Abstract
Acinetobacter baumannii is an opportunistic pathogen and a major cause of nosocomial infections worldwide. This study aimed to isolate and characterize phages with lytic activity against multidrug-resistant A. baumannii strains to enable antibacterial alternatives. Eight phages (AKO8a, PS118, B612, MCR, IDQ7, 89P13, CRL20, and CIM23) were isolated and subjected to genomic, phylogenetic, and functional analyses. Antibacterial activity was assessed in vitro against A. baumannii strain AbAK04 by measuring optical density over 17 h at multiplicities of infection (MOIs) of 0.1, 1, and 10, using a repeated-measures design with time as a crossed factor and MOI as a nested factor. Tukey’s post-hoc test identified significant bacterial growth reductions of 57–72% (p < 0.001). Specifically, phages PS118 and 89P13 reduced growth by 71% at MOI 10; CIM23, B612, and CRL20 achieved 68% reduction at MOI 1; and MCR reduced growth by 64% at MOIs 0.1 and 1. Notably, lytic phage MCR encodes a glycosyl hydrolase family 58 (GH58) enzyme, potentially contributing to its antibacterial activity. Genomic analyses confirmed absence of virulence and antibiotic resistance genes, with all phages classified as novel species within the Kagunavirus genus. These findings support the use of these phages as promising candidates for in vivo evaluation.
1. Introduction
The advent of antibiotics revolutionized medicine, but their misuse and overuse have accelerated the emergence of antimicrobial resistance (AMR), a major global health concern [1,2]. AMR is now ranked among the top ten global health threats [3], with projections of 10 million deaths annually by 2050 [4,5]. Acinetobacter baumannii, a top-priority pathogen included in the ESKAPE group (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) [6,7,8], has been implicated in hospital-acquired infections, rapid resistance gene acquisition, and rising carbapenem resistance worldwide [9]. In Mexico, A. baumannii was the fourth most isolated pathogen in 2023, with 550 healthcare-associated infections [10]. Specifically, in the state of Sinaloa in Northwestern Mexico, A. baumannii was identified as the first most common causative agent in primary healthcare infections, comprising 13.48% of total reported cases in January 2025 [11].
The use of phages has become a promising alternative to combat AMR, particularly against A. baumannii. Phages were discovered around the same time as antibiotics. Phages specifically target and kill bacteria [12]. Phage-based intervention reports have recently increased, reflecting rapid progress in translating this technology from laboratory study to clinical application in patients [13]. However, challenges remain in the development, acceptance, and approval of phages for widespread clinical use [14,15,16]. To ensure treatment viability, phages must undergo rigorous characterization before application [12]. In particular, phages can follow either a lytic cycle (virulent phages) or a lysogenic cycle (temperate phages). In the lytic cycle, phages replicate and lyse the host cell to release new virions [17]. In the lysogenic cycle, phages integrate into the host genome as prophages, remaining dormant until reactivation triggers the lytic cycle [18,19]. The full potential of temperate phages as antimicrobial agents in combating AMR has been investigated since these phages are easier to isolate and are distinct mechanistically in the synergy between antibiotics and the phages [20,21,22,23]. However, their therapeutic use is controversial due to risks of lysogeny and horizontal gene transfer [24], requiring thorough genomic and phenotypic characterization essential to ensure safety [25], such as testing for spontaneous phage release, especially when sequencing data are limited [26].
Only a few phages capable of infecting A. baumannii have been identified, including both lytic and temperate phages such as 5W, ABMM1, ΦFG02, and ΦCO01 [12,27,28]. Notably, the temperate phage ABMM1 effectively kills A. baumannii at a high multiplicity of infection and reduces infection severity in a zebrafish model, demonstrating its potential as an alternative treatment [27]. Lytic phage ΦFG02 drives A. baumannii AB900 to evolve into a phage-resistant, capsule-deficient form that resensitizes to ceftazidime, illustrating phage therapy’s potential to restore antibiotic effectiveness [29]. However, these documented phages are limited by factors such as host range, stability, and bacterial defense evasion. Similarly, Bagińska et al. [30] isolated twelve A. baumannii phages, eleven of which were temperate, and only one was lytic. Their study did not include a comprehensive analysis of the phages’ genetic composition nor assess their therapeutic potential in vivo, emphasizing the need for further research in these areas. Phage Indie, on the other hand, reduces A. baumannii resistance to ceftazidime, enhancing its activity and demonstrating its promise as an antimicrobial agent in phage–antibiotic synergy in vitro [31].
Given the growing concern over AMR and the urgent need for alternative treatments due to the limited effectiveness of current intervention alternatives against multidrug-resistant A. baumannii, the present study aimed to isolate and conduct a genomic and functional characterization of the recovered phages to further assess their safety as alternative antimicrobial agents. These findings documented the development of a well-characterized phage library targeting clinical bacterial isolates and provided enabling approaches for future in vivo trials in conjunction with the effective use of phages against multidrug-resistant bacterial pathogens.
2. Results
2.1. Antimicrobial Susceptibility Testing
All tested clinical isolates exhibited multidrug resistance, defined as resistance to three or more antimicrobial classes. Resistance profiles varied across species but were particularly pronounced in A. baumannii strains, which consistently resisted β-lactams, aminoglycosides, and fluoroquinolones [8]. A summary of the resistance patterns for all tested strains is presented in Figure 1.
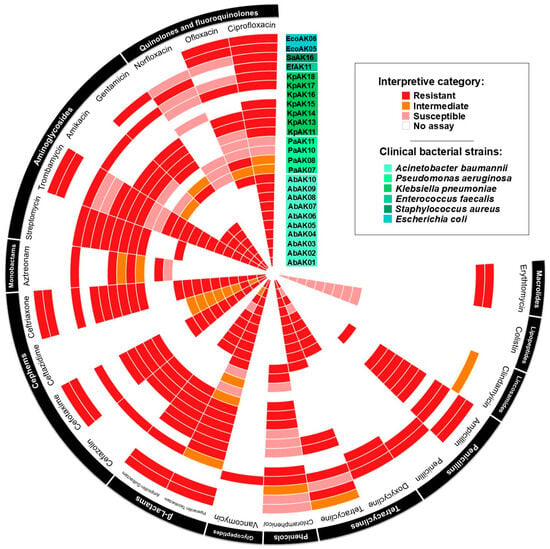
Figure 1.
Antimicrobial resistance pattern of clinical strains of Acinetobacter baumannii, other ESKAPE pathogens, and Gram-negative pathogens. The antimicrobial resistance profiles were determined by measuring the diameter of the inhibition zone using the Kirby–Bauer disk diffusion assay. The tested antimicrobials were specific to each bacterial genus according to CLSI protocols.
2.2. Phage Isolation, Plaque Characterization, and Host Range Analysis
From the analysis of sixteen wastewater samples collected from treatment plants in Sinaloa, Mexico, the initial objective was to isolate phages active against members of the ESKAPE group of pathogens. A total of twenty-two phages were isolated, all showing lytic activity against multidrug-resistant A. baumannii. All phages produced clear plaques of varying diameters on trypticase soy agar plates. Host range analysis using ten clinical A. baumannii strains, thirteen ESKAPE pathogens (P. aeruginosa, K. pneumoniae, E. faecalis, S. aureus), and two Gram-negative pathogens (E. coli) confirmed that the recovered phages exclusively infect their host bacterium, A. baumannii strain AbAK04 (Table 1). Among them, eight phages exhibited high infective production and plating efficiency on A. baumannii strain AbAK04, with titers ranging from 6.0 × 108 to 1.0 × 1010 plaque-forming units (PFU)/mL, making them suitable candidates for evaluating their antibacterial potential (Figure 2).

Table 1.
Summary of characteristics of the different Acinetobacter baumannii-specific phages.
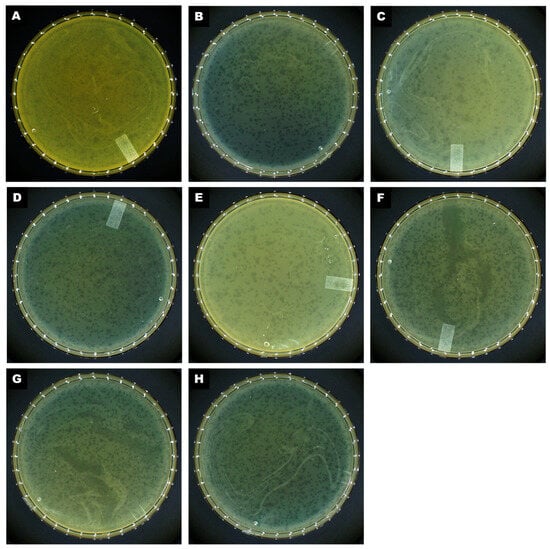
Figure 2.
