Lycorine Inhibits Influenza Virus Replication by Affecting Nascent Nucleoporin Nup93 Synthesis
Abstract
1. Introduction
2. Results
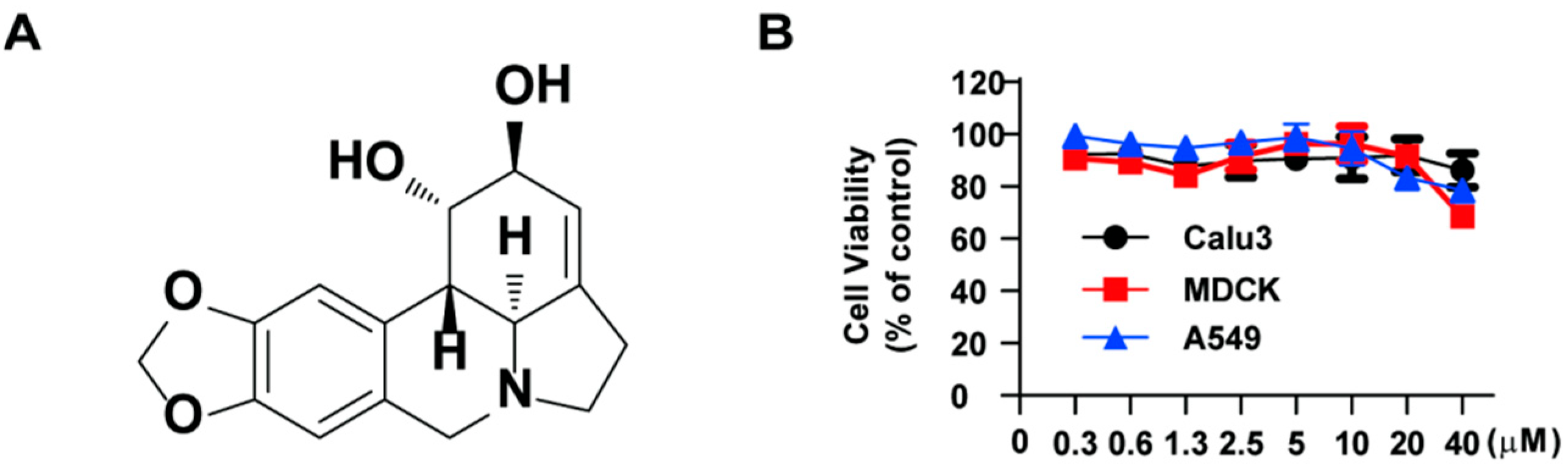
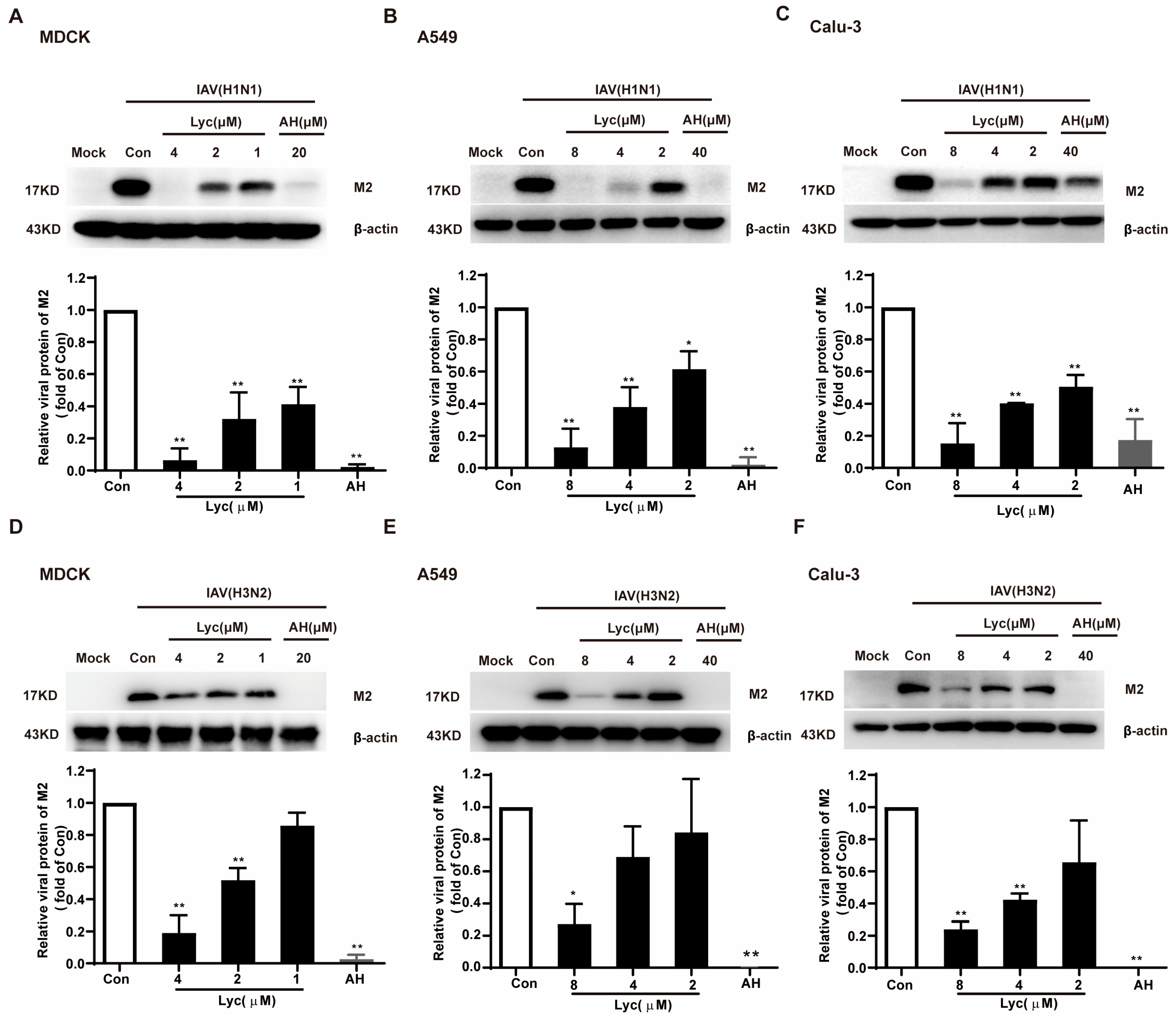
2.1. Lycorine Inhibits the Replication of Influenza Virus
2.2. Lycorine Affects the Multiple Stages of the Viral Replication Cycle Following Internalization
2.3. Lycorine Inhibits Nuclear Export of IAV NP
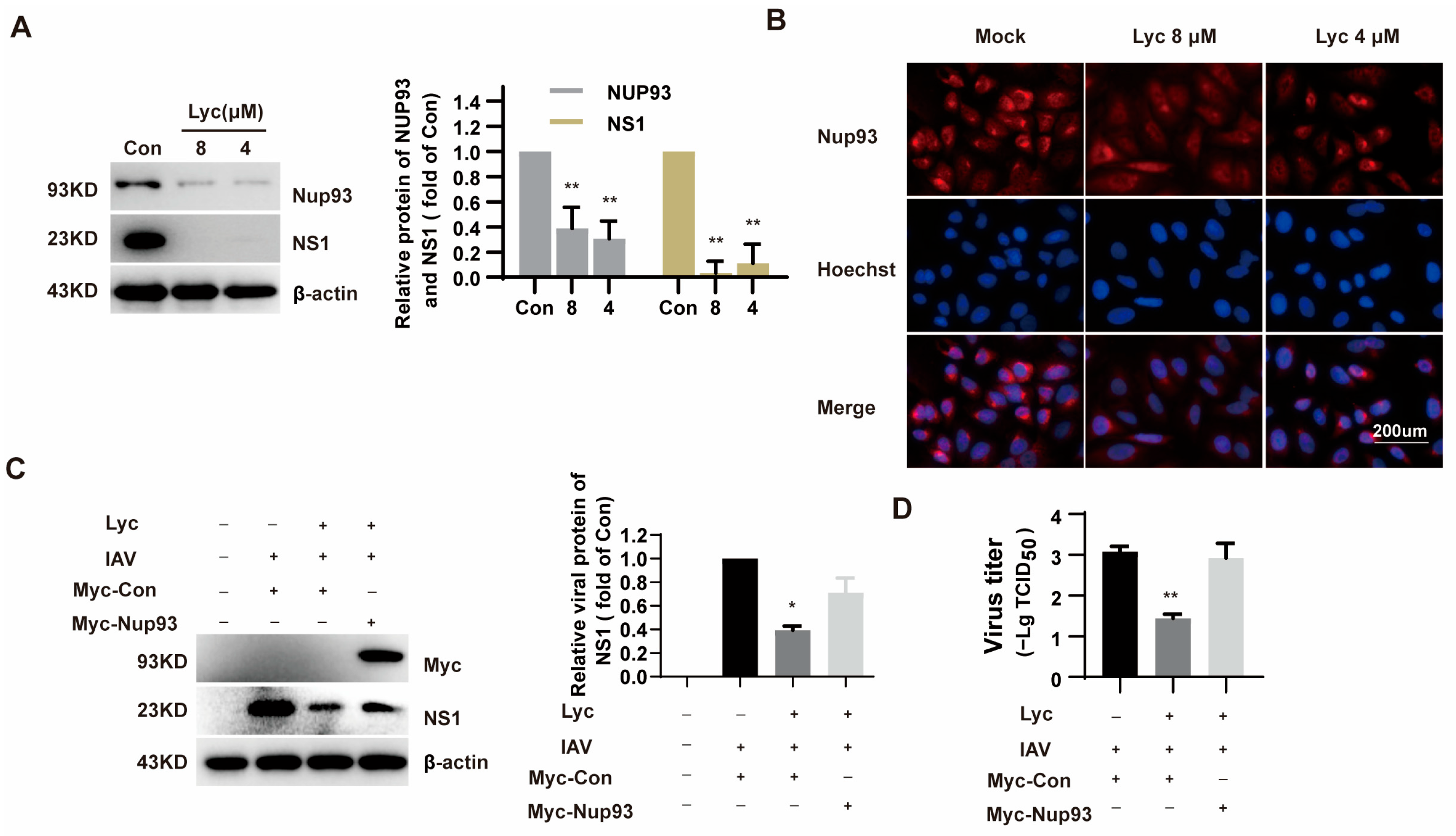
2.4. Lycorine Affects the Expression of Nup93
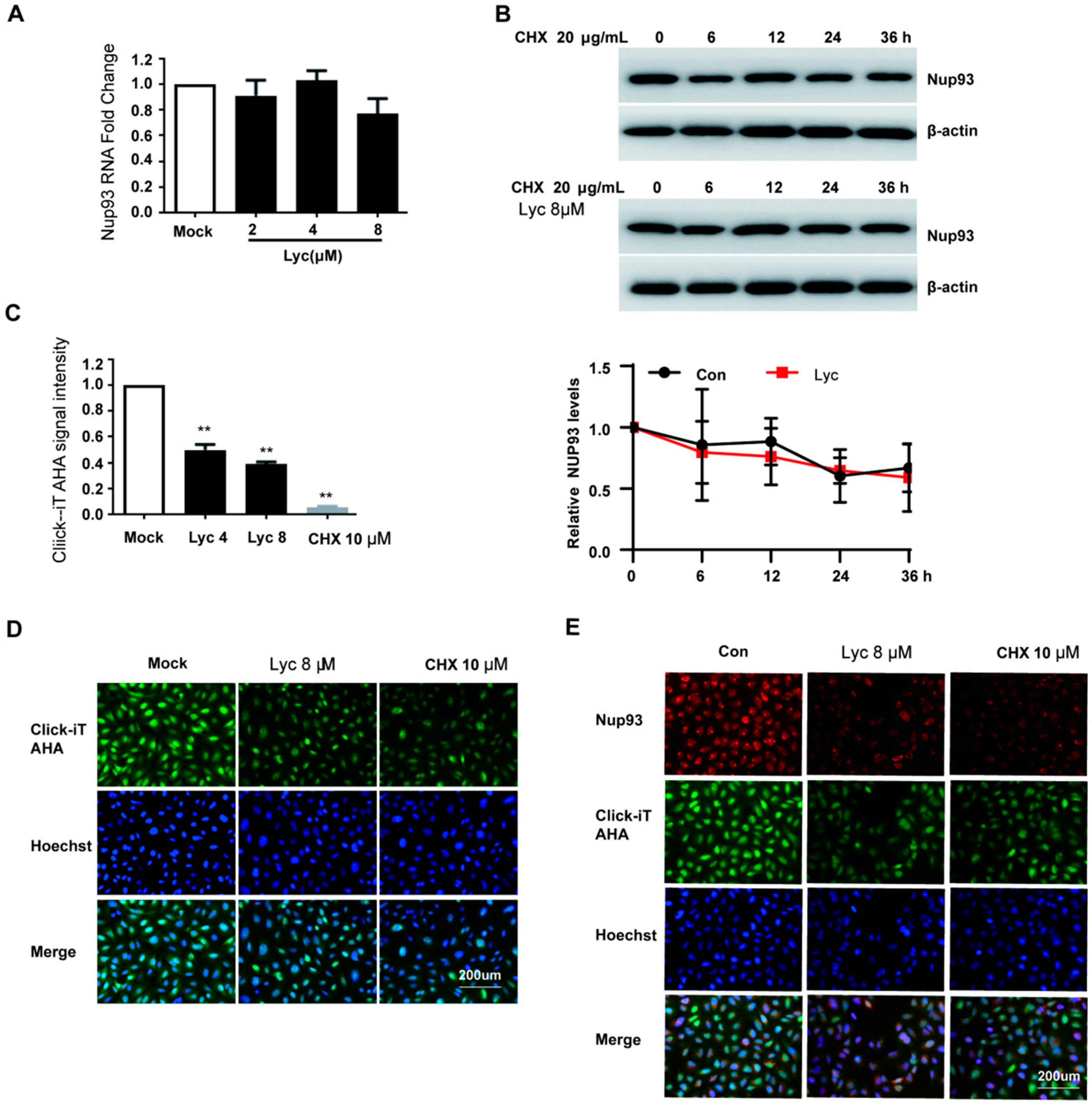
2.5. Lycorine Inhibits the Nascent Synthesis of Nup93 Protein
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Virus
4.2. Compounds
4.3. Cytotoxicity Assay
4.4. Anti-IAV Assay
4.5. Western Blot
4.6. Real-Time Quantitative PCR Analysis
4.7. Virus Titer Determination
4.8. Time-of-Addition Assay and Time-Course Assay
4.9. Immunofluorescence
4.10. Nascent Protein Synthesis Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Mayank, A.; Lal, S.K. Molecular events leading to the creation of a pandemic influenza virus. Indian J. Microbiol. 2009, 49, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Donchet, A.; Vassal-Stermann, E.; Gérard, F.C.A.; Ruigrok, R.W.H.; Crépin, T. Differential Behaviours and Preferential Bindings of Influenza