Biological and Medicinal Properties of Chrysanthemum boreale Makino and Its Bioactive Products
Abstract
1. Introduction
2. The Plant Chrysanthemum boreale Makino
2.1. Distribution and Morphology
2.2. Genomic Content
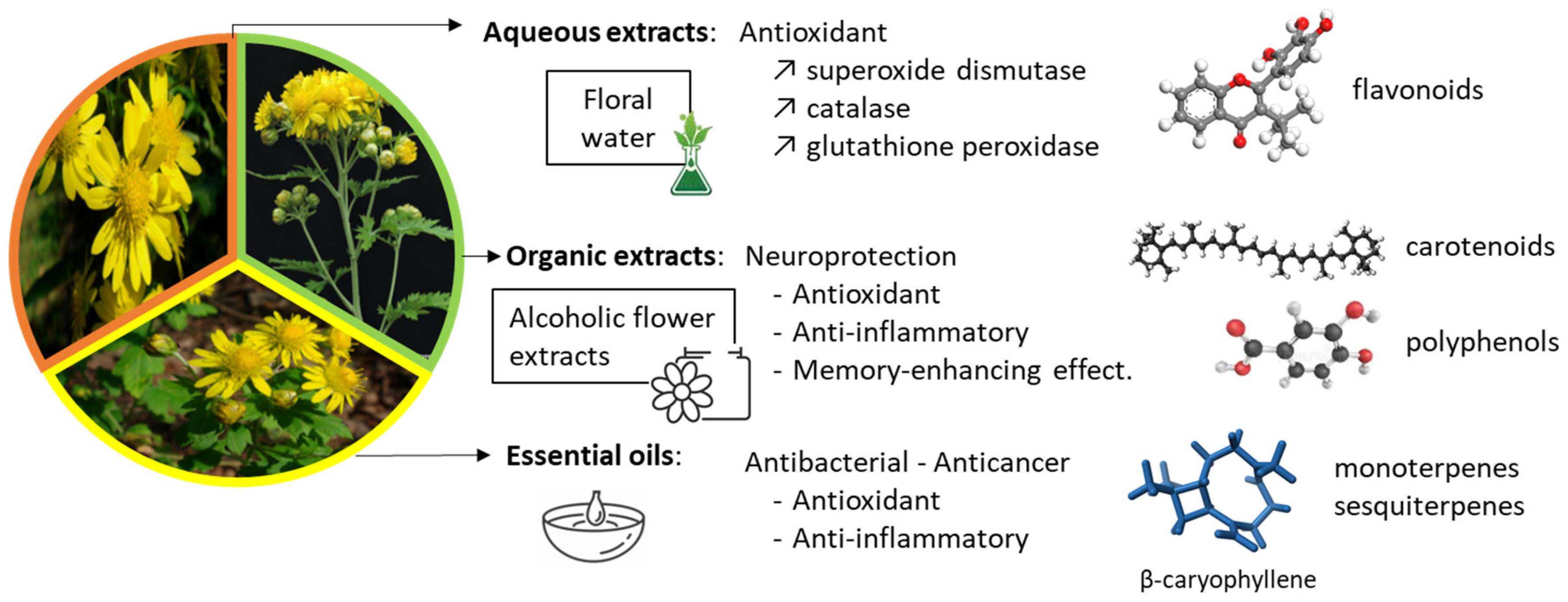
3. Plant Extracts and Essential Oils
3.1. Aqueous Extracts
| Activity Types | Main Observations | References |
|---|---|---|
| Hepatoprotection | Reduction of CCl4-induced hepatic damages in mice with an AE orally given. The AE decreased the levels of serum liver enzymes (aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, and alkaline phosphatase). | [53] |
| Antioxidant activity | Oral administration of the AE increased activity of antioxidant enzymes (SOD, catalase, and glutathione peroxidase), and the concentration of dopamine in brain of Parkinson-type mice. | [54] |
| Vascular modeling | A floral water from C. boreale inhibited migration and proliferation of aortic smooth muscle cells. The extract modulated the MAPK pathway through inhibition of PDGFR-β. | [56] |
| Anti-atopic dermatitis | Reduction in skin symptom severity and inflammation in a mouse model of atopic dermatitis. The methanolic extract decreased expression of TNF-α, IL-4, and the level of serum IgE in mice orally treated with the plant extract. | [59] |
| Anti-diabetic | A water extract of C. boreale flowers showed a marked inhibitory activity of angiotensin-converting enzyme (ACE). Guanosine was identified as the main inhibitor. | [55] |
| Skin regeneration | Effect of a hydrosoluble extract of C. boreale on skin regeneration. The hydrosol promoted proliferation and migration of human HaCat skin keratinocytes. | [60] |
| Plant growth Inhibition | C. boreale AE reduced plant germination, root hair development, and growth of various plants (allelopathic effect). | [52] |
3.2. Organic Extracts
| Activity Types | Main Observations | References |
|---|---|---|
| Neuronal protection | Reduction in neuronal damages in vitro with a methanolic extract of C. boreale. | [63] |
| Antioxidant activity | Potent antioxidant activity of a methanolic extract of flowers from C. boreale. | [68] |
| Protection against retinal damages | An ethyl acetate fraction prepared from C. boreale flowers showed potent antioxidant activity in retinal pigment epithelium cells. | [69] |
| Anti-inflammatory activity | Marked inhibition of NO production and iNOS expression with a methanolic extract of C. boreale. | [64] |
| Antibacterial Effects | A chloroform extract revealed antibacterial effects against selected bacterial strains. Sesquiterpenoid lactones were identified from the extract. | [65] |
| Cytotoxic activities | Antiproliferative activity of a chloroform extract of C. boreale against K562 human myeloid leukemia cells and isolation of an active substance. | [67] |
3.3. Essential Oils
| Activity Types | Main Observations | References |
|---|---|---|
| Antibacterial activity | Activity of an EO from C. boreale against selected bacteria., including Staphylococcus aureus and Streptococcus pyogenes(Gram-(+)) and Escherichia coli (Gram-(−)). | [70] |
| Anti-biofilm formation | Inhibition of biofilm formation and bacterial adherence by the EO. | [79] |
| Skin regeneration | Stimulation of keratinocyte proliferation and promotion of wound closure with a C. boreale EO. | [76] |
| Anti-atopic dermatitis | Anti-inflammatory effects, with inhibition of IL-6 production in HaCaT cells. | [90] |
| Anticancer effects | Inhibition of proliferation and induction of apoptosis of KB cells in vitro with C. boreale EO. | [75] |
| Anti-obesity | Inhibition of lipid accumulation in 3T3-L1 cells by a C. boreale EO, via suppression of activation of the adipogenic transcription factors PPAR-γ, C/EBPα, and SREBP-1. Antiadipogenic and lipolysis effects. | [91] |
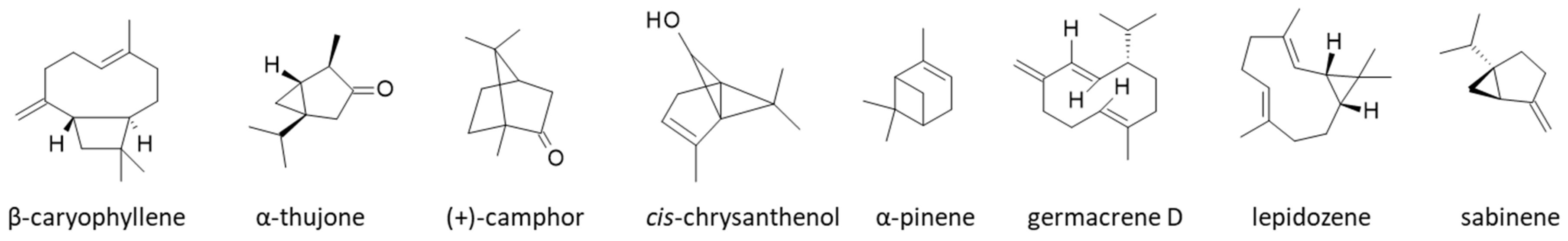
| Prevention of muscle atrophy | C. boreale EO reduces skeletal muscle atrophy and the monoterpene sabinene is primarily responsible for the effect via regulation of the MAPK/MuRF-1 pathway. | [92] |
4. Bioactive Substances Isolated from Chrysanthemum boreale
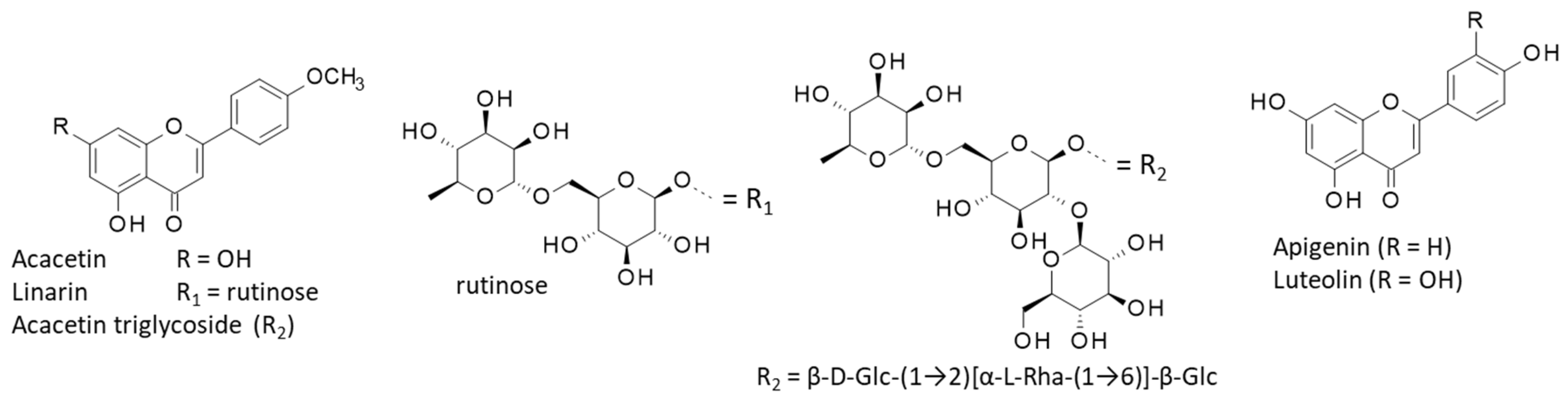
4.1. Flavonoids
4.2. Sesquiterpene Lactones (SLs)
4.2.1. Monomeric SLs

4.2.2. Dimeric SLs

4.3. Other Compounds
5. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| AChE | acetylcholinesterase |
| AE | aqueous extracts |
| AR | aldose reductase |
| COX-2 | cyclooxygenase-2 |
| EO | essential oil |
| Hsp70 | heat shock protein 70 |
| iNOS | inducible nitric oxide synthase |
| NF-κB | nuclear factor kappa-light-chain enhancer of activated B cells |
| NO | nitric oxide |
| OE | organic extracts |
| PGE2 | prostaglandin E2 |
| ROS | reactive oxygen species |
| SL | sesquiterpene lactone |
| SOD | superoxide dismutase |
| TAK1 | transforming growth factor-beta-activated kinase 1 |
References
- Darras, A. Overview of the Dynamic Role of Specialty Cut Flowers in the International Cut Flower Market. Horticulturae 2021, 7, 51. [Google Scholar] [CrossRef]
- Pan, B.; Du, Y.; Chen, Q.; Wang, Y.; Chen, L.; Li, H.; Huang, C.; Gao, K. China’s chrysanthemum in the global market: Evaluating the international competitiveness and influencing factors. Front. Sustain. Food Syst. 2025, 9, 1521709. [Google Scholar] [CrossRef]
