Involvement of Matrix Metalloproteinases (MMP-2 and MMP-9), Inflammasome NLRP3, and Gamma-Aminobutyric Acid (GABA) Pathway in Cellular Mechanisms of Neuroinflammation in PTSD
Abstract
1. Introduction
2. Results
2.1. Biomarker Profiles in PTSD: Age-Stratified Analysis
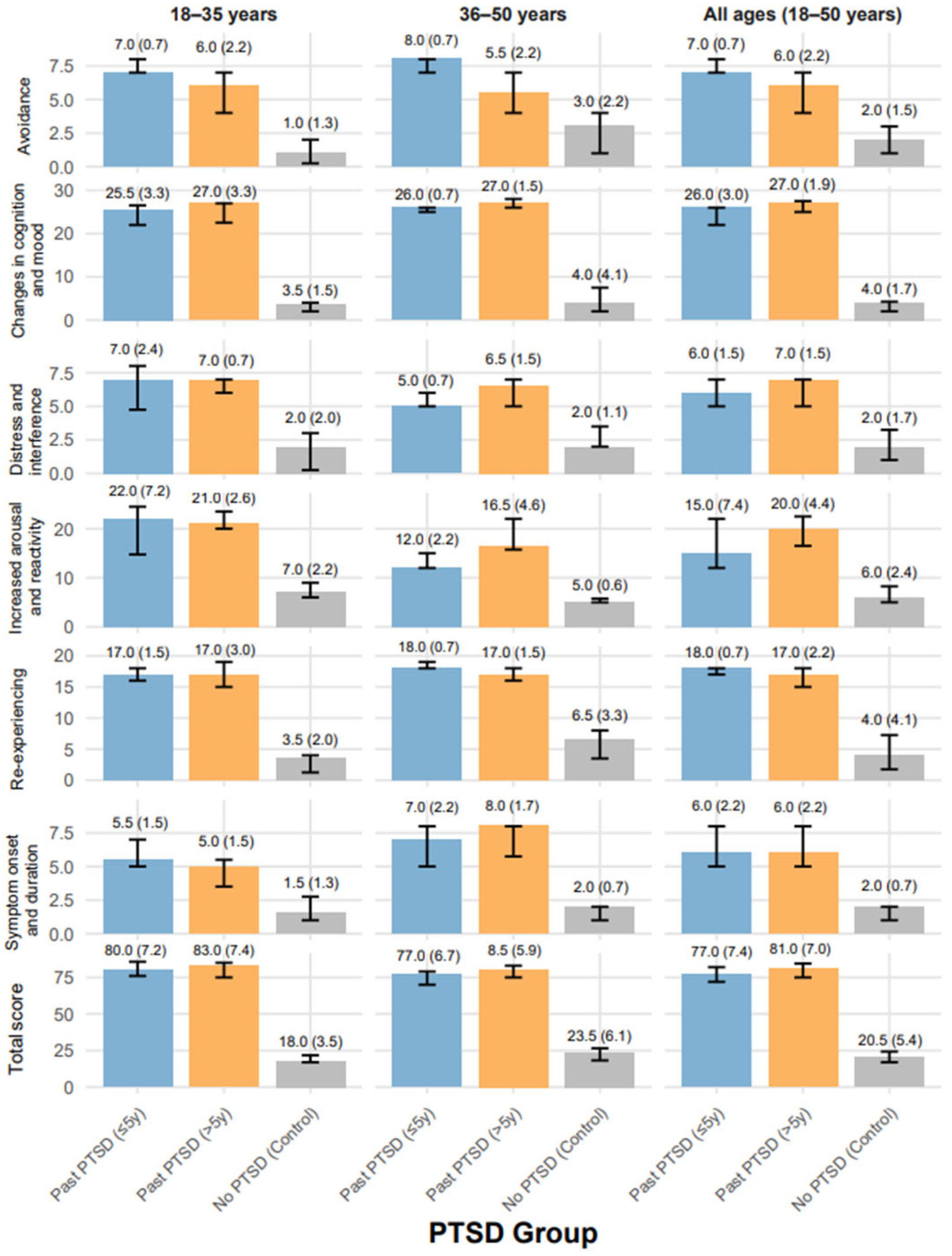
2.2. PSSI-5 Scores Across PTSD Groups by Age Distribution
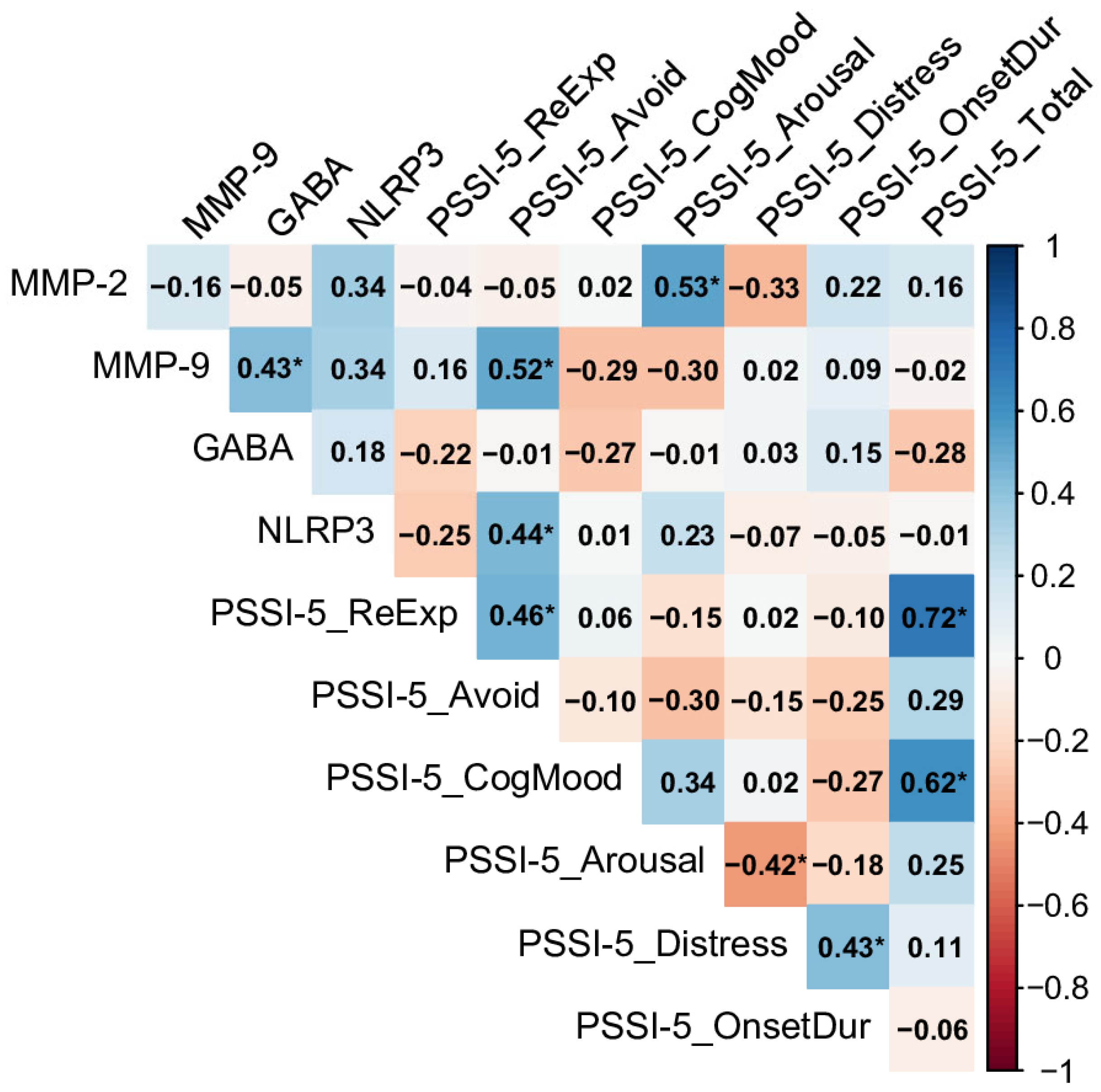
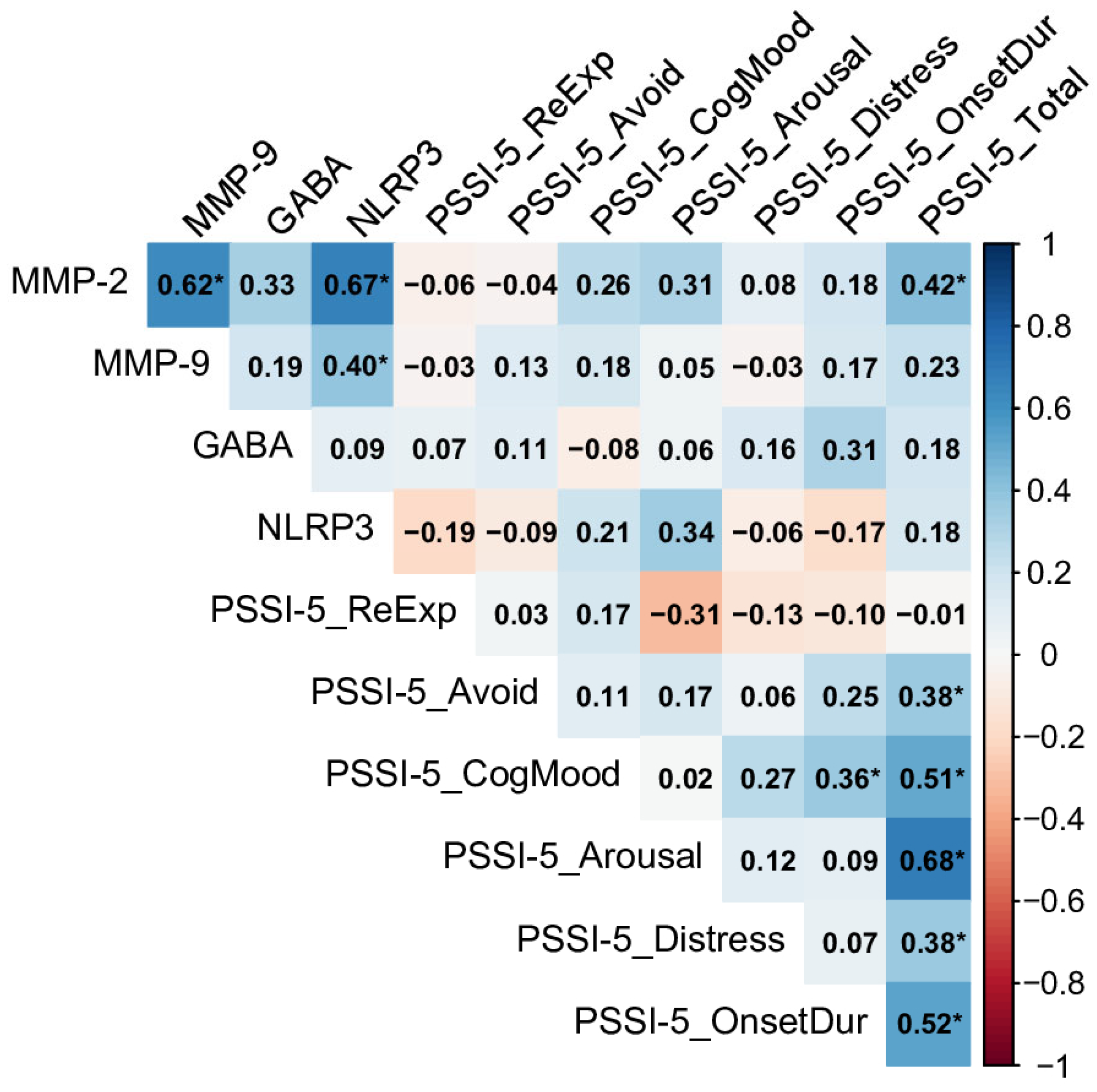
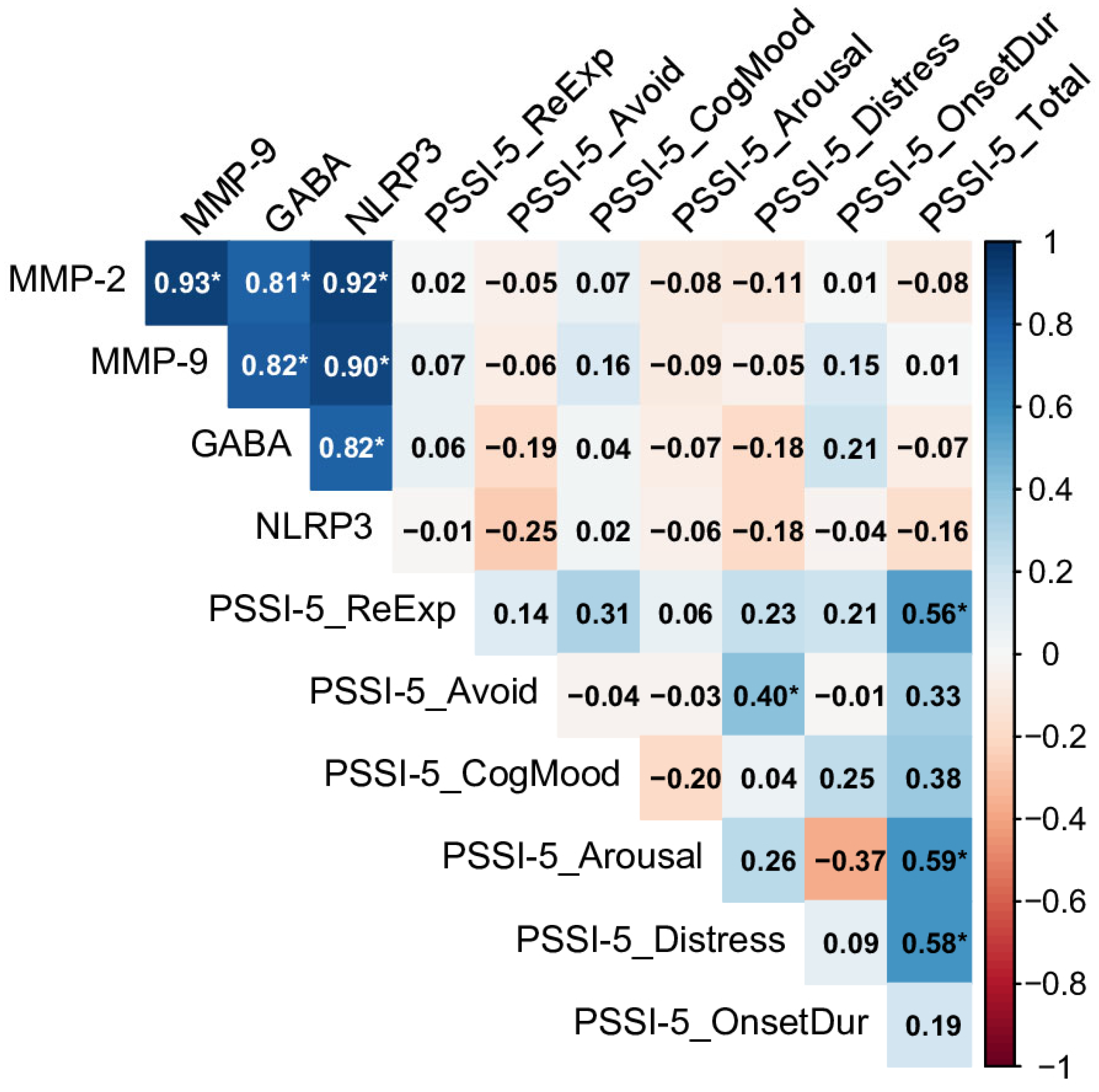
2.3. Relationships Between Biomarkers and PSSI-5 Domains and Total Score Across Control and PTSD Groups
3. Discussion
4. Material and Methods
4.1. Characteristics of the Participants
4.2. Clinical Interview
4.3. Blood Sampling
4.4. Biomarkers Evaluations
4.5. Statistical Analysis
Characteristics of the Statistical Tool
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTSD | Post-traumatic stress disorder |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| PSSI-5 | Symptom Scale–Interview Version for DSM-5 |
| CNS | Central Nervous System |
| MMP | Matrix metalloproteinase |
| BBB | Blood–brain barrier |
| MMP | Matrix metalloproteinase |
| ECM | Extra cellular matrix |
| DAMPs | Danger-associated molecular patterns |
| NLRP3 | NOD-Like Receptor Pyrin Domain-Containing Protein 3 |
| CARD, ASC | Apoptosis-associated speck-like protein containing a caspase re-cruitment domain |
| GABA | Gamma-aminobutyric acid |
| VCAM-1 | Vascular cell adhesion molecule |
| ROS | Reactive oxygen species |
| ELISA kits | Enzyme-linked immunosorbent assay kits |
References
- Wu, L.; Jin, M. Clinical Significance of Serum MMP-9, S100-β and GFAP in Patients with Mental Disorders after Traumatic Brain Injury. Actas Esp. Psiquiatr. 2025, 531, 11–18. [Google Scholar] [CrossRef]
- Watling, S.E.; Rhind, S.G.; Warsh, J.; Green, D.; McCluskey, T.; Tong, J.; Truong, P.; Chavez, S.; Richardson, J.D.; Kish, S.J.; et al. Exploring brain glutathione and peripheral blood markers in posttraumatic stress disorder: A combined [1H]MRS and peripheral blood study. Front. Psychiatry 2023, 14, 1195012. [Google Scholar] [CrossRef]
- Grochecki, P.; Smaga, I.; Wydra, K.; Marszalek-Grabska, M.; Slowik, T.; Kedzierska, E.; Listos, J.; Gibula-Tarlowska, E.; Filip, M.; Kotlinska, J.H. Impact of Mephedrone on Fear Memory in Adolescent Rats: Involvement of Matrix Metalloproteinase-9 (MMP-9) and N-Methyl-D-aspartate (NMDA) Receptor. Int. J. Mol. Sci. 2023, 24, 1941. [Google Scholar] [CrossRef]
- Holder, N.; Batten, A.; Shiner, B.; Neylan, T.C.; Maguen, S. Reliable symptom worsening among veterans receiving cognitive processing therapy or prolonged exposure therapy for posttraumatic stress disorder in routine Veterans Health Administration care. J. Affect. Disord. 2025, 387, 119472. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, Y.; He, X.; Li, L.; Yue, Z.; Liang, Y.; Huang, Y. Effects of MMP2 and its inhibitor TIMP2 on DNA damage, apoptosis and senescence of human lens epithelial cells induced by oxidative stress. J. Bioenerg. Biomembr. 2024, 56, 619–630. [Google Scholar] [CrossRef]
- Martinelli, S.; Anderzhanova, E.A.; Bajaj, T.; Wiechmann, S.; Dethloff, F.; Weckmann, K.; Heinz, D.E.; Ebert, T.; Hartmann, J.; Geiger, T.M.; et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat. Commun. 2021, 12, 4643. [Google Scholar] [CrossRef]
