Plasma Concentrations of Matrilysins (MMP-7, MMP-26) and Stromelysins (MMP-3, MMP-10) as Diagnostic Biomarkers in High-Grade Serous Ovarian Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Plasma Levels for Tested Parameters in Serous High-Grade Serous Ovarian Carcinoma Patients and in Control Groups
2.2. Correlation by Spearman’s Method
2.3. Diagnostic Criteria of Tested MMPs: CA 125 and HE-4
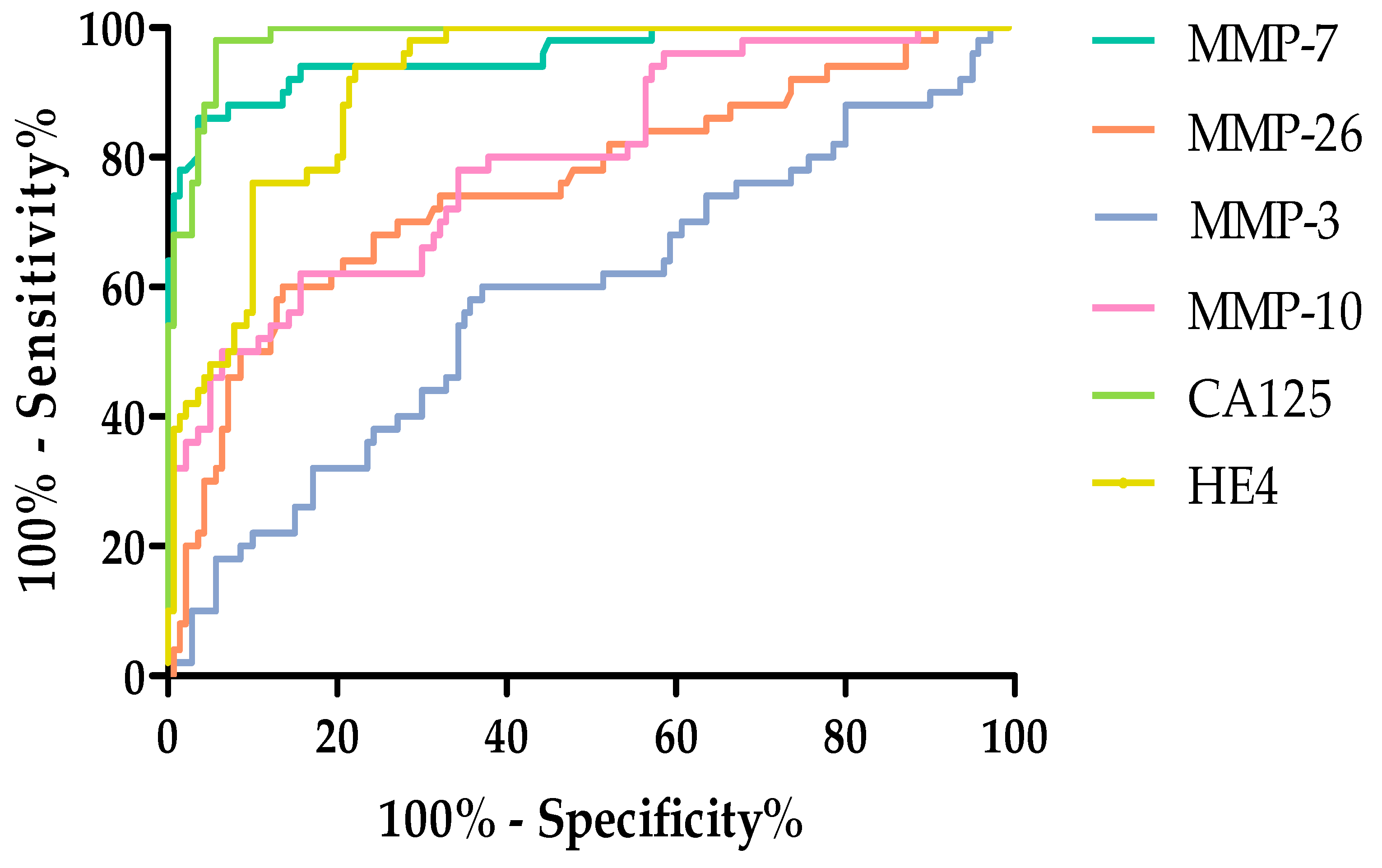
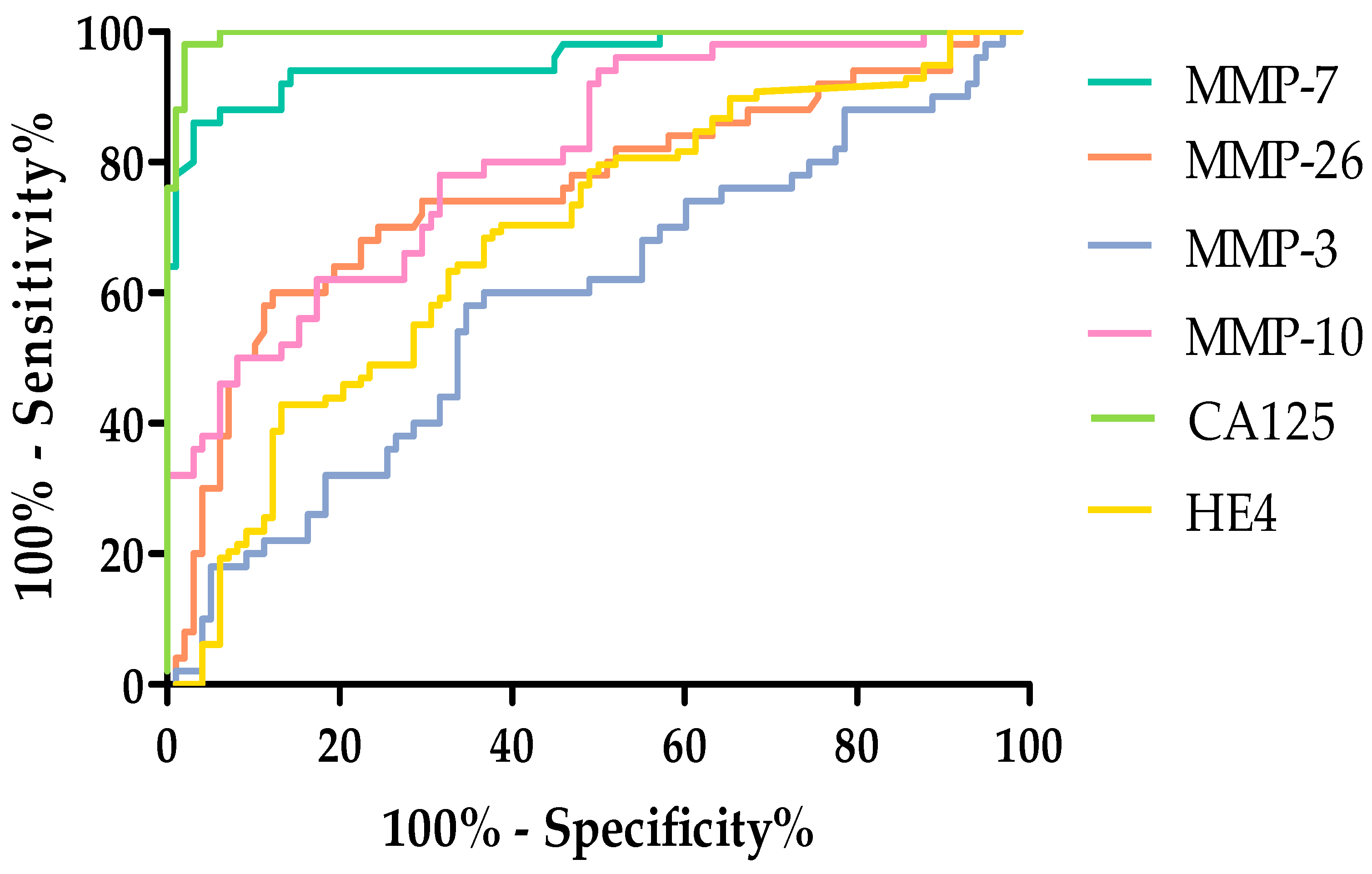
2.4. Evaluation of the Diagnostic Power of Tests by ROC Function
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Biochemical Analyses
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMP-7 | Metalloproteinase 7 |
| MMP-3 | Metalloproteinase 3 |
| MMP-10 | Metalloproteinase 10 |
| MMP-26 | Metalloproteinase 26 |
| CA125 | Cancer antigen 125 |
| HE4 | Human epididymis protein 4 |
| OC | Ovarian cancer |
| BL | Begin lesion |
| HW | Healthy women |
| MMPs | Matrix metalloproteinases |
| ELISA | Enzyme-linked immunosorbent assay |
| CMIA | Chemiluminescent Microparticle Immuno Assay |
| FIGO | International Federation of Gynecology and Obstetrics |
| USG | Medical ultrasound |
| SE | Diagnostic sensitivity |
| SP | Diagnostic specificity |
| PPV | Diagnostic positive predictive value |
| NPV | Diagnostic negative predictive value |
| ACC | Diagnostic accuracy |
References
- World Ovarian Cancer Coalition. World Ovarian Cancer Coalition Atlas. 2023. Available online: https://worldovariancancercoalition.org/about-ovarian-cancer/what-is-ovarian-cancer/ (accessed on 11 May 2025).
- Kicman, A.; Niczyporuk, M.; Kulesza, M.; Motyka, J.; Ławicki, S. Utility of Matrix Metalloproteinases in the Diagnosis. Monitoring and Prognosis of Ovarian Cancer Patients. Cancer Manag. Res. 2022, 14, 3359–3382. [Google Scholar] [CrossRef] [PubMed]
- Atallah, G.A.; Abd Aziz, N.H.; Teik, C.K.; Shafiee, M.N.; Kampan, N.C. New Predictive Biomarkers for Ovarian Cancer. Diagnostics 2021, 11, 465. [Google Scholar] [CrossRef]
- Kicman, A.; Gacuta, E.; Kulesza, M.; Będkowska, E.G.; Marecki, R.; Klank-Sokołowska, E.; Knapp, P.; Niczyporuk, M.; Ławicki, S. Diagnostic Utility of Selected Matrix Metalloproteinases (MMP-2. MMP-3. MMP-11. MMP-26). HE4. CA125 and ROMA Algorithm in Diagnosis of Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 6265. [Google Scholar] [CrossRef] [PubMed]
