Abstract
Epigenetic modifications play a crucial role in gene regulation and have been implicated in various physiological processes and disease conditions. DNA methylation (DNAm) has been implicated in the etiology and progression of many stress-related psychiatric behaviors, such as depression. The ability to manipulate DNAm may provide a means to reverse and treat such disorders. Although CRISPR-based technologies have enabled locus-specific DNAm editing, their clinical applicability may be limited due to immunogenicity concerns and off-target effects. In this study, we introduce a novel approach for targeted DNAm manipulation using single-stranded methylated DNA probes. The probes were designed against the GRE of FKBP5 and the promoter region of MAOA. In both human embryonic kidney HEK293 and mouse pituitary AtT-20 cells, transfection with their respective methylated probes significantly increased DNAm at targeted CpG sites in a persistent and dose-dependent manner. Importantly, the induced methylation effectively attenuated glucocorticoid-induced upregulation of FKBP5 gene expression. Alteration of methylation was specific to single-stranded probes, as double-stranded methylated probes and unmethylated probes showed no significant effects. Some limitations include the need to further characterize factors that influence probe efficiency, such as probe length and CpG density; develop an efficient in vivo probe delivery system; and perform a more extensive consideration of possible off-target effects. Despite these limitations, our findings suggest that methylated DNA probes have the potential to function as a simple tool for targeted epigenetic manipulation and serve as a safer alternative to CRISPR-based epigenome editing tools for the treatment of stress-related disorders such as depression.
1. Introduction
Epigenetic modifications, such as DNA methylation (DNAm), can lead to alterations in the nuclear architecture and landscape, which can in turn affect the accessibility of transcription and regulatory factors to genes. DNAm typically occurs at the 5′ end of cytosine residues within CpG dinucleotides and is generally associated with transcriptional repression, which is mediated by the binding of methyl-CpG binding proteins and recruitment of histone-modifying enzymes []. Aberrant DNAm has been linked to many diseases, from cancer to neurodegenerative disorders, but also psychiatric diseases such as depression, anxiety, and PTSD [,,,,].
Epigenetic mechanisms can also mediate the impact of adverse environmental conditions on gene function. For instance, environmental stressors can cause epigenetic changes in the brain [,]. In such instances, it has been shown that the glucocorticoid (GC) receptor that binds to cortisol can directly alter DNAm of genes that are targets of GC signaling, thus raising the possibility of mitigating disease symptoms by potentially reversing these epigenetic marks.
Targeted manipulation of DNAm at specific gene loci can modulate gene function and provide new therapeutic strategies for disorders associated with aberrant epigenetic regulation. In plants, small RNAs have been shown to direct DNAm and gene silencing []. Studies have shown the possibility of using pharmacological methods, such as DNMT inhibitors, to impact DNAm in mammalian systems. However, these drugs target the epigenetic machinery and are not locus-specific []. Although recent advances in CRISPR-based technologies have enabled locus-specific DNAm or epigenome editing in mammalian systems [,,], these methods may have limited clinical applicability due to their immunogenicity [,,] and off-targeting [,]. However, CRISPR-based therapies are now on the horizon for the treatment of debilitating diseases such as sickle cell disease [], cancer [], and, most recently, a rare condition called carbamoyl phosphate synthase 1 (CPS1) deficiency []. These therapies must weigh the benefits of genetic manipulation against potential disease burden caused by the therapy itself. Until these technologies are further refined to minimize their own disease burden and gain traction for the treatment of more common disorders, safer methods need to be explored.
In this study, we sought to develop a simpler tool for site-specifically altering DNAm. We asked whether a single-stranded methylated DNA probe can induce DNAm of its complementary target in the cell. The benefits of such a technology are that a longer probe (>20 nucleotides) would reduce off-target effects, while its considerably smaller DNA size compared to a viral vector would enable its encapsulation in naturally occurring lipid particles such as extracellular vesicles that have low immunogenicity and can cross the blood–brain barrier []. We tested the ability of a simple DNA probe to add DNAm onto its target genomic DNA by investigating the action of glucocorticoids on the epigenome.
Previously, we have shown that of the thousands of genomic regions in blood and brain tissues that undergo DNAm changes in response to chronic GC exposure, more than 70% are loss of DNAm events []. Therefore, the ability of the glucocorticoid receptor (GR) to promote the loss of DNAm of its targets provides a useful system for testing alternative epigenetic tools that can restore DNAm. Also, a successful implementation of such a tool can serve as a proof-of-concept demonstration of a novel therapeutic tool for the treatment of depression and stress-related disorders.
We focused on the FK506 binding protein 5 (FKBP5) gene, which encodes a co-chaperone of the glucocorticoid receptor and has been identified as a key regulator of the stress response []. It is thought that GC-induced loss of DNAm and increase in FKBP5 levels lead to attenuated intracellular GC signaling and GC resistance, which are comorbid in more than 50% of cases of depression []. As such, genetic and epigenetic variations in FKBP5 have been linked to depressive symptoms [,]. At the molecular level, chronic exposure to stress or excess glucocorticoids can induce the persistent demethylation of intronic glucocorticoid response elements (GREs) in the FKBP5 gene []. This demethylation allows for increased binding of the GR to GREs and a more robust transcription of FKBP5, which in turn leads to decreased sensitivity to GCs and GC resistance [,]. Epigenetic alterations in FKBP5 have been linked to several stress-related psychiatric disorders such as PTSD, anxiety, and alcohol abuse [,,].
We tested a probe designed against the conserved intronic GRE of human and mouse FKBP5. Our findings suggest that methylated DNA probes may serve as a promising tool for targeted epigenetic manipulation and have potential therapeutic applications for mitigating the impact of excess stress or GC exposure in psychiatric disorders such as depression and non-psychiatric disorders associated with aberrant DNAm patterns. Further, it may be refined to target other genes in a simpler and safer way that can circumvent some of the limitations posed by CRISPR and other epigenome-editing technologies.
