Renal and Vascular Effects of the Allosteric Transglutaminase 2 Modulator LDN-27219 in One-Kidney DOCA–Salt Mice
Abstract
1. Introduction
2. Results
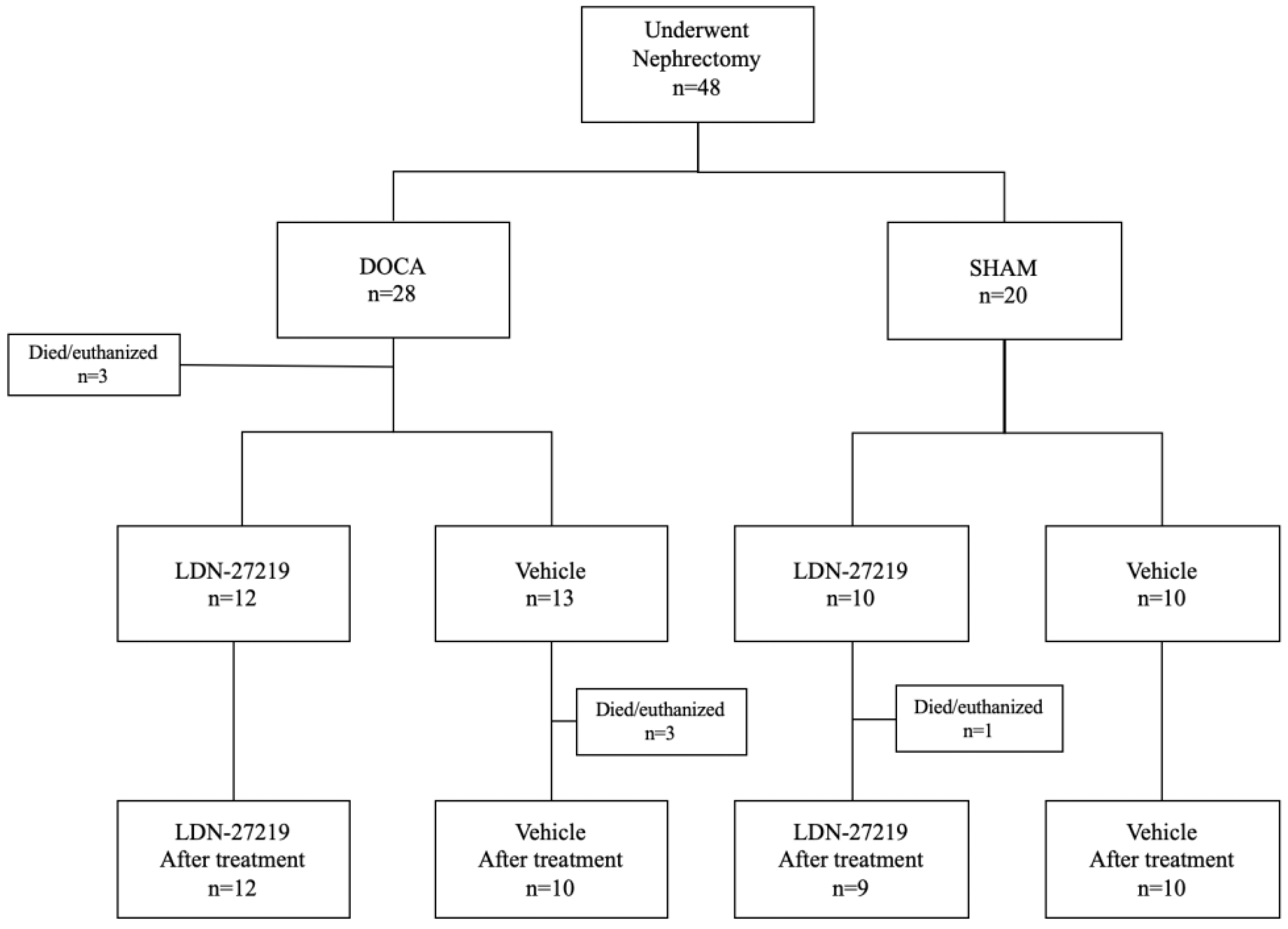
2.1. Animals
2.2. Kidney Function and Blood Pressure
2.3. Renal Fibrosis
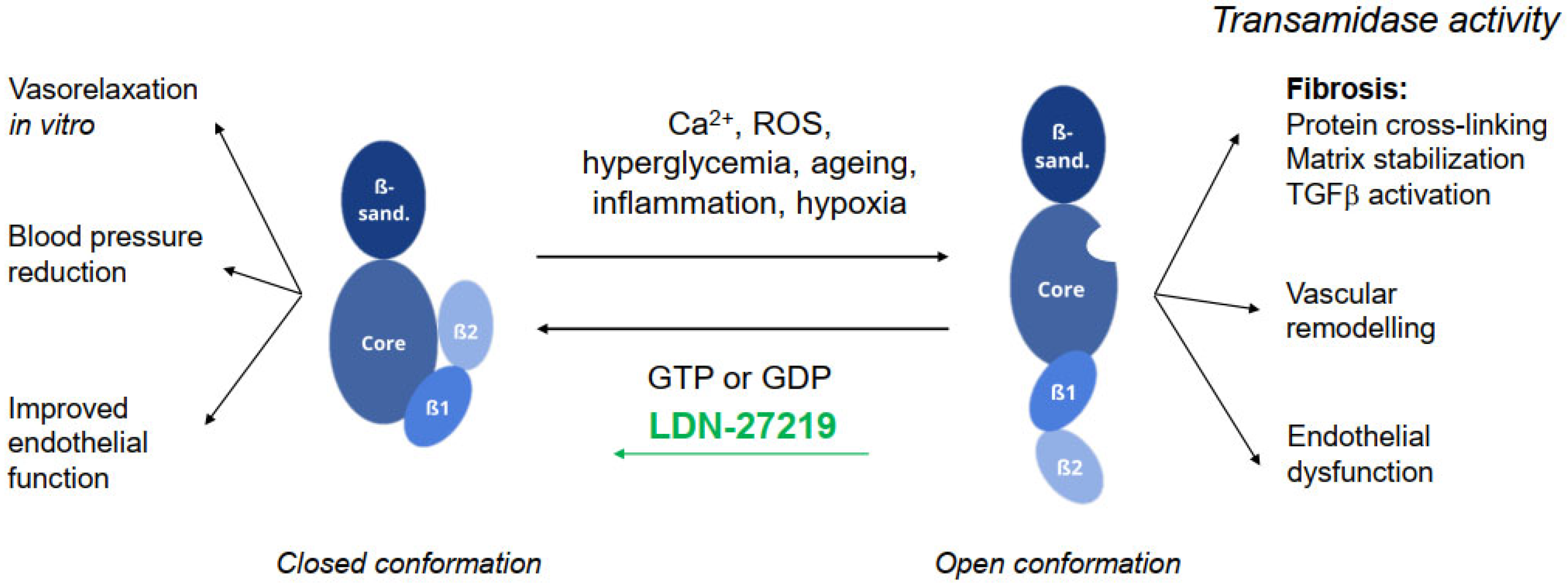
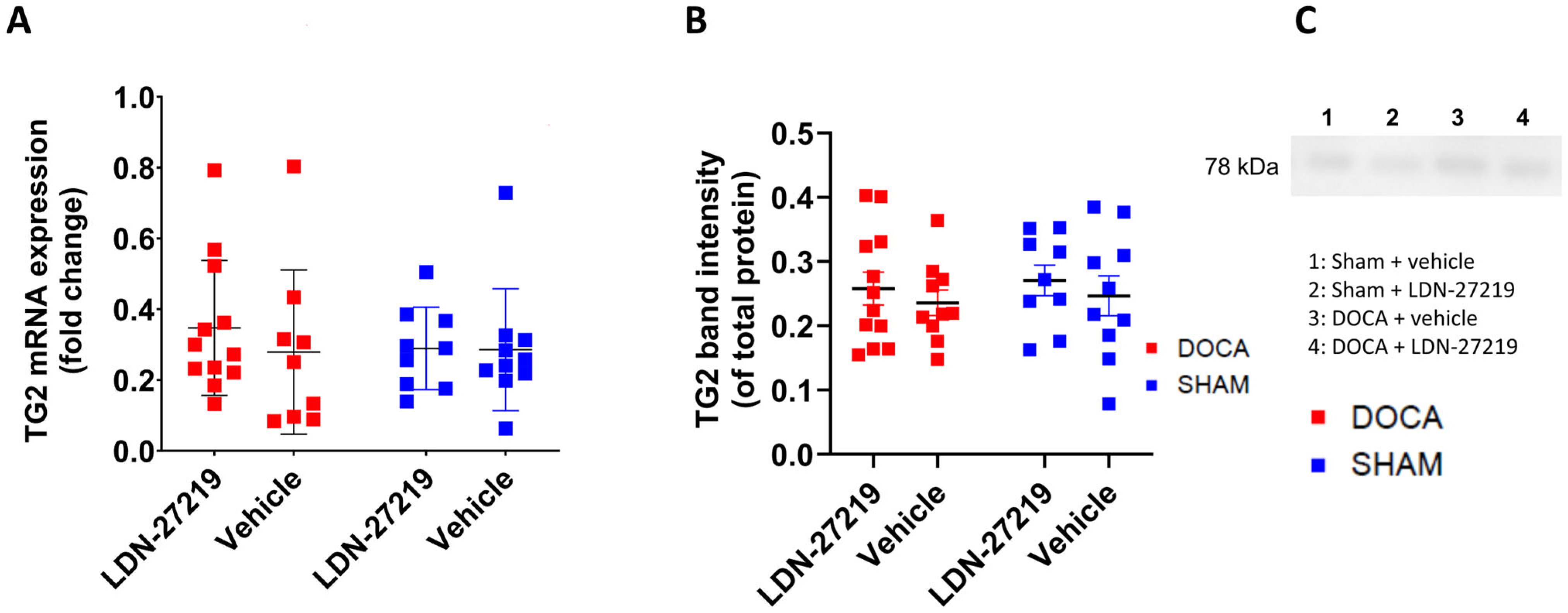
2.4. TG2 mRNA, Protein Expression, TG2 Conformation and Transamidase Activity
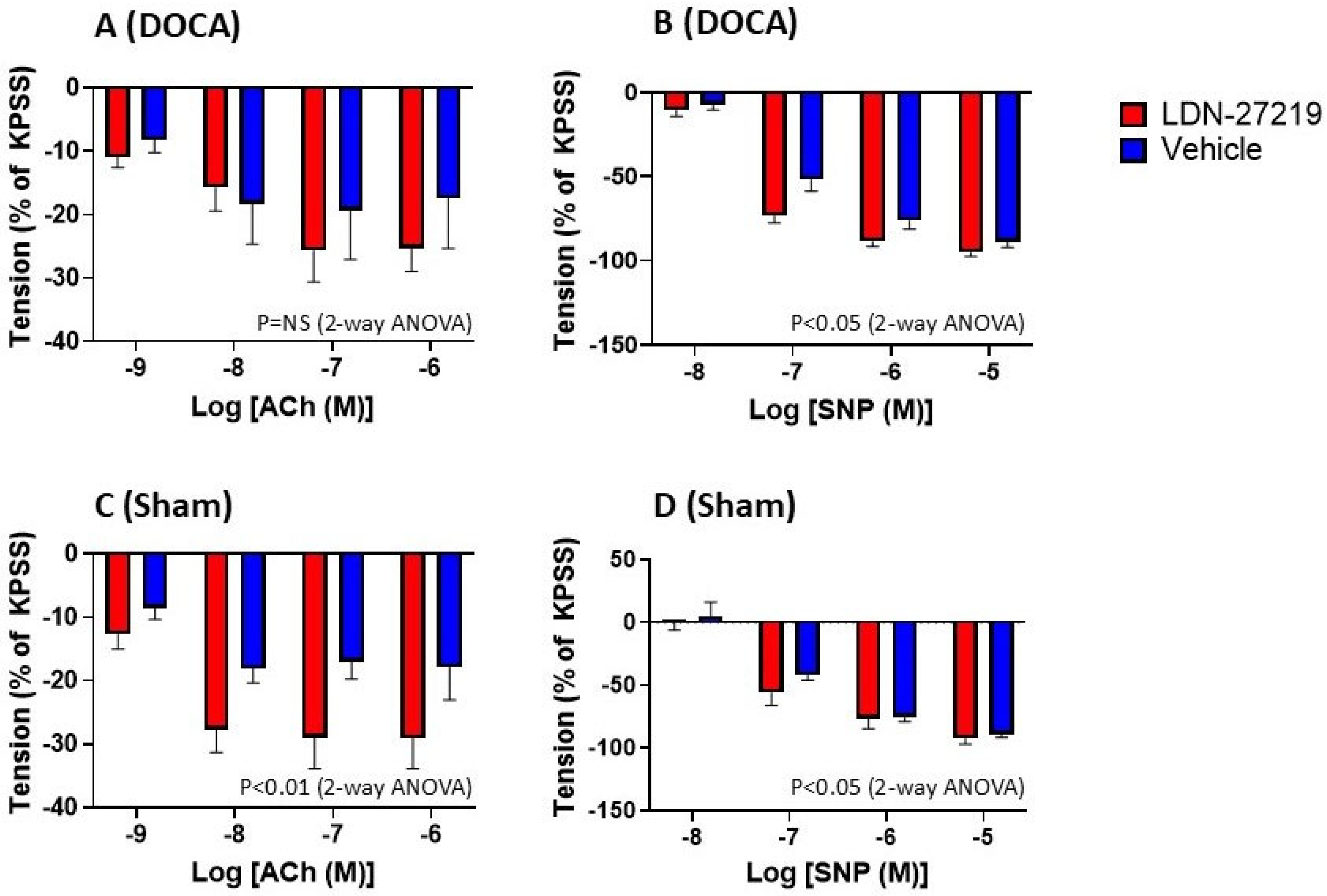
2.5. Vascular Function
3. Discussion
3.1. The One-Kidney DOCA Model
3.2. LDN-27219
3.3. Albuminuria, Renal Fibrosis, and Fibrosis Markers
3.4. TG2 Transamidase Activity and Conformation
3.5. Vascular Effects of LDN-27219 Treatment
4. Methods
4.1. Animals
4.2. Blood Pressure Measurement
4.3. Nephrectomy and DOCA Pellet Insertion
4.4. Treatment
4.5. Sample Collection
4.6. Transamidase Activity Assay
4.7. Renal Histology
4.8. qPCR of Renal Tissue Samples
4.9. Western Blotting
4.10. Assessment of TG2 Conformation
4.11. Vascular Function
4.12. Statistical Analyses
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| BP | blood pressure |
| BPA | biotinamide pentylamine |
| DOCA | deoxycorticosterone acetate |
| MAP | mean arterial pressure |
| NA | noradrenaline |
| NO | nitric oxide |
| PSS | physiological saline solution |
| SNP | sodium nitroprusside |
| TGF-β | transforming growth factor beta |
| TG2 | transglutaminase 2 |
References
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Soltani, F.; Kaartinen, M.T. Transglutaminases in fibrosis—Overview and recent advances. Am. J. Physiol. Cell Physiol. 2023, 325, C885–C894. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Furutani, Y.; Hitomi, K.; Kojima, S. Transglutaminase 2 has opposing roles in the regulation of cellular functions as well as cell growth and death. Cell Death Dis. 2016, 7, e2244. [Google Scholar] [CrossRef]