- Kicman, A.; Gacuta, E.; Marecki, R.; Kicman., M.S.; Kulesza., M.; Klank-Sokołowska., E.; Knapp., P.; Niczyporuk., M.; Szmitkowski., M.; Ławicki., S. Diagnostic Utility of Metalloproteinases from Collagenase Group (MMP-1. MMP-8 and MMP-13) in Biochemical Diagnosis of Ovarian Carcinoma. Cancers 2024, 16, 3969. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Zhuo, L.; Sun, K.; Meng, F.; Zhou, M.; Sun, J. Prediction of Prognosis and Treatment Response in Ovarian Cancer Patients from Histopathology Images Using Graph Deep Learning: A Multicenter Retrospective Study. Eur. J. Cancer 2024, 199, 113532. [Google Scholar] [CrossRef]
- Gęca, K.; Litwiński, J.; Ostrowski, T.; Świetlicka, I.; Polkowski, W.P.; Skórzewska, M. Exploring the Survival Determinants in Recurrent Ovarian Cancer: The Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Cancers 2024, 16, 2150. [Google Scholar] [CrossRef]
- Kulesza, M.; Guszczyn, T.; Kicman, A.; Ławicki, S. Title Changes in Plasma Levels of Selected Matrix Metalloproteinases (MMPs) Enzymes in Patients with Osgood–Schlatter Disease (OSD). J. Clin. Med. 2024, 13, 5655. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, M.; Kicman, A.; Motyka, J.; Guszczyn, T.; Ławicki, S. Importance of Metalloproteinase Enzyme Group in Selected Skeletal System Diseases. Int. J. Mol. Sci. 2023, 24, 17139. [Google Scholar] [CrossRef]
- Lubowicka, E.; Gacuta, E.; Zajkowska, M.; Głażewska, E.K.; Przylipiak, A.; Chrostek, L.; Zbucka-Krętowska, M.; Ławicki, S. The plasma levels and diagnostic utility of matrix metalloproteinase-9 and CA 125 in cervical cancer patients. Pol. Merkur. Lekarski. 2017, 43, 10–14. [Google Scholar]
- Sidorkiewicz, I.; Piskór, B.; Dąbrowska, E.; Guzińska-Ustymowicz, K.; Pryczynicz, A.; Zbucka-Krętowska, M.; Ławicki, S. Plasma Levels and Tissue Expression of Selected Cytokines. Metalloproteinases and Tissue Inhibitors in Patients with Cervical Cancer. Anticancer Res. 2019, 39, 6403–6412. [Google Scholar] [CrossRef]
- Padežnik, T.; Oleksy, A.; Cokan, A.; Takač, I.; Sobočan, M. Changes in the Extracellular Matrix in Endometrial and Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 5463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef] [PubMed]
