Impact of Combined Exposure to Copper Nanoparticles, Copper Oxide Nanoparticles, and Pesticides on the Metabolic Activity of Nitrobacter winogradskyi
Abstract
1. Introduction
2. Results
2.1. Metabolic Activity
2.2. IC50 Estimation of the Effects of Several Concentrations of Pesticides with Nanoparticles
2.3. The Loewe Additive Model and Scheirer–Ray–Hare Test
2.4. MALDI TOF/TOF MS
2.5. MALDI TOF/TOF MS Data Analysis
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Culture Conditions
4.3. Metabolic Activity Assay
4.4. Protein Profile of N. winogradskyi Exposed to CuNPs and Pesticides
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Dou, F.; Li, C.; Ma, X.; Ma, L.Q. Impacts of metallic nanoparticles and transformed products on soil health. Crit. Rev. Environ. Sci. Technol. 2021, 51, 973–1002. [Google Scholar] [CrossRef]
- Panagos, P.; Ballabio, C.; Lugato, E.; Jones, A.; Borrelli, P.; Scarpa, S.; Orgiazzi, A.; Montanarella, L. Potential sources of anthropogenic copper inputs to European agricultural soils. Sustainability 2018, 10, 2380. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture: A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 2017, 7, 28–40. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Fernández-Baldo, M.A.; Bertolino, F.A.; Durán, N.; Seabra, A.B.; Tortella, G.R. The nanotechnology among us: Are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit. Rev. Biotechnol. 2019, 39, 157–172. [Google Scholar] [CrossRef]