Nucleoproteins on Importins-α. Viruses 2020, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Sharma, K.; Saxena, S.K.; Sharma, J.G.; Rajput, R.; Kumar, B. Unravelling the interaction between Influenza virus and the nuclear pore complex: Insights into viral replication and host immune response. Virusdisease 2024, 35, 231–242. [Google Scholar] [CrossRef]
- Fernandez-Martinez, J.; Rout, M.P. One Ring to Rule them All? Structural and Functional Diversity in the Nuclear Pore Complex. Trends Biochem. Sci. 2021, 46, 595–607. [Google Scholar] [CrossRef]
- Li, B.; Clohisey, S.M.; Chia, B.S.; Wang, B.; Cui, A.; Eisenhaure, T.; Schweitzer, L.D.; Hoover, P.; Parkinson, N.J.; Nachshon, A.; et al. Genome-wide CRISPR screen identifies host dependency factors for influenza A virus infection. Nat. Commun. 2020, 11, 164. [Google Scholar] [CrossRef]
- Şenbaş Akyazi, B.; Pirinçal, A.; Kawaguchi, A.; Nagata, K.; Turan, K. Interaction of influenza A virus NS2/NEP protein with the amino-terminal part of Nup214. Turk. J. Biol. Turk Biyol. Derg. 2020, 44, 82–92. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.; Chen, Z. Human cellular protein nucleoporin hNup98 interacts with influenza A virus NS2/nuclear export protein and overexpression of its GLFG repeat domain can inhibit virus propagation. J. Gen. Virol. 2010, 91, 2474–2484. [Google Scholar] [CrossRef]