- Ali, M.A.; Michel, H.E.; Menze, E.T.; Tadros, M.G.; Wahdan, S.A. The potential neuroprotective effect of empagliflozin against depressive-like behavior induced by chronic unpredictable mild stress in rats: Involvement of NLRP3 inflammasome. Eur. J. Pharmacol. 2025, 998, 177525. [Google Scholar] [CrossRef]
- Yang, L.; Xing, W.; Shi, Y.; Hu, M.; Li, B.; Hu, Y.; Zhang, G. Stress-induced NLRP3 inflammasome activation and myelin alterations in the hippocampus of PTSD rats. Neuroscience 2024, 555, 156–166. [Google Scholar] [CrossRef]
- McDermott, K.; Rush, C.; Pham, T.; Hooker, J.; Louis, C.; Rochon, E.A.; Vranceanu, A.M. PTSD symptoms, pain catastrophizing, and pain outcomes after acute orthopedic injury. Pain Med. 2024, 25, 758–767. [Google Scholar] [CrossRef]
- Davis, L.L.; Hamner, M.B. Post-traumatic stress disorder: The role of the amygdala and potential therapeutic interventions—A review. Front. Psychiatry 2024, 15, 1356563. [Google Scholar] [CrossRef] [PubMed]
- Shelef, L.; Itzhaky, L.; Bechor, U.; Tatsa-Laur, L.; Mann, J.J. Relationships of DSM-5 PTSD symptom clusters to suicidal ideation and death ideation in outpatient military veterans. Psychiatry Res. 2024, 339, 115993. [Google Scholar] [CrossRef]
- Chevalier, C.M.; Krampert, L.; Schreckenbach, M.; Schubert, C.F.; Reich, J.; Novak, B.; Schmidt, M.V.; Rutten, B.P.F.; Schmidt, U. MMP9 mRNA is a potential diagnostic and treatment monitoring marker for PTSD: Evidence from mice and humans. Eur. Neuropsychopharmacol. 2021, 51, 20–32. [Google Scholar] [CrossRef]
- Blanco, I.; Deasy, S.; Amontree, M.; Gabriel, M.; Caccavano, A.; Vicini, S.; Glasgow, E.; Conant, K. MMP-2/9 inhibition modulates sharp wave abundance, inhibitory proteoglycan sulfation, and fear memory in juvenile zebrafish: Relevance to affective disorders. bioRxiv 2025, bioRxiv:2025.02.25.640105. [Google Scholar] [CrossRef]
- Ghelfi, L.; Mongan, D.; Susai, S.R.; Föcking, M.; Cotter, D.R.; Cannon, M. Plasma levels of matrix metalloproteinases in early psychosis, anxiety and depression: Evidence from the ALSPAC cohort. Brain Behav. Immun. 2025, 124, 137–143. [Google Scholar] [CrossRef]
- Li, Q.; Michaud, M.; Shankar, R.; Canosa, S.; Schwartz, M.; Madri, J.A. MMP-2: A modulator of neuronal precursor activity and cognitive and motor behaviors. Behav. Brain Res. 2017, 333, 74–82. [Google Scholar] [CrossRef]
- Soylu, K.O.; Yemisci, M.; Karatas, H. The link between spreading depolarization and innate immunity in the central nervous system. J. Headache Pain 2025, 26, 25. [Google Scholar] [CrossRef]
- Clancy, K.J.; Devignes, Q.; Kumar, P.; May, V.; Hammack, S.E.; Akman, E.; Casteen, E.J.; Pernia, C.D.; Jobson, S.A.; Lewis, M.W.; et al. Circulating PACAP levels are associated with increased amygdala-default mode network resting-state connectivity in posttraumatic stress disorder. Neuropsychopharmacology 2023, 48, 1245–1254. [Google Scholar] [CrossRef]
- Wellington, N.J.; Boųcas, A.P.; Lagopoulos, J.; Quigley, B.L.; Kuballa, A.V. Molecular pathways of ketamine: A systematic review of immediate and sustained effects on PTSD. Psychopharmacology 2025, 242, 1197–1243. [Google Scholar] [CrossRef]
- He, H.; Zhang, X.; He, H.; Xu, G.; Li, L.; Yang, C.; Liu, Y.E.; You, Z.; Zhang, J. Microglial priming by IFN-γ involves STAT1-mediated activation of the NLRP3 inflammasome. CNS Neurosci. Ther. 2024, 30, e70061. [Google Scholar] [CrossRef]