- Shirsath, P. Hybrid Chrysanthemum Seeds Market Report 2025 (Global Edition). March 2025, Report ID: CMR142807. Available online: https://www.cognitivemarketresearch.com/hybrid-chrysanthemum-seeds-market-report (accessed on 15 June 2025).
- Kogei Art Kyoto. The Chrysanthemum and Japan: A Timeless Connection in the Days Leading to Choyo no Sekk. 6 September 2024. Available online: https://kogeiart.kyoto.jp/articles/post-2754/?srsltid=AfmBOoqWfHqrVxGqF2az1eKd0QgHJWz13tnrXJfs48c_i7Lt9iIOBhXk (accessed on 15 June 2025).
- Chen, Q.; Gao, K.; Xu, Y.; Sun, Y.; Pan, B.; Chen, D.; Luo, C.; Cheng, X.; Liu, H.; Huang, C. Research advance on cold tolerance in chrysanthemum. Front. Plant Sci. 2023, 14, 1259229. [Google Scholar] [CrossRef] [PubMed]
- Mekapogu, M.; Kwon, O.K.; Song, H.Y.; Jung, J.A. Towards the Improvement of Ornamental Attributes in Chrysanthemum: Recent Progress in Biotechnological Advances. Int. J. Mol. Sci. 2022, 23, 12284. [Google Scholar] [CrossRef]
- Hao, D.C.; Song, Y.; Xiao, P.; Zhong, Y.; Wu, P.; Xu, L. The genus Chrysanthemum: Phylogeny, biodiversity, phytometabolites, and chemodiversity. Front. Plant Sci. 2022, 13, 973197. [Google Scholar] [CrossRef]
- Su, J.; Jiang, J.; Zhang, F.; Liu, Y.; Ding, L.; Chen, S.M.; Chen, F. Current achievements and future prospects in the genetic breeding of chrysanthemum: A review. Hortic. Res. 2019, 6, 109. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Kulus, D. Chrysanthemum biotechnology: Discoveries from the recent literature. Folia Hort. 2014, 26, 67–77. [Google Scholar] [CrossRef]
- Eisa, E.A.; Tilly-Mándy, A.; Honfi, P.; Shala, A.Y.; Gururani, M.A. Chrysanthemum: A Comprehensive Review on Recent Developments on In Vitro Regeneration. Biology 2022, 11, 1774. [Google Scholar] [CrossRef]
- Sharma, N.; Radha Kumar, M.; Kumari, N.; Puri, S.; Rais, N.; Natta, S.; Dhumal, S.; Navamaniraj, N.; Chandran, D.; Mohankumar, P.; et al. Phytochemicals, therapeutic benefits and applications of chrysanthemum flower: A review. Heliyon 2023, 9, e20232. [Google Scholar] [CrossRef]
- Gu, J.; Scotti, F.; Reich, E.; Kirchhof, R.; Booker, A.; Heinrich, M. Chrysanthemum species used as food and medicine: Understanding quality differences on the global market. South Afr. J. Bot. 2022, 148, 123–134. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C.; Zhou, J.; Zhou, F.; Gui, A.; Chu, H.; Shao, Q. Chrysanthemum morifolium as a traditional herb: A review of historical development, classification, phytochemistry, pharmacology and application. J. Ethnopharmacol. 2024, 330, 118198. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, S.; Samiei, L.; Shakeri, A. Chrysanthemum, an ornamental genus with considerable medicinal value: A comprehensive review. South Afr. J. Bot. 2022, 144, 23–43. [Google Scholar] [CrossRef]
- Hang, H.; Chen, R.; Wang, C.; Sun, Y.; Du, D. A review of the extraction processes and biological characteristics of Chrysanthemum polysaccharides. Int. J. Biol. Macromol. 2025, 285, 138224. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Hu, W.J.; Yu, A.Q.; Wu, L.H.; Yang, D.Q.; Kuang, H.X.; Wang, M. Review of polysaccharides from Chrysanthemum morifolium Ramat.: Extraction, purification, structural characteristics, health benefits, structural-activity relationships and applications. Int. J. Biol. Macromol. 2024, 278, 134919. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, T.; Wang, D.; Liu, Q. Exploring the antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory properties of Chrysanthemum morifolium and Chrysanthemum indicum: A narrow review. Front. Pharmacol. 2025, 16, 1538311. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, Y.; Li, D.; Chen, Y. Chrysanthemum indicum L.: A Comprehensive Review of its Botany, Phytochemistry and Pharmacology. Am. J. Chin. Med. 2020, 48, 871–897. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Deng, C.; Chen, J.; Han, W.; Yang, X.; Wang, Z.; Dai, S. The mechanisms of optimal nitrogen conditions to accelerate flowering of Chrysanthemum vestitum under short day based on transcriptome analysis. J. Plant Physiol. 2023, 285, 153982. [Google Scholar] [CrossRef]
- Liu, Y.; Anderson, N.O. Phenotypic differences among and within extant populations of Chrysanthemum arcticum L. and C. a. subsp. arcticum. BMC Plant Biol. 2022, 22, 517. [Google Scholar] [CrossRef]
- Shen, C.Z.; Zhang, C.J.; Chen, J.; Guo, Y.P. Clarifying Recent Adaptive Diversification of the Chrysanthemum-Group on the Basis of an Updated Multilocus Phylogeny of Subtribe Artemisiinae (Asteraceae: Anthemideae). Front. Plant Sci. 2021, 12, 648026. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Yan, H.; Liu, J.; Jiang, J.; Mao, Z.; Wang, T. Chrysanthemum classification method integrating deep visual features from both the front and back sides. Front. Plant Sci. 2025, 15, 1463113. [Google Scholar] [CrossRef]
- Cai, Z.; He, M.Y.; Li, C.; Qi, H.N.; Bai, R.B.; Yang, J.; Zhang, C. Identification of chrysanthemum using hyperspectral imaging based on few-shot class incremental learning. Comput. Electron. Agric. 2023, 215, 108371. [Google Scholar] [CrossRef]
- Chrysanthemum boreale (Makino) Makino. Botanical Magazine. [Shokubutsu-gaku zasshi]. Tokyo 1909, 23, 20. Available online: https://www.ipni.org/n/193330-1 (accessed on 15 June 2025).
