Construction of Microsphere Culture System for Human Mesenchymal Stem Cell Aggregates
Abstract
1. Introduction
2. Results
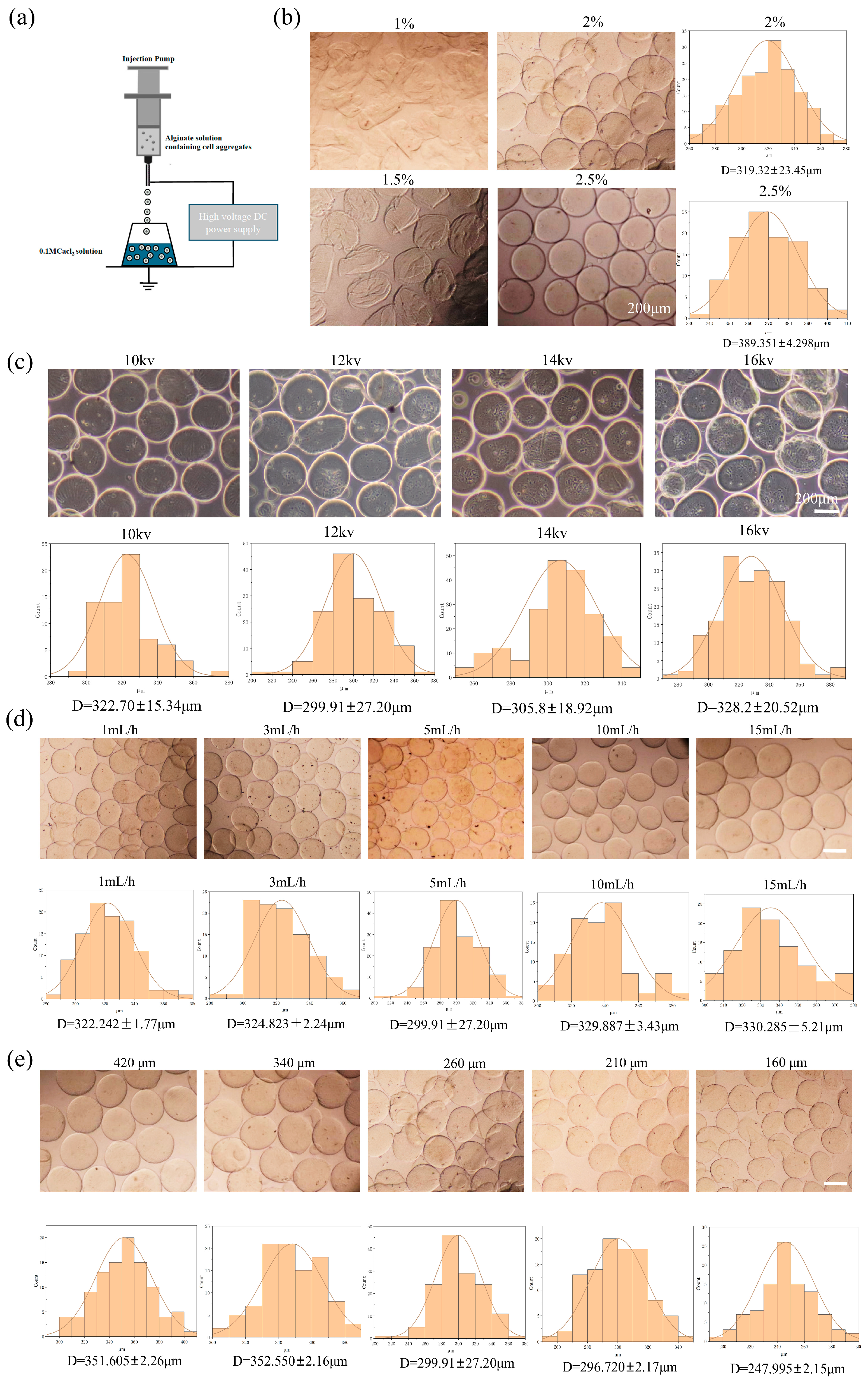
2.1. The Construction of the Alg-HM Culture System for the Culture of hMSCs
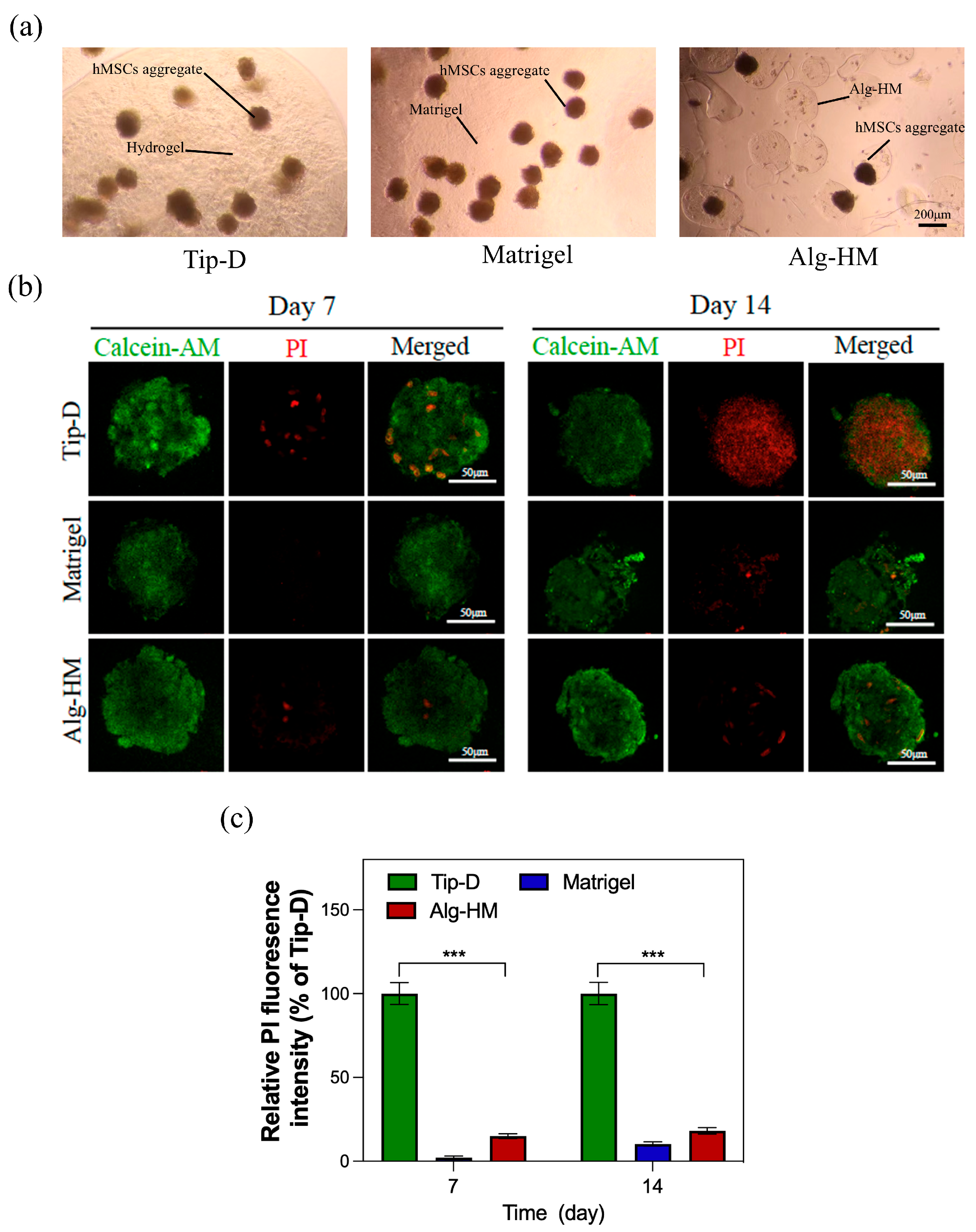
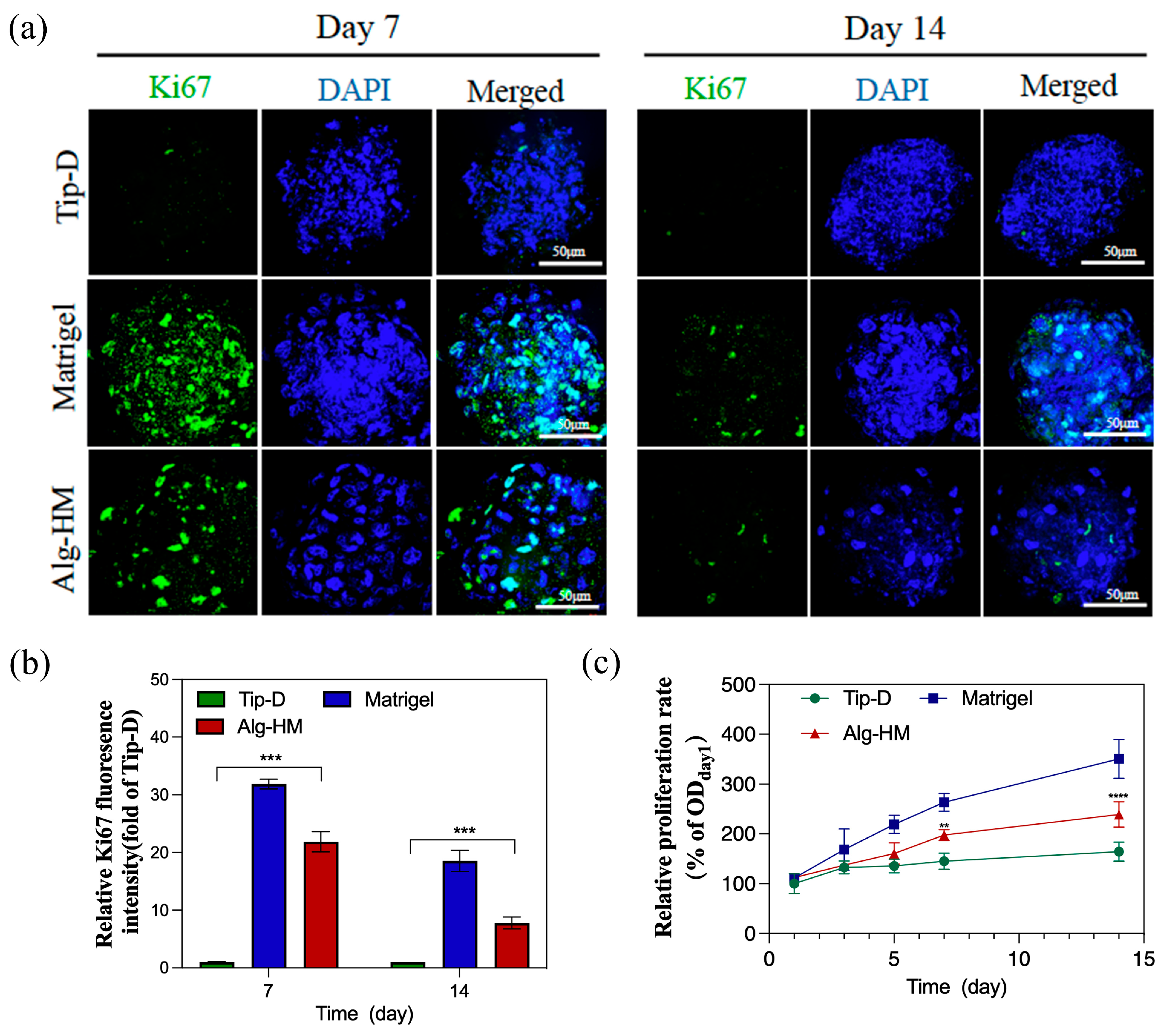
2.2. The Alg-HM Culture System Significantly Reduces the Central Necrosis and Increases the Proliferation Activity of the hMSC Aggregate
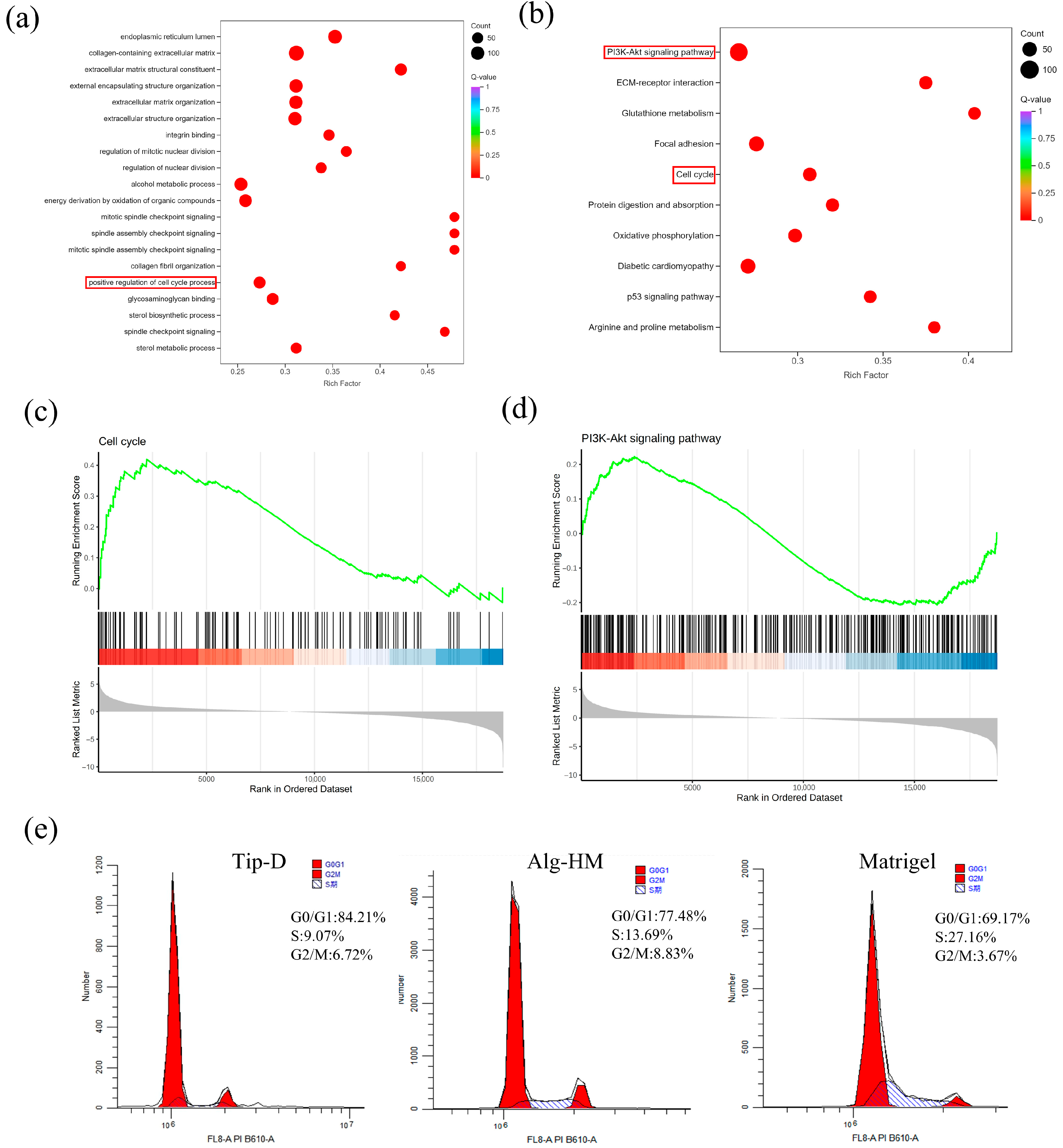
2.3. The RNA Sequencing Results Showed That the Alg-HM Culture System Can Significantly Activate the Signaling Pathways Related to Cell Proliferation in hMSCs
3. Discussion
4. Materials and Methods
4.1. Preparation of the Alg-HM Culture System
4.2. Cultivation of hMSC and Preparation of hMSC Aggregates
4.3. Construction of hMSC Aggregate Culture System
4.4. The Cell Counting Kit-8(CCK-8) Assay Was Used to Determine Cell Viability
4.5. Calcein-AM/PI Cell Staining
4.6. Ki-67 Cell Proliferation Detection
4.7. RNA Sequencing Analysis
4.8. Cell Cycle Assay
