Abstract
Fungi are ubiquitous organisms that are capable of transient or persistent colonization in humans. Their polymorphic nature and complex host–mycobiome interactions remain incompletely understood. Emerging evidence highlights the role of resident fungi in modulating immune responses and adapting to host changes, which can trigger a shift from commensalism to parasitism, particularly in immunocompromised individuals. This study evaluated the effects of two major β-glucans—zymosan and curdlan—on the expression of pattern recognition receptors (Dectin1, Dectin2, TLR2, TLR4) in human peripheral blood mononuclear cells (PBMCs). It also examined their impact on reactive oxygen species (ROS) production, cytokine/chemokine gene expression, and antioxidant enzyme expression. Both β-glucans significantly increased the mRNA levels of all tested receptors and enhanced ROS generation. Curdlan downregulated key antioxidant enzymes (SOD1, CAT, GPX1), while zymosan markedly upregulated SOD1. These findings demonstrate that the β-glucans zymosan and curdlan have a substantial influence on PBMC reactivity and oxidative stress responses. Further studies are needed to deepen our understanding of host–fungal interactions and their implications in health and disease.
1. Introduction
The mycobiome, a component of the microbiome community, comprises fungi shaped by their quantitative and qualitative composition, temporal or local variability, and the sum of interactions among them and between members of other subgroups, such as the bacteriome and virome. External factors, e.g., nutrients, alcohol, and medications, act as environmental variables that significantly impact this collectivity. The intestines have received the most attention regarding the human mycobiome; however, different areas, such as the skin, the respiratory tract, the vagina, and oral or nasal cavities, have also been inhabited [1,2]. In a healthy state, resident fungi and their host maintain a mutualistic relationship [1,2,3]. An expanding amount of data underscores the role of symbiotic fungi in shaping immunity by facilitating the development of peripheral lymphoid organs [4] and affecting immune cell responses [5,6]. Nevertheless, the nature of this connection is not as thoroughly understood, and fungi’s polymorphic nature is a crucial virulence factor that plays a vital role in the shift from relative tolerance to destruction [7].
The efficiency of the host defense system depends significantly on pattern recognition receptors (PRRs), which identify microbial signals known as pathogen-associated molecular patterns (PAMPs). Four major subfamilies of PRRs are currently acknowledged: cytoplasmic sensors, including RIG-I-like receptors (RLRs) and NOD-like receptors (NLRs), along with cell membrane-anchored Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) [8]. Dectin-1, Dectin-2, Mincle, and Mannose Receptor (MR) from the CLR family, along with TLR2, TLR4, and TLR6 from the TLRs, are the most effective in sensing fungi. Alongside membrane receptors, soluble PRRs such as mannose-binding lectins (MBLs) derived from CLRs are essential for fungi detection [9]. The interaction of fungal components, especially carbohydrates like β-glucans, chitin, and mannose-based structures [10] with PRRs establishes the foundation for the immunological cascade [11]. The recognition of fungi by PRRs, particularly Dectin-1 and Dectin-2, enhances phagocytosis, increases the production of reactive oxygen species (ROS) [12], generates proinflammatory cytokines [13], and regulates Th1 and Th17 responses [14]. The structural differences between β-glucans used in this study are summarized in Table 1 [15,16].

Table 1.
Comparative characteristics of β-glucans used in the study.
In contrast to the bacterial microbiome, which remains relatively stable over time, the composition of the mycobiome varies significantly [1]. In certain circumstances, fungal colonizers can become Trojan horses, disrupting advantageous relationships with mammalian hosts. Fungi’s morphological plasticity contributes considerably to their success as both commensals and pathogens [7]. Several fungal genera are commonly found as commensals in the human microbiota, including Candida, Malassezia, Saccharomyces, and Cladosporium [17]. Candida albicans is the most well-characterized and prevalent fungal commensal, especially in the gastrointestinal and genitourinary tracts [18]. Under conditions of dysbiosis or immune suppression, some commensal fungi, such as C. albicans, can shift toward pathogenicity and cause localized or even systemic infections [19]. In contrast, other clinically relevant fungal pathogens, such as Aspergillus fumigatus or Cryptococcus neoformans, are not typically part of the human commensal mycobiota but can cause severe opportunistic infections, particularly in immunocompromised individuals [19]. Dectin-1 expression affects the makeup of the gut microbiota, and colonization of opportunistic pathogenic fungi that harm beneficial ones is one effect of receptor lack [20]. Also, during fungal dysbiosis, Dectin-1 regulates antifungal immunity to prevent the overgrowth of opportunistic fungi [21]. The limited number of research papers suggests that disturbances in the mycobiota composition may lead to the unfavorable cohabitation of bacteria and fungi, which could be associated with pathological conditions such as Crohn’s disease or cystic fibrosis [1,22]. The dysbiosis experiments demonstrate that the relationship between commensal fungi and the bacteriome is a delicate balance that can easily be disrupted. When the microbiome is unsettled, the commensal components of the mycobiome can become unregulated and pathogenic [22]. An imbalanced gut microbiome compromises the intestinal barrier and disrupts cellular immunity, especially neutrophils, leading to the translocation of intestinal-colonizing fungi across the epithelial barrier into the bloodstream [2,23].
Our previous report demonstrated that β-glucans and mannans, such as curdlan, zymosan, and mannan, can modulate the activity of peripheral blood mononuclear cells (PBMCs) by inducing the production of key cytokines and chemokines, including IFN-γ, CCL3, GM-CSF, IL-1β, and CXCL8. Additionally, our findings suggest that the Syk signaling pathway is involved in this response, as its inhibition significantly reduced cytokine and chemokine expression [24]. In light of these results, this study was designed to clarify the interaction of β-glucans (zymosan and curdlan) with PRRs in human PBMCs and examine the immune effects of this interaction.
2. Results
2.1. The Effect of Curdlan and Zymosan on TLR2, TLR4, Dectin1, and Dectin2 mRNA Expression on PBMCs
Firstly, we examined the effect of both β-glucans (at 1 μg/mL and 10 μg/mL) on the mRNA expression of TLR2, TLR4, Dectin1, and Dectin2 on PBMCs (Figure 1). As calculated after RT-qPCR, curdlan induced an increase in the mRNA expression level of TLR2 (1.6- and 2.4-fold; both p > 0.05) (Figure 1A), TLR4 (1.7- and 2.6-fold; p < 0.01 and p > 0.05, respectively) (Figure 1B), Dectin1 (3.9- and 4.3-fold; both p > 0.05) (Figure 1C), and Dectin2 (3.9- and 3.9-fold; both p > 0.05) (Figure 1D) in PBMCs stimulated with 1 and 10 µg/mL, respectively, when compared to non-treated control cells. Similarly, zymosan also increased the mRNA expression levels of TLR2 (1.6- and 4.5-fold; p > 0.05 and p < 0.05, respectively) (Figure 1A), TLR4 (1.5- and 2.3-fold; both p < 0.05) (Figure 1B), Dectin1 (1.7- and 4.5-fold; p < 0.05, p < 0.0001, respectively) (Figure 1C), and Dectin2 (2.3- and 2.5-fold; p < 0.05, p < 0.01, respectively) (Figure 1D) in PBMCs stimulated with 1 and 10 µg/mL, respectively, when compared to unstimulated control cells. In the case of both stimulants, the concentration of 10 µg/mL induced a statistically significantly greater increase in the mRNA expression of the investigated receptors than the concentration of 1 µg/mL, except for the mRNA expression of Dectin2. Both concentrations of curdlan—1 and 10 µg/mL—induced a similar increase in Dectin2 mRNA expression.