2. Results
2.1. The Use of Methylated, Single-Stranded Probe to Induce Target-Specific DNAm
To test whether a simple DNA probe can target-specifically alter DNAm, we generated a probe against specific CpG sites within the glucocorticoid response element (GRE) of the human FKBP5 gene. In vitro-methylated, single- or double-stranded DNA probes were generated and transfected into human HEK293 cells. Probes were designed with sufficiently short lengths to avoid amplification by subsequent epigenetic assays (Figure 1). Two days post-transfection, cells were analyzed for DNAm levels of the FKBP5 GRE by bisulfite pyrosequencing. Pyrosequencing analysis showed an increase of 20.5% in DNAm at CpG-1 (p = 7.0 × 10−4) and an increase of 15.0% at CpG-2 (p = 4.9 × 10−4) in 500 ng of methylated, single-stranded probe compared to untransfected samples (Figure 2A). We also observed a dose–response when we performed transfections with only 250 ng of probe, with CpG-1 showing a more modest increase of 8.6% (p = 0.0066) and CpG-2 showing an increase of 7.6% (p = 1.1 × 10−4) compared to untransfected samples. Samples transfected with unmethylated, single-stranded, or methylated, double-stranded probes did not lead to an appreciable increase in DNAm (p > 0.05). Representative pyrosequencing tracing is shown in Figure 2B.
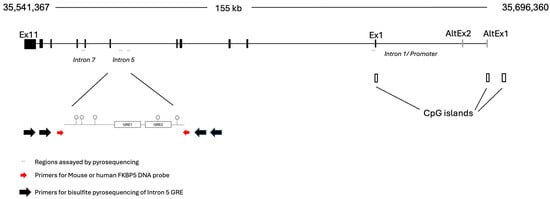
Figure 1.
Genomic organization of FKBP5. The human FKBP5 locus is located on the negative strand of chromosome 6. For this study, three intronic regions indicated by thin horizontal gray lines have been tested. Two sets of large black arrows represent the outside and inside PCR primers used for bisulfite pyrosequencing. These black arrows flank the primers used for generating the probe (red arrows), which cannot be amplified by the pyrosequencing primers. The intron 5 GREs are composed of the main GRE formed by GRE1 and GRE2, for which the DNA probe was designed, and an adjacent GRE that was tested as a negative control region.
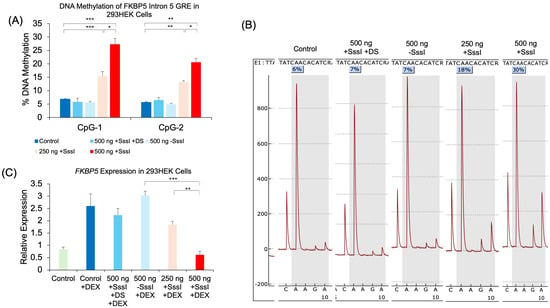
Figure 2.
Dose-dependent DNAm and gene expression changes following transfection of methylated DNA probes against FKBP5. (A) 293HEK cells were transfected with a single-stranded unmethylated probe (500 ng − SssI), a single-stranded methylated probe at two different concentrations (250 ng and 500 ng + SssI), or a double-stranded methylated probe (500 ng + SssI +DS). Untransfected cells served as controls (Control). DNAm levels of five CpG sites at the conserved glucocorticoid response element (GRE) of human FKBP5 intron 5 were analyzed by bisulfite pyrosequencing. Data for the first two CpGs are shown. (B) Typical pyrograms obtained from bisulfite pyrosequencing are shown for each group at CpG-1. The percent DNAm determination occurs when R (or A/G) is dispensed, and it corresponds to the reverse complement of T/C (T for unmethylated and C for methylation CpG, respectively). Each pyrogram represents % methylation from one sample. (C) FKBP5 expression was measured by qRT-PCR in the same groups of 293HEK cells as in (A) treated with 1 μM dexamethasone (DEX) for four hours prior to collection. Bar graphs represent the mean ± SEM, and unpaired two-tailed Student’s t-tests were performed with n = 4 per group. *** p < 0.001, ** p < 0.01, and * p < 0.05.
2.2. The Effect of Probe-Induced DNAm on Gene Expression
We also investigated whether the increase in DNAm was associated with differential gene expression. Previous studies have shown that while DNAm changes may not immediately result in changes in gene expression, they can modulate the level to which a gene can respond to a stimulus. In this case, the GREs of FKBP5 are responsive to glucocorticoids in a methylation-sensitive way []. Treatment of the 293HEK cells with dexamethasone (DEX) caused a significant increase in FKBP5 expression (2.5-fold, p = 0.036). While transfection with unmethylated, single-stranded or methylated, double-stranded probes did not lead to an appreciable attenuation of the DEX-induced increase in expression, methylated, single-stranded probes significantly reduced the DEX-induced increase in FKBP5. Specifically, cells treated with methylated, single-stranded 500 ng of probe DNA showed a 76.2% reduction in expression compared to that of unmethylated, single-stranded 500 ng of probe DNA treated with DEX. Furthermore, we observed a dose–response with methylated, single-stranded 250 ng of probe DNA, where a 29.2% reduction in expression was observed compared to unmethylated, single-stranded 500 ng of probe DNA treated with DEX (Figure 2C).
2.3. Persistence of Probe-Induced DNAm Patterns and Accumulation of DNAm Following Multiple Probe Transfections
Previously, we demonstrated that glucocorticoid-induced DNA methylation persisted throughout development []. This persistence was replicated in a simpler mouse neuronal cell line treated with DEX []. Therefore, we tested whether DNA probe-induced increase in DNA methylation can persist through several weeks. Cells transfected once with 500 ng of methylated DNA probe showed an appreciable increase in DNAm across all 5 CpGs compared to unmethylated DNA probe (p < 0.038) at Week 1 (W1). Further culturing the transfected cells for four weeks showed that those transfected once with a methylated DNA probe retained their DNA methylation patterns at CpG-1, CpG-3, and CpG-5 (p < 0.0054) compared to cells transfected with an unmethylated DNA probe at Week 1 or Week 4 (Figure 3A). We observed no significant increase in DNAm at CpG-2 at Week 1 and hence no significant increase at Week 4. At CpG-4, we observed a significant increase in DNAm by 10.4%, but this probe-induced increase in DNAm showed a decay of 5.2% by Week 4 (p = 0.003). We also tested whether repeated transfections could have a cumulative effect on DNA methylation. 293HEK cells were transfected once, twice, three times, or four times with the same amount of DNA probes, each transfection taking place three days following the previous transfection. Results showed a cumulative effect on CpGs 3, 4, and 5, with a more profound increase in DNAm at CpGs 1 and 2 following the third and fourth transfections (Figure 3B).
