Intestinal Immune System Expression of Coho Salmon Challenged with Oxytetracycline: In Vivo and In Vitro Approach
Abstract
1. Introduction
2. Results
2.1. In Vitro Experiments
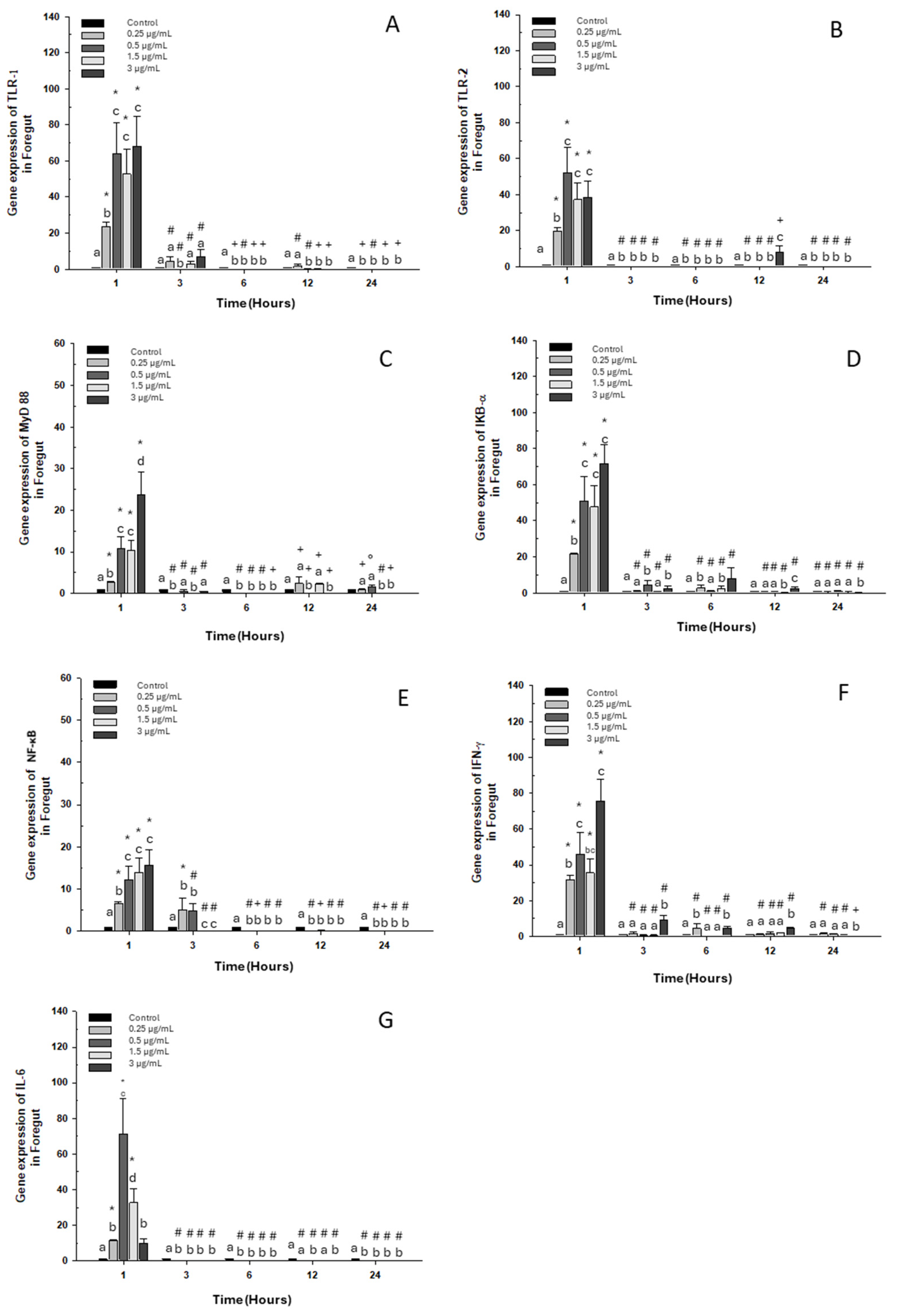
2.1.1. Foregut
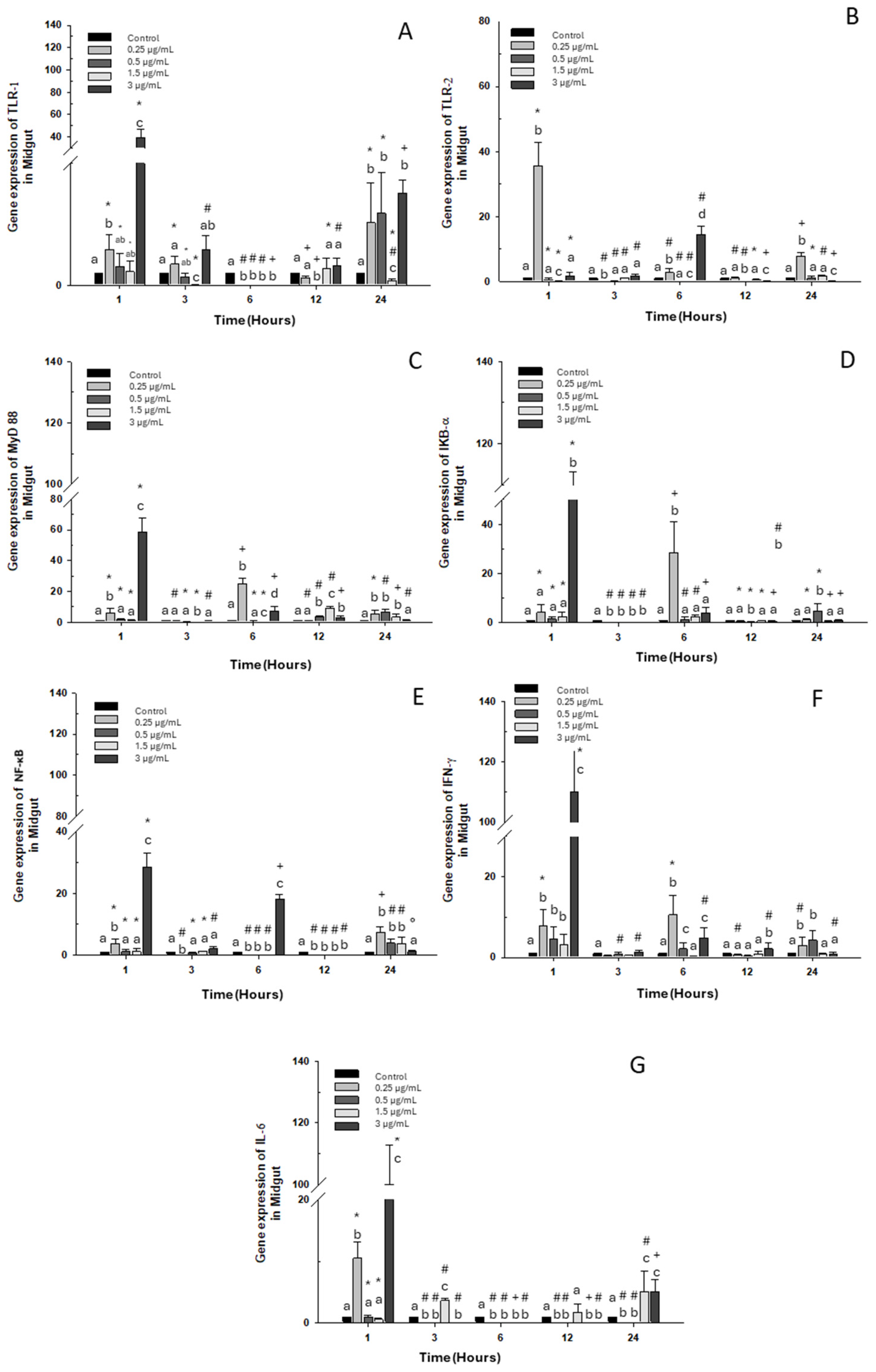
2.1.2. Midgut
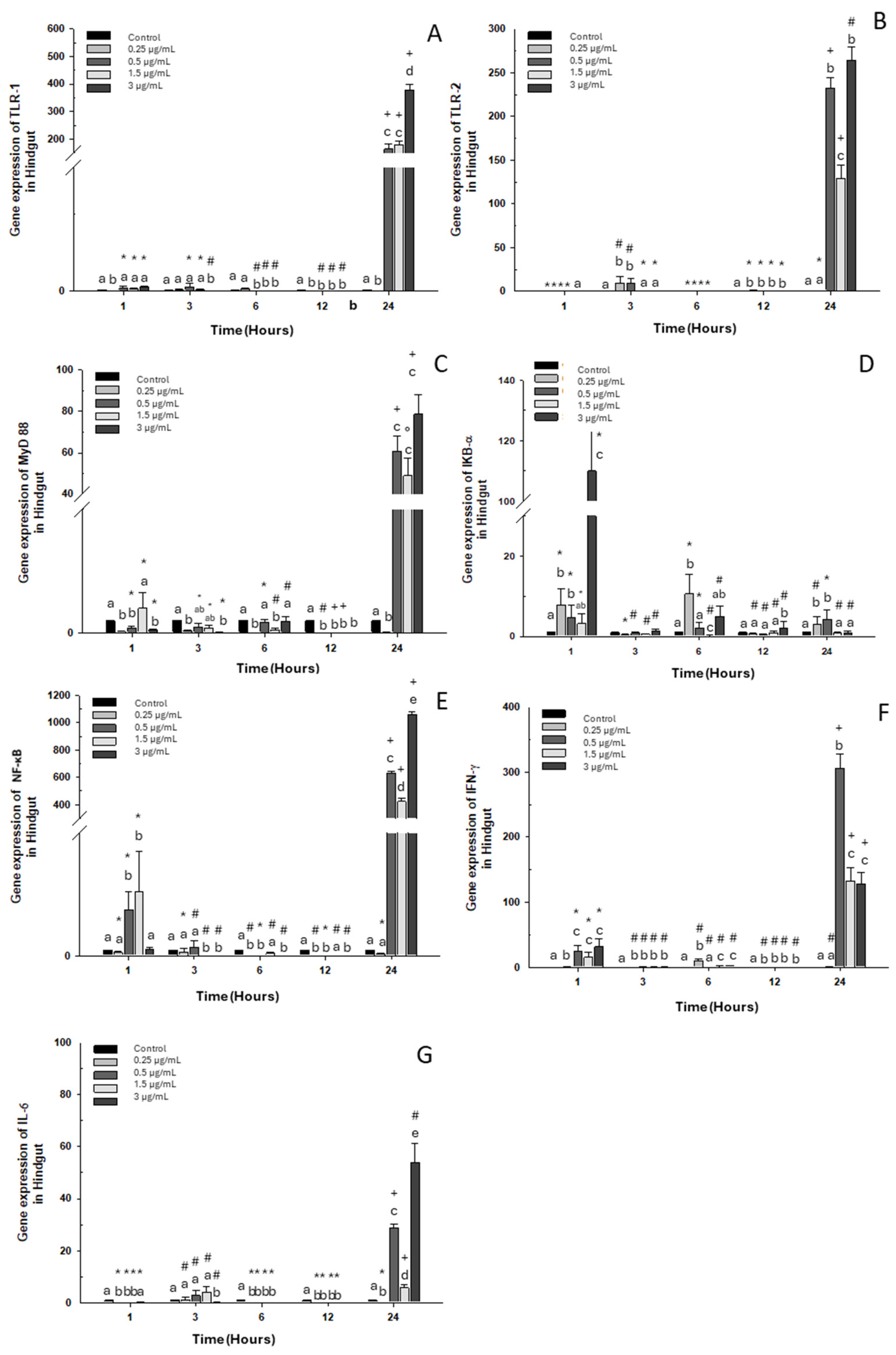
2.1.3. Hindgut
2.2. In Vivo Experiment
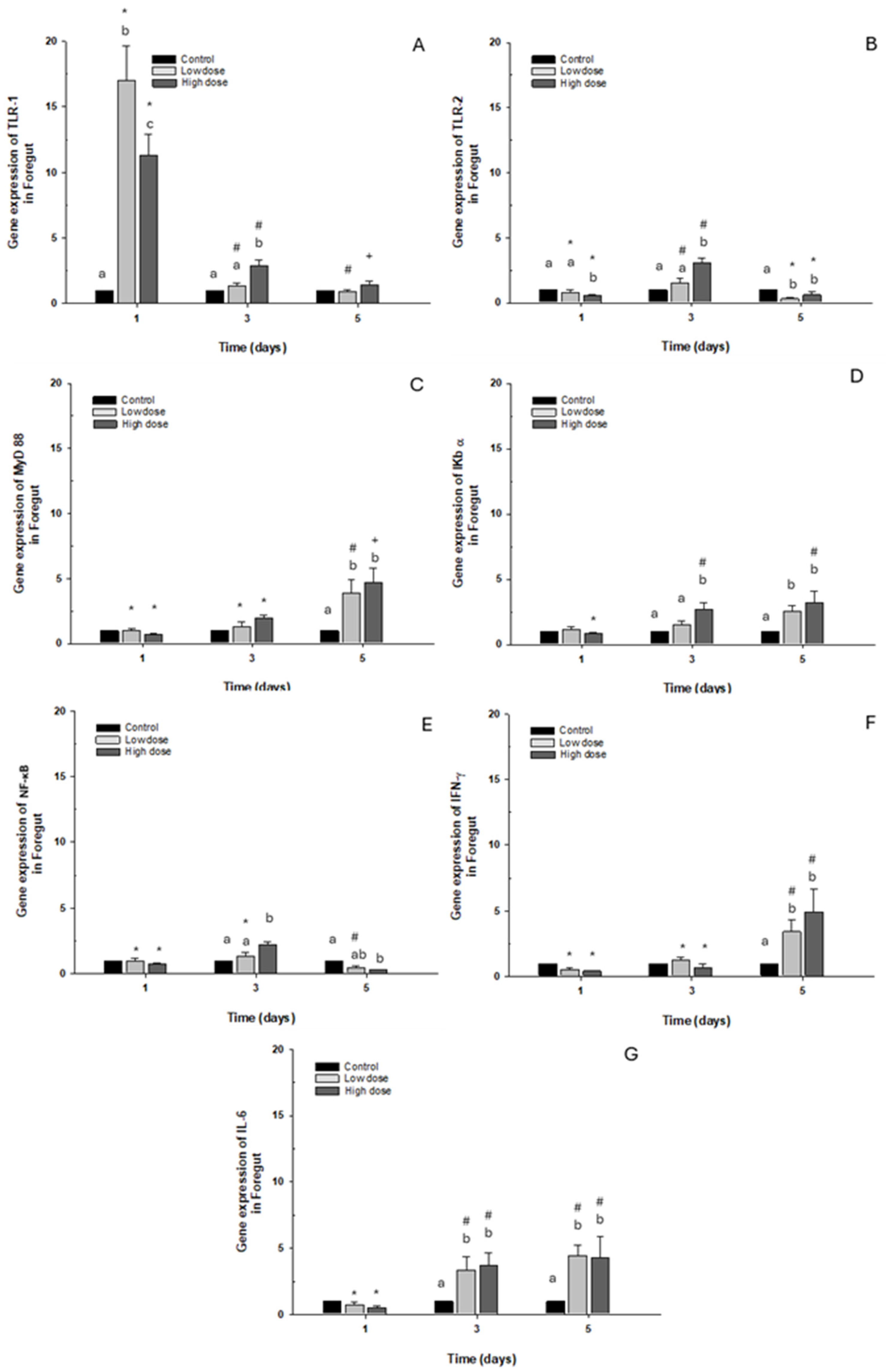
2.2.1. Foregut
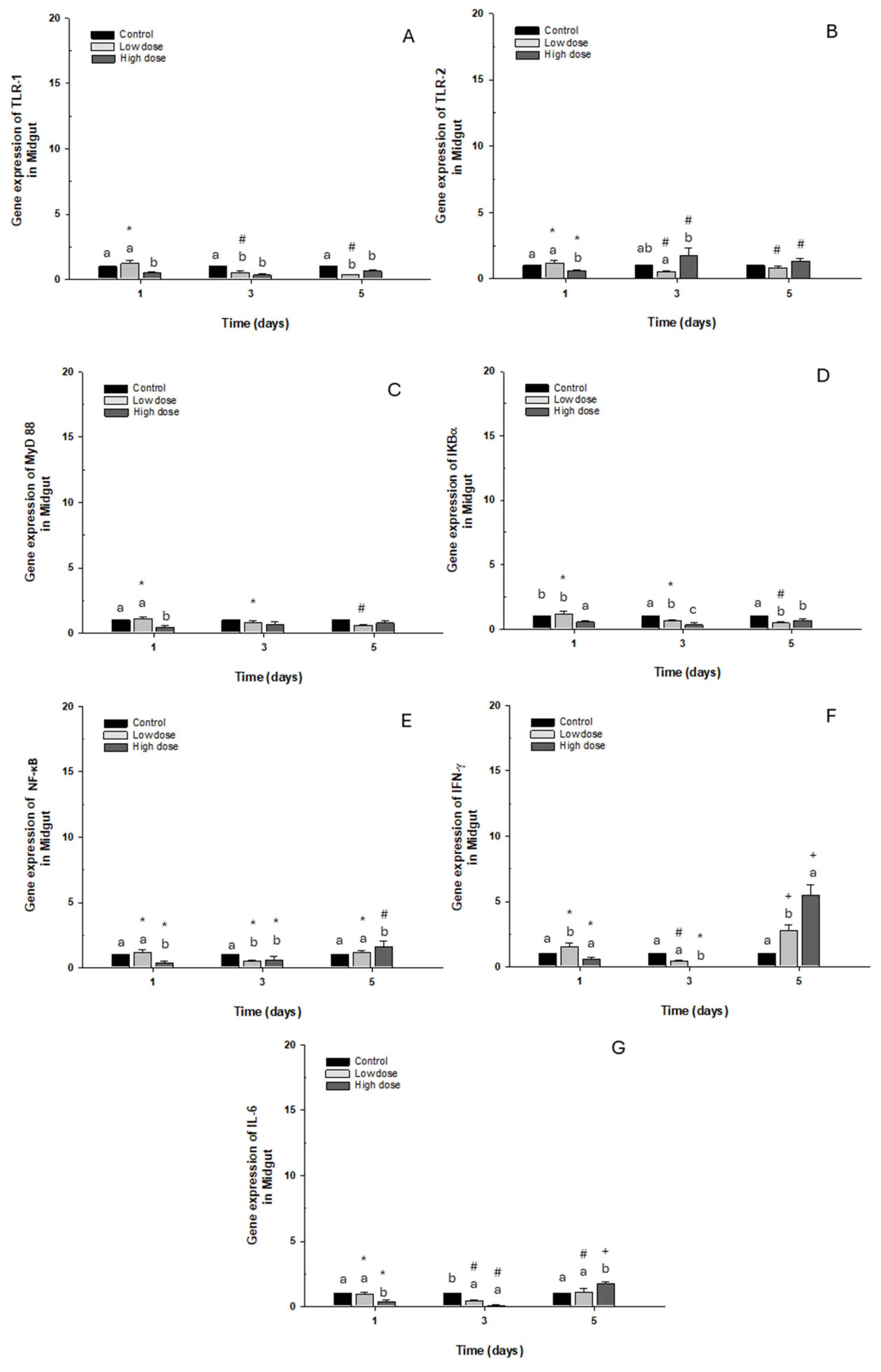
2.2.2. Midgut
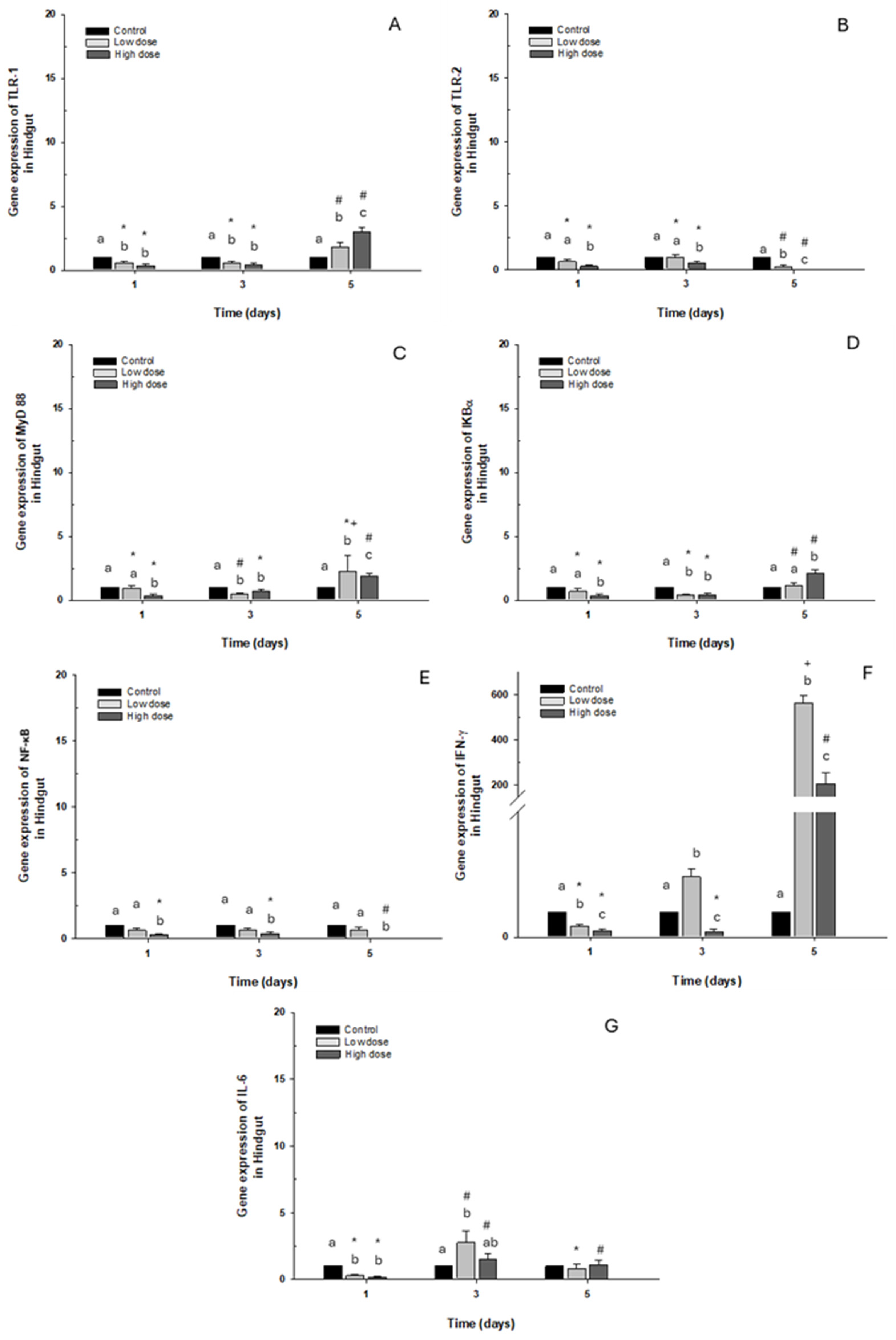
2.2.3. Hindgut
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Intestinal Primary Cell Culture Preparation
4.3. In Vitro Experimental Treatment
4.4. In Vivo Experiment
4.5. Total RNA Extraction
4.6. RT-qPCR Analysis
4.7. Statistical Treatment of Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamada, A.; Torre, C.; Drancourt, M.; Ghigo, E. Trained immunity carried by non-immune cells. Front. Microbiol. 2019, 9, 3225. [Google Scholar]
- Romo, M.R.; Pérez-Martínez, D.; Ferrer, C.C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J. Chapter 24: The adaptive immune system. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Meseguer, J.; Lopez-Ruiz, A.; Garcia-Ayala, A. Reticulo-endothelial stroma of the head-kidney from the seawater teleost gilthead seabream (Sparus aurata L.): An ultrastructural and cytochemical study. Anat. Rec. 1995, 241, 303–309. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Whyte, S.K. The innate immune response of finfish. A review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Banu, H.; Prakash, A.; Tripathi, G. Immune system of fish: An evolutionary perspective. In Antimicrobial Immune Response; IntechOpen: London, UK, 2021. [Google Scholar]
- Martínez, D.P.; Oliver, C.; Santibañez, N.; Coronado, J.L.; Oyarzún-Salazar, R.; Enriquez, R.; Vargas-Chacoff, L.; Romero, A. PAMPs of Piscirickettsia salmonis Trigger the Transcription of Genes Involved in Nutritional Immunity in a Salmon Macrophage-Like Cell Line. Front. Immunol. 2022, 13, 849752. [Google Scholar] [CrossRef]