Morphology of plaques formed on a double-layered agar plate by (A) CRL20, (B) 89P13, (C) AKO8a, (D) PS118, (E) IDQ7, (F) MCR, (G) CIM23, and (H) B612.
2.3. Comparative Genomics and Phylogenetic Analysis of Acinetobacter baumannii-Targeting Phages as a Promising Alternative Intervention
Genome sequencing of the eight selected phages (AKO8a, PS118, B612, MCR, IDQ7, 89P13, CRL20, and CIM23) was performed for further characterization of the phages, and a temperate lifestyle was confirmed with PhageAI version 0.10.0 software. The bioinformatic analysis revealed that the phages’ genetic material consisted of double-stranded DNA and exhibited variations in genome length, GC content, and number of open reading frames (ORFs) (Table 2). Noteworthy, no virulence or antibiotic resistance genes were detected in any of the phage genomes through searches of the ABRIcate databases, and no tRNA genes were identified using tRNAscan-SE version 2.0 software.

Table 2.
Summary of the genomic characteristics of phages.
The number of ORFs with similarity to known genes varied among the eight examined phages. Phage AKO8a had the highest proportion, with 149 ORFs matching known function genes and only eight predicted as hypothetical proteins. In contrast, the remaining phages had between 34 and 39 ORFs associated with known functions (Table 2). The number of hypothetical proteins ranged from 105 in phage MCR to 123 in PS118, with most phages carrying over 100 hypothetical ORFs. Predicted ORFs in the phage genomes exhibited between 98% and 100% similarity with Escherichia coli phages, such as vB_EcoS_XY1 (YP_010749717.1), JSSK01 (WFD55412.1), vB_EcoS-phiEc3 (YP_010749508.1), K1ind2 (YP_009597365.1), and K1H (YP_009168857.1).
All predicted ORFs were clustered into seven functional modules: DNA metabolism, hypothetical proteins, structure, packaging, lysogeny control, and additional functions, as shown in Figure 3A. Interestingly, genomic maps revealed a putative immunity protein, QCF69_gp43 associated with lysogeny in five phages (PS118, B612, IDQ7, 89P13, CIM23) (Figures S1 and S2). By contrast, the genomes of phages MCR, AKO8a, and CRL20 lacked any lysogeny-related genes. The analysis also demonstrated that all phage genomes encoded proteins for DNA metabolism (e.g., DNA polymerase, helicase, HNH endonuclease, adenine-specific methyltransferase), structural components (e.g., tail fiber, major capsid, minor tail, and assembly chaperones), and genome packaging (small and large terminase subunits). Related to lysis functions, all phages encoded lysozyme, endopeptidase, endolysin, holin and o-spanin. Of the eight phages analyzed, only phage MCR encoded a glycosyl hydrolase family 58 (GH58) corresponding to ORF62, which contains N-terminal domains of the phage endosialidase (pfam PF12218), and a catalytic beta propeller domain of phage endosialidase (pfam PF12217).
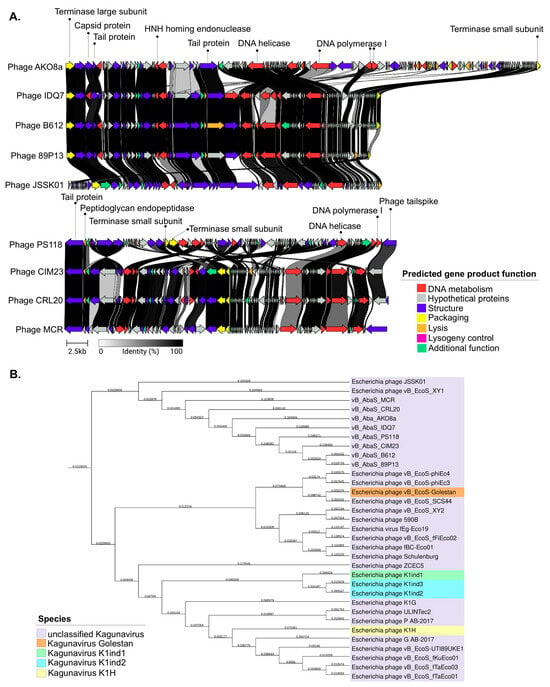
Figure 3.
Genomic characterization of phages within the Kagunavirus genus. (A): Phage comparison with the phage JSSK01 reference genome performed using the Clinker tool. Homologous regions between phages are shown with gray shading. Colored arrows represent ORFs based on their predicted function according to their module. (B): Phylogenetic tree of phages constructed using Genome-BLAST Distance Phylogeny within the Kagunavirus genus, Guernseyvirinae subfamily.
Subsequent BLASTN nucleotide analysis of the phage genomes revealed a close sequence similarity to 12 E. coli phages, with more than 80% nucleotide identity to the eight selected phages. Phage JSSK01 (OQ442786.1) was identified as the closest relative, showing more than 80% coverage and more than 95% identity. The phylogenetic tree confirmed that E. coli phages were potentially the closest ancestors (Figure 3B). Intergenomic comparisons using VIRIDIC software (available online: https://rhea.icbm.uni-oldenburg.de/viridic/; accessed on 8 April 2025) (Figure 4) placed all eight phages within the genus Kagunavirus, with more than 70% similarity to E. coli phages JSSK01 and vB_EcoS_XY1, suggesting that the phages examined in the present study represent a novel, unclassified species. Following the International Committee on Taxonomy of Viruses (ICTV) criteria, the phages were designated as A. baumannii phages vB_AbaS_AKO8a, vB_AbaS_PS118, vB_AbaS_B612, vB_AbaS_MCR, vB_AbaS_IDQ7, vB_AbaS_89P13, vB_AbaS_CRL20, and vB_AbaS_CIM23. Phage common names are AKO8a, PS118, B612, MCR, IDQ7, 89P13, CRL20, and CIM23, respectively. Classification was confirmed using PhageAI version 0.10.0 software with a 99.2% probability, aligning with ICTV taxonomy.
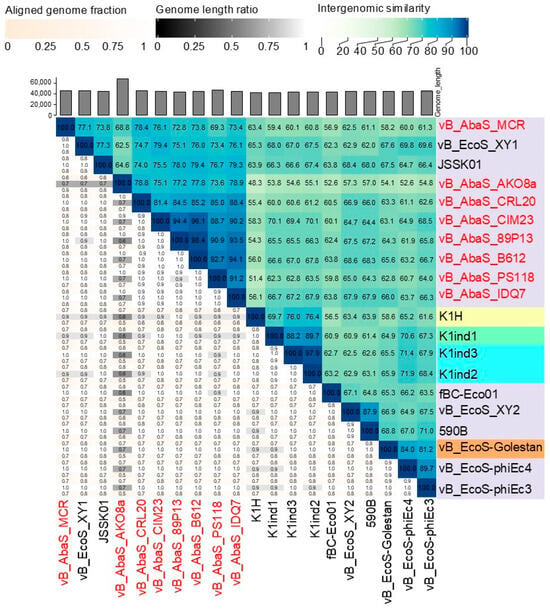
Figure 4.
Taxonomic assignment of Acinetobacter phages through comparative genomics. Heatmap of VIRIDIC software. The values of percentage identity range from 0 (0%, white) to 1 (100%, blue).
2.4. Evaluating Lysogenic Activity in Phages
Based on the bioinformatics analysis, the temperate lifestyle of the recovered phages was further examined by using the genome-based predictions to obtain evidence of the phage’s replication strategy. To further characterize their ability to induce rapid infections in the target bacterial strains, the phage lifestyle was validated using lysogenicity assays. In particular, these assays determined whether a phage integrated into the host genome, indicating a lysogenic lifestyle [26]. Alternatively, the phages were assessed for following a strictly lytic cycle, which involves immediate replication, production of new phage particles, and lysis of the host cell without integration [32].
The assay results demonstrated that phages PS118, B612, IDQ7, 89P13, and CIM23 did not form “mesas” at the tested concentrations (104, 106, and 108) over 96 h of co-incubation with A. baumannii strain AbAK04, indicating an inability to lysogenize the host under the tested conditions (Figure S3). This suggests that these phages were unable to establish lysogeny. In contrast, phages AKO8a, CRL20, and MCR formed “mesas” at a concentration of 104 after 96 h of co-incubation with A. baumannii strain AbAK04 (Figure S4A). Subsequent patch plate assays revealed that colonies of A. baumannii strain AbAK04 appeared to harbor prophages (MCR-17, AKO8a-9, AKO8a-10, AKO8a-11, CRL20-3, CRL20-4, CRL20-5, CRL20-6, CRL20-7). Even after repeating this assay multiple times, the A. baumannii colonies showed no signs of any spontaneous lysis (Figure S4B).