- Tyler, R.E.; Bluitt, M.N.; Van Voorhies, K.J.; Liu, W.; Magee, S.N.; Pitrolo, E.R.; Cordero, V.L.; Ornelas, L.C.; Krieman, C.G.; Bender, B.N.; et al. The persistent effects of predator odor stressor enhance interoceptive sensitivity to alcohol through GABAA receptor adaptations in the prelimbic cortex in male, but not female rats. bioRxiv 2024, bioRxiv:2024.10.30.621141. [Google Scholar] [CrossRef]
- Bonomi, R.; Hillmer, A.T.; Woodcock, E.; Bhatt, S.; Rusowicz, A.; Angarita, G.A.; Carson, R.E.; Davis, M.T.; Esterlis, I.; Nabulsi, N.; et al. Microglia-mediated neuroimmune suppression in PTSD is associated with anhedonia. Proc. Natl. Acad. Sci. USA 2024, 121, e2406005121. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, F.; Yang, L.; Tuolihong, L.; Wang, X.; Du, Z.; Zhang, Y.; Yin, X.; Li, Y.; Lu, K.; et al. Involvement of the GABAergic system in PTSD and its therapeutic significance. Front. Mol. Neurosci. 2023, 16, 1052288. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G. Biomarkers and treatments for mood disorders encompassing the neurosteroid and endocannabinoid systems. J. Neuroendocrinol. 2023, 35, e13226. [Google Scholar] [CrossRef]
- Sarkar, I.; Snippe-Strauss, M.; Tenenhaus Zamir, A.; Benhos, A.; Richter-Levin, G. Individual behavioral profiling as a translational approach to assess treatment efficacy in an animal model of post-traumatic stress disorder. Front. Neurosci. 2022, 16, 1071482. [Google Scholar] [CrossRef]
- Li, X.; Gu, Y.; Qi, M.; Chen, R.; Xiao, D.; Yuan, P.; Xiang, H.; Shi, X. Neurobiochemical biomarkers and other risk factors for post-traumatic acute stress disorder. J. Psychiatr. Res. 2023, 157, 276–284. [Google Scholar] [CrossRef]
- Kim, E.G.; Chang, W.; Shin, S.; Adhikari, A.S.; Seol, G.H.; Song, D.Y.; Min, S.S. Maternal separation in mice leads to anxiety-like/aggressive behavior and increases immunoreactivity for glutamic acid decarboxylase and parvalbumin in the adolescence ventral hippocampus. Korean J. Physiol. Pharmacol. 2023, 27, 113–125. [Google Scholar] [CrossRef]
- Broussot, L.; Contesse, T.; Costa-Campos, R.; Glangetas, C.; Royon, L.; Fofo, H.; Lorivel, T.; Georges, F.; Fernandez, S.P.; Barik, J. A non-canonical GABAergic pathway to the VTA promotes unconditioned freezing. Mol. Psychiatry 2022, 27, 4905–4917. [Google Scholar] [CrossRef]
- Feklicheva, I.; Boks, M.P.; de Kloet, E.R.; Chipeeva, N.; Maslennikova, E.; Pashkov, A.; Korobova, S.; Komelkova, M.; Kuznetsova, Y.; Platkovski, P.; et al. Biomarkers in PTSD-susceptible and resistant veterans with war experience of more than ten years ago: FOCUS ON cortisol, thyroid hormones, testosterone and GABA. J. Psychiatr. Res. 2022, 148, 258–263. [Google Scholar] [CrossRef]
- Rosso, I.M.; Silveri, M.M.; Olson, E.A.; Eric Jensen, J.; Ren, B. Regional specificity and clinical correlates of cortical GABA alterations in posttraumatic stress disorder. Neuropsychopharmacology 2022, 47, 1055–1062. [Google Scholar] [CrossRef]
- Vargas, L.D.S.; Lima, K.R.; Mello-Carpes, P.B. Infralimbic and prelimbic prefrontal cortex activation is necessary to the enhancement of aversive memory extinction promoted by reactivation. Brain Res. 2021, 1770, 147630. [Google Scholar] [CrossRef]
- Enomoto, S.; Kato, T.A. Stress Mediated Microglial Hyper-Activation and Psychiatric Diseases. Brain Nerve 2021, 73, 795–802. [Google Scholar] [CrossRef]
- Brackbill, R.M.; Hadler, J.L.; DiGrande, L.; Ekenga, C.C.; Farfel, M.R.; Friedman, S.; Perlman, S.E.; Stellman, S.D.; Walker, D.J.; Wu, D.; et al. Asthma and Posttraumatic Stress Symptoms 5 to 6 Years Following Exposure to the World Trade Center Terrorist Attack. JAMA 2009, 302, 502–516. [Google Scholar] [CrossRef]