4.9. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, R.; Wan, Z.; Yang, C.; Shen, X.; Wang, R.; Zhang, H.; Yang, R.; Li, J.; Song, Y.; Su, H. Advances and clinical challenges of mesenchymal stem cell therapy. Front. Immunol. 2024, 15, 1421854. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.C.; Park, G.T.; Moon, H.J.; Choi, E.B.; Lim, M.J.; Yoon, J.W.; Lee, N.; Kwon, S.M.; Lee, B.J.; Kim, J.H. Hybrid spheroids containing mesenchymal stem cells promote therapeutic angiogenesis by increasing engraftment of co-transplanted endothelial colony-forming cells in vivo. Stem Cell Res. Ther. 2023, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kawasaki, H.; Wells, A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J. Biomed. Biotechnol. 2010, 2010, 795385. [Google Scholar] [CrossRef]
- Shimazawa, Y.; Kusamori, K.; Tsujimura, M.; Shimomura, A.; Takasaki, R.; Takayama, Y.; Shimizu, K.; Konishi, S.; Nishikawa, M. Intravenous injection of mesenchymal stem cell spheroids improves the pulmonary delivery and prolongs in vivo survival. Biotechnol. J. 2022, 17, e2100137. [Google Scholar] [CrossRef] [PubMed]
- Vorwald, C.E.; Ho, S.S.; Whitehead, J.; Leach, J.K. High-Throughput Formation of Mesenchymal Stem Cell Spheroids and Entrapment in Alginate Hydrogels. Methods Mol. Biol. 2018, 1758, 139–149. [Google Scholar] [CrossRef]