To confirm that the host colonies did not harbor integrated prophages, we performed additional confirmatory analyses (Figure S4C,D), such as spot tests with culture supernatants as well as homoimmunity assays. Both types of assays yielded negative results indicative of the absence of lysogeny because no lysis zones were observed in the supernatant spot test, suggesting a lack of spontaneous phage release. Also, no protection from superinfection was detected in the homoimmunity assay, ruling out the presence of functional prophages. In summary, these results collectively confirm that phages AKO8a, CRL20, and MCR do not establish lysogeny in A. baumannii strain AbAK04 under the tested conditions, and instead, the examined phages follow a strictly lytic replication cycle.
2.5. Antibacterial Activity, One-Step Growth Curve, and Phage Adsorption Rate
After 17 h of co-incubation with A. baumannii strain AbAK04, the evaluated phage MOI values significantly reduced bacterial growth in liquid culture, as determined by absorbance measurements, with reductions ranging from 57% to 72% (Figure 5). The repeated-measures design indicated that there were significant differences (p < 0.001) in bacterial growth among the different MOIs. Specifically, phages PS118 (Figure 5A) and 89P13 (Figure 5H) reduced bacterial levels by 71% at MOI 10. Phages CIM23 (Figure 5D), B612 (Figure 5G), and CRL20 (Figure 5E) achieved a 68% reduction at MOI 1. Meanwhile, phage MCR (Figure 5C) reduced the bacterial population by 64% at MOIs of 0.1 and 1. In summary, all of these results confirm the antibacterial activity of the examined phages.
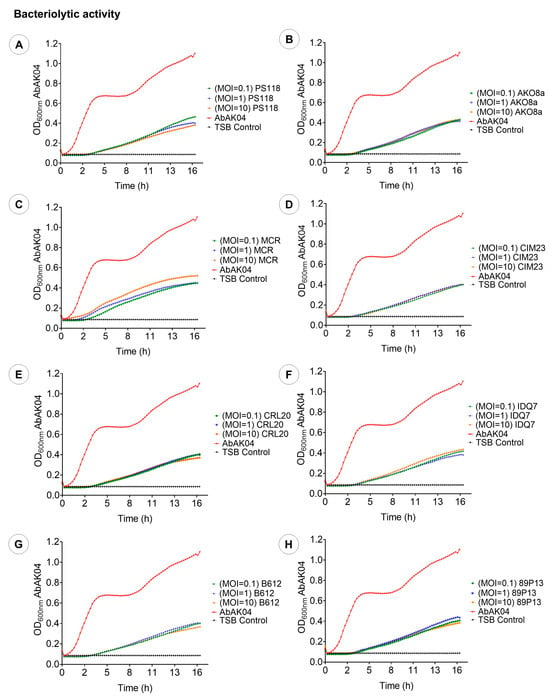
Figure 5.
Bacteriolytic activity of eight phages at various MOIs (10, 1, and 0.1). (A): PS118. (B): AKO8a. (C): MCR. (D): CIM23. (E): CRL20. (F): IDQ7. (G): B612. (H): 89P13. Each graph presents the mean values of three tests (n = 3) ± standard deviation (SD), error bars represent the standard deviation.
Following the growth reduction assays, the next experimental approach aimed to investigate the replication dynamics of the phages, focusing on their latency period, burst size, and adsorption rate to determine how efficiently the examined phages replicated and interacted with the bacterial host. The results from these assays demonstrated that the lysogenic phages PS118, B612, IDQ7, 89P13, and CIM23, which encoded the putative immunity protein QCF69_gp43, did not replicate after three trials, and no latency period, burst size, or adsorption rate results were obtained. In contrast, the lytic phages CRL20, AKO8a, and MCR exhibited a latency period of 20 min, with burst sizes of 11, 13, and 21 PFU/cell, respectively (Figure 6A). Notably, the burst size was calculated based on the total number of phages released at the plateau stage of the one-step growth curve (lysis time ~50 min), since not all cells lyse synchronously immediately after the latency period. Adsorption assays showed that 60% of CRL20, 80% of AKO8a, and 61% of MCR phages adhered to A. baumannii strain AbAK04 within 5 min (Figure 6B).
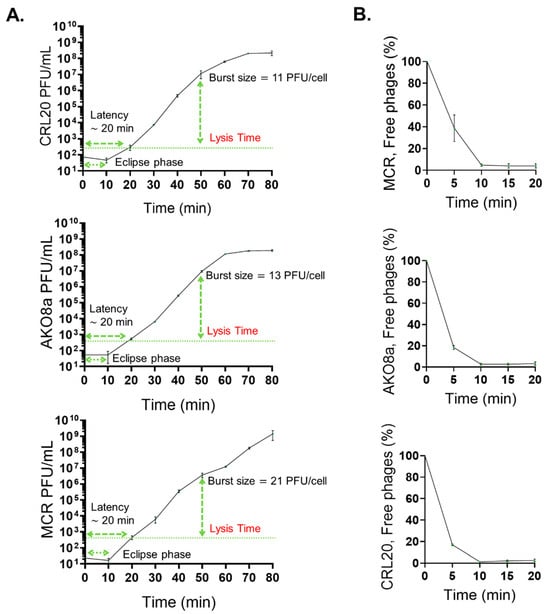
Figure 6.
Lytic activity assays. (A): One-step growth curve for lytic phages AKO8a, MCR, and CRL20. The latency time corresponds to the time before newly formed phages are first detected in the supernatant. The lysis time, however, is defined as the time point at which phage release reaches a plateau, reflecting the completion of cell lysis in the majority of the population. Burst size was calculated at this plateau. (B): Adsorption curve for lytic phages AKO8a, MCR, and CRL20. Each graph presents the mean values of three tests (n = 3) ± standard deviation (SD).
3. Discussion
Phages have gained significant interest in recent years for their potential as antimicrobial agents against multidrug-resistant pathogens, offering a promising alternative to combat antimicrobial resistance [33,34,35]. Phages are seen as a promising solution to combat A. baumannii [9,29,36], currently the most critical nosocomial pathogen [7,8]. However, isolating virulent A. baumannii phages remains a significant challenge. The bacterium employs a variety of defense mechanisms to evade phage infection, including inhibition of phage adsorption, CRISPR-Cas systems, restriction-modification systems, and superinfection immunity, all of which hinder successful phage infection and replication [37,38,39]. These challenges are combined by extensive genetic diversity and genetic mosaicism among phages, which complicates the identification of lytic candidates with antibacterial activity [40,41]. Therefore, most recent studies have reported the isolation of temperate and lytic phages as alternative antimicrobial agents against A. baumannii [12,27,28,30,42]. To address this limitation, establishing large, well-characterized phage libraries has been proposed as a viable strategy [41]. Phages with clinical potential should undergo thorough genomic and phenotypic characterization and be deposited in centralized phage banks accessible to the global medical community to support the development of effective phage-based treatments.
Given the urgent need to isolate and characterize novel phages with antimicrobial potential [43], the present study conducted a screen of phages from wastewater infecting multidrug-resistant A. baumannii. By targeting specifically multidrug-resistant A. baumannii, a total of eight phages were subsequently selected for further characterization with the ultimate goal of enabling a highly specific therapeutic approach by minimizing unintended effects on beneficial microbiota and reducing the risk of resistance selection in other bacterial species. The specificity of these eight phages could be particularly advantageous for personalized phage therapy, where tailored treatments can be designed based on the infecting strain, optimizing efficacy while avoiding collateral impacts on the patient’s microbiome [44]. Currently, there is no standardized criterion for defining broad versus narrow host ranges [43,45]. While phages in natural environments, such as wastewater, coevolve with bacteria and may gain the ability to infect multiple strains or genera [46], the precision of phages with a very specific host range offers a targeted and controlled strategy for managing infections, particularly in the context of multidrug-resistant pathogens, as is A. baumannii.
The genomic classification of the examined eight phages as a new species within the Kagunavirus genus was justified by their nucleotide identity being less than 95% compared to the reference phages, supporting their classification based on established species delimitation criteria for phages [47]. While comprehensive, future studies incorporating transmission electron microscopy (TEM) imaging would further confirm and expand the biological characterization of these phages. Moreover, the phage genomic analysis, conducted in the present study, revealed an absence of antibiotic resistance and virulent genes, suggesting that the recovered phages could serve as a promising antimicrobial alternative highlighting their potential as a safe and effective solution against multidrug-resistant A. baumannii [16]. Additionally, the observed absence of tRNA genes indicated phage dependence on the host bacteria for protein synthesis [48].