- Pontigo, J.P.; Saravia, J.; Oyarzún, R.; González, M.P.; Hawes, C.; Morera, F.J.; Pino, J.; Wadsworth, S.; Muñoz, J.L.P.; Vargas-Chacoff, L. Modulation of the Expression of Immune-related gene in Atlantic and Coho Salmon during Infestation with the Sea Lice Caligus rogercresseyi. Fishes 2019, 4, 42. [Google Scholar] [CrossRef]
- Cuesta, A.; Vargas-Chacoff, L.; García-López, A.; Arjona, F.; Martínez-Rodríguez, G.; Meseguer, J.; Mancera, J.M.; Esteban, M.A. Effect of sex-steroid hormones, testosterone and estradiol, on humoral immune parameters of gilthead seabream. Fish Shellfish Immunol. 2007, 23, 693–700. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Martínez, D.; Oyarzún, R.; Nualart, D.; Olavarría, V.; Yáñez, A.; Bertrán, C.; Ruiz-Jarabo, I.; Mancera, J. Combined effects of high stocking density and Piscirickettsia salmonis treatment on the immune system, metabolism and osmoregulatory responses of the Sub-Antarctic notothenioid fish Eleginops maclovinus. Fish Shellfish Immunol. 2014, 40, 424–434. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J. Effects of pH, temperature and salinity on immune parameters of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol. 2000, 10, 387–391. [Google Scholar] [CrossRef]
- Martínez, D.; De Lázaro, O.; Cortés, P.; Oyarzún-Salazar, R.; Paschke, K.; Vargas-Chacoff, L. Hypoxia modulates the transcriptional immunological response in Oncorhynchus kisutch. Fish Shellfish Immunol. 2020, 106, 1042–1051. [Google Scholar] [CrossRef]
- Saravia, J.; Paschke, K.; Pontigo, J.P.; Nualart, D.; Navarro, J.M.; Vargas-Chacoff, L. Effects of temperature on the innate immune response on Antarctic and sub-Antarctic fish Harpagifer antarcticus and Harpagifer bispinis challenged with two immunostimulants, LPS and Poly I:C: In vivo and in vitro approach. Fish Shellfish Immunol. 2022, 130, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Truscott, K.N.; White, K. The influence of metal and temperature stress on the immune system of crabs. Funct. Ecol. 1990, 4, 455–461. [Google Scholar] [CrossRef]
- Kazmi, S.; Wang, L.; Cai, Y.; Wang, Z. Temperature effects in single or combined with chemicals to the aquatic organisms: An overview of thermo-chemical stress. Ecol. Indic. 2022, 143, 109354. [Google Scholar] [CrossRef]