- Collighan, R.J.; Griffin, M. Transglutaminase 2 cross-linking of matrix proteins: Biological significance and medical applications. Amino Acids 2009, 36, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Park, H.H. Structural aspects of transglutaminase 2: Functional, structural, and regulatory diversity. Apoptosis 2017, 22, 1057–1068. [Google Scholar] [CrossRef]
- Begg, G.E.; Carrington, L.; Stokes, P.H.; Matthews, J.M.; Wouters, M.A.; Husain, A.; Lorand, L.; Iismaa, S.E.; Graham, R.M. Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc. Natl. Acad. Sci. USA 2006, 103, 19683–19688. [Google Scholar] [CrossRef]
- Han, B.G.; Cho, J.W.; Cho, Y.D.; Jeong, K.C.; Kim, S.Y.; Lee, B.I. Crystal structure of human transglutaminase 2 in complex with adenosine triphosphate. Int. J. Biol. Macromol. 2010, 47, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.H.; Lee, D.S.; Choi, K.; Jeong, E.M.; Kim, I.G.; Kim, Y.W.; Chun, J.N.; Jeon, J.H.; Park, H.H. Crystal structure of transglutaminase 2 with GTP complex and amino acid sequence evidence of evolution of GTP binding site. PLoS ONE 2014, 9, e107005. [Google Scholar] [CrossRef]
- Stamnaes, J.; Cardoso, I.; Iversen, R.; Sollid, L.M. Transglutaminase 2 strongly binds to an extracellular matrix component other than fibronectin via its second C-terminal beta-barrel domain. FEBS J. 2016, 283, 3994–4010. [Google Scholar] [CrossRef] [PubMed]
- Iismaa, S.E.; Chung, L.; Wu, M.J.; Teller, D.C.; Yee, V.C.; Graham, R.M. The core domain of the tissue transglutaminase Gh hydrolyzes GTP and ATP. Biochemistry 1997, 36, 11655–11664. [Google Scholar] [CrossRef]