- Będkowska, G.E.; Gacuta, E.; Zajkowska, M.; Głażewska, E.K.; Osada, J.; Szmitkowski, M.; Chrostek, L.; Dąbrowska, M.; Ławicki, S. Plasma Levels of MMP-7 and TIMP-1 in Laboratory Diagnostics and Differentiation of Selected Histological Types of Epithelial Ovarian Cancers. J. Ovarian Res. 2017, 10, 39. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Da, C.-M.; Liao, B.; Zhang, H.-H. Roles of Matrix Metalloproteinase-7 (MMP-7) in Cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef]
- Vos, M.C.; Van Der Wurff, A.A.M.; Bulten, J.; Kruitwagen, R.; Feijen, H.; Van Kuppevelt, T.H.; Hendriks, T.; Massuger, L.F.A.G. Limited Independent Prognostic Value of MMP-14 and MMP-2 Expression in Ovarian Cancer. Diagn. Pathol. 2016, 11, 34. [Google Scholar] [CrossRef]
- Jeleniewicz, W.; Cybulski, M.; Nowakowski, A.; Stenzel-Bembenek, A.; Guz, M.; Marzec-Kotarska, B.; Kotarski, J.; Stepulak, A. MMP-2 mRNA Expression in Ovarian Cancer Tissues Predicts Patients’ Response to Platinum-Taxane Chemotherapy. Anticancer Res. 2019, 39, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Qian, J.; Zhu, F.; Wu, F.; Zhao, H.; Zhu, H. The Prognostic Values of Matrix Metalloproteinases in Ovarian Cancer. J. Int. Med. Res. 2020, 48, 0300060519825983. [Google Scholar] [CrossRef]
- Sillanpää, S.M.; Anttila, M.A.; Voutilainen, K.A.; Ropponen, K.M.; Sironen, R.K.; Saarikoski, S.V.; Kosma, V. Prognostic Significance of Matrix Metalloproteinase-7 in Epithelial Ovarian Cancer and Its Relation to Β-catenin Expression. Int. J. Cancer 2006, 119, 1792–1799. [Google Scholar] [CrossRef]
- Acar, A.; Onan, A.; Coskun, U.; Uner, A.; Bagriacik, U.; Atalay, F.; Unsal, D.K.; Guner, H. Clinical Significance of Serum MMP-2 and MMP-7 in Patients with Ovarian Cancer. Med. Oncol. 2008, 25, 279–283. [Google Scholar] [CrossRef]
