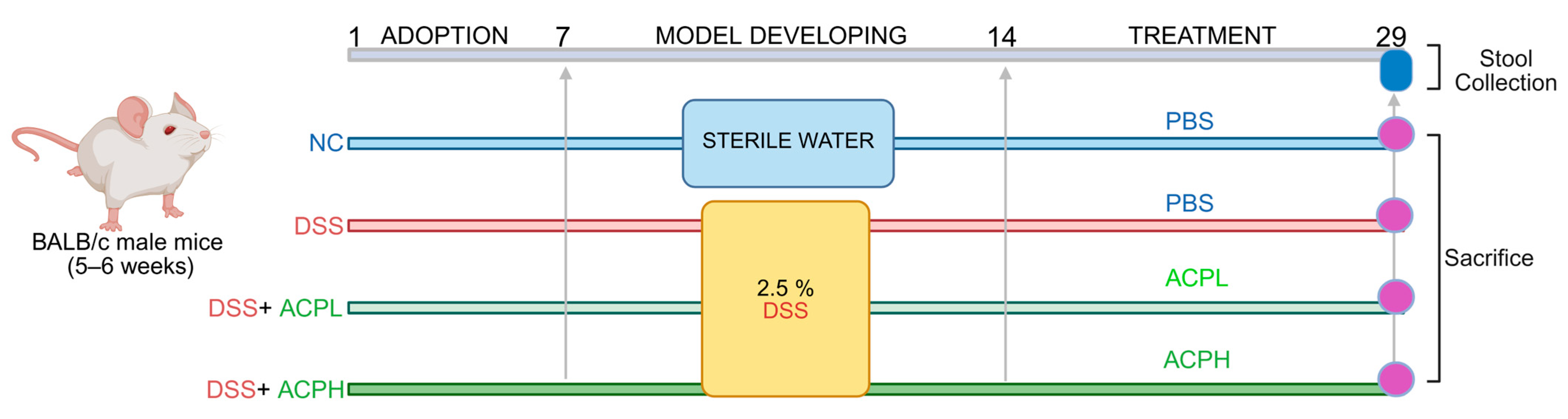
4.6. Experimental Design and Animal Housing
4.7. Animal Modeling and Treatment Protocol
4.8. Disease Activity Index
4.9. Colonic Histopathological Evaluation
4.10. Evaluation of Mucus Epithelium Thickness and Goblet Cells
4.11. IHC for Mucin-2
4.12. Immunofluorescent Staining for Macrophage Markers in Colon Tissue
4.13. Therapeutic Impact of ACP on DSS-Induced Colitis and Gut–Liver Axis at the mRNA Level
4.14. Evaluation of Hepatic Inflammation: LPS, MPO Activity, and Cytokine Levels
4.15. Western Blot Analysis
4.16. Fecal Microbial DNA Isolation and 16S rRNA Gene Sequencing
4.17. Biostatistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, L.; Ha, C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.D.; Yu, A.P.; Wu, E.Q.; Xie, J.; Mulani, P.M.; Chao, J. Systematic review: The costs of ulcerative colitis in Western countries. Aliment. Pharmacol. Ther. 2010, 31, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, R.; Tremaine, W.J.; Harmsen, W.S.; Loftus, E.V., Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin. Gastroenterol. Hepatol. 2017, 15, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Kotze, P.G.; Underwood, F.E.; Damião, A.O.M.C.; Ferraz, J.G.P.; Saad-Hossne, R.; Toro, M.; Iade, B.; Bosques-Padilla, F.; Teixeira, F.V.; Juliao-Banos, F.; et al. Progression of inflammatory bowel diseases throughout Latin America and the Caribbean: A systematic review. Clin. Gastroenterol. Hepatol. 2020, 18, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.S.; Chandran, P.; Sundby, M.; Visioli, F.; Gonçalves, F.d.C.; Burks, S.R.; Paz, A.H.; Frank, J.A. Therapeutic ultrasound attenuates DSS-induced colitis through the cholinergic anti-inflammatory pathway. EBioMedicine 2019, 45, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.T.; Amos, G.C.; Murphy, A.R.; Murch, S.; Wellington, E.M.; Arasaradnam, R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Cheng, S.; Li, L.; Liu, Y.; Wang, D.; Liu, G. Natural anti-inflammatory compounds as drug candidates for inflammatory bowel disease. Front. Pharmacol. 2021, 12, 684486. [Google Scholar] [CrossRef] [PubMed]
- Do, K.-H.; Ko, S.-H.; Kim, K.B.; Seo, K.; Lee, W.-K. Comparative study of intestinal microbiome in patients with ulcerative colitis and healthy controls in Korea. Microorganisms 2023, 11, 2750. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mao, T.; Lu, X.; Wang, M.; Yun, Y.; Jia, Z.; Shi, L.; Jiang, H.; Li, J.; Shi, R. A potential therapeutic approach for ulcerative colitis: Targeted regulation of macrophage polarization through phytochemicals. Front. Immunol. 2023, 14, 1155077. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-M.; Wang, Q.-M.; Huang, B.-Y.; Mai, C.-T.; Wang, C.-L.; Wang, T.-T.; Zhang, X.-J. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol. Res. 2021, 172, 105796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Zhang, Q.; Yang, J.; Liu, G. Roles of macrophages on ulcerative colitis and colitis-associated colorectal cancer. Front. Immunol. 2023, 14, 1103617. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.G. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J. Autoimmun. 