- Martínez, D.; Vargas-Lagos, C.; Oyarzún, R.; Loncoman, C.A.; Pontigo, J.P.; Yáñez, A.J.; Vargas-Chacoff, L. Temperature modulates the immunological response of the sub-antarctic notothenioid fish Eleginops maclovinus injected with Piscirickettsia salmonis. Fish Shellfish Immunol. 2018, 82, 492–503. [Google Scholar] [CrossRef]
- Lu, M.; Su, M.; Liu, N.; Zhang, J. Effects of environmental salinity on the immune response of the coastal fish Scatophagus argus during bacterial infection. Fish Shellfish Immunol. 2022, 124, 401–410. [Google Scholar] [CrossRef]
- Tort, L.; Padrós, F.; Rotllant, J.; Crespo, S. Winter syndrome in the gilthead sea bream Sparus aurata: Immunological and histopathological features. Fish Shellfish Immunol. 1998, 8, 37–47. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller, J.D. Stress and immune system in fish. In Biology and Physiology of Freshwater Neotropical Fish; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–114. [Google Scholar]
- Iida, M.; Nguyen, H.T.; Takahashi, F.; Bak, S.-M.; Kanda, K.; Iwata, H. Effects of exposure to oxytetracycline on the liver proteome of red seabream (Pagrus major) in a real administration scenario. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109325. [Google Scholar] [CrossRef] [PubMed]
- Shulgina, L.V.; Yakush, E.V.; Shulgin, Y.P.; Shenderyuk, V.V.; Chukalova, N.N.; Baholdina, L.P. Antibiotics in aquaculture and their ecological significance. A review. Izvestiya TINRO 2015, 181, 216–230. (In Russian) [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108840. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Tafalla, C.; Novoa, J.M.; Alvarez, A.; Figueras, A. In vivo and in vitro effect of oxytetracycline treatment on the immune response of turbot, Scophthalmus maximus (L.). J. Fish Dis. 1999, 22, 271–276. [Google Scholar] [CrossRef]
- Serezli, H.; Cagirgan, I.; Okumus, S.; Akhan, F.; Balta, I. The effect of oxytetracycline on non-specific immune response in sea bream (Sparus aurata L. 1758). Turk. J. Vet. Anim. Sci. 2005, 29, 31–35. [Google Scholar]