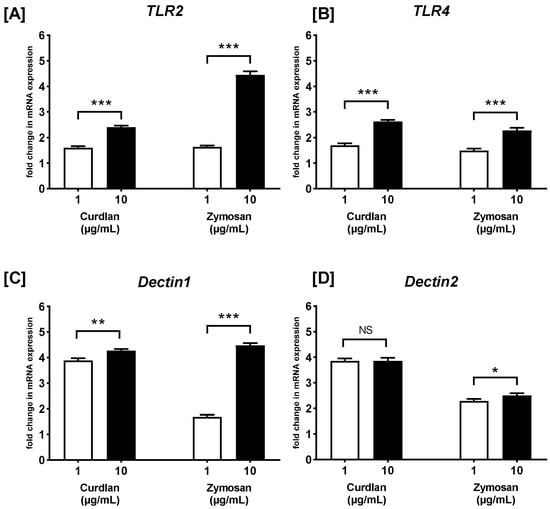
Figure 1.
The effects of curdlan and zymosan on (A) TLR2, (B) TLR4, (C) Dectin1, and (D) Dectin2 mRNA expression in PBMCs, evaluated by RT-qPCR. PBMCs were incubated with curdlan and zymosan at 1 or 10 μg/mL concentrations or with medium alone for 72 h. Total mRNA was extracted, and cDNA was synthesized from mRNA. RT-qPCR was then performed to evaluate the expression levels of the four receptors. The expression levels were normalized to the transcript level of the housekeeping gene ACTB, and the relative mRNA expression was calculated using the 2−ΔΔCq method. The results are presented as the mean ± SD of three independent experiments conducted in duplicate. * p < 0.05, ** p < 0.01, *** p < 0.001; NS—not significant.
2.2. The Effects of R406, SB203580, Laminarin, and TAK-242 on Curdlan- and Zymosan-Induced TLR2, TLR4, Dectin1, and Dectin2 mRNA Expression on PBMCs
To examine whether the studied receptors are involved in the β-glucan-mediated response in PBMCs, SB203580, as well as the p38 MAPK inhibitor, R406, Syk kinase inhibitor, TAK-242, TLR4 antagonist, and laminarin, Dectin-1 antagonist were used (Figure 2). We observed that PBMC pretreatment with SB203580 did not affect (p > 0.05) the curdlan- and zymosan-stimulated mRNA levels of TLR2 (Figure 2A). In turn, pretreatment of PBMCs with R406 caused statistically significant suppression (p < 0.001) of the curdlan- and zymosan-stimulated mRNA expression levels of TLR4; however, TAK-242 did not affect the level of mRNA expression induced by curdlan or zymosan (Figure 2B). We also demonstrated that PBMC pretreated with R406 or laminarin resulted in statistically significant suppression of curdlan- and zymosan-mediated Dectin1 (p < 0.01) (Figure 2C) and Dectin2 (p < 0.001) (Figure 2D) mRNA expression when compared to non-primed PBMCs.
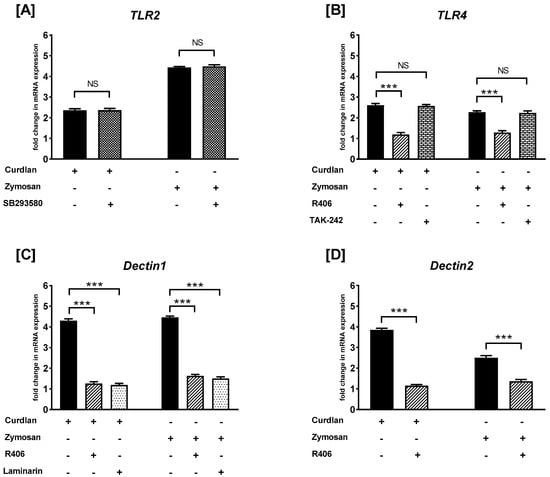
Figure 2.
The effects of R406, SB203580, laminarin, and TAK-242 on curdlan- and zymosan-induced (A) TLR2, (B) TLR4, (C) Dectin1, and (D) Dectin2 mRNA expression in PBMCs, evaluated by RT-qPCR. PBMCs were preincubated with R406 at 2 μM, SB203580 at 10 μM, laminarin at 100 μg/mL, TAK-242 at 5 ng/mL, or medium alone for 30 min before stimulation with curdlan or zymosan at 10 μg/mL. Total mRNA was extracted and used for cDNA synthesis. RT-qPCR was conducted to assess the expression of the receptors at the mRNA level. The expression levels were normalized to the transcript level of the housekeeping gene ACTB, and relative mRNA expression was calculated using the 2−ΔΔCq method. The results are presented as the mean ± SD of three separate experiments performed in duplicate. *** p < 0.001; NS—not significant.
2.3. The Effects of Curdlan and Zymosan on PBMC Cytokine/Chemokine mRNA Expression
Next, RT-qPCR was carried out, and the fold change of cytokine/chemokine mRNA expression in curdlan- and zymosan-stimulated PBMCs compared to the non-stimulated cells was assessed (Figure 3 and Figure 4). We observed that the IL17 mRNA levels were 1.2- and 2.3-fold higher in the PBMCs stimulated with 1 and 10 µg/mL of curdlan, respectively, when compared to the non-treated control cells (both p > 0.05) (Figure 3A). Moreover, the mRNA levels for IL17 were slightly upregulated when the cells were exposed to 1 and 10 µg/mL of zymosan, as follows: 1.6- and 2-fold (both p > 0.05) (Figure 4A). IL22 gene expression was narrowly increased from 1.1 to 1.4 times in the PBMCs challenged with 1 and 10 µg/mL of curdlan (both p > 0.05) (Figure 3B), while the increase only from 1.5 to 1.7 times was induced after stimulation with zymosan at 1 and 10 µg/mL (both p > 0.05) (Figure 4B). As can be seen in Figure 3C, the increase in the level of IL23 mRNA was insubstantial when the PBMCs were treated with 1 and 10 µg/mL of curdlan (1.1- and 1.2-fold; both p > 0.05). In contrast, exposure of the PBMCs to zymosan at concentrations of 1 and 10 µg/mL resulted in very significant IL23 mRNA expression upregulation (31- and 51-fold; both p > 0.05) in comparison to the non-stimulated cells (Figure 4C). We also found that the levels of IL6 transcription in the PBMCs stimulated with 1 and 10 µg/mL of curdlan were comparable to unstimulated cells (1.1- and 1.2-fold; both p > 0.05) (Figure 3D), while the relevant increase from 11 to 23 times was induced after stimulating the PBMCs with zymosan at 1 (p > 0.05) and 10 µg/mL (p < 0.05) (Figure 4D). As in the case of IL6, we documented that the mRNA expression level of TNF in PBMCs treated with 1 and 10 µg/mL of curdlan were comparable to unstimulated cells (1.1- and 1.2-fold; both p > 0.05) (Figure 3E), while exposure of these cells to zymosan at a concentration of 1 and 10 µg/mL resulted in the most considerable among all tested cytokines/chemokines’ TNF mRNA expression upregulation (45- and 55-fold; both p < 0.05) (Figure 4E). For both the PBMCs treated with curdlan and zymosan at concentrations of 1 and 10 µg/mL, we documented no change in CCL2 mRNA expression (1.1- and 1.2-fold; both p < 0.05) compared to the cells untreated with these stimulants (Figure 3F and Figure 4F). Simultaneously, in the case of the PBMCs being challenged with 1 and 10 µg/mL of curdlan, we observed a slight enhancement of TGFB1 mRNA transcript levels (1.1- and 1.25-fold; both p < 0.05) (Figure 3G); however, the PBMCs treated with zymosan at concentrations of 1 and 10 µg/mL resulted in an 8- and 13-fold increase (both p < 0.05) of TGFB1 mRNA expression, respectively (Figure 4G). In the case of curdlan, a concentration of 10 µg/mL induced a statistically significantly greater increase in the mRNA expression of the investigated mediators than a concentration of 1 µg/mL for IL17 (p < 0.001) and IL22 (p < 0.01). Additionally, we observed this relationship for IL6, IL23 (p < 0.001), TNF (p < 0.01), and IL17 (p < 0.05) after stimulation with zymosan.
