Abstract
This review offers a synthesis of the current understanding of the impact of low-dose thallium (Tl) on public health, specifically emphasizing its diverse effects on various populations and organs. The article integrates insights into the cytotoxic effects, genotoxic potential, and molecular mechanisms of thallium in mammalian cells. Thallium, a non-essential heavy metal present in up to 89 different minerals, has garnered attention due to its adverse effects on human health. As technology and metallurgical industries advance, various forms of thallium, including dust, vapor, and wastewater, can contaminate the environment, extending to the surrounding air, water sources, and soil. Moreover, the metal has been identified in beverages, tobacco, and vegetables, highlighting its pervasive presence in a wide array of food sources. Epidemiological findings underscore associations between thallium exposure and critical health aspects such as kidney function, pregnancy outcomes, smoking-related implications, and potential links to autism spectrum disorder. Thallium primarily exerts cellular toxicity on various tissues through mitochondria-mediated oxidative stress and endoplasmic reticulum stress. This synthesis aims to shed light on the intricate web of thallium exposure and its potential implications for public health, emphasizing the need for vigilant consideration of its risks.
1. Introduction
Thallium (Tl) is a blue-white heavy metal of the rare earth group (atomic number 81, atomic mass 204.38), also known as a structural biochemistry/post-transition metal. It is soft and ductile, with an appearance similar to tin, and it exists in two oxidation states (I and III), with (Tl+) being more stable than (Tl3+). Thallium is primarily found in sulfide minerals associated with low-temperature hydrothermal mineralization, with as many as 89 known thallium minerals recorded [1]. Thallium has a wide range of applications, including in semiconductors, scintillation camera imaging, optical fibers glass, high-temperature superconducting materials, and coatings [2,3]. In recent years, numerous countries have experienced thallium contamination incidents, primarily attributed to the extraction and metallurgical activities of sulfide ores [4,5]. The industrial processes have led to the dispersion of dust, vapors, or liquid contaminants into the surrounding air, water sources, and soil, subsequently infiltrating the biosphere [6].
The data provided in this review were obtained through searches conducted using the commonly used scientific research engine Web of Science, accessed at https://clarivate.com/webofsciencegroup/solutions/web-of-science/ on 4 January 2024. During the retrieval process, the keyword “thallium” was used under the condition of “all fields”. The search was filtered within the Web of Science categories “Public Environmental Occupational Health”, “Environmental Sciences”, and “Toxicology”, respectively, and articles were carefully reviewed in chronological order. Preference was given to the latest papers, and articles were selected based on the presence of keywords in the title or abstract. Furthermore, relevant references cited in the articles were explored to further extend the search. In total, approximately 1500 articles were reviewed, and 78 articles were recruited.
2. Thallium Exposure in Daily Life
2.1. Thallium in Beverages
Recent research has revealed thallium contamination in drinking water in many countries and regions [7,8]. Tea leaves grown in different regions have also been found to be affected by thallium contamination [9]. Furthermore, the presence of thallium has been detected in tea prepared under various conditions [10]. In addition, the presence of thallium has also been detected in coffee beans [11]. Table 1 provides a compilation of the latest studies on this topic.

Table 1.
The content of thallium in beverages. The concentrations of thallium in drinking water, tea leaves, tea, and coffee beans in different regions.
2.2. Thallium in Tobaccos
There are approximately 1.1 billion smokers globally, making the thallium content in tobacco an important public health issue [12]. Furthermore, non-smokers may be exposed to secondhand and thirdhand smoke, highlighting the importance of the investigation [13]. In recent years, the presence of thallium in tobacco has been discovered, and there is a correlation between its presence and the cultivation location [14,15]. Interestingly, counterfeit cigarettes were found to contain higher levels of thallium compared to authentic brands [16]. Previous research has indicated a significant difference in thallium content when comparing the residences of smokers and non-smokers [13]. Table 2 provides a compilation of the latest studies on this topic.

Table 2.
The thallium content within tobacco products. The concentration of thallium in tobacco across different regions and brands.
2.3. Thallium in Vegetables
After thallium contaminates the soil, it can be absorbed by plants [17]. Previous studies have indicated that thallium is present in vegetables from various regions, with the type of plant being a significant factor influencing absorption. Studies indicate that, based on estimated transfer factors and bioconcentration factors, watercress, snow peas, chard, and pak choi exhibit a high enrichment capability for thallium [18,19,20]. In summary, thallium exposure to the human body may occur through various pathways. Table 3 compiles data that exceed the maximum permissible level (MPL) [21].

Table 3.
The thallium (Tl) content in vegetables exceeds the MPL (0.5 mg/kg). Concentrations of thallium in vegetables from different regions and varieties in China. MPL: maximum permissible level.