- Caipang, C.M.; Lazado, C.; Brinchmann, F.; Berg, I.; Kiron, V. In vivo modulation of immune response and antioxidant defense in Atlantic cod, Gadus morhua following oral administration of oxolinic acid and florfenicol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Lunden, S.; Miettinen, L.G.; Lonnstrom, E.M.; Lilius, C.; Bylund, G. Effect of florfenicol on the immune response of rainbow trout (Oncorhynchus mykiss). Vet. Immunol. Immunopathol. 1999, 67, 317–325. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Figueroa, D.; Nualart, D.; Muñoz, J.L. The oxytetracycline and florfenicol effect on the immune system and oxidative stress response of the SHK-1 cell line of Salmo salar. Fishes 2024, 9, 493. [Google Scholar] [CrossRef]
- Guardiola, R.; Cerezuela, J.; Meseguer, M.A.; Esteban, M.A. Modulation of the immune parameters and expression of genes of gilthead seabream (Sparus aurata L.) by dietary administration of oxytetracycline. Aquaculture 2012, 334, 51–57. [Google Scholar] [CrossRef]
- Rombout, J.H.; Taverne-Thiele, A.J.; Villena, M.I. The gut associated lymphoid tissue (GALT) of carp (Cyprinus carpio L.): An immunocytochemical analysis. Dev. Comp. Immunol. 1993, 17, 55–66. [Google Scholar] [CrossRef]
- Abelli, L.; Picchietti, S.; Romano, N.; Mastrolia, L.; Scapigliati, G. Immunohistochemistry of gut-associated lymphoid tissue of the sea bass Dicentrarchus labrax. Fish Shellfish Immunol. 1997, 7, 235–245. [Google Scholar] [CrossRef]
- Scapigliati, G.; Romano, N.; Buonocore, F.; Picchietti, S.; Baldassini, M.R.; Prugnoli, D.; Galice, A.; Meloni, S.; Secombes, C.; Mazzini, M. The immune system of sea bass, Dicentrarchus labrax, reared in aquaculture. Dev. Comp. Immunol. 2002, 26, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zanuzzo, F.; Sandrelli, R.; Peroni, F.; Hall, J.R.; Rise, M.L.; Gamperl, A.K. Atlantic salmon (Salmo salar) bacterial and viral innate immune responses are not impaired by florfenicol or tetracycline administration. Fish Shellfish Immunol. 2022, 123, 298–313. [Google Scholar] [CrossRef]
- Navarrete, P.; Mardones, P.; Opazo, R.; Espejo, R.; Romero, J. Oxytetracycline treatment reduces bacterial diversity of intestinal microbiota of Atlantic salmon. J. Aquat. Anim. Health 2008, 20, 177–183. [Google Scholar] [CrossRef]