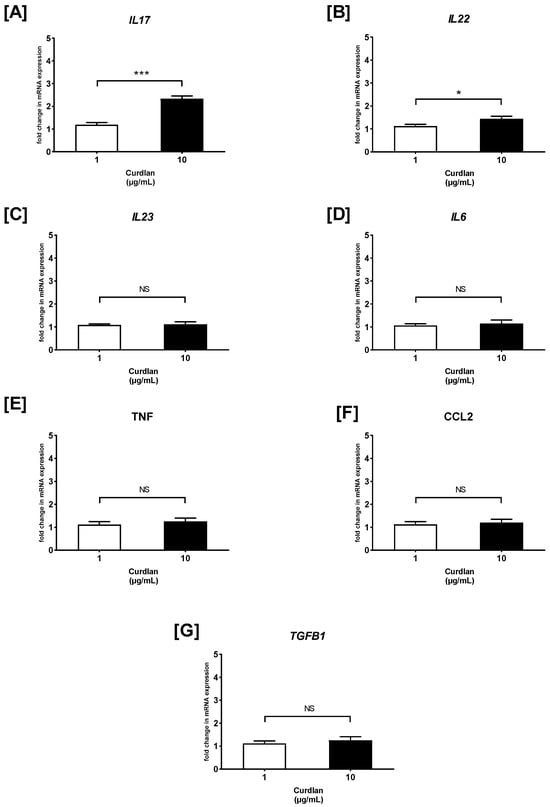
Figure 3.
The effects of curdlan on (A) IL17, (B) IL22, (C) IL23, (D) IL6, (E) TNF, (F) CCL2, and (G) TGFB1 mRNA expression in PBMCs evaluated by RT-qPCR. In the first step, PBMCs were cultured for 72 h with curdlan at 1 and 10 μg/mL or medium alone. Afterwards, total mRNA was extracted and used for cDNA synthesis, followed by a RT-qPCR assay. The expression levels of genes encoding selected cytokines/chemokines were normalized to the transcript level of the housekeeping gene ACTB, and relative mRNA expression was calculated using the 2−ΔΔCq method. The results are shown as the mean ± SD of three separate experiments performed in duplicate. * p < 0.05, *** p < 0.001; NS—not significant.
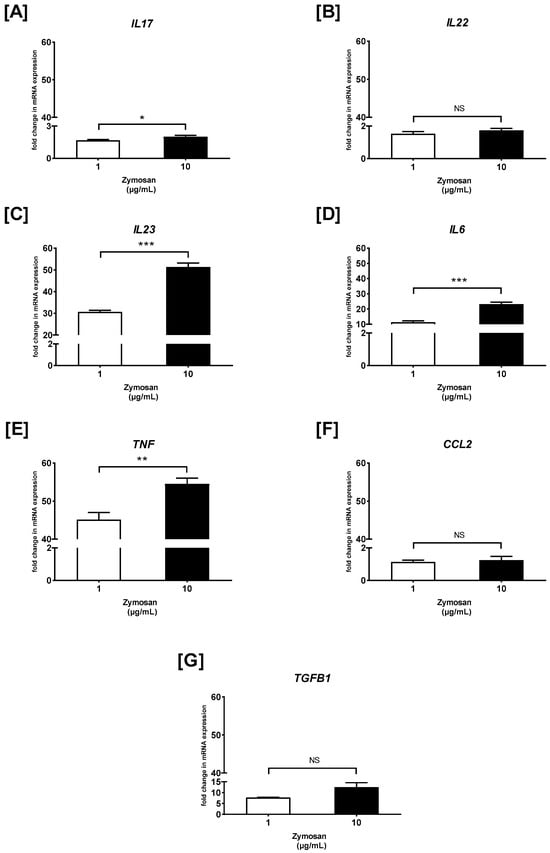
Figure 4.
The effects of zymosan on (A) IL17, (B) IL22, (C) IL23, (D) IL6, (E) TNF, (F) CCL2, and (G) TGFB1 mRNA expression in PBMCs evaluated by RT-qPCR. In the first step, PBMCs were cultured for 72 h with zymosan at 1 and 10 μg/mL or medium alone. Afterwards, total mRNA was extracted and used for cDNA synthesis, followed by a RT-qPCR assay. The expression levels of genes encoding selected cytokines/chemokines were normalized to the transcript level of the housekeeping gene ACTB, and relative mRNA expression was calculated using the 2−ΔΔCq method. The results are shown as the mean ± SD of three separate experiments performed in duplicate. * p < 0.05, ** p < 0.01, *** p < 0.001; NS—not significant.
2.4. The Effects of R406 and Laminarin on Curdlan- and Zymosan-Induced PBMC Responses
As shown in Figure 5A, the PBMCs preincubated with R406 or laminarin caused a substantial suppression of curdlan-induced IL17 mRNA expression. We also documented that the PBMCs preincubated with R406, but not with laminarin, reduced the curdlan-mediated mRNA levels of IL22 and TNF (Figure 5B,E). Additionally, we established that pre-stimulation of PBMCs with R406 or laminarin did not lead to inhibition in the curdlan-mediated mRNA expression of IL23 (Figure 5C), IL6 (Figure 5D), CCL2 (Figure 5F), and TGFB1 (Figure 5G).
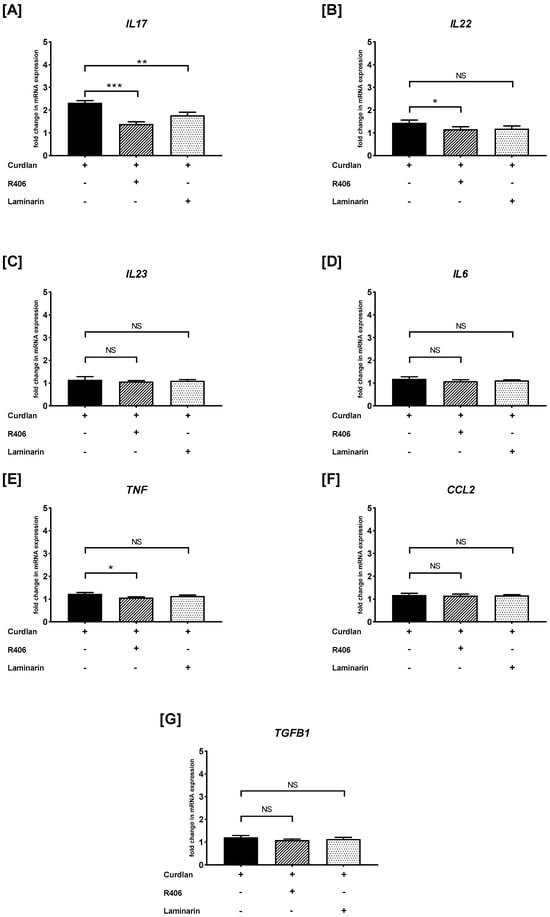
Figure 5.
The effects of R406 and/or laminarin on curdlan-induced (A) IL17, (B) IL22, (C) IL23, (D) IL6, (E) TNF, (F) CCL2, and (G) TGFB1 mRNA expressions in PBMCs. PBMCs were pretreated for 30 min with R406 at 2 μM or laminarin at 100 μg/mL, or medium alone, before stimulation with curdlan at a concentration of 10 μg/mL. Total mRNA was extracted and used for cDNA synthesis, followed by RT-qPCR to evaluate cytokine and chemokine mRNA expression. The expression levels were normalized to the transcript level of the housekeeping gene ACTB, and relative mRNA expression was calculated using the 2−ΔΔCq method. The results are shown as the mean ± SD of three separate experiments performed in duplicate. * p < 0.05, ** p < 0.01, *** p < 0.001; NS—not significant.