3. Epidemiological Investigation of Thallium
3.1. The Effects of Thallium on Human Kidney Health
Chronic kidney disease (CKD) has become a serious public health problem, and exposure to heavy metals is a risk factor for CKD [22]. Previous studies have shown a significant relationship between kidney function and Tl (Table 4). A study was conducted on 5037 elderly individuals aged over 65 in Yinchuan City, Ningxia, China. Using an eGFR < 80 mL/min/1.73 m2 as the standard, a total of 1631 individuals exhibited abnormal eGFR values, and there was a negative correlation between Tl content and abnormal eGFR [23]. Similar results were also observed in other studies. A study of 934 patients with essential hypertension in Wuhan, China, revealed a positive correlation between Tl content in the urine of male patients and eGFR [24]. Interestingly, a study involving 592 elderly individuals aged over 60 with diabetes in Fuyang City, Anhui Province, China, indicated a negative correlation between Tl concentration in urine and CKD [25]. Furthermore, a survey of the urine of 512 adolescents aged 11–16 years in Mexico revealed a positive correlation between urine Tl concentration and eGFR [26]. Based on geographical proximity, a survey was conducted on 2069 residents living near the petrochemical plant in Yunlin, Taiwan. The results showed that, in comparison to the low-exposure group residing farther from the petrochemical plant, the high-exposure group had higher urinary thallium concentrations, significantly reduced eGFR, and a higher risk of developing CKD [27], which means that Tl enters the human body due to industrial activities. To summarize, there is a notable correlation between Tl exposure and renal function. A total of 27,733 participants aged over 20 from the National Health and Nutrition Examination Survey conducted between 2003 and 2012 were analyzed. The results showed that decreased renal function was related to decreased excretion of Tl in urine [28]. Tl in urine may become a biomarker of kidney function.

Table 4.
The effects of Tl levels in urine on kidney functions in humans.
3.2. The Effects of Thallium on the Health of Children and Pregnant Women
Heavy metals pose a significant risk to fetuses [29], with Tl being able to enter the fetus through the maternal placenta [30,31]. Previous studies have shown that pregnant women and their children will be affected by Tl [Table 5]. Exposure of pregnant women to Tl is associated with an increased risk of preterm birth [32]. A study conducted in Anhui Province, China, found that umbilical cord serum Tl levels were associated with reduced height and weight in young girls [33]. The current study has unveiled a significant negative association between maternal Tl exposure during early pregnancy and cord blood leukocyte mtDNAcn [34]. Furthermore, maternal exposure to Tl during delivery is associated with shortened telomere length in newborns [35]. Tl not only affects the fetus but also alters the metabolic system of pregnant women. Urinary Tl shows a positive correlation with acetate, scyllo-inositol, formate, and dimethylamine. Conversely, it exhibits a negative correlation with trans-aconitate and N-acetyl neuraminic acid. Positive associations with pregnanolone-3-glucuronide were particularly robust in the first trimester, while negative associations were observed with estrogen metabolites in the third trimester [36]. It is worth noting that serum Tl concentration is significantly associated with the risk of gestational diabetes mellitus [37]. In summary, Tl presents potential risks for both children and pregnant women.

Table 5.
Effects on human health in pregnant women and children associated with thallium (Tl) levels in urine, umbilical cord serum, and blood: overview of published studies.
3.3. The Effects of Thallium on the Health of Smokers
Previous research has revealed the presence of thallium in cigarette components [13,14], further unveiling a clear correlation between thallium content and the specific brands and geographical origins of these tobacco products [15,16]. These findings emphasize the importance of investigating the impact of smoking populations and thallium content on public health. The study has reported a statistically significant elevation in the average urinary thallium levels among smokers compared to the control group [38,39]. Moreover, smokers exhibited higher serum thallium concentrations compared to non-smokers [40]. Notably, a significant association was observed between the duration of illicit opioid substance use and both urinary and hair thallium concentrations [38]. Higher urinary Tl levels are associated with poorer lung function [41]. Furthermore, another study has demonstrated an association between urinary Tl and the exacerbation of lung function decline, especially among smokers [42]. Research on secondhand and thirdhand smoke exposure has found a correlation between elevated levels of Tl and the residences of smokers [16]. To summarize, there exists a significant correlation between Tl exposure and lung function, with smokers being exposed to higher levels of Tl through cigarette smoking, potentially resulting in more severe lung damage. Table 6 provides an updated compilation of papers published on this subject.

Table 6.
The effects on human health of smoker of Tl in urine/blood.
3.4. Thallium Causes Psychological, Metabolic, and Other Effects
Research has indicated that environmental toxicants, especially heavy metals, are associated with autism spectrum disorders (ASD) [43,44,45]. Similarly, children diagnosed with ASD exhibited higher Tl levels on average [46]. Another study found similar results, indicating a positive correlation between urinary Tl levels in adults and ASD [47]. Moreover, brain research revealed that serum Tl levels are associated with subjective memory impairment and Alzheimer’s disease [48]. Regarding metabolism, urinary Tl levels are associated with a decrease in thyroid hormone (T4) levels [49]. Urinary Tl concentration is positively correlated with an increase in serum bilirubin levels, a marker of hepatic function [41]. In terms of body composition, urinary Tl is positively correlated with body mass index (BMI) and waist circumference [50]. It is worth noting that urinary thallium levels are significantly correlated with levels of the urinary DNA damage marker, 8-hydroxy-2-deoxyguanosine (8-OHdG) [51]. The effects of thallium exposure on the body are comprehensive and multifaceted, potentially posing risks to public health. Table 7 presents a summary of the psychological, metabolic, and other effects of Tl.