- Tatsukawa, H.; Takeuchi, T.; Shinoda, Y.; Hitomi, K. Identification and characterization of substrates crosslinked by transglutaminases in liver and kidney fibrosis. Anal. Biochem. 2020, 604, 113629. [Google Scholar] [CrossRef]
- Pinilla, E.; Comerma-Steffensen, S.; Prat-Duran, J.; Rivera, L.; Matchkov, V.V.; Buus, N.H.; Simonsen, U. Transglutaminase 2 Inhibitor LDN 27219 Age-Dependently Lowers Blood Pressure and Improves Endothelium-Dependent Vasodilation in Resistance Arteries. Hypertension 2021, 77, 216–227. [Google Scholar] [CrossRef]
- Steppan, J.; Bergman, Y.; Viegas, K.; Armstrong, D.; Tan, S.; Wang, H.; Melucci, S.; Hori, D.; Park, S.Y.; Barreto, S.F.; et al. Tissue Transglutaminase Modulates Vascular Stiffness and Function Through Crosslinking-Dependent and Crosslinking-Independent Functions. J. Am. Heart Assoc. 2017, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, Y.J.; Jeon, H.Y.; Han, E.T.; Park, W.S.; Hong, S.H.; Kim, Y.M.; Ha, K.S. The vicious cycle between transglutaminase 2 and reactive oxygen species in hyperglycemic memory-induced endothelial dysfunction. FASEB J. 2019, 33, 12655–12667. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.S. Transglutaminase 2 in diabetes mellitus: Unraveling its multifaceted role and therapeutic implications for vascular complications. Theranostics 2024, 14, 2329–2344. [Google Scholar] [CrossRef] [PubMed]
- Engholm, M.; Pinilla, E.; Mogensen, S.; Matchkov, V.; Hedegaard, E.R.; Chen, H.; Mulvany, M.J.; Simonsen, U. Involvement of transglutaminase 2 and voltage-gated potassium channels in cystamine vasodilatation in rat mesenteric small arteries. Br. J. Pharmacol. 2016, 173, 839–855. [Google Scholar] [CrossRef]
- Case, A.; Stein, R.L. Kinetic analysis of the interaction of tissue transglutaminase with a nonpeptidic slow-binding inhibitor. Biochemistry 2007, 46, 1106–1115. [Google Scholar] [CrossRef]