- Comeras, L.B.; Hörmer, N.; Mohan Bethuraj, P.; Tasan, R.O. NPY Released From GABA Neurons of the Dentate Gyrus Specially Reduces Contextual Fear Without Affecting Cued or Trace Fear. Front. Synaptic. Neurosci. 2021, 13, 635726. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zion, Z.; Simon, A.J.; Rosenblatt, M.; Korem, N.; Duek, O.; Liberzon, I.; Shalev, A.Y.; Hendler, T.; Levy, I.; Harpaz-Rotem, I.; et al. Connectome-Based Predictive Modeling of PTSD Development Among Recent Trauma Survivors. JAMA Netw Open 2025, 8, e250331. [Google Scholar] [CrossRef]
- van Zuiden, M.; Engel, S.; Karchoud, J.F.; Wise, T.J.; Sijbrandij, M.; Mouthaan, J.; Olff, M.; van de Schoot, R. Sex-differential PTSD symptom trajectories across one year following suspected serious injury. Eur. J. Psychotraumatol. 2022, 13, 2031593. [Google Scholar] [CrossRef]
- Lima, B.B.; Hammadah, M.; Wilmot, K.; Pearce, B.D.; Shah, A.; Levantsevych, O.; Kaseer, B.; Obideen, M.; Gafeer, M.M.; Kim, J.H.; et al. posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav. Immun. 2019, 75, 26–33. [Google Scholar] [CrossRef]
- Tripathi, K.; Demiray, Y.E.; Kliche, S.; Jing, L.; Hazra, S.; Hazra, J.D.; Richter-Levin, G.; Stork, O. Reducing glutamic acid decarboxylase in the dorsal dentate gyrus attenuates juvenile stress induced emotional and cognitive deficits. Neurobiol. Stress 2021, 15, 100350. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.B.; Pinna, G.; Barros, H.M.T. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Hunter, S.C.; Dhinojwala, M.; Ghebrezadik, D.; Guo, J.; Inoue, K.; Young, L.J.; Dias, B.G. Incerto-thalamic modulation of fear via GABA and dopaminę. Neuropsychopharmacology 2021, 46, 1658–1668. [Google Scholar] [CrossRef]
- Arditte Hall, K.A.; DeLane, S.E.; Anderson, G.M.; Lago, T.R.; Shor, R.; Wang, W.; Rasmusson, A.M.; Pineles, S.L. Plasma gamma-aminobutyric acid (GABA) levels and posttraumatic stress disorder symptoms in trauma-exposed women: A preliminary report. Psychopharmacology 2021, 238, 1541–1552. [Google Scholar] [CrossRef]
- Albrecht, A.; Ben-Yishay, E.; Richter-Levin, G. Behavioral profiling reveals an enhancement of dentate gyrus paired pulse inhibition in a rat model of PTSD. Mol. Cell. Neurosci. 2021, 111, 103601. [Google Scholar] [CrossRef]
- Waguespack, H.F.; Aguilar, B.L.; Malkova, L.; Forcelli, P.A. Inhibition of the Deep and Intermediate Layers of the Superior Colliculus Disrupts Sensorimotor Gating in Monkeys. Front. Behav. Neurosci. 2020, 14, 610702. [Google Scholar] [CrossRef]
- Gaspersz, R.; Lamers, F.; Wittenberg, G.; Beekman, A.T.F.; van Hemert, A.M.; Schoevers, R.A.; Penninx, B.W.J.H. The role of anxious distress in immune dysregulation in patients with major depressive disorder. Transl. Psychiatry 2017, 7, 1268. [Google Scholar] [CrossRef] [PubMed]
- Gebresenbet, E.A.; Zegeye, S.; Biratu, T.D. Prevalence and associated factors of depression and posttraumatic stress disorder among trauma patients: Multi-centered cross-sectional study. Front. Psychiatry 2025, 16, 1447232. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.M.; Bovin, M.J.; Günak, M.M.; Lawrence, K.A.; Pietrzak, R.H. Psychometric properties, factor structure, and functional correlates of the PTSD checklist for DSM-5 in a U.S. national sample of older veterans. Int. Psychogeriatr. 2025, 37, 100027. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.A.; Chen, Q.; Roberts, A.L.; Winning, A.; Rimm, E.B.; Gilsanz, P.; Glymour, M.M.; Tworoger, S.S.; Koenen, K.C.; Kubzansky, L.D. Cross-Sectional and Longitudinal Associations of Chronic Posttraumatic Stress Disorder With Inflammatory and Endothelial Function Markers in Women. Biol. Psychiatry 2017, 82, 875–884. [Google Scholar] [CrossRef]