- Cesarz, Z.; Funnell, J.L.; Guan, J.; Tamama, K. Soft Elasticity-Associated Signaling and Bone Morphogenic Protein 2 Are Key Regulators of Mesenchymal Stem Cell Spheroidal Aggregates. Stem Cells Dev. 2016, 25, 622–635. [Google Scholar] [CrossRef]
- Yen, B.L.; Hsieh, C.C.; Hsu, P.J.; Chang, C.C.; Wang, L.T.; Yen, M.L. Three-Dimensional Spheroid Culture of Human Mesenchymal Stem Cells: Offering Therapeutic Advantages and In Vitro Glimpses of the In Vivo State. Stem Cells Transl. Med. 2023, 12, 235–244. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Rasouli, M.; Safari, F.; Kanani, M.H.; Ahvati, H. Principles of Hanging Drop Method (Spheroid Formation) in Cell Culture. Methods Mol. Biol. 2025, 2879, 289–300. [Google Scholar] [CrossRef]
- Huang, G.S.; Dai, L.G.; Yen, B.L.; Hsu, S.H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choe, G.; Oh, S.; Lee, J.Y. In Situ Formation of Proangiogenic Mesenchymal Stem Cell Spheroids in Hyaluronic Acid/Alginate Core-Shell Microcapsules. ACS Biomater. Sci. Eng. 2020, 6, 6938–6948. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Lee, J.H.; Huh, Y.S.; Takayama, S. Alginate Microencapsulation for Three-Dimensional In Vitro Cell Culture. ACS Biomater. Sci. Eng. 2021, 7, 2864–2879. [Google Scholar] [CrossRef]
