The Effect of Parental Faecal Microbiome Transplantation from Children with Autism Spectrum Disorder on Behavior and Gastrointestinal Manifestations in the Male Offspring of Shank3 Mice
Abstract
1. Introduction
2. Results
2.1. Behavior
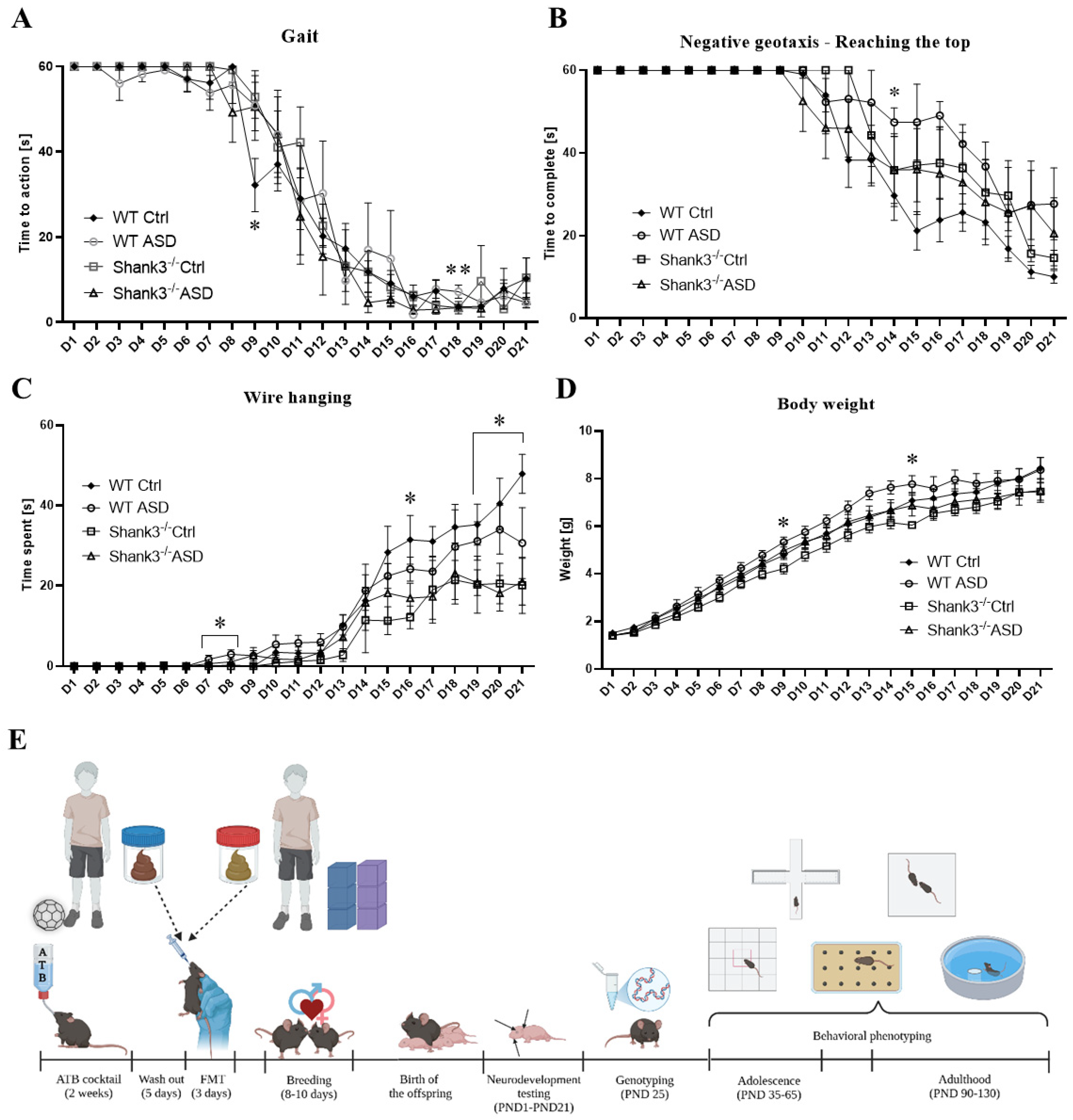
2.1.1. Neurodevelopmental Milestones
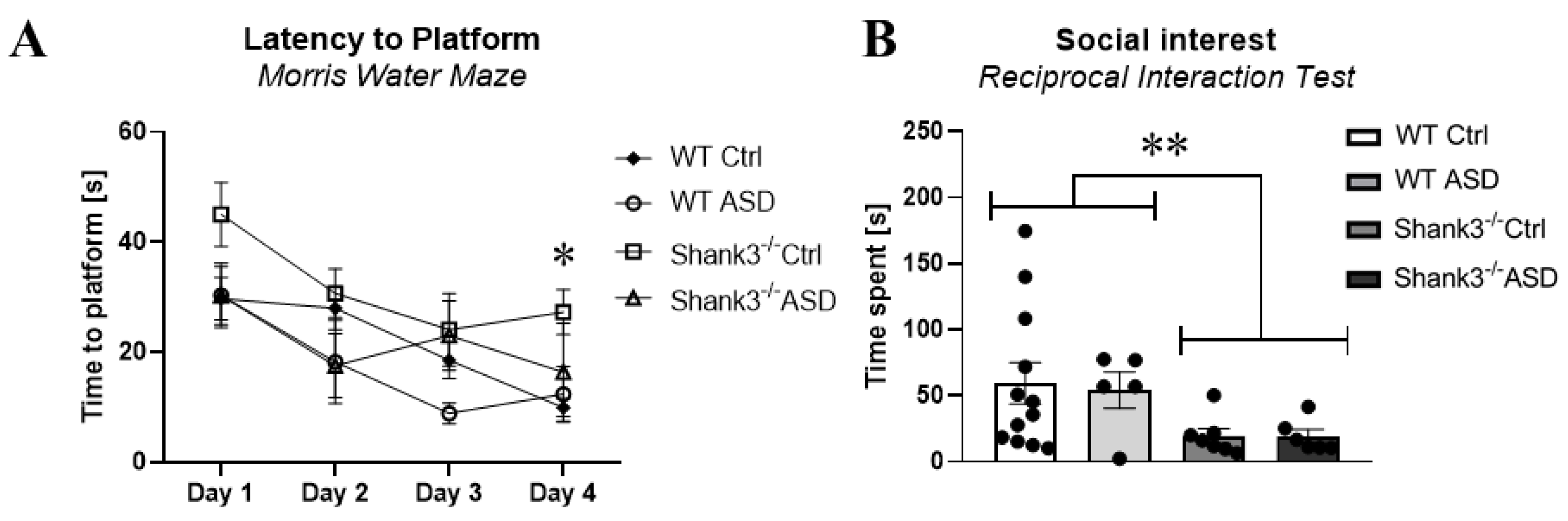
2.1.2. Behavioral Testing in Adolescence
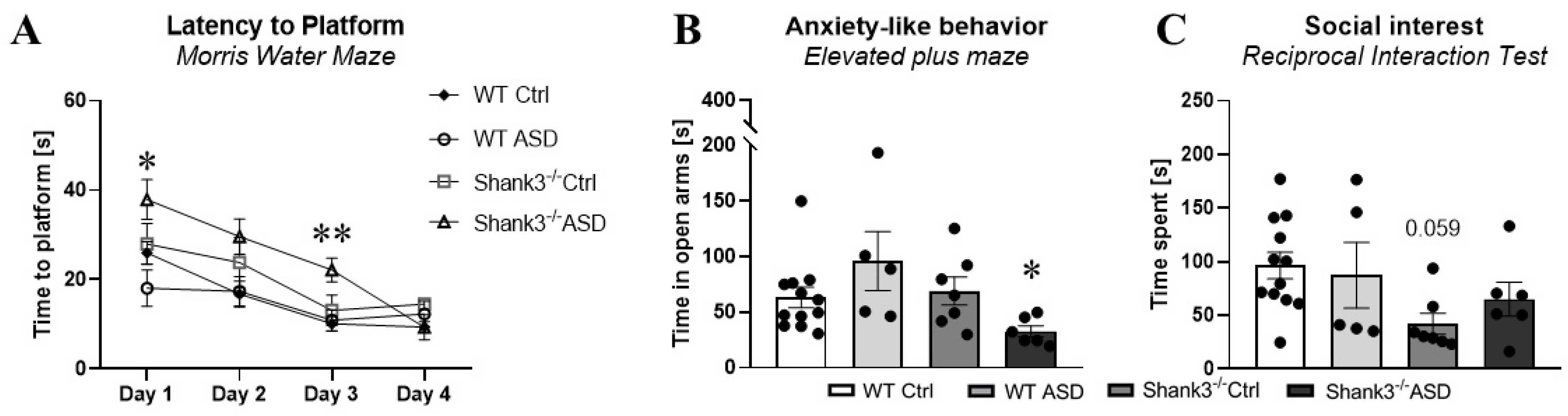
2.1.3. Behavioral Testing in Adulthood
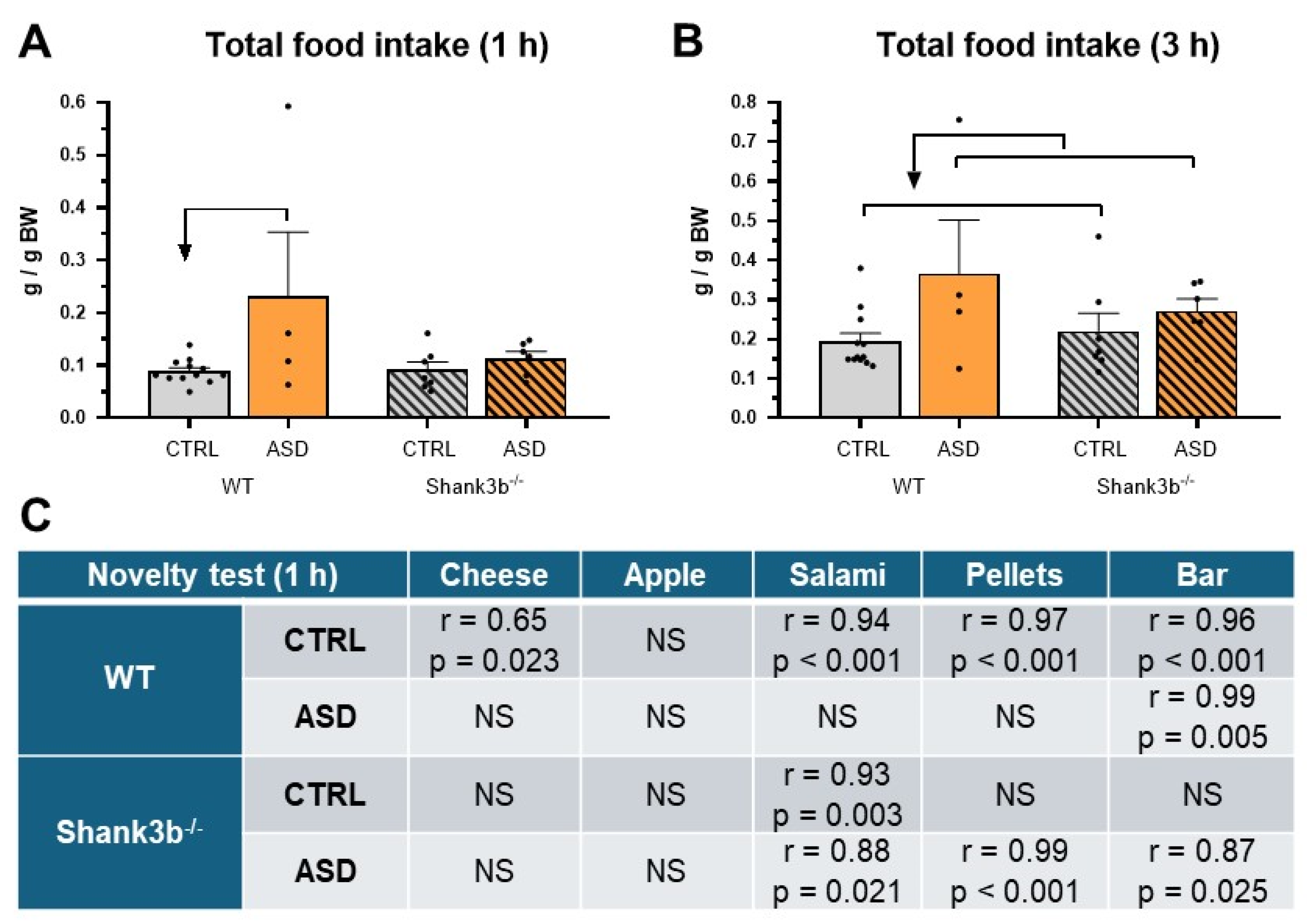
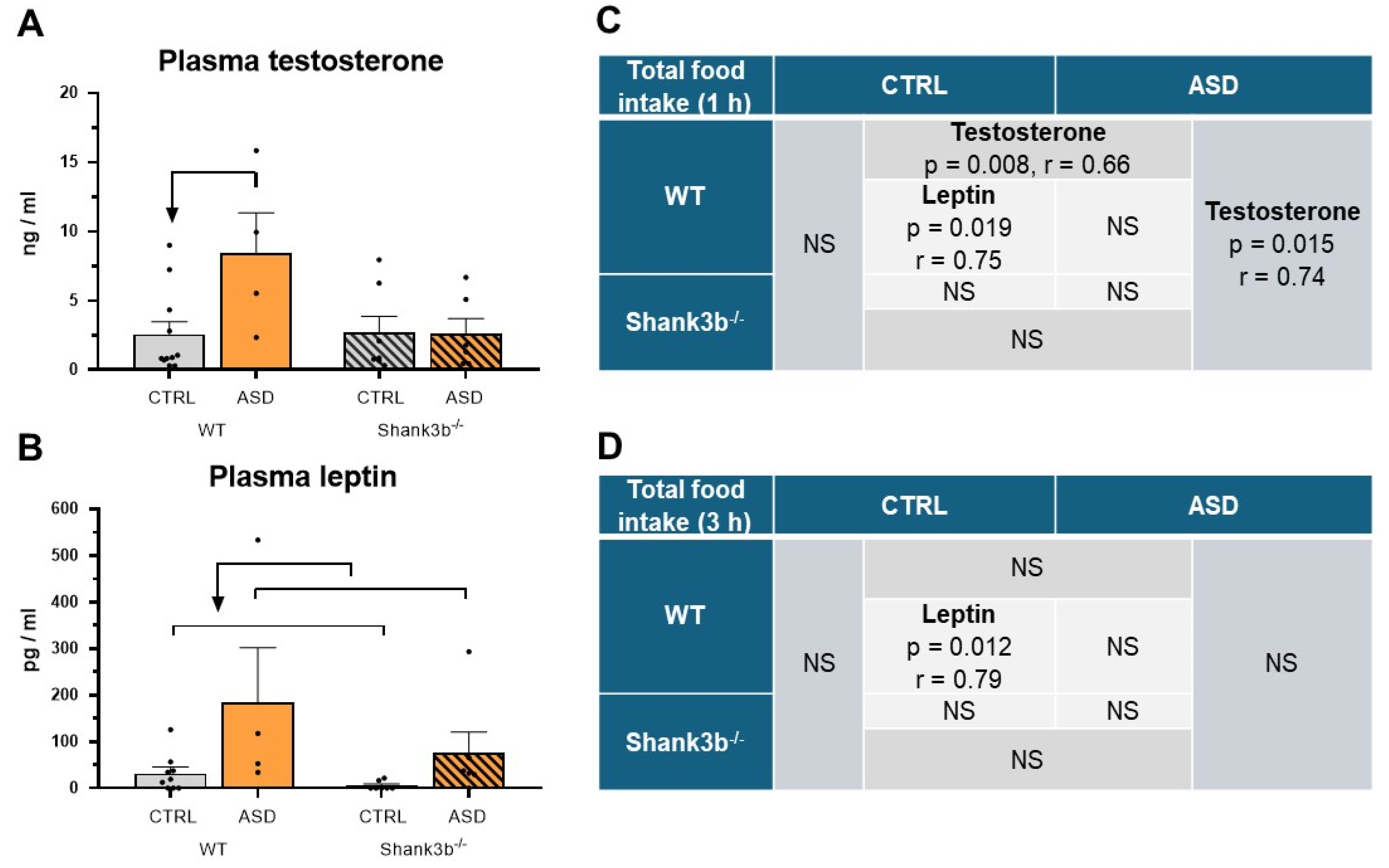
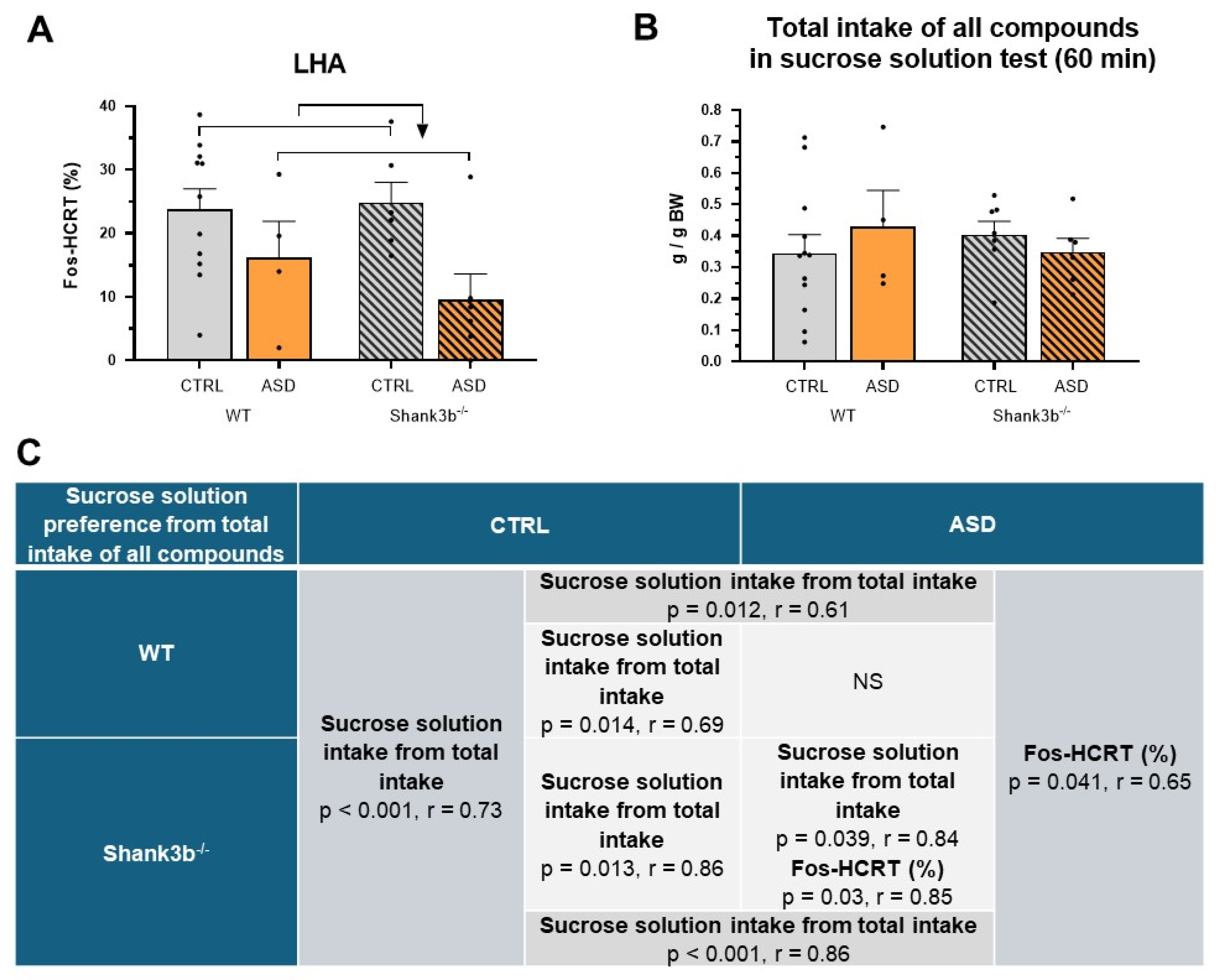
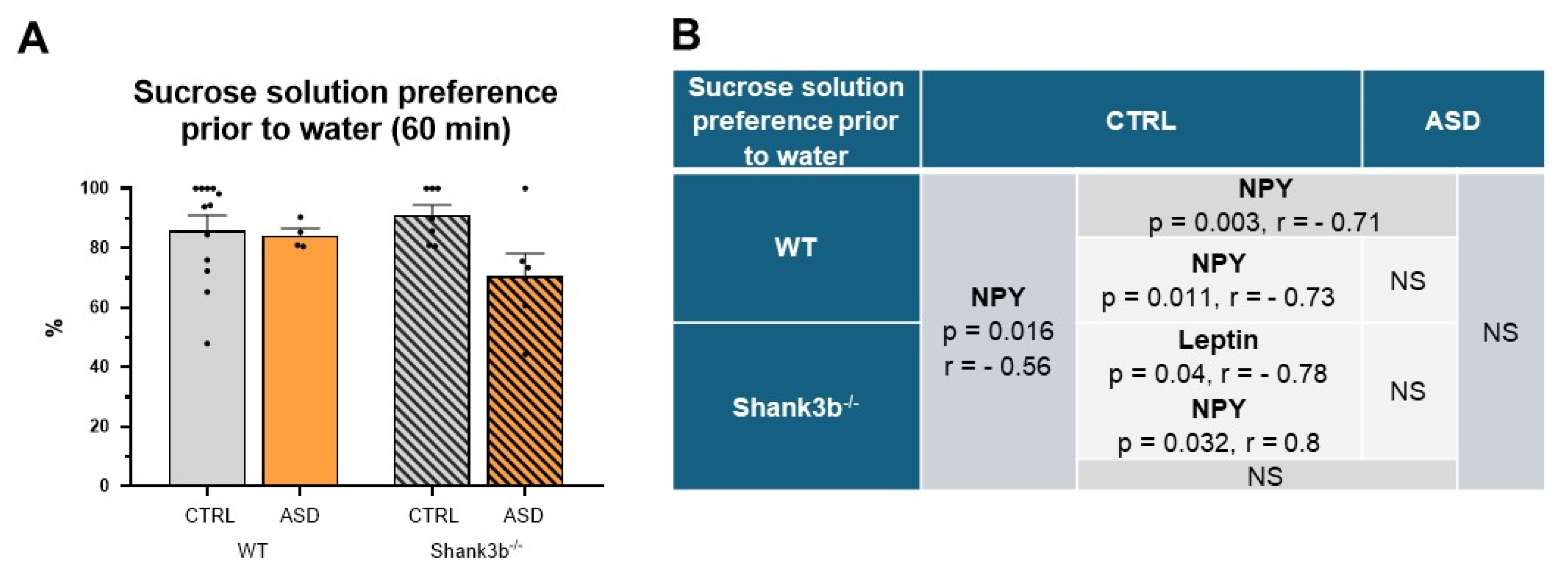
2.2. Food Intake and Preference Studies
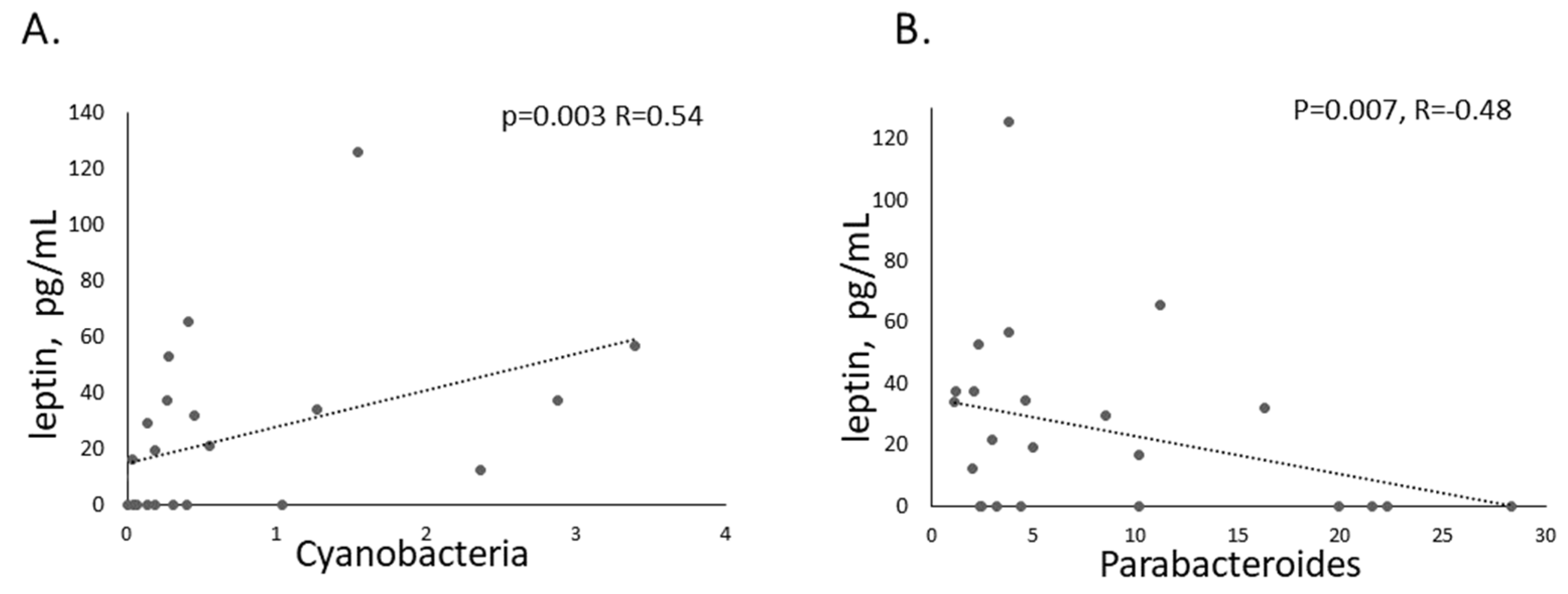
2.3. Microbiota
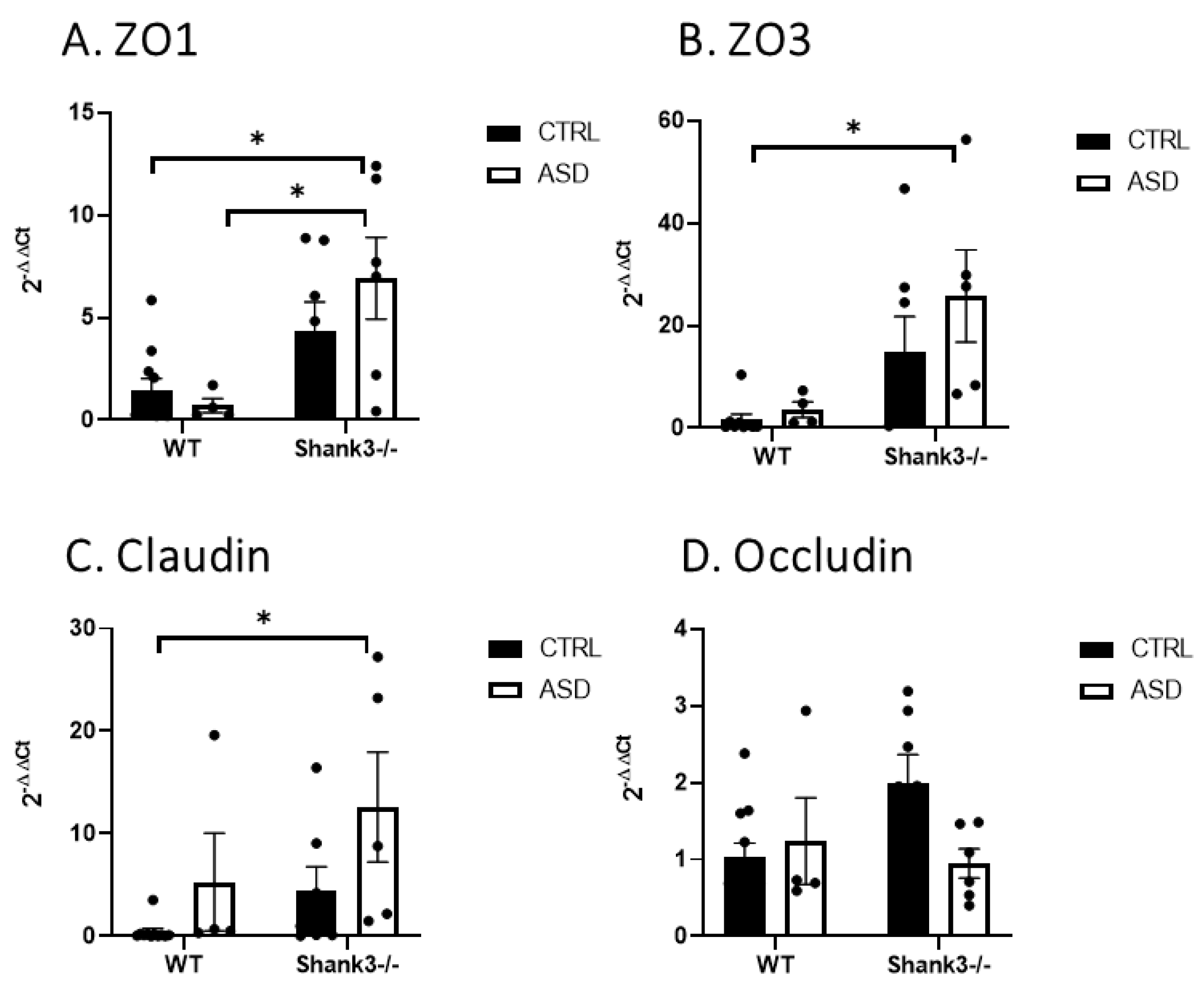
2.4. Permeability of the Gut
3. Discussion
4. Materials and Methods
4.1. Selection of Donors
4.2. Experimental Animals
4.3. Fecal Microbiota Transplant Preparation
4.4. Assessment of Behavior in Mice
4.4.1. Neurodevelopment
4.4.2. Behavioral Phenotyping
4.5. Food Intake and Preference
4.5.1. Voluntary Food Intake and Preference in 24 H Lasting Sweet Solution and Sweet–Fat Solution Test
4.5.2. Food Intake and Preference Induced by Overnight Fasting During the Novelty Test and 60 Min Lasting Sweet Solution Test
4.6. Blood Analysis
4.7. Fos and Fos-HCRT Immunohistochemistry
4.8. RT-PCR
4.9. Microbiota Analysis
4.10. Statistical Analysis
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Donovan, S.M. Microbiome and nutrition in autism spectrum disorder: Current knowledge and research needs. Nutr. Rev. 2016, 74, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Cheroni, C.; Caporale, N.; Testa, G. Autism spectrum disorder at the crossroad between genes and environment: Contributions, convergences, and interactions in ASD developmental pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef]