- Tapia-Paniagua, S.; Vidal, S.; Lobo, C.; De La Banda, I.G.; Esteban, M.; Balebona, M.; Moriñigo, M. Dietary administration of the probiotic SpPdp11: Effects on the intestinal microbiota and immune-related gene expression of farmed Solea senegalensis treated with oxytetracycline. Fish Shellfish Immunol. 2015, 46, 449–458. [Google Scholar] [CrossRef]
- Reda, R.M.; Ibrahim, R.; Ahmed, N.; El-Bouhy, Z. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Fu, S.; Qian, K.; Tu, X.; Lu, J.; Yao, T.; Ye, L.; Ye, J. Comparative analysis of intestinal structure, enzyme activity, intestinal microbiota and gene expression in different segments of pufferfish (Takifugu obscurus). Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101341. [Google Scholar] [CrossRef]
- Calduch-Giner, J.A.; Sitja-Bobadilla, A.; Perez-Sanchez, J. Gene expression profiling reveals functional specialization along the intestinal tract of a carnivorous teleostean fish (Dicentrarchus labrax). Front. Physiol. 2016, 7, 359. [Google Scholar]
- Díaz-Ibarrola, D.; Martínez, D.; Vargas-Lagos, C.; Saravia, J.; Vargas-Chacoff, L. Transcriptional modulation of immune genes in gut of Sub-Antarctic notothenioid fish Eleginops maclovinus challenged with Francisella noatunensis subsp. noatunensis. Fish Shellfish Immunol. 2022, 124, 56–65. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Kondera, E.; Bojarski, B.; Ługowska, K.; Kot, B.; Witeska, M. Effects of oxytetracycline and gentamicin therapeutic doses on hematological, biochemical and hematopoietic parameters in Cyprinus carpio juveniles. Animals 2020, 10, 2278. [Google Scholar] [CrossRef]
- Bancroft, A.; McKenzie, A.; Grencis, R. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 1998, 160, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Steenwinckel, V.; Louahed, J.; Lemaire, M.; Sommereyns, C.; Warnier, G.; McKenzie, A.; Brombacher, F.; Van Snick, J.; Renauld, J. IL-9 promotes IL-13-dependent Paneth cell hyperplasia and upregulation of innate immunity mediators in intestinal mucosa. J. Immunol. 2009, 182, 4737–4743. [Google Scholar] [CrossRef]