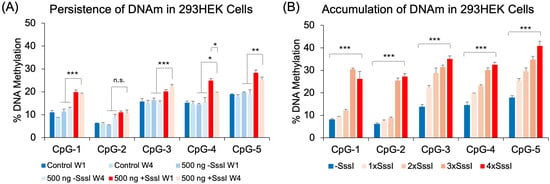
Figure 3.
Persistence and accumulation of DNA methylation. (A) 293HEK cells were transfected with the unmethylated (−SssI) and methylated (+SssI) FKBP5 DNA probe at Week 1 (W1), after which the cells were cultured for an additional four weeks (W4) before analysis by bisulfite pyrosequencing. (B) 293HEK cells were transfected with unmethylated (−SssI) and methylated (+SssI) FKBP5 DNA probes consecutively every three days and expanded, while 50% of the cells were collected for analysis. Bar graphs represent the mean ± SEM, and unpaired two-tailed Student’s t-tests were performed with n = 4 per group. *** p < 0.001, ** p < 0.01, and * p < 0.05.
2.4. Effect of DNA Probes in Non-Targeted Regions
We also tested the effect of our targeted probe on non-targeted regions. Chromosome conformation capture experiments have demonstrated physical interactions between GREs and the promoter [,], and it has been speculated that GREs that are scattered across several intronic regions of a glucocorticoid-responsive gene interact in the 3-D space of the nucleus to coordinate glucocorticoid-induced gene expression. It may be possible that other genomic regions in proximity with the region targeted by the probe may be exposed to the DNA methylation machinery. Therefore, we assayed the DNAm levels of a GRE immediately adjacent to the probe-targeted GRE in intron 5, a GRE in intron 7, and a GRE immediately downstream of the first exon in intron 1. DNAm analysis showed that most of the CpGs tested in introns 5 and 7 were too hypermethylated to undergo probe-induced DNA methylation (Figure 4A,B). CpG-6 in intron 7, which was strangely hypomethylated compared to other CpGs (<2% for all treatment groups), did not show any increase in DNAm. Further, hypomethylated intron 1 CpGs showed no differences among untransfected cells, cells treated with the unmethylated probe, and cells treated with the methylated probe. The only exception was at CpG-1, where cells transfected with the methylated probe showed a small 1.3% increase in DNAm compared to the other groups (p = 0.004, Figure 4C).
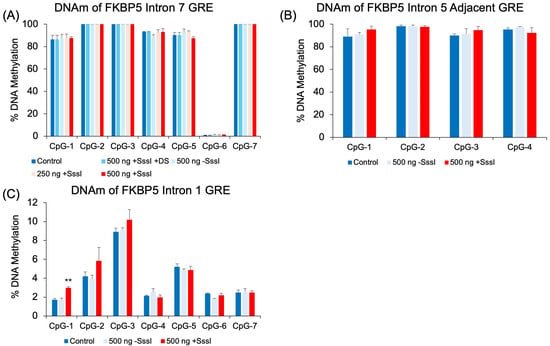
Figure 4.
DNAm analysis of human FKBP5 GREs at additional intronic regions. Experimentally verified glucocorticoid response elements (GREs) at intron 7 (A), intron 5 (B), and 1 (C) were evaluated in probe-transfected 293 HEK samples for non-specific epigenetic effects. There were no significant differences in cells transfected with unmethylated vs. methylated DNA probes, except at CpG-1 of Intron 1 (C). Bar graphs represent the mean ± SEM, and unpaired two-tailed Student’s t-tests were performed with n = 4 per group. ** p < 0.01.
2.5. Epigenetic and Transcriptional Effects of Methylated, Single-Stranded Probe in a Mouse Pituitary Cell Line
We also tested mouse pituitary cells to determine whether the findings in 293HEK can be recapitulated in a different cell type and species. Mouse AtT-20 cells were transfected with the mouse version of the probe against the conserved Fkbp5 intron 5 GRE. Results showed that the use of methylated, single-stranded probes increased DNAm at three of the four CpGs when compared to unmethylated single-stranded probes: CpG-2 (9.5%, p = 0.003), CpG-3 (6.1%, p = 0.003), and CpG-4 (7.2%, p = 0.03). There were no appreciable differences in DNAm between unmethylated, single-stranded probes and methylated, double-stranded probes (Figure 5A). We then tested whether transfected DNA probes can modulate DEX-inducibility as the human probes had in 293HEK cells. Fkbp5 expression analysis showed that samples treated with no DNA probe, a methylated double-stranded probe, or an unmethylated single-stranded probe had similar levels of gene induction by DEX treatment (7.8-, 6.3-, and 6.7-fold induction, respectively, and p < 1.3 × 10−4). However, samples transfected with methylated, single-stranded probe showed a significant reduction in DEX-induced expression compared to samples transfected with unmethylated, single-stranded probe (42.5% reduction, p = 6.4 × 10−5, Figure 5B).
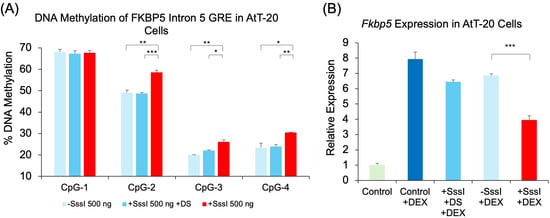
Figure 5.
DNAm and gene expression analysis following transfection of methylated DNA probes against mouse Fkbp5. (A) AtT-20 cells were transfected with single-stranded unmethylated probe (500 ng − SssI), single-stranded methylated probe (500 ng + SssI), or double-stranded methylated probe (500 ng + SssI +DS). Untransfected cells served as controls (Control). DNAm levels of four CpG sites at the conserved glucocorticoid response element (GRE) of mouse Fkbp5 intron 5 were analyzed by bisulfite pyrosequencing. Data for all four GRE CpGs are shown. (B) Fkbp5 expression was measured by qRT-PCR in the same groups of AtT-20 cells as in (A) treated with 1 μM dexamethasone (DEX) for four hours prior to collection. Bar graphs represent the mean ± SEM, and unpaired two-tailed Student’s t-tests were performed with n = 4 per group. *** p < 0.001, ** p < 0.01, and * p < 0.05.