- Foa, E.B.; McLean, C.P.; Zang, Y.; Zhong, J.; Powers, M.B.; Kauffman, B.Y.; Rauch, S.; Porter, K.; Knowles, K. Psychometric properties of the Posttraumatic Diagnostic Scale for DSM-5 (PDS-5). Psychol. Assess. 2016, 28, 1166–1171. [Google Scholar] [CrossRef]
- Lotzin, A.; Laskowsky, I. Feasibility of a breath robot intervention to reduce sleep problems in posttraumatic stress disorder: Protocol for a randomized controlled study. Pilot Feasibility Stud. 2024, 10, 24. [Google Scholar] [CrossRef]
- Rahmat, S.; Velez, J.; Farooqi, M.; Smiley, A.; Prabhakaran, K.; Rhee, P.; Dornbush, R.; Ferrando, S.; Smolin, Y. Post-traumatic stress disorder can be predicted in hospitalized blunt trauma patients using a simple screening tool. Trauma Surg. Acute Care Open 2021, 6, e000623. [Google Scholar] [CrossRef]
- Kredlow, M.A.; Fitzgerald, H.E.; Carpenter, J.K.; Taghian, N.R.; Otto, M.W.; Hofmann, S.G.; Phelps, E.A. Recurrent negative autobiographical memories and mental health. J. Mood Anxiety Disord. 2024, 8, 100074. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.R.; Jaeb, M.; Dunsmoor, J.E.; Stowe, Z.N.; Cisler, J.M. Decoding threat neurocircuitry representations during traumatic memory recall in PTSD. Neuropsychopharmacology 2025, 50, 568–575. [Google Scholar] [CrossRef]
- Rattel, J.A.; Danböck, S.; Miedl, S.F.; Liedlgruber, M.; Wilhelm, F.H. Hitting the Rewind Button: Imagining Analogue Trauma Memories in Reverse Reduces Distressing Intrusions. Cognit. Ther. Res. 2024, 48, 932–943. [Google Scholar] [CrossRef]
- Kuan, P.F.; Clouston, S.; Yang, X.; Kotov, R.; Bromet, E.; Luft, B.J. Molecular linkage between post-traumatic stress disorder and cognitive impairment: A targeted proteomics study of World Trade Center responders. Transl. Psychiatry 2020, 10, 269. [Google Scholar] [CrossRef]
- Bomyea, J.; Caudle, M.M.; Bartolovich, A.L.; Simmons, A.N.; Jak, A.J.; Golshan, S. Randomized controlled trial of computerized working memory training for Veterans with PTSD. J. Psychiatr. Res. 2025, 181, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Al-Roub, A.; Akhter, N.; Al-Rashed, F.; Wilson, A.; Alzaid, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. TNFα induces matrix metalloproteinase-9 expression in monocytic cells through ACSL1/JNK/ERK/NF-kB signaling pathways. Sci. Rep. 2023, 13, 14351. [Google Scholar] [CrossRef] [PubMed]
- Potik, D.; Einat, T.; Idisis, Y. Posttraumatic Stress Disorder Symptom Clusters, Exposure to Potentially Morally Injurious Events, and Aggression Among Army Veterans. Clin. Psychol. Psychother. 2024, 31, e3056. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.; Ma, L.; Kong, F. Sodium aescinate improve behavioral performance by inhibiting dorsal raphe nucleus NLRP3 inflammasome in Post-traumatic stress disorder Rat Model. Biochem. Biophys. Res. Commun. 2023, 671, 166–172. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Lu, Y.; Li, X.; Liao, Y.; Peng, Z.; Li, Y.; Hou, L.; Yuan, Z.; Cheng, J. Key Mechanisms and Potential Targets of the NLRP3 Inflammasome in Neurodegenerative Diseases. J. Neuroinflammation 2020, 17, 205. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Gan, Y.; Mi, Z.; Luo, S.; Lei, J.; Fang, Q.; Li, H. Tabersonine ameliorates depressive-like behavior by inhibiting NLRP3 inflammasome activation in a mouse model. Neuropharmacology 2025, 273, 110432. [Google Scholar] [CrossRef]