- Tomova, A.; Pivovarciova, A.; Babinska, K.; Mravec, B. Intestinal microbiota and the brain: Multilevel interactions in health and disease. Ceskoslov. Fysiol. 2015, 64, 23–34. [Google Scholar]
- Martin, C.R.; Mayer, E.A. Gut-Brain Axis and Behavior. Nestle Nutr. Inst. Workshop Ser. 2017, 88, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota-brain axis in neurological disorder. Front. Neurosci. 2023, 17, 1225875. [Google Scholar] [CrossRef]
- Soto, M.; Herzog, C.; Pacheco, J.A.; Fujisaka, S.; Bullock, K.; Clish, C.B.; Kahn, C.R. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol. Psychiatry 2018, 23, 2287–2301. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Coury, D.L.; Ashwood, P.; Fasano, A.; Fuchs, G.; Geraghty, M.; Kaul, A.; Mawe, G.; Patterson, P.; Jones, N.E. Gastrointestinal conditions in children with autism spectrum disorder: Developing a research agenda. Pediatrics 2012, 130 (Suppl. S2), S160–S168. [Google Scholar] [CrossRef] [PubMed]
- Babinská, K.; Slobodníková, L.; Jánošíková, D.; Lakatošová, S.; Bakoš, J.; Ostatníková, D. The effects of probiotic administration on gastrointestinal functions in children with autism. Act. Nerv. Super. Rediviva 2012, 54, 82. [Google Scholar]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabro, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Finegold, S.M. Desulfovibrio species are potentially important in regressive autism. Med. Hypotheses 2011, 77, 270–274. [Google Scholar] [CrossRef]