Based on ORF classification, the recovered phages in the present study were found to encode genes associated with several functional modules, highlighting their potential antibacterial activity. Lytic enzymes were identified as key factors in bacterial elimination [31]. Among them, lysozymes play a crucial role by lysing host cells to release phage particles [49], along with endolysins and endopeptidases that degrade bacterial peptidoglycan [50]. Genes encoding holins, membrane proteins responsible for disrupting the bacterial membrane, and spanins, which facilitate outer membrane lysis, were also detected [19,51]. The DNA metabolism module included proteins with helix-turn-helix domains for DNA binding [52] and adenine-specific methyltransferases, which regulate gene expression via DNA methylation [53]. HNH endonucleases, located near terminase genes, were present and are involved in DNA packaging and homologous recombination [54]. Additionally, transcriptional regulators encoded by these temperate phages may trigger the lytic cycle in response to DNA damage [55], potentially influencing bacterial processes such as virulence, metabolism, antibiotic resistance, and stress responses [56].
Of particular interest, this study revealed that the five temperate phages (PS118, B612, IDQ7, 89P13, and CIM23) carried a superinfection immunity protein, QCF69_gp43, which may play a role in regulating phage–phage interactions [9]. Superinfection immunity is a mechanism that allows a prophage-infected bacterium to resist subsequent infections by other phages, typically through repressor proteins that block the replication of invading phages [57,58,59]. While such mechanisms can be beneficial for the long-term persistence of phages and may help prevent secondary phage infections in chronic scenarios, temperate phages are not suitable for therapeutic use under current clinical guidelines [60,61]. This is due to their unpredictable induction into the lytic cycle [62], inability to rapidly lyse host bacteria, and, above all, their potential to mediate horizontal gene transfer [24], including virulence or resistance determinants, to otherwise avirulent bacterial populations [20]. Although the temperate phages described in this study lack detectable antibiotic resistance or virulence genes and do not carry key lysogeny-related genes (e.g., integrases, transposases, repressors) [57,63,64], their use as therapeutic agents is not recommended unless these phages are genetically engineered to eliminate the lysogeny-associated superinfection immunity protein QCF69_gp43 prior to any in vivo application as an antibacterial agent.
The antimicrobial activity of phages AKO8a, PS118, B612, MCR, IDQ7, 89P13, CRL20, and CIM23 was confirmed through in vitro bacteriolytic assays, with some showing comparable lytic efficacy to previously characterized phages such as vB_AbaM_ABMM [27], Abp95 [65], Abgy202141 [66], and vAbaIN10 [67]. Notably, five of these phages (PS118, B612, IDQ7, 89P13, and CIM23) harbor genes associated with lysogeny, yet were still able to significantly reduce bacterial populations, suggesting their potential as antimicrobial agents. Further investigation into possible genetic modification could enhance their suitability for phage-based therapeutic strategies.
The genome analysis for phages AKO8a, MCR, and CRL20 revealed a lack of lysogeny-associated genes, classifying them as lytic phages with potential as antimicrobial agents against multidrug-resistant A. baumannii. One-step growth curves and adsorption data for the lytic phages AKO8a, MCR, and CRL20 revealed smaller burst sizes and shorter latency periods compared to the temperate phage øCO01, but similar to the lytic phage øFG02 [12]. Phage AKO8a had an adsorption period comparable to vB_AbaM_ABMM1 [27], while phages MCR and CRL20 showed approximately 30% lower adsorption rates than other Acinetobacter phages [68]. The slower adsorption rates observed for MCR and CRL20 may suggest distinct mechanisms of bacterial recognition and infection [69]. These findings underscore the potential of phages with diverse adsorption kinetics to enhance therapeutic approaches, positioning them as promising candidates for future in vivo assays. Interestingly, the presence of a glycosyl hydrolase family 58 (GH58) enzyme in the phage MCR suggests that it may function as an endosialidase, facilitating phage infection by degrading the bacterial capsule. This enzyme is commonly found in phages that infect bacteria with polysialic acid capsules, such as E. coli K1 phages [70]. A. baumannii displays a wide variety of capsule types (K loci), responsible for producing distinct polysaccharides, some potentially containing sialic acid-like components [71,72]. Further research and experimental validation are thus necessary to clarify how the tailspike proteins of phage MCR recognize and degrade these surface structures, such as in the phage ABPH49 [73].
In conclusion, the phages characterized in this study, particularly the three lytic phages MCR, AKO8a, and CRL20, demonstrate potential as biological antimicrobial agents, warranting future in vivo assays. This work contributes to expanding intervention libraries against multidrug-resistant A. baumannii, a globally critical pathogen, and represents an important step toward the standardization of phage therapy as an antimicrobial strategy worldwide.
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions, and Storage
The ten clinical A. baumannii strains, thirteen ESKAPE pathogens (P. aeruginosa, K. pneumoniae, E. faecalis, and S. aureus), and two Gram-negative pathogens (E. coli) used in this study were obtained from the strain collection at a public hospital in Culiacán, Sinaloa, Mexico. The bacterial identity of each clinical isolate was confirmed through Gram-staining and Vitek®2 (bioMérieux, Durham, NC, USA). An endpoint polymerase chain reaction (PCR) was performed to confirm the species of the bacterial host of the phages, using species-specific primers for A. baumannii [74], F: 5′-TAATGCTTTGATCGGCCTTG-3′ and R: 5′-TGGATTGCACTTCATCTTGG-3′; for P. aeruginosa [75], F: 5′-CTGGGTCGAAAGGTGGTTGTTATC-3′ and R: 5′-GCGGCTGGTGCGGCTGAGTC-3′; for E. faecium [76], F: 5′-GAAAAAACAATAGAAGAATTAT-3′ and R: 5′-TGCTTTTTTGAATTCTTCTTTA-3′; for S. aureus [77], F: 5′-GCGATTGATGGTGATACGGTT-3′ and R: 5′-AGCCAAGCCTTGACGAACTAAAGC-3′; for K. pneumoniae [78], F: 5′-CAACCATGGTGGTCGATTAG-3′ and R: 5′-TGGTAGCCATATCCCTTTGG-3′; and for E. coli [79]. The amplified products were analyzed using 1% agarose gels supplemented with 0.04 μL/mL GelRed nucleic acid stain (Phoenix Research, Candler, NC, USA), and the nucleotide sequences of the amplicons were confirmed through conventional Sanger DNA sequencing (Elim Biopharmaceuticals, Inc., Hayward, CA, USA). All bacterial strains were propagated in tryptic soy broth (TSB; Oxoid Ltd., Hants, UK) at 37 °C for 24 h and were stored at −80 °C in 20% (v/v) glycerol.
4.2. Antimicrobial Susceptibility Profile
The antimicrobial resistance in the ten clinical A. baumannii strains, thirteen ESKAPE pathogens, and two Gram-negative pathogens was performed using the disk diffusion method according to Clinical and Laboratory Standards Institute guidelines (CLSI) [80], with minor modifications. The Kirby–Bauer method was applied on Mueller Hinton agar plates for each tested bacterial isolate. Twenty-three antibiotics, classified into twelve distinct classes, were tested with an appropriate disk (Oxoid Ltd., Basingstoke, UK) at the following amounts: ciprofloxacin (CIP, 5 μg), ofloxacin (OFX, 5 μg), norfloxacin (NOR, 10 μg) [Quinolones and fluoroquinolones], gentamicin (GM, 10 μg), amikacin (AN, 30 μg), tobramycin (NN, 10 μg), streptomycin (S, 10 μg) [Aminoglycosides], aztreonam (ATM, 30 μg) [Monobactams], ceftriaxone (CRO, 30 μg), cefotaxime (CTX, 30 μg), cefozolin (CZ, 30 μg), ceftazidime (CFZ, 30 μg) [Cephems], ampicillin-sulbactam (SAM, 10/10 μg), piperacillin-tazobactam (TZP, 100/10) [β-Lactams], vancomycin (VAN, 30 μg) [Glycopeptides], chloramphenicol (C, 30 μg) [Phenicols], tetracycline (TE, 30 μg), doxycycline (D, 30 μg) [Tetracyclines], penicillin (P, 10 μg), ampicillin (AM, 10 μg) [Penicillins], clindamicina (CC, 2 μg) [Lincosamides], colistin (CT) [Lipopeptides], erythromicyn (E, 15 μg) [Macrolides]. Escherichia coli strain ATCC 25922 was employed as positive control for all experiments, each consisting of three replicates per condition tested.
