Advances and Prospects of Fowl Adenoviruses Vaccine Technologies in the Past Decade
Abstract
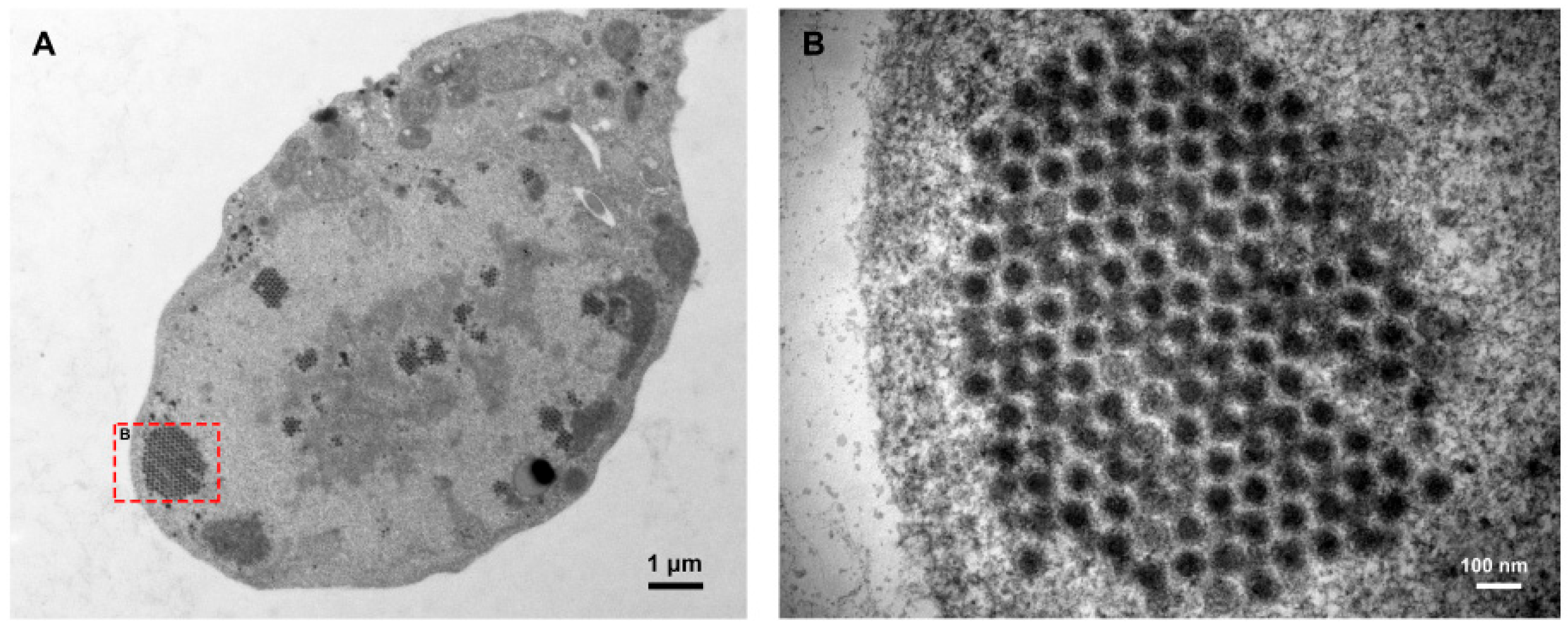
1. Introduction
2. Inactivated Vaccines
3. Live Attenuated Vaccines
| Strain | Origin | Dosage (/Bird) | Inoculation Route | Survival Rate (%) | Reference |
|---|---|---|---|---|---|
| rR188I | LMH | 105 PFU | IM | 100 | [34] |
| FAdV-4/QT35 | QT35 | 5 × 104 TCID50 | IM | 100 | [39] |
| FA4-EGFP | LMH | 106 TCID50 | IM | 100 | [40] |
| FAdV4-EGFP-rF2 | LMH-F2 | 2.5 × 104 TCID50 | IM | 100 | [54] |
| FAdV4-RFP-F1 | LMH | 2 × 105 TCID50 | IM | 100 | [55] |
| FAdV4-F/8a-rF2 | LMH | 106 TCID50 | IM | 100 | [56] |
| rHN20 | LMH | 106 PFU | IN | 100 | [57] |
| rHN20 | LMH | 106 PFU | IM | 100 | [57] |
| rHN20 | LMH | 106 PFU | SC | 100 | [57] |
4. Viral Vector Vaccines
5. Subunit Vaccines
6. Vaccine Design and Prospects
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benko, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarria, M.; Hess, M.; Jones, M.S.; Kajan, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Wang, S.; Zhang, F.; Zhao, C.; Chen, Q.; Zhao, R.; Guo, P.; Ju, L.; Li, J.; Hou, G.; et al. Molecular epidemiology analysis of fowl adenovirus detected from apparently healthy birds in eastern China. BMC Vet. Res. 2023, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Cuzange, A.; Ruigrok, R.W.; Chroboczek, J.; Jacrot, B. The avian adenovirus penton: Two fibres and one base. J. Mol. Biol. 1995, 252, 379–385. [Google Scholar] [CrossRef]
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef]
- Davison, A.J.; Akter, P.; Cunningham, C.; Dolan, A.; Addison, C.; Dargan, D.J.; Hassan-Walker, A.F.; Emery, V.C.; Griffiths, P.D.; Wilkinson, G.W.G. Homology between the human cytomegalovirus RL11 gene family and human adenovirus E3 genes. J. Gen. Virol. 2003, 84, 657–663. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Q.; Li, T.; Geng, T.; Chen, H.; Xie, Q.; Shao, H.; Wan, Z.; Qin, A.; Ye, J. Fiber-1, not fiber-2, directly mediates the infection of the pathogenic serotype 4 fowl adenovirus via its shaft and knob domains. J. Virol. 2020, 94, e00954-00920. [Google Scholar] [CrossRef]
- Niczyporuk, J.S. Deep analysis of Loop L1 HVRs1-4 region of the hexon gene of adenovirus field strains isolated in Poland. PLoS ONE 2018, 13, e0207668. [Google Scholar] [CrossRef]
- Yu, G.; Lin, Y.; Dou, Y.; Tang, Y.; Diao, Y. Prevalence of Fowl Adenovirus Serotype 4 and Co-Infection by Immunosuppressive Viruses in Fowl with Hydropericardium Hepatitis Syndrome in Shandong Province, China. Viruses 2019, 11, 517. [Google Scholar] [CrossRef]
- Staff, P.O. Correction: Crystal structure of the fibre head domain of the atadenovirus snake adenovirus 1. PLoS ONE 2015, 10, e0120613. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Vidovszky, M.Z.; Ballmann, M.Z.; Sanz-Gaitero, M.; Singh, A.K.; Harrach, B.; Benko, M.; van Raaij, M.J. Crystal structure of the fibre head domain of bovine adenovirus 4, a ruminant atadenovirus. Virol. J. 2015, 12, 81. [Google Scholar] [CrossRef]