- Ohori-Morita, Y.; Ashry, A.; Niibe, K.; Egusa, H. Current perspectives on the dynamic culture of mesenchymal stromal/stem cell spheroids. Stem Cells Transl. Med. 2025, 14, szae093. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Chai, Y.; Zhuo, C.; Xu, Y.; Xue, T.; Shao, D.; Tao, Y.; Li, M. 3D Printing of a Vascularized Mini-Liver Based on the Size-Dependent Functional Enhancements of Cell Spheroids for Rescue of Liver Failure. Adv. Sci. 2024, 11, e2309899. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef]
- Passemard, S.; Szabó, L.; Noverraz, F.; Montanari, E.; Gonelle-Gispert, C.; Bühler, L.H.; Wandrey, C.; Gerber-Lemaire, S. Synthesis Strategies to Extend the Variety of Alginate-Based Hybrid Hydrogels for Cell Microencapsulation. Biomacromolecules 2017, 18, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ge, Y.; Wu, Y.; Feng, Y.; Liu, H.; Cao, W.; Xie, J.; Zhang, J. High-Voltage Electrostatic Field Hydrogel Microsphere 3D Culture System Improves Viability and Liver-like Properties of HepG2 Cells. Int. J. Mol. Sci. 2024, 25, 1081. [Google Scholar] [CrossRef]