2016, 66, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, R.; Wen, S.; Li, Q.; Lai, X.; Zhang, Z.; Sun, L.; Sun, S.; Cao, F. Tea (Camellia sinensis) ameliorates DSS-induced colitis and liver injury by inhibiting TLR4/NF-κB/NLRP3 inflammasome in mice. Biomed. Pharmacother. 2023, 158, 114136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Farid, A.; Elamir, A. Dual role of melatonin as an anti-colitis and anti-extra intestinal alterations against acetic acid-induced colitis model in rats. Sci. Rep. 2022, 12, 6344. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Lu, Q.; Tang, M.; Tao, L.; Zhao, H.; Zhang, C.; Yu, Y.; Wu, X.; Liu, H.; Cui, R. Periplaneta americana extract ameliorates dextran sulfate sodi-um-induced ulcerative colitis via immunoregulatory and PI3K/AKT/NF-κB signaling pathways. Inflammopharmacology 2022, 30, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Niu, G.-C.; Li, C.-Y.; Guo, J.-B.; Song, J.; Li, H.; Zhang, X.-L. Mechanism of ulcerative colitis-aggravated liver fibrosis: The activation of hepatic stellate cells and TLR4 signaling through gut-liver axis. Front. Physiol. 2021, 12, 695019. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Csak, T. Inflammasomes in liver diseases. J. Hepatol. 2012, 57, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 2021, 8, 2681. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-T.; Meng, S.-Y.; Pan, B.-R. Drug therapy for ulcerative colitis. World J. Gastroenterol. WJG 2004, 10, 2311. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Wędrychowicz, A.; Zając, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.; Yang, Z.; Hou, Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019, 138, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural re-sources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.Z.; Mishra, A.K.; Kausar, T.; Mahanta, S.; Sarma, B.; Kumar, V.; Mishra, P.K.; Panda, J.; Baek, K.H.; Mohanta, Y.K. Exploring edible mushrooms for diabetes: Un-veiling their role in prevention and treatment. Molecules 2023, 28, 2837. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Zhang, T.; Xin, Y.; Huang, X.-J.; Yin, J.-Y.; Nie, S.-P. Comprehensive evaluation of alkali-extracted polysaccharides from Agrocybe cylindracea: Comparison on structural characterization. Carbohydr. Polym. 2021, 255, 117502. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Du, H.; Hu, Q.; Yang, W.; Pei, F.; Xiao, H. Health benefits of edible mushroom polysaccharides and associated gut mi-crobiota regulation. Crit. Rev. Food Sci. Nutr. 2021, 62, 6646–6663. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, Y.; Wang, Y.; Wang, X.; Xu, J.; Zhao, H.; Zheng, D.; Sun, L.; Tai, G.; Zhou, Y.; et al. β-1,6-Glucan from Pleurotus eryngii modulates the immunity and gut microbiota. Front. Immunol. 2022, 13, 859923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, M.; Liu, C. A comprehensive review of secondary metabolites from the genus Agrocybe: Biological activities and pharmacological implications. Mycology 2024, 15, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jing, H.; Zhang, J.; Che, G.; Zhou, M.; Gao, Z.; Li, S.; Ren, Z.; Hao, L.; Liu, Y.; et al. Optimization of Mycelia selenium polysaccharide extraction from Agrocybe cylindracea SL-02 and assessment of their antioxidant and anti-ageing activities. PLoS ONE 2016, 11, e0160799. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, J.; Yang, H.; Yang, Z.; Xue, H.; Wu, F.; Wang, Z.; Xie, L.; Chen, Y. Acetylation modification and antioxidant activity of polysaccharides from Agrocybe cylindracea. J. Food Meas. Charact. 2022, 16, 1911–1919. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Liu, Z.; Gao, Y.; Wu, B.; Xu, H. Fe3O4 nanoparticle-coated mushroom source biomaterial for Cr (VI) polluted liquid treatment and mechanism research. R. Soc. Open Sci. 2018, 5, 171776. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, G.; Zhao, H.; Dong, N.; Wu, B.; Chen, Y.; Lu, Q. Natural-derived polysaccharides from plants, mushrooms, and sea-weeds for the treatment of inflammatory bowel disease. Front. Pharmacol. 2021, 12, 651813. [Google Scholar]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Bian, Z.; Qiu, H.; Wang, Y.; Wang, Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann. New York Acad. Sci. 2017, 1401, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Alioui, Y.; Ullah, H.; Ali, S.; Rahman, M.U.; Elkharti, M.; Farooqui, N.A.; Rehman, A.U.; Ilyas, M.; Alsholi, D.M.; Siddiqi, N.Z.; et al. Polysaccharides derived from golden mushroom (Cantharellus cibarius Fr.) modulate gut microbiota and enhance intestinal barrier function to ameliorate dextran sulfate sodium-induced colitis in mice. Front. Pharmacol. 2024, 15, 1498625. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Joseph, T.P.; Aliya, S.; Song, S.; Saleem, M.Z.; Nisar, M.A.; Wang, Y.; Meyiah, A.; Ma, Y.; Xin, Y. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J. Funct. Foods 2020, 64, 103641. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Li, P.; Yu, J.; Mu, G.; Li, X.; Tuo, Y. Saccharomyces cerevisiae I4 showed alleviating effects on dextran sulfate sodium-induced colitis of balb/c mice. Foods 2022, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, Y.; Xiao, J.; You, L.; Pedisić, S.; Liao, L. The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria Lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food Chem. Toxicol. 2021, 149, 112001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Cheng, H.; Liu, H.; Li, L.; Chen, Z.; Chen, S.; Wang, C.; Wang, D. Neuroprotection of low-molecular-weight galactan obtained from Cantharellus cibarius Fr. against Alzheimer’s disease. Carbohydr. Polym. 2023, 316, 121033. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Deng, T.; Ali, M.; Farooqui, N.A.; Alsholi, D.M.; Siddiqui, N.Z.; Rehman, A.U.; Ali, S.; Ilyas, M.; Wang, L.; et al. Sea conch peptides hydrolysate alleviates DSS-induced colitis in mice through immune modulation and gut microbiota restoration. Molecules 2023, 28, 6849. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of intestinal barrier dysfunction in gut dysbiosis and diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, H.; Zhang, Z.; Zhang, Q.; Tang, J.; Zhou, J.; Wang, Y.; Peng, W. The Protective Role of Scorias spongiosa polysaccharide-based microcapsules on intestinal barrier integrity in DSS-induced colitis in mice. Foods 2023, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Diling, C.; Xin, Y.; Chaoqun, Z.; Jian, Y.; Xiaocui, T.; Jun, C.; Ou, S.; Yizhen, X. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 2017, 8, 85838–85857. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; van Bergenhenegouwen, J.; Overbeek, S.; van de Kant, H.J.G.; Garssen, J.; Folkerts, G.; Vos, P.; Morgan, M.E.; Kraneveld, A.D.; Fuss, I.J. Bifidobacterium breve attenuates murine dextran sodium sulfate-induced colitis and increases regulatory T cell responses. PLoS ONE 2014, 9, e95441. [Google Scholar] [CrossRef] [PubMed]