- Heberling, C.A.; Dhurjati, P.S.; Sasser, M. Hypothesis for a systems connectivity model of Autism Spectrum Disorder pathogenesis: Links to gut bacteria, oxidative stress, and intestinal permeability. Med. Hypotheses 2013, 80, 264–270. [Google Scholar] [CrossRef]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Tomova, A.; Soltys, K.; Kemenyova, P.; Karhanek, M.; Babinska, K. The Influence of Food Intake Specificity in Children with Autism on Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 2797. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl. Environ. Microbiol. 2011, 77, 6718–6721. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e1617. [Google Scholar] [CrossRef] [PubMed]
- Babinská, K.; Tomova, A.; Celušáková, H.; Babková, J.; Repiská, G.; Kubranská, A.; Filčíková, D.; Siklenková, L.; Ostatníková, D. Fecal calprotectin levels correlate with main domains of the autism diagnostic interview-revised (ADI-R) in a sample of individuals with autism spectrum disorders from Slovakia. Physiol. Res. 2017, 66 (Suppl. S4), S517–S522. [Google Scholar] [CrossRef]
- de Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424. [Google Scholar] [CrossRef]
- Altieri, L.; Neri, C.; Sacco, R.; Curatolo, P.; Benvenuto, A.; Muratori, F.; Santocchi, E.; Bravaccio, C.; Lenti, C.; Saccani, M.; et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 2011, 16, 252–260. [Google Scholar] [CrossRef]
- D’Eufemia, P.; Celli, M.; Finocchiaro, R.; Pacifico, L.; Viozzi, L.; Zaccagnini, M.; Cardi, E.; Giardini, O. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996, 85, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayadhi, L.; Zayed, N.; Bhat, R.S.; Moubayed, N.M.S.; Al-Muammar, M.N.; El-Ansary, A. The use of biomarkers associated with leaky gut as a diagnostic tool for early intervention in autism spectrum disorder: A systematic review. Gut Pathog. 