- Kim, M.H.; Banerjee, D.; Celik, N.; Ozbolat, I.T. Aspiration-assisted freeform bioprinting of mesenchymal stem cell spheroids within alginate microgels. Biofabrication 2022, 14, 024103. [Google Scholar] [CrossRef]
- Abbas, S.E.M.; Maged, G.; Wang, H.; Lotfy, A. Mesenchymal Stem/Stromal Cells Microencapsulation for Cell Therapy. Cells 2025, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Pangjantuk, A.; Kaokaen, P.; Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. 3D culture of alginate-hyaluronic acid hydrogel supports the stemness of human mesenchymal stem cells. Sci. Rep. 2024, 14, 4436. [Google Scholar] [CrossRef]
- Qu, J.; Wang, L.; Niu, L.; Lin, J.; Huang, Q.; Jiang, X.; Li, M. Porous Silk Fibroin Microspheres Sustainably Releasing Bioactive Basic Fibroblast Growth Factor. Materials 2018, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, Y.; Liu, B. Preparation of chitosan microcarriers by high voltage electrostatic field and freeze drying. J. Biosci. Bioeng. 2019, 128, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef]
- Maji, S.; Lee, H. Engineering Hydrogels for the Development of Three-Dimensional In Vitro Models. Int. J. Mol. Sci. 2022, 23, 2662. [Google Scholar] [CrossRef]