- Tanaka, R. On the speciation and karyotypes in diploid and tetraploid species of chrysanthemum. Cytologia 1960, 25, 43–58. [Google Scholar] [CrossRef]
- Summer’s Web Garden. Chrysanthemum boreale. Available online: http://flowers.la.coocan.jp/Asteraceae/Chrysanthemum%20seticuspe%20boreale.htm (accessed on 15 June 2025).
- Lee, H.K.; Sivanesan, I.; Jeong, B.R. Growth and Morphological Characteristics of Wild Clones of Chrysanthemum boreale Mak. Korean Soc. Hortic. Sci. 2007, 48, 413–417. [Google Scholar]
- Chung, Y.S.; Jun, T.H.; Lee, Y.G.; Jung, J.A.; Won, S.Y.; Hwang, Y.J.; Silva, R.R.; Choi, S.C.; Kim, C.S. A genetic linkage map of wild Chrysanthemum species indigenous to Korea and its challenges. Int. J. Agric. Biol. 2018, 20, 2708–2716. [Google Scholar]
- Nguyen, T.K.; Dang, L.M.; Song, H.-K.; Moon, H.; Lee, S.J.; Lim, J.H. Wild Chrysanthemums Core Collection: Studies on Leaf Identification. Horticulturae 2022, 8, 839. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, C.H.; Kim, K.S. Phylogenetic analysis of Korean native Chrysanthemum species based on morphological characteristics. Sci. Hortic. 2014, 175, 278–289. [Google Scholar] [CrossRef]
- Im, S.I.; Bae, J.E.; Choi, S.H. Analysis of aroma components from flower tea of German chamomile and Chrysanthemum boreale Makino. Korean J. Food Cook. Sci. 2006, 22, 768–773. [Google Scholar]
- Lee, K.D.; Lee, Y.B.; Yang, M.S.; Kim, P.J. Effect of Soil Amendment Application on Yields and Effective Components of Chrysanthemum boreale M. Korean J. Soil Sci. Fertil. 2002, 35, 27–37. [Google Scholar]
- Lee, K.R.; Yang, M.S.; Lee, Y.B.; Kim, P.J. Effects of nitrogen fertilization on the yield and effective components of Chrysanthemum boreale M. Korean J. Soil Sci. Fertil. 2002, 35, 38–46. [Google Scholar]
- Lee, K.D.; Yang, M.S. Effects of pig manure application on nitrogen uptake, yield and active component of Chrysanthemum boreale M. Korean J. Med. Crop Sci. 2003, 11, 371–376. [Google Scholar]
- Lee, K.D.; Yang, M.S. Soil amendment effects on the yield and terpene contents of the flowerhead of Chrysanthemum boreale M. Agrochimica 2006, 50, 62–71. [Google Scholar]
- Lee, K.D.; Yang, M.S.; Jung, Y.K.; Sohn, B.K.; Cho, J.S.; Lee, S.T.; Kim, P.J. Effect of NPK fertilization on the yields and effective component of Chrysanthemum boreale M. J. Korean Soc. Agric. Chem. Biotechnol. 2003, 46, 134–139. [Google Scholar]
- Pathania, S.; Dhiman, S.R.; Kashyap, B.; Kumar, A.; Kaushal, R.; Gupta, R.K.; Alkahtani, J.; AlMunqedhi, B.M. Assessing the influence of planting time and fertilization on growth, flowering, yield and soil properties of chrysanthemum. J. King Saud Univ. Sci. 2024, 36, 103418. [Google Scholar] [CrossRef]
- Lee, K.D.; Yang, M.S. Changes in Mineral and Terpene Concentration Following Calcium Fertilization of Chrysanthemum boreale M. Res. J. Agric. Biol. Sci. 2005, 1, 222–226. [Google Scholar]
- Lee, K.D.; Yang, M.S.; Supanjani, S.; Smith, D.L. Fertilizer effect on the yield and terpene components from the flowerheads of Chrysanthemum boreale M. (Compositae). Agron. Sustain. Dev. 2005, 25, 205–211. [Google Scholar] [CrossRef]
- Won, S.Y.; Jung, J.A.; Kim, J.S. The complete mitochondrial genome sequence of Chrysanthemum boreale (Asteraceae). Mitochondrial DNA B Resour. 2018, 3, 529–530. [Google Scholar] [CrossRef]
- Won, S.Y.; Jung, J.A.; Kim, J.S. The complete chloroplast genome of Chrysanthemum boreale (Asteraceae). Mitochondrial DNA B Resour. 2018, 3, 549–550. [Google Scholar] [CrossRef]
- Won, S.Y.; Kwon, S.J.; Lee, T.H.; Jung, J.A.; Kim, J.S.; Kang, S.H.; Sohn, S.H. Comparative transcriptome analysis reveals whole-genome duplications and gene selection patterns in cultivated and wild Chrysanthemum species. Plant Mol. Biol. 2017, 95, 451–461. [Google Scholar] [CrossRef]
- Tyagi, S.; Jung, J.; Kim, J.S.; Won, S.Y. A comparative analysis of the complete chloroplast genomes of three Chrysanthemum boreale strains. PeerJ 2020, 8, e9448. [Google Scholar] [CrossRef]
- Abd El-Twab, M.H.; Kondo, K. Isolation of chromosomes and mutation in the interspecific hybrid between Chrysanthemum boreale and C. vestitum using fluorescence in situ hybridization and genomic in situ hybridization. Chromosome Bot. 2007, 2, 19–24. [Google Scholar] [CrossRef]
- Bala, A.; Bala, M.; Khare, V. A review on cytological study in Chrysanthemum species. J. Pharmacog. Phytochem. 2020, 9, 549–553. [Google Scholar]
- Hoang, T.K.; Hwang, Y.J.; Lim, J.H. Chemical polyploidization of Chrysanthemum boreale. Plant Cell Tissue Organ Cult. 2020, 140, 677–683. [Google Scholar] [CrossRef]
- Won, S.Y.; Hwang, Y.J.; Jung, J.A.; Kim, J.S.; Kang, S.H.; Sohn, S.H. Identification of repetitive DNA sequences in the Chrysanthemum boreale genome. Sci. Hortic. 2018, 236, 238–243. [Google Scholar] [CrossRef]
- Cuyacot, A.R.; Won, S.Y.; Park, S.K.; Sohn, S.H.; Lee, J.; Kim, J.S.; Kim, H.H.; Lim, K.B.; Hwang, Y.J. The chromosomal distribution of repetitive DNA sequences in Chrysanthemum boreale revealed a characterization in its genome. Sci. Hortic. 2016, 198, 438–444. [Google Scholar] [CrossRef]
- van Lieshout, N.; van Kaauwen, M.; Kodde, L.; Arens, P.; Smulders, M.J.M.; Visser, R.G.F.; Finkers, R. De novo whole-genome assembly of Chrysanthemum makinoi, a key wild chrysanthemum. G3 2022, 12, jkab358. [Google Scholar] [CrossRef]
- Hoang, T.; Wang, Y.; Hwang, Y.J.; Lim, J.H. Analysis of the morphological characteristics and karyomorphology of wild Chrysanthemum species in Korea. Hortic. Environ. Biotechnol. 2020, 61, 359–369. [Google Scholar] [CrossRef]
- Park, S.K.; Lim, J.H.; Shin, H.K.; Jung, J.A.; Kwon, Y.S.; Kim, M.S.; Kim, K.S. Identification of Chrysanthemum genetic resources resistant to white rust caused by Puccinia horiana. Plant Breed. Biotechnol. 2014, 2, 184–193. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kil, B.S.; Woo, W.H. Allelopathic effects of Chrysanthemum boreale on seed germination and seedling growth of the selected plants. Korean J. Ecol. 2000, 23, 431–434. [Google Scholar]
- Jeon, J.R.; Park, J.R. Effects of Chrysanthemum boreale M. Water Extract on Serum Liver Enzyme Activities and Kupffer Cells of Carbon Tetrachloride-Induced Rats. Food Sci. Biotechnol. 2005, 14, 290–296. [Google Scholar]
- Kim, S.H.; Choi, J.W. Antioxidant Activity of Water Extract of Chrysanthemum boreale against MPTP-induced Mice Models. J. Physiol. Pathol. Korean Med. 2013, 27, 49–56. [Google Scholar]