2021, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Babinska, K.; Celusakova, H.; Belica, I.; Szapuova, Z.; Waczulikova, I.; Nemcsicsova, D.; Tomova, A.; Ostatnikova, D. Gastrointestinal Symptoms and Feeding Problems and Their Associations with Dietary Interventions, Food Supplement Use, and Behavioral Characteristics in a Sample of Children and Adolescents with Autism Spectrum Disorders. Int. J. Environ. Res. Public. Health 2020, 17, 6372. [Google Scholar] [CrossRef]
- Bandini, L.G.; Anderson, S.E.; Curtin, C.; Cermak, S.; Evans, E.W.; Scampini, R.; Maslin, M.; Must, A. Food selectivity in children with autism spectrum disorders and typically developing children. J. Pediatr. 2010, 157, 259–264. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Food selectivity in autism spectrum disorders: A systematic review. J. Child. Neurol. 2014, 29, 1554–1561. [Google Scholar] [CrossRef]
- Tomova, A.; Soltys, K.; Repiska, G.; Palkova, L.; Filcikova, D.; Minarik, G.; Turna, J.; Prochotska, K.; Babinska, K.; Ostatnikova, D. Specificity of gut microbiota in children with autism spectrum disorder in Slovakia and its correlation with astrocytes activity marker and specific behavioural patterns. Physiol. Behav. 2020, 214, 112745. [Google Scholar] [CrossRef] [PubMed]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. Bioessays 2014, 36, 940–949. [Google Scholar] [CrossRef]
- Näslund, E.; Hellström, P.M. Appetite signaling: From gut peptides and enteric nerves to brain. Physiol. Behav. 2007, 92, 256–262. [Google Scholar] [CrossRef]