- Oriana, F. Subcloning, Enzymatic Characterization, and In Silico Docking of Transglutaminase 2. Master’s Thesis, Brandeis University, Waltham, MA, USA, 2009. [Google Scholar] [CrossRef]
- Shweke, N.; Boulos, N.; Jouanneau, C.; Vandermeersch, S.; Melino, G.; Dussaule, J.C.; Chatziantoniou, C.; Ronco, P.; Boffa, J.J. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am. J. Pathol. 2008, 173, 631–642. [Google Scholar] [CrossRef]
- Prat-Duran, J.; Pinilla, E.; Norregaard, R.; Simonsen, U.; Buus, N.H. Transglutaminase 2 as a novel target in chronic kidney disease—Methods, mechanisms and pharmacological inhibition. Pharmacol. Ther. 2021, 222, 107787. [Google Scholar] [CrossRef]
- Johnson, T.S.; Fisher, M.; Haylor, J.L.; Hau, Z.; Skill, N.J.; Jones, R.; Saint, R.; Coutts, I.; Vickers, M.E.; El Nahas, A.M.; et al. Transglutaminase inhibition reduces fibrosis and preserves function in experimental chronic kidney disease. J. Am. Soc. Nephrol. 2007, 18, 3078–3088. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Furini, G.; Schroeder, N.; Huang, L.; Boocock, D.; Scarpellini, A.; Coveney, C.; Tonoli, E.; Ramaswamy, R.; Ball, G.; Verderio, C.; et al. Proteomic Profiling Reveals the Transglutaminase-2 Externalization Pathway in Kidneys after Unilateral Ureteric Obstruction. J. Am. Soc. Nephrol. 2018, 29, 880–905. [Google Scholar] [CrossRef]
- Prat-Duran, J.; De Araujo, I.; Juste, N.; Pinilla, E.; Rios, F.J.; Montezano, A.C.; Touyz, R.M.; Simonsen, U.; Norregaard, R.; Buus, N.H. Pharmacological modulation of transglutaminase 2 in the unilateral ureteral obstruction mouse model. Eur. J. Pharmacol. 2024, 984, 177037. [Google Scholar] [CrossRef]
