Chemopreventive Effects of Bioactive Peptides Derived from Black Soldier Fly Larvae Protein Hydrolysates in a Rat Model of Early-Stage Colorectal Carcinogenesis
Abstract
1. Introduction
2. Results
2.1. Effect of ASBP, ASBP-AHS, and ASBP-AHF on Physiological Parameters and Liver Function Enzymes
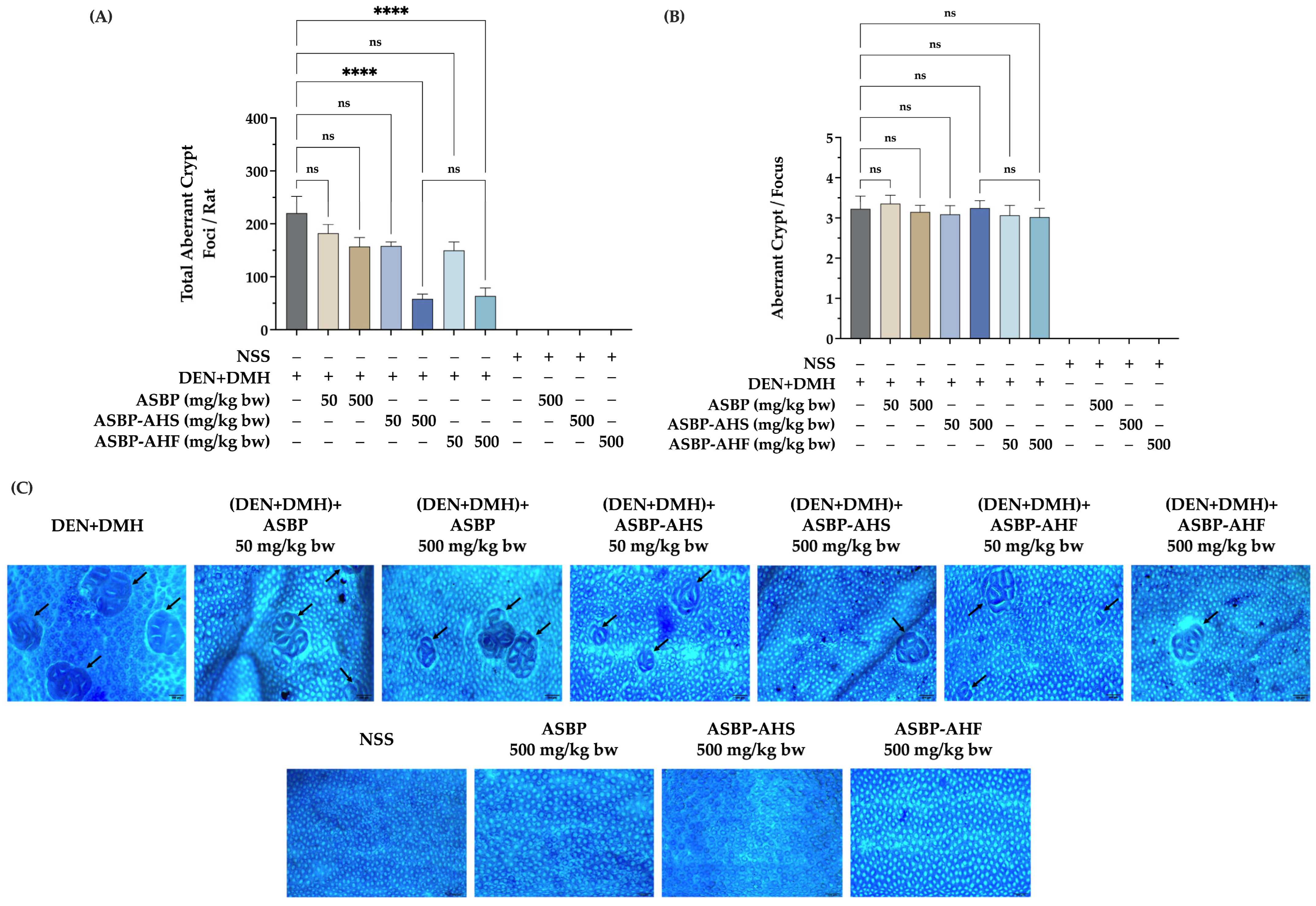
2.2. Effects of ASBP, ASBP-AHS, and ASBP-AHF on Preneoplastic Lesions in the Colon Tissues of DEN and DMH-Treated Rats
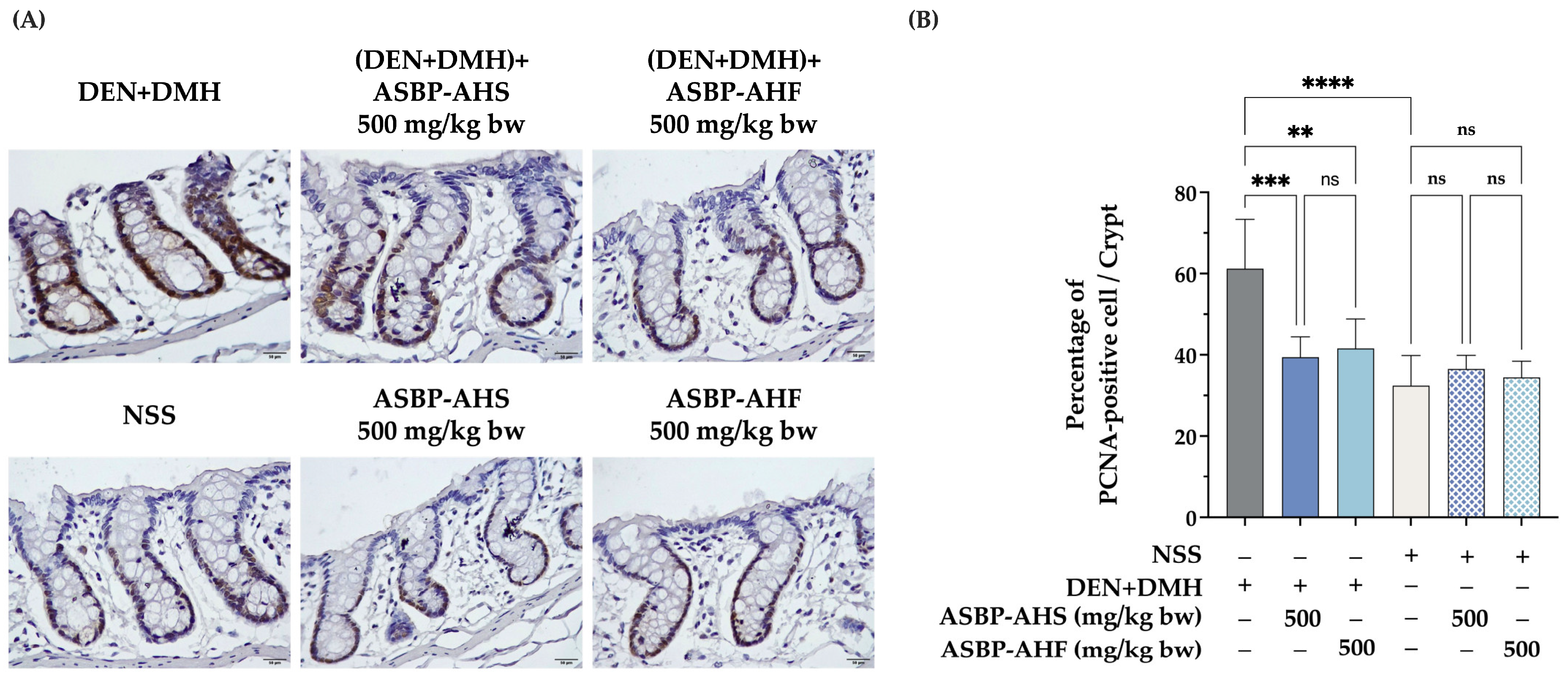
2.3. Reduction in Proliferating Cell Nuclear Antigen-Positive Cells by ASBP-AHS and ASBP-AHF in Early DEN and DMH-Induced Colon Carcinogenesis
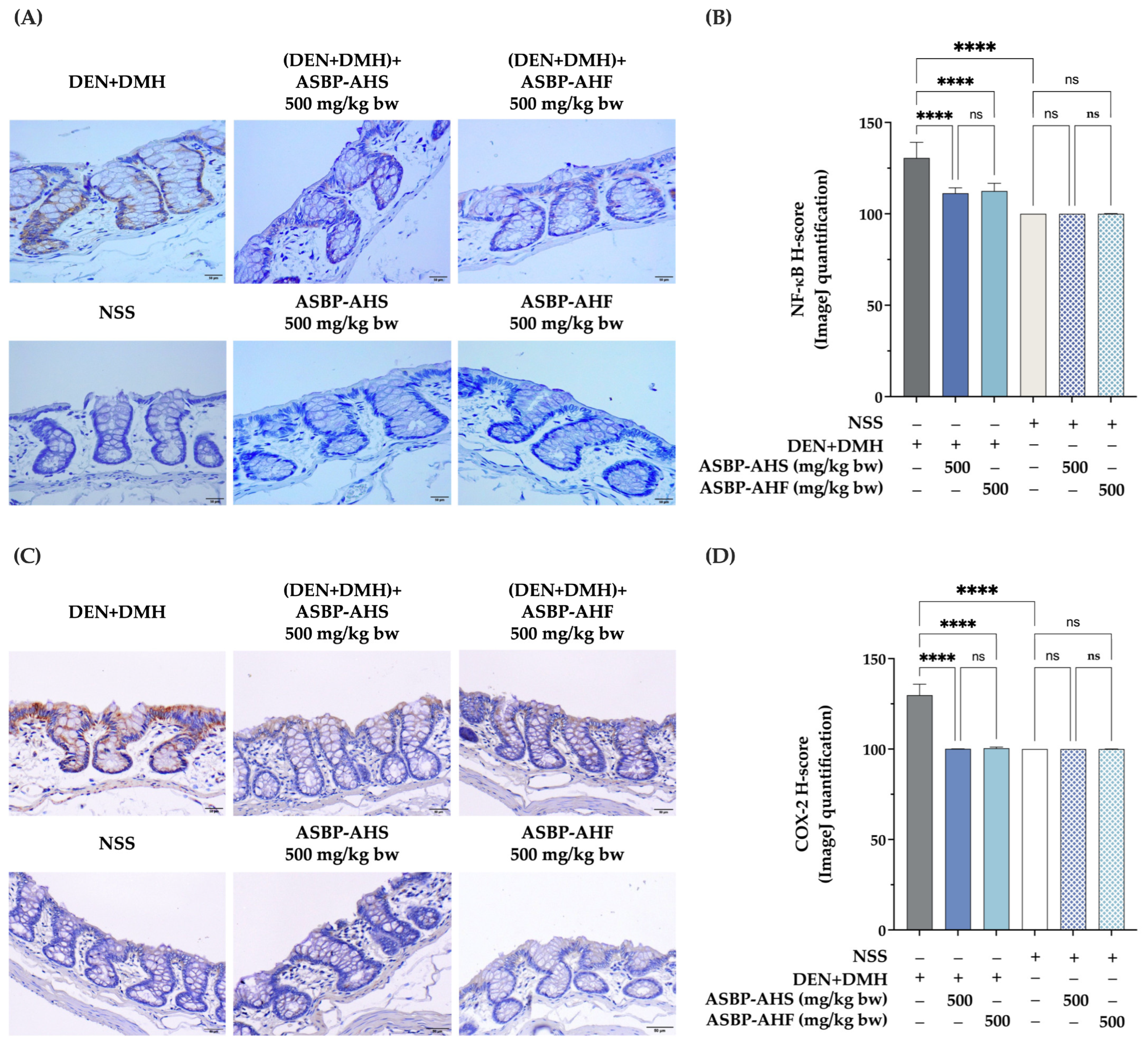
2.4. Suppression of NF-κB and COX-2 Expression by ASBP-AHS and ASBP-AHF in Early DEN and DMH-Induced Colon Carcinogenesis
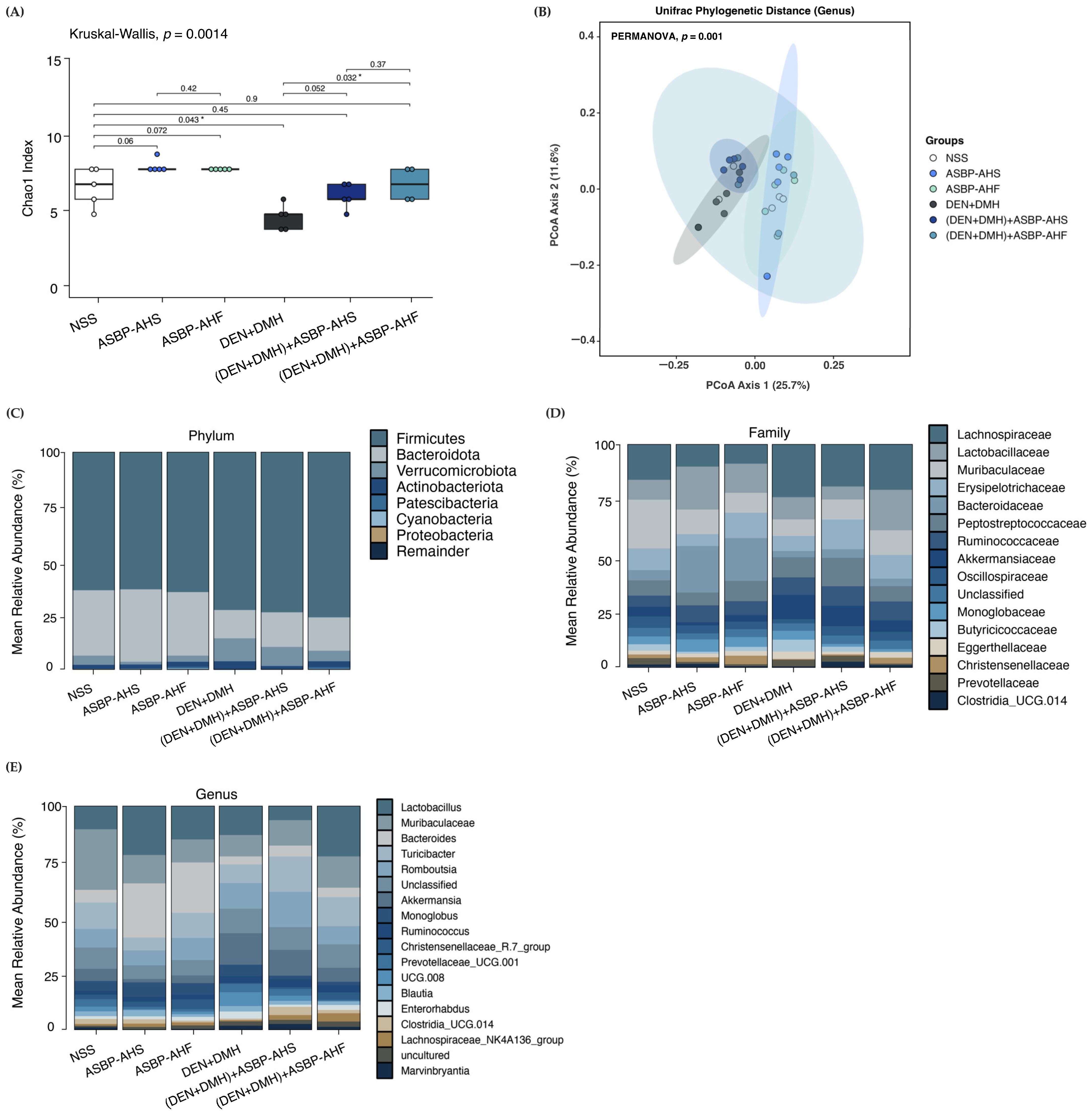
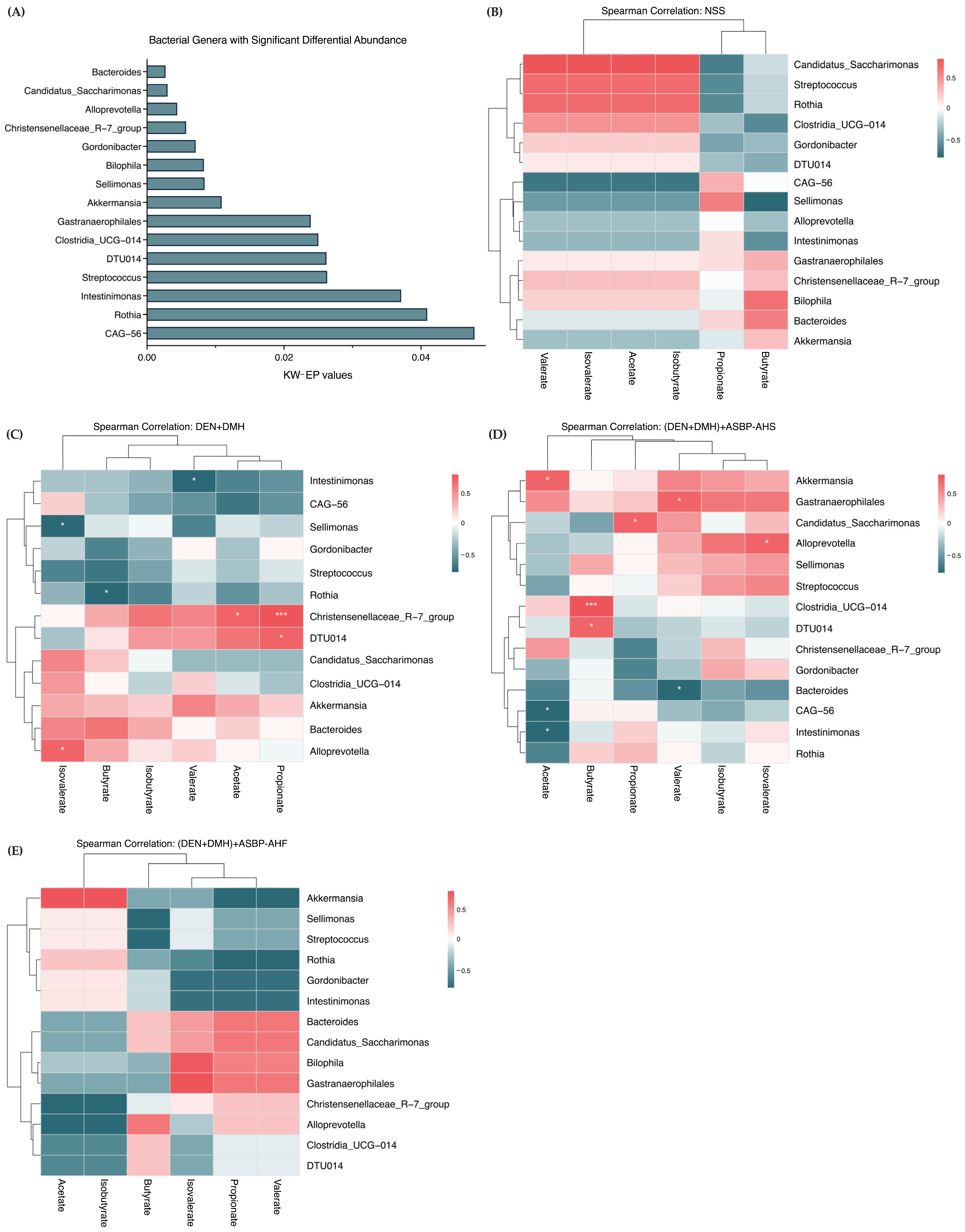
2.5. Modulation of Gut Microbiota by ASBP-AHS and ASBP-AHF in DEN and DMH-Treated Rats
2.6. Effect of ASBP-AHS and ASBP-AHF on SCFA Levels in DEN + DMH-Treated Rats
2.7. Correlation Between Gut Microbial Genera and SCFA Production Following ASBP-AHS and ASBP-AHF Intervention
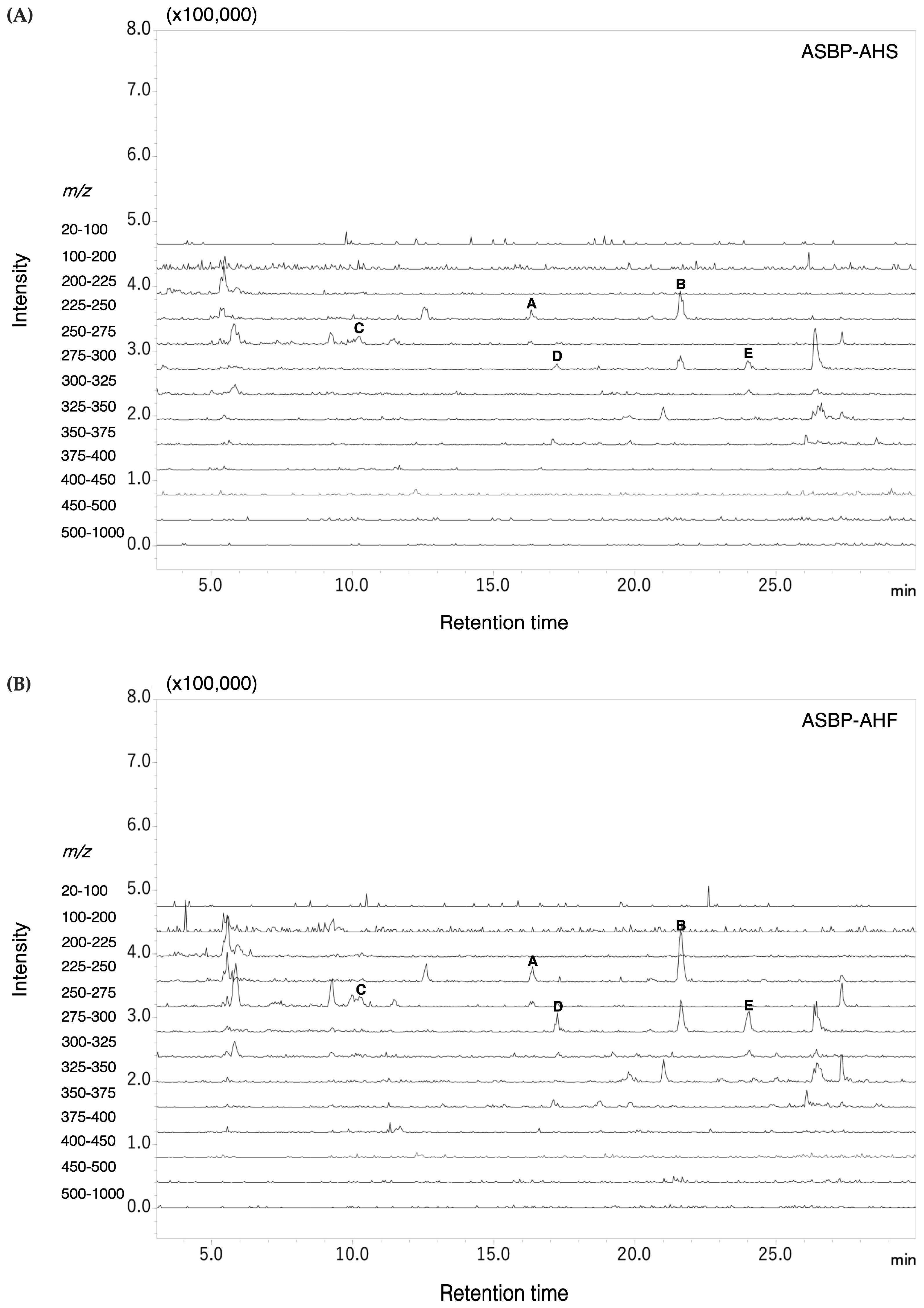
2.8. Pyroglutamyl Peptides Identified in ASBP-AHS and ASBP-AHF
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Samples
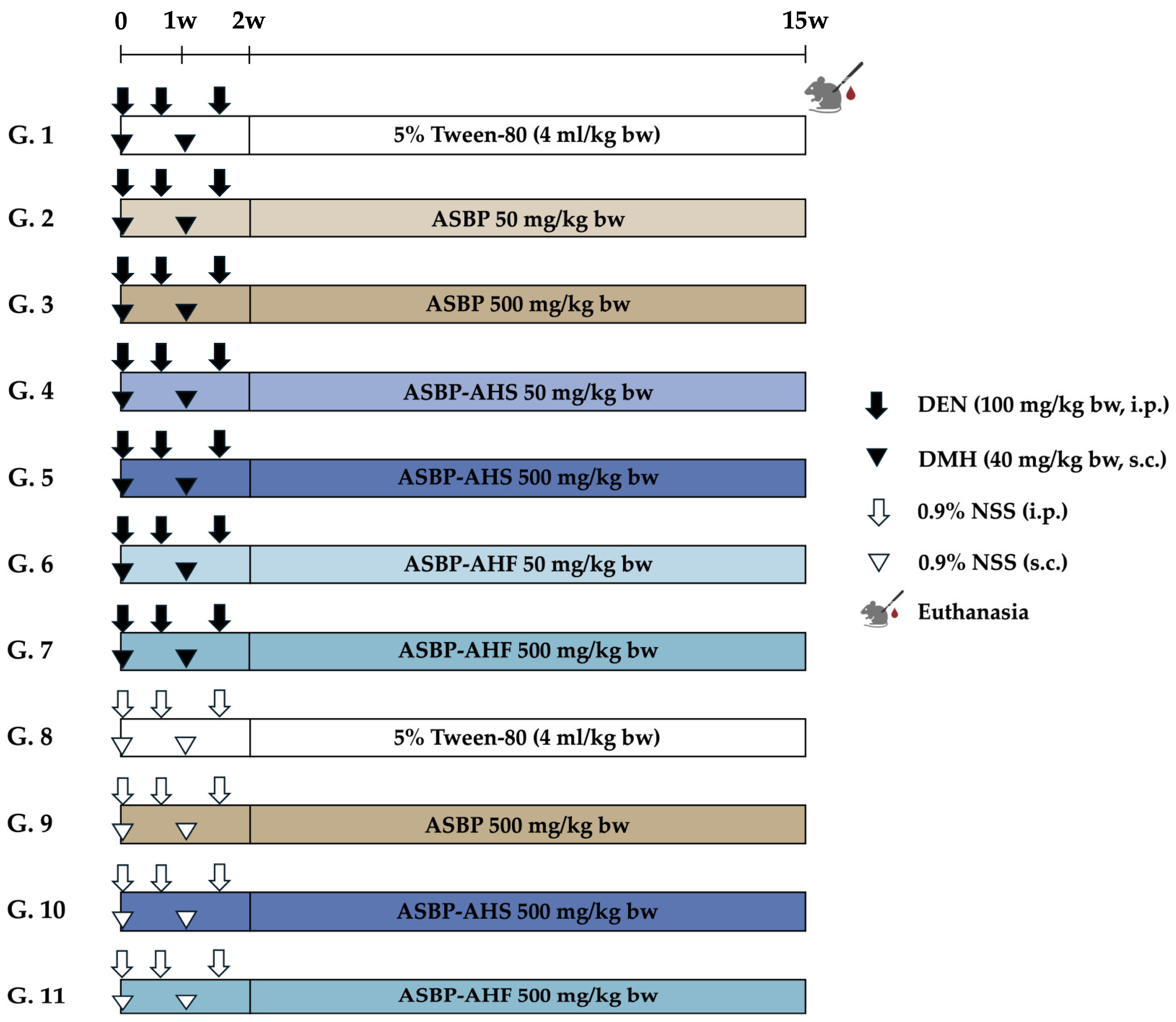
4.3. Animals and Experimental Protocol
4.4. Determination of Preneoplastic Lesions in Colon Tissues