- Kim, J.; Lee, S.H.; Sun, N.; Choung, D.H.; Kim, W.K.; Lee, S.; Song, K.B. Isolation of an Angiotensin Converting Enzyme Inhibitory Substance from Chrysanthemum boreale Makino. J. Food Sci. 2003, 68, 816–819. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Yoon, M.S.; Yu, H.J.; Park, J.H.; Kim, B.; Lee, H.M. Chrysanthemum boreale flower floral water inhibits platelet-derived growth factor-stimulated migration and proliferation in vascular smooth muscle cells. Pharm. Biol. 2015, 53, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hwang, I.G.; Nho, J.W.; Chang, Y.D.; Lee, C.H.; Woo, K.S.; Jeong, H.S. Quality Characteristics and Antioxidant Activity of Chrysanthemum indicum L., Chrysanthemum boreale M. and Chrysanthemum zawadskii K. Powdered Teas. J. Korean Soc. Food Sci. Nutr. 2009, 38, 824–831. [Google Scholar] [CrossRef]
- You, K.; Bang, C.; Lee, K.; Ham, I. CHJ Anti-inflammatory effects of Chrysanthemum boreale flower. Korea J. Herbol. 2011, 26, 31–37. [Google Scholar]
- Yang, G.; Lee, K.; An, D.G.; Lee, M.H.; Ham, I.H.; Choi, H.Y. Effect of Chrysanthemi borealis flos on atopic dermatitis induced by 1-chloro 2,4-dinitrobenzene in NC/Nga mouse. Immunopharmacol. Immunotoxicol. 2012, 34, 413–418. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hwang, D.I.; Yoon, M.S.; Choi, I.H.; Lee, H.M. Effect of Hydrosol Extracted from Chrysanthemum boreale Makino Flower on Proliferation and Migration in Human Skin Keratinocyte. J. Soc. Cosmet. Sci. Korea 2016, 42, 95–101. [Google Scholar] [CrossRef]
- Park, N.Y.; Lee, G.D.; Jeong, Y.J.; Kwon, J.H. Optimization of extraction conditions for physicochemical properties of ethanol extracts from Chrysanthemum boreale. J. Korean Soc. Food Sci. Nutr. 1998, 27, 585–590. [Google Scholar]
- Lee, S.; Jang, G.; Jung, J.; Park, S.; Lee, J.; Lee, Y.; Lee, J.; Ji, Y.; Choi, J.; Kim, G. Memory-Improving Activity of the Flower Extract from Chrysanthemum boreale (Makino) Maskino in Scopolamine-Treated Rodents. Processes 2023, 11, 159. [Google Scholar] [CrossRef]
- Song, P.; Choi, S.Y.; Hwang, J.S.; Park, H.C.; Kim, K.K.; Son, H.J.; Hong, C.O.; Kim, Y.J.; Kim, W.; Lee, K.M. Chrysanthemum boreale Makino Inhibits Oxidative Stress-Induced Neuronal Damage in Human Neuroblastoma SH-SY5Y Cells by Suppressing MAPK-Regulated Apoptosis. Molecules 2022, 27, 5498. [Google Scholar] [CrossRef]
- Kim, Y.; Sung, J.; Sung, M.; Choi, Y.; Jeong, H.S.; Lee, J. Involvement of heme oxygenase-1 in the anti-inflammatory activity of Chrysanthemum boreale Makino extracts on the expression of inducible nitric oxide synthase in RAW264.7 macrophages. J. Ethnopharmacol. 2010, 131, 550–554. [Google Scholar] [CrossRef]
- Yang, M.S.; Nam, S.H. Antibacterial Activities of Extracts from Chrysanthemum boreale M. Appl. Biol. Chem. 1995, 38, 269–272. [Google Scholar]
- Jang, D.S.; Nam, S.H.; Choi, S.U.; Yang, M.S. Antibacterial Activity of Some Chrysanthemum spp. Appl. Biol. Chem. 1996, 39, 315–319. [Google Scholar]
- Yang, M.S.; Nam, S.H. Isolation of Cytotoxic Substances from Chysanthemum boreale M. Appl. Biol. Chem 1995, 38, 273–277. [Google Scholar]
- Doan, T.T.M.; Uy, N.P.; Tran, G.H.; Lee, S.; Lim, J.H. Antioxidant Activity of the Chrysanthemum Family and Quantitative Analysis of Phenolic Compounds by HPLC/UV. J. Biol. Regul. Homeost. Agents 2024, 38, 137–148. [Google Scholar]
- Kim, M.J.; Kim, D.H.; Kwak, H.S.; Yu, I.S.; Um, M.Y. Protective Effect of Chrysanthemum boreale Flower Extracts against A2E-Induced Retinal Damage in ARPE-19 Cell. Antioxidants 2022, 11, 669. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, Y.H.; Yu, H.H.; Jeong, S.I.; Cha, J.D.; Kil, B.S.; You, Y.O. Antibacterial activity and chemical composition of essential oil of Chrysanthemum boreale. Planta Med. 2003, 69, 274–277. [Google Scholar] [CrossRef]
- Chang, K.M.; Kim, G.H. Volatiles of Chrysanthemum zawadskii var. latilobum K. Prev. Nutr. Food Sci. 2012, 17, 234–238. [Google Scholar] [CrossRef]
- Mallmann, M.P.; Oliveira, M.S. Beta-caryophyllene in psychiatric and neurological diseases: Role of blood-brain barrier. Vitam. Horm. 2024, 126, 125–168. [Google Scholar]
- Chung, K.S.; Hong, J.Y.; Lee, J.H.; Lee, H.J.; Park, J.Y.; Choi, J.H.; Park, H.J.; Hong, J.; Lee, K.T. β-Caryophyllene in the Essential Oil from Chrysanthemum boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mancinelli, R.; Gullì, M.; Eufemi, M.; Mammola, C.L.; Mazzanti, G.; Di Giacomo, S. Chemopreventive Potential of Caryophyllane Sesquiterpenes: An Overview of Preliminary Evidence. Cancers 2020, 12, 3034. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.D.; Jeong, M.R.; Lee, Y.E. Induction of Apoptosis in Human Oral Epidermoid Carcinoma Cells by Essential Oil of Chrysanthemum boreale Makino. Food Sci. Biotechnol. 2005, 14, 350–354. [Google Scholar]
- Kim, D.Y.; Won, K.J.; Yoon, M.S.; Hwang, D.I.; Yoon, S.W.; Park, J.H.; Kim, B.; Lee, H.M. Chrysanthemum boreale Makino essential oil induces keratinocyte proliferation and skin regeneration. Nat. Prod. Res. 2015, 29, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.H.; Hwang, D.I.; Kim, D.Y.; Kim, H.B.; Lee, H.M. A Study on the Anti-wrinkle Properties of Cosmetics Containing Essential Oil from Chrysanthemum boreale MAKINO. J. Life Sci. 2019, 29, 442–446. [Google Scholar]
- Hong, C.U. Essential Oil Composition of Chrysanthemum boreale and Chrysanthemum indicum. Appl. Biol Chem. 2002, 45, 108–113. [Google Scholar]
- Kim, B.S.; Park, S.J.; Kim, M.K.; Kim, Y.H.; Lee, S.B.; Lee, K.H.; Choi, N.Y.; Lee, Y.R.; Lee, Y.E.; You, Y.O. Inhibitory Effects of Chrysanthemum boreale Essential Oil on Biofilm Formation and Virulence Factor Expression of Streptococcus mutans. Evid.-Based Complement. Altern. Med. 2015, 2015, 616309. [Google Scholar]
- Park, B.I.; You, Y.O.; Mo, J.S.; An, S.Y.; Choi, N.Y.; Kim, K.J. Anti-cariogenic Properties of α-Pinene, a Monoterpene in Plant Essential Oil. Int. J. Oral Biol. 2017, 42, 25–31. [Google Scholar] [CrossRef]
- Seung, W.K. Identification of the Change in Volatile Compound Composition According to the Flowering Stages of Wild Chrysanthemum Species Using HS-SPME-GC-MS. Flower Res. J. 2021, 29, 138–145. [Google Scholar]
- Kim, S.J.; Ha, T.J.; Kim, J.Y.; Nam, J.H.; Yoo, D.L.; Suh, J.T.; Kim, K.S. Volatile Flavor Compounds in the Leaves of Fifteen Taxa of Korean Native Chrysanthemum Species. Hortic. Sci. Technol. 2014, 32, 558–570. [Google Scholar] [CrossRef][Green Version]
- Bae, S.M.; Lee, S.C. Effect of Hot-air Drying Temperature on Volatile Compounds in Chrysanthemum boreale M. Flowers. Korean J. Food Sci. Technol. 2008, 40, 466–469. [Google Scholar]