2.6. Additional Genomic Target of DNAm Probe: MAOA
To demonstrate the broader applicability of our methylated probe approach beyond the FKBP5 gene, we tested the regulatory intronic region of Monoamine Oxidase A (MAOA) [], a gene whose encoded protein metabolizes monoamine neurotransmitters such as dopamine, serotonin, and norepinephrine. Following the transfection of methylated single-stranded probes targeting the CpG-dense MAOA intronic region into 293HEK cells, we observed significant increases in DNAm across multiple CpG sites compared to cells transfected with unmethylated probes: CpG-1 (10.1%, p = 0.01), CpG-5 (12.7%, p = 0.04), CpG-6 (11.2%, p = 0.02), CpG-8 (9.3%, p = 0.006), CpG-10 (11.8%, p = 0.006), and CpG-12 (14.3%, p = 0.01) (Figure 6A). A one-way ANOVA revealed a significant difference between the two treatments (F(1, 26) = 6.25, p = 0.019), where methylation levels were higher in the methylated probe-treated group (M = 58.68, SD = 7.52) compared to the unmethylated probe-treated group (M = 52.31, SD = 10.25). Consistent with the increased methylation, we observed a corresponding decrease in MAOA gene expression. qRT-PCR analysis revealed that cells transfected with methylated probes showed a 27.3% reduction in MAOA expression compared to cells transfected with unmethylated probes (p = 0.041) (Figure 6B).
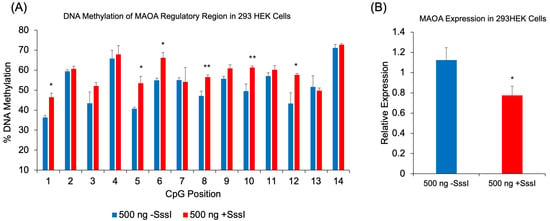
Figure 6.
DNAm and gene expression analysis following transfection of methylated DNA probes against MAOA. (A) 293HEK cells were transfected with a single-stranded unmethylated probe (-SssI) or a single-stranded methylated probe (+SssI). DNAm levels of 14 CpG sites at a regulatory region of human MAOA were analyzed by bisulfite pyrosequencing. (B) MAOA expression was measured by qRT-PCR in the same groups of 293HEK cells. Bar graphs represent the mean ± SEM, and unpaired two-tailed Student’s t-tests were performed with n = 4 per group. * p < 0.05 and ** p < 0.01. A one-way ANOVA was used to test for overall differences across all 14 CpGs, followed by Tukey’s post hoc test for pairwise comparisons.
3. Discussion
In this study, we introduced a simple approach for inducing targeted DNAm using methylated DNA probes. Our approach consisted of PCR amplification against a region of interest, which in this case was a methylation-sensitive, glucocorticoid response element in the intronic region of the FKBP5 gene []. The PCR product was first in vitro methylated using the bacterial methyltransferase M.SssI and denatured to yield single-stranded DNA probes. Using DNA probes against the FKBP5 GRE, we observed appreciable increases in DNAm across at least two of the five CpGs across the GRE in the human 293HEK cells. The observed increase in DNAm was specific to the single-stranded methylated probe, as the double-stranded methylated probe and single-stranded unmethylated probe did not induce an increase in DNAm. Importantly, the increase in DNAm at these CpGs was able to attenuate the DEX-induced increase in FKBP5 levels. Further, gene regulation by the DNA probe was dose-dependent, as the introduction of half the amount of DNA probe resulted in a reduced addition of DNAm and reduced attenuation of FKBP5 induction by DEX compared to the full amount of DNA probe.
We also observed the persistent effects of our DNA probes on genomic target methylation, as a single administration of the DNA probe lasted through at least a four-week period, suggesting robustness of the probe-induced DNA methylation. This phenomenon is reminiscent of glucocorticoid-induced loss of DNAm, which persisted for at least four weeks in the mouse brain and in a DEX-treated neuronal cell line [,]. It should be noted that the reversal of DNAm induction observed at CpG-4 in Figure 3A is not new, as some of the CpGs that lost DNAm by glucocorticoid treatment also showed a similar reversion to baseline by the fourth week []. In addition, repeated introduction of the probe had a cumulative effect on DNA methylation, although the increase in DNAm with successive transfections was not at the same magnitude as that following the first transfection.
Investigation of other GREs in intronic regions that have been thought to physically interact in 3D space with the intronic GRE (intron 5) targeted by the probe did not show any significant changes in DNAm, emphasizing the specificity of probe targeting. One exception to the negative results at other GREs is the increase in DNAm at one CpG in intron 1 (Figure 4C). However, the magnitude of the difference was less than 2%, which is less than the 2.98% mean coefficient of variation obtained from the FKBP5 Intron 1 pyrosequencing assay.
We then tested whether the probe-induced DNAm was unique to human cells by testing the mouse version of the Fkbp5 probe in the AtT-20 mouse pituitary cell line. Although more subtle than that observed in the human cell lines, the increase in DNAm was significant. Similarly, this increase in DNAm was able to thwart DEX-induction of Fkbp5. There are few factors that can potentially explain the difference in the magnitude of the probe-induced increase in DNAm, including cell-type and species-specific differences, growth rate differences in cells, and different levels of DNAm-modifying enzymes. Growth rate may play an important role, as some methylation-altering events, such as glucocorticoid-induced loss of methylation, depend on cell proliferation [].
An additional genomic region was tested to further generalize the probe-induced increase in DNAm. This time we designed a probe against an important regulatory region of MAOA, whose methylation levels correlated with enzyme levels determined by PET brain imaging []. In addition, the MAOA enzyme metabolizes the neurotransmitter serotonin and is thus targeted by a class of antidepressants called monoamine inhibitors (MAOI). Although significant increases in DNAm were observed across many CpGs, many CpGs were impervious to the probe. The sub-optimal increase in DNAm at these CpGs may be due to the sequence of the regulatory region in MAOA. It is also possible that CpG-dense regions may be harder to modify, as the MAOA probe covers approximately the same base pairs of DNA as that for FKBP5 but contains almost three times more CpGs. Additional work is needed to further examine the relationship between probe CpG density and DNAm efficiency.