4.5. Effects of ASBP-AHS and ASBP-AHF on Cell Proliferation, Apoptosis, and Pro-Inflammatory
Cytokine Expression in Colon Tissues
4.6. Immunohistochemistry Image Analysis and Histological Score Quantification
4.7. Measurement of SCFAs in Rat Feces
4.8. Analysis of Composition of Fecal Intestinal Microbiota in Rat
4.9. Determination of the Bioactive Peptides Using LC-MS/MS
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Aberrant crypt foci |
| ACN | Acetonitrile |
| ASBP | Alkali-soluble Black Soldier Fly larvae protein |
| ASBP-AH | ASBP–Alcalase hydrolysate |
| ASBP-AHF | ASBP-AH prepared by freeze-drying |
| ASBP-AHS | ASBP-AH prepared by spray-drying |
| COX-2 | Cyclooxygenase-2 |
| DEN | Diethylnitrosamine |
| DMH | 1,2-Dimethylhydrazine |
| IHC | Immunohistochemistry |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| NF-κB | Nuclear factor kappa B |
| PCNA | Proliferating cell nuclear antigen |
| pGlu | Pyroglutamyl |
| pGlu-Glu | Pyroglutamyl-glutamic acid |
| pGlu-Leu | Pyroglutamyl-leucine |
| pGlu-Phe | Pyroglutamyl-phenylalanine |
| pGlu-Tyr | Pyroglutamyl-tyrosine |
| pGlu-Val | Pyroglutamyl-valine |
| SCFA | Short-chain fatty acid |
References
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome in Colorectal Cancer Progression. Front. Immunol. 2021, 12, 807648. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Feliu, J.; Espinosa, E.; Basterretxea, L.; Paredero, I.; Llabrés, E.; Jiménez-Munárriz, B.; Antonio-Rebollo, M.; Losada, B.; Pinto, A.; Custodio, A.B.; et al. Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy. Cancers 2021, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical implications of chemotherapeutic agent organ toxicity on perioperative care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef]
- Bidram, M.; Ganjalikhany, M.R. Bioactive peptides from food science to pharmaceutical industries: Their mechanism of action, potential role in cancer treatment and available resources. Heliyon 2024, 10, e40563. [Google Scholar] [CrossRef]
- Budchart, P.; Khamwut, A.; Sinthuvanich, C.; Ratanapo, S.; Poovorawan, Y.; T-Thienprasert, N.P. Partially Purified Gloriosa superba Peptides Inhibit Colon Cancer Cell Viability by Inducing Apoptosis Through p53 Upregulation. Am. J. Med. Sci. 2017, 354, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Xu, F.; Han, Y.; Hernández-Ledesma, B.; Xiao, H. Inhibitory Effects of Peptide Lunasin in Colorectal Cancer HCT-116 Cells and Their Tumorsphere-Derived Subpopulation. Int. J. Mol. Sci. 2020, 21, 537. [Google Scholar] [CrossRef]
- Shailubhai, K.; Yu, H.H.; Karunanandaa, K.; Wang, J.Y.; Eber, S.L.; Wang, Y.; Joo, N.S.; Kim, H.D.; Miedema, B.W.; Abbas, S.Z.; et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000, 60, 5151–5157. [Google Scholar]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Dhieb, D.; Osman, H.A.; Mekideche, H. The Gut Microbiota and Colorectal Cancer: Understanding the Link and Exploring Therapeutic Interventions. Biology 2025, 14, 251. [Google Scholar] [CrossRef]