Similarly, we examined the effects of R406 and laminarin on the zymosan-induced PBMC response (Figure 6). We found that preincubation of PBMCs with either R406 or laminarin significantly reduced zymosan-induced IL17 mRNA synthesis (p < 0.01) (Figure 6A). The most potent inhibition of zymosan-mediated mRNA expression after pretreatment with R406 and laminarin was observed for IL22 (p < 0.001) (Figure 6B), IL23 (p < 0.001) (Figure 6C), IL6 (p < 0.001) (Figure 6D), and TNF (p < 0.001) (Figure 6E). Conversely, PBMCs preincubated with laminarin, but not with R406, resulted in a statistically significant reduction (p < 0.01) in zymosan-stimulated mRNA expression of CCL2 (Figure 6F). Furthermore, the level of zymosan-induced TGFB1 mRNA transcript was decreased in cells pretreated with R406 (p < 0.001) and laminarin (p < 0.01) (Figure 6G).
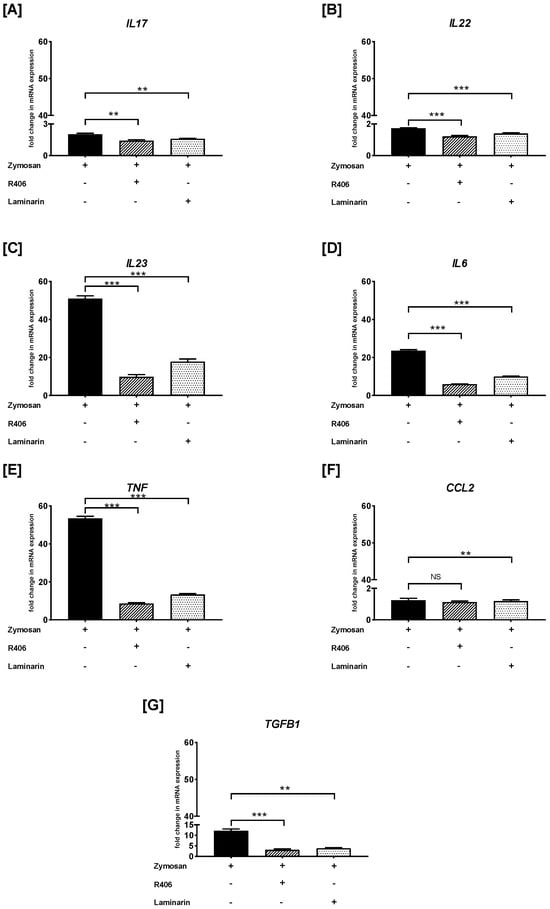
Figure 6.
The effects of R406 and/or laminarin on zymosan-induced (A) IL17, (B) IL22, (C) IL23, (D) IL6, (E) TNF, (F) CCL2, and (G) TGFB1 mRNA expression in PBMCs. PBMCs were pretreated for 30 min with R406 at 2 μM or laminarin at 100 μg/mL, or medium alone for 30 min before stimulation with zymosan at a concentration of 10 μg/mL. Total mRNA was extracted and used for cDNA synthesis, followed by RT-qPCR to evaluate cytokine and chemokine mRNA expression. The expression levels were normalized to the transcript level of the housekeeping gene ACTB, and relative mRNA expression was calculated using the 2−ΔΔCq method. The results are shown as the mean ± SD of three separate experiments performed in duplicate. ** p < 0.01, *** p < 0.001; NS—not significant.
2.5. The Impact of Curdlan and Zymosan on ROS Production by PBMCs
Next, we examined the production of ROS by PBMCs in response to β-glucans. As shown in Figure 7, the stimulation of PBMCs with curdlan and zymosan significantly increased ROS generation compared to the non-stimulated cells. The statistical analysis revealed that ROS production in PBMCs stimulated with curdlan at 1 and 10 µg/mL doses was significantly higher than that in unstimulated PBMCs (p < 0.05 and p < 0.0001, respectively). Moreover, statistically significant increases in ROS production were observed in PBMCs stimulated with zymosan at concentrations of 10 µg/mL compared to the unstimulated control cells (p < 0.001).
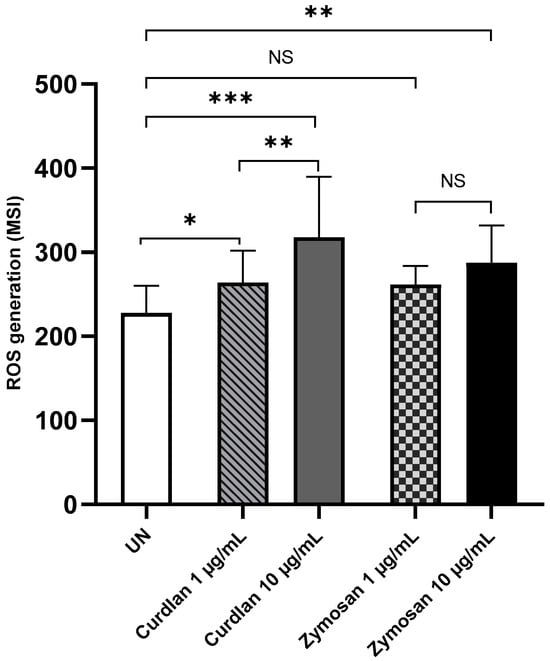
Figure 7.
Curdlan- and zymosan-induced ROS production by PBMCs. PBMCs were incubated with curdlan and zymosan at 1 and 10 μg/mL or medium alone for 72 h. CellROX™ Green Reagent was used at a concentration of 5 μM for 30 min. The results are shown as the mean ± SD of four independent experiments, and each experiment was carried out in duplicate samples. UN—unstimulated cells; * p < 0.05, ** p < 0.01, *** p < 0.0001; NS—not significant.
2.6. The Effects of Curdlan and Zymosan on SOD1, CAT, and GPX1 mRNA Expression on PBMCs
To investigate the effects of β-glucans on oxidative stress-related enzymes, we measured the mRNA expression levels of SOD1, CAT, and GPX1 in PBMCs treated with curdlan and zymosan at 10 μg/mL. As illustrated in Figure 8, treatment with curdlan resulted in a reduction in the expression of all three analyzed antioxidant enzymes compared to the untreated control. Specifically, the expression of SOD1 was reduced to 0.57-fold (p > 0.05), CAT to 0.74-fold (p > 0.05), and GPX1 to 0.14-fold (p > 0.05) in response to curdlan stimulation. In contrast, zymosan stimulation resulted in varying effects on these genes. While the expression of GPX1 was only slightly decreased (0.92-fold; p > 0.05) and CAT was significantly downregulated (0.49-fold; p > 0.05), a remarkable increase was seen in SOD1 expression, which was upregulated to 3.22-fold (p < 0.01) compared to the untreated control. This indicates that zymosan significantly activates SOD1 expression while simultaneously decreasing CAT and moderately affecting GPX1.
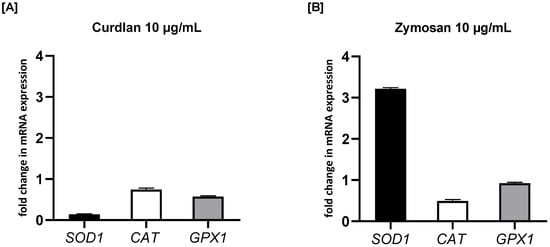
Figure 8.