- Basting, T.; Lazartigues, E. DOCA-Salt Hypertension: An Update. Curr. Hypertens. Rep. 2017, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- O′Donaughy, T.L.; Qi, Y.; Brooks, V.L. Central action of increased osmolality to support blood pressure in deoxycorticosterone acetate-salt rats. Hypertension 2006, 48, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Hartner, A.; Cordasic, N.; Klanke, B.; Veelken, R.; Hilgers, K.F. Strain differences in the development of hypertension and glomerular lesions induced by deoxycorticosterone acetate salt in mice. Nephrol. Dial. Transplant. 2003, 18, 1999–2004. [Google Scholar] [CrossRef]
- Schenk, J.; McNeill, J.H. The pathogenesis of DOCA-salt hypertension. J. Pharmacol. Toxicol. Methods 1992, 27, 161–170. [Google Scholar] [CrossRef]
- Tauber, P.; Sinha, F.; Berger, R.S.; Gronwald, W.; Dettmer, K.; Kuhn, M.; Trum, M.; Maier, L.S.; Wagner, S.; Schweda, F. Empagliflozin Reduces Renal Hyperfiltration in Response to Uninephrectomy, but Is Not Nephroprotective in UNx/DOCA/Salt Mouse Models. Front. Pharmacol. 2021, 12, 761855. [Google Scholar] [CrossRef]
- Ling, Y.H.; Krishnan, S.M.; Chan, C.T.; Diep, H.; Ferens, D.; Chin-Dusting, J.; Kemp-Harper, B.K.; Samuel, C.S.; Hewitson, T.D.; Latz, E.; et al. Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt-induced hypertension. Pharmacol. Res. 2017, 116, 77–86. [Google Scholar] [CrossRef]
- Belanger, K.M.; Crislip, G.R.; Gillis, E.E.; Abdelbary, M.; Musall, J.B.; Mohamed, R.; Baban, B.; Elmarakby, A.; Brands, M.W.; Sullivan, J.C. Greater T Regulatory Cells in Females Attenuate DOCA-Salt-Induced Increases in Blood Pressure Versus Males. Hypertension 2020, 75, 1615–1623. [Google Scholar] [CrossRef]