- Gershtein, E.S.; Levkina, N.V.; Digayeva, M.A.; Laktionov, K.P.; Tereshkina, I.V.; Kushlinsky, N.E. Matrix Metalloproteinases 2. 7. and 9 and Tissue Inhibitor of Metalloproteinases-1 in Tumors and Serum of Patients with Ovarian Neoplasms. Bull. Exp. Biol. Med. 2010, 149, 628–631. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Bauerschlag, D.; Zhou, Y.; Sapinoso, L.M.; Ching, K.; Frierson, H.; Bräutigam, K.; Sehouli, J.; Stickeler, E.; Könsgen, D.; et al. An Integrated Clinical-Genomics Approach Identifies a Candidate Multi-Analyte Blood Test for Serous Ovarian Carcinoma. Clin. Cancer Res. 2007, 13, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Li, Y.; Zhang, L.; Deng, Y.; Qi, D.; Li, Y.; Li, W. Roles of Matrix Metalloproteinase-26 in the Growth. Invasion and Angiogenesis of Breast Cancer. Oncol. Lett. 2012, 4, 832–836. [Google Scholar] [CrossRef]
- Ripley, D.; Tunuguntla, R.; Susi, L.; Chegini, N. Expression of Matrix Metalloproteinase-26 and Tissue Inhibitors of Metalloproteinase-3 and -4 in Normal Ovary and Ovarian Carcinoma. Int. J. Gynecol. Cancer 2006, 16, 1794–1800. [Google Scholar] [CrossRef]
- Ławicki, P.; Malinowski, P.; Motyka, J.; Ławicki, M.; Kicman, A.; Kulesza, M.; Gacuta, E.; Guszczyn, T.; Januszkiewicz, M.; Zbucka-Krętowska, M.; et al. Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients. J. Clin. Med. 2023, 12, 2618. [Google Scholar] [CrossRef]
- Bogusiewicz, M.; Rechberger, T.; Jakimiuk, A.J.; Skorupski, P.; Jakowicki, J.A.; Postawski, K. Evaluation of matrix metalloproteinases-1 and -3 concentrations in the tunica albuginea. the apical wall of atretic follicles and the corpus luteum of normal human ovaries. Gynecol. Endocrinol. 2000, 14, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Machaliński, B.; Menkiszak, J.; Sompolska-Rzechuła, A. Suitability Assessment of Baseline Concentration of MMP3. TIMP3. HE4 and CA125 in the Serum of Patients with Ovarian Cancer. J. Ovarian Res. 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Sütő, R.; Pócsi, M.; Szabó, Z.; Fejes, Z.; Ivády, G.; Kerekes, G.; Fagyas, M.; Nagy, A.; Szentkereszty, Z.; Kappelmayer, J.; et al. Elevated Level of Serum Human Epididymis Protein 4 (HE4) Predicts Disease Severity and Mortality in COVID-19 Pneumonia. BMC Pulm. Med. 2023, 23, 512. [Google Scholar] [CrossRef]
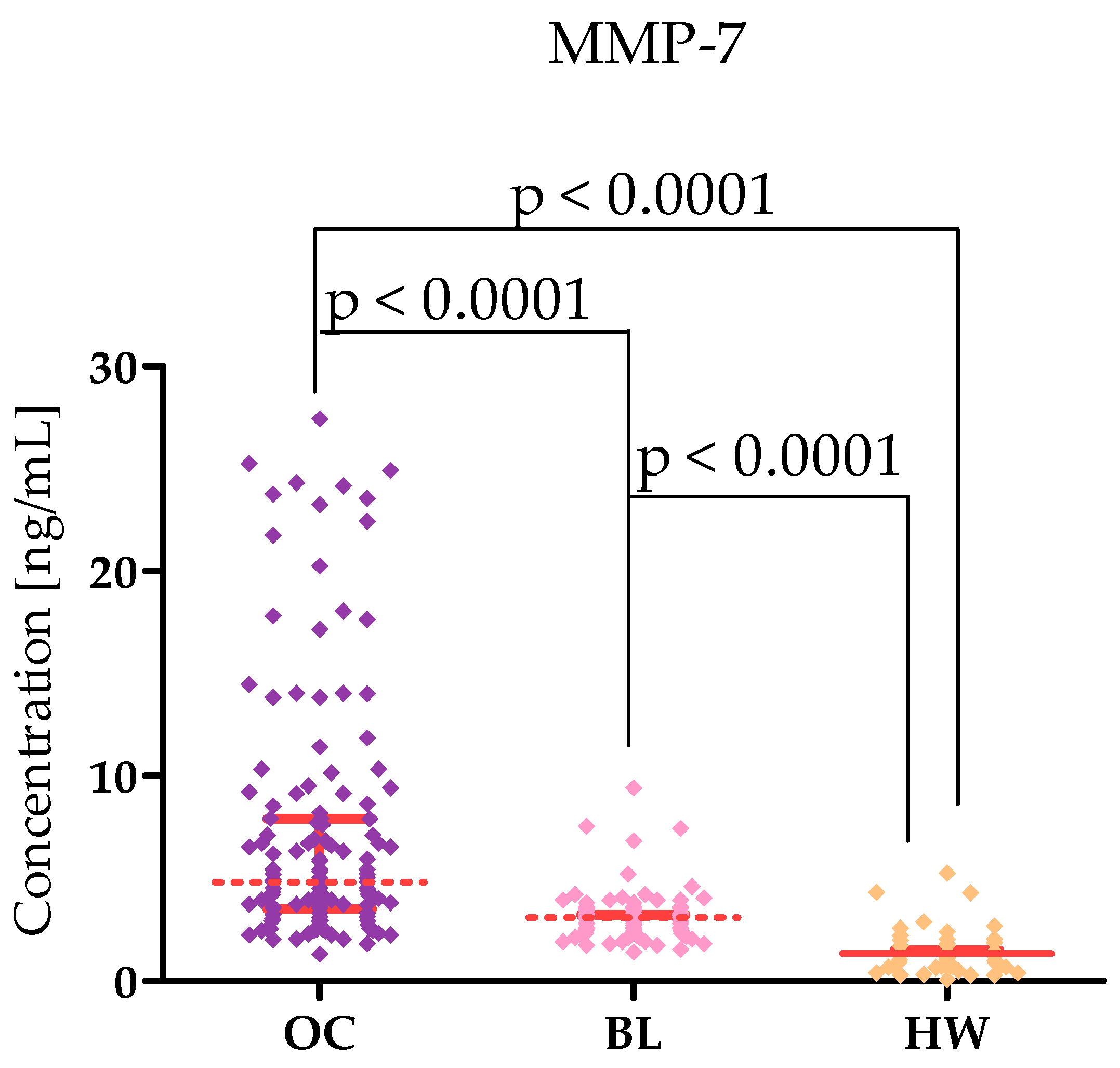

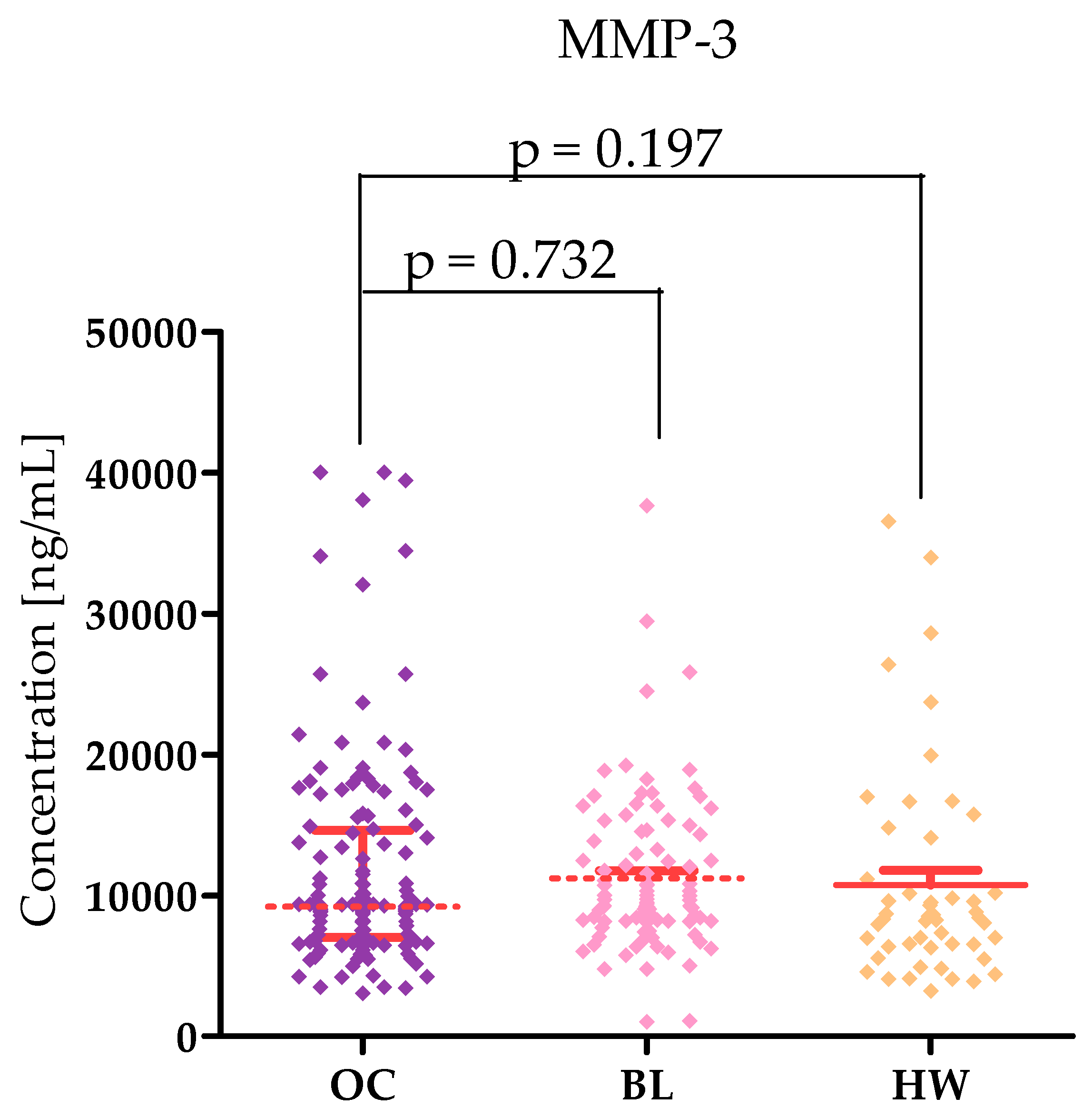
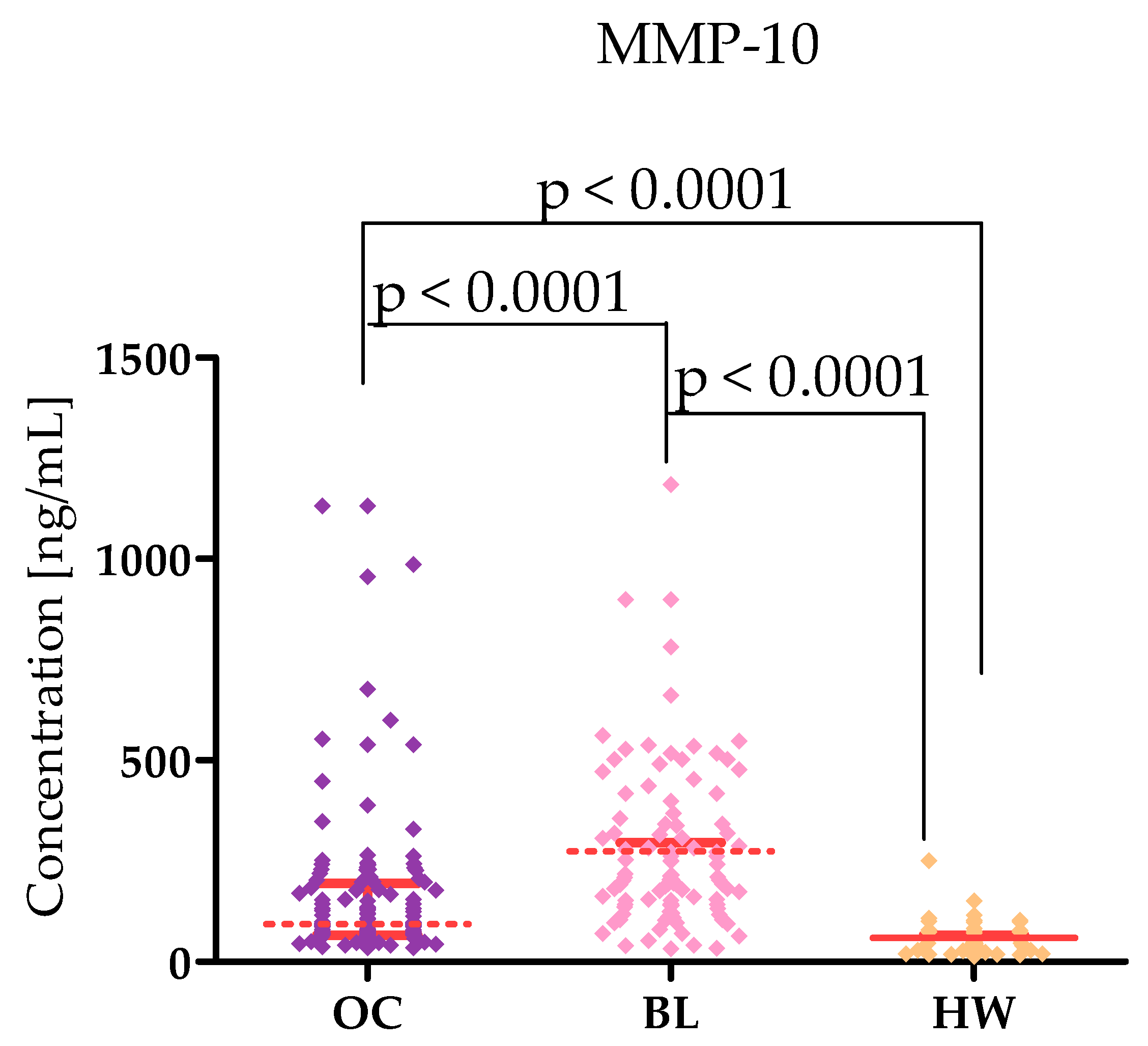

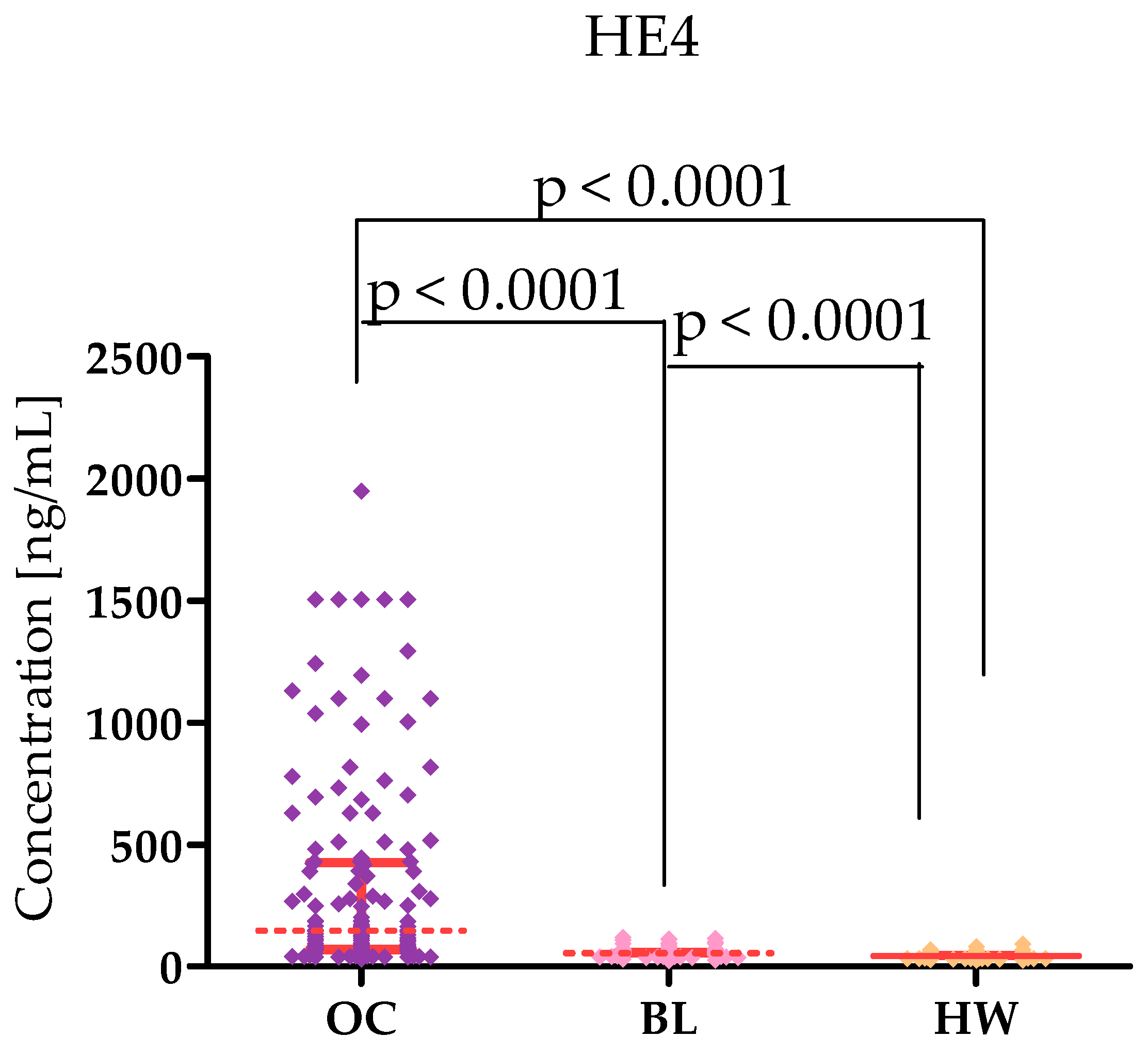