Table 7.
The psychological, metabolic, and other effects caused by thallium.
4. Molecular Toxicity and Adverse Reactions Caused by Thallium
The toxicity of thallium (Tl) is related to two of its chemical properties. Firstly, the toxic mechanism of Tl+ is associated with potassium (K+) ions [52]. The ionic radius of Tl+ is similar to that of K+ [53]. In the reduced state, the ionic radius of Tl+ is 1.76 Å, while that of K+ is 1.60 Å [54]. Tl+ will compete with K+ on potassium channels, interfering with potassium-dependent biological functions [2]. In aquatic toxicology research, the toxicity of Tl (I) depends on the concentration of potassium ions (K+) in freshwater environments [55]. The second aspect of Tl toxicity arises from its reaction with sulfhydryl (-SH) groups in proteins and mitochondrial membranes [2]. Thallium (Tl) exhibits a high affinity for sulfur (S) ligands, forming complexes with protein thiol groups [56]. In rats, Tl decreases the activity of Cu–Zn superoxide dismutase (SOD), and this characteristic is associated with it [57]. Furthermore, the toxicity of thallium (Tl) is similar to many heavy metals in that it inhibits heavy metal toxicity by binding to the thiol (-SH) groups on glutathione (GSH) [58,59].
4.1. Mitochondria-Mediated Oxidative Stress
The toxicity induced by thallium is comprehensive and wide-ranging, with the most common effect being the induction of oxidative stress. In the study of hepatotoxicity, the mitochondria isolated from rat Hepatocyte were cultured with Tl (I). The results indicate that Tl (I) induces a significant increase in the oxidative stress parameters of mitochondria, a decrease in the ATP/ADP ratio, mitochondrial membrane potential (MMP) collapse, and the release of cytochrome c [60]. Additionally, the study found that both Tl (I) and Tl (III) induce the formation of reactive oxygen species (ROS), lipid peroxidation, and mitochondrial membrane potential collapse in isolated rat liver cells [61]. Further mechanistic studies revealed that both Tl (I) and Tl (III) induce the cascade activation of caspase enzymes and the appearance of apoptotic phenotypes. However, treatment with mitochondrial permeability transition (MPT) pore closure agents (cyclosporin A and carnitine), ATP generators (L-glutamine, fructose, and xylitol), and antioxidants (α-tocopherol or deferoxamine) inhibited caspase-3 activation and apoptosis [62]. Previous reports have indicated that thallium (Tl) can pass through the blood–brain barrier, causing neurotoxicity. A series of studies were conducted using rat pheochromocytoma (PC12) cells as a model due to their similarities with sympathetic neurons. The results show that both Tl (I) and Tl (III) lead to concentration- and time-dependent decreases in cell viability, reduced glutathione levels, and increased levels of oxidants in the cytoplasm. Additionally, this results in a significant increase in mitochondrial H2O2 steady-state levels and a decrease in membrane potential [63]. Furthermore, it was discovered that the extent of cell apoptosis induced by Tl (I) and Tl (III) varies in the presence of epidermal growth factor (EGF). The results suggest that EGF partially mitigates toxicity by inhibiting the sustained activation of the MAPK signaling cascade, and indicate that p38 plays a crucial role as a mediator in Tl (III)-induced apoptosis in PC12 cells [64]. Using the HN9.10e cell line, which is a fusion of mouse hippocampal neuroblasts and neuroblastoma cells, it was observed that Tl (I) caused neurite shortening, loss of matrix adhesion, and an increase in cytoplasmic calcium. Additionally, dose-dependent increases in mitochondrial reactive oxygen species (mtROS) levels and decreases in transmembrane mitochondrial potential (ΔΨm) were observed [65]. In the exploration of neurotoxic mechanisms, exposure of E17-E18 Wistar rat primary hippocampal neurons to Tl (I) resulted in a significant decrease in cell viability, elevated levels of reactive oxygen species (ROS), and a notable increase in apoptosis rates. Electron microscopy revealed mitochondrial swelling and vacuolar degeneration. Additionally, the Nrf2-Keap1 signaling pathway demonstrated a protective role against Tl (I)-induced cytotoxicity, accompanied by a significant decrease in the level of the mitochondrial fusion protein Mitofusin 2 (Mfn2). Notably, the activation of the Nrf2-Keap1 signaling pathway through t-BHQ (Nrf2 and II phase detoxifying agent) maintained the functionality of Mfn2, contributing to the reduction of damage caused by Tl (I) [66]. To conclude, thallium induces mitochondrial-mediated oxidative stress, leading to damage, and subsequently triggers apoptosis. Table 8 provides an updated compilation of papers published on this subject.

Table 8.
Thallium induces mitochondria-mediated oxidative stress and apoptosis.