We posit that of the three epigenetic mechanisms, i.e., DNA methylation, histone modifications, and RNA-mediated silencing, DNA methylation is the most stable, as methyltransferases faithfully copy methylation patterns during cell replication across development and cell lineage maintenance []. In contrast, histone modifications lack locus specificity or basepair resolution and are prone to off-target effects such as cell toxicity arising from broad effects of histone modifications over multiple loci []. Importantly, depending on the chromatin state, there can be rapid turnover of histone modifications on timescales of minutes to hours [,]. Likewise, RNA-mediated silencing involving shRNAs, siRNAs, or microRNAs is also of finite duration, as these silencing RNAs have a half-life of 28–220 h, and their continued presence is required for gene silencing [,]. RNA-mediated silencing can also be prone to immune activation due to the double-strandedness of silencing RNAs and off-target effects due to their relatively short nucleotide length [,]. Our results suggest that the DNAm-based tool may be a viable therapeutic tool given the persistence of induced DNAm and specific genomic targeting due to its longer nucleotide length.
Our current study highlights the potential of this method to modulate stress response pathways, as demonstrated by the targeted methylation of the FKBP5 gene. FKBP5 is known to be influenced by glucocorticoid exposure and methylation status, with demethylation of the glucocorticoid response element leading to increased gene expression upon subsequent glucocorticoid exposure []. By inducing targeted methylation of this region, we were able to attenuate the glucocorticoid-induced upregulation of FKBP5 expression, suggesting that our approach could be used to fine-tune stress response pathways and potentially mitigate the detrimental effects of chronic stress or glucocorticoid exposure.
The success of our method in both human and mouse cell lines indicates its potential for translation to in vivo models and eventual clinical applications. However, several challenges need to be addressed before this approach can be fully realized in vivo. First, the efficiency of probe delivery and cellular uptake may vary depending on the cell type and target tissue, requiring optimization of tissue uptake methods and probe design. Second, the long-term stability and persistence of the induced methylation changes need to be evaluated in vivo to determine the durability of the therapeutic effects. The safety and potential off-target effects of the methylated probes must also be thoroughly assessed in animal models before considering human applications. Finally, we will need a suitable delivery device that can tissue-specifically deliver DNA probes for in vivo studies.
Small extracellular vesicles (EVs) such as exosomes hold immense therapeutic potential as delivery devices, as their vesicle cargo space can easily accommodate a ~200 bp DNA fragment and their cell and tissue uptake efficiency can be modified []. Recent advances in the EV field have provided promising directions on how surface proteins of EVs can be engineered to increase tissue and cell uptake efficiency by overexpressing certain surface proteins obtained from donor cells on the surface of EVs, which can more efficiently target similar tissue types [,,,]. This “like-attracts-like” phenomenon is thought to be mediated by core ligands and homing peptides that fuse with similar transmembrane proteins on the EV surface to confer affinity to target cells [,]. Alternatively, click chemistry has enabled the conjugation of peptides of interest on the surface of EVs to increase their delivery to target tissues [,]. Such modifications will enable the efficient delivery of small molecules, such as our DNA probes, to brain tissues.
There are several limitations to the study. We have not tested several important factors that may influence the efficacy of the DNA probe. These include CpG density, length of the probe, and cell growth rate. First, it is unclear whether a highly CpG-dense region is less likely to become methylated compared to a genomic region with lower CpG density. The MAOA region, which harbors 14 CpGs over ~170 bps, was significantly more CpG-dense than the FKBP5 GRE with 5 CpGs over ~200 bps of DNA. Second, it is unclear whether the length of the probe can affect targeting and methylation efficiency. While longer DNA probes can provide better target specificity compared to 20 nucleotide-long guide RNAs used in CRISPR, longer sequences can also contain small regions of high complementarity that can hinder efficient targeting. As such, additional non-targeted regions need to be examined in greater detail. Third, it remains unclear whether cell proliferation rate can affect target DNA methylation. We have only used two cell lines that undergo robust cell division in culture. Additional cell lines, especially those of neuronal origins, need to be tested to be useful in vivo for animal models of stress and depression.
Despite these challenges, our study provides a proof-of-concept demonstration for the use of methylated DNA probes as a targeted epigenetic therapy. The ability to induce site-specific DNAm changes can open new avenues for the treatment of a wide range of diseases characterized by aberrant methylation patterns. As our understanding of the epigenetic basis of diseases continues to grow, the development of targeted epigenetic therapies, such as the one presented here, will become increasingly important. In conclusion, we have developed a novel method for targeted induction of DNAm using methylated DNA probes. This approach offers a promising tool for modulating gene expression and function, with potential therapeutic applications in various diseases characterized by aberrant methylation patterns.
4. Methods
4.1. Probe Design and Amplification
Methylated DNA probes targeting the conserved glucocorticoid response element (GRE) in intron 5 of the human (chr6: 35,601,961–35,602,194; GRCh38/hg38, 256 bp) and mouse (chr17: 28,639,321–28,639,560; GRCm39/mm39, 239 bp) of FKBP5 were designed. A separate DNA probe designed against an intronic, regulatory region of the human MAOA gene (chrX: 43,656,383–43,656,553; GRCh38/hg38, 170 bp) was also tested []. Additional tests for non-specific effects of the human FKBP5 probe investigated another adjacent region in intron 5 (chr6: 35,610,962–35,611,313), intron 1 (chr6: 35,687,767–35,688,045), and intron 7 (chr6: 35,590,524–35,591,014). For both human and mouse probes, PCR primers targeted smaller regions than those analyzed by bisulfite sequencing to preclude the amplification of probe DNA during methylation analysis. Primers used for generating the probes are shown in Table 1. The genomic organization of the human FKBP5 locus is shown in Figure 1.

Table 1.
Sequence of primers for probe design and pyrosequencing.