- Reddy, V.S.; Nemerow, G.R. Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection. Proc. Natl. Acad. Sci. USA 2014, 111, 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, W.; Gao, D.; Li, Y.; Yang, X.; Liu, H.; Yao, H.; Chen, L.; Wang, C.; Zhao, J. Genetic characterization of novel fowl Aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerg. Microbes Infect. 2016, 5, e117. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, L.; Wang, Y.; Zhang, Y.; Qi, X.; Liu, C.; Gao, Y.; Wang, X.; Cui, H. The first whole genome sequence and pathogenicity characterization of a fowl adenovirus 4 isolated from ducks associated with inclusion body hepatitis and hydropericardium syndrome. Avian Pathol. 2017, 46, 571–578. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, Y.; Shi, Z.; Liu, L.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Cui, H.; Wang, X. Different Dynamic Distribution in Chickens and Ducks of the Hypervirulent, Novel Genotype Fowl Adenovirus Serotype 4 Recently Emerged in China. Front. Microbiol. 2017, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dou, Y.; Zheng, X.; Tang, Y.; Zhang, M.; Zhang, Y.; Wang, Z.; Diao, Y. Hydropericardium Hepatitis Syndrome Emerged in Cherry Valley Ducks in China. Transbound. Emerg. Dis. 2017, 64, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, M.; Gao, Z.; Li, M.; Cao, J.; Ye, H.; Song, S.; Yan, L. Pathogenicity and virus shedding ability of fowl adenovirus serotype 4 to ducks. Vet. Microbiol. 2022, 264, 109302. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, H.; Diao, Y.; Li, X.; Zhang, S.; Gao, B.; Tang, Y.; Hu, J.; Diao, Y. Pathogenicity of fowl adenovirus (FAdV) serotype 4 strain SDJN in Taizhou geese. Avian Pathol. 2019, 48, 477–485. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Deng, Y.; Yang, X.; Li, Y.; Wang, Z.; Chang, H.; Liu, H. Molecular characterization and pathogenicity of a fowl adenovirus serotype 4 isolated from peacocks associated with hydropericardium hepatitis syndrome. Infect. Genet. Evol. 2021, 90, 104766. [Google Scholar] [CrossRef]
- Mohamed, M.H.A.; El-Sabagh, I.M.; Abdelaziz, A.M.; Al-Ali, A.M.; Alramadan, M.; Lebdah, M.A.; Ibrahim, A.M.; Al-Ankari, A.S. Molecular characterization of fowl Aviadenoviruses species D and E associated with inclusion body hepatitis in chickens and falcons indicates possible cross-species transmission. Avian Pathol. 2018, 47, 384–390. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, B.; Li, S.; Ren, X.; Hong, Y.; Liao, J.; Yu, D.; Ren, T.; Liao, M.; Xu, C. Genetic characterization of fowl adenovirus serotype 4 isolates in Southern China reveals potential cross-species transmission. Infect. Genet. Evol. 2019, 75, 103928. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yang, Q.; Wu, M.J.; Zhang, Z.; Song, J.; Wang, W.; Yang, J.; Ji, J.; Zhang, Y.; Dai, H.; et al. Genomic and Pathologic Characterization of the First FAdV-C Serotype 4 Isolate from Black-Necked Crane. Viruses 2023, 15, 1653. [Google Scholar] [CrossRef]
- Niczyporuk, J.S.; Kozdrun, W.; Czekaj, H.; Piekarska, K.; Stys-Fijol, N. Isolation and molecular characterization of Fowl adenovirus strains in Black grouse: First reported case in Poland. PLoS ONE 2020, 15, e0234532. [Google Scholar] [CrossRef]
- Borkenhagen, L.K.; Fieldhouse, J.K.; Seto, D.; Gray, G.C. Are adenoviruses zoonotic? A systematic review of the evidence. Emerg. Microbes Infect. 2019, 8, 1679–1687. [Google Scholar] [CrossRef]