- Akcay, E.; Karatas, H. P2X7 receptors from the perspective of NLRP3 inflammasome pathway in depression: Potential role of cannabidiol. Brain Behav. Immun. Health 2024, 41, 100853. [Google Scholar] [CrossRef]
- Gross, G.M.; Spiller, T.R.; Duek, O.; Pietrzak, R.H.; Harpaz-Rotem, I. Clinical significance of novel 8-factor model of DSM-5 PTSD in national VA PTSD residential treatment data: Internally- v. externally-cued intrusions. J. Affect. Disord. 2023, 328, 255–260. [Google Scholar] [CrossRef]
- Tanriverdi, B.; Gregory, D.F.; Olino, T.M.; Ely, T.D.; Harnett, N.G.; van Rooij, S.J.H.; Lebois, L.A.M.; Seligowski, A.V.; Jovanovic, T.; Ressler, K.J.; et al. Hippocampal Threat Reactivity Interacts with Physiological Arousal to Predict PTSD Symptoms. J. Neurosci. 2022, 42, 6593–6604. [Google Scholar] [CrossRef]
- Colak, M.; Sireli, O. Mediator role of cognitive distortions in the relationship between posttraumatic stress disorder symptoms and peritraumatic stress levels. J. Affect. Disord. 2025, 379, 282–288. [Google Scholar] [CrossRef]
- Adonis, M.; Loucaides, M.; Sullman, M.J.M.; Lajunen, T. The protective role of self compassion in trauma recovery and its moderating impact on post traumatic symptoms and post traumatic growth. Sci. Rep. 2025, 15, 8145. [Google Scholar] [CrossRef] [PubMed]
- Sanger, B.D.; Alarachi, A.; McNeely, H.E.; McKinnon, M.C.; McCabe, R.E. Brain Fog and Cognitive Dysfunction in Posttraumatic Stress Disorder: An Evidence-Based Review. Psychol. Res. Behav. Manag. 2025, 18, 589–606. [Google Scholar] [CrossRef]
- Konrad, A.C.; Miu, A.C.; Trautmann, S.; Kanske, P. Neural correlates and plasticity of explicit emotion regulation following the experience of trauma. Front. Behav. Neurosci. 2025, 19, 1523035. [Google Scholar] [CrossRef] [PubMed]
- Allbaugh, L.J.; Marinack, L.; Pickover, A.M.; Powers, A.; Marshall Lee, E.D.; Cloitre, M.; Kaslow, N.J. Understanding emotion dysregulation in PTSD—GAD comorbidity. J. Anxiety Disord. 2025, 110, 102985. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.D.; Comer, J.S.; Keane, T.M.; Checko Scioli, E.; Pincus, D.B. Intensive Treatment of Chronic Pain and PTSD: The PATRIOT Program. Behav. Sci. 2024, 14, 1103. [Google Scholar] [CrossRef]
- Deng, J.; Lin, X.; Zheng, Y.; Su, S.; Liu, X.; Yuan, K.; Shi, L.; Bao, Y.; Lu, L. Manipulating critical memory periods to treat psychiatry disorders. Sci. Bull. 2023, 68, 2477–2486. [Google Scholar] [CrossRef]
- R Core Team: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2024. Available online: https://www.R-project.org/ (accessed on 18 April 2025).
- Harrell, F., Jr. Hmisc: Harrell Miscellaneous. R Package, Version 5.2-0. 2024. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 18 April 2025).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package, Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 18 April 2025).
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.; Wiernik, B. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption. CRAN. 2024. Available online: https://easystats.github.io/report (accessed on 18 April 2025).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. R Package, Version 1.1-3. 2022. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 18 April 2025).