- Rodrigues, S.; Bland, G.D.; Gao, X.; Rodrigues, S.M.; Lowry, G.V. Investigation of pore water and soil extraction tests for characterizing the fate of poorly soluble metal-oxide nanoparticles. Chemosphere 2021, 267, 128885. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Ahmed, B.; Sushkova, S.; Singh, R.; Soldatov, M.; Laratte, B.; Fedorenko, A.; Mandzhieva, S.; Blicharska, E.; et al. Interaction of copper-based nanoparticles to soil, terrestrial, and aquatic systems: Critical review of the state of the science and future perspectives. Environ. Contam. 2019, 252, 51–96. [Google Scholar]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2016, 67, erw449. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Reed, P. Nitrobacter as an indicator of toxicity in wastewater. SWS Contract Report 1983, 326, 31. [Google Scholar]
- Williams, J.O.; Dilosi, L.B. Response of chemolithotrophic Nitrobacter, Nitrosomonas to toxicity of organophosphate and pyrethroid pesticides. Asian J. Biol. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Simonin, M.; Martins, J.M.F.; Le Roux, X.; Uzu, G.; Calas, A.; Richaume, A. Toxicity of TiO2 nanoparticles on soil nitrification at environmentally relevant concentrations: Lack of classical dose–response relationships. Nanotoxicology 2017, 11, 247–255. [Google Scholar] [CrossRef]
- VandeVoort, A.; Arai, Y. Macroscopic observation of soil nitrification kinetics impacted by copper nanoparticles: Implications for micronutrient nanofertilizer. Nanomaterials 2018, 8, 927. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Cea, M.; Rodríguez-Rodríguez, C.; Seguel, A.; Parada, J. Sorption parameters of carbendazim and iprodione in the presence of copper nanoparticles in two different soils. J. Soil Sci. Plant Nutr. 2019, 19, 469–476. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Diez, M.C.; Cea, M.; Sant’Ana da Silva, A.; Rodríguez-Rodríguez, C.E.; Tortella, G.R. Combined pollution of copper nanoparticles and atrazine in soil: Effects on dissipation of the pesticide and on microbiological community profiles. J. Hazard. Mater. 2019, 361, 228–236. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Sousa, D.Z.; Martínez, M.; Fernández-Baldo, M.A.; Tortella, G.R. Short term changes in the abundance of nitrifying microorganisms in a soil–plant system simultaneously exposed to copper nanoparticles and atrazine. Sci. Total Environ. 2019, 670, 1068–1074. [Google Scholar] [CrossRef]
- Deni, J.; Penninckx, M.J. Nitrification and autotrophic nitrifying bacteria in a hydrocarbon-polluted soil. Appl. Environ. Microbiol. 1999, 65, 4008–4013. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.A.; Zam, S.Z.; Akram, M.; Shin, S.; Yeom, I.T. Coagulation and dissolution of CuO nanoparticles in the presence of dissolved organic matter under different pH values. Sustainability 2019, 11, 2825. [Google Scholar] [CrossRef]
- Wu, F.; Harper, B.J.; Crandon, L.E.; Harper, S.L. Assessment of Cu and CuO nanoparticle ecological responses using laboratory small-scale microcosms. Environ. Sci. Nano 2020, 7, 105–115. [Google Scholar] [CrossRef]
- Ishihara, J.; Mekubo, T.; Kusaka, C.; Kondo, S.; Aiba, H.; Ishikawa, S.; Ogasawara, N.; Oshima, T.; Takahashi, H. Critical role of the periplasm in copper homeostasis in Gram-negative bacteria. Biosystems 2023, 231, 104980. [Google Scholar] [CrossRef]