| Tested Parameters | Ovarian Cancer (OC) Stage I–II Median Range 95 Percentile 5 Percentile | Ovarian Cancer (OC) Stage III–IV Median Range 95 Percentile 5 Percentile | Statistical Significance |
|---|---|---|---|
| MMP-7 [ng/mL] | 4.500 1.800–27.40 24.28 2.002 | 4.800 1.300–25.20 2.200 20.20 | p < 0.0001 |
| MMP-26 [pg/mL] | 9240 5850–47,950 44,383 6092 | 9220 3578–47,950 23,455 6175 | p < 0.0001 |
| MMP-3 [pg/mL] | 9065 4159–39,441 25,378 4224 | 9328 3033–40,000 34,304 4179 | p = 0.197 |
| MMP-10 [ng/mL] | 87.06 38.90–1131 566.3 50.29 | 103.8 33.60–1131 551.4 41.40 | p < 0.0001 |
| CA125 [U/mL] | 105.1 17.10–1000 411.6 19.46 | 495.7 23.00–4113 2063 38.82 | p < 0.0001 |
| HE4 [U/mL] | 182.4 28.90–1944 1222 36.35 | 140.4 35.57–1500 1500 37.93 | p < 0.0001 |
| Parameter | MMP-7 | MMP-26 | MMP-3 | MMP-10 | CA125 | HE4 |
|---|---|---|---|---|---|---|
| Serous Ovarian Cancer | ||||||
| MMP-7 | - | r = −0.2519 p = 0.0119 | r = −0.1741 p = 0.0849 | r = −0.08830 p = 0.3848 | r = 0.02867 p = 0.9775 | r = 0.07760 p = 0.4452 |
| MMP-26 | r = 0.02042 p = 0.8107 | - | r = −0.2086 p = 0.0134 | r = −0.2312 p = 0.0060 | r = 0.1725 p = 0.0415 | r = −0.2164 p = 0.0102 |
| MMP-3 | r = −0.06679 p = 0.4330 | r = −0.02095 p = 0.8060 | - | r = 0.1963 p = 0.0201 | r = 0.07582 p = 0.3732 | r = 0.04334 p = 0.6112 |
| MMP-10 | r = −0.2030 p = 0.0161 | r = 0.2168 p = 0.0101 | r = 0.1963 p = 0.0201 | - | r = 0.07967 p = 0.3494 | r = 0.2131 p = 0.0115 |
| Benign lesion | ||||||
| MMP-7 | - | r = 0.1666 p = 0.0993 | r = 0.03748 p = 0.7126 | r = −0.1047 p = 0.3025 | r = −0.04681 p = 0.6455 | r = 0.02135 p = 0.8339 |
| MMP-26 | r = 0.1666 p = 0.0993 | - | r = 0.126 p =0.952 | r = 0.03624 p = 0.7218 | r = 0.05994 p = 0.9530 | r = −0.1387 p = 0.1709 |
| MMP-3 | r = 0.03748 p = 0.7126 | r = 0.05994 p = 0.9530 | - | r = 0.1118 p = 0.2704 | r = 0.04293 p = 0.6731 | r = −0.06714 p = 0.5090 |
| MMP-10 | r = −0.1047 p = 0.3025 | r = 0.03624 p = 0.7218 | r = 0.1118 p = 0.2704 | - | r = −0.05854 p = 0.5649 | r = −0.1463 p = 0.1486 |
| Healthy women | ||||||
| MMP-7 | - | r = 0.1145 p = 0.4284 | r = −0.2479 p = 0.0825 | r = 0.2146 p = 0.1346 | r = 0.2308 p = 0.1069 | r = 0.1665 p = 0.365 |
| MMP-26 | r = 0.1145 p = 0.4284 | - | r = 0.07582 p = 0.6007 | r = −0.1023 p = 0.4797 | r = −0.03011 p = 0.9836 | r = 0.07276 p = 0.6193 |