4.2. Thallium Induces Cellular Toxicity by Eliciting Endoplasmic Reticulum Stress
As widely recognized, the Nrf2-Keap1 signaling pathway plays a crucial role in the unfolded protein response [67], and its activation provides protection against the toxicity induced by Tl (I) [66]. Tl (I) and Tl (III) induce increased expression of ATF-6 and IRE-1 in Madin–Darby Canine Kidney cells, leading to XBP-1 splicing and nuclear translocation, resulting in cellular endoplasmic reticulum stress [68]. In hepatopancreatic cells of the environmental pollution-sensitive indicator organism Gammarus pulex, exposure to Tl (I) resulted in increased numbers of lipid droplets, lysosomes, and autophagic vacuoles. Additionally, fragmentation and expansion of the rough endoplasmic reticulum (RER) were observed [69]. In summary, thallium induces endoplasmic reticulum (ER) stress. Table 9 provides an updated compilation of papers published on this subject.

Table 9.
Thallium induces cellular toxicity by eliciting endoplasmic reticulum stress.
4.3. Genotoxicity and other Adverse Effects of Thallium
In the study of genetic toxicity, exposure of human lymphocytes to Tl (I) and Tl (III) significantly increases structural chromosomal abnormalities with or without gaps, and raises the percentage of cells with abnormalities without gaps [70]. Exposure of human blood cells to Tl (I) increases the length of the comet assay [71]. Furthermore, Tl (I) causes a decrease in both the viability and motility of mature mouse sperm, along with an increase in fragmented DNA [72]. In mouse neuroblastoma cells (Neuro-2A), acetylcholinesterase activity is highly sensitive to inhibition by Tl [73]. In the study of immunotoxicity, Tl (I) suppresses the expression of genes involved in B cell development, enhances the production of thymic CD4+ T cells, and promotes the migration of initial CD4+ T cells and recent thymic emigrants (RTE) from the thymus to the spleen [74]. To summarize, thallium can induce DNA damage, leading to genotoxicity. Table 8 presents a summary of the molecular toxicity and adverse reactions of Tl. Table 10 provides an updated compilation of papers published on this subject.

Table 10.
The genotoxicity and other adverse effects of thallium.
4.4. The Toxicity and Adverse Reactions of Thallium in Mammalian Models
In rat experiments, Tl (I) leads to a reduction in glomerular filtration rate (GFR) and urine volume, an increase in proteinuria, and a decrease in plasma amino acid concentration, indicating kidney toxicity [75,76]. Tl (I) induces increased lipid peroxidation in the hypothalamus, cerebellum, and striatum, a decrease in GSH in the striatum, reduced Cu,Zn-SOD activity in the hypothalamus and striatum, and results in impaired motor function in the animals [57,77]. Exposure to Tl (I) during pregnancy in female CD-1 mice results in offspring with trunk curvature, tail abnormalities, rotation defects in the forelimbs and hindlimbs, and delayed skeletal ossification [78]. In summary, in addition to cellular-level experiments, thallium has also been found to induce varying degrees of toxicity in animal models, particularly evident in the kidneys and nervous system. Table 11 provides an updated compilation of papers published on this subject.

Table 11.
The toxicity and adverse reactions of thallium in mammalian models.
5. Conclusions
Thallium emissions from smelting plants and technology manufacturing processes enter rivers through wastewater and accumulate in soil, or they enter organisms through the air. Thallium exposure pathways include drinking water, beverages, cigarettes, and vegetables. Multiple epidemiological studies have found that thallium has a significant impact on renal function, with this phenomenon observed in elderly, young, or diseased populations. Children and pregnant women are highly sensitive populations. Research has found that thallium increases the risk of premature birth and impacts child development. Due to the presence of thallium in cigarettes, smokers have higher serum thallium concentrations, which are correlated with lung damage. Furthermore, it has been found that thallium levels in urine are associated with autism. Moreover, thallium is correlated with many metabolites including thyroid hormone (T4), bilirubin levels, and 8-hydroxy-2′-deoxyguanosine (8-OHdG), and it is even associated with parameters such as body mass index (BMI) and waist circumference. The toxicity induced by thallium is extensive. Although the mechanisms of thallium toxicity are not yet fully understood, the most common is mitochondria-mediated oxidative stress. Studies using various cell lines, including hepatocytes (rat), pheochromocytoma (rat), murine hippocampal neuroblasts, and primary hippocampal neurons, have found that thallium can cause mitochondrial damage, including increased oxidative stress, changes in mitochondrial membrane potential (ΔΨm), and decreased ATP/ADP ratio, and even induce cell apoptosis. Recent studies have found that thallium can induce cell toxicity by causing endoplasmic reticulum stress, including increased expression of ATF-6 and IRE-1, XBP-1 splicing, and nuclear translocation. It is worth noting that the Nrf2-Keap1 signaling pathway, which plays an important role in the unfolded protein response, can protect against Tl (I)-induced toxicity when activated. In vitro studies have shown that thallium can induce various adverse reactions, including genetic toxicity, decreased sperm motility, and abnormal migration of immune cells. Animal studies have found consistent results with the epidemiological findings: Tl (I) reduces glomerular filtration rate (GFR), urine volume, and plasma amino acid concentrations, while increasing proteinuria, leading to renal toxicity. Additionally, it increases lipid peroxidation in the brain and impairs motor function in animals, resulting in neurotoxicity. Exposure of mice to Tl (I) during pregnancy leads to developmental defects in offspring, indicating the presence of developmental toxicity (Figure 1). However, most countries have not established limits for Tl in agricultural products, animal feed, and human food, thereby increasing the potential public health risks.