- Zaph, C.; Troy, A.; Taylor, B.; Berman-Booty, L.; Guild, K.J.; Du, Y.; Yost, E.; Gruber, A.; May, M.; Greten, F. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 2007, 446, 552–556. [Google Scholar] [CrossRef]
- Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011, 32, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.L.; McCormick, B.A. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res. 2011, 343, 5–12. [Google Scholar] [CrossRef]
- Ouellette, A.J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 2010, 26, 547–553. [Google Scholar] [CrossRef]
- Clevers, H.C.; Bevins, C.L. Paneth cells: Maestros of the small intestinal crypts. Annu. Rev. Physiol. 2013, 75, 289–311. [Google Scholar] [CrossRef] [PubMed]
- Lunder, T.; Aas, H.; Kaur, M. Environmental factors influencing immune responses in farmed fish. Aquac. Res. 2020, 51, 1–10. [Google Scholar]
- Georgopoulou, U.; Vernier, J. Local immunological response in the posterior intestinal segment of the rainbow trout after oral administration of macromolecules. Dev. Comp. Immunol. 1986, 10, 529–537. [Google Scholar] [CrossRef]
- Muñoz, J.L.; Martínez, D.; Nualart, D.P.; Mardones, O.; Delmoral, I.; Morera, F.; Vargas-Chacoff, L. Antibiotic oxytetracycline is affecting the dynamics of serotonergic response in brain of coho salmon. Aquaculture 2025, 603, 742376. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Nualart, D.P.; Dann, F.; Oyarzún-Salazar, R.; Morera, F.J.; Vargas-Chacoff, L. Immune transcriptional response in head kidney primary cell cultures isolated from the three most important species in Chilean salmonids aquaculture. Biology 2023, 12, 924. [Google Scholar] [CrossRef] [PubMed]
- Namdari, R.; Abedini, S.; Law, F.C.P. A comparative tissue distribution study of oxytetracycline in rainbow trout, Oncorhynchus mykiss (Walbaum), and chinook salmon, Oncorhynchus tshawytscha (Walbaum). Aquac. Res. 1999, 30, 279–286. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Pedro, A.V.F.; Martínez, D.; Pontigo, J.P.; Vargas-Lagos, C.; Hawes, C.; Wadsworth, S.; Morera, F.J.; Vargas-Chacoff, L.; Yáñez, A.J. Transcriptional activation of genes involved in oxidative stress in Salmo salar challenged with Piscirickettsia salmonis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 229, 18–25. [Google Scholar] [CrossRef]