- Furusawa, Y.; Yamada, S.; Kawaoka, Y. Host Factor Nucleoporin 93 Is Involved in the Nuclear Export of Influenza Virus RNA. Front. Microbiol. 2018, 9, 1675. [Google Scholar] [CrossRef]
- Min, J.S.; Kwon, S.; Jin, Y.H. SARS-CoV-2 RdRp Inhibitors Selected from a Cell-Based SARS-CoV-2 RdRp Activity Assay System. Biomedicines 2021, 9, 996. [Google Scholar] [CrossRef]
- Wang, H.; Guo, T.; Yang, Y.; Yu, L.; Pan, X.; Li, Y. Lycorine Derivative LY-55 Inhibits EV71 and CVA16 Replication Through Downregulating Autophagy. Front. Cell. Infect. Microbiol. 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lao, Z.; Xu, J.; Li, Z.; Long, H.; Li, D.; Lin, L.; Liu, X.; Yu, L.; Liu, W.; et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology 2020, 546, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, L.F.; Wang, Q.Y.; Shang, L.Q.; Shi, P.Y.; Yin, Z. Anti-dengue-virus activity and structure-activity relationship studies of lycorine derivatives. ChemMedChem 2014, 9, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.H.; Zhang, X.L.; Lao, G.J.; Su, G.M.; Wang, L.; Li, Y.L.; Ye, W.C.; He, J. Tandem mass tag-based quantitative proteomic analysis of lycorine treatment in highly pathogenic avian influenza H5N1 virus infection. PeerJ 2019, 7, e7697. [Google Scholar] [CrossRef]