- Smith, P.M.; Ferguson, A.V. Neurophysiology of hunger and satiety. Dev. Disabil. Res. Rev. 2008, 14, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.C.; Cole, S.L.; Berridge, K.C. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: Interactions between homeostatic and reward circuitry. Front. Syst. Neurosci. 2015, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef] [PubMed]
- Berkel, S.; Marshall, C.R.; Weiss, B.; Howe, J.; Roeth, R.; Moog, U.; Endris, V.; Roberts, W.; Szatmari, P.; Pinto, D.; et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010, 42, 489–491. [Google Scholar] [CrossRef]
- Kathuria, A.; Nowosiad, P.; Jagasia, R.; Aigner, S.; Taylor, R.D.; Andreae, L.C.; Gatford, N.J.F.; Lucchesi, W.; Srivastava, D.P.; Price, J. Stem cell-derived neurons from autistic individuals with SHANK3 mutation show morphogenetic abnormalities during early development. Mol. Psychiatry 2018, 23, 735–746. [Google Scholar] [CrossRef]
- Reichova, A.; Bacova, Z.; Bukatova, S.; Kokavcova, M.; Meliskova, V.; Frimmel, K.; Ostatnikova, D.; Bakos, J. Abnormal neuronal morphology and altered synaptic proteins are restored by oxytocin in autism-related SHANK3 deficient model. Mol. Cell Endocrinol. 2020, 518, 110924. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef]
- Szabó, J.; Renczés, E.; Borbélyová, V.; Ostatníková, D.; Celec, P. Assessing sociability using the Three-Chamber Social Interaction Test and the Reciprocal Interaction Test in a genetic mouse model of ASD. Behav. Brain Funct. 2024, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Ma, K.; Wang, Z.J.; Hu, Z.; Matas, E.; Wei, J.; Yan, Z. Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat. Neurosci. 2018, 21, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Pillerová, M.; Drobná, D.; Szabó, J.; Renczés, E.; Borbélyová, V.; Ostatníková, D.; Celec, P.; Tóthová, Ľ. Neuromotor Development in the Shank3 Mouse Model of Autism Spectrum Disorder. Brain Sci. 2022, 12, 872. [Google Scholar] [CrossRef]