- Schweigert, N.; Acero, J.L.; von Gunten, U.; Canonica, S.; Zehnder, A.J.B.; Eggen, R.I.L. DNA degradation by the mixture of copper and catechol is caused by DNA-copper-hydroperoxo complexes, probably DNA-Cu(I)OOH. Environ. Mol. Mutagen. 2000, 36, 5–12. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Ong, S.-K.; Sato, C. Effects of heavy metals on nitrifying bacteria. Water Sci. Technol. 1997, 36, 69–74. [Google Scholar] [CrossRef]
- Fischer, J.; Evlanova, A.; Philippe, A.; Filser, J. Soil properties can evoke toxicity of copper oxide nanoparticles towards springtails at low concentrations. Environ. Pollut. 2021, 270, 116084. [Google Scholar] [CrossRef]
- Lang, M.; Cai, Z. Effects of chlorothalonil and carbendazim on nitrification and denitrification in soils. J. Environ. Sci. 2009, 21, 458–467. [Google Scholar] [CrossRef]
- Ding, H.; Zheng, X.; Zhang, J.; Zhang, Y.; Yu, J.; Chen, D. Influence of chlorothalonil and carbendazim fungicides on the transformation processes of urea nitrogen and related microbial populations in soil. Environ. Sci. Pollut. Res. 2019, 26, 31133–31141. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Wen, C.-Y.; Chiu, T.-C.; Yen, J.-H. Effect of fungicide iprodione on soil bacterial community. Ecotoxicol. Environ. Saf. 2004, 59, 127–132. [Google Scholar] [CrossRef]
- Rai, J.P.N. Effects of long-term 2,4-D application on microbial populations and biochemical processes in cultivated soil. Biol. Fertil. Soils 1992, 13, 187–191. [Google Scholar]
- Vasileiadis, S.; Puglisi, E.; Papadopoulou, E.S.; Pertile, G.; Suciu, N.; Pappolla, R.A.; Tourna, M.; Karas, P.A.; Papadimitriou, F.; Kasiotakis, A.; et al. Blame it on the metabolite: 3,5-dichloroaniline rather than the parent compound is responsible for the decreasing diversity and function of soil microorganisms. Appl. Environ. Microbiol. 2018, 84, e01536-18. [Google Scholar] [CrossRef]