4.3. Phage Isolation, Propagation, and Titration
To investigate the potential of phages against ESKAPE pathogens, initial efforts were focused on A. baumannii, a clinically relevant member of this group and a critical-priority multidrug-resistant pathogen [7,8]. Phages were isolated using A. baumannii as the bacterial host to facilitate the enrichment and recovery of lytic phages from environmental sources. A total of 16 samples of hospital sewage and wastewater were collected for this purpose. For the phage identification method, hospital wastewater samples were pretreated before being used in the phage propagation assay, following the procedures described previously [81]. Bacterial cultures were prepared using a previously described enrichment [48] method with some modifications. Briefly, 1 mL of wastewater was mixed with 30 mL of double-strength TSB (Oxoid Ltd., Hants, UK) and 1 mL of bacterial culture. The mixture was then incubated at 37 °C for 18 h with gentle shaking at 50 rpm. After incubation, the mixture was centrifuged at 8000× g for 10 min at 4 °C using a Megafuge16R (Thermo Fisher Scientific Inc., Waltham, MA, USA), and the supernatants were filtered through a 0.22 μm nylon membrane (Pall Corp., New York, NY, USA).
The presence of phages was determined using a spot assay on a soft agar bacterial lawn. Briefly, serial dilutions of the phage-containing supernatant (105 to 108 PFU/mL) were prepared. A bacterial lawn was first established by mixing 100 µL of an overnight culture of the host strain with 3 mL of soft agar (TSB with 0.5% agar) and pouring the mixture onto TSA plates (Oxoid Ltd., Hants, UK). After solidification, 10 µL of each diluted and filtered supernatant was spotted onto the lawn and allowed to dry. Plates were then incubated at 37 °C for 18 h. The appearance of clear lysis zones (plaques) confirmed the presence of lytic phages. For purifying the selected phages, the isolated plaque was diluted in 1000 µL of SM buffer (50 mM Tris-Cl, pH 7.5, 100 mM NaCl, 8 mM MgSO4·7H2O), centrifuged at 8000× g for 10 min at 4 °C using a Sorvall Legend Micro17R (Thermo Fisher Scientific Inc., Waltham, MA, USA), and then filtered through an Acrodisc™ syringe filter with a 0.2 μm nylon membrane (Sigma Aldrich, Edo. de México, Mexico). This purification process was repeated at least three times. For determining the phage titer, a series of dilutions was subjected to the double-layer agar method and the phage titer was calculated by dividing the number of plaques observed by the volume of phages added, multiplied by the dilution factor, and expressed as plaque-forming units (PFU) per mL. The purified phages were stored either at 4 °C in SM buffer for immediate use or at −80 °C with 20% (v/v) glycerol for longer storage.
4.4. Host Range of Phage and Efficiency of Plating
The host range of the purified phage was determined using the double-layer agar method, as previously described [82] with minor modifications. Bacterial lawns of the ten clinical A. baumannii strains, thirteen ESKAPE pathogens, and two Gram-negative pathogens were prepared independently by combining 1 mL of the overnight bacterial cultures with 3 mL of soft agar (TSB with 0.5% agar) and pouring this mixture onto TSA agar plates. After the bacterial lawn solidified, 10 μL of diluted phage solution (105 PFU/mL) was applied to each bacterial lawn. The phages were evaluated by the appearance of clear zones of bacterial lysis following overnight incubation at 37 °C. Based on the characteristics of the plaques observed, the bacteriolytic activity of the phages was categorized according to the previous study [12] with some modifications, including no lysis, phage resistance, partial lysis, nebula lysis, and productive infection. The development of clear plaques on a soft agar layer indicated a productive infection.
The twenty-two isolated phages were further evaluated for productive infection using the efficiency of plating (EOP) method evaluated by Mirzaei et al. [83] with some modifications. Each phage was tested in triplicate at two dilutions (105 and 106 PFU/mL) against A. baumannii strain AbAK04 which had previously shown lysis in spot assays. The testing conditions were the same as those used in the spot assays, using bacterial cultures in the stationary growth phase and the double-layer agar method. In brief, the bacterial strains were incubated for 18 h at 37 °C. Subsequently, 1000 µL of each bacterial culture was combined with 100 µL of diluted phage lysate and used in double-layer agar assays. After overnight incubation at 37 °C, the number of PFU was counted for each combination. The EOP was calculated by dividing the average PFU in the target bacteria by the average PFU in the host bacteria. Based on the EOP values, the strains were classified into the following categories: “high efficiency” (EOP ≥ 0.5), “medium efficiency” (0.1 ≤ EOP < 0.5), “low efficiency” (0.001 < EOP < 0.1), and “inefficient” (EOP ≤ 0.001).
4.5. Phage DNA Extraction, Sequencing and Genome Assembly, Detection of Lysogeny
Phage genomic DNA was extracted using the phenol–chloroform technique as described by Sambrook & Russell [84]. One mL of the phage suspension was treated with 10 μL of DNase I/RNase A (10 mg/mL) at 37 °C for 30 min, followed by the addition of 50 μL of SDS (10%), 40 μL of EDTA (0.5 M), and 2.5 μL of proteinase K (20 mg/mL) and incubation at 56 °C for 2 h. An equal volume of phenol–chloroform (1:1, v/v) was added, and the mixture was centrifuged at 3500× g for 10 min. The recovered aqueous layer was carefully transferred and mixed with another equal volume of phenol–chloroform (1:1, v/v), and this process was repeated three more times. After centrifugation at 3500× g for 10 min, the final aqueous phase was combined with 200 μL of 3 M sodium acetate and ethanol, and the sample was placed at −20 °C overnight before being centrifuged at 15,000× g for 30 min. The DNA pellet was washed with 70% ethanol, air dried, dissolved in 20 μL of nuclease-free water, and stored at −20 °C. The final DNA concentration and quality were measured with a NanoDrop 2000c spectrometer (Thermo Scientific, Wilmington, NC, USA), and were assessed by gel electrophoresis, respectively.
For sequencing the phage genomes, DNA libraries were constructed following the manufacturer’s protocol with the Nextera XT Library Preparation Kit (Illumina, San Diego, CA, USA). After the library preparation, DNA quantification was performed using a Qubit 2.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), and the phage genome sequencing was carried out using the Illumina MiniSeq System at CIAD Mazatlán (Centro de Investigación en Alimentación y Desarrollo, Mazatlán Unit) with a 2 × 150 bp paired-end approach over 300 cycles. The raw sequencing data were processed for quality control using fastp version 0.23.0 [85], and the assembly of the genome was conducted using SPAdes version 3.15.5 [86], applying a coverage threshold of 10x to construct the contigs.
4.6. Bioinformatics Analysis
Contigs were annotated using the PHANNOTATE algorithm within PATRIC version 3.36.16.1 (available online: https://www.patricbrc.org/app/Annotatio; 8 August 2024). Phage identification was performed using BLASTN to compare closely related reference sequences in the NCBI database. These reference genomes sequence alignments were performed to the isolated phages with Geneious version 9.1.8 (available online: https://www.geneious.com/; 29 January 2025). Phage lifestyles were classified with PhageAI version 0.10.0 software (available online: https://phage.ai/; 3 February 2025). Virulence and antibiotic resistance genes were annotated using ABRIcate version 0.8.13 (available online: https://github.com/tseemann/abricate; 17 February 2025), from databases such as NCBI, CARD, ARG-ANNOT, Resfinder, MEGARES, and VFDB. tRNA genes were predicted using tRNAscan-SE version 2.0 [87]. Open reading frames (ORFs) were identified using the ORF search tool (available online: https://www.ncbi.nlm.nih.gov/orffinder/; 19 February 2025) and protein functions were analyzed with BLASTp on the NCBI server (available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi; 21 February 2025), using a non-redundant protein database with a score threshold >50 and an e-value <1.0 × 10−3. The characteristics of the ORFs were further analyzed using Artemis Comparison Tool version 18.2.0. [88] and DNAPlotter version 18.2.0 [89], respectively. For the phylogenetic analysis, alignments were compiled using the VICTOR platform (available online: https://ggdc.dsmz.de/victor.php; 24 February 2025) to explore phylogenetic relationships by creating phylogenomic trees using the Genome-BLAST remote phylogeny method. The results in the Newick format were then used to construct a phylogenetic tree with iTOL version 6.9.1 (available online: http://itol.embl.de; 13 March 2025). Intergenomic similarities between viral genomes were assessed using VIRIDIC software (https://rhea.icbm.uni-oldenburg.de/viridic/; accessed 8 April 2025) with standard BLASTN settings. Additionally, the clinker pipeline, version 0.0.28 [90] was used to perform a comparative analysis of the phages when compared to their reference genomes. For the identification of ORF62, which encodes the tailspike endosialidase, the analysis was performed using InterProScan (available online: https://www.ebi.ac.uk/interpro/search/sequence/; 27 April 2025) [22].