- Wada, S.; Sato, K.; Ohta, R.; Wada, E.; Bou, Y.; Fujiwara, M.; Kiyono, T.; Park, E.Y.; Aoi, W.; Takagi, T.; et al. Ingestion of low dose pyroglutamyl leucine improves dextran sulfate sodium-induced colitis and intestinal microbiota in mice. J. Agric. Food Chem. 2013, 61, 8807–8813. [Google Scholar] [CrossRef]
- Fan, X.; Guo, H.; Teng, C.; Yang, X.; Qin, P.; Richel, A.; Zhang, L.; Blecker, C.; Ren, G. Supplementation of quinoa peptides alleviates colorectal cancer and restores gut microbiota in AOM/DSS-treated mice. Food Chem. 2023, 408, 135196. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Tedeschi, T.; Faccini, A.; Pratesi, F.; Folli, C.; Migliorini, P.; Jacobs, J.; Depraetere, S.; Caligiani, A.; Sforza, S. Shotgun proteomics, in-silico evaluation and immunoblotting assays for allergenicity assessment of lesser mealworm, black soldier fly and their protein hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar] [CrossRef]
- Praseatsook, K.; Vachiraarunwong, A.; Taya, S.; Setthaya, P.; Sato, K.; Wanibuchi, H.; Wongpoomchai, R.; Dejkriengkraikul, P.; Gi, M.; Yodkeree, S. Anticancer and Antioxidant Effects of Bioactive Peptides from Black Soldier Fly Larvae (Hermetia illucens). Nutrients 2025, 17, 645. [Google Scholar] [CrossRef] [PubMed]