- Klein, K.; Tenno, T. Estimating the impact of inhibitory substances on activated sludge denitrification process. Water Pract. Technol. 2019, 14, 863–871. [Google Scholar] [CrossRef]
- Suárez-Ojeda, M.E.; Guisasola, A.; Carrera, J. Inhibitory impact of quinone-like compounds over partial nitrification. Chemosphere 2010, 80, 474–480. [Google Scholar] [CrossRef]
- Tang, N.H.; Blum, D.J.W.; Nirmalakhandan, N.; Speece, R.E. QSAR parameters for toxicity of organic chemicals to Nitrobacter. J. Environ. Eng. 1992, 118, 17–37. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Bendixen, K.; Karlson, U. Detection of Microbial Growth on Polycyclic Aromatic Hydrocarbons in Microtiter Plates by Using the Respiration Indicator WST-1. Appl. Environ. Microbiol. 2002, 68, 2683–2689. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Y.; Qin, Q.; Xu, J.; Wang, B.; Chen, J.; Liu, B.; Zhang, W.; Qiao, L. MALDI-TOF characterization of protein expression mutation during morphological changes of bacteria under the impact of antibiotics. Anal. Chem. 2019, 91, 2352–2359. [Google Scholar] [CrossRef]
- Hasan, N.; Ahmad, F.; Wu, H.F. Monitoring the heat stress response of Escherichia coli via NiO nanoparticle assisted MALDI-TOF mass spectrometry. Talanta 2013, 103, 38–46. [Google Scholar] [CrossRef]
- Navratilova, J.; Praetorius, A.; Gondikas, A.; Fabienke, W.; von der Kammer, F.; Hofmann, T. Detection of engineered copper nanoparticles in soil using single particle ICP-MS. Int. J. Environ. Res. Public Health 2015, 12, 15756–15768. [Google Scholar] [CrossRef]