| Primer | Nucleotide Sequences (5′→3′) | Efficiency (%) Foregut | Efficiency (%) Midgut | Efficiency (%) Hindgut | GenBank No/Reference |
|---|---|---|---|---|---|
| TLR-1 Fw | TCCGGAGACGTTTCATCCCA | 101.6 | 101.7 | 102.91 | MF945984 |
| TLR-1 Rv | GAGGTTCAGCGCTAACAGCA | ||||
| TLR-2 Fw | GGGTCTAACTGGGAAGCAGC | 100.1 | 100.2 | 100.1 | Martinez et al., 2020 [13] |
| TLR-2 Rv | AACGGAATGAGACGGATGGG | ||||
| MyD 88 Fw | CTTTCACAACCACCGAAGCC | 102.71 | 100.3 | 101.6 | Martinez et al., 2018 [17] |
| MyD 88 Rv | TACAAACCGAAACCGCTCCT | ||||
| INF-γ Fw | GCCGTGTGTTGGTTTTTGATTT | 102.72 | 100.1 | 100.1 | Martinez et al., 2018 [17] |
| INF-γ Rv | GTGTCTGTCTGACTGATGGTGA | ||||
| NFκ-b Fw | AAAGTGCCAGTACCAAGCCC | 100.1 | 101.6 | 102.5 | Martinez et al., 2018 [17] |
| NFκ-b Rv | CATGCTGATGAGCTACTGTTGTT | ||||
| IL-6 Fw | GAGCTACGTAACTTCCTGGTTGAC | 102.1 | 100.3 | 102.1 | Martinez et al., 2020 [13] |
| IL-6 Rv | GCAAGTTTCTACTCCAGGCCTGAT | ||||
| IkB-α Fw | TCTGCCACCAGCTGTATGA | 102.5 | 100.1 | 100.1 | BT074199.1 |
| IkB-αRv | TCTGCCCGAATGTAATGTCA | ||||
| 18S Fw | GTCCGGGAAACCAAAGTC | 101.6 | 102.5 | 102.1 | Pedro et al., 2019 [61] |
| 18S Fw | TTGAGTCAAATTAAGCCGCA |
| Tissue | Genes | In Vitro | In Vivo | ||||
|---|---|---|---|---|---|---|---|
| Time | Doses | Time × Doses | Time | Doses | Time × Doses | ||
| Foregut | TLR-1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Midgut | TLR-1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Hindgut | TLR-1 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | NS |
| Foregut | TLR-2 | <0.0001 | NS | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Midgut | TLR-2 | <0.0001 | <0.0001 | <0.0001 | NS | 0.0077 | <0.0001 |
| Hindgut | TLR-2 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Foregut | MyD 88 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Midgut | MyD 88 | <0.0001 | <0.0001 | <0.0001 | NS | <0.0001 | 0.0004 |