- Lai, J.; He, X.; Zhang, R.; Zhang, L.; Chen, L.; He, F.; Li, L.; Yang, L.; Ren, T.; Xiang, B. Chicken Interferon-Alpha and -Lambda Exhibit Antiviral Effects against Fowl Adenovirus Serotype 4 in Leghorn Male Hepatocellular Cells. Int. J. Mol. Sci. 2024, 25, 1681. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, G.; Niu, Y.; Cai, Y.; Liu, S. Airborne Transmission of a Serotype 4 Fowl Adenovirus in Chickens. Viruses 2019, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhou, J.; Chen, Z.; Chen, C.; Wang, Z.; Yang, P.; Fu, G.; Liu, X.; Huang, Y.; Wan, C. Mechanistic insights into the kidney injury in chickens induced by hypervirulent fowl adenovirus serotype 4. Microbiol. Spectr. 2025, 13, e00058-00025. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Chen, P.; Zhang, S.; Sun, J.; Yuan, W. Pathogenicity and molecular characterization of a fowl adenovirus 4 isolated from chicken associated with IBH and HPS in China. BMC Vet. Res. 2018, 14, 400. [Google Scholar] [CrossRef]
- Huixin, L.; Juan, W.; Liye, Q.; Zongxi, H.; Shengwang, L. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect. Genet. Evol. 2016, 45, 230–241. [Google Scholar] [CrossRef]
- Ye, J.; Liang, G.; Zhang, J.; Wang, W.; Song, N.; Wang, P.; Zheng, W.; Xie, Q.; Shao, H.; Wan, Z.; et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg. Microbes Infect. 2016, 5, e50. [Google Scholar] [CrossRef]
- Zhao, J.; Ruan, S.; Guo, Y.; He, Z.; Xu, M.; Zhang, G. Serological and phylogenetic analysis indicating prevalence of fowl adenovirus in China. Vet. Rec. 2018, 182, 381. [Google Scholar] [CrossRef]
- Hou, L.; Chi, Z.; Zhang, Y.; Xue, Q.; Zhai, T.; Chang, S.; Wang, J.; Zhao, P. Natural co-infection of fowl adenovirus type E-8b and avian hepatitis E virus in parental layer breeders in Hebei, China. Virol. Sin. 2023, 38, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Dong, G.; Zhang, Y.; Tian, S.; Cui, Z.; Chang, S.; Zhao, P. Co-infection of fowl adenovirus with different immunosuppressive viruses in a chicken flock. Poult. Sci. 2018, 97, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, A.; Wang, Y.; Cui, H.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Li, K.; Gao, L.; et al. A Single Amino Acid at Residue 188 of the Hexon Protein Is Responsible for the Pathogenicity of the Emerging Novel Virus Fowl Adenovirus 4. J. Virol. 2021, 95, e0060321. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, J.; Chen, Z.; Chen, C.; Yang, P.; Wang, Z.; Fu, G.; Wan, C.; Huang, Y. Hypervirulent fowl adenovirus serotype 4 elicits early innate immune response and promotes virus-induced cellular autophagy in the spleen. Poult. Sci. 2024, 103, 103831. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Schachner, A.; Mitra, T.; Heidl, S.; Liebhart, D.; Hess, M. Fowl adenovirus (FAdV) fiber-based vaccine against inclusion body hepatitis (IBH) provides type-specific protection guided by humoral immunity and regulation of B and T cell response. Vet. Res. 2020, 51, 143. [Google Scholar] [CrossRef]
- Schachner, A.; Gonzalez, G.; Endler, L.; Ito, K.; Hess, M. Fowl Adenovirus (FAdV) Recombination with Intertypic Crossovers in Genomes of FAdV-D and FAdV-E, Displaying Hybrid Serological Phenotypes. Viruses 2019, 11, 1094. [Google Scholar] [CrossRef]
- Kim, M.S.; Lim, T.H.; Lee, D.H.; Youn, H.N.; Yuk, S.S.; Kim, B.Y.; Choi, S.W.; Jung, C.H.; Han, J.H.; Song, C.S. An inactivated oil-emulsion fowl Adenovirus serotype 4 vaccine provides broad cross-protection against various serotypes of fowl Adenovirus. Vaccine 2014, 32, 3564–3568. [Google Scholar] [CrossRef]