- Sjoberg, D.; Whiting, K.; Curry, M.; Lavery, J.; Larmarange, J. Reproducible Summary Tables with the gtsummary Package. R J. 2024, 13, 570–580. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix. (Version 0.94). 2024. Available online: https://github.com/taiyun/corrplot (accessed on 18 April 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 18 April 2025).
- Wickham, H. Stringr: Simple, Consistent Wrappers for Common String Operations. R Package, Version 1.5.1. 2023. Available online: https://CRAN.R-project.org/package=stringr (accessed on 18 April 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 18 April 2025).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data. R Package, Version 1.3.1. 2024. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 18 April 2025).

| Characteristic | N | Past PTSD (≤5 y) (N = 33) | Past PTSD (>5 y) (N = 31) | No PTSD (Control) (N = 28) | p |
|---|---|---|---|---|---|
| MMP-2, ng/mL | |||||
| All ages (18–50 years) | 92 | 21.66 a (16.90–23.80) | 5.34 b (2.81–12.55) | 1.75 c (1.37–2.11) | <0.001 |
| 18–35 years | 45 | 23.09 a (19.96–24.03) | 4.88 b (3.42–10.35) | 1.92 c (1.56–2.15) | <0.001 |
| 36–50 years | 47 | 20.74 a (15.48–23.27) | 5.78 b (2.61–12.51) | 1.42 c (1.11–1.96) | <0.001 |
| MMP-9, ng/mL | <0.001 | ||||
| All ages (18–50 years) | 92 | 418.70 a (341.08–558.19) | 175.00 b (66.64–306.66) | 48.99 c (39.77–58.23) | <0.001 |
| 18–35 years | 45 | 390.13 a (351.56–476.93) | 150.73 b (70.97–289.14) | 47.64 c (42.47–52.88) | <0.001 |
| 36–50 years | 47 | 460.07 a (341.08–558.19) | 197.50 b (65.47–320.99) | 51.00 c (36.51–60.64) | <0.001 |
| GABA, nmol/L | <0.001 | ||||
| All ages (18–50 years) | 92 | 54.33 a (43.68–65.87) | 133.73 b (84.67–191.29) | 406.94 c (259.67–454.33) | <0.001 |
| 18–35 years | 45 | 52.29 a (45.32–63.68) | 106.42 b (86.42–165.31) | 406.94 c (319.98–521.89) | <0.001 |
| 36–50 years | 47 | 60.40 a (36.30–65.87) | 160.14 b (84.79–245.58) | 406.53 c (230.99–452.47) | <0.001 |
| NLRP3, pg/mL | <0.001 | ||||
| All ages (18–50 years) | 92 | 791.50 a (693.23–832.14) | 282.80 b (176.51–543.72) | 111.41 c (87.50–131.37) | <0.001 |
| 18–35 years | 45 | 810.84 a (798.15–842.00) | 240.74 b (186.69–544.02) | 115.33 c (107.32–130.73) | <0.001 |
| 36–50 years | 47 | 693.23 a (615.89–788.30) | 339.08 b (164.60–536.33) | 100.56 c (68.02–130.13) | <0.001 |
| Characteristic | N | Past PTSD (≤5 y) (N = 33) | Past PTSD (>5 y) (N = 31) | No PTSD (Control) (N = 28) | p |
|---|---|---|---|---|---|
| PSSI-5 Re-experiencing | |||||
| All ages (18–50 years) | 92 | 18.00 a (17.00–18.00) | 17.00 b (15.00–18.00) | 4.00 c (1.75–7.25) | <0.001 |
| 18–35 years | 45 | 17.00 a (16.00–18.00) | 17.00 a (15.00–19.00) | 3.50 b (1.25–4.00) | <0.001 |
| 36–50 years | 47 | 18.00 a (18.00–19.00) | 17.00 a (16.00–18.00) | 6.50 b (3.50–8.00) | <0.001 |
| PSSI-5 Avoidance | <0.001 | ||||
| All ages (18–50 years) | 92 | 7.00 a (7.00–8.00) | 6.00 b (4.00–7.00) | 2.00 c (1.00–3.00) | <0.001 |
| 18–35 years | 45 | 7.00 a (7.00–8.00) | 6.00 a (4.00–7.00) | 1.00 b (0.25–2.00) | <0.001 |
| 36–50 years | 47 | 8.00 a (7.00–8.00) | 5.50 b (4.00–7.00) | 3.00 c (1.00–4.00) | <0.001 |
| PSSI-5 Changes in Cognition and Mood | <0.001 | ||||
| All ages (18–50 years) | 92 | 26.00 a (22.00–26.00) | 27.00 a (25.00–27.50) | 4.00 b (2.00–4.25) | <0.001 |
| 18–35 years | 45 | 25.50 a (22.00–26.50) | 27.00 a (22.50–27.00) | 3.50 b (2.00–4.00) | <0.001 |