- Kshetri, R.; Beavers, J.O.; Hyde, R.; Ewa, R.; Schwertman, A.; Porcayo, S.; Richardson, B.D. Behavioral decline in Shank3. Mol. Autism 2024, 15, 52. [Google Scholar] [CrossRef]
- Wu, S.; Wang, J.; Zhang, Z.; Jin, X.; Xu, Y.; Si, Y.; Liang, Y.; Ge, Y.; Zhan, H.; Peng, L.; et al. Shank3 deficiency elicits autistic-like behaviors by activating p38α in hypothalamic AgRP neurons. Mol. Autism 2024, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Love, C.; Sominsky, L.; O’Hely, M.; Berk, M.; Vuillermin, P.; Dawson, S.L. Prenatal environmental risk factors for autism spectrum disorder and their potential mechanisms. BMC Med. 2024, 22, 393. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Sun, Z.; Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.A.; Litonjua, A.A.; Weiss, S.T.; Liu, Y.Y. Revealing the importance of prenatal gut microbiome in offspring neurodevelopment in humans. eBioMedicine 2023, 90, 104491. [Google Scholar] [CrossRef]
- Orchanian, S.B.; Hsiao, E.Y. The microbiome as a modulator of neurological health across the maternal-offspring interface. J. Clin. Investig. 2025, 135, e184314. [Google Scholar] [CrossRef]
- ADDMNS. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 2014, 63 (Suppl. S2), 1–21. [Google Scholar]
- Grabrucker, A.M. Autism Spectrum Disorders; Exon Publications: Brisbane, Australia, 2021; ISBN 13: 978-0-6450017-8-5. Available online: https://www.ncbi.nlm.nih.gov/books/NBK573612/ (accessed on 14 April 2025).