- Schonewille, E.; Jaspers, R.; Paul, G.; Hess, M. Specific-pathogen-free chickens vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of neutralizing antibodies. Avian Dis. 2010, 54, 905–910. [Google Scholar] [CrossRef]
- Xie, Q.; Cao, S.; Zhang, W.; Wang, W.; Li, L.; Kan, Q.; Fu, H.; Geng, T.; Li, T.; Wan, Z.; et al. A novel fiber-2-edited live attenuated vaccine candidate against the highly pathogenic serotype 4 fowl adenovirus. Vet. Res. 2021, 52, 35. [Google Scholar] [CrossRef]
- Ruan, S.; Zhao, J.; Yin, X.; He, Z.; Zhang, G. A subunit vaccine based on fiber-2 protein provides full protection against fowl adenovirus serotype 4 and induces quicker and stronger immune responses than an inactivated oil-emulsion vaccine. Infect. Genet. Evol. 2018, 61, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; He, L.; Zhu, E.; Fang, T.; Yue, J.; Wen, M.; Wang, K.; Cheng, Z. A fowl adenovirus serotype 4 (FAdV-4) Fiber2 subunit vaccine candidate provides complete protection against challenge with virulent FAdV-4 strain in chickens. Vet. Microbiol. 2021, 263, 109250. [Google Scholar] [CrossRef]
- Marouf, S.; Ibrahim, H.M.; El-Naggar, M.S.; Swelum, A.A.; Alqhtani, A.H.; El-Saadony, M.T.; El-Tarabily, K.A.; Salem, H.M. Inactivated pentavalent vaccine against mycoplasmosis and salmonellosis for chickens. Poult. Sci. 2022, 101, 102139. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, Q.; Zhao, Y.; Hu, Y.X.; Zhang, G.Z. Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS ONE 2015, 10, e0133073. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, Y.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Cui, H.; Wang, X. An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China. Viruses 2017, 9, 216. [Google Scholar] [CrossRef]
- Meng, K.; Yuan, X.; Yu, J.; Zhang, Y.; Ai, W.; Wang, Y. Identification, Pathogenicity of Novel Fowl Adenovirus Serotype 4 SDJN0105 in Shandong, China and Immunoprotective Evaluation of the Newly Developed Inactivated Oil-emulsion FAdV-4 Vaccine. Viruses 2019, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yao, K.C.; Liu, Y.Y.; You, G.J.; Li, S.Y.; Liu, P.; Zhao, Q.; Wen Rui Wu, Y.P.; Huang, X.B.; Cao, S.J.; et al. Isolation and molecular characterization of prevalent Fowl adenovirus strains in southwestern China during 2015-2016 for the development of a control strategy. Emerg. Microbes Infect. 2017, 6, e103. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xie, Q.; Zhang, W.; Zhang, J.; Wang, W.; Lian, M.; Zhao, Z.; Ren, D.; Xie, S.; Lin, Y.; et al. A novel recombinant FAdV-4 virus with fiber of FAdV-8b provides efficient protection against both FAdV-4 and FAdV-8b. Viruses 2022, 14, 376. [Google Scholar] [CrossRef]
- Wang, B.; Song, M.; Song, C.; Zhao, S.; Yang, P.; Qiao, Q.; Cong, Y.; Wang, Y.; Wang, Z.; Zhao, J. An inactivated novel chimeric FAdV-4 containing fiber of FAdV-8b provides full protection against hepatitis-hydropericardium syndrome and inclusion body hepatitis. Vet. Res. 2022, 53, 75. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yang, M.F.; Cui, B.A.; Cui, P.; Sheng, M.; Chen, G.; Wang, S.J.; Geng, J.W. Construction and immunogenicity of a recombinant fowlpox vaccine coexpressing S1 glycoprotein of infectious bronchitis virus and chicken IL-18. Vaccine 2010, 28, 8112–8119. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, A.; Jiang, N.; Qi, X.; Gao, Y.; Cui, H.; Liu, C.; Zhang, Y.; Li, K.; Gao, L.; et al. A novel inactivated bivalent vaccine for chickens against emerging hepatitis-hydropericardium syndrome and infectious bursal disease. Vet. Microbiol. 2022, 266, 109375. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.D.; Li, Y.; Cai, X.H.; Yin, X. Viral Live-Attenuated Vaccines (LAVs): Past and Future Directions. Adv. Sci. 2024, 12, e2407241. [Google Scholar] [CrossRef]