- Parra, B.; Tortella, G.R.; Cuozzo, S.; Martínez, M. Negative effect of copper nanoparticles on the conjugation frequency of conjugative catabolic plasmids. Ecotoxicol. Environ. Saf. 2019, 169, 662–668. [Google Scholar] [CrossRef]
- Hobbie, J.E.; Daley, R.J.; Jasper, S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 1977, 33, 1225–1228. [Google Scholar] [CrossRef]
- Kent, R.D.; Vikesland, P.J. Evaluación controlada de la disolución de nanopartículas de plata mediante microscopía de fuerza atómica. Environ. Sci. Technol. 2012, 46, 6977–6984. [Google Scholar] [CrossRef]
- De Peretti, A.I.F.; De Moro, G.B.M.; Ghittoni, N.E.; De Duffard, A.M.E.; Duffard, R.O. Effects of 2,4-dichlorophenoxyacetic acid on Rhizobium sp. in pure culture. Environ. Toxicol. Water Qual. 1987, 2, 217–228. [Google Scholar] [CrossRef]
- Briceño, G.; Lamilla, C.; Leiva, B.; Levio, M.; Donoso-Piñol, P.; Schalchli, H.; Gallardo, F.; Diez, M.C. Pesticide-tolerant bacteria isolated from a biopurification system to remove commonly used pesticides to protect water resources. PLoS ONE 2020, 15, e0234865. [Google Scholar] [CrossRef]
- Božik, M.; Cejnar, P.; Šašková, M.; Nový, P.; Maršík, P.; Klouček, P. Stress response of Escherichia coli to essential oil components—Insights on low-molecular-weight proteins from MALDI-TOF. Sci. Rep. 2018, 8, 13042. [Google Scholar] [CrossRef] [PubMed]
- Scheirer, C.J.; Ray, W.S.; Hare, N. The analysis of ranked data derived from completely randomized factorial designs. Biometrics 1976, 32, 429. [Google Scholar] [CrossRef]
- Van der Borght, K.; Tourny, A.; Bagdziunas, R.; Thas, O.; Nazarov, M.; Turner, H.; Verbist, B.; Ceulemans, H. BIGL: Biochemically intuitive generalized Loewe null model for prediction of the expected combined effect compatible with partial agonism and antagonism. Sci. Rep. 2017, 7, 17935. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Wiley: New York, NY, USA, 2017; pp. 1–15. [Google Scholar]