4.2. In Vitro Methylation of DNA Probes
Purified FKBP5 probes were subjected to in vitro methylation using the bacterial CpG methyltransferase (M.SssI, New England Biolabs, Ipswich, MA, USA) []. One µg of the probe was incubated with 4 units of M.SssI, 160 µM S-adenosylmethionine (SAM), and 1X NEBuffer 2 in a total reaction volume of 100 µL at 37 °C for two 1 h cycles followed by 20 min at 65 °C for enzyme inactivation. A negative control reaction (FKBP5 SssI-) was performed in parallel, where the M.SssI enzyme was replaced with an equal volume of water. Following in vitro methylation, probes were purified again and eluted in 20 µL of TE buffer. The concentrations of the methylated (FK SssI+) and unmethylated (FK SssI-) probes were measured using Qubit 4 (Thermo Fisher Scientific, Waltham, MA, USA). Similar reactions were performed for the mouse Fkbp5 and human MAOA probes.
4.3. Cell Culture and Transfection
To induce demethylation at the GRE of the endogenous FKBP5 gene, we chose cell lines that we have previously shown to undergo DEX or glucocorticoid-induced loss of methylation [,]. Human embryonic kidney 293 (293HEK) and mouse pituitary AtT-20 cells were purchased from Atcc.org and were treated with 1 µM dexamethasone (DEX) for 5 days and cultured for an additional 5 days without any DEX. This would allow CpGs to undergo persistent loss of DNA methylation, which would then be restored by introducing methylated probes. Separate wells of cells were left untreated as negative controls or transfection reagent-only controls. Prior to transfection, cells were seeded in 24-well plates at a density of 2 × 105 cells per well and allowed to adhere overnight in DMEM free of antibiotics. The transfection of the methylated and unmethylated probes was performed in quadruplicate (500 ng of probe per well) using X-tremeGENE 360 Transfection Reagent (MilliporeSigma, Burlington, MA, USA) according to the manufacturer’s instructions. Cells were fed fresh DMEM media one day after transfection and harvested on the second day for collecting gDNA. For the assessment of glucocorticoid-induced gene expression, a subset of the transfected 293HEK cells was treated with 1 µM DEX for 4 h prior to harvesting. Cells were harvested for total RNA extraction using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s instructions.
4.4. DNA Extraction and Methylation Analysis by Bisulfite Pyrosequencing
Genomic DNA was extracted from 293HEK and AtT-20 cells using the DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer’s instructions. The extracted DNA was bisulfite-converted using the EZ DNAm-Gold Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. For bisulfite pyrosequencing, bisulfite-converted DNA and a pair of outside primers were used to PCR amplify the genomic region of interest. Two μLs of outside PCR products were used for a second PCR reaction using inside PCR primers. One of the Inside primers was biotinylated and allowed for the isolation of and primer extension on a PSQ HS 96 pyrosequencer (Qiagen) according to the manufacturer’s instructions, and CpG sites were quantified, from 0% to 100% methylation, using the Pyro Q-CpG software. Primers used for assessing DNAm levels of FKBP5 and MAOA genes are shown in Table 1. We determined the coefficient of variation (COV) using pyrosequencing assays for the MAOA, FKBP5 Intron 1, and Intron 5 loci. This was performed by running six replicates of bisulfite PCR products on the same pyrosequencing plate and measuring the variation in %DNAm for multiple CpGs. From these assays, we determined the COV to be 3.66% for MAOA, 2.98% for FKBP5 Intron 1 GRE, and 3.27% for FKBP5 Intron 5 GRE.
4.5. Gene Expression Analysis
Reverse Transcription Quantitative Real-Time PCR (RT-qPCR) was performed to assess the impact of targeted DNAm on gene expression. Total RNA from 293HEK or AtT-20 RNA samples was extracted using the RNeasy Mini Kit, and complementary DNA (cDNA) was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions. FKBP5 and MAOA expression analysis was performed using Taqman probes and the QuantStudio5 Real-Time PCR System (Thermo Fisher Scientific). For the determination of relative expression values, the −ΔΔCt method was used, where triplicate Ct values for each cDNA sample were averaged and subtracted from those derived from ACTB [].
4.6. Statistics and Data Analysis
Data are presented as mean ± SEM, with the number of replicates (N) indicated in the figure legends. Prior to hypothesis testing, data distributions were assessed for normality using the Shapiro–Wilk test. For comparisons between two groups (e.g., methylated vs. unmethylated probe or DEX-treated vs. vehicle control), unpaired two-tailed Student’s t-tests were performed. For experiments involving multiple CpG sites, e.g., the MAOA locus, one-way ANOVA was used to test for overall differences, followed by Tukey’s post hoc test for pairwise comparisons. All analyses were conducted in GraphPad Prism v.9. A p-value < 0.05 was considered statistically significant.