- Vilela, J.; Rohaim, M.A.; Munir, M. Application of CRISPR/Cas9 in Understanding Avian Viruses and Developing Poultry Vaccines. Front. Cell Infect. Microbiol. 2020, 10, 581504. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, W.; Kan, Q.; Mu, Y.; Zhang, W.; Chen, J.; Li, L.; Fu, H.; Li, T.; Wan, Z.; et al. FAdV-4 without Fiber-2 Is a Highly Attenuated and Protective Vaccine Candidate. Microbiol. Spectr. 2022, 10, e0143621. [Google Scholar] [CrossRef]
- Mu, Y.; Xie, Q.; Wang, W.; Lu, H.; Lian, M.; Gao, W.; Li, T.; Wan, Z.; Shao, H.; Qin, A.; et al. A Novel Fiber-1-Edited and Highly Attenuated Recombinant Serotype 4 Fowl Adenovirus Confers Efficient Protection Against Lethal Challenge. Front. Vet. Sci. 2021, 8, 759418. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, Y.; Jiang, H.; Xu, Z.; Guo, Y.; Cao, X.; Li, T.; Wan, Z.; Shao, H.; Qin, A.; et al. Efficient cross-protection against serotype 4/8a fowl adenoviruses (FAdVs): Recombinant FAdV-4 with FAdV-8a Fiber. Microbiol. Spectr. 2023, 11, e0246223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, Q.; Guo, R.; Liu, A.; Xu, Z.; Gao, Y.; Cui, H.; Liu, C.; Qi, X.; Zhang, Y.; et al. Immunogenicity of Novel Live Vaccine Based on an Artificial rHN20 Strain against Emerging Fowl Adenovirus 4. Viruses 2021, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, P.; Yu, X.; Cheng, Z.; Shen, Z.; Liu, Z.; Xiao, Y. Research progress on the vaccine against group I fowl Aviadenovirus. Chin. J. Anim. Infect. Dis. 2020, 28, 108–116. [Google Scholar] [CrossRef]
- Corredor, J.C.; Pei, Y.; Nagy, E. Fowl Adenovirus-Based Vaccine Platform. Methods Mol. Biol. 2017, 1581, 29–54. [Google Scholar] [CrossRef]
- Wang, H.; Tian, J.; Zhao, J.; Zhao, Y.; Yang, H.; Zhang, G. Current Status of Poultry Recombinant Virus Vector Vaccine Development. Vaccines 2024, 12, 630. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, Y.; Liu, A.; Cui, H.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Li, K.; Gao, L.; et al. Development of a Novel Avian Vaccine Vector Derived From the Emerging Fowl Adenovirus 4. Front. Microbiol. 2021, 12, 780978. [Google Scholar] [CrossRef] [PubMed]
- Ackford, J.G.; Corredor, J.C.; Pei, Y.; Krell, P.J.; Bedecarrats, G.; Nagy, E. Foreign gene expression and induction of antibody response by recombinant fowl adenovirus-9-based vectors with exogenous promoters. Vaccine 2017, 35, 4974–4982. [Google Scholar] [CrossRef]
- Pei, Y.; Corredor, J.C.; Griffin, B.D.; Krell, P.J.; Nagy, E. Fowl Adenovirus 4 (FAdV-4)-Based Infectious Clone for Vaccine Vector Development and Viral Gene Function Studies. Viruses 2018, 10, 97. [Google Scholar] [CrossRef]
- Vemula, S.V.; Ahi, Y.S.; Swaim, A.M.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS ONE 2013, 8, e62496. [Google Scholar] [CrossRef]