| MMP-3 | r = −0.2479 p = 0.0825 | r = 0.07582 p = 0.6007 | - | r = 0.1427 p = 0.3228 | r = 0.03492 p = 0.8098 | r = 0.08869 p = 0.5402 |
| MMP-10 | r = 0.2146 p = 0.1346 | r = −0.1023 p = 0.4797 | r = 0.1427 p = 0.3228 | - | r = −0.1389 p = 0.3362 | r = −0.1787 p = 0.2144 |
| SE | SP | PPV | NPV | ACC | ||
|---|---|---|---|---|---|---|
| MMP-3 | value [%] | 64% | 60% | 76.2% | 45.45% | 62.67% |
| 95 cl [%] | 53.79–73.36% | 45.18–73.59% | 68.85–82.25% | 37.10–54.08% | 54.40–70.42% | |
| MMP-7 | value [%] | 94% | 86% | 93.07% | 87.76% | 91.33% |
| 95 cl [%] | 87.40–97.77% | 73.26–94.18% | 87.09–96.40% | 76.60–94.01% | 85.64–95.30% | |
| MMP-10 | value [%] | 81% | 62% | 81% | 62% | 74.67% |
| 95 cl [%] | 71.93–88.16% | 47.17–75.35% | 74.71–86.02% | 50.76–72.09% | 66.93–81.41% | |
| MMP-26 | value [%] | 82% | 60% | 80.39% | 62.5% | 74.67% |
| 95 cl [%] | 73.05–88.97% | 45.18–73.59% | 74.26–85.35% | 50.88–72.84% | 66.93–81.41% | |
| CA125 | value [%] | 93% | 98% | 98.94% | 87.5% | 94.67% |
| 95 cl [%] | 86.11–97.14% | 89.35–99.95% | 93.03–99.85% | 77.39–93.47% | 89.76–97.67% | |
| HE-4 | value [%] | 75% | 94% | 96.15% | 65.28% | 81.33% |
| 95 cl [%] | 65.34–83.12% | 83.45–98.75% | 89.24–98.69% | 57.07–72.67% | 74.16–87.22% | |
| Stage I–II | ||||||
|---|---|---|---|---|---|---|
| SE | SP | PPV | NPV | ACC | ||
| MMP-3 | value [%] | 58.33% | 60.00% | 41.18% | 75.00% | 59.46% |
| 95 cl [%] | 36.64–77.89% | 45.18–73.59% | 30.24–53.06% | 63.97–83.53% | 47.41–70.73% | |
| MMP-7 | value [%] | 91.67% | 86.00% | 75.86% | 95.56% | 87.84% |
| 95 cl [%] | 73.00–98.97% | 73.26–94.18% | 61.01–86.33% | 85.02–98.79% | 78.16–94.29% | |
| MMP-10 | value [%] | 91.67% | 62.00% | 53.66% | 93.94% | 71.62% |
| 95 cl [%] | 73.00–98.97% | 47.17–75.35% | 44.34–62.73% | 80.16–98.35% | 59.95–81.50% | |
| MMP-26 | value [%] | 75.00% | 60.00% | 47.37% | 83.33% | 64.86% |
| 95 cl [%] | 53.29–90.23% | 45.18–73.59% | 37.38–57.57% | 70.69–91.20% | 52.89–75.61% | |
| CA125 | value [%] | 83.33% | 98.00% | 95.24% | 92.45% | 93.24% |
| 95 cl [%] | 62.62–95.26% | 89.35–99.95% | 74.02–99.29% | 83.34–96.77% | 84.93–97.77% | |
| HE-4 | value [%] | 83.33% | 94.00% | 86.96% | 92.16% | 92.16% |
| 95 cl [%] | 62.62–95.26% | 83.45–98.75% | 68.69–95.30% | 82.73–96.65% | 81.48–96.11% | |