- Xiao, Z.; Fan, B.; Niu, Y.; Wu, M.; Liu, J.; Ma, S. Characterization of odor-active compounds of various Chrysanthemum essential oils by gas chromatography-olfactometry, gas chromatography-mass spectrometry and their correlation with sensory attributes. J. Chromatogr. B 2016, 1009–1010, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, T.; Fan, Q.; Qi, X.; Zhang, F.; Fang, W.; Jiang, J.; Chen, F.; Chen, S. Identification of floral scent in chrysanthemum cultivars and wild relatives by gas chromatography-mass spectrometry. Molecules 2015, 20, 5346–5359. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Park, S.M.; Kim, B.; Lee, H.M. Chemical Composition, Antioxidant and Anti-melanogenic Activities of Essential Oils from Chrysanthemum boreale Makino at Different Harvesting Stages. Chem. Biodivers. 2018, 15, e1700506. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Lee, S.Y.; Choi, I.H.; Kim, B.; Mee, H.M. Essential oil from Chrysanthemum boreale flowers modulates SNARE protein-linked mast cell response and skin barrier proteins and ameliorates atopic dermatitis-like lesions in mice. Hortic. Environ. Biotechnol. 2022, 63, 287–298. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Kim, N.Y.; Kim, B.; Lee, H.M. 1-Iodohexadecane Alleviates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice: Possible Involvements of the Skin Barrier and Mast Cell SNARE Proteins. Molecules 2022, 27, 1560. [Google Scholar] [CrossRef]
- Bail, J.; Lee, W.Y.; Kim, J.Y.; Pak, C.H. Changes in essential oil contents and growth of Chrysanthemum boreale treated by uniconazole. J. Korean Soc. Hortic. Sci. 2002, 43, 364–368. [Google Scholar]
- Jeong, J.H.; Nguyen, T.K.N.; Choi, M.J.; Nguyen, L.T.H.; Shin, H.M.; Lee, B.W.; Yang, I.J. A Study on the Activities of Five Natural Plant Essential Oils on Atopic Dermatitis. J. Soc. Cosmet. Sci. Korea 2021, 47, 23–30. [Google Scholar]
- Hwang, D.I.; Choi, I.H.; Kim, D.Y.; Park, S.M.; Kim, H.B.; Li, Y.L.; Lee, H.M. Inhibitory Effects of Chrysanthemum boreale Makino on 3T3-L1 Preadipocyte Differentiation and Down-regulation of Adipogenesis and Lipogenesis. J. Life Sci. 2019, 29, 332–336. [Google Scholar]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.J. Sabinene Prevents Skeletal Muscle Atrophy by Inhibiting the MAPK-MuRF-1 Pathway in Rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef]
- Nugroho, A.; Lim, S.C.; Choi, J.; Park, H.J. Identification and quantification of the sedative and anticonvulsant flavone glycoside from Chrysanthemum boreale. Arch. Pharm. Res. 2013, 36, 51–60. [Google Scholar] [CrossRef]
- Oinonen, P.P.; Jokela, J.K.; Hatakka, A.I.; Vuorela, P.M. Linarin, a selective acetylcholinesterase inhibitor from Mentha arvensis. Fitoterapia 2006, 77, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Cheriet, T.; Mancini, I.; Seghiri, R.; Benayache, F.; Benayache, S. Chemical constituents and biological activities of the genus Linaria (Scrophulariaceae). Nat. Prod. Res. 2015, 29, 1589–1613. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.; Park, J.H.; Choi, J.S.; Park, K.S.; Hong, J.P.; Park, H.J. Structure determination and quantification of a new flavone glycoside with anti-acetylcholinesterase activity from the herbs of Elsholtzia ciliata. Nat. Prod. Res. 2019, 33, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Fernández, S.; Wasowski, C.; Paladini, A.C.; Marder, M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004, 77, 399–404. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Taghrir, H.; Boveiri Dehsheikh, A.; Zomorodian, K.; Irajie, C.; Mahmoodi Sourestani, M.; Iraji, A. Linarin, a Glycosylated Flavonoid, with Potential Therapeutic Attributes: A Comprehensive Review. Pharmaceuticals 2021, 14, 1104. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, J.J.; Islam, M.N.; Jin, S.E.; Min, B.S.; Lee, J.H.; Sohn, H.S.; Choi, J.S. Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch. Pharm. Res. 2012, 35, 1021–1035. [Google Scholar] [CrossRef]
- Veitch, N.C.; Elliott, P.C.; Kite, G.C.; Lewis, G.P. Flavonoid glycosides of the black locust tree, Robinia pseudoacacia (Leguminosae). Phytochemistry 2010, 71, 479–486. [Google Scholar] [CrossRef]
- Uehara, A.; Kadota, Y.; Iwashina, T. Flavonoids from the Leaves of Chrysanthemum seticuspe f. seticuspe in the Imperial Palace—Chemotaxonomical Comparison with Chrysanthemum seticuspe f. boreale. Mem. Natl. Mus. Nat. Sci. 2014, 49, 23–28. [Google Scholar]
- Uehara, A.; Nakata, M.; Kitajima, J.; Iwashina, T. Internal and external flavonoids from the leaves of Japanese Chrysanthemum species (Asteraceae). Biochem. Syst. Ecol. 2012, 41, 142–149. [Google Scholar] [CrossRef]
- Shin, K.H.; Kang, S.S.; Seo, E.A.; Shin, S.W. Isolation of aldose reductase inhibitors from the flowers of Chrysanthemum boreale. Arch. Pharmacal. Res. 1995, 18, 64–68. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, K.-T.; Choi, H.-E.; Ha, T.J.; Nam, J.H.; Hong, S.Y.; Chang, D.C.; Kim, K.S. Anti-inflammatory effects of flavonoids in Korean Chrysanthemum species via suppression of inducible nitric oxide synthase and cyclooxygenase-2 in LPS-induced RAW 264.7 macrophages. Food Sci. Biotechnol. 2015, 24, 975–985. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, J.W.; Park, J.H.; Choi, Y.S.; Kwak, H.S.; Kim, D.H.; Choi, Y.R.; Yoo, I.S. Use of Chrysanthemum boreale Extract for Eye Health. Korean Patent KR20200022926A, 4 March 2020. [Google Scholar]
- Yang, M.S.; Park, K.H.; Jang, D.S.; Choi, S.U.; Nam, S.H.; Shiro, M. Cumambrin A in Chrysanthemum boreale Makino. Preparation, X-ray crystal structure and 13C- and 1H-NMR study of cumambrin A. Korean J. Pharmacogn. 1996, 27, 207–211. [Google Scholar]
- Jang, D.S.; Park, K.H.; Kim, H.M.; Hong, D.H.; Chun, H.K.; Kho, Y.H.; Yang, M.S. Biological activities of sesquiterpene lactones isolated from several compositae plants. Part 1 cytotoxicity against cancer cell lines. Korean J. Pharmacogn. 1998, 29, 243–247. [Google Scholar]
- Jang, D.S.; Park, K.H.; Lee, J.R.; Ha, T.J.; Park, Y.B.; Nam, S.H.; Yang, M.S. Antimicrobial Activities of Sesquiterpene Lactones Isolated from Hemisteptia lyrata, Chrysanthemum zawadskii and Chrysanthemum boreale. J. Korean Soc. Agric. Chem. Biotechnol. 1999, 42, 176–179. [Google Scholar]
- Jiang, S.; Wang, M.; Jiang, Z.; Zafar, S.; Xie, Q.; Yang, Y.; Liu, Y.; Yuan, H.; Jian, Y.; Wang, W. Chemistry and Pharmacological Activity of Sesquiterpenoids from the Chrysanthemum Genus. Molecules 2021, 26, 3038. [Google Scholar] [CrossRef]
- Wen, B.; Hexum, J.K.; Widen, J.C.; Harki, D.A.; Brummond, K.M. A redox economical synthesis of bioactive 6,12-guaianolides. Org. Lett. 2013, 15, 2644–2647. [Google Scholar] [CrossRef]
- Lee, J.R.; Yang, M.S.; Lee, J.; Hwang, S.W.; Kho, Y.H.; Park, K.H. New guaianolides from leaves and stems of Chrysanthemum boreale. Planta Med. 2003, 69, 880–882. [Google Scholar]
- Lee, J.R.; Yang, M.S.; Jang, D.S.; Ha, T.J.; Park, K.M.; Lee, C.H.; Kho, Y.H.; Park, K.H. A new guaianolide as apoptosis inhibitor from Chrysanthemum boreale. Planta Med. 2001, 67, 585–587. [Google Scholar] [CrossRef]