- Punvittayagul, C.; Chariyakornkul, A.; Chewonarin, T.; Jarukamjorn, K.; Wongpoomchai, R. Augmentation of diethylnitrosamine-induced early stages of rat hepatocarcinogenesis by 1,2-dimethylhydrazine. Drug Chem. Toxicol. 2019, 42, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Adrianto, A.A.; Riwanto, I.; Sadhana, U.; Setyawan, H.; Mahati, E.; Widyarini, S.; Wandita, A.A.A.; Paramita, D.K. Morphological Changes and Inflammation Preceded the Pathogenesis of 1,2-Dimethylhydrazine-Induced Colorectal Cancer. Asian Pac. J. Cancer Prev. 2024, 25, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Vinayagam, R.; Arokia Vijaya Anand, M.; Isa, N.M.; Ponnaiyan, R. Biochemical and molecular aspects of 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis: A review. Toxicol Res. 2020, 9, 2–18. [Google Scholar] [CrossRef]
- Sun, T.; Liu, S.; Zhou, Y.; Yao, Z.; Zhang, D.; Cao, S.; Wei, Z.; Tan, B.; Li, Y.; Lian, Z.; et al. Evolutionary biologic changes of gut microbiota in an ‘adenoma-carcinoma sequence’ mouse colorectal cancer model induced by 1, 2-Dimethylhydrazine. Oncotarget 2017, 8, 444–457. [Google Scholar] [CrossRef]
- Gazme, B.; Boachie, R.; Tsopmo, A.; Udenigwe, C. Occurrence, properties and biological significance of pyroglutamyl peptides derived from different food sources. Food Sci. Hum. Wellness 2019, 8, 268–274. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Cancer Burden Growing Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 17 April 2024).