- Alsholi, D.M.; Yacoub, G.S.; Rehman, A.U.; Ullah, H.; Khan, A.I.; Deng, T.; Siddiqui, N.Z.; Alioui, Y.; Farooqui, N.A.; Elkharti, M.; et al. Lactobacillus rhamnosus attenuates cisplatin-induced intestinal mucositis in mice via modulating the gut microbiota and improving intestinal inflammation. Pathogens 2023, 12, 1340. [Google Scholar] [CrossRef] [PubMed]
- Vent-Schmidt, J.; Han, J.M.; MacDonald, K.G.; Levings, M.K. The role of FOXP3 in regulating immune responses. Int. Rev. Immunol. 2014, 33, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal barrier in human health and disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Bravo, E.; Palleschi, S.; Aspichueta, P.; Buqué, X.; Rossi, B.; Cano, A.; Napolitano, M.; Ochoa, B.; Botham, K.M. Swertia mussotii prevents high-fat diet-induced non-alcoholic fatty liver disease in rats by inhibiting expression the TLR4/MyD88 and the phosphorylation of NF-κB. Pharm. Biol. 2022, 60, 1960–1968. [Google Scholar]
- Chami, B.; Martin, N.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G. Gut–liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A Polysaccharide Isolated from Dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Thipart, K.; Gruneck, L.; Phunikhom, K.; Sharpton, T.J.; Sattayasai, J.; Popluechai, S. Dark-purple rice extract modulates gut microbiota composition in acetic acid–and indomethacin-induced inflammatory bowel disease in rats. Int. Microbiol. 2023, 26, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-H.; Duan, J.-A.; Jiang, S.; Feng, N.-N.; Qiu, W.-Q.; Ling, Y. Polysaccharides from Chrysanthemum morifolium Ramat ameliorate colitis rats by modulating the intestinal microbiota community. Oncotarget 2017, 8, 80790–80803. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, Y.; Chen, B.; Zhang, G.; Ou, S.; Luo, J.; Peng, X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Mei, Y.; Zhang, H.; Li, J.; Zhang, M.; Li, Y.; Yang, W.; Liu, Y.; Liang, Y. Ameliorative effect of an acidic polysaccharide from Phellinus linteus on ulcerative colitis in a DSS-induced mouse model. Int. J. Biol. Macromol. 2024, 265, 130959. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Dong, N.; Fang, Q.; Zhang, Y.; Chen, C.; Cui, S.W.; Nie, S. Polysaccharides from natural Cordyceps sinensis attenuated dextran sodium sulfate-induced colitis in C57BL/6J mice. Food Funct. 2023, 14, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.W.; Wang, R.; Yao, L.W.; Zhou, Y.F.; Sun, P.L.; Zheng, B.; Chen, Y.F. Anti-inflammatory effects of Mytilus coruscus polysaccharide on RAW264. 7 cells and DSS-induced colitis in mice. Mar. Drugs 2021, 19, 468. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Liu, R.; Yang, Z.; Ma, A. Polysaccharide from Pleurotus tuber-regium mycelium improves DSS-induced colitis in mice by regulating inflammatory cytokines, oxidative stress and gut microbiota. Food Funct. 