The effects of (A) curdlan and (B) zymosan on SOD1, CAT, and GPX1 mRNA expression in PBMCs evaluated by RT-qPCR. PBMCs were incubated with curdlan or zymosan at 10 μg/mL or with medium alone for 72 h. Total mRNA was extracted and used for cDNA synthesis, followed by RT-qPCR to assess the expression of the three enzymes at the mRNA level. The expression levels were normalized to the transcript level of the housekeeping gene ACTB, and relative mRNA expression was calculated using the 2−ΔΔCq method. The results are presented as the mean ± SD of three independent experiments conducted in duplicate.
3. Discussion
Our understanding of the interactions between fungi and immune cells remains comparatively limited. Molecules derived from fungi that modulate immune responses, specifically β-glucans, mannans, and chitin, demonstrate multidirectional effects through PRRs, influencing phagocytosis, ROS production, and the secretion of various cytokines from immune cells in both humans and rodents [11], as documented in our previous studies [24,25,26,27,28]. These immunologically active molecules are found in the cell walls of various pathogenic fungi, including Candida albicans (β-glucans, mannans), Aspergillus fumigatus (β-glucans, chitin), and Cryptococcus neoformans (β-glucans, chitin) [29,30]. The most well-known interaction occurs through transmembrane Dectin-1, which specifically recognizes β-(1,3)(1,6)-D-glucans [31]. The identification of β-glucans through Dectin-1 induces epigenetic modifications within immune cells, resulting in a more effective response during infections, a phenomenon commonly referred to as trained immunity [13]. There is a considerable deficiency in knowledge regarding Dectin-2’s role in recognizing fungal molecular patterns. The specific ligand associated with Dectin-2 remains unidentified; however, it is likely to interact with the α-mannans present in Candida albicans [32]. Equally significant are the TLRs that identify zymosan through TLR2, mannan via TLR4, and chitin, which both TLR2 and TLR4 recognize [11].
In this study, we examined the influence of β-glucans, specifically curdlan and zymosan, on freshly isolated human PBMCs. Initially, we examined their impact on the expression of Dectin1, Dectin2, TLR2, and TLR4 in PBMCs. A significant finding of this study was that curdlan and zymosan, in concentrations of 1 μg/mL or 10 μg/mL, enhanced the mRNA expression levels of all examined receptors in the respective cell type. These data follow, to some extent, what Li et al. [33] discovered: insoluble β-glucan from the Candida albicans cell wall (100 µg/mL) and zymosan (50 µg/mL) increased the mRNA expression of Dectin1 in human THP-1 monocytes. Zymosan also enhanced the TLR2 mRNA expression in these cells; however, no change in the TLR2 mRNA levels was observed after treatment with insoluble β-glucan. Our results align with Glaser et al.’s [34] notification that increased TLR2 expression in monocytes stimulated with zymosan at a 1 µg/mL concentration. Bonfim et al. [35] confirmed this finding after stimulating monocytes with zymosan at 10 μg/mL, with a focus on TLR2 and Dectin1. Apetrei et al. [36] documented that curdlan can enhance the expression of Dectin1 on monocytes at a concentration of 20 μg/mL.
Activating specific cell signaling pathways via antigen–receptor interactions represents a fundamental mode of cell communication. A wide range of PRRs, such as some members of the TLR and CLR families, play an essential role in antifungal immunity, and representatives from both receptor subtypes may interact to recognize specific fungal cell wall components. It should be underlined that Dectin-1 and TLR2 are very closely situated in the membrane surface of monocytes or macrophages and could synergistically affect human PBMCs [37]. Some authors suggest that the signaling pathways of Dectin-1 cooperate with TLR2 in recognizing β-glucans [38,39,40]. We found a noticeable boost in β-glucan-mediated TLR2 mRNA expression levels, and we also saw similar encouraging results with Dectin1 expression. These results and those previously mentioned substantiate that relatively low concentrations of fungal antigens enhance cellular sensitivity and may trigger a signal for immune hyperactivation. The cooperation of the signaling pathways related to these receptors may lead to faster and more effective recognition of fungal antigens and a more robust immune response, promoting the development of inflammation.
Laminarin acts as a Dectin-1 antagonist in various cell types. Our study demonstrates that PBMCs preincubated with laminarin and/or a Syk kinase inhibitor, a key molecule in CLR-mediated cell responses, downregulated β-glucan-induced Dectin1 and Dectin2 mRNA expression. These findings may confirm the participation of the studied β-glucans in enhancing the expression of Dectin1 and Dectin2 on the surface of immunocompetent cells. Previously, laminarin has been shown to reduce the zymosan-mediated surface expression of Dectin1 on cultured bone marrow-derived murine mast cells [41].
The responsiveness of various cell populations to β-glucans may depend on the specific local environment in which they are activated [42]. Experimental data indicate that fungi such as Candida albicans can alter immune responses by modulating the reactivity of immunocompetent cells [43]. It is unequivocal that cytokines and chemokines play a pivotal role in orchestrating numerous antifungal defense mechanisms within the host [44]. They facilitate the mobilization of immune cells to the site of infection, enhance phagocytosis, and regulate the mechanisms associated with acquired immunity. For instance, TNF and IL-17 are crucial for maintaining the activity of neutrophils and macrophages [43,45].
Furthermore, it has been shown that IL-17 plays a vital role in the antifungal defense of mucous membranes, while IL-23 enhances its production by T lymphocytes [46,47]. IL-22, synthesized by Th17 cells, contributes to host defense and protects against candidiasis [34]. TGF-β is necessary for the differentiation of Th17 cells [43]. There is also evidence that CCL2 and IL-6 affect defensive strategies against fungi [48,49]. In a compelling study conducted by Quintin et al. [50], it was demonstrated that C. albicans and fungal cell wall β-glucans triggered the functional reprogramming of monocytes, leading to an increased production of cytokines, both in vivo and in vitro. Thus, it has been demonstrated that monocytes can be trained to exhibit an enhanced and prolonged response to microbial components following prior exposure to C. albicans or β-glucans. Additionally, specific cytokines such as TNF, IL-6, IL-1, and CXCL8 were secreted by human dendritic cells (DCs), monocytes/macrophages, and neutrophils in response to β-glucans [39,51,52,53,54,55].
Considering that cytokines and chemokines serve as essential humoral agents that affect cellular activity, it is crucial to investigate whether components associated with fungi modify the gene expression levels of multiple cytokines and chemokines, particularly those engaged in antifungal defense, in PBMCs. In the present study, we documented that curdlan induces a modest increase in the mRNA expression of IL17, IL22, IL23, IL6, TNF, CCL2, and TGFB1, with the most notable increase observed for IL17. At the same time, curdlan also caused a decrease in the expression of antioxidant enzymes, including SOD1, CAT, and GPX1. Based on these findings, curdlan may exert immunomodulatory effects in vitro, suggesting its potential relevance in conditions characterized by excessive inflammation. However, further studies are needed to explore its role in disease-specific contexts.
Dillon et al. [56] demonstrated that zymosan, acting through TLR2 and Dectin-1, modulates DCs’ IL-10 secretion and alters macrophage TGF-β production. According to research, the cooperative activity of these receptors also increases the production of proinflammatory cytokines, including TNF, IL-6, and IL-1β, by immunocompetent cells or enhances the phagocytic activity of macrophages [57,58,59]. Here, we confirmed this observation in the case of a PBMC challenge with zymosan, as we showed increased mRNA expression levels of both TNF and IL6. Furthermore, zymosan triggers a significant rise in mRNA levels for IL23, along with an exceptional TGFB1 level.
We also demonstrated that zymosan induces expression changes more significantly than curdlan; we noted the highest increase in IL23 and TNF expression. Other reports suggest that alveolar macrophages can produce TNF in response to zymosan depletion [60]. De Graaff et al. [15] noted that when human M(IL-4) macrophages were exposed to zymosan (500 μg/mL), CCL2 mRNA expression remained unchanged; however, the secretion of IL-6 and TNF increased. They also demonstrated that following exposure to curdlan, the secretion of IL-6 and TNF from human M(IL-4) macrophages was elevated.