- Requena, T.; Miguel, M.; Garcés-Rimón, M.; Martínez-Cuesta, M.C.; López-Fandiño, R.; Peláez, C. Pepsin egg white hydrolysate modulates gut microbiota in Zucker obese rats. Food Funct. 2017, 8, 437–443. [Google Scholar] [CrossRef]
- Psarianos, M.; Aghababaei, F.; Schlüter, O.K. Bioactive compounds in edible insects: Aspects of cultivation, processing and nutrition. Food Res. Int. 2025, 203, 115802. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Yu, X.; Li, H.; Ji, L.; Sun, Y.; Jiang, X.; Li, X.; Liu, H. Effects of freeze-drying and spray-drying on the physical and chemical properties of Perinereis aibuhitensis hydrolysates: Sensory characteristics and antioxidant activities. Food Chem. 2022, 382, 132317. [Google Scholar] [CrossRef] [PubMed]
- Taya, S.; Dissook, S.; Ruangsuriya, J.; Yodkeeree, S.; Boonyapranai, K.; Chewonarin, T.; Wongpoomchai, R. Thai Fermented Soybean (Thua-Nao) Prevents Early Stages of Colorectal Carcinogenesis Induced by Diethylnitrosamine and 1,2-Dimethylhydrazine Through Modulations of Cell Proliferation and Gut Microbiota in Rats. Nutrients 2024, 16, 3506. [Google Scholar] [CrossRef]
- Singai, C.; Pitchakarn, P.; Taya, S.; Phannasorn, W.; Wongpoomchai, R.; Wongnoppavich, A. Chemopreventive Potential of Phyllanthus emblica Fruit Extract against Colon and Liver Cancer Using a Dual-Organ Rat Carcinogenesis Model. Pharmaceuticals 2024, 17, 818. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Chantana, W.; Pitchakarn, P.; Subhawa, S.; Chantarasuwan, B.; Temviriyanukul, P.; Chewonarin, T. Ficus dubia latex extract prevent DMH-induced rat early colorectal carcinogenesis through the regulation of xenobiotic metabolism, inflammation, cell proliferation and apoptosis. Sci. Rep. 2022, 12, 15472. [Google Scholar] [CrossRef]
- Negroni, A.; Cucchiara, S.; Stronati, L. Apoptosis, Necrosis, and Necroptosis in the Gut and Intestinal Homeostasis. Mediat. Inflamm. 2015, 2015, 250762. [Google Scholar] [CrossRef]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Molina-Frechero, N.; Mori-Estevez, A.D.; Sánchez-Acuña, G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med. Oral. Patol. Oral. Cir. Bucal 2013, 18, e174-9. [Google Scholar] [CrossRef]
- Noble, P.; Vyas, M.; Al-Attar, A.; Durrant, S.; Scholefield, J.; Durrant, L. High levels of cleaved caspase-3 in colorectal tumour stroma predict good survival. Br. J. Cancer 2013, 108, 2097–2105. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Venkatachalam, K.; Namasivayam, N. Anti-inflammatory and anticancer effects of p-methoxycinnamic acid, an active phenylpropanoid, against 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Mol. Cell. Biochem. 2019, 451, 117–129. [Google Scholar] [CrossRef]
- Guo, C.; Kumar, A.; Liao, C. Pterostilbene Exhibited the Anticancer Effect Against 1, 2-Dimethylhydrazine (DMH)-Induced Colorectal Cancer via Alteration of Oxidative Stress, Inflammation and Gut Microbiota. J. Biochem. Mol. Toxicol. 2025, 39, e70123. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Yan, M.; Zhihua, M.; Zhaoping, L.; Zhenzhen, H.; Chenyi, Z.; Chen, L. Pea Starch-Lauric Acid Complex Alleviates Dextran Sulfate Sodium-Induced Colitis in C57BL/6J Mice. Nutr. Cancer 2023, 75, 1673–1686. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Kropp, C.; Le Corf, K.; Relizani, K.; Tambosco, K.; Martinez, C.; Chain, F.; Rawadi, G.; Langella, P.; Claus, S.P.; Martin, R. The Keystone commensal bacterium Christensenella minuta DSM 22607 displays anti-inflammatory properties both in vitro and in vivo. Sci. Rep. 2021, 11, 11494. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Zhong, Y.; Nyman, M.; Fåk, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef]
- Fan, Z.; Ke, X.; Jiang, L.; Zhang, Z.; Yi, M.; Liu, Z.; Cao, J.; Lu, M.; Wang, M. Genomic and biochemical analysis reveals fermented product of a putative novel Romboutsia species involves the glycometabolism of tilapia. Aquaculture 2024, 581, 740483. [Google Scholar] [CrossRef]
- Sebastià, C.; Folch, J.M.; Ballester, M.; Estellé, J.; Passols, M.; Muñoz, M.; García-Casco, J.M.; Fernández, A.I.; Castelló, A.; Sánchez, A.; et al. Interrelation between gut microbiota, SCFA, and fatty acid composition in pigs. mSystems 2024, 9, e0104923. [Google Scholar] [CrossRef]
- He, Y.; Qin, X.; Liao, C.; Lima, R.L.S.; Hou, Q.; Lei, J.; Lai, Y.; Jiang, Q.; Wang, B.; Zhang, B. Genistein alleviates colitis by suppressing inflammation and modulating colonic Marvinbryantia formatexigens abundance and metabolites. Curr. Res. Food Sci. 2025, 10, 101016. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Pourshafie, M.R.; Rohani, M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun. Inflamm. Dis. 2022, 10, e635. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, Z. Causal associations among gut microbiota, 1400 plasma metabolites, and asthma: A two-sample Mendelian randomization study. Front. Mol. Biosci. 2024, 11, 1370919. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-R.; Chou, T.-S.; Huang, C.-Y.; Hsiao, J.-K. A potential probiotic—Lachnospiraceae NK4A136 group: Evidence from the restoration of the dietary pattern from a high-fat diet. Med. Environ. Sci. Biol. 2020. preprint. [Google Scholar]
- Yin, Y.; Cai, J.; Zhou, L.; Xing, L.; Zhang, W. Dietary oxidized beef protein alters gut microbiota and induces colonic inflammatory damage in C57BL/6 mice. Front. Nutr. 2022, 9, 980204. [Google Scholar] [CrossRef]