- Tian, K.Y.; Guo, H.F.; Li, N.; Zhang, Y.H.; Wang, Z.; Wang, B.; Yang, X.; Li, Y.T.; Zhao, J. Protection of chickens against hepatitis-hydropericardium syndrome and Newcastle disease with a recombinant Newcastle disease virus vaccine expressing the fowl adenovirus serotype 4 fiber-2 protein. Vaccine 2020, 38, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Entrican, G.; Francis, M.J. Applications of platform technologies in veterinary vaccinology and the benefits for one health. Vaccine 2022, 40, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, Q.; Chu, Z.; Wang, P.; Luo, C.; Zhang, Y.; Fang, X.; Qiu, L.; Dang, R.; Yang, Z. Immune protection efficacy of FAdV-4 surface proteins fiber-1, fiber-2, hexon and penton base. Virus Res. 2018, 245, 1–6. [Google Scholar] [CrossRef]
- Schachner, A.; Marek, A.; Jaskulska, B.; Bilic, I.; Hess, M. Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis-hydropericardium syndrome (HHS). Vaccine 2014, 32, 1086–1092. [Google Scholar] [CrossRef]
- Liu, W.; Liu, M.; Wang, S.; Tang, Z.; Liu, J.; Song, S.; Yan, L. The Development of a Novel Fiber-2 Subunit Vaccine against Fowl Adenovirus Serotype 4 Formulated with Oil Adjuvants. Vaccines 2024, 12, 263. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Z.; Liu, L.; Li, Y.; Gao, W.; Song, X.; Li, X. Knob domain of Fiber 2 protein provides full protection against fowl adenovirus serotype 4. Virus Res. 2023, 330, 199113. [Google Scholar] [CrossRef]
- Shah, M.S.; Ashraf, A.; Rahman, M.; Khan, M.I.; Qureshi, J.A. A subunit vaccine against hydropericardium syndrome using adenovirus penton capsid protein. Vaccine 2012, 30, 7153–7156. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Pan, X.; Zhi, W.; Chen, H.; Bai, B.; Ma, C.; Ma, D. Probiotics Surface-Delivering Fiber2 Protein of Fowl Adenovirus 4 Stimulate Protective Immunity Against Hepatitis-Hydropericardium Syndrome in Chickens. Front. Immunol. 2022, 28, 919100. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.A.; Dastjerdi, P.Z.; Omar, A.R. Virus-like particles in poultry disease: An approach to effective and safe vaccination. Front. Vet. Sci. 2024, 11, 1405605. [Google Scholar] [CrossRef] [PubMed]
- Tufail, S.; Shah, M.A.; Zafar, M.; Asif, T.A.; Shehzad, A.; Shah, M.S.; Habib, M.; Saleemi, M.K.; Muddassar, M.; Mirza, O.; et al. Identification of potent epitopes on hexon capsid protein and their evaluation as vaccine candidates against infections caused by members of Adenoviridae family. Vaccine 2021, 39, 3560–3564. [Google Scholar] [CrossRef]
- Xinglong, W.; Qiuxia, T.; Li, Q.; Zengqi, Y. Penton-dodecahedron of fowl adenovirus serotype 4 as a vaccine candidate for the control of related diseases. Vaccine 2019, 37, 839–847. [Google Scholar] [CrossRef]
- Gupta, A.; Ahmed, K.A.; Ayalew, L.E.; Popowich, S.; Kurukulasuriya, S.; Goonewardene, K.; Gunawardana, T.; Karunarathna, R.; Ojkic, D.; Tikoo, S.K.; et al. Immunogenicity and protective efficacy of virus-like particles and recombinant fiber proteins in broiler-breeder vaccination against fowl adenovirus (FAdV)-8b. Vaccine 2017, 35, 2716–2722. [Google Scholar] [CrossRef]
- Faiza, A.; Soban, T.; Majid Ali, S.; Muhammad, S.S.; Mudasser, H.; Osman, M.; Mazhar, I.; Moazur, R. In silico epitope prediction and immunogenic analysis for penton base epitope-focused vaccine against hydropericardium syndrome in chicken. Virus Res. 2019, 273, 197750. [Google Scholar] [CrossRef]