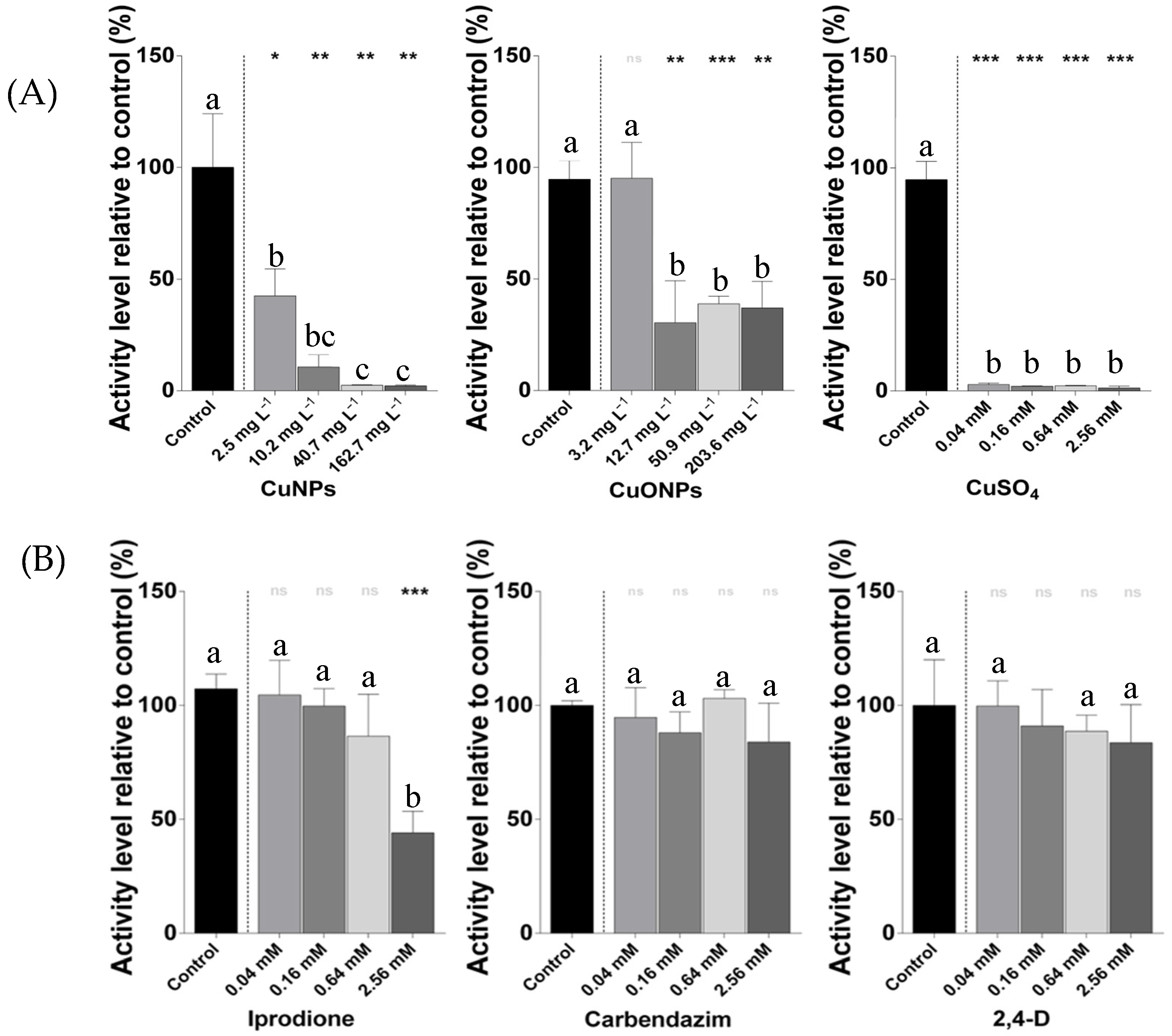
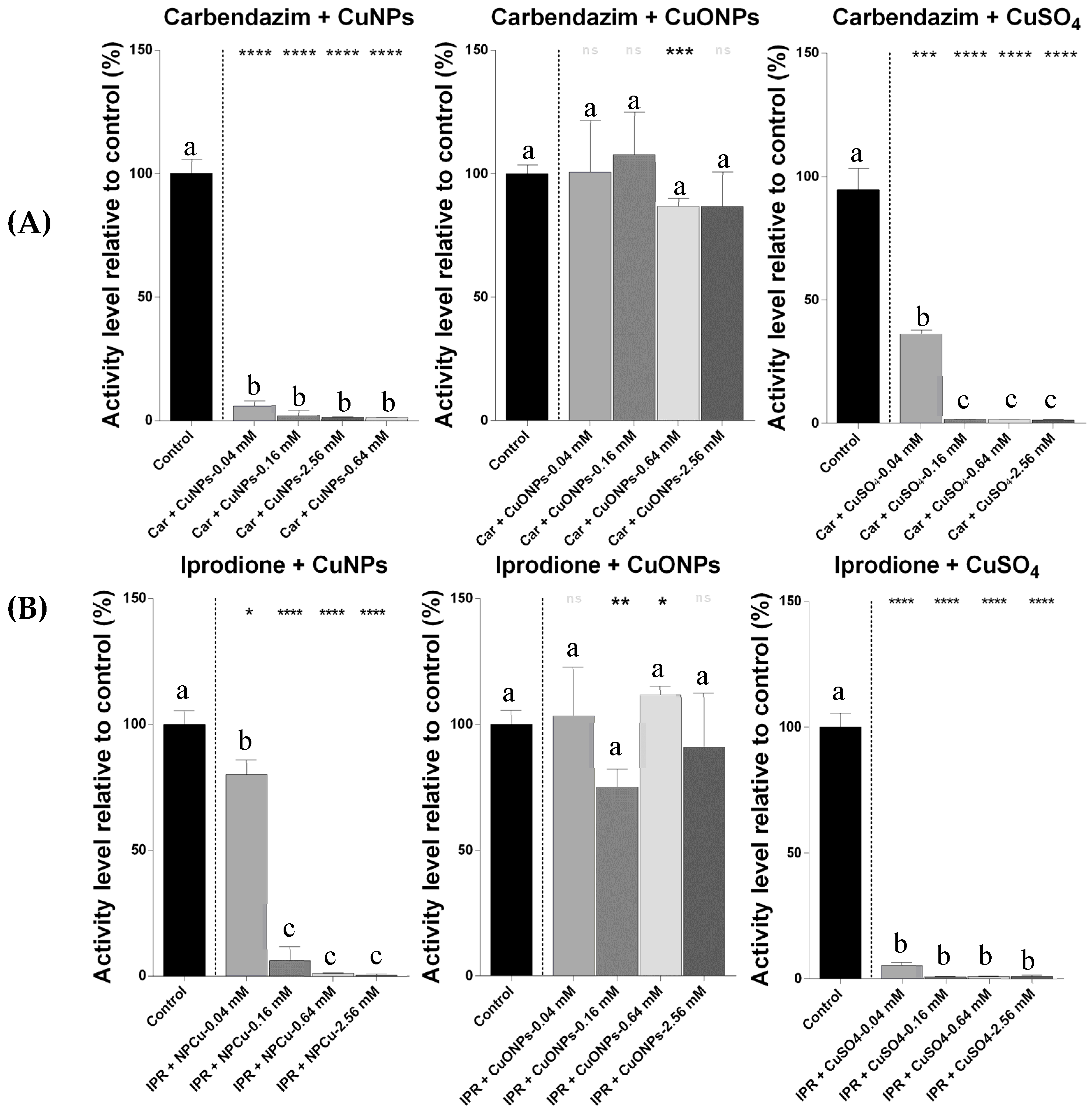
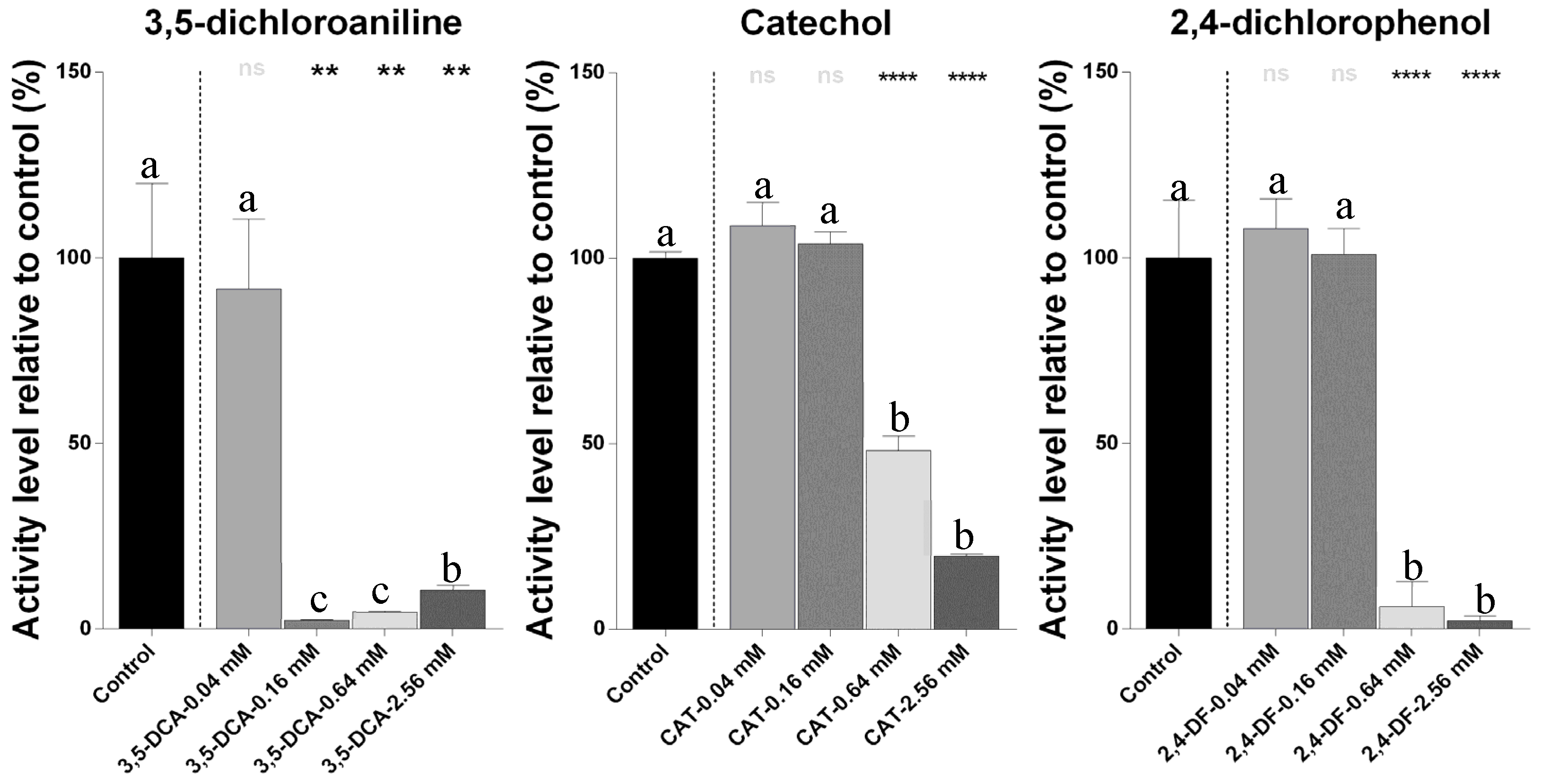
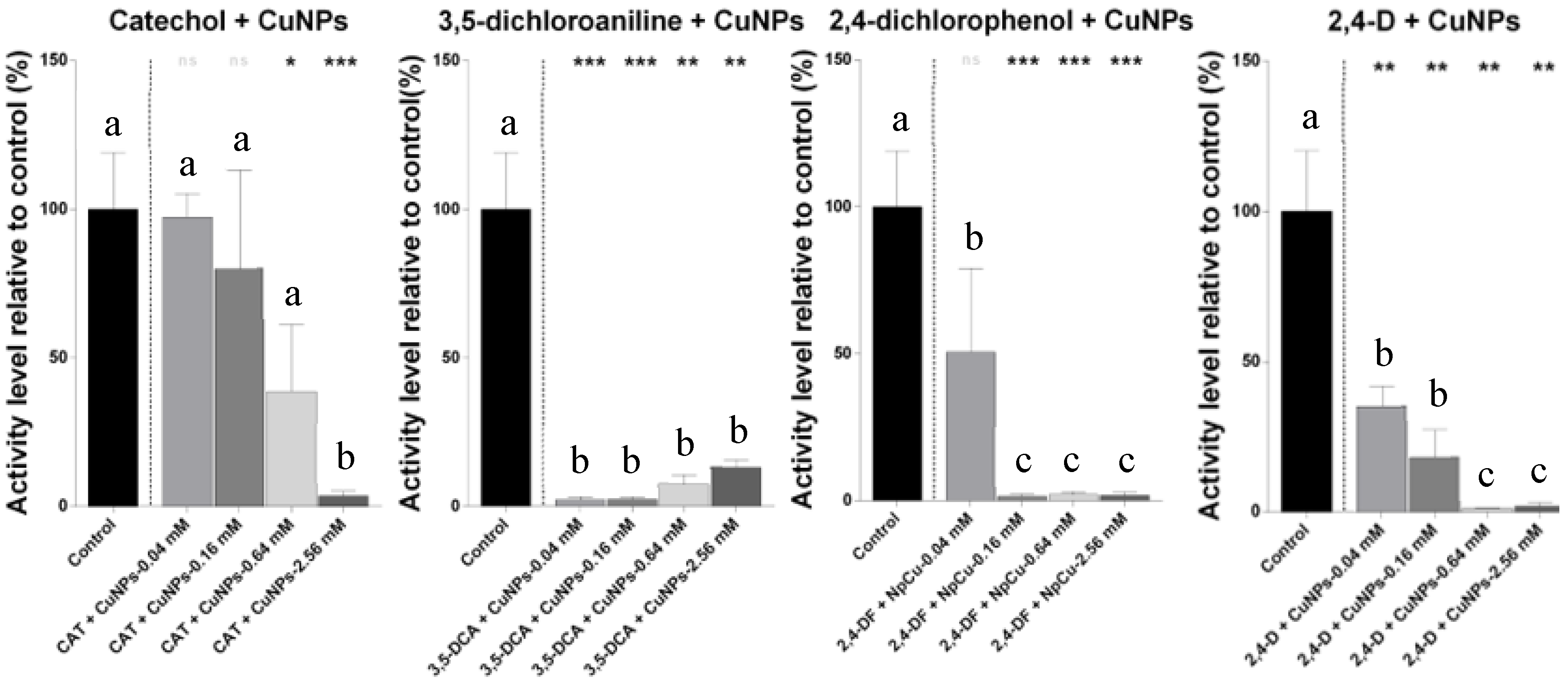
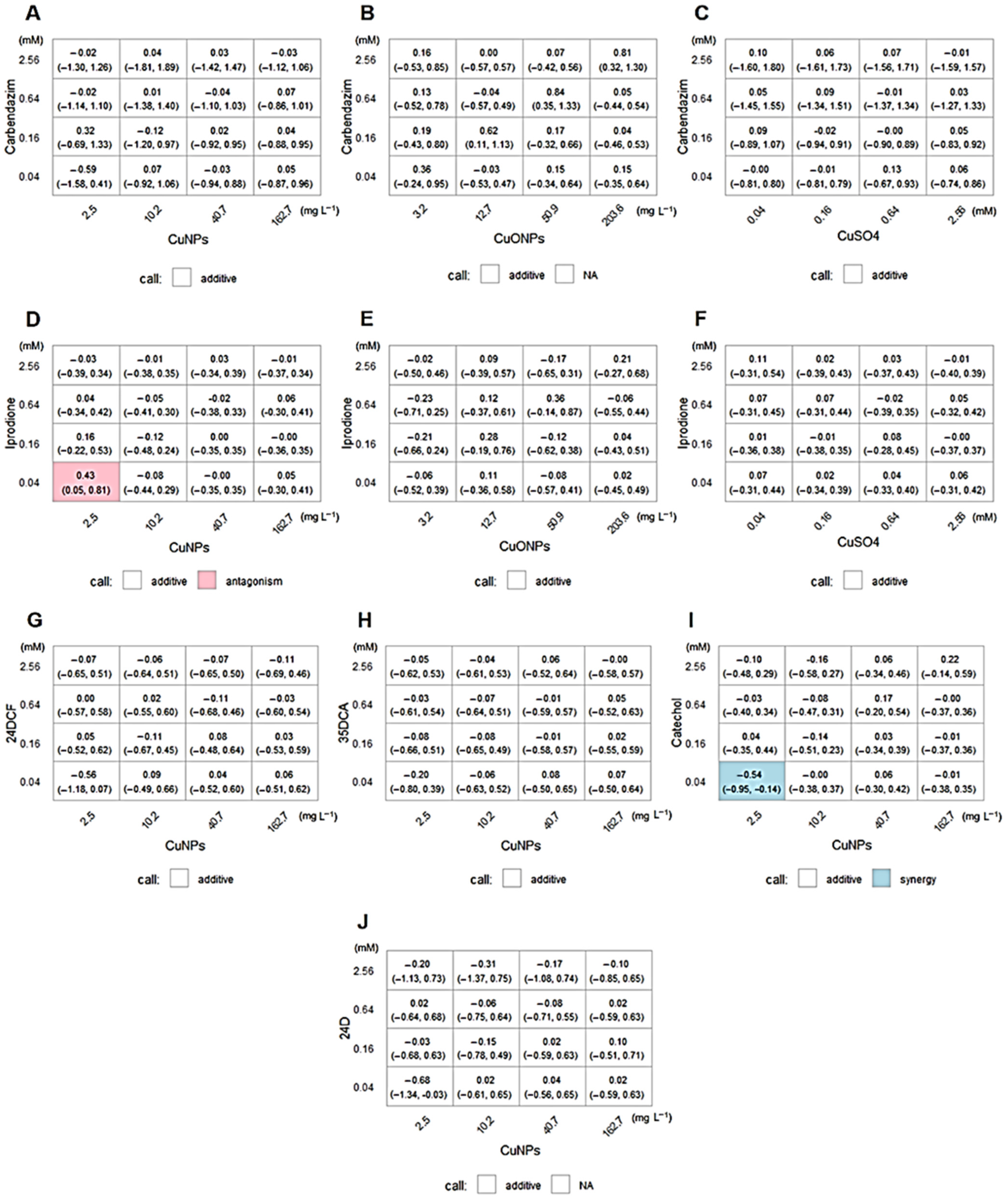
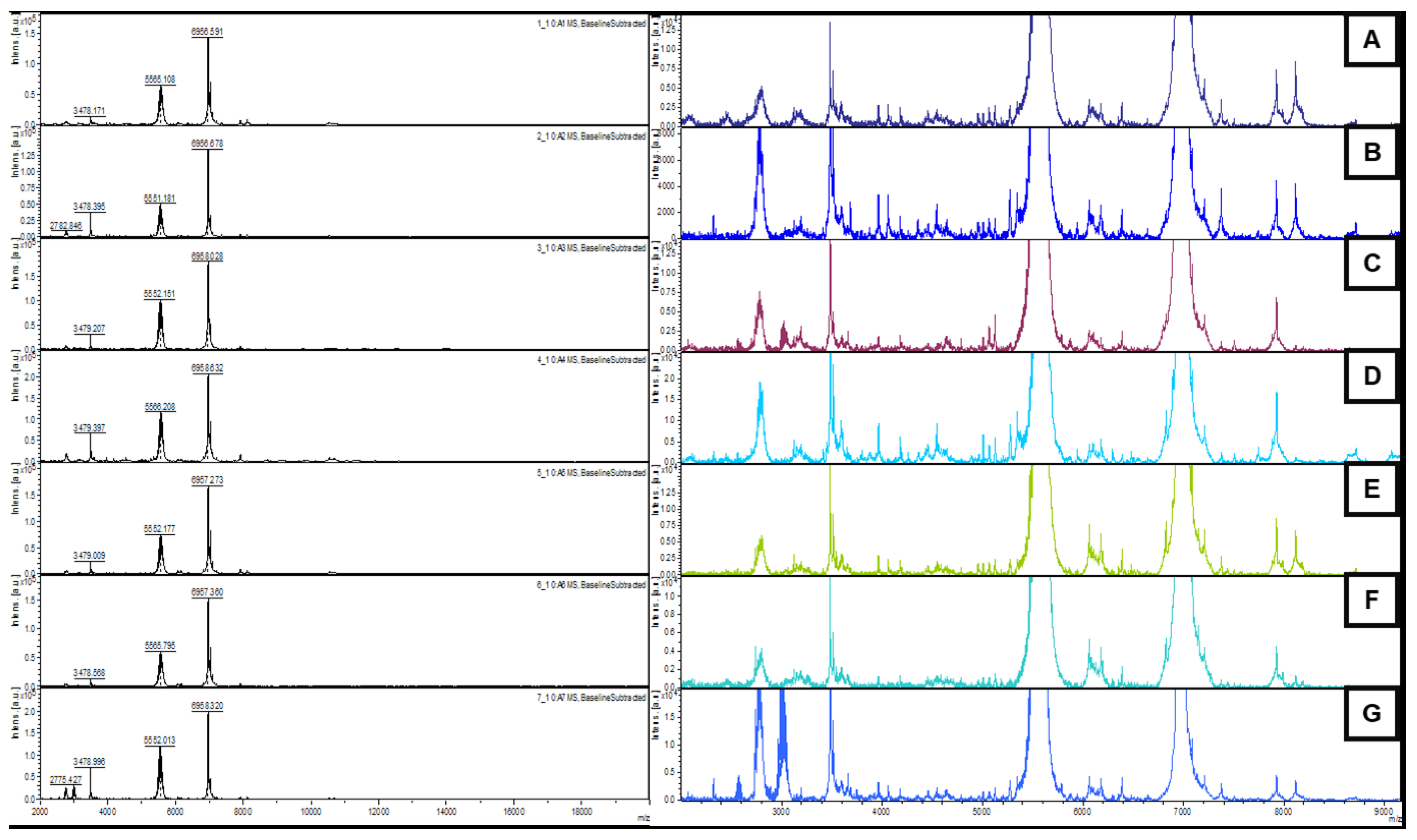
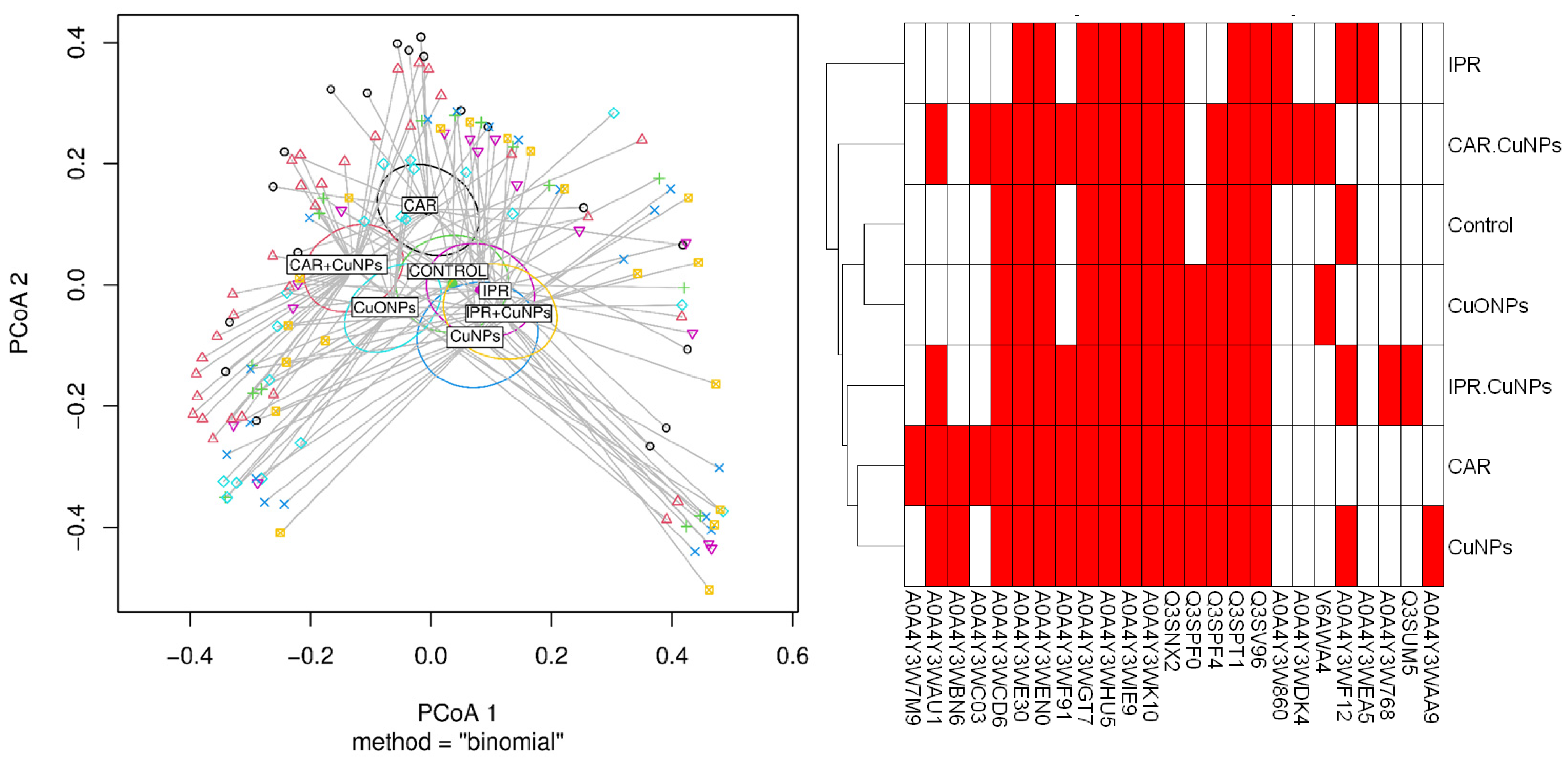
| Evaluated Compounds | Mixed Treatment Type | Estimated IC50 * |
|---|---|---|
| CuNPs | Copper-based comp. | ≤2.5 mg L−1 |
| CuONPs | “ | 31.81 mg L−1 |
| CuSO4 | “ | ≤0.04 mM |
| Carbendazim | Pesticide (fungicide) | ≥2.56 mM |
| Iprodione | “ | 0.83 mM |
| 2,4-D | Pesticide (herbicide) | ≥2.56 mM |
| 3,5-dichloroaniline | Pesticide derivate | 0.26 mM |
| Catechol | “ | 0.74 mM |
| 2,4-dichlorophenol | “ | 0.85 mM |
| Carbendazim + CuNPs (1:1) | Fungicide + copper-based comp. | ≤0.04 mM + ≤2.5 mg L−1 |
| Carbendazim + CuONPs (1:1) | “ | 0.92 mM + 73.18 mg L−1 |
| Carbendazim + CuSO4 (1:1) | “ | ≤0.04 mM |
| Iprodione + CuNPs (1:1) | “ | ≤0.04 mM + ≤2.5 mg L−1 |
| Iprodione + CuONPs (1:1) | “ | 1.26 mM + 100.22 mg L−1 |
| Iprodione + CuSO4 (1:1) | “ | ≤0.04 mM |
| 2,4-D + CuNPs (1:1) | Herbicide + copper-based comp. | ≤0.04 mM + ≤2.5 mg L−1 |
| 3,5-dichloroaniline + CuNPs (1:1) | Pesticide derivate + copper-based comp. | 0.08 mM + 5.08 mg L−1 |
| Catechol + CuNPs (1:1) | “ | 0.46 mM + 29.23 mg L−1 |