- Huang, L.; Haylor, J.L.; Hau, Z.; Jones, R.A.; Vickers, M.E.; Wagner, B.; Griffin, M.; Saint, R.E.; Coutts, I.G.; El Nahas, A.M.; et al. Transglutaminase inhibition ameliorates experimental diabetic nephropathy. Kidney Int. 2009, 76, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Gross, V.; Lippoldt, A.; Bohlender, J.; Bader, M.; Hansson, A.; Luft, F.C. Cortical and medullary hemodynamics in deoxycorticosterone acetate-salt hypertensive mice. J. Am. Soc. Nephrol. 1998, 9, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.N.; Ritter, M.L.; Wackman, K.K.; Oliveira, V.; Balapattabi, K.; Grobe, C.C.; Brozoski, D.T.; Reho, J.J.; Nakagawa, P.; Mouradian, G.C., Jr.; et al. Cardiometabolic effects of DOCA-salt in male C57BL/6J mice are variably dependent on sodium and nonsodium components of diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R467–R485. [Google Scholar] [CrossRef]
- Wang, Q.; Hummler, E.; Nussberger, J.; Clement, S.; Gabbiani, G.; Brunner, H.R.; Burnier, M. Blood pressure, cardiac, and renal responses to salt and deoxycorticosterone acetate in mice: Role of Renin genes. J. Am. Soc. Nephrol. 2002, 13, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Wyss, C.; Wang, Q.; Golshayan, D.; Nussberger, J.; Burnier, M.; Lehr, H.A.; Schaefer, S.C. Potassium restores vasorelaxation of resistance arterioles in non-hypertensive DOCA/salt fed mice. Microvasc. Res. 2012, 84, 340–344. [Google Scholar] [CrossRef]
- Jandu, S.K.; Webb, A.K.; Pak, A.; Sevinc, B.; Nyhan, D.; Belkin, A.M.; Flavahan, N.A.; Berkowitz, D.E.; Santhanam, L. Nitric oxide regulates tissue transglutaminase localization and function in the vasculature. Amino Acids 2013, 44, 261–269. [Google Scholar] [CrossRef]
- Schaertl, S.; Prime, M.; Wityak, J.; Dominguez, C.; Munoz-Sanjuan, I.; Pacifici, R.E.; Courtney, S.; Scheel, A.; Macdonald, D. A profiling platform for the characterization of transglutaminase 2 (TG2) inhibitors. J. Biomol. Screen. 2010, 15, 478–487. [Google Scholar] [CrossRef]