- Hu, J.; Li, G.; Wang, X.; Cai, L.; Rong, M.; Li, H.; Xie, M.; Zhang, Z.; Rong, J. Development of a subunit vaccine based on fiber2 and hexon against fowl adenovirus serotype 4. Virus Res. 2021, 305, 198552. [Google Scholar] [CrossRef]
- Schachner, A.; De Luca, C.; Heidl, S.; Hess, M. Recombinantly Expressed Chimeric Fibers Demonstrate Discrete Type-Specific Neutralizing Epitopes in the Fowl Aviadenovirus E (FAdV-E) Fiber, Promoting the Optimization of FAdV Fiber Subunit Vaccines towards Cross-Protection in vivo. Microbiol. Spectr. 2022, 10, e0212321. [Google Scholar] [CrossRef]
- De Luca, C.; Schachner, A.; Heidl, S.; Hess, M. Vaccination with a fowl adenovirus chimeric fiber protein (crecFib-4/11) simultaneously protects chickens against hepatitis-hydropericardium syndrome (HHS) and inclusion body hepatitis (IBH). Vaccine 2022, 40, 1837–1845. [Google Scholar] [CrossRef]
- Liu, S.; Dong, X.; Lei, B.; Zhang, W.; Wang, X.; Yuan, W.; Zhao, K. A novel subunit vaccine based on Fiber1/2 knob domain provides full protection against fowl adenovirus serotype 4 and induces stronger immune responses than a Fiber2 subunit vaccine. Poult. Sci. 2024, 103, 103888. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Li, B.; Li, J.; Shen, Y.; Jiang, Y.; Cui, W.; Tang, L. Immunoprotection of FliBc chimeric fiber2 fusion proteins targeting dendritic cells against Fowl adenovirus serotype 4 infection. Poult. Sci. 2024, 103, 103474. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.H.; Sun, L.; Tu, K.H.; Teng, Q.Y.; Xue, J.; Zhang, G.Z. Experimental co-infection of variant infectious bursal disease virus and fowl adenovirus serotype 4 increases mortality and reduces immune response in chickens. Vet. Res. 2021, 52, 61. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, N.; Jing, D.; Liu, X.; Zeng, Z.; Wang, J.; Xiao, F.; Zhang, H.; Chi, H.; Wan, C.; et al. Characterization and evaluation of an oral vaccine via nano-carrier for surface immunogenic protein (Sip) delivery against Streptococcus agalactiae infection. Int. J. Biol. Macromol. 2023, 235, 123770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Sun, B.; Shi, C.; Sun, Y.; Jin, Z.; Hu, G. Intranasal immunization with O-2′-Hydroxypropyl trimethyl ammonium chloride chitosan nanoparticles loaded with Newcastle disease virus DNA vaccine enhances mucosal immune response in chickens. J. Nanobiotechnol. 2021, 19, 240. [Google Scholar] [CrossRef]
- Xu, C.; Dobson, H.E.; Yu, M.; Gong, W.; Sun, X.; Park, K.S.; Kennedy, A.; Zhou, X.; Xu, J.; Xu, Y.; et al. STING agonist-loaded mesoporous manganese-silica nanoparticles for vaccine applications. J. Control. Release 2023, 357, 84–93. [Google Scholar] [CrossRef]
- Hills, R.A.; Tan, T.K.; Cohen, A.A.; Keeffe, J.R.; Keeble, A.H.; Gnanapragasam, P.N.P.; Storm, K.N.; Rorick, A.V.; West, A.P., Jr.; Hill, M.L.; et al. Proactive vaccination using multiviral Quartet Nanocages to elicit broad anti-coronavirus responses. Nat. Nanotechnol. 2024, 19, 1216–1223. [Google Scholar] [CrossRef]
- Zhou, D.; Cheng, R.; Yao, Y.; Zhang, G.; Li, X.; Wang, B.; Wang, Y.; Yu, F.; Yang, S.; Liu, H.; et al. An attachment glycoprotein nanoparticle elicits broadly neutralizing antibodies and protects against lethal Nipah virus infection. NPJ Vaccines 2024, 9, 158. [Google Scholar] [CrossRef]