Author Contributions
N.M.—conceptualization, investigation, funding acquisition, and original draft preparation; J.G.—investigation and validation; R.A.L.–investigation and validation; A.G.—investigation and validation; and R.S.L.—conceptualization, investigation, funding acquisition, and original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Woodrow Wilson Fellowship (Johns Hopkins University) granted to N.M. and the George Browne Genetics Cell Laboratory Fund granted to R.S.L.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, W.; Kim, J.; Yun, J.-M.; Ohn, T.; Gong, Q. MeCP2 regulates gene expression through recognition of H3K27me3. Nat. Commun. 2020, 11, 3140. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef]
- Shireby, G.; Dempster, E.L.; Policicchio, S.; Smith, R.G.; Pishva, E.; Chioza, B.; Davies, J.P.; Burrage, J.; Lunnon, K.; Vellame, D.S.; et al. DNA methylation signatures of Alzheimer’s disease neuropathology in the cortex are primarily driven by variation in non-neuronal cell-types. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yang, B.; Rothschild, G.; Mann, J.J.; Sanford, L.D.; Tang, X.; Huang, C.; Wang, C.; Zhang, W. Epigenetic regulation in major depression and other stress-related disorders: Molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target. Ther. 2023, 8, 309. [Google Scholar] [CrossRef]
- Persaud, N.S.; Cates, H.M. The Epigenetics of Anxiety Pathophysiology: A DNA Methylation and Histone Modification Focused Review. Eneuro 2022, 10. [Google Scholar] [CrossRef]
- Katrinli, S.; Wani, A.H.; Maihofer, A.X.; Ratanatharathorn, A.; Daskalakis, N.P.; Montalvo-Ortiz, J.; Núñez-Ríos, D.L.; Zannas, A.S.; Zhao, X.; Aiello, A.E.; et al. Epigenome-wide association studies identify novel DNA methylation sites associated with PTSD: A meta-analysis of 23 military and civilian cohorts. Genome Med. 2024, 16, 147. [Google Scholar] [CrossRef]
- Rahman, M.F.; McGowan, P.O. Cell-type-specific epigenetic effects of early life stress on the brain. Transl. Psychiatry 2022, 12, 326. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Xu, H.; Ellenbroek, B.; Dai, J.; Wang, L.; Yan, C.; Wang, W. The Changes of Histone Methylation Induced by Adolescent Social Stress Regulate the Resting-State Activity in mPFC. Research 2023, 6, 0264. [Google Scholar] [CrossRef]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA Methylation. PLoS Genet 2020, 16, e1009034. [Google Scholar] [CrossRef] [PubMed Central]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA methyltransferase inhibitors combination therapy for the treatment of solid tumor: Mechanism and clinical application. Clin. Epigenetics 2021, 13, 166. [Google Scholar] [CrossRef]
- Liu, X.S.; Wu, H.; Ji, X.; Stelzer, Y.; Wu, X.; Czauderna, S.; Shu, J.; Dadon, D.; Young, R.A.; Jaenisch, R. Editing DNA Methylation in the Mammalian Genome. Cell 2016, 167, 233–247.e17. [Google Scholar] [CrossRef] [PubMed]
- Vojta, A.; Dobrinić, P.; Tadić, V.; Bočkor, L.; Korać, P.; Julg, B.; Klasić, M.; Zoldoš, V. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016, 44, 5615–5628. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tao, Y.; Gao, X.; Zhang, L.; Li, X.; Zou, W.; Ruan, K.; Wang, F.; Xu, G.-L.; Hu, R. A CRISPR-based approach for targeted DNA demethylation. Cell Discov. 2016, 2, 16009. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Koo, T.; Jee, H.-G.; Cho, H.-Y.; Lee, G.; Lim, D.-G.; Shin, H.S.; Kim, J.-S. CRISPR RNAs trigger innate immune responses in human cells. Genome Res. 2018, 28, 367–373. [Google Scholar] [CrossRef]
- Ewaisha, R.; Anderson, K.S. Immunogenicity of CRISPR therapeutics—Critical considerations for clinical translation. Front. Bioeng. Biotechnol. 2023, 11, 1138596. [Google Scholar] [CrossRef]
- Hakim, C.H.; Kumar, S.R.P.; Pérez-López, D.O.; Wasala, N.B.; Zhang, D.; Yue, Y.; Teixeira, J.; Pan, X.; Zhang, K.; Million, E.D.; et al. Cas9-specific immune responses compromise local and systemic AAV CRISPR therapy in multiple dystrophic canine models. Nat. Commun. 2021, 12, 6769. [Google Scholar] [CrossRef]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Höijer, I.; Emmanouilidou, A.; Östlund, R.; van Schendel, R.; Bozorgpana, S.; Tijsterman, M.; Feuk, L.; Gyllensten, U.; Hoed, M.D.; Ameur, A. CRISPR-Cas9 induces large structural variants at on-target and off-target sites in vivo that segregate across generations. Nat. Commun. 2022, 13, 627. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; De La Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Fraietta, J.A.; Davis, M.M.; Cohen, A.D.; Weber, K.L.; Lancaster, E.; Mangan, P.A.; Kulikovskaya, I.; Gupta, M.; Chen, F.; et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020, 367, eaba7365. [Google Scholar] [CrossRef]
- Musunuru, K.; A Grandinette, S.; Wang, X.; Hudson, T.R.; Briseno, K.; Berry, A.M.; Hacker, J.L.; Hsu, A.; A Silverstein, R.; Hille, L.T.; et al. Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease. N. Engl. J. Med. 2025, 392, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, X.; Chandra, S.; Lyon, C.; Ning, B.; Jiang, L.; Fan, J.; Hu, T.Y. Extracellular vesicles: Emerging tools as therapeutic agent carriers. Acta Pharm. Sin. B 2022, 12, 3822–3842. [Google Scholar] [CrossRef]
- Seifuddin, F.; Wand, G.; Cox, O.; Pirooznia, M.; Moody, L.; Yang, X.; Tai, J.; Boersma, G.; Tamashiro, K.; Zandi, P.; et al. Genome-wide Methyl-Seq analysis of blood-brain targets of glucocorticoid exposure. Epigenetics 2017, 12, 637–652. [Google Scholar] [CrossRef]
- Scammell, J.G.; Denny, W.B.; Valentine, D.L.; Smith, D.F. Overexpression of the FK506-Binding Immunophilin FKBP51 Is the Common Cause of Glucocorticoid Resistance in Three New World Primates. Gen. Comp. Endocrinol. 2001, 124, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.C.; Davis, J.M. DST Studies in Psychotic Depression: A Meta-Analysis. Am. J. Psychiatry 1997, 154, 1497–1503. [Google Scholar] [CrossRef]
- Binder, E.B.; Salyakina, D.; Lichtner, P.; Wochnik, G.M.; Ising, M.; Pütz, B.; Papiol, S.; Seaman, S.; Lucae, S.; Kohli, M.A.; et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004, 36, 1319–1325. [Google Scholar] [CrossRef]