| Hindgut | MyD 88 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | NS | 0.0156 |
| Foregut | IkB-α | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0006 |
| Midgut | IkB-α | 0.0037 | 0.0111 | <0.0001 | 0.0008 | <0.0001 | 0.0001 |
| Hindgut | IkB-α | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0048 | <0.0001 |
| Foregut | INF-γ | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0105 | <0.0001 |
| Midgut | INF-γ | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Hindgut | INF-γ | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Foregut | IL-6 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0019 |
| Midgut | IL-6 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0103 | <0.0001 |
| Hindgut | IL-6 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | 0.0002 |
| Foregut | NFκ-b | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | <0.0001 |
| Midgut | NFκ-b | <0.0001 | <0.0001 | <0.0001 | 0.0063 | NS | NS |
| Hindgut | NFκ-b | <0.0001 | <0.0001 | <0.0001 | NS | <0.0001 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nualart, D.; Muñoz, J.L.P.; Vargas-Chacoff, L. Intestinal Immune System Expression of Coho Salmon Challenged with Oxytetracycline: In Vivo and In Vitro Approach. Int. J. Mol. Sci. 2025, 26, 6330. https://doi.org/10.3390/ijms26136330
Nualart D, Muñoz JLP, Vargas-Chacoff L. Intestinal Immune System Expression of Coho Salmon Challenged with Oxytetracycline: In Vivo and In Vitro Approach. International Journal of Molecular Sciences. 2025; 26(13):6330. https://doi.org/10.3390/ijms26136330
Chicago/Turabian StyleNualart, Daniela, José Luis P. Muñoz, and Luis Vargas-Chacoff. 2025. "Intestinal Immune System Expression of Coho Salmon Challenged with Oxytetracycline: In Vivo and In Vitro Approach" International Journal of Molecular Sciences 26, no. 13: 6330. https://doi.org/10.3390/ijms26136330
APA StyleNualart, D., Muñoz, J. L. P., & Vargas-Chacoff, L. (2025). Intestinal Immune System Expression of Coho Salmon Challenged with Oxytetracycline: In Vivo and In Vitro Approach. International Journal of Molecular Sciences, 26(13), 6330. https://doi.org/10.3390/ijms26136330