| Stage III–IV | ||||||
|---|---|---|---|---|---|---|
| SE | SP | PPV | NPV | ACC | ||
| MMP-3 | value [%] | 66.22% | 60.00% | 71.01% | 54.55% | 63.71% |
| 95 cl [%] | 54.28–76.81% | 45.18–73.59% | 62.71–78.12% | 44.80–63.96% | 54.60–72.15% | |
| MMP-7 | value [%] | 96.00% | 86.00% | 91.14% | 93.48% | 92.00% |
| 95 cl [%] | 88.75–99.17% | 73.26–94.18% | 83.78–95.34% | 82.47–97.76% | 85.78–96.10% | |
| MMP-10 | value [%] | 78.67% | 60.00% | 74.68% | 65.22% | 71.20% |
| 95 cl [%] | 67.68–87.29% | 45.18–73.59% | 67.31–80.86% | 53.46–75.37% | 62.42–78.95% | |
| MMP-26 | value [%] | 85.33% | 60.00% | 76.19% | 73.17% | 75.20% |
| 95 cl [%] | 75.27–92.44% | 45.18–73.59% | 69.23–81.99% | 60.16–83.12% | 66.68–82.49% | |
| CA125 | value [%] | 97.33% | 98.00% | 98.65% | 96.08% | 97.60% |
| 95 cl [%] | 90.70–99.68% | 89.35–99.95% | 91.29–99.80% | 86.19–98.97% | 93.15–99.50% | |
| HE-4 | value [%] | 73.33% | 94.00% | 94.83% | 70.15% | 81.60% |
| 95 cl [%] | 61.86–82.89% | 83.45–98.75% | 85.85–98.23% | 61.60–77.49% | 73.68–87.96% | |
| Parameters | AUC | SEAUC | 95% CI | p (AUC = 0.5) |
|---|---|---|---|---|
| MMP-7 | 0.9566 | 0.01756 | 0.9222–0.9911 | <0.0001 |
| MMP-26 | 0.7550 | 0.04279 | 0.6711–0.8389 | <0.0001 |
| MMP-3 | 0.5821 | 0.04903 | 0.4860–0.6783 | 0.08502 |
| MMP-10 | 0.7939 | 0.03749 | 0.7204–0.8673 | <0.0001 |
| CA125 | 0.9840 | 0.006660 | 0.9709–0.9971 | <0.0001 |
| HE4 | 0.9136 | 0.01971 | 0.8749–0.9522 | <0.0001 |
| Stage I–II | ||||
|---|---|---|---|---|
| Parameters | AUC | SEAUC | 95% CI | p (AUC = 0.5) |
| MMP-7 | 0.9548 | 0.01942 | 0.9167–0.9928 | <0.0001 |
| MMP-26 | 0.7555 | 0.04967 | 0.6581–0.8528 | <0.0001 |
| MMP-3 | 0.5004 | 0.05920 | 0.3844–0.6165 | 0.9943 |
| MMP-10 | 0.7939 | 0.03749 | 0.7204–0.8673 | <0.0001 |
| CA125 | 0.9840 | 0.006660 | 0.9709–0.9971 | <0.0001 |
| HE4 | 0.9179 | 0.03020 | 0.8587–0.9772 | <0.0001 |
| Stage III–IV | ||||
| Parameters | AUC | SEAUC | 95% CI | p (AUC = 0.5) |
| MMP-7 | 0.9574 | 0.01791 | 0.9223–0.9926 | <0.0001 |
| MMP-26 | 0.7548 | 0.04513 | 0.6663–0.8433 | <0.0001 |
| MMP-3 | 0.5865 | 0.05051 | 0.4875–0.6855 | 0.08568 |
| MMP-10 | 0.8065 | 0.03683 | 0.7343–0.8787 | <0.0001 |
| CA125 | 0.9955 | 0.003221 | 0.9892–1.002 | <0.0001 |