Our study also demonstrated that zymosan elicited an increase in ROS production, coinciding with an upregulation of SOD1 expression. This observation suggests the activation of compensatory mechanisms within the antioxidant system. This study provides further evidence that β-glucans induce PBMC responses in a manner dependent on Dectin-1. Pre-stimulation of PBMCs with laminarin, which acts as a Dectin-1 antagonist, inhibited the expression of the studied cytokine transcripts, particularly in response to zymosan.
In conclusion, our investigation has yielded substantial evidence that model β-glucans—zymosan and curdlan—significantly influence the reactivity of human PBMCs. Our research demonstrates that zymosan elicits a more pronounced immune response compared to curdlan, as evidenced by an increase in cytokine mRNA transcripts for IL23, IL6, TNF, and TGFB1, accompanied by a marked elevation in ROS production. These cytokines are known to participate in inflammatory and antifungal immune responses, and their expression in PBMCs highlights the immunomodulatory potential of β-glucans. Additional research is imperative to elucidate the role of these molecules in regulating innate immune function. A more comprehensive understanding of fungal–host interactions may facilitate the advancement of preventive strategies or more efficacious therapies, particularly for individuals in whom dysregulation of the mycobiome contributes to disease manifestation.
4. Materials and Methods
4.1. Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated by centrifugation through Histopaque-1077 (Sigma-Aldrich, Saint Louis, MO, USA) from buffy coats obtained from healthy, anonymous donors, received as waste material from the Regional Center for Blood Donation and Blood Treatment in Lodz, Poland. Four milliliters of buffy coat were gently mixed with 5 mL of phosphate-buffered saline (PBS, 1×; Santa Cruz Biotechnology, Dallas, TX, USA) containing 2 mM ethylenediaminetetraacetic acid (EDTA, pH 8.0; Sigma-Aldrich) and were carefully layered onto 5 mL of Histopaque-1077. The PBS/EDTA buffer was prepared by combining 50 mL of 10× PBS, 2 mL of 0.5 M EDTA, and 448 mL of distilled water, resulting in final concentrations of 1× PBS and 2 mM EDTA. The cells were centrifuged at room temperature at 400× g for 30 min. The second opaque layer was aspirated and transferred directly to a clean tube, then washed three times with the previously prepared PBS/EDTA buffer (1× PBS containing 2 mM EDTA). A Bürker chamber (Marienfeld Superior™, Lauda-Königshofen, Germany) was utilized to count the number of PBMCs obtained. This study was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki, as established by the World Medical Association (1964) and its subsequent amendments, and received approval from the Committee for Bioethics in Medicine at the Medical University of Lodz, Poland (RNN/39/20/KE).
4.2. Cell Culture
PBMCs were suspended in RPMI-1640 (supplemented with 1% penicillin/streptomycin (Sigma-Aldrich), 2 mM L-glutamate (Gibco, Waltham, MA, USA), and 10% heat-inactivated fetal bovine serum (FBS) (Gibco) to achieve a density of 1 × 106 cells per mL, then in vitro cultures were established in 48-well sterile (non-pyrogenic) polystyrene flat-bottom plates (Corning, Tewksbury, MA, USA). The cells were cultured at 37 °C in a humidified atmosphere with 5% CO2, both in the presence and absence of β-glucans: zymosan A (Sigma-Aldrich, Cat. No. Z4250), prepared from Saccharomyces cerevisiae yeast cell wall (water-insoluble), and curdlan (Sigma-Aldrich, Cat. No. C7821), a water-insoluble linear β-(1,3)-glucan from Alcaligenes faecalis, with a purity of ≥98% (TLC). Both glucans were used at concentrations of 1 μg/mL or 10 μg/mL. Stock suspensions were prepared according to the manufacturer’s instructions. The cells were harvested after 72 h of incubation.
4.3. RNA Extraction, cDNA Synthesis, and Quantitative RT-PCR (RT-qPCR)
Total RNA was extracted from 5 × 106 PBMCs using TRI Reagent® (Sigma-Aldrich). The RNA concentration (A260) and purity (A260/A280) were measured with a NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The RNA samples were immediately frozen at −80 °C and stored until laboratory analysis. Following the manufacturer’s protocol, complementary DNA (cDNA) was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The primers were designed using Primer3 Software and were confirmed through the Primer-BLAST online tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 3 June 2025)) developed by NCBI and sourced from Genomed (Warsaw, Poland). The sequences of the primers are detailed in Table 2. The CFX Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) was utilized to conduct RT-qPCR. Gene expression was quantified in duplicates using SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad Laboratories). The reaction mixture comprised 2 μL of forward and reverse primers (500 nM), 1 μL of cDNA template, 5 μL of SsoAdvanced™ Universal SYBR® Green Supermix, and 2 μL of PCR-grade water (Gibco), resulting in a final volume of 10 μL. The PCR cycling conditions included an initial step at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 10 s, and then annealing/extension at 60 °C for 10 s, in accordance with the manufacturer’s recommendations for the SsoAdvanced™ Universal SYBR® Green Supermix (Cat. No. #1725271). The fold changes of the analyzed samples were calculated using the ΔΔCt method with CFX Maestro™ software (version 4.1.2433.1219) [61]. The reference gene, human ACTB, was employed to normalize the expression of mRNAs.

Table 2.
Sequences of primers used in the study.
4.4. Treatment of PBMCs with Signaling Pathway Inhibitors
To analyze the role of the signaling molecules in curdlan- and zymosan-induced responses, purified PBMCs were prestimulated with a Syk inhibitor, R406 (InvivoGen, San Diego, CA, USA), at a concentration of 2 μM, a Dectin-1 antagonist, laminarin (Sigma-Aldrich), at a concentration of 100 μg/mL, a TLR4 antagonist, TAK-242 (Sigma-Aldrich), at a concentration of 5 ng/mL, and a p38 MAPK inhibitor, (SB203580) (InvivoGen), at a concentration of 10 μM, or with medium alone for 30 min in a humidified atmosphere containing 5% CO2 at 37 °C, before the primary procedure was conducted. The concentrations of the Syk inhibitor, laminarin, TAK-242, and the p38 MAPK inhibitor were initially based on the literature and were ultimately selected during preliminary experiments [62,63,64,65]. None of the chosen concentrations impacted PBMC viability, as determined by the trypan blue exclusion assay.
4.5. ROS Production Measurement
PBMCs suspended in RPMI-1640 medium were exposed to curdlan and zymosan at final concentrations of 1 or 10 μg/mL or to a medium alone for spontaneous ROS generation. The cells were then incubated for 72 h at 37 °C in a 5% CO2 humidified atmosphere. Next, Invitrogen™ CellROX™ Green Reagent (Thermo Fisher Scientific) was added at a final concentration of 5 μM, and the cells were incubated again at 37 °C for 30 min. Following this, a triple-wash procedure was performed using 1× PBS through centrifugation at 150× g for 5 min at 20 °C. Fluorescence intensity was measured using the FLUOstar Omega Microplate Reader (BMG Labtech, Ortenberg, Germany), with the excitation at 485 nm and emission at 520 nm. The ROS levels were quantified as the mean signal intensity (MSI).
4.6. Statistical Analysis
Statistical data analysis was conducted using STATISTICA 13.1 software (Statsoft Inc., Tulsa, OK, USA). Simple descriptive statistics (means and standard deviations (SD)) were computed for all continuous variables. The normality of distribution was assessed using the Shapiro–Wilk test. The Student’s t-test was used to identify statistically significant differences across all examined parameters, except for ROS production, for which the Mann–Whitney U test was employed. The significance level was established at p < 0.05 (two-sided).