- Anada, T.; Fukuda, J.; Sai, Y.; Suzuki, O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 2012, 33, 8430–8441. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Smith, A.M.; Senior, J.J. Alginate Hydrogels with Tuneable Properties. Adv. Biochem. Eng. Biotechnol. 2021, 178, 37–61. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Li, S.; Sang, M.; Cui, T.; Xie, J. Construction of Microsphere Culture System for Human Mesenchymal Stem Cell Aggregates. Int. J. Mol. Sci. 2025, 26, 6435. https://doi.org/10.3390/ijms26136435
Lv C, Li S, Sang M, Cui T, Xie J. Construction of Microsphere Culture System for Human Mesenchymal Stem Cell Aggregates. International Journal of Molecular Sciences. 2025; 26(13):6435. https://doi.org/10.3390/ijms26136435
Chicago/Turabian StyleLv, Chenlong, Shangkun Li, Min Sang, Tingting Cui, and Jinghui Xie. 2025. "Construction of Microsphere Culture System for Human Mesenchymal Stem Cell Aggregates" International Journal of Molecular Sciences 26, no. 13: 6435. https://doi.org/10.3390/ijms26136435
APA StyleLv, C., Li, S., Sang, M., Cui, T., & Xie, J. (2025). Construction of Microsphere Culture System for Human Mesenchymal Stem Cell Aggregates. International Journal of Molecular Sciences, 26(13), 6435. https://doi.org/10.3390/ijms26136435