- Avolio, E.; Olivito, I.; Rosina, E.; Romano, L.; Angelone, T.; De Bartolo, A.; Scimeca, M.; Bellizzi, D.; D’Aquila, P.; Passarino, G.; et al. Modifications of Behavior and Inflammation in Mice Following Transplant with Fecal Microbiota from Children with Autism. Neuroscience 2022, 498, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wei, H.; Chen, J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems 2021, 6, e01343-20. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Hou, W.; Bi, D.; Yin, F.; Gao, Y.; Huang, D.; Li, Y.; Cao, Z.; Yan, Y.; et al. Fecal microbiota transplantation improves VPA-induced ASD mice by modulating the serotonergic and glutamatergic synapse signaling pathways. Transl. Psychiatry 2023, 13, 17. [Google Scholar] [CrossRef]
- Zheng, L.; Jiao, Y.; Zhong, H.; Tan, Y.; Yin, Y.; Liu, Y.; Liu, D.; Wu, M.; Wang, G.; Huang, J.; et al. Human-derived fecal microbiota transplantation alleviates social deficits of the BTBR mouse model of autism through a potential mechanism involving vitamin B. mSystems 2024, 9, e0025724. [Google Scholar] [CrossRef]
- Goo, N.; Bae, H.J.; Park, K.; Kim, J.; Jeong, Y.; Cai, M.; Cho, K.; Jung, S.Y.; Kim, D.H.; Ryu, J.H. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020, 262, 118497. [Google Scholar] [CrossRef]
- Kahathuduwa, C.N.; West, B.D.; Blume, J.; Dharavath, N.; Moustaid-Moussa, N.; Mastergeorge, A. The risk of overweight and obesity in children with autism spectrum disorders: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Pirník, Z.; Szadvári, I.; Borbélyová, V.; Tomova, A. Altered sex differences related to food intake, hedonic preference, and FosB/deltaFosB expression within central neural circuit involved in homeostatic and hedonic food intake regulation in Shank3B mouse model of autism spectrum disorder. Neurochem. Int. 2024, 181, 105895. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Kwong, C.; Hansen, R.; Hertz-Picciotto, I.; Croen, L.; Krakowiak, P.; Walker, W.; Pessah, I.N.; Van de Water, J. Brief report: Plasma leptin levels are elevated in autism: Association with early onset phenotype? J. Autism Dev. Disord. 2008, 38, 169–175. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.M.; Guo, M.; Du, Y.; Chen, Y.W.; Xiong, X.Y.; Cheng, Y. Altered leptin level in autism spectrum disorder and meta-analysis of adipokines. BMC Psychiatry 2024, 24, 479. [Google Scholar] [CrossRef]
- Hasan, Z.A.; Al-Kafaji, G.; Al-Sherawi, M.I.; Razzak, R.A.; Eltayeb, D.; Cristina, S.; Moiz, B. Investigation of Serum Levels of Leptin, Ghrelin and Growth Hormone in Bahraini Children with Autism. Int. Arch. Transl. Med. 2019, 5, 007. [Google Scholar] [CrossRef]
- Elias, C.F.; Aschkenasi, C.; Lee, C.; Kelly, J.; Ahima, R.S.; Bjorbaek, C.; Flier, J.S.; Saper, C.B.; Elmquist, J.K. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 1999, 23, 775–786. [Google Scholar] [CrossRef]
- Moorman, D.E.; James, M.H.; Kilroy, E.A.; Aston-Jones, G. Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur. J. Neurosci. 2016, 43, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria-From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Yousefi, R.; Mottaghi, A.; Saidpour, A. Spirulina platensis effectively ameliorates anthropometric measurements and obesity-related metabolic disorders in obese or overweight healthy individuals: A randomized controlled trial. Complement. Ther. Med. 2018, 40, 106–112. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e225. [Google Scholar] [CrossRef]
- Krajmer, P.; Spajdel, M.; Kubranska, A.; Ostatnikova, D. 2D:4D finger ratio in Slovak autism spectrum population. Bratisl. Lek. Listy 2011, 112, 377–379. [Google Scholar]
- Quartier, A.; Chatrousse, L.; Redin, C.; Keime, C.; Haumesser, N.; Maglott-Roth, A.; Brino, L.; Le Gras, S.; Benchoua, A.; Mandel, J.L.; et al. Genes and Pathways Regulated by Androgens in Human Neural Cells, Potential Candidates for the Male Excess in Autism Spectrum Disorder. Biol. Psychiatry 2018, 84, 239–252. [Google Scholar] [CrossRef]
- Baron-Cohen, S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Mu, C.; Qiao, D.; Chen, H.; Zhao, Y.; Cui, H.; Zhang, R.; Li, S. Androgen levels in autism spectrum disorders: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1371148. [Google Scholar] [CrossRef]
- McGivern, R.F.; Holcomb, C.; Poland, R.E. Effects of prenatal testosterone propionate treatment on saccharin preference of adult rats exposed to ethanol in utero. Physiol. Behav. 1987, 39, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Park, Y.H.; Sim, M.; Kim, S.A.; Joung, H.; Shin, D.M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 2019, 170, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Kushak, R.I.; Winter, H.S. Intestinal microbiota, metabolome and gender dimorphism in autism spectrum disorders. Res. Autism Spectr. Disord. 2018, 49, 65–74. [Google Scholar] [CrossRef]
- Esnafoglu, E.; Cırrık, S.; Ayyıldız, S.N.; Erdil, A.; Ertürk, E.Y.; Daglı, A.; Noyan, T. Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J. Pediatr. 2017, 188, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight junctions: From molecules to gastrointestinal diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef]
- Geirnaert, A.; Steyaert, A.; Eeckhaut, V.; Debruyne, B.; Arends, J.B.; Van Immerseel, F.; Boon, N.; Van de Wiele, T. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe 2014, 30, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Xie, X.Q.; Zhou, L.; Guan, Q.; Ren, Y.; Mao, Y.; Shi, J.S.; Xu, Z.H.; Geng, Y. Desulfovibrio fairfieldensis-Derived Outer Membrane Vesicles Damage Epithelial Barrier and Induce Inflammation and Pyroptosis in Macrophages. Cells 2022, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Bockmann, J.; Steinestel, K.; Boeckers, T.M.; Grabrucker, A.M. Altered Intestinal Morphology and Microbiota Composition in the Autism Spectrum Disorders Associated SHANK3 Mouse Model. Int. J. Mol. Sci. 2019, 20, 2134. [Google Scholar] [CrossRef]