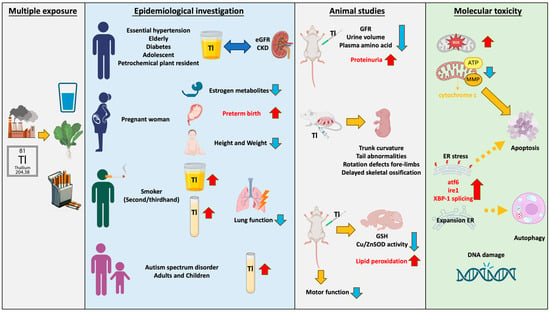
Figure 1.
Thallium exhibits diverse contamination pathways and toxicity. Thallium pollution in the environment occurs through dust, steam, and wastewater. It enters organisms through sources such as drinking water, tobacco, and vegetables. Ingesting thallium in daily life can lead to damage in various organs and tissues. The red arrow pointing upwards indicates an increase; the blue arrow pointing downwards indicates a decrease; double arrows represent correlation; dashed arrows represent possible mechanisms.
Author Contributions
Y.C.: investigation, writing—original draft. C.-K.C.: validation, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by grants from the Taiwan Ministry of Science and Technology (MOST 111-2314-B-002-236-MY3) and National Science and Technology Council, Taiwan (NSTC 112-2314-B-002-273-MY3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors gratefully acknowledge the support of the Taiwan Ministry of Science and Technology and National Science and Technology Council, Taiwan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, F.; Gu, S. Secondary Sulfate Minerals from Thallium Mineralized Areas: Their Formation and Environmental Significance. Minerals 2021, 11, 855. [Google Scholar] [CrossRef]
- Peter, A.L.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Qi, J.; Liu, J.; Wang, J.; Lin, K.; Ouyang, Q.; Zhang, X.; Wei, X.; Xiao, T.; El-Naggar, A.; et al. Thallium isotopic compositions as tracers in environmental studies: A review. Environ. Int. 2022, 162, 107148. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Dong, X.; Yin, M.; Tsang, D.C.W.; Sun, J.; Liu, J.; Song, G.; Liu, Y. Temporal sedimentary record of thallium pollution in an urban lake: An emerging thallium pollution source from copper metallurgy. Chemosphere 2020, 242, 125172. [Google Scholar] [CrossRef] [PubMed]
- Vaněk, A.; Chrastný, V.; Komárek, M.; Penížek, V.; Teper, L.; Cabala, J.; Drábek, O. Geochemical position of thallium in soils from a smelter-impacted area. J. Geochem. Explor. 2013, 124, 176–182. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef] [PubMed]
- Campanella, B.; Onor, M.; D’Ulivo, A.; Giannecchini, R.; D’Orazio, M.; Petrini, R.; Bramanti, E. Human exposure to thallium through tap water: A study from Valdicastello Carducci and Pietrasanta (northern Tuscany, Italy). Sci. Total Environ. 2016, 548–549, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, L.; D’Orazio, M.; Doveri, M.; Lelli, M.; Petrini, R.; Giannecchini, R. Groundwater and potentially toxic elements in a dismissed mining area: Thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). J. Geochem. Explor. 2019, 197, 84–92. [Google Scholar] [CrossRef]
- Li, F.; Jing, M.; Ma, F.; Wang, W.; Li, M. Comparison and Risk Assessment of Macroelements and Trace Metals in Commercial Teas from Different Regions of China. Biol. Trace Elem. Res. 2023, 201, 1503–1519. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, G.; Liu, K.; Yu, R.; Lu, Q.; Zhang, Y. Potential exposure to metals and health risks of metal intake from Tieguanyin tea production in Anxi, China. Environ. Geochem. Health 2019, 41, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Albals, D.; Al-Momani, I.F.; Issa, R.; Yehya, A. Multi-element determination of essential and toxic metals in green and roasted coffee beans: A comparative study among different origins using ICP-MS. Sci. Prog. 2021, 104, 368504211026162. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Drago, G.; Perrino, C.; Canepari, S.; Ruggieri, S.; L’Abbate, L.; Longo, V.; Colombo, P.; Frasca, D.; Balzan, M.; Cuttitta, G.; et al. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM(2.5). Environ. Res. 2018, 165, 71–80. [Google Scholar] [CrossRef]
- Karbowska, B.; Zembrzuski, W. Determining Thallium in a Commercial Tobacco Brand Available in Poland. Pol. J. Environ. Stud. 2016, 25, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Polzin, G.M.; Zhang, L.; Watson, C.H.; Paschal, D.C.; Ashley, D.L. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem. Toxicol. 2006, 44, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Polzin, G.M.; Watson, C.H.; Ashley, D.L. Cadmium, lead, and thallium in smoke particulate from counterfeit cigarettes compared to authentic US brands. Food Chem. Toxicol. 2007, 45, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Pavlickova, J.; Zbiral, J.; Smatanova, M.; Habarta, P.; Houserova, P.; Kuban, V. Uptake of thallium from naturally-contaminated soils into vegetables. Food Addit. Contam. 