- Nio, Y.; Ookawara, M.; Yamasaki, M.; Hanauer, G.; Tohyama, K.; Shibata, S.; Sano, T.; Shimizu, F.; Anayama, H.; Hazama, M.; et al. Ameliorative effect of phosphodiesterase 4 and 5 inhibitors in deoxycorticosterone acetate-salt hypertensive uni-nephrectomized KKAy mice. FASEB J. 2020, 34, 14997–15014. [Google Scholar] [CrossRef]
- Zhang, J.; Guttmann, R.P.; Johnson, G.V. Tissue transglutaminase is an in situ substrate of calpain: Regulation of activity. J. Neurochem. 1998, 71, 240–247. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Greenberg, C.S. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J. Biol. Chem. 1987, 262, 1901–1906. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]





| DOCA | Sham | p-value (Effect of DOCA) | |||
|---|---|---|---|---|---|
| LDN-27219 (n = 12) | Vehicle (n = 10) | LDN-27219 (n = 9) | Vehicle (n = 10) | ||
| Weight before treatment (g) | 23.3 ± 1.8 | 24.0 ± 1.7 | 24.1 ± 1.0 | 23.5 ± 1.2 | 0.81 |
| Weight after treatment (g) | 24.9 ± 2.3 | 24.5 ± 1.7 | 24.7 ± 1.4 | 24.4 ± 1.5 | 0.87 |
| Heart (mg/g) | 5.14 ± 0.15 | 5.54 ± 0.14 | 5.02 ± 0.14 | 4.81 ± 0.11 | 0.006 |
| Liver (mg/g) | 5.74 ± 0.12 | 5.87 ± 0.17 | 5.22 ± 0.14 | 4.86 ± 0.07 | <0.0001 |
| Kidney (mg/g) | 1.08 ± 0.03 | 1.08 ± 0.03 | 0.88 ± 0.05 | 0.85 ± 0.01 | <0.0001 |
| Spleen (mg/g) | 5.54 ± 0.82 | 3.94 ± 0.54 | 2.91 ± 0.30 | 2.77 ± 0.38 | 0.006 |
| 24 h urine volume (mL) | 7.9 ± 2.7 * | 12.3 ± 5.9 | 1.5 ± 0.7 | 1.6 ± 1.6 | <0.0001 |
| P-urea (mmol/L) | 5.7 ± 0.4 | 5.1 ± 0.8 | 9.4 ± 1.9 | 9.2 ± 1.4 | 0.0003 |
| MAP at inclusion (mmHg) | 77.6 ± 2.3 | 84.8 ± 3.5 | 87.8 ± 3.9 | 80.9 ± 3.8 | 0.38 |
| MAP before treatment (mmHg) | 82.7 ± 3.2 | 88.1 ± 2.3 | 91.4 ± 4.8 | 82.1 ± 4.1 | 0.70 |
| MAP after treatment (mmHg) | 80.4 ± 7.6 | 85.2 ± 3.1 | 83.3 ± 4.3 | 83.6 ± 2.5 | 0.90 |