4.7. Phage-Mediated Antibacterial Activity Assays
In vitro antibacterial activity was evaluated using a 96-well microplate format using bacterial cultures in the stationary growth phase (108 CFU/mL) and phage suspensions at a multiplicity of infection (MOI) of 10, 1, and 0.1 [91]. The positive control consisted of 200 μL of bacterial culture, and the negative control was 200 μL of TSB. For each experimental condition, 100 μL of TSB and 80 μL of the phage dilution were mixed to achieve the desired MOI, followed by the addition of 20 µL of the bacterial culture in a final volume of 200 µL per well. The microplate was incubated at 37 °C for 17 h using a Synergy HT microplate reader (Biotek, Inc., Winooski, VT, USA), and absorbance measurements were taken at a wavelength of 600 nm every 15 min after orbital shaking before each measurement. The lysogenic behavior of a phage was verified by several experimental tests, especially when no genes associated with lysogeny are detected in the phage genome and it is classified as temperate by some bioinformatics program. The lysogenic activity of the isolated phages was detected in triplicate, according to a study described previously [26].
For performing the one-step growth curve, the burst size was determined from triplicate experiments by calculating the ratio of the final count of released phage particles to the initial count of infected bacterial cells, divided by the number of infected cells (phage titer at 0 min—phage titer at 0 min with chloroform), as in previous studies [48]. The adsorption test was performed in triplicate using a modified version of the method described by Park and Park [92]. In detail, bacterial cultures (1 mL) in the stationary growth phase (108 CFU/mL) were subjected to centrifugation at 8000× g for 10 min, resuspended in TSB and further diluted (107 CFU/mL) in 9 mL of fresh TSB. The bacterial suspension was then mixed with 1 mL of the phage solution (MOI = 0.1) and further incubated at 37 °C for 5 min. After incubation, the samples were centrifuged at 15,000× g for 1 min to eliminate free unabsorbed phages, and the phage titer was calculated by the double-layer agar method, which corresponded to time point 0. The procedure above was repeated every 5 min for a total of 20 min. The percentage of free phages was evaluated by calculating the number of phages at a specific time divided by the initial number of phages multiplied by 100.
4.8. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 8.0.1 (GraphPad Software, Inc., La Jolla, CA, USA) and Minitab® 19, Minitab Statistical Software (Minitab, LLC., State College, PA, USA). Quantitative values were reported as the mean ± standard deviation. For the bacteriolytic activity assay, a repeated-measures design was utilized by defining the MOI as a nested factor and time as a crossed factor. Group comparisons were then conducted using Tukey’s post-hoc test. Probability values less than 0.05 were considered significant for all statistical analyses.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26136141/s1.
Author Contributions
A.K.O.-O.: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Visualization, Writing—original draft preparation, and Writing—review and editing. B.Q. and J.P.G.-G.: Conceptualization, Validation, Writing—review and editing, Supervision. N.C.-d.C.; J.B.V.-T.: Validation, Writing—review & editing, Supervision. C.C.-Q.: Conceptualization, Validation, Writing—review and editing, Supervision, Project administration, and Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the Ciencia de Frontera 2023 [Grant no. CF-2023-G-645], by the Consejo Nacional de Humanidades, Ciencias y Tecnologías (Conahcyt) of Mexico to A.K.O.O. [Grant No. 1083340], and by the United States Department of Agriculture (USDA), Agricultural Research Service (ARS), CRIS Project Number 2030-42000-055-00D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The complete genome sequence of vB_AbaS_PS118, vB_AbaS_MCR, vB_AbaS_IDQ7, vB_AbaS_CRL20, vB_AbaS_CIM23, vB_AbaS_B612, vB_AbaS_AKO8a, and vB_AbaS_89P13 have been submitted to the GenBank database and the accession numbers PQ997946, PQ997947, PQ997948, PQ997949, PQ997950, PQ997951, PQ997952, and PQ997953 has been assigned, respectively.
Acknowledgments
The authors thank Bertram Lee (with the USDA-ARS), as well as Juan Daniel Lira-Morales, Cynthia Cristina Rivas-Lopez, Viviana Guadalupe Hernandez-Navarro, Manuel Acosta, André Fierro-Ruvalcaba, Célida Isabel Martínez-Rodríguez, and Miriam Vega-Rodríguez with CIAD-LANIIA for providing excellent technical assistance. Additionally, the authors thank José Basilio Heredia, Erick Paul Gutierrez-Grijalva, and Laura Aracely Contreras-Angulo with the Laboratorio de Alimentos Funcionales y Nutracéuticos (LAFN) for providing access to the Synergy HT microplate reader and to Laboratorio Regional de Inocuidad Alimentaria (LARIA) for allowing use of Vitek®2. Host bacterial strains were provided by the Instituto Mexicano del Seguro Social Culiacán (IMSS).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lobanovska, M.P.; Pilla, G. Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.M.; Canton, R.; Perez-Cobas, A.E.; Fernandez-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; E Wool, E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Attili, A.R.; Nocera, F.P.; Sisto, M.; Linardi, M.; Gigli, F.; Ngwa, V.N.; Fiorito, F.; Cerracchio, C.; Meligrana, M.C.T.; Bonacucina, E.; et al. Evidence and antibiotic resistance profiles of clinical Acinetobacter calcoaceticus-Acinetobacter baumannii (ACB) and non-ACB complex members in companion animals: A 2020–2022 retrospective study. Comp. Immunol. Microbiol. Infect. Dis. 2024, 109, 102185. [Google Scholar] [CrossRef]
- Thacharodi, A.; Vithlani, A.; Hassan, S.; Alqahtani, A.; Pugazhendhi, A. Carbapenem-resistant Acinetobacter baumannii raises global alarm for new antibiotic regimens. iScience 2024, 27, 111367. [Google Scholar] [CrossRef]
- Sati, H.; Carrara, E.; Savoldi, A.; Hansen, P.; Garlasco, J.; Campagnaro, E.; Boccia, S.; Castillo-Polo, J.A.; Magrini, E.; Garcia-Vello, P.; et al. The WHO Bacterial Priority Pathogens List 2024: A prioritisation study to guide research, development, and public health strategies against antimicrobial resistance. Lancet Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Luo, J.; Xie, L.; Yang, M.; Liu, M.; Li, Q.; Wang, P.; Fan, J.; Jin, J.; Luo, C. Synergistic Antibacterial Effect of Phage pB3074 in Combination with Antibiotics Targeting Cell Wall against Multidrug-Resistant Acinetobacter baumannii. In Vitro and Ex Vivo. Microbiol. Spectr. 2023, 11, e0034123. [Google Scholar] [CrossRef]
- RHOVE. Boletin Estatal Panorama Epidemiológico de las Infecciones Asociadas a la Atención de la Salud; Secretaría de Salud México. Red Hospitalaria de Vigilancia Epidemiológica (RHOVE): Mexico City, Mexico, 2023. [Google Scholar]
- RHOVE-Sinaloa. Boletin Estatal Panorama Epidemiológico de las Infecciones Asociadas a la Atención de la Salud; Secretaría de Salud de Sinaloa: Culiacán, Mexico, 2025. [Google Scholar]
- Gordillo Altamirano, F.; Forsyth, J.H.; Patwa, R.; Kostoulias, X.; Trim, M.; Subedi, D.; Archer, S.K.; Morris, F.C.; Oliveira, C.; Kielty, L.; et al. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat. Microbiol. 2021, 6, 157–161. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Iredell, J.; Danis-Wlodarczyk, K.; Kebriaei, R.; Abedon, S.T. Translating phage therapy into the clinic: Recent accomplishments but continuing challenges. PLoS Biol. 2023, 21, e3002119. [Google Scholar] [CrossRef] [PubMed]