2006, 23, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, X.; Wang, J.; Xiao, T.; Chen, D.; Sheng, G.; Yin, M.; Lippold, H.; Wang, C.; Chen, Y. Thallium contamination in arable soils and vegetables around a steel plant-A newly-found significant source of Tl pollution in South China. Environ. Pollut. 2017, 224, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, N.; Zhang, W.; Wei, X.; Tsang, D.C.W.; Sun, Y.; Luo, X.; Bao, Z.; Zheng, W.; Wang, J.; et al. Thallium contamination in farmlands and common vegetables in a pyrite mining city and potential health risks. Environ. Pollut. 2019, 248, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Wang, Y.; Tsang, D.C.W.; Yang, X.; Beiyuan, J.; Yin, M.; Xiao, T.; Jiang, Y.; Lin, W.; et al. Emerging risks of toxic metal(loid)s in soil-vegetables influenced by steel-making activities and isotopic source apportionment. Environ. Int. 2021, 146, 106207. [Google Scholar] [CrossRef] [PubMed]
- Umweltqualität, F. Maximum Emission Values–Maximum Thallium Emission Values for Livestock (Richtlinie 2310 Blatt 29 (E)); Kommission Reinhaltung der Luft im VDI und DIN–Normenausschuss KRdL: Düsseldorf, Germany, 1998. [Google Scholar]
- Kazancioglu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, R.; He, P.; Zhang, Z.; Dai, Y.; Li, M.; Liu, Z.; Yang, H.; Guan, S.; Sun, J. Association between urinary metal concentrations and abnormal estimated glomerular filtration rate in Chinese community-dwelling elderly: Exploring the mediating effect of triglycerides. Ecotoxicol. Environ. Saf. 2023, 259, 114966. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, K.; Jiang, S.; Liu, D.; Zhou, H.; Zhong, R.; Zeng, Q.; Cheng, L.; Miao, X.; Tong, Y.; et al. Association of co-exposure to heavy metals with renal function in a hypertensive population. Environ. Int. 2018, 112, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Hu, B.; Meng, X.L.; Sun, L.; Li, H.B.; Xu, P.R.; Cheng, B.J.; Sheng, J.; Tao, F.B.; Yang, L.S.; et al. The associations between urinary metals and metal mixtures and kidney function in Chinese community-dwelling older adults with diabetes mellitus. Ecotoxicol. Environ. Saf. 2021, 226, 112829. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Vargas, G.G.; Silbergeld, E.K.; Rothenberg, S.J.; Fadrowski, J.J.; Rubio-Andrade, M.; Parsons, P.J.; Steuerwald, A.J.; Navas-Acien, A.; Guallar, E. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ. Res. 2014, 132, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.H.; Ke, D.Y.; Wang, J.E.; Chan, C.C. Associations between renal functions and exposure of arsenic and polycyclic aromatic hydrocarbon in adults living near a petrochemical complex. Environ. Pollut. 2020, 256, 113457. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003–2012. Environ Int 2018, 121, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Sabra, S.; Malmqvist, E.; Saborit, A.; Gratacos, E.; Gomez Roig, M.D. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLoS ONE 2017, 12, e0185645. [Google Scholar] [CrossRef] [PubMed]
- Ziskoven, R.; Achenbach, C.; Schulten, H.R.; Roll, R. Thallium determinations in fetal tissues and maternal brain and kidney. Toxicol. Lett. 1983, 19, 225–231. [Google Scholar] [CrossRef]
- Li, X.; Li, A.; Zhang, W.; Liu, X.; Liang, Y.; Yao, X.; Song, M. A pilot study of mothers and infants reveals fetal sex differences in the placental transfer efficiency of heavy metals. Ecotoxicol. Environ. Saf. 2019, 186, 109755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xia, W.; Zhang, B.; Pan, X.; Liu, W.; Jin, S.; Huo, W.; Liu, H.; Peng, Y.; Sun, X.; et al. Predictors of thallium exposure and its relation with preterm birth. Environ. Pollut. 2018, 233, 971–976. [Google Scholar] [CrossRef]
- Qi, J.; Lai, Y.; Liang, C.; Yan, S.; Huang, K.; Pan, W.; Feng, L.; Jiang, L.; Zhu, P.; Hao, J.; et al. Prenatal thallium exposure and poor growth in early childhood: A prospective birth cohort study. Environ. Int. 2019, 123, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shu, Y.; Song, L.; Liu, B.; Zhang, L.; Wang, L.; Liu, Y.; Bi, J.; Xiong, C.; Cao, Z.; et al. Prenatal exposure to thallium is associated with decreased mitochondrial DNA copy number in newborns: Evidence from a birth cohort study. Environ. Int. 2019, 129, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, L.; Song, L.; Liu, B.; Liu, Y.; Bi, J.; Liu, Q.; Chen, K.; Li, Y.; Xia, W.; et al. The association between prenatal exposure to thallium and shortened telomere length of newborns. Chemosphere 2021, 265, 129025. [Google Scholar] [CrossRef] [PubMed]