- He, J.; Qi, W.B.; Wang, L.; Tian, J.; Jiao, P.R.; Liu, G.Q.; Ye, W.C.; Liao, M. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir. Viruses 2013, 7, 922–931. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Wang, R.; Zhang, Z.R.; Zhang, Y.N.; Deng, C.L.; Zhang, B.; Shang, L.Q.; Ye, H.Q. In Vitro Inhibition of Alphaviruses by Lycorine. Virol. Sin. 2021, 36, 1465–1474. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, M.; Yu, S.; Zhang, C.; Fang, T.; Liu, D.; Jia, B.; Zhu, M.; Wang, B.; Wang, Q.; et al. Antiviral and virucidal activities of lycorine on duck tembusu virus in vitro by blocking viral internalization and entry. Poult. Sci. 2021, 100, 101404. [Google Scholar] [CrossRef]
- Jin, Y.H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H.; et al. Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomed. Int. J. Phytother. Phytopharm. 2021, 86, 153440. [Google Scholar] [CrossRef]
- Agrawal, T.; Siddqui, G.; Dahiya, R.; Patidar, A.; Madan, U.; Das, S.; Asthana, S.; Samal, S.; Awasthi, A. Inhibition of early RNA replication in Chikungunya and Dengue virus by lycorine: In vitro and in silico studies. Biochem. Biophys. Res. Commun. 2024, 730, 150393. [Google Scholar] [CrossRef]