- Green, S.I.; Clark, J.R.; Santos, H.H.; Weesner, K.E.; Salazar, K.C.; Aslam, S.; Campbell, J.W.; Doernberg, S.B.; Blodget, E.; Morris, M.I.; et al. A Retrospective, Observational Study of 12 Cases of Expanded-Access Customized Phage Therapy: Production, Characteristics, and Clinical Outcomes. Clin. Infect. Dis. 2023, 77, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S.; et al. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Verbeken, G. Magistral Phage Preparations: Is This the Model for Everyone? Clin. Infect. Dis. 2023, 77, S360–S369. [Google Scholar] [CrossRef]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temperate phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef]
- Meng, L.H.; Ke, F.; Zhang, Q.Y.; Zhao, Z. Functional Analysis of the Endopeptidase and Holin From Planktothrix. agardhii. Cyanophage PaV-LD. Front. Microbiol. 2022, 13, 849492. [Google Scholar] [CrossRef]
- Al-Anany, A.M.; Fatima, R.; Hynes, A.P. Temperate phage-antibiotic synergy eradicates bacteria through depletion of lysogens. Cell Rep. 2021, 35, 109172. [Google Scholar] [CrossRef]
- Al-Anany, A.M.; Fatima, R.; Nair, G.; Mayol, J.T.; Hynes, A.P. Temperate phage-antibiotic synergy across antibiotic classes reveals new mechanism for preventing lysogeny. mBio 2024, 15, e0050424. [Google Scholar] [CrossRef]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The protein sequence classification resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Gummalla, V.S.; Zhang, Y.; Liao, Y.T.; Wu, V.C.H. The Role of Temperate Phages in Bacterial Pathogenicity. Microorganisms 2023, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Schmieger, H. Method for host-independent detection of generalized transducing bacteriophages in natural habitats. Appl. Environ. Microbiol. 2001, 67, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, F.L.G.; Barr, J.J. Screening for Lysogen Activity in Therapeutically Relevant Bacteriophages. Bio. Protoc. 2021, 11, e3997. [Google Scholar] [CrossRef]
- Mardiana, M.; Teh, S.H.; Tsai, Y.C.; Yang, H.H.; Lin, L.C.; Lin, N.T. Characterization of a novel and active temperate phage vB_AbaM_ABMM1 with antibacterial activity against Acinetobacter baumannii infection. Sci. Rep. 2023, 13, 11347. [Google Scholar] [CrossRef]
- Peng, W.; Zeng, F.; Wu, Z.; Jin, Z.; Li, W.; Zhu, M.; Wang, Q.; Tong, Y.; Chen, L.; Bai, Q. Isolation and genomic analysis of temperate phage 5W targeting multidrug-resistant Acinetobacter baumannii. Arch. Microbiol. 2021, 204, 58. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Kostoulias, X.; Subedi, D.; Korneev, D.; Peleg, A.Y.; Barr, J.J. Phage-antibiotic combination is a superior treatment against Acinetobacter baumannii. in a preclinical study. EBioMedicine 2022, 80, 104045. [Google Scholar] [CrossRef]
- Baginska, N.; Harhala, M.A.; Cieslik, M.; Orwat, F.; Weber-Dabrowska, B.; Dabrowska, K.; Gorski, A.; Jonczyk-Matysiak, E. Biological Properties of 12 Newly Isolated Acinetobacter baumannii-Specific Bacteriophages. Viruses 2023, 15, 231. [Google Scholar] [CrossRef]
- Orozco-Ochoa, A.K.; Gonzalez-Gomez, J.P.; Quinones, B.; Castro-Del Campo, N.; Valdez-Torres, J.B.; Chaidez-Quiroz, C. Bacteriophage Indie resensitizes multidrug-resistant Acinetobacter baumannii to antibiotics in vitro. Sci. Rep. 2025, 15, 11578. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Yu, M.; Chen, Y.L.; Jin, M. The Life Cycle Transitions of Temperate Phages: Regulating Factors and Potential Ecological Implications. Viruses 2022, 14, 1904. [Google Scholar] [CrossRef]
- Gu Liu, C.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- North, O.I.; Brown, E.D. Phage-antibiotic combinations: A promising approach to constrain resistance evolution in bacteria. Ann. N. Y. Acad. Sci. 2021, 1496, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Ferry, T.; Abdel, M.P. Phage Therapy as a Novel Therapeutic for the Treatment of Bone and Joint Infections. Clin. Infect. Dis. 2023, 77, S407–S415. [Google Scholar] [CrossRef]
- Engeman, E.; Freyberger, H.R.; Corey, B.W.; Ward, A.M.; He, Y.; Nikolich, M.P.; Filippov, A.A.; Tyner, S.D.; Jacobs, A.C. Synergistic Killing and Re-Sensitization of Pseudomonas aeruginosa to Antibiotics by Phage-Antibiotic Combination Treatment. Pharmaceuticals 2021, 14, 184. [Google Scholar] [CrossRef]
- Azam, A.H.; Tanji, Y. Bacteriophage-host arm race: An update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl. Microbiol. Biotechnol. 2019, 103, 2121–2131. [Google Scholar] [CrossRef]
- Ambroa, A.; Blasco, L.; Lopez, M.; Pacios, O.; Bleriot, I.; Fernandez-Garcia, L.; Gonzalez de Aledo, M.; Ortiz-Cartagena, C.; Millard, A.; Tomas, M. Genomic Analysis of Molecular Bacterial Mechanisms of Resistance to Phage Infection. Front. Microbiol. 2021, 12, 784949. [Google Scholar] [CrossRef]
- Tu, Q.; Pu, M.; Li, Y.; Wang, Y.; Li, M.; Song, L.; Li, M.; An, X.; Fan, H.; Tong, Y. Acinetobacter Baumannii Phages: Past, Present and Future. Viruses 2023, 15, 673. [Google Scholar] [CrossRef]
- Leptihn, S.; Loh, B. Complexity, challenges and costs of implementing phage therapy. Future Microbiol. 2022, 17, 643–646. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Hua, X.; Yu, Y.; Leptihn, S.; Loh, B. Therapeutic evaluation of the Acinetobacter baumannii. phage Phab24 for clinical use. Virus Res. 2022, 320, 198889. [Google Scholar] [CrossRef]
- Badawy, S.; Baka, Z.A.M.; Abou-Dobara, M.I.; El-Sayed, A.K.A.; Skurnik, M. Biological and molecular characterization of fEg-Eco19, a lytic bacteriophage active against an antibiotic-resistant clinical Escherichia coli isolate. Arch. Virol. 2022, 167, 1333–1341. [Google Scholar] [CrossRef]
- Ross, A.; Ward, S.; Hyman, P. More Is Better: Selecting for Broad Host Range Bacteriophages. Front. Microbiol. 2016, 7, 1352. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Qi, B. Advancing Phage Therapy: A Comprehensive Review of the Safety, Efficacy, and Future Prospects for the Targeted Treatment of Bacterial Infections. Infect. Dis. Rep. 2024, 16, 1127–1181. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.; Wong, C.W.Y.; Wang, S.; Delaquis, P. How Broad Is Enough: The Host Range of Bacteriophages and Its Impact on the Agri-Food Sector. Phage 2021, 2, 83–91. [Google Scholar] [CrossRef]
- Lopez-Cuevas, O.; Castro-Del Campo, N.; Leon-Felix, J.; Gonzalez-Robles, A.; Chaidez, C. Characterization of bacteriophages with a lytic effect on various Salmonella serotypes and Escherichia coli O157:H7. Can. J. Microbiol. 2011, 57, 1042–1051. [Google Scholar] [CrossRef]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A Roadmap for Genome-Based Phage Taxonomy. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Orozco-Ochoa, A.K.; Gonzalez-Gomez, J.P.; Castro-Del Campo, N.; Lira-Morales, J.D.; Martinez-Rodriguez, C.I.; Gomez-Gil, B.; Chaidez, C. Characterization and genome analysis of six novel Vibrio parahaemolyticus phages associated with acute hepatopancreatic necrosis disease (AHPND). Virus Res. 2023, 323, 198973. [Google Scholar] [CrossRef]
- Fastrez, J. Phage lysozymes. EXS 1996, 75, 35–64. [Google Scholar] [CrossRef]
- Petrzik, K. Peptidoglycan Endopeptidase from Novel Adaiavirus Bacteriophage Lyses Pseudomonas aeruginosa Strains as Well as Arthrobacter. globiformis. and A. pascens. Bacteria. Microorganisms. 2023, 11, 1888. [Google Scholar] [CrossRef]
- Kongari, R.; Rajaure, M.; Cahill, J.; Rasche, E.; Mijalis, E.; Berry, J.; Young, R. Phage spanins: Diversity, topological dynamics and gene convergence. BMC Bioinform. 2018, 19, 326. [Google Scholar] [CrossRef]
- Greive, S.J.; Fung, H.K.; Chechik, M.; Jenkins, H.T.; Weitzel, S.E.; Aguiar, P.M.; Brentnall, A.S.; Glousieau, M.; Gladyshev, G.V.; Potts, J.R.; et al. DNA recognition for virus assembly through multiple sequence-independent interactions with a helix-turn-helix motif. Nucleic Acids Res. 2016, 44, 776–789. [Google Scholar] [CrossRef]