| 36–50 years | 47 | 26.00 a (25.00–26.00) | 27.00 a (26.00–28.00) | 4.00 b (2.00–7.50) | <0.001 |
| PSSI-5 Increased Arousal and Reactivity | <0.001 | ||||
| All ages (18–50 years) | 92 | 15.00 a (12.00–22.00) | 20.00 b (16.50–22.50) | 6.00 c (5.00–8.25) | <0.001 |
| 18–35 years | 45 | 22.00 a (14.75–24.50) | 21.00 b (20.00–23.50) | 7.00 b (6.00–9.00) | <0.001 |
| 36–50 years | 47 | 12.00 a (12.00–15.00) | 16.50 b (15.75–22.00) | 5.00 c (5.00–5.75) | <0.001 |
| PSSI-5 Distress and Interference | <0.001 | ||||
| All ages (18–50 years) | 92 | 6.00 a (5.00–7.00) | 7.00 a (5.00–7.00) | 2.00 b (1.00–3.25) | <0.001 |
| 18–35 years | 45 | 7.00 a (4.75–8.00) | 7.00 a (6.00–7.00) | 2.00 b (0.25–3.00) | <0.001 |
| 36–50 years | 47 | 5.00 a (5.00–6.00) | 6.50 a (5.00–7.00) | 2.00 b (2.00–3.50) | <0.001 |
| PSSI-5 Symptom Onset and Duration | <0.001 | ||||
| All ages (18–50 years) | 92 | 6.00 a (5.00–8.00) | 6.00 a (5.00–8.00) | 2.00 b (1.00–2.00) | <0.001 |
| 18–35 years | 45 | 5.50 a (5.00–7.00) | 5.00 a (3.50–5.50) | 1.50 b (1.00–2.75) | <0.001 |
| 36–50 years | 47 | 7.00 a (5.00–8.00) | 8.00 a (5.75–8.00) | 2.00 b (1.00–2.00) | <0.001 |
| PSSI-5 Total | <0.001 | ||||
| All ages (18–50 years) | 92 | 77.00 a (72.00–82.00) | 81.00 a (75.00–84.50) | 20.50 b (17.00–24.25) | <0.001 |
| 18–35 years | 45 | 80.00 a (76.00–85.75) | 83.00 a (75.00–85.00) | 18.00 b (17.00–21.75) | <0.001 |
| 36–50 years | 47 | 77.00 a (70.00–79.00) | 80.50 a (75.00–83.00) | 23.50 b (18.25–26.50) | <0.001 |
| Characteristic | Total (N = 92) | Past PTSD (≤5 y) (N = 33) | Past PTSD (>5 y) (N = 31) | No PTSD (Control) (N = 28) | p |
|---|---|---|---|---|---|
| Age, years | 34.0 (27.0–41.0) | 34.0 (31.0–41.0) | 36.0 (29.5–41.0) | 33.5 (24.3–41.5) | 0.524 |
| Employment in hazardous conditions, years | 10.0 (6.0–14.0) | 11.0 (7.0–14.0) | 10.0 (7.5–15.0) | 10.0 (3.0–14.0) | 0.418 |
| BMI, kg/m2 | 23.0 (22.0–26.0) | 22.0 a (22.0–24.0) | 22.0 a (20.0–24.5) | 25.0 b (22.0–28.3) | 0.011 |
| Daily cigarette consumption, cigarettes/day | 5.0 (0.0–20.0) | 5.0 b (5.0–20.0) | 5.0 ab (2.5–20.0) | 1.0 a (0.0–6.3) | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzesińska, A.; Ogłodek, E.A. Involvement of Matrix Metalloproteinases (MMP-2 and MMP-9), Inflammasome NLRP3, and Gamma-Aminobutyric Acid (GABA) Pathway in Cellular Mechanisms of Neuroinflammation in PTSD. Int. J. Mol. Sci. 2025, 26, 5662. https://doi.org/10.3390/ijms26125662
Grzesińska A, Ogłodek EA. Involvement of Matrix Metalloproteinases (MMP-2 and MMP-9), Inflammasome NLRP3, and Gamma-Aminobutyric Acid (GABA) Pathway in Cellular Mechanisms of Neuroinflammation in PTSD. International Journal of Molecular Sciences. 2025; 26(12):5662. https://doi.org/10.3390/ijms26125662
Chicago/Turabian StyleGrzesińska, Anna, and Ewa Alicja Ogłodek. 2025. "Involvement of Matrix Metalloproteinases (MMP-2 and MMP-9), Inflammasome NLRP3, and Gamma-Aminobutyric Acid (GABA) Pathway in Cellular Mechanisms of Neuroinflammation in PTSD" International Journal of Molecular Sciences 26, no. 12: 5662. https://doi.org/10.3390/ijms26125662
APA StyleGrzesińska, A., & Ogłodek, E. A. (2025). Involvement of Matrix Metalloproteinases (MMP-2 and MMP-9), Inflammasome NLRP3, and Gamma-Aminobutyric Acid (GABA) Pathway in Cellular Mechanisms of Neuroinflammation in PTSD. International Journal of Molecular Sciences, 26(12), 5662. https://doi.org/10.3390/ijms26125662