Author Contributions
Conceptualization, E.K., J.A. and P.Ż.; methodology, E.K.; formal analysis, E.K., J.A., S.R., M.J. and A.G.-B.; investigation, E.K., S.R. and P.Ż.; writing—original draft preparation, E.K., J.A. and P.Ż.; visualization, E.K., A.G.-B. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Lodz, grant number 503/6-127-07/503-61-001, and the National Science Centre in Poland, grant number 2021/05/X/NZ6/00492.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Committee for Bioethics in Medicine at the Medical University of Lodz, Poland (RNN/39/20/KE).
Informed Consent Statement
Informed consent was not required as the study used anonymized buffy coats obtained as waste material from the Regional Center for Blood Donation and Blood Treatment in Lodz, Poland.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to their inclusion in an ongoing research project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Belvoncikova, P.; Splichalova, P.; Videnska, P.; Gardlik, R. The human mycobiome: Colonization, composition and the role in health and disease. J. Fungi 2022, 8, 1046. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, L.; Zhai, B. The mycobiome: Interactions with host and implications in diseases. Curr. Opin. Microbiol. 2023, 75, 102361. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Yang, Y.; Zhong, W.; He, F.; Li, J. The mycobiome as integral part of the gut microbiome: Crucial role of symbiotic fungi in health and disease. Gut Microbes 2024, 16, 2440111. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zheng, W.; Zhao, G.; Zhang, H.; Wang, X.; Guo, Y.; Qin, C.; Shi, Y. Peripheral lymphoid volume expansion and maintenance are controlled by gut microbiota via RALDH+ dendritic cells. Immunity 2016, 44, 330–342. [Google Scholar] [CrossRef]
- Ruchti, F.; LeibundGut-Landmann, S. New insights into immunity to skin fungi shape our understanding of health and disease. Parasite Immunol. 2023, 45, e12948. [Google Scholar] [CrossRef]
- Gutierrez, M.W.; van Tilburg Bernardes, E.; Changirwa, D.; McDonald, B.; Arrieta, M.C. Molding immunity-modulation of mucosal and systemic immunity by the intestinal mycobiome in health and disease. Mucosal Immunol. 2022, 15, 573–583. [Google Scholar] [CrossRef]
- Hall, R.A.; Noverr, M.C. Fungal interactions with the human host: Exploring the spectrum of symbiosis. Curr. Opin. Microbiol. 2017, 40, 58–64. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Riwes, M.M.; Leather, H.; Neal, D.; Bennett, C.; Sugrue, M.; Cline, C.; Stokes, J.; Hiemenz, J.; Hsu, J.; Wingard, J.R. Association of mannose-binding lectin levels and invasive fungal disease in hematologic malignancy patients receiving myelosuppressive chemotherapy or allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 1228–1232. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 2019, 89, 24–33. [Google Scholar] [CrossRef]
- Mentrup, T.; Stumpff-Niggemann, A.Y.; Leinung, N.; Schlosser, C.; Schubert, K.; Wehner, R.; Tunger, A.; Schatz, V.; Neubert, P.; Gradtke, A.C.; et al. Phagosomal signalling of the C-type lectin receptor Dectin-1 is terminated by intramembrane proteolysis. Nat. Commun. 2022, 13, 1880. [Google Scholar] [CrossRef]
- Pedro, A.R.V.; Lima, T.; Fróis-Martins, R.; Leal, B.; Ramos, I.C.; Martins, E.G.; Cabrita, A.R.J.; Fonseca, A.J.M.; Maia, M.R.G.; Vilanova, M.; et al. Dectin-1-Mediated Production of Pro-Inflammatory Cytokines Induced by Yeast β-Glucans in Bovine Monocytes. Front. Immunol. 2021, 12, 689879. [Google Scholar] [CrossRef]
- Carvalho, A.; Giovannini, G.; De Luca, A.; D’Angelo, C.; Casagrande, A.; Iannitti, R.G.; Ricci, G.; Cunha, C.; Romani, L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell. Mol. Immunol. 2012, 9, 276–286. [Google Scholar] [CrossRef]
- de Graaff, P.; Berrevoets, C.; Rösch, C.; Schols, H.A.; Verhoef, K.; Wichers, H.J.; Debets, R.; Govers, C. Curdlan, zymosan and a yeast-derived β-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunol. Immunother. 2021, 70, 547–561. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal fungi in health and disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Mem. Inst. Oswaldo Cruz 2020, 115, e200430. [Google Scholar] [CrossRef]
- Mata-Martínez, P.; Bergón-Gutiérrez, M.; Del Fresno, C. Dectin-1 signaling update: New perspectives for trained immunity. Front. Immunol. 2022, 13, 812148. [Google Scholar] [CrossRef]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Sam, Q.H.; Chang, M.W.; Chai, L.Y. The Fungal Mycobiome and Its Interaction with Gut Bacteria in the Host. Int. J. Mol. Sci. 2017, 18, 330. [Google Scholar] [CrossRef]
- Sprague, J.L.; Kasper, L.; Hube, B. From intestinal colonization to systemic infections: Candida albicans translocation and dissemination. Gut Microbes 2022, 14, 2154548. [Google Scholar] [CrossRef]
- Kozłowska, E.; Brzezińska-Błaszczyk, E.; Rasmus, P.; Żelechowska, P. Fungal β-glucans and mannan stimulate peripheral blood mononuclear cells to cytokine production in Syk-dependent manner. Immunobiology 2020, 225, 151985. [Google Scholar] [CrossRef]
- Żelechowska, P.; Różalska, S.; Wiktorska, M.; Brzezińska-Błaszczyk, E.; Agier, J. Curdlan stimulates tissue mast cells to synthesize pro-inflammatory mediators, generate ROS, and migrate via Dectin-1 receptor. Cell Immunol. 2020, 351, 104079. [Google Scholar] [CrossRef]
- Żelechowska, P.; Brzezińska-Błaszczyk, E.; Różalska, S.; Agier, J.; Kozłowska, E. Mannan activates tissue native and IgE-sensitized mast cells to proinflammatory response and chemotaxis in TLR4-dependent manner. J. Leukoc. Biol. 2021, 109, 931–942. [Google Scholar] [CrossRef]
- Żelechowska, P.; Brzezińska-Błaszczyk, E.; Różalska, S.; Agier, J.; Kozłowska, E. Native and IgE-primed rat peritoneal mast cells exert pro-inflammatory activity and migrate in response to yeast zymosan upon Dectin-1 engagement. Immunol. Res. 2021, 69, 176–188. [Google Scholar] [CrossRef]
- Żelechowska, P.; Brzezińska-Błaszczyk, E.; Agier, J.; Kozłowska, E. Different effectiveness of fungal pathogen-associated molecular patterns (PAMPs) in activating rat peritoneal mast cells. Immunol. Lett. 2022, 248, 7–15. [Google Scholar] [CrossRef]
- Derkacz, D.; Krasowska, A. Alterations in the level of ergosterol in Candida albicans’ plasma membrane correspond with changes in virulence and result in triggering diversed inflammatory response. Int. J. Mol. Sci. 2023, 24, 3966. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. The role of dectin-1 in health and disease. Immunobiology 2021, 226, 152071. [Google Scholar] [CrossRef]