- Park, K.H.; Yang, M.S.; Park, M.K.; Kim, S.C.; Yang, C.H.; Park, S.J.; Lee, J.R. A new cytotoxic guaianolide from Chrysanthemum boreale. Fitoterapia 2009, 80, 54–56. [Google Scholar] [CrossRef]
- Lee, J.R.; Park, M.K. Structural Analysis and Biological Activities of Sesquiterpene Lactones Isolated from the Leaves and Stems of Chrysanthemum boreale Makino. J. Environ. Sci. Int. 2017, 26, 1285–1295. [Google Scholar] [CrossRef]
- Lee, J.R.; Park, M.K. Isolation of Guaianolides with ACAT Inhibitory Activity from the Leaves and Stems of Chrysanthemum boreale Makino. J. Environ. Sci. Int. 2017, 26, 1275–1284. [Google Scholar] [CrossRef]
- Lee, H.K.; Hong, Y.G. Effect of cumambrin A Treatment on Blood Pressure in Spontaneously Hypertensive Rats. J. Korean Soc. Phys. Ther. 2002, 14, 297–307. [Google Scholar]
- Hong, Y.G.; Yang, M.S.; Pak, Y.B. Effect of cumambrin A treatment on blood pressure in spontaneously hypertensive rats. Korean J. Pharmacogn. 1999, 30, 226–230. [Google Scholar]
- Hong, Y.G.; Yang, M.S.; Pak, Y.B. Effect of Cumambrin A on the Relaxation of Rat Aorta. Korean J. Pharmacogn. 2005, 36, 17–20. [Google Scholar]
- Zhou, L.; Liu, Q.; Hong, G.; Song, F.; Zhao, J.; Yuan, J.; Xu, J.; Tan, R.X.; Tickner, J.; Gu, Q.; et al. Cumambrin A prevents OVX-induced osteoporosis via the inhibition of osteoclastogenesis, bone resorption, and RANKL signaling pathways. FASEB J. 2019, 33, 6726–6735. [Google Scholar] [CrossRef]
- Li, J.; Yoshida, Y.; Kurita, M.; Usuki, T. Cynaropicrin and inhibition of NF-kappaB activation: A structure activity relationship study. Bioorg. Med. Chem. Lett. 2019, 29, 1518–1521. [Google Scholar] [CrossRef]
- Hu, B.; Wu, F.; Shi, Z.; He, B.; Zhao, X.; Wu, H.; Yan, S. Dehydrocostus lactone attenuates osteoclastogenesis and osteoclast-induced bone loss by modulating NF-kappaB signalling pathway. J. Cell. Mol. Med. 2019, 23, 5762–5770. [Google Scholar] [CrossRef]
- Hayata, M.; Watanabe, N.; Kamio, N.; Tamura, M.; Nodomi, K.; Tanaka, K.; Iddamalgoda, A.; Tsuda, H.; Ogata, Y.; Sato, S.; et al. Cynaropicrin from Cynara scolymus L. suppresses Porphyromonas gingivalis LPS-induced production of inflammatory cytokines in human gingival fibroblasts and RANKL-induced osteoclast differentiation in RAW264.7 cells. J. Nat. Med. 2019, 73, 114–123. [Google Scholar] [CrossRef]
- Zhu, M.; Shan, J.; Xu, H.; Xia, G.; Xu, Q.; Quan, K.; Liu, X.; Dai, M. Glaucocalyxin A suppresses osteoclastogenesis induced by RANKL and osteoporosis induced by ovariectomy by inhibiting the NF-κB and Akt pathways. J. Ethnopharmacol. 2021, 276, 114176. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, P.; Bennett, S.; Charlesworth, O.; Tan, R.; Peng, X.; Gu, Q.; Kujan, O.; Xu, J. The therapeutic effect and mechanism of parthenolide in skeletal disease, cancers, and cytokine storm. Front. Pharmacol. 2023, 14, 1111218. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jang, W.G. Zaluzanin C (ZC) induces osteoblast differentiation through regulating of osteogenic genes expressions in early stage of differentiation. Bioorg. Med. Chem. Lett. 2017, 27, 4789–4793. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Ang, E.; Dai, L.; Yang, X.; Ye, D.; Chen, H.; Zhou, L.; Yang, M.; Teguh, D.; Tan, R.; et al. Natural Germacrane Sesquiterpenes Inhibit Osteoclast Formation, Bone Resorption, RANKL-Induced NF-kappaB Activation, and IkappaBalpha Degradation. Int. J. Mol. Sci. 2015, 16, 26599–26607. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yuan, G.; Lin, X.; Liu, Q.; Xu, J.; Lian, Z.; Song, F.; Zheng, J.; Xie, D.; Chen, L.; et al. Dehydrocostus lactone (DHC) suppresses estrogen deficiency-induced osteoporosis. Biochem. Pharmacol. 2019, 163, 279–289. [Google Scholar] [CrossRef]
- Tanaka, Y.T.; Tanaka, K.; Kojima, H.; Hamada, T.; Masutani, T.; Tsuboi, M.; Akao, Y. Cynaropicrin from Cynara scolymus L. suppresses photoaging of skin by inhibiting the transcription activity of nuclear factor-kappa B. Bioorg. Med. Chem. Lett. 2013, 23, 518–523. [Google Scholar] [CrossRef]
- Hay, A.J.; Hamburger, M.; Hostettmann, K.; Hoult, J.R. Toxic inhibition of smooth muscle contractility by plant-derived sesquiterpenes caused by their chemically reactive alpha-methylenebutyrolactone functions. Br. J. Pharmacol. 1994, 112, 9–12. [Google Scholar] [CrossRef]
- Gan, Z.; Huang, J.; Xu, M.; Yuan, X.; Shang, X.; Chen, X.; Chen, K. Micheliolide prevents estrogen deficiency-induced bone loss via inhibiting osteoclast bone resorption. Aging 2023, 15, 10732–10745. [Google Scholar] [CrossRef]
- Lee, H.I.; Lee, G.R.; Lee, J.; Kim, N.; Kwon, M.; Kim, H.J.; Kim, N.Y.; Park, J.H.; Jeong, W. Dehydrocostus lactone inhibits NFATc1 via regulation of IKK, JNK, and Nrf2, thereby attenuating osteoclastogenesis. BMB Rep. 2020, 53, 218–222. [Google Scholar] [CrossRef]
- Wang, L.C.; Liao, L.X.; Lv, H.N.; Liu, D.; Dong, W.; Zhu, J.; Chen, J.F.; Shi, M.L.; Fu, G.; Song, X.M.; et al. Highly Selective Activation of Heat Shock Protein 70 by Allosteric Regulation Provides an Insight into Efficient Neuroinflammation Inhibition. EBioMedicine 2017, 23, 160–172. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L.; Merfort, I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001, 276, 39713–39720. [Google Scholar] [CrossRef]
- García-Piñeres, A.J.; Lindenmeyer, M.T.; Merfort, I. Role of cysteine residues of p65/NF-kappaB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci. 2004, 75, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Rüngeler, P.; Castro, V.; Mora, G.; Gören, N.; Vichnewski, W.; Pahl, H.L.; Merfort, I.; Schmidt, T.J. Inhibition of transcription factor NF-kappaB by sesquiterpene lactones: A proposed molecular mechanism of action. Bioorg. Med. Chem. 1999, 7, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, J.; Yang, L.; Yan, Y.; Chen, M.; Li, B.; Xu, Z.; Qin, T.; Qu, S.; Wang, L.; et al. Micheliolide exerts effects in myeloproliferative neoplasms through inhibiting STAT3/5 phosphorylation via covalent binding to STAT3/5 proteins. Blood Sci. 2023, 5, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef]
- Matos, M.S.; Anastácio, J.D.; Nunes Dos Santos, C. Sesquiterpene Lactones: Promising Natural Compounds to Fight Inflammation. Pharmaceutics 2021, 13, 991. [Google Scholar] [CrossRef]