- Abdellatif, B.; McVeigh, C.; Bendriss, G.; Chaari, A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; Dilavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Lord, C.; Pickles, A.; McLennan, J.; Rutter, M.; Bregman, J.; Folstein, S.; Fombonne, E.; Leboyer, M.; Minshew, N. Diagnosing autism: Analyses of data from the Autism Diagnostic Interview. J. Autism Dev. Disord. 1997, 27, 501–517. [Google Scholar] [CrossRef]
- Reygner, J.; Charrueau, C.; Delannoy, J.; Mayeur, C.; Robert, V.; Cuinat, C.; Meylheuc, T.; Mauras, A.; Augustin, J.; Nicolis, I.; et al. Freeze-dried fecal samples are biologically active after long-lasting storage and suited to fecal microbiota transplantation in a preclinical murine model of. Gut Microbes 2020, 11, 1405–1422. [Google Scholar] [CrossRef]
- Ikhtaire, S.; Shajib, M.S.; Reinisch, W.; Khan, W.I. Fecal calprotectin: Its scope and utility in the management of inflammatory bowel disease. J. Gastroenterol. 2016, 51, 434–446. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Z.Y.; Hu, Y.Y.; Bi, Y.W.; Yang, J.M.; Zou, W.J.; Song, Y.L.; Li, S.; Shen, T.; Li, S.J.; et al. The gut microbiota regulates autism-like behavior by mediating vitamin B. Microbiome 2020, 8, 120. [Google Scholar] [CrossRef]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017, 28, 1450–1461. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Šarayová, V.; Mihalovičová, L.; Miláček, D.; Gurecká, R.; Šebeková, K. Neurodevelopmental testing of mice in the neonatal period does not affect their locomotor activity, depressive- and anxiety-like behaviour in adolescence. Behav. Brain Res. 2021, 404, 113170. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Borbélyová, V.; Renczés, E.; Chovanec, M.; Mego, M.; Celec, P. Transient effects of chemotherapy for testicular cancer on mouse behaviour. Sci. Rep. 2020, 10, 10224. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar] [CrossRef]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. 2011, 53, 2920. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Pirnik, Z.; Maixnerová, J.; Matysková, R.; Koutová, D.; Zelezná, B.; Maletínská, L.; Kiss, A. Effect of anorexinergic peptides, cholecystokinin (CCK) and cocaine and amphetamine regulated transcript (CART) peptide, on the activity of neurons in hypothalamic structures of C57Bl/6 mice involved in the food intake regulation. Peptides 2010, 31, 139–144. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 1997. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Delroisse, J.M.; Boulvin, A.L.; Parmentier, I.; Dauphin, R.D.; Vandenbol, M.; Portetelle, D. Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by realtime PCR. Microbiol. Res. 2008, 163, 663–670. [Google Scholar] [CrossRef]
- Bekele, A.Z.; Koike, S.; Kobayashi, Y. Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiol. Lett. 2010, 305, 49–57. [Google Scholar] [CrossRef]
- Tang, J.; Iliev, I.D.; Brown, J.; Underhill, D.M.; Funari, V.A. Mycobiome: Approaches to analysis of intestinal fungi. J. Immunol. Methods 2015, 421, 112–121. [Google Scholar] [CrossRef]
| ZO3 | ZO1 | Claudin | Occludin | |
|---|---|---|---|---|
| Dubosiella | −0.44 | −0.37 | −0.42 | −0.33 |
| p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | |
| Butyricicoccus | −0.47 | −0.42 | −0.36 | |
| NS | p < 0.01 | p < 0.05 | p < 0.05 | |
| Colidextribacter | −0.47 | −0.45 | −0.41 | |
| p < 0.05 | p < 0.01 | p < 0.05 | NS | |
| Roseburia | −0.50 | −0.48 | −0.54 | |
| p < 0.01 | p < 0.01 | p < 0.01 | NS | |
| Lachnoclostridium | −0.44 | −0.37 | −0.39 | −0.36 |
| p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | |
| Lactobacillus | −0.33 | |||
| NS | NS | NS | p < 0.05 | |
| Desulfovibrio | 0.55 | 0.40 | 0.50 | |
| p < 0.01 | p < 0.05 | p < 0.01 | NS | |
| Turicibacter | 0.38 | 0.35 | ||
| p < 0.05 | NS | p < 0.05 | NS | |
| Parabacteroides | 0.45 | |||
| NS | p < 0.01 | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borbélyová, V.; Szabó, J.; Sušienková, P.; Potvin, J.; Belvončíková, P.; Groß, T.; Jančovičová, A.; Bačová, Z.; Rašková, B.; Szadvári, I.; et al. The Effect of Parental Faecal Microbiome Transplantation from Children with Autism Spectrum Disorder on Behavior and Gastrointestinal Manifestations in the Male Offspring of Shank3 Mice. Int. J. Mol. Sci. 2025, 26, 5927. https://doi.org/10.3390/ijms26135927
Borbélyová V, Szabó J, Sušienková P, Potvin J, Belvončíková P, Groß T, Jančovičová A, Bačová Z, Rašková B, Szadvári I, et al. The Effect of Parental Faecal Microbiome Transplantation from Children with Autism Spectrum Disorder on Behavior and Gastrointestinal Manifestations in the Male Offspring of Shank3 Mice. International Journal of Molecular Sciences. 2025; 26(13):5927. https://doi.org/10.3390/ijms26135927
Chicago/Turabian StyleBorbélyová, Veronika, Jakub Szabó, Petronela Sušienková, Judith Potvin, Paulína Belvončíková, Tim Groß, Alžbeta Jančovičová, Zuzana Bačová, Barbara Rašková, Ivan Szadvári, and et al. 2025. "The Effect of Parental Faecal Microbiome Transplantation from Children with Autism Spectrum Disorder on Behavior and Gastrointestinal Manifestations in the Male Offspring of Shank3 Mice" International Journal of Molecular Sciences 26, no. 13: 5927. https://doi.org/10.3390/ijms26135927
APA StyleBorbélyová, V., Szabó, J., Sušienková, P., Potvin, J., Belvončíková, P., Groß, T., Jančovičová, A., Bačová, Z., Rašková, B., Szadvári, I., Antal, M., Pirník, Z., Karhánek, M., Šoltys, K., Gardlík, R., Celec, P., Ostatníková, D., & Tomova, A. (2025). The Effect of Parental Faecal Microbiome Transplantation from Children with Autism Spectrum Disorder on Behavior and Gastrointestinal Manifestations in the Male Offspring of Shank3 Mice. International Journal of Molecular Sciences, 26(13), 5927. https://doi.org/10.3390/ijms26135927