- Chen, D.; Cai, J.; Yin, J.; Jiang, J.; Jing, C.; Zhu, Y.; Cheng, J.; Di, Y.; Zhang, Y.; Cao, M.; et al. Lycorine-derived phenanthridine downregulators of host Hsc70 as potential hepatitis C virus inhibitors. Future Med. Chem. 2015, 7, 561–570. [Google Scholar] [CrossRef]
- Vrijsen, R.; Vanden Berghe, D.A.; Vlietinck, A.J.; Boeyé, A. Lycorine: A eukaryotic termination inhibitor? J. Biol. Chem. 1986, 261, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Santos, A.; Alonso, G.; Vazquez, D. Inhibitors of protein synthesis in eukarytic cells. Comparative effects of some amaryllidaceae alkaloids. Biochim. Biophys. Acta 1976, 425, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Rabut, G.; Doye, V.; Ellenberg, J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat. Cell Biol. 2004, 6, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.H.; Wang, H.; Han, M.Q.; Wang, D.; Hu, Y.X.; Zhou, K.; Li, Y. Nucleoporin 85 interacts with influenza A virus PB1 and PB2 to promote its replication by facilitating nuclear import of ribonucleoprotein. Front. Microbiol. 2022, 13, 895779. [Google Scholar] [CrossRef]




| Genes | Sequence (5′-3′) |
|---|---|
| Influenza M2-F | GACCRATCCTGTCACCTCTGAC |
| Influenza M2-R | GGGCATTYTGGACAAAKCGTCTACG |
| Influenza HA-F | CAAACAGAAGACGGAGGACTACCAC |