- Maitre, L.; Robinson, O.; Martinez, D.; Toledano, M.B.; Ibarluzea, J.; Marina, L.S.; Sunyer, J.; Villanueva, C.M.; Keun, H.C.; Vrijheid, M.; et al. Urine Metabolic Signatures of Multiple Environmental Pollutants in Pregnant Women: An Exposome Approach. Environ. Sci. Technol. 2018, 52, 13469–13480. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liang, C.; Yan, S.; Li, Z.; Huang, K.; Xia, X.; Hao, J.; Zhu, P.; Tao, F. Association between serum thallium in early pregnancy and risk of gestational diabetes mellitus: The Ma’anshan birth cohort study. J. Trace Elem. Med. Biol. 2019, 52, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Molavi, N.; Ghaderi, A.; Banafshe, H.R. Determination of thallium in urine, blood, and hair in illicit opioid users in Iran. Hum. Exp. Toxicol. 2020, 39, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; NasehGhafoori, P.; Rasouli-Azad, M.; Sehat, M.; Mehrzad, F.; Nekuei, M.; Aaseth, J.; Banafshe, H.R.; Mehrpour, O. Examining of Thallium in Cigarette Smokers. Biol. Trace Elem. Res. 2018, 182, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, S.; Amirabadizadeh, A.; Ataei, M.; Ataei, H.; Zardast, M.; Shariatmadari, M.R.; Mousavi-Mirzaei, S.M.; Mehrpour, O. Comparison of serum concentrations of essential and toxic elements between cigarette smokers and non-smokers. Environ. Sci. Pollut. Res. Int. 2021, 28, 37672–37678. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Li, Z.C.; Zhou, Y.; Dong, C.Y.; Kuang, H.X.; Zheng, T.; Xiang, M.D.; Chen, X.C.; Li, H.Y.; Zeng, X.W.; et al. Associations between trace level thallium and multiple health effects in rural areas: Chinese Exposure and Response Mapping Program (CERMP). Sci. Total Environ. 2023, 862, 160466. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, X.; Bai, Y.; Feng, W.; Wang, S.; Chen, Z.; Fu, W.; Li, G.; Chen, W.; Wang, G.; et al. Effect of thallium exposure and its interaction with smoking on lung function decline: A prospective cohort study. Environ. Int. 2019, 127, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental toxicants and autism spectrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Li, Y.; Liu, Y.; Zhao, Z. Blood Mercury, Arsenic, Cadmium, and Lead in Children with Autism Spectrum Disorder. Biol. Trace Elem. Res. 2018, 181, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Huang, D.; Xu, C.; Wang, J.; Jin, Y. Hair levels of heavy metals and essential elements in Chinese children with autism spectrum disorder. J. Trace Elem. Med. Biol. 2021, 66, 126748. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol. Trace Elem. Res. 2013, 151, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Howsmon, D.P.; Kruger, U.; Geis, E.; Gehn, E.; Fimbres, V.; Pollard, E.; Mitchell, J.; Ingram, J.; Hellmers, R.; et al. Significant Association of Urinary Toxic Metals and Autism-Related Symptoms-A Nonlinear Statistical Analysis with Cross Validation. PLoS ONE 2017, 12, e0169526. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef] [PubMed]
- Yorita Christensen, K.L. Metals in blood and urine, and thyroid function among adults in the United States 2007-2008. Int J Hyg Environ Health 2013, 216, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.A.; Elobeid, M.; Ruden, D.M.; Allison, D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int. J. Environ. Res. Public. Health 2010, 7, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Nishimoto, N. Thallium–poisoner’s poison: An overview and review of current knowledge on the toxicological effects and mechanisms. Curr. Res. Toxicol. 2024, 6, 100157. [Google Scholar] [CrossRef] [PubMed]
- Rusznyák, I.; György, L.; Ormai, S.; Millner, T. On some potassium-like qualities of the thallium ion. Experientia 1968, 24, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Rinklebe, J.; Shaheen, S.M.; El-Naggar, A.; Wang, H.; Du Laing, G.; Alessi, D.S.; Sik Ok, Y. Redox-induced mobilization of Ag, Sb, Sn, and Tl in the dissolved, colloidal and solid phase of a biochar-treated and un-treated mining soil. Environ. Int. 2020, 140, 105754. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Tan, S.W.; Cheng, C.J.; Chen, P.J. Revealing the toxicity of monovalent and trivalent thallium to medaka fish in controlled exposure conditions. Aquat. Toxicol. 2022, 250, 106258. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Cvjetko, I.; Pavlica, M. Thallium toxicity in humans. Arh. Hig. Rada Toksikol. 2010, 61, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Arzate, S.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Maldonado, P.D.; Vazquez-Roman, B.; Rios, C.; Santamaria, A. Delayed effects of thallium in the rat brain: Regional changes in lipid peroxidation and behavioral markers, but moderate alterations in antioxidants, after a single administration. Food Chem. Toxicol. 