- Meng, F.C.; Wang, Z.; Peng, S.J.; Zhou, G.G.; Khalid, A.; Mao, J.X.; Wang, G.W.; Liao, Z.H.; Chen, M. Recent advances of sesquiterpenoid dimers from Compositae: Distribution, chemistry and biological activities. Phytochem. Rev. 2024, 23, 625–655. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Yan, J.J.; Liu, T.T.; Gao, J.; Huang, H.L.; Sun, C.P.; Huo, X.K.; Deng, S.; Zhang, B.J.; Ma, X.C. Natural sesquiterpenoid oligomers: A chemical perspective. Eur. J. Med. Chem. 2020, 203, 112622. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Y.; Cui, H.; Huang, D.; Zhou, J.; Wu, T.; Chen, Y.; Shi, L.; Xu, J. Chrysanolide A, an unprecedented sesquiterpenoid trimer from the flowers of Chrysanthemum indicum L. RSC Adv. 2013, 3, 10168–10172. [Google Scholar] [CrossRef]
- Tarasov, V.A.; Abdullaev, N.D.; Kasymov, S.Z.; Sidyakin, G.P. Cumambrin A—A sesquiterpene lactone from Handelia trichophylla. Chem. Nat. Compd. 1974, 10, 826. [Google Scholar] [CrossRef]
- Tarasov, V.A.; Kasymov, S.É.; Sidyakin, G.P. Sesquiterpene lactones of Handelia trichophylla. Chem. Nat. Compd. 1976, 12, 105–106. [Google Scholar] [CrossRef]
- Abdullaev, N.D.; Yagudaev, M.R.; Tarasov, V.A.; Kasimov, S.; Sidyakin, G.P. 13C NMR spectra of handelin and its derivatives. Chem. Nat. Compd. 1979, 15, 285–289. [Google Scholar] [CrossRef]
- Tashkhodzhaev, B.; Karimov, Z. Spatial structure of dimeric guanolides. Crystal and molecular structure of the diguanolide handelin. Chem. Nat. Compd 1994, 30, 186–192. [Google Scholar] [CrossRef]
- Chen, Z.N.; Xu, P.J. Structural determination of yejuhua lactone, isolated from Chrysanthemum indicum L. Yao Xue Xue Bao 1987, 22, 67–69. [Google Scholar] [PubMed]
- Haruna, M.; Kato, M.; Ito, K.; Nikai, T.; Sugihara, H.; Muratat, H. Angeloylcumambrin-B, an antimicrobial sesquiterpene lactone from Chrysanthemum ornatum var. Spontaneum. Phytochemistry 1981, 20, 2583–2584. [Google Scholar] [CrossRef]
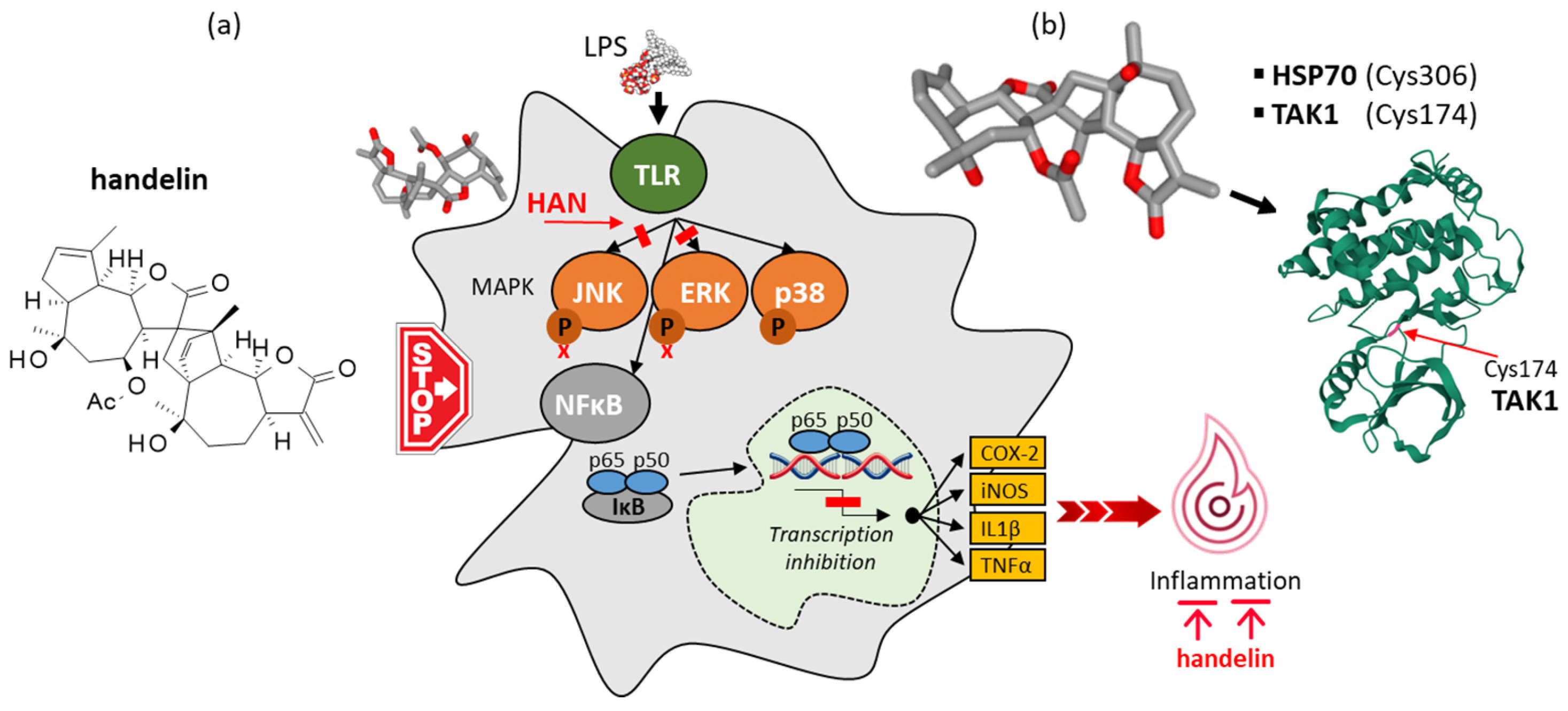
- Kang, S.S.; Kim, J.S.; Son, K.H.; Lee, C.O.; Kim, Y.H. Isolation of handelin from Chrysanthemum boreale. Arch. Pharm. Res. 1996, 19, 406–410. [Google Scholar] [CrossRef]
- Pyee, Y.; Chung, H.J.; Kim, J.S.; Choi, T.J.; Kang, S.S.; Lee, S.K. Anti-inflammatory activity of handelin through the modulation of NF-κB signaling and pro-inflammatory cytokine productions. Planta Medica 2012, 78, PD86. [Google Scholar] [CrossRef]
- Pyee, Y.; Chung, H.J.; Choi, T.J.; Park, H.J.; Hong, J.Y.; Kim, J.S.; Kang, S.S.; Lee, S.K. Suppression of inflammatory responses by handelin, a guaianolide dimer from Chrysanthemum boreale, via downregulation of NF-κB signaling and pro-inflammatory cytokine production. J. Nat. Prod. 2014, 77, 917–924. [Google Scholar] [CrossRef]
- Benedik, N.S.; Proj, M.; Steinebach, C.; Sova, M.; Sosič, I. Targeting TAK1, Evolution of inhibitors, challenges, and future directions. Pharmacol. Ther. 2025, 267, 108810. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, H.Y. The novel TAK1 inhibitor handelin inhibits NF-kappaB and AP-1 activity to alleviate elastase-induced emphysema in mice. Life Sci. 2023, 319, 121388. [Google Scholar] [CrossRef]
- Totzke, J.; Scarneo, S.A.; Yang, K.W.; Haystead, T.A.J. TAK1, a potent tumour necrosis factor inhibitor for the treatment of inflammatory diseases. Open Biol. 2020, 10, 200099. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Hu, Q.; Liu, L.; Yuan, Y.; Zhang, C.; Tang, J.; Shen, X. Eupalinolide B attenuates lipopolysaccharide-induced acute lung injury through inhibition of NF-κB and MAPKs signaling by targeting TAK1 protein. Int. Immunopharmacol. 2022, 111, 109148. [Google Scholar] [CrossRef]
- Toviwek, B.; Gleeson, D.; Gleeson, M.P. QM/MM and molecular dynamics investigation of the mechanism of covalent inhibition of TAK1 kinase. Org. Biomol. Chem. 2021, 19, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Gurbani, D.; Weisberg, E.L.; Hunter, J.C.; Li, L.; Jones, D.S.; Ficarro, S.B.; Mowafy, S.; Tam, C.P.; Rao, S.; et al. Structure-guided development of covalent TAK1 inhibitors. Bioorg. Med. Chem. 2017, 25, 838–846. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, L.; Liu, X.; Chen, H.; Liu, K.; Huang, N.; Wang, Y. Epoxymicheliolide prevents dextran sulfate sodium-induced colitis in mice by inhibiting TAK1-NF-kappaB pathway and activating Keap1-NRF2 signaling in macrophages. Int. Immunopharmacol. 2022, 113, 109404. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Park, K.H.; Yang, M.S. Germacranolides from flowers of Chrysanthemum boreale Makino. Korean J. Pharmacogn. 1998, 29, 67–70. [Google Scholar]
- Jang, D.S.; Yang, M.S.; Ha, T.J.; Park, K.H. Structural analogues of cumambrin B from the flower of Chrysanthemum boreale. Arch Pharm. Res. 1998, 21, 591–594. [Google Scholar] [CrossRef]
- Duan, Y.; Xiang, Y.; Chu, J.; Lin, X.; He, M.; Zhang, C.; Sun, S.; Huang, L. Handelin Reduces Ultraviolet A-Induced Photoaging by Inhibiting Reactive Oxygen Species Generation and Enhancing Autophagy. Tohoku J. Exp. Med. 2023, 259, 189–198. [Google Scholar] [CrossRef]
- Chu, J.; Xiang, Y.; Lin, X.; He, M.; Wang, Y.; Ma, Q.; Duan, J.; Sun, S. Handelin protects human skin keratinocytes against ultraviolet B-induced photodamage via autophagy activation by regulating the AMPK-mTOR signaling pathway. Arch. Biochem. Biophys. 2023, 743, 109646. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, J.; Lan, X.; Zeng, W.; Zhou, J.; Huang, T.E.; Xiao, W.L.; Wang, Q.Q.; Sun, S.; Su, W.; et al. Handelin extends lifespan and healthspan of Caenorhabditis elegans by reducing ROS generation and improving motor function. Biogerontology 2022, 2, 115–128. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, B.H.; Wang, X.; Huang, C.P.; Xu, S.M.; Wang, J.L.; Huang, T.E.; Xiao, W.L.; Tian, X.L.; Lan, X.Q.; et al. Handelin alleviates cachexia- and aging-induced skeletal muscle atrophy by improving protein homeostasis and inhibiting inflammation. J. Cachexia Sarcopenia Muscle 2024, 15, 173–188. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Sun, S.; Wang, X.; Guan, Y.; Mi, Q.; Zeng, W.; Xiang, H.; Zhu, H.; Zou, X.; et al. Autophagy and Hsp70 activation alleviate oral epithelial cell death induced by food-derived hypertonicity. Cell Stress Chaperones 2020, 25, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Z.; Wang, C.; Tian, X.; Huo, X.; Wang, Y.; Sun, C.; Feng, L.; Ma, J.; Zhang, B.; et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-kappaB/COX-2 signaling pathway by targeting IKKbeta. Am. J. Cancer Res. 2017, 7, 1270–1284. [Google Scholar] [PubMed]
- Yan, Y.; Yang, M.; Jiao, Y.; Li, L.; Liu, Z.; Shi, J.; Shen, Z.; Peng, G. Drug screening identified that handelin inhibits feline calicivirus infection by inhibiting HSP70 expression in vitro. J. Gen. Virol. 2024, 105, 1936. [Google Scholar] [CrossRef]
- Mouawad, N.; Capasso, G.; Ruggeri, E.; Martinello, L.; Severin, F.; Visentin, A.; Facco, M.; Trentin, L.; Frezzato, F. Is It Still Possible to Think about HSP70 as a Therapeutic Target in Onco-Hematological Diseases? Biomolecules 2023, 13, 604. [Google Scholar] [CrossRef] [PubMed]