| HE4 | 0.6843 | 0.03817 | 0.6095–0.7591 | <0.0001 |
| Studied Group | Number of Patients | Age (Median) (Range) | Group Size | ||
|---|---|---|---|---|---|
| Premenopausal | Postmenopausal | ||||
| High-Grade Serous Ovarian Carcinoma in total | High-Grade Serous Ovarian Carcinoma in total | 100 | 60 26–91 | 22 | 78 |
| Stage I | 7 | 51 26–70 | 4 | 3 | |
| Stage II | 17 | 46 36–74 | 9 | 8 | |
| Stage I–II | 24 | 48 26–74 | 13 | 11 | |
| Stage III | 38 | 61 36–91 | 4 | 34 | |
| Stage IV | 38 | 64 34–80 | 5 | 33 | |
| Stage III–IV | 76 | 63 34–91 | 9 | 67 | |
| Control groups | Benign ovarian tumour (cystis serous) | 73 | 48 25–80 | 37 | 36 |
| Healthy women | 50 | 47 32–69 | 24 | 26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewa, G.; Ławicki, P.; Grabowska, H.; Ławicki, M.; Kulesza, M.; Kicman, A.; Malinowski, P.; Ławicki, S. Plasma Concentrations of Matrilysins (MMP-7, MMP-26) and Stromelysins (MMP-3, MMP-10) as Diagnostic Biomarkers in High-Grade Serous Ovarian Cancer Patients. Int. J. Mol. Sci. 2025, 26, 5661. https://doi.org/10.3390/ijms26125661
Ewa G, Ławicki P, Grabowska H, Ławicki M, Kulesza M, Kicman A, Malinowski P, Ławicki S. Plasma Concentrations of Matrilysins (MMP-7, MMP-26) and Stromelysins (MMP-3, MMP-10) as Diagnostic Biomarkers in High-Grade Serous Ovarian Cancer Patients. International Journal of Molecular Sciences. 2025; 26(12):5661. https://doi.org/10.3390/ijms26125661
Chicago/Turabian StyleEwa, Gacuta, Paweł Ławicki, Hanna Grabowska, Michał Ławicki, Monika Kulesza, Aleksandra Kicman, Paweł Malinowski, and Sławomir Ławicki. 2025. "Plasma Concentrations of Matrilysins (MMP-7, MMP-26) and Stromelysins (MMP-3, MMP-10) as Diagnostic Biomarkers in High-Grade Serous Ovarian Cancer Patients" International Journal of Molecular Sciences 26, no. 12: 5661. https://doi.org/10.3390/ijms26125661
APA StyleEwa, G., Ławicki, P., Grabowska, H., Ławicki, M., Kulesza, M., Kicman, A., Malinowski, P., & Ławicki, S. (2025). Plasma Concentrations of Matrilysins (MMP-7, MMP-26) and Stromelysins (MMP-3, MMP-10) as Diagnostic Biomarkers in High-Grade Serous Ovarian Cancer Patients. International Journal of Molecular Sciences, 26(12), 5661. https://doi.org/10.3390/ijms26125661