2005, 43, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Thallium Use, Toxicity, and Detoxification Therapy: An Overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, J.F.; Chen, P.J.; Huang, Y.T.; Liu, B.H. Thallium exposure interfered with heart development in embryonic zebrafish (Danio rerio): From phenotype to genotype. Sci. Total Environ. 2023, 878, 162901. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.R.; Mashayekhi, V.; Aslani, M.; Hosseini, M.J. Toxicity of thallium on isolated rat liver mitochondria: The role of oxidative stress and MPT pore opening. Environ. Toxicol. 2015, 30, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Eskandari, M.R.; Daraei, B. A comparison of hepatocyte cytotoxic mechanisms for thallium (I) and thallium (III). Environ. Toxicol. 2010, 25, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.R.; Pourahmad, J.; Daraei, B. Thallium(I) and thallium(III) induce apoptosis in isolated rat hepatocytes by alterations in mitochondrial function and generation of ROS. Toxicol. Environ. Chem. 2010, 93, 145–156. [Google Scholar] [CrossRef]
- Hanzel, C.E.; Verstraeten, S.V. Thallium induces hydrogen peroxide generation by impairing mitochondrial function. Toxicol. Appl. Pharmacol. 2006, 216, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.T.L.; Marotte, C.; Verstraeten, S.V. Epidermal growth factor prevents thallium(I)- and thallium(III)-mediated rat pheochromocytoma (PC12) cell apoptosis. Arch. Toxicol. 2017, 91, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, E.; Onor, M.; Colombaioni, L. Neurotoxicity Induced by Low Thallium Doses in Living Hippocampal Neurons: Evidence of Early Onset Mitochondrial Dysfunction and Correlation with Ethanol Production. ACS Chem. Neurosci. 2019, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Sun, Y.; Long, J.; Sui, X.; Yang, J.; Wang, Q.; Wang, S.; He, H.; Luo, Y.; Qiu, Z.; et al. Involvement of the Nrf2-Keap1 signaling pathway in protection against thallium-induced oxidative stress and mitochondrial dysfunction in primary hippocampal neurons. Toxicol. Lett. 2020, 319, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003, 23, 7198–7209. [Google Scholar] [CrossRef] [PubMed]
- Morel Gomez, E.; Casali, C.I.; Fernandez, M.D.C.; Verstraeten, S.V. Tl(I) and Tl(III) induce reticulum stress in MDCK cells. Environ. Toxicol. Pharmacol. 2023, 101, 104192. [Google Scholar] [CrossRef] [PubMed]
- Ozgul Ozalp, F.; Kutlu, M.; Iscan, A. The Effects of Thallium Acetate on Hepatopancreatic Cells of Gammarus pulex (Crustacea: Amphipoda). Ekoloji 2011, 20, 15–20. [Google Scholar] [CrossRef]
- Rodríguez-Mercado, J.J.; Mosqueda-Tapia, G.; Altamirano-Lozano, M.A. Genotoxicity assessment of human peripheral Lymphocytes induced by thallium(I) and thallium(III). Toxicol. Environ. Chem. 2017, 99, 987–998. [Google Scholar] [CrossRef]
- Rodriguez-Mercado, J.J.; Hernandez-de la Cruz, H.; Felipe-Reyes, M.; Jaramillo-Cruz, E.; Altamirano-Lozano, M.A. Evaluation of cytogenetic and DNA damage caused by thallium(I) acetate in human blood cells. Environ. Toxicol. 2015, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Alonzo, R.; Núñez-Tapia, F.A.; Moreno-Godínez, M.E.; Talavera-Mendoza, O.; Ortuño-Pineda, C.; Quintanilla-Vega, B.; Solís-Heredia, M.J.; Rodríguez-Mercado, J.J.; Urióstegui-Acosta, M. Effect of quality sperm and DNA damage by thallium exposure in mice mature sperm. Toxicol. Lett. 2016, 259, S232–S233. [Google Scholar] [CrossRef]
- Repetto, G.; Sanz, P.; Repetto, M. In vitro effects of thallium on mouse neuroblastoma cells. Toxicol. Vitr. 1994, 8, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, L.; Yao, H.; Su, Q.; Ye, J. Thallium exposure induces changes in B and T cell generation in mice. Toxicology 2023, 492, 153532. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.; Appenroth, D. Renal amino acid transport in immature and adult rats during thallium-induced nephrotoxicity. Toxicology 1996, 106, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, D.; Gambaryan, S.; Winnefeld, K.; Leiterer, M.; Bräunlich, H. Functional and morphological aspects of thallium-induced nephrotoxicity in rats. Toxicology 1995, 96, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Galván-Arzate, S.; Martínez, A.; Medina, E.; Santamaría, A.; Ríos, C. Subchronic administration of sublethal doses of thallium to rats: Effects on distribution and lipid peroxidation in brain regions. Toxicol. Lett. 2000, 116, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Barrera, L.; Rodriguez-Mercado, J.J.; Mateos-Nava, R.A.; Vazquez-Martinez, Y.; Altamirano-Lozano, M.A. Effect on the offspring of pregnant females CD-1 mice treated with a single thallium(I) application. Reprod. Toxicol. 2019, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).