- Tozzi, L.; Farrell, C.; Booij, L.; Doolin, K.; Nemoda, Z.; Szyf, M.; Pomares, F.B.; Chiarella, J.; O'Keane, V.; Frodl, T. Epigenetic Changes of FKBP5 as a Link Connecting Genetic and Environmental Risk Factors with Structural and Functional Brain Changes in Major Depression. Neuropsychopharmacology 2017, 43, 1138–1145. [Google Scholar] [CrossRef]
- Lee, R.S.; Tamashiro, K.L.K.; Yang, X.; Purcell, R.H.; Harvey, A.; Willour, V.L.; Huo, Y.; Rongione, M.; Wand, G.S.; Potash, J.B. Chronic Corticosterone Exposure Increases Expression and Decreases Deoxyribonucleic Acid Methylation of Fkbp5 in Mice. Endocrinology 2010, 151, 4332–4343. [Google Scholar] [CrossRef]
- Cox, O.H.; Song, H.Y.; Garrison-Desany, H.M.; Gadiwalla, N.; Carey, J.L.; Menzies, J.; Lee, R.S. Characterization of glucocorticoid-induced loss of DNA methylation of the stress-response gene Fkbp5 in neuronal cells. Epigenetics 2021, 16, 1377–1397. [Google Scholar] [CrossRef]
- Wochnik, G.M.; Rüegg, J.; Abel, G.A.; Schmidt, U.; Holsboer, F.; Rein, T. FK506-binding Proteins 51 and 52 Differentially Regulate Dynein Interaction and Nuclear Translocation of the Glucocorticoid Receptor in Mammalian Cells. J. Biol. Chem. 2005, 280, 4609–4616. [Google Scholar] [CrossRef]
- Miller, O.; Shakespeare-Finch, J.; Bruenig, D.; Mehta, D. DNA methylation of NR3C1 and FKBP5 is associated with posttraumatic stress disorder, posttraumatic growth, and resilience. Psychol. Trauma Theory Res. Pract. Policy 2020, 12, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Kremer, T.L.; Chen, J.; Buhl, A.; Berhe, O.; Bilek, E.; Geiger, L.S.; Ma, R.; Moessnang, C.; Reichert, M.; Reinhard, I.; et al. Multimodal Associations of FKBP5 Methylation with Emotion-Regulatory Brain Circuits. Biol. Psychiatry 2024, 96, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, H.; Yan, A.; Yin, F.; Qiao, X. DNA Methylation in Alcohol Use Disorder. Int. J. Mol. Sci. 2023, 24, 10130. [Google Scholar] [CrossRef]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.C.; Pariante, C.M.; Pace, T.W.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-specific FKBP5 DNA demethylation mediates gene–childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef]
- Shumay, E.; Logan, J.; Volkow, N.D.; Fowler, J.S. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics 2012, 7, 1151–1160. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, J.; Chen, Q.; Yang, S.; Wang, Z.; Xiao, B.; Lai, Z.; Jing, Y.; Li, Y.; Li, X. Regulation of de novo and maintenance DNA methylation by DNA methyltransferases in postimplantation embryos. J. Biol. Chem. 2024, 301, 107990. [Google Scholar] [CrossRef]
- Shi, M.-Q.; Xu, Y.; Fu, X.; Pan, D.-S.; Lu, X.-P.; Xiao, Y.; Jiang, Y.-Z. Advances in targeting histone deacetylase for treatment of solid tumors. J. Hematol. Oncol. 2024, 17, 37. [Google Scholar] [CrossRef]
- Kimura, H.; Cook, P.R. Kinetics of core histones in living human cells: Little exchange of H3 and H4 and some rapid exchange of H2B. J. Cell Biol. 2001, 153, 1341–1353. [Google Scholar] [CrossRef] [PubMed Central]
- Kassem, S.; Ferrari, P.; Hughes, A.L.; Soudet, J.; Rando, O.J.; Strubin, M. Histone exchange is associated with activator function at transcribed promoters and with repression at histone loci. Sci. Adv. 2020, 6, eabb0333. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H. Factors influencing degradation kinetics of mRNAs and half-lives of microRNAs, circRNAs, lncRNAs in blood in vitro using quantitative PCR. Sci. Rep. 2022, 12, 7259. [Google Scholar] [CrossRef]
- Marzi, M.J.; Ghini, F.; Cerruti, B.; de Pretis, S.; Bonetti, P.; Giacomelli, C.; Gorski, M.M.; Kress, T.; Pelizzola, M.; Muller, H.; et al. Degradation dynamics of microRNAs revealed by a novel pulse-chase approach. Genome Res. 2016, 26, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Ga, Y.J.; Kim, S.H.; Cho, Y.H.; Kim, J.W.; Kim, C.; Yeh, J.-Y. Small interfering RNA (siRNA)-based therapeutic applications against viruses: Principles, potential, and challenges. J. Biomed. Sci. 2023, 30, 88. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, J.; Meister, G. siRNA Specificity: RNAi Mechanisms and Strategies to Reduce Off-Target Effects. Front. Plant Sci. 2021, 11, 526455. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Koh, E.; Lee, E.J.; Nam, G.-H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.-S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Xitong, D.; Xiaorong, Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene 2016, 575, 377–384. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Y.; Li, X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J. Drug Target. 2019, 28, 129–141. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.-A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, T.; Lin, H.; Chen, Y.; Lin, Y.; Le, D.; Huang, Y.; Wang, A.H.; Lee, C.; Ling, T. Small Extracellular Vesicles Engineered Using Click Chemistry to Express Chimeric Antigen Receptors Show Enhanced Efficacy in Acute Liver Failure. J. Extracell. Vesicles 2025, 14, e70044. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Bruno, S.; Rosenwasser, N.; Gorgun, C.; Reverberi, D.; Gagliani, M.C.; Cortese, K.; Grange, C.; Bussolati, B.; Quarto, R.; et al. Standardized Method to Functionalize Plasma-Extracellular Vesicles via Copper-Free Click Chemistry for Targeted Drug Delivery Strategies. ACS Appl. Bio Mater. 2024, 7, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Renbaum, P.; Abrahamove, D.; Fainsod, A.; Wilson, G.G.; Rottem, S.; Razin, A. Cloning, characterization, and expression in Escherichia coli of the gene coding for the CpG DNA methylase from Spiroplasma sp. strain MQ1(M.SssI). Nucleic Acids. Res. 1990, 18, 1145–1152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duis, J.; Cox, O.H.; Ji, Y.; Seifuddin, F.; Lee, R.S.; Wang, X. Effect of Genotype and Maternal Affective Disorder on Intronic Methylation of FK506 Binding Protein 5 in Cord Blood DNA. Front. Genet. 2018, 9, 648. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).