| Influenza HA-R | ATTACCTTGCTCCTGCCACTTGC |
| GAPDH-F(H) | GGTGGTCTCCTCTGACTTCAACA |
| GAPDH-R(H) | GTTGCTGTAGCCAAATTCGTTGT |
| Gapdh-F(D) | AGTCAAGGCTGAGAACGGGAAACT |
| Gapdh-R(D) | TCCACAACATACTCAGCACCAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Wang, H.; Wang, K.; Wu, S.; Jiang, J.; Li, Y. Lycorine Inhibits Influenza Virus Replication by Affecting Nascent Nucleoporin Nup93 Synthesis. Int. J. Mol. Sci. 2025, 26, 5358. https://doi.org/10.3390/ijms26115358
Yan H, Wang H, Wang K, Wu S, Jiang J, Li Y. Lycorine Inhibits Influenza Virus Replication by Affecting Nascent Nucleoporin Nup93 Synthesis. International Journal of Molecular Sciences. 2025; 26(11):5358. https://doi.org/10.3390/ijms26115358
Chicago/Turabian StyleYan, Haiyan, Huiqiang Wang, Kun Wang, Shuo Wu, Jiandong Jiang, and Yuhuan Li. 2025. "Lycorine Inhibits Influenza Virus Replication by Affecting Nascent Nucleoporin Nup93 Synthesis" International Journal of Molecular Sciences 26, no. 11: 5358. https://doi.org/10.3390/ijms26115358
APA StyleYan, H., Wang, H., Wang, K., Wu, S., Jiang, J., & Li, Y. (2025). Lycorine Inhibits Influenza Virus Replication by Affecting Nascent Nucleoporin Nup93 Synthesis. International Journal of Molecular Sciences, 26(11), 5358. https://doi.org/10.3390/ijms26115358