- Platt, C.D.; Ma, J.K.; Chalouni, C.; Ebersold, M.; Bou-Reslan, H.; Carano, R.A.; Mellman, I.; Delamarre, L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc. Natl. Acad. Sci. USA 2010, 107, 4287–4292. [Google Scholar] [CrossRef]

| Strain | Origin | Adjuvant | Dosage (/Bird) | Inoculation Route | Survival Rate (%) | Reference |
|---|---|---|---|---|---|---|
| K531/07 | CEL | ISA 70 | 5 × 105 TCID50 | IM | 80 | [38] |
| SB15 | LMH | Oil | 1 × 106 TCID50 | SC | 100 | [41] |
| HLJFAd15 | CEL | Oil | 1 × 106 TCID50 | IM | 100 | [45] |
| SDJN0105 | CEL | Oil | 1 × 106 TCID50 | IM | 100 | [46] |
| CH/GZXF/1602 | CEK | Oil | 1 × 106 TCID50 | SC | 100 | [47] |
| FA4-F8b | LMH | Oil | 1 × 106 TCID50 | IM | 100 | [48] |
| rFAdV-4-fiber/8b | LMH | ADJ501 | 1 × 106 TCID50 | IM | 100 | [49] |
| FAdV-4 rHN20-vvIBDV-VP2 | LMH | Oil | 3 × 106 PFU | IM | 100 | [51] |
| Proteins | Adjuvant | Dose | Inoculation Route | Survival Rate (%) | Reference |
|---|---|---|---|---|---|
| rFiber2 | FCA | 50 μg | IM | 80 | [42] |
| rFiber2 | FCA | 100 μg | IM | 100 | [42] |
| rFiber2 | FCA | 150 μg | IM | 100 | [42] |
| Fiber2 | FCA | 2.5 μg | SC | 100 | [41] |
| Fiber1 | FCA | 100 μg | ND | 100 | [67] |
| Fiber2 | FCA | 50 μg | ND | 100 | [67] |
| Penton | FCA | 200 μg | ND | 90 | [67] |
| Hexon | FCA | 200 μg | ND | 95 | [67] |
| Fiber2 | Marcol 52 white oil | 10 μg | SC | 100 | [69] |
| Fiber2 | Marcol 52 white oil | 5 μg | SC | 90 | [69] |
| Penton | Marcol 52 white oil | 200 μg | SC | 100 | [69] |
| Penton | Marcol 52 white oil | 100 μg | SC | 70 | [69] |
| Penton base | FCA | 200 μL | SC | 90 | [71] |
| His6-PreS-penton base1−225 | Montanide ISA71 VG | 500 μL | SC | 50 | [77] |
| His6-PreS-penton base FL | Montanide ISA71 VG | 500 μL | SC | 50 | [77] |
| Enterococcus faecalis/ pTX8048-DCpep-Fiber2-CWA | N/A | 5.0 × 1.0 × 109 CFU | OG | 100 | [72] |
| Pt-Dds | N/A | 0.5 μg | ND | 100 | [75] |
| rFH | MontanideTM ISA71 VG | ≥5 μg | IM | 100 | [78] |
| HBc-hexon(Asp348-Phe369) | MontanideTM ISA71 VG | 100 μg | SC | 90 | [74] |
| HBc-hexon (Ser19-Pro82) | MontanideTM ISA71 VG | 100 μg | SC | 70 | [74] |
| HBc-hexon(Gly932-Phe956) | MontanideTM ISA71 VG | 100 μg | SC | 40 | [74] |
| Fib-8a | GERBU Adjuvant P | 50 μg | IM | 94 | [36] |
| Fiber1/2 knob domain | FCA | 10 μg | IM | 100 | [81] |
| Fiber2 | FCA | 10 μg | IM | 100 | [81] |
| FliBc-fiber2-SP | white oil | 50 μg | IM | 100 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Yang, P.; Zhou, J.; Liu, X.; Huang, Y.; Wan, C. Advances and Prospects of Fowl Adenoviruses Vaccine Technologies in the Past Decade. Int. J. Mol. Sci. 2025, 26, 6434. https://doi.org/10.3390/ijms26136434
Zhu C, Yang P, Zhou J, Liu X, Huang Y, Wan C. Advances and Prospects of Fowl Adenoviruses Vaccine Technologies in the Past Decade. International Journal of Molecular Sciences. 2025; 26(13):6434. https://doi.org/10.3390/ijms26136434
Chicago/Turabian StyleZhu, Chunhua, Pei Yang, Jiayu Zhou, Xiaodong Liu, Yu Huang, and Chunhe Wan. 2025. "Advances and Prospects of Fowl Adenoviruses Vaccine Technologies in the Past Decade" International Journal of Molecular Sciences 26, no. 13: 6434. https://doi.org/10.3390/ijms26136434
APA StyleZhu, C., Yang, P., Zhou, J., Liu, X., Huang, Y., & Wan, C. (2025). Advances and Prospects of Fowl Adenoviruses Vaccine Technologies in the Past Decade. International Journal of Molecular Sciences, 26(13), 6434. https://doi.org/10.3390/ijms26136434