2024, 15, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.Z.; Rehman, A.U.; Yousuf, W.; Khan, A.I.; Farooqui, N.A.; Zang, S.; Xin, Y.; Wang, L. Effect of crude polysaccharide from seaweed, Dictyopteris divaricata (CDDP) on gut microbiota restoration and anti-diabetic activity in streptozotocin (STZ)-induced T1DM mice. Gut Pathog. 2022, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related sub-stances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, J.; Gu, T.; Sun, Q.; Xu, W.; Peng, Y. Chicoric acid effectively mitigated dextran sulfate sodium (DSS)-induced colitis in BALB/c mice by modulating the gut microbiota and fecal metabolites. Int. J. Mol. Sci. 2024, 25, 841. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total. Environ. 2021, 750, 143085. [Google Scholar] [CrossRef] [PubMed]


| Components | Concentration (mg/kg) | Percentage (%) |
|---|---|---|
| Mannuronic acid | 1521.43 | 0.48 |
| Mannose | 4964.29 | 1.56 |
| Ribose | 3221.43 | 1.01 |
| Glucuronic acid | 8650 | 2.72 |
| Galacturonic acid | 2707.14 | 0.85 |
| Glucose | 276,950 | 87.13 |
| Galactose | 18,782.14 | 5.91 |
| Xylose | 75 | 0.02 |
| Arabinose | 128.57 | 0.04 |
| Fucose | 850 | 0.27 |
| Score | Significance | |
|---|---|---|
| Regeneration | 4 | No tissue repair |
| 3 | Surface epithelium not intact | |
| 2 | Regeneration with crypt depletion | |
| 1 | Almost complete regeneration | |
| 0 | Complete regeneration or normal tissue | |
| Inflammation | 3 | Severe |
| 2 | Moderate | |
| 1 | Slight | |
| 0 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.; Naveed, M.; Rahman, M.U.; Alioui, Y.; Ansari, I.; Ali, S.; Ghaleb, E.; Farooqui, N.A.; Abusidu, M.; Xin, Y.; et al. Agrocybe cylindracea Polysaccharides Ameliorate DSS-Induced Colitis by Restoring Intestinal Barrier Function and Reprogramming Immune Homeostasis via the Gut–Liver Axis. Int. J. Mol. Sci. 2025, 26, 6805. https://doi.org/10.3390/ijms26146805
Atta A, Naveed M, Rahman MU, Alioui Y, Ansari I, Ali S, Ghaleb E, Farooqui NA, Abusidu M, Xin Y, et al. Agrocybe cylindracea Polysaccharides Ameliorate DSS-Induced Colitis by Restoring Intestinal Barrier Function and Reprogramming Immune Homeostasis via the Gut–Liver Axis. International Journal of Molecular Sciences. 2025; 26(14):6805. https://doi.org/10.3390/ijms26146805
Chicago/Turabian StyleAtta, Aamna, Muhammad Naveed, Mujeeb Ur Rahman, Yamina Alioui, Immad Ansari, Sharafat Ali, Eslam Ghaleb, Nabeel Ahmed Farooqui, Mohammad Abusidu, Yi Xin, and et al. 2025. "Agrocybe cylindracea Polysaccharides Ameliorate DSS-Induced Colitis by Restoring Intestinal Barrier Function and Reprogramming Immune Homeostasis via the Gut–Liver Axis" International Journal of Molecular Sciences 26, no. 14: 6805. https://doi.org/10.3390/ijms26146805
APA StyleAtta, A., Naveed, M., Rahman, M. U., Alioui, Y., Ansari, I., Ali, S., Ghaleb, E., Farooqui, N. A., Abusidu, M., Xin, Y., & Feng, B. (2025). Agrocybe cylindracea Polysaccharides Ameliorate DSS-Induced Colitis by Restoring Intestinal Barrier Function and Reprogramming Immune Homeostasis via the Gut–Liver Axis. International Journal of Molecular Sciences, 26(14), 6805. https://doi.org/10.3390/ijms26146805