| DOCA | Sham | |||
|---|---|---|---|---|
| LDN-27219 (n = 12) | Vehicle (n = 10) | LDN-27219 (n = 9) | Vehicle (n = 8) | |
| Internal diameter (µm) | 205 ± 27 | 202 ± 41 | 191 ± 54 | 235 ± 58 |
| Contraction to KPSS (mN) | 5.1 ± 0.7 | 4.5 ± 0.5 | 4.6 ± 0.5 | 5.3 ± 0.6 |
| Contraction to KPSS (mN) repeated | 4.5 ± 0.5 | 4.1 ± 0.5 | 4.3 ± 0.6 | 5.1 ± 0.7 |
| Contraction before ACh (mN) | 3.6 ± 0.4 | 3.8 ± 0.6 | 4.3 ± 0.6 | 5.0 ± 0.5 |
| Contraction before SNP (mN) | 2.9 ± 0.3 | 3.0 ± 0.4 | 2.7 ± 0.5 | 3.7 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mees, I.; Prat-Duran, J.; Comerma-Steffensen, S.; Simonsen, U.; Pinilla, E.; Buus, N.H. Renal and Vascular Effects of the Allosteric Transglutaminase 2 Modulator LDN-27219 in One-Kidney DOCA–Salt Mice. Int. J. Mol. Sci. 2025, 26, 5724. https://doi.org/10.3390/ijms26125724
Mees I, Prat-Duran J, Comerma-Steffensen S, Simonsen U, Pinilla E, Buus NH. Renal and Vascular Effects of the Allosteric Transglutaminase 2 Modulator LDN-27219 in One-Kidney DOCA–Salt Mice. International Journal of Molecular Sciences. 2025; 26(12):5724. https://doi.org/10.3390/ijms26125724
Chicago/Turabian StyleMees, Ian, Judit Prat-Duran, Simon Comerma-Steffensen, Ulf Simonsen, Estéfano Pinilla, and Niels Henrik Buus. 2025. "Renal and Vascular Effects of the Allosteric Transglutaminase 2 Modulator LDN-27219 in One-Kidney DOCA–Salt Mice" International Journal of Molecular Sciences 26, no. 12: 5724. https://doi.org/10.3390/ijms26125724
APA StyleMees, I., Prat-Duran, J., Comerma-Steffensen, S., Simonsen, U., Pinilla, E., & Buus, N. H. (2025). Renal and Vascular Effects of the Allosteric Transglutaminase 2 Modulator LDN-27219 in One-Kidney DOCA–Salt Mice. International Journal of Molecular Sciences, 26(12), 5724. https://doi.org/10.3390/ijms26125724