- Page, J.D.; Chaney, S.G.; Hall, I.H.; Lee, K.H.; Holbrook, D.J. Inhibition of inosine monophosphate dehydrogenase by sesquiterpene lactones. Biochim. Biophys. Acta. 1987, 926, 186–194. [Google Scholar] [CrossRef]
- Bailly, C. Covalent binding of withanolides to cysteines of protein targets. Biochem. Pharmacol. 2024, 226, 116405. [Google Scholar] [CrossRef]
- Zhang, F.; Ishiuchi, K.; Sugiyama, A.; Ohsawa, M.; Makino, T. B-ring-homo-tonghaosu, isolated from Chrysanthemum morifolium capitulum, acts as a peroxisome proliferator-activated receptor-γ agonist. J. Nat. Med. 2019, 73, 497–503. [Google Scholar] [CrossRef]
- Wu, B.; Feast, G.C.; Thompson, A.L.; Robertson, J. Synthesis of stereoisomers of Artemisia and Chrysanthemum bis(acetylenic) enol ether spiroacetals. J. Org. Chem. 2012, 77, 10623–10630. [Google Scholar] [CrossRef]
- Lee, J.R.; Park, M.K. Isolation of New Sesquiterpene lactone and 3-(3-Methylbutanoly) 2-(2,4-hexadiyuylidene)-1,6-dioxaspirp[4,5]decane with Cytotoxic Activity from Chrysanthemum boreale Makino. Proc. Korean Environ. Sci. Soc. Conf. 2012, 21, 553–555. [Google Scholar]
- Lee, J.R.; Park, M.K. A Guaianolides as Apoptosis Inhibitor from flowers of Chrysanthemum boreale Makino. Proc. Korean Environ. Sci. Soc. Conf. 2012, 21, 556–558. [Google Scholar]
- Liu, L.L.; Wang, R.; Shi, Y.P. Chrysindins A-D, polyacetylenes from the flowers of Chrysanthemum indicum. Planta Med. 2011, 77, 1806–1810. [Google Scholar] [CrossRef] [PubMed]
- Marco, J.A.; Sanz, J.F. New sesquiterpene lactones and acetylenes from Chrysanthemum lavandulifolium. Tetrahedron 1990, 46, 6931–6938. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Bang, M.H.; Song, M.C.; Kim, S.U.; Chang, Y.J.; Baek, N.I. Isolation of β-sitosterol and zingerone 4-O-β-D-glucopyranoside from Chrysanthemum boreale Makino. Korean J. Med. Crop Sci. 2005, 13, 284–287. [Google Scholar]
- Kim, J.S.; Ki, D.W.; Lee, I.K.; Yun, B.S. Pullusurfactins A–C, new biosurfactants produced by Aureobasidium pullulans A11231-1-58 from Chrysanthemum boreale Makino. J. Antibiot. 2023, 76, 741–745. [Google Scholar] [CrossRef]
- Kim, D.K.; Shim, S.K.; Lee, S.C.; Bae, D.W.; Kim, H.K. Occurrence of Blossom Blight of Chrysanthemum boreale Caused by Didymella chrysanthemi. Plant Pathol. J. 2001, 17, 347–349. [Google Scholar]
- Khoiriyah, N.; Susilowati, D.; Mintyastuti, D.S.; Ali, R.M.; Forgenie, D. The technical efficiency of chrysanthemum flower farming: A stochastic frontier analysis. Anjoro 2023, 4, 50–59. [Google Scholar] [CrossRef]
- Liu, C.F.; Chen, Y.J.; Chen, P.A.; Kuo, C.C.; Chen, K.H.; Chen, C.H.; Su, T.C.; Chen, I.Z.; Chang, Y.S. Impact of Temperature on Growth, Photosynthetic Efficiency, Yield, and Functional Components of Bud-Leaves and Flowers in Edible Chrysanthemum (Chrysanthemum morifolium Ramat). Horticulturae 2025, 11, 448. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, S.; Liu, Y.; Daniyal, M.; Jian, Y.; Peng, C.; Shen, J.; Liu, S.; Wang, W. The flower head of Chrysanthemum morifolium Ramat. (Juhua): A paradigm of flowers serving as Chinese dietary herbal medicine. J. Ethnopharmacol. 2020, 261, 113043. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Harada, T.; Iwahashi, F. In vitro and in planta investigation of succinate dehydrogenase inhibitor resistance conferred by target-site amino acid substitutions in Puccinia horiana, chrysanthemum white rust. Pest Manag. Sci. 2024, 80, 2874–2880. [Google Scholar] [CrossRef]
- Chae, S.C. An Up-To-Date Review of Phytochemicals and Biological Activities in Chrysanthemum Spp. Biosci. Biotechnol. Res. Asia 2016, 13, 13954. Available online: https://www.biotech-asia.org/?p=13954 (accessed on 15 June 2025).
- Reed, C.; Régamey, F. The Chrysantheme Papers. In The Pink Notebook of Madame Chrysantheme and Other Documents of French Japonisme, 1st ed.; University of Hawai’i Press: Honolulu, HI, USA, 2010. [Google Scholar]
- Chang, S.H.; Kim, W.T.; Choi, H.Y.; Ham, I.; Yang, G.; Bang, C.S. Compound Comprising Extracts or Fractions of Chrysanthemum boreale Makino Having Anti-Inflammation Activity. WO2011065657, 3 June 2011. [Google Scholar]
- Lee, H.M.; Kim, D.Y.; Hwang, D.I.; Yoon, S.W.; Lee, S.J. Composition Comprising Chrysanthemum boreale Makino Essential Oil Having Anti-Oxidant and Anti-Obesity. KR101685773B1, 12 December 2016. [Google Scholar]
- Loh, Z.H.; Chua, C.L.L.; Mah, S.H. Chapter 3—Essential oils as potential skin antiinflammatory agent and their mechanisms of action. Stud. Nat. Prod. Chem. 2023, 76, 67–111. [Google Scholar]
- Jiang, Y.; Zhang, W.; Chen, X.; Wang, W.; Köllner, T.G.; Chen, S.; Chen, F.; Chen, F. Diversity and Biosynthesis of Volatile Terpenoid Secondary Metabolites in the Chrysanthemum Genus. Crit. Rev. Plant Sci. 2021, 40, 422–445. [Google Scholar] [CrossRef]
- Xu, D.; Li, S.; Wang, L.; Jiang, J.; Zhao, L.; Huang, X.; Sun, Z.; Li, C.; Sun, L.; Li, X.; et al. TAK1 inhibition improves myoblast differentiation and alleviates fibrosis in a mouse model of Duchenne muscular dystrophy. J. Cachexia Sarcopenia Muscle 2021, 12, 192–208. [Google Scholar] [CrossRef]
- Ma, G.H.; Chen, K.X.; Zhang, L.Q.; Li, Y.M. Advance in biological activities of natural guaiane-type sesquiterpenes. Med. Chem. Res. 2019, 28, 1339–1358. [Google Scholar] [CrossRef]
- Ma, L.F.; Chen, Y.L.; Shan, W.G.; Zhan, Z.J. Natural disesquiterpenoids: An update. Nat. Prod Rep. 2020, 37, 999–1030. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, F.; Zhao, H.; Song, J.; Song, M.; Zhu, J.; Wang, Y.; Man Hoi, M.P.; Lin, L.; Zhang, Q. Dimeric guaianolide sesquiterpenoids from the flowers of Chrysanthemum indicum ameliorate hepatic steatosis through mitigating SIRT1-mediated lipid accumulation and ferroptosis. J. Adv. Res. 2025; in press. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C. Biological and Medicinal Properties of Chrysanthemum boreale Makino and Its Bioactive Products. Int. J. Mol. Sci. 2025, 26, 5956. https://doi.org/10.3390/ijms26135956
Bailly C. Biological and Medicinal Properties of Chrysanthemum boreale Makino and Its Bioactive Products. International Journal of Molecular Sciences. 2025; 26(13):5956. https://doi.org/10.3390/ijms26135956
Chicago/Turabian StyleBailly, Christian. 2025. "Biological and Medicinal Properties of Chrysanthemum boreale Makino and Its Bioactive Products" International Journal of Molecular Sciences 26, no. 13: 5956. https://doi.org/10.3390/ijms26135956
APA StyleBailly, C. (2025). Biological and Medicinal Properties of Chrysanthemum boreale Makino and Its Bioactive Products. International Journal of Molecular Sciences, 26(13), 5956. https://doi.org/10.3390/ijms26135956