- Ohara, T.; Mori, T. Antiproliferative Effects of Short-chain Fatty Acids on Human Colorectal Cancer Cells via Gene Expression Inhibition. Anticancer Res. 2019, 39, 4659–4666. [Google Scholar] [CrossRef]
- Leonov, G.E.; Varaeva, Y.R.; Livantsova, E.N.; Starodubova, A.V. The Complicated Relationship of Short-Chain Fatty Acids and Oral Microbiome: A Narrative Review. Biomedicines 2023, 11, 2749. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid–Producing Microbiota in the Gut. Diabetes Care 2016, 40, 54–62. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; Farooq, U.; Liu, B.; Wang, Z.; Sun, H.; Cui, Y.; Li, D.; Shi, Y. Chronological dynamics of the gut microbiome in response to the pasture grazing system in geese. Microbiol. Spectr. 2024, 12, e04188-23. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kajiwara, S.; Sato, K. Metabolic fate of peptides in a rice protein hydrolysate in rat intestine and blood after oral administration. J. Food Bioact. 2022, 20, 40–55. [Google Scholar] [CrossRef]
- Hirai, S.; Horii, S.; Matsuzaki, Y.; Ono, S.; Shimmura, Y.; Sato, K.; Egashira, Y. Anti-inflammatory effect of pyroglutamyl-leucine on lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2014, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, T.; Wada, S.; Ohta, R.; Wada, E.; Takagi, T.; Naito, Y.; Yoshikawa, T. Identification of pyroglutamyl peptides with anti-colitic activity in Japanese rice wine, sake, by oral administration in a mouse model. J. Funct. Foods 2016, 27, 612–621. [Google Scholar] [CrossRef]
- Shirako, S.; Kojima, Y.; Tomari, N.; Nakamura, Y.; Matsumura, Y.; Ikeda, K.; Inagaki, N.; Sato, K. Pyroglutamyl leucine, a peptide in fermented foods, attenuates dysbiosis by increasing host antimicrobial peptide. NPJ Sci. Food 2019, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Berraquero-García, C.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M.; García-Moreno, P.J. Encapsulation of Bioactive Peptides by Spray-Drying and Electrospraying. Foods 2023, 12, 2005. [Google Scholar] [CrossRef]
- Hu, M.; Du, Y.; Li, W.; Zong, X.; Du, W.; Sun, H.; Liu, H.; Zhao, K.; Li, J.; Farooq, M.Z.; et al. Interplay of Food-Derived Bioactive Peptides with Gut Microbiota: Implications for Health and Disease Management. Mol. Nutr. Food Res. 2024, 68, e2400251. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, S.; Kishimoto, Y.; Takahashi, K.; Kaminogawa, S.; Hosono, A. Continuous intake of resistant maltodextrin enhanced intestinal immune response through changes in the intestinal environment in mice. Biosci. Microbiota Food Health 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Fuhrich, D.G.; Lessey, B.A.; Savaris, R.F. Comparison of HSCORE assessment of endometrial beta3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal. Quant. Cytopathol. Histpathol. 2013, 35, 210–216. [Google Scholar]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Cizkova, K.; Foltynkova, T.; Gachechiladze, M.; Tauber, Z. Comparative Analysis of Immunohistochemical Staining Intensity Determined by Light Microscopy, ImageJ and QuPath in Placental Hofbauer Cells. ACTA Histochem. ET Cytochem. 2021, 54, 21–29. [Google Scholar] [CrossRef]
- Wen, Z.; Luo, D.; Wang, S.; Rong, R.; Evers, B.M.; Jia, L.; Fang, Y.; Daoud, E.V.; Yang, S.; Gu, Z.; et al. Deep Learning–Based H-Score Quantification of Immunohistochemistry-Stained Images. Mod. Pathol. 2024, 37, 100398. [Google Scholar] [CrossRef] [PubMed]
- Phannasorn, W.; Pharapirom, A.; Thiennimitr, P.; Guo, H.; Ketnawa, S.; Wongpoomchai, R. Enriched Riceberry Bran Oil Exerts Chemopreventive Properties through Anti-Inflammation and Alteration of Gut Microbiota in Carcinogen-Induced Liver and Colon Carcinogenesis in Rats. Cancers 2022, 14, 4358. [Google Scholar] [CrossRef] [PubMed]
- Wijanarti, S.; Gu, R.; Chen, L.; Liu, W.; Cai, M.; Suzuki, R.; Sato, K. Substrate specificity of exopeptidases in small intestinal mucosa determines the structure of food-derived collagen peptides in rat lumen and blood. J. Food Bioact. 2024, 26, 29–41. [Google Scholar] [CrossRef]
- Pharapirom, A.; Setthaya, P.; Vachiraarunwong, A.; Jongjareonrak, A.; Sato, K.; Wongpoomchai, R.; Ruangsuriya, J. Identification of novel cancer chemopreventive peptides from bran residue of Riceberry cultivated in Northern Thailand. J. Funct. Foods 2024, 120, 106391. [Google Scholar] [CrossRef]
| Groups | Treatments | Body Weight (g) | Consumption (g/Day) | ||
|---|---|---|---|---|---|
| Initial | Final | Food | Water | ||
| 1 | DEN + DMH | 123.33 ± 12.11 | 482.50 ± 33.87 | 20.71 ± 2.43 | 25.63 ± 3.67 |
| 2 | (DEN + DMH) + ASBP 50 mg/kg bw | 120.63 ± 7.29 | 469.38 ± 24.99 | 19.13 ± 2.20 | 25.25 ± 3.56 |
| 3 | (DEN + DMH) + ASBP 500 mg/kg bw | 124.38 ± 5.63 | 458.75 ± 29.73 | 19.17 ± 2.17 | 26.08 ± 3.53 |
| 4 | (DEN + DMH) + ASBP-AHS 50 mg/kg bw | 121.25 ± 6.94 | 461.88 ± 37.60 | 19.38 ± 2.28 | 25.28 ± 2.74 |
| 5 | (DEN + DMH) + ASBP-AHS 500 mg/kg bw | 117.50 ± 8.86 | 466.88 ± 46.13 | 19.54 ± 2.16 | 25.27 ± 3.31 |
| 6 | (DEN + DMH) + ASBP-AHF 50 mg/kg bw | 118.00 ± 7.58 | 447.00 ± 40.87 | 19.56 ± 2.61 | 25.16 ± 3.28 |
| 7 | (DEN + DMH) + ASBP-AHF 500 mg/kg bw | 125.83 ± 7.36 | 475.83 ± 41.76 | 20.00 ± 2.75 | 25.25 ± 3.48 |
| 8 | NSS | 127.86 ± 8.59 | 500.00 ± 32.79 | 19.92 ± 2.03 | 26.42 ± 5.02 |
| 9 | ASBP 500 mg/kg bw | 127.00 ± 11.51 | 452.00 ± 44.0 | 18.86 ± 2.07 | 25.16 ± 2.28 |
| 10 | ASBP-AHS 500 mg/kg bw | 133.00 ± 8.37 | 483.00 ± 34.2 | 19.42 ± 2.39 | 25.76 ± 3.75 |