- Sato, K.; Yang, X.L.; Yudate, T.; Chung, J.S.; Wu, J.; Luby-Phelps, K.; Kimberly, R.P.; Underhill, D.; Cruz, P.D., Jr.; Ariizumi, K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 2006, 281, 38854–38866. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.H.; Chen, Q.; Zhou, W.Q.; Yu, M.W.; Lü, G.X.; Lü, X.L.; Shen, Y.N.; Liu, W.D.; Wu, S.X. Insoluble beta-glucan from the cell wall of Candida albicans induces immune responses of human THP-1 monocytes through Dectin-1. Chin. Med. J. 2009, 122, 496–501. [Google Scholar]
- Glaser, K.; Kern, D.; Speer, C.P.; Schlegel, N.; Schwab, M.; Thome, U.H.; Härtel, C.; Wright, C.J. Imbalanced inflammatory responses in preterm and term cord blood monocytes and expansion of the CD14+CD16+ subset upon Toll-like receptor stimulation. Int. J. Mol. Sci. 2023, 24, 919. [Google Scholar] [CrossRef]
- Bonfim, C.V.; Mamoni, R.L.; Blotta, M.H. TLR-2, TLR-4 and Dectin-1 expression in human monocytes and neutrophils stimulated by Paracoccidioides brasiliensis. Med. Mycol. 2009, 47, 722–733. [Google Scholar] [CrossRef]
- Apetrei, N.S.; Călugăru, A.; Bădulescu, M.M.; Lupu, A.R.; Moscovici, M.; Mocanu, G.; Mihai, D.; Szegli, G.; Cremer, L. The effects of some Curdlan derivatives on Dectin-1 expression and cytokine production in human peripheral blood mononuclear cells. Roum. Arch. Microbiol. Immunol. 2010, 69, 61–66. [Google Scholar]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef]
- Ferwerda, G.; Meyer-Wentrup, F.; Kullberg, B.J.; Netea, M.G.; Adema, G.J. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell. Microbiol. 2008, 10, 2058–2066. [Google Scholar] [CrossRef]
- Kelly, E.K.; Wang, L.; Ivashkiv, L.B. Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J. Immunol. 2010, 184, 5545–5552. [Google Scholar] [CrossRef]
- Yang, Z.; Marshall, J.S. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology 2009, 214, 321–330. [Google Scholar] [CrossRef]
- Antachopoulos, C.; Roilides, E. Cytokines and fungal infections. Br. J. Haematol. 2005, 129, 583–596. [Google Scholar] [CrossRef]
- Trautwein-Weidner, K.; Gladiator, A.; Nur, S.; Diethelm, P.; LeibundGut-Landmann, S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015, 8, 221–231. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef]
- Zhang, S. The role of transforming growth factor β in T helper 17 differentiation. Immunology 2018, 155, 24–35. [Google Scholar] [CrossRef]
- Nur, S.; Sparber, F.; Lemberg, C.; Guiducci, E.; Schweizer, T.A.; Zwicky, P.; Becher, B.; LeibundGut-Landmann, S. IL-23 supports host defense against systemic Candida albicans infection by ensuring myeloid cell survival. PLoS Pathog. 2019, 15, e1008115. [Google Scholar] [CrossRef]
- De Luca, A.; Zelante, T.; D’Angelo, C.; Zagarella, S.; Fallarino, F.; Spreca, A.; Iannitti, R.G.; Bonifazi, P.; Renauld, J.C.; Bistoni, F.; et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010, 3, 361–373. [Google Scholar] [CrossRef]
- Moradi, A.; El-Shetehy, M.; Gamir, J.; Austerlitz, T.; Dahlin, P.; Wieczorek, K.; Künzler, M.; Mauch, F. Expression of a fungal lectin in Arabidopsis enhances plant growth and resistance toward microbial pathogens and a plant-parasitic nematode. Front. Plant Sci. 2021, 12, 657451. [Google Scholar] [CrossRef]
- Peng, L.; Zhong, J.; Xiao, Y.; Wang, B.; Li, S.; Deng, Y.; He, D.; Yuan, J. Therapeutic effects of an anti-IL-6 antibody in fungal keratitis: Macrophage inhibition and T cell subset regulation. Int. Immunopharmacol. 2020, 85, 106649. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Arruda-Silva, F.; Bianchetto-Aguilera, F.; Gasperini, S.; Polletti, S.; Cosentino, E.; Tamassia, N.; Cassatella, M.A. Human neutrophils produce CCL23 in response to various TLR-agonists and TNFα. Front. Cell. Infect. Microbiol. 2017, 7, 176. [Google Scholar] [CrossRef]
- Camilli, G.; Eren, E.; Williams, D.L.; Aimanianda, V.; Meunier, E.; Quintin, J. Impaired phagocytosis directs human monocyte activation in response to fungal derived β-glucan particles. Eur. J. Immunol. 2018, 48, 757–770. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Hill Gaston, J.S.; Goodall, J.C. β-Glucan size controls Dectin-1-mediated immune responses in human dendritic cells by regulating IL-1β production. Front. Immunol. 2017, 8, 791. [Google Scholar] [CrossRef]
- Kankkunen, P.; Teirilä, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. 2010, 184, 6335–6342. [Google Scholar] [CrossRef]
- Lemoine, S.; Jaron, B.; Tabka, S.; Ettreiki, C.; Deriaud, E.; Zhivaki, D.; Le Ray, C.; Launay, O.; Majlessi, L.; Tissieres, P.; et al. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J. Allergy Clin. Immunol. 2015, 136, 1355–1368. [Google Scholar] [CrossRef]
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; van Dyke, T.; et al. Yeast zymosan, a stimulus for TLR2 and Dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Smith, A.J.; Graves, B.; Child, R.; Rice, P.J.; Ma, Z.; Lowman, D.W.; Ensley, H.E.; Ryter, K.T.; Evans, J.T.; Williams, D.L. Immunoregulatory activity of the natural product laminarin varies widely as a result of its physical properties. J. Immunol. 2018, 200, 788–799. [Google Scholar] [CrossRef]
- Yadav, M.; Schorey, J.S. The beta-glucan receptor Dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 2006, 108, 3168–3175. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Shimada, T.; Wolf, A.J.; Hsu, Y.M.; Becker, C.A.; Lin, X.; Underhill, D.M. Differential use of CARD9 by Dectin-1 in macrophages and dendritic cells. J. Immunol. 2009, 182, 1146–1154. [Google Scholar] [CrossRef]
- Wawrocki, S.; Kielnierowski, G.; Rudnicka, W.; Seweryn, M.; Druszczynska, M. Interleukin-18, functional IL-18 receptor and IL-18 binding protein expression in active and latent tuberculosis. Pathogens 2020, 9, 451. [Google Scholar] [CrossRef]
- Pandori, W.J.; Lima, T.S.; Mallya, S.; Kao, T.H.; Gov, L.; Lodoen, M.B. Toxoplasma gondii activates a Syk-CARD9-NF-κB signaling axis and gasdermin D-independent release of IL-1β during infection of primary human monocytes. PLoS Pathog. 2019, 15, e1007923. [Google Scholar] [CrossRef]
- Rego, C.M.A.; Francisco, A.F.; Boeno, C.N.; Paloschi, M.V.; Lopes, J.A.; Silva, M.D.S.; Santana, H.M.; Serrath, S.N.; Rodrigues, J.E.; Lemos, C.T.L.; et al. Inflammasome NLRP3 activation induced by Convulxin, a C-type lectin-like isolated from Crotalus durissus terrificus snake venom. Sci. Rep. 2022, 12, 4706. [Google Scholar] [CrossRef]
- Hounkpe, B.W.; Moraes, C.R.P.; dos Santos, M.N.N.; Costa, F.F.; De Paula, E.V. Heme induces mRNA expression and activation of tissue factor by TLR4-dependent mechanisms. Exp. Biol. Med. 2022, 247, 1414–1420. [Google Scholar]
- Brand, P.; Plochmann, S.; Valk, E.; Zahn, S.; Saloga, J.; Knop, J.; Becker, D. Activation and translocation of p38 mitogen-activated protein kinase after stimulation of monocytes with contact sensitizers. J. Investig. Dermatol. 2002, 119, 99–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).