- Zinoviev, V.V.; Evdokimov, A.A.; Gorbunov, Y.A.; Malygin, E.G.; Kossykh, V.G.; Hattman, S. Phage T4 DNA [N6-adenine] methyltransferase: Kinetic studies using oligonucleotides containing native or modified recognition sites. Biol. Chem. 1998, 379, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, D.; Huang, Y.; Zhu, X.; Rui, M.; Wan, T.; Zheng, X.; Shen, Y.; Chen, X.; Ma, K.; et al. Structural and functional characterization of deep-sea thermophilic bacteriophage GVE2 HNH endonuclease. Sci. Rep. 2017, 7, 42542. [Google Scholar] [CrossRef]
- Mascolo, E.; Adhikari, S.; Caruso, S.M.; deCarvalho, T.; Folch Salvador, A.; Serra-Sagrista, J.; Young, R.; Erill, I.; Curtis, P.D. The transcriptional regulator CtrA controls gene expression in Alphaproteobacteria phages: Evidence for a lytic deferment pathway. Front. Microbiol. 2022, 13, 918015. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, S.; Shin, M.; Kim, J. Synergistic Antimicrobial Effects of Phage vB_AbaSi_W9 and Antibiotics against Acinetobacter baumannii. Infection. Antibiotics 2024, 13, 680. [Google Scholar] [CrossRef]
- Donnelly-Wu, M.K.; Jacobs, W.R., Jr.; Hatfull, G.F. Superinfection immunity of mycobacteriophage L5: Applications for genetic transformation of mycobacteria. Mol. Microbiol. 1993, 7, 407–417. [Google Scholar] [CrossRef]
- Elois, M.A.; Silva, R.D.; Pilati, G.V.T.; Rodriguez-Lazaro, D.; Fongaro, G. Bacteriophages as Biotechnological Tools. Viruses 2023, 15, 349. [Google Scholar] [CrossRef]
- Mavrich, T.N.; Hatfull, G.F. Evolution of Superinfection Immunity in Cluster A Mycobacteriophages. mBio 2019, 10. [Google Scholar] [CrossRef]
- Fowoyo, P.T. Phage Therapy: Clinical Applications, Efficacy, and Implementation Hurdles. Open Microbiol. J. 2024, 18. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Zhang, S.; Di, L.; Qi, Y.; Qian, X.; Wang, S. Treatment of infections caused by carbapenem-resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2024, 14, 1395260. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Pirnay, J.P.; De Vos, D. Chapter 4 Guidelines to Compose an Ideal Bacteriophage Cocktail. In Bacteriophage Therapy; Springer: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Huang, L.; Huang, S.; Jiang, L.; Tan, J.; Yan, X.; Gou, C.; Chen, X.; Xiang, L.; Wang, D.; Huang, G.; et al. Characterisation and sequencing of the novel phage Abp95, which is effective against multi-genotypes of carbapenem-resistant Acinetobacter baumannii. Sci. Rep. 2023, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, X.; Zhou, J.; Wang, L.; Wang, Q.; Qi, X.; Liu, J.; Zhao, D.; Hsiang, T.; Jiang, Y. Isolation, characterization and therapeutic evaluation of a new Acinetobacter virus Abgy202141 lysing Acinetobacter baumannii. Front. Microbiol. 2024, 15, 1379400. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, I.; Debarbieux, L.; Sow, O.; Sambe Ba, B.; Diagne, M.M.; Cisse, A.; Fall, C.; Dieye, Y.; Dia, N.; Constantin de Magny, G.; et al. Isolation and characterization of Acinetobacter phage vAbaIN10 active against carbapenem-resistant Acinetobacter baumannii (CRAB) isolates from healthcare-associated infections in Dakar, Senegal. J. Glob. Antimicrob. Resist. 2025, 41, 151–158. [Google Scholar] [CrossRef]
- Badawy, S.; Pajunen, M.I.; Haiko, J.; Baka, Z.A.M.; Abou-Dobara, M.I.; El-Sayed, A.K.A.; Skurnik, M. Identification and Functional Analysis of Temperate Siphoviridae Bacteriophages of Acinetobacter baumannii. Viruses 2020, 12, 604. [Google Scholar] [CrossRef]
- Abedon, S.T. Bacteriophage Adsorption: Likelihood of Virion Encounter with Bacteria and Other Factors Affecting Rates. Antibiotics 2023, 12, 723. [Google Scholar] [CrossRef]
- Schwarzer, D.; Stummeyer, K.; Gerardy-Schahn, R.; Muhlenhoff, M. Characterization of a novel intramolecular chaperone domain conserved in endosialidases and other bacteriophage tail spike and fiber proteins. J. Biol. Chem. 2007, 282, 2821–2831. [Google Scholar] [CrossRef]
- Roshini, J.; Patro, L.P.P.; Sundaresan, S.; Rathinavelan, T. Structural diversity among Acinetobacter baumannii. K-antigens and its implication in the in silico serotyping. Front. Microbiol. 2023, 14, 1191542. [Google Scholar] [CrossRef]
- Singh, J.K.; Adams, F.G.; Brown, M.H. Diversity and Function of Capsular Polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2018, 9, 3301. [Google Scholar] [CrossRef]
- Evseev, P.V.; Sukhova, A.S.; Tkachenko, N.A.; Skryabin, Y.P.; Popova, A.V. Lytic Capsule-Specific Acinetobacter Bacteriophages Encoding Polysaccharide-Degrading Enzymes. Viruses 2024, 16, 771. [Google Scholar] [CrossRef]
- Falah, F.; Shokoohizadeh, L.; Adabi, M. Molecular identification and genotyping of Acinetobacter baumannii. isolated from burn patients by PCR and ERIC-PCR. Scars Burn. Heal. 2019, 5, 2059513119831369. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Kim, M.H.; Cho, M.S.; Kim, B.K.; Kim, J.Y.; Kim, C.; Park, D.S. Improved PCR for identification of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2013, 97, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.H.; Hwang, S.Y.; Moon, B.Y.; Park, Y.K.; Shin, S.; Hwang, C.Y.; Park, Y.H. Occurrence of antimicrobial resistance and virulence genes, and distribution of enterococcal clonal complex 17 from animals and human beings in Korea. J. Vet. Diagn. Investig. 2012, 24, 924–931. [Google Scholar] [CrossRef]
- Hamdan-Partida, A.; González García, S.; Bustos-Martínez, J. Identificación de Staphylococcus aureus utilizando como marcadores los genes nucA y femB. Ciencias Clínicas 2015, 16, 37–41. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; Emeka, P.M. Genetic fingerprinting and profile analysis of virulence genes in XDR clinical isolates of Klebsiella Pneumoniae. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5233–5244. [Google Scholar] [CrossRef]
- Muhammed, N.a.A.; Farhan, M.B.; Shabeeb, Z.A. Detection of luxS Gene in Serratia marcescens and rpoS Gene in Enterobacter cloacae Isolates Using PCR Reaction. J. Al-Nahrain Univ. Sci. 2018, 21, 115–120. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Document M100; Clinical and Laboratory Standards Institute. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2020. [Google Scholar]
- Liu, Y.; Mi, Z.; Mi, L.; Huang, Y.; Li, P.; Liu, H.; Yuan, X.; Niu, W.; Jiang, N.; Bai, C.; et al. Identification and characterization of capsule depolymerase Dpo48 from Acinetobacter baumannii phage IME200. PeerJ 2019, 7, e6173. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Wang, H.; Wang, S.; Fang, R.; Li, X.; Xing, J.; Wu, Q.; Li, Z.; Song, N. Characterization and efficacy against carbapenem-resistant Acinetobacter baumannii of a novel Friunavirus phage from sewage. Front. Cell. Infect. Microbiol. 2024, 14, 1382145. [Google Scholar] [CrossRef]
- Mirzaei, M.K.; Nilsson, A.S. Correction: Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0127606. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Extraction of Bacteriophage lambda DNA from Large-scale Cultures Using Proteinase K and SDS. Cold Spring Harb. Protoc. 2006, 2006, pdb.prot3972. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods Mol. Biol. 2019, 1962, 1–14. [Google Scholar] [CrossRef]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef]
- Carver, T.; Thomson, N.; Bleasby, A.; Berriman, M.; Parkhill, J. DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 2009, 25, 119–120. [Google Scholar] [CrossRef]
- Gilchrist, C.L.M.; Chooi, Y.H. clinker & clustermap.js: Automatic generation of gene cluster comparison figures. Bioinformatics 2021, 37, 2473–2475. [Google Scholar] [CrossRef]
- Gonzalez-Gomez, J.P.; Rodriguez-Arellano, S.N.; Gomez-Gil, B.; Vergara-Jimenez, M.J.; Chaidez, C. Genomic and biological characterization of bacteriophages against Enterobacter cloacae, a high-priority pathogen. Virology 2024, 595, 110100. [Google Scholar] [CrossRef]
- Park, D.W.; Park, J.H. Characterization and Food Application of the Novel Lytic Phage BECP10: Specifically Recognizes the O-polysaccharide of Escherichia coli O157:H7. Viruses 2021, 13, 1469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).