| 11 | ASBP-AHF 500 mg/kg bw | 134.00 ± 6.52 | 487.00 ± 30.1 | 19.56 ± 3.50 | 25.97 ± 6.19 |
| Groups | Treatments | Liver | Kidney | Spleen | |||
|---|---|---|---|---|---|---|---|
| Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | Absolute (g) | Relative (%) | ||
| 1 | DEN + DMH | 13.98 ± 1.42 | 2.89 ± 0.16 | 2.95 ± 0.29 | 0.61 ± 0.06 | 0.85 ± 0.07 | 0.18 ± 0.02 |
| 2 | (DEN + DMH) + ASBP 50 mg/kg bw | 13.11 ± 0.54 | 2.80 ± 0.20 | 2.71 ± 0.26 | 0.58 ± 0.05 | 0.83 ± 0.13 | 0.18 ± 0.03 |
| 3 | (DEN + DMH) + ASBP 500 mg/kg bw | 12.88 ± 0.56 | 2.82 ± 0.17 | 2.75 ± 0.19 | 0.60 ± 0.03 | 0.84 ± 0.07 | 0.18 ± 0.02 |
| 4 | (DEN + DMH) + ASBP-AHS 50 mg/kg bw | 13.18 ± 1.24 | 2.86 ± 0.22 | 2.79 ± 0.28 | 0.60 ± 0.06 | 0.85 ± 0.09 | 0.19 ± 0.02 |
| 5 | (DEN + DMH) + ASBP-AHS 500 mg/kg bw | 12.83 ± 1.48 | 2.75 ± 0.13 | 2.73 ± 0.30 | 0.59 ± 0.04 | 0.84 ± 0.17 | 0.18 ± 0.04 |
| 6 | (DEN + DMH) + ASBP-AHF 50 mg/kg bw | 12.88 ± 2.06 | 2.87 ± 0.28 | 2.67 ± 0.18 | 0.60 ± 0.05 | 0.74 ± 0.08 | 0.17 ± 0.02 |
| 7 | (DEN + DMH) + ASBP-AHF 500 mg/kg bw | 12.70 ± 1.05 | 2.67 ± 0.07 | 2.85 ± 0.41 | 0.60 ± 0.05 | 0.75 ± 0.10 | 0.16 ± 0.01 |
| 8 | NSS | 12.72 ± 1.06 | 2.55 ± 0.24 | 2.85 ± 0.48 | 0.57 ± 0.07 | 0.74 ± 0.09 | 0.15 ± 0.01 |
| 9 | ASBP 500 mg/kg bw | 12.88 ± 0.56 | 2.82 ± 0.17 | 2.75 ± 0.19 | 0.60 ± 0.03 | 0.84 ± 0.07 | 0.18 ± 0.02 |
| 10 | ASBP-AHS 500 mg/kg bw | 12.36 ± 0.98 | 2.56 ± 0.07 | 2.71 ± 0.18 | 0.56 ± 0.03 | 0.69 ± 0.05 | 0.14 ± 0.01 |
| 11 | ASBP-AHF 500 mg/kg bw | 12.41 ± 1.31 | 2.54 ± 0.13 | 2.54 ± 0.14 | 0.52 ± 0.02 | 0.71 ± 0.09 | 0.15 ± 0.03 |
| Groups | Treatments | AST (U/L) | ALT (U/L) |
|---|---|---|---|
| 1 | DEN + DMH | 151.17 ± 41.52 | 66.83 ± 18.91 ** |
| 2 | (DEN + DMH) + ASBP 50 mg/kg bw | 133.13 ± 36.73 | 44.75 ± 9.02 |
| 3 | (DEN + DMH) + ASBP 500 mg/kg bw | 134.75 ± 39.12 | 58.25 ± 13.36 * |
| 4 | (DEN + DMH) + ASBP-AHS 50 mg/kg bw | 146.75 ± 35.75 | 68.50 ± 24.05 *** |
| 5 | (DEN + DMH) + ASBP-AHS 500 mg/kg bw | 132.75 ± 29.95 | 59.75 ± 14.61 * |
| 6 | (DEN + DMH) + ASBP-AHF 50 mg/kg bw | 158.40 ± 46.15 * | 76.00 ± 27.16 *** |
| 7 | (DEN + DMH) + ASBP-AHF 500 mg/kg bw | 114.67 ± 44.32 | 47.67 ± 18.96 |
| 8 | NSS | 89.57 ± 23.39 | 29.86 ± 3.48 |
| 9 | ASBP 500 mg/kg bw | 78.40 ± 6.11 | 32.40 ± 1.52 |
| 10 | ASBP-AHS 500 mg/kg bw | 79.20 ± 3.63 | 27.00 ± 2.12 |
| 11 | ASBP-AHF 500 mg/kg bw | 73.60 ± 4.56 | 31.60 ± 3.21 |
| SCFA (µmol/g Feces) | Treatments | |||
|---|---|---|---|---|
| NSS | DEN + DMH | (DEN + DMH) + ASBP-AHS 500 mg/kg bw | (DEN + DMH) + ASBP-AHF 500 mg/kg bw | |
| Acetate | 40.42 ± 15.98 a | 10.11 ± 11.32 b | 39.12 ± 3.34 a | 18.71 ± 7.34 b |
| Propionate | 63.38 ± 3.41 a | 8.04 ± 5.35 c | 39.31 ± 5.91 b | 6.15 ± 4.34 c |
| Butyrate | 16.19 ± 0.07 a | 4.00 ± 0.26 b | 5.70 ± 2.46 b | 5.44 ± 1.72 b |
| Isobutyrate | 37.04 ± 20.06 a | 6.15 ± 7.43 b | 13.67 ± 0.45 ab | 17.12 ± 7.90 ab |
| Isovalerate | 4.91 ± 3.53 a | 1.28 ± 0.11 ab | 2.70 ± 1.23 ab | 0.98 ± 0.43 b |
| Valerate | 17.53 ± 5.65 a | 2.61 ± 0.88 b | 10.77 ± 7.07 ab | 3.01 ± 2.83 b |
| Position | Pyroglutamyl Peptides | Precursor Ion (m/z) | Retention Time (min) | Precursor Scan (m/z) |
|---|---|---|---|---|
| A | Pyroglutamyl-valine (pGlu-Val) | 229 | 16.3 | 72 (* Val), 84 (* pGlu), 118 (y1), 183 (a2), 229 |
| B | Pyroglutamyl-leucine (pGlu-Leu) | 243 | 22 | 84 (* pGlu), 86 (* Leu), 132 (y1), 197 (a2), 243 |
| C | Pyroglutamyl-glutamic acid (pGlu-Glu) | 259 | 10 | 84 (* pGlu), 102 (* Glu), 148 (y1), 241 (b1), 259 |
| D | Pyroglutamyl-tyrosine (pGlu-Tyr) | 293 | 17 | 84 (* pGlu), 136 (* Tyr), 182 (y1), 292 (y2 + ACN) |
| E | Pyroglutamyl-phenylalanine (pGlu-Phe) | 277 | 24 | 84 (* pGlu), 120 (* Phe), 166 (y1), 231 (a2), 276 (y2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praseatsook, K.; Vachiraarunwong, A.; Sato, K.; Dissook, S.; Wanibuchi, H.; Taya, S.; Wongpoomchai, R.; Dejkriengkraikul, P.; Gi, M.; Yodkeeree, S. Chemopreventive Effects of Bioactive Peptides Derived from Black Soldier Fly Larvae Protein Hydrolysates in a Rat Model of Early-Stage Colorectal Carcinogenesis. Int. J. Mol. Sci. 2025, 26, 5955. https://doi.org/10.3390/ijms26135955
Praseatsook K, Vachiraarunwong A, Sato K, Dissook S, Wanibuchi H, Taya S, Wongpoomchai R, Dejkriengkraikul P, Gi M, Yodkeeree S. Chemopreventive Effects of Bioactive Peptides Derived from Black Soldier Fly Larvae Protein Hydrolysates in a Rat Model of Early-Stage Colorectal Carcinogenesis. International Journal of Molecular Sciences. 2025; 26(13):5955. https://doi.org/10.3390/ijms26135955
Chicago/Turabian StylePraseatsook, Kwanchanok, Arpamas Vachiraarunwong, Kenji Sato, Sivamoke Dissook, Hideki Wanibuchi, Sirinya Taya, Rawiwan Wongpoomchai, Pornngarm Dejkriengkraikul, Min Gi, and Supachai Yodkeeree. 2025. "Chemopreventive Effects of Bioactive Peptides Derived from Black Soldier Fly Larvae Protein Hydrolysates in a Rat Model of Early-Stage Colorectal Carcinogenesis" International Journal of Molecular Sciences 26, no. 13: 5955. https://doi.org/10.3390/ijms26135955
APA StylePraseatsook, K., Vachiraarunwong, A., Sato, K., Dissook, S., Wanibuchi, H., Taya, S., Wongpoomchai, R., Dejkriengkraikul, P., Gi, M., & Yodkeeree, S. (2025). Chemopreventive Effects of Bioactive Peptides Derived from Black Soldier Fly Larvae Protein Hydrolysates in a Rat Model of Early-Stage Colorectal Carcinogenesis. International Journal of Molecular Sciences, 26(13), 5955. https://doi.org/10.3390/ijms26135955