| 2,4-dichlorophenol + CuNPs (1:1) | “ | ≤0.04 mM + ≤2.5 mg L−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajardo, R.; Rubilar, O.; López-Mena, E.; Sanchez-Ante, G.; Fincheira, P.; Martinez, M.; Schoebitz, M.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Bardelhe, L.; et al. Impact of Combined Exposure to Copper Nanoparticles, Copper Oxide Nanoparticles, and Pesticides on the Metabolic Activity of Nitrobacter winogradskyi. Int. J. Mol. Sci. 2025, 26, 6391. https://doi.org/10.3390/ijms26136391
Gajardo R, Rubilar O, López-Mena E, Sanchez-Ante G, Fincheira P, Martinez M, Schoebitz M, Tighe-Neira R, Inostroza-Blancheteau C, Bardelhe L, et al. Impact of Combined Exposure to Copper Nanoparticles, Copper Oxide Nanoparticles, and Pesticides on the Metabolic Activity of Nitrobacter winogradskyi. International Journal of Molecular Sciences. 2025; 26(13):6391. https://doi.org/10.3390/ijms26136391
Chicago/Turabian StyleGajardo, Roberto, Olga Rubilar, Edgar López-Mena, Gildardo Sanchez-Ante, Paola Fincheira, Miguel Martinez, Mauricio Schoebitz, Ricardo Tighe-Neira, Claudio Inostroza-Blancheteau, Leonardo Bardelhe, and et al. 2025. "Impact of Combined Exposure to Copper Nanoparticles, Copper Oxide Nanoparticles, and Pesticides on the Metabolic Activity of Nitrobacter winogradskyi" International Journal of Molecular Sciences 26, no. 13: 6391. https://doi.org/10.3390/ijms26136391
APA StyleGajardo, R., Rubilar, O., López-Mena, E., Sanchez-Ante, G., Fincheira, P., Martinez, M., Schoebitz, M., Tighe-Neira, R., Inostroza-Blancheteau, C., Bardelhe, L., & Tortella-Fuentes, G. (2025). Impact of Combined Exposure to Copper Nanoparticles, Copper Oxide Nanoparticles, and Pesticides on the Metabolic Activity of Nitrobacter winogradskyi. International Journal of Molecular Sciences, 26(13), 6391. https://doi.org/10.3